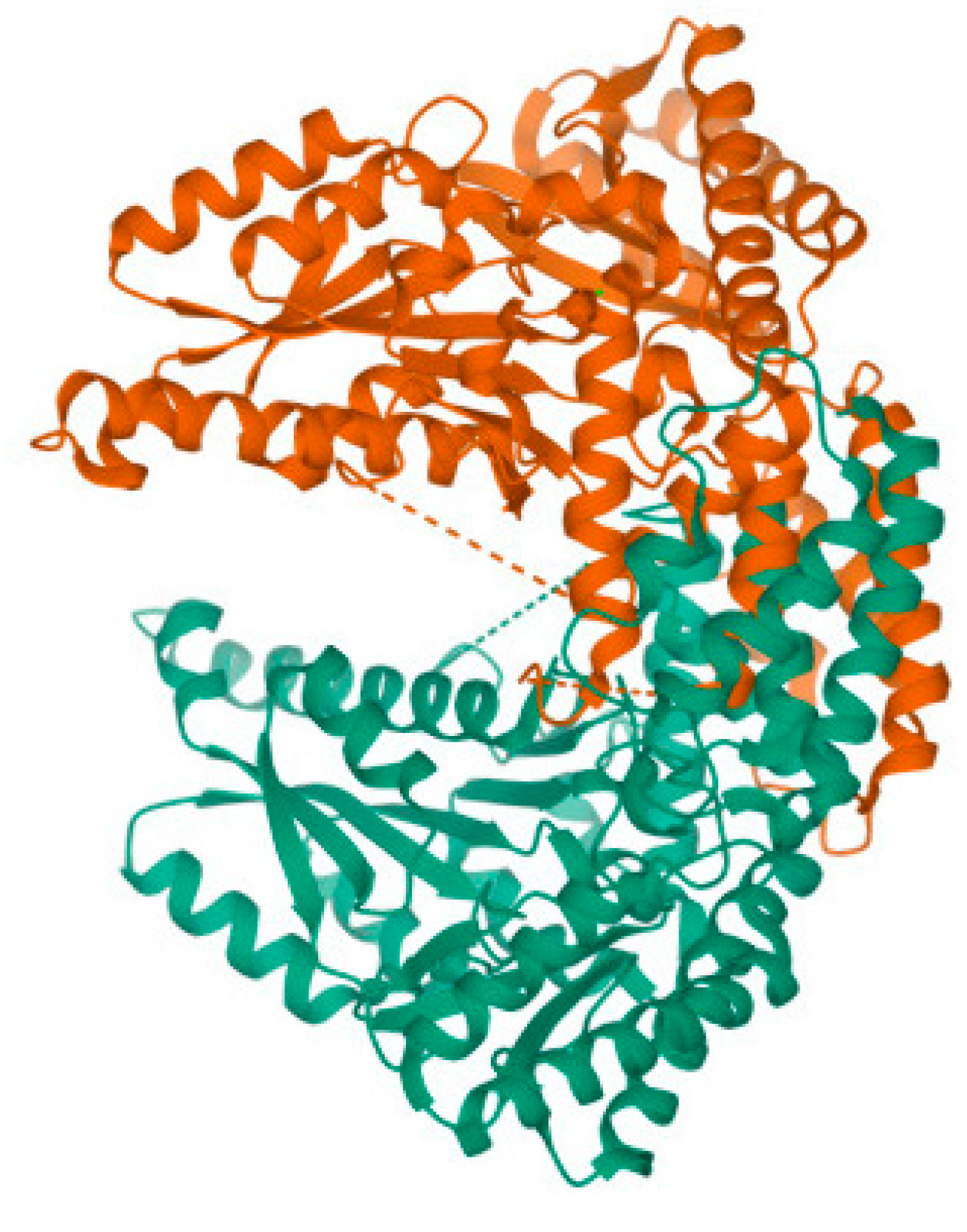
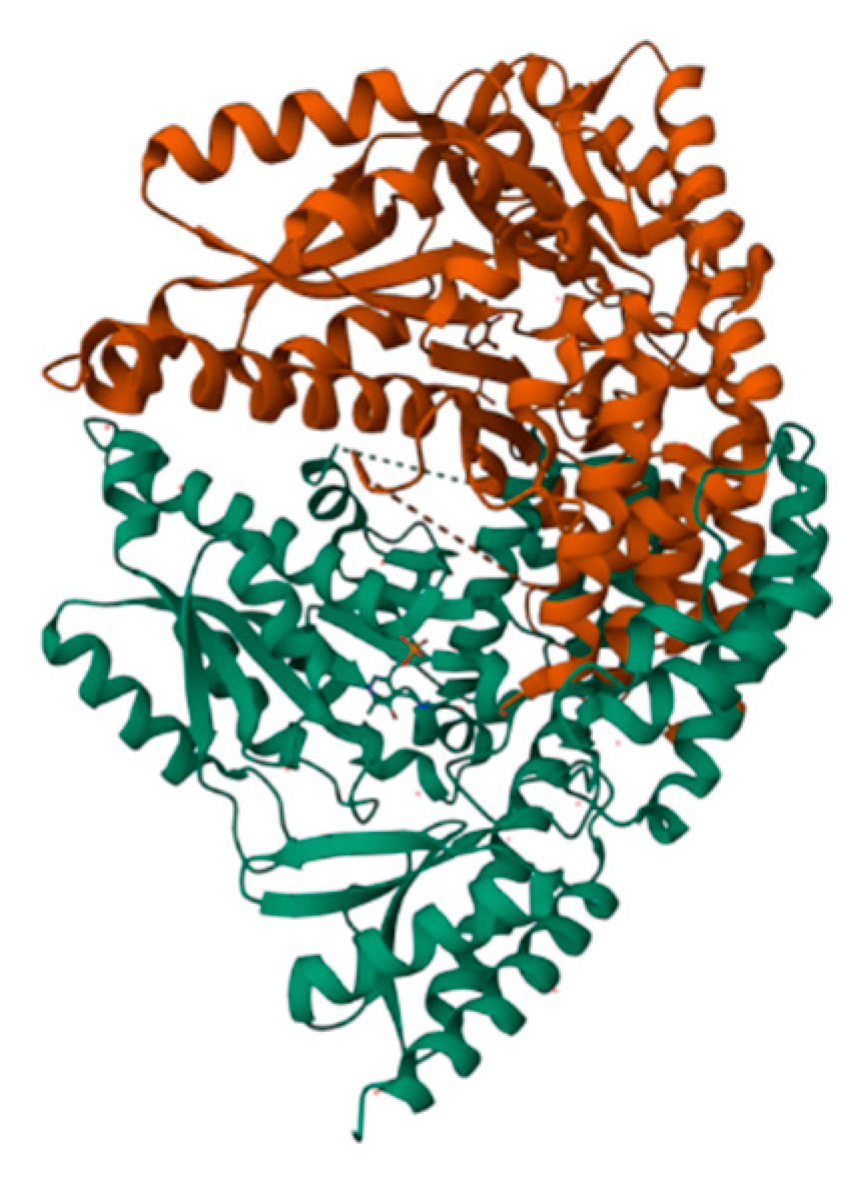
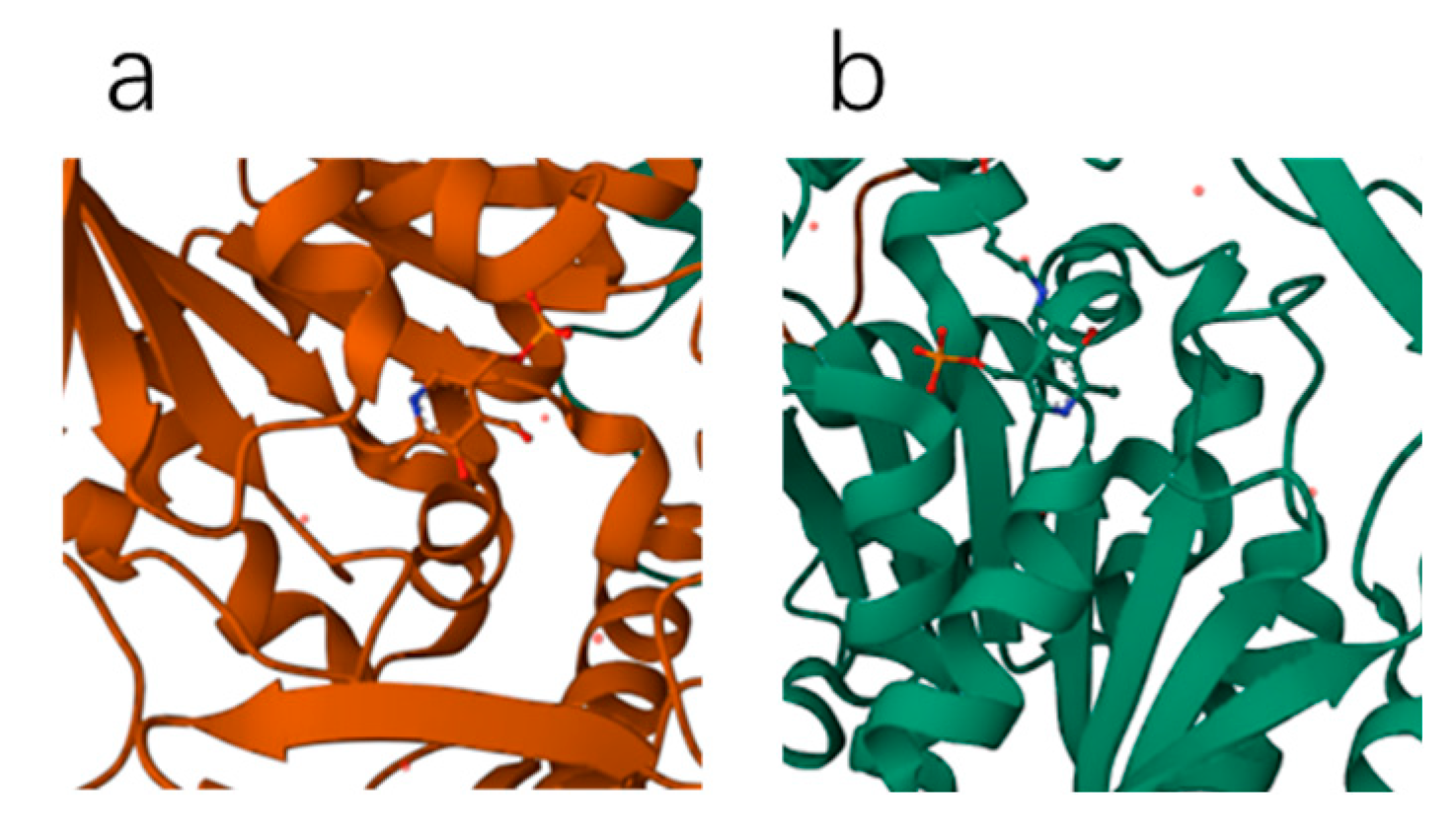
3.1. Parkinson’s Disease and L-DOPA
Among the elderly population, Parkinson’s disease has a significantly high incidence rate. Up to the present, no fully curative medication has been discovered for this disease, rendering it an incurable condition. The underlying cause of Parkinson’s disease is an imbalance between dopamine and acetylcholine, with an increase in the excitatory effects of acetylcholine. The primary pathological characteristic involves the degeneration and death of dopaminergic neurons in the substantia nigra and the aggregation of α-synuclein in the brainstem neurons, forming Lewy bodies. In simple terms, Parkinson’s disease is a neurodegenerative disorder characterized by a reduction in dopamine secretion. Patients with Parkinson’s disease exhibit symptoms such as resting tremors, muscular rigidity, facial stiffness, and abnormal posture and gait. Additionally, a portion of patients (20% to 40%) may experience cognitive impairment, neuropsychiatric symptoms, and dementia during the course of the disease. As a result, Parkinson’s disease is considered a neurological disorder.
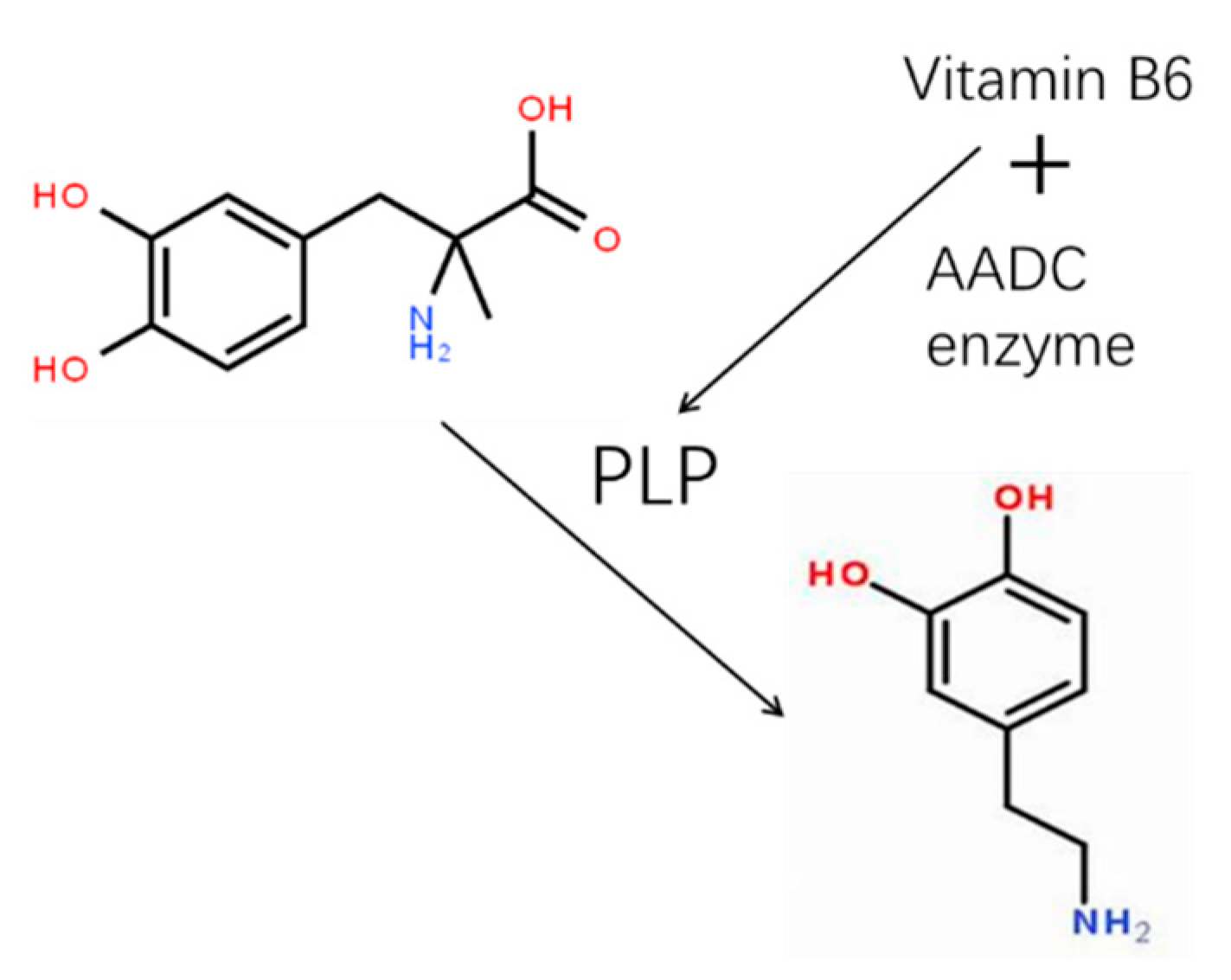
However, Parkinson’s patients cannot directly take dopamine to alleviate their symptoms. This is because dopamine cannot penetrate the blood-brain barrier to reach the brain. The blood-brain barrier is a structure composed of vascular endothelial cells and neuroglial cells, which restricts certain substances from entering the brain from the bloodstream, thus protecting the brain from harmful substances. Due to its large molecular size and strong polarity, dopamine cannot pass through the blood-brain barrier and, therefore, cannot be administered orally. To treat Parkinson’s disease, precursor drugs that can pass through the blood-brain barrier, such as L-DOPA, are typically used. Once metabolized, L-DOPA is converted into dopamine within the brain.
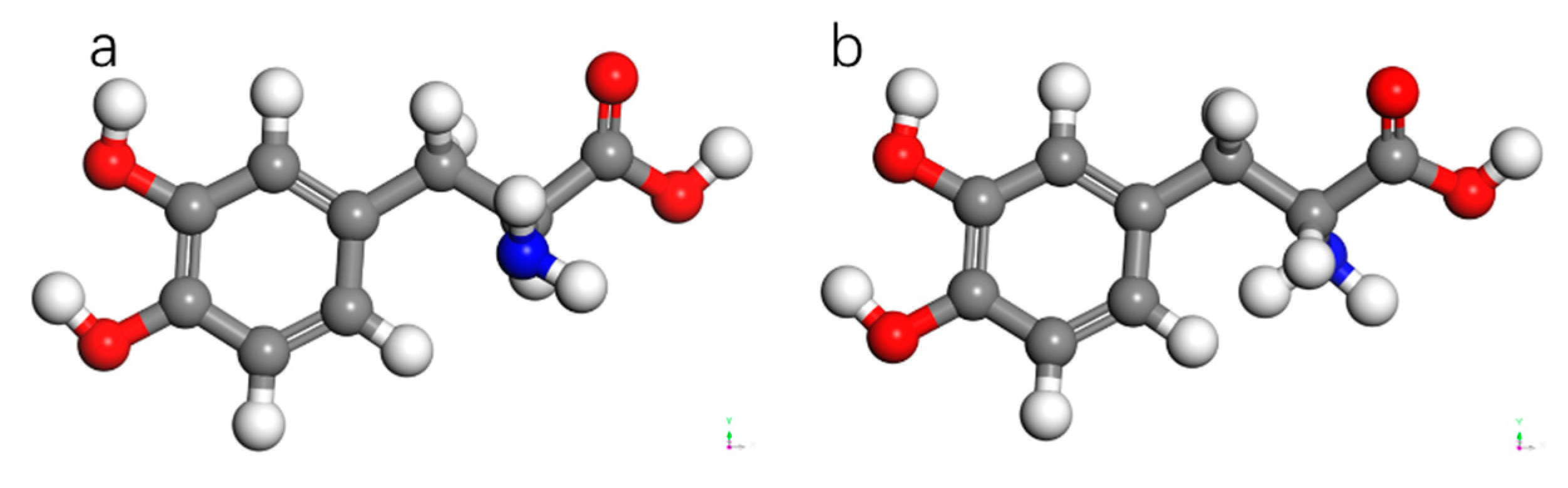
DOPA can be considered one of the earliest discovered chiral drugs in human history. Its corresponding chiral isomer is D-DOPA. If no artificial intervention is made during the pharmaceutical manufacturing process, an equal number of L-DOPA and D-DOPA molecules are naturally produced. However, only L-DOPA can be catalyzed by AADC enzyme. If an excessive amount of D-DOPA is ingested, it cannot be metabolized by the human body, leading to vascular blockage. Therefore, in the pharmaceutical manufacturing process, L-DOPA is selectively chosen for production.
