Submitted:
31 July 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Set-Up and Delivery
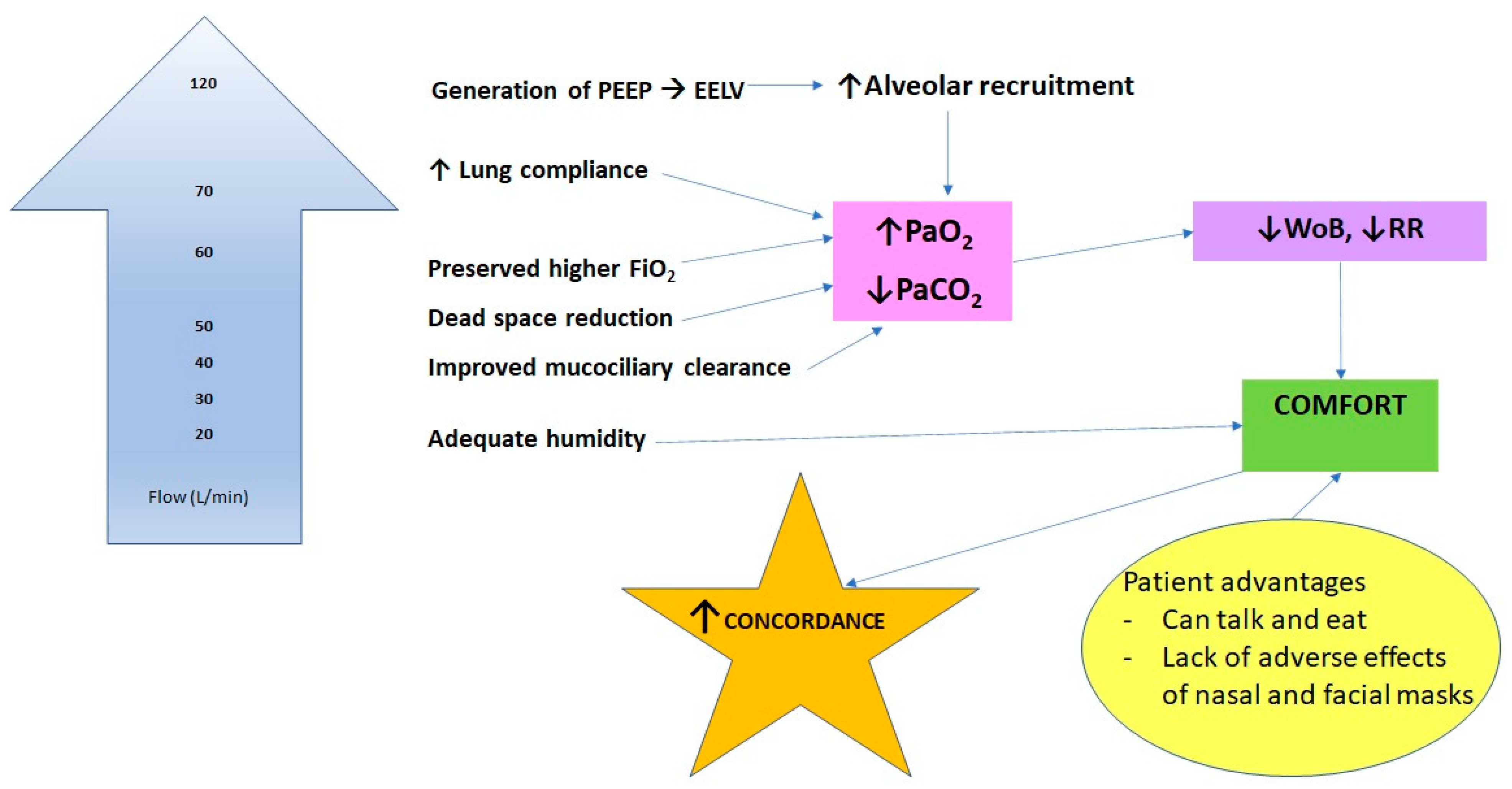
Physiological Effects of HFNC
Clinical Applications
HFNC in the peri-operative setting
Use of HFNC in COVID-19
HFNC in the Emergency Department
HFNC vs NIV in the acute setting
HFNC in immunocompromised patients
HFNC at home
HFNC in the palliative care setting
Conclusion
List of Abbreviations
| HFNC | High-flow Nasal Cannula oxygen therapy |
| COPD | Chronic Obstructive Pulmonary Disease |
| AECOPD | Acute Exacerbation of Chronic Obstructive Pulmonary Disease |
| NIV | Non-invasive ventilation |
| FiO2 | Fraction of inspired O2 |
| PaO2 | Partial pressure of Oxygen in Arterial Blood |
| PaCO2 | Partial pressure of Carbon Dioxide in Arterial Blood |
| ptCO2 | Transcutaneous carbon dioxide monitoring |
| TV | Tidal Volume |
| P/F | PaO2: FiO2 |
| ICU | Intensive Care Unit |
| RECOVERY-RS | Randomised Evaluation of Covid-19 Therapy – Respiratory Support |
| LTOT | Long-term oxygen therapy |
| mMRC score | Modified Medical Research Council dyspnoea score |
| HRQoL | Health Related Quality of Life |
| EQ-5D-5L | European quality of life five dimensional questionnaire five level score |
| SGRQ | St George’s Respiratory Questionnaire |
| 6MWT | 6-Minute Walk Test |
| QALY | Quality Adjusted Life Year |
References
- Delorme, M.; Bouchard, P.A.; Simon, M.; Simard, S.; Lellouche, F. Effects of High-Flow Nasal Cannula on the Work of Breathing in Patients Recovering From Acute Respiratory Failure*. Critical Care Medicine. 2017, 45, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Ricard, J.D.; Roca, O.; Lemiale, V.; Corley, A.; Braunlich, J.; Jones, P.; et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Medicine. 2020, 46, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, R.F.; Hart, N.; Kaltsakas, G. High-flow therapy: physiological effects and clinical applications. Breathe. 2020, 16, 200224. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.E.; Williams, A.B.; Gerard, C.; Hockey, H. Evaluation of a Humidified Nasal High-Flow Oxygen System, Using Oxygraphy, Capnography and Measurement of Upper Airway Pressures. Anaesthesia and Intensive Care. 2011, 39, 1103–1110. [Google Scholar] [CrossRef]
- Williams, R.; Rankin, N.; Smith, T.; Galler, D.; Seakins, P. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Critical Care Medicine. 1996, 24, 1920–1929. [Google Scholar] [CrossRef]
- Nishimura, M. High-Flow Nasal Cannula Oxygen Therapy Devices. Respiratory Care. 2019, 64, 735–742. [Google Scholar] [CrossRef]
- Cirio, S.; Piran, M.; Vitacca, M.; Piaggi, G.; Ceriana, P.; Prazzoli, M.; et al. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respiratory Medicine. 2016, 118, 128–132. [Google Scholar] [CrossRef]
- Möller, W.; Celik, G.; Feng, S.; Bartenstein, P.; Meyer, G.; Eickelberg, O.; et al. Nasal high flow clears anatomical dead space in upper airway models. Journal of Applied Physiology. 2015, 118, 1525–1532. [Google Scholar] [CrossRef]
