Introduction
Recent advancements in DNA sequencing technologies have revolutionised our understanding of the plant microbiome, revealing its incredible diversity and the dynamic interactions between plants and their microbial inhabitants (Srivastava et al., 2022). The plant microbiome consists of diverse microorganisms, including bacteria, fungi, archaea, and viruses, that reside in and around plant tissues. Studies on model plants demonstrated bacteria and fungi's complex and highly specific composition in plant parts such as the rhizosphere, root endosphere, and aboveground tissues (Lundberg et al., 2012). Moreover, comparative studies across various plant species have revealed significant variations in the composition of root-associated microbial communities, indicating host-specific selection and assembly processes (Mendes et al., 2013).
The plant microbiome is not a static entity but undergoes dynamic changes influenced by various factors (Lebeis, 2014). Studies have shown that the plant's microbial community structure and composition change over time, indicating specific colonisation and succession patterns (Bulgarelli et al., 2013). Additionally, abiotic stresses such as drought, temperature extremes, and nutrient availability have been found to impact the dynamics of the plant microbiome (Berendsen et al., 2012). These dynamic interactions suggest a co-evolutionary relationship between plants and their microbial partners, wherein microbes adapt to changing environmental conditions and help the plant cope with stresses (Dubey et al., 2019).
Understanding the factors that drive the diversity of the plant microbiome is crucial for comprehending the functional roles of different microbial taxa in plant-microbe interactions. The diverse microbial communities associated with plants play a crucial role in shaping plant health, growth, and response to environmental stresses. They contribute to nutrient acquisition, hormone synthesis, pathogen defence, and tolerance to abiotic stresses (Levy et al., 2018). The interplay between the innate immunity of plants and the plant microbiota is an active area of research, providing insights into the mechanisms underlying plant-microbe interactions (Hacquard et al., 2017). This chapter emphasises the microbiome's effect on plant growth, development, stress response and overall health and vigour. The potential implications of microbiome research in promoting sustainable agriculture and developing innovative crop protection and development measures have also been highlighted.
1. Plant-Microbe Interactions:
Plants have evolved intricate mechanisms to interact with and harness the benefits of microorganisms in their surrounding environment (Van et al., 2008). These interactions can be mutualistic or antagonistic, and understanding their dynamics and outcomes is crucial for unravelling the intricate web of plant-microbe relationships. Mutualistic interactions between plants and microbes profoundly impact plant health and growth (Pieterse et al., 2014). For example, the association between plants and Arbuscular Mycorrhizal Fungi (AMF) enhances nutrient uptake, especially phosphorus, in exchange for carbohydrates provided by the plant. Similarly, nitrogen-fixing bacteria, such as Rhizobia, form symbiotic associations with legume plants, providing them with a source of fixed nitrogen while obtaining carbohydrates from the plant.
Table 1 highlights the plant microbiome interactions. Other examples include the association of plants with plant growth-promoting rhizobacteria (PGPR) that enhance nutrient availability, produce phytohormones, and suppress pathogens (Bulgarelli et al., 2012).
Plants also face constant challenges from pathogenic microbes in their environment, and they have evolved sophisticated defence mechanisms to combat these invaders. The plant immune system detects and responds to pathogenic microorganisms. Additionally, plants can induce systemic acquired resistance (SAR) or priming, where prior exposure to beneficial microbes or non-pathogenic microorganisms enhances the plant's defence against subsequent pathogen attacks (Mine et al., 2014).
Microbes can influence various aspects of plant physiology. They can enhance nutrient acquisition, promote root and shoot growth, and regulate plant hormonal balance (Yang et al., 2009). Furthermore, plant-associated microbes can modulate the plant's response to abiotic stresses, such as drought, salinity, and temperature extremes. They can also shape the plant's secondary metabolite production, influencing traits like flavour, aroma, and disease resistance.
1.1. Microbial Communities Associated with Plants:
The microbial communities associated with plants, including the rhizosphere, root endosphere, and aboveground parts, form complex and dynamic ecosystems (Peiffer et al., 2013). These communities consist of diverse microorganisms, including bacteria, fungi, archaea, and viruses, interacting with each other and the host plant. Combined or individual inoculation of these useful microbes have enhanced the nutrional and floral qualities in crops like Potato, Zinnia elegans, and Gazania rigens (Saini et al., 2019, 2020,2021). Understanding the composition and function of these microbial communities is essential for comprehending their roles in plant health, nutrient acquisition, and stress tolerance.
Several studies have employed high-throughput sequencing technologies, such as amplicon sequencing and metagenomics, to characterise the microbial communities associated with various plant species (Edwards et al., 2015). These studies have revealed distinct community structures and compositions across different plant compartments and species, suggesting host-specific factors' influence in shaping the microbiome.
1.2. Symbiotic relationship with plants (e.g., mycorrhizal fungi, nitrogen-fixing bacteria)
Plants form mutualistic symbiotic relationships with various microorganisms, such as mycorrhizal fungi and nitrogen-fixing bacteria, which play crucial roles in nutrient acquisition and plant growth (Hartman et al., 2017). Most plant species' roots form a symbiotic association with mycorrhizal fungus. facilitating the exchange of nutrients between the fungus and the plant (Schlaeppi et al., 2016). There are two main types of mycorrhizal associations: arbuscular mycorrhizal (AM) and ectomycorrhizal (ECM). Both associations enhance the plant's uptake of nutrients, particularly phosphorus, from the soil. The mycorrhizal fungi receive organic carbon compounds from the plant in return (Dhiman et al., 2022).
Nitrogen-fixing bacteria, such as rhizobia, establish a symbiotic relationship with leguminous plants, enabling them to transform atmospheric nitrogen into a form that the plant can use. The bacteria colonise the plant's root nodules, forming a specialised organ where nitrogen fixation occurs. This symbiotic relationship plays a vital role in enhancing the nitrogen status of plants and can have significant implications for sustainable agriculture and ecosystem functioning.
2. Influence of Microbiome Interaction with Plant Physiology
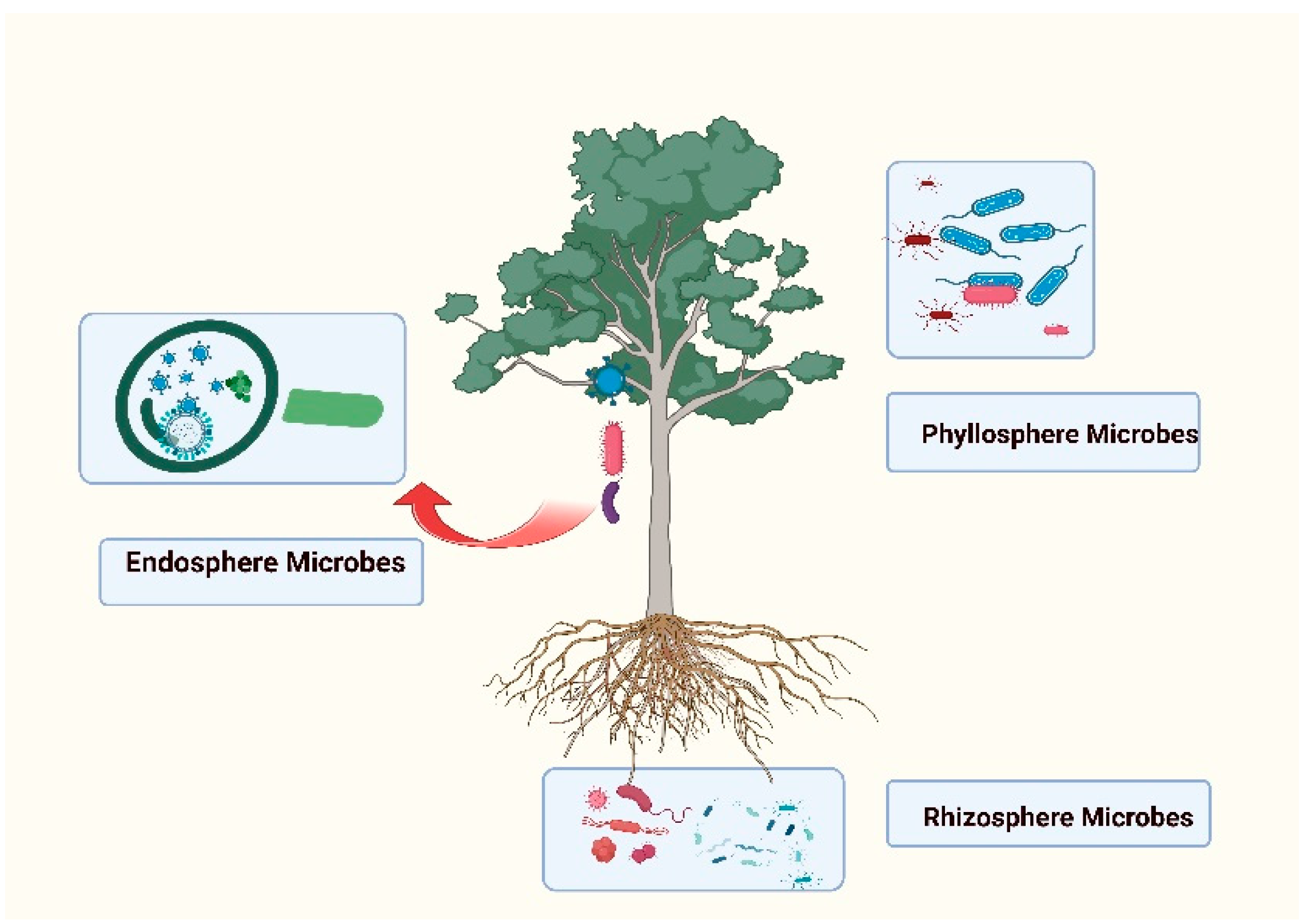
The relationship between the plant microbiome and plant physiology is complex and multifaceted. The plant microbiome, which refers to the community of microorganisms living in and around plant tissues, profoundly influences various aspects of plant physiology. (Colla et al., 2017). Throughout the evolution of life on Earth, it has become evident that microorganisms have formed close associations with plants, specifically with their various tissues and organs. The plant microbiome encompasses all the microorganisms that are associated with plants, including those residing on the surfaces of leaves (phyllosphere), in the soil surrounding the roots (rhizosphere), and within the plant's internal tissues (endosphere) (Srivastava et al., 2021).
