Submitted:
01 August 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. Treatment
2.3. Outcomes and Definitions
2.4. Statistical analysis
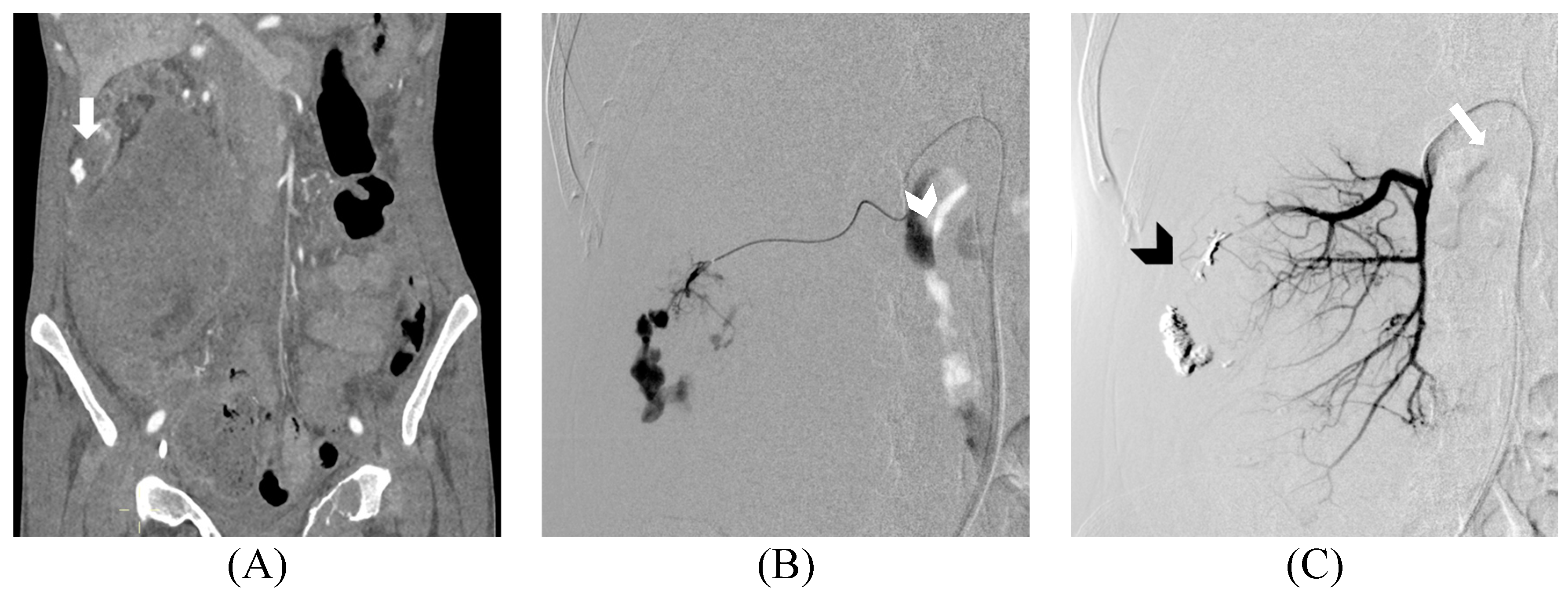
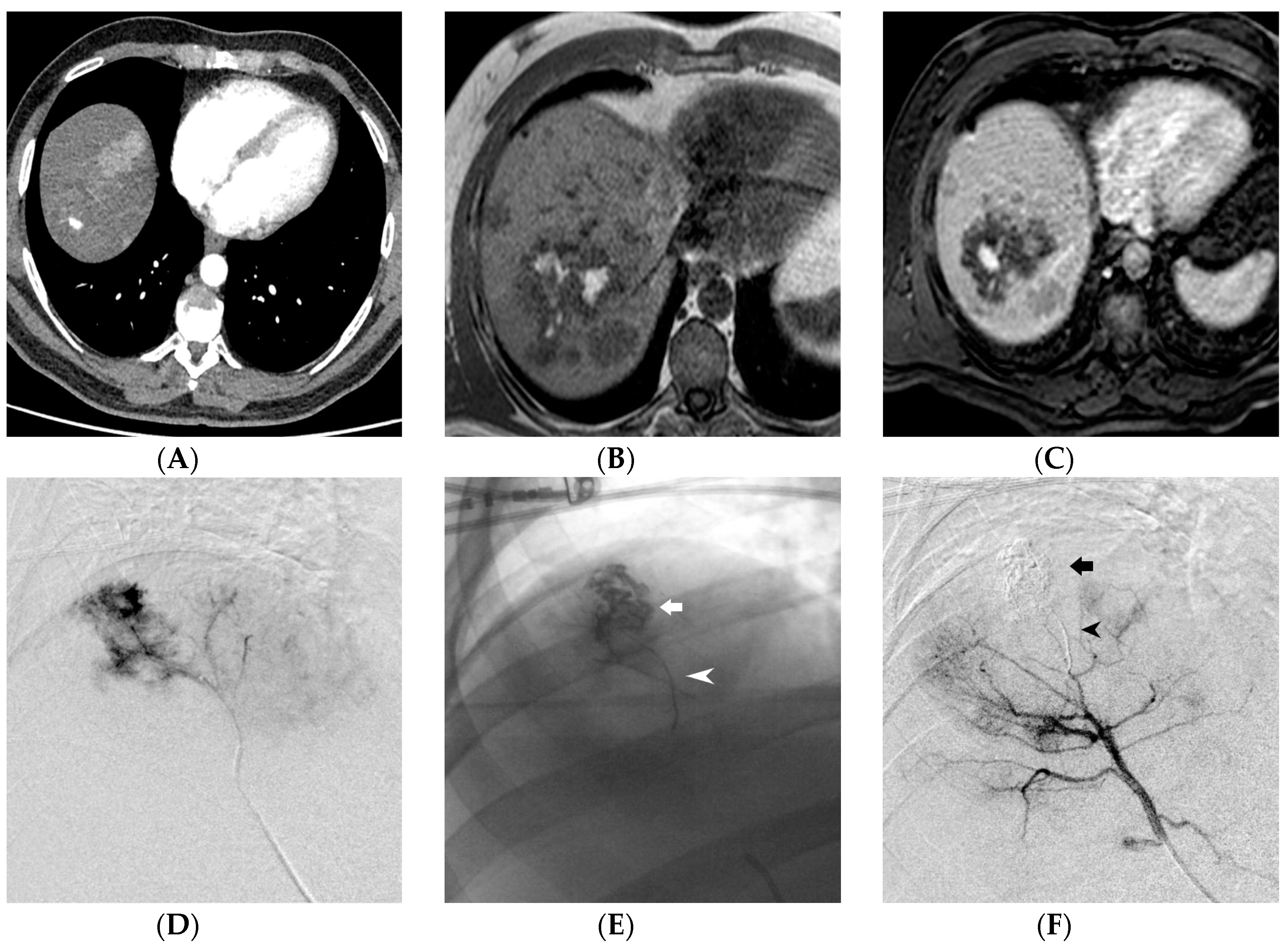
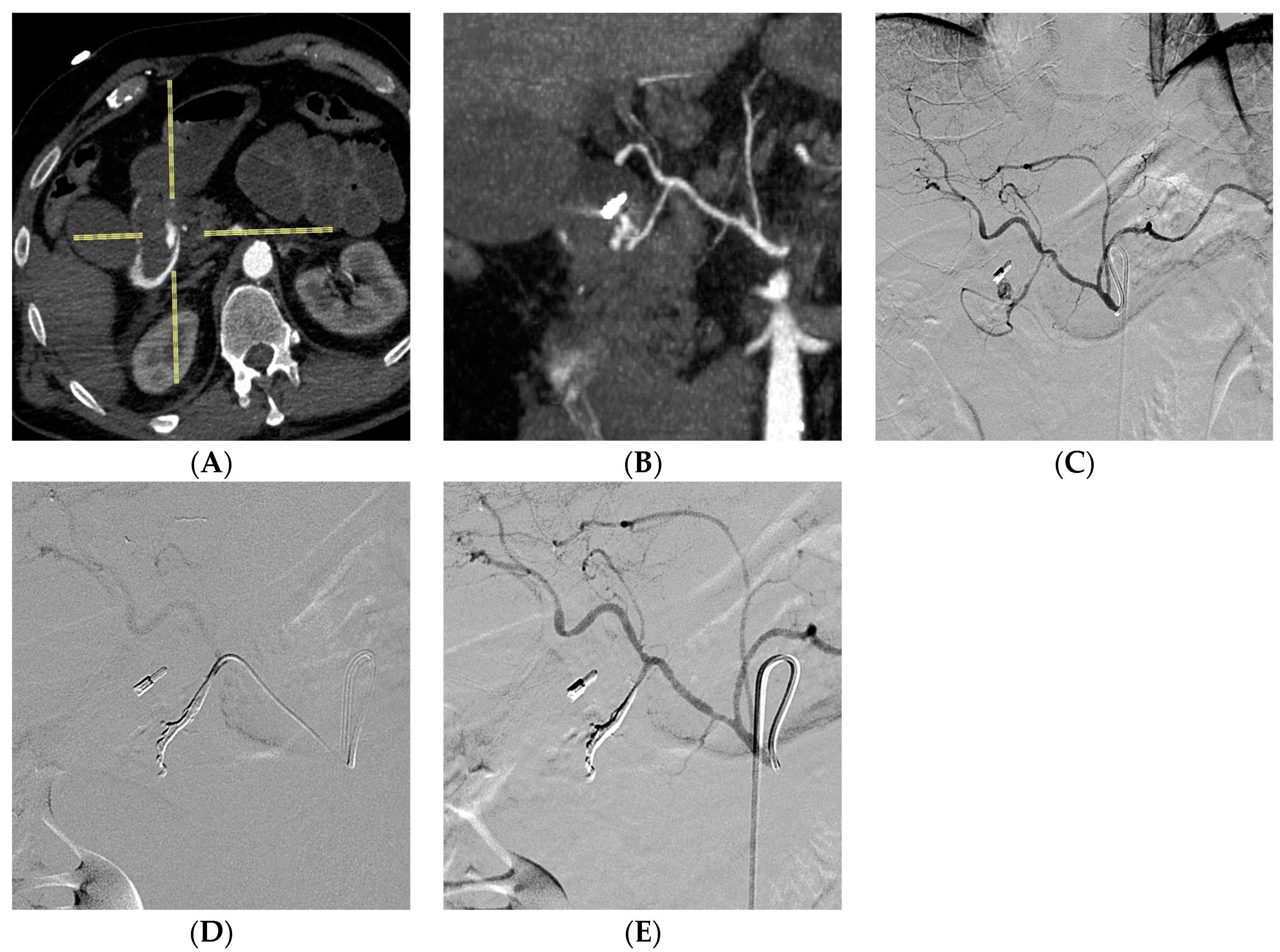
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gabelmann A, Görich J, Merkle EM. Endovascular treatment of visceral artery aneurysms. J Endovasc Ther 2002;9:38–47. [CrossRef]
- Belli A-M, Markose G, Morgan R. The role of interventional radiology in the management of abdominal visceral artery aneurysms. Cardiovasc Intervent Radiol 2012;35:234–43. [CrossRef]
- Lu M, Weiss C, Fishman EK, Johnson PT, Verde F. Review of visceral aneurysms and pseudoaneurysms. J Comput Assist Tomogr 2015;39:1–6. [CrossRef]
- Saad NEA, Saad WEA, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics 2005;25 Suppl 1:S173-189. [CrossRef]
- Shuaib W, Tiwana MH, Vijayasarathi A, Sadiq MF, Anderson S, Amin N, et al. Imaging of vascular pseudoaneurysms in the thorax and abdomen. Clin Imaging 2015;39:352–62. [CrossRef]
- Jesinger RA, Thoreson AA, Lamba R. Abdominal and pelvic aneurysms and pseudoaneurysms: imaging review with clinical, radiologic, and treatment correlation. Radiographics 2013;33:E71-96. [CrossRef]
- Saba L, Anzidei M, Lucatelli P, Mallarini G. The multidetector computed tomography angiography (MDCTA) in the diagnosis of splenic artery aneurysm and pseudoaneurysm. Acta Radiol 2011;52:488–98. [CrossRef]
- Corvino F, Giurazza F, Ierardi AM, Lucatelli P, Basile A, Corvino A, et al. Splenic Artery Pseudoaneurysms: The Role of ce-CT for Diagnosis and Treatment Planning. Diagnostics (Basel) 2022;12:1012. [CrossRef]
- Hagspiel KD, Flors L, Hanley M, Norton PT. Computed tomography angiography and magnetic resonance angiography imaging of the mesenteric vasculature. Tech Vasc Interv Radiol 2015;18:2–13. [CrossRef]
- Keeling AN, McGrath FP, Lee MJ. Interventional radiology in the diagnosis, management, and follow-up of pseudoaneurysms. Cardiovasc Intervent Radiol 2009;32:2–18. [CrossRef]
- Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K. Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol 2005;185:741–9. [CrossRef]
- Madhusudhan KS, Venkatesh HA, Gamanagatti S, Garg P, Srivastava DN. Interventional Radiology in the Management of Visceral Artery Pseudoaneurysms: A Review of Techniques and Embolic Materials. Korean J Radiol 2016;17:351–63. [CrossRef]
- Barrionuevo P, Malas MB, Nejim B, Haddad A, Morrow A, Ponce O, et al. A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg 2019;70:1694–9. [CrossRef]
- Regus S, Lang W. Rupture Risk and Etiology of Visceral Artery Aneurysms and Pseudoaneurysms: A Single-Center Experience. Vasc Endovascular Surg 2016;50:10–5. [CrossRef]
- Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, Rockman C, et al. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. Journal of Vascular Surgery 2020;72:3S-39S. [CrossRef]
