1. Introduction
For the last decades, the development of new ceramic and semiconducting thin films by vacuum processes has become one of the most challenging tasks for creating innovative functional materials. If binary compounds associating a single metal with a light element such as boron, carbon, nitrogen or oxygen, have been expansively developed by various deposition methods [
1,
2,
3,
4], the addition of a third element to form ternary compounds still remains pertinent nowadays. These numerous combinations with three different elements may lead to the formation of an homogeneous single phase or even multi-phase disperse system exhibiting diverse types of designs at the micro- and nanoscale such as nanocomposite structures, solid solutions, or sometimes nanostructured architectures [
5,
6,
7]. The strategy implemented to grow these ternary thin films obviously depends on the targeted deposited materials. Many studies report on investigations focused on the fabrication of compounds made of two metals with a metalloid element like oxygen or nitrogen, leading to oxides and nitrides, respectively. [
8,
9,
10,
11,
12]. Both types of materials can be produced by the sputtering method involving either a ceramic target, or a metallic one and consequently a reactive atmosphere. Since the latter typically exhibits non-linear phenomena of operating parameters (target voltage, deposition rate and so on), they intrinsically restrain some achievable compositions and thus, some physical properties of as-deposited thin films. Some original approaches (high pumping speed, feedback control systems, …) have been developed to cope with the main drawbacks of the reactive sputtering process. They finally led to get tunable oxide (or nitride) thin films by means of pertinent adjustments of deposition parameters.
Considering ternary compounds involving a metal combined with two light elements such as oxygen and nitrogen, namely Me-O
2-N
2 systems, to prepare oxynitride thin films, the reactive sputtering process becomes even more complex and tricky to control [
13,
14,
15]. For these thin films, one of the challenging tasks is the high reactivity of oxygen towards the sputtered metal (compared to that of nitrogen), which may limit some reachable compositions and, thus reducing the range of final properties. An original method, namely RGPP – Reactive Gas Pulsing Process, was proposed twenty years ago to easily manage the reactive sputtering process pulsing the most reactive specie, i.e., oxygen gas, and fabricate oxynitride coatings with tunable oxygen and nitrogen concentrations from pure nitrides to pure oxides [
16]. This method was and is still successfully applied to obtain oxides, nitrides and oxynitrides with adjustable metalloid concentrations [
17,
18,
19,
20,
21,
22]. The resulting properties showed a wide range of behaviors in-between that of nitrides (high hardness, wear resistance, …) and oxides (optical transparency, high electrical resistivity, …) which are strongly dependent on the oxygen and nitrogen amounts in the films [
23,
24,
25,
26,
27]. However, deposition rates of these oxynitrides still remain in an order of magnitude much lower than that of metallic rates due to the full poisoning phenomena of the metallic target surface by the reactive gases (alternation of the reactive sputtering process between nitridation and oxidation). As a result, despite the strong potential of RGPP for a possible transfer at an industrial scale, the quite low deposition rate of oxynitride thin films is still a drawback and reaching rates close to that of metal would be a strength of the technique.
In this work, we prepare Ti-O-N thin films by reactive sputtering implementing the RGPP technique. The starting point is a pure Ti metallic target sputtered in a reactive atmosphere composed of oxygen and nitrogen gases. Compared to previous published studies [
22], we report on controlling the reactive sputtering process where both reactive gases are independently and periodically pulsed during the deposition stage. This is the main original approach of the study since to the best of our knowledge, a simultaneous pulsing injection of two reactive gases has never been reported in reactive sputtering processes. In this work, the same O
2 and N
2 pulsing periods are used and a constant delay is applied between the starting points of nitrogen and oxygen injections. Duty cycles related to each gas are systematically investigated from 0 to 100% of each pulsing period of both reactive gases. Deposition rate of titanium oxynitride thin films are measured as function of both duty cycles showing rates in-between that of the pure Ti metal and those of TiO
