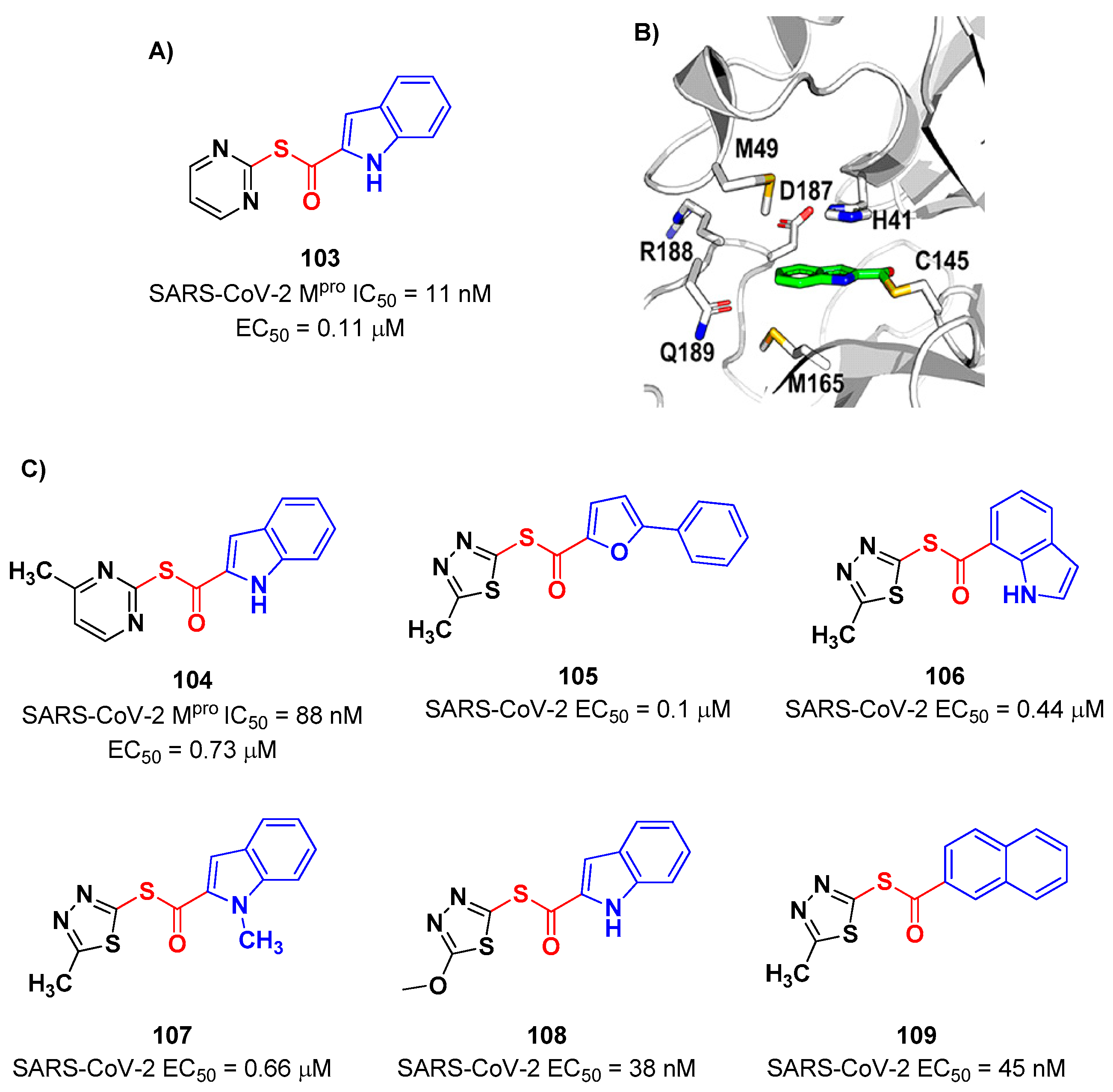
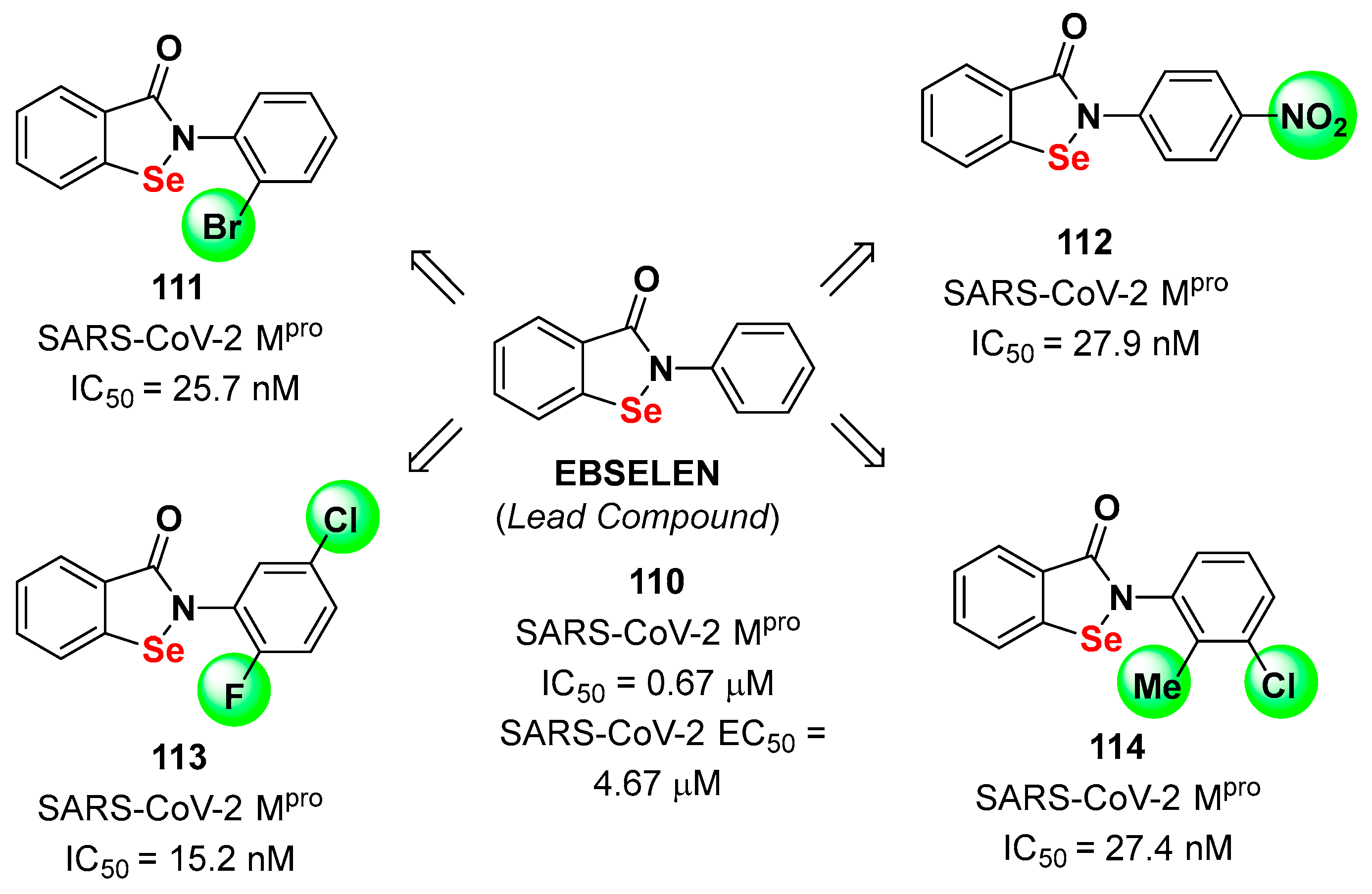
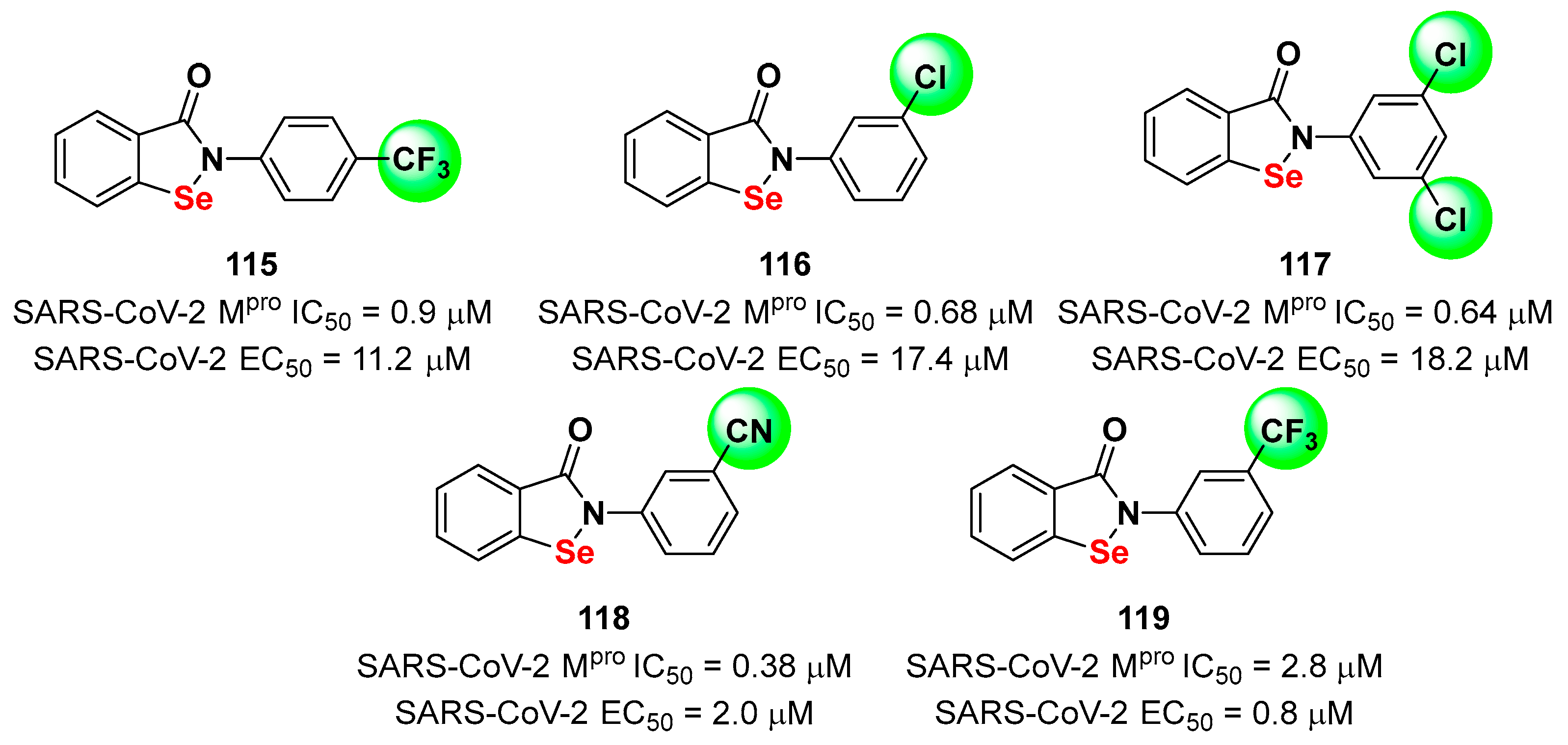
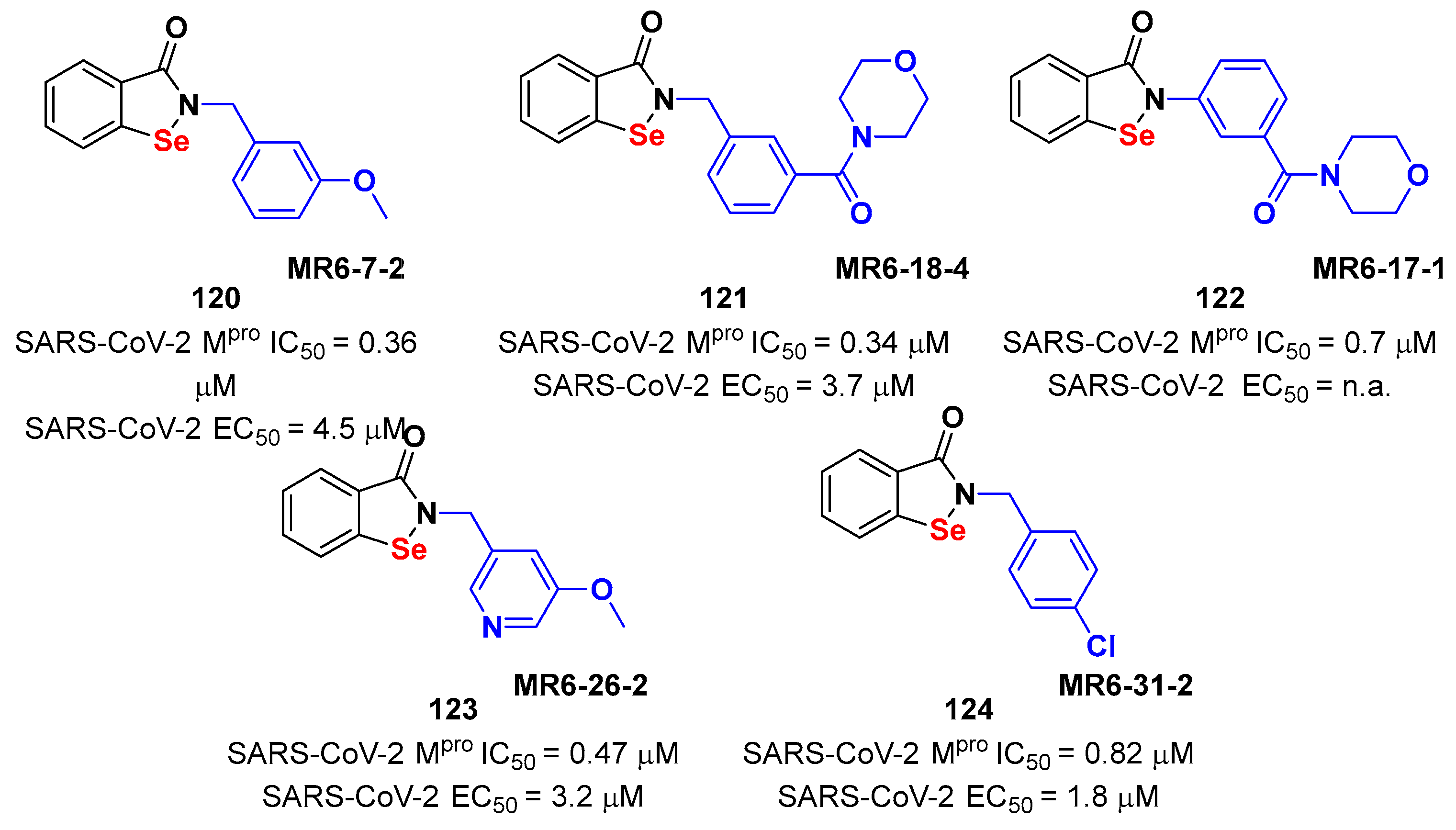
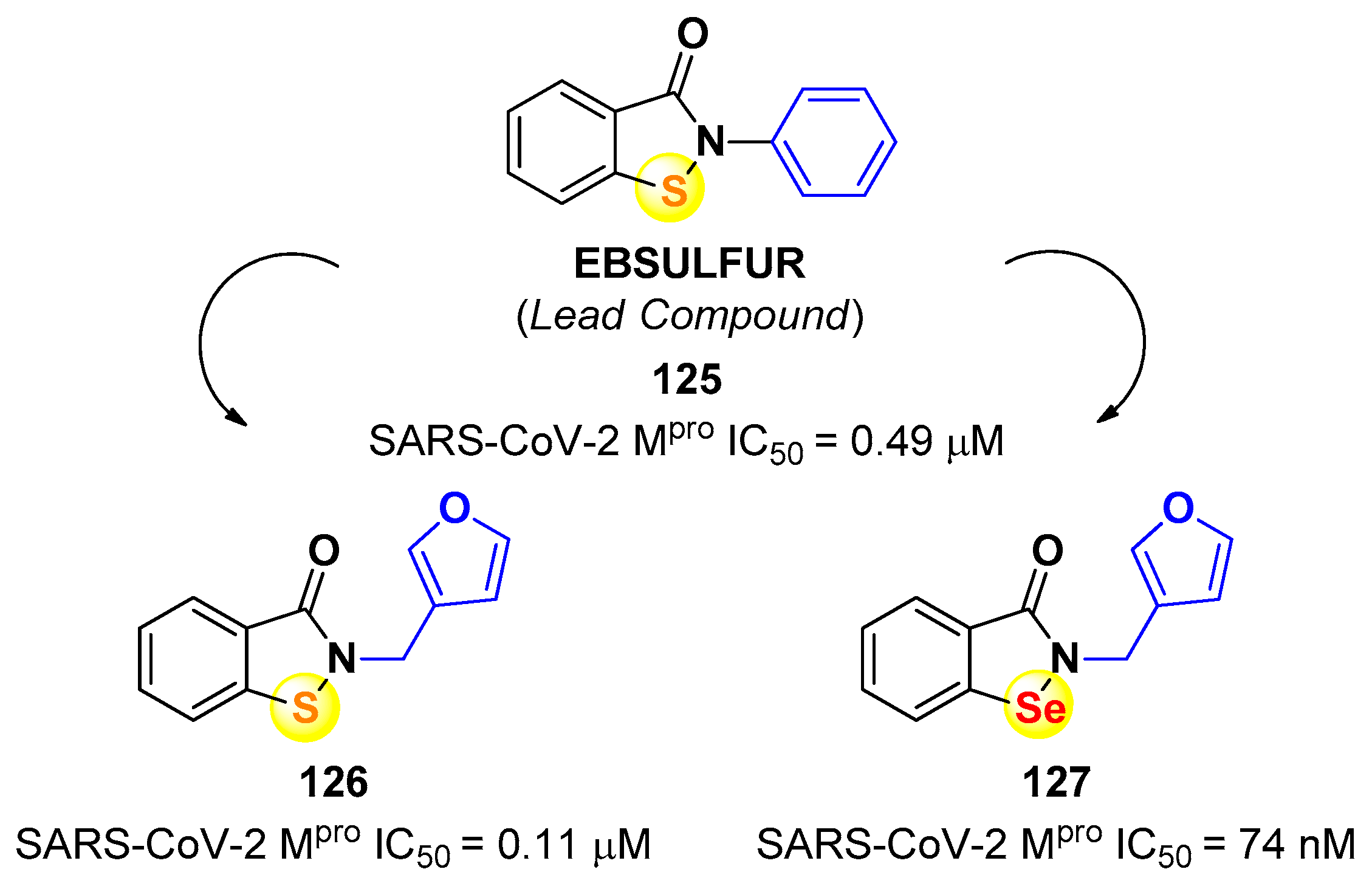
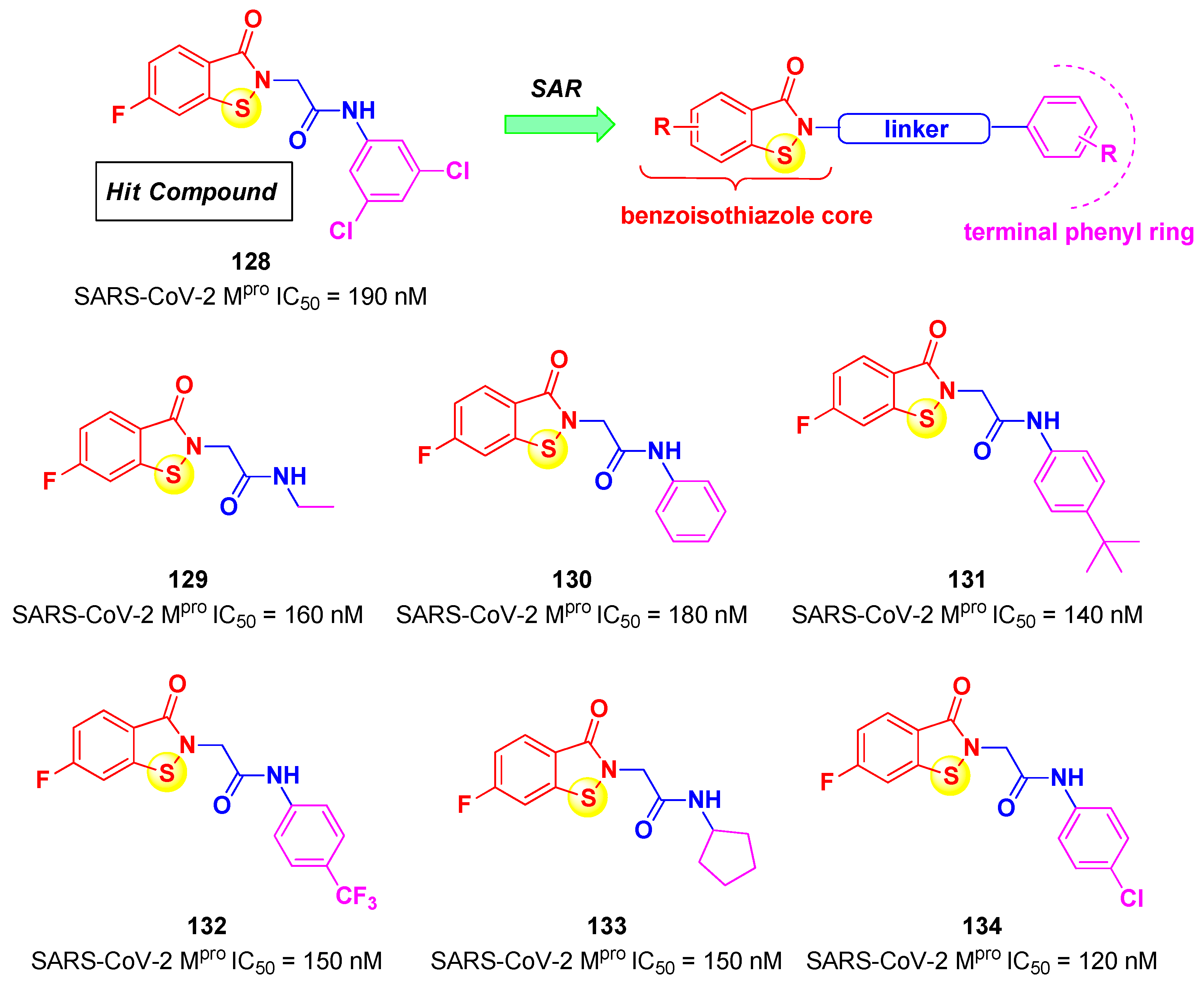
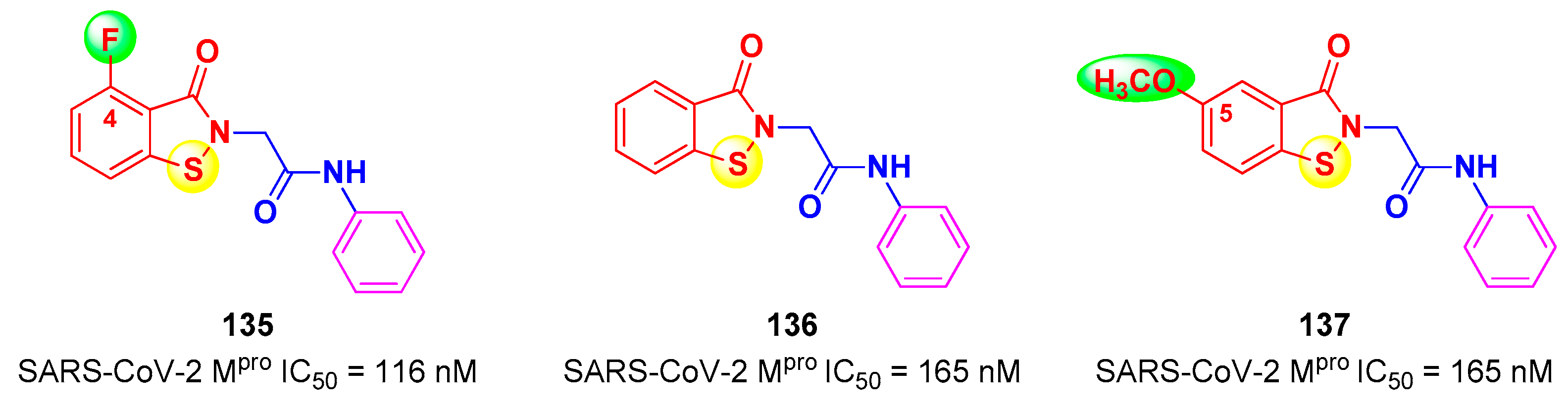
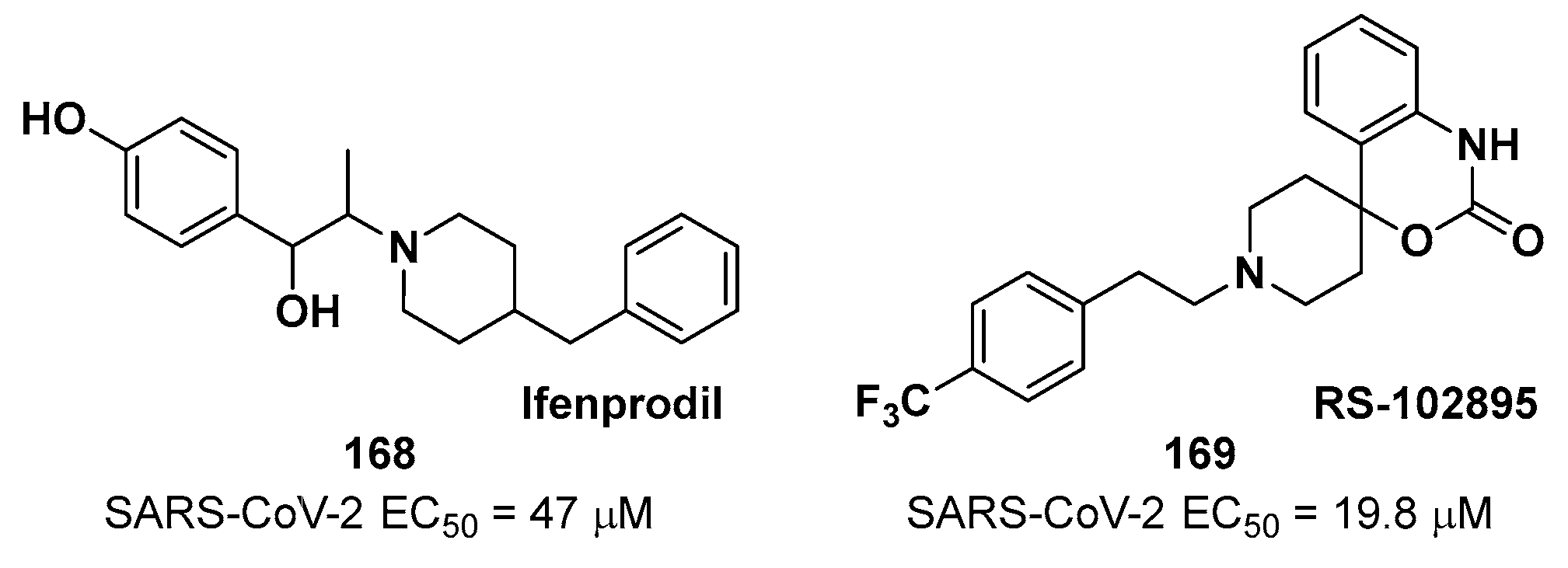
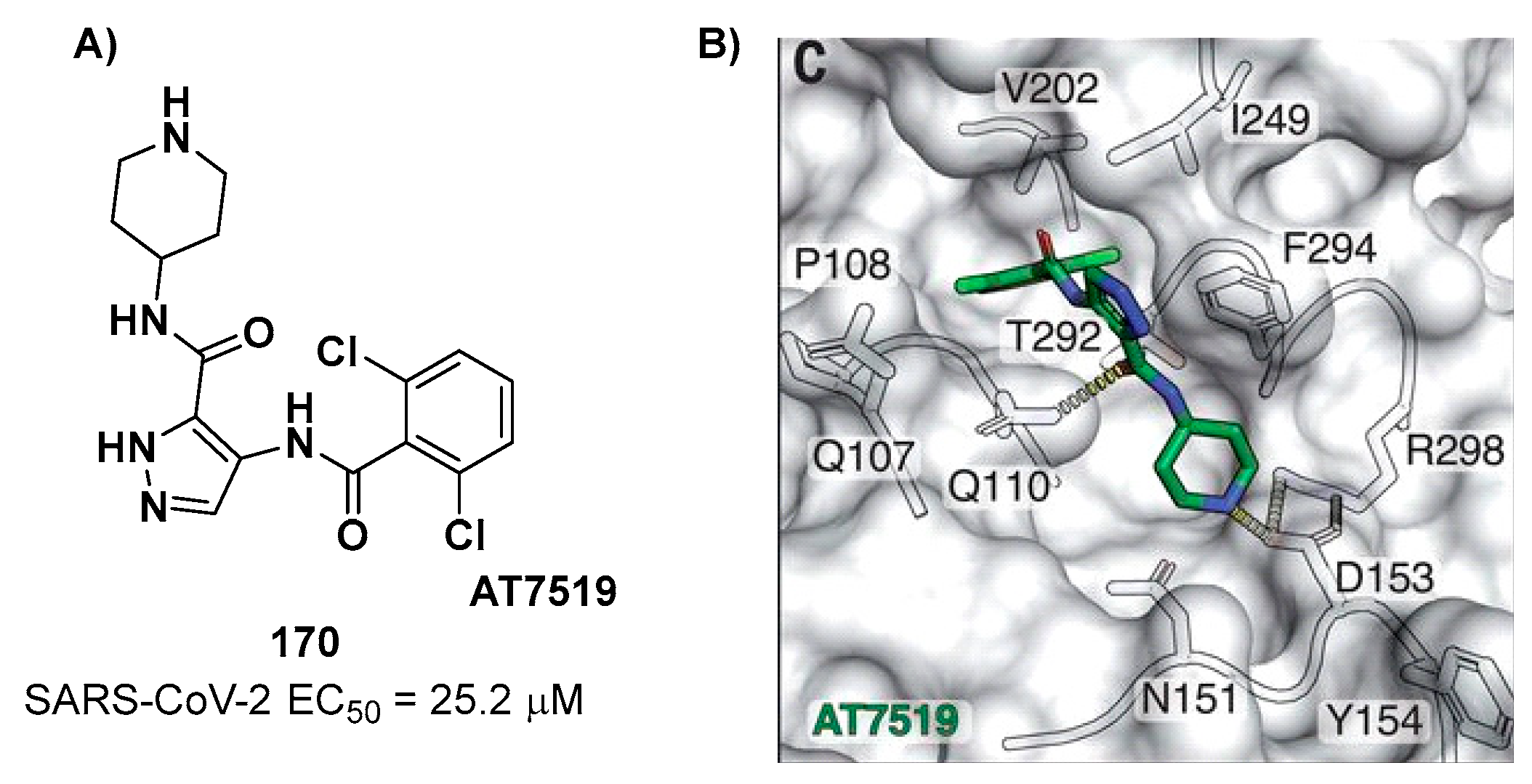
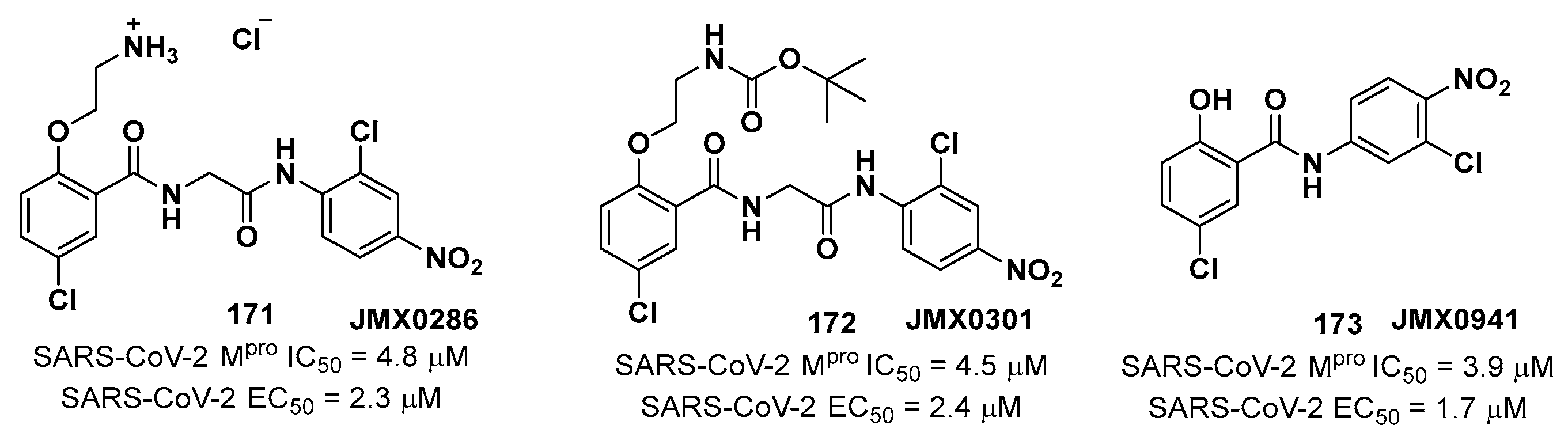
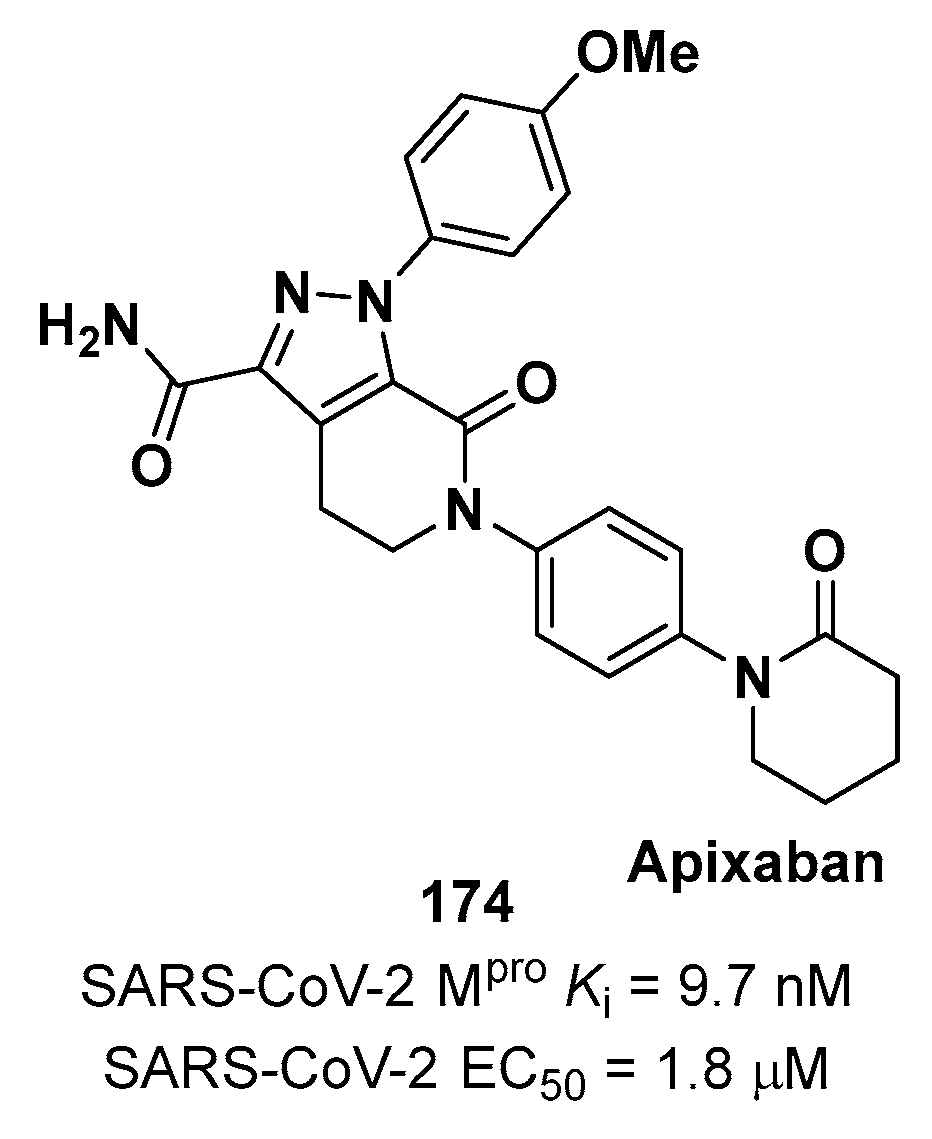
4. Nitriles and Nirmatrelvir
The nitrile warhead has a moderate electrophilic character. The electron-poor carbon of the nitrile group is able to undergo nucleophilic attack from the thiol group of the SARS-CoV-2 M
pro Cys145 residue to afford a reversible covalent thioimidate adduct [
16]. In 2013, Chuck
et al. demonstrated that nitrile-based peptidomimetics have broad-spectrum inhibition towards human coronaviruses M
pro [
23]. This function has been taken into consideration by Pfizer’s scientists to develop the first oral drug for the treatment of COVID-19) [
24]. This drug contains a SARS-CoV-2 M
pro bearing a nitrile warhead in P1’ called
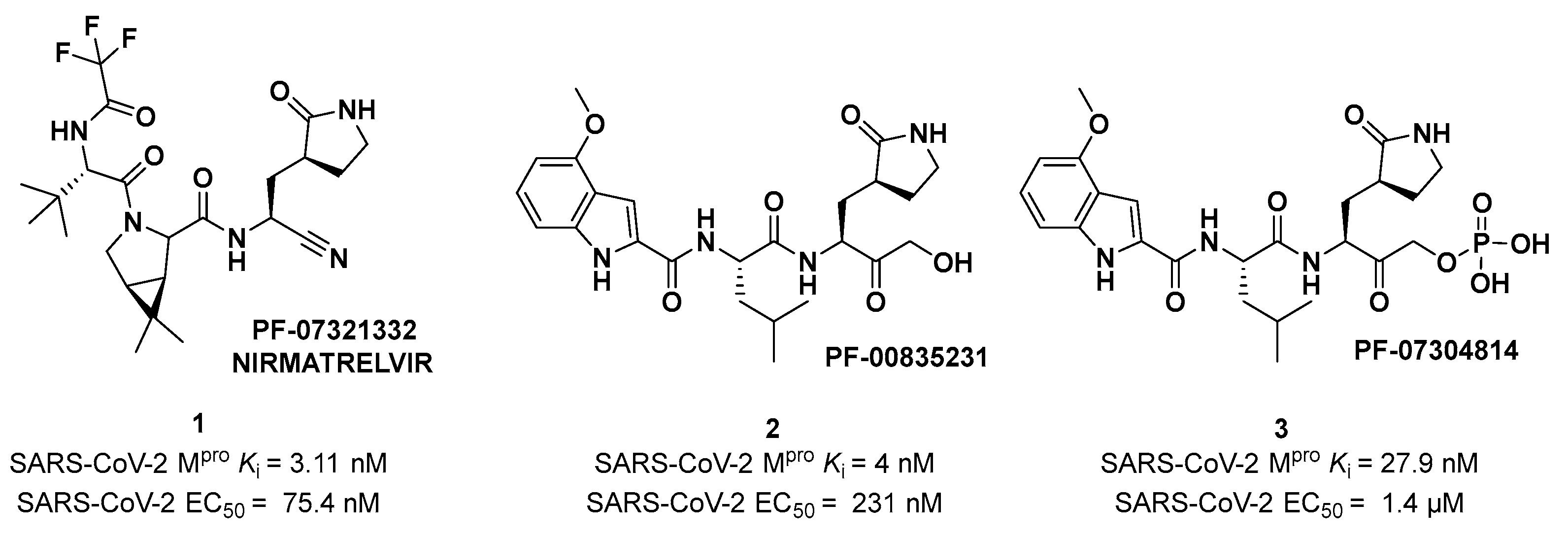
Nirmatrelvir (compound
1 in
Figure 4). Beyond the P1’ warhead,
Nirmatrelvir embodies a P1 γ-lactam moiety that resembles the Gln residue of the native substrate and acts as a recognition motif for the S1 pocket. The introduction of the dimethylcyclopropyl proline moiety at the P2 position was made to achieve a deep fit within the S2 subsite. The
N-terminal recognition unit is composed of a trifluoroacetamide group which provides interaction with the S3/S4 subpocket and gives the compound better pharmacokinetic properties. The discovery and development of
Nirmatrelvir started from two hit compounds:
PF-00835231 (
2 in
Figure 4), an α-hydroxy ketone-based peptidomimetic, and the corresponding phosphate prodrug
PF-07304814 (
3 in
Figure 4), developed by Pfizer Inc. against SARS-CoV during the 2002 SARS outbreak [
24]. Both compounds were selected as SARS-CoV-2 M
pro inhibitors for
in vitro and
in vivo biological evaluation, showing promising results as intravenously administered drug candidates. In particular,
PF-00835231 showed high potency (
Ki = 4 nM) and good antiviral activity (EC
50 = 231 nM) [
25].
Owen
et al. designed and synthesized a series of
PF-00835231 analogues in order to improve its pharmacokinetic properties and obtain compounds suitable for oral administration [
25]. The rationale of the design was to decrease the number of H-bond donors (HBD) without affecting the drug-target interaction. The first modification involved the α-hydroxy ketone warhead, replaced with two new electrophilic moieties, namely a benzothiazole-7-yl ketone and a nitrile group. The nitrile derivate (
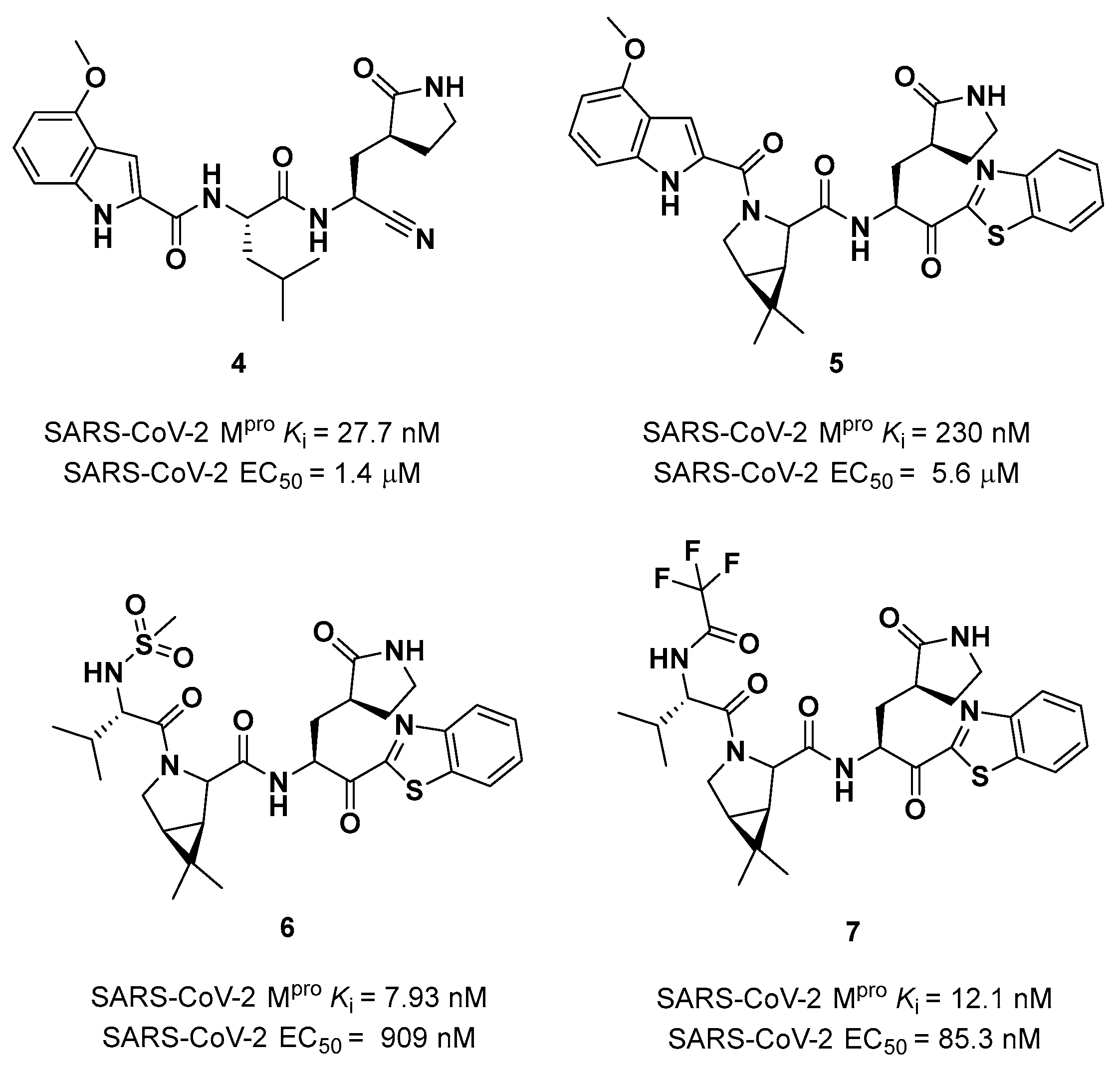
4 in
Figure 5) showed higher oral absorption in rat models but lower enzymatic inhibitory and cell-based antiviral activity than
2 (
Ki = 27.7 nM; EC
50 = 1.4 µM) [
25]. Another HBD that was not critical for the drug-target interaction was the leucine residue at P2, replaced with a cyclically modified proline residue (6,6-dimethyl-3-azabicyclo[3.1.0]hexane) able to fit inside the S2 pocket. This modification, combined with the benzothiazole-7-yl ketone warhead in P1’ position, led to compound
5 (
Figure 5). In this case, the cell permeability increased, while enzimatic inhibitory and cell-based antiviral activity decreased as compared to compound
4 (
Ki = 230 nM; EC
50 = 5.6 µM) probably due to the loss of a key H-bond with Gln189 in the S2 subsite. To regain potency, further investigations led to the replacement of the indole ring in P3 to reach a better fit with the S3 subsite. Two branched acyclic moieties were proposed: a methanesulfonamide and a trifluoroacetamide functionality (compounds
6 and
7, respectively;
Figure 5). Compound
6 and
7 showed comparable
Ki values (12.1 nM
vs 7.93 nM) but different antiviral activity (
6: EC
50 = 909 nM;
7: EC
50 = 85.3 nM). Moreover,
7 showed much higher oral absorption than
6 in rat and monkey
in vivo models. The combination of the nitrile warhead in P1’ with the trifluoroacetamide moiety in P3 eventually led to the identification of
PF-07321332,
i.e. Nirmatrelvir (
1 in
Figure 4).
