Submitted:
05 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
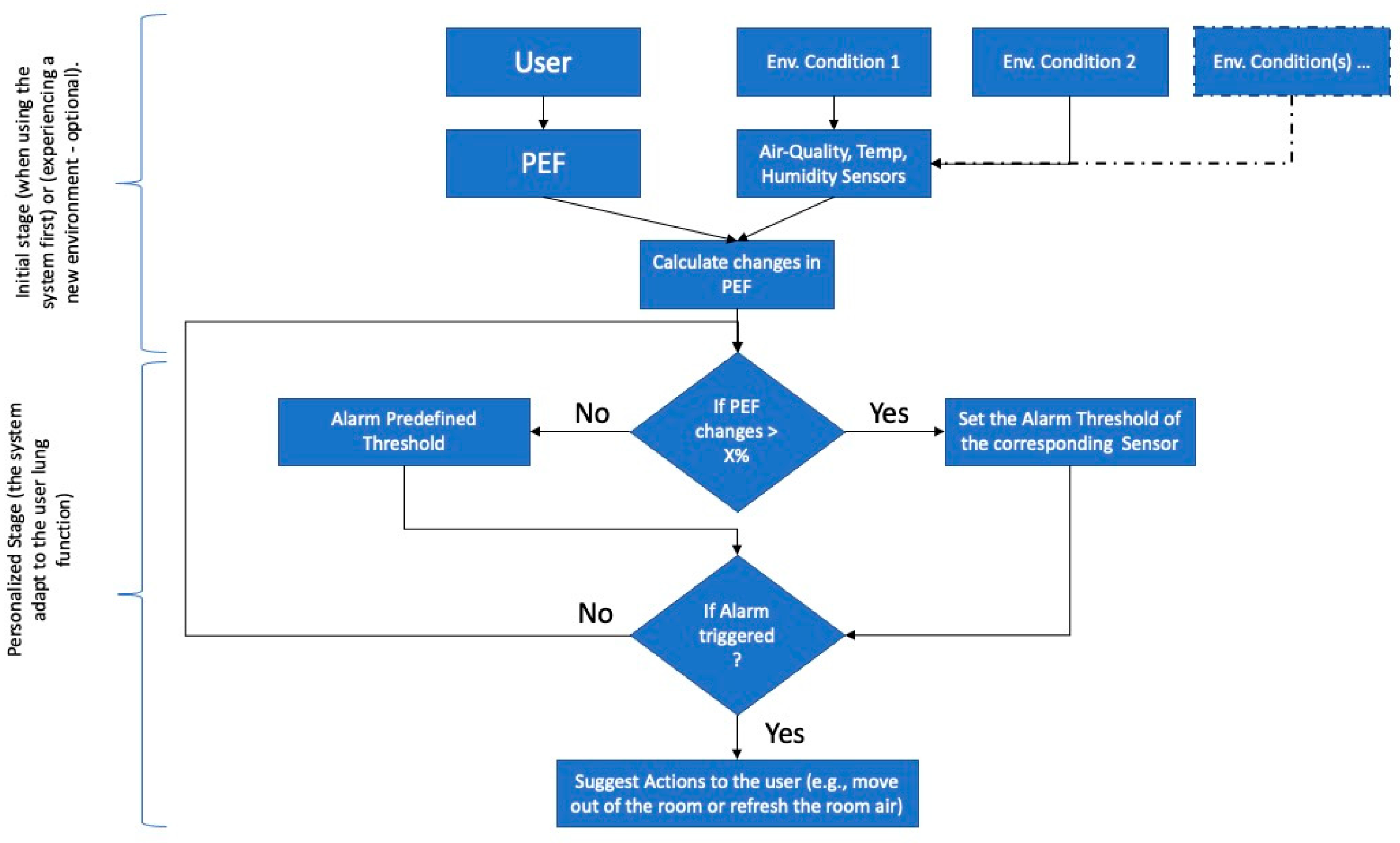
2. Methodology
2.1. System Overview
2.2. Air-Quality Sensors
2.3. Lungs Function Tester
2.4. Collection of Environmental Data and PEF
3. Results and Discussion
3.1. PEF in Standard Environment Condition
3.2. PEF in different Environment Condition
3.3. Personalized Threshold Identification
3.4. Predicting PEF Values in Different Environmental Conditions
3.5. Insights and Limitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global Strategy for Asthma Management and Prevention: GINA Executive Summary. European Respiratory Journal 2008, 31, 143–178. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. The Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- 2022 GINA Main Report. Available online: https://ginasthma.org/gina-reports/ (accessed on 4 July 2023).