2 compound. Similarly, the optical transmittance in the visible range of Ti-O-N thin films show a gradual and well-controlled transition from absorbent to transparent behaviors. A special emphasis is put on the poisoning state of the Ti target surface assuming real time measurements of the target voltage and total sputtering pressure during the deposition stage. It is shown that alternations between 2 or 3 sputtering modes (elemental, nitrided, oxidized) can be produced as a function of O
2 and N
2 duty cycles. These alternations are then correlated with evolution of deposition rate and optical transparency of titanium oxynitride thin films.
2. Materials and Methods
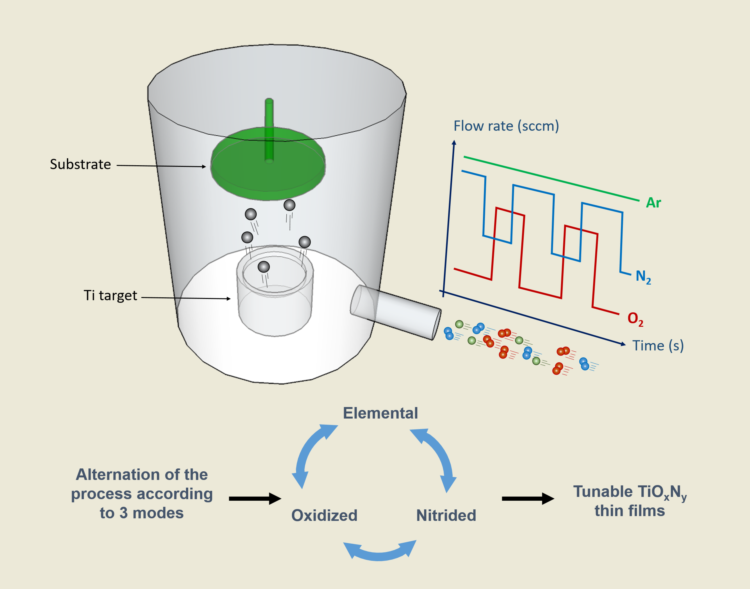
Titanium oxynitride thin films were prepared by DC reactive magnetron sputtering. The sputtering machine was a 60 L vacuum reactor evacuated by a turbomolecular pump backed with a mechanical primary pump achieving an ultimate pressure of 5 10
-8 mbar. A metallic titanium target (51 mm diameter and purity 99.6 at. %) was sputtered in an atmosphere composed of argon, oxygen and nitrogen gases. The substrate was fixed, grounded and located at 65 mm in front of the Ti target. The argon flow rate was kept constant at
qAr = 2.6 sccm and a constant pumping speed
SAr = 13 L s
-1 was used, leading to an argon partial pressure close to 3.0 10
-3 mbar. Before any deposition, a pre-sputtering time of 15 min was applied to the Ti target to remove some contamination layers on the target surface and stabilize the process with a Ti target current intensity I
Ti = 200 mA corresponding to a current density J
Ti = 100 Am
-2. For such operating conditions, the Ti target potential in pure argon sputtering atmosphere reached about U
Ti = 305 V. Afterwards, nitrogen and/or oxygen gases were introduced using independent pulsing signals for each reactive gas. To this aim, a home-made computer controlled, namely, the reactive gas pulsing process (RGPP), was developed to accurately manage reactive gas mass flow rate
vs. time [
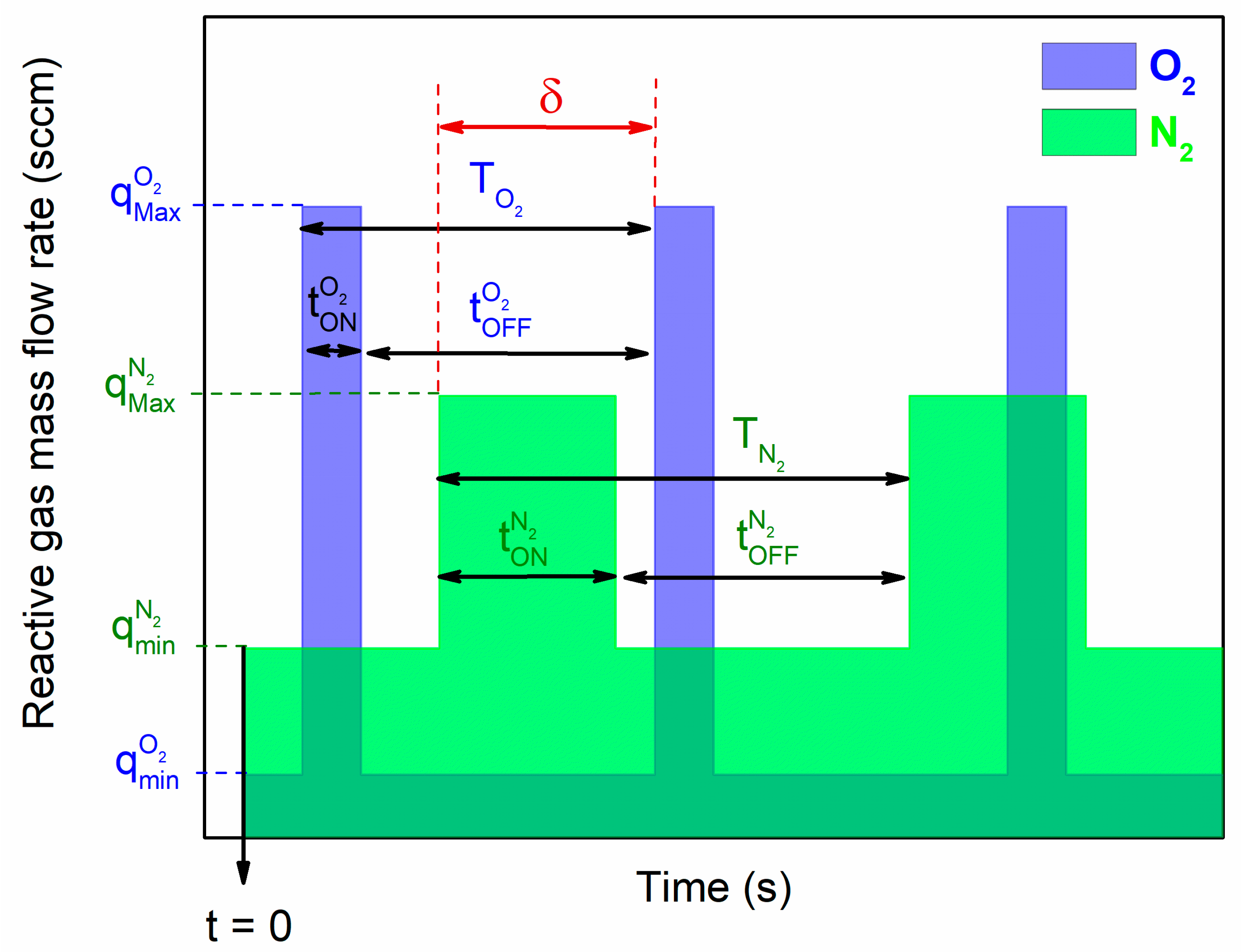
22]. In this paper, both gases were separately injected following a rectangular pulsing signal (
Figure 1).
Except injection times of each reactive gas (i.e., t
O2ON and t
N2ON related to O
2 and N
2 gases, respectively) all the other pulsing parameters described in
Figure 1 were kept constant. Minimum O
2 and N
2 flow rates (q
O2min and q
N2min, respectively) were both fixed at 0 sccm in order to completely stop the oxygen and nitrogen injections during the corresponding t
O2OFF and t
N2OFF times, respectively. For maximum O
2 and N
2 flow rates, q
O2Max = 2.0 sccm and q
N2Max = 0.8 sccm. These maximum values correspond to the amount of reactive gas (O
2 or N
2) required to fully poisoned (oxidized or nitrided, respectively) the Ti target when the reactive gas is constantly and solely injected in the process. In order to better control the reactive process, the pulsing period of both gases were set to T
O2 = T
N2 = T = 45 s. This choice is based-on previous investigations showing that the reactive sputtering process involving one Ti metallic target sputtered in a reactive atmosphere (especially O
2), can alternate between poisoned and elemental modes by playing on the time of injection of the reactive gas (i.e., t
ON time) [
28]. Last but not least concerns the delay time, namely δ, defined as the time between the starting point of N
2 and O
2 pulsing. This δ parameter was chosen at δ = 34 s (i.e., 75% of T) in order to allow an adjustment of the kinetics of the reactive process by playing only with times of injections of each reactive gas, especially so as to allow the process to be restored to the elemental mode during the t
O2OFF time, and before the injection of N
2 (i.e., before the starting point of the t
N2ON time). Since the main purpose of this study is focused on the deposition of tunable titanium oxynitride thin films, t
O2ON and t
N2ON times were systematically changed from 0 to 100 % of the pulsing period T. In other words, the duty cycles of O
2 and N
2 gases defined as α
O2 = t
O2ON/T and α
N2 = t
N2ON/T were gradually modified. For all depositions, real time measurements of the Ti target voltage and total sputtering pressure were recorded. These parameters give information about the state of poisoning of the Ti target surface.