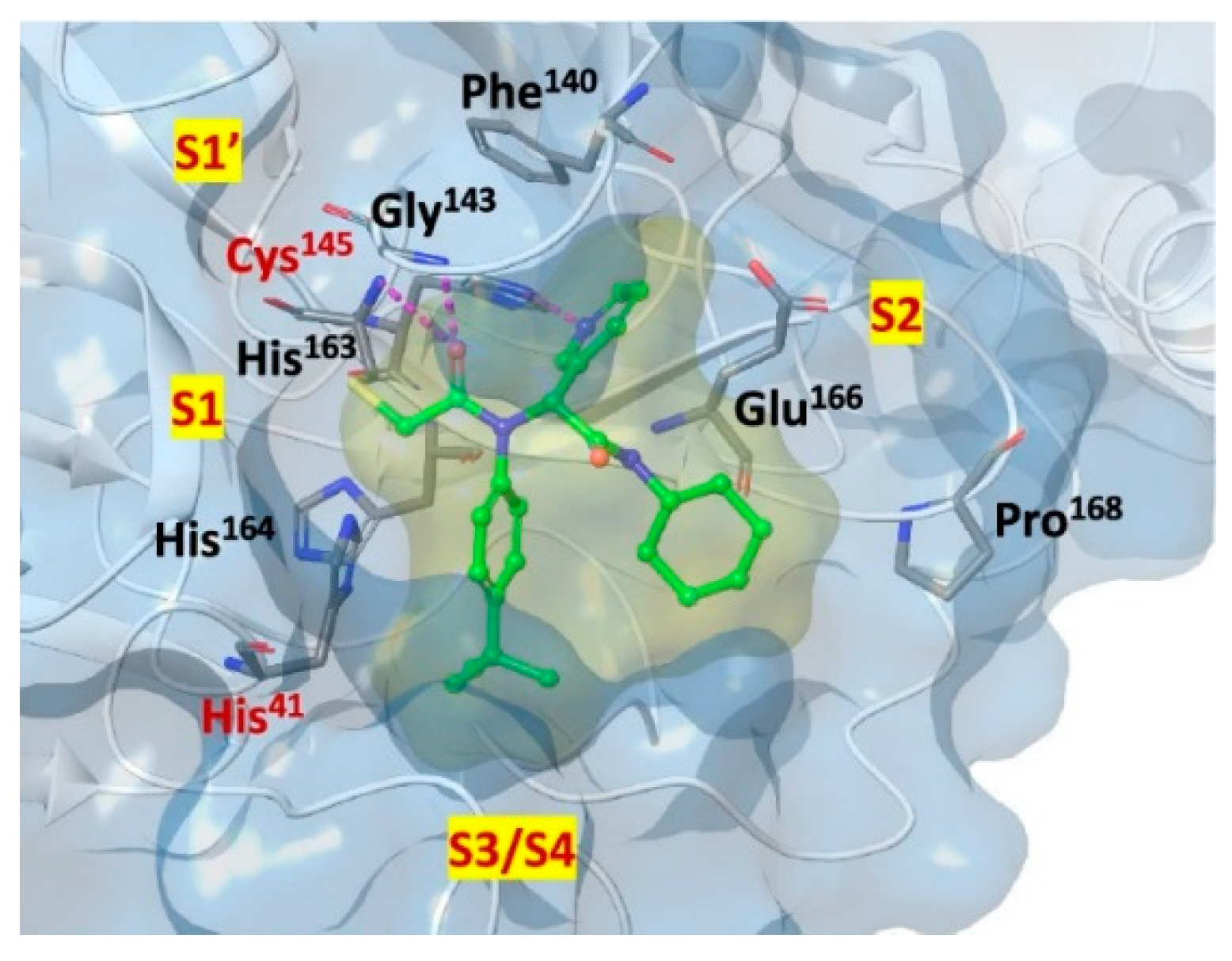
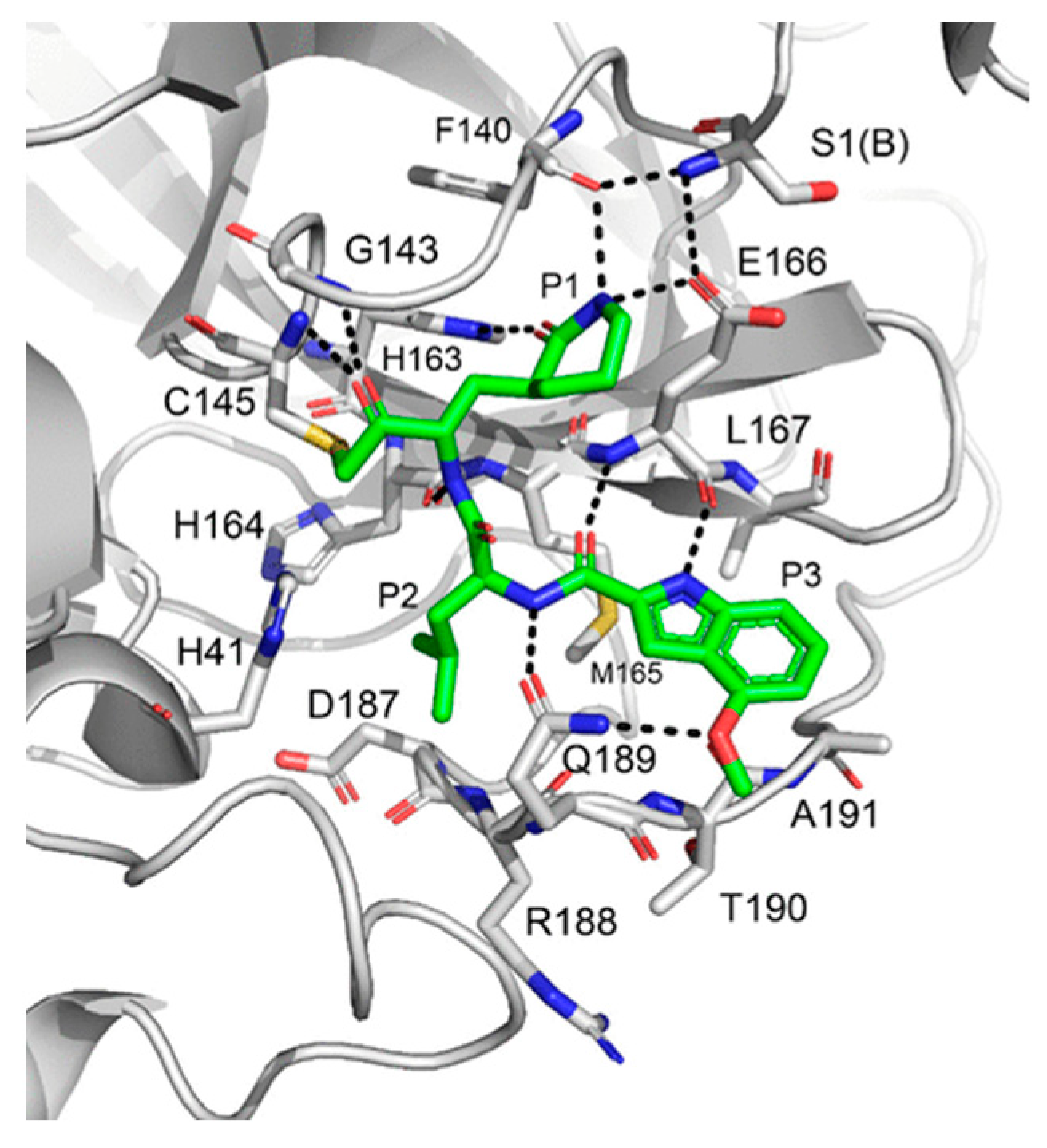
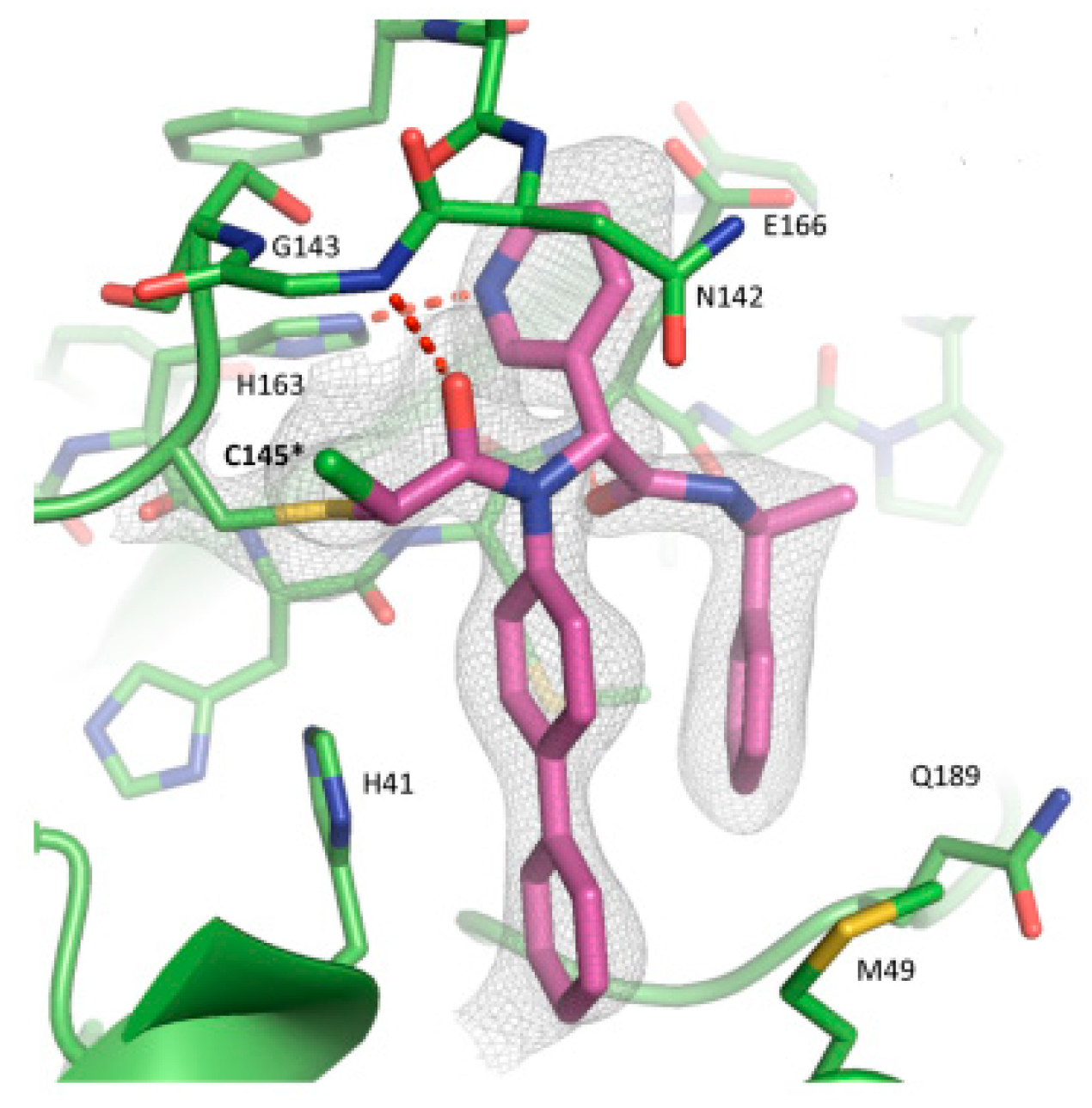
The co-crystal structure of
Nirmatrelvir with SARS-CoV-2 M
pro clarified its mechanism of action. The nitrile warhead in P1’ forms a reversible covalent bond with Cys145 at S1’, with the formation of the thioimidate adduct that establishes H-bond with the Gly143 residue. The γ-lactam mojety interacts with His163 and Glu166 in the S1 pocket, while the aliphatic dimethyl-bicycloproline fits inside the S2 pocket via the formation of several van der Waals interactions with the surrounding apolar residues. Moreover, the carbonyl oxygen of the trifluoromethyl acetamide acts as an H-bond acceptor toward the Gln189 residue, while the trifluoromethyl group establishes interactions with the S4 pocket (
Figure 6) [
16].
The nitrile moiety was selected over the benzothiazole-7-yl ketone warhead due to several features:
i) superior water solubility allowing for high concentration solutions of a drug candidate in pre-clinical trials;
ii) reduced tendency of epimerization of the near P1 stereocenter;
iii) easier synthesis for a scale up process [
24].
Nirmatrelvir showed superior enzimatic inhibitory and antiviral activity (
Ki = 3.11 nM; EC
50 = 74.5 nM) as compared to the parent compounds (
Figure 4). The introduction of a structure with less HBDs and a trifluoroacetamide moiety assured good gut permeability, oral bioavailability and proper drug clearance in
in vivo rat models.
In vivo evaluations in monkey models showed a decrease in oral bioavailability and gut permeability linked to the first pass metabolism [
25]. To decrease the first pass metabolism,
Nirmatrelvir is co-administered with the anti-HIV-1 drug
Ritonavir, which is also an inhibitor of CYP450. Remarkable results in preclinical and clinical studies led this new combination to be authorized by the Food and Drug Administration (FDA) [
26], European Medicine Agency (EMA) [
27] and UK
Medicines and Healthcare products Regulatory Agency (MHRA) as the only orally administered drug to treat COVID-19 [
28] under the commercial name of
Paxlovid®.
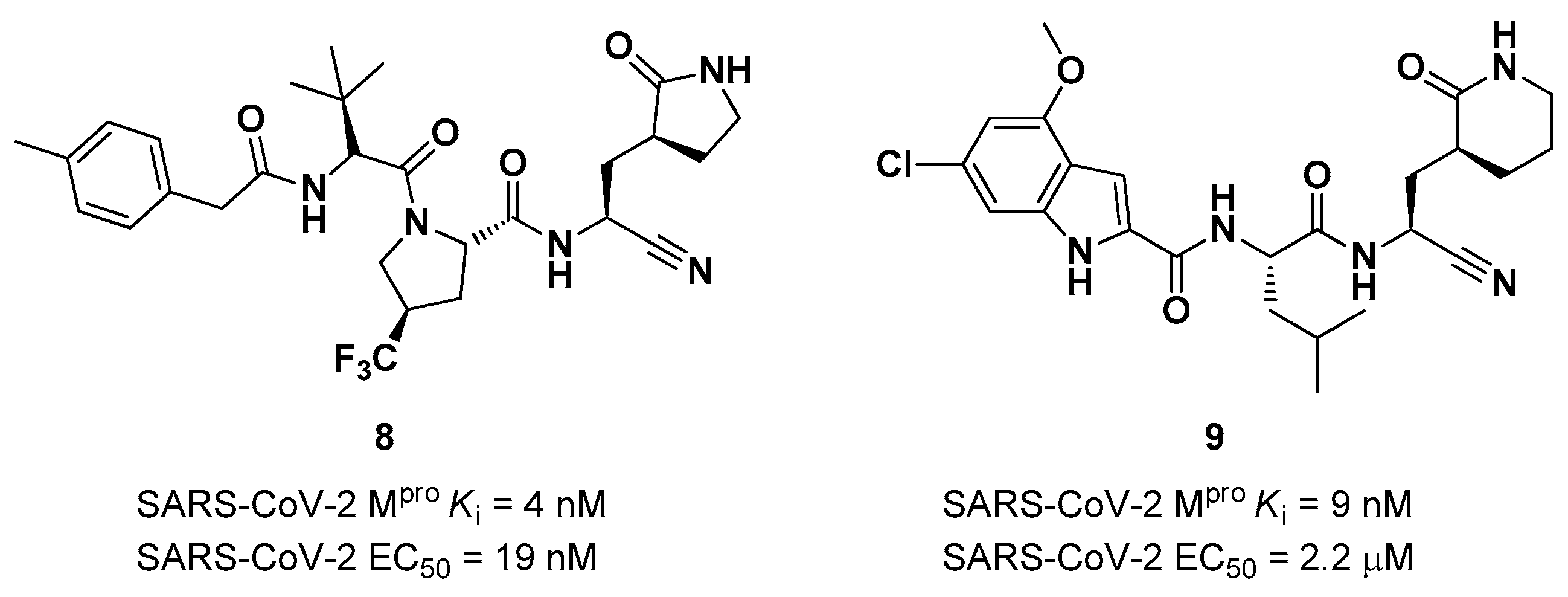
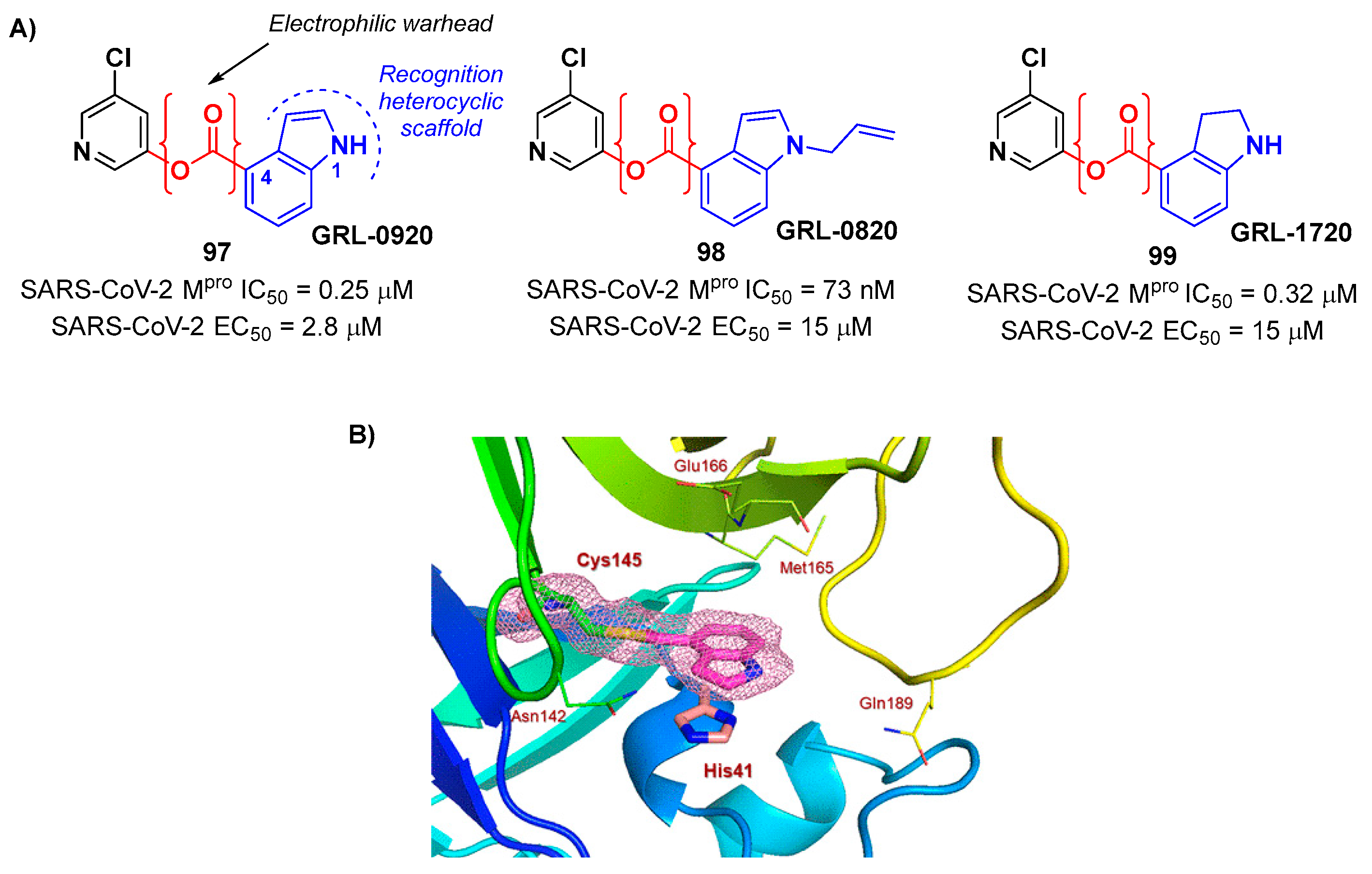
The success of the nitrile warhead on
Nirmatrelvir lead Pfizer scientists to investigate other nitrile-based compounds as inhibitors of SARS-CoV-2 M
pro, resumed in a new patent published on 16
th December 2021, identifying compound
8 (
Figure 7) as a new SARS-CoV-2 M
pro inhibitor (
Ki = 4 nM; EC
50 = 19 nM) [
29]. Bai
et al. explored other nitrile analogs, investigating the 4-methoxyindole mojety at P3 [
30]. Among all products, compound
9 (
Figure 7), having a 6-chloro-4-methyloxyindole function at P3, showed the best results in terms of
in vitro enzymatic inhibitory and antiviral activity (IC
50 = 9 nM; EC
50 = 2.2 μM) [
22].
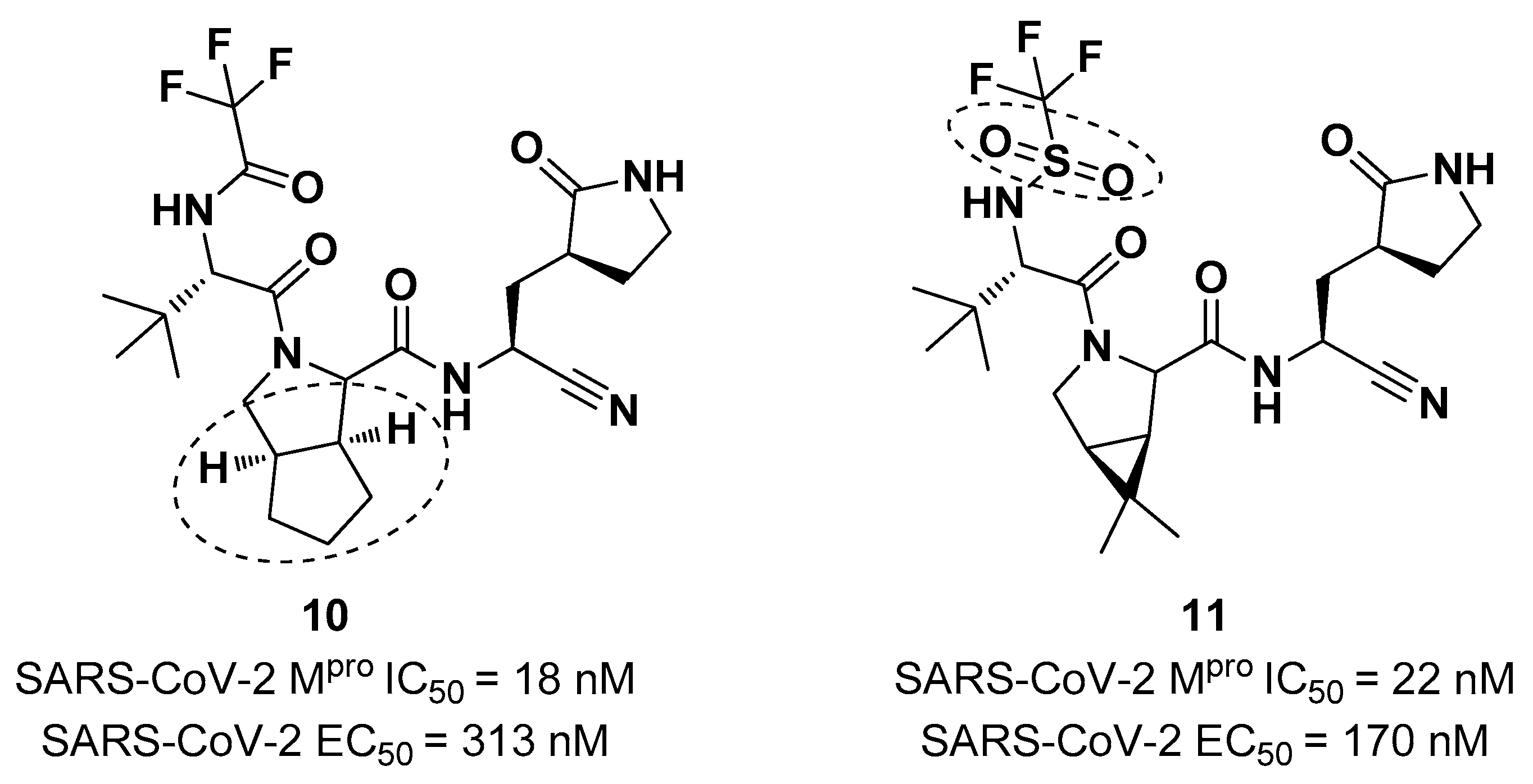
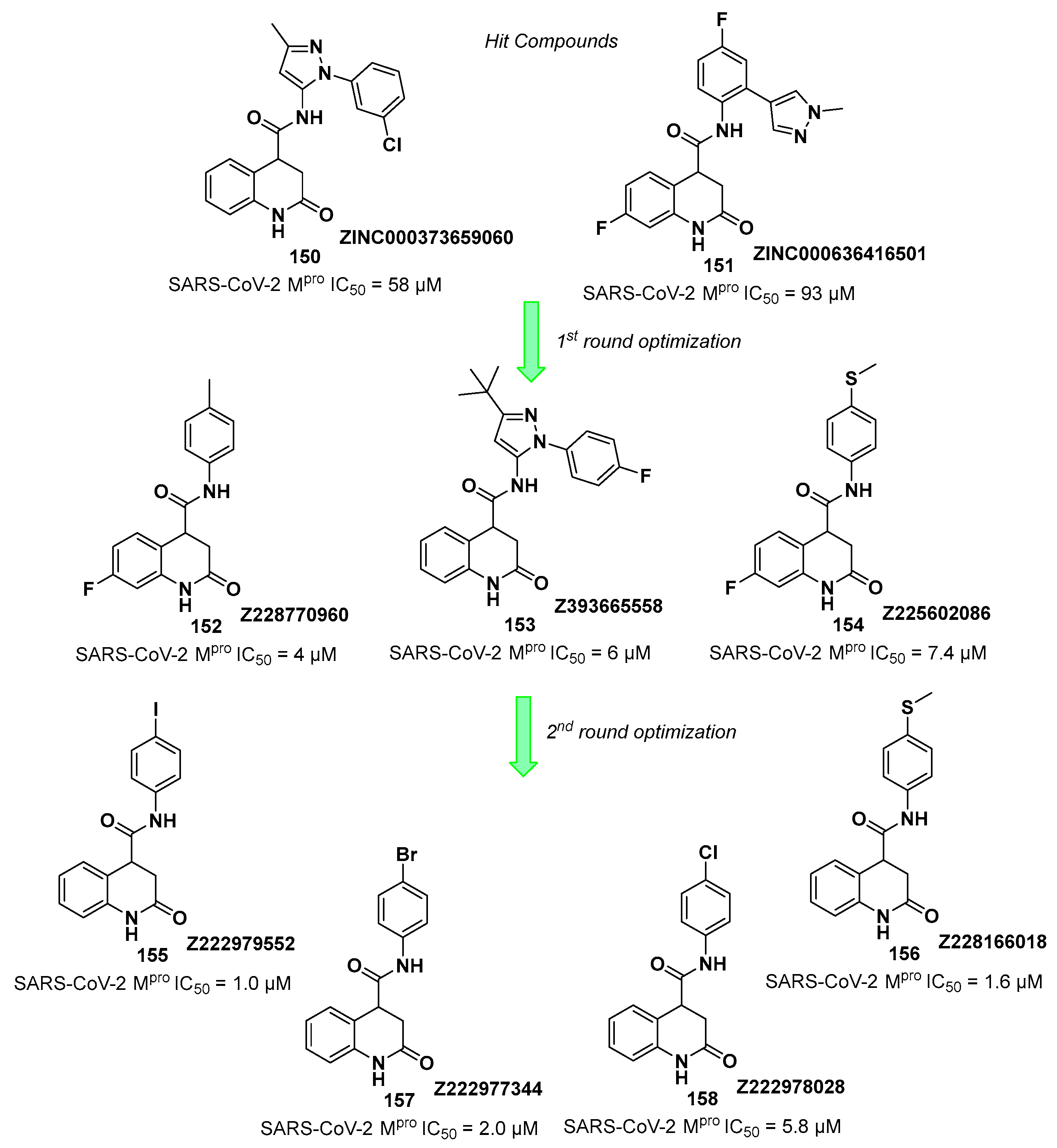
Starting from
Nirmatrelvir, Zhu
et al. developed a series of novel SARS-CoV-2 M
pro inhibitors by optimizing the P2 and P4 positions of the peptide framework [
31]. Within this series, compounds
10 and
11 (
Figure 8) exhibited optimal inhibitory activity toward SARS-CoV-2 M
pro with IC
50 values of 18 nM and 22 nM, respectively. Compound
10 derived from an optimization study of the P2 site in which the dimethyl-bicycloproline of
Nirmatrelvir was replaced with a cyclopentenyl proline. Compound
11 derived from an optimization attempt of the P4 site with the aim of improving the metabolic stability. In particular, the trifluoromethyl group of
Nirmatrelvir was replaced with a trifluoromethanesulfonyl group. Compounds
10 and
11 exhibited higher antiviral activity than
Nirmatrelvir against SARS-CoV-2 infected VeroE6 cells, with EC
50 values of 313 nM and 170 nM, respectively. They also exhibited better metabolic stability than
Nirmatrelvir and similar PK properties [
31].
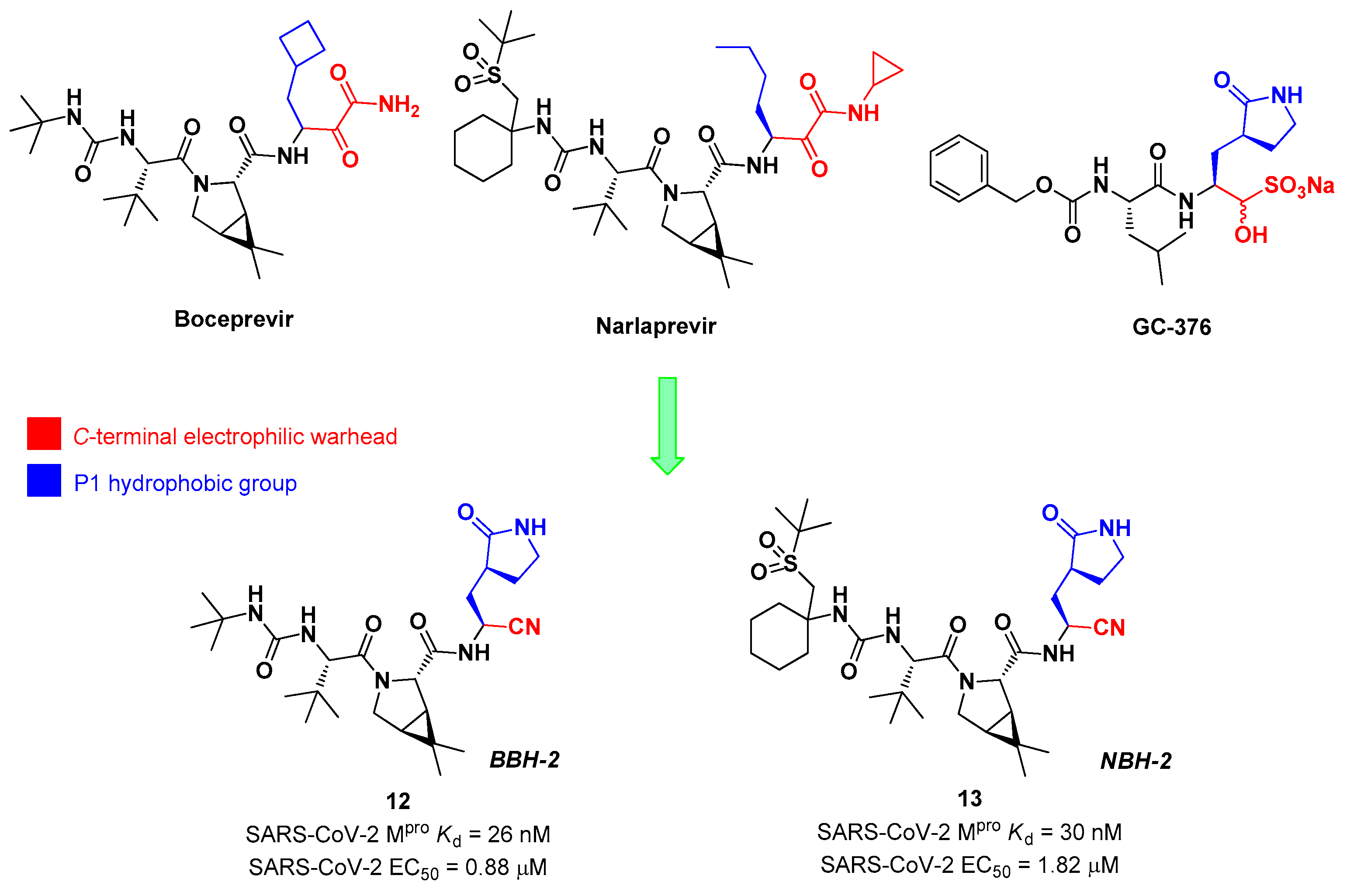
Inspired by the structures of hepatitis C virus NS3/4A serine protease inhibitors
Narlaprevir and
Boceprevir, and SARS-CoV M
pro inhibitors such as
GC-376 (
Figure 9), Kneller
et al. developed three SARS-CoV-2 M
pro inhibitors bearing a nitrile warhead [
32]. Compound
12 and
13 (the two most active derivatives), called
BBH-2 and
NBH-2, respectively (
Figure 9), were designed by replacing the
C-terminal di-ketoamide group of
Boceprevir and
Narlaprevir, respectively, with a nitrile warhead, whereas the hydrophobic P1 groups present in the serine protease inhibitors were replaced in both derivatives with a γ-lactam ring as per
GC-376.
These compounds have been shown to have a high binding affinity toward M
pro, comparable to that of
Nirmatrelvir (
Kd values of 26 nM and 30 nM for
12 and
13, respectively, and 7 nM for
Nirmatrelvir). Furthermore, the antiviral activity of these inhibitors were measured in SARS-CoV-2 M
pro infected VeroE6 TMPRSS cells in presence of a P-glycoprotein inhibitor (CP-100346), exhibiting EC
50 values of 0.88 and 1.82 μM for
12 and
13, respectively [
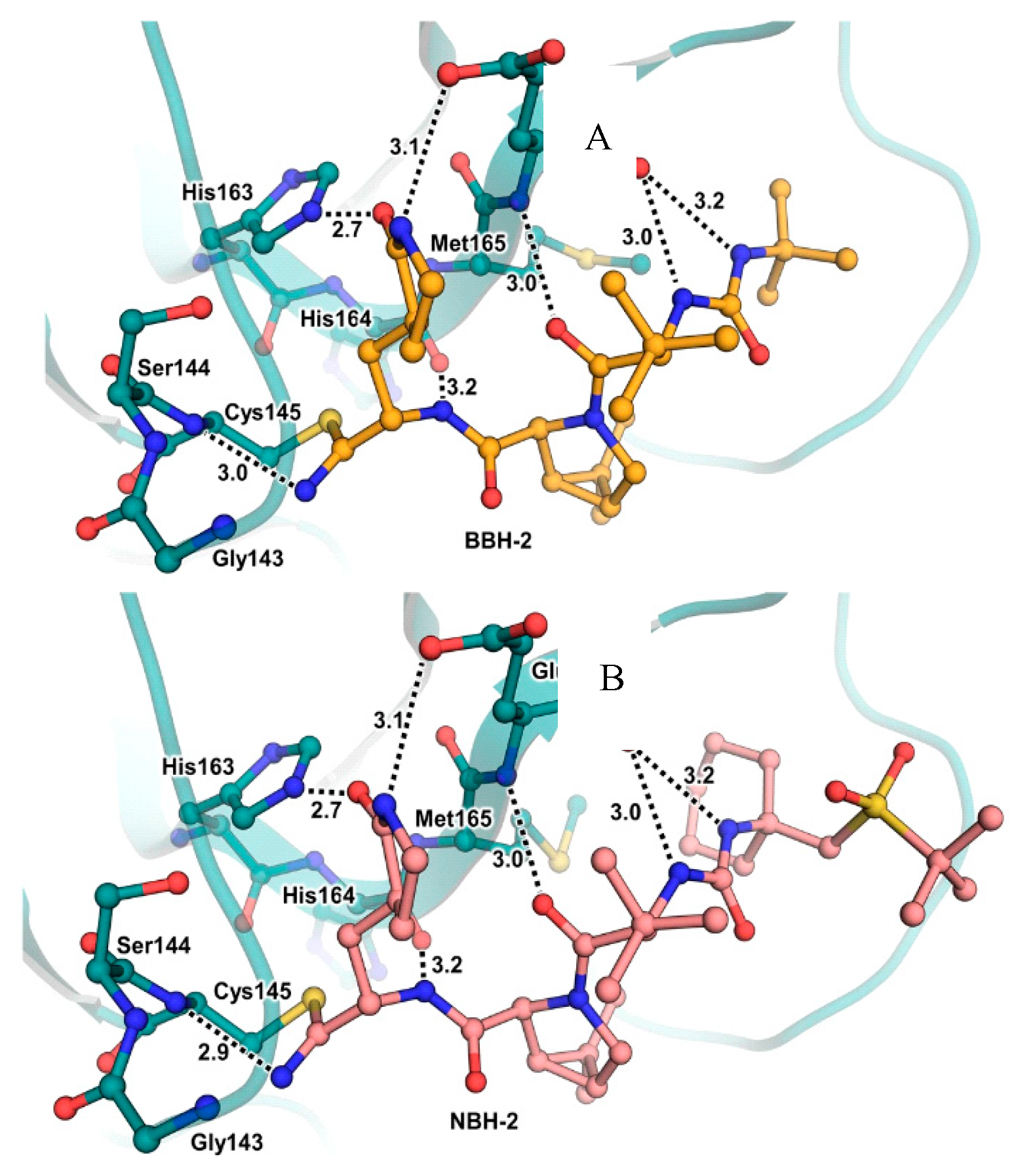
32]. No cytotoxicity was detected at 10 μM. The X-ray crystal structures of SARS-CoV-2 M
pro in complex with
12 and
13 showed that these two inhibitors bind in the same way the active site, forming a covalent bond between Cys145 and the nitrile warhead, and the newly formed thioimidate group is involved in an H-bond with the Cys145 backbone. The γ-lactam ring at P1 forms H-bonds with His163 and Glu166 main chains and an H-bond with His163 side chain. The other interactions are hydrophobic or involve H-bond with Glu166 [
32].
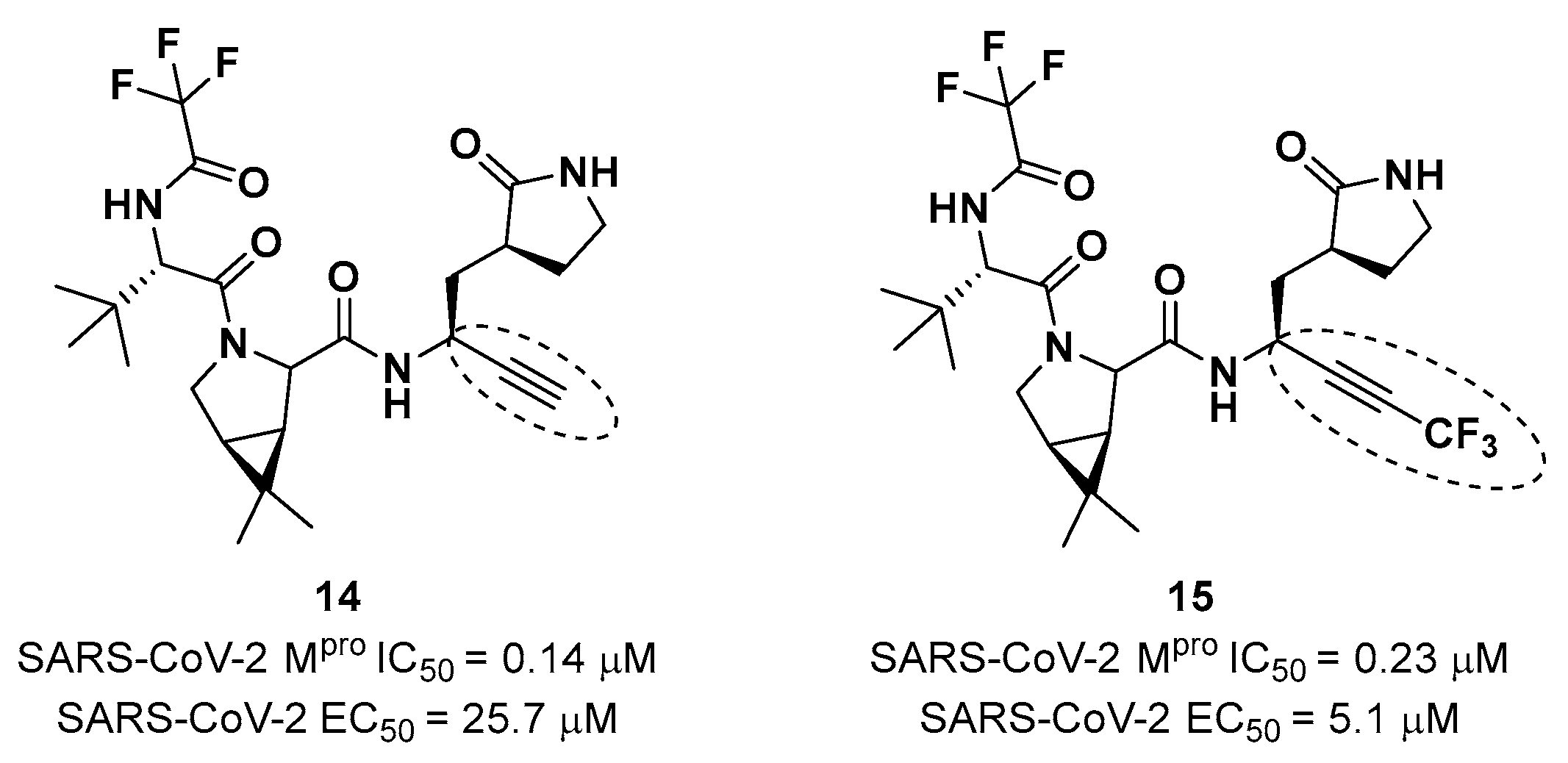
Brewitz
et al. developed different
Nirmatrelvir derivatives by accomplishing the isoelectronic replacement nitrile/alkyne group [
33]. The most promising compounds of this series turned out to be the alkyne derivate
14 and the CF
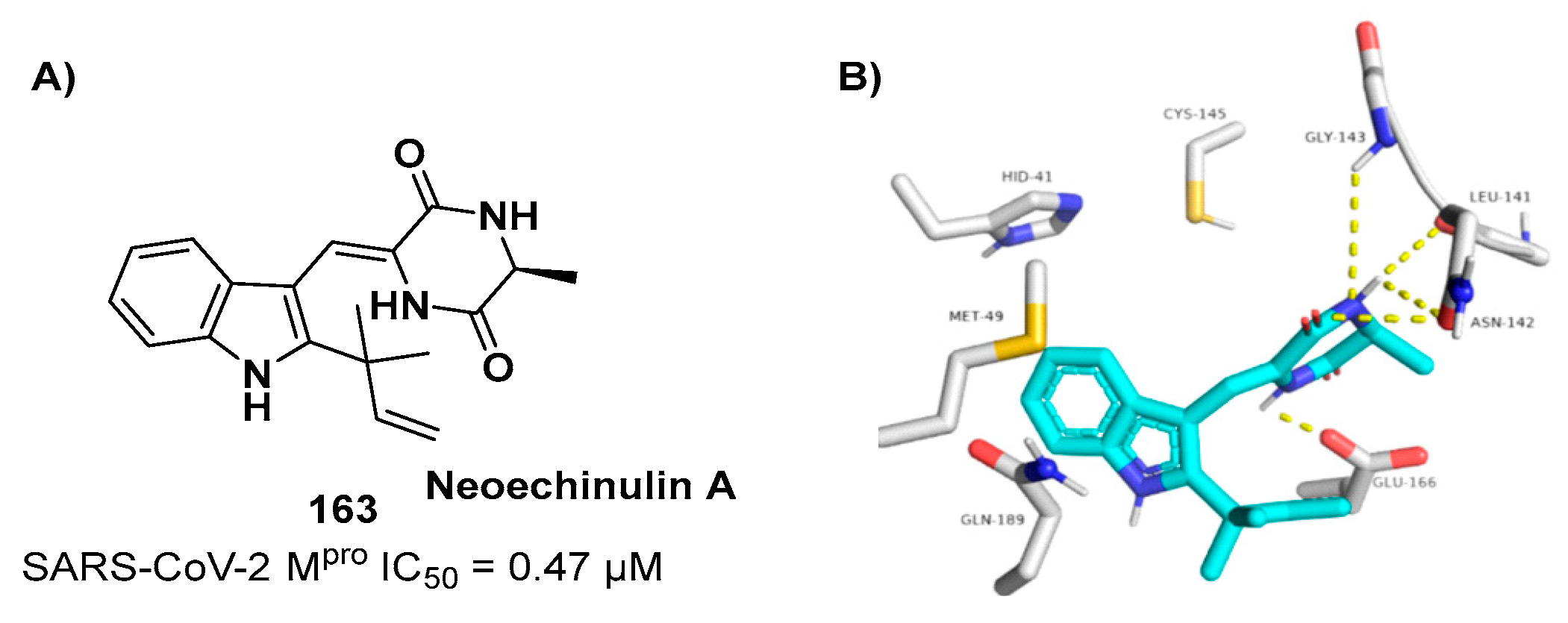
3-capped alkyne
15 (
Figure 11). They displayed noteworthy SARS-CoV-2 M
pro inhibitory and antiviral activity, high selectivity (no activity against SARS-CoV-2 PL
pro) and low cytotoxicity profile. MS analyses and crystallographic studies demonstrated that, unlike nitrile derivates which inhibit M
pro in a reversable manner, these alkyne derivatives inhibit M
pro by apparent irreversible covalent mechanism forming an internal vinyl thioether adduct with the Cys145 residue [
33].
