Submitted:
30 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
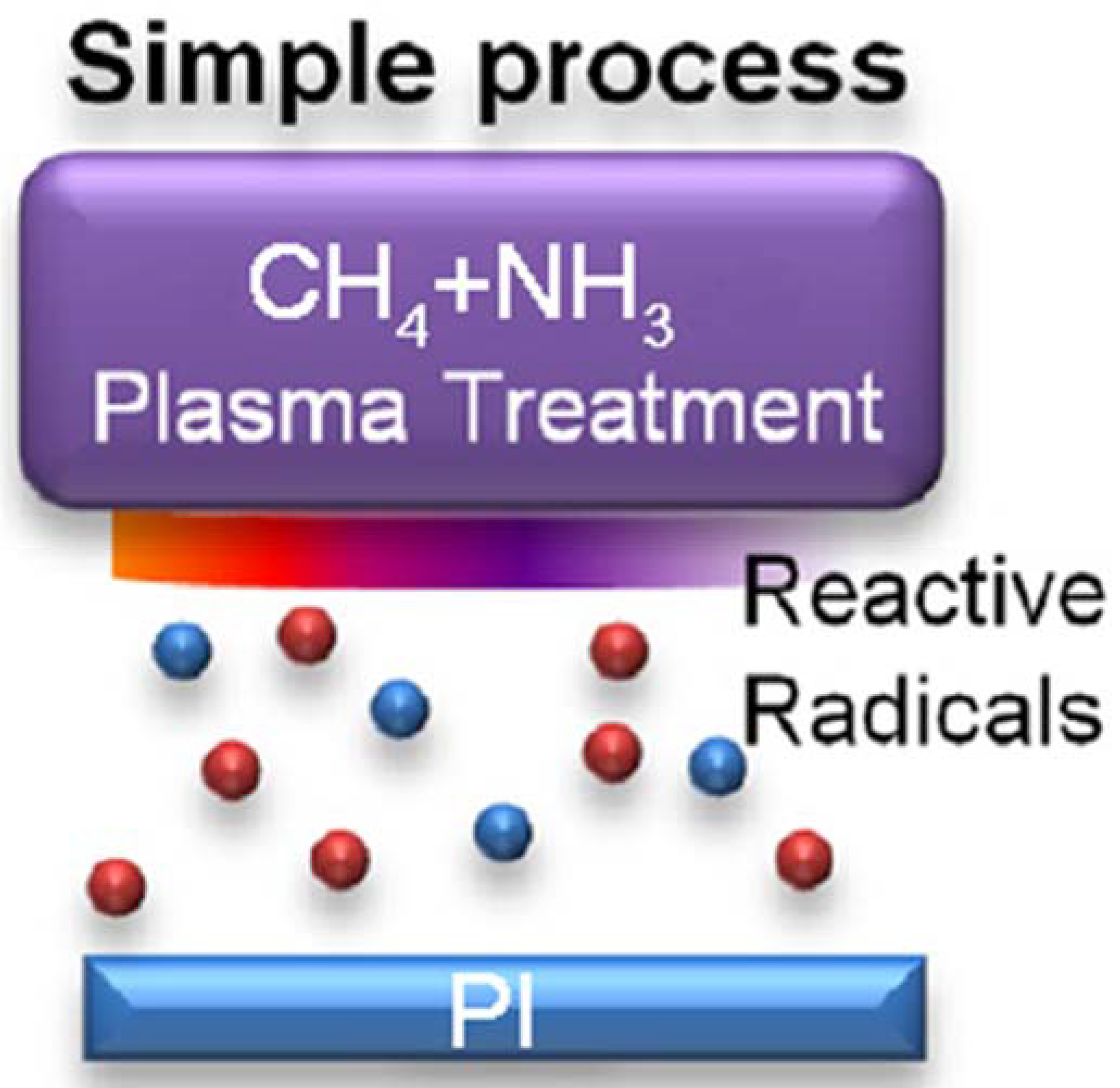
1. Introduction
2. Experimental Section
2.1. Material Preparation
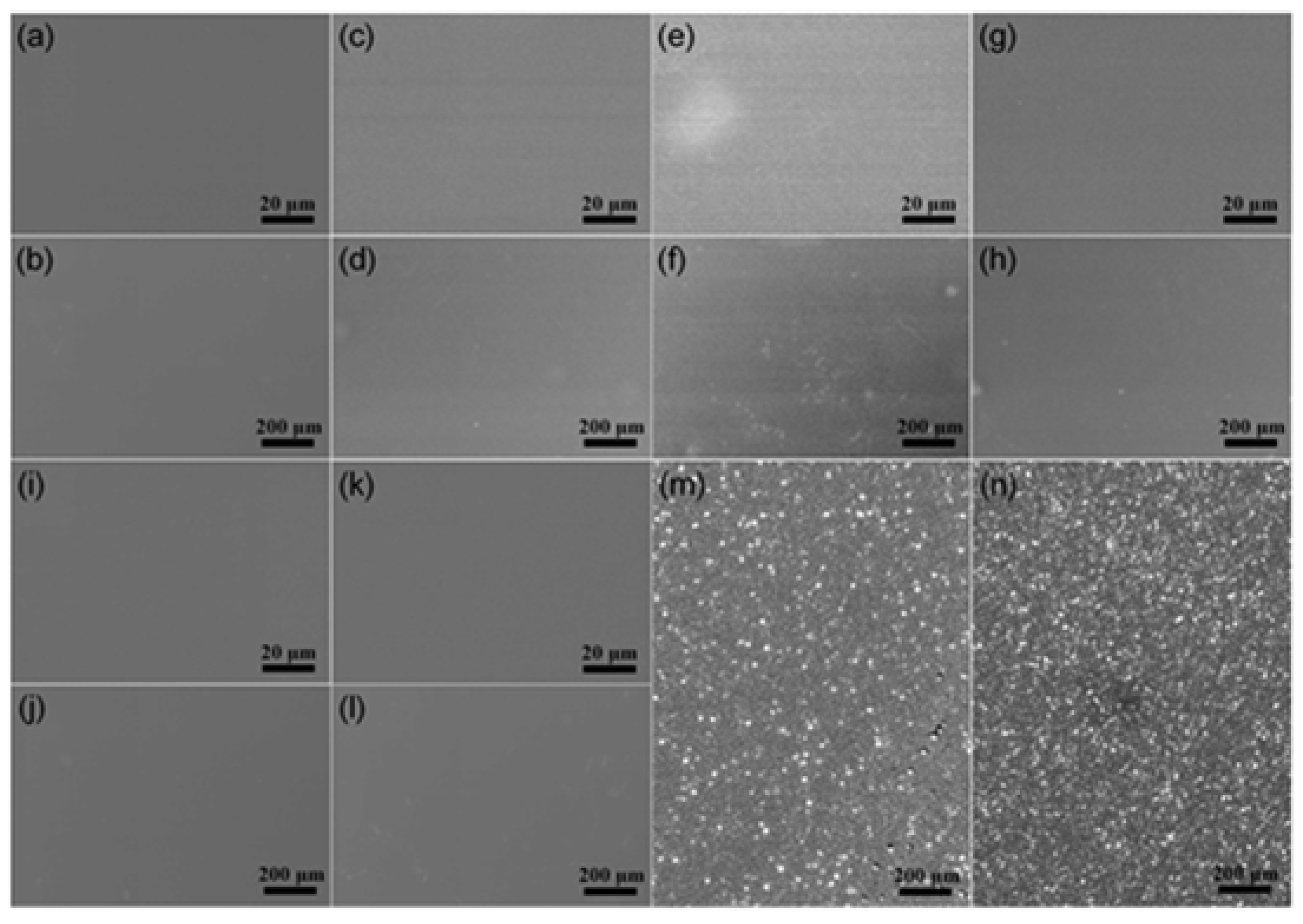
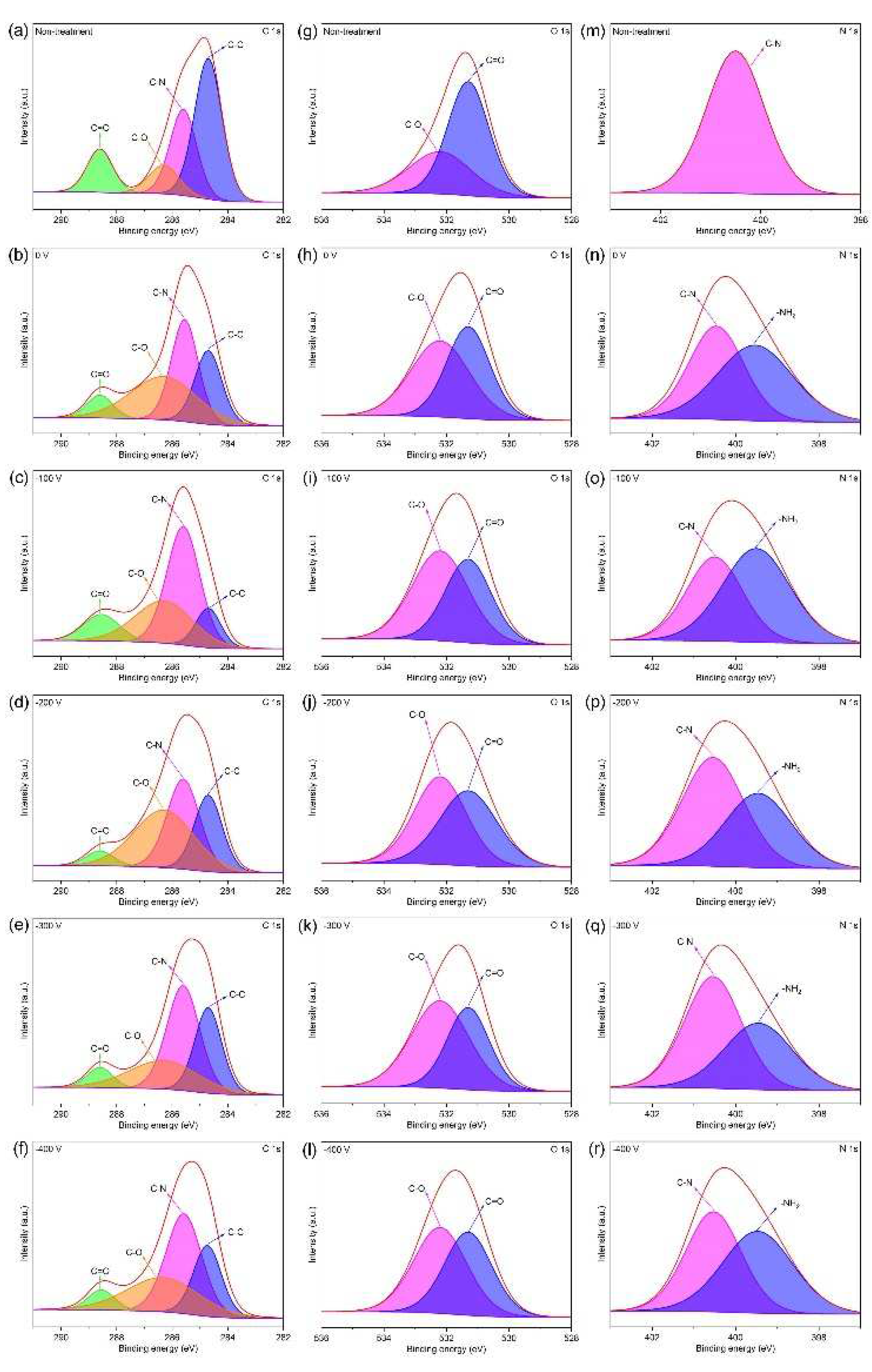
2.2. Morphological and Structural Characterization
2.3. Mechanical Property Characterization
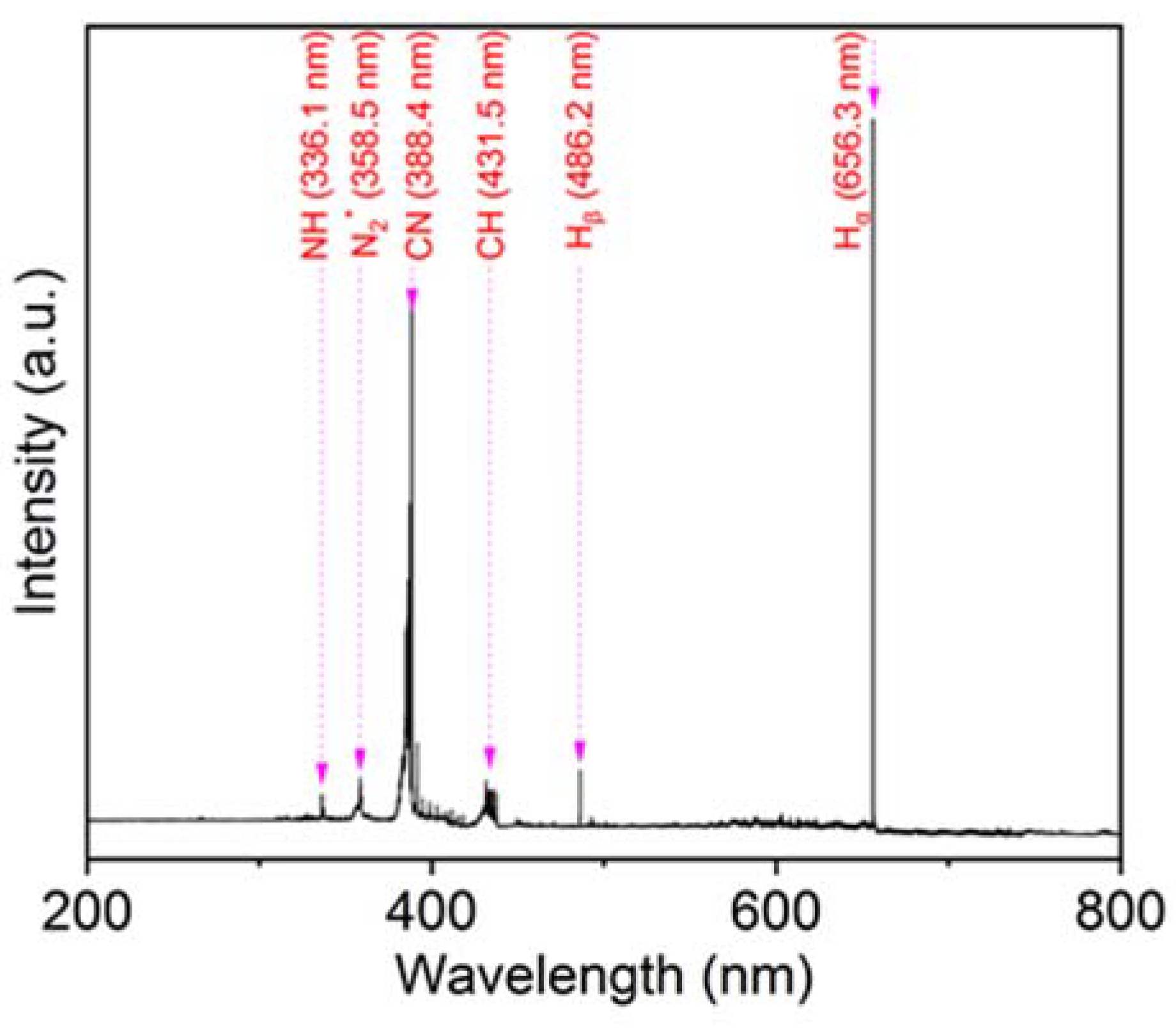
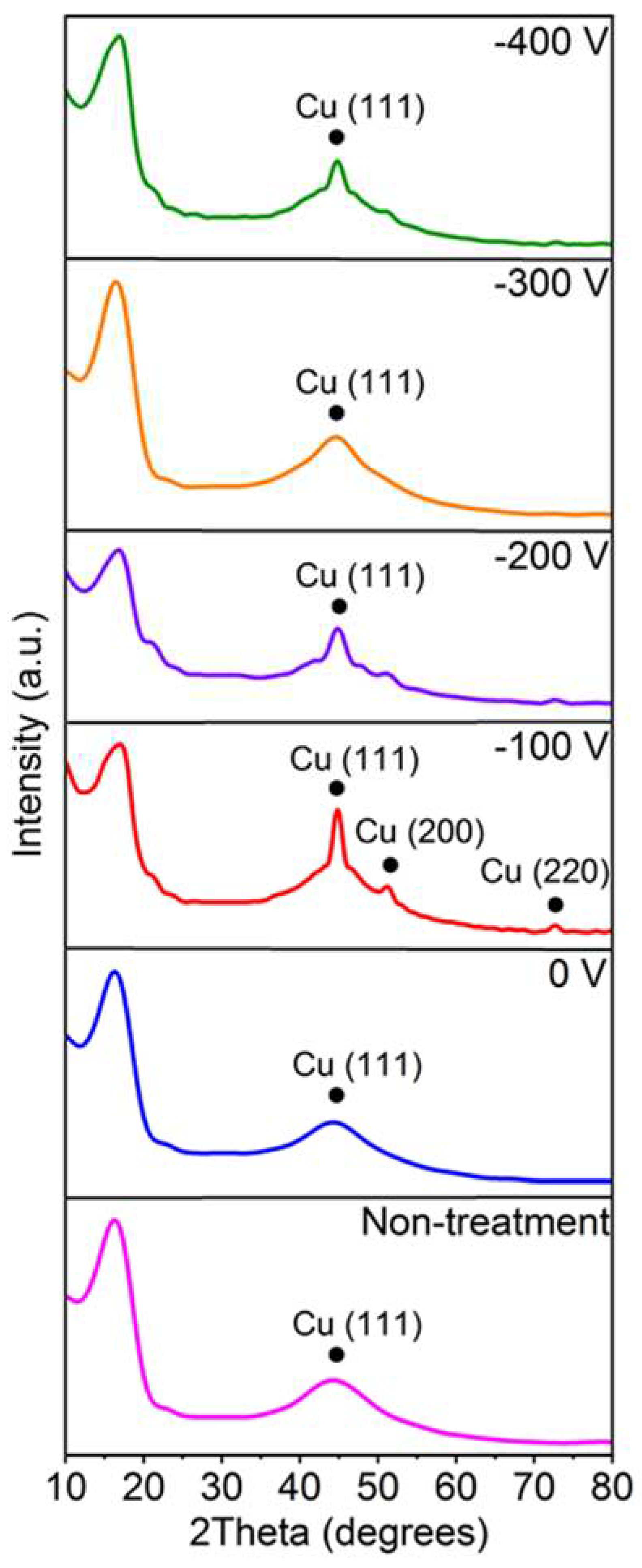
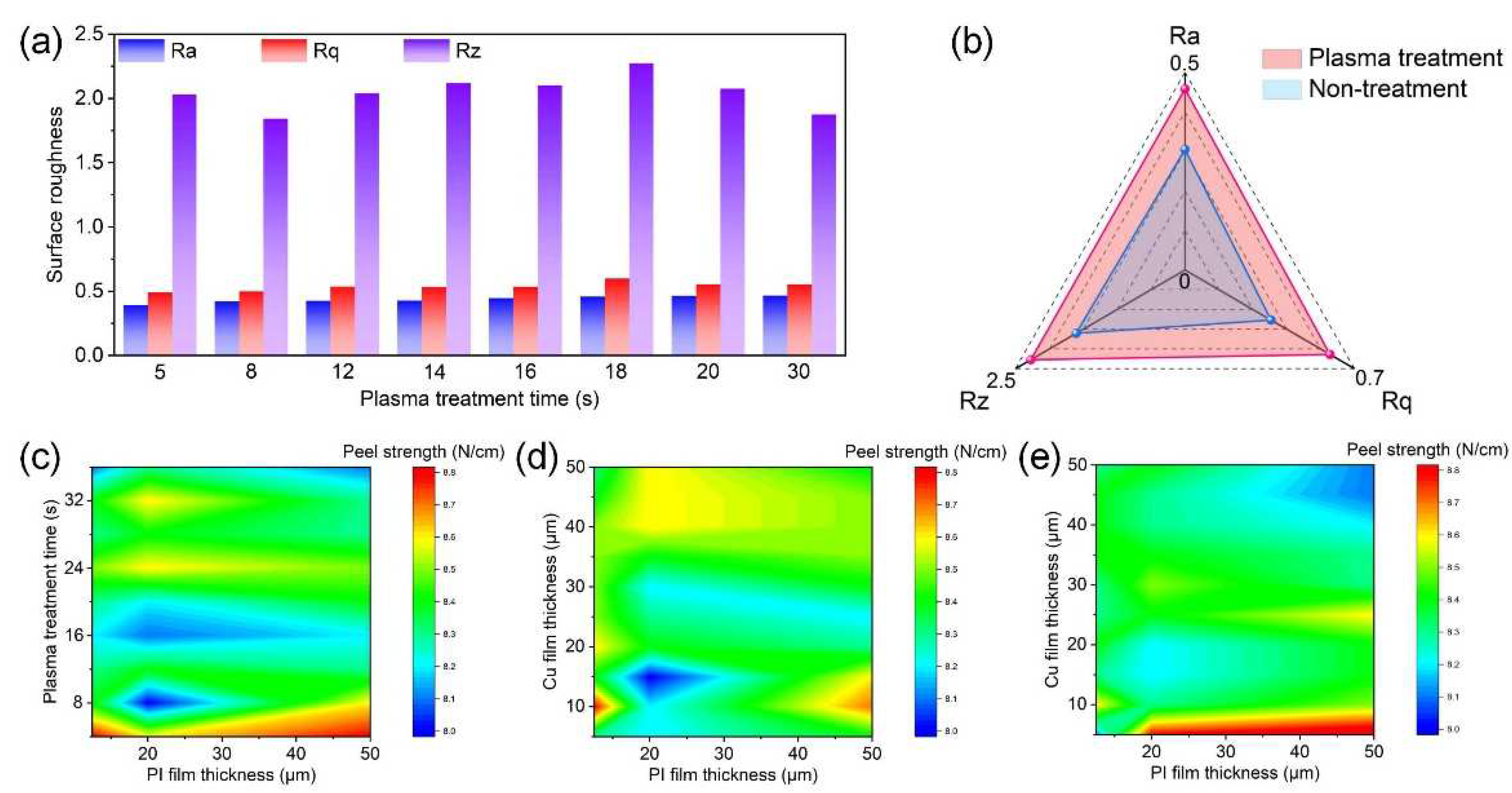
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cen-Puc, M.; Schander, A.; Gleason, M.G.V.; Lang, W. An assessment of surface treatments for adhesion of polyimide thin films. Polymers 2021, 13, 1955. [Google Scholar] [CrossRef]
- Liu, T.J.; Chen, C.H.; Wu, P.Y.; Lin, C.H.; Chen, C.M. Efficient and adhesiveless metallization of flexible polyimide by functional grafting of carboxylic acid groups. Langmuir 2019, 35, 7212–7221. [Google Scholar] [CrossRef]