Films were deposited on glass substrates ultrasonically cleaned in acetone, ethanol and deionized water for 10 min and dried in an oven at 60 ̊C for 20 min. The film’s thickness was measured by profilometry, and the deposition time was adjusted in order to prepare Ti-O-N films with a thickness of 400 nm. Deposition rate was determined from the film’s thickness and the corresponding deposition time. Optical transmittance spectra were measured on film/ glass substrate system in the visible range using a PerkinElmer Lambda 900 spectrophotometer. The average transmittance was computed at 633 nm assuming the curve passing through the inflection points of interference fringes [
28]. At a wavelength of 633 nm, it is representative of the transparency of the material in the visible region.
3. Results
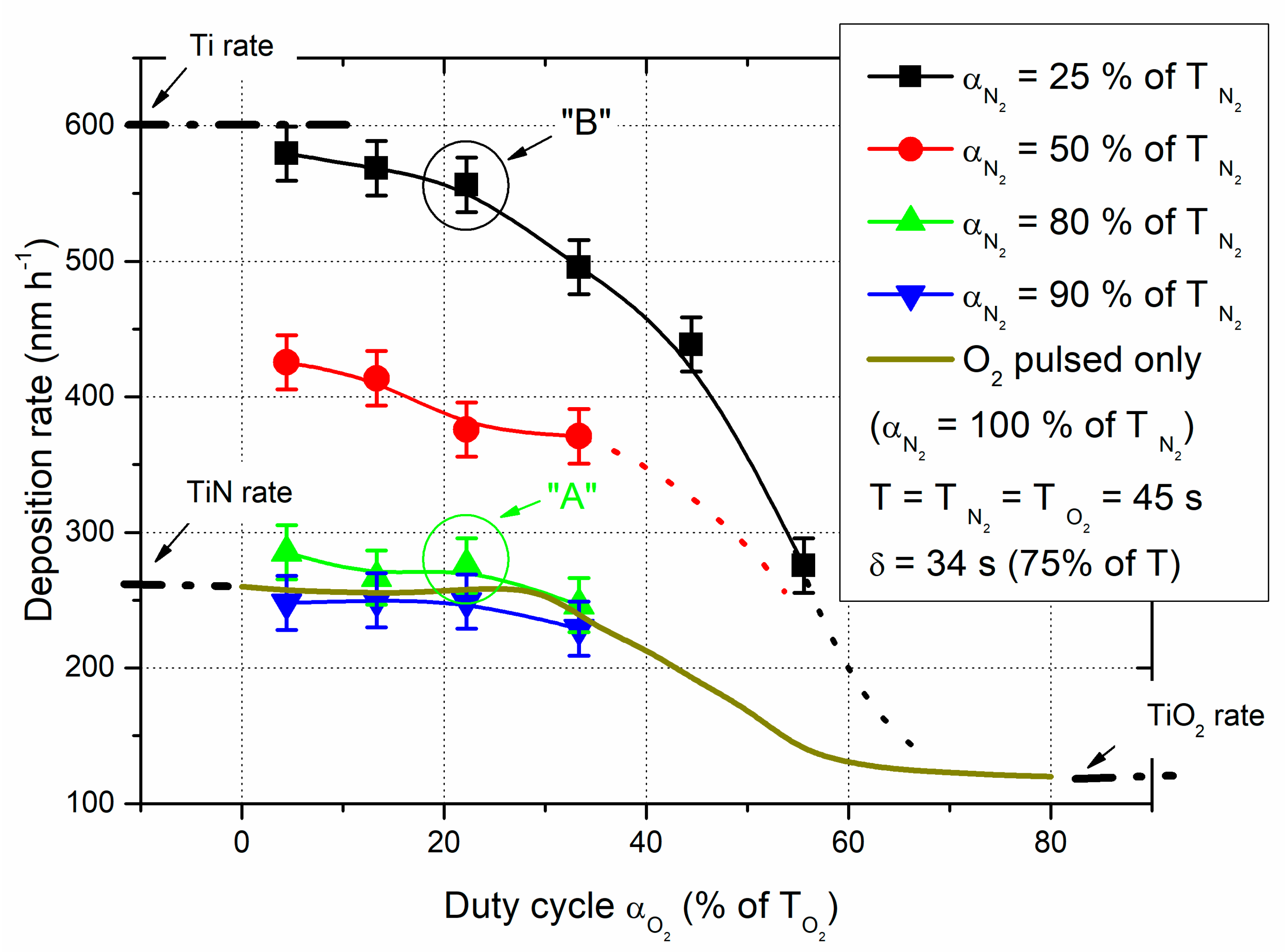
The deposition rate of Ti-O-N films was first measured as function of the oxygen duty cycle α
O2 and for various nitrogen duty cycles α
N2 (
Figure 2).
Without pulsing the nitrogen gas and based on previous results [
28], a continuous evolution of the deposition rate
vs. oxygen duty cycle α
O2 is clearly measured. The typical feature of the conventional reactive sputtering process exhibiting a sudden drop of the deposition rate as a function of the reactive gas flow rate is not produced by RGPP. Deposition rate is nearly constant up to α
O2 = 33% of T
O2 and close that of TiN rate (around 260 nm h
-1). For O
2 duty cycles higher than 70% of T
O2, the rate regularly decreases and tends to be stable at 120 nm h
-1, the lowest rate obtained for the deposition of TiO
2 thin films prepared with a constant supply of the oxygen gas. As RGPP is applied to the deposition of titanium oxynitride thin films, instabilities of the reactive sputtering process can be avoided and the sudden change of many experimental parameters for some given nitrogen and oxygen flow rates, particularly the abrupt drop of the deposition rate due to poisoning of the target surface by oxygen, is circumvented. A smooth evolution is obtained instead of non-linear phenomena as the oxygen duty cycle increases, especially for oxygen duty cycles included between 33 and 70% of T
O2.
Pulsing the nitrogen gas at the same time as the oxygen gas does not lead to significant changes of the deposition rate
vs. oxygen duty cycle α
O2 for nitrogen duty cycle α
N2 in-between 80-100% of the N
2 pulsing period. Deposition rate remains nearly constant up to α
O2 = 33% of T
O2 with values very close to that of the reactive process implementing the single O
2 pulsing. Afterwards (not shown in
Figure 2 for clarity), a similar smooth drop of the rate is measured as the oxygen duty cycle changes from 33 to 70% of the oxygen pulsing period. For α
O2 < 33% of T
O2, despite short t
O2ON times (in the order of a few seconds) and due to the high reactivity of titanium towards oxygen, the process avalanches rapidly in the oxidized sputtering mode. During the t
O2OFF time, it is long enough to restore the process in the nitrided sputtering mode. As a result, the process alternates between the nitrided and the oxidized sputtering mode assuming oxygen duty cycles lower than 70% of T
O2. For higher α
O2, the t
O2OFF time becomes too short and the process is completed trapped in the oxidized sputtering mode.