Figure 1. Depicts the microbiome associated with the plants.
Rhizospheric Microbiome:
The rhizosphere refers to the soil region directly influenced by plant roots by releasing substances such as exudates, mucilage, and sloughed cells. These root exudates contain diverse compounds, including organic acids, sugars, amino acids, fatty acids, vitamins, growth factors, hormones, and antimicrobial substances. The rhizosphere provides a highly favourable environment for plant and microbial growth compared to the surrounding bulk soil (Alford et at., 2010).
The interactions and exchanges within the rhizosphere contribute to optimal plant growth and enhance soil health by facilitating the efficient cycling of various nutrients. The rhizosphere can be divided into three distinct layers:
- i
The endo-rhizosphere, the outermost plant root surface layer, exhibits intense microbial activities and nutrient transformations.
- ii
The rhizoplane represents the actual interface between the root and the soil. This intermediate zone is in direct contact with the root epidermis and the mucilage surrounding the root.
- iii
The ecto-rhizosphere is the rhizosphere's outermost layer that extends into the bulk soil. This region also influences microbial activities and nutrient dynamics, albeit to a lesser extent than the endo-rhizosphere (Berendsen et al., 2012).
Overall, these complex and dynamic interactions of the microbiome within the rhizosphere contribute to enhanced plant growth and the efficient cycling of nutrients, ultimately promoting the health and productivity of both plants and the surrounding soil ecosystem (Yadav et al., 2023).
Phyllospheric Microbiome:
The phyllosphere refers to the aboveground portion of plants with limited nutrient availability. The composition of the phyllospheric microbiome is influenced by various abiotic factors, including temperature changes, moisture levels, radiation, wind, and precipitation. (Bashir et al., 2022). Consequently, the phyllospheric microbiome exhibits greater dynamics than the microbiome found in the rhizosphere. Leaves secrete organic acids, sugars, and phytohormones through stomata, leaf hairs, and veins, which attract microbes and facilitate colonisation on the leaf surface. (Bringel & Couée 2015). It is estimated that approximately 107 microbes can be found per square centimetre on the leaf surface. By employing conventional culture-based approaches and advanced techniques, researchers have discovered that plant microbiome bacterial populations primarily comprise Actinobacteria, Proteobacteria, Firmicutes, Bacteroidetes, and occasionally cyanobacteria. (Chaudhary et al., 2017) Additionally, fungi and oomycetes are commonly found in these microbial communities. The microbes residing in the phyllosphere and rhizosphere are often called epiphytes.
Endospheric Microbiome:
The microorganisms that reside within the cells or between the cells of plant tissues are known as endophytes, and collectively they form the endophytic microbiome. Some endophytes spend their entire life cycle or a portion of it within the host plant. (Vandenkoornhuyse et al., 2015). The term "endophyte" encompasses a complex network of interactions, including interactions between the various microorganisms that make up the endophyte community and the plant's defence mechanisms that prevent these fungi from becoming pathogens (Gupta et al., 2021). Endophytes can colonise various plants' parts, such as stems, leaves, roots, and even seeds, without causing harm or infection to their host plant. Endophytic fungi belong to many different ecological and phylogenetic groups in a highly diverse environment. Endophytic microbes mostly belong to the Ralstonia, Burkholderia, Pseudomonas, Staphylococcus, Mesorhizobium, Propionibacterium, Dyella, Bacillus, and Ascomycetes; the rest are very rare (Compant et al., 2021).
The relationship between the plant microbiome and plant physiology is complex and varied. The plant microbiome, comprising microorganisms that inhabit plant tissues, plays a significant role in influencing various aspects of plant physiology. (Van et al., 2016). There are several physiological processes which get affected by plant microbiome, and a few of them are as follows;
2.1. Plant growth promotion
Plant Growth Promoting Microbes (PGPMs) is a subset of the plant microbiome. PGPMs are beneficial microorganisms that establish symbiotic or mutualistic relationships with plants, enhancing plant growth, development, and overall health. (Baig et al., 2012). PGPMs promote plant growth through multiple mechanisms. They can improve nutrient availability and uptake by fixing atmospheric nitrogen, solubilising phosphorus, and enhancing the release of other essential nutrients. Some PGPMs produce growth-promoting substances such as phytohormones (auxins, cytokinins, gibberellins) that stimulate plant growth and development. PGPMs play a crucial role in facilitating the uptake of mineral nutrients, such as nitrogen and phosphorus, by regulating plant physiological processes. Their utilisation, particularly in rhizobia inoculants, has shown promising results in improving crop productivity (Zaidi and Khan, 2005). However, further research is needed to enhance the understanding of the complex interactions within the rhizosphere and develop effective strategies for successful colonisation and establishment of inoculants in the plant microbiome.
Examples of plant growth-promoting microbes include:
- (i).
Rhizobium spp: These nitrogen-fixing bacteria form symbiotic associations with legume plants, converting atmospheric nitrogen into a form that plants can utilise.
- (ii).
Azospirillum spp: These bacteria colonise the root surface and enhance plant growth by producing growth-promoting substances such as auxins, cytokinins, and gibberellins.
- (iii).
Bacillus: Some species of Bacillus, such as Bacillus subtilis and Bacillus pumilus, promote plant growth by producing enzymes that solubilise phosphate, enhancing plant nutrient availability.
- (iv).
Trichoderma spp: These fungi are known for their biocontrol abilities, suppressing plant pathogens and promoting plant growth through the production of antifungal compounds and induction of systemic resistance in plants.
- (v).
Mycorrhizal fungi: These fungi form mutualistic associations with plant roots, increasing nutrient uptake, especially phosphorus, and improving plant tolerance to environmental stresses.
- (vi).
Pseudomonas spp: Certain species of Pseudomonas, like Pseudomonas fluorescens, have plant growth-promoting properties and can suppress plant diseases by producing antibiotics and siderophores (Turner et al.2013).
2.2. Nutrient mobilisation
Microorganisms in the plant microbiome play a crucial role in mobilising nutrients, particularly those that are less available or in complex forms in the soil (Peiffer et al., 2013). Certain microorganisms can solubilise minerals, such as phosphate-solubilising bacteria, which convert insoluble phosphorus into a plant-accessible form. Similarly, microbial activity in the rhizosphere can enhance the release of bound nutrients, such as iron and zinc, from soil particles, making them more accessible to plant roots (Fageria & Stone, 2006). This nutrient mobilisation by the microbiome increases plant nutrient availability, leading to improved nutrient uptake and utilisation (Saini et al., 2020).
2.3. Role in carbon sequestration
The plant microbiome contributes to carbon sequestration through various mechanisms. Microbes enhance plant productivity by facilitating nutrient uptake, nutrient cycling, and hormone regulation, leading to increased carbon assimilation through photosynthesis. Additionally, microbes play a role in the decomposition of plant residues, promoting soil organic matter formation and carbon sequestration. Carbon reserves in various ecosystems. In terrestrial environments, cultivable lands and microorganisms associated with plants play a vital role in absorbing atmospheric carbon and reserving it in the soil. (Lehmann & Kleber 2015). Plants contribute to carbon sequestration by releasing approximately 40% of the carbon produced through photosynthesis into the soil via their roots. Microbes in the soil then utilise this carbon for their growth, resulting in carbon storage in the primary soil carbon pool. The plant microbiome, comprising bacteria, fungi, archaea, and algae, contributes uniquely to carbon sequestration through various metabolic pathways.
Prokaryotic bacteria in the soil play an active role in capturing carbon by utilising the carbon released through plant root exudates. Soil fungi, characterised by their enduring hyphae, also contribute significantly to carbon storage, accounting for around 54-900 kg ha−1 of carbon. (Liang & Zhu 2021). Soil fungi produce glomalin, a soil protein that contributes 5% to soil carbon storage. However, improper agricultural practices can adversely affect soil carbon storage, impacting soil properties, microbial communities, and other soil components. (Grover et al. 2015). Minimising disturbances in arable land can enhance soil carbon storage and promote the proliferation of native plant-associated microbial communities, including autotrophic microbes in the leaf surface (phyllosphere) and within plant tissues (endophytes) that utilise atmospheric CO2. Despite its importance, the role of the plant microbiome in carbon sequestration remains largely unexplored.