5. Aldehydes
The aldehyde functional group is considered the most widely used electrophilic warhead among covalent SARS-CoV-2 M
pro inhibitors, due to the susceptibility of the electron poor carbonyl carbon to undergo nucleophilic addition by the thiol group of the Cys145 residue, with the formation of a reversible hemi-thioacetal adduct [
2]. The latter highly resembles the tetrahedral intermediate of the hydrolisis of the endogenous substrate, ensuring a longer residence time and an enhanced I-E complex stability due to several H-bonds between the adduct and residues of the S1’ pocket. Furthermore, the hemithioacetal adduct is able to stabilize the ligand-target interaction acting as a H-bond donor toward the Cys145 backbone. This critical H-bond is normally provided in the natural substrate by a carbonyl oxygen of the amide group next to a Gln residue [
34]. Thus, this critical interaction can be provided only by carbonyl- and ketoamide-based inhibitors, and this could explain their higher efficacy in comparison to other electrophilic warheads. The first peptidomimetics bearing an aldehyde warhead targeting SARS-CoV-2 M
pro were compounds
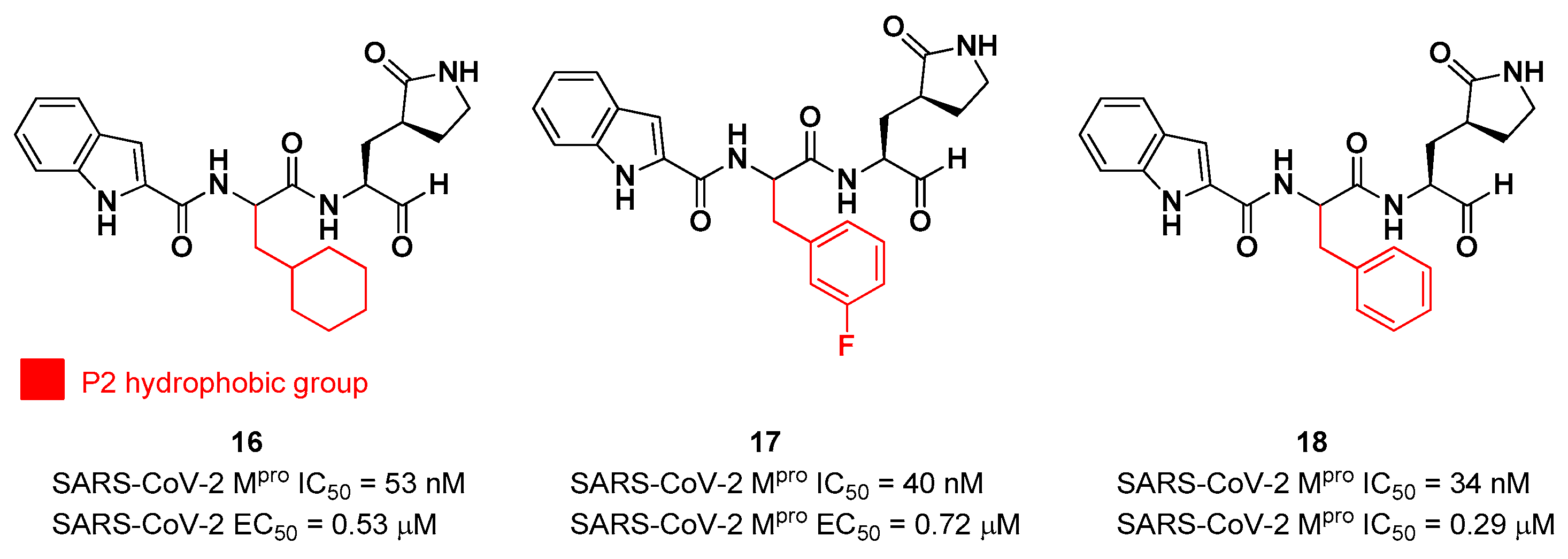
16 and
17 (
Figure 16), designed and synthesized by Dai
et al. [
35]. The work was mainly devoted to explore the enzyme S2 site. Compound
16 has a cyclohexyl ring at P2, while in compound
17 we find a 3-fluorophenyl ring. Besides the aldehyde warhead at P1’, other common features are the 2-indole moiety at P3 and the (
S)-γ-lactam group at P1. Also compound
18 (
Figure 12) was synthetized later on by Dai
et al., and it turned out to be a broad-spectrum M
pro inhibitor of enterovirus and SARS-CoV-2 [
36].
The prementioned aldehyde derivatives exhibited a high enzymatic inhibitory and antiviral activity (
16: IC
50 = 53 nM and EC
50 = 0.53 μM;
17: IC
50 = 40 nM and EC
50 = 0.72 μM;
18: IC
50 = 34 nM and EC
50 = 0.29 μM) [
36]. From the X-ray analysis of the structures of SARS-CoV-2 M
pro in complex with compounds
17 and
18, it was possible to observe the main interactions involved in the mechanism of action: a C-S covalent bond was detected between the carbonyl carbon of the warhead and the thiol group of the Cys145 of S1’ pocket; this adduct is stabilized by an additional H-bond between the thio-hemiacetal hydroxyl group and the Cys145 backbone. The (
S)-γ- lactam moiety at P2 is deeply inserted into the S2 subsite forming several H-bonds with key residues of this pocket, while the indole group at P3 interacts via a H-bond with the Glu166 residue located in the surface of S3/S4 sites. The binding modes of compounds
16 and
17 are quite similar, except for the interaction with S3, involving two different P3 moieties. In compound
17, the 3-fluorophenyl group undergoes a downward rotation in the S2 pocket, unlike the cyclohexyl substituent in
16, due to an additional H-bond involving the fluorine atom and the Gln189 redisue and several hydrophobic interactions of the aromatic ring with the surrounding residues. Compound
16 was chosen as a potential drug candidate due to its better pharmacokinetic properties [
35].
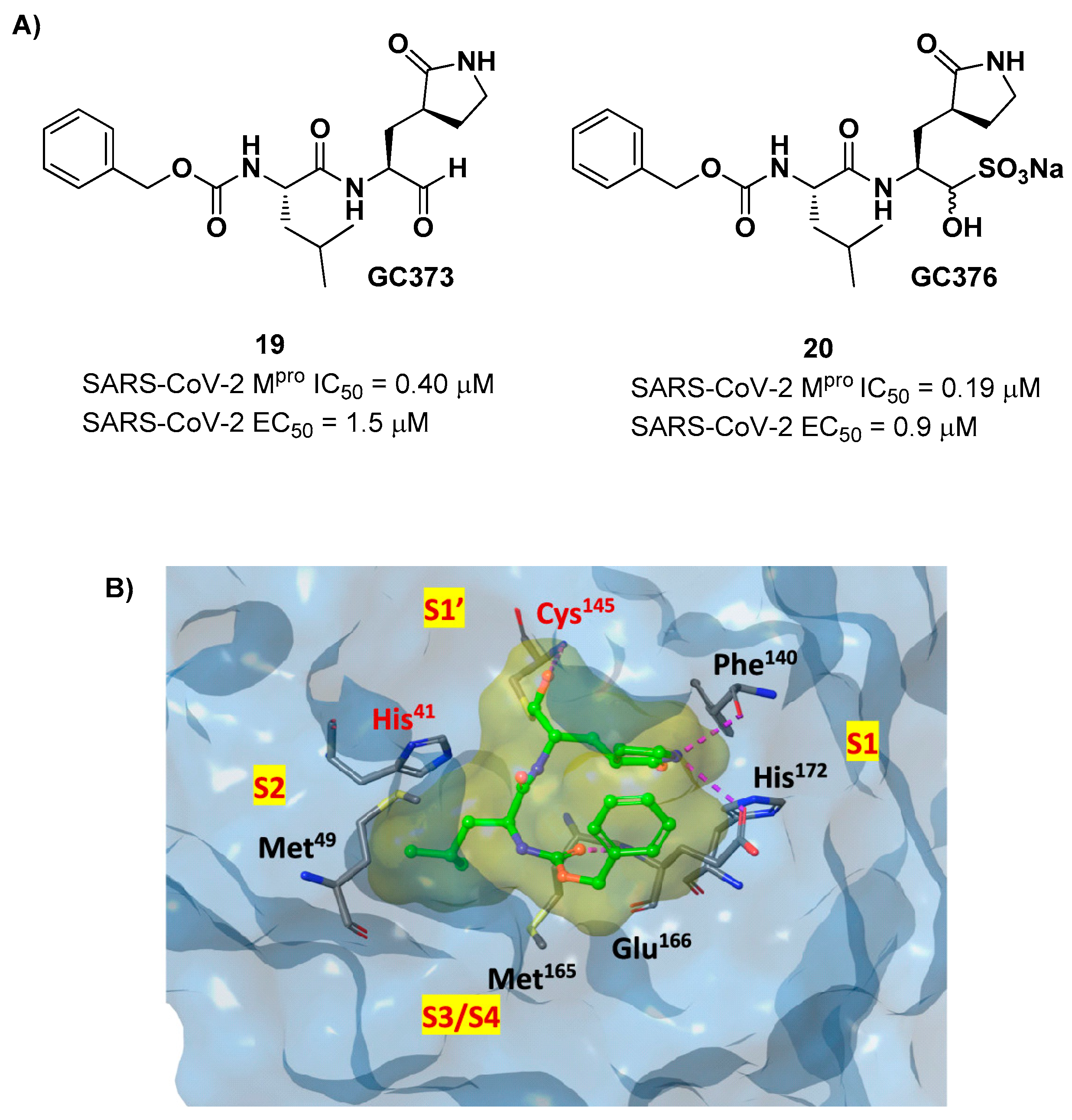
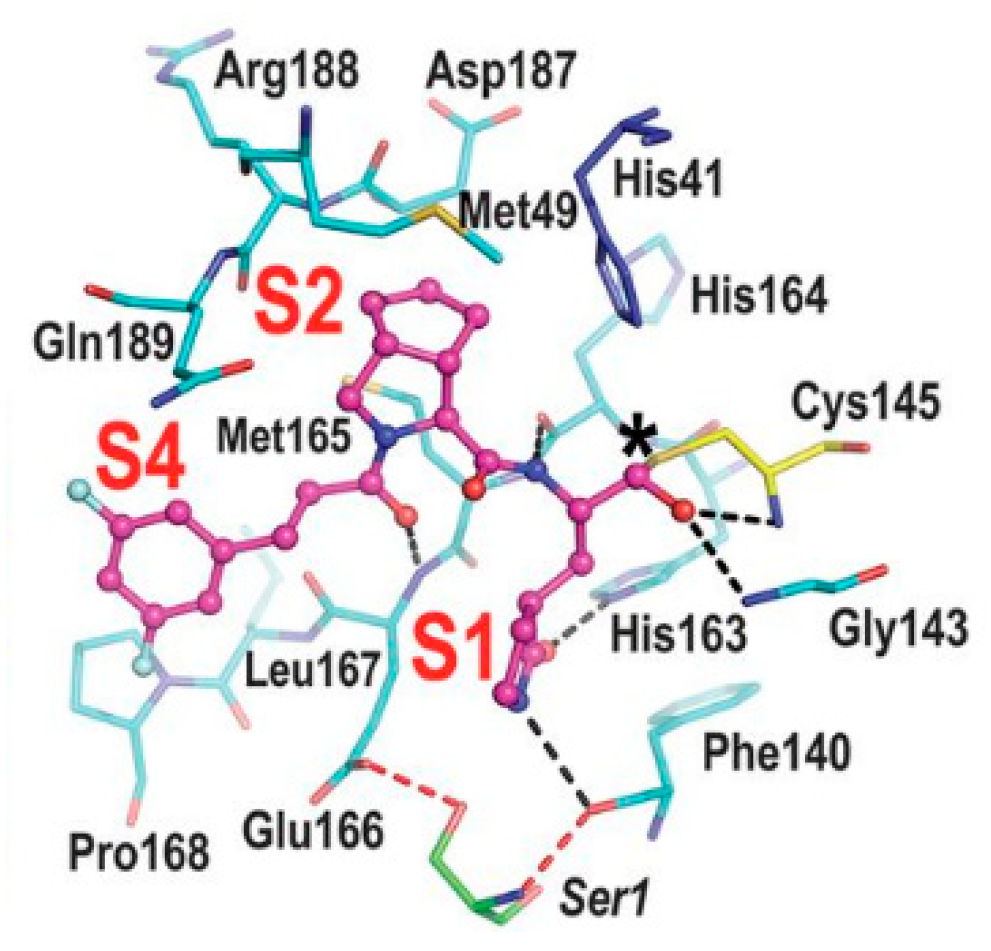
Other examples of aldehyde-based SARS-CoV-2 M
pro inhibitors are
GC373 (
i.e. compound
19 in
Figure 13A) and its bisulfite prodrug
GC376 (
i.e. compound
20 in
Figure 13A) [
37]. These compounds were initially used to treat feline infectious peritonitis, caused by feline coronaviruses FCoV, and then they were repurposed as SARS-CoV-2 M
pro inhibitors. In both compounds there is a benzyl moiety at P3, a leucine residue at P2 and a (
S)-γ-lactam group at P1.
19 and
20 inhibited the activity of SARS-CoV-2 M
pro with an IC
50 value of 0.40 and 0.19 μM, respectively. The X-ray crystal structure of SARS-CoV-2 M
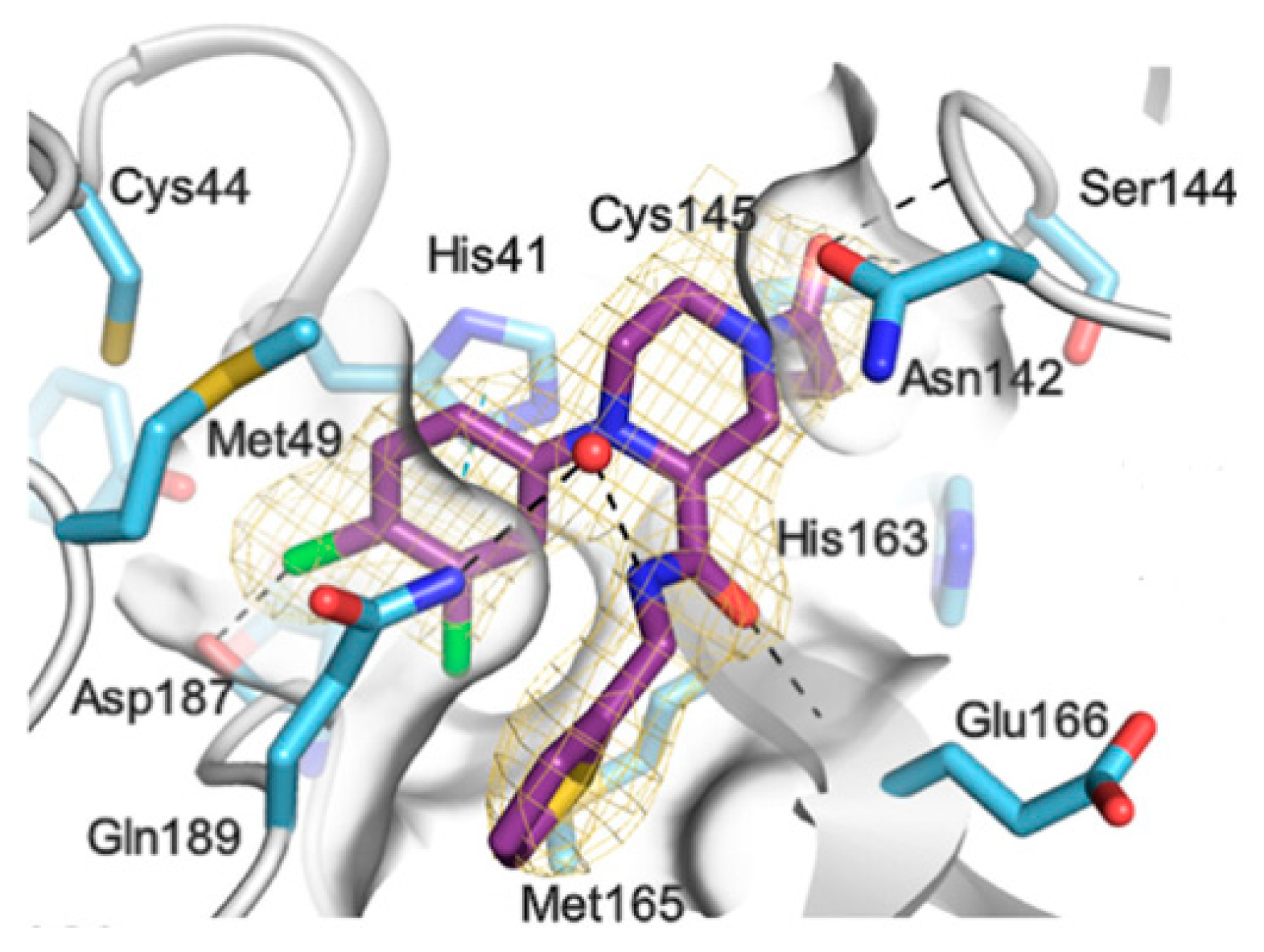
pro in complex with both compounds confirmed the ability of the prodrug
20 to provide the aldehyde function since they showed an identical binding mode: the aldehyde warhead reacted covalently with Cys145 thiol group to give a hemithioacetal adduct which is stabilized by several H-bonds inside the oxyanionic hole with the residues of Gly143, Ser144 and Cys145 (
Figure 13B) [
37]. The P1 γ- lactam moiety forms a H-bond with His163 and Glu166 and interacts with the main chain of Phe140, while the P2 leucine is inserted into the P2 pocket establishing hydrophobic interactions with Met149, His41 and Met49. The benzyl group at P3 interacts with the S3/S4 superficial sites by means of hydrophobic interactions. Both compounds exhibited high
in vitro antiviral activity observed in VeroE6 cell infected with SARS-CoV-2 (
19: EC
50 = 1.5 μM;
20: EC
50 = 0.9 μM) and low cell toxicity (CC
50 > 100 μM for both compounds). Nevertheless, the use of the bisulfite prodrug
20 has been preferred due to better outcomes in terms of bioavailability.
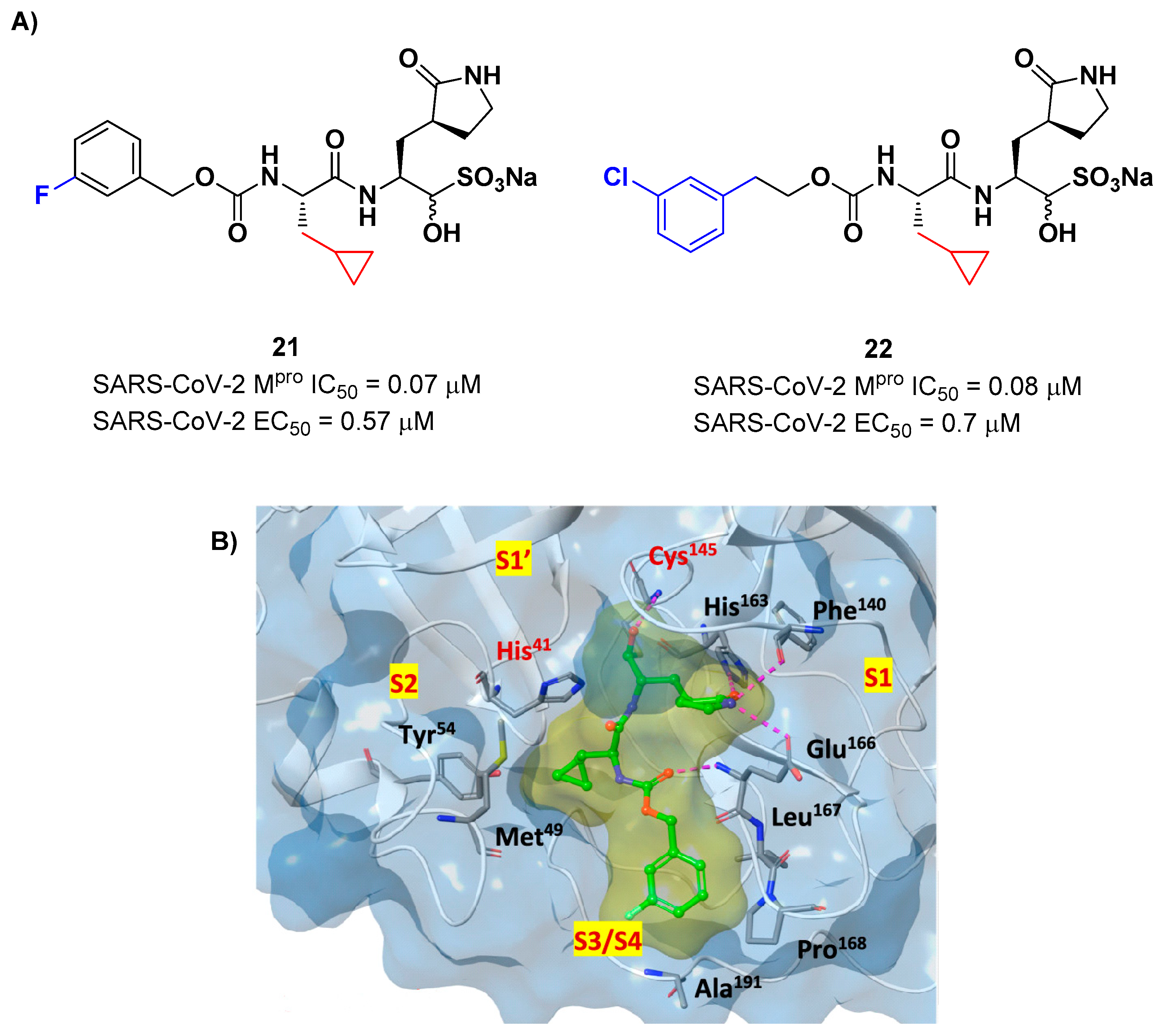
Structural modifications of
20 were conducted in order to improve the
in vitro antiviral activity against SARS-CoV-2 [
37]. Specifically, its P2 position was redesigned by substituting the leucine side chain with a constrained cyclopropylmethyl ring, while its benzyl group at P3 was decorated with a
meta-F substituent (
i.e. compound
21 in
Figure 14A) or elongated with a supplementary methylene group that connects a 3-chlorophenyl ring to the carbamate linkage (
i.e. compound
22 in
Figure 14A) [
38]. Both compounds displayed improved
in vitro inhibitory activity against M
pro as well as antiviral activity in comparison to the lead (
21: IC
50 = 0.07 and EC
50 = 0.57 μM;
22: IC
50 = 0.08 μM and EC
50 = 0.7 μM)[
38]. The X-ray structure of the complex between M
pro and
21 indicated that the P2 cyclopropyl fragment is able to fit more deeply within the S2 subsite, while the substitution at P3 with a halogenated phenyl ring allowed the inhibitor to fit deeper the S3/S4 pocket instead of remaining on the surface of the protease [
38].
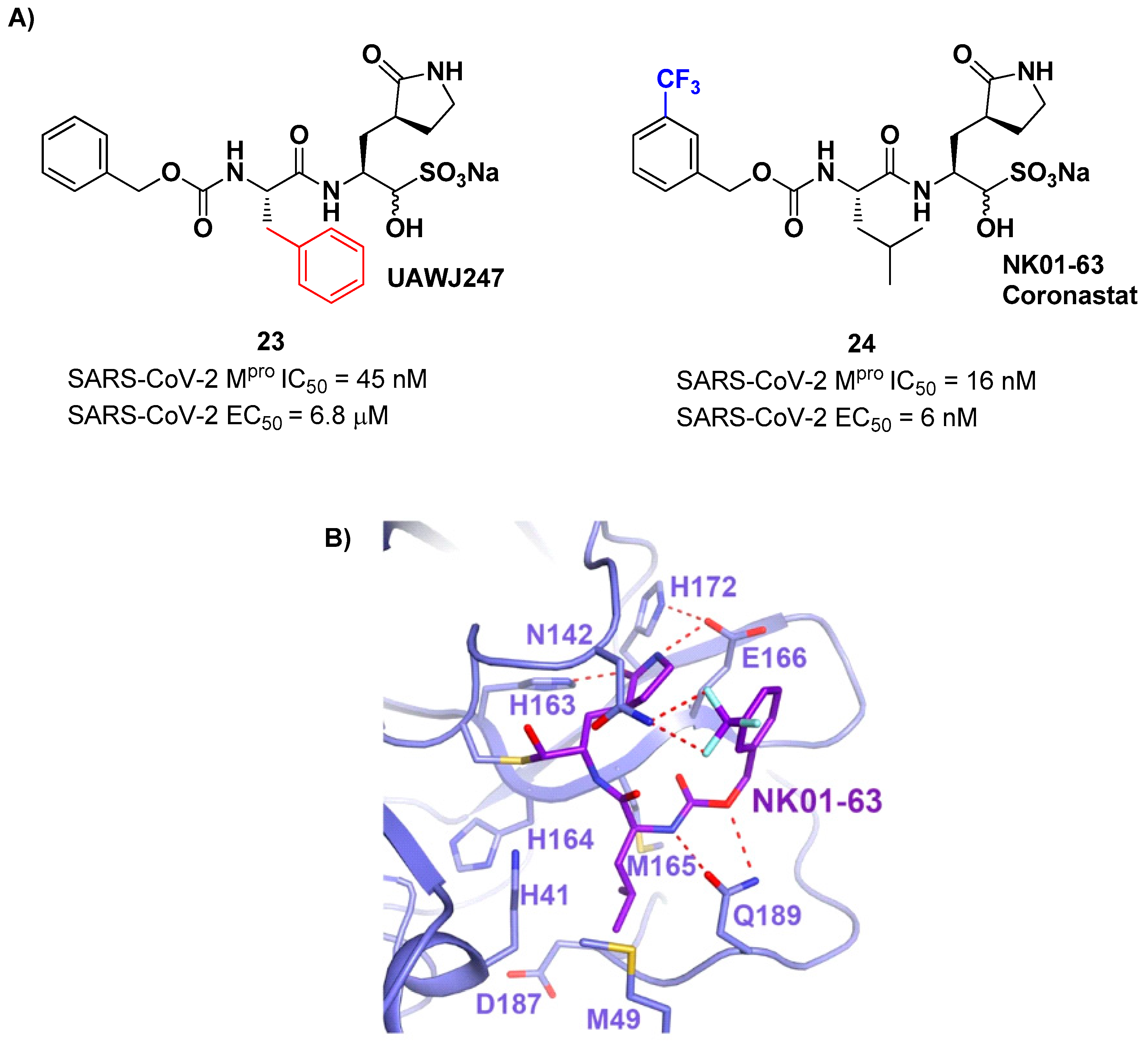
Other analogs of
20 with improved
in vitro enzymatic inhibitory and antiviral activity are represented by compound
23 and
24 (
Figure 15) [
39,
40]. In compound
23, also called
UAWJ247, structural variation in comparison to
20 involved the P2 position, where a phenyl ring replaced the isobutyl moiety. This compound exhibited an enzymatic inhibitory activity comparable to
20 (IC
50 = 45 nM) and a moderate antiviral activity. Compound
24, also called
NK01-63 or
Coronastat, contains a 3-trifluoromethylbenzyl group as a replacement of the benzyl group at P3 present in
20. Compounds
24 showed a potent inhibitory activity against SARS-CoV-2 M
pro (IC
50 = 16 nM) and an excellent antiviral activity (EC
50 = 6 nM in Huh7
ACE2 infected cell with SARS-CoV-2). The improvement in potency and antiviral activity of
24 can be explained by the presence of two additional H-bonds provided by the trifluoromethyl substituent at P3 with the Asn142 residue found in S3/S4 pocket, as observed in the X-ray structure [
39,
40].
Another interesting approach was undertaken to enhance the interaction inside the S2 pockect. It involved the introduction of a bicyclic proline derivative, a motif present in the structure of two potent inhibitors of HCV protease,
i.e. Boceprevir (
Figure 9) and
Telaprevir (
Figure 16). In particular, the proline residue was fused with three- (
Boceprevir) or five-membered (
Telaprevir) carbocyclic rings to form constrained bicyclic synthons [
41]. The most interesting compounds are depicted in
Figure 16. The aldehyde derivative
25 (also
MI-09 in
Figure 16) contains the same P2 fragment extrapolated from
Boceprevir and connected to a
para-OMe benzyl carbamate functional group, while the aldehyde derivatives
26 and
27 (also
MI-23 and
MI-30, respectively, in
Figure 16) are endowed with the P2 fragment of
Telaprevir and variously halogenated benzyl carbamate moieties at P3 [
41].
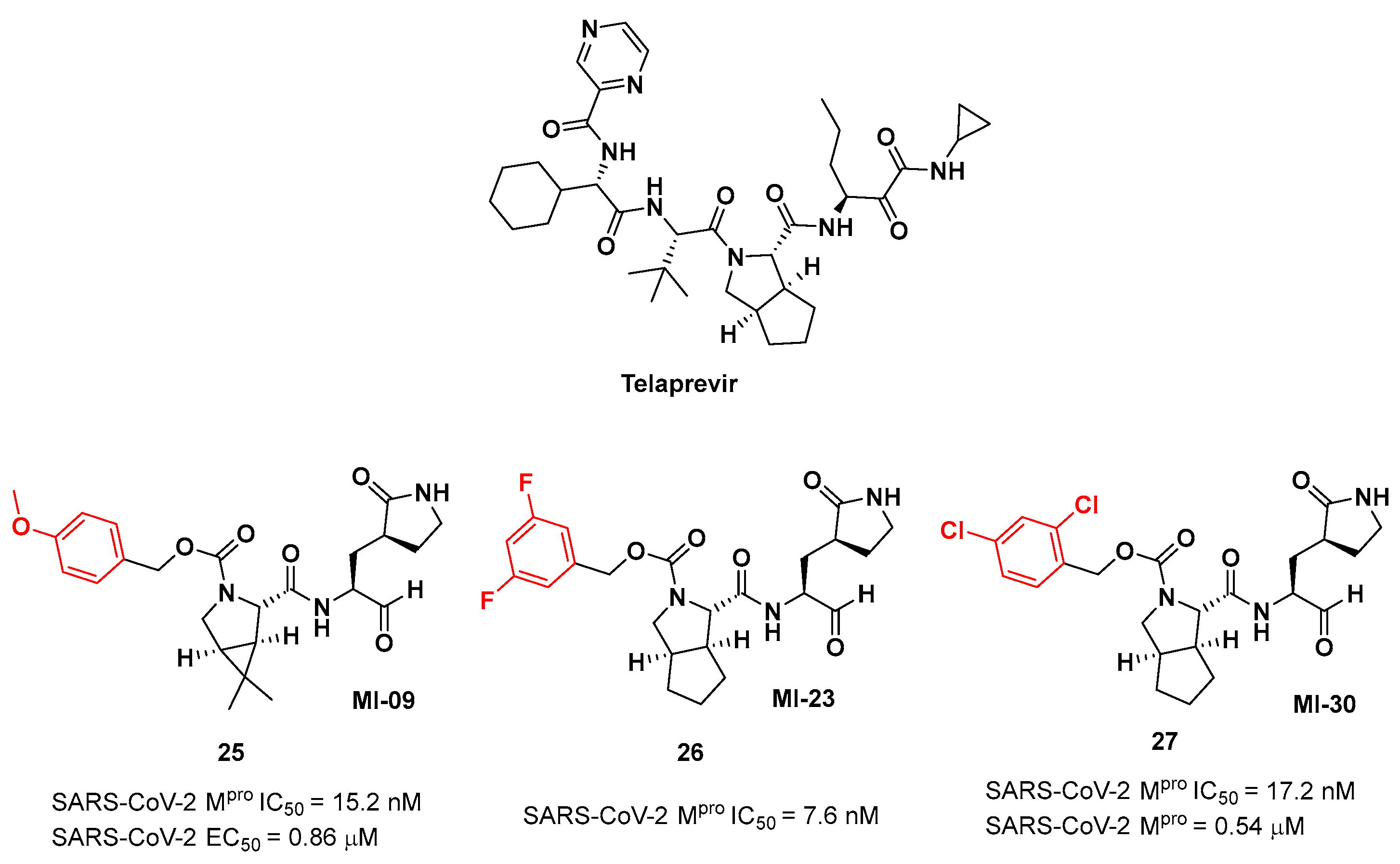
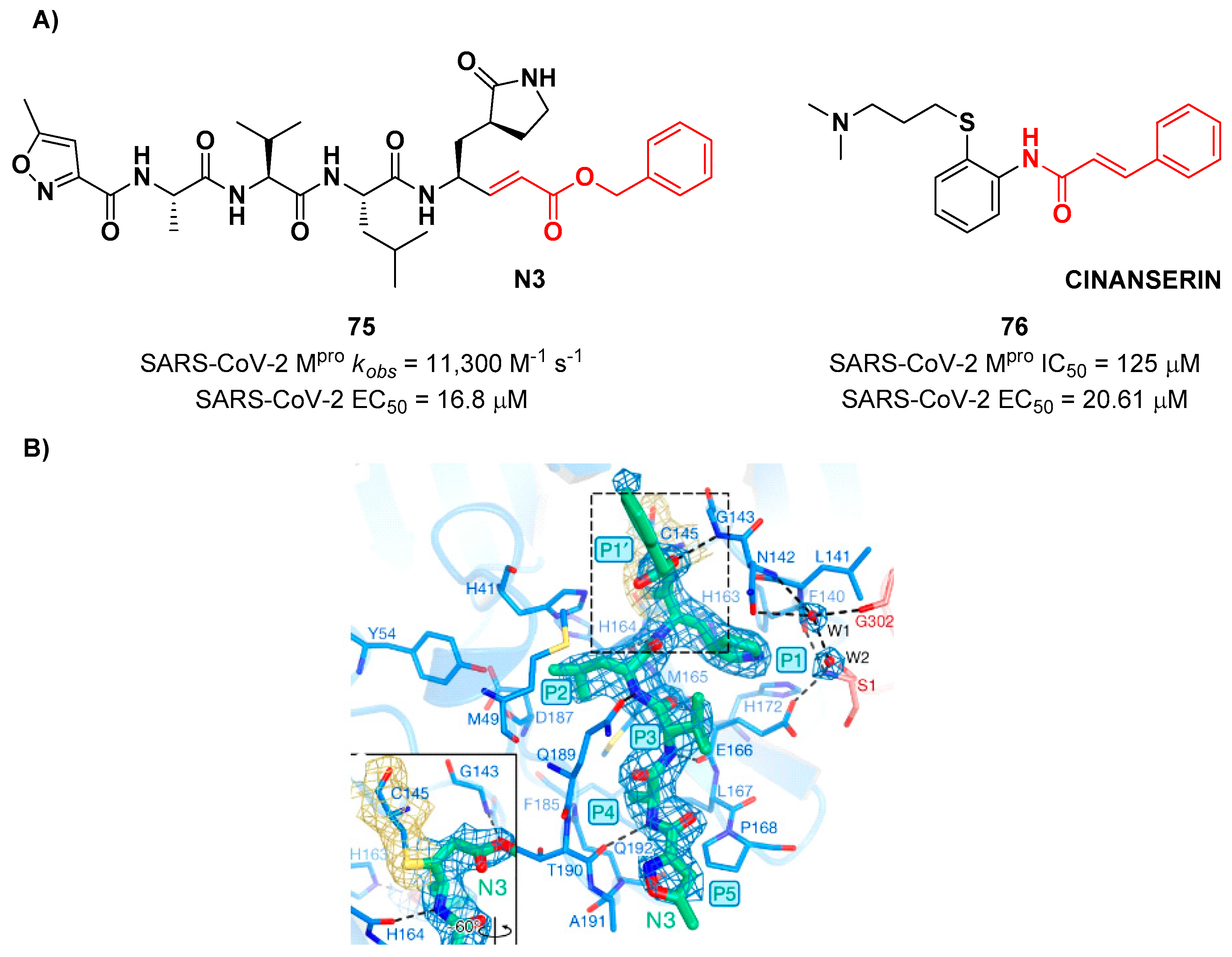
Figure 16.
Chemical structure of Telaprevir and chemical structure and biological activity of compounds 25-27.
Figure 16.
Chemical structure of Telaprevir and chemical structure and biological activity of compounds 25-27.
25-27 showed inhibitory activity against SARS-CoV-2 M
pro in the nanomolar range. The X-ray analysis of the complex
26/M
pro showed that the bicyclic proline is in its
trans-exo conformation, providing a deep fit within the S2 pocket (
Figure 17) [
41].
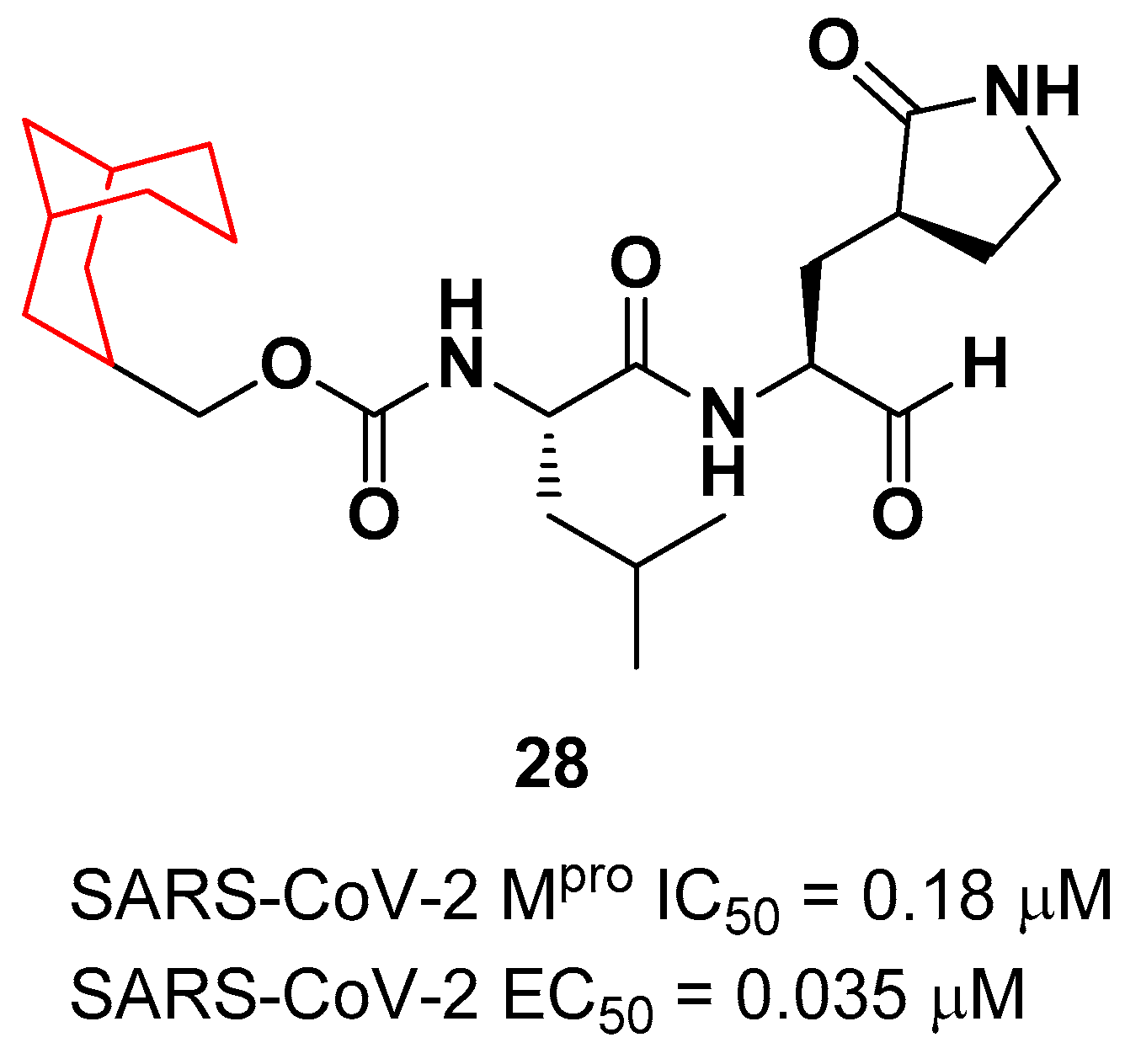
Another example of
GC376 derivative emerged from a study based on activity-guide optimization [
42]. Compound
28 (
Figure 18) is a novel dipeptidyl structure, bearing a constrained bicyclic ring in P3 position. This compound exhibited an inhibitory activity against SARS-CoV-2 M
pro in the micromolar range (IC
50 = 0.18 μM ) and antiviral activity in the sub-micromolar range (EC
50 = 0.035 μM; VeroE6 infected cells).