- Ibrahim, N.M.; Almarzouqi, F.I.; Al Melaih, F.A.; Farouk, H.; Alsayed, M.; AlJassim, F.M. Prevalence of Asthma and Allergies among Children in the United Arab Emirates: A Cross-Sectional Study. World Allergy Organization Journal 2021, 14, 100588. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.A.; Kobeisy, S.A.N.; AlKhater, S.A.; Alharbi, A.S.; Alqwaiee, M.M.; Alotaibi, F.N.; Alawam, K.A.; Alahmadi, T.S.; Al-Somali, F.M.; Almaghamsi, T.M.; et al. Childhood Asthma Awareness in Saudi Arabia: Five-Year Follow-Up Study. Journal of Asthma and Allergy 2020, 13, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Tarraf, H.; Aydin, O.; Mungan, D.; Albader, M.; Mahboub, B.; Doble, A.; Lahlou, A.; Tariq, L.; Aziz, F.; El Hasnaoui, A. Prevalence of Asthma among the Adult General Population of Five Middle Eastern Countries: Results of the SNAPSHOT Program. BMC Pulm Med 2018, 18, 68. [Google Scholar] [CrossRef]
- Szefler, S.J.; Chipps, B. Challenges in the Treatment of Asthma in Children and Adolescents. Annals of Allergy, Asthma & Immunology 2018, 120, 382–388. [Google Scholar] [CrossRef]
- Janssens, T.; Ritz, T. Perceived Triggers of Asthma: Key to Symptom Perception and Management. Clinical & Experimental Allergy 2013, 43, 1000–1008. [Google Scholar] [CrossRef]
- L Miller, R. Trigger Control to Enhance Asthma Management - UpToDate. Available online: https://www.uptodate.com/contents/trigger-control-to-enhance-asthma-management (accessed on 27 May 2023).
- Alharbi, E.T.; Nadeem, F.; Cherif, A. Predictive Models for Personalized Asthma Attacks Based on Patient’s Biosignals and Environmental Factors: A Systematic Review. BMC Medical Informatics and Decision Making 2021, 21, 345. [Google Scholar] [CrossRef]
- Tibble, H.; Tsanas, A.; Horne, E.; Horne, R.; Mizani, M.; Simpson, C.R.; Sheikh, A. Predicting Asthma Attacks in Primary Care: Protocol for Developing a Machine Learning-Based Prediction Model. BMJ Open 2019, 9, e028375. [Google Scholar] [CrossRef]