- Spicuzza, L.; Schisano, M. High-flow nasal cannula oxygen therapy as an emerging option for respiratory failure: the present and the future. Therapeutic Advances in Chronic Disease. 2020, 11, 204062232092010. [Google Scholar] [CrossRef]
- Sztrymf, B.; Messika, J.; Mayot, T.; Lenglet, H.; Dreyfuss, D.; Ricard, J.D. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: A prospective observational study. Journal of Critical Care. 2012, 27, 324–e9. [Google Scholar] [CrossRef]
- Itagaki, T.; Okuda, N.; Tsunano, Y.; Kohata, H.; Nakataki, E.; Onodera, M.; et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients. Respiratory Care. 2014, 59, 70–74. [Google Scholar] [CrossRef]
- Pisani, L.; Astuto, M.; Prediletto, I.; Longhini, F. High flow through nasal cannula in exacerbated COPD patients: a systematic review. Pulmonology. 2019, 25, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Di mussi, R.; Spadaro, S.; Stripoli, T.; Volta, C.A.; Trerotoli, P.; Pierucci, P.; et al. High-flow nasal cannula oxygen therapy decreases postextubation neuroventilatory drive and work of breathing in patients with chronic obstructive pulmonary disease. Critical Care. 2018, 22. [Google Scholar] [CrossRef]
- Longhini, F.; Pisani, L.; Lungu, R.; Comellini, V.; Bruni, A.; Garofalo, E.; et al. High-Flow Oxygen Therapy After Noninvasive Ventilation Interruption in Patients Recovering From Hypercapnic Acute Respiratory Failure. Critical Care Medicine. 2019, 47, e506–11. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.F.; Spooner, A.J.; Dunster, K.R.; Anstey, C.M.; Corley, A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016, 71, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Prescott, E.; Lange, P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. American Journal of Respiratory and Critical Care Medicine. 1996, 153, 1530–1535. [Google Scholar]
- Hasani, A.; Chapman, T.; McCool, D.; Smith, R.; Dilworth, J.; Agnew, J. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chronic Respiratory Disease. 2008, 5, 81–86. [Google Scholar] [CrossRef]
- Roca, O.; Riera, J.; Torres, F.; Masclans, J. High-flow oxygen therapy in acute respiratory failure. Respiratory Care. 2010, 55, 408–413. [Google Scholar]
- Roca, O.; Pérez-Terán, P.; Masclans, J.R.; Pérez, L.; Galve, E.; Evangelista, A.; et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: High flow nasal cannula in heart failure. Journal of Critical Care. 2013, 28, 741–746. [Google Scholar] [CrossRef]
- Makdee, O.; Monsomboon, A.; Surabenjawong, U.; Praphruetkit, N.; Chaisirin, W.; Chakorn, T.; et al. High-Flow Nasal Cannula Versus Conventional Oxygen Therapy in Emergency Department Patients With Cardiogenic Pulmonary Edema: A Randomized Controlled Trial. Annals of Emergency Medicine. 2017, 70, 465–472. [Google Scholar] [CrossRef]