As shown in
Figure 4, the specific process by which L-DOPA is converted into dopamine in the human body remains elusive. However, it is generally believed that biologically active form of vitamin B6 - pyridoxal phosphate (PLP) - participates in the catalytic reaction. PLP serves as a coenzyme and forms a covalent bond with amino acid residues in AADC enzyme, thereby forming a catalytic intermediate that facilitates the decarboxylation of L-DOPA. Specifically, PLP undergoes a keto-enol tautomerization with the carboxyl group of L-DOPA, generating a keto-enol intermediate, which then undergoes decarboxylation to produce dopamine and CO2. In this process, PLP continuously receives and releases carboxyl and amino groups, thus enabling the conversion of L-DOPA to dopamine.
3.3. Theoretical Analysis of Results
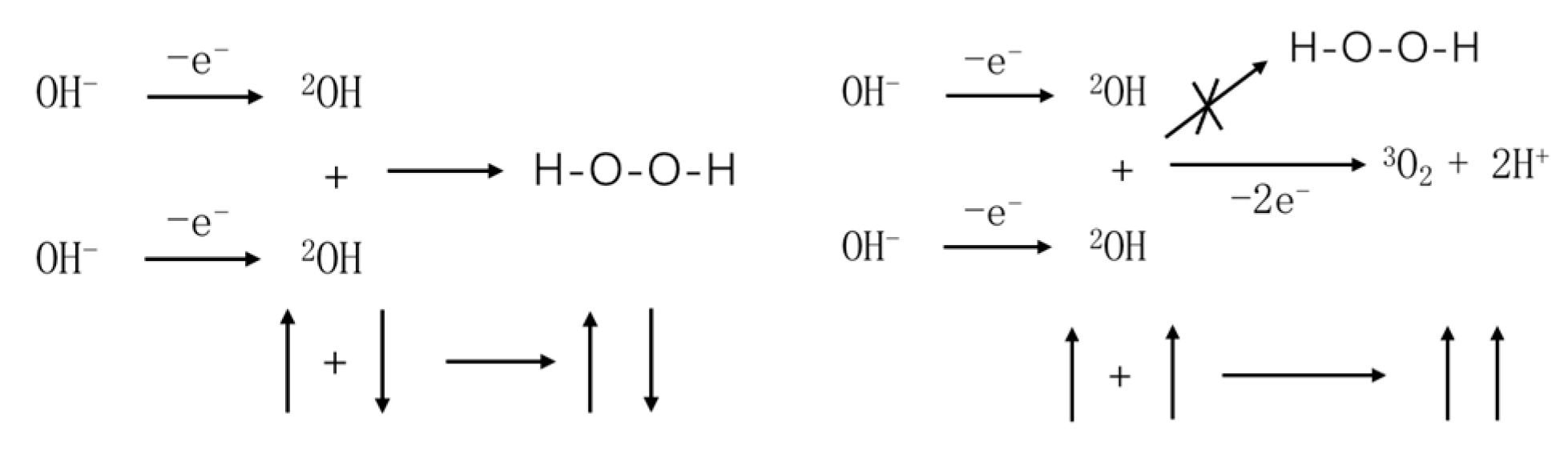
Section 3.2.3 demonstrated that the interaction energy between AADC enzyme and D-DOPA molecules is greater than that between AADC enzyme and L-DOPA molecules. This observation suggests that AADC enzyme may exhibit chiral selectivity. However, it is noteworthy that L-DOPA and D-DOPA share identical chemical structures, indicating that their valence electron arrangements and chemical properties are exactly the same. Consequently, the underlying reason for this disparity can only be attributed to the electron spin of L-DOPA and D-DOPA molecules.
According to the theoretical analysis presented in
Section 2, non-polar molecules undergo polarization currents during chemical reactions, resulting in electron redistribution within the molecules, whereas AADC enzyme is a levorotatory protein molecule. Based on the CISS effect, valence electrons with spin-up are more likely to accumulate near the negative charge center of the molecule, while valence electrons with spin-down are more distributed around the positive charge center. For stable, low-energy chemical bonding with AADC enzyme, the spin direction of the reactant’s valence electrons must be antiparallel to that of AADC enzyme. Since covalent bond formation involves the sharing of electron pairs, molecules that can form stable structures with AADC enzyme must carry valence electrons with the opposite spin direction to the negative charge center of AADC, i.e., spin-down valence electrons. Conversely, substances that form unstable interactions with AADC enzyme are expected to carry a higher proportion of spin-up valence electrons.
Therefore, a natural conjecture is that L-DOPA molecules carry valence electrons with spin-down, while D-DOPA molecules carry valence electrons with spin-up. Due to this characteristic, L-DOPA molecules are more prone to forming covalent bonds with AADC enzyme, making them more easily anchored at the catalytic site of AADC. S. By the same token, D-DOPA molecules require a transition to the triplet state surface when reacting with AADC enzyme. This transition necessitates one of the valence electrons to undergo a transition and emit to an energy level orbital that intersects with the other electron, forming an exchange anti-symmetric coupling orbital wave function, thus enabling the formation of an unstable chemical bond. Consequently, the interaction potential energy between D-DOPA molecules and AADC enzyme increases, generating an energy barrier. The barrier represents the energy required for the valence electron to transition to the nearest neighboring orbital. As a result, the difference in spin orientations of the electrons carried by the two molecules leads to the macroscopic phenomenon of chiral selectivity of AADC enzyme. Furthermore, it has been found that when spin in enzyme molecules is preferentially guided in a particular direction, charge recombination is reduced, which in turn improves charge transfer, thus enhancing the reaction rate [
19].
3.3.1. Calculation of Charge Transfer in the Interaction of L-DOPA and D-DOPA with Peptide Chains
To validate the hypothesis proposed in
Section 3.3, it is necessary to calculate the charge transfer between the enzyme molecule and L-DOPA, as well as D-DOPA, during the occurrence of the reaction. However, directly calculating the charge transfer between the large AADC enzyme molecule and L-DOPA or D-DOPA is impractical due to the sheer size of the enzyme. As AADC enzyme is composed of levorotatory peptide chains, for the convenience of computation, we calculated the charge transfer between levorotatory peptide chain molecules consisting of different numbers of amino acid units and L-DOPA or D-DOPA molecules. Prior to that, we need to analyze the active sites of L-DOPA and D-DOPA when undergoing a chemical reaction.
To determine the active sites, we calculated the Fukui indices of L-DOPA and D-DOPA molecules using the DMol3 [
20,
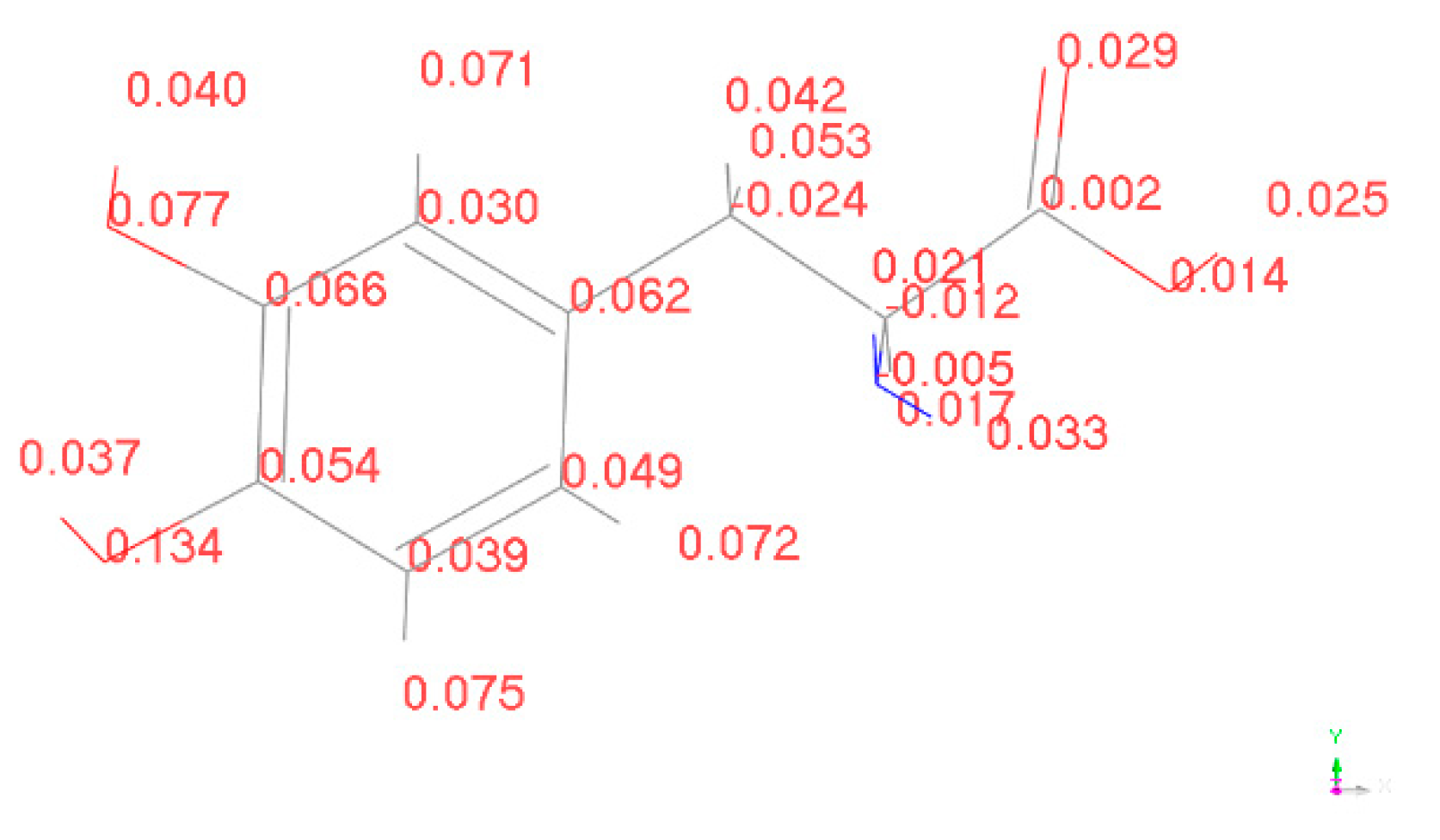
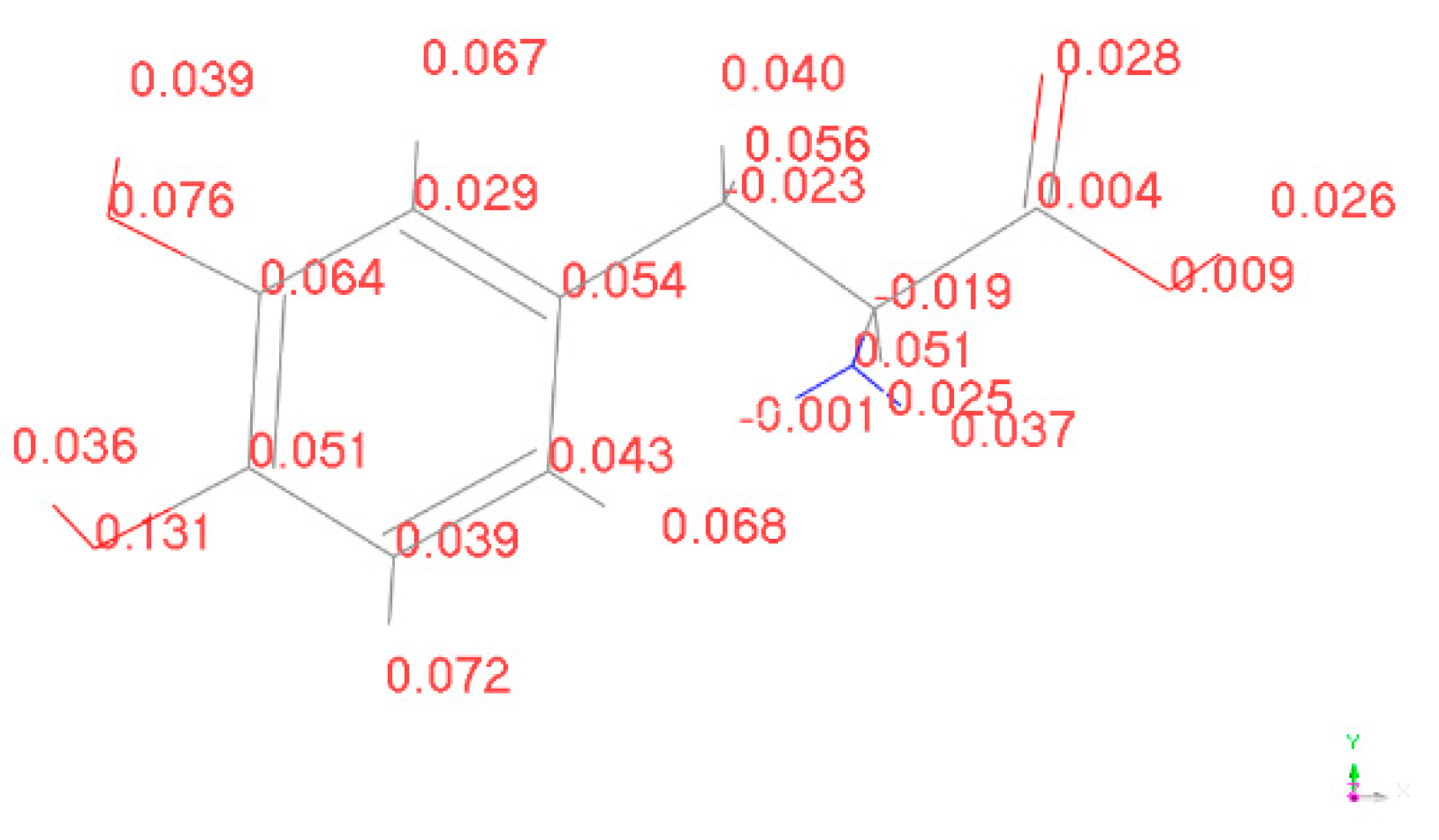
21] module in MS. The Fukui index provides a qualitative judgment of which functional group in the molecule is most likely to be involved in a chemical reaction. The larger the Fukui index of an atom, the higher its chemical reactivity will be. The Fukui indices are categorized into nucleophilic attack index (f+), electrophilic attack index (f-), and radical attack index (f0). The calculated results of the electrophilic attack index for L-DOPA and D-DOPA molecules are shown in the following figure:
The higher the electrophilic attack index, the more susceptible the atom is to losing electrons to other molecules. From
Figure 15 and
Figure 16, it can be observed that both L-DOPA and D-DOPA have the same sites that are prone to electron loss during the chemical reaction, specifically located on the oxygen atom of the benzene ring in the lower-left region of the figures. The electrophilic attack index at this site is 0.134 for L-DOPA and 0.131 for D-DOPA.
