Submitted:
31 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strains, Materials and Culture
2.2. Passage Culture and Viable Count
2.2.1. Solid-Liquid-Liquid Circulation Subculture
2.2.2. Solid-Liquid-Liquid Passage Culture
2.2.3. Solid-Liquid-Liquid Continuous Passage Culture
2.2.4. Determination of Cell Concentration
2.3. Data Analysis
3. Results
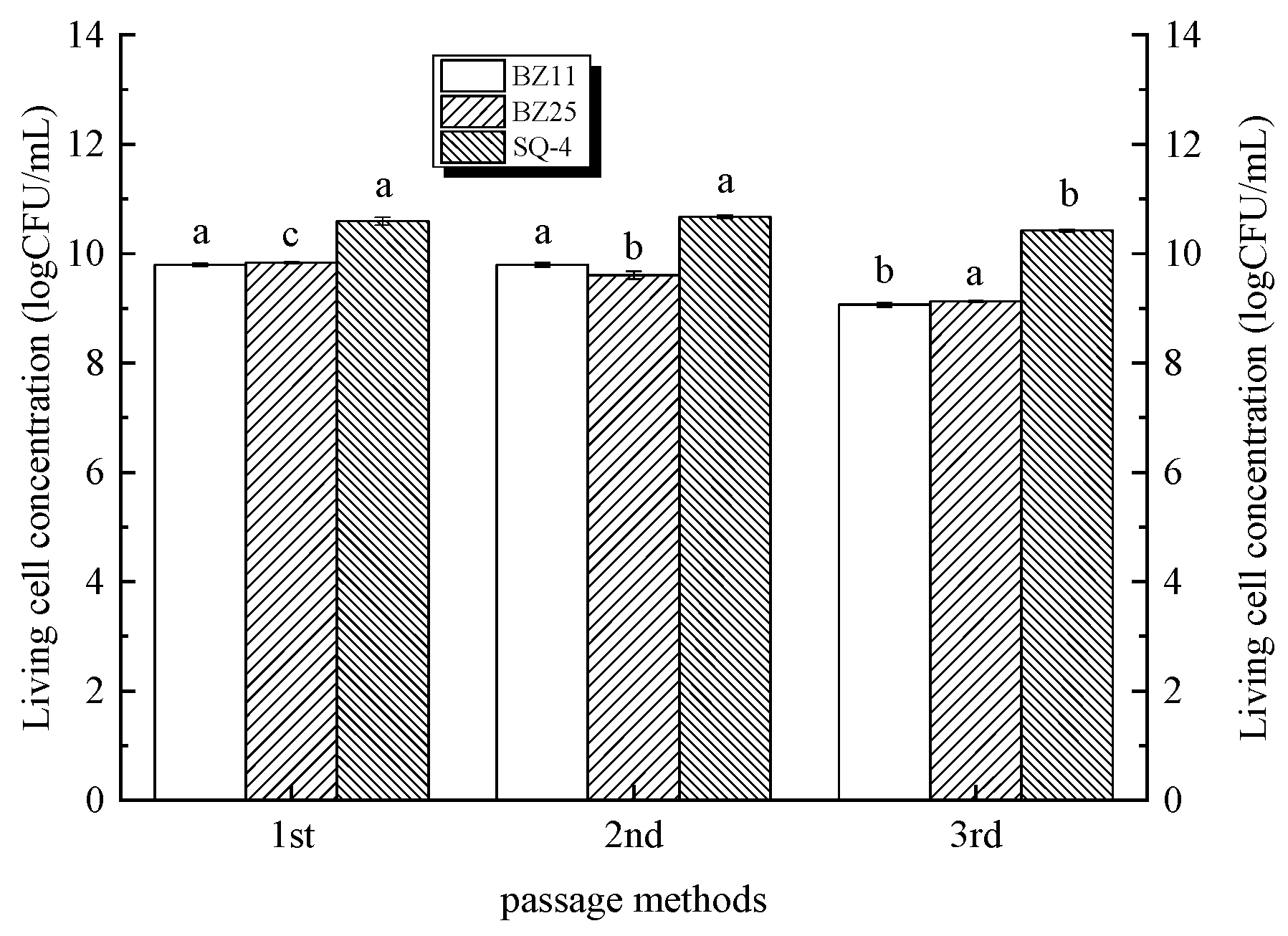
3.1. Strain Growth Characteristics with the First Method of Passage Culture (Figure 2)
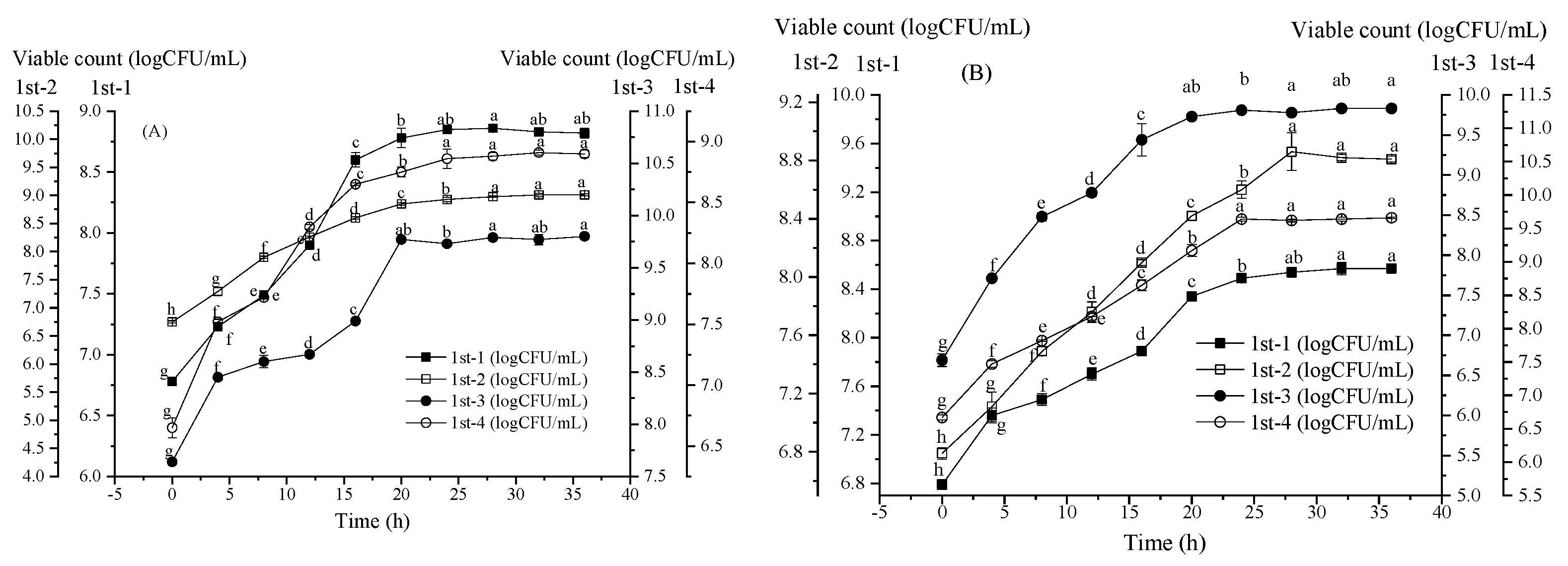
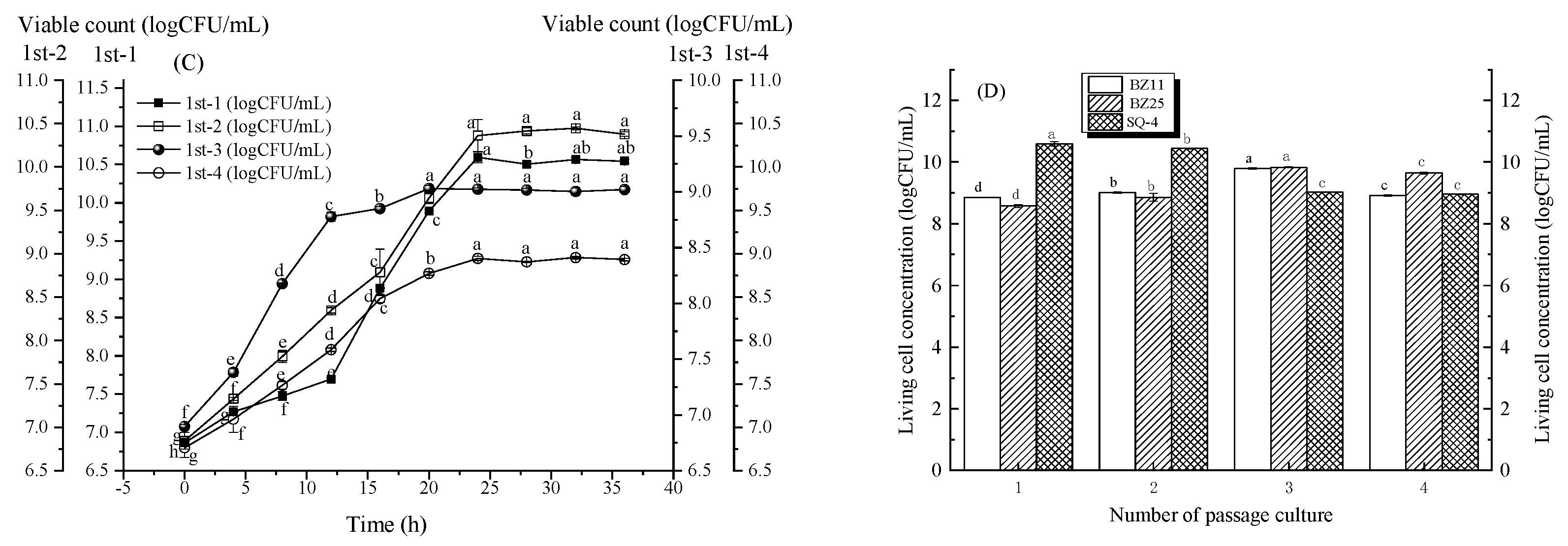
3.2. Change in Strain Growth with the Second Method of Passage Culture
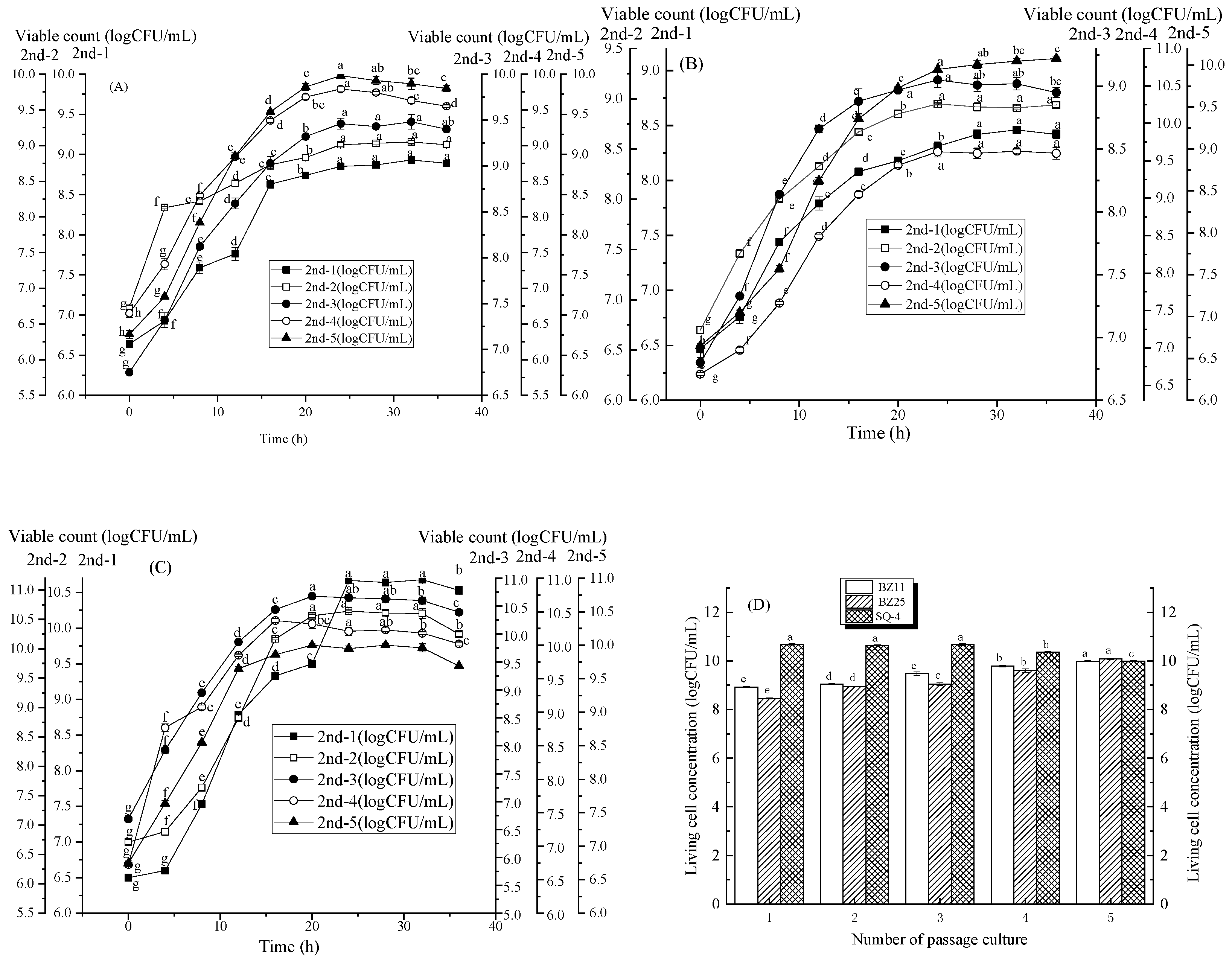
3.3. Change in Strain Growth with the Third Method of Passage Culture
4. Discussion
5. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Declarations of interest
References
- Oliveira, M.E.G.d.; Garcia, E.F.; Oliveira, C.E.V.d.; Gomes, A.M.P.; Pintado, M.M.E.; Madureira, A.R.M.F.; Conceição, M.L.d.; EgyptoQueiroga, R.d.C.R.d.; Souza, E.L.d. Addition of probiotic bacteria in a semi-hard goat cheese (coalho): Survival to simulated gastrointestinal conditions and inhibitory effect against pathogenic bacteria. Food Research International 2014, 64. [Google Scholar] [CrossRef]