- Pitton MB, Dappa E, Jungmann F, Kloeckner R, Schotten S, Wirth GM, et al. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur Radiol 2015;25:2004–14. [CrossRef]
- Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, et al. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2007;45:276–83; discussion 283. [CrossRef]
- Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, Saeger HD. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery 2005;137:323–8. [CrossRef]
- Chadha M, Ahuja C. Visceral artery aneurysms: diagnosis and percutaneous management. Semin Intervent Radiol 2009;26:196–206. [CrossRef]
- Ghoneim TP, Thornton RH, Solomon SB, Adamy A, Favaretto RL, Russo P. Selective arterial embolization for pseudoaneurysms and arteriovenous fistula of renal artery branches following partial nephrectomy. J Urol 2011;185:2061–5. [CrossRef]
- Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions—Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. Journal of Vascular and Interventional Radiology 2019;30:1168-1184.e1. [CrossRef]
- Fankhauser GT, Stone WM, Naidu SG, Oderich GS, Ricotta JJ, Bjarnason H, et al. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011;53:966–70. [CrossRef]
- Khalil A, Fartoukh M, Bazot M, Parrot A, Marsault C, Carette M-F. Systemic arterial embolization in patients with hemoptysis: initial experience with ethylene vinyl alcohol copolymer in 15 cases. AJR Am J Roentgenol 2010;194:W104-110. [CrossRef]
- Rennert J, Herold T, Schreyer AG, Banas B, Jung EM, Feuerbach S, et al. [Evaluation of a liquid embolization agent (Onyx) for transcatheter embolization for renal vascular lesions]. Rofo 2009;181:996–1001. [CrossRef]
- Urbano J, Manuel Cabrera J, Franco A, Alonso-Burgos A. Selective arterial embolization with ethylene-vinyl alcohol copolymer for control of massive lower gastrointestinal bleeding: feasibility and initial experience. J Vasc Interv Radiol 2014;25:839–46. [CrossRef]
- Dariushnia SR, Redstone EA, Heran MKS, Cramer HR, Ganguli S, Gomes AS, et al. Society of Interventional Radiology Quality Improvement Standards for Percutaneous Transcatheter Embolization. Journal of Vascular and Interventional Radiology 2021;32:476.e1-476.e33. [CrossRef]
- Loffroy R, Guiu B, D’Athis P, Mezzetta L, Gagnaire A, Jouve J-L, et al. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol 2009;7:515–23. [CrossRef]
- Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. Journal of Vascular and Interventional Radiology 2017;28:1432-1437.e3. [CrossRef]
- Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology Clinical Practice Guidelines. Journal of Vascular and Interventional Radiology 2003;14:S199–202. [CrossRef]
- Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol 2017;40:1141–6. [CrossRef]
- Minici R, Ammendola M, Manti F, Siciliano MA, Minici M, Komaei I, et al. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization (DSM-TACE) in the Downstaging of Intermediate-Stage Hepatocellular Carcinoma (HCC) in Patients With a Child-Pugh Score of 8-9. Front Pharmacol 2021;12:634087. [CrossRef]