On the other hand, nitrogen duty cycles lower than 80% of the pulsing period give rise to a relevant enhancement of the deposition rate. For α
N2 = 50% of T
N2 and considering the lowest oxygen duty cycles (e.g., α
O2 < 20% of T
O2), it is worth noting that the deposition rate is strongly boosted and exceeds 400 nm h
-1. This enhanced deposition rate is even greater for α
N2 = 25% of T
N2 since it tends to be close to the Ti metallic rate (close to 600 nm h
-1). For such operating conditions, both t
O2ON and t
N2ON times are shorter than ten seconds and both t
O2OFF and t
N2OFF times are long enough to restore the process in the elemental mode, mainly (it will be more discussed later from Ti target potential
vs. time measurements). Since the sputtering yield of titanium oxide is lower than that of titanium nitride, which is itself lower than that of pure titanium [
29], one can expect the highest deposition rates when the process runs predominantly in the elemental sputtering mode. For the shortest nitrogen duty cycles, it is also interesting remarking that the deposition rate is very sensitive to the oxygen injection, as shown for α
N2 = 25% of T
N2 in
Figure 2. The small decrease of rate measured up to α
O2 = 20% of T
O2 becomes more marked in between 25-60% of T
O2 with a halved rate, and finally with a trend to achieve TiO
2- like rate. The oxidation of the Ti target surface prevails even for the very short nitrogen injection times, where the process is trapped in the oxidized sputtering mode.
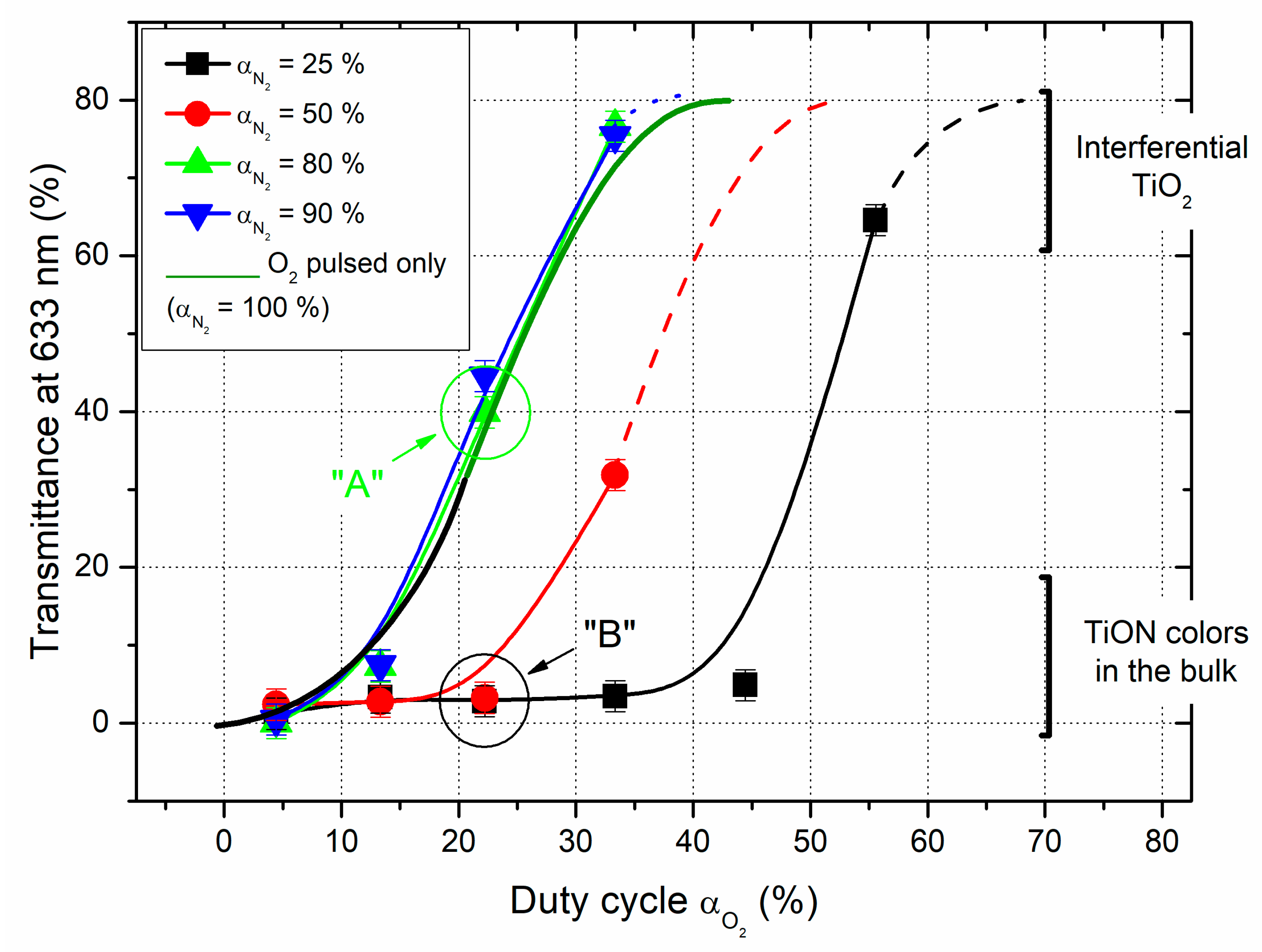
Ti-O-N films sputter-deposited on glass substrates also exhibit optical transmittance in the visible range, which strongly depends on oxygen and nitrogen pulsing conditions (
Figure 3). With a constant nitrogen flow rate and pulsing only the oxygen gas, absorbent Ti-O-N films are produced for oxygen duty cycles lower than a few % of T
O2. Films become gradually transparent for α
O2 > 10% of T
O2 and then completely interferential with a TiO
2-like behaviors for α
O2 > 40% of T
O2 where a further increase of oxygen duty cycle does not change the optical transparency with an average of 80% of transmittance at 633 nm. As a result, the most interesting range of oxygen duty cycles leading to relevant changes of the Ti-O-N optical properties is in-between 10-40% of T
O2. Assuming the evolution of deposition rate
vs. α
O2 earlier discussed from results in
Figure 2, this range is located before the significant drop of the rate.