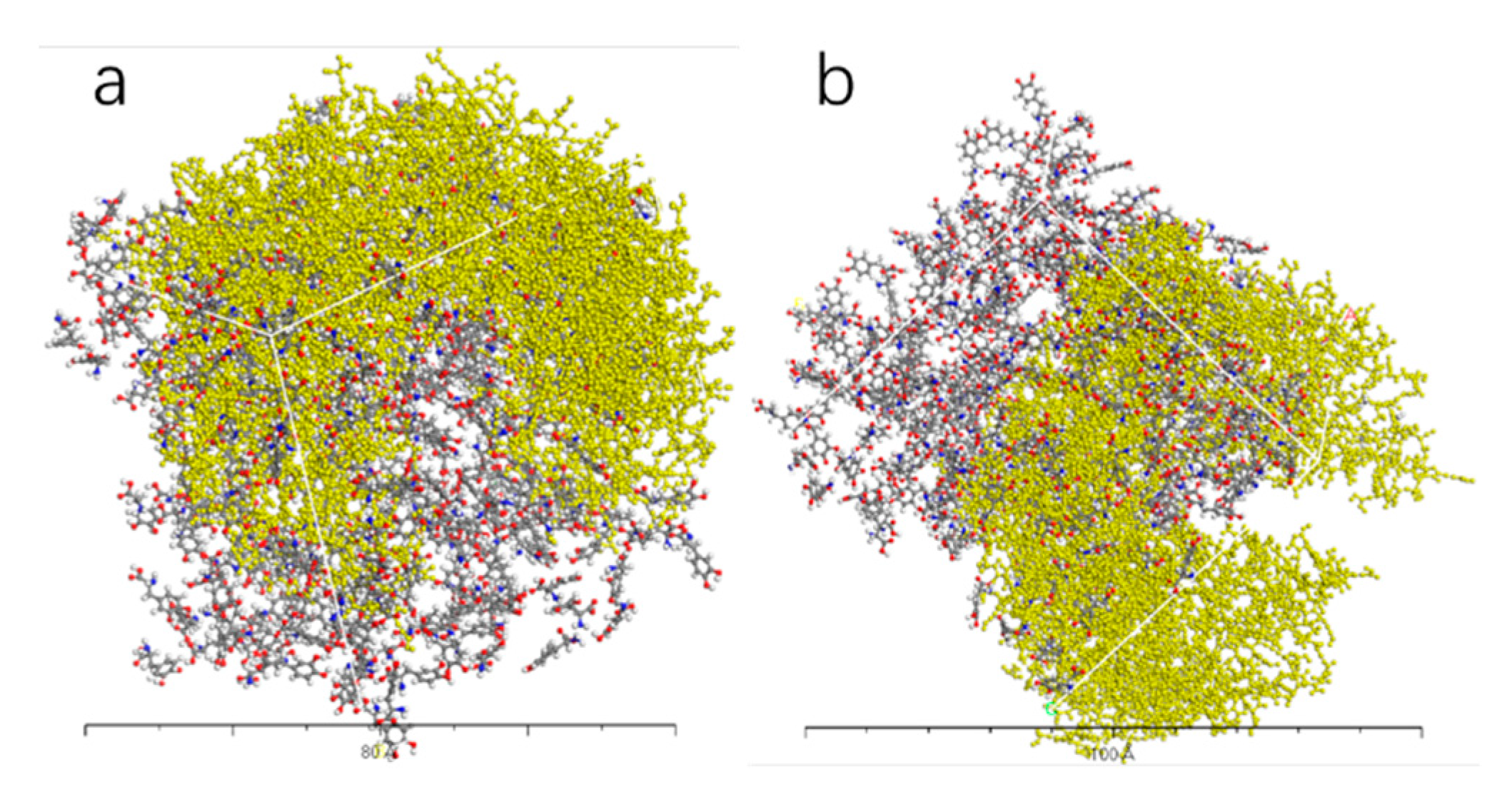
Based on the results, in the subsequent calculations, we positioned the benzene ring of L-DOPA and D-DOPA away from the levorotatory peptide chain to ensure that it is close to the side of the peptide chain. For comprehensive analysis, we also configured levorotatory peptide chains composed of 10, 15, 20, and 25 amino acid units, ensuring that the pitch and radius of each peptide chain are consistent. Additionally, we set the distance between the centers of L-DOPA and D-DOPA molecules and the centers of the peptide chain molecules to simulate the molecular approach during the chemical reaction. Due to the different exposed functional groups at the ends of the peptide chains, separate considerations are needed. Thus, we need to separately calculate the scenarios in which the N-terminus or the C-terminus of the peptide chains approaches L-DOPA and D-DOPA molecules.
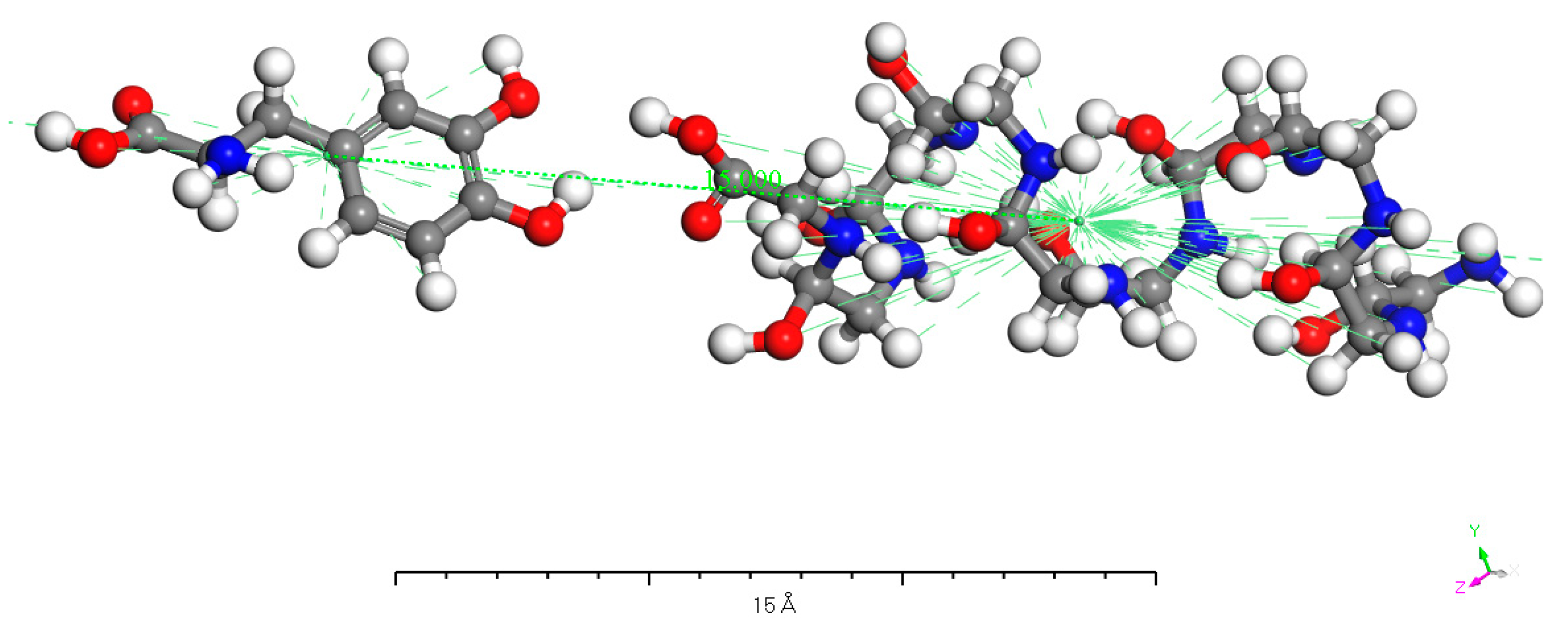
As shown in
Figure 17, this is one of the computed charge transfer models described above. The figure depicts the situation where the benzene ring side of the D-DOPA molecule approaches the C-terminus of a levorotatory peptide chain composed of 10 amino acids. We set the distance between the centers of the two molecules to be 15 angstroms. However, this distance is not the final value as the model needs to undergo structural optimization before calculating the electron population of the molecules. After structural optimization, the two molecules will be in the lowest energy state, which represents the most probable real-world scenario. At this point, the distance between the centers of the two molecules will change.
Due to the plethora of atoms and their valence electrons, it is difficult to discern the trend of charge transfer by directly analyzing the charge transfer data of each atom. Therefore, a method akin to calculating the center of mass of an object can be used to compute the positions of the positive and negative charge centers of the molecules. The calculation formula is as follows:
After performing the above calculation for each molecule, the positions of the positive and negative charge centers of the molecules are obtained. Then, the difference between the two position vectors is calculated and the magnitude of the resulting vector represents the distance between the positive and negative centers of the molecule in that specific scenario, which indicates the polarization strength of the molecule.
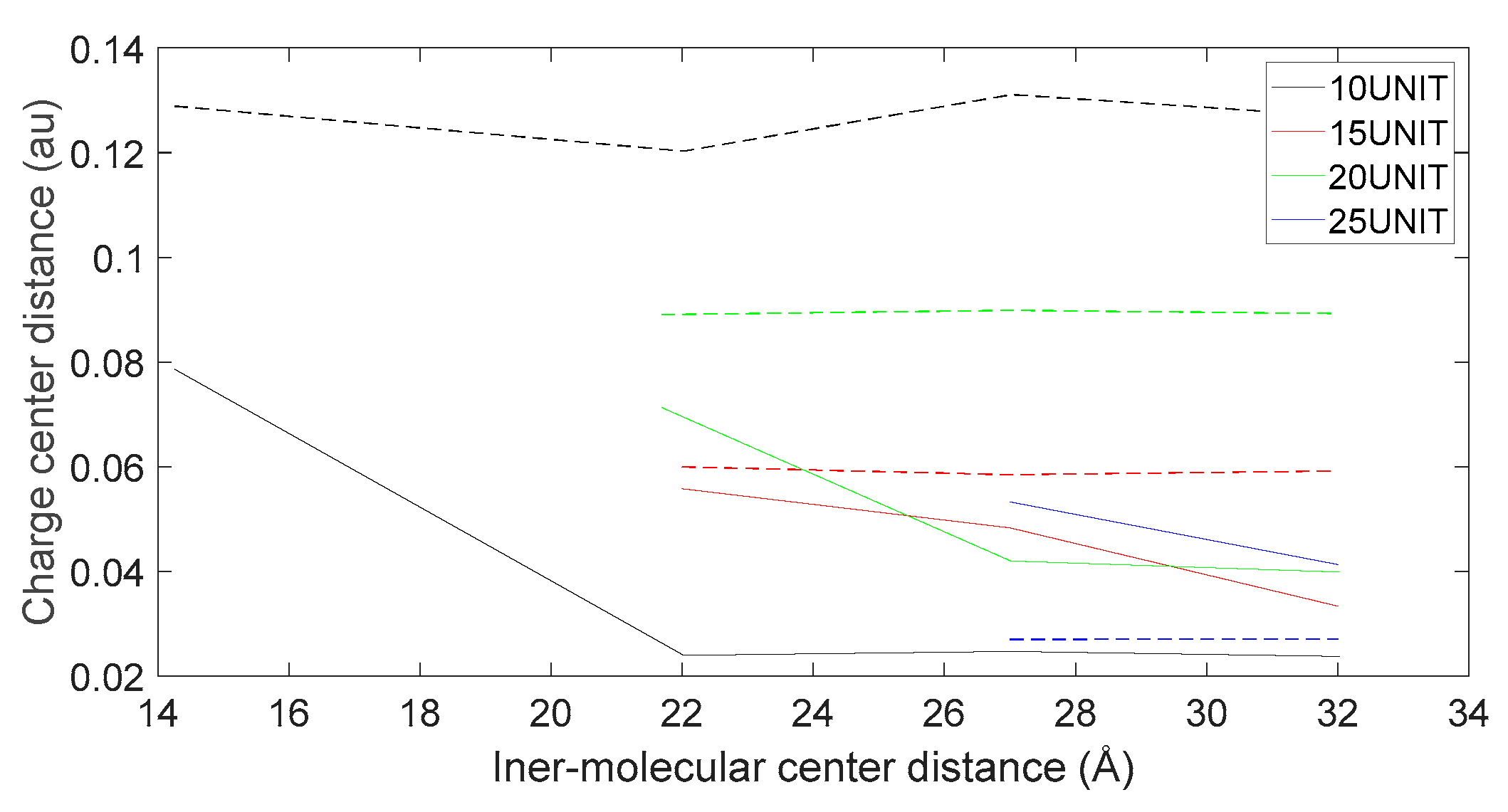
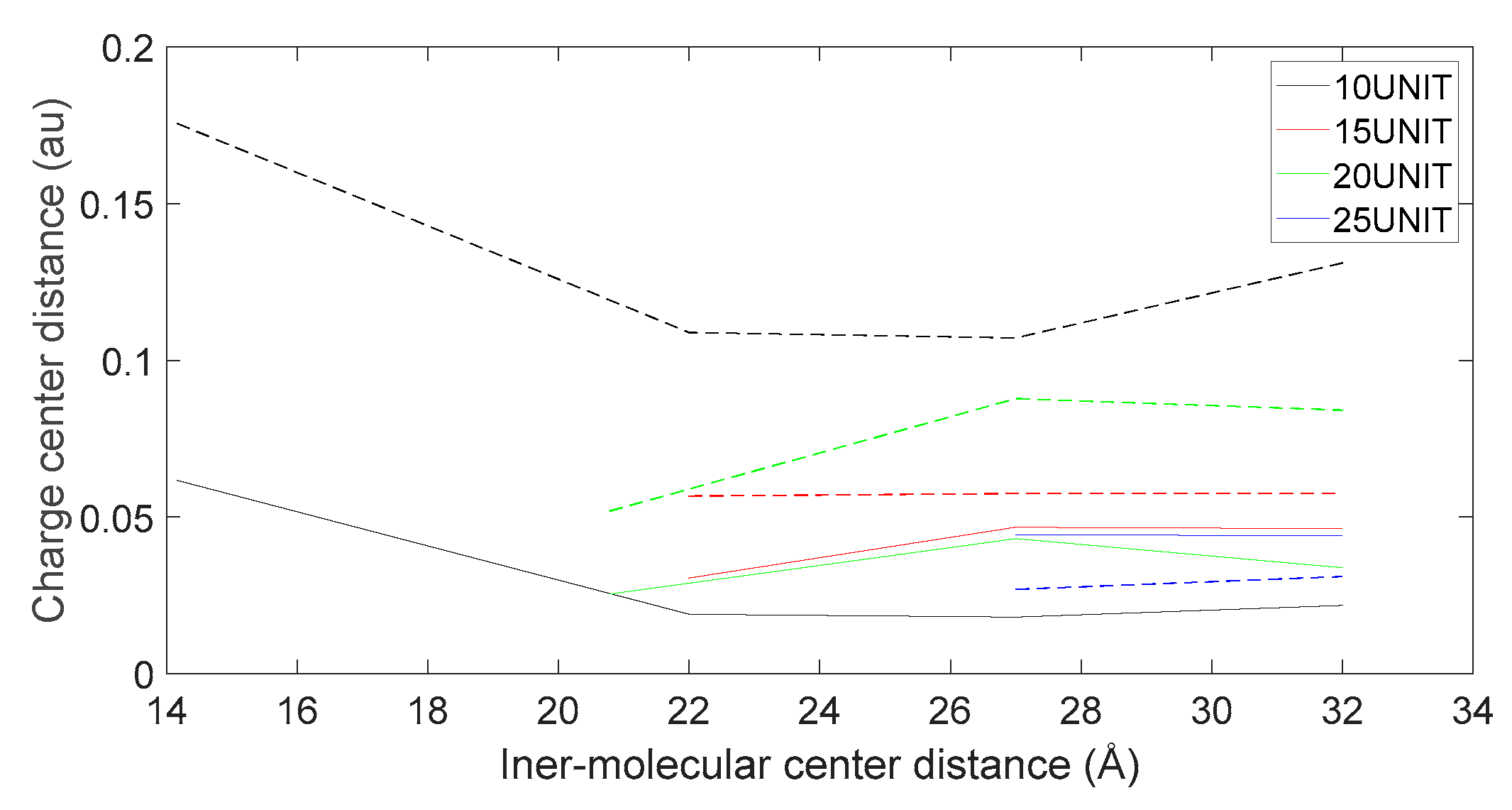
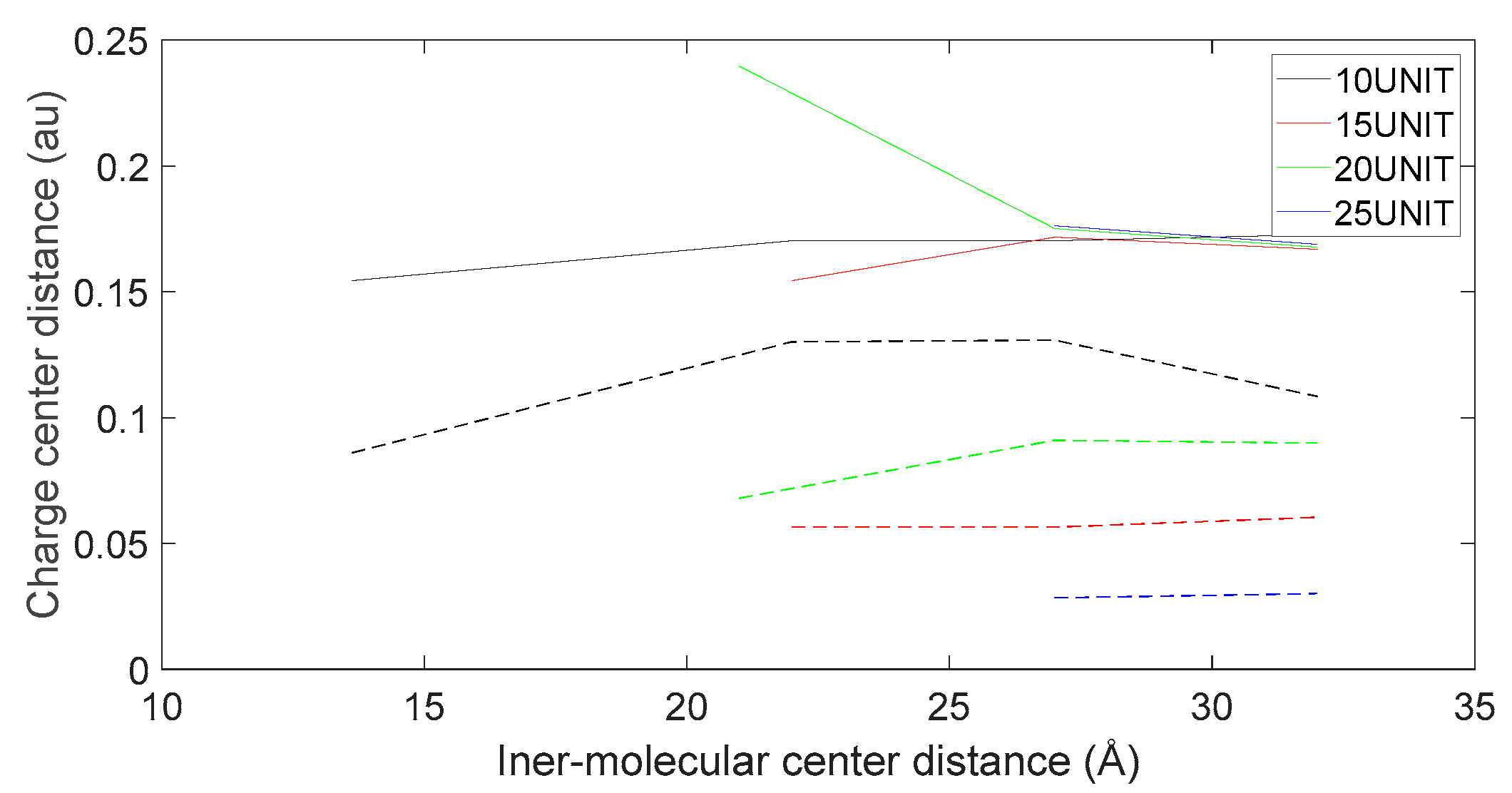
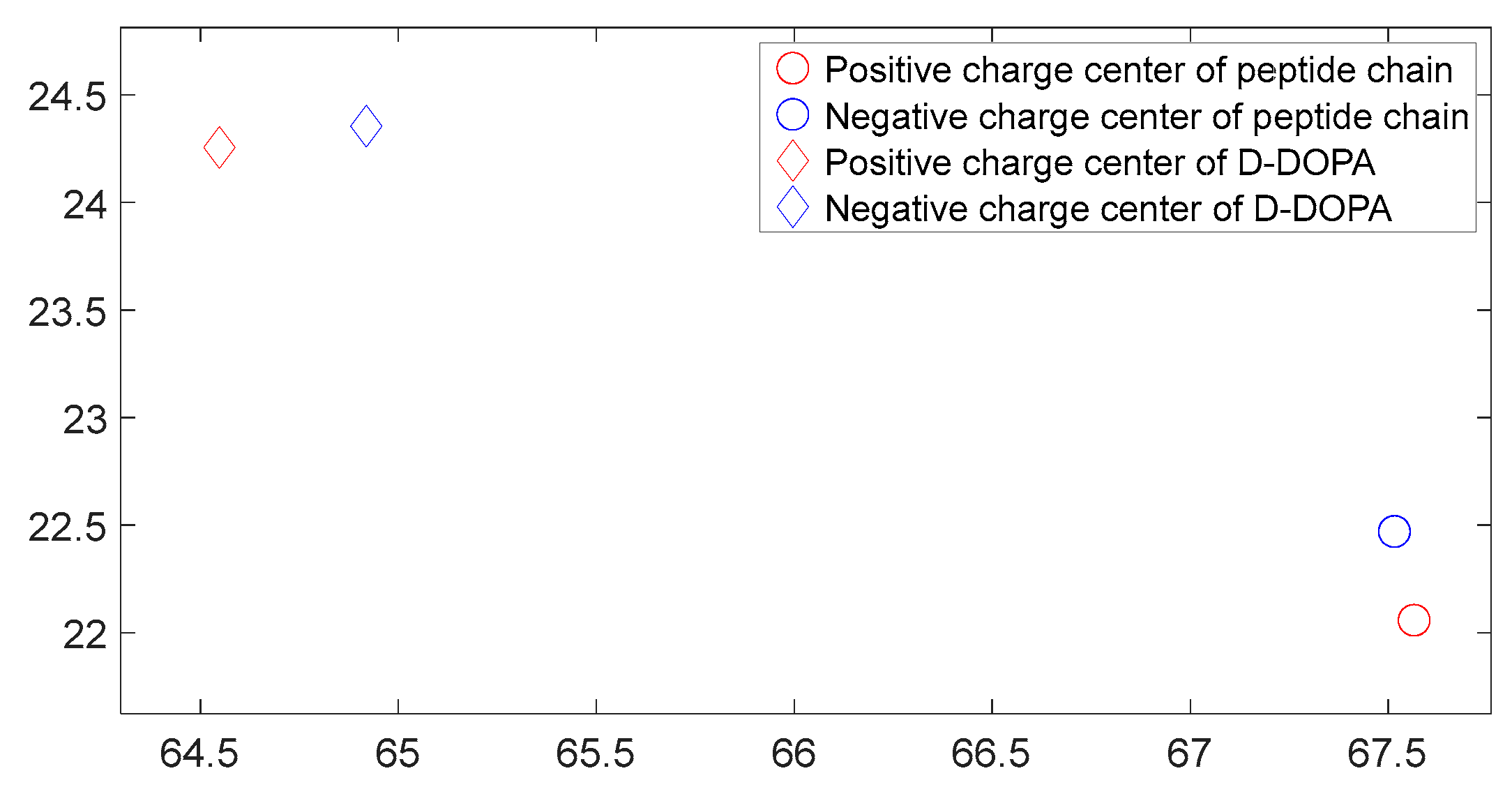
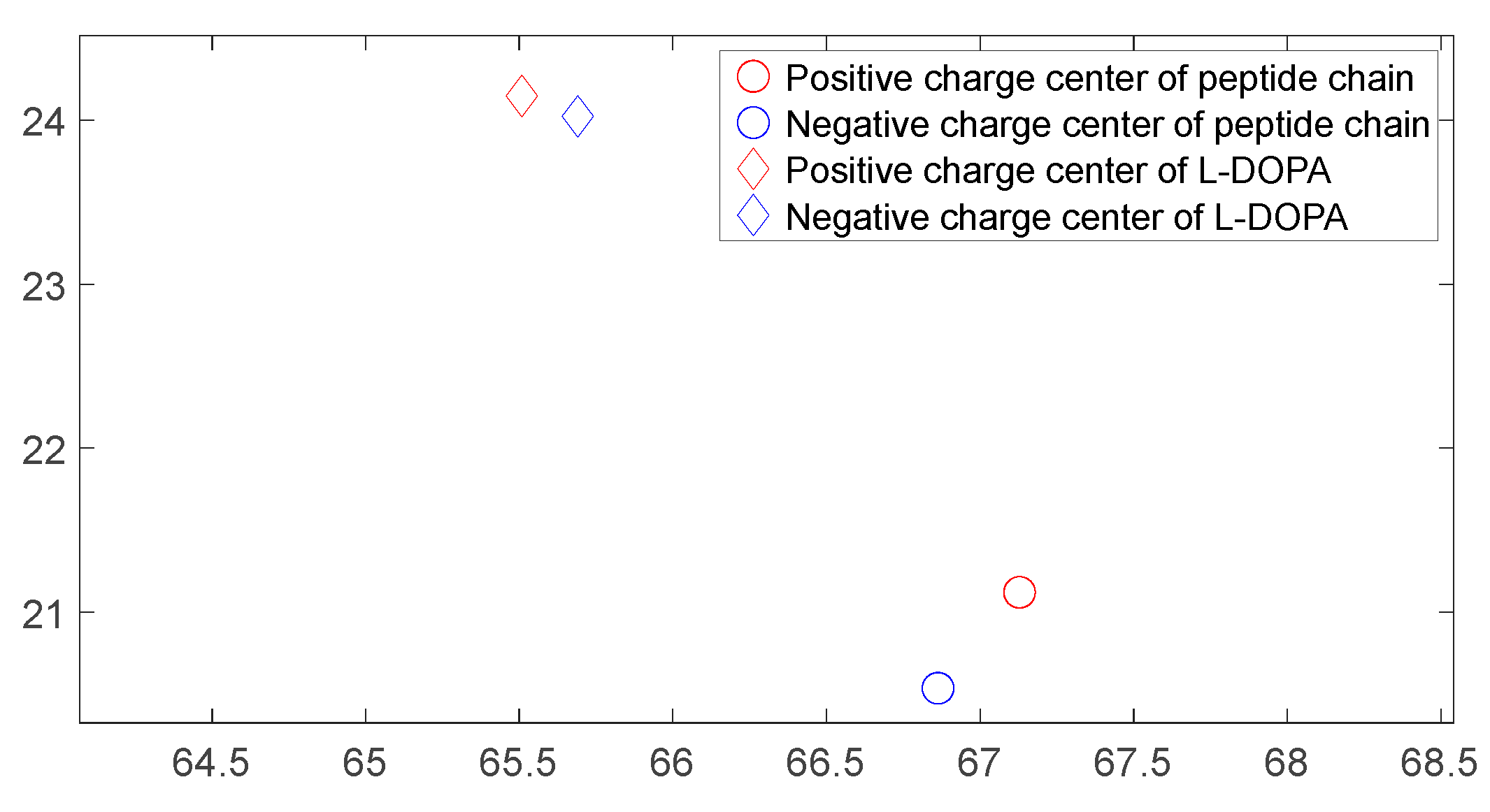
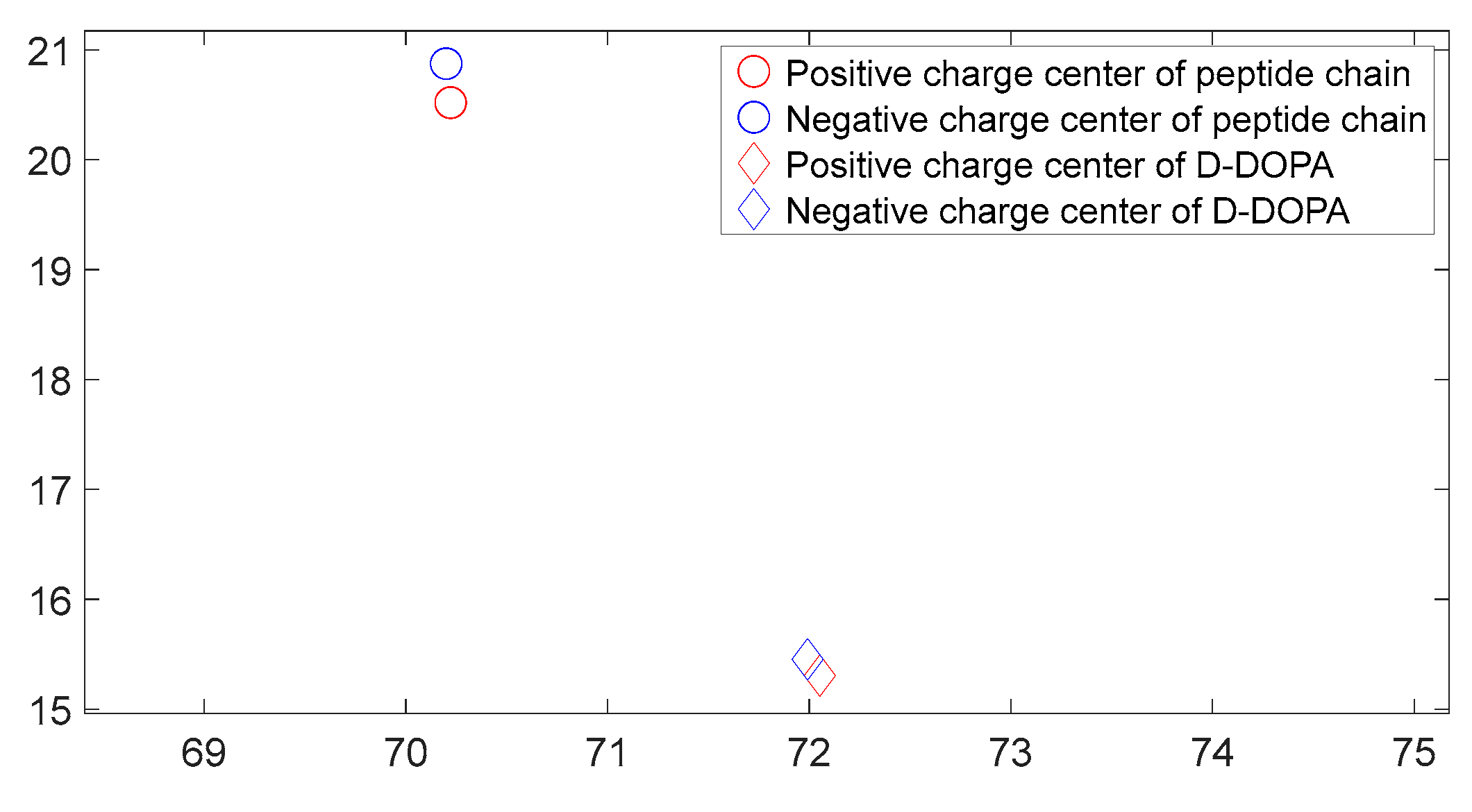
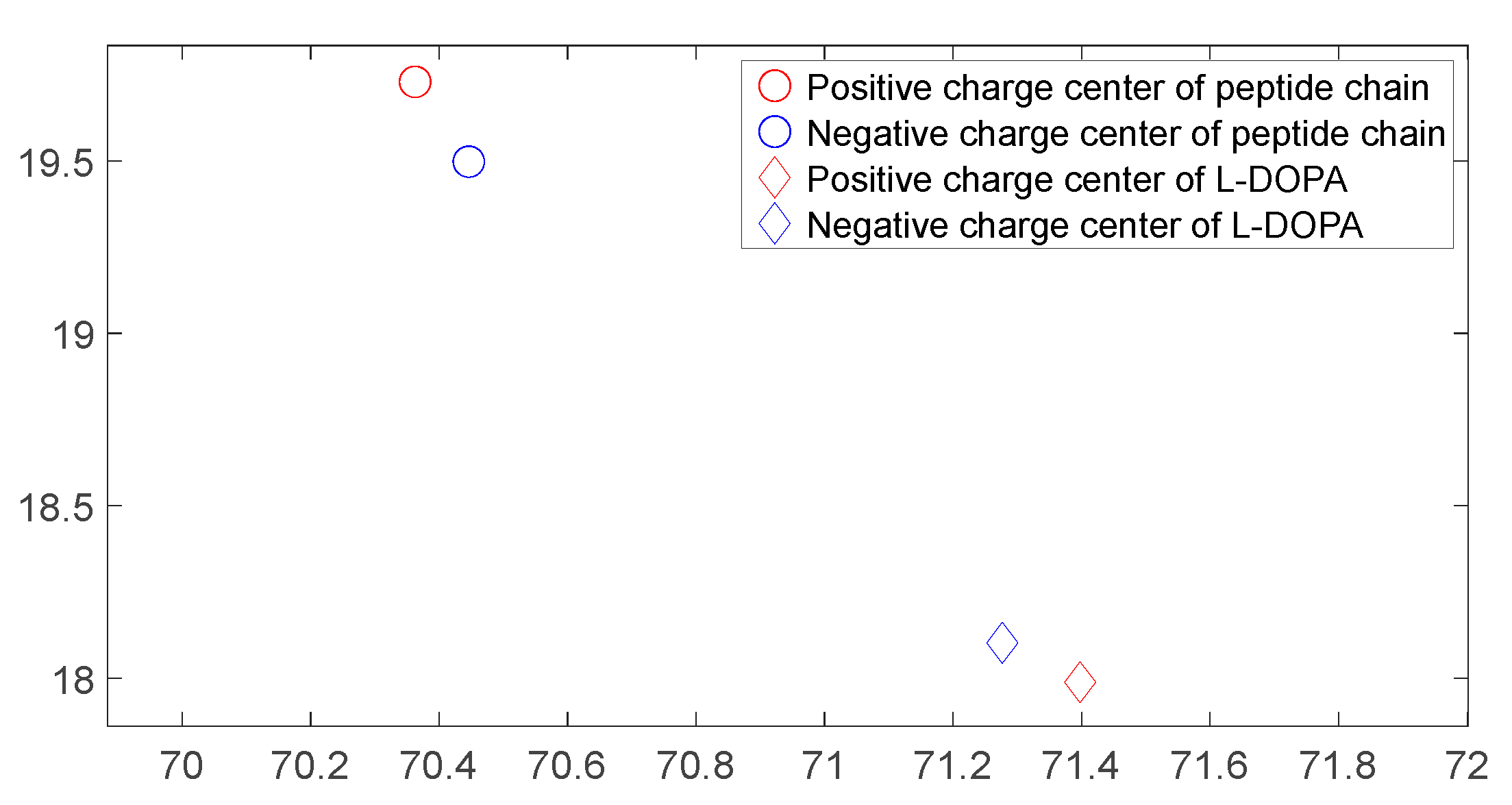
As shown in
Figure 18,
Figure 19,
Figure 20 and
Figure 21, it can be observed that the distance between the positive and negative charge centers of the D-DOPA molecule is smaller compared to that of the peptide molecule. By contrast, the distance between the positive and negative charge centers of the L-DOPA molecule is larger compared to the peptide molecule. Furthermore, the displacement distance of the positive and negative charge centers of the L-DOPA molecule is greater than that of D-DOPA. These findings indicate that L-DOPA is more prone to polarization compared to D-DOPA, while the difference in the positive and negative charge centers of the peptide molecule between the L-DOPA and D-DOPA comparison groups is not significant. This is attributed to the significantly larger molecular weight of the peptide molecule compared to L-DOPA and D-DOPA, which results in the presence of a larger number of electrons. Consequently, the changes in distance between the two molecules have a much greater impact on the variation in the positive and negative charge centers of the peptide molecule compared to the effects introduced by the different DOPA molecules.