- Minici R, Ammendola M, Manti F, Siciliano MA, Giglio E, Minici M, et al. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization as a Bridging Therapy in Patients with Early Stage Hepatocellular Carcinoma and Child-Pugh Stage B Eligible for Liver Transplant. Front Pharmacol 2021;12:634084. [CrossRef]
- Costa D, Ielapi N, Minici R, Peluso A, Bracale UM, Andreucci M, et al. Risk Factors for Bleeding Varicose Veins in Patients with Chronic Venous Disease. Medicina 2023;59:1034. [CrossRef]
- Minici R, Mercurio M, Iannò B, Galasso O, Gasparini G, Laganà D. Advantages of the Use of Axial Traction Magnetic Resonance Imaging (MRI) of the Shoulder in Patients with Suspected Rota-Tor Cuff Tears: An Exploratory Pilot Study. Healthcare 2023;11:724. [CrossRef]
- Ammendola M, Filice F, Battaglia C, Romano R, Manti F, Minici R, et al. Left hemicolectomy and low anterior resection in colorectal cancer patients: Knight-griffen vs. transanal purse-string suture anastomosis with no-coil placement. Front Surg 2023;10:1093347. [CrossRef]
- Rossi R, Talarico M, Schepis F, Coppi F, Sgura FA, Monopoli DE, et al. Effects of sildenafil on right ventricle remodelling in Portopulmonary hypertension. Pulm Pharmacol Ther 2021;70:102071. [CrossRef]
- Rossi R, Talarico M, Pascale A, Pascale V, Minici R, Boriani G. Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics (Basel) 2022;12:2572. [CrossRef]
- Cernigliaro M, Stanca C, Galbiati A, Spinetta M, Coda C, Negroni D, et al. Innovation in Acute Ischemic Stroke Patients over 80 y/o—A Retrospective Monocentric Study on Mechanical Thrombectomy of Consecutive Patients: Is Age an Adequate Selection Criterion? Journal of Clinical Medicine 2023;12:3688. [CrossRef]
- Zabicki B, Limphaibool N, Holstad MJV, Juszkat R. Endovascular management of pancreatitis-related pseudoaneurysms: A review of techniques. PLoS One 2018;13:e0191998. [CrossRef]
- Balderi A, Antonietti A, Ferro L, Peano E, Pedrazzini F, Fonio P, et al. Endovascular treatment of visceral artery aneurysms and pseudoaneurysms: our experience. Radiol Med 2012;117:815–30. [CrossRef]
- Nagaraja R, Govindasamy M, Varma V, Yadav A, Mehta N, Kumaran V, et al. Hepatic artery pseudoaneurysms: a single-center experience. Ann Vasc Surg 2013;27:743–9. [CrossRef]
- Leyon JJ, Littlehales T, Rangarajan B, Hoey ET, Ganeshan A. Endovascular embolization: review of currently available embolization agents. Curr Probl Diagn Radiol 2014;43:35–53. [CrossRef]
- Minici R, Paone S, Talarico M, Zappia L, Abdalla K, Petullà M, et al. Percutaneous treatment of vascular access-site complications: a ten years’ experience in two centres. CVIR Endovasc 2020;3:29. [CrossRef]
- Rappaport AM, Schneiderman JH. The function of the hepatic artery. Rev Physiol Biochem Pharmacol 1976;76:129–75. [CrossRef]
- Li L, Zhang Y, Chen Y, Zhu K-S, Chen D-J, Zeng X-Q, et al. A multicentre retrospective study of transcatheter angiographic embolization in the treatment of delayed haemorrhage after percutaneous nephrolithotomy. Eur Radiol 2015;25:1140–7. [CrossRef]
