1. Introduction
Biological parameters extracted from electrical signals from the nervous system, muscles, heart, and other parts of the body have been used for years to analyze the human body and its behavior. In addition, electrical signals from cancer cell lines, normal cells and viruses, among others, have been widely used for the detection of various diseases. Single-cell parameters such as cellular and cytoplasmic conductivity, relaxation frequency, and membrane capacitance are important. The physiological state of cells can change in response to stimulation, and therefore, the health state of cells can be characterized by measuring some specific cell markers [
1]. Electrical parameters belong to the original markers of cell markers, which can identify diseases, make early diagnosis and prevention. The electrical properties of cells are often used to describe cell viability, growth and identification, and these properties are closely related to cell structure and chemical composition [
2]. Therefore, it is possible to obtain the dielectric properties of cells by quantitative analysis of their electrical parameters. The electrical properties of cells can provide key data that can provide insight into their complex physiological state. Electrical properties are important in cell quantification, isolation, capture, and single-cell characterization studies. Single-cell electrical manipulation can safely manipulate cells without causing harm, and analytical and numerical polarization models resulting from electric fields can be used to describe and characterize the dielectric electrophoretic behavior of cells [
3]. Single-cell analysis enables the manipulation of individual cells to specific spatial regions for single-cell analysis, as well as the ability to isolate and characterize rare cells. In addition to the detection of membrane, cytoplasm and nuclear antigens, single-cell analysis can also detect whole cells and cellular components, such as organelles, nuclei, DNA, RNA, chromosomes, cytokines, hormones, and protein content, which can also be studied by single-cell manipulation. Single-cell analysis also enables further exploration of cell proliferation and cell cycle [
4].
There are many techniques that can be used to characterize biomaterials, such as scanning tunneling microscopy (STM), nanotechnology, microstrip cavity resonance measurement, dielectric impedance measurement (DEPIM), etc. Cellular techniques can be divided into single cell analysis and cell identification. Single-cell analysis uses patch clamp and nanoprobes, and the latest technologies include microfluidic, micro electrical impedance spectroscopy and electrical rotation.
Most of the current articles are limited to the introduction of specific single-cell sequencing methods, and there is no systematic review of the various operations and applications of single-cell analysis. In this review, we have reviewed the methods for single cell sorting and isolation and discuss their advantages and disadvantages and possible application scenarios. This article next reviewed single-cell operations, several methods for extracting electrical parameters are summarized, and their structural characteristics and application fields are introduced. Next, this article illustrates the specific analysis and application of single-cell sequencing. Finally, we summarize the current shortcomings and problems of single-cell sequencing, provide solutions, and tentatively give future application directions and prospects.
2. Single Cell Sorting and Separation
In general, the biological samples used for testing are often a mixture of numerous normal and unimportant cells, with only a small proportion of cells of real research and reference value. Overall analysis of biological samples provides only average information on mixed cell populations, while a small proportion of significant cell subpopulation information would be lost in the background. The single cell sorting technique allows for the precise selection of certain cells of greater value from a mixed population of multiple cell types and amounts, greatly facilitating the subsequent single cell analysis for this cell. The selection of the sorting method depends to a large extent on the sample to be separated and the subsequent analytical operations to be performed. Common methods are micropipette isolation, the separation and sorting system, the cell selector sorting system, DEPArray sorting system and the microfluidics.
Micropipette isolation is an earlier technique for single cell isolation, which is conducted under a high-power microscope using micromechanical manipulators or visual tweezers [
5]. This method is low-cost, accurate and can effectively control the selection, transfer, and release of target cells, but it is time-consuming, has low throughput and easy to cause mechanical damage to target cells. This method is suitable for isolating a small number of target cells from the whole cell population [
6].
As the study of living cells is more valuable, it is important to maintain the physiological activity of the cells in single cell analysis. As the application of single cell isolation techniques has expanded further, the use of micropipette isolation methods, which can cause damage to cells, has declined and techniques that can be performed non-destructively are becoming more widely used. The separation and sorting system (dual electrophoretic array system) is a semi-automatic sorting system that separates rare cells from the mixed cell population by fluorescent labeling and places the sorted and captured target cells in appropriate locations for subsequent sequencing analysis [
7]. The DEPArray sorting system (Di-Electro-Phoretic Array system) is also a semiautomatic sorting system which separates rare cells from a mixed cell sample. Single cells are captured by the microelectrodes arranged on the chip. Then the microelectrodes are controlled to move the target cells to a certain position on the chip and transfer them to suitable media to complete following sequencing analysis. The cell selector sorting system automatically separates rare cells from mixed cell populations using a multifunctional robotic system. It automatically searches and realizes single cell sorting, and mechanically separates target cells or clones directly without affecting cell vitality. It can conduct cell sorting by observing cell images in real time with high precision [
8]. However, the technologies mentioned have the shared disadvantages of the are time consuming and small sample. Therefore, the samples always need to be divided and enriched before they could be input to the system [
9].