By calculating the charge distribution of the molecules in their individual states and when in proximity to each other, and subsequently taking the difference between the two results, we can obtain the absolute value of charge transfer during the approaching process of the molecules. The computational results are presented as follows:
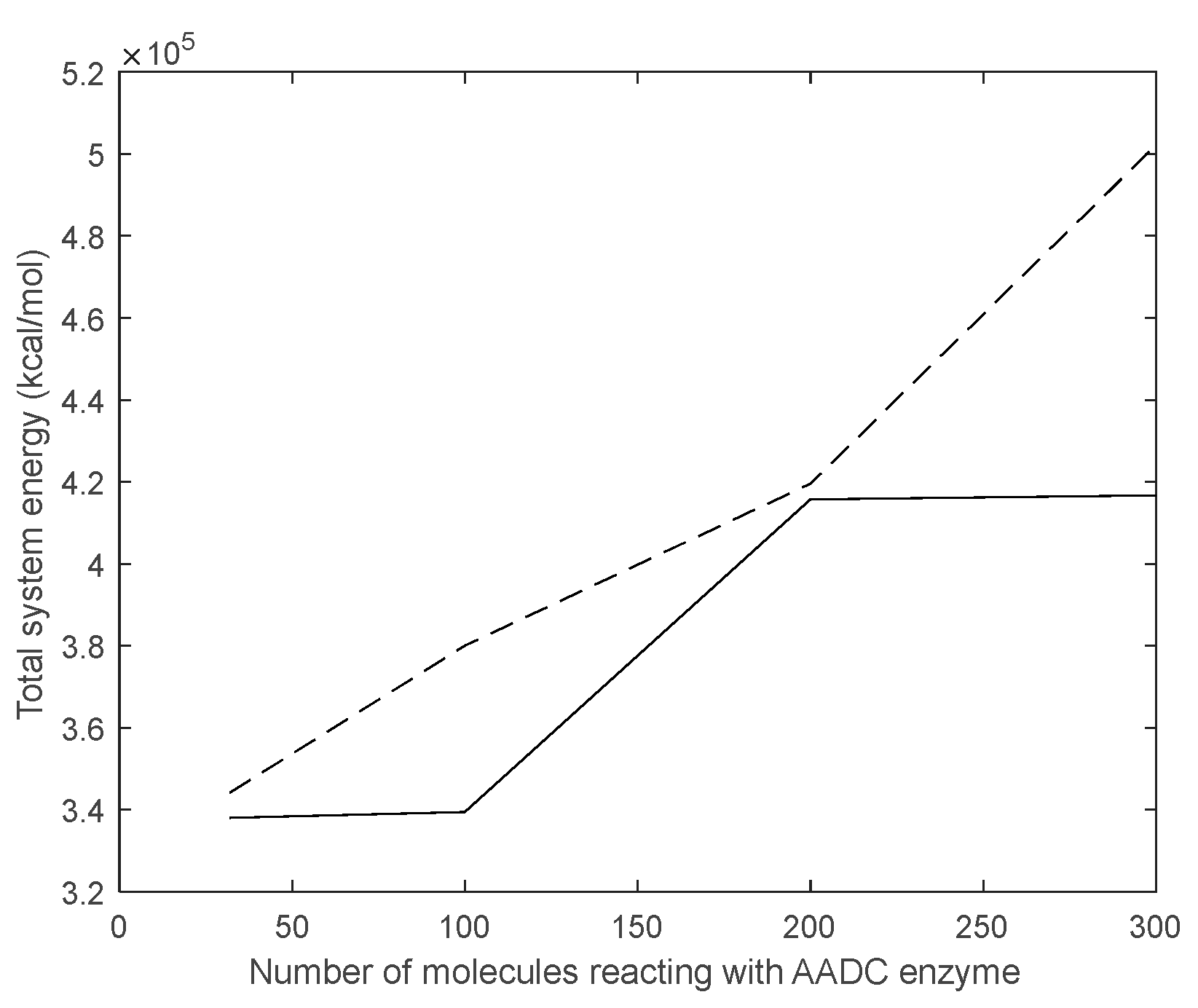
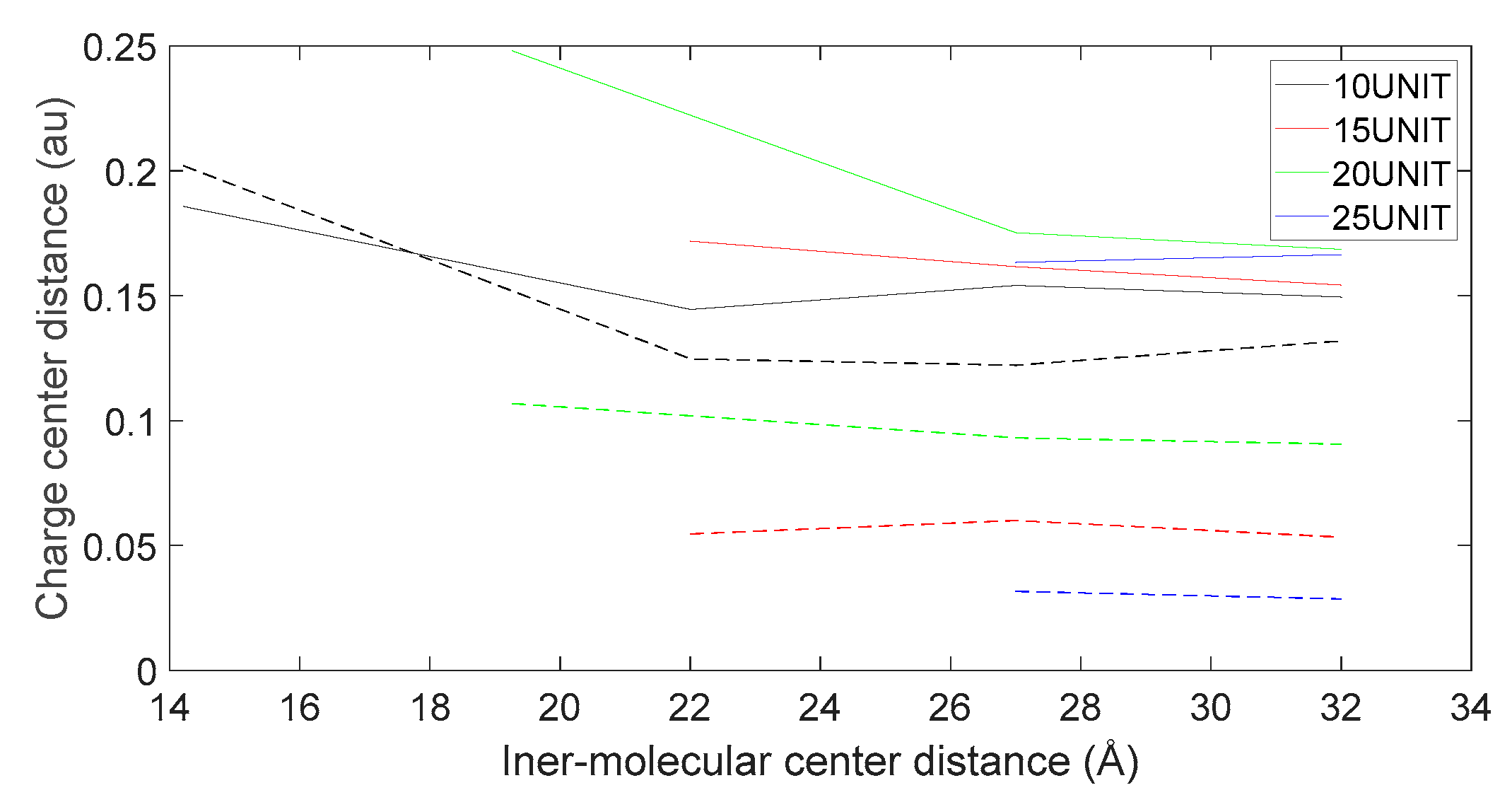
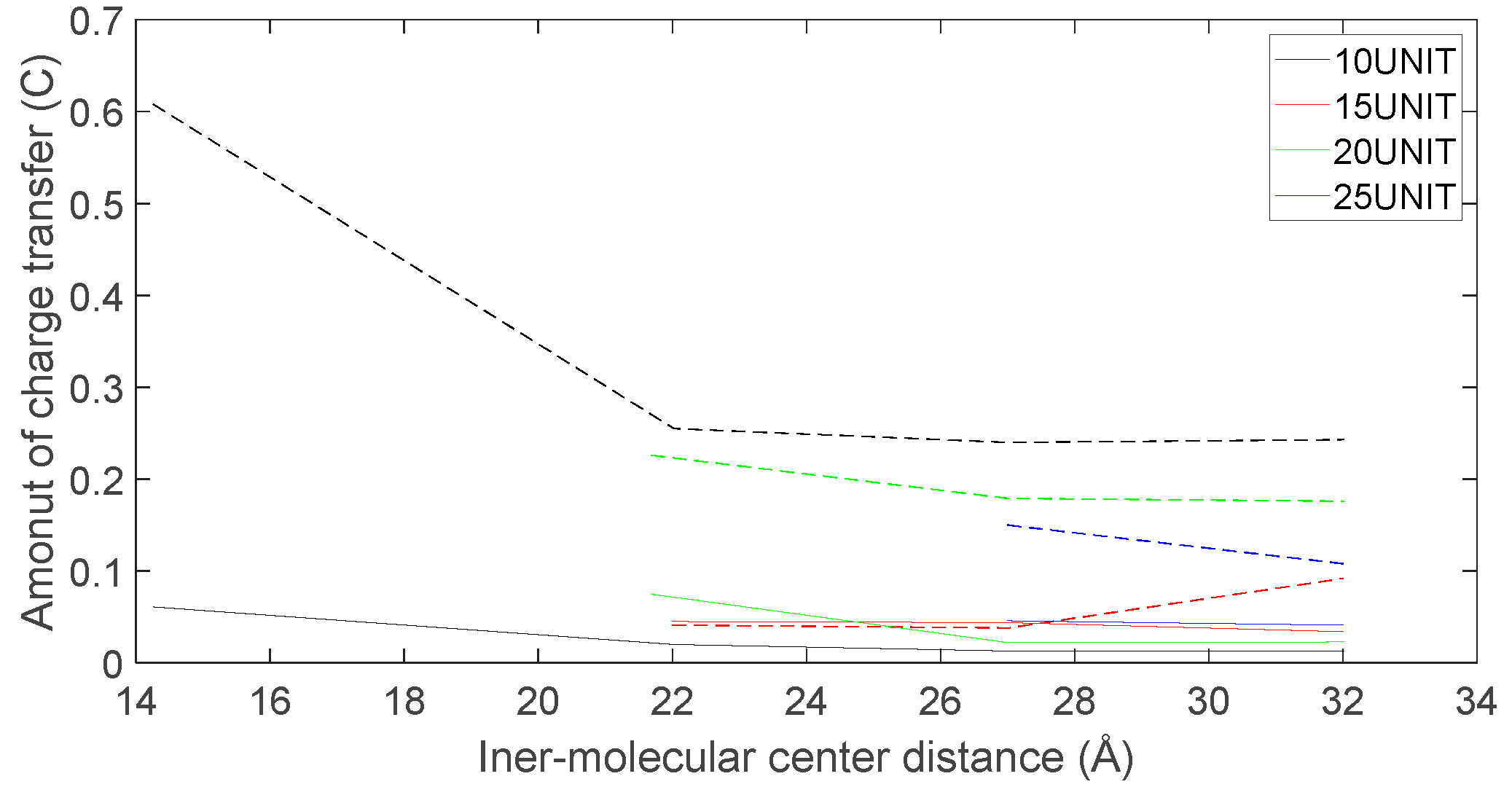
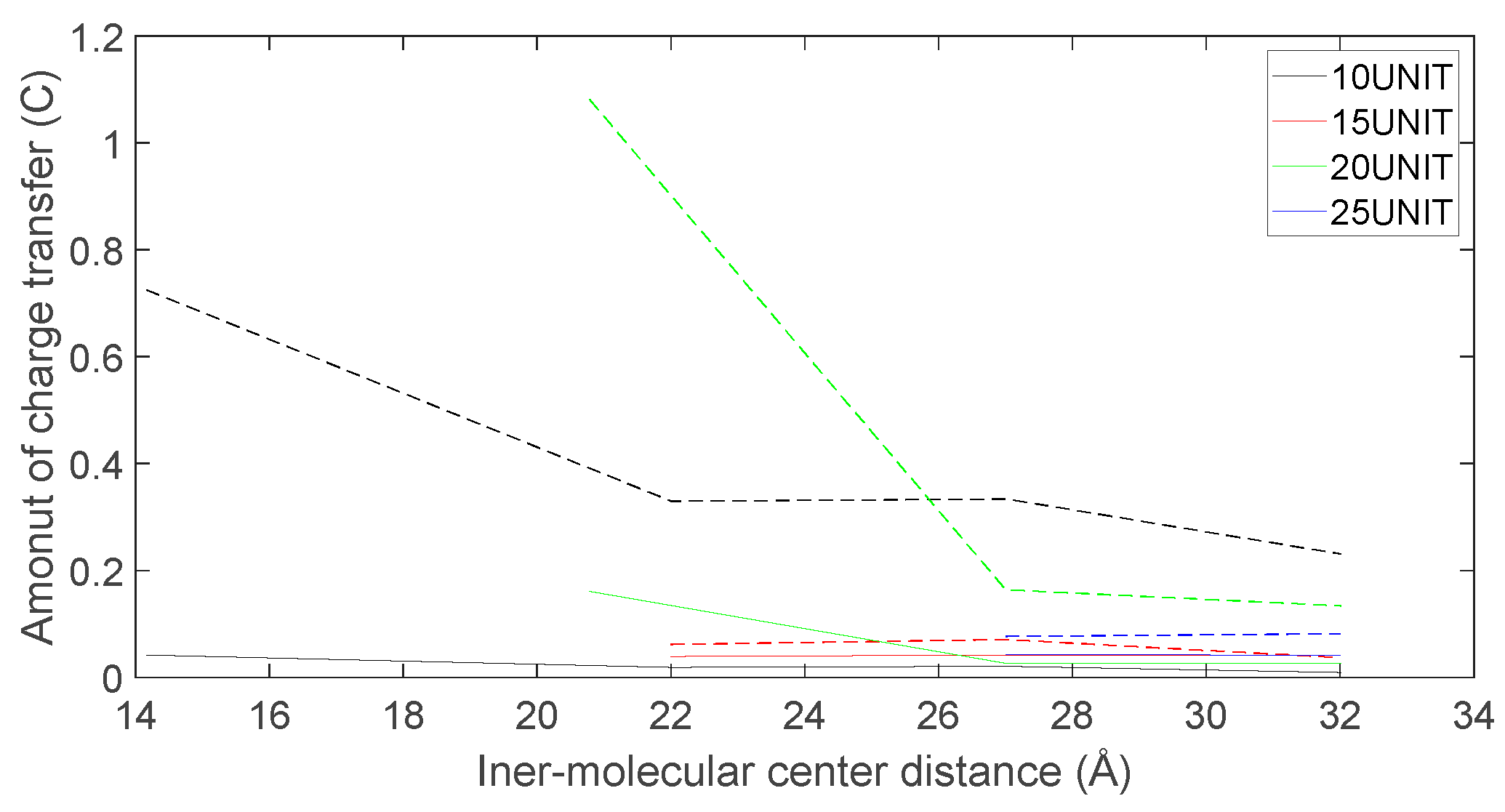
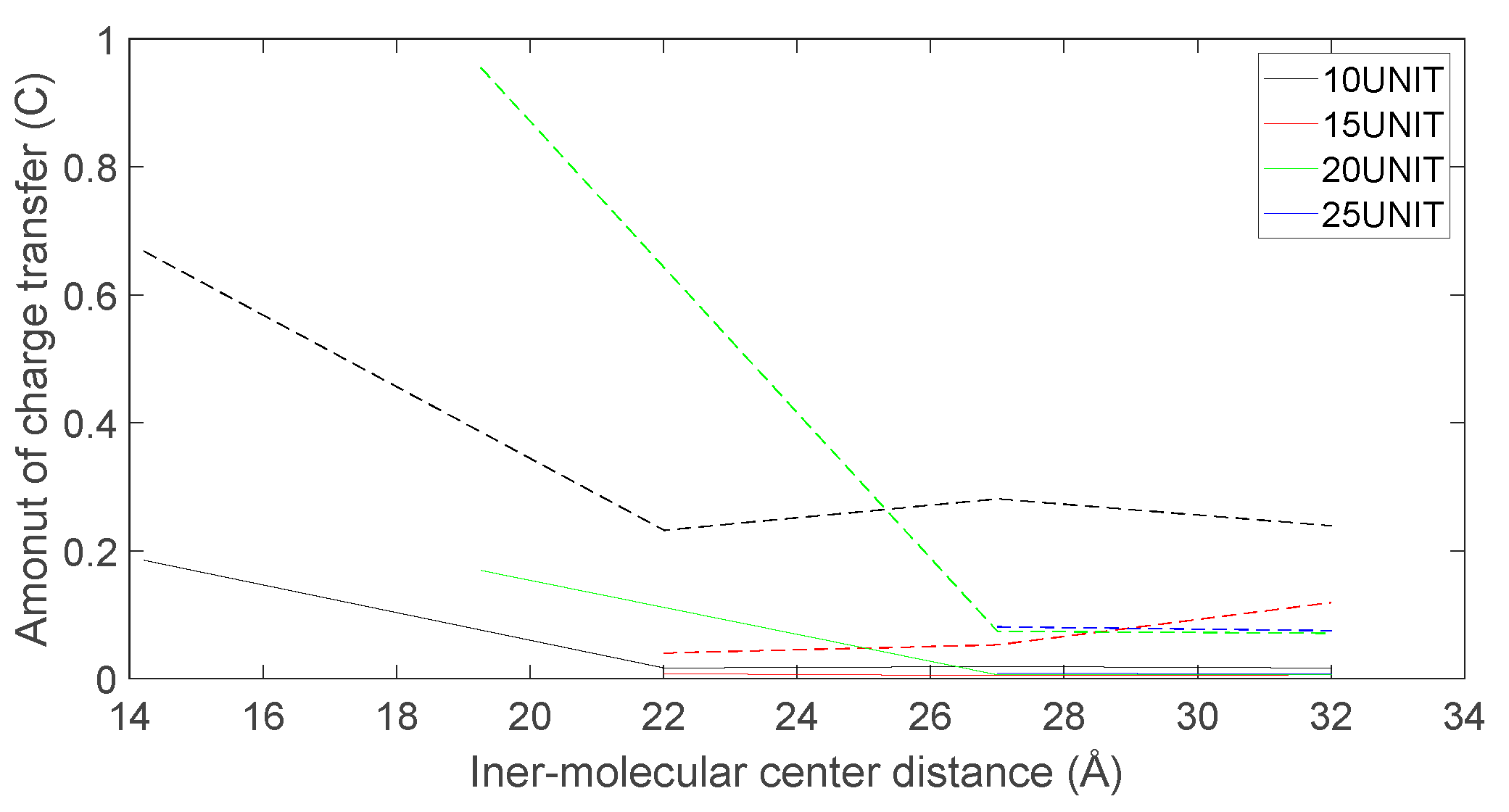
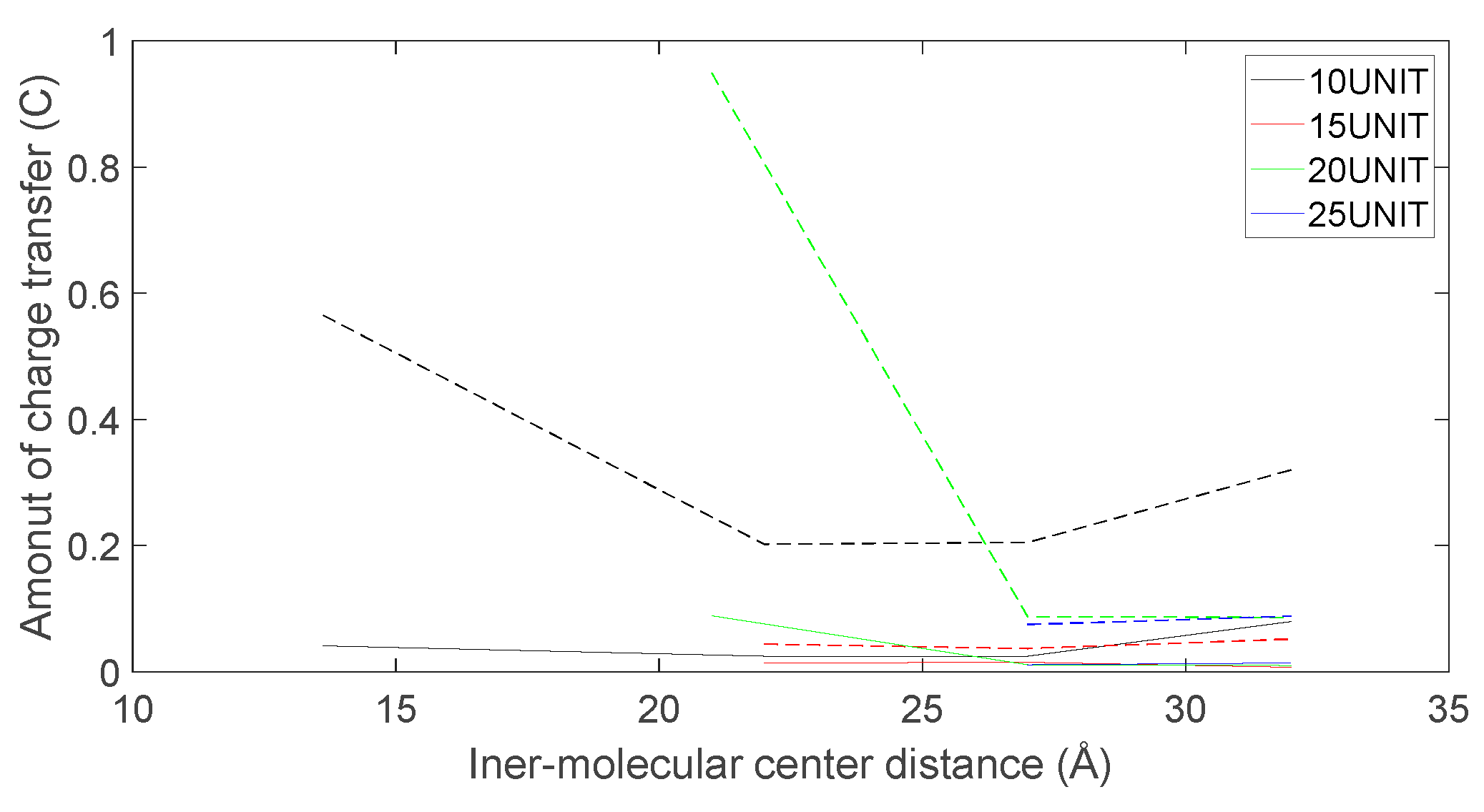
As depicted in
Figure 22,
Figure 23,
Figure 24 and
Figure 25, the following conclusions can be drawn: Firstly, the charge transfer of D-DOPA molecules increases with the rise in the peptide chain’s molecular weight whereas the charge transfer of L-DOPA molecules decreases with the decline in the peptide chain’s molecular weight. Secondly, the charge transfer of the peptide chain with both L-DOPA and D-DOPA molecules increases as the distance between them decreases. Moreover, the charge transfer of the peptide chain is generally greater than that of L-DOPA or D-DOPA. Furthermore, D-DOPA molecules are more prone to charge transfer compared to L-DOPA molecules, consistent with the analysis of charge centers. As the distance between the two molecules decreases, the difference in charge transfer between the peptide chain and L-DOPA, D-DOPA molecules becomes more pronounced.