As previously noticed for deposition rate vs. αO2, pulsing the nitrogen gas does not necessarily induce relevant changes in the optical transmittance of the films. For nitrogen duty cycles higher than 80% of TN2, the gradual and smooth change of optical transmittance as a function of αO2 is obtained for the same range of oxygen duty cycles (i.e., in-between 10-40% of TO2).
The regular absorbent-to-transparent transition is clearly shifted to higher oxygen duty cycles for Ti-O-N thin films prepared with nitrogen duty cycles lower than 50% of T
N2. It is even more noticeable for α
N2 = 25% of T
N2 since films prepared up to α
O2 = 45% of T
O2 still exhibit an optical transmittance at 633 nm lower than a few %. This range of oxygen duty cycles correlates with the highest deposition rates (close to the Ti metallic rate) and before the substantial drop of the rate corresponding to a predominant poisoning of the Ti target surface by the oxygen gas, as report in
Figure 2. These results also support that adjusting simultaneously and independently both reactive gas duty cycles allows an extension of the reactive sputtering conditions suitable to produce absorbent Ti-O-N thin films with colors in the bulk.
A real time measurement of the Ti target potential U
Ti (and total sputtering pressure P
Sput.) is a simple and valuable approach to follow instabilities of the reactive sputtering process [
16]. This potential
vs. time recording is typical of the poisoning state of the target surface by the reactive gas and characterizes the kinetics of poisoning. In this study, these measurements have been first performed for operating conditions corresponding to the deposition of semi-transparent and absorbent Ti-O-N films, as indicated by point “A” and “B” in
Figure 2 and
Figure 3, respectively.
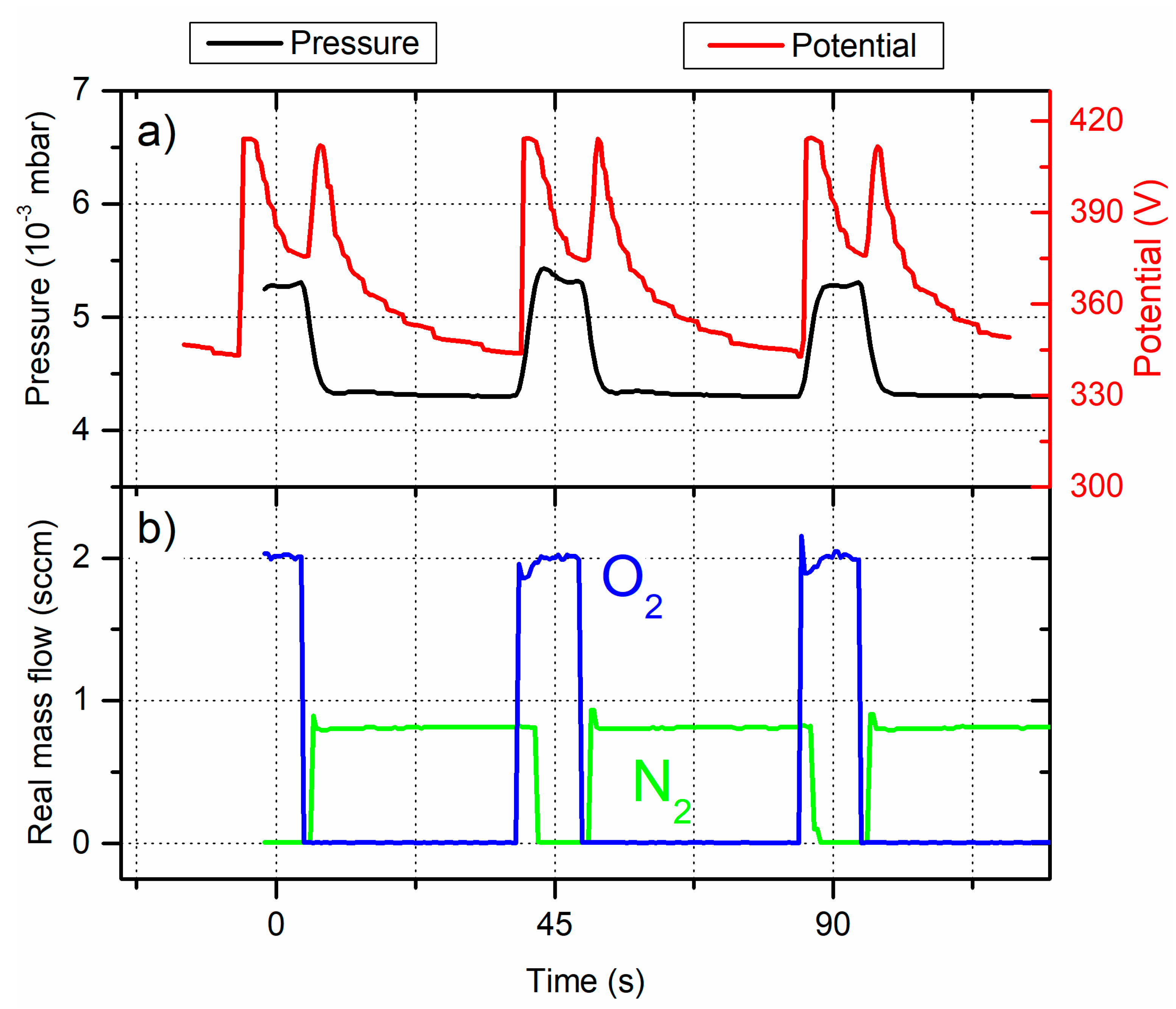
Figure 4a shows some typical measurements of U
Ti and P
Sput. vs. time for films prepared with α
O2 = 22% of T
O2 and α
N2 = 80% of T
N2 (semi-transparent films – point “A”). Real mass flow rates of both reactive gases are also given in order to show how injection times and nature of the reactive gas influence the process. They also illustrate that the response time of mass flowmeters are very short (less than 1 second) to introduce and/or to stop reactive gas injections despite of unsuitable overshoots and adjustments of setpoints at the beginning of injections.
For these pulsing conditions, it is first important noticing that the sputtering process is always in reactive mode although the interval starting at the end of t
O2ON and finishing at the beginning of t
N2ON produces a short gap (for 1 to 2 s as shown in
Figure 4b) without any reactive gas injection. This leads a total sputtering pressure of 4.3×10
-3 mbar corresponding to a nitrided sputtering mode. The Ti target potential is also influenced by O
2 and N
2 pulsing (
Figure 4a). When oxygen is introduced (beginning of t
O2ON time), U
Ti abruptly rises from 344 V (nitrided mode) to 415 V in a few hundred milliseconds, then drops down to 375 V till the end of the oxygen injection. This is assigned to the high reactivity of oxygen towards titanium, which is enhanced when the reactive process runs in the nitrided mode [
15]. In addition, diffusion of reactive ionic species, chemisorption, knock-in [
30] as well as implantation in the Ti target [
31,
32] all induce surface modifications. The Ti target behavior is then strongly affected by the formation of some complex compounds (certainly a mixture of titanium oxides, nitrides and oxynitrides a few nm thick) reducing the sputtering yield and inducing the typical hysteresis phenomena of the reactive sputtering process.