2.4. Role in water use efficiency
The plant microbiome improves water use efficiency, especially under water-limited conditions (Agler et al., 2016). Certain microorganisms in the microbiome can enhance the root system's capacity to extract water from the soil, increase water uptake, and reduce water loss through transpiration. This is achieved through various mechanisms, including producing extracellular polysaccharides that improve soil water-holding capacity, synthesising plant hormones that regulate stomatal conductance, and forming biofilms that reduce water evaporation from the leaf surface. By enhancing water use efficiency, the microbiome helps plants cope with drought stress and optimise water resources for growth and development. The interaction between the plant microbiome and physiology is a complex process that can substantially impact plant growth, development, and overall physiological functions. The microbiome influences plant physiology through multiple mechanisms: nutrient acquisition, hormone regulation, stress tolerance, and defence responses (Malhi et al., 2021).
Microorganisms in the plant microbiome play a crucial role in enhancing nutrient availability and uptake by the plant (Liu et al., 2020). For example, mycorrhizal fungi form symbiotic associations with plant roots and improve nutrient uptake, particularly phosphorus. Additionally, some bacteria in the microbiome can solubilise minerals, fix atmospheric nitrogen, or enhance the availability of other essential nutrients, further benefiting plant nutrient acquisition.
The plant microbiome can influence plant physiology by modulating hormone levels and signalling pathways. Certain microorganisms produce or metabolise plant hormones, such as auxins, cytokinins, gibberellins, and abscisic acid, which regulate plant growth and development (Ansari, 2018). The microbiome can promote or inhibit hormone synthesis, affecting root elongation, shoot growth, flowering, and fruit development. Moreover, microbial-derived signals can trigger systemic responses in plants, leading to physiological changes beyond the site of microbial colonisation.
Microorganisms in the plant microbiome can enhance plant tolerance to various environmental stresses, including drought, salinity, pathogens, and pests. These stress-tolerant microorganisms can produce protective compounds, activate defence mechanisms, and promote stress-responsive gene expression in plants. For instance, certain bacteria and fungi produce antimicrobial compounds that inhibit pathogen growth, while others can induce systemic resistance in plants, priming them to respond more effectively to subsequent pathogen attacks.
The microbiome can shape plant immune responses and defence mechanisms against pathogens and pests (Teixeira et al., 2019). Beneficial microorganisms in the microbiome can trigger the plant's innate immune system, producing antimicrobial compounds, pathogenesis-related proteins, and activation of defence-related signalling pathways. The microbiome can also prime the plant's systemic acquired resistance, enabling a faster and stronger defence response upon subsequent pathogen encounters. Moreover, competition for resources and space within the microbiome can limit the colonisation and establishment of harmful pathogens.
3. Influence of Microbiome Interaction on Stress Tolerance
The influence of microbiome interaction on stress tolerance has been extensively studied and documented (Noman et al., 2021). The plant microbiome enhances plant tolerance to various abiotic stresses (Surówka et al., 2020). For example, the microbiome can alleviate nutrient stress by enhancing nutrient uptake and mobilisation through certain bacteria mechanisms, such as mycorrhizal associations and nutrient solubilisation (Pii et al., 2016). Hormone regulation by the microbiome, including the production or metabolism of stress-related hormones, is another important pathway for enhancing stress tolerance (Chaudhry & Sidhu, 2022). Signalling molecules produced by microorganisms in the microbiome, such as volatile organic compounds and small peptides, can activate stress tolerance mechanisms in plants (Stone et al., 2018). Additionally, the microbiome can activate plant defence responses, induce systemic resistance, and compete with harmful pathogens, thus enhancing biotic stress resistance (Ulrich et al., 2019).
3.1. Abiotic stress tolerance
The role of the microbiome in abiotic stress tolerance has been investigated for specific stresses such as drought, salinity, temperature extremes, and heavy metal toxicity (Balfagón et al., 2020). Under drought stress, the microbiome can improve water uptake and retention, regulate stomatal conductance, and promote the synthesis of osmoprotectants (Zolla et al., 2013). In the case of salinity stress, the microbiome contributes to salinity tolerance by promoting ion homeostasis, modulating ion transporters, and detoxifying salt-induced reactive oxygen species (ROS) (Verma et al., 2021). The microbiome also influences temperature stress tolerance by producing heat shock proteins and cryoprotective compounds (Ali et al., 2022). Moreover, the microbiome can alleviate heavy metal toxicity through metal immobilisation, chelation, and detoxification (Irfan et al., 2023). Understanding the role of the microbiome in stress tolerance provides valuable insights for developing strategies to enhance plant resilience. The potential application of the plant microbiome in mitigating abiotic stress and improving crop productivity has been recognised (Hussain et al., 2018). Harnessing the potential of the microbiome can lead to the development of sustainable agricultural practices and the cultivation of stress-tolerant crop varieties. The microbiome enhances plant tolerance to abiotic stresses, which are non-living factors that can negatively impact plant growth and productivity. Abiotic stresses include drought, salinity, temperature extremes, and heavy metal toxicity. The microbiome influences abiotic stress tolerance through various mechanisms, such as nutrient acquisition, hormone regulation, osmotic adjustment, antioxidant production, and modulation of stress-responsive genes (Stone et al., 2018).
3.1.1. Drought
Drought stress occurs when plants experience limited water availability, reducing growth and physiological activity (Zolla et al., 2013). The microbiome can enhance drought tolerance by improving water uptake and retention in the root zone. Certain microorganisms in the microbiome produce exopolysaccharides that increase soil water-holding capacity and enhance water availability to plants. Additionally, the microbiome can regulate plant stomatal conductance and promote the synthesis of osmoprotectants, such as proline and sugars, which help maintain cellular water balance and protect against dehydration. The bacterium Pseudomonas fluorescens produces the enzyme ACC deaminase, which breaks down ACC, an ethylene precursor. Ethylene is a plant hormone that promotes leaf abscission and senescence, which can lead to plant death under drought conditions. ACC deaminase can help plants maintain their water status and survive drought and its mechanism by reducing ethylene levels. Certain rhizobacteria, such as Bacillus and Pseudomonas species, have been found to promote drought tolerance in plants. These bacteria produce compounds like exopolysaccharides that improve soil structure and water-holding capacity, enhancing water retention around plant roots. They also induce the expression of stress-related genes in plants, triggering physiological responses that improve water use efficiency. Mycorrhizal fungi form mutualistic associations with plant roots, and they can enhance drought tolerance by improving water and nutrient uptake (Srivastava et al., 2022). The fungal hyphae extend the root system, increasing the surface area for water absorption. Additionally, mycorrhizal fungi can produce glomalin, a glycoprotein that helps bind soil particles together, improving soil structure and water retention. Endophytic fungi reside within plant tissues without causing harm and can confer drought tolerance to their host plants. These fungi can produce compounds that scavenge reactive oxygen species (ROS) produced under drought stress and can cause cellular damage. By reducing ROS levels, endophytic fungi help maintain plant cell integrity and enhance drought tolerance. Actinobacteria, such as Streptomyces species, are known for producing secondary metabolites with diverse functions. Some actinobacteria associated with plants produce drought-protective compounds, such as trehalose, which acts as an osmoprotectant and helps plants retain water during drought conditions. These bacteria can also produce enzymes that break down organic matter, releasing nutrients beneficial for plant growth under drought stress. Non-pathogenic fungi, such as Trichoderma species, have been shown to enhance drought tolerance in plants. They produce plant growth-promoting substances, including enzymes and phytohormones, which help plants cope with water scarcity. These fungi also induce systemic resistance in plants, priming them for better defence against drought stress.
It is important to note that the effectiveness of different microbial species in enhancing drought stress tolerance may vary depending on the plant species, soil conditions, and environmental factors.