- Ram, S.; Zhang, W.; Williams, M.; Pengetnze, Y. Predicting Asthma-Related Emergency Department Visits Using Big Data. IEEE J Biomed Health Inform 2015, 19, 1216–1223. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, J.C.-Y.; Tseng, V.S. A Novel Data Mining Mechanism Considering Bio-Signal and Environmental Data with Applications on Asthma Monitoring. Computer Methods and Programs in Biomedicine 2011, 101, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L.; Bacharier, L.B.; Bateman, E.; Boulet, L.-P.; Brightling, C.; Buhl, R.; Brusselle, G.; Cruz, A.A.; Drazen, J.M.; Duijts, L.; et al. Key Recommendations for Primary Care from the 2022 Global Initiative for Asthma (GINA) Update. npj Prim. Care Respir. Med. 2023, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Cardet, J.C.; Chung, K.F.; Diver, S.; Ferreira, D.S.; Fitzpatrick, A.; Gaga, M.; Kellermeyer, L.; Khurana, S.; Knight, S.; et al. Management of Severe Asthma: A European Respiratory Society/American Thoracic Society Guideline. European Respiratory Journal 2020, 55. [Google Scholar] [CrossRef]
- Singh, V.; Meena, P.; Sharma, B.B. Asthma-like Peak Flow Variability in Various Lung Diseases. Lung India 2012, 29, 15–18. [Google Scholar] [CrossRef]
- Merchant, R.K.; Inamdar, R.; Quade, R.C. Effectiveness of Population Health Management Using the Propeller Health Asthma Platform: A Randomized Clinical Trial. The Journal of Allergy and Clinical Immunology: In Practice 2016, 4, 455–463. [Google Scholar] [CrossRef]
- Fleming, L.; Murray, C.; Bansal, A.T.; Hashimoto, S.; Bisgaard, H.; Bush, A.; Frey, U.; Hedlin, G.; Singer, F.; van Aalderen, W.M.; et al. The Burden of Severe Asthma in Childhood and Adolescence: Results from the Paediatric U-BIOPRED Cohorts. Eur Respir J 2015, 46, 1322–1333. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.H. Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control. Diabetes Metab J 2023, 47, 27–41. [Google Scholar] [CrossRef]
- Borrelli, N.; Grimaldi, N.; Papaccioli, G.; Fusco, F.; Palma, M.; Sarubbi, B. Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients. Int J Environ Res Public Health 2023, 20, 5775. [Google Scholar] [CrossRef]
- Faragli, A.; Abawi, D.; Quinn, C.; Cvetkovic, M.; Schlabs, T.; Tahirovic, E.; Düngen, H.-D.; Pieske, B.; Kelle, S.; Edelmann, F.; et al. The Role of Non-Invasive Devices for the Telemonitoring of Heart Failure Patients. Heart Fail Rev 2021, 26, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Sarkar, S.; Martin, S.S. Digital Health Technology and Mobile Devices for the Management of Diabetes Mellitus: State of the Art. Diabetologia 2019, 62, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.; Greene, G.; MacHale, E.; Seheult, J.; Mokoka, M.; D’Arcy, S.; Taylor, T.; Murphy, D.M.; Hunt, E.; Lane, S.J.; et al. A Randomised Clinical Trial of Feedback on Inhaler Adherence and Technique in Patients with Severe Uncontrolled Asthma. Eur Respir J 2018, 51, 1701126. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Holgate, S.T.; Pawankar, R.; Ledford, D.K.; Cecchi, L.; Al-Ahmad, M.; Al-Enezi, F.; Al-Muhsen, S.; Ansotegui, I.; Baena-Cagnani, C.E.; et al. Meteorological Conditions, Climate Change, New Emerging Factors, and Asthma and Related Allergic Disorders. A Statement of the World Allergy Organization. World Allergy Organ J 2015, 8, 25. [Google Scholar] [CrossRef]
- Tiesler, C.M.T.; Thiering, E.; Tischer, C.; Lehmann, I.; Schaaf, B.; von Berg, A.; Heinrich, J. Exposure to Visible Mould or Dampness at Home and Sleep Problems in Children: Results from the LISAplus Study. Environ Res 2015, 137, 357–363. [Google Scholar] [CrossRef]
- Tiotiu, A.; Ioan, I.; Wirth, N.; Romero-Fernandez, R.; González-Barcala, F.-J. The Impact of Tobacco Smoking on Adult Asthma Outcomes. Int J Environ Res Public Health 2021, 18, 992. [Google Scholar] [CrossRef]
- Bush, A.; Fleming, L. Diagnosis and Management of Asthma in Children. BMJ 2015, 350, h996. [Google Scholar] [CrossRef]
- AG, M. PF 100 - Asthma Monitor. Available online: https://www.microlife.com/consumer-products/respiratory-care/digital-peak-flow-meter/pf-100 (accessed on 27 May 2023).
- WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Available online: https://www.who.int/publications-detail-redirect/9789240034228 (accessed on 5 July 2023).
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric Reference Values from a Sample of the General U.S. Population. Am J Respir Crit Care Med 1999, 159, 179–187. [Google Scholar] [CrossRef]
- A, H.M. Correlation-Based Feature Subset Selection for Machine Learning. Thesis submitted in partial fulfillment of the requirements of the degree of Doctor of Philosophy at the University of Waikato 1998. [Google Scholar]
- Shevade, S.K.; Keerthi, S.S.; Bhattacharyya, C.; Murthy, K.R.K. Improvements to the SMO Algorithm for SVM Regression. IEEE Transactions on Neural Networks 2000, 11, 1188–1193. [Google Scholar] [CrossRef]