- Spiliopoulos S, Sabharwal T, Karnabatidis D, Brountzos E, Katsanos K, Krokidis M, et al. Endovascular treatment of visceral aneurysms and pseudoaneurysms: long-term outcomes from a multicenter European study. Cardiovasc Intervent Radiol 2012;35:1315–25. [CrossRef]
- Song H-H, Won Y-D, Kim Y-J. Transcatheter N-butyl cyanoacrylate embolization of pseudoaneurysms. J Vasc Interv Radiol 2010;21:1508–11. [CrossRef]
- Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol 2008;25:204–15. [CrossRef]
- Loffroy R, Rao P, Ota S, De Lin M, Kwak B-K, Krause D, et al. Packing technique for endovascular coil embolisation of peripheral arterial pseudo-aneurysms with preservation of the parent artery: safety, efficacy and outcomes. Eur J Vasc Endovasc Surg 2010;40:209–15. [CrossRef]
- Kulkarni CB, Moorthy S, Pullara SK, Kannan RR. Endovascular Treatment of Aneurysm of Splenic Artery Arising from Splenomesentric Trunk Using Stent Graft. Korean J Radiol 2013;14:931–4. [CrossRef]
- Künzle S, Glenck M, Puippe G, Schadde E, Mayer D, Pfammatter T. Stent-graft repairs of visceral and renal artery aneurysms are effective and result in long-term patency. J Vasc Interv Radiol 2013;24:989–96. [CrossRef]
- Gong T, Tsauo J, Ding M, Jin L, Duan F, Yu Y, et al. Transcatheter arterial embolization for cancer-related non-variceal upper gastrointestinal bleeding: A multicenter retrospective study of 107 patients. Diagnostic and Interventional Imaging 2023;104:60–6. [CrossRef]
- Sfyroeras GS, Dalainas I, Giannakopoulos TG, Antonopoulos K, Kakisis JD, Liapis CD. Flow-diverting stents for the treatment of arterial aneurysms. J Vasc Surg 2012;56:839–46. [CrossRef]
- Hardman RL, Taussky P, Kim R, O’Hara RG. Post-Transplant Hepatic Artery Pseudoaneurysm Treated with the Pipeline Flow-Diverting Stent. Cardiovasc Intervent Radiol 2015;38:1043–6. [CrossRef]
- Rabuffi P, Bruni A, Antonuccio EGM, Ambrogi C, Vagnarelli S. Treatment of visceral artery aneurysms and pseudoaneurysms with the use of cerebral flow diverting stents: initial experience. CVIR Endovasc 2020;3:48. [CrossRef]
- Venturini M, Piacentino F, Coppola A, Bettoni V, Macchi E, De Marchi G, et al. Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives. J Clin Med 2021;10:2520. [CrossRef]
- Laganà D, Carrafiello G, Mangini M, Dionigi G, Caronno R, Castelli P, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006;59:104–11. [CrossRef]
- Roberts KJ, McCulloch N, Forde C, Mahon B, Mangat K, Olliff SP, et al. Emergency treatment of haemorrhaging coeliac or mesenteric artery aneurysms and pseudoaneurysms in the era of endovascular management. Eur J Vasc Endovasc Surg 2015;49:382–9. [CrossRef]
- Piacentino F, Fontana F, Curti M, Macchi E, Coppola A, Ossola C, et al. Non-Adhesive Liquid Embolic Agents in Extra-Cranial District: State of the Art and Review of the Literature. J Clin Med 2021;10:4841. [CrossRef]