Technologies of microfluidics provide alternative pathways in single cell analysis with larger sample scale and higher efficiency. Microfluidics can complete single cell sorting, cleavage, and amplification, and has the characteristics of high throughput, small reaction volume, less pollution and little influence on sequencing. However, the disadvantages of high cost and low capture rate for viscous and non-spherical cells are also unmissable [
10]. Among the microfluidic technologies, the droplet microfluidic method is gaining increasing attention, which is widely used in many different circumstances. This approach uses many droplets formed by reagents in water-in-oil. Every individual mixed droplet is an independent reaction unit, different droplets are separated and connected by continuous flow of inert oil. Therefore, the chemical reactions of different droplets are isolated. There would be no interference among droplets [
11]. The advantage of this technology is that single cell operations including lysis, separation and extraction can be easily performed in droplets. Such operations also have a high throughput. However, there are currently some bottlenecks in droplet microfluidics, as processes such as washing, and buffer exchange are still difficult to implement on a large scale [
12].
Besides, valve devices are also commonly used in microfluidic technologies. The valves connect microfluidic chambers, which play an important role in flow control and cell confinement [
13]. These valves make it easy to add and remove reagents, capture, mix, split, lyse and analytes to be analyzed in different reaction chambers for a range of different analyses and manipulations. Furthermore, as such operations are performed at the single cell level, each cell can be precisely selected for analysis. In addition, the opening and closing of the valves can be controlled automatically by a computer programs, thus greatly increasing the automation scale of the analysis work and saving time and labor costs [
14].
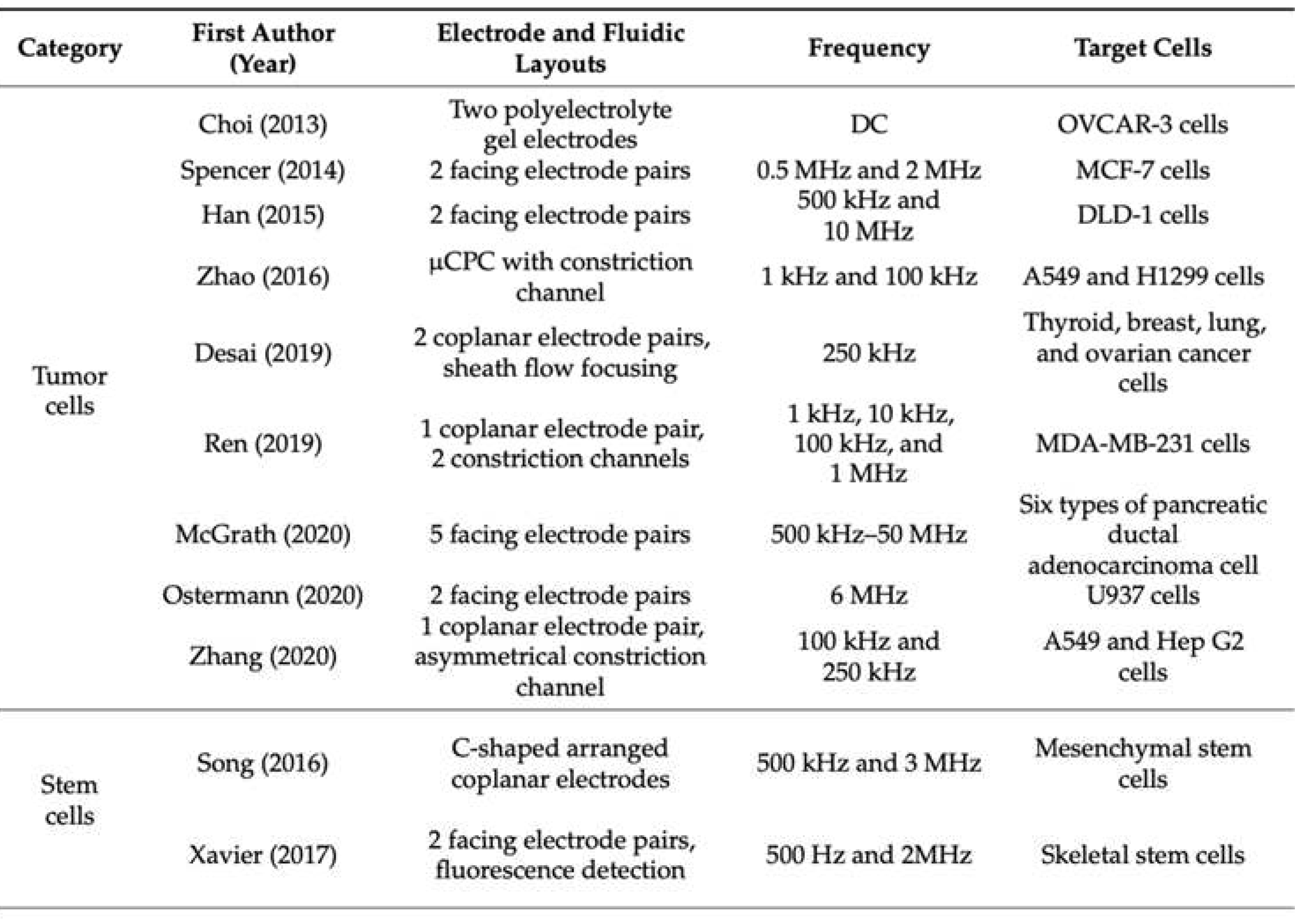
Table 1.
An aggregation of recent advances of cell electrical impedance research.
Table 1.
An aggregation of recent advances of cell electrical impedance research.