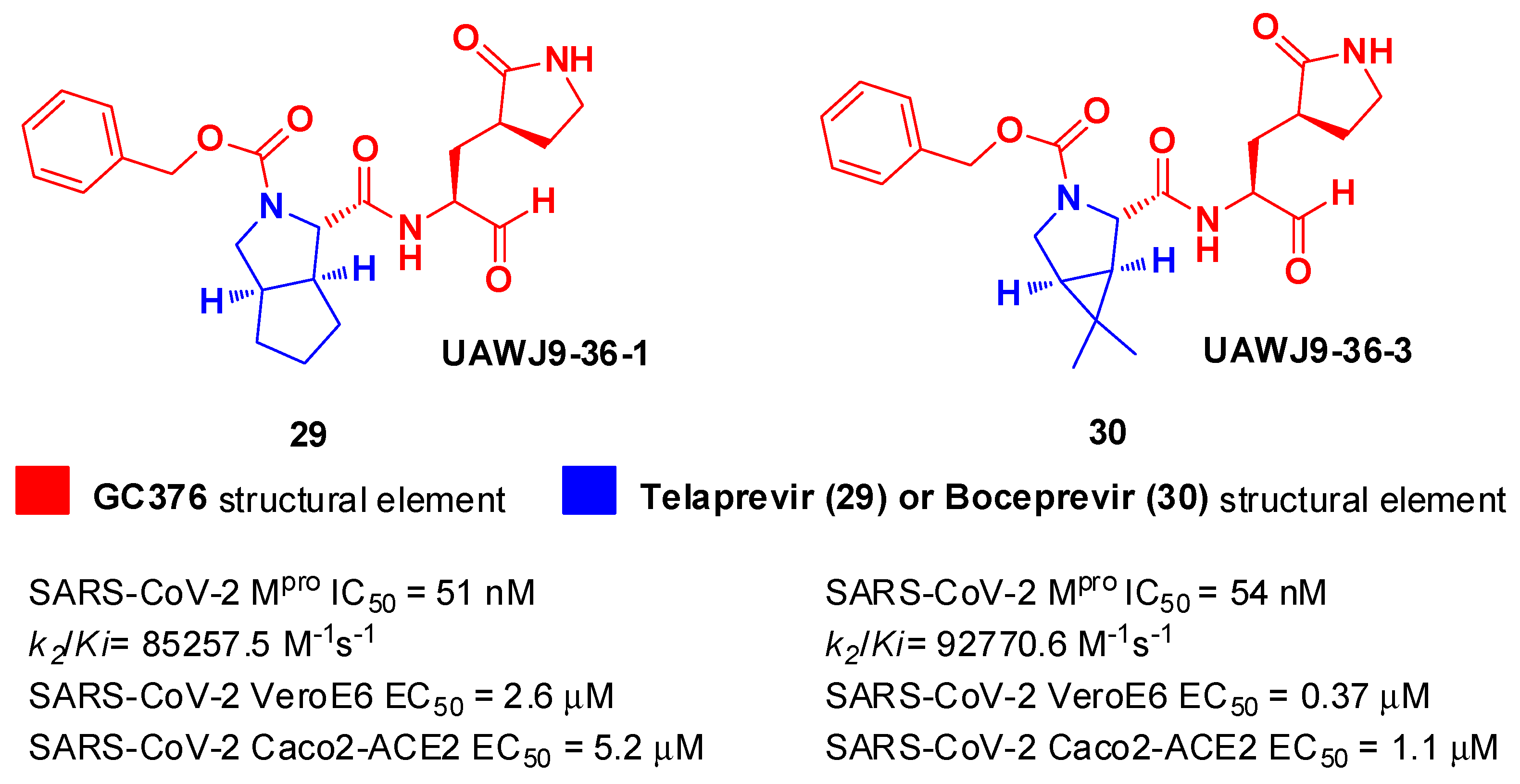
In parallel, Xia
et al. designed and synthetized compound
29 and
30 (
Figure 19), called
UAWJ9-36-1 and
UAWJ9-36-3, respectively, by retaining the P2 bicyclic proline synthons found in
Telaprevir and
Boceprevir [
43].
29 was designed as a hybrid form of
GC376 and
Telaprevir, whereas
30 was designed as a hybrid form of
GC376 and
Boceprevir.
Compounds
29 and
30 underwent enzymatic assays against all seven human coronavirus M
pros, providing a similar inhibition profile against SARS-CoV-2 M
pro (and overall for all M
pros) in comparison with
GC-376 (
29: IC
50 = 51 nM with a
k2/Ki = 85257.5 M
-1s
-1;
30: IC
50 = 54 nM with a
k2/Ki = 92770.6 M
-1s
-1). The antiviral activity was assessed against two different cell lines (VeroE6 and Caco2-ACE2) infected with SARS-CoV-2, hCoV-OC43, hCoV-229E, and hCoV-NL63.
30 showed an improved antiviral activity against SARS-CoV-2 as compared to
GC-376 (EC
50 = 0.37 μM on VeroE6 and EC
50 = 1.1 μM on Caco2-ACE2). The X-ray structures of SARS-CoV-2 M
pro in complex with both compounds confirmed the capacity of the bicyclic prolines to fit within the S2 pocket (
Figure 20) [
43].
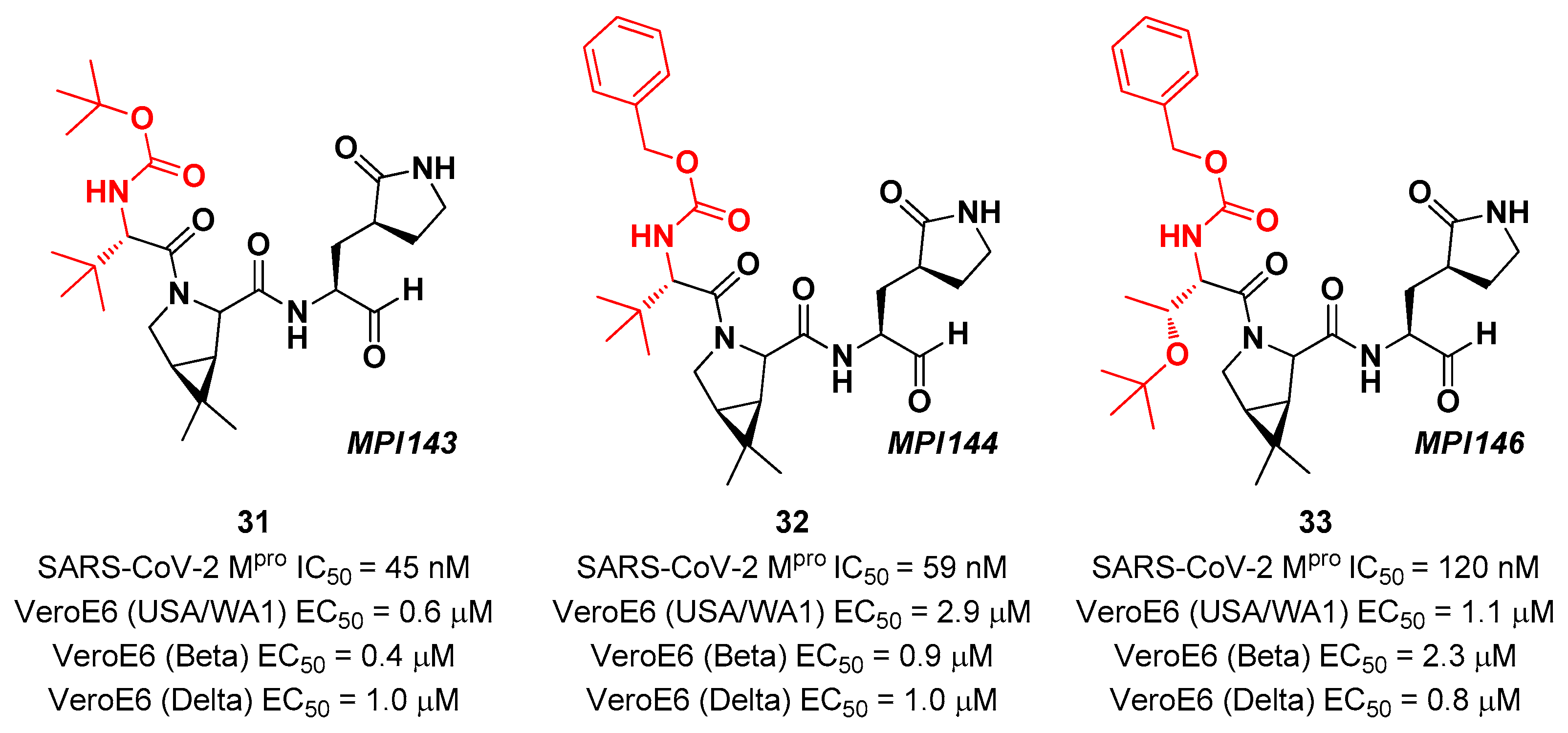
Other
Boceprevir analogs with aldehyde warhead at P1’ came from the work of Alugubelli
et al. [
44]. Among the 19 synthesized compounds, the most promising derivatives were compound
31,
32 and
33 (
Figure 21), called
MPI43,
MPI44 and
MPI46, respectively, displaying high potency
in vitro and
in cellulo assays with IC
50 values in the range 45–120 nM
(in vitro) and EC
50 values in the range 0.14 – 0.31 µM (
in cellulo) [
44]. These compounds were also evaluated for their antiviral efficacy against VeroE6 cells infected with USA-WA1/2020, Beta and Delta strains of SARS-CoV-2. Interestingly, these three compounds bear a P4 N-terminal carbamate moiety, which seems to be critical for high cellular and antiviral potency and low cytotoxicity [
44].
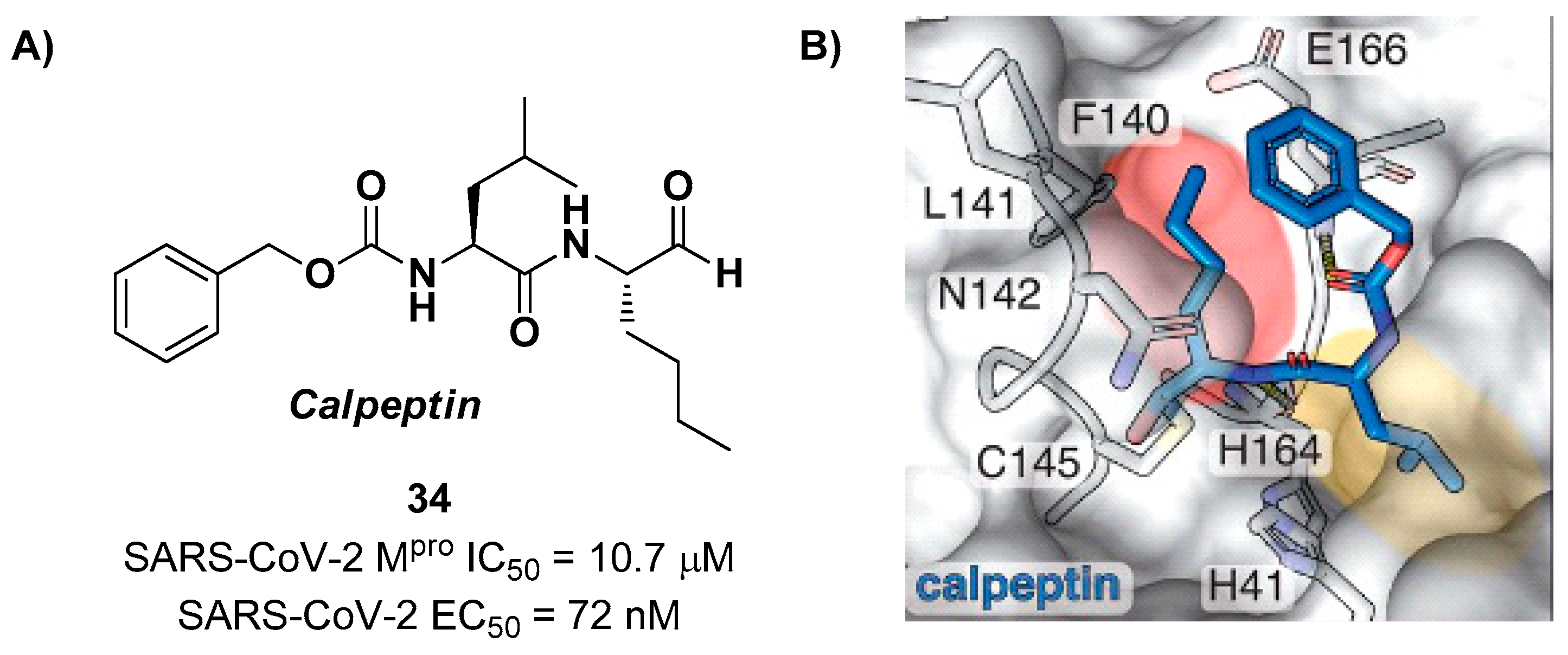
Another aldehyde-based compound was found by Günther
et al. [
45], using a large-scale X-ray crystallography to screen a library of more than 5,000 compounds among approved drugs or drugs in clinical trials. The selected compounds were then tested for their antiviral activity against SARS-CoV-2 in VeroE6 cells. Among them,
Calpeptin (
34 in
Figure 22A) showed the highest antiviral activity, with an EC
50 value of 72 nM and known enzymatic inhibitory activity (IC
50 = 10.7 μM) [
45,
46]. This compound contains an aldehyde warhead at P1’, a
n-Leu residue at P1, a Leu residue at P2 and a Boc group at P3. The X-ray crystal structure of SARS-CoV-2 M
pro in complex with
34 showed the standard thiohemiacetal covalent adduct Cys145/aldehyde warhead (
Figure 22B).
34 also inhibits cathepsin L, making it a good anti-COVID-19 drug candidate as a dual inhibitor [
45].
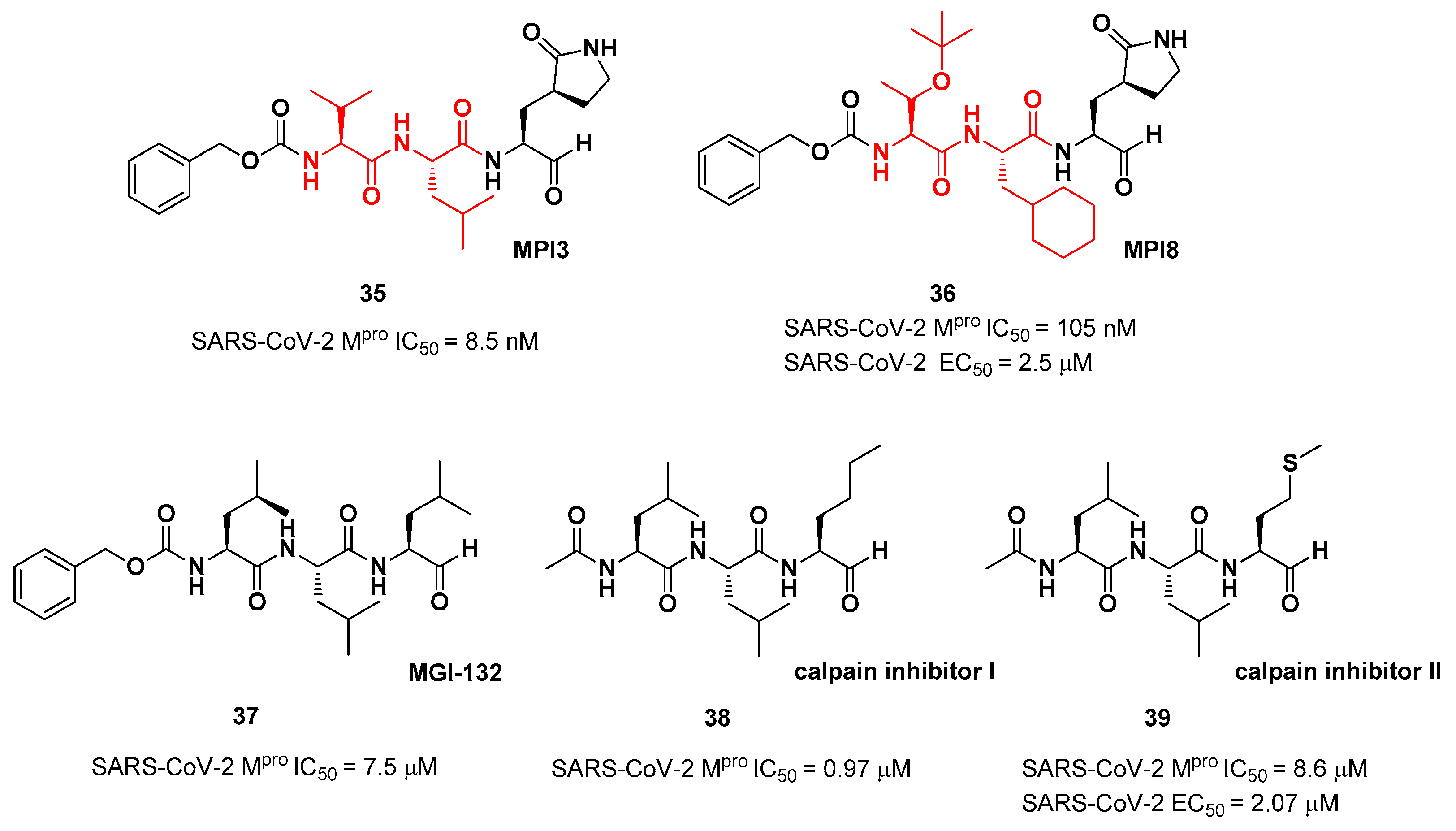
The aldehyde-based inhibitors discussed so far are dipeptidyl derivatives. However, there are some examples of notable tripeptidyl derivatives (
Figure 23). Compound
35 and
36, called
MPI3 and
MPI8, respectively, are the most active tripeptides designed and synthetized by Yang
et al. [
47]. Both compounds have the aldehyde warhead at P1’ and a (
S)-γ-lactam group at P1.
35 is endowed with
l-Leu at P2 and a Cbz-protected
l-Val at P3, while in
36 we have a cyclohexyl ring at P2 and a Cbz-protected
t-Bu Thr at P3. In the
in vitro enzymatic inhibitory activity assay,
35 turned out to be the most active compound displaying an IC
50 value of 8.5 nM. Conversely, in the
in vitro antiviral test
35 turned out to be less active than
36 (EC
50 = 2.5 μM in VeroE6 cells) in probably due to the presence of all natural amino acids that can be hydrolyzed by cellular proteases [
47]. Such a high potency could be related to the dual inhibition of SARS-Co-2 M
pro (IC
50 = 105 nM) and cathepsin L (IC
50 = 1.2 nM). This assumption led to consideration of the tripeptidyl proteasome inhibitor
MGI-132 (compound
37 in
Figure 23) [
48], and
calpain/cathepsin inhibitor I and
II (compounds
38 and
39, respectively, in
Figure 23) as potential SARS-CoV-2 M
pro inhibitors [
39].
In vitro enzymatic inhibition and cell-based antiviral tests brought about interesting results, in particular for the
calpain inhibitor II. This could represent an interesting starting point for the development of dual inhibitors of calpain/cathepsin and SARS-CoV-2 M
pro.
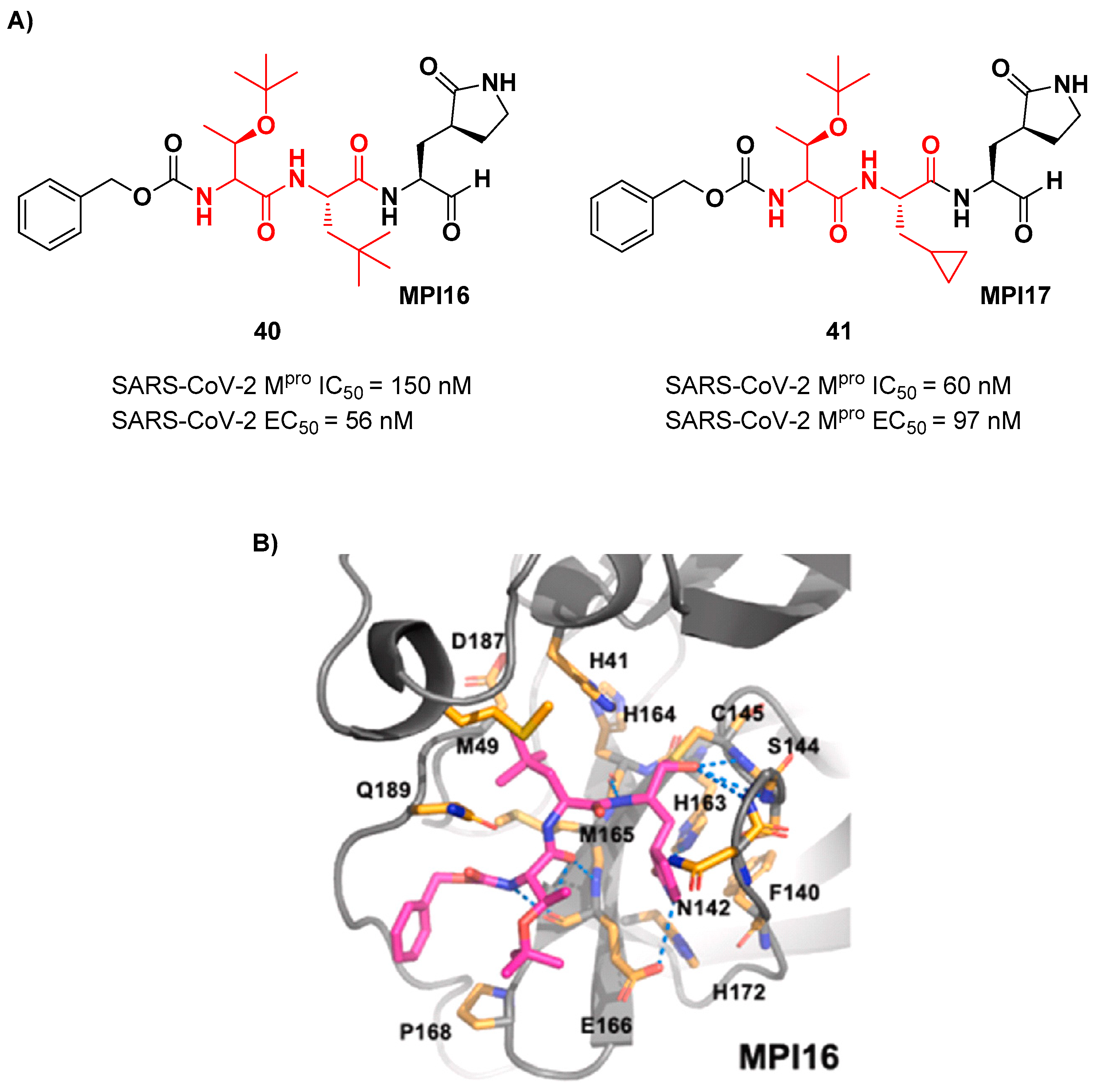
Ma
et al. developed another series of tripeptidyl derivatives maintaining the aldehyde warhead at P1’ and the (
S)-γ-lactam group at P1, and then varying the chemical composition at both P2 and P3 positions [
49]. The most interesting compounds were
40 and
41, also called
MPI16 and
MPI17 (
Figure 24). At P3 position an
O-
t-butyl-Thr residue is present in both inhibitors, while at P2 a
t-butyl group and a cyclopropyl ring are in
40 and
41, respectively. These compounds showed excellent enzymatic inhibitory activity (
40: IC
50 = 150 nM;
41: IC
50 = 60 nM), high potency in the cell-based assays (
40: EC
50 = 56 nM;
41: EC
50 = 97 nM) and high antiviral activity against different variants of SARS-CoV-2, suggesting that the
O-
t-butyl-Thr residue at P3 can be a key structural element for future design. From the X-ray structure of the SARS-CoV-2 M
pro in complex with
40 emerged that the
O-
t-butyl-Thr resudue may furnish supplementary Van der Waals interactions with Pro168 and Glu166 [
49].
6. Ketones
The ketone group is extensively expoilted as electrophilic warhead in the design of cysteine protease inhibitors including SARS-CoV-2 M
pro inhibitors. Ketones are less reactive than aldehydes; then electron withdrawing groups (especially halogens) in the α position are often needed to increase the electrophilic character of carbonyl carbon and make it more susceptible to undergo nucleophilic attack. The first example of ketone-based inhibitor was the already cited
PF-00835231 (
2 in
Figure 4), an α-hydroxy ketone-based peptidomimetic, and its corresponding phosphate prodrug
PF-07304814 (
3 in
Figure 4), developed by Pfizer Inc. against SARS-CoV infection during the 2002 SARS outbreak and reproposed as SARS-CoV-2 M
pro inhibitors [
24].
2 showed potent inhibitory activity toward SARS-CoV M
pro (
Ki = 4 nM in SARS CoV-1 protease FRET assay) [
50], as well as toward SARS-CoV-2 M
pro (IC
50 = 6.9 nM;
Ki = 0.27 nM [
25]). Successively, the antiviral activity of
2 was assessed on epithelial VeroE6 cells infected with SARS-CoV-2 showing promising results (EC
50 = 231 nM). Both
2 and
3 entered
in vivo preclinical studies showing comparable enzimatic inhibitory and antiviral activity.
3 displayed superior solubility and pharmacokinetics properties as compared to
2. The X-ray structure of the covalent complex SARS-CoV-2 M
pro/
2 evidenced the key interactions with the target: the carbonyl carbon of the α-hydroxy ketone moiety at P1’ binds covalently the Cys145 thiol group to form an hemithioketal adduct that, together with the α-hydroxy group, establishes additional H-bonds with His41 and Gly143 within the S1 subpocket. The γ-lactam moiety at P1 and the Leu resdue at P2 insert into subsites S1 and S2, respectively, while the P3 indole moiety estabilishes Van der Waals interaction with residues at S3 [
25].
Taking into consideration the structures of the already published SARS-CoV-2 inhibitors
PF-00835231 and
GC-376, Bai
et al. explored different α-acyloxymethyl ketones bearing a six-membered lactam moiety at P1, in order to mimic the Gln residue [
51]. The most promising compounds turned out to be
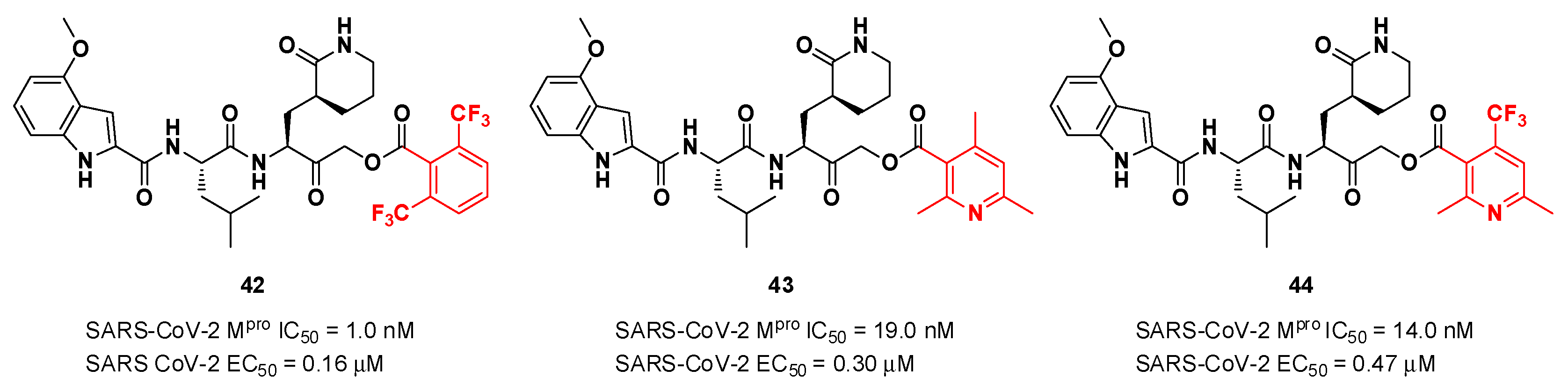
42,
43 and
44 (
Figure 25), which bear a 2,6-bis(trifluoro)methylbenzoate, 2,4,6-trimethylnicotinate and 4-trifluoromethyl-2,6-dimethylnicotinate fragment as
C-terminal α-acyloxy moiety, respectively.
Compound
42, which was already investigated by Kratz
et al. as inactivator of cathepsin B [
52], showed the best results in terms of enzymatic inhibitory activity against SARS-CoV-2 M
pro (IC
50 = 1.0 nM) and excellent SARS-CoV-2 replication inhibition
in vitro (EC
50 = 0.16 μM) [
51]. In regard to the two nicotinic-based inhibitors,
43 exhibited a better inhibition profile (IC
50 = 19.0 nM; EC
50 = 0.30 μM). The irreversible mechanism of action of
43 was confirmed by X-ray analysis (
Figure 26): the attack of the Cys145 thiol group to the α-carbon of the ketone group and the loss of the 2,4,6-trimethylnicotinate as a leaving group. The six-membered lactam inserts into the S1 pocket establishing the same H-bonds as for the five-membered lactam of
GC-376. Similar interactions involving other positions are in accordance with the already discussed
GC-376 [51].
Other remarkable ketone-based SARS-CoV-2 M
pro inhibitors are represented by the
PF-00835231 benzothiazolyl analogs
5,
6 and
7 (
Figure 5), already discussed in the section of
Nirmatrelvir [
25]. Many other benzothiazolyl ketones with anti-SARS-CoV activity have been repurposed as SARS-CoV-2 M
pro inhibitors. One example is represented by the compound
45 (
Figure 27) developed by Hattori
et al. [
53], also called
YH-53 [54].
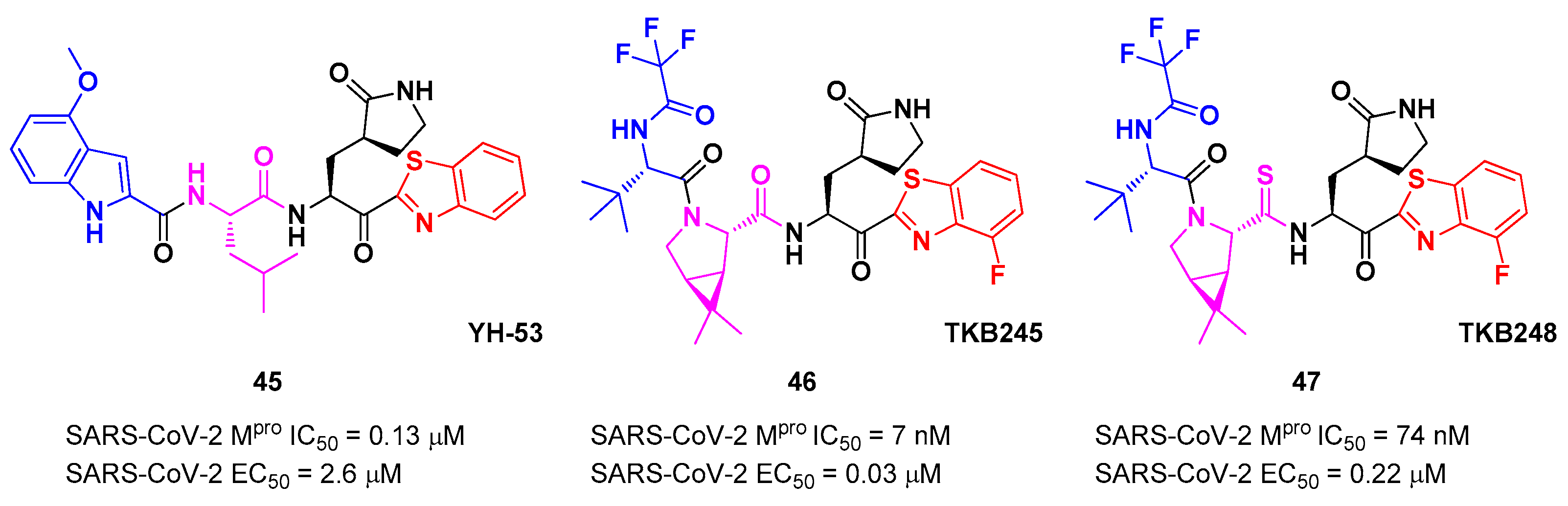
45 bears a P1’ benzothiazolyl ketone, a P1
γ-lactame moiety, a P2 Leu residue and a P3 indole substituent. The SARS-CoV-2 M
pro assays reported for this compound an IC
50 of 0.13 μM and an antiviral activity of 2.6 μM assessed in VeroE6 cells [
53]. Starting from this lead compound, Higashi-Kuwata
et al. considered the introduction of fluorine atoms to improve pharmacokinetics properties, due to the higher lipophilicity of the C-F bond compared to C-H bond [
55]. Among several modifications, they explored 4-fluorinated benzothiazole ketones, obtaining compounds
46 and
47 (
Figure 27), named
TKB245 and
TKB248, respectively, as the most promising M
pro inhibitors. By replacing the 4-methoxy-indole ring at P3 with the trifluoroacetyl
l-α-
tert-butyl Gly, and the Leu residue at P2 with the 6,6-dimethyl-3-azabicyclohexane fragment, they achieved
46 which exhibited an improved enzymatic inhibitory and antiviral activity with respect to
45 (IC
50 = 7 nM μM; EC
50 = 0.03 μM). To reduce the hydrolysis rate of this compound and improve its pharmacokinetic properties, the carbonyl group at P2 was replaced with a thiocarbonyl group affording compound
47, (named
TKB248). Pharmacokinetic studies evidenced higher T
1/2 for
47 as compared to
46 (4.34 h
vs 3.82 h
), although the enzymatic inhibitory and antiviral activity were lower (IC
50 = 74 nM; EC
50 = 0.22 μM) [
55].
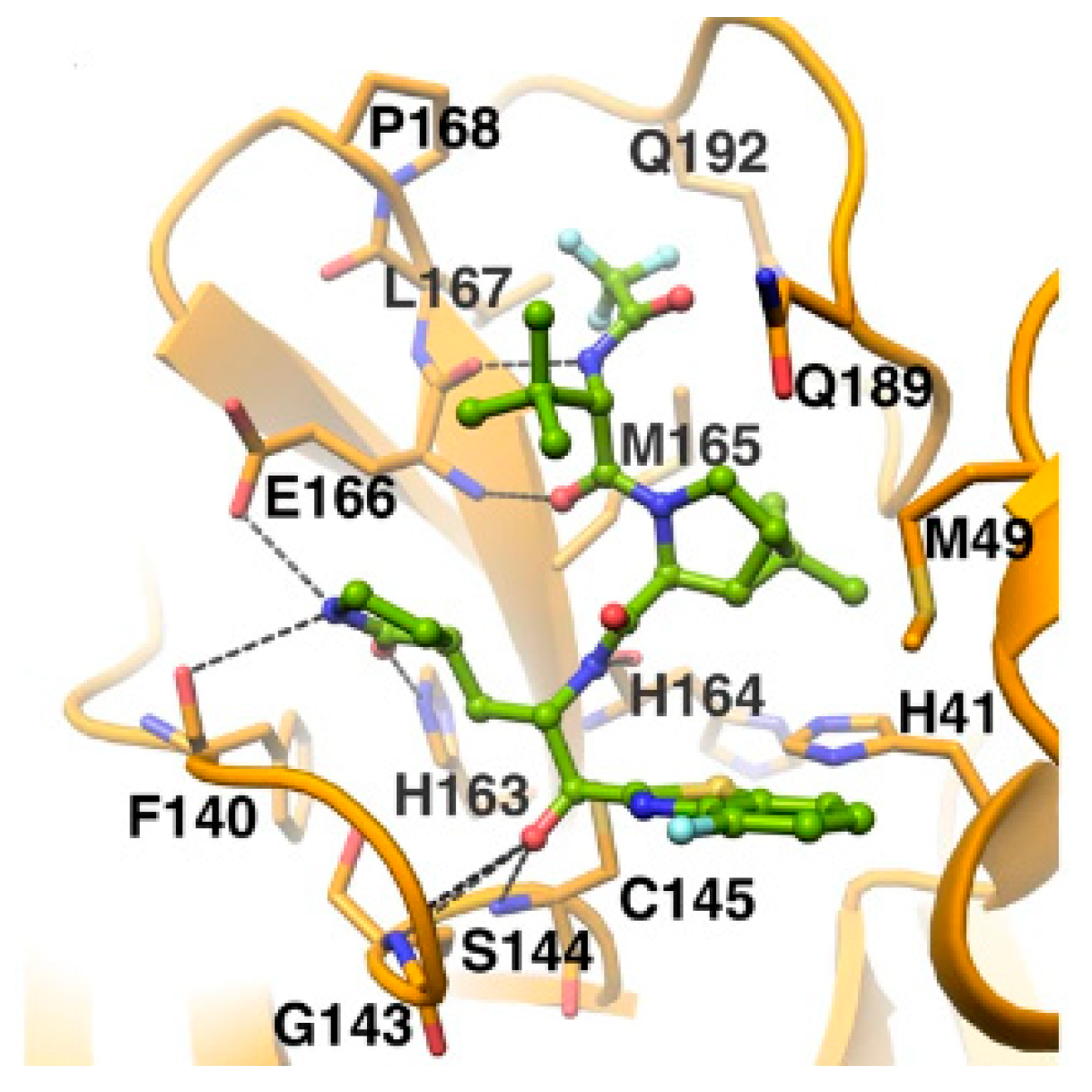
The
in vivo evaluation of efficacy and pharmacokinetic parameters of both compounds were performed on human ACE2-knocked-in mice exposed to SARS-CoV-2, showing promising results. From the mass spectrometric analysis, it was found that both compounds promote dimerization of M
pro, which is bound to these inhibitors, preventing the entrance of the natural substrate. To elucidate the inhibition mechanism, the X-ray structures of
46 and
47 in complex with SAR-CoV-2 M
pro were obtained, showing that both compounds have identical binding mode with the active site [
55]. As an example,
Figure 28 shows the co-crystal structure of
46 with SAR-CoV-2 M
pro: the carbonyl carbon at P1’ forms a covalent thiohemiacetal adduct with Cys145 and the thiohemiacetal-OH establish H-bonds with Gly143 and Ser144, while the 4-fluorobenzothiazole ring fills the S1’ subsite with the 4-fluorine atom that points out of the subpocket. At the P1 position the γ-lactame ring establishes H-bonds with His-163, Glu166 and Phe140, while the centered amide group forms another H-bond with His164. The dimethyl-bicyclo[3.1.0]-proline moiety fits into the S2 pocket, while the trifluoromethylacetamide group at P3 forms halogen interactions with residues of the S3 pocket [
55].
Yang
et al. reported novel benzothiazolyl-based peptidomimetics acting as SARS-CoV-2 M
pro inhibitors, which were designed starting from
Nirmatrelvir [
56]. They firstly introduced the [2.2.1]azabicylic ring at P2 position and the benzothiazolyl warhead at the
C-terminus, then they began to explore different substitutions at P3. The most promising derivative was the compound
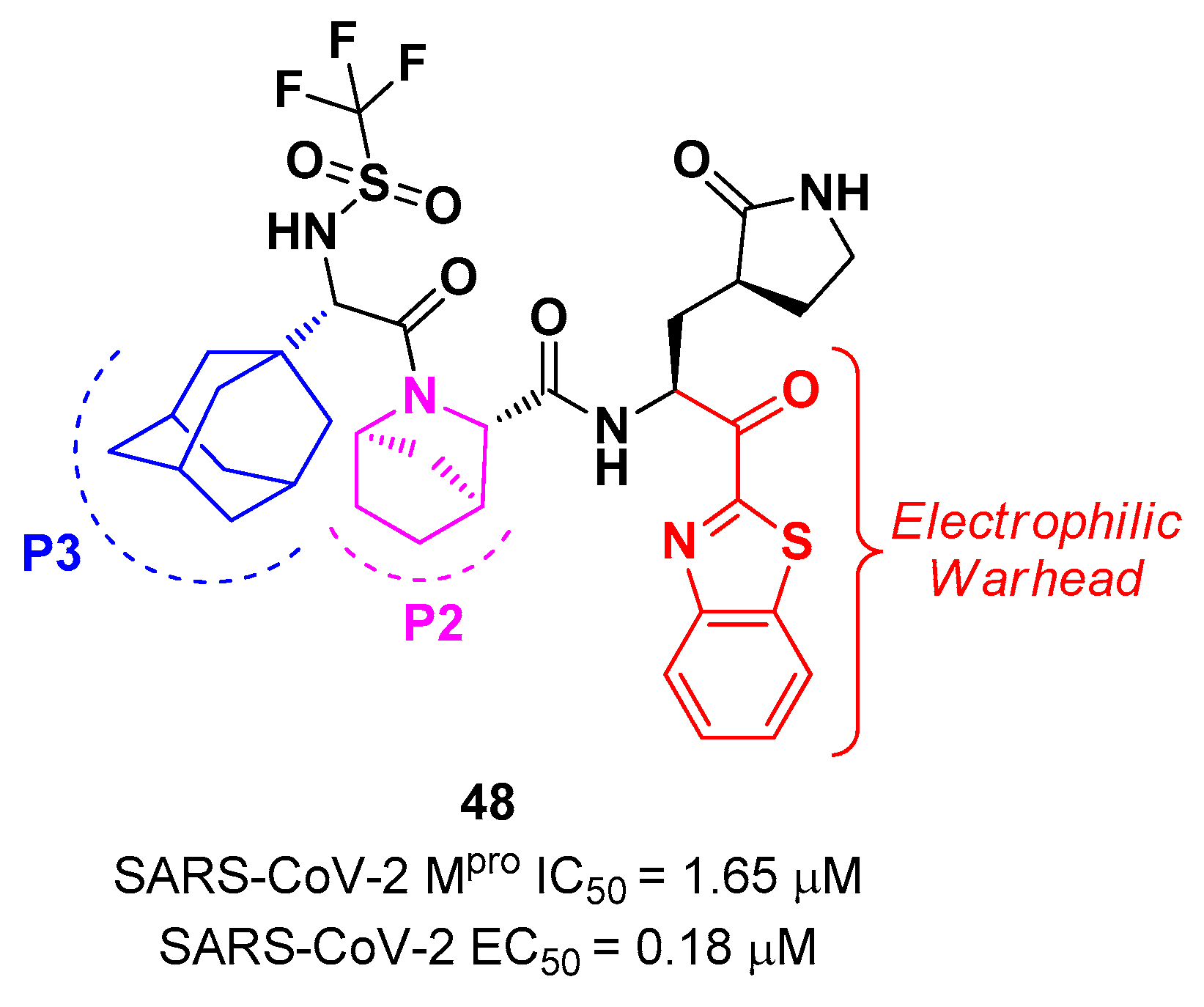
48 (
Figure 29), containing a trifluoromethanesulfonamide group at the
N-terminus and an adamantly group at P3.
48 exhibited an interesting inhibitory activity against SARS-CoV-2 M
pro (IC
50 = 1.65 μM), a good antiviral activity against SARS-CoV-2 infected VeroE6 cells (EC
50 = 0.18 μM) and low cytotoxicity. Most importantly, the PK properties and target selectivity of compound
48 were superior than those of other derivatives [
56].