- Yang, S.; Wu, D.; Qi, S.; Cui, G.; Jin, R.; Wu, Z. Fabrication of highly reflective and conductive double-surface-silvered layers embedded on polymeric films through all-wet process at room temperature. J. Phys. Chem. B 2009, 113, 9694–9701. [Google Scholar] [CrossRef]
- Ho, S.M.; Wang, T.H.; Chen, H.L.; Chen, K.M.; Lian, S.M.; Hung, A. Metallization of polyimide film by wet process. J. Appl. Polym. Sci. 1994, 51, 1373–1380. [Google Scholar] [CrossRef]
- Ng, J.H.G.; Desmulliez, M.P.Y.; Lamponi, M.; Moffat, B.G.; McCarthy, A.; Suyal, H.; Walker, A.C.; Prior, K.A.; Hand, D.P. A direct-writing approach to the micro-patterning of copper onto polyimide. Circuit World 2009, 35, 3–17. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yao, W.; Wei, Y. Properties of Cu film and Ti/Cu film on polyimide prepared by ion beam techniques. Appl. Surf. Sci. 2010, 256, 7010–7017. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Noh, B.I.; Yoon, J.W.; Choi, J.H.; Jung, S.B. Effect of Cr thickness on adhesion strength of Cu/Cr/polyimide flexible copper clad laminate fabricated by roll-to-roll process. Mater. Trans. 2010, 51, 85–89. [Google Scholar] [CrossRef]
- Lin, F.; Li, W.; Tang, Y.; Shao, H.; Su, C.; Jiang, J.; Chen, N. High-performance polyimide filaments and composites improved by O2 plasma treatment. Polymers 2018, 10, 695. [Google Scholar] [CrossRef]
- Qu, H.; Wang, Z.; Cang, D. Flexible bandpass filter fabricated on polyimide substrate by surface modification and in situ self-metallization technique. Polymers 2019, 11, 2068. [Google Scholar] [CrossRef]