3. Single Cell Manipulation
After a specific single cell is isolated, it needs to be manipulated by a specific method to obtain the interesting biological parameters. At the single cell level, many cell characteristics are of interest to study [
15]. For instance, there are no two cells from a shared genetic group that are totally the same. Such differences are expressed as phenotype heterogeneity at the single-cell level, which is essential for accurate explaining of diagnostic and treatment results of diseases [
16]. The heterogeneity among genetically identical cells also plays an important role in the analysis of cancer metastasis [
17], drug resistance [
18] and stem cell differentiation [
19]. Methods for implementing electrical impedance measurements in microfluidic devices generally include Electrical impedance sensing (EIS), Impedance flow cytometry (IFC), Cell-based electrical impedance (CEI), microelectron-mechanical systems (MEMS) and Integrated microelectrode array (IMA).
The common feature of single-cell analysis technology is to try to achieve high throughput and label-free without damaging the cells. In general, fluorescent labelling techniques require many sample preparation steps and the removal of the labelled dye are always indispensable before subsequent processing can take place, whereas labelless methods eliminate these steps. However, it is important to note that although label-free cell isolation methods based on intrinsic characteristics such as cell-cell morphology, size and deformability can provide information of cell phenotype, these methods are inferior in specificity and sensitivity to the fluorescent antibody-labelled methods used in conventional stream cytometry [
20]. Therefore, although classic technologies such as patch clamp and fluorescent probes are quite effective tools, some new approaches such as EIS and IMA are in some ways superior.
EIS is a powerful tool that allows rapid, noninvasive, and label-free access to the electrical parameters of single cells. The electrical parameters of single cells, including equivalent cell resistance, membrane capacitance, and cytoplasmic conductivity, are closely related to biophysical properties and dynamic activities of cells such as size, morphology, membrane integrity, growth state, and proliferation [
21]. With miniaturization, low cost, geometric size comparable to cell size, and flexible structure design, microfluidic technology is a powerful tool for single-cell analysis, providing operation and analysis methods at the single-cell level [
22]. EIS sensing is used to select the most sensitive frequency for subsequent high-speed analysis or long-term monitoring of cell behavior and phenotype. The measured EIS can characterize various cellular physiological processes such as adhesion, growth, division, differentiation, proliferation, and cellular structure formation. The electrical impedance properties of single cells can reveal the complex physiological state of cells [
23]. Single-cell electrical impedance-based biosensors can detect a variety of biological parameters and do not rely on fluorescent labeling. At lower frequencies (100 KHZ to 1 MHz) electrical impedance refers to the size information of the cell, at higher frequencies (about a few MHz) electrical impedance refers to the membrane capacitance of the cell, and at higher frequencies electrical impedance refers to the conductance of intracellular organelles of the cell [
24]. To accurately classify different cell subsets, it is necessary to use the combination of multi-frequency impedance signals and a variety of biophysical parameters to enrich the characteristic information of different cells, which is conducive to improving the phenotypic resolution [
25]. Electrical impedance integrated microfluidic devices have been widely used for cell-based single-cell analysis.
Integrated microelectrode array (IMA) biosensors could also be explored in single cells analysis. The micro-processing and single-cell level operation of IMA chips are realized by surface chemical modification of IMA chips. Important sensing parameters were identified, including specific cell membrane capacity, cell membrane resistivity, and cell-substrate average separation. Analysis of the frequency dependent properties of the single cell covered microelectrode impedance and the IMA sensor circuit response revealed a frequency band in which the electrical properties of single cells can be determined for cellular biosensing applications [
26].
IFC is a high-throughput single-cell analysis method with miniaturization, low peripheral requirements, and flexible integration of query units [
27]. IFC measures the change of the response current caused by a single cell passing through the schema-shaped electrode in the microfluidic channel. The distribution of the AC electric field in the channel determines the sensitivity of the IFC device, so the electrode configuration should be concerned [
28].