In the same way, the total sputtering pressure increases and stabilizes to 5.3×10-3 mbar, corresponding to the oxidized mode of the reactive sputtering process. Stopping the oxygen injection and starting the nitrogen one gives rise to a second sharp peak of the Ti target potential, again at about 415 V. It is closely linked to the return of the process in the nitrided mode with a speeder implementation since the second peak is harper than the first one. As a result, the reactive sputtering process is not at steady state conditions but periodically alternates between the nitrided and oxidized mode. For such operating parameters, it predominates in the nitrided mode since UTi still decreases at the end of the tO2ON time (despite a nearly constant pressure), whereas potential tends to become constant when nitrogen is solely introduced (end of the tN2ON time).
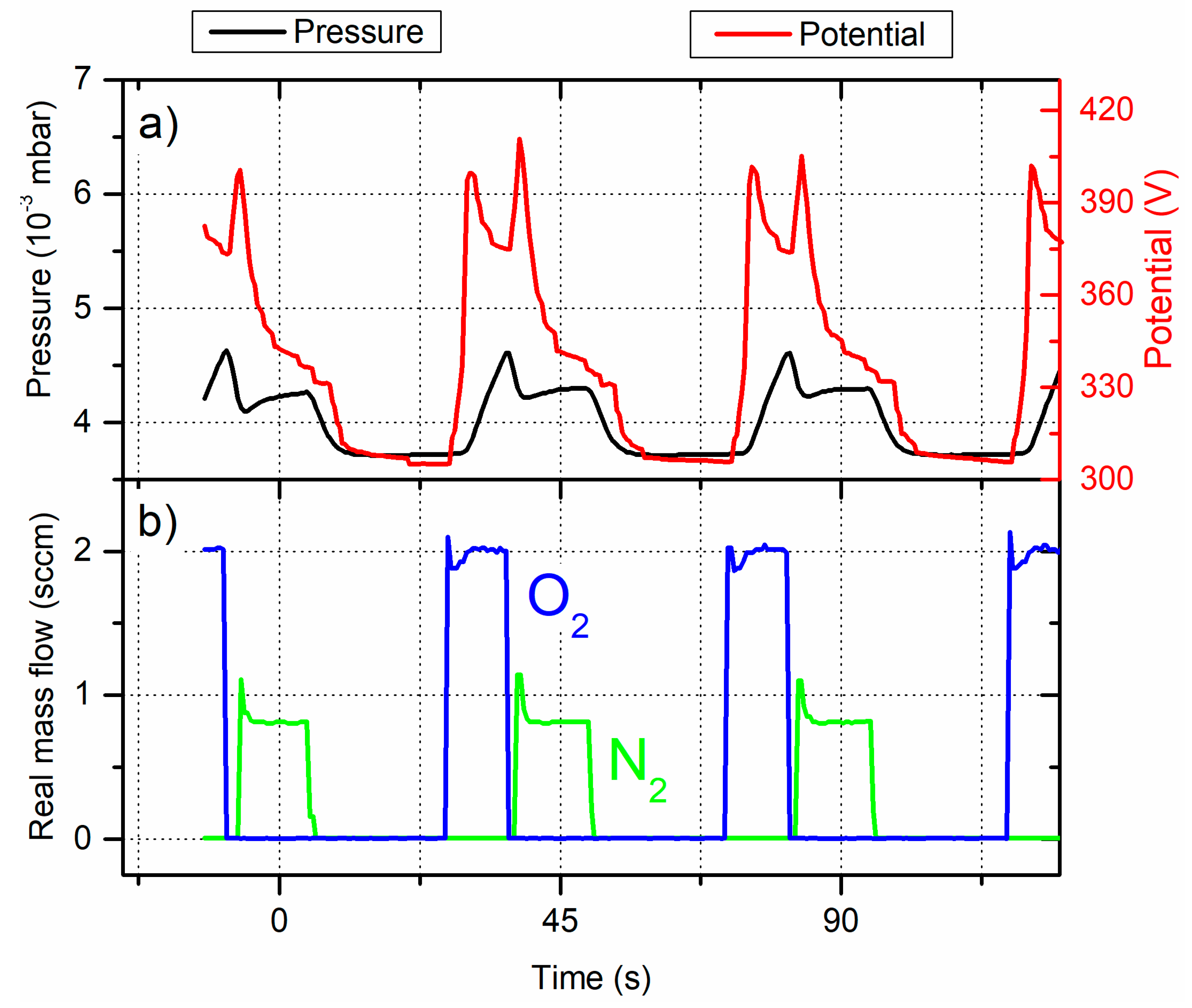
Keeping the same oxygen injection time (i.e., α
O2 = 22% of T
O2) and reducing that of nitrogen to α
N2 = 25% of T
N2 extends the time of sputtering without reactive gas injection. Applying these experimental parameters also allow supplying one reactive gas at a time (
Figure 5).
Before injecting reactive gases, UTi is the lowest and reaches 305 V and the total sputtering pressure is 3.7×10-3 mbar at the end of the tO2OFF time. The process is in the elemental sputtering mode. Pulsing O2 at first produces again an sudden increase of the Ti target potential to more than 405 V with a sharp peak at the beginning of the tO2ON time. The total sputtering pressure continues to grow until the end of the tO2ON time and a longer O2 injection would avalanche the process in the oxidized mode only. Stopping O2 injection does not completely stabilize UTi and introducing N2 leads to the same peak of the target potential, but narrower. Pressure tends to 4.3×10-3 mbar during the tN2ON time, which means that the process is in the nitrided mode. Similarly, when the two reactive gases are stopped, potential as well as pressure both reduce and come back to values obtained before pulsing O2 (305 V and 3.7×10-3 mbar, respectively) , and so the process is restored to the elemental mode. These results show that reducing O2 injection time does not completely trap the reactive sputtering process in the poisoned mode by O2 or N2. Therefore, a precise adjustment of reactive gas duty cycles are two key parameters to alternate the process between 3 modes: oxidized, nitrided and elemental.