- Lewis, S.A.; Weiss, S.T.; Britton, J.R. Airway Responsiveness and Peak Flow Variability in the Diagnosis of Asthma for Epidemiological Studies. Eur Respir J 2001, 18, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, O.F.C., Alvarez-Jimenez, R., de Grooth, HJ. et al. Breathing variability—implications for anaes-thesiology and intensive care. Crit Care 2021, 25, 280. [CrossRef] [PubMed]

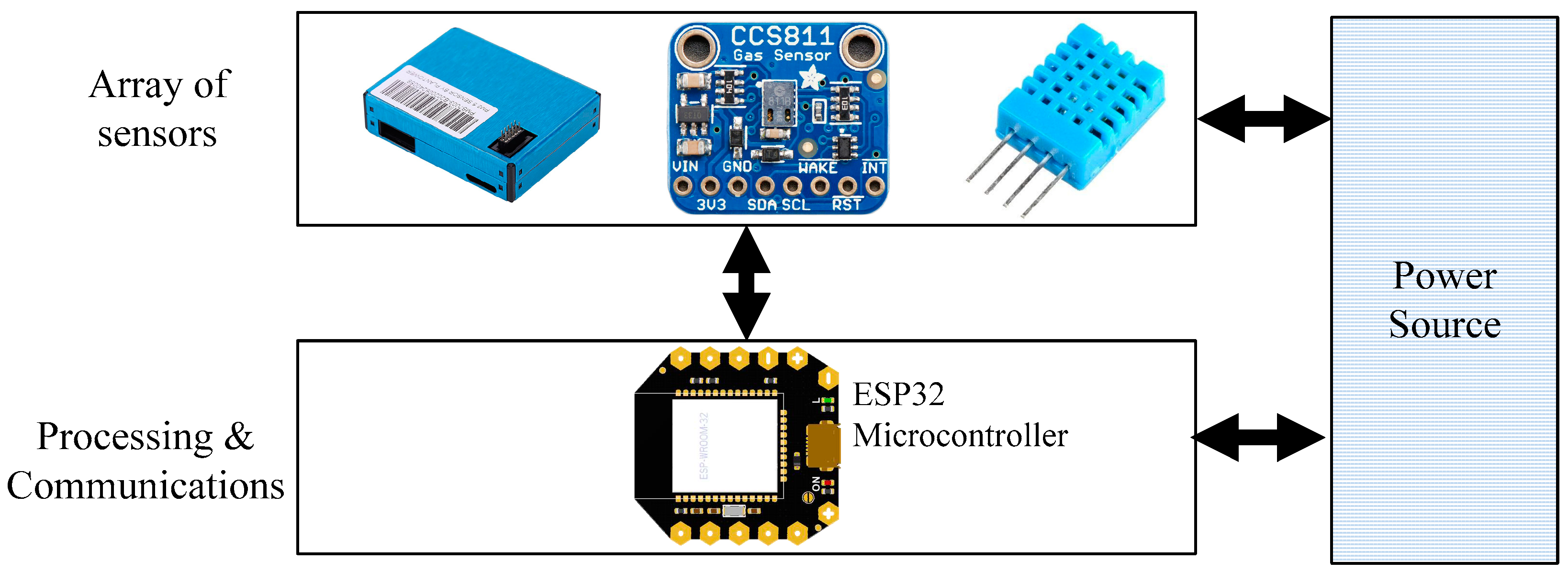
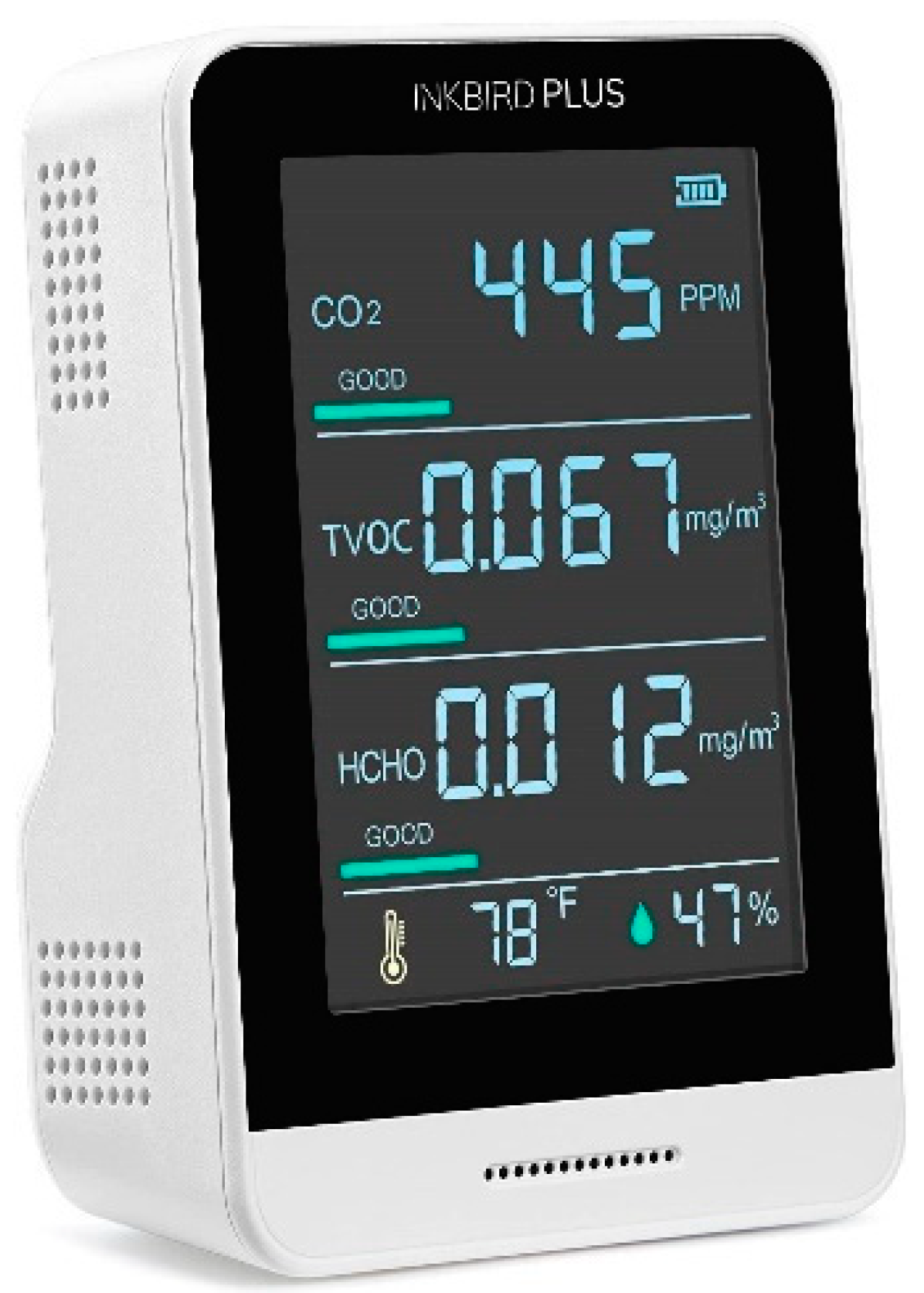
| Measured value | Sensor Model | Range of the customized Device | Range of InkbirdPlus (Commercial Device) |
Effect on human |
|---|---|---|---|---|
| Temperature | DHT11 | 0 – 50˚C | 0-99˚C | |
| Humidity | DHT11 | 20 – 80% | 20-99% | |
| CO2 | CCS811 module | 0 – 5,000 ppm | 350-2,000ppm | <500 (Normal); 500-1000 (Little uncomfortable); 1000-2500 (Tired); 2500-5000 (unhealthy) |
| TVOC | CCS811 module | 0 – 6,000 (PPM) | 0 – 2,000 (PPM) | <50 (Normal); 50-750 (Anxious, uncomfortable); 750-6000 (depressive, headache); >6000 (headache and other nerve problems) |
| Air-quality Sensor | PM2.5 module | 0 – 1,000 ug/m3 | 0-1,000 ug/m3) | < 12 μg/m³ (Good), 12.1 to 40.4 μg/m³ Slightly to Moderately Polluted, >40.0 μg/m³ (Seriously Polluted) |
| No. | Gender | Age | Hight | Weight |
|---|---|---|---|---|
| 1 | M | 23 | 168 | 78 |
| 2 | M | 22 | 170 | 76 |
| 3 | F | 23 | 162 | 58 |
| 4 | M | 24 | 171 | 77 |
| 5 | F | 24 | 157 | 59 |
| 6 | M | 23 | 169 | 75 |
| 7 | F | 25 | 160 | 58 |
| 8 | M | 23 | 174 | 79 |
| 9 | F | 23 | 161 | 63 |
| 10 | F | 24 | 159 | 59 |
| 11 | F | 22 | 156 | 61 |
| 12 | F | 23 | 163 | 60 |
| No | PEF(L/min) | PM10 (µg/m3) | PM2.5 (µg/m3) | TVOC (µg/m3) | CO2 (ppm) | Temperature (°C) | Humidity (%) |
|---|---|---|---|---|---|---|---|
| 1 | 520 | 15/13 | 8/8 | 270/290 | 600/580 | 21/21 | 45/44 |
| 2 | 580 | 20/18 | 10/11 | 300/280 | 650/630 | 22/22 | 50/50 |
| 3 | 450 | 18/15 | 9/10 | 280/285 | 700/670 | 23/23 | 55/55 |
| 4 | 560 | 22/20 | 9/8 | 320/300 | 750/720 | 24/24 | 60/60 |
| 5 | 455 | 17/17 | 9/9 | 260/300 | 800/760 | 20/20 | 42/42 |
| 6 | 550 | 20/21 | 10/10 | 280/268 | 750/730 | 23/23 | 44/44 |
| 7 | 420 | 22/22 | 9/10 | 260/260 | 730/780 | 24/24 | 50/50 |
| 8 | 550 | 20/20 | 9/8 | 300/290 | 690/700 | 23/23 | 50/49 |
| 9 | 445 | 18/16 | 9/11 | 280/300 | 660/700 | 24/24 | 52/52 |