These findings indicate that molecules carrying more charges exhibit larger internal charge transfer during chemical reactions. In the process of chemical reactions, due to the substantial molecular weight of the enzyme molecule, the CISS effect is expected to be significantly marked within the enzyme molecule.
3.3.2. Reaction Trends and CISS Effect
In
Section 3.3.1, we analyzed the general trends of charge transfer for L-DOPA and D-DOPA when they approach peptide chains of different lengths. To provide more specific insights, we conducted separate calculations using data from electronic charge distributions for interactions between a peptide composed of 10 amino acids and L-DOPA, as well as D-DOPA. The results were used to create spatial representations of the positions of positive and negative charge centers when the molecules approach each other:
As shown in
Figure 26,
Figure 27,
Figure 28 and
Figure 29, these four graphs represent the positions of charge centers when the distance between the two molecules is consistent. These graphs are projections on the X-Y plane in three-dimensional space. From
Figure 26 and
Figure 27, it can be observed that the angle between the lines connecting the charge centers of the interacting molecules is significant. Thus, we can infer that the C-terminus of the polypeptide chain has a repulsive effect on both L-DOPA and D-DOPA, meaning that L-DOPA and D-DOPA are more inclined to react with the N-terminus of the polypeptide. Through these four graphs, we also find that both L-DOPA and D-DOPA approach the polypeptide with negative charge centers, which is consistent with the predictions based on the Fukui indices in
Section 3.3.1. Furthermore, it can be observed that D-DOPA approaches the positive charge center of the polypeptide, while L-DOPA approaches the negative charge center. This finding aligns with the analysis in
Section 3.3, indicating that L-DOPA tends to form covalent bonds with the polypeptide by sharing electrons whereas D-DOPA, due to charge-spin repulsion, can only approach the positive charge center of the polypeptide. This is because when the polypeptide approaches D-DOPA, the electrons with parallel spins are transferred to the molecule’s negative charge center, leaving behind the electrons with compatible spins at the positive charge center.
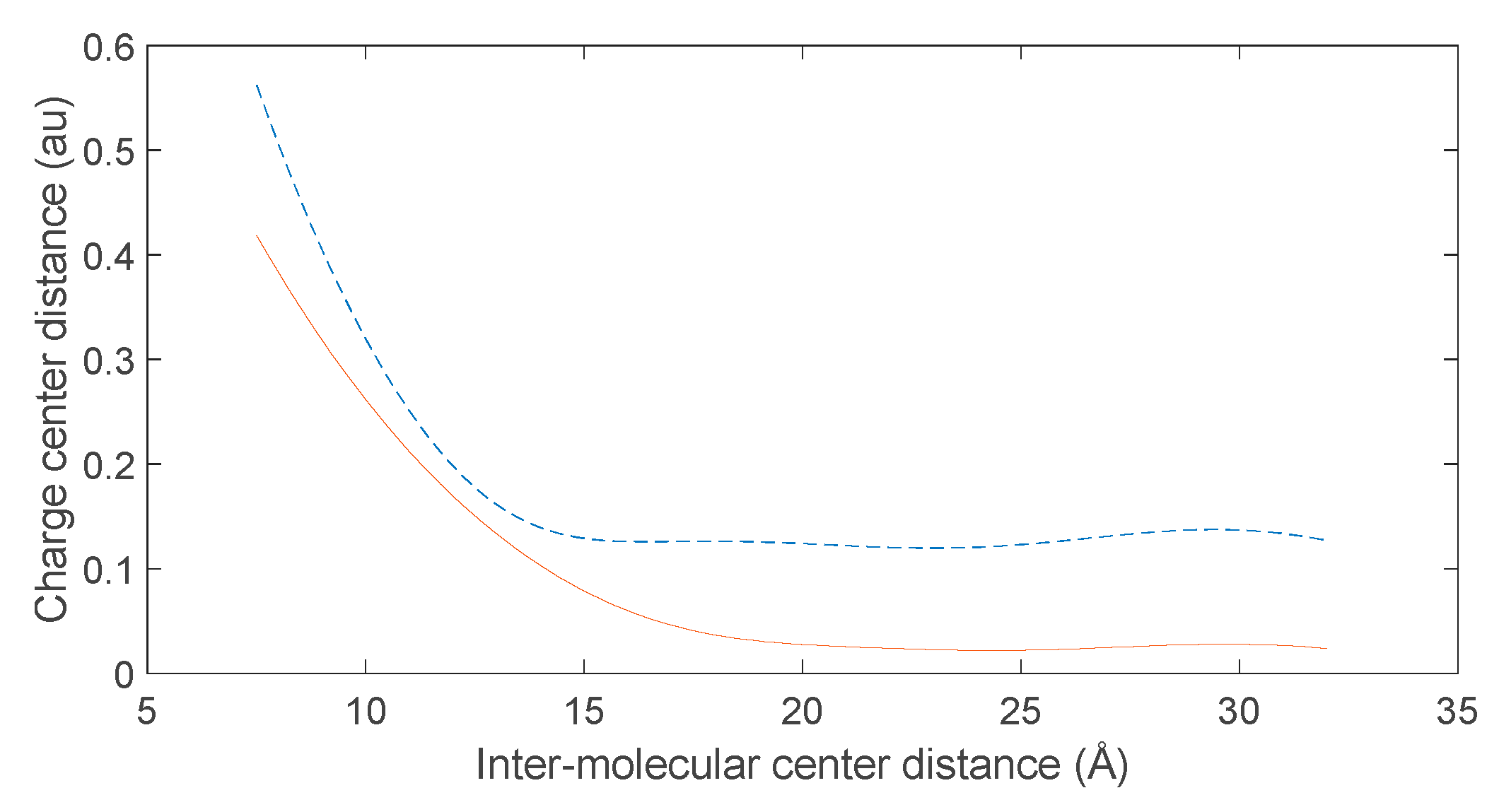
Based on the electronic population calculation data when the C-terminus of a 10-amino acid polypeptide molecule approaches D-DOPA, we performed polynomial fitting using the least squares method to establish the relationship between the distance of the positive and negative charge centers of the two molecules and the distance between their centers. As shown in
Figure 30, it can be observed that initially, when the two molecules are relatively far apart, the distance between the positive and negative charge centers is very close. However, as the distance between them approaches a certain value, the distance between the charge centers suddenly increases.
According to equations (3) and (4) in
Section 2.3, the intermolecular forces between nonpolar molecules are attractive, and the strength of these attractive forces increases with the polarization intensity of the molecules. Therefore, the rate of approach between nonpolar molecules during the reaction becomes faster. From
Figure 30, it can be deduced that when the two molecules approach each other rapidly, the distance between their charge centers increases rapidly. Since positively charged atoms in nonpolar molecules are nearly stationary, this implies that the speed of electron transfer between the molecules increases as they come closer.
If one of the interacting molecules possesses a chiral structure, according to equations (1) and (2) in
Section 2.2, it can be observed that the increased speed of electron transfer leads to a significant CISS effect.
Based on the above analysis, it can be concluded that when chiral molecules are involved as reactants, the CISS effect becomes very prominent when the two molecules are in close proximity. However, when the two molecules are relatively far apart, the manifestation of the CISS effect will be less significant.
3.4. Spin Theoretical Analysis and Verification of L-DOPA and D-DOPA
Based on the theoretical analysis in
Section 3.3, it can be inferred that L-DOPA carries more electrons with spin oriented downward, while D-DOPA carries more electrons with spin oriented upward. To validate this inference, we employed the CASTEP [
22] computational package to calculate the electronic spin state distribution of these two molecules. In order to achieve higher accuracy in the calculations, we considered the spin-orbit interaction of electrons. However, it should be noted that CASTEP supports calculations only for crystal cell structures.
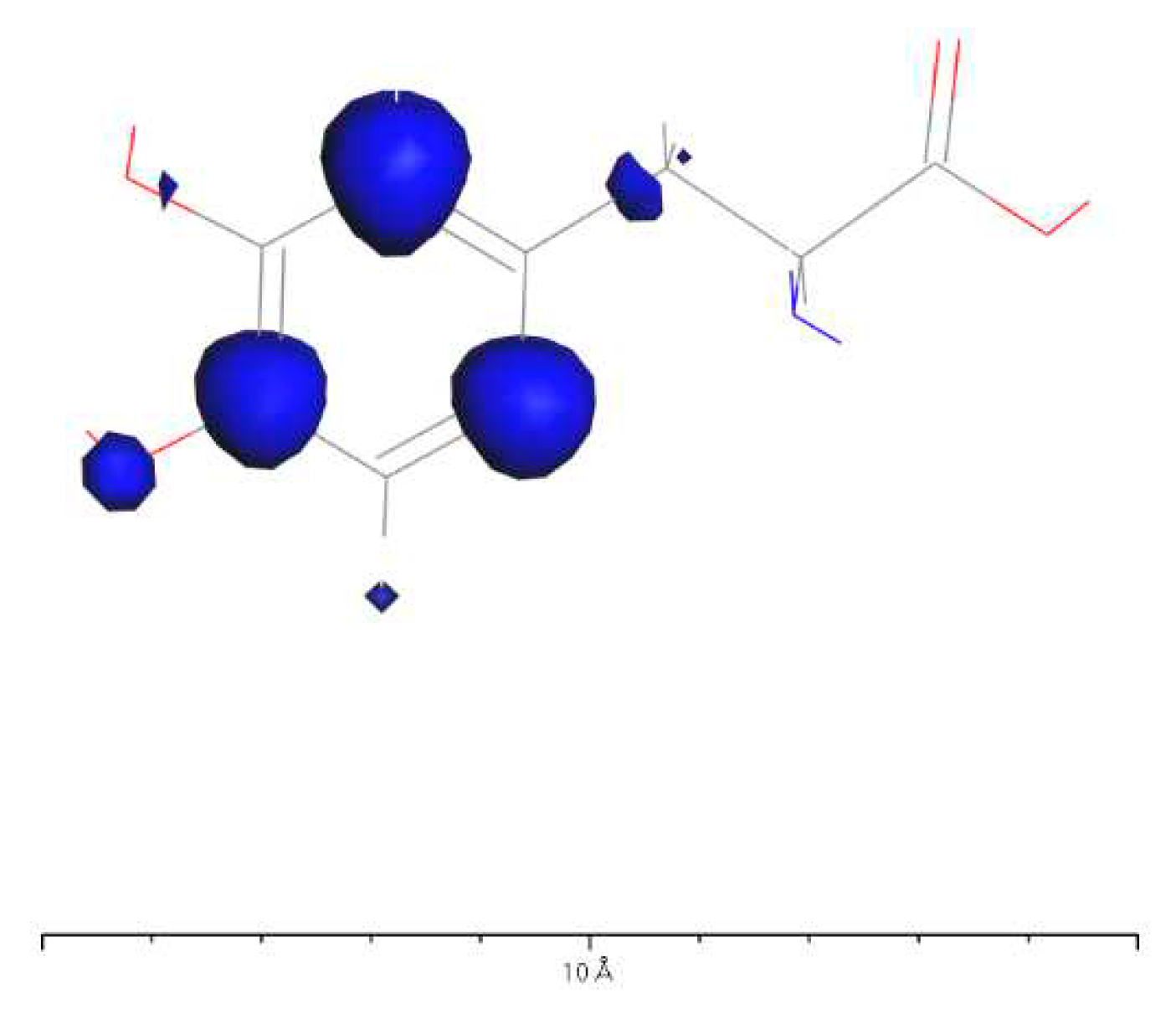
As illustrated in
Figure 31, we placed L-DOPA and D-DOPA molecules in sufficiently large crystal cells, ensuring that the intermolecular interactions can be neglected during the computations.