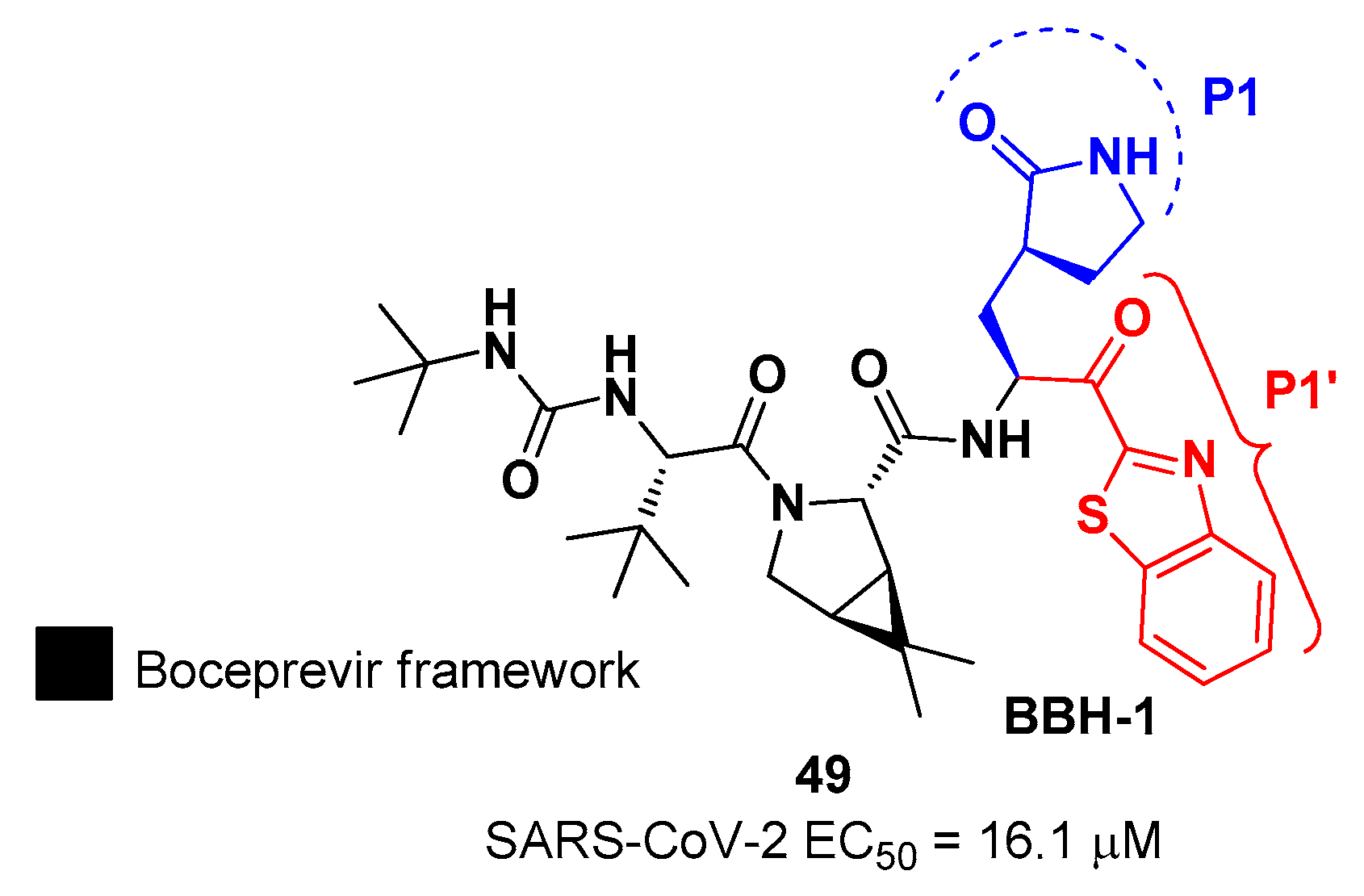
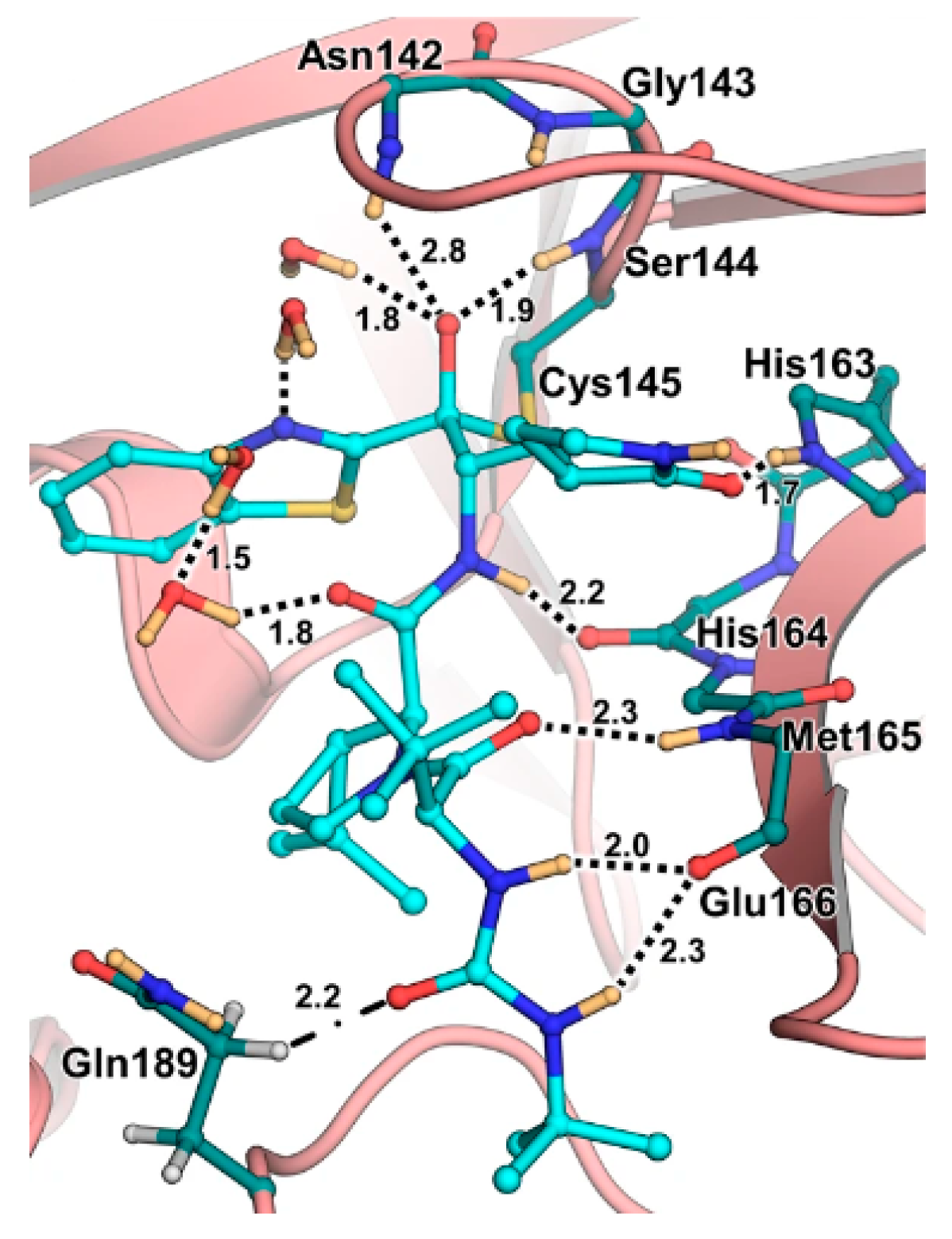
In the already mentioned work of Kneller
et al., beside the development of nitrile-based compounds, the authors designed a novel SARS-CoV-2 M
pro inhibitor bearing a benzothiazolyl ketone warhead [
32].
49, called
BBH-1, was obtained by replacing the P1’ ketoamide group of
Boceprevir with benzothiazolyl ketone warhead, while the hydrophobic P1 group was replaced with a γ-lactam ring found in
GC-376 (
Figure 30).
The antiviral activity of this inhibitor was determined in SARS-CoV-2 M
pro infected VeroE6 TMPRSS cells in presence of a P-glycoprotein inhibitor, exhibiting EC
50 value of 1.54 μM, while the same experiment in absence of the P-glycoprotein inhibitor displayed EC
50 value of 16.1 μM. The X-ray/neutron (XN) crystal structure of SARS-CoV-2 M
pro in complex with
49 was obtained (
Figure 31), in order to get insights on the binding mode of the inhibitor and the protonation state of
49-M
pro complex. The XN structure revealed the formation of a covalent bond between the Cys145 residue and the ketone warhead with the newly formed hemithioketal group unprotonated and its negatively charged oxygen inserted into the oxyanion hole of the target. The alkoxy anion is hydrated by a water molecule and stabilized by an H-bond with the Cys145 backbone. Water molecules interact also with the aromatic rings of the benzothiazolyl moiety, which due to its bulkiness pushes the His41 residue away from its original position causing the elimination of the catalytic water molecule from the active site and deprotonation of His164. The γ-lactam of the P1 group inserts into the substrate binding subsite S1 cavity, forming an H-bond between carbonyl oxygen and His163 sidechain. The remaining amide groups and urea group of
49 are involved in several H-bonds with His164 and Glu166, while the carbonyl oxygen of the urea moiety forms an H-bond with the side chain of Gln189 [
32].
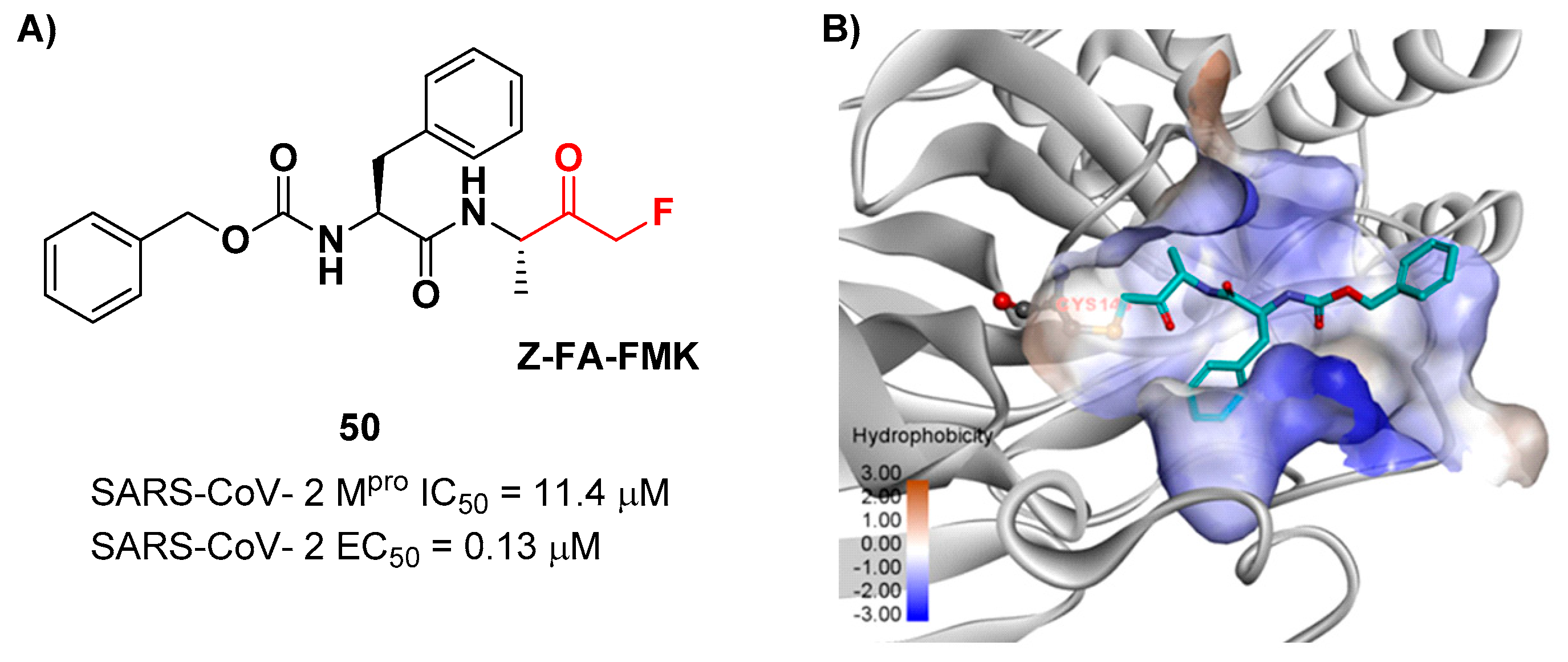
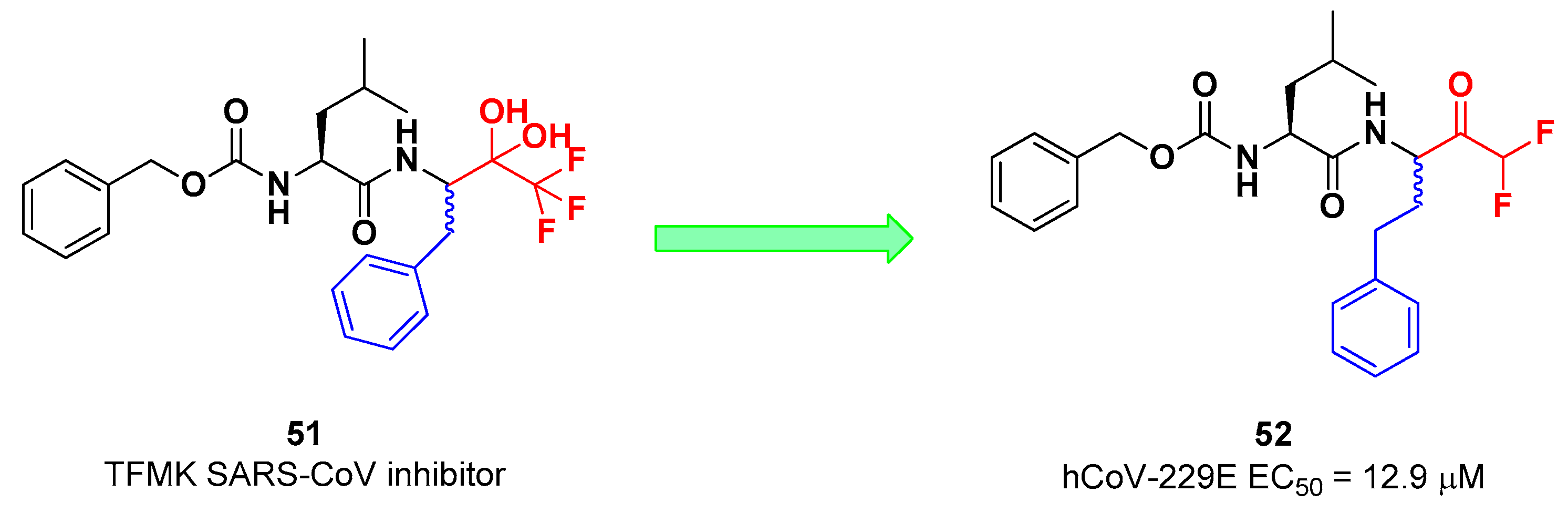
Another interesting example of ketone-based SARS-CoV-2 M
pro inhibitors is represented by fluoromethyl ketones (FMKs). The electron-withdrawing effect exerted by the fluorine atoms in α-position of a methylketones enhances the electrophilicity of the carbonyl carbon toward nucleophilic attack of the thiol group of cysteinyl proteases [
2]. Based on the number of fluorine atoms in α-position, it is possible to distinguish mono-fluoromethyl ketones (MFMKs), di-fluoromethyl ketones (DFMKs), and tri-fluoromethyl ketones (TFMKs) [
57]. Zhu
et al. reported a MFMK as SARS-CoV-2 M
pro inhibitor from a quantitative high throughput screening of existing compounds [
58]. This compound, called
Z-FA-FMK (
50 in
Figure 32A), is also an irreversible inhibitor of caspase-3.
50 showed a potent enzymatic inhibitory activity toward M
pro (IC
50 = 11.4 μM) and high antiviral activity (EC
50 = 0.13 μM), obtained from SARS-CoV-2 infected VeroE6 cells exploiting a CPE reduction assay [
58]. Docking models of
50 with SARS-CoV-2 M
pro showed that the thiol group of Cys145 performs a nucleophilic attack at the α-position of the methylketone group in an S
N2-like reaction, where the fluorine atom acts as a leaving group (
Figure 32B) [
2]. However, MFMKs are easily catabolized forming toxic metabolites such as fluoroacetate, a feature that compromises their therapeutic utility [
57].
On the other hand, DFMKs and TFMKs seem to be more prone to the therapeutic use as they enhance the electrophilicity of the carbonyl group without manifesting metabolic issues [
2]. In fact, TFMKs have been already investigated as SARS-CoV M
pro inhibitors, in particular with the development of compound
51, as reported Shao
et al. (
Figure 33) [
59]. Normally they act as reversible covalent inhibitors toward the target but, in their hydrated form, they can also act as transition-state competitive analogues [
2]. Citarella
et al. synthetized a new dipeptidyl DFMK as SARS-CoV-2 M
pro inhibitor (
i.e. compound
52;
Figure 33) [
60], based on the corresponding TFMKs SARS-CoV M
pro inhibitor reported by Shao
et al., using a direct and chemoselective difluoromethyl unit transfer reaction [
61,
62].
52 bears a Cbz-Leu-HomoPhe sequence as peptide framework linked to a
C-terminal DFMK moiety. Compound
52 exhibited important antiviral activity toward MRC5 cell monolayer infected with hCoV-229E (EC
50 = 12.9 μM).
In silico studies showed that the two fluorine atom are able to establish halogen bond with residues of the S1’ pocket [
60].
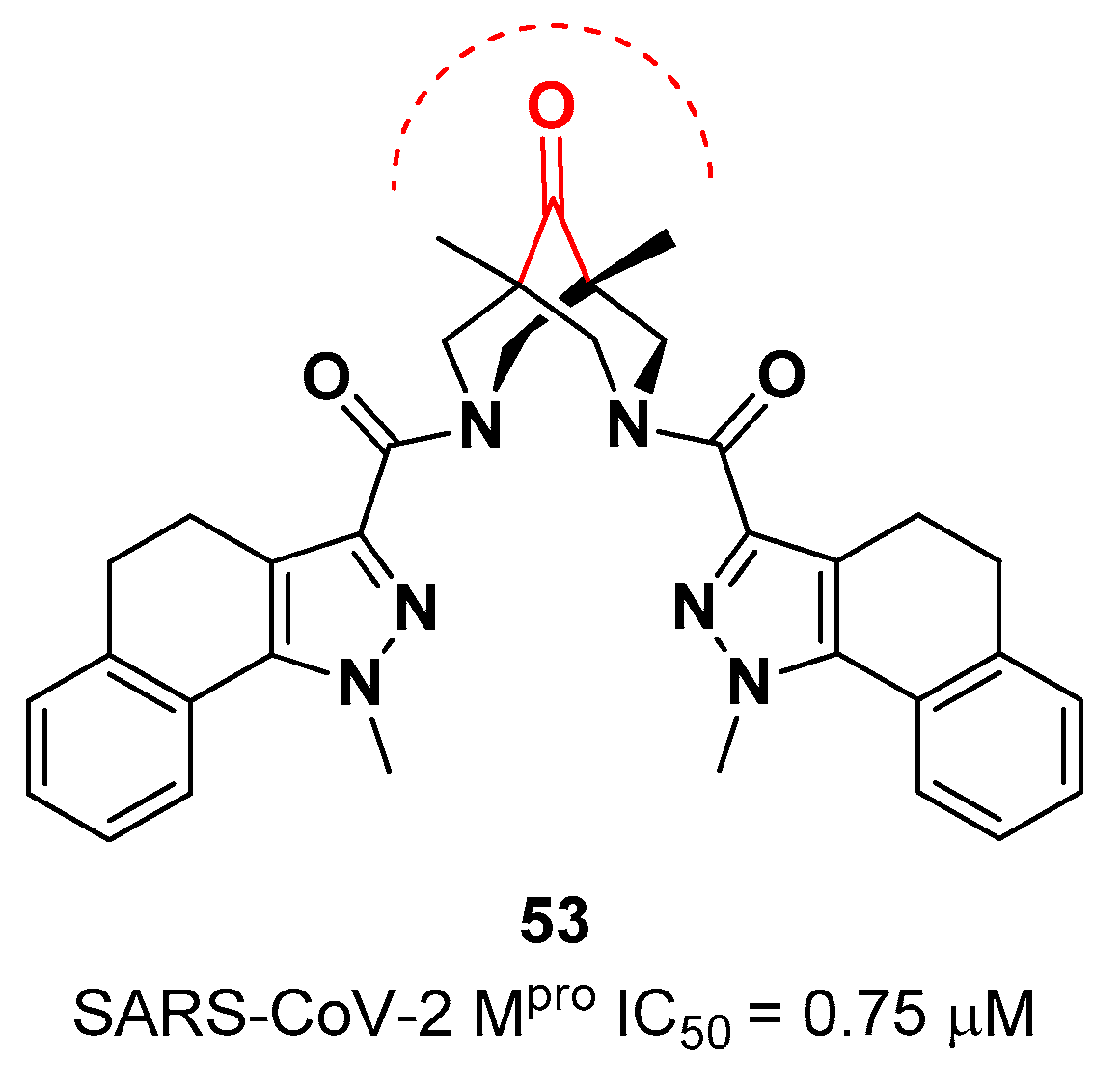
The ketone warhead was also applied in a non-peptidic compound, in particular in a bispidine-based ketone
53 (
Figure 34).
In silico studies suggested that the central carbonylic function act as an electrophilic warhead during the formation of the covalent adduct with Cys145. Enzymatic inhibitory activity of compound
53 toward SARS-CoV-2 M
pro showed noteworthy results (IC
50 = 0.75
μM) [
63]
.
7. α-Haloacetamides
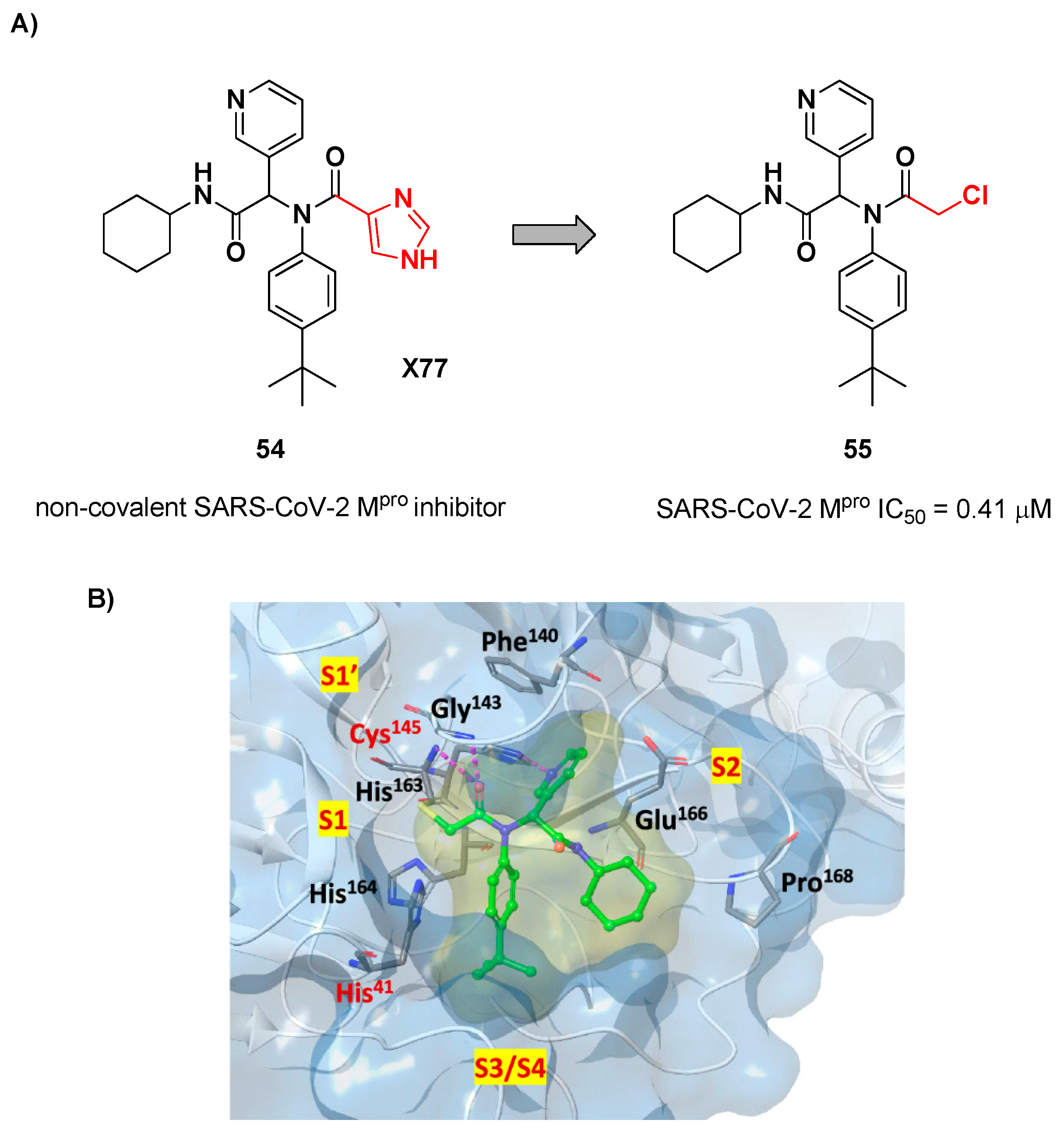
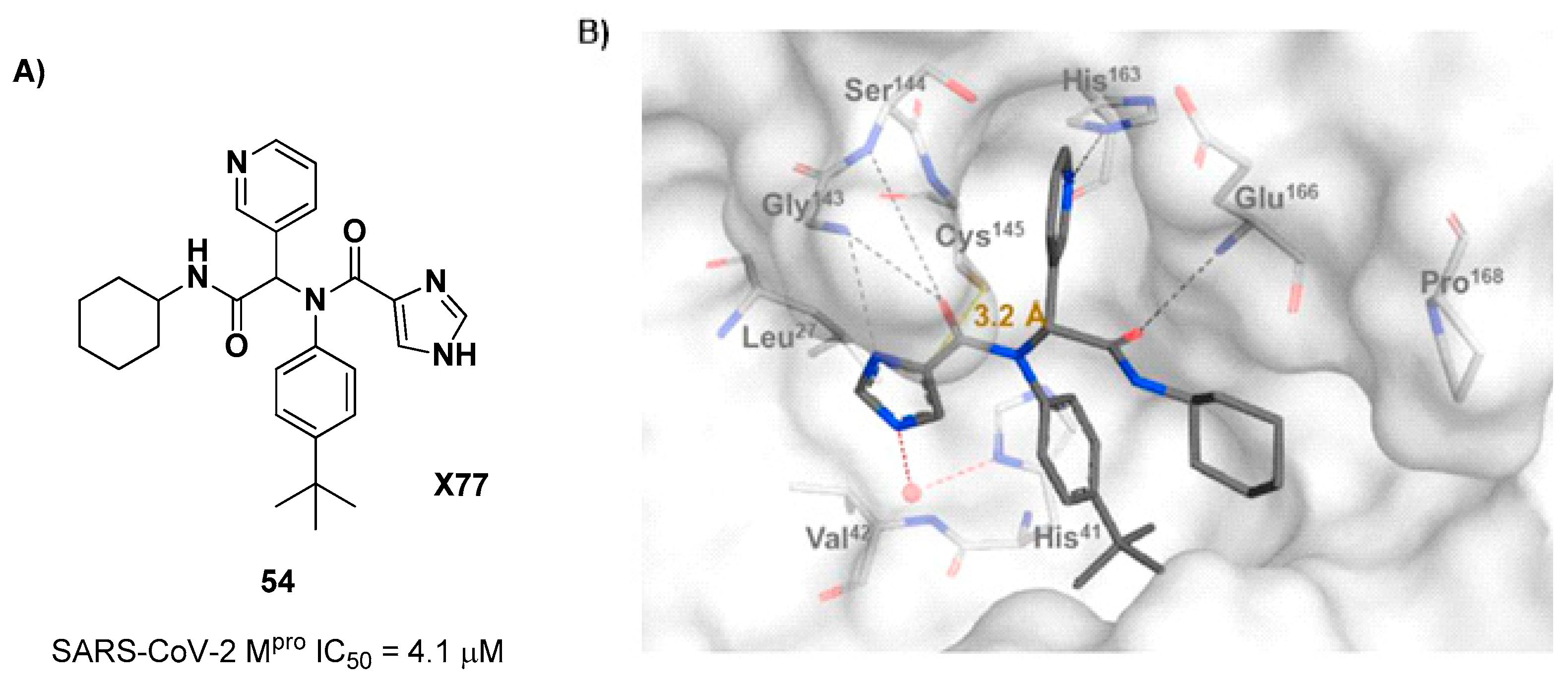
The design of new SARS-CoV-2 M
pro inhibitors during these years has been also focused on α-haloacetamide-based compounds. The halogen atoms in α-position act as leaving groups during the cysteinyl nucleophilic attack on C-X, leading to an irreversible inhibition of the enzyme due to the formation of a S-C covalent bond. A first example of of α-haloacetamide SARS-CoV-2 M
pro inhibitor derives from the work of Stille
et al. [
64]. Starting from the non-covalent SARS-CoV-2 M
pro inhibitor
54 (
Figure 35A), called
X77, Stille and co-workers carried out a study of covalent docking by replacing the P1’ imidazole ring with different electrophile warheads. From this study the most promising compound turned out to be
55 (
Figure 35A), that showed an interesting enzymatic inhibitory activity against SARS-CoV-2 M
pro (IC
50 = 0.41 μM and
KI = 16 μM). Structure-activity relationship (SAR) analysis of the entire panel of synthesized compounds revealed that the pyridine ring is fundamental to achieve inhibitory activity, the
tert-butyl group is beneficial in terms of potency while the replacement of C-Cl/ C-F is detrimental [
64]. The co-crystal structure of SARS-CoV-2 M
pro/
55 is showed in
Figure 35B: a covalent bond is formed between the thiol group of Cys145 and α-carbon, while the pyridyne and
tert-butylphenyl rings fit into the S2 and S4 subpockets, respectively.
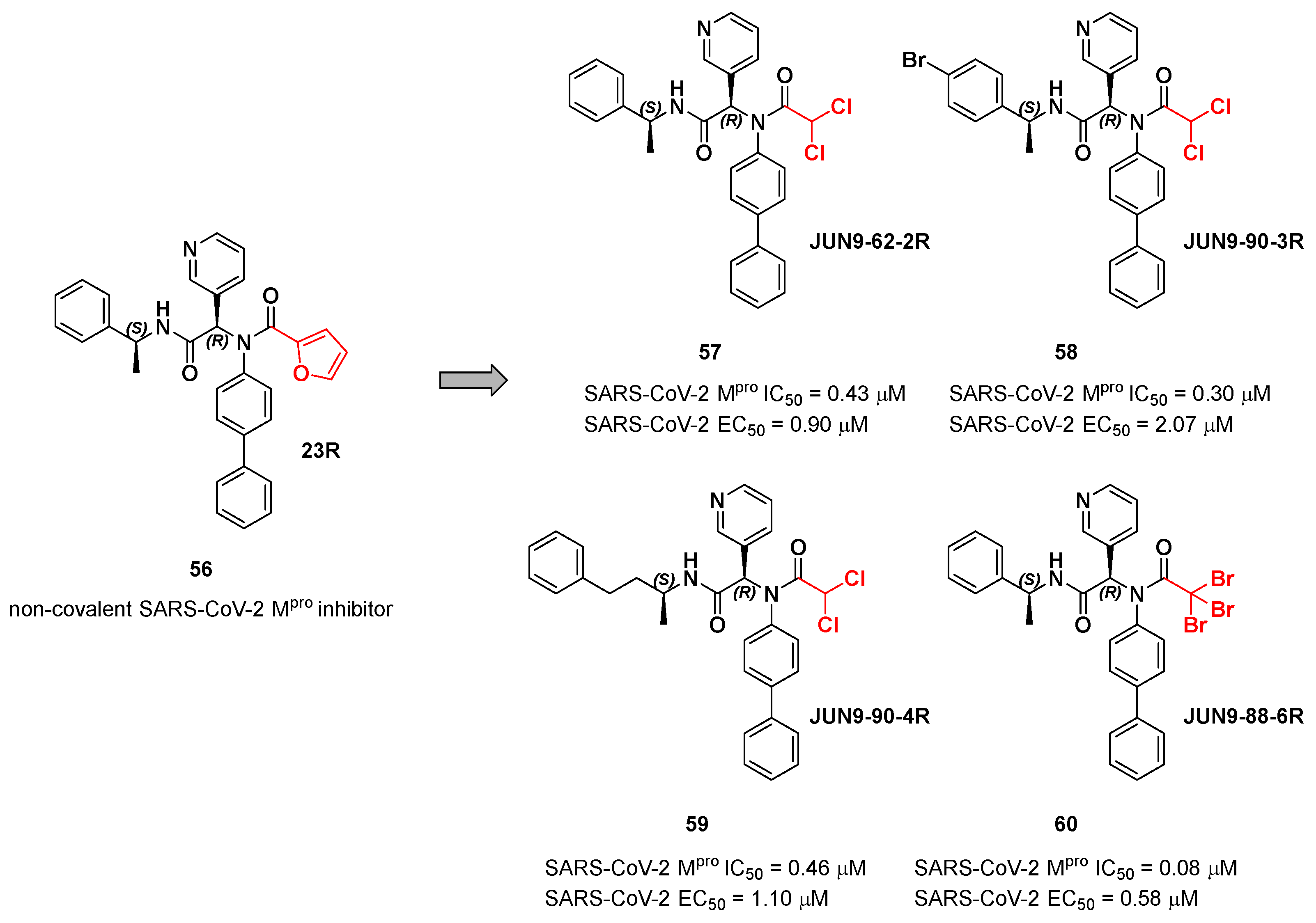
The same approach of the previous work was used by Wang
et al. for the design of new SARS-CoV-2 α-haloacetamide inhibitors [
65]. Starting from the non-covalent inhibitor
56 (
Figure 36), called
23R, they replaced its P1’ furyl ring with a series of di- and tri-haloacetamides. The most promising compounds of this series turned out to be
57,
58,
59 and
60, called
Jun9- 62-2R,
Jun9-90-3R,
Jun9-90-4R, and
Jun9-88-6R, respectively. Compounds
57,
58 and
59 are endowed with an α,α-dichloroacetamide moiety as an electrophilic warhead at P1’, while
60 contains an α,α,α-tribromoacetamide [
65]. All compounds exhibited outstanding and selective M
pro inhibition (IC
50 range 0.08–0.46 μM) and good antiviral efficacy on Caco2-hACE2 cells infected with SARS-CoV-2 (EC
50 range 0.58–2.07 μM).
The co-crystal structure of SARS-CoV-2 M
pro with compound
57 is shown in
Figure 37 and confirms the ability of this electrophile warhead to establish a covalent bond with the Cys145 residue. Moreover, the
R configuration of pyridine ring at P1 position seems to be fundamental for the fitting inside the S1 subsite.
Based on compound
56, a new series of α-chloro-fluoroacetamide (CFA) was proposed as covalent SARS-CoV-2 M
pro inhibitors by Yamane
et al. [
66]. This weak electrophilic warhead for cysteine –SH group was firstly explored in the design of tyrosine kinase inhibitors, demonstrating high selectivity with respect to other warheads. The CFA-S bond is reversable and can be hydrolyzed under neutral conditions to regenerate the cysteine residue [
67]. For this reason, off-target reactions with cysteine proteases and related drawbacks are limited. The most effective compound of this series was
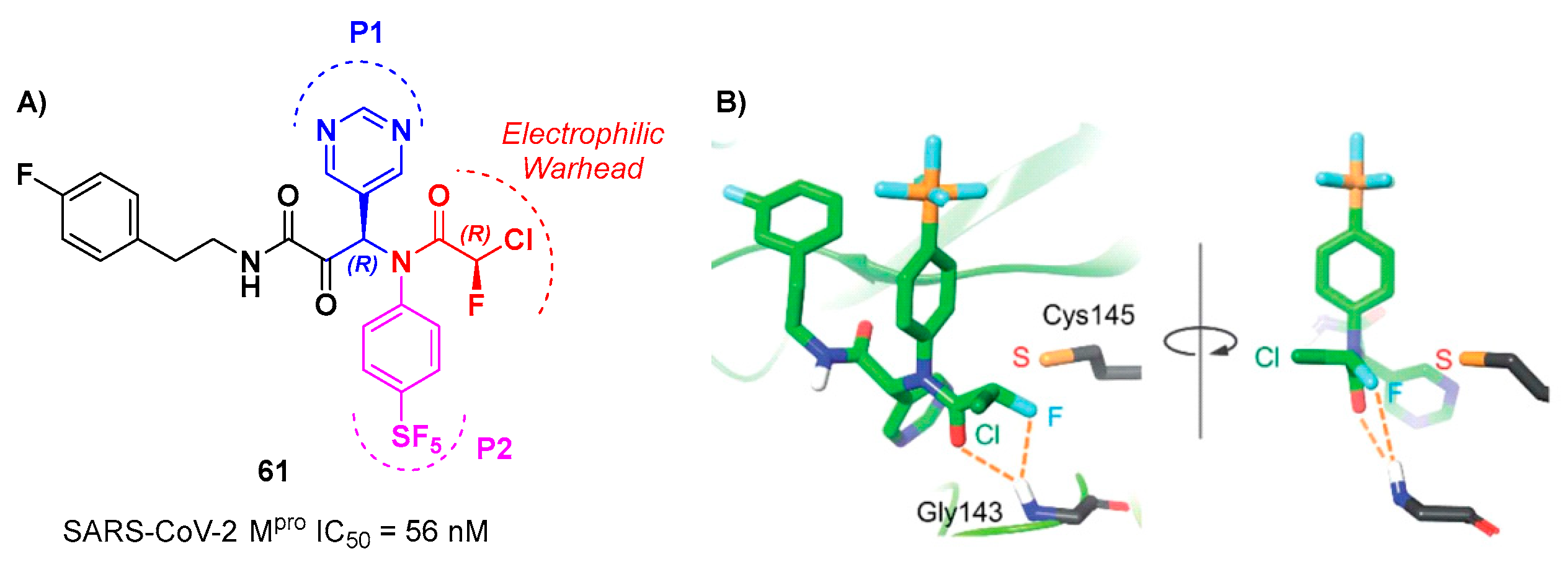
61 (
Figure 38A), which contains a pyrimidine ring at P1 and a phenyl-pentafluorosulfanyl group at P2 [
66]. Furthermore, it contains two stereocenters, thus four stereisomers. From the biological evaluation emerged the only the isomer with (
R,R)-configuration exhibits outstanding and selective enzymatic inhibitory activity (IC
50 = 56 nM). Docking simulations were performed for all stereoisomers of 61 (Figure 38B): the most stable pose of (
R,
R)-61 suggests that the fluorine atom of the CFA group is able to establish a H-bond with the backbone -NH of the Gly143 residue in the oxyanion hole at S1’, inducing the activation of the inhibitor towards the Cys145 catalytic residue [
66].
Other α-haloacetamide-based SARS-CoV-2 M
pro inhibitors were discovered by Xiong
et al. [
68]. They first performed a virtual screening of a set of commercial non-peptidic compounds from the ChemDiv database, followed by covalent docking. From this study, 8 compounds were selected for the enzymatic inhibitory activity tests toward SARS-CoV-2 M
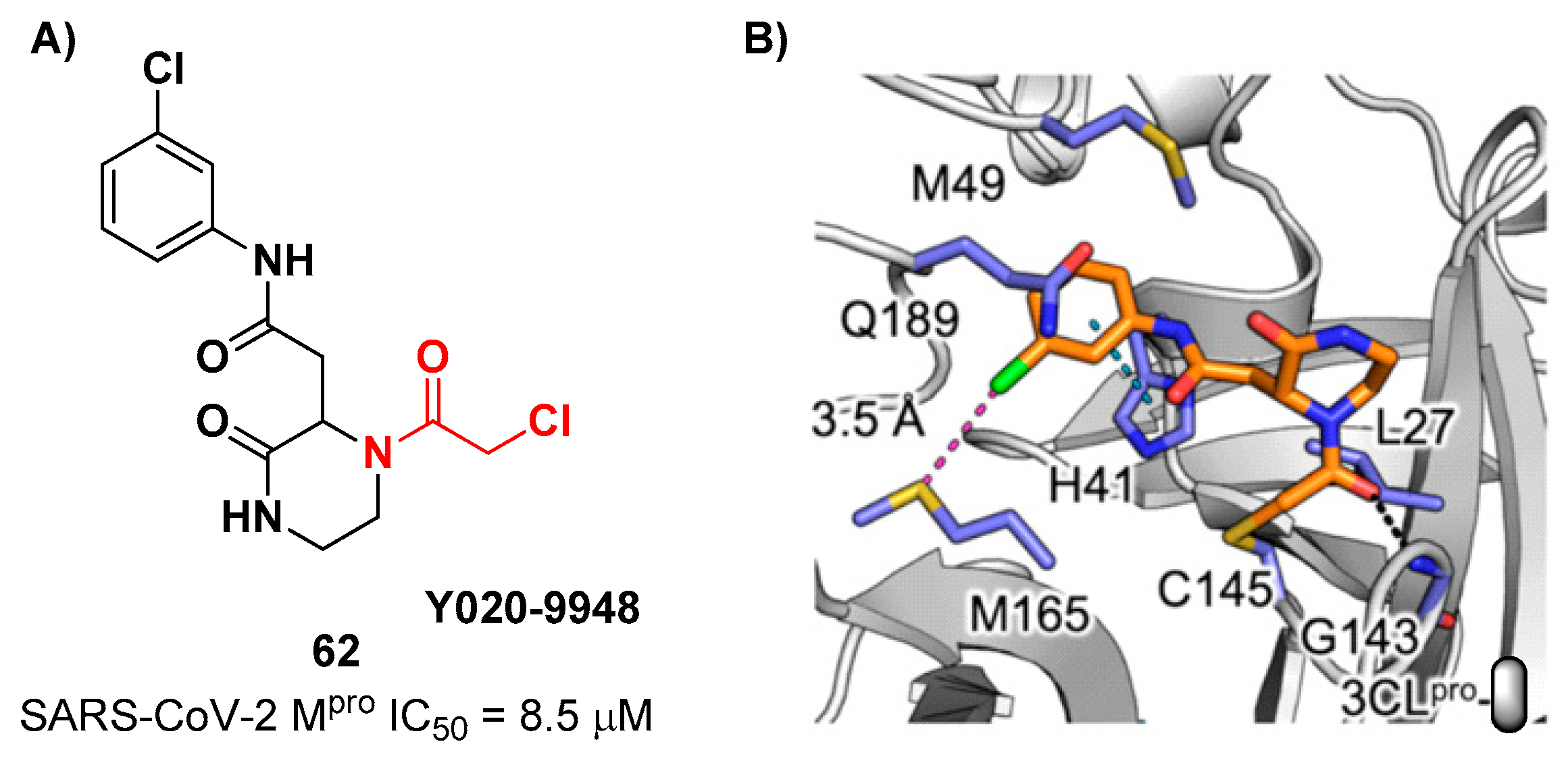
pro . Among them, 3 compounds showed remarkable activity with the piperazin-2-one-based compound
62 (
Figure 39), called
Y020-9948, as the most promising derivative (IC
50 = 8.5 μM). The discovery of this new non-peptidyl structure active toward M
pro led Xiong and co-workers to resolve the X-ray crystal structure of the complex SARS-CoV-2 M
pro/
62, in order to understand the binding mode and in view of future developments of more potent analogs. From the X-ray analysis it is possible to observe the formation of a covalent bond between the Cys145 –SH group and the methylene group of the electrophilic warhead. Furthermore, the carbonyl carbon of the warhead establishes a H-bond with the Gly143 residue, while the
meta-chlorophenyl moiety interacts by π–π stacking with His41 and a halogen-bond with the sulfur atom of the Met165 residue [
68].