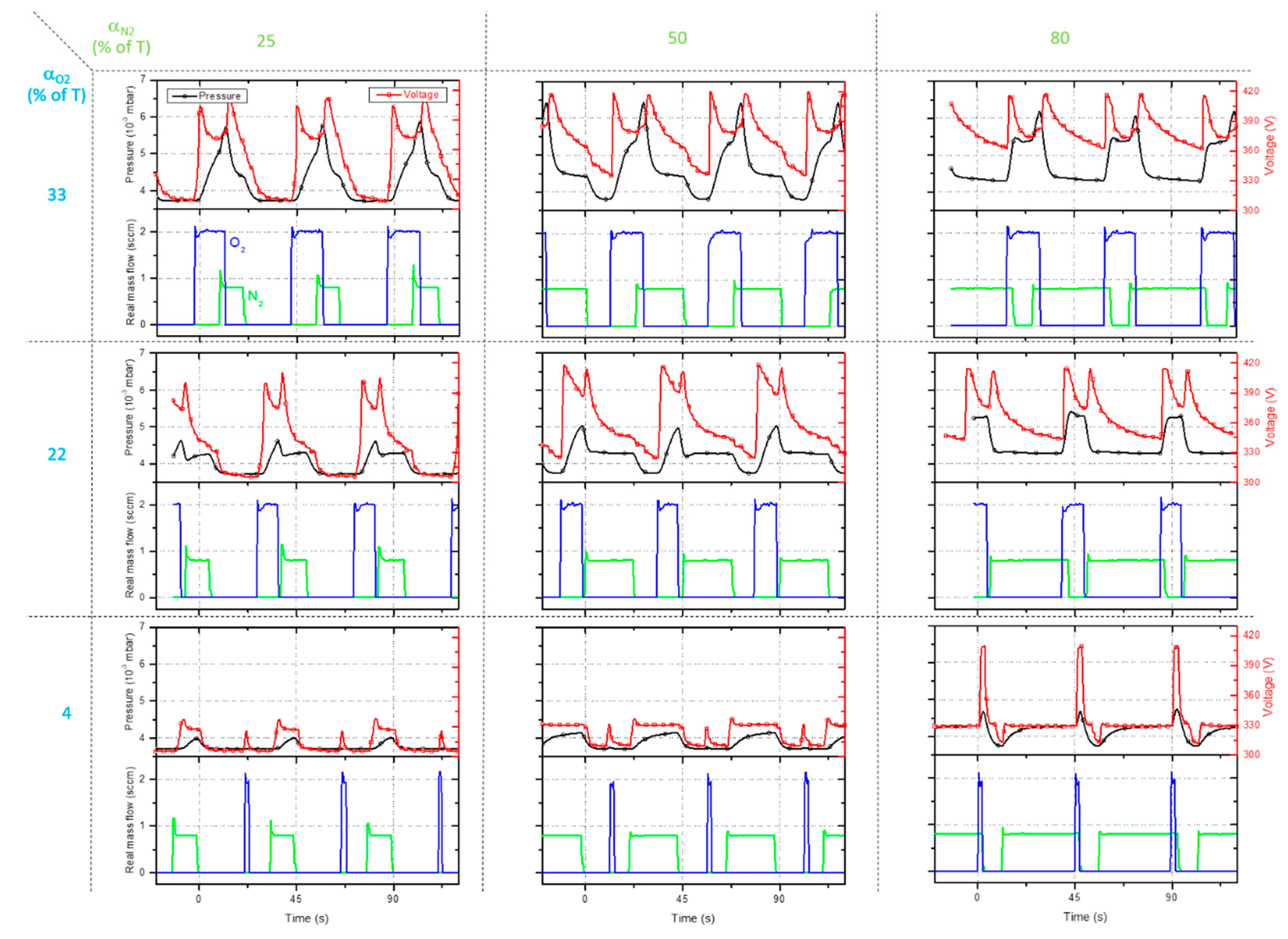
Both duty cycles have been systematically changed from 0 to 100% of the same pulsing period T = T
O2 = T
N2 = 45 s, and real time measurements of potential and pressure have also been recorded. Some examples are given in
Figure 6 in order to illustrate the tunability of the reactive sputtering between the different modes by changing only α
O2 and α
N2.
For low duty cycles like αO2 = 4% of TO2 and αN2 = 25% of TN2, tO2OFF and tO2OFF times are both long enough to restore the process in the elemental mode since the Ti target potential is constant and reaches 305 V before introducing O2 or N2 with a total sputtering pressure of 3.7×10-3 mbar. The very short introduction of oxygen gives rise to small peaks of UTi without a noticeable change of pressure. On the other hand, the pulsing time of nitrogen is longer for αN2 = 25 and 50% of TN2 to set the process in the nitride mode with UTi stabilizing at 332 V and pressure tending to 4.3×10-3 mbar. These pulsing conditions show that the oxidized state is not achieved and the process mainly alternates between nitrided and elemental modes. A further increase of tN2ON time (αN2 = 80% of TN2) allows a more oxidized state (UTi overpasses 405 V with a corresponding peak of pressure), but reduces the time kept in the elemental mode. The nitrided mode predominates for αO2 = 4% of TO2 and αN2 = 80% of TN2, and these parameters of the process between the 3 modes (increasing more αN2 traps the process in the nitrided mode).
This alternance between the 3 modes is even more obtained with α
O2 = 22% of T
O2 and α
N2 = 25% of T
N2, or even α
O2 = 33% of T
O2 and α
N2 = 50% of T
N2, as shown in
Figure 6. Assuming a single pulsing period (45 s), the shape of U
Ti vs. time and pressure
vs. time are very similar for these sputtering conditions. Two sharp peaks of the Ti target potential are observed due to oxidized then nitrided mode. They are followed by a shoulder and a trend to achieve the lowest and stable value of 305 V assigned to the return to the elemental mode. Extending both duty cycles leads to an overlapping of O
2 and N
2 pulsing signals where the oxidized state prevails over the nitrided one since oxygen is more reactive towards titanium. It particularly reduces the time for restoring the process in the elemental mode and an alternation between oxidized and nitrided modes is only possible. These conditions are particularly established for α
O2 = 22% of T
O2 and α
N2 = 80% of T
N2 (or α
O2 = 33% of T
O2 and α
N2 = 80% of T
N2, as illustrated in
Figure 6) where the Ti target potential never reaches 305 V and the total sputtering pressure cannot go below 4.3×10
-3 mbar.
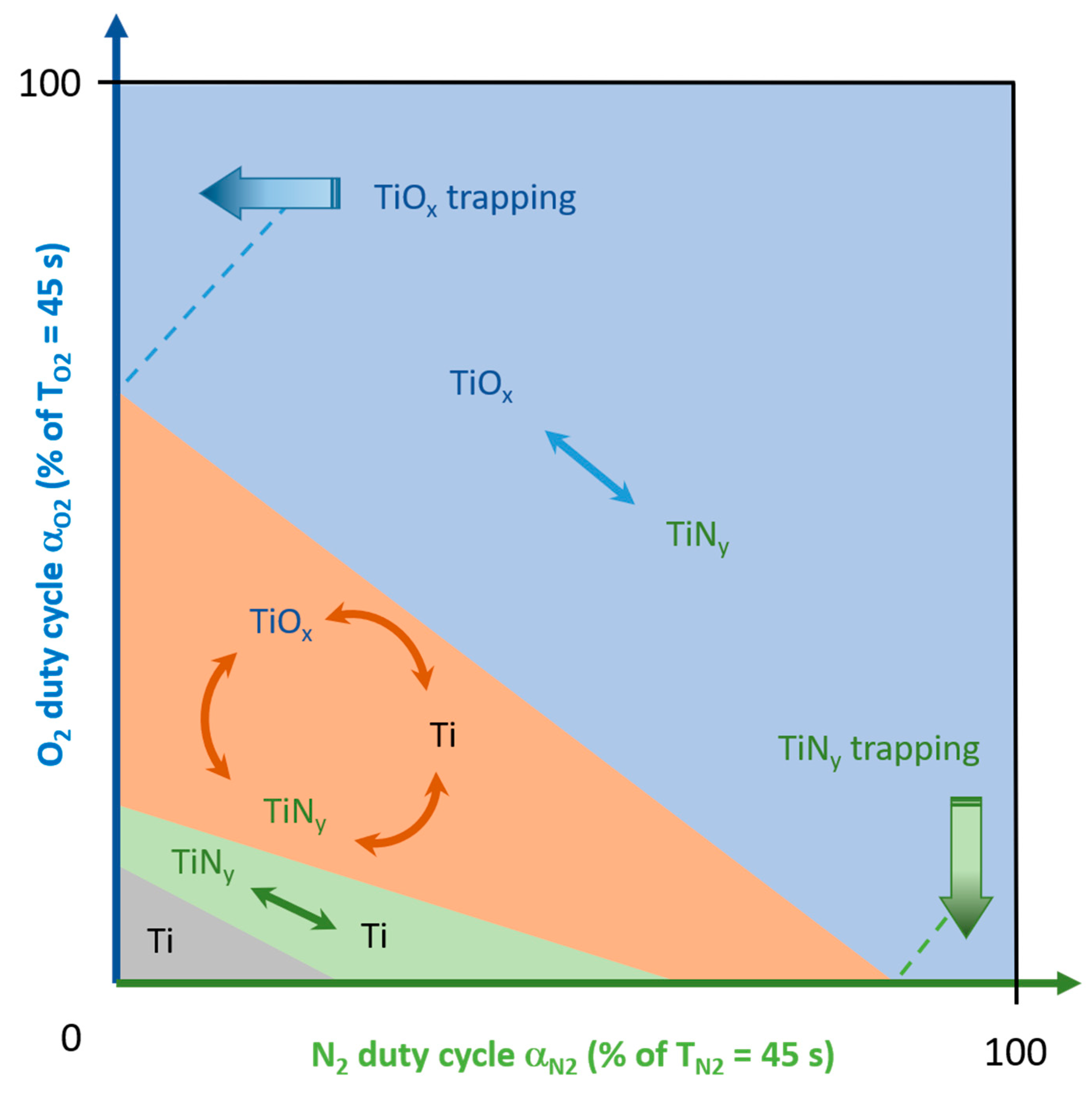
Using all real time measurements of Ti target potential and total sputtering pressure
vs. time systematically recorded for duty cycles varying from 0 to 100% of T, a qualitative 2D diagram is built. It defines boundaries of the reactive sputtering process alternations between 1,2 or 3 modes taking into account O
2 and N
2 duty cycles only (
Figure 7).
This kind of 2D diagram showing the state of the reactive sputtering process
vs. reactive gas supply has ever been suggested and built for the deposition of titanium oxynitride thin films by conventional method [
15] (i.e., constant supply of O
2 and N
2), but to the best of our knowledge never implementing two reactive gases pulsed by RGPP. This diagram represents how the different modes of the reactive sputtering process can be favored as a function of O
2 and N
2 pulsing injections, and so one can deduce the type of titanium oxynitride thin films that can be sputter-deposited. For operating conditions used in this study, the process is mainly in elemental sputtering mode for the lowest O
2 and N
2 duty cycles (grey zone, namely Ti in
Figure 7) since t
ON times are too short to completely poison the Ti target surface by oxygen and/or nitrogen. This correlates with the highest deposition rates of Ti-O-N films (close to that of Ti) previously reported in
Figure 2. Increasing duty cycles (especially for N
2) leads to an alternation of the process between elemental and nitrided modes (green zone, TiN
y ↔ Ti in
Figure 7). Injecting for a longer time one of the reactive gas or both gives rise to a periodic alternation of the process between the 3 modes: elemental, nitrided and oxidized ones (orange zone, TiO
x ↔ TiN
y ↔ Ti in
Figure 7). This zone is connected to drop of the deposition rate
vs. α
O2 formerly reported in
Figure 2, and also to the gradual transition from absorbent to transparent Ti-O-N films showed from optical transmittance in
Figure 3. For the highest duty cycles, the process is then fully in compound sputtering mode and may by pulsed between oxidized and nitrided modes (blue zone, TiO
x ↔ TiN
y in
Figure 7). It corresponds to the deposition of interferential thin films produced with the lowest deposition rates. It is worth noting that the process can be trapped either in nitrided, or oxidized sputtering mode when duty cycle values are opposite from each other, i.e., α
O2 tending to 100% of T
O2 (and inversely α
N2 tending to 0% of T
N2) and
vice versa.
Although sizes boundaries between zones still remain qualitative and are not accurately defined, they allow the occurrence of sputtering regimes and the range of transitions between 1, 2 or 3 modes. As similarly reported for instabilities of the reactive sputtering process involving one metallic target with one reactive gas (basic system by conventional reactive sputtering), boundaries of the different zones depend on the reactivity of the metal towards oxygen and nitrogen species, geometry and dimensions of the sputtering chamber, experimental parameters such as pumping speed, target current and so on. As a result, playing with parameters influencing sizes and features of instabilities may favor a zone than another one and then a given sputtering mode.