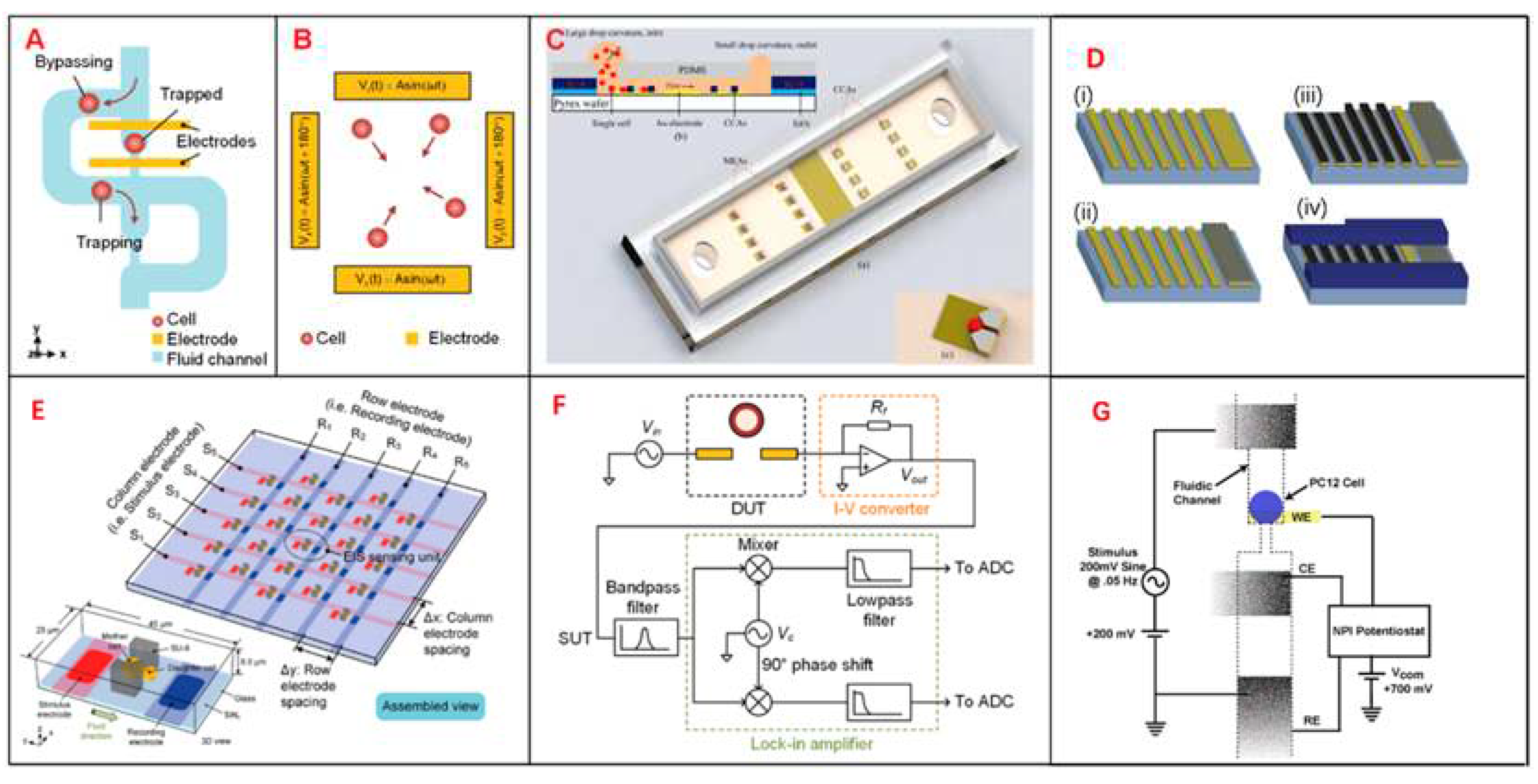
Figure 1.
Different designs of EIS devices, schematics of single-cell EIS sensing system, multilayer microelectromechanical system (MEMS) device for Single-Cell Electric Impedance Spectroscopy and Electrochemical Analysis, IFC devices analysis for tumor. (A) Schematics of μ-fluidic traps, which can immobilize single cells with a cellular EIS sensing device. (B) A quadrupole electric device to gather cells towards the center. (C) A 3D single-cell culturing device, which can reveal HeLa cell migration. (D) The production process of MEMS. (i) The first layer, deposit and arrange the Ti/Au layer. (ii) The second layer, sputter deposit Ag layer and chloride. (iii) Electroplate platinum black to select electrodes. (iv) Spin SU-8 2010 to a ∼12-μm thickness and subsequently arrange it. (E) A microelectrode array device to fix single cell for EIS measurement. (F) Schematics of single-cell impedance sensing system with lock-in amplifier. (G) A circuit model to analyze the release of neurotransmitter of single PC12 cell during electrical stimulation simultaneously.
Figure 1.
Different designs of EIS devices, schematics of single-cell EIS sensing system, multilayer microelectromechanical system (MEMS) device for Single-Cell Electric Impedance Spectroscopy and Electrochemical Analysis, IFC devices analysis for tumor. (A) Schematics of μ-fluidic traps, which can immobilize single cells with a cellular EIS sensing device. (B) A quadrupole electric device to gather cells towards the center. (C) A 3D single-cell culturing device, which can reveal HeLa cell migration. (D) The production process of MEMS. (i) The first layer, deposit and arrange the Ti/Au layer. (ii) The second layer, sputter deposit Ag layer and chloride. (iii) Electroplate platinum black to select electrodes. (iv) Spin SU-8 2010 to a ∼12-μm thickness and subsequently arrange it. (E) A microelectrode array device to fix single cell for EIS measurement. (F) Schematics of single-cell impedance sensing system with lock-in amplifier. (G) A circuit model to analyze the release of neurotransmitter of single PC12 cell during electrical stimulation simultaneously.
In single-cell studies targeting biological processes, the real-time nature of the measurement results is of great importantance. Although EIS, IFC and other methods are label-free, high-throughput and unharmful to cells, their measurement results are not as good as the CEI method in real time analysis. CEI biosensors have been extensively explored, and the technique does not require cellular manipulation and provides real-time dynamic measurements of receptor-mediated cellular changes. An electro biosensor measures the impedance of a cell grown on a surface embedded with a electrode when the cell is exposed to an electric field generated by a continuous sweep of an AC voltage over a range of frequencies. Impedance depends on the number, size, and shape of cells on the electrode surface, the distance between cells and the electrode surface, and cell-cell contacts [
29]. CEI assays provide more comprehensive efficacy predictions than many other assays since both activating signaling events and down-regulation events contribute to the overall CEI response. Meanwhile, due to the comprehensive nature of the detected response, CEI can also detect biased compounds and monitor cellular toxicity of compounds in real-time [
30]. Screening with CEI has less bias because the readings are not concentrated in one pathway. CEI assays can be used for initial screening of known targets, especially smaller compound libraries, and for understudied targets where downstream signaling pathways are still unclear [
29].