- Park, Y.J.; Yu, D.M.; Ahn, J.H.; Choi, J.H.; Hong, Y.T. Surface modification of polyimide films by an ethylenediamine treatment for a flexible copper clad laminate. Macromol. Res. 2012, 20, 168–173. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, E.J.; Kwon, S.H. Influence of surface treatment of polyimide film on adhesion enhancement between polyimide and metal films. Bull. Korean Chem. Soc. 2007, 28, 188–192. [Google Scholar]
- Yun, H.K.; Cho, K.; Kim, J.K. Adhesion improvement of epoxy resin/ polyimide joints by amine treatment of polyimide surface. Polymer 1997, 38, 827–834. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.S.; Lee, H.K. Improvement of adhesion between copper layer and polyimide films modified with alkaline potassium permanganate and/or alkali surface treatments. Macromol. Symp. 2007, 249–250, 586–590. [Google Scholar] [CrossRef]
- Ruoff, A.L.; Kramer, E.J.; Li, C.Y. Improvement of adhesion of copper on polyimide by reactive ion-beam etching. IBM J. Res. Develop. 1988, 32, 626–630. [Google Scholar] [CrossRef]
- Bouhamed, A.; Kia, A.M.; Naifar, S.; Dzhagan, V.; Müller, C.; Zahn, D.R.T.; Choura, S.; Kanoun, O. Tuning the adhesion between polyimide substrate and MWCNTs/epoxy nanocomposite by surface treatment. Appl. Surf. Sci. 2017, 422, 420–429. [Google Scholar] [CrossRef]
- Lai, Y.H.; Sil, M.C.; Chen, C.M. Surface composite engineering of polyimide to create amine functionalities for autocatalytic metallization. Appl. Surf. Sci. 2021, 541, 148500. [Google Scholar] [CrossRef]
- Yu, Z.J.; Kang, E.T.; Neoh, K.G. Electroless plating of copper on polyimide films modified by surface grafting of tertiary and quaternary amines polymers. Polymer 2002, 43, 4137–4146. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Masumoto, M. Improved adhesion between kapton film and copper metal by plasma graft polymerization of vinylimidazole. Macromolecules 1996, 29, 1642–1648. [Google Scholar] [CrossRef]