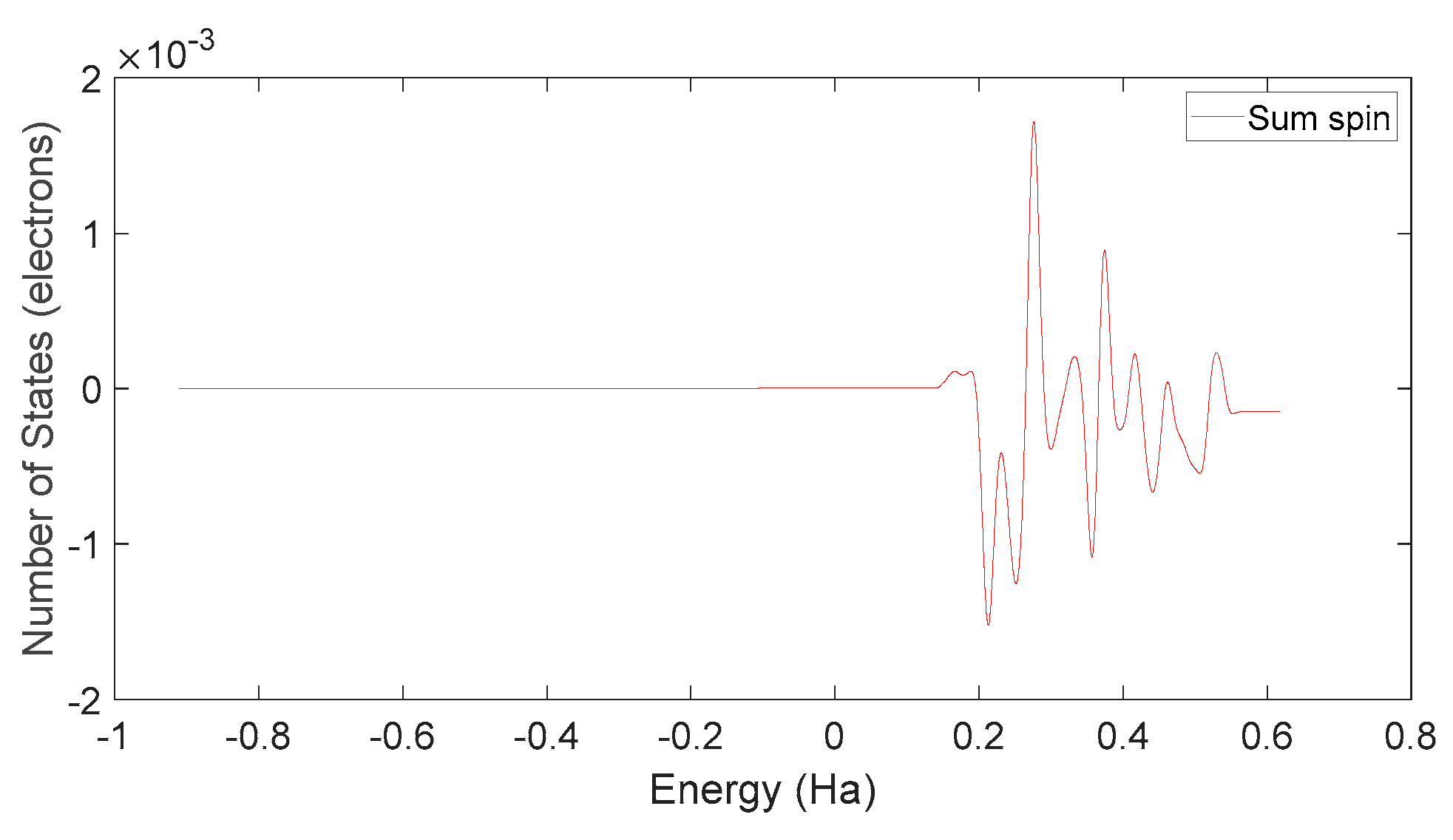
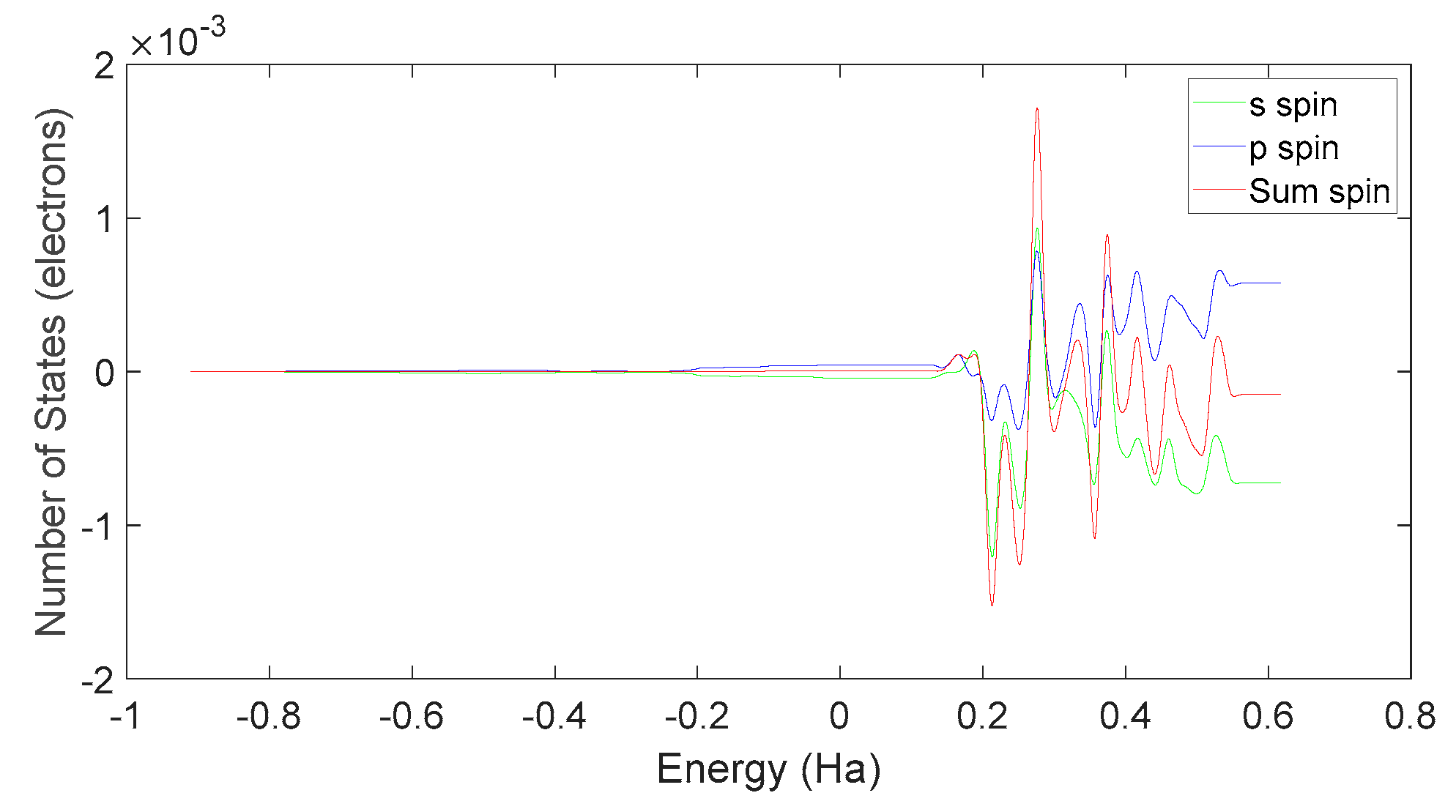
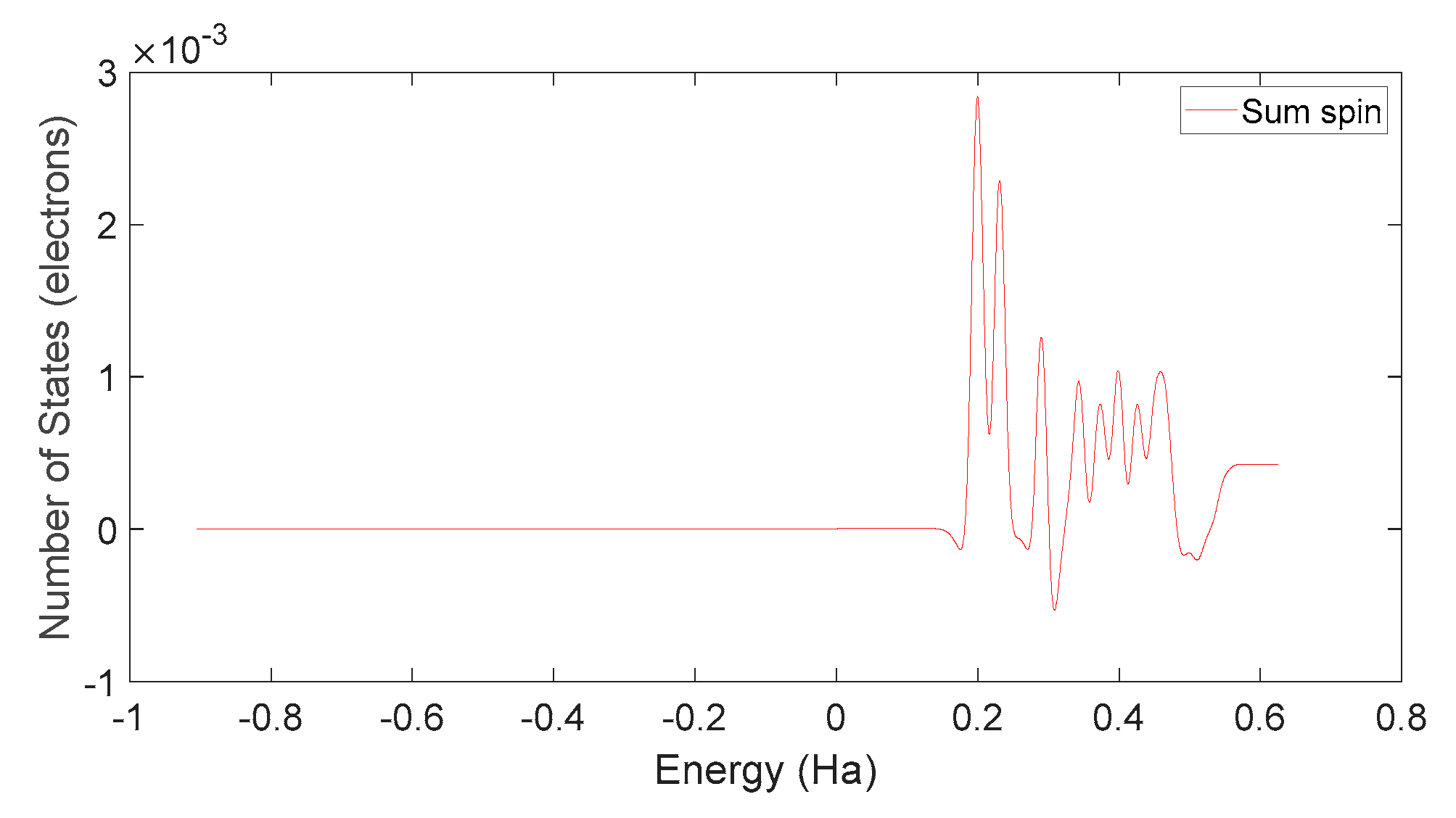
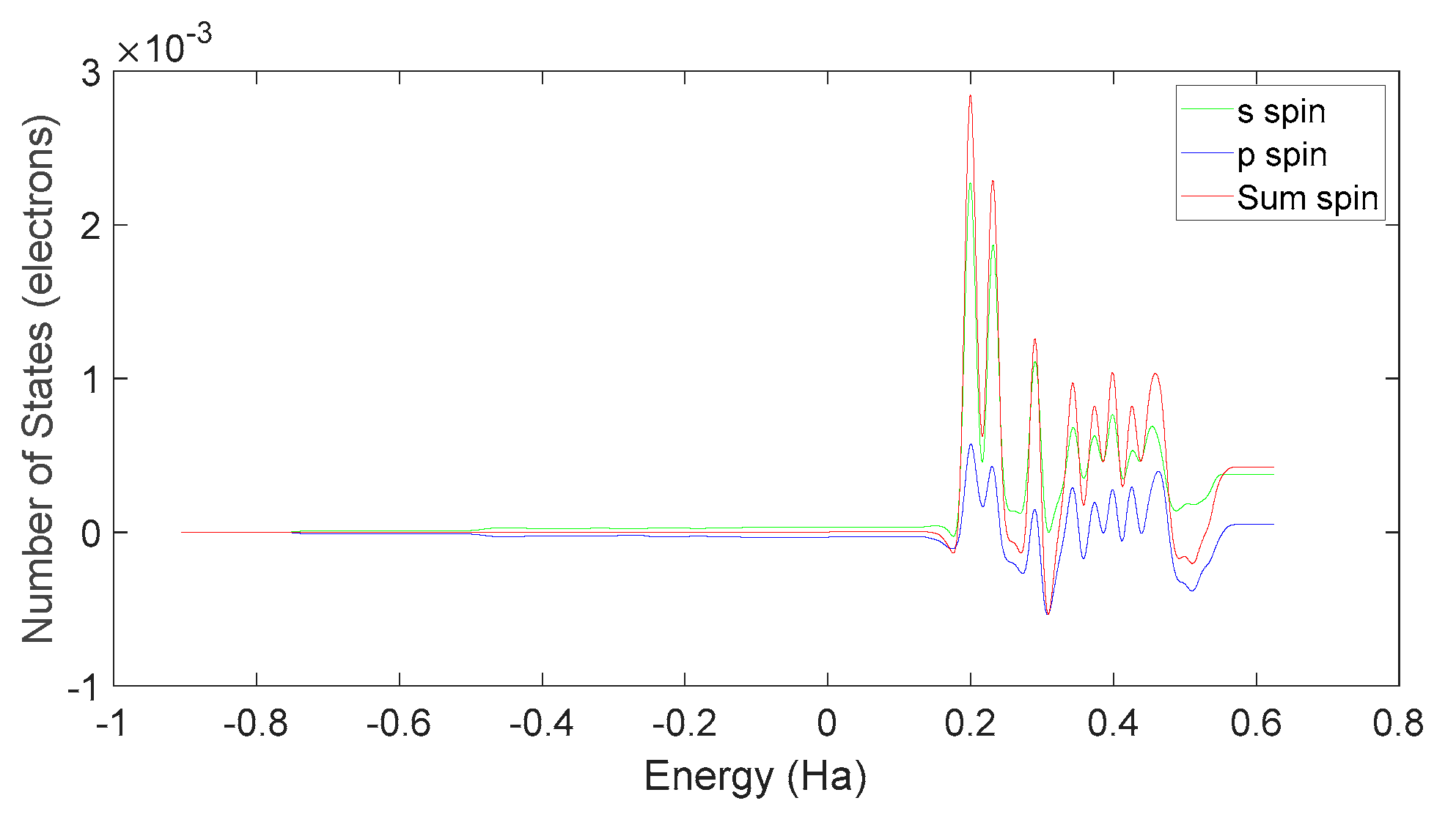
Based on
Figure 34 and
Figure 37, it can be observed that in L-DOPA molecules, the majority of higher-energy electrons have a spin orientation predominantly downward, while in D-DOPA molecules, the majority of higher-energy electrons have a spin orientation predominantly upward. This indicates that in L-DOPA molecules, the majority of valence electrons have a spin orientation downward, whereas in D-DOPA molecules, the majority of valence electrons have a spin orientation upward.
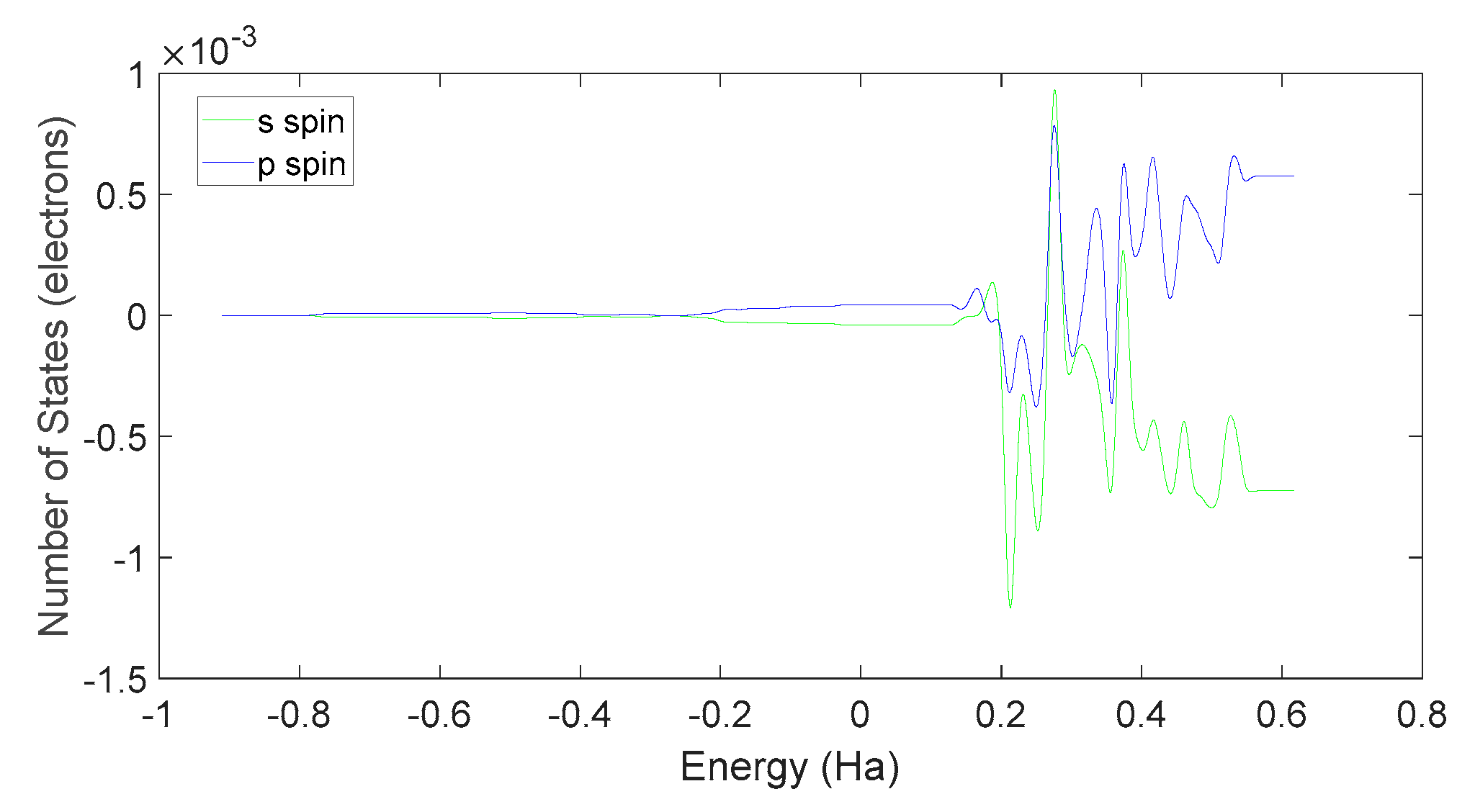
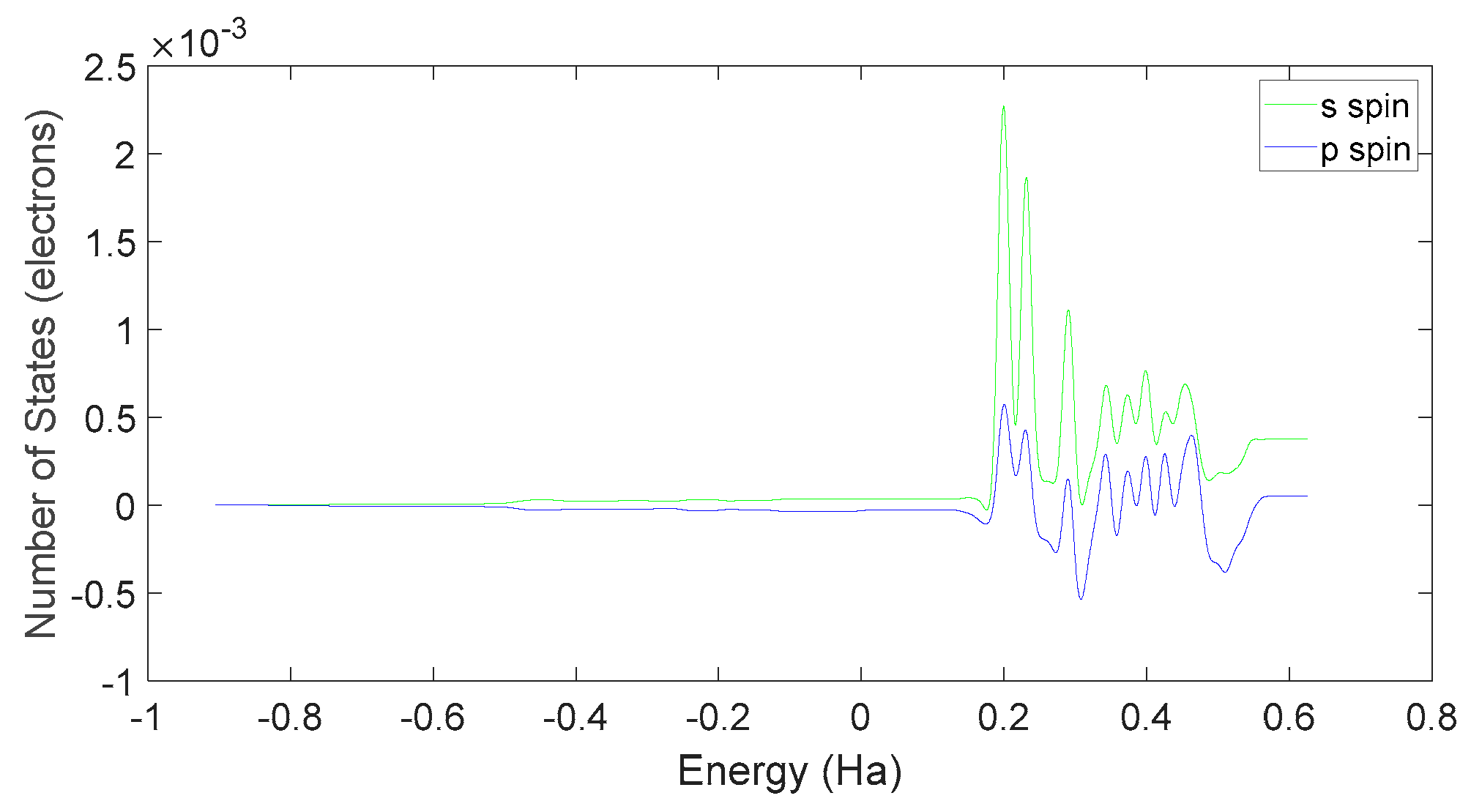
Further examination on
Figure 35 and
Figure 38 reveals that the downward spin orientation of valence electrons in L-DOPA molecules is mainly contributed by the P orbital, whereas the upward spin orientation of valence electrons in D-DOPA molecules is jointly provided by the S and P orbitals. As demonstrated in
Figure 32 and
Figure 33, the atoms primarily responsible for providing the spin-polarized electrons are oxygen and carbon atoms. Among these, the oxygen atom identified in
Section 3.3.1 using Fukui indices analysis is included. The difference in electron spin orientations between L-DOPA and D-DOPA molecules is also consistent with the optical rotation of chiral substances.