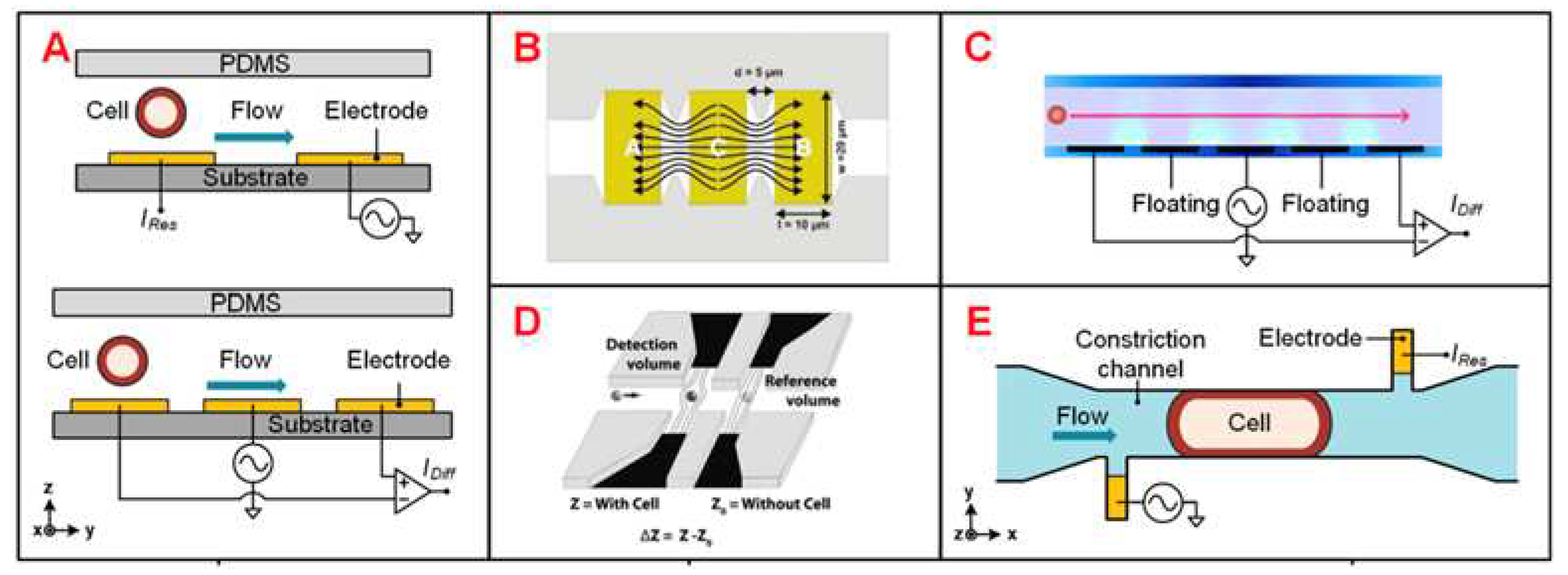
Figure 2.
Different IFC devices design. (A) Schematics of coplanar electrode configuration for absolute and differential measurement. (B) The coplanar electrodes, which has larger electrode area exposed to the medium. (C) Coplanar electrodes with two extra floating electrodes. (D) Electrodes of liquid material. (E) Asymmetrical liquid electrodes, with the constriction channel in which cells flow.
Figure 2.
Different IFC devices design. (A) Schematics of coplanar electrode configuration for absolute and differential measurement. (B) The coplanar electrodes, which has larger electrode area exposed to the medium. (C) Coplanar electrodes with two extra floating electrodes. (D) Electrodes of liquid material. (E) Asymmetrical liquid electrodes, with the constriction channel in which cells flow.
On the other hand, microfluidic technology also has diverse applications in single cell manipulation. In recent years researchers have placed increasing emphasis on the efficiency of single-cell manipulation. A higher degree of automation is therefore becoming increasingly important for single-cell manipulation techniques. MEMS is one typical example. MEMS technology, in particular, enables the study of individual cells as simplified semi-automated high-throughput methods. With the rapid development of MEMS technology, a number of micro-scale devices have already been constructed for bioanalysis at single-cell scale analysis. In some cases, MEMS methods have inherent scaling advantages [
31] and have been used to study various cell types, including red blood cells [
32], lymphocytes, and others.