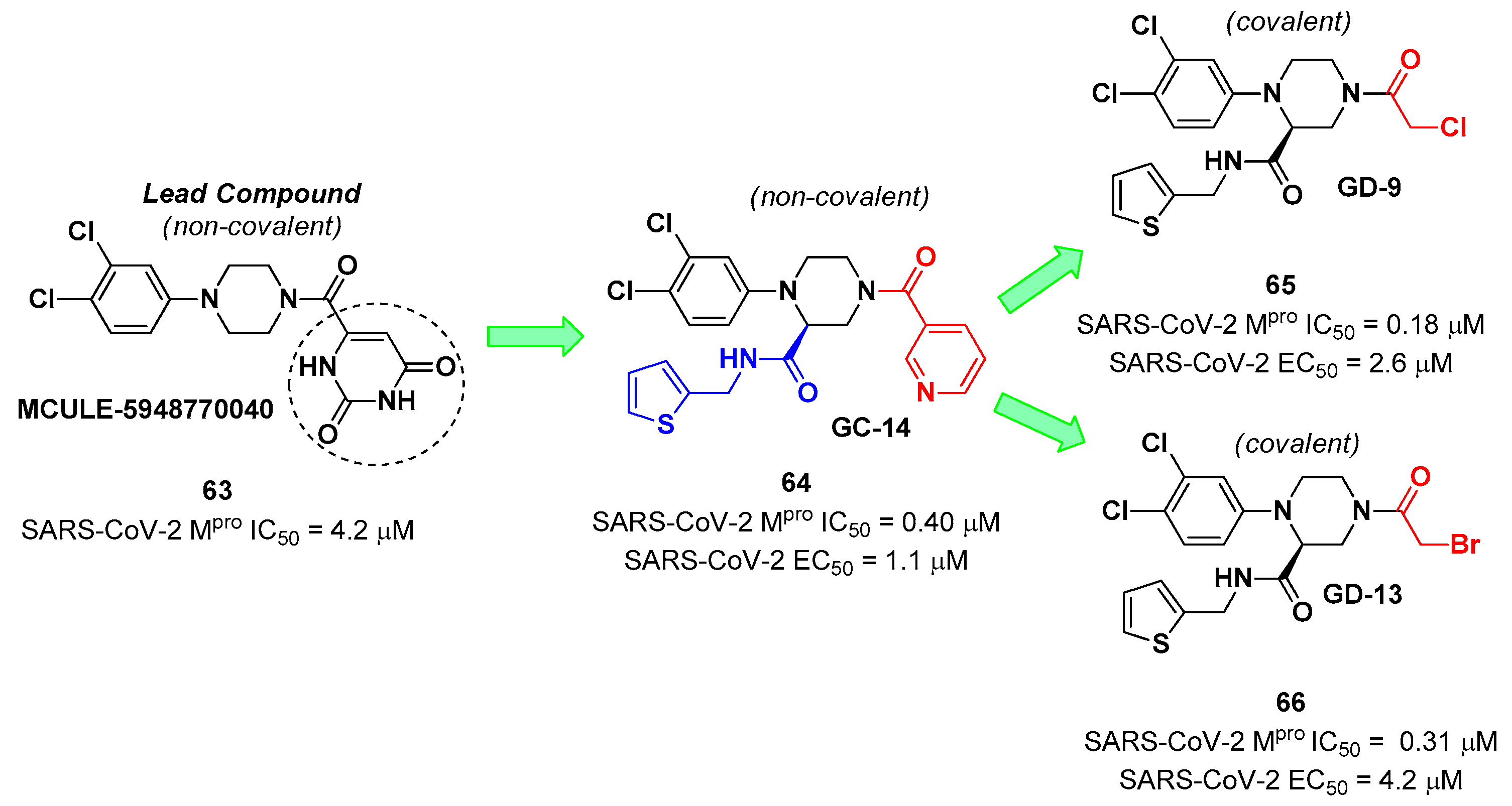
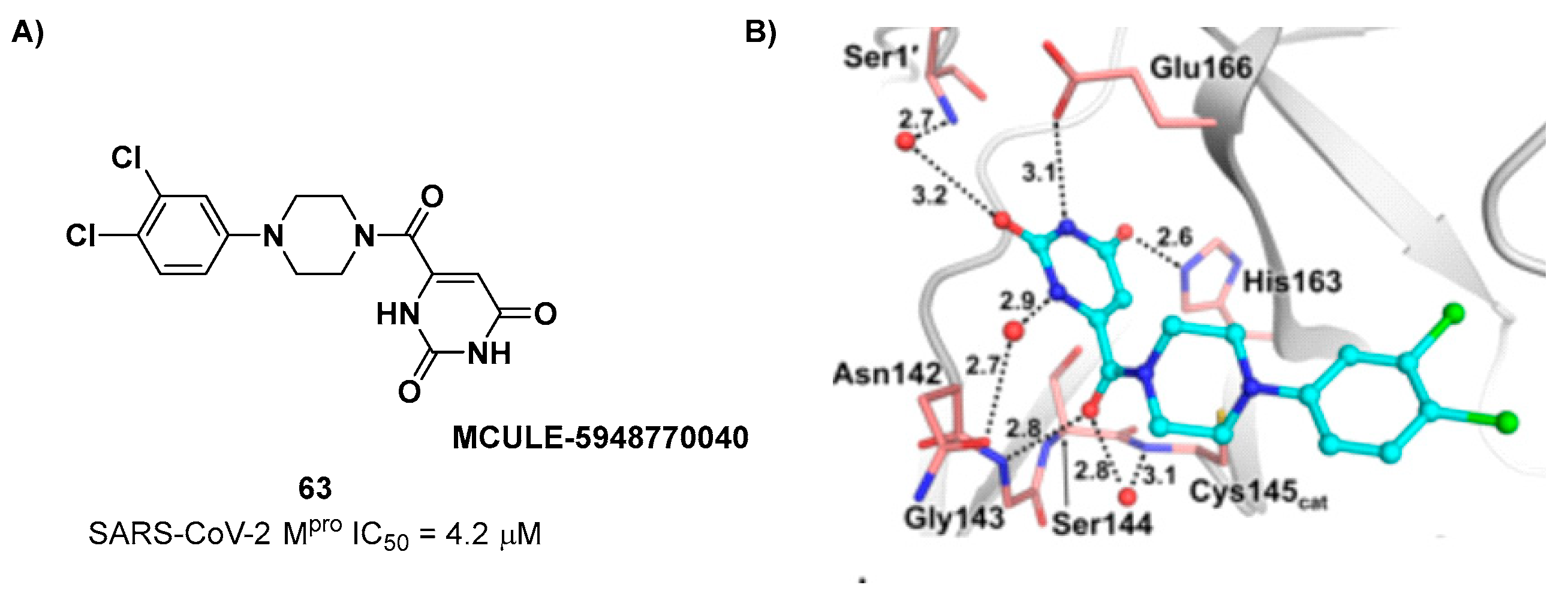
The non-covalent inhibitor
63 (
Figure 40), called
MCULE-5948770040, was discovered
via high-throughput virtual screening by Clyde
et al. [
69]. It showed moderate inhibitory activity against M
pro (IC
50 = 4.2 μM). From the X-ray structure of the complex SARS-CoV-2 M
pro/
63 it can be observed that the P1-uracil and P2-dichlorobenzene moieties are inserted into the S1 and S2 subsites, respectively, linked through the piperazine ring which lays above the Cys145 catalytic residue, while the S3/S4 subsites remain unoccupied [
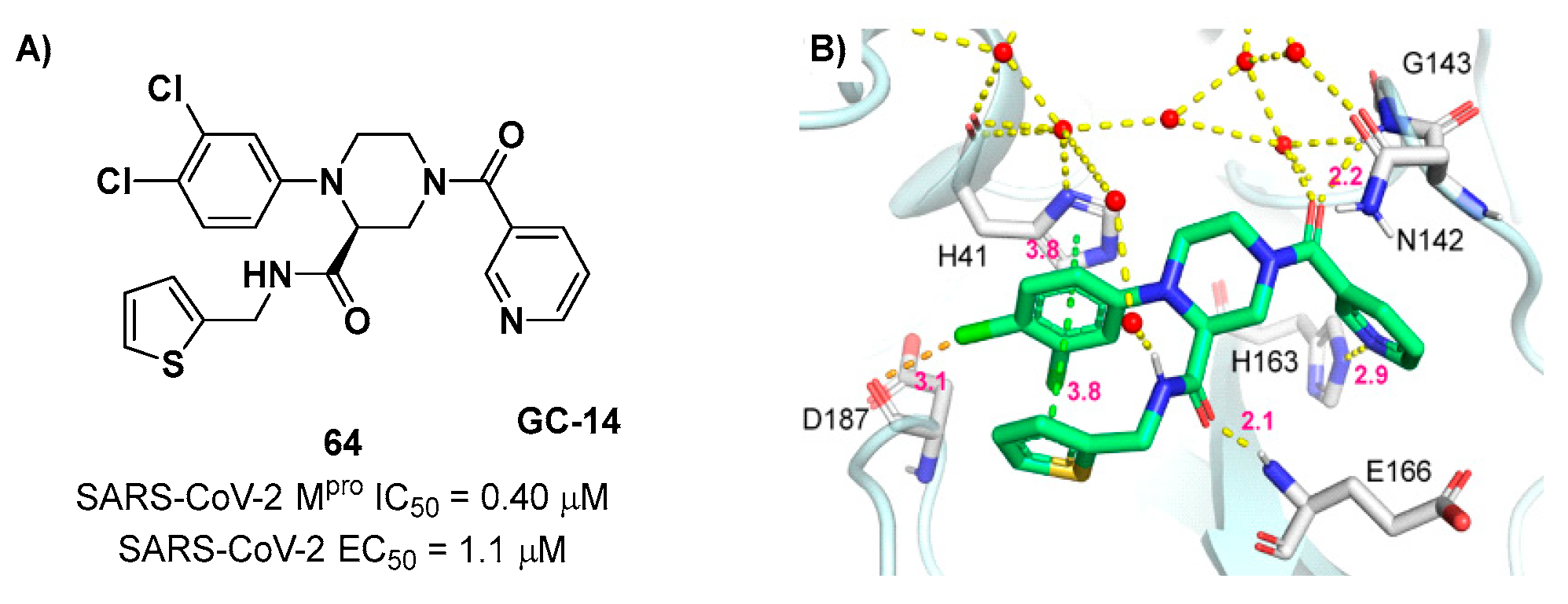
69]. Starting from this piperazine-based hit compound, Gao
et al. designed a panel of parent compounds from which ,
64 (
Figure 40), called
GC-14, emerged as the most promising derivative [
70]. It differs from the reference compound by the presence of a
N-(thiophen-2-ylmethyl)acetamide group hanging on the piperazine core and a 3-pyridin ring in place of the pyrimidine-2,4(1
H,3
H)-dione nucleus at the
C-terminus.
64 acted as a potent non-covalent inhibitor of SARS-CoV-2 M
pro, and also showed potent
in vitro antiviral activity against SARS-CoV-2 (IC
50 = 0.4 μM and EC
50 = 1.1 μM) [
69]. On the basis of these encouraging results, Gao
et al. decided to introduce different electrophilic warheads on the piperazine ring of
64, in order to develop covalent inhibitors [
71]. 30 novel compounds came out from this work where the electrophilic warheads were linked to the piperazine ring via amide or sulfonamide bonds [
71]. Among them, the most promising inhibitors were the α-chloroacetamide
65 (IC
50 = 0.18 μM) and the α-bromoacetamide
66 (IC
50 = 0.31 μM;
Figure 40), called
GD-9 and
GD-13, respectively (
Figure 40).
In vitro antiviral activity of these new potent SARS-CoV-2 M
pro inhibitors were also evaluated on VeroE6 cells infected with SARS-CoV-2, showing quite good results for compound
65 (EC
50 = 2.6 μM), but selectivity profile (CC
50 = 12.5 μM) [
71].
The X-ray co-crystalized structure of SARS-CoV-2 M
pro with compound
66 confirmed the irreversible covalent binding mode which occurs between the Cys145 thiol group and the methylene carbon of the α-bromoacetamide moiety. The thiophen-2-ylmethyl substituent is surface-exposed and formed hydrophobic contacts with the Gln189 residue, the halogenated phenyl ring is inserted into the S2 pocket forming π–π stacking interactions with the His41 residue and a halogen-bond involving the
para-chloro substituent and the Asp187 residue (
Figure 41) [
71].
Other interesting non-peptidyl SARS-CoV-2 M
pro inhibitors containing an α-chloroacetamide warhead are represented by the pyrazoline-based compounds
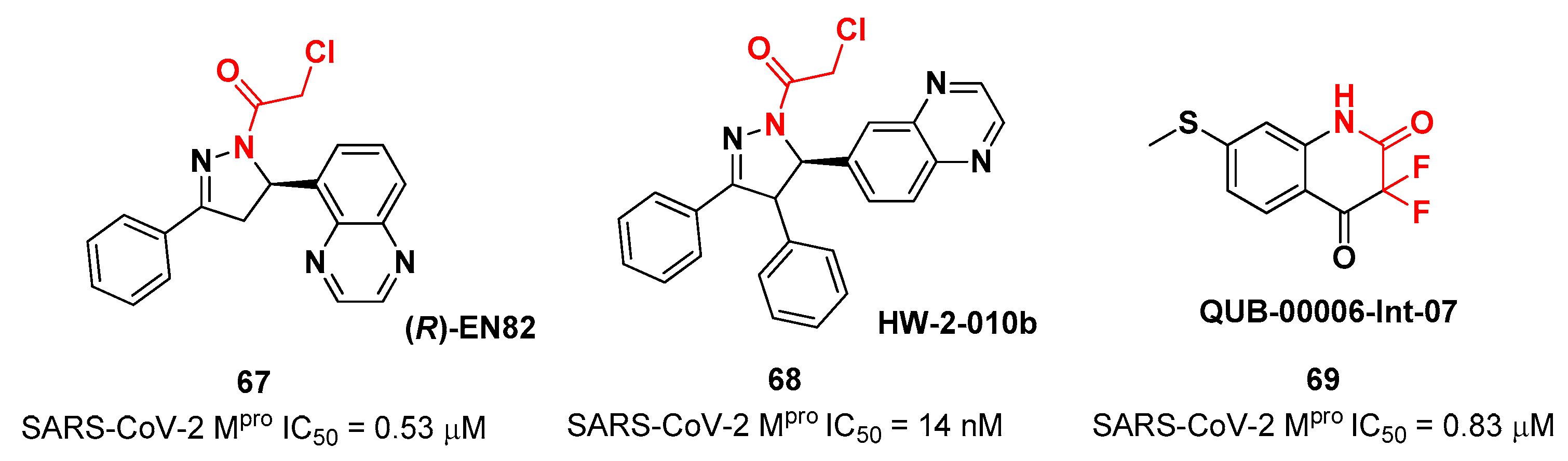
67 and
68 and the difluorinated amide
69 (
Figure 42). Compound
67, called
(R)-EN82, was developed by Moon
et al. and underwent activity-based protein profiling, a preliminary screening test applied on a library of 582 chloroacetamides and acrylamides [
72]. From this first screening, 4 compounds emerged as promising antiviral candidates. Within this selection,
67 turned out to be the most active compound with an IC
50 value of 0.53 μM (
Figure 42). Afterwards, Moon and co-workers carried out an exhaustive SAR analysis of this class of derivatives by exploring the effect of different substituents at C-4 pyrazoline-ring. This work led to the discovery of compound
68, called
cis-HW-2-010B, which showed inhibitory activity against SARS-CoV-2 M
pro in the nanomolar range (IC
50 = 14 nM;
Figure 42) [
72]. In another study,
El Khoury Léa et al. applied advanced in silico techniques for the design and synthesis of the cyclic α,α-difluoro-amide 69 (Figure 42), called QUB-00006-Int-07, acting as a covalent SARS-CoV-2Mpro inhibitor (IC50 = 0.83 μM) [
73]
.
8. α-Ketoamides
Another important electrophilic warhead that has been extensively explored in the design of SARS-CoV-2 M
pro inhibitors is the α-ketoamide moiety. The α-carbonyl group undergoes nucleophilic attack by the thiol group of the Cys145 catalytic residue, leading to the formation of a reversible covalent C-S bond. Furthermore, the α-ketoamide warhead establishes additional H-bonds with residues surrounding the active site, stabilizing the whole drug-target interaction. Examples of α-ketoamide-based M
pro inhibitors came from the outstanding works of Zhang
et al. [
74,
75]. As a first approach, they designed and synthetized a new series of peptidomimetic α-ketoamides acting as broad-spectrum inhibitors of enteroviruses, alpha- and beta-CoVs M
pro [
74]. Among them, the most interesting derivative was
70 that showed noteworthy enzymatic inhibitory activity against SARS-CoV-2 M
pro (IC
50 = 0.67 μM) and antiviral activity (performed on human Calu-3 lung cells) in the micromolar range (EC
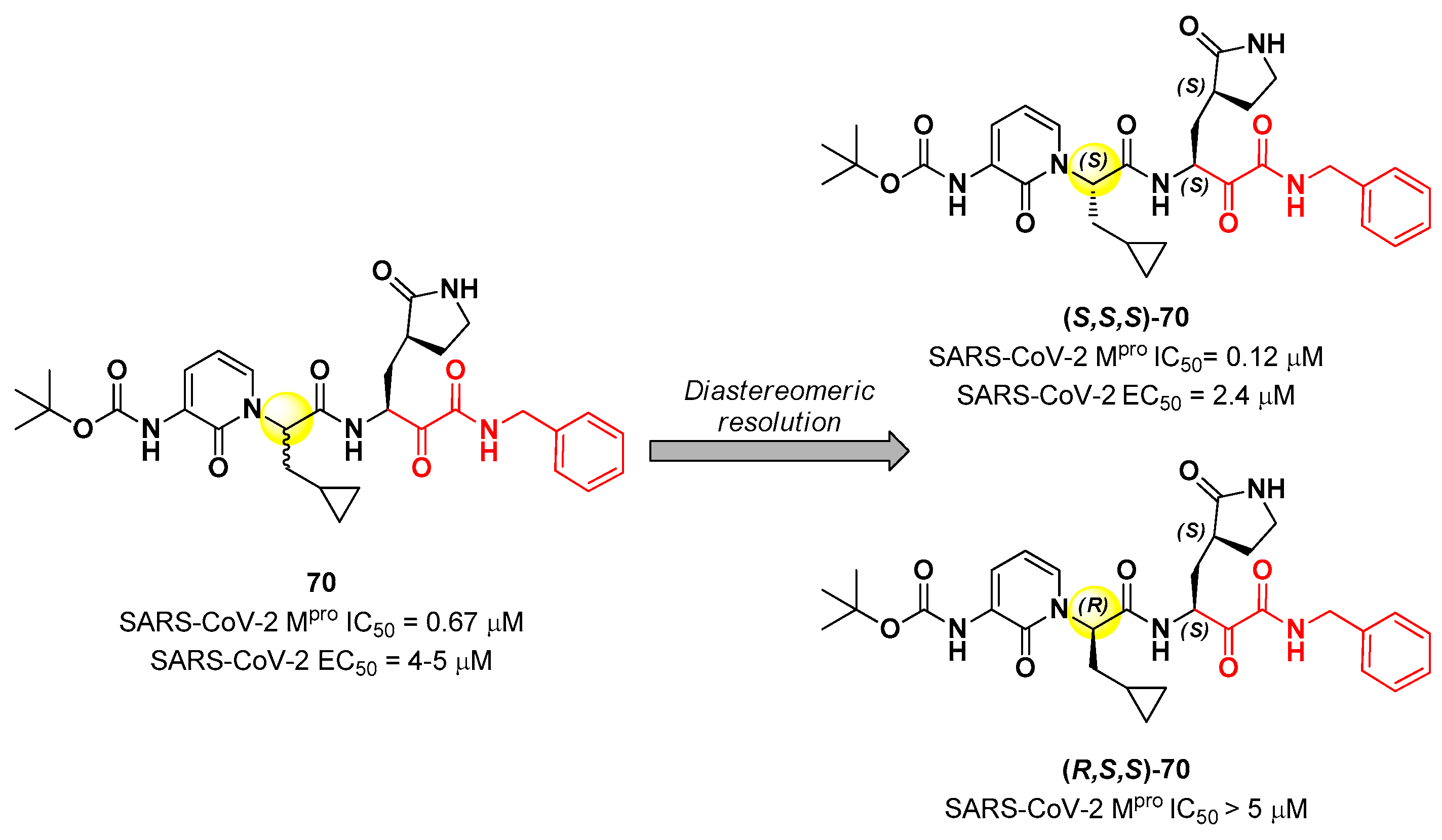
50 = 4 – 5 µM). Cooper
et al. demonstrated that the synthesis used to afford compound
70 led to two diastereomers differing for a single chiral center in P2 moiety (
Figure 43) [
76]. The diastereomeric resolution of
70, followed by the evaluation of the enzymatic inhibitory activity of the single isomers against M
pro led to the discovery that the diastereomer (
S,
S,
S)-
70 is more active than the diastereomer (
R,
S,
S)-
70 (IC
50 = 0.12 μM for (
S,
S,
S)-
70; IC
50 > 5 μM for (
R,
S,
S)-
70). The antiviral activity of (
S,
S,
S)-
70 was then assessed in different cell lines, finding that in human Calu-3 lung cells infected with SARS-CoV-2 it exhibited an EC
50 value of 2.4 μM [
76].
Another SARS-CoV-2 M
pro inhibitor bearing an α-ketoamide warhead was
71 (
Figure 44A), a non-peptidic compound reported by Quan
et al. [
77]. This compound was obtained by multiple optimization rounds driven by
in vitro and
in vivo assays, involving the P1, P2, and P3 group of a bis-amide bearing an α-ketoamide warhead. Afterwards, different groups linked to the α-ketoamide moiety at P1’ position were explored.
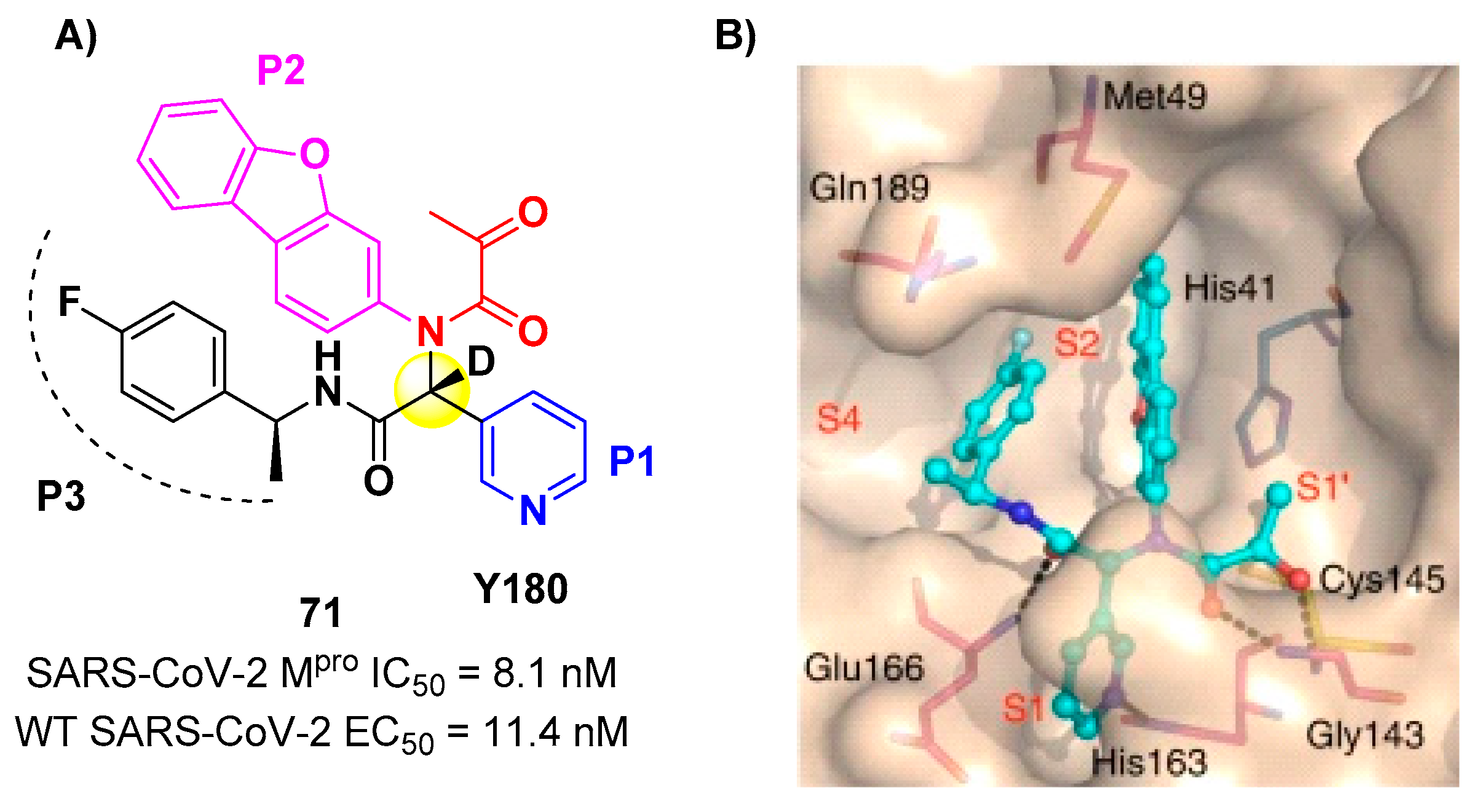
71 is made up by an α-acetylketoamide at P1’, a pyridyne ring at P1, a dibenzo(
b,d)furan group at P2 and a 4-fluorophenyl ring at P3. During the optimization steps, the configuration of the stereocenter linking the P1–P2 units was explored, finding that the (
R)-epimers are always more active than the (
S)-epimers. However, it was found that the (
R)-epimers are prone to epimerization
in vivo. In order to reduce such R/S interconversion, at the stereocenter of
71 was inserted a deuterium atom.
71 exhibited a potent inhibitory activity against SARS-CoV-2 M
pro (IC
50 = 8.1 nM) and outstanding oral bioavailability (92.9%, 31.9% and 85.7% in mice, rats and dogs, respectively). The
in vitro antiviral activity of
71 was assessed on wild-type SARS-CoV-2 and three emerging variants: B.1.1.7 (Alpha), B.1.617.1 (Kappa) and P.3 (Theta), displaying EC
50 values of 11.4 nM, 20.3 nM, 34.4 nM and 23.7 nM, respectively. Finally, the
in vivo antiviral activity of the orally-administered
71 was evaluated against Alpha and Kappa variants in infected K18-hACE2 transgenic mouse model, showing potent antiviral activity [
77]. The X-ray co-crystal structure of SARS-CoV-2 M
pro in complex with
71 was also obtained (
Figure 44B), showing a covalent bond between terminal ketone moiety and the Cys145 thiol group. The thioemiketal adduct is stabilized by two H-bonds formed by the thioemiketal-OH and the oxygen of the α-keto moiety with Cys145-amide and Gly143-backbone, respectively. The pyridyne ring at P1 fits into the S1 pocket forming a H-bond with the His163 residue. The dibenzo[
b,d]furan group at P2 is deeply inserted into the S2 pocket forming π -π interactions with the His41 residue. Moreover, this group is further stabilized through hydrophobic interactions with the S2 His41 and the 4-fluorophenyl ring at P3. The latter is also stabilized by hydrophobic interactions involving Met49 and Gln189 [
77].
An α-ketoamide-based SARS-CoV-2 M
pro inhibitor currently on phase III clinical trials is
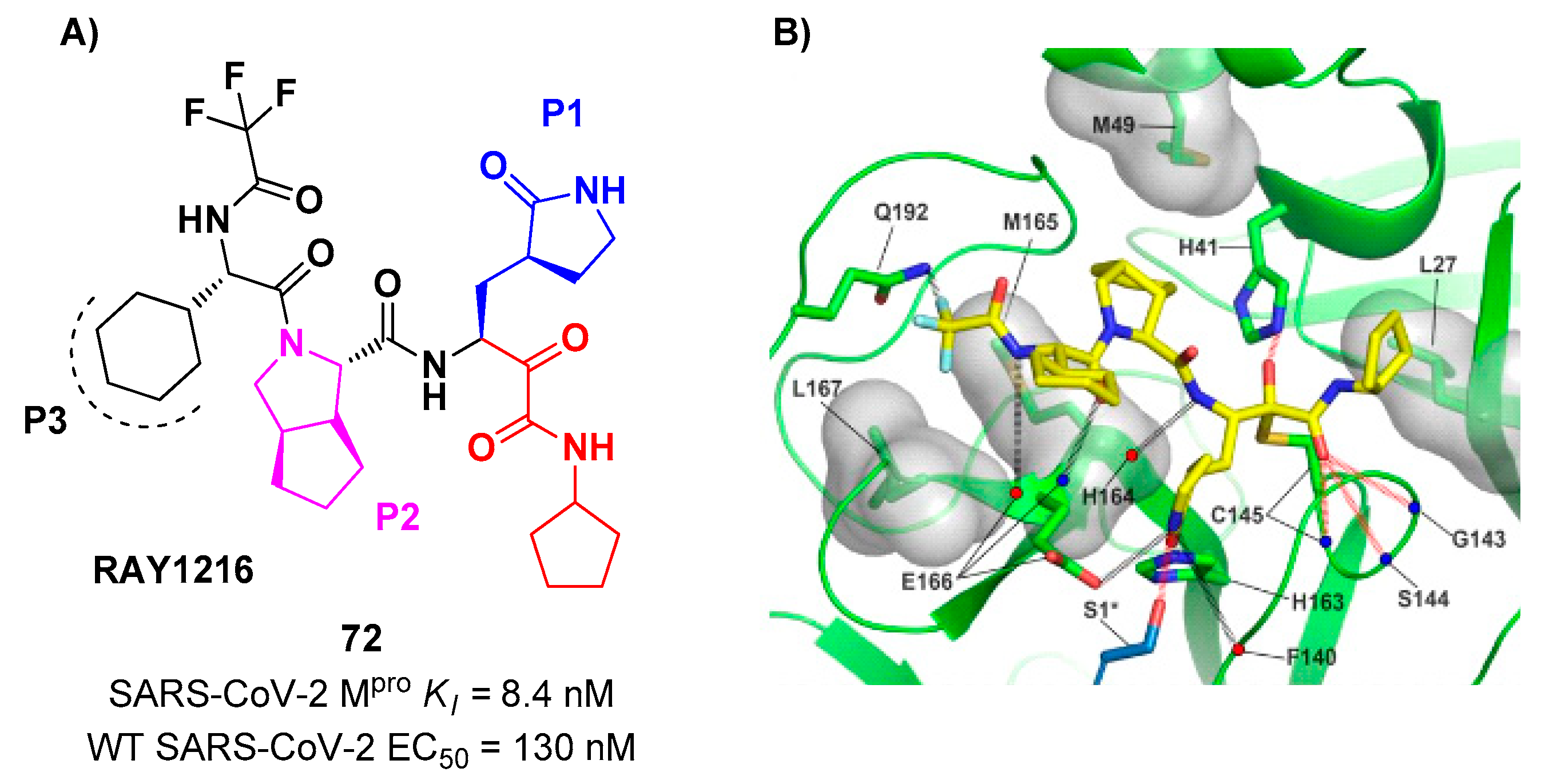
72 (
Figure 45A)
, called
RAY1216, [
78]. The design of this compound started from several SAR-optimization rounds of
Nirmatrelvir involving the P1, P2, P3, and P4 moieties and the nitrile warhead at P1’. In particular, the α-ketoamide warhead at P1’ has been functionalized with a cyclopentyl ring. Moreover, the inhibitor bears a γ-lactam moiety at P1, a cyclopentylproline at P2 (previously used in
Telaprevir), a cyclohexylglycine at P3 and a tri-fluoroacetamide group at P4.
72 exibited a
Ki = 8.4 nM in the enzymatic assay (a value comparable with
Nirmatrelvir) [
78]. The antiviral assays were performed on VeroE6 cell infected with different variants of SARS-CoV-2: the obtained EC
50 were 95 nM for WT SARS-CoV-2, 130 nM for Alpha variant, 277 nM for Beta variant, 97 nM for Delta variant, 86 nM for Omicron BA.1 variant and 158 nM for Omicron BA.5 variant. The
in vivo antiviral activity of
72 was tested at different doses in a human ACE2 transgenic mouse model, displaying similar efficacy to
Nirmatrelvir. Pharmacokinetic properties of
72 were evaluated and compared with
Nirmatrelvir on different animal species (mice, rats and cynomolgus macaques), showing shorter elimination half-live and faster plasma clearance. The X-ray structure of
72 bound to SARS-CoV-2 M
pro showed the following (
Figure 45B):
i) in general, the structural elements in common with
Nirmatrelvir maintain the same interactions with the target;
ii) the formation of a covalent bond between the Cys145 residue and the α-ketoamide warhead is confirmed;
iii) the thioemiketal-OH establish a H-bond with His41;
iv) the α-ketoamide carbonyl oxygen accepts H-bonds from the oxyanion hole residues;
v) the cyclopentyl moiety establishes hydrophobic interactions with Leu27;
vi) P2 cyclopentylproline fits into the S2 subsite;
vii) the P3 appers to have no important interactions with the target [
78].
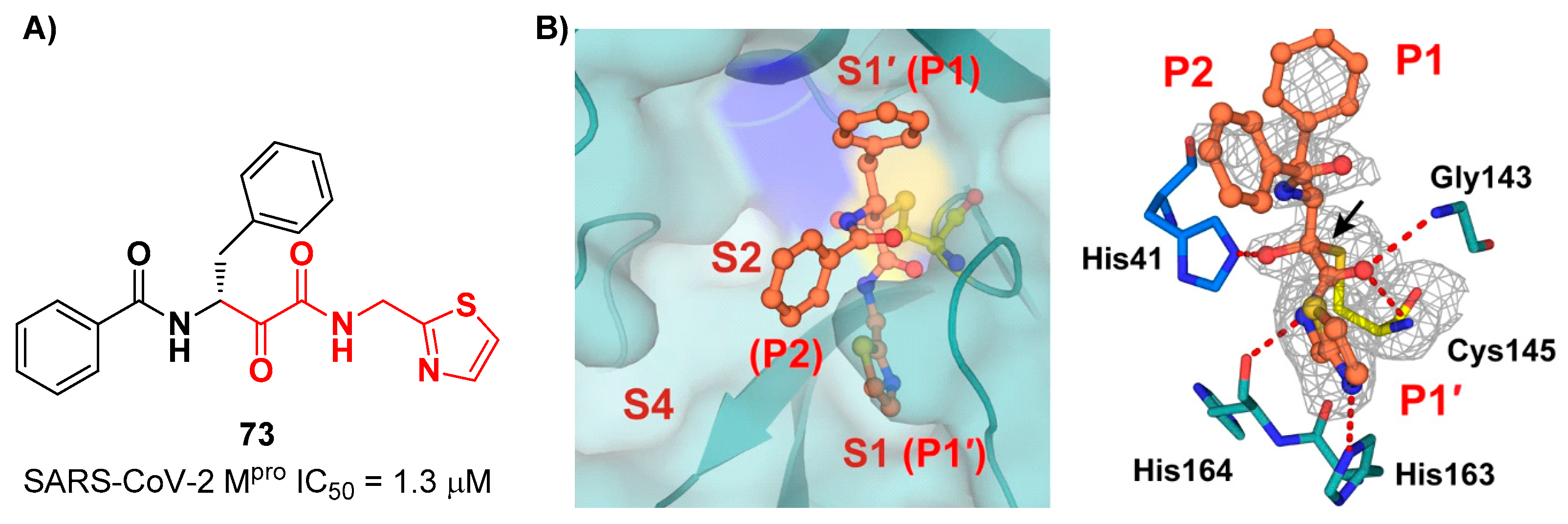
A recent paper from Huang
et al. proposed a novel α-ketoamide-based inhibitor with potent activity against SARS-CoV-2 Omicron variants [
79]. They started with an
in vitro screening of in-house library containing more than 30,000 compounds.
73 (
Figure 46A) was identified as a new hit compound, showing an IC
50 value of 1.3 μM. From the X-ray analysis of the complex SARS-CoV-2 M
pro/
73 (
Figure 46B) emerged that the α-ketoamide warhead binds covalently the Cys145 catalytic residue, interacting with several H-bonds with His41, His164 and residues of the oxyanion hole. The P1 benzyl and P1’ thiazole moieties occupy the S1 and S1’ subsites, respectively, while the P2 phenyl does not fit inside the S2 pocket, but it was observed exposed to the solvent region [
53].
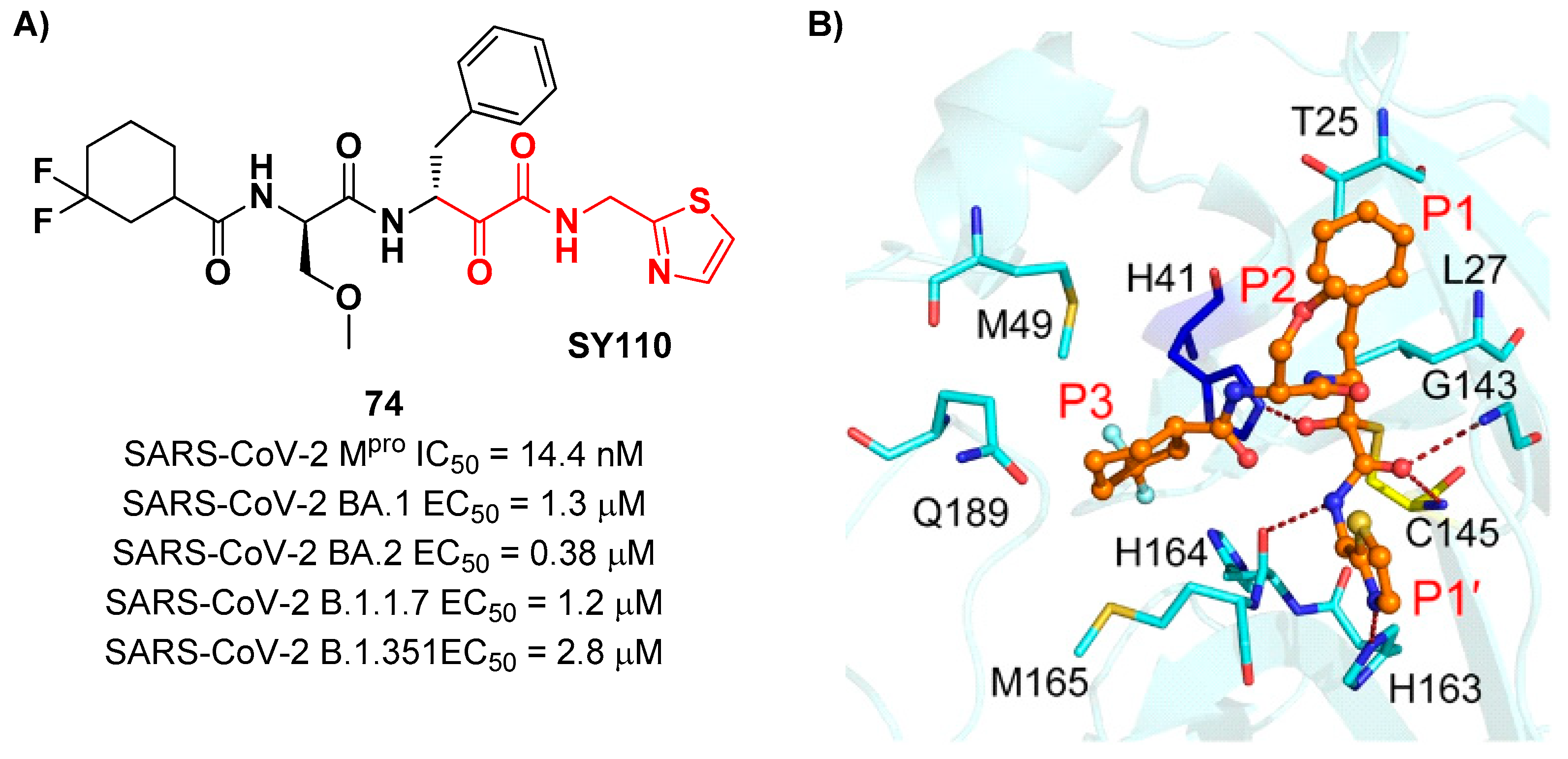
The same research group performed a structural optimization of
73 focusing on three portions: the P1’ thiazole ring, P1 and P2 phenyl rings. Each position was investigated keepin the other two fixed. The most active compound turned out to be
74 (
Figure 47A), called
SY110 [
79]
. This compound was selected based on its enzymatic inhibition activity, low cytotoxicity profile and PK parameters evaluated in Sprague-Dawley rats, displaying an IC
50 value of 14.4 nM and higher area under the curve and oral bioavailability as compared to other derivatives. Furthermore,
74 exhibited potent antiviral activity in plaque reduction assays against SARS-CoV-2 Omicron BA.1, its sub-lineages B.1.1.7, B.1.351 and BA.2, and against SARS-CoV-1 and MERS-CoV, demonstrating a pan-coronavirus antiviral efficacy (EC
50 = 1.3, 0.38, 1.2, 2.8, 0.45 and 6.3 μM against SARS-CoV-2 Omicron BA.1, BA.2, B.1.1.7, B.1.351, SARS-CoV-1 and MERS-CoV).
In vivo experiments on Omicron-infected K18-hACE2 mouse models demonstrated the ability of
74 to alleviate the virus-induced pathology and, most importantly, to partially overcome
Nirmatrelvir-resistance M
pro mutants in FRET assay [
79]. X-ray co-crystal structure of SARS-CoV-2 M
pro in complex with
74 showed an atypical binding mode (
Figure 47B):
i) the α-ketoamide warhead was found covalently bound to the Cys145 catalytic residue in the (
R)-configuration, forming several H-bonds with His41, Cys145 and Gly163;
ii) the P1’ thiazole moiety occupies the S1’ subsite establishing a H-bond with His163;
iii) the P1 benzyl moiety occupies the P1 pocket, establishing hydrophobic interactions with Thr25 and Leu27;
iv) the P2 chiral ether is exposed to the solvent region near to a flexible loop;
v) the P3 3,3-difluorocyclohexyl ring establishes hydrophobic interactions with the residues Met49, Met165 and Gln189 [
79].
9. Michael acceptors
Michael acceptors (MAs) such as α,β-unsaturated carbonyl, esters, vinyl, sulfonamides and nitriles have been widely used in the development of SARS-CoV-2 M
pro inhibitors. These groups exploit the electrophilic unsaturated β-position to form an irreversible covalent adduct with Cys145 through a Michael addition mechanism. The first example of MA as SARS-CoV-2 M
pro inhibitor is compound
75 (
Figure 48A), called
inhibitor N3, a peptidomimetic derivative previously reported as proteases inhibitor of SARS-CoV and MERS-CoV [
80]. This pseudo-tetrapeptide contains a vinyl group and a benzyl-ester moiety at P1’ (the MA warhead), a γ-lactam ring at P1, a Leu residue at P2, a Val residue at P3 and a
N-terminal Ala residue (P4) capped with an isoxazol-3-yl group (P5). As expected,
75 showed a time-dependent enzymativ inhibition with
kobs = 11,300 M
-1s
-1, while the plaque antiviral assays on SARS-CoV-2 infected VeroE6 cells showed an EC
50 of 16.8 μM. The X-ray crystal structure of SARS-CoV-2 M
pro in complex with
75 (
Figure 48B) confirmed the formation of a covalent bond between β-vinyl carbon and Cys145 –SH group, while the P1’ benzyl group fits into the S1’ subsite forming van der Waals interactions with Thr24 and Thr25. The P1 γ-lactam ring fits into the S1 subpocket and is H-bonded with His163 and Glu166. P2 Leu side chain is deeply inserted into the S2 pocket, while the P3 Val side chain is solvent-exposed. The P4 Ala side chain forms hydrophobic interactions with Met165, Leu167, Phe185 and Gln192, while the isoxazole ring at P5 establishes van der Waals interactions with Phe168. In the same study, another compound was taken into consideration for drug repurposing,
i.e.
CINANSERIN (
76 in
Figure 48A), a well-characterized hydroxytryptamine receptor antagonist discovered in 1960 (
Figure 48A).
76 displayed an IC
50 value of 125 μM and an EC
50 value of 20.6 μM on infected VeroE6 cells [
80]. Also in this case, the presence of an α-β unsaturated amide was supposed to be reactive toward catalytic Cys145
via Michael addition [
22].
Iketani
et al. discovered compound
77 (
Figure 49A)
, by an
in vitro screening of existing SARS-CoV M
pro inhibitors [
81]. It is characterized by a pseudo-tripeptide structure containing an acrylic ethyl ester moiety at P1’, a γ-lactam ring at P1, a Leu residue at P2 and a
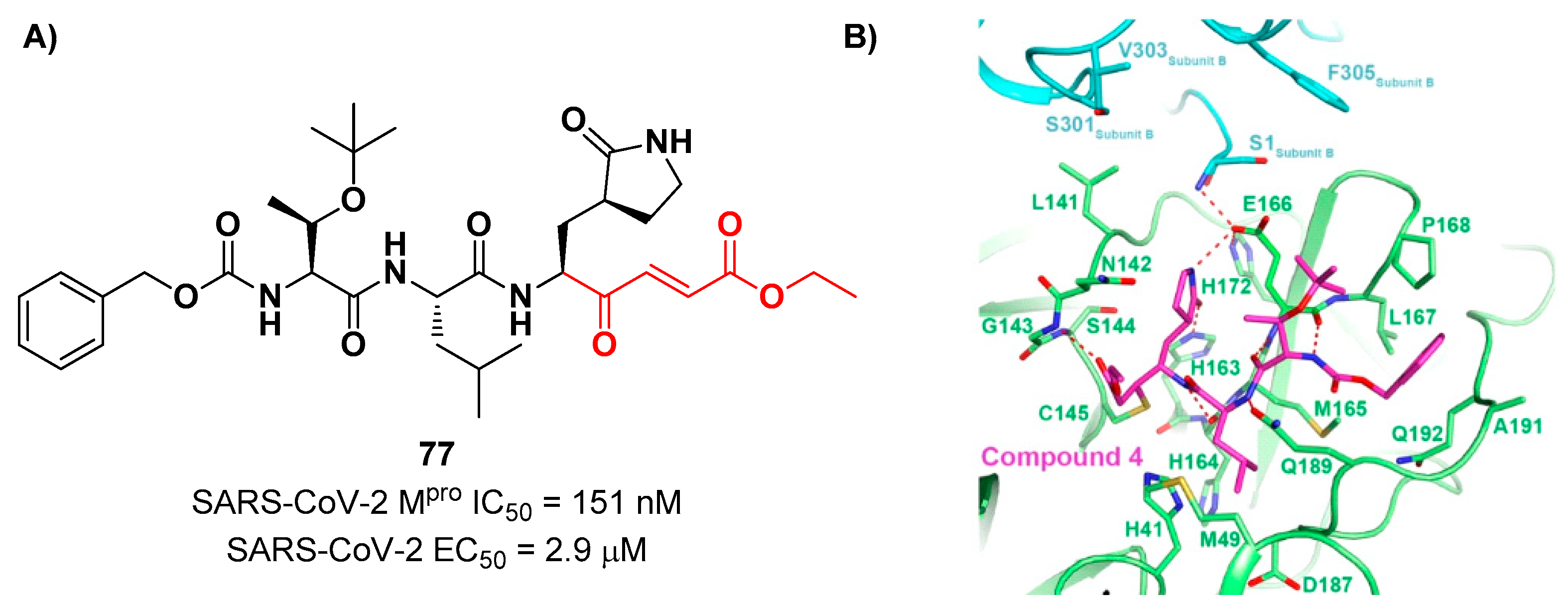
N-Boc-
O-
tert-butyl-Thr at P3 position.