- Silveira, E.O.d.; Neto, J.H.L.; Silva, L.A.d.; Raposo, A.E.S.; Magnani, M.; Cardarelli, H.R. The effects of inulin combined with oligofructose and goat cheese whey on the physicochemical properties and sensory acceptance of a probiotic chocolate goat dairy beverage. LWT - Food Science and Technology 2015, 62. [Google Scholar] [CrossRef]
- Pattra, C.; Suvarnakuta, J.S.; Chutinun, P.; Sunthorn, K.; Kaemwich, J. Effects of the food manufacturing chain on the viability and functionality of Bifidobacterium animalis through simulated gastrointestinal conditions. PloS one 2016, 11. [Google Scholar] [CrossRef]
- Mitsuharu, M.; Hifumi, O.; Yoshimi, B. H+-ATPase activity in Bifidobacterium with special reference to acid tolerance. International journal of food microbiology 2004, 93, 109–113. [Google Scholar] [CrossRef]
- Meile, L.; Ludwig, W.; Rueger, U.; Gut, C.; Kaufmann, P.; Dasen, G.; Wenger, S.; Teuber, M. Bifidobacterium lactis sp. nov., a Moderately Oxygen Tolerant Species Isolated from Fermented Milk. Systematic and Applied Microbiology 1997, 20. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, B.; Xiao, D.; Ding, X.; Zeng, H.; Song, L. Research on the processing technology and quality of brown rice germinated with hydrogen-rich water. Science and Technology of Food Industry 2021, 42, 145–153. [Google Scholar] [CrossRef]
- Odebrecht, D.C.; Crhistine, S.M.; Dias, d.M.C.A.R.; Maisonnave, A.A.C. Application of propidium monoazide coupled with quantitative PCR to evaluate cell viability of Bifidobacterium animalis subsp. lactis in a non-dairy probiotic beverage. Annals of Microbiology 2020, 70. [Google Scholar] [CrossRef]
- Astashkina, A.P.; Khudyakova, L.I.; Kolbysheva, Y.V. Microbiological quality control of probiotic products. Procedia Chemistry 2014, 10. [Google Scholar] [CrossRef]
- Machado, D.; Palmeira-de-Oliveira, A.; Cerca, N. Optimization of culture conditions for Gardnerella vaginalis biofilm formation. Journal of Microbiological Methods 2015, 118. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-G.; Son, J.-W.; Kim, H.-J.; Park, C.-S.; Lee, J.-K.; Ji, G.E.; Oh, D.-K. High concentration cultivation of Bifidobacteriumbifidum in a submerged membrane bioreactor. Biotechnology Progress 2006, 22, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-K.; Kim, J.-Y.; Lee, K.-Y.; Heo, T.-R. High cell density cultivation of Bifidobacterium longum using a calcium carbonate-alginate beads system. J. Microbiol. Biotechnol. 2002, 12, 444–448. [Google Scholar]
- Lu, Y.; Xing, S.; He, L.; Li, C.; Wang, X.; Zeng, X.; Dai, Y. Characterization, high-density fermentation, and the production of a directed vat set starter of Lactobacilli used in the food industry: a review. 2022, 11, 3063. [Google Scholar] [CrossRef] [PubMed]
- Rujiao, Z.; Laping, H.; Ling, Z.; Cuiqin, L.; Qiujin, Z. Screening of cholesterol-lowering Bifidobacterium from guizhou Xiang Pigs, and evaluation of its tolerance to oxygen, acid, and bile. Korean journal for food science of animal resources 2016, 36. [Google Scholar] [CrossRef]
- de-Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. Journal of Applied Bacteriology 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Y.; Li, L.; Li, B.; Li, Y.; Zhou, S.; Shirtliff, M.E.; Xu, Z.; Peters, B.M. Discovery and control of culturable and viable but non-culturable cells of a distinctive Lactobacillus harbinensis strain from spoiled beer. Scientific Reports 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Montoro, B.P.; Benomar, N.; Gómez, N.C.; Ennahar, S.; Horvatovich, P.; Knapp, C.W.; Gálvez, A.; Abriouel, H. Proteomic analysis of Lactobacillus pentosus for the identification of potential markers involved in acid resistance and their influence on other probiotic features. Food Microbiology 2018, 72. [Google Scholar] [CrossRef]
- Zhao, M.; Su, X.Q.; Nian, B.; Chen, L.J.; Zhang, D.L.; Duan, S.M.; Wang, L.Y.; Shi, X.Y.; Jiang, B.; Jiang, W.W.; et al. Integrated meta-omics approaches to understand the microbiome of spontaneous fermentation of traditional Chinese Pu-erh Tea. mSystems 2019, 4, e00680–00619. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.E.; Button, J.E.; Santarelli, M.; Dutton, R.J. Cheese rind communities provide tractable systems for in situ andiIn vitro studies of microbial diversity. Cell 2014, 158. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