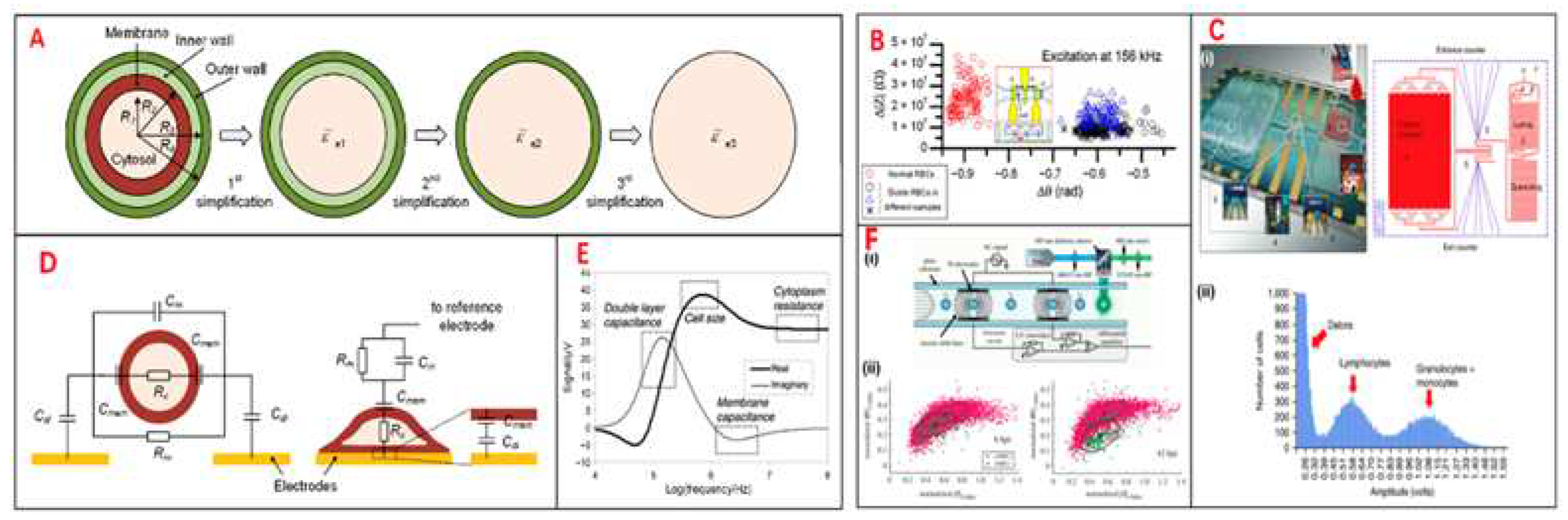
Figure 3.
Electrical impedance sensing technique for single-cell analysis. (A) Electrical model and equivalent circuit models (ECMs) of a single cell. The mode is simplified to a homogeneous sphere. (B) Measurement of electric impedance for normal and sickle RBCs at 156 kHz. (C) (i) Pictures of immunocapture biochip. (ii) Pulse amplitudes of impedance signals which shows the cell size of distribution of cells. (D) The cell mode could be suspended between a pair of sensors and adhered on a sensor. (E) Simulation results of an ECM model, which shows different frequency domains related to cell parameters. (F) Schematics of IFC device. Measurement of impedance of infected and uninfected RBCs in 6 and 42h.
Figure 3.
Electrical impedance sensing technique for single-cell analysis. (A) Electrical model and equivalent circuit models (ECMs) of a single cell. The mode is simplified to a homogeneous sphere. (B) Measurement of electric impedance for normal and sickle RBCs at 156 kHz. (C) (i) Pictures of immunocapture biochip. (ii) Pulse amplitudes of impedance signals which shows the cell size of distribution of cells. (D) The cell mode could be suspended between a pair of sensors and adhered on a sensor. (E) Simulation results of an ECM model, which shows different frequency domains related to cell parameters. (F) Schematics of IFC device. Measurement of impedance of infected and uninfected RBCs in 6 and 42h.
4. Single Cell Analysis and Application
In general, living cells are usually of greater research interest due to their more significant biological properties and applications. Therefore, whether analysis can be performed while maintaining the activity of the target cells is a hot issue in single cell technology. Most single cell analysis techniques have the advantage of not damaging the cell cytosol. For example, single cell sequencing is a non-invasive method for tumor diagnosis and prognosis prediction. Single cell sequencing can obtain comprehensive information, assist in early diagnosis of tumors, select the best therapeutic drugs, and monitor recurrence. The integrated analysis of single cell sequencing with other omics can also provide valuable information that can promote the development of precision medicine in cancer. Besides, some researchers have combined on-chip oxygen control to a single IFC chip for sickle cell disease diagnosis and monitoring, the living single cell-based analysis results prove to be accurate and detailed [
33].