3.1.2. Salinity
Salinity stress arises when plants encounter high salt levels in the soil, leading to osmotic stress and ion toxicity (Verma et al., 2021). The microbiome contributes to salinity tolerance by promoting ion homeostasis and osmotic adjustment. Some microorganisms can modulate ion transporters in plant roots, reducing the uptake of toxic ions like sodium (Na+) and enhancing the uptake of essential nutrients. Moreover, the microbiome can produce enzymes that detoxify salt-induced reactive oxygen species (ROS) and enhance antioxidant defence mechanisms, minimising oxidative damage caused by salinity stress. The fungus Aspergillus niger can produce osmolytes, such as proline and glycinebetaine, which help to protect plants from the effects of salinity. These osmolytes help maintain the plant cells' water balance and prevent them from plasmolysing. Halotolerant bacteria: Certain bacteria, such as Halomonas, Halobacillus, and Bacillus species, are known for tolerating high salinity conditions. These bacteria can colonise the plant roots and rhizosphere, promoting salt tolerance by producing enzymes that detoxify salt ions, facilitating the uptake of essential nutrients, and enhancing water and nutrient availability in saline soils. A study by Marasco et al. (2012) demonstrated the role of halotolerant bacteria in enhancing salt tolerance in wheat plants. Use of silicon alongwith Arbuscular mycorrhizal fungi (AMF) facilitated the growth of vegetable and fruits like watermelon in saline conditions (Bijalwan et al., 2021). AMF are known to form symbiotic associations with the roots of many plant species. These fungi are crucial in enhancing salt tolerance by improving nutrient uptake, particularly phosphorus, and facilitating water absorption. They also produce osmoprotectant compounds, such as trehalose, which help plants maintain cellular water balance under saline conditions. A study by Wu et al. (2019) demonstrated the positive effect of AMF colonisation on salt tolerance in tomato plants. Like drought, Some actinobacteria have been shown to enhance salt tolerance in plants through mechanisms such as the production of plant growth-promoting substances, modulation of plant hormone levels, and stress-related gene expression. A study by Nadeem et al. (2014) investigated the role of actinobacteria in promoting salt tolerance in canola plants. Some salt-tolerant yeasts, such as Saccharomyces cerevisiae and Candida versatile, can form beneficial associations with plant roots and improve salt tolerance. These yeasts promote plant growth, enhance nutrient uptake, and produce osmoprotectants that protect plant cells from salt-induced damage.
3.1.3. Temperature extremes
Temperature extremes, such as heat and cold stress, can disrupt plant physiological processes and impact growth and development. The microbiome plays a role in temperature stress tolerance by influencing plants' heat and cold acclimation responses. Certain microorganisms can produce heat shock proteins and other molecular chaperones that protect proteins from denaturation under heat stress (Balfagón et al., 2020). Similarly, the microbiome can enhance cold acclimation by modulating the synthesis of antifreeze proteins and other cryoprotective compounds, improving plant survival under freezing temperatures. The bacterium Bacillus subtilis produces the enzyme superoxide dismutase, which helps to scavenge reactive oxygen species (ROS). Plants under heat stress produce ROS, and they can damage plant cells. Superoxide dismutase helps to reduce ROS levels and protect plants from heat damage. Trichoderma fungi have been found to enhance plant tolerance to both heat and cold stresses. They do so by inducing the expression of stress-related genes in plants, producing protective compounds. Trichoderma spp. also promotes root growth and nutrient uptake, which can help plants withstand temperature extremes. The fungus Trichoderma virens produces the protein cryoprotectin, which helps to protect plant cells from cold damage. Cryoprotectins are proteins that help to prevent water from freezing inside plant cells. This is important because when water freezes, it expands and can damage plant cells. Pseudomonas fluorescens has been shown to enhance plant heat tolerance by producing a heat-shock protein called HspA. Heat-shock proteins act as molecular chaperones, properly folding proteins and preventing their denaturation under high temperatures. Pseudomonas fluorescens colonisation can lead to increased levels of HspA in plants, thereby improving their heat stress resilience. Some species of Arthrobacter bacteria have been found to enhance plant cold tolerance. They produce antifreeze proteins that lower the freezing point of plant tissues, protecting them from frost damage. Arthrobacter sp. can colonise plant surfaces and contribute to improved cold stress resilience.
3.1.4. Role in alleviating heavy metal toxicity
Heavy metal toxicity poses a significant threat to plant growth and ecosystem health. The microbiome can alleviate heavy metal toxicity by various mechanisms, including metal immobilisation, chelation, and detoxification (Irfan et al., 2023). Certain microorganisms in the microbiome can produce metal-chelating compounds, such as phytochelatins and organic acids, which sequester heavy metals and prevent their uptake by plants. Additionally, some microorganisms possess metal-oxidising or metal-reducing capabilities, facilitating the transformation of toxic metals into less harmful forms.
- (a).
Producing chelating agents: Some microbes produce chelating agents that can bind to heavy metals and make them less toxic. For example, the bacterium Pseudomonas fluorescens produces the chelating agent pyochelin, which can bind to lead and cadmium, thus, making the heavy metals less toxic to the plant and helping to prevent them from being absorbed by the roots.
- (b).
Detoxifying heavy metals: Some microbes can detoxify them by breaking them into less toxic forms. For example, Rhodococcus erythropolis can produce the enzyme metallothionein, which binds to heavy metals and prevents them from being toxic to the plant. This enzyme is particularly effective at detoxifying cadmium.
- (c).
Inducing systemic resistance: Some microbes can induce systemic resistance in plants, which helps them to defend themselves against the toxic effects of heavy metals. For example, the fungus Trichoderma harzianum can produce a compound that activates the plant's defence system and helps it to tolerate heavy metals. This compound helps the plant produce antioxidants that protect it from the damage caused by heavy metals.
- (a).
Changing the expression of metal transporter proteins: Some microbes can change the expression of metal transporter proteins in plants, which can help to reduce the uptake of heavy metals by the plant. For example, the bacterium Bacillus subtilis can produce a compound that reduces the expression of a metal transporter protein called ZIP1, which helps reduce the plant's cadmium uptake.
- (b).
Competition for nutrients: Microbes can compete with heavy metals for nutrients, such as iron and zinc and make it more difficult for heavy metals to be taken up by the plant
Numerous studies provide resolving metal toxicity via microbiomes like arbuscular mycorrhizal (AM) fungi; they enhance plant tolerance to heavy metals by sequestering metals within their mycelium, preventing their uptake by plant roots. For example, studies have shown that AM fungi, such as
Rhizophagus irregularis, can reduce the accumulation of heavy metals like cadmium (Cd) and lead (Pb) in plants. Pseudomonas bacteria have been found to alleviate heavy metal toxicity in plants. These bacteria possess metal-chelating properties and can produce siderophores, which are small molecules that bind to heavy metals and make them less available for plant uptake.
Pseudomonas putida, for instance, has been shown to reduce the toxicity of metals like copper (Cu) and zinc (Zn) in plants.
Bacillus subtilis is known for its plant growth-promoting abilities and potential to mitigate heavy metal toxicity. It can produce organic acids and enzymes that enhance the solubility and availability of nutrients, thus helping plants overcome metal-induced nutrient imbalances. It has improved plants' growth and metal tolerance to heavy metals like chromium (Cr) and nickel (Ni).
Saccharomyces cerevisiae has been extensively studied for its ability to immobilise heavy metals and reduce their toxicity in plants. It can sequester metals like cadmium (Cd) and copper (Cu) through adsorption onto its cell walls. When applied to contaminated soils, it can effectively reduce the bioavailability and uptake of heavy metals by plants.
Glomus intraradices (AMF) have been found to enhance plant tolerance to heavy metals such as arsenic (As). It forms a symbiotic association with plant roots and helps in the immobilisation and sequestration of arsenic, reducing its uptake and translocation within the plant. These examples illustrate the diverse mechanisms different microorganisms employ to alleviate heavy metal toxicity in plants.
Table 2 elaborates on the microbes utilised to combat plants' abiotic stress.
3.2. Biotic stress resistance
In addition to abiotic stresses, plants face challenges from various pathogens and pests. The microbiome can enhance plant resistance to biotic stresses by inducing systemic acquired resistance and producing antimicrobial compounds. Beneficial microorganisms in the microbiome can activate defence-related signalling pathways, producing pathogenesis-related proteins, phytoalexins, and other antimicrobial compounds that inhibit pathogen growth. The microbiome can compete with pathogens for resources and space, limiting their colonisation and establishment. Bacillus subtilis is a beneficial bacterium known to induce systemic resistance in plants. It activates plant defences by producing lipopeptides, such as surfactin and fengycin, which trigger immune responses and inhibit the growth of fungal pathogens like Fusarium spp. and Botrytis cinerea. Trichoderma species, such as Trichoderma harzianum, have been widely studied for their antagonistic activity against plant pathogens. They compete for nutrients and space, produce antifungal compounds like chitinases and β-1,3-glucanases, and parasitise other fungi, including pathogenic species like Rhizoctonia solani and Pythium spp. Indirect effects on pests contribute to biological control. For example, plants inoculated with entomopathogenic fungi like Beauveria bassiana attract predatory insects such as ladybugs and lacewings, which feed on aphids and other pests. These beneficial insects help regulate pest populations in an eco-friendly manner. Priming and tolerance induction: Some plant-associated bacteria, like Pseudomonas fluorescens, can prime plants for enhanced defence responses. P. fluorescens alongwith AMF effectively control Meloidogyne javanica infestation and increased nutrient absorption in eggplant species (Sharma et al., 2021, 2022). They activate defence-related genes, such as those that produce pathogenesis-related proteins and phytohormones like jasmonic acid and ethylene. This priming allows plants to respond more rapidly and effectively to subsequent pathogen attacks.
4. Role of microbiome interaction in improving plant health
The interaction between the microbiome and plants is crucial in improving plant health. The microbiome influences various aspects of plant physiology, nutrient availability, disease resistance, and stress tolerance, all of which contribute to overall plant health and productivity. Microorganisms in the plant microbiome participate in nutrient cycling processes, such as decomposition and mineralisation of organic matter, nutrient fixation, and nutrient solubilisation (Bhat et al., 2023). These activities help release nutrients to plants, promoting optimal nutrient uptake and utilisation. Improved nutrient availability contributes to healthy plant growth, development, and vigour. The microbiome can protect plants from diseases by preventing pathogen colonisation, inhibiting pathogen growth, and activating plant defence mechanisms. Beneficial microorganisms in the microbiome can compete with pathogens for resources and space, produce antimicrobial compounds, and trigger the plant's immune responses. This reduces the incidence and severity of plant diseases, improving plant health and reducing reliance on chemical pesticides.