- López-Martínez L, Molina-Nuevo JD, Pedrosa-Jiménez MJ, Juliá-Mollá E. Spontaneous Haematomas in Anticoagulated Covid-19 Patients: Diagnosis and Treatment by Embolization. Cardiovasc Intervent Radiol 2022;45:1001–6. [CrossRef]
- van Vugt R, Bosscha K, van Munster IP, de Jager CPC, Rutten MJCM. Embolization as treatment of choice for bleeding peptic ulcers in high-risk patients. Dig Surg 2009;26:37–42. [CrossRef]
- Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol 2001;12:195–200. [CrossRef]
- Minici R, Venturini M, Fontana F, Guzzardi G, Pingitore A, Piacentino F, et al. Efficacy and Safety of Ethylene-Vinyl Alcohol (EVOH) Copolymer-Based Non-Adhesive Liquid Embolic Agents (NALEAs) in Transcatheter Arterial Embolization (TAE) of Acute Non-Neurovascular Bleeding: A Multicenter Retrospective Cohort Study. Medicina 2023;59:710. [CrossRef]
- Tipaldi MA, Orgera G, Krokidis M, Rebonato A, Maiettini D, Vagnarelli S, et al. Trans Arterial Embolization of Non-variceal Upper Gastrointestinal Bleeding: Is the Use of Ethylene-Vinyl Alcohol Copolymer as Safe as Coils? Cardiovasc Intervent Radiol 2018;41:1340–5. [CrossRef]
- Huang Y-S, Chang C-C, Liou J-M, Jaw F-S, Liu K-L. Transcatheter arterial embolization with N-butyl cyanoacrylate for nonvariceal upper gastrointestinal bleeding in hemodynamically unstable patients: results and predictors of clinical outcomes. J Vasc Interv Radiol 2014;25:1850–7. [CrossRef]
- Yonemitsu T, Kawai N, Sato M, Sonomura T, Takasaka I, Nakai M, et al. Comparison of hemostatic durability between N-butyl cyanoacrylate and gelatin sponge particles in transcatheter arterial embolization for acute arterial hemorrhage in a coagulopathic condition in a swine model. Cardiovasc Intervent Radiol 2010;33:1192–7. [CrossRef]
- Minici R, Fontana F, Venturini M, Guzzardi G, Siciliano A, Piacentino F, et al. Transcatheter Arterial Embolization (TAE) in the Management of Bleeding in the COVID-19 Patient. Medicina 2023;59:1062. [CrossRef]
- Abdulmalak G, Chevallier O, Falvo N, Di Marco L, Bertaut A, Moulin B, et al. Safety and efficacy of transcatheter embolization with Glubran®2 cyanoacrylate glue for acute arterial bleeding: a single-center experience with 104 patients. Abdom Radiol (NY) 2018;43:723–33. [CrossRef]
- Yonemitsu T, Kawai N, Sato M, Tanihata H, Takasaka I, Nakai M, et al. Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and n-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J Vasc Interv Radiol 2009;20:1176–87. [CrossRef]
- Sapoval M, Vidal V, Déan C, Del Giudice C, Tradi F, Chevallier O, et al. Safety and Efficacy of Peripheral Embolization with EASYX Liquid Embolic Agent: A Multicenter Prospective Study. J Vasc Interv Radiol 2021;32:1136–43. [CrossRef]
- Minici R, Ammendola M, Talarico M, Luposella M, Minici M, Ciranni S, et al. Endovascular recanalization of chronic total occlusions of the native superficial femoral artery after failed femoropopliteal bypass in patients with critical limb ischemia. CVIR Endovasc 2021;4:68. [CrossRef]
- Né R, Chevallier O, Falvo N, Facy O, Berthod P-E, Galland C, et al. Embolization with ethylene vinyl alcohol copolymer (Onyx®) for peripheral hemostatic and non-hemostatic applications: a feasibility and safety study. Quant Imaging Med Surg 2018;8:280–90. [CrossRef]
- Minici R, Serra R, Giurdanella M, Talarico M, Siciliano MA, Carrafiello G, et al. Efficacy and Safety of Distal Radial Access for Transcatheter Arterial Chemoembolization (TACE) of the Liver. Journal of Personalized Medicine 2023;13:640. [CrossRef]
- Kim PH, Tsauo J, Shin JH, Yun S-C. Transcatheter Arterial Embolization of Gastrointestinal Bleeding with N-Butyl Cyanoacrylate: A Systematic Review and Meta-Analysis of Safety and Efficacy. J Vasc Interv Radiol 2017;28:522-531.e5. [CrossRef]
- Minici R, Serra R, Ierardi AM, Petullà M, Bracale UM, Carrafiello G, et al. Thoracic endovascular repair for blunt traumatic thoracic aortic injury: Long-term results. Vascular 2022:17085381221127740. [CrossRef]
- Bracale UM, Peluso A, Panagrosso M, Cecere F, Del Guercio L, Minici R, et al. Ankle-Brachial Index evaluation in totally percutaneous approach vs. femoral artery cutdown for endovascular aortic repair of abdominal aortic aneurysms. Chirurgia 2022;35. [CrossRef]
- Minici R, Serra R, De Rosi N, Ciranni S, Talarico M, Petullà M, et al. Endovascular treatment of femoro-popliteal occlusions with retrograde tibial access after failure of the antegrade approach. Catheter Cardiovasc Interv 2023. [CrossRef]
- Regine R, Palmieri F, De Siero M, Rescigno A, Sica V, Cantarela R, et al. Embolization of traumatic and non-traumatic peripheral vascular lesions with Onyx. Interv Med Appl Sci 2015;7:22–9. [CrossRef]
- Minici R, Serra R, Maglia C, Guzzardi G, Spinetta M, Fontana F, et al. Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers. Journal of Personalized Medicine 2023;13:812. [CrossRef]
- Sun CJ, Wang CE, Wang YH, Xie LL, Liu TH, Ren WC. Transcatheter arterial embolization of acute gastrointestinal tumor hemorrhage with Onyx. Indian J Cancer 2015;51 Suppl 2:e56-59. [CrossRef]
- Minici R, Fontana F, Venturini M, Guzzardi G, Piacentino F, Spinetta M, et al. A Multicenter Retrospective Cohort Study Evaluating the Clinical Outcomes of Patients with Coagulopathy Undergoing Transcatheter Arterial Embolization (TAE) for Acute Non-Neurovascular Bleeding. Medicina 2023;59:1333. [CrossRef]
- Minici R, Guzzardi G, Venturini M, Fontana F, Coppola A, Spinetta M, et al. Transcatheter Arterial Embolization (TAE) of Cancer-Related Bleeding. Medicina 2023;59:1323. [CrossRef]
- Khalil A, Parrot A, Fartoukh M, Djibre M, Tassart M, Carette M-F. Pulmonary artery occlusion with ethylene vinyl alcohol copolymer in patients with hemoptysis: initial experience in 12 cases. AJR Am J Roentgenol 2012;198:207–12. [CrossRef]
- Loffroy R, Midulla M, Falvo N, Chevallier O. Ethylene Vinyl Alcohol Copolymer as First Hemostatic Liquid Embolic Agent for Non-variceal Upper Gastrointestinal Bleeding Patients: Pros and Cons. Cardiovasc Intervent Radiol 2018;41:1808–9. [CrossRef]
- Kolber MK, Shukla PA, Kumar A, Silberzweig JE. Ethylene Vinyl Alcohol Copolymer (Onyx) Embolization for Acute Hemorrhage: A Systematic Review of Peripheral Applications. Journal of Vascular and Interventional Radiology 2015;26:809–15. [CrossRef]
- Loffroy R, Favelier S, Genson P-Y, Guiu B. Onyx for embolization of life-threatening hemoptysis: a promising but luxury embolic agent! Cardiovasc Intervent Radiol 2012;35:221; author reply 222. [CrossRef]
- Lv X, Li Y, Jiang C, Wu Z. The Incidence of Trigeminocardiac Reflex in Endovascular Treatment of Dural Arteriovenous Fistula with Onyx. Interv Neuroradiol 2010;16:59–63. [CrossRef]
- Simon SD, Reig AS, Archer KJ, Mericle RA. Biomechanical attributes of microcatheters used in liquid embolization of intracranial aneurysms. Journal of NeuroInterventional Surgery 2012;4:211–4. [CrossRef]
- Pop R, Mertz L, Ilyes A, Mihoc D, Richter JS, Manisor M, et al. Beam hardening artifacts of liquid embolic agents: comparison between Squid and Onyx. Journal of NeuroInterventional Surgery 2019;11:706–9. [CrossRef]
- Larzon T, Mathisen SR. Internal sealing of acute aortic bleeding with a catheter-delivered liquid to solid embolic agent (Onyx). Vascular 2010;18:106–10. [CrossRef]
- Guillon R, Garcier JM, Abergel A, Mofid R, Garcia V, Chahid T, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003;26:256–60. [CrossRef]
| Variables | All patients (n=38) | |||
|---|---|---|---|---|
| Unruptured VAP (n=21) | Ruptured VAP (n=17) | P value | ||
| Age (years) | 55.7 (±23.9) | 53.7 (±25.5) | 58.2 (±22.3) | 0.547 |
| Sex (M/F) | 11 (28.9%) / 27 (71.1%) | 7 (33.3%) / 14 (66.7%) | 4 (23.5%) / 13 (76.5%) | 0.721 |
| BMI | 25.7 (±4) | 25.8 (±4) | 25.6 (±4.1) | 0.918 |
| eGFR (mL/min) | 71.4 (±22.2) | 75.8 (±19.8) | 66.1 (±24.5) | 0.290 |
| INR | 1.26 (±0.3) | 1.26 (±0.3) | 1.25 (±0.3) | 0.965 |
| aPTT (s) | 40.2 (±5.5) | 40 (±5.5) | 40.5 (±5.4) | 0.768 |
| Platelet count (No. x103/μL) | 341 (±91.8) | 335.4 (±98.2) | 348 (±85.6) | 0.692 |
| Coagulopathy | 9 (23.7%) | 6 (28.6%) | 3 (17.6%) | 0.476 |
| Baseline Hemoglobin (g/dl) | 7.7 (±0.8) | 7.4 (±0.5) | 8 (±1) | 0.093 |
| Antiplatelet therapy | 9 (23.7%) | 5 (23.8%) | 4 (23.5%) | 1 |
| Anticoagulant therapy | 11 (28.9%) | 5 (23.8%) | 6 (35.3%) | 0.491 |
| Hemodynamic instability | 7 (18.4%) | 0 (0%) | 7 (41.2%) | 0.002 |
| Symptomatic Pseudoaneurysm | 24 (63.2%) | 7 (33.3%) | 17 (100%) | <0.001 |
| CT-angiography execution | 37 (97.4%) | 21 (100%) | 16 (94.1%) | 0.447 |
| VAP max diameter (cm) | 3.9 (±1.2) | 4.4 (±1.2) | 3.3 (±0.9) | 0.005 |
| Variables | All patients (n=38) | |||
|---|---|---|---|---|
| Unruptured VAP (n=21) | Ruptured VAP (n=17) | P value | ||
Etiology
|
16 (42.1%) 9 (23.7%) 12 (31.6%) 1 (2.6%) |
8 (38.1%) 6 (28.6%) 6 (28.6%) 1 (4.8%) |
8 (47.1%) 3 (17.6%) 6 (35.3%) 0 (0%) |
0.660 |
Site of the pseudoaneurysm
|
13 (34.2%) 7 (18.4%) 1 (2.6%) 4 (10.5%) 3 (7.9%) 4 (10.5%) 6 (15.8%) |
7 (33.3%) 4 (19%) 0 (0%) 3 (14.3%) 1 (4.8%) 2 (9.5%) 4 (19%) |
6 (35.3%) 3 (17.6%) 1 (5.9%) 1 (5.9%) 2 (11.8%) 2 (11.8%) 2 (11.8%) |
0.830 |
Endovascular treatment timing
|
14 (36.8%) 24 (63.2%) |
14 (66.7%) 7 (33.3%) |
0 (0%) 17 (100%) |
<0.001 |
|
18 (47.4%) 20 (52.6%) |
11 (52.4%) 10 (47.6%) |
7 (41.2%) 10 (58.8%) |
0.532 |
EVOH viscosity (centiStokes)
|
2 (5.3%) 6 (15.8%) 7 (18.4%) 23 (60.5%) |
1 (4.8%) 4 (19%) 2 (9.5%) 14 (66.7%) |
1 (5.9%) 2 (11.8%) 5 (29.4%) 9 (52.9%) |
0.449 |
| Intraoperative contrast medium (mL) | 33.6 (±12) | 30.9 (±11.9) | 36.9 (±11.6) | 0.100 |
| Volume of contrast to creatinine clearance ratio | 0.58 (±0.54) | 0.45 (±0.29) | 0.74 (±0.73) | 0.064 |
Vascular access site
|
34 (89.5%) 2 (5.3%) 2 (5.3%) |
18 (85.7%) 1 (4.8%) 2 (9.5%) |
16 (94.1%) 1 (5.9%) 0 (0%) |
0.424 |
Sheath diameter
|
7 (18.4%) 27 (71.1%) 4 (10.5%) |
4 (19%) 16 (76.2%) 1 (4.8%) |
3 (17.6%) 11 (64.7%) 3 (17.6%) |
0.435 |
| CT-to-groin time (min) | 143.8 (±179) | 199.2 (±225.9) | 75.3 (±35.6) | 0.150 |
| Procedure time (min) | 39.7 (±10.3) | 41 (±11.6) | 38.1 (±8.5) | 0.444 |
| CT-to-embolization time (min) | 186.4 (±182.4) | 245.9 (±229) | 112.8 (±31.5) | 0.177 |
| Fluoroscopy time (min) | 14.6 (±5.9) | 15.1 (±6) | 14 (±5.8) | 0.744 |
| Cumulative air kerma (mGy) | 218 (±68.2) | 206.3 (±68.1) | 232.3 (±67.6) | 0.246 |
| Dose area product (DAP) (Gy/cm2) | 34.7 (±10.1) | 33.7 (±10) | 35.9 (±10.3) | 0.537 |
| Variables | All patients (n=38) | |||
|---|---|---|---|---|
| Unruptured VAP (n=21) | Ruptured VAP (n=17) | P value | ||
| Technical success | 38 (100%) | 21 (100%) | 17 (100%) | 1 |
| Clinical success | 35 (92.1%) | 19 (90.5%) | 16 (94.1%) | 0.679 |
| Rebleeding | 5 (13.2%) | 3 (14.3%) | 2 (11.8%) | 0.819 |
Repeated XA
|
33 (86.8%) 2 (5.3%) 3 (7.9%) |
18 (85.7%) 1 (4.8%) 2 (9.5%) |
15 (88.2%) 1 (5.9%) 1 (5.9%) |
0.911 |
Imaging follow-up modality
|
34 (89.5%) 1 (2.6%) 3 (7.9%) |
18 (85.7%) 0 (0%) 3 (14.3%) |
16 (94.1%) 1 (5.9%) 0 (0%) |
0.154 |
Vascular access site hemostasis
|
19 (50%) 19 (50%) |
11 (52.4%) 10 (47.6%) |
8 (47.1%) 9 (52.9%) |
0.744 |
| Units of packed red blood cells transfused per patient | 1.6 (±2.1) | 0.6 (±0.7) | 2.9 (±2.5) | <0.001 |
| Non-target embolization | 1 (2.6%) | 1 (4.8%) | 0 (0%) | 0.362 |
| Procedure-related complication Rate | 7 (18.4%) | 4 (19%) | 3 (17.6%) | 0.912 |
| End-organ infarction Rate | 4 (10.5%) | 2 (9.5%) | 2 (11.8%) | 0.823 |
| Vascular access-site complication (VASC) Rate | 2 (5.3%) | 1 (4.8%) | 1 (5.9%) | 0.878 |
Procedure-related Complications (SIR classification)
|
31 (81.6%) 7 (18.4%) 0 (0%) |
17 (81%) 4 (19%) 0 (0%) |
14 (82.4%) 3 (17.6%) 0 (0%) |
0.912 |
Procedure-related Complications (CIRSE classification)
|
31 (81.6%) 1 (2.6%) 6 (15.8%) |
17 (81%) 1 (4.8%) 3 (14.3%) |
14 (82.4%) 0 (0%) 3 (17.6%) |
0.644 |
| 30-day bleeding-related mortality | 1 (2.6%) | 1 (4.8%) | 0 (0%) | 0.362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