| 10 | 450 | 19/20 | 8/9 | 260/290 | 650/680 | 20/20 | 44/44 |
| 11 | 435 | 18/20 | 8/9 | 280/290 | 660/670 | 23/23 | 48/48 |
| 12 | 445 | 19/19 | 9/10 | 300/290 | 680/680 | 23/23 | 45/45 |
| PEF | PM10 | PM2.5 | TVOC | CO2 | Temp. | Humidity | |
|---|---|---|---|---|---|---|---|
| R-squared values | 1.00 | 0.15 | 0.52* | 0.38 | 0.05 | 0.01 | 0.03 |
| PM2.5 (µg/m3) | Temperature (°C) | Humidity (%) | ||
|---|---|---|---|---|
| Env.1: Good AQ | Avg. | 9.09 | 22.45 | 49.09 |
| SD | 1.04 | 1.51 | 5.34 | |
| Env.2: Medium AQ | Avg. | 15.26 | 22.80 | 49.00 |
| SD | 0.5 | 0.79 | 1.98 | |
| Env.3: S_Hot-Temp | Avg. | 10 | 32.00 | 54 |
| SD | 1.67 | 1.42 | 1.08 | |
| Env.4: S_High-HM | Avg. | 8.5 | 36 | 67 |
| SD | 1.3 | 1.82 | 1.34 |
| Good AQ | Medium AQ | Hot-Temp | High-HM | Medium AQ | Hot-Temp | High-HM | |
|---|---|---|---|---|---|---|---|
| No. | PEF(L/min) | PEF(L/min) | PEF(L/min) | PEF(L/min) | PEF(L/min) Diff % | PEF(L/min) Diff % | PEF(L/min) Diff % |
| 1 | 520 | 512 | 515 | 516 | -1.5% | -1.0% | -0.8% |
| 2 | 580 | 567 | 569 | 578 | -2.3% | -1.9% | -0.3% |
| 3 | 450 | 428* | 446 | 448 | -4.9%* | -0.9% | -0.5% |
| 4 | 560 | 549 | 555 | 558 | -2.0% | -0.9% | -0.3% |
| 5 | 455 | 461 | 450 | 455 | -1.3% | -1.2% | -0.1% |
| 6 | 550 | 539 | 545 | 546 | -2.0% | -0.9% | -0.8% |
| 7 | 420 | 411 | 416 | 418 | -2.2% | -1.0% | -0.5% |
| 8 | 550 | 538 | 548 | 547 | -2.2% | -0.3% | -0.5% |
| 9 | 445 | 435 | 444 | 444 | -2.2% | -0.2% | -0.3% |
| 10 | 450 | 430* | 449 | 446 | -4.5%* | -0.3% | -0.8% |
| 11 | 435 | 426 | 432 | 432 | -2.0% | -0.6% | -0.6% |
| 12 | 445 | 436 | 441 | 441 | -2.0% | -0.9% | -0.8% |
| Input Attributes | Target Attribute | Attribute Evaluator | CfsSubsetEva | Selected attributes (ordered) | LR* | MLP* | SMOreg | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | RMSE | CC | RMSE | CC | RMSE | |||||
| 12 | PEF(L/min) | Search Method | BestFirst | PM2.5, Gender, Weight | 0.42 | 47.53 | 0.73 | 49.92 | 0.79 | 33.09 |
| 16 | PEF_M_AQ | Search Method | BestFirst | PEF(L/min), PM2.5_1, Condition, CO2 | 0.44 | 45.75 | 0.85 | 29.70 | 0.90 | 23.10 |
| 12 | PEF_M_AQ | Search Method | BestFirst | PEF(L/min), PM2.5_1, Condition, CO2 | 0.98 | 9.83 | 0.88 | 27.76 | 0.96 | 13.49 |
| *MLP = Multilayer Perceptron, *LR= Linear Regression | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