Single cell technology can also be used in biological tissue biopsies. Circulating tumor cells (CTCS) are tumor cells that originate from epithelial primary or metastatic tumors and enter the bloodstream with high viability and potential for metastasis. CTCS liquid biopsy can monitor tumor progression in real time [
34]. Tumor metastasis is one of the main causes of death in cancer patients. CTCS can provide early warning of tumor heterogeneity and drug resistance and identify the mechanism of tumor occurrence and development [
35]. Current methods for detecting tumor metastasis are mainly based on imaging, but early tumor metastasis is difficult to detect at the cellular level, even when viewed under high-resolution images. However, through high-resolution imaging combined with dynamic monitoring of the number and nature of CTCS, potential clues of tumor lesion metastasis can be found, providing potential for early targeted therapy [
36]. Often, high-throughput sequencing analyses of tumor tissues ignore their heterogeneity because they target mixed samples of millions of cells that only reflect the overall genomic characteristics of the cells, while ignoring genetic material from low-abundance but functionally important cells such as CTCS and cancer stem cells [
37]. The separation of CTCS can be divided into two steps: enrichment and capture. The technology is mainly based on cell surface labeling and microfluidic chips. Enrichment based on cell surface markers generally includes positive and negative selection. This method utilizes antibodies such as anti-epithelial cell adhesion molecules (EpCAM) and cytokeratin (CK) to capture and enrich tumor cells from the epithelium, while leucocyte derived antibodies are utilized to eliminate white blood cells [
38]. Based on the enrichment of microfluidic chips, peripheral blood mononuclear cells (PBMCs) of tumor patients were first isolated according to the biological and physical characteristics of tumor cells. The PBMCs then slowly passed through a microfluidic chip coated with EpCAM antibodies under slow laminar flow control. EpCAM+ cells will be captured and bound to the bottom of the chip, while other lymphocytes will flow out with the fluid [
39]. CTCs are mainly used in the following aspects: to reduce the interference of tumor heterogeneity, and to compare the differences of single-cell genome, transcriptome and epigenome between tumor primary site and metastatic site, peripheral blood CTCs and metastatic lymph nodes [
40]. Single-cell transcriptome sequencing analysis of CTCS can be used to understand the therapeutic effect of tumors, as well as the genetic heterogeneity, evolution, and drug resistance of tumor cells. Surface markers and cell size of CTCS vary from tumor to tumor. Most CTCS are aggregated, which is also a significant feature of tumor stem cells, and the presence of CTCS in peripheral blood of tumor patients can indicate the progression of the tumor [
41]. For rare peripheral blood CTCS detection, traditional single cell sorting methods such as fluorescence activated cell sorting (FACS) are no longer suitable. Instead, micromanipulation, microfluidic technology, and cell selection systems are used.
Single cell technology can also be combined with viral recombinant technology for the precise treatment of human diseases. Studies have demonstrated gene therapy at the single-cell level, presented experimental and computational methods for parallel characterization of recombinant adeno-associated virus (rAAVs) tropism, and enabled safe and precise gene delivery vectors. Recombinant AAVs (rAAVs) are the gene delivery vector of choice for many studies because of their broad viral tropism, ability to transect dividing and nondividing cells, and their ability to ensure long-term transgene expression with stability and persistence [
42]. However, rAAV has poor target specificity and a relatively low therapeutic index for systemic gene therapy [
43], and optimized AAV gene delivery vectors that can be used for cell type-specific delivery are urgently needed [
44]. Single-cell RNA sequencing (scRNA-seq) enables comprehensive analysis of the transcriptome at the entire cell type level. High-throughput single-cell transcriptome analysis can further understand AVV. Studies provided tropism information beyond several AAV variants and beyond the predominant cell types.
Beyond disease research, single-cell sequencing technology can be used to reveal the development of various human tissues such as cortical cells of the nervous system. Single-cell sequencing has been used to analyze single cells in the developing human prefrontal cortex from 8 to 26 weeks of gestation, identifying cell types and subtypes within major categories [
45].
IFC device mentioned above is one of the most widely used single cell analysis techniques. IFC was used to classify two tumor cell lines A549 and H1299 (human lung alveolar-like cell line commonly used in non-small cell lung cancer in respiratory research) according to different cell membrane capacitance and cytoplasmic conductivity [
46]. Other researchers have used IFC to isolate lung cancer DTCs (LC-DTCs) from red blood cells, peripheral blood mononuclear cells (PBMCs) and normal lung cells based on impedance amplitude [
47]. Another investigator developed an IFC device to isolate individual PDAC tumor cells against xenograft [
48]. They found that the phase of impedance signals in six PDAC cells was correlated with specific gene expression. The combination of individual intrinsic bioelectrical markers such as membrane capacitance, cytoplasmic conductivity and cell diameter can significantly improve the classification accuracy of both A459 and HEPG2 tumor cells. Besides, some researchers used a commercial IFC device and found that necrotic and surviving U937 human lymphoma cells could be clearly distinguished based on the phase of the impedance signal [
49]. In addition to its application to research directly related to human, IFC equipment has been widely used for the detection, isolation, and activity analysis of unicellular microorganisms. Studies have demonstrated that IFC devices can detect bacteria based on cell size [
50], can accurately measure the diameter of different bacteria [
45], and can achieve higher sensitivity and detection throughput of bacterial size [
51].