Figure 32.
Spin electron distribution in L-DOPA molecule.
Figure 32.
Spin electron distribution in L-DOPA molecule.
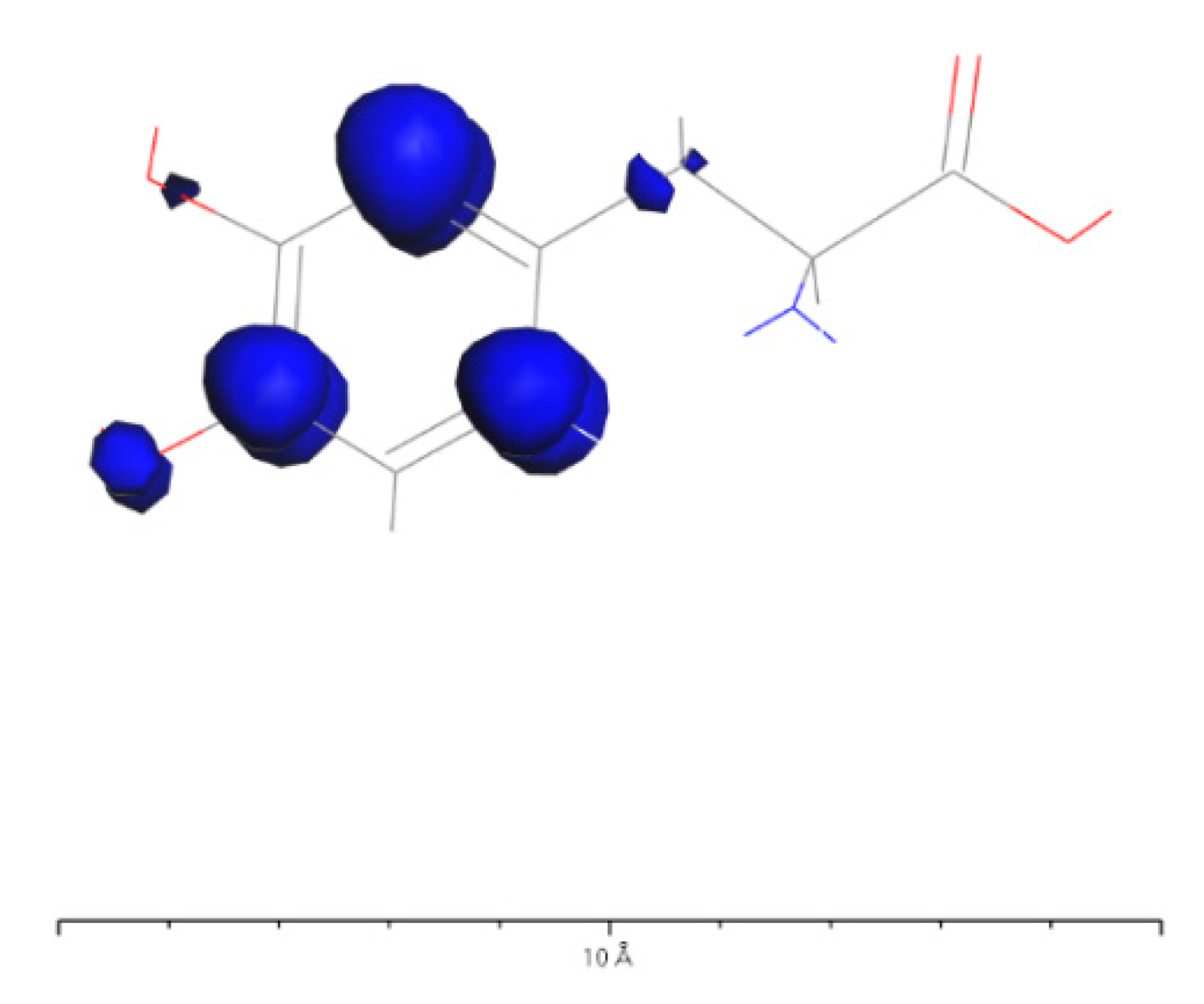
Figure 33.
Spin electron distribution in D-DOPA molecule.
Figure 33.
Spin electron distribution in D-DOPA molecule.
Figure 34.
Total spin state density in L-DOPA.
Figure 34.
Total spin state density in L-DOPA.
Figure 35.
Spin state density of S orbital and P orbital in L-DOPA.
Figure 35.
Spin state density of S orbital and P orbital in L-DOPA.
Figure 36.
Electron spin state density in L-DOPA.
Figure 36.
Electron spin state density in L-DOPA.
Figure 37.
Total spin state density in D-DOPA.
Figure 37.
Total spin state density in D-DOPA.
Figure 38.
Spin state density of S orbital and P orbital in D-DOPA.
Figure 38.
Spin state density of S orbital and P orbital in D-DOPA.
Figure 39.
Electron spin state density in D-DOPA.
Figure 39.
Electron spin state density in D-DOPA.
As is well-known in the field of chemistry, chiral molecules often exhibit optical rotation. Currently, there are numerous theories explaining optical rotation [
23]. When a solution of L-DOPA is irradiated with polarized light, the plane of polarization of light will rotate in the left-handed direction, whereas when a solution of D-DOPA is irradiated with polarized light, the plane of polarization will rotate in the right-handed direction.
This optical rotation does not require an external magnetic field, indicating that it is unrelated to the splitting of energy levels in a magnetic field. A beam of polarized light can be considered as a superposition of left-handed polarized light and right-handed polarized light. When the frequencies and intensities of the left-handed and right-handed polarized light are the same, the plane of polarization of the resulting beam of polarized light will remain unchanged. The optical rotation observed in L-DOPA and D-DOPA molecules are attributed to their different abilities to absorb left-handed and right-handed polarized light.
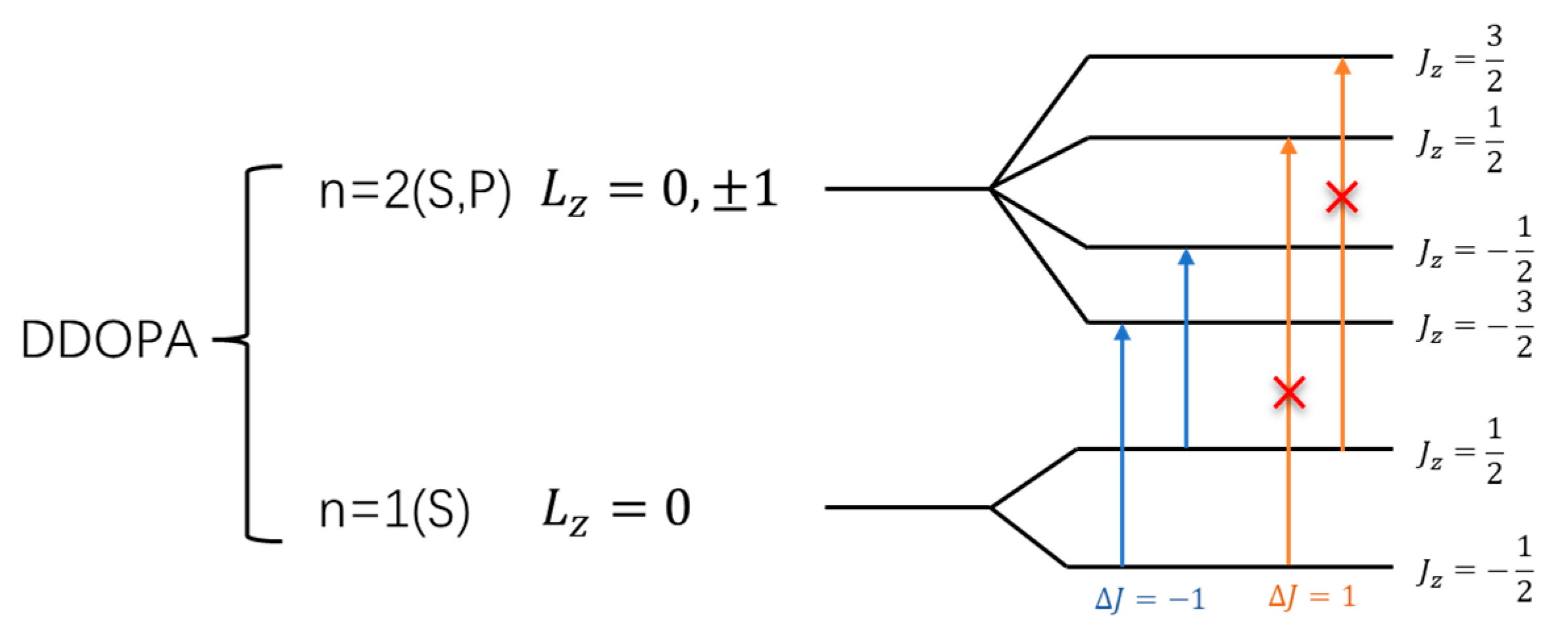
In both L-DOPA and D-DOPA molecules, the atoms with relatively high atomic numbers are oxygen and carbon. The electronic configurations of oxygen and carbon atoms are as follows: Oxygen -
, Carbon -
. As a result, their orbital energy levels can be depicted as shown in
Figure 40 and 41. The electrons in atoms possess orbital angular momentum
, which, when coupled with spin
, gives rise to the total angular momentum
. This results in the energy level diagram shown in the figure. It is worth noting that the coupled energy levels in the figure are shown together in the molecule since there is no external magnetic field to induce the Zeeman effect. Therefore, the energy levels depicted in
Figure 40 and 41 are not separated by differences in energy levels.
When falling on the atoms, the light that satisfies the energy level difference will be absorbed. Although electronic transitions can occur between energy levels with the same principal quantum number, these transitions do not absorb light due to their small energy differences. Thus, light absorption transitions occur between energy levels with different principal quantum numbers, specifically when electrons transition from the n=1 level to the n=2 level, as depicted in the figure.
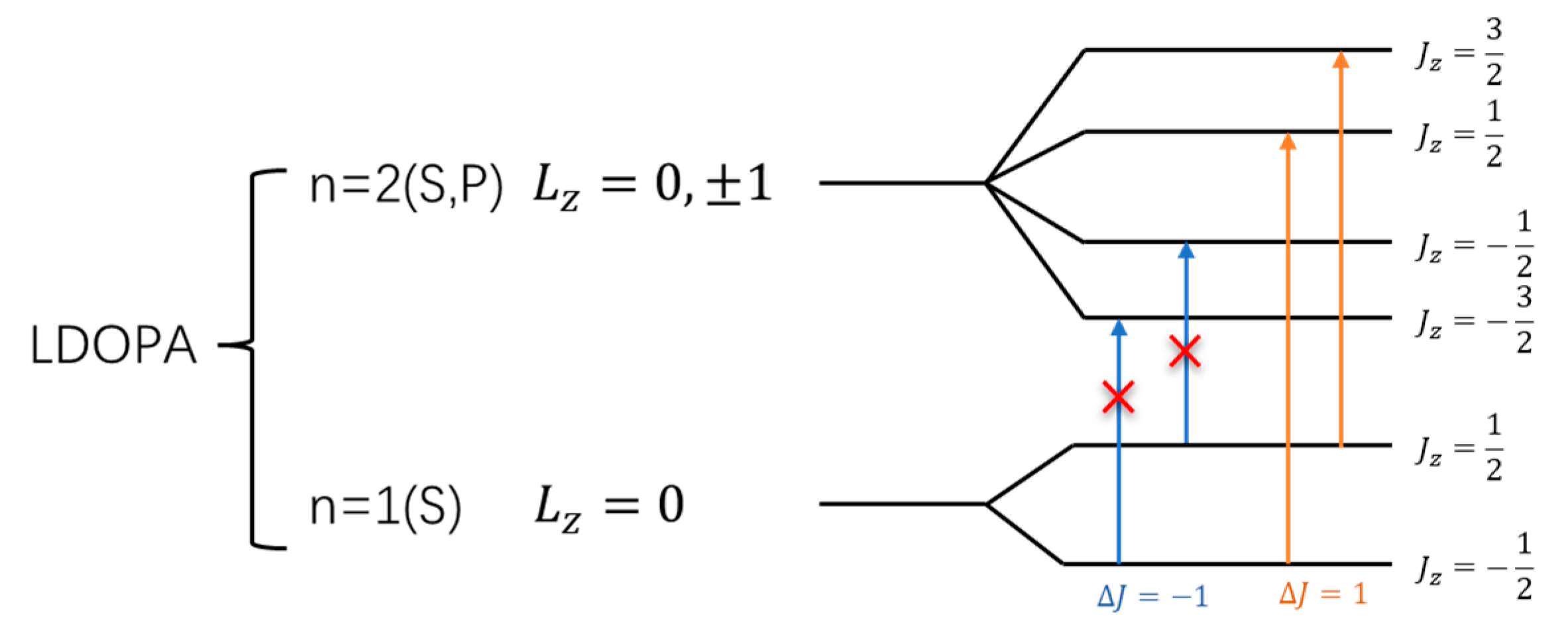
However, when electrons interact with photons, they must satisfy the conservation of angular momentum. Therefore, after absorbing right-circularly polarized light, electrons will undergo transitions with
, as indicated by the orange arrows in
Figure 40 and 41. Similarly, after absorbing left-circularly polarized light, electrons will undergo transitions with
, as indicated by the blue arrows in
Figure 40 and 41.
When the total electron spin on the atomic energy level is 0, the likelihood of absorbing left-circularly polarized light is the same as that of absorbing right-circularly polarized light. Based on the analysis in
Figure 34,
Figure 35,
Figure 36,
Figure 37,
Figure 38 and
Figure 39, as shown in
Figure 40, most of the spin-down valence electrons in the L-DOPA molecule occupy states with
and
. Similarly, as shown in
Figure 41, most of the spin-up valence electrons in the D-DOPA molecule occupy states with
and
. Given the Pauli exclusion principle, these already occupied states cannot accommodate more electrons, and thus, these transitions cannot occur. Consequently, the transitions with red crosses on the arrows in
Figure 40 and 41 are less likely to occur for these molecules.
From the figures, it can be observed that L-DOPA molecules are more inclined to undergo transitions with , which means they are more likely to absorb right-circularly polarized light. On the other hand, D-DOPA molecules are more prone to undergo transitions with , making them more likely to absorb left-circularly polarized light.
By and large, the preferential absorption of right-circularly polarized light by L-DOPA molecules leads to a reduction in the intensity of right-circularly polarized light after transmission, causing the polarization plane of light to rotate to the left (left-handed rotation). Conversely, D-DOPA causes the polarization plane of light to rotate to the right (right-handed rotation). This phenomenon also explains the origins of their names.
Figure 32 reveals that most of the electrons with spin states in L-DOPA molecules are distributed on the benzene ring. This indicates that before the decarboxylation is completed to form a covalent bond, the benzene ring of L-DOPA molecules will be fixed on the AADC enzyme molecule. This can be likened to securing a patient to an operating table during surgery. Once the L-DOPA molecule is firmly fixed, PLP completes the decarboxylation with the exposed carboxyl group. After that, the L-DOPA molecule transforms into dopamine and the electron spin structure changes. Consequently, the dopamine molecule dissociates from AADC and goes on to exert its effects on other neurotransmitters. This is analogous to a patient recovering after surgery and leaving the hospital without turning back.
Based on the analysis above, it can be fully demonstrated that the chirality selectivity of enzyme molecules for the two chiral forms of dopamine is due to the different electron spin states they carry, which substantiates the theoretical analysis in
Section 3.3.