Microorganisms in the plant microbiome can induce systemic resistance, a phenomenon in which the plant's defence responses are primed and activated, resulting in enhanced resistance against a broad range of pathogens. This priming effect can be achieved by producing signalling molecules, activating defence-related genes, and modulation of hormone signalling pathways. Induced systemic resistance improves the plant's ability to fend off pathogen attacks, even in distant tissues, improving plant health and disease resilience. Certain microorganisms in the microbiome produce growth-promoting substances, such as auxins, cytokinins, and gibberellins, which stimulate plant growth, root development, and overall plant vigour. These microorganisms can also regulate plant hormone levels, influencing various physiological processes, including flowering, fruiting, and senescence. Proper hormone regulation contributes to balanced plant growth, improved crop yield, and overall plant health. The microbiome can aid in the detoxification of harmful compounds, such as pollutants and xenobiotics, that can accumulate in the plant's tissues. Certain microorganisms can degrade or transform these compounds, reducing their toxicity and impact on plant health. This detoxification process contributes to the overall well-being of the plant and its ability to thrive in contaminated environments.
4.1. Bioinoculants and biofertiliser
Bioinoculants and bio fertilisers contain beneficial microorganisms or microbial consortia that are applied to plants or soils to enhance plant growth, nutrient uptake, and soil health. Bioinoculants typically consist of specific strains of bacteria, fungi, or other microorganisms that have proven beneficial effects on plant growth and nutrient availability. These are live microorganism consortia; when administered to plants or soil, they form a symbiotic or mutualistic connection with the plant, resulting in favourable impacts on plant growth and development (Santoyo et al.,2021).
Mycorrhizal fungi are extensively used as bio fertilisers for sustainable forestry and agroforestry practices. These fungi establish a symbiotic relationship with the roots of plants and form hyphae that extend into deep soil to absorb nutrients. They also enhance nutrient efficiency, as phosphorus absorption is difficult for plants due to its low solubility. These fungi increase the surface area and enhance nutrient absorption, thus acting as a good fertiliser. The recent area of research revolves around the Arbuscular mycorrhizal (AM) fungi which are widely dispersed soil fungi that create symbiotic relationships with most plants. They have been extensively explored in agriculture, horticulture, and forestry endeavours, as well as for environmental revitalisation to boost crop output and health while limiting the use of agrochemicals. AM fungi improve the soil structure and aeration as fungal hyphae promote soil aggregation and help in nutrient cycling and organic matter decomposition, thereby improving overall soil health (Nichols, 2008). They can induce systemic resistance in plants and protect them against pathogens. They help plants against drought stress by improving the water intake through their hyphal network. Nitrogen-fixing bacteria are potential microbiomes that stimulate plant health by providing a sustainable nitrogen source and reducing dependency on chemical fertilisers. Co-inoculation of AMF and Pseudomonas fluorescens produced synergistic effects in sesame crop as they enhanced the morphological and mineral content of this oil producing crop ( Yadav et al., 2021a). Similar similar were obtained by Yadav et al., (2021b) in carrot under saline conditions where nutrient uptake increased to several folds by using AMF and Pseudomonas fluorescens.
Biofertilisers and bioinoculants increase plant growth and yield by improving nutrient uptake, resilience to drought, pathogen suppression, and soil health. Plants inoculated with bioinoculants and bio fertilisers frequently have higher biomass, improved root development, and exacerbated overall vigour. Using bioinoculants and biofertilisers in agriculture can reduce the reliance on synthetic fertilisers and pesticides, improve soil fertility, and contribute to sustainable and environmentally friendly agricultural practices (Kour et al., 2020).
4.2. Microbiome engineering
The modification and engineering of microbial populations associated with plants to promote desired plant traits, such as increased nutrient use efficiency, disease resistance, and stress tolerance, is known as Microbiome engineering. This can be achieved by introducing beneficial microorganisms, manipulating existing microbial communities, or modifying microbial functions and interactions. Microbiome engineering techniques include the application of specific microbial inoculants, the use of prebiotics or probiotics to promote the growth of beneficial microorganisms, and the genetic modification of microorganisms to enhance desired traits.
It opens up new avenues for producing bioinoculants and biofertilisers that can boost plant development, intake of nutrients, resistance to infection, and stress tolerance (Afridi et al., 2022). Plant microbiomes can be altered to improve plant-microbe interactions by using synthetic microbial consortia, genetic engineering approaches, ecological modulation, and the integration of omics technology. Various strategies are employed to engineer the plants' microbiome, like genetic engineering approaches. Disease-resistant genes, stress-tolerance genes, secondary metabolite-producing genes, nitrogen-fixing genes and the genes which help in nutrient assimilation can be introduced or modified into the plants. Microbial inoculation, amendment of the soil, and plant-associated microbiome modifications can all be used to change the microbiome's composition and function in favour of intended plant-microbe encounters. The formulation and production of synthetic microbial consortia is another microbiome engineering method. These consortiums comprised selected microorganisms with complementary roles and associations with plants, making it possible to establish synergistic effects that improve plant performance by meticulously choosing and mixing helpful microorganisms. Phosphorus solubilisation, nitrogen fixation, plant hormones synthesis, and disease suppression are all possible using synthetic microbial consortia (Kenneth et al.,2019). Microbiome engineering also involves the alteration of existing microbe communities through ecological modulation. This strategy selectively enriches or decreases specific microbial populations to favour positive interactions with plants. Ensuring the stability and permanence of engineered microbial communities under various environmental circumstances, like addressing safety-related issues, ramping up production for the commercialisation process, and complying with regulatory requirements, are some of the key concerns in this regard. Microbiome engineering holds significant potential for improving plant health, enhancing crop productivity, and developing resilient agricultural systems (Kaul et al., 2021).
4.3. Food security and sustainable agriculture
Food security and agricultural sustainability are inextricably linked and mutually dependent concepts. Sustainable agriculture endorses the sustainable development and effective use of resources by using measures such as soil conservation, managing water resources, and agroforestry. This helps to sustain agricultural land efficiency and fertility, maintaining a constant food supply in the long term. Food security entails producing adequate food and promising equal distribution and accessibility. The microbiome associated with the crops plays a crucial role in ensuring food safety by improving the overall productivity of the crops (Sessitsch & Mitter, 2015). The microbiome can increase crop yields, reduce crop losses, and enhance food production by improving nutrient availability, disease resistance, and stress tolerance. Microbiome-based strategies, such as biofertilisers, bioinoculants, and microbiome engineering, can promote sustainable agriculture practices by reducing the reliance on chemical inputs, minimising environmental impacts, and improving soil health. Additionally, the microbiome's influence on plant resilience and adaptation to changing climatic conditions can help ensure food security in the face of climate change and other environmental challenges. Understanding and harnessing the potential of the microbiome in agriculture is critical for developing sustainable and resilient food systems that can meet the growing global demand for food while minimising environmental degradation (Chouhan et al., 2021).
5. Role of the Microbiome in enhancing plant resilience to climate change
The microbiome plays an significant role in boosting plant resilience to climate change, which is characterized by rising temperatures, shifting rainfall patterns, and an increase in the incidence of adverse weather conditions.The interactions between plants and microorganisms in the microbiome contribute to various adaptive mechanisms that help plants withstand and recover from the impacts of climate change. Here are some key roles of the microbiome in enhancing plant resilience to climate change:
Enhanced Stress Tolerance: The microbiome can improve plant tolerance to climate-related stresses, such as heatwaves, drought, and flooding. Beneficial microorganisms in the microbiome can enhance stress tolerance by improving water uptake and retention, regulating plant hormone levels, producing osmoprotectants, and activating stress-responsive genes. These mechanisms enable plants to maintain physiological functions and withstand the adverse effects of extreme temperatures and water availability, thus enhancing their resilience to climate change (Roy et al., 2021).
Nutrient Cycling and Adaptation: Microorganisms in the plant microbiome play a critical role in nutrient cycling and adaptation to changing environmental conditions. As climate patterns shift, the availability and cycling of nutrients in the soil can be affected. The microbiome facilitates nutrient acquisition and mobilisation by decomposing organic matter, solubilising minerals, and fixing atmospheric nitrogen. This nutrient cycling helps plants adapt to changing nutrient availability, ensuring their continued growth and productivity under varying climatic conditions (Philippot et al., 2013).
Disease Resistance: Climate change can alter the dynamics of plant diseases, leading to new disease outbreaks and increased susceptibility to pathogens. The microbiome contributes to plant disease resistance by promoting the growth of beneficial microorganisms that compete with pathogens for resources and produce antimicrobial compounds. Additionally, the microbiome can induce systemic acquired resistance, priming plants for enhanced defence responses against pathogens, which enhances the plant's ability to resist and recover from disease outbreaks, thus bolstering their resilience to climate-driven changes in pathogen dynamics (Velásquez et al., 2018).