- Cortegiani, A.; Accurso, G.; Mercadante, S.; Giarratano, A.; Gregoretti, C. High flow nasal therapy in perioperative medicine: from operating room to general ward. BMC Anesthesiology. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Badiger, S.; John, M.; Fearnley, R.A.; Ahmad, I. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. British Journal of Anaesthesia. 2015, 115, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Renda, T.; Corrado, A.; Iskandar, G.; Pelaia, G.; Abdalla, K.; Navalesi, P. High-flow nasal oxygen therapy in intensive care and anaesthesia. British Journal of Anaesthesia. 2018, 120, 18–27. [Google Scholar] [CrossRef]
- Frat, J.P.; Ricard, J.D.; Quenot, J.P.; Pichon, N.; Demoule, A.; Forel, J.M.; et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. The Lancet Respiratory Medicine. 2019, 7, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Monnin, M.; Girard, M.; Conseil, M.; Cisse, M.; Carr, J.; et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Medicine. 2016, 42, 1877–1887. [Google Scholar] [CrossRef]
- Hernández, G.; Vaquero, C.; González, P.; Subira, C.; Frutos-Vivar, F.; Rialp, G.; et al. Effect of Postextubation High-Flow Nasal Cannula vs Conventional Oxygen Therapy on Reintubation in Low-Risk Patients: A Randomized Clinical Trial. JAMA. 2016, 315, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Paugam-Burtz, C.; Godet, T.; Khoy-Ear, L.; Rozencwajg, S.; Delay, J.M.; et al. Effect of early postextubation high-flow nasal cannula vs conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: a French multicentre randomised controlled trial (OPERA). Intensive Care Medicine. 2016, 42, 1888–1898. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, H.; Zhang, R.; Ye, X.; Wei, J. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Critical Care. 2019, 23. [Google Scholar] [CrossRef]
- Douglas, N.; Ng, I.; Nazeem, F.; Lee, K.; Mezzavia, P.; Krieser, R.; et al. A randomised controlled trial comparing high-flow nasal oxygen with standard management for conscious sedation during bronchoscopy. Anaesthesia. 2017, 73, 169–176. [Google Scholar] [CrossRef]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients With Acute Hypoxemic Respiratory Failure and COVID-19. JAMA. 2022, 327, 546. [Google Scholar] [CrossRef]
- Burnim, M.S.; Wang, K.; Checkley, W.; Nolley, E.P.; Xu, Y.; Garibaldi, B.T. The Effectiveness of High-Flow Nasal Cannula in Coronavirus Disease 2019 Pneumonia. Critical Care Medicine Publish Ahead of Print. 2021. [Google Scholar]
- Tinelli, V.; Cabrini, L.; Fominskiy, E.; Franchini, S.; Ferrante, L.; Ball, L.; et al. High Flow Nasal Cannula Oxygen vs. Conventional Oxygen Therapy and Noninvasive Ventilation in Emergency Department Patients: A Systematic Review and Meta-Analysis. The Journal of Emergency Medicine. 2019, 57, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Mankodi, D.; Shaharyar, S.; Ravindranathan, S.; Danckers, M.; Herscovici, P.; et al. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: A systematic review. Respiratory Medicine. 2016, 121, 100–108. [Google Scholar] [CrossRef]
- Schwabbauer, N.; Berg, B.; Blumenstock, G.; Haap, M.; Hetzel, J.; Riessen, R. Nasal high–flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiology. 2014, 14. [Google Scholar] [CrossRef]