77 demonstrated to inhibit SARS-CoV-2 M
pro with an IC
50 value of 151 nM, while kinetic studies showed an inactivation rate (
kinact/
Ki) of 4.13 × 10
5 M
−1s
−1. Its ability to inhibit SARS-CoV-2 viral replication was tested in infected VeroE6 cells in CPE assays, showing an EC
50 value of 2.9 μM. Also for this compound, crystallographic studies confirmed the MA standard covalent binding mode (
Figure 49B) [
81].
Other examples of MAs as SARS-CoV-2 M
pro inhibitors came from a virtual screening campaign of an in-house database of ligands containing different MAs warheads, such as vinyl sulfones, vinyl amides, vinyl esters vinyl ketones, vinyl nitriles and vinyl phosphonates [
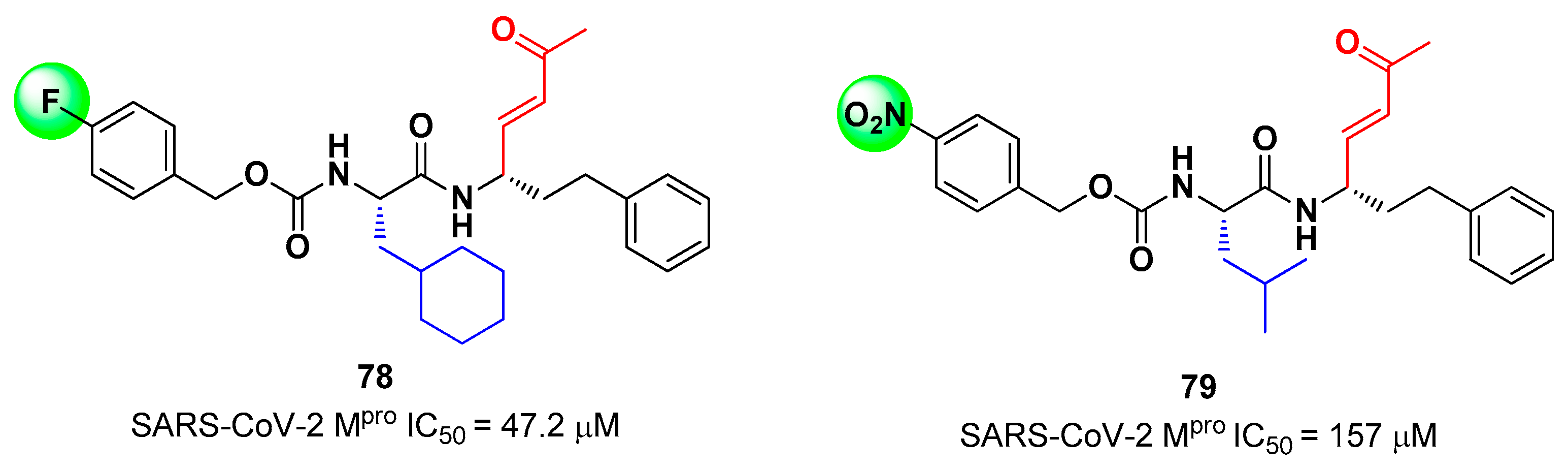
82]. This strategy allowed to identify two compounds,
78 and
79 (
Figure 50), containing a vinyl ketone moiety. Enzyme-inhibition assays revealed that both compounds were able to modertely inhibit SARS-CoV-2 M
pro, with an IC
50 of 47.2 μM and 157.5 μM, respectively. Docking and molecular dynamics studies validated the covalent inhibition at the active site
via Michael addition underlining the importance of the aliphatic residue at P2 and the aromatic ring with EWG groups in
para position at P3 [
82].
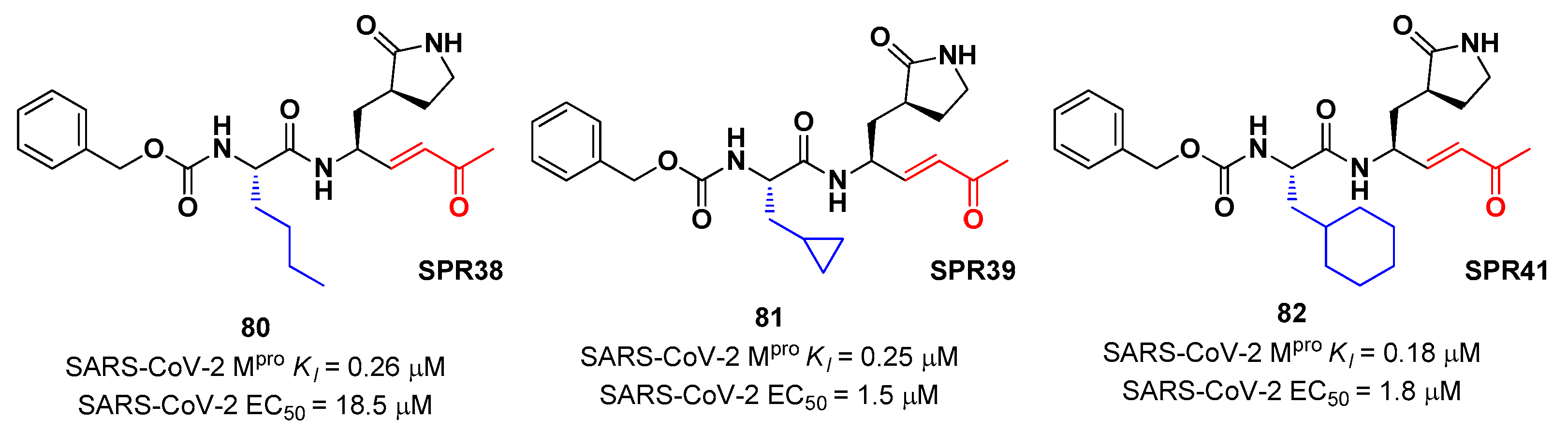
Lead optimization of
78 and
79 were performed by the same authors in a second work, consisting in the introduction of the γ-lactam moiety at P1 (as for most potent SARS-CoV-2 M
pro inhibitors) and different moieties at P2 [
83]. From the enzymatic assays compounds
80,
81 and
82 (
Figure 51), called respectively
SPR38,
SPR39 and
SPR41, turned out to be the most promising derivativrs exhibiting activity in the sub-micromolar range (
Ki = 0.18–0.26 μM). These compounds were also selected for cross-reactivity tests towards human cathepsin L and B showing activity in the micromolar range with the exception of
82, which displayed a
Ki value of 0.25 μM against cathepsin L and was claimed as a dual inhibitor. The detected antiviral activity rangrd from 1.5 to 18.5 μM.
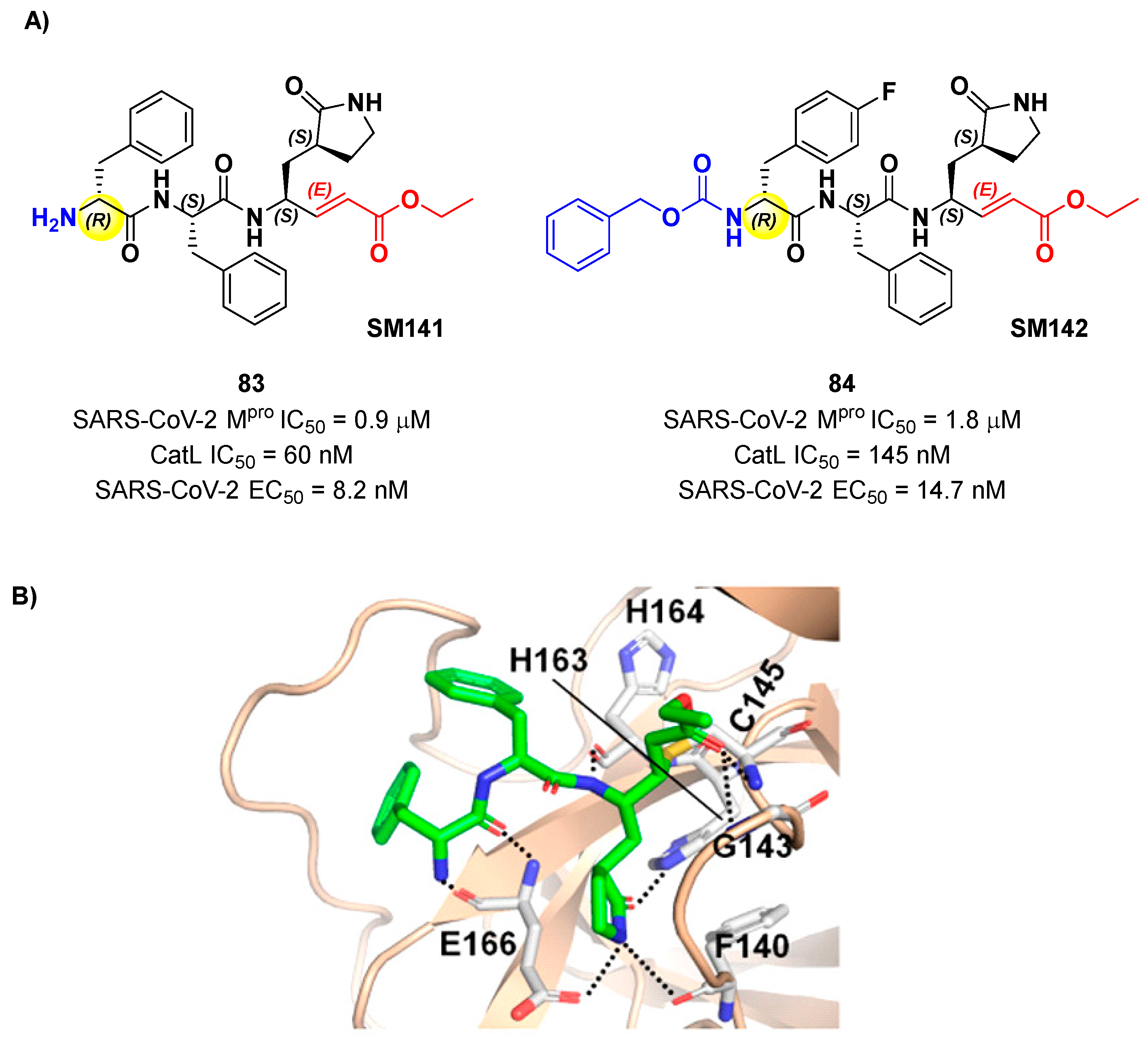
Another lead optimization work (starting from a set of probes bearing an α-chloromethyl ketone warhead at P1’) was performed by Mondal
et al. and led to the identification of compounds
83 and
84 (
Figure 52A) as promising MAs-based SARS-CoV-2 M
pro inhibitors [
84]. The new MA warhead replced the α-chloromethyl ketone moiety as the latter is associated with the already mentioned high reactivity and cytotoxicity.
83 and
84 showed inhibitory activity in micromolar range and antiviral activity in the nanomolar range (
83: IC
50 = 0.9 μM and EC
50 = 8.2 nM;
84: IC
50 = 1.8 μM and EC
50 = 14.7 nM). From a structural point of view,
84 contains a
N-terminal Cbz group and a
para-fluoro-
d-Phe residue at P3, while
83 contains a free terminal amine and
l- and
d-Phe at P2 and P3, respectively. The high antiviral activity (as compared to the enzymatic inhibition activity) of these compounds may be explained by a dual-inhibition of M
pro and other proteases involved in SARS-CoV-2 replication cycle. Further investigation confirmed this hypothesis; indeed they inhibited cathepsin L in the nanomolar range (IC
50 = 60 nM for
83, IC
50 = 145 nM for
84). The good cytotoxic profile of these compounds led to
in vivo investigations: the results showed that
83 and
84 inhibit the viral replication in SARS-CoV-2 infected K18-hACE2 mice when administered intraperitoneally, indicating them as potential anti-SARS-CoV-2. The crystal structure of the complex M
pro/
83 (
Figure 52B) confirmed the formation of the Michael adduct with Cys145 and the fit of the γ-lactam ring into the S1 pocket [
84].
Citarella and co-workers explored the cinnamic ester moiety as a MA warhead for the covalent inhibition of SARS-CoV-2 M
pro [
85]. In this work, they replaced the epoxyketone warhead in an already existing M
pro inhibitor, obtaining a pseudo-dipeptide with a backbone functionalized with different fragments at its
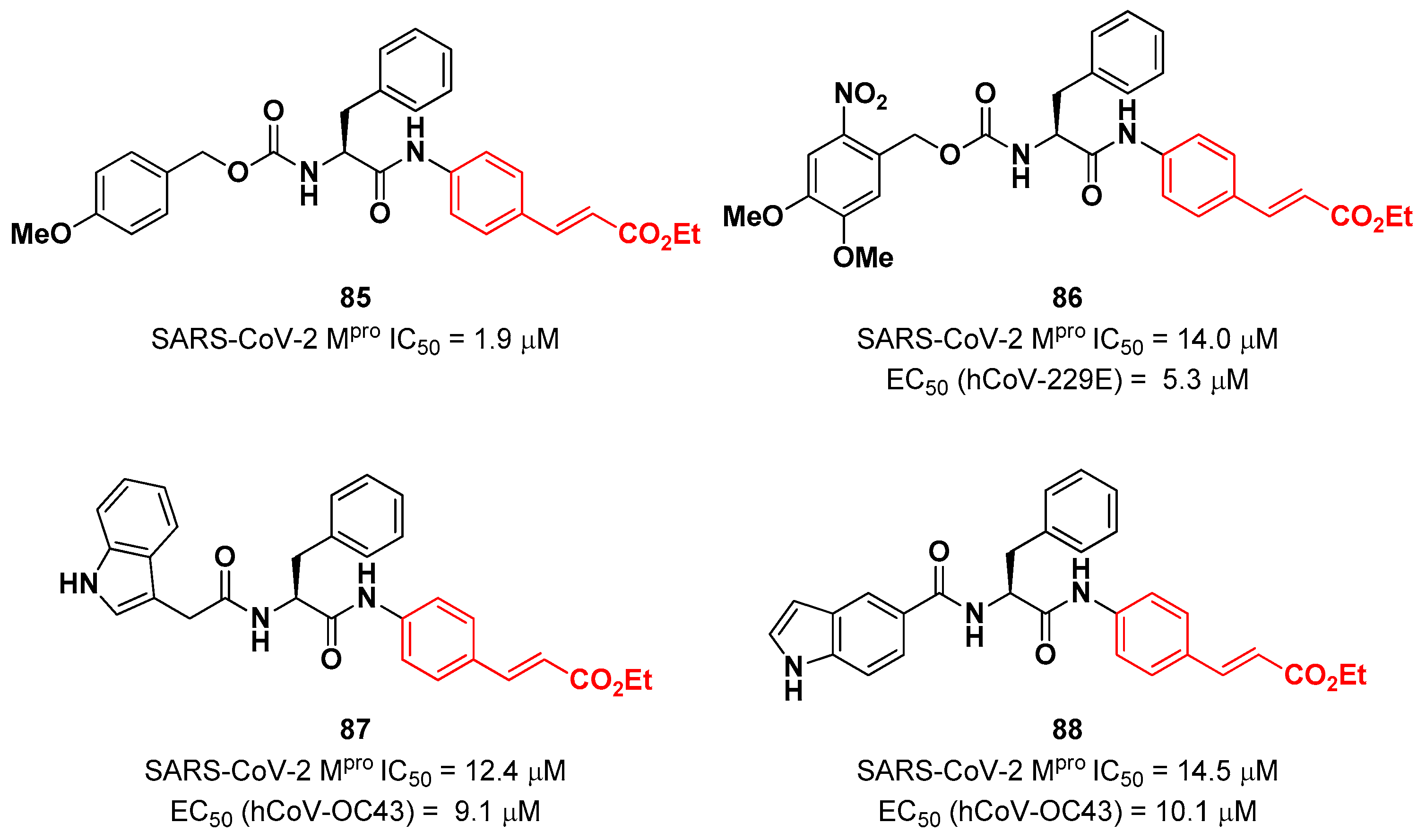
N-terminus. From the enzymatic inhibition activity test the most promising compound turned out to be the carbamate derivate 85 (
Figure 53), containing a
p-OMe substituent on the
N-terminal phenyl ring. The antiviral activity test on representative hCoVs demonstrated that 86 displayed EC
50 values within the low micromolar range against hCoV-229E replication (α-CoV), while compounds 87 and 88 (
Figure 53), both containing an indole moiety at the
N-terminus, exhibited an interesting antiviral activity against hCoV-OC43 replication (β-CoV). Docking studies and mass experiments suggested the formation of a Michael adduct between the β position of the cinnamic ester and Cys145 [
85].
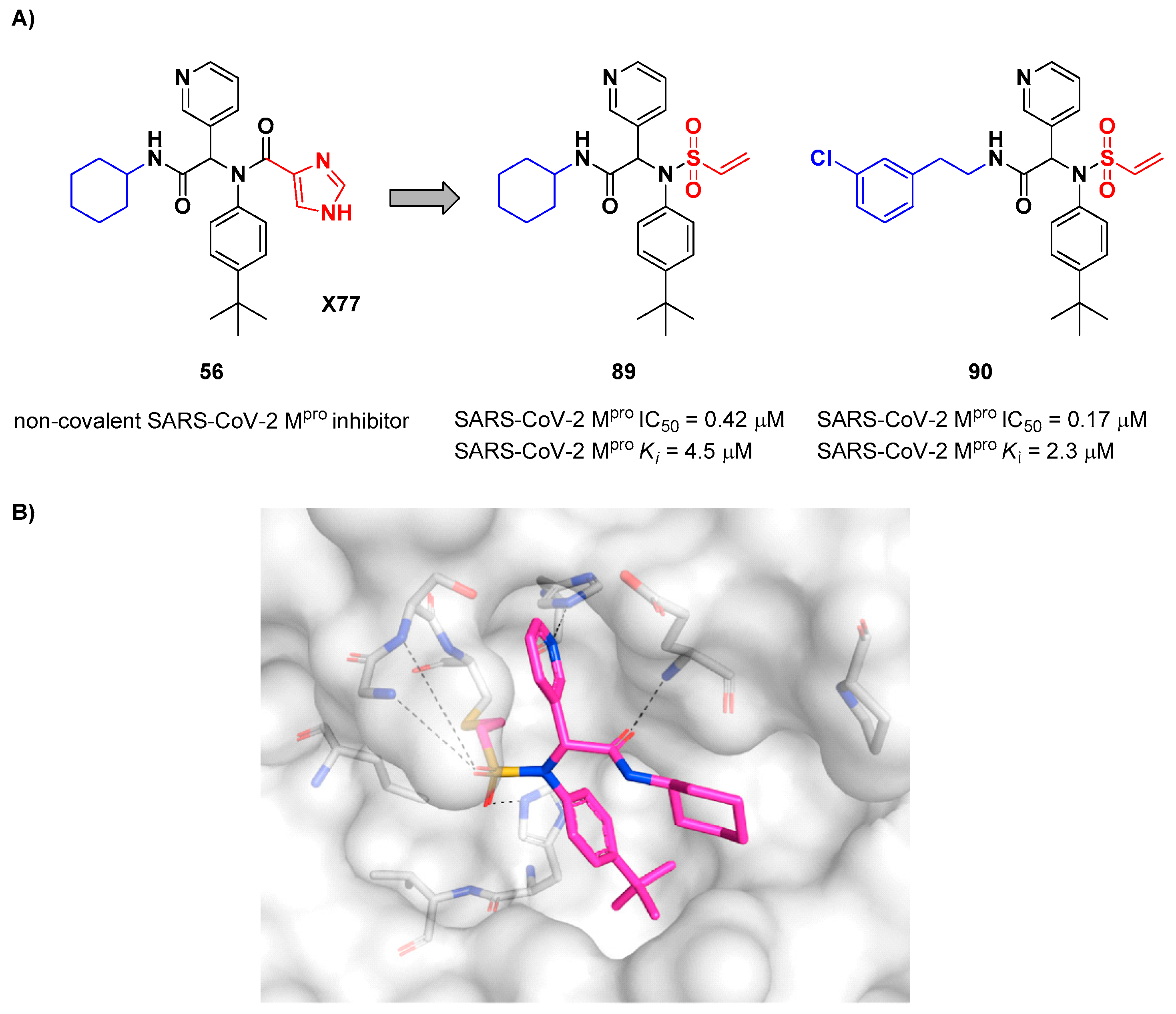
Stille
et al. (already mentioned in the section of α-haloacetamides) developed a library of covalent inhibitors with different electrophile warheads, by replacing the P1’ imidazole ring in compound
54 (
Figure 35A and
Figure 54A) [
64]. Compounds
89 and
90, bearing a vinyl sulphone moiety as a warhead, were selected as promising SARS-CoV-2 M
pro inhibitors as they showed enzymatic inhibitory activity in the sub-micromolar (IC
50 = 0.42 μM and
Ki = 4.5 μM for
89; IC
50 = 0.17 μM and
KI = 2.3 μM for
90).
89 differs from
90 in having of a cyclohexyl group at the
N-terminus in place of a 2-(
meta-chlorophenyl)ethyl group. The binding of
89 was confirmed by X-ray crystallography (
Figure 54B)[
64].
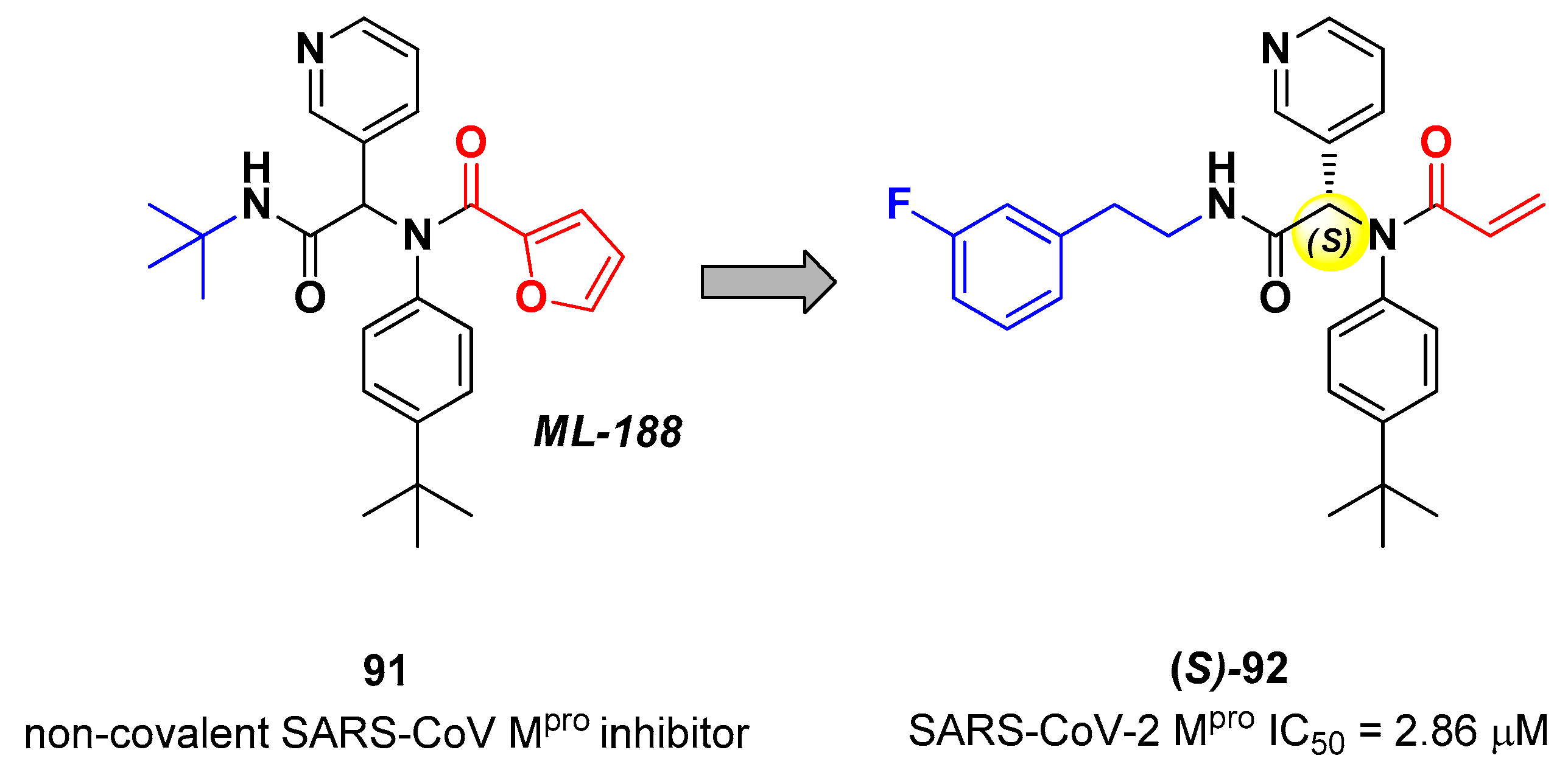
Zaidman
et al. designed MAs as SARS-CoV-2 inhibitors exploiting an automated pipeline, called
Covalentizer, able to suggest new covalent inhibitors from non-covalent compounds [
86]. Starting from the non-covalent SARS-CoV M
pro inhibitor
91 (
Figure 55), called
ML-188, they obtained a library of SARS-CoV-2 M
pro inhibitors by replacing its furan ring at P1’ with an acrylamide warhead. The most active compound was
92 (
Figure 55), which, apart from the P1’ position, differs from
91 for
N-terminus (
meta-fluorophenetylamide moiety). This compound, obtained as a racemic mixture, exhibited M
pro inhibition in the micromolar range (IC
50 = 2.95 μM). After chiral chromatography separation and test on single isomers, the (
S)
-enantiomer turned out to be by far the most derivative ((
S)-
92: IC
50 = 2.86 μM; (
R)-
92: IC
50 = 86.3 μM). The X-ray crystal structure of (
S)-
92 in complex with SARS-CoV-2 M
pro confirmed the covalent binding mode at the acrylamide warhead, with the
p-
tert-butylphenyl group fitting into the S2 subsite and the fluorophenyl moiety establishing hydrophobic interactions with Met165 and Gln189 in the S4 cleft [
86].
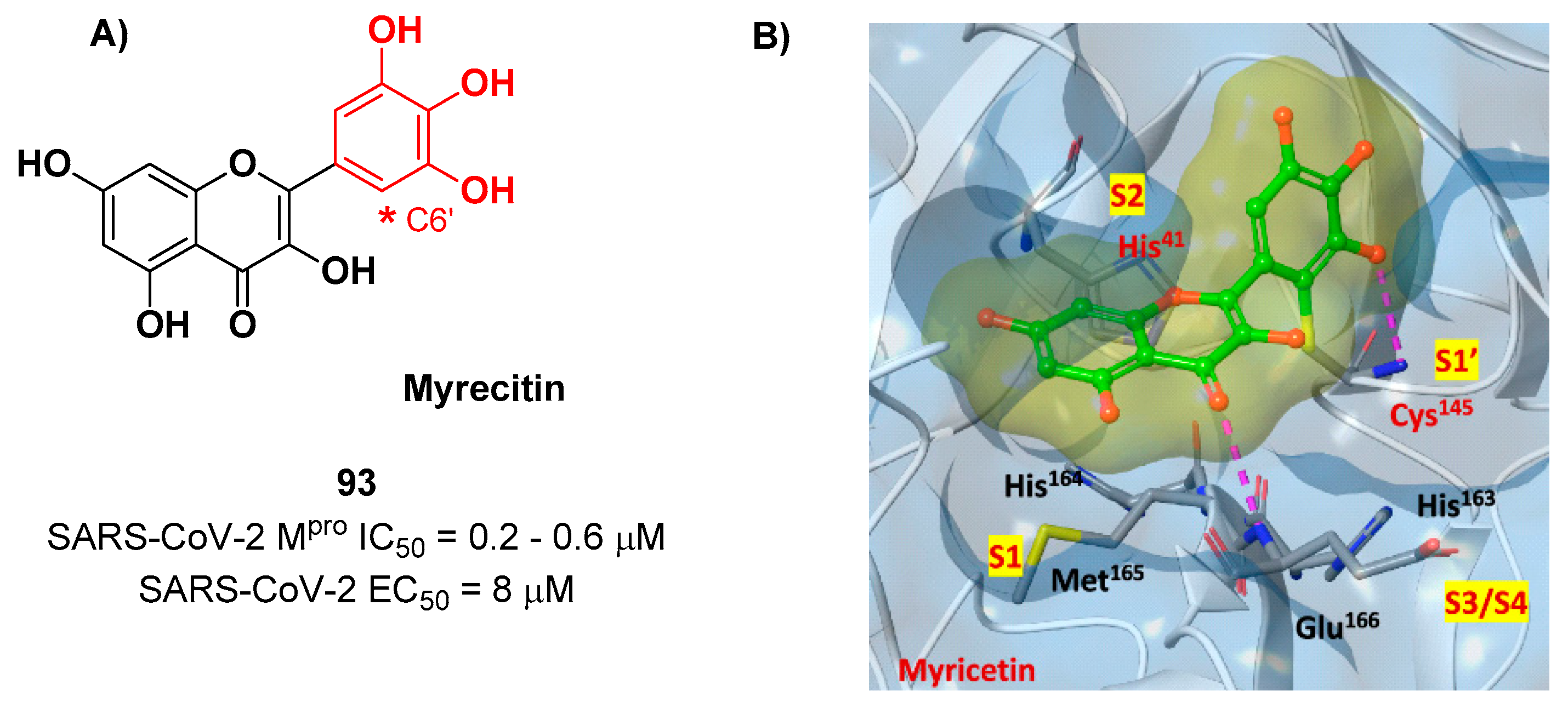
Also flavonoids were identified by
in vitro screening as SARS-CoV-2 M
pro inhibitors acting as Mas.
Myrecitin (
93 in
Figure 56) is one of the most important examples of M
pro inhibitors belonging to natural products [
87]. Its pyrogallic motif
in vivo undergoes oxidation to give an
ortho-quinone function able to exert Michael’s reactivity towards Cys145 [
88]. The X-ray crystallography confirmed such hypothesis (
Figure 56), highlighting the presence of a covalent bond between C6’ and Cys145 –SH group.
In vitro evaluations revealed high potency in the enzymatic assay (IC
50 value 0.2– 0.6 μM) and micromolar antiviral activity in the cell-based (EC
50 = 8 μM).
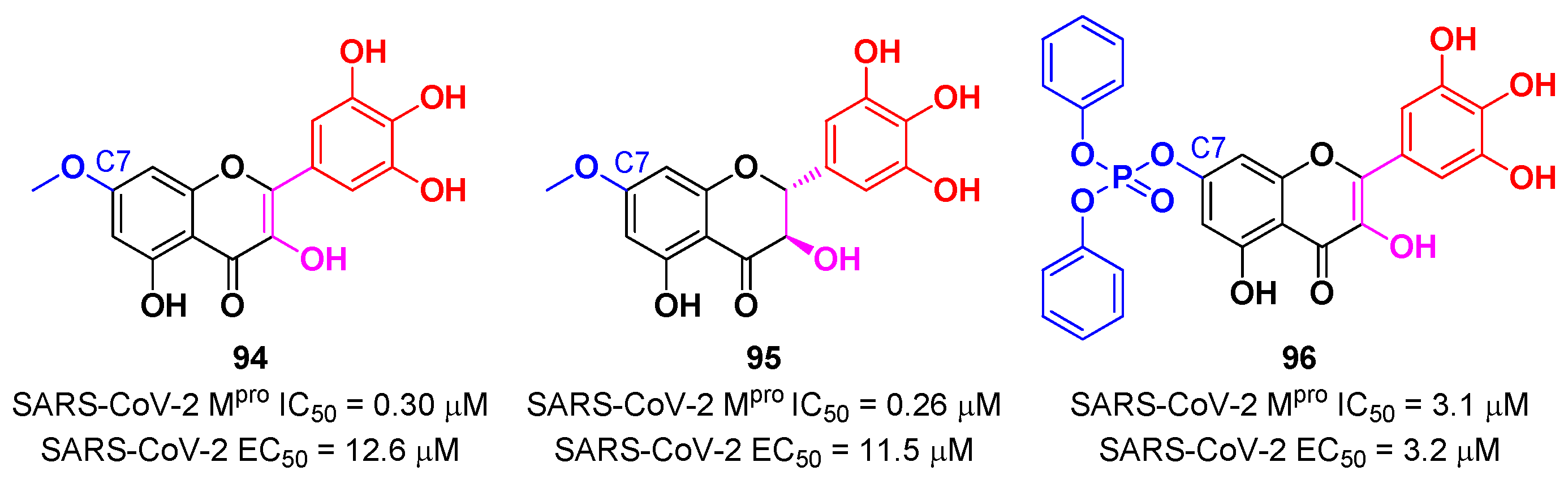
In view of these promising results, several derivates of
Myrecitin were synthetized (
Figure 57) [
88]. The introduction of a
para-methyl group at its C7 led to compounds
94 and
95, which showed higher potency (IC
50 = 0.30 μM for compound
94; IC
50 = 0.26 μM for compound
95) and good antiviral activity (EC
50 = 12.6 μM for
94; EC
50 = 11.5 μM for
95). The addition of a phosponate group in the same position led to compound
96, which exhibited good enzymatic inhibitory activity (IC
50 = 3.1 μM) and the highest antiviral efficacy (EC
50 = 3.2 μM) among these series of derivates [
88].
12. Non-covalent inhibitors
Non-covalent inhibitors do not possess an electrophilic warhead and generally display less reactivity and improved selectivity as compared to covalent inhibitors. They establish only secondary interactions with the active site such as H-bonds, hydrophobic stackings, Van der Waals forces, electrostatic interactions and salt bridges [
99]. Some examples of SARS-CoV-2 M
pro non-covalent inhibitors have been already introduced throughout the article as they may have acted as lead structures for the development of covalent inhbiitors. These include compound
54 (
X77),
56 (
23R),
63 (
MCULE-5948770040) and
64 (
GC-14).
54 (
Figure 68A), previously identified as SARS-CoV M
pro inhibitor [
100], was investigated by Stille
et al. in complex with SARS-CoV-2 M
pro via X-ray analysis [
64]. The imidazole-amide moiety interacts via H-bonds with His41 and Gly143, while it is positioned at 3.2 Å from Cys145-SH. The pyridyne ring fits into the S2 pocket forming a H-bond with His163 side chain, while the
tert-butylphenyl group is deeply inserted into the S4 subpocket. The carbonyl oxygen of the cyclohexyl-amide moiety accepts a H-bond from the –NH function of Glu166 (
Figure 68B). Its enzymatic inhibitory activity was in the micromolar range (IC
50 = 4.1 μM) [
64].
Kitamura
et al. developed
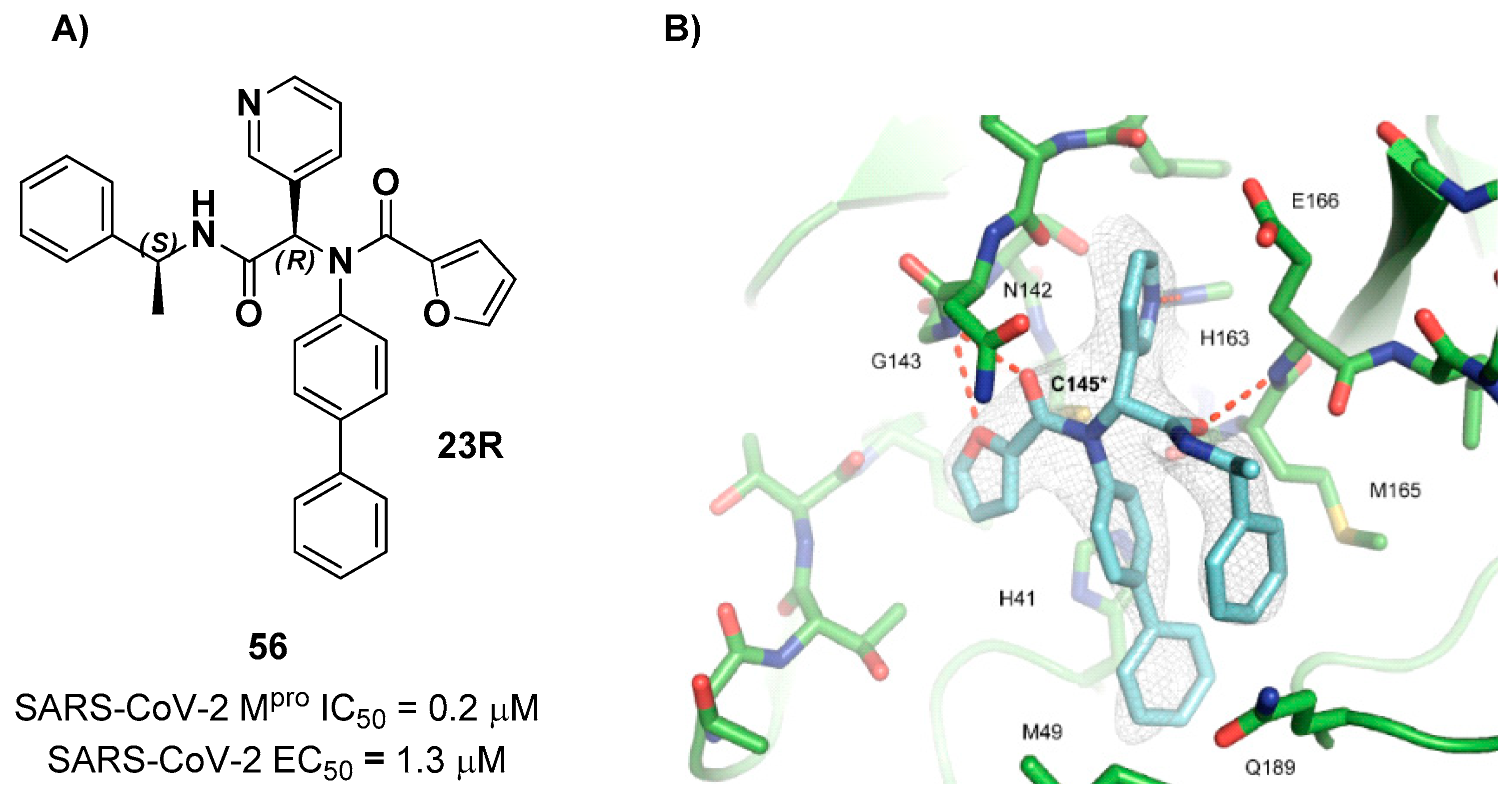
56 (compound
23R in
Figure 69A) which displayed an IC
50 value of 0.2 μM in the enzymatic test and an EC
50 value of 1.3 μM in the antiviral assay. The X-ray crystal structure of SARS-CoV-2 M
pro in complex with
56 is reported in
Figure 69B. The furylamide moiety at P1’ forms a bifurcated H-bond with Gly143, the biphenyl group at P2 fits into the S2 pocket forming hydrophobic interactions, while the pyridine ring at P1 occupies the S1 pocket and establishes several hydrophobic interactions and a H-bond with the His163 residue. The amide group that links the pyridine ring to the α-methylbenzyl group establishes a H-bond with the main chain of Glu166n, while the phenyl ring of the α-methylbenzyl moiety is partially positioned in both the S2 and S4 pockets forming π- π interactions with the external phenyl of the biphenyl group [
101].
As already mentioned, the non-covalent inhibitor
63 (
Figure 70A) was discovered
via high-throughput virtual screening by Clyde
et al. and it was the starting point for the design of piperazine-based covalent compounds [
69]. From the X-ray structure of the complex SARS-CoV-2 M
pro/
63 it can be observed that the P1-uracil and P2-dichlorobenzene moieties are inserted into the S1 and S2 subsites, respectively, linked through the piperazine ring which lays above the catalytic Cys145, while the S3/S4 subsites remain unoccupied (
Figure 70B) [
69].
Gao
et al. performed several modifications of
63 in order to occupy each pocket of the active site of M
pro, and he most promising compound of this work turned out to be
64 (
Figure 71A) [
70]. The co-crystal structure of complex M
pro/
64 (
Figure 71B) showed that the nicotinic group at P1 fits into the S1 subsite and establishes a H-bond with the His163 residue. The dichlorophenyl group at P2 in inserted into the S2 pocket forming π- π interactions His41 side chain and a halogen bond between the chlorine atom at C-4 and Asp187 backbone. The 2-methylthiophene group at P4 fits into the S4 pocket forming a H-bond with the Glu166 residue, while the piperazine ring shows the same binding mode as
63. Additionally, the P4 thiophene, P2 3,4-dichlorophenyl ring and imidazole side chain of His41 are involved in a sandwich-like π- π interactions, similarly to
56 [
70].
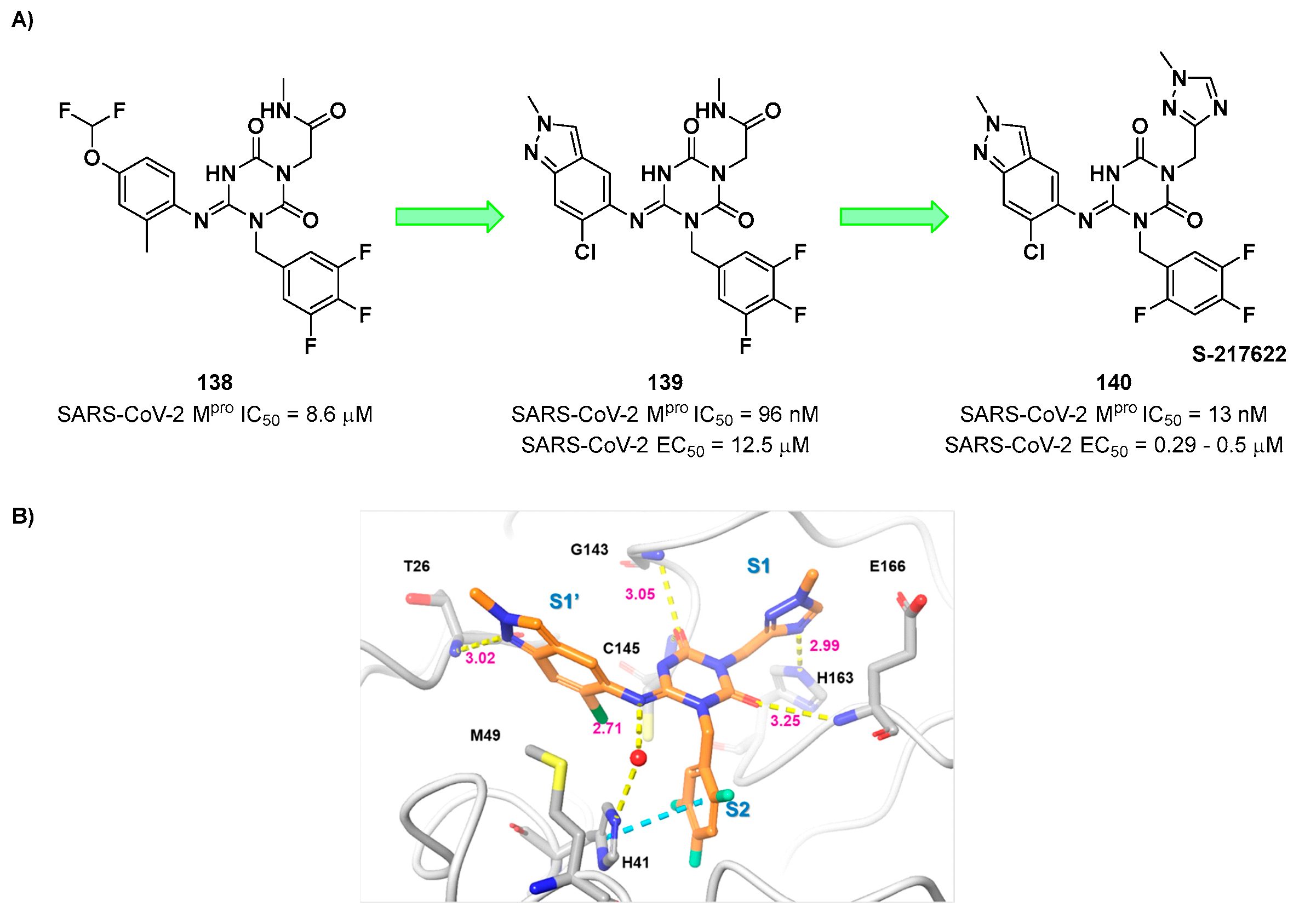
In early 2022, Unoh
et al. discovered
S-217622 (compound
140 in
Figure 72A), the first oral non-covalent, nonpeptidic SARS-CoV-2 M
pro inhibitor clinical candidate [
102]. This compound was obtained
via screening of an in-house library subjected to a docking-based virtual screening with predefined filters (a hydrophobic moiety in S2 and two H-bond acceptors in S1) followed by biological screening. The obtained hit compound
138 (
Figure 72A) was characterized by good
in vitro inhibitory activity (IC
50 = 8.6 μM) and optimal
in vitro and
in vivo PK profile for oral administration. The X-ray crystal structure of SARS-CoV-2 M
pro in complex with
138 was resolved to understand important binding interactions to maintain for a further hit optimization [
102]. To optimize the interaction at S1’, the 4-difluoromethoxy-2-methylbenzene group was replaced with a 6-chloro-2-methyl-2
H-indazole scaffold, which maintained a H-bond with Thr26, achieving compound
139 (
Figure 72A). This compound displayed an outstanding improvement in the inhibitory activity (IC
50 = 96 nM) and a good antiviral activity against infected VeroE6 cells (EC
50 = 12.5 μM), maintaining the favorable PK properties as
138 [
102]. Additional modifications entailed the replacement of the methyl-amide function at P1 with a 1-methyl-1
H-1,2,4-triazole ring, affording the clinical candidate
140 (
Figure 72A)
. This compound displayed excellent enzymatic inhibitory and antiviral activity (IC
50 = 13 nM; EC
50 = 0.37 μM on WT SARS-CoV-2) and optimal PK profile, especially in monkey and dog models.