Restoration of Disturbed Ecosystems: Climate change can exacerbate ecosystem disturbances, such as wildfires, hurricanes, and deforestation. The microbiome is critical in ecosystem restoration by facilitating plant establishment, nutrient cycling, and soil recovery. Beneficial microorganisms in the microbiome can aid in soil stabilisation, enhance soil fertility, and promote plant growth in disturbed areas. They contribute to reestablishing functional ecosystems and recovering vegetation following climate-related disturbances (Delgado et al., 2017).
Genetic Adaptation: The microbiome can influence the genetic adaptation of plants to changing climatic conditions. Microorganisms in the microbiome can interact with the plant's genetic machinery, influencing gene expression and regulating physiological processes. This interaction can facilitate selecting and expressing adaptive traits in response to climate change. Through epigenetic modifications and other regulatory mechanisms, the microbiome can contribute to the plant's ability to adapt and thrive under new environmental conditions (Chen et al., 2022).
Conclusion and the way forward
Over the past few years, plant microbiome research has made significant progress through collaborative efforts across various disciplines. Plants and their associated microbiome share an intricate relationship that helps plants sustain various environmental stress, leading to improved adaptation. Further research into microbiome interactions in the context of plant physiology and immunity to environmental stresses delivers an enormous opportunity for expanding our understanding of plant-microbe relationships and unleashing their potential for sustainable agriculture and resilient environments.
References
- Afridi, Muhammad Siddique, Javed, Muhammad Ammar, Ali, Sher, De Medeiros, Flavio Henrique Vasconcelos, Ali, Baber, Salam, Abdul, Sumaira,, Marc, Romina Alina, Alkhalifah, Dalal Hussien M., Selim, Samy, and et al. 2022. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Frontiers in Plant Science 13: 899464. [CrossRef]
- Agler, M. T., Ruhe, J., Kroll, S., Morhenn, C., Kim, S. T., Weigel, D., &Kemen, E. M. (2016). Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS biology, 14(1), e1002352. [CrossRef]
- Alford, É. R., Pilon-Smits, E. A., &Paschke, M. W. (2010). Metallophytes—a view from the rhizosphere. Plant and soil, 337, 33-50. [CrossRef]
- Ali, Sajad, Tyagi, Anshika, Park, Suvin, Mir, Rakeeb A., Mushtaq, Muntazir, Bhat, Basharat, Mahmoudi, Henda, and Hanhong Bae. 2022. Deciphering the plant microbiome to improve drought tolerance: Mechanisms and perspectives. Environmental and Experimental Botany, 201. [CrossRef]
- Ansari, M. I. (2018). Plant microbiome and its functional mechanism in response to environmental stress. International Journal of Green Pharmacy (IJGP), 12(01).
- Baig, Saeeda. 2012. Reviewing personal bacteria - the human microbiome project.., 22.
- Balfagón, Damián, Zandalinas, Sara I., Mittler, Ron, and Aurelio Gómez-Cadenas. 2020. High temperatures modify plant responses to abiotic stress conditions. Physiologia Plantarum 170: 335–344. [CrossRef]
- Balfagón, Damián, Zandalinas, Sara I., Mittler, Ron, and Aurelio Gómez-Cadenas. 2020. High temperatures modify plant responses to abiotic stress conditions. Physiologia Plantarum 170: 335–344. [CrossRef]
- Barea, J. M. (2000). Rhizosphere and mycorrhiza of field crops. In Biological resource management connecting Science and Policy (pp. 81-92). Berlin, Heidelberg: Springer Berlin Heidelberg.
- Bashir, I., War, A. F., Rafiq, I., Reshi, Z. A., Rashid, I., &Shouche, Y. S. (2022). Phyllosphere microbiome: Diversity and functions. Microbiological Research, 254, 126888. [CrossRef]
- Basu, Anirban, Prasad, Priyanka, Das, Subha Narayan, Kalam, Sadaf, Sayyed, R. Z., Reddy, M. S., and Hesham El Enshasy. 2021. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 13: 1140. [CrossRef]
- Berendsen, Roeland L., Pieterse, Corné M.J., and Peter A.H.M. Bakker. 2012. The rhizosphere microbiome and plant health. Trends in Plant Science 17: 478–486. [CrossRef]
- Bhardwaj, D., Ansari, M. W., Sahoo, R. K., &Tuteja, N. (2014). Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microbial cell factories, 13, 1-10. [CrossRef]
- Bhat, Mudasir Ahmad, Mishra, Awdhesh Kumar, Jan, Saima, Bhat, Mujtaba Aamir, Kamal, Mohammad Azhar, Rahman, Safikur, Shah, Ali Asghar, and Arif Tasleem Jan. 2023. Plant Growth Promoting Rhizobacteria in Plant Health: A Perspective Study of the Underground Interaction. Plants 12: 629. [CrossRef]
- Bijalwan, Priyanka, Jeddi, Kaouthar, Saini, Ishan, Sharma, Meenakshi, Kaushik, Prashant, and Kamel Hessini. 2021. Mitigation of saline conditions in watermelon with mycorrhiza and silicon application. Saudi Journal of Biological Sciences 28: 3678–3684. [CrossRef]
- Bringel, F., &Couée, I. (2015). Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Frontiers in microbiology, 6, 486. [CrossRef]
- Bulgarelli, Davide, Rott, Matthias, Schlaeppi, Klaus, van Themaat, Emiel Ver Loren, Ahmadinejad, Nahal, Assenza, Federica, Rauf, Philipp, Huettel, Bruno, Reinhardt, Richard, Schmelzer, Elmon, and et al. 2012. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488: 91–95. [CrossRef]
- Bulgarelli, Davide, Schlaeppi, Klaus, Spaepen, Stijn, van Themaat, Emiel Ver Loren, and Paul Schulze-Lefert. 2013. Structure and Functions of the Bacterial Microbiota of Plants. Annual Review of Plant Biology 64: 807–838. [CrossRef]
- Chaudhary, Deepika, Kumar, Rakesh, Sihag, Khushboo, and Anju Kumari. 2017. Phyllospheric microflora and its impact on plant growth: A review. Agricultural Reviews 38: 51–59. [CrossRef]
- Chaudhry, Smita, and Gagan Preet Singh Sidhu. 2021. Climate change regulated abiotic stress mechanisms in plants: a comprehensive review. Plant Cell Reports 41: 1–31. [CrossRef]
- Chen, Chen, Wang, Miao, Zhu, Jingzhi, Tang, Yongwei, Zhang, Hanchao, Zhao, Qiming, Jing, Minyu, Chen, Yahua, Xu, Xihui, Jiang, Jiandong, and et al. 2022. Long-term effect of epigenetic modification in plant–microbe interactions: modification of DNA methylation induced by plant growth-promoting bacteria mediates promotion process. Microbiome 10: 1–19. [CrossRef]
- Chouhan, Gowardhan Kumar, Verma, Jay Prakash, Jaiswal, Durgesh Kumar, Mukherjee, Arpan, Singh, Saurabh, Pereira, Arthur Prudêncio de Araujo, Liu, Hongwei, Abd_Allah, Elsayed Fathi, and Brajesh Kumar Singh. 2021. Phytomicrobiome for promoting sustainable agriculture and food security: Opportunities, challenges, and solutions. Microbiological Research 248: 126763. [CrossRef]
- Colla, G., Hoagland, L., Ruzzi, M., Cardarelli, M., Bonini, P., Canaguier, R., &Rouphael, Y. (2017). Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Frontiers in plant science, 8, 2202. [CrossRef]
- Compant, S., Cambon, M. C., Vacher, C., Mitter, B., Samad, A., &Sessitsch, A. (2021). The plant endosphere world–bacterial life within plants. Environmental Microbiology, 23(4), 1812-1829. [CrossRef]
- Delgado-Baquerizo, Manuel, Maestre, Fernando T., Reich, Peter B., Jeffries, Thomas C., Gaitan, Juan J., Encinar, Daniel, Berdugo, Miguel, Campbell, Colin D., and Brajesh K. Singh. 2016. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nature Communications 7: 10541–10541. [CrossRef]
- Dhiman, Mamta, Sharma, Lakshika, Kaushik, Prashant, Singh, Abhijeet, and Madan Mohan Sharma. 2022. Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature. Sustainability 14: 10220. [CrossRef]
- Diagne, N., Ngom, M., Djighaly, P. I., Fall, D., Hocher, V., &Svistoonoff, S. (2020). Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity, 12(10), 370. [CrossRef]
- Dubey, Anamika, Malla, Muneer Ahmad, Khan, Farhat, Chowdhary, Kanika, Yadav, Shweta, Kumar, Ashwani, Sharma, Satyawati, Khare, Pramod K., and Mohammad Latif Khan. 2019. Soil microbiome: a key player for conservation of soil health under changing climate. Biodiversity and Conservation 28: 2405–2429. [CrossRef]
- Edwards, Joseph, Johnson, Cameron, Santos-Medellín, Christian, Lurie, Eugene, Podishetty, Natraj Kumar, Bhatnagar, Srijak, Eisen, Jonathan A., and Venkatesan Sundaresan. 2015. Structure, variation, and assembly of the root-associated microbiomes of rice. Proceedings of the National Academy of Sciences of the United States of America 112: E911–E920. [CrossRef]
- Egamberdieva, D., Wirth, S., Jabborova, D., Räsänen, L. A., & Liao, H. (2017). Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. Journal of Plant Interactions, 12(1), 100-107. [CrossRef]
- Fageria, N. K., and L. F. Stone. 2006. Physical, Chemical, and Biological Changes in the Rhizosphere and Nutrient Availability. Journal of Plant Nutrition 29: 1327–1356. [CrossRef]
- Glick, Bernard R.. 2012. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012: 1–15. [CrossRef]
- Gonzalez-Chavez, C., D'Haen, Jan, Vangronsveld, J., and J.C. Dodd. 2002. Copper sorption and accumulation by the extraradical mycelium of different Glomus spp. (arbuscular mycorrhizal fungi) isolated from the same polluted soil. Plant and Soil 240: 287–297. [CrossRef]
- Gorbushina, Anna A.. 2006. Fungal activities in subaerial rock-inhabiting microbial communities., 267–288. [CrossRef]
- Grover, M., Maheswari, M., Desai, S., Gopinath, K. A., &Venkateswarlu, B. (2015). Elevated CO2: Plant associated microorganisms and carbon sequestration. Applied Soil Ecology, 95, 73-85. [CrossRef]
- Gupta, Rupali, Anand, Gautam, Gaur, Rajeeva, and Dinesh Yadav. 2021. Plant–microbiome interactions for sustainable agriculture: a review. Physiology and Molecular Biology of Plants 27: 165–179. [CrossRef]
- Hacquard, Stéphane, Spaepen, Stijn, Garrido-Oter, Ruben, and Paul Schulze-Lefert. 2017. Interplay Between Innate Immunity and the Plant Microbiota. Annual Review of Phytopathology 55: 565–589. [CrossRef]
- Hartman, K., van der Heijden, M. G., Roussely-Provent, V., Walser, J. C., &Schlaeppi, K. (2017). Deciphering composition and function of the root microbiome of a legume plant. Microbiome, 5(1), 1-13. [CrossRef]
- Hussain, Syed Sarfraz, Mehnaz, Samina, and Kadambot H. M. Siddique. 2018. Harnessing the Plant Microbiome for Improved Abiotic Stress Tolerance., 21–43. [CrossRef]
- Irfan, M., Aslam, H., Maqsood, A., Tazeen, S. K., Mahmood, F., & Shahid, M. (2023). Changes in Plant Microbiome in Response to Abiotic Stress. In Plant Microbiome for Plant Productivity and Sustainable Agriculture (pp. 99-119). Singapore: Springer Nature Singapore.