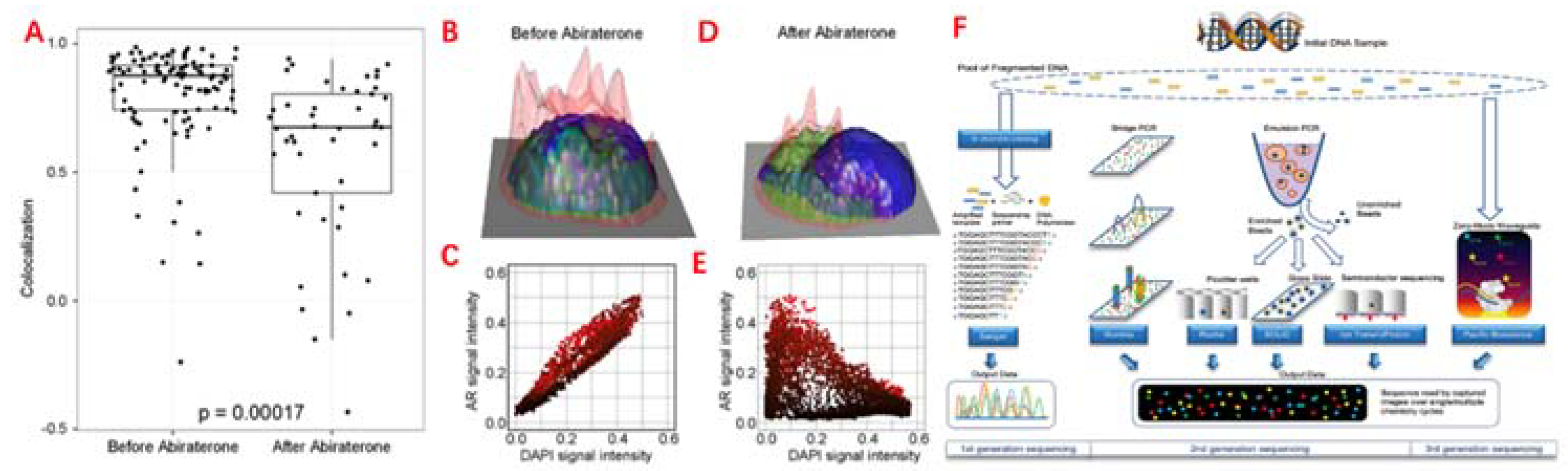
Figure 3.
Single cell analysis applications in CTC. (A) Comparison of the AR subcellular localization in each CTC identified in the blood prior, before and after 9 weeks of abiraterone treatment, which reveals the intracellular colocalization relationship between the AR and DAPI signals of CTC. (B)&(E) Height map of AR signal intensity of single CTC, before and after 9 weeks of treatment. (C)&(E) AR versus DAPI signal intensities for each pixel inside the single CTC, reveal the relationship of nuclear exclusion as negative correlation and nuclear localization as positive correlation. (F) Different sequencing approaches. Technical features include sample preparation, sequencing chemistries, and data output formats.
Figure 3.
Single cell analysis applications in CTC. (A) Comparison of the AR subcellular localization in each CTC identified in the blood prior, before and after 9 weeks of abiraterone treatment, which reveals the intracellular colocalization relationship between the AR and DAPI signals of CTC. (B)&(E) Height map of AR signal intensity of single CTC, before and after 9 weeks of treatment. (C)&(E) AR versus DAPI signal intensities for each pixel inside the single CTC, reveal the relationship of nuclear exclusion as negative correlation and nuclear localization as positive correlation. (F) Different sequencing approaches. Technical features include sample preparation, sequencing chemistries, and data output formats.
5. Conclusions
In this review, we have summarized the methods for single cell sorting and isolation, the specific cell sorting procedures were introduced. This article next reviewed single-cell operations, including impedance flow cytometry, electrical impedance sensing, and microfluidic technology. Next, this article illustrates the specific analysis and application of single-cell sequencing. Single-cell sequencing can analyze a variety of cells including CTCS, LC-DTCs, rAAVs, and can also be used for MEMS, EIS, IMA, and to determine cell types and subtypes. Finally, this article summarizes the current shortcomings and problems of single-cell sequencing, provides solutions, and tentatively gives future application directions and prospects.
The electrical manipulation and application of single cells has been studied for many years, but further developments are needed to demonstrate reliability and efficiency for the continuous separation and purification of particles with high throughput and purity. Single-cell operations and applications require continued adoption of innovations that increase sensitivity while reducing cost, scale, and complexity. How to improve the performance of current single-cell manipulation methods and simultaneously isolate and characterize single cells, and whether to provide a universal platform for medium characterization and single cell isolation, may be the direction of future researchers to focus on. Electrical manipulation and application of single cells involves modeling, designing, and manipulating these intersecting mathematical, physical, and hardware disciplines that are relatively unfamiliar to researchers in the biological sciences [
3]. In addition, many engineers have difficulty preventing cell death in the device when performing experiments because they are not exposed to basic cell biology and do not know how to properly study and maintain healthy cell lines. Therefore, if automated computers can be put into cell electrical analysis, and cell death caused by various reasons can be reduced, the work of various fields can be expanded, and talents in various research fields can cooperate, perhaps the electrical operation, analysis and application of single cells will have a broader prospect.
In the future, researchers might design microchips which could be used for battery electrical performance analysis to improve the potential for single-cell analysis, which is expected to diagnose diseases by rapidly characterizing cell electrical properties. Researchers can also explore more efficient fluorescent dyes to develop such as quantum dots and high-tech flow cytometers, including spectroscopic and microfluidic flow cytometers [
4]. In general, the electrical manipulation of single cells plays an extremely important role in driving the booming drug development business. As the research progresses, more single cell manipulation methods will be developed and applied in practice. It is hoped that this review will provide new ideas and references for scholars to make the cause of human health flourish.
Author Contributions
Conceptualization, W.Z., J.W., H.L., S.L., and X.L.; writing—original draft writing, W.Z., J.W., X.L., and B.L.; writing—review and editing, W.Z., J.W., H.L., X.L., C.L., and S.L.; supervision, C.L. and S.L. All authors have read and agreed to the published version of the manuscript.