140 demonstrated also to inhibit the viral replication of a series of SARS-CoV-2 variants (WT, Alpha, Beta, Gamma, Delta and Omicron with EC
50 values from 0.29 to 0.5 μM) as well as different human coronaviruses (SARS-CoV, MERS-CoV, hCoV-229E and hCoV-OC43 with EC
50 values from 74 nM to 5.5 μM) [
102]. These promising results prompted Unoh and co-workers to test
140 in vivo in mice models infected with SARS-CoV-2 Gamma strain:
140 was administered orally immediately and 12 hours after infection, exhibiting rapid efficacy measured after 24 hours. These promising results in pre-clinical studies make the SARS-CoV-2 M
pro inhibitor
140 a potential clinical candidate for oral administration. The X-ray crystal structure of SARS-CoV-2 M
pro complexed with
140 showed that the 1-methyl-1
H-1,2,4-triazole group occupy the S1 pocket establishing a H-bond with the His163 backbone. The 2,4,5-trifluorobenzylic group at P2 fits into the S2 pocket forming a face-to-face π-π interactions with a rotated His41 residue, while the 6-chloro-2-methyl-2
H-indazole scaffold at P1′ maintains the H-bond with Thr26 and establishes hydrophobic interactions with the Met49 residue (
Figure 72B) [
102].
Another contribution in this field was made by Yang
et al. which performed a multiple conformational-based virtual screening on a library of more than 8,000 compounds [
103]. This screening was based on nine different conformations of SARS-CoV-2 M
pro substrate-binding site, and the 49 top-ranked compounds were then evaluated for their binding profiles. Six compounds were able to bind M
pro; therefore, they were selected for
in vitro evaluation of their enzymatic inhibitory activity on FRET assays. All of them were able to inhibit M
pro with IC
50 values ranging from 0.69 to 2.05 μM. The two most promising compounds from the enzymatic inhibitory assay, namely compound
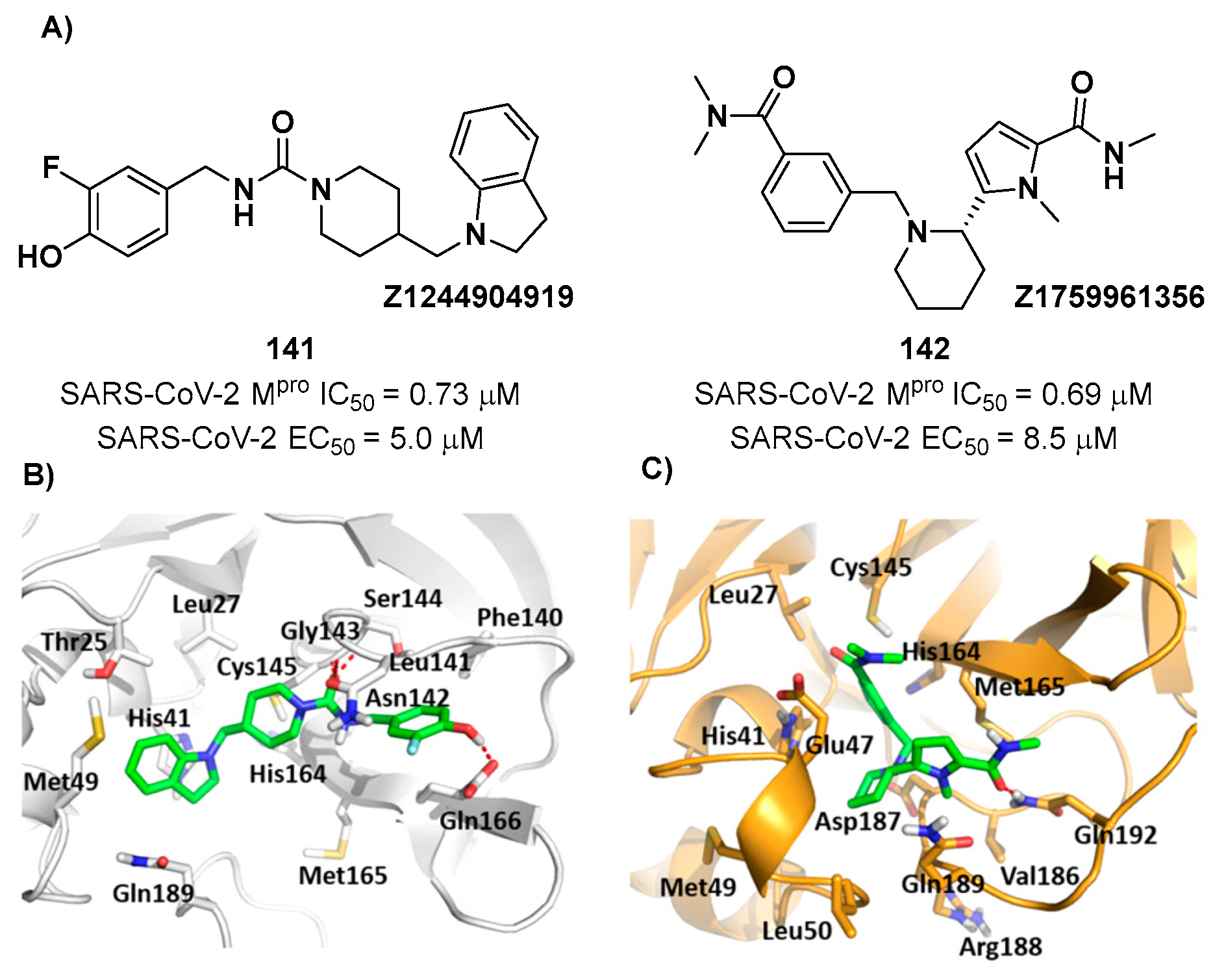
141 (IC
50 = 0.73 μM)
, called
Z1244904919, and compound
142 (IC
50 = 0.69 μM)
, called
Z1759961356, were selected for the evaluation of their antiviral activity on SARS-CoV-2 infected VeroE6 cells in plaque reduction assays, displaying EC
50 values of 5.0 and 8.5 μM, respectively (
Figure 73A). The hypothetical binding modes between these compounds and SARS-CoV-2 M
pro were elucidated by MD simulation and binding free energy analysis and is depicted in
Figure 73B and
Figure 73C: in
141, the fluorophenol moiety fits intothe S1 pocket, while the piperidine ring interacts with the residues of the S4 pocket and connect with the indoline nucleus which, in turns, establishes interactions with the residues of the S2 and S3 sites.
142 has a higher binding affinity due to suggested additional interactions with the His164 and Met165 in the S1 subsite and Asn47 in the S2 subsite [
103].
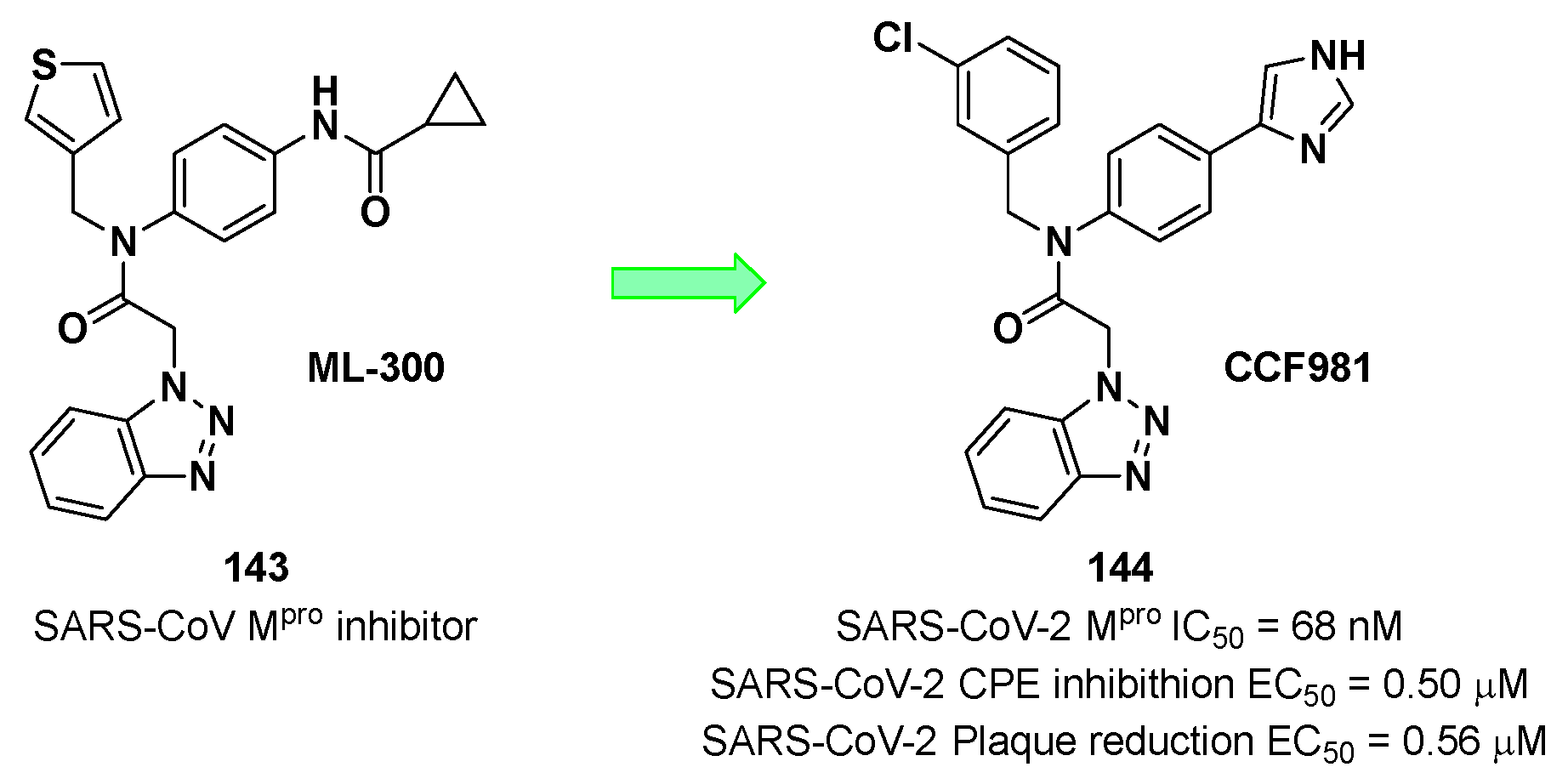
Han
et al. developed a novel non-covalent SARS-CoV-2 M
pro inhibitor starting from the already known benzotriazole-based SARS-CoV inhibitor
ML-300 (
143 in
Figure 74) [
104]. The optimization of
143 led to compound
144, called
CCF981 (
Figure 74), the most potent compound of the work with an IC
50 value of 68 nM against SARS-CoV-2 M
pro and EC
50 values in virus infected VeroE6 cells of 0.50 and 0.56 μM in cytopathic effect (CPE) inhibition and in a plaque reduction assay, respectively.
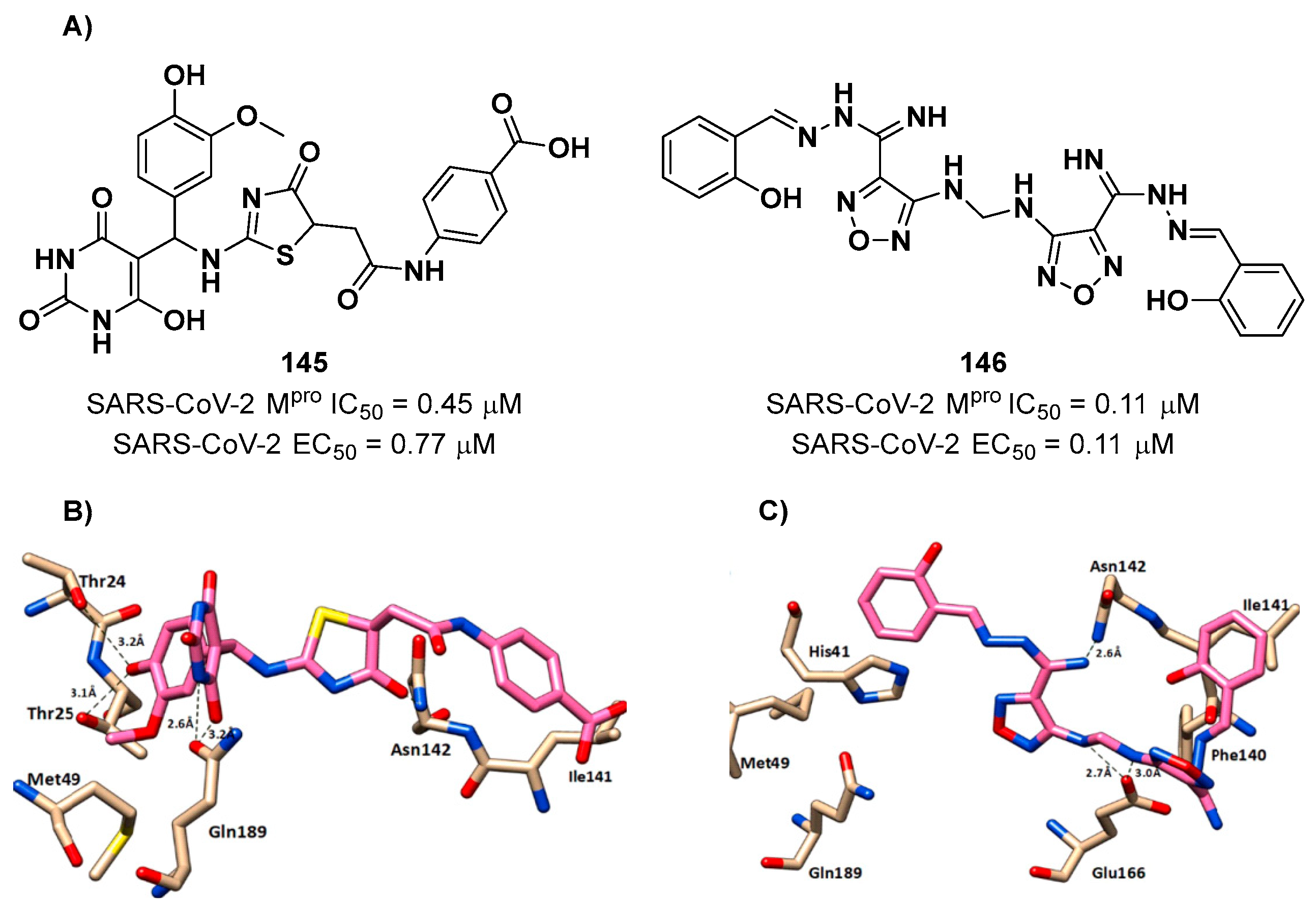
Another interesting compound was obtained by Elsegini
et al. through a pharmacophore-based virtual screening against M
pro, PL
pro and human furin protease, an enzyme involved in the cleavage of spike protein during viral entry, with the aim to select potential dual inhibitor to maximize the antiviral efficacy [
105]. After mapping more than 500,000 compounds, only 16 of them were selected for biological evaluation. Among them, compounds
145 and
146 showed inhibition activity against M
pro and human furin protease in the sub-micromolar range, but activity against PL
pro in the nanomolar range (
Figure 75A). Both compounds exhibited a promising antiviral activity when tested
in vitro against NRC-03-nhCoV, the SARS-CoV-2 strain isolated in Egypt, in VeroE6 cells, exhibiting EC
50 values of 0.77 and 0.11 µM, respectively. Docking studies suggested that both compounds form H-bonds with Asn142 and hydrophobic interactions with Met49 and Ile41; compound
145 forms H-bonds with Thr24, Thr25, and Gln189, while compounds
146 forms an H-bond with Glu166 and a π-π interaction with His41 (
Figure 75B and
Figure 75C) [
105].
Luttens
et al. were able to identify non-covalent M
pro inhibitors through a virtual screening applied on ultralarge chemical libraries [
107]. This strategy brought about the selection of top-ranked compounds for enzyme inhibition assay. Three of them were able to inhibit SARS-CoV-2 M
pro. The hydantoin-based compounds
147 and
148 (
Figure 76) were co-crystalized with SARS-CoV-2 M
pro and the X-ray crystal structures (PDB: 7B2U and PDB: 7AU4) showed that the hydantoin carbonyl groups interacts with the Gly143 and Glu166 backbone
via H-bonds, while the substituents on the hydantoin core fits into S2 and S1 pockets. Furthermore, compounds
147 and
148 were subjected to hit-to-lead optimization driven by guided by docking predictions and determined crystal structures [
107]. This strategy was focused on the maintenance of the hydantoin core and variation of the groups at P1 and P2 positions. From this study emergerd that the isoquinoline and spyro-cyclic scaffolds were the best chemical frameworks for the P1 and P2 position, respectively. Compound
149 (
Figure 76), characterized by an
ortho-chlorophenyl ring as aromatic tail at the P2 position, displayed the best inhibitory activity against SARS-CoV-2 M
pro with IC
50 = 77 nM and
KD = 38 nM. This compound was also subjected to CPE-based assays performed in infected Huh7 cells, showing an EC
50 value of 0.11 μM.
149 was also screened against SARS-CoV and MERS-CoV in CPE-based assays, demonstrating a broad-spectrum activity against coronaviruses (SARS-CoV-1 EC
50 = 0.39 μM in VeroE6 cells, MERS-CoV EC
50 = 0.20 μM in Huh7 cells) [
107].
Another important non-covalent SARS-CoV-2 M
pro inhibitor was developed by Rossetti
et al. by examination of hits derived from two
in silico screening studies (REAL Space and ZINC compound libraries) on two different structures of M
pro [
108]. From the top-ranked hits, a total of 486 compounds were selected on the basis of fragment screening, drug-likeness and chemical diversity. Only five of them were effective M
pro inhibitors. The top two are characterized by a dihydro-quinolinone core (
i.e. 150 and
151;
Figure 77). They underwent a first round of chemical structure similarity searches that led to the discovery to three significantly more potent derivatives,
i.e. 152,
153 and
154 (
Figure 77), called
Z228770960,
Z393665558 and
Z225602086, respectively, with two of them able to enhance the melting temperature of M
pro in a thermal-shift assay. Further optimizations were obtained after a second step of chemical structure similarity searches(
i.e. 155-158, called
Z222979552,
Z228166018,
Z222977344 and
Z222978028, respectively;
Figure 77) [
108].
The co-crystal structure of the most potent derivative
155 with SARS-CoV-2 M
pro confirmed the non-covalent mechanism of action (
Figure 78): the dihydro-quinolinone core forms several H-bonds with Glu166, His163, His172, while the carbonyl oxygen of the amide bond accepts two H-bonds from Cys145 thiol and Glu166 backbone, respectively. The
para-iodobenzene ring establishes π-π interactions with the His41 residue and additional hydrophobic interaction with Asn142, Met49 and Met165 [
108].
Other quinazolin-4-one-based non-covalent inhibitors of SARS-CoV-2 M
pro are compounds
160 and
161 (
Figure 79A), proposed by Zhang
et al. [
109], which were developed starting from the lead
Baicalein (
159 in
Figure 79A) by replacing its chromen-4-one ring and varying the substitution pattern in C2 and N3 positions. The most interesting compound (
160) contains a phenyl group both at C2 and N3 position and posses higher activity as compared to
Baicalein [
56]. Furthermore,
160 and
161 showed optimal selectivity against other human proteases and low cytotoxicity [
109]. The co-crystal structure of SARS-CoV-2 M
pro/
161 is reported in
Figure 79B: the three hydroxy groups form a H-bond pattern with the backbone of Gly143, Ser144 and Gly145, and with the imidazole ring of the His163 residue via a water molecule, while this aromatic portion forms hydrophobic interactions with Cys145 Asn142 and His41. The carbonyl oxygen of the quinazolin-4-one core forms a H-bond with the Glu166 backbone, while the 3’-methyl-4’-fluorophenyl substituent at N3 position deeply fits into the S2 pocket. The
sec-butyl substituent at C2 position matches a newly formed cavity called S2c pocket, a result of a ligand-induced conformation change involving the side chains of Met49 and Gln189 [
109].
Using a similar rational approach, Citarella
et al. designed a novel SARS-CoV-2 M
pro inhibitor characterized by a trifluoromethyl diazirine ring [
110]. In the context of developing fluorinated functionalities able to target druggable enzymes [
111], they replaced the epoxyketone warhead in an already existing M
pro inhibitor with a trifluoromethyl diazirine ring to afford compound
162, called
MPD112 (
Figure 80A). This compound showed
in vitro inhibition activity against SARS-CoV-2 M
pro at low micromolar level (IC
50 = 4.1 μM), no cytotoxicity and selectivity toward M
pro against PL
pro. Furthermore, mass experiments with SARS-CoV-2 M
pro revealed no covalent interaction between target and ligand, suggesting a non-covalent mechanism of inhibition. A molecular docking approach was exploited to shed light on the binding mode of
162 within the target active site (
Figure 80B): docking results showed that the trifluoromethyl diazirine ring is located in close proximity to the Cys145 thiol group forming a halogen bond with the side chain of Thr26. The benzyl carbamate moiety establishes several hydrophobic interactions with Phe140, Glu166 and a H-bond with the oxygen of the Glu166 backbone. The central phenyl group forms hydrophobic interactions with Pro168, Leu167 and Gln189 [
110].
In early 2022, Alhadrami
et al. reported the indole alkaloid
Neoechinulin A (
163 in
Figure 81A) [
112], isolated from the Red Sea fungus
Aspergillus fumigatus, as a potential SARS-CoV-2 M
pro inhibitor through bio-guided screening. This compound showed a promising inhibitory activity with an IC
50 value of 0.47 μM. The mechanism of inhibition was elucidated using docking and molecular dynamics simulations:
163 is characterized by a diketopiperidine nucleus which forms four hydrogen bonds with the Leu141, Asn142 and Gly143 residues of the S1′ sub-pocket and with Glu166 residue of the S2 sub-pocket. Important hydrophobic interactions were also observed with the His41 residue (
Figure 81B) [
112].
Zhang
et al. demonstrated that the natural product
Shikonin (
164 in
Figure 82A) is a broad spectrum anti-coronaviruses agent, with micromolar IC
50 values against the M
pro of SARS-CoV-2, SARS-CoV, MERS-CoV, hCoV-HKU1, hCoV-NL63, and hCoV-229E [
113]. The X-ray crystal structure of SARS-CoV-2 M
pro in complex with
Shikonin was determined by Li
et al. in a previous work [
114]. They found out that the presence of
Shikonin in the active site cleft of the enzyme causes a conformational change in the catalytic dyad His41-Cys45 and establishes a face-to-face π-π interaction involving the His41 residue of the target and the naphtoquinone ring of the inhibitor. One of the two –OH groups of
Shikonin establishes H-bonds with the Cys145 –SH group and His164 backbone, while the
iso-hexenyl group establishes H-bonds with Arg188 and Gln189 residues. Another important difference is the change in position of a flexible loop, including Cys44 to Tyr54, Asp187 to Ala191, and Leu141 to Ser144.
Zhang
et al. discovered two 9,10-dihydrophenanthrene derivatives as novel SARS-CoV-2 M
pro inhibitors through an
in vitro screening of an in-house compound library based on the same scaffold [
115]. The first round of optimization pointed out the importance of the
para-bromo phenyl group at R1 and the pyridyne ring at R3 for the inhibitory activity. Then, these two units were maintained for further optimizations carried out on the pyridine ring. The aliphatic –OH group was taken into account for obtaining hydrolyzable esters. The enzymatic assay revealed that compounds
165 and
166 (
Figure 83), called
C1 and
C2, were the most promising derivatives with IC
50 values of 1.5 μM and 1.8 μM, respectively. Kinetic studies provided
Ki values of 6.1 μM and 7.6 μM, for
165 and
166, respectively,and suggested a dose-dependent mixed-inhibition mechanism of SARS-CoV-2 M
pro, which means that these inhibitors can bind at least two sites in the target protein, probably the substrate-binding site and the dimer interface. Indeed, molecular docking simulations confirmed this bimodal binding mechanism for
165 [
115].
Figure 1.
(A) Crystal structure of SARS-CoV-2 Mpro (PDB: 7ALH) enlightening the two monomers (monomer A is depicted in orange, monomer B in cyan); (B) Crystal structure of SARS-CoV-2 Mpro monomer (PDB: 1P9S) enlightening the three domains (domain I is depicted in blue, domain II in green, domain III in orange).
Figure 1.
(A) Crystal structure of SARS-CoV-2 Mpro (PDB: 7ALH) enlightening the two monomers (monomer A is depicted in orange, monomer B in cyan); (B) Crystal structure of SARS-CoV-2 Mpro monomer (PDB: 1P9S) enlightening the three domains (domain I is depicted in blue, domain II in green, domain III in orange).
Figure 2.
Active site of the SARS-CoV-2 Mpro with position of the catalytic dyad, oxyanion hole and subsites S1'-S5.
Figure 2.
Active site of the SARS-CoV-2 Mpro with position of the catalytic dyad, oxyanion hole and subsites S1'-S5.
Figure 3.
Schematic representation of the allosteric sites of Mpro. The allosteric sites are located near the dimerization site (allosteric site 1) or between domains II and III (allosteric site 2). The active site in each monomer is indicated with a yellow circle.
Figure 3.
Schematic representation of the allosteric sites of Mpro. The allosteric sites are located near the dimerization site (allosteric site 1) or between domains II and III (allosteric site 2). The active site in each monomer is indicated with a yellow circle.
Figure 4.
Chemical structure and biological activity of Pfizer inhibitors Nirmatrelvir, 2 and 3.
Figure 4.
Chemical structure and biological activity of Pfizer inhibitors Nirmatrelvir, 2 and 3.
Figure 5.
Chemical structure and biological activity of compounds derived from 2.
Figure 5.
Chemical structure and biological activity of compounds derived from 2.
Figure 6.
Crystal structure of the complex of SARS-CoV-2 Mpro/Nirmatrelvir (PDB: 7MLF).
Figure 6.
Crystal structure of the complex of SARS-CoV-2 Mpro/Nirmatrelvir (PDB: 7MLF).
Figure 7.
Chemical structure and biological activity of compounds 8 and 9.
Figure 7.
Chemical structure and biological activity of compounds 8 and 9.
Figure 8.
Chemical structure of compounds 10 and 11 developed by Zhu et al. The part marked with dotted circle highlights the modifications with respect to Nirmatrelvir.
Figure 8.
Chemical structure of compounds 10 and 11 developed by Zhu et al. The part marked with dotted circle highlights the modifications with respect to Nirmatrelvir.
Figure 9.
Chemical structure and biological activity of Boceprevir, Narlaprevir, GC-376, 12 and 13. The colors highlight the chemical features the authors work on for the design of new compounds.
Figure 9.
Chemical structure and biological activity of Boceprevir, Narlaprevir, GC-376, 12 and 13. The colors highlight the chemical features the authors work on for the design of new compounds.
Figure 10.
X-ray crystal structures of SARS-CoV-2 Mpro in complex with 12 (A)(PDB: 7TEH) and 13 (B)(PDB:7TFR).
Figure 10.
X-ray crystal structures of SARS-CoV-2 Mpro in complex with 12 (A)(PDB: 7TEH) and 13 (B)(PDB:7TFR).
Figure 11.
Chemical structure and biological activity of compounds 14 and 15. The part marked with dotted circle highlights the isoelectronic replacement of the nitrile group.
Figure 11.
Chemical structure and biological activity of compounds 14 and 15. The part marked with dotted circle highlights the isoelectronic replacement of the nitrile group.
Figure 12.
Chemical structure and biological activity of the first aldehyde derivatives (16-18) as inhibitors of SARS-CoV-2 Mpro developed by Dai et al.
Figure 12.
Chemical structure and biological activity of the first aldehyde derivatives (16-18) as inhibitors of SARS-CoV-2 Mpro developed by Dai et al.
Figure 13.
A) Chemical structure and biological activity of compounds 19 and 20; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 19 (PDB: 6WTJ).
Figure 13.
A) Chemical structure and biological activity of compounds 19 and 20; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 19 (PDB: 6WTJ).
Figure 14.
A) Chemical structure and biological activity of compounds 21 and 22. Those parts marked with colors indicate modifications with respect the lead structure 20; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 21 (PDB: 7LCO).
Figure 14.
A) Chemical structure and biological activity of compounds 21 and 22. Those parts marked with colors indicate modifications with respect the lead structure 20; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 21 (PDB: 7LCO).
Figure 15.
A) Chemical structure and biological activity of compounds 23 and 24. Those parts marked with colors indicate modifications with respect the lead structure 20; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 24 (PDB: 7TIZ).
Figure 15.
A) Chemical structure and biological activity of compounds 23 and 24. Those parts marked with colors indicate modifications with respect the lead structure 20; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 24 (PDB: 7TIZ).
Figure 17.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 26 (PDB: 7D3I).
Figure 17.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 26 (PDB: 7D3I).
Figure 18.
Chemical structure and biological activity of compound 28.
Figure 18.
Chemical structure and biological activity of compound 28.
Figure 19.
Chemical structure and biological activity of compounds 29 and 30.
Figure 19.
Chemical structure and biological activity of compounds 29 and 30.
Figure 20.
A) X-ray structure of SARS-CoV-2Mpro in complex with compound 29 (PDB: 7LIH); B) X-ray structure of SARS-CoV-2Mpro in complex with compound 30 (PDB: 7LIY).
Figure 20.
A) X-ray structure of SARS-CoV-2Mpro in complex with compound 29 (PDB: 7LIH); B) X-ray structure of SARS-CoV-2Mpro in complex with compound 30 (PDB: 7LIY).
Figure 21.
Chemical structure and biological activity of compounds 31-33.
Figure 21.
Chemical structure and biological activity of compounds 31-33.
Figure 22.
A) Chemical structure and biological activity of compound 34; B) X-ray structure of SARS-CoV-2 Mpro in complex with 34 (PDB: 7AKU).
Figure 22.
A) Chemical structure and biological activity of compound 34; B) X-ray structure of SARS-CoV-2 Mpro in complex with 34 (PDB: 7AKU).
Figure 23.
Chemical structure and biological activity of compounds 35-39.
Figure 23.
Chemical structure and biological activity of compounds 35-39.
Figure 24.
A) Chemical structure and biological activity of compounds 40 and 41; B) X-ray structures of SARS-CoV Mpro in complex with compound 40 (PDB: 7RVQ).
Figure 24.
A) Chemical structure and biological activity of compounds 40 and 41; B) X-ray structures of SARS-CoV Mpro in complex with compound 40 (PDB: 7RVQ).
Figure 25.
Chemical structure and biological activity of compounds 42, 43 and 44.
Figure 25.
Chemical structure and biological activity of compounds 42, 43 and 44.
Figure 26.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 43 (PDB: 7MBI).
Figure 26.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 43 (PDB: 7MBI).
Figure 27.
Chemical structure and biological activity of compounds 45, 46 and 47.
Figure 27.
Chemical structure and biological activity of compounds 45, 46 and 47.
Figure 28.
X-ray structure of SARS-CoV-2 Mpro in complex with 46 (PBD: 8DOX).
Figure 28.
X-ray structure of SARS-CoV-2 Mpro in complex with 46 (PBD: 8DOX).
Figure 29.
Chemical structure and biological activity of compound 48.
Figure 29.
Chemical structure and biological activity of compound 48.
Figure 30.
Chemical structure and biological activity of compound 49.
Figure 30.
Chemical structure and biological activity of compound 49.
Figure 31.
X-ray/neutron (XN) crystal structure of SARS-CoV-2 Mpro in complex with 49 (PDB: 7TD1).
Figure 31.
X-ray/neutron (XN) crystal structure of SARS-CoV-2 Mpro in complex with 49 (PDB: 7TD1).
Figure 32.
A) Chemical structure and biological activity of compound 50; B) Predicted binding mode of 50 with SARS-CoV-2Mpro.
Figure 32.
A) Chemical structure and biological activity of compound 50; B) Predicted binding mode of 50 with SARS-CoV-2Mpro.
Figure 33.
Chemical structure and biological activity of compounds 51 and 52.
Figure 33.
Chemical structure and biological activity of compounds 51 and 52.
Figure 34.
Chemical structure and biological activity of compound 53.
Figure 34.
Chemical structure and biological activity of compound 53.
Figure 35.
A) Chemical structure and biological activity of compounds 54 and 55; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 55 (PDB: 7MLF).
Figure 35.
A) Chemical structure and biological activity of compounds 54 and 55; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 55 (PDB: 7MLF).
Figure 36.
Chemical structure and biological activity of compounds 56–60.
Figure 36.
Chemical structure and biological activity of compounds 56–60.
Figure 37.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 57 (PDB: 7RN1).
Figure 37.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 57 (PDB: 7RN1).
Figure 38.
A) Chemical structure and biological activity of compound 61; B) Docking of SARS-CoV-2 Mpro in complex with compound 61.
Figure 38.
A) Chemical structure and biological activity of compound 61; B) Docking of SARS-CoV-2 Mpro in complex with compound 61.
Figure 39.
A) Chemical structure and biological activity of compound 62; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 62 (PBD: 7VVT).
Figure 39.
A) Chemical structure and biological activity of compound 62; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 62 (PBD: 7VVT).
Figure 40.
Chemical structure and biological activity of compounds 63-66.
Figure 40.
Chemical structure and biological activity of compounds 63-66.
Figure 41.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 66 (PDB: 8B56).
Figure 41.
X-ray structure of SARS-CoV-2 Mpro in complex with compound 66 (PDB: 8B56).
Figure 42.
Chemical structure and biological activity of compounds 67-69.
Figure 42.
Chemical structure and biological activity of compounds 67-69.
Figure 43.
Structure and biological activity of 70 and resolution of the two diastereomers.
Figure 43.
Structure and biological activity of 70 and resolution of the two diastereomers.
Figure 44.
A) Chemical structure and biological activity of compound 71; B) X-ray structure of SARS-CoV-2 Mpro in complex with 71 (PDB: 7FAZ).
Figure 44.
A) Chemical structure and biological activity of compound 71; B) X-ray structure of SARS-CoV-2 Mpro in complex with 71 (PDB: 7FAZ).
Figure 45.
A) Chemical structure and biological activity of compound 72; B) X-ray structure of SARS-CoV-2 Mpro with 72 (PDB: 8IGN).
Figure 45.
A) Chemical structure and biological activity of compound 72; B) X-ray structure of SARS-CoV-2 Mpro with 72 (PDB: 8IGN).
Figure 46.
A) Chemical structure and biological activity of compound 73; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 73 (PDB: 8HHT).
Figure 46.
A) Chemical structure and biological activity of compound 73; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 73 (PDB: 8HHT).
Figure 47.
A) Chemical structure and biological activity of compound 74; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 74 (PDB: 8HHU).
Figure 47.
A) Chemical structure and biological activity of compound 74; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 74 (PDB: 8HHU).
Figure 48.
A) Chemical structure and biological activity of compounds 75 and 76; B) X-ray structure of SARS-CoV-2 Mpro in complex with 75 (PDB: 6LU7).
Figure 48.
A) Chemical structure and biological activity of compounds 75 and 76; B) X-ray structure of SARS-CoV-2 Mpro in complex with 75 (PDB: 6LU7).
Figure 49.
A) Chemical structure and biological activity of compound 77; B) X-ray structure of SARS-CoV-2 Mpro in complex with 77 (PDB: 7JT7).
Figure 49.
A) Chemical structure and biological activity of compound 77; B) X-ray structure of SARS-CoV-2 Mpro in complex with 77 (PDB: 7JT7).
Figure 50.
Chemical structure and biological activity of compounds 78 and 79.
Figure 50.
Chemical structure and biological activity of compounds 78 and 79.
Figure 51.
Chemical structure and biological activity of compounds 80-82.
Figure 51.
Chemical structure and biological activity of compounds 80-82.
Figure 52.
A) Chemical structure and biological activity of compounds 83 and 84; B) X-ray structure of SARS-CoV-2Mpro in complex with 83 (PDB: 7MB0).
Figure 52.
A) Chemical structure and biological activity of compounds 83 and 84; B) X-ray structure of SARS-CoV-2Mpro in complex with 83 (PDB: 7MB0).
Figure 53.
Chemical structure and biological activity of compounds 85-88.
Figure 53.
Chemical structure and biological activity of compounds 85-88.
Figure 54.
A) Chemical structure and biological activity of compounds 89 and 90; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 89 (PDB: 7MLG).
Figure 54.
A) Chemical structure and biological activity of compounds 89 and 90; B) X-ray structure of SARS-CoV-2 Mpro in complex with compound 89 (PDB: 7MLG).
Figure 55.
Chemical structure and biological activity of compounds 91 and 92.
Figure 55.
Chemical structure and biological activity of compounds 91 and 92.
Figure 56.
A) Chemical structure and biological activity of compound 93; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 93 (PDB: 7B3E).
Figure 56.
A) Chemical structure and biological activity of compound 93; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 93 (PDB: 7B3E).
Figure 57.
Chemical structure and biological activity of compounds 94-96.
Figure 57.
Chemical structure and biological activity of compounds 94-96.
Figure 58.
A) Chemical structure and biological activity of compounds 97-99; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 97 (PDB: 7RBZ).
Figure 58.
A) Chemical structure and biological activity of compounds 97-99; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 97 (PDB: 7RBZ).
Figure 59.
Chemical structure and biological activity of compounds 100 and 101.
Figure 59.
Chemical structure and biological activity of compounds 100 and 101.
Figure 60.
A) Chemical structure and biological activity of compound 102; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 102 (PDB: 7JT0).
Figure 60.
A) Chemical structure and biological activity of compound 102; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 102 (PDB: 7JT0).
Figure 61.
A & C) Chemical structure and biological activity of compounds 103-109; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 103 (PDB: 7X6K).
Figure 61.
A & C) Chemical structure and biological activity of compounds 103-109; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 103 (PDB: 7X6K).
Figure 62.
Chemical structure and biological activity of compounds 110-114.
Figure 62.
Chemical structure and biological activity of compounds 110-114.
Figure 63.
Chemical structure and biological activity of compounds 115-119.
Figure 63.
Chemical structure and biological activity of compounds 115-119.
Figure 64.
Chemical structure and biological activity of compounds 120-124.
Figure 64.
Chemical structure and biological activity of compounds 120-124.
Figure 65.
Chemical structure and biological activity of Ebsulfur, 126 and 127.
Figure 65.
Chemical structure and biological activity of Ebsulfur, 126 and 127.
Figure 66.
Chemical structure and biological activity of compounds 128-134.
Figure 66.
Chemical structure and biological activity of compounds 128-134.
Figure 67.
Chemical structure and biological activity of compounds 135-137.
Figure 67.
Chemical structure and biological activity of compounds 135-137.
Figure 68.
A) Chemical structure and biological activity of the compound 54; B) X-Ray crystal structure of SARS-CoV-2 Mpro in complex with 54 (PDB: 6W63), highlighting binding site interactions.
Figure 68.
A) Chemical structure and biological activity of the compound 54; B) X-Ray crystal structure of SARS-CoV-2 Mpro in complex with 54 (PDB: 6W63), highlighting binding site interactions.
Figure 69.
A) Chemical structure and biological activity of the compound 56; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 56 (PDB: 7KX5).
Figure 69.
A) Chemical structure and biological activity of the compound 56; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 56 (PDB: 7KX5).
Figure 70.
A) Chemical structure and biological activity of compound 63; B) X-ray structure of SARS-CoV-2 Mpro in complex with 63 (PDB: 7LTJ).
Figure 70.
A) Chemical structure and biological activity of compound 63; B) X-ray structure of SARS-CoV-2 Mpro in complex with 63 (PDB: 7LTJ).
Figure 71.
A) Chemical structure and biological activity of compound 64; B) X-ray structure of SARS-CoV-2 Mpro in complex with 64 (PDB: 8ACL).
Figure 71.
A) Chemical structure and biological activity of compound 64; B) X-ray structure of SARS-CoV-2 Mpro in complex with 64 (PDB: 8ACL).
Figure 72.
A) Chemical structure and biological activity of compounds 138-140; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 140 (PDB: 7VU6).
Figure 72.
A) Chemical structure and biological activity of compounds 138-140; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 140 (PDB: 7VU6).
Figure 73.
A) Chemical structure and biological activity of compounds 141 and 142; B) Docking of SARS-CoV-2 Mpro in complex with 141; C) Docking of SARS-CoV-2 Mpro in complex with 142.
Figure 73.
A) Chemical structure and biological activity of compounds 141 and 142; B) Docking of SARS-CoV-2 Mpro in complex with 141; C) Docking of SARS-CoV-2 Mpro in complex with 142.
Figure 74.
Chemical structure and biological activity of compounds 143 and 144.
Figure 74.
Chemical structure and biological activity of compounds 143 and 144.
Figure 75.
A) Chemical structure and biological activity of compounds 145 and 146. B) Docking of SARS-CoV-2 Mpro in complex with 145; C) Docking of SARS-CoV-2 Mpro in complex with 146.
Figure 75.
A) Chemical structure and biological activity of compounds 145 and 146. B) Docking of SARS-CoV-2 Mpro in complex with 145; C) Docking of SARS-CoV-2 Mpro in complex with 146.
Figure 76.
Chemical structure and biological activity of compounds 147-149.
Figure 76.
Chemical structure and biological activity of compounds 147-149.
Figure 77.
Chemical structure and biological activity of compounds 150-158.
Figure 77.
Chemical structure and biological activity of compounds 150-158.
Figure 78.
X-ray crystal structure of SARS-CoV-2 Mpro in complex with 155 (PDB:7P2G).
Figure 78.
X-ray crystal structure of SARS-CoV-2 Mpro in complex with 155 (PDB:7P2G).
Figure 79.
A) Chemical structure and biological activity of compounds 159 - 161. B) X-ray crystal structure of 161 in complex with SARS-CoV-2 Mpro (PDB: 8I4S).
Figure 79.
A) Chemical structure and biological activity of compounds 159 - 161. B) X-ray crystal structure of 161 in complex with SARS-CoV-2 Mpro (PDB: 8I4S).
Figure 80.
A) Chemical structure and biological activity of compound 159; B) SARS-CoV-2 Mpro/162 complex suggested by molecular docking.
Figure 80.
A) Chemical structure and biological activity of compound 159; B) SARS-CoV-2 Mpro/162 complex suggested by molecular docking.
Figure 81.
A) Chemical structure and biological activity of compound 163. B) Docking of SARS-CoV-2 Mpro in complex with 163.
Figure 81.
A) Chemical structure and biological activity of compound 163. B) Docking of SARS-CoV-2 Mpro in complex with 163.
Figure 82.
A) Chemical structure and biological activity of compound 164; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 164 (PDB: 7CA8).
Figure 82.
A) Chemical structure and biological activity of compound 164; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 164 (PDB: 7CA8).
Figure 83.
Chemical structure and biological activity of compound 165 and 166.
Figure 83.
Chemical structure and biological activity of compound 165 and 166.
Figure 84.
A) Chemical structure and biological activity of compound 167; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 167 (PDB: 7AXM).
Figure 84.
A) Chemical structure and biological activity of compound 167; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 167 (PDB: 7AXM).
Figure 85.
Chemical structure and biological activity of compounds 168 and 169.
Figure 85.
Chemical structure and biological activity of compounds 168 and 169.
Figure 86.
A) Chemical structure and biological activity of compound 170; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 170 (PDB: 7AGA).
Figure 86.
A) Chemical structure and biological activity of compound 170; B) X-ray crystal structure of SARS-CoV-2 Mpro in complex with 170 (PDB: 7AGA).
Figure 87.
Chemical structure and biological activity of compounds 171-173.
Figure 87.
Chemical structure and biological activity of compounds 171-173.
Figure 88.
Chemical structure and biological activity of compound 174.
Figure 88.
Chemical structure and biological activity of compound 174.