- Kaul, Sanjana, Choudhary, Malvi, Gupta, Suruchi, and Manoj K. Dhar. 2021. Engineering Host Microbiome for Crop Improvement and Sustainable Agriculture. Frontiers in Microbiology, 12. [CrossRef]
- Kenneth, O. C., Nwadibe, E. C., Kalu, A. U., &Unah, U. V. (2019). Plant growth promoting rhizobacteria (PGPR): a novel agent for sustainable food production. Am J Agric Biol Sci, 14(35), 54. [CrossRef]
- Kour, Divjot, Rana, Kusam Lata, Yadav, Ajar Nath, Yadav, Neelam, Kumar, Manish, Kumar, Vinod, Vyas, Pritesh, Dhaliwal, Harcharan Singh, and Anil Kumar Saxena. 2019. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatalysis and Agricultural Biotechnology 23: 101487. [CrossRef]
- Lau, S. E., Teo, W. F. A., Teoh, E. Y., & Tan, B. C. (2022). Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discover Food, 2(1), 9. [CrossRef]
- Lebeis, S. L. (2014). The potential for give and take in plant–microbiome relationships. Frontiers in plant science, 5, 287. [CrossRef]
- Lehmann, Johannes, and Markus Kleber. 2015. The contentious nature of soil organic matter. Nature 528: 60–68. [CrossRef]
- Levy, A., Salas Gonzalez, I., Mittelviefhaus, M., Clingenpeel, S., Herrera Paredes, S., Miao, J., &Dangl, J. L. (2018). Genomic features of bacterial adaptation to plants. Nature genetics, 50(1), 138-150. [CrossRef]
- Liang, Chao, and Xuefeng Zhu. 2021. The soil Microbial Carbon Pump as a new concept for terrestrial carbon sequestration. Science China Earth Sciences 64: 545–558. [CrossRef]
- Liu, Hongwei, Brettell, Laura E., Qiu, Zhiguang, and Brajesh K. Singh. 2020. Microbiome-Mediated Stress Resistance in Plants. Trends in Plant Science 25: 733–743. [CrossRef]
- Lundberg, D. S., Lebeis, S. L., Paredes, S. H., Yourstone, S., Gehring, J., Malfatti, S., &Dangl, J. L. (2012). Defining the core Arabidopsis thaliana root microbiome. Nature, 488(7409), 86-90. [CrossRef]
- Malhi, Gurdeep Singh, Kaur, Manpreet, Kaushik, Prashant, Alyemeni, Mohammed Nasser, Alsahli, Abdulaziz Abdullah, and Parvaiz Ahmad. 2021. Arbuscular mycorrhiza in combating abiotic stresses in vegetables: An eco-friendly approach. Saudi Journal of Biological Sciences 28: 1465–1476. [CrossRef]
- Marasco, R., Rolli, E., Ettoumi, B., Vigani, G., Mapelli, F., Borin, S., &Daffonchio, D. (2012). A drought resistance-promoting microbiome is selected by root system under desert farming. PloS one, 7(10), e48479. [CrossRef]
- Mendes, R., Garbeva, P., &Raaijmakers, J. M. (2013). The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS microbiology reviews, 37(5), 634-663. [CrossRef]
- Mine, A., Sato, M., & Tsuda, K. (2014). Toward a systems understanding of plant–microbe interactions. Frontiers in plant science, 5, 423. [CrossRef]
- Nadeem, Sajid Mahmood, Ahmad, Maqshoof, Zahir, Zahir Ahmad, Javaid, Arshad, and Muhammad Ashraf. 2014. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnology Advances 32: 429–448. [CrossRef]
- Naeem, A., Woertz, J. R., & Fein, J. B. (2006). Experimental measurement of proton, Cd, Pb, Sr, and Zn adsorption onto the fungal species Saccharomyces cerevisiae. Environmental science & technology, 40(18), 5724-5729. [CrossRef]
- Nichols, Kristine A.. 2008. Indirect Contributions of AM Fungi and Soil Aggregation to Plant Growth and Protection., 177–194. [CrossRef]
- Noman, Muhammad, Ahmed, Temoor, Ijaz, Usman, Shahid, Muhammad, Azizullah,, Li, Dayong, Manzoor, Irfan, and Fengming Song. 2021. Plant–Microbiome Crosstalk: Dawning from Composition and Assembly of Microbial Community to Improvement of Disease Resilience in Plants. International Journal of Molecular Sciences 22: 6852. [CrossRef]
- Pandit, A., Adholeya, A., Cahill, D., Brau, L., &Kochar, M. (2020). Microbial biofilms in nature: unlocking their potential for agricultural applications. Journal of applied microbiology, 129(2), 199-211. [CrossRef]
- Peiffer, Jason A., Spor, Aymé, Koren, Omry, Jin, Zhao, Tringe, Susannah Green, Dangl, Jeffery L., Buckler, Edward S., and Ruth E. Ley. 2013. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proceedings of the National Academy of Sciences of the United States of America 110: 6548–6553. [CrossRef]
- Philippot, Laurent, Raaijmakers, Jos M., Lemanceau, Philippe, and Wim H. van der Putten. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nature Reviews Microbiology 11: 789–799. [CrossRef]
- Pieterse, Corné M.J., Zamioudis, Christos, Berendsen, Roeland L., Weller, David M., Van Wees, Saskia C.M., and Peter A.H.M. Bakker. 2014. Induced Systemic Resistance by Beneficial Microbes. Annual Review of Phytopathology 52: 347–375. [CrossRef]
- Pii, Youry, Borruso, Luigimaria, Brusetti, Lorenzo, Crecchio, Carmine, Cesco, Stefano, and Tanja Mimmo. 2016. The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiology and Biochemistry 99: 39–48. [CrossRef]
- Podolich, O., Ardanov, P., Zaets, I., Pirttilä, A. M., &Kozyrovska, N. (2015). Reviving of the endophytic bacterial community as a putative mechanism of plant resistance. Plant and Soil, 388, 367-377. [CrossRef]
- Rajkumar, Mani, Ae, Noriharu, Prasad, Majeti Narasimha Vara, and Helena Freitas. 2010. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends in Biotechnology 28: 142–149. [CrossRef]
- Rolli, E., Marasco, R., Vigani, G., Ettoumi, B., Mapelli, F., Deangelis, M. L. &Daffonchio, D. (2015). Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environmental microbiology, 17(2), 316-331. [CrossRef]
- Roy, Swarnendu, Chakraborty, Arka Pratim, and Rakhi Chakraborty. 2021. Understanding the potential of root microbiome influencing salt-tolerance in plants and mechanisms involved at the transcriptional and translational level. Physiologia Plantarum 173: 1657–1681. [CrossRef]
- Saini, Ishan, Aggarwal, Ashok, and Prashant Kaushik. 2019. Inoculation with Mycorrhizal Fungi and Other Microbes to Improve the Morpho-Physiological and Floral Traits of Gazania rigens (L.) Gaertn. Agriculture 9: 51. [CrossRef]
- Saini, Ishan, Kaushik, Prashant, Al-Huqail, Asma A., Khan, Faheema, and Manzer H. Siddiqui. 2021. Effect of the diverse combinations of useful microbes and chemical fertilizers on important traits of potato. Saudi Journal of Biological Sciences 28: 2641–2648. [CrossRef]
- Saini, Ishan, Yadav, Vinod K, Aggarwal, Ashok, and Prashant Kaushik. 2020. Effect of superphosphate, urea and bioinoculants on Zinnia elegans Jacq.. Experiment 58: 730-737. [CrossRef]
- Santos-Medellín, Christian, Edwards, Joseph, Liechty, Zachary, Nguyen, Bao, and Venkatesan Sundaresan. 2017. Drought Stress Results in a Compartment-Specific Restructuring of the Rice Root-Associated Microbiomes. Mbio, 8. [CrossRef]
- Santoyo, Gustavo, Guzmán-Guzmán, Paulina, Parra-Cota, Fannie Isela, Santos-Villalobos, Sergio de Los, Orozco-Mosqueda, Ma. del Carmen, and Bernard R. Glick. 2021. Plant Growth Stimulation by Microbial Consortia. Agronomy 11: 219. [CrossRef]
- Schlaeppi, Klaus, Bender, S. Franz, Mascher, Fabio, Russo, Giancarlo, Patrignani, Andrea, Camenzind, Tessa, Hempel, Stefan, Rillig, Matthias C., and Marcel G. A. Heijden. 2016. High-resolution community profiling of arbuscular mycorrhizal fungi. New Phytologist 212: 780–791. [CrossRef]
- Sessitsch, A., &Mitter, B. (2015). 21st century agriculture: integration of plant microbiomes for improved crop production and food security. Microbial biotechnology, 8(1), 32.