- Thompson, W.B. Fundamentals of Plasma Physics (Book); merican Scientist, 1981. [Google Scholar]
- Boswell, R.W. Very efficient plasma generation by whistler waves near the lower hybrid frequency. Plasma Phys. Control. Fusion 1984, 26, 1147. [Google Scholar] [CrossRef]
- Sadeghi, N.; Margot, J.; Gottscho, R.A.; Lee, T.C.J. Limits to ion energy control in high density glow discharges: Measurement of absolute metastable ion concentrations. J. Appl. Phys. 1993, 73, 7188. [Google Scholar]
- Light, M.; Chen, F.F. Helicon wave excitation with helical antennas. Phys. Plasmas 1994, 2, 1084–1093. [Google Scholar] [CrossRef]
- Arnush, D.; Chen, F.F. Generalized theory of helicon waves. II. Excitation and absorption. Phys. Plasmas 1998, 5, 1239–1254. [Google Scholar] [CrossRef]
- Shamrai, K.P.; Pavlenko, V.P.; Taranov, V.B. Excitation, conversion and damping of waves in a helicon plasma source driven by an m = 0 antenna. Plasma Phys. Control. Fusion 1997, 39, 505. [Google Scholar] [CrossRef]
- Kamiya, S.; Furuta, H.; Omiya, M. Adhesion energy of Cu/polyimide interface in flexible printed circuits. Surf. Coat. Technol. 2007, 202, 1084–1088. [Google Scholar] [CrossRef]
- Guo, Z.F.; Yi, Y.H.; Wang, L.; Yan, J.H.; Guo, H.C. Pt/TS-1 catalyst promoted C−N coupling reaction in CH4−NH3 plasma for HCN synthesis at low temperature. ACS Catal. 2018, 8, 10219–10224. [Google Scholar] [CrossRef]
- Kim, J.K.; Kang, K.H.; Lee, J.M. Electrical properties of transferred graphene films on pre-treated polyimide substrate by inductively coupled plasma. Carbon 2016, 109, 282–289. [Google Scholar] [CrossRef]
- Yang, C.H.; Lee, S.C.; Wu, J.M.; Lin, T.C. The properties of copper films deposited on polyimide by nitrogen and oxygen plasma pre-treatment. Appl. Surf. Sci. 2005, 252, 1818–1825. [Google Scholar] [CrossRef]
- Wu, P.H.; Lai, Y.Z.; Zhang, Y.P.; Sil, M.C.; Lee, P.H.H.; Wei, T.C.; Chen, C.M. Organosiloxane monolayers terminated with amine groups as adhesives for Si metallization. ACS Appl. Nano Mater. 2020, 3, 3741–3749. [Google Scholar] [CrossRef]
- Liao, Y.; Cao, B.; Wang, W.C.; Zhang, L.Q.; Wu, D.Z.; Jin, R.G. A facile method for preparing highly conductive and reflective surface-silvered polyimide films. Appl. Surf. Sci. 2009, 255, 8207–8212. [Google Scholar] [CrossRef]
- Qiu, W.L.; Leisen, J.E.; Liu, Z.Y.; Quan, W.Y.; Koros, W.J. Key features of polyimide-derived carbon molecular sieves. Angew. Chem. Int. Ed. 2021, 60, 22322–22331. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, B.L.; Wang, Z.C.; Guo, J