- Vargas, F.; Saint-Leger, M.; Boyer, A.; Bui, N.H.; Hilbert, G. Physiologic Effects of High-Flow Nasal Cannula Oxygen in Critical Care Subjects. Respiratory Care. 2015, 60, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. New England Journal of Medicine. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Ling, B.; Zhu, Q.; Hu, Y.; Tan, D.; et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. International Journal of Chronic Obstructive Pulmonary Disease. 2019, 14, 1229–1237. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, L.; Yu, H.; Su, X.; Tiankui Shuai Zhu, L.; et al. High-Flow Nasal Cannula Oxygen Therapy versus Non-Invasive Ventilation for AECOPD Patients After Extubation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Chronic Obstructive Pulmonary Disease. 2022, 17, 1987–1999. [Google Scholar] [CrossRef]
- Azoulay, E.; Lemiale, V.; Mokart, D.; Nseir, S.; Argaud, L.; Pène, F.; et al. Effect of High-Flow Nasal Oxygen vs Standard Oxygen on 28-Day Mortality in Immunocompromised Patients With Acute Respiratory Failure. JAMA. 2018, 320, 2099. [Google Scholar] [CrossRef]
- Frat, J.P.; Ragot, S.; Girault, C.; Perbet, S.; Prat, G.; Boulain, T.; et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. The Lancet Respiratory Medicine. 2016, 4, 646–652. [Google Scholar] [CrossRef]
- Hardinge, M.; Annandale, J.; Bourne, S.; Cooper, B.; Evans, A.; Freeman, D.; et al. British Thoracic Society guidelines for home oxygen use in adults: accredited by, N. I.C.E. Thorax. 2015, 70 (Suppl 1), i1–43. [Google Scholar] [CrossRef]
- Storgaard, L.H.; Hockey, H.; Laursen, B.S.; Weinreich, U.M. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. International Journal of Chronic Obstructive Pulmonary Disease. 2018, 13, 1195–1205. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American journal of respiratory and critical care medicine 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Kikuchi, T.; Horie, T.; Shiraki, A.; Kitajima, T.; Kadowaki, T.; et al. Domiciliary High-Flow Nasal Cannula Oxygen Therapy for Patients with Stable Hypercapnic Chronic Obstructive Pulmonary Disease. A Multicenter Randomized Crossover Trial. Annals of the American Thoracic Society. 2018, 15, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.F.; Kind, P.; Parkin, D.; et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Bonnevie, T.; Elkins, M.; Paumier, C.; Medrinal, C.; Combret, Y.; Patout, M.; et al. Nasal High Flow for Stable Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2019, 16, 368–377. [Google Scholar] [CrossRef]
- Nagata, K.; Horie, T.; Chohnabayashi, N., Jinta; et al. Home High-Flow Nasal Cannula Oxygen Therapy for Stable Hypercapnic COPD: A Randomized Clinical Trial. American Journal of Respiratory and Critical Care Medicine. 2022, 206, 1326–1335. [Google Scholar] [CrossRef]
- Horvath, C.; Baty, F.; Kähler, C.J. Long term home high flow nasal cannula therapy in hypercapnic and hypoxic patients after non-invasive ventilation failure – a retrospective analysis. European Respiratory Journal. 2019.