- Sharma, Meenakshi, Delta, Anil K., Dhanda, Parmdeep S., Kaushik, Prashant, Mohanta, Yugal K., Saravanan, Muthupandian, and Tapan K. Mohanta. 2022. AMF and PSB applications modulated the biochemical and mineral content of the eggplants. Journal of Basic Microbiology 62: 1371–1378. [CrossRef]
- Sharma, Meenakshi, Saini, Ishan, Kaushik, Prashant, Aldawsari, Mona Mohammed, Al Balawi, Thamer, and Pravej Alam. 2021. Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi Journal of Biological Sciences 28: 3685–3691. [CrossRef]
- Srivastava, A. K., Das, A. K., Jagannadham, P. T. K., Bora, P., Ansari, F. A., &Bhate, R. (2022). Bioprospecting microbiome for soil and plant health management amidst huanglongbing threat in citrus: a review. Frontiers in Plant Science, 13, 858842.
- Srivastava, A. K., Kashyap, P. L., Srivastava, M., & Wiley, J. (Eds.). (2021). The Plant Microbiome in Sustainable Agriculture. Wiley Blackwell.
- Srivastava, Ashutosh, Sharma, Vijay Kumar, Kaushik, Prashant, El-Sheikh, Mohamed A., Qadir, Shaista, and Sheikh Mansoor. 2022. Effect of silicon application with mycorrhizal inoculation on Brassica juncea cultivated under water stress. PLoS ONE 17: e0261569. [CrossRef]
- Stone, Bram W. G., Weingarten, Eric A., and Colin R. Jackson. 2018. The Role of the Phyllosphere Microbiome in Plant Health and Function., 533–556. [CrossRef]
- Surówka, E., Rapacz, M., &Janowiak, F. (2020). Climate change influences the interactive effects of simultaneous impact of abiotic and biotic stresses on plants. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses, 1-50.
- Teixeira, P. J. P., Colaianni, N. R., Fitzpatrick, C. R., &Dangl, J. L. (2019). Beyond pathogens: microbiota interactions with the plant immune system. Current opinion in microbiology, 49, 7-17.
- Tiwari, Shalini, Prasad, Vivek, Chauhan, Puneet S., and Charu Lata. 2017. Bacillus amyloliquefaciens Confers Tolerance to Various Abiotic Stresses and Modulates Plant Response to Phytohormones through Osmoprotection and Gene Expression Regulation in Rice. Frontiers in Plant Science 8: 1510. [CrossRef]
- Turner, T. R., James, E. K., & Poole, P. S. (2013).. Genome biology, 14(6), 1-10.https://doi.org/10.1186/gb-2013-14-6-209.
- Ulrich, Danielle E. M., Sevanto, Sanna, Ryan, Max, Albright, Michaeline B. N., Johansen, Renee B., and John M. Dunbar. 2019. Plant-microbe interactions before drought influence plant physiological responses to subsequent severe drought. Scientific Reports 9: 1–10. [CrossRef]
- van der Heijden, Marcel G. A., Bardgett, Richard D., and Nico M. van Straalen. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters 11: 296–310. [CrossRef]
- Van Der Heijden, M. G., Bruin, S. D., Luckerhoff, L., Van Logtestijn, R. S., &Schlaeppi, K. (2016). A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. The ISME journal, 10(2), 389-399. [CrossRef]
- Vandenkoornhuyse, Philippe, Quaiser, Achim, Duhamel, Marie, Le Van, Amandine, and Alexis Dufresne. 2015. The importance of the microbiome of the plant holobiont. New Phytologist 206: 1196–1206. [CrossRef]
- Velásquez, André C., Castroverde, Christian Danve M., and Sheng Yang He. 2018. Plant–Pathogen Warfare under Changing Climate Conditions. Current Biology 28: R619–R634. [CrossRef]
- Verma, Hariom, Kumar, Dharmendra, Kumar, Vinod, Kumari, Madhuree, Singh, Sandeep Kumar, Sharma, Vijay Kumar, Droby, Samir, Santoyo, Gustavo, White, James F., and Ajay Kumar. 2021. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 9: 1729. [CrossRef]
- Verma, Hariom, Kumar, Dharmendra, Kumar, Vinod, Kumari, Madhuree, Singh, Sandeep Kumar, Sharma, Vijay Kumar, Droby, Samir, Santoyo, Gustavo, White, James F., and Ajay Kumar. 2021. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 9: 1729. [CrossRef]
- Vurukonda, S. S. K. P., Vardharajula, S., Shrivastava, M., &SkZ, A. (2016). Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiological research, 184, 13-24. [CrossRef]
- Wu, Qiang-Sheng, He, Jia-Dong, Srivastava, A K, Zhang, Fei, and Ying-Ning Zou. 2019. Development of propagation technique of indigenous AMF and their inoculation response in citrus. The Indian Journal of Agricultural Sciences, 89. [CrossRef]
- Yadav, Alpa, Batra, Divya, Kaushik, Prashant, and Tapan K. Mohanta. 2023. Abundance and distribution of arbuscular mycorrhizal fungi associated with oil-yielding plants. Journal of Basic Microbiology 63: 814–827. [CrossRef]
- Yadav, Alpa, Saini, Ishan, Kaushik, Prashant, Ansari, Mushtaq Ahmad, Khan, Mohammad Rashid, and Nazrul Haq. 2021. Effects of arbuscular mycorrhizal fungi and P-solubilizing Pseudomonas fluorescence (ATCC-17400) on morphological traits and mineral content of sesame. Saudi Journal of Biological Sciences 28: 2649–2654. [CrossRef]
- Yadav, Vinod Kumar, Jha, Radha Krishna, Kaushik, Prashant, Altalayan, Fahad H., Al Balawi, Thamer, and Pravej Alam. 2021. Traversing arbuscular mycorrhizal fungi and Pseudomonas fluorescens for carrot production under salinity. Saudi Journal of Biological Sciences 28: 4217–4223. [CrossRef]
- Yang, Jungwook, Kloepper, Joseph W., and Choong-Min Ryu. 2009. Rhizosphere bacteria help plants tolerate abiotic stress. Trends in Plant Science 14: 1–4. [CrossRef]
- Zaidi, Almas, and Saghir Khan. 2005. Interactive Effect of Rhizotrophic Microorganisms on Growth, Yield, and Nutrient Uptake of Wheat. Journal of Plant Nutrition 28: 2079–2092. [CrossRef]
- Zolla, G., Badri, D. V., Bakker, M. G., Manter, D. K., &Vivanco, J. M. (2013). Soil microbiomes vary in their ability to confer drought tolerance to Arabidopsis. Applied soil ecology, 68, 1-9. [CrossRef]
- Zolla, G., Badri, D. V., Bakker, M. G., Manter, D. K., &Vivanco, J. M. (2013). Soil microbiomes vary in their ability to confer drought tolerance to Arabidopsis. Applied soil ecology, 68, 1-. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).