- McKinstry, S.; Singer, J.; Baarsma, J.P.; Weatherall, M.; Beasley, R.; Fingleton, J. Nasal high-flow therapy compared with non-invasive ventilation in COPD patients with chronic respiratory failure: A randomized controlled cross-over trial. Respirology. 2019. [CrossRef]
- Huang, H.W.; Sun, X.M.; Shi, Z.H.; Chen, G.Q.; Chen, L.; Friedrich, J.O.; et al. Effect of High-Flow Nasal Cannula Oxygen Therapy Versus Conventional Oxygen Therapy and Noninvasive Ventilation on Reintubation Rate in Adult Patients After Extubation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Intensive Care Medicine. 2017, 33, 609–623. [Google Scholar] [CrossRef]
- Epstein, A.J.; Hartridge-Lambert, S.K.; Ramaker, J.S.; Voigt, L.; Portlock, C.S. Humidified High-Flow Nasal Oxygen Utilization in Patients with Cancer at Memorial Sloan-Kettering Cancer Center. Journal of Palliative Medicine. 2011, 14, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, N.; Fracchia, C. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. A narrative review. Pulmonology. 2019, 25, 289–298. [Google Scholar] [PubMed]
- Wilson, M.T.; Mittal, A.; Dobler, C.C.;Curtis, J.R.; et al. High-Flow Nasal Cannula Oxygen in Patients with Acute Respiratory Failure and Do-Not-Intubate or Do-Not-Resuscitate Orders: A Systematic Review. Journal of Hospital Medicine. 2020, 15, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Messika, J.; Ben Ahmed, K.; Gaudry, S.; Miguel-Montanes, R.; Rafat, C.; Sztrymf, B.; et al. Use of High-Flow Nasal Cannula Oxygen Therapy in Subjects With ARDS: A 1-Year Observational Study. Respiratory Care. 2014, 60, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Steele, P.; Dabscheck, E.; Smallwood, N. Nasal High Flow Therapy For Symptom Management in People Receiving Palliative Care. Journal of Pain and Symptom Management. 2021. [CrossRef]
- Hui, D.; Morgado, M.; Chisholm, G.; Withers, L.; Nguyen, Q.; Finch, C.; et al. High-Flow Oxygen and Bilevel Positive Airway Pressure for Persistent Dyspnea in Patients With Advanced Cancer: A Phase II Randomized Trial. Journal of Pain and Symptom Management. 2013, 46, 463–473. [Google Scholar] [CrossRef]
- Shah, N.; Mehta, Z.; Mehta, Y. High-Flow Nasal Cannula Oxygen Therapy in Palliative Care #330. Journal of Palliative Medicine. 2017, 20, 679–680. [Google Scholar]
- Murphy, P.B.; Rehal, S.; Arbane, G.; Bourke, S.; Calverley, P.M.A.; Crook, A.M.; et al. Effect of Home Noninvasive Ventilation With Oxygen Therapy vs Oxygen Therapy Alone on Hospital Readmission or Death After an Acute COPD Exacerbation. JAMA [Internet]. 2017, 317, 2177. Available online: https://jamanetwork.com/journals/jama/fullarticle/2627985. [CrossRef]
- Gassama, A.; Mukherjee, D.; Ahmed, U.; Coelho, S.; Daniels, M.; Mukherjee, R. The Effect of Telemonitoring (TM) on Improving Adherence with Continuous Positive Airway Pressure (CPAP) in Obstructive Sleep Apnoea (OSA): A Service Improvement Project (SIP). Healthcare. 2022, 10, 465. [Google Scholar] [CrossRef]
| Clinical Application | Setting of Application | Physiological Effects |
|---|---|---|
| Acute respiratory failure | ED, ICU, hospital ward | Maintains higher FiO2, improves lung compliance, improves alveolar recruitment, increases PaO2, decreases PaCO2, reduces WoB, improves patient comfort |
| Acute exacerbation of COPD | ED, ICU, HDU, hospital ward | Increases dead space wash out, leading to improved gas exchange and reduction in PaCO2 |
| Stable hypercapnic COPD | Home | Reduces exacerbations and PaCO2, leading to improved quality of life |
| Support during exercise in COPD | Home, pulmonary rehabilitation | Improves oxygenation, leading to less dyspnoea, leading to increased exercise tolerance |
| Cardiogenic pulmonary oedema | ED, ICU, HDU, hospital ward | Improves oxygenation, decreases afterload |
| Prevention of reintubation | Theatres, ICU, HDU, hospital ward | Improves gas exchange similar to NIV |
| Preoxygenation during airway procedures | Theatres | Increased apnoea time |
| Breaks from positive airway pressure | ICU, hospital ward | Patients can eat and talk |
| Immunocompromised patients | ED, ICU, hospital ward | Improves gas exchange similar to NIV |
| Palliative patients | ED, hospital ward, ICU/HDU if clinically appropriate, home, hospice | Decreases breathlessness |
| Bronchiectasis and cystic fibrosis | ICU, hospital ward, home | Improves mucociliary clearance, leading to improved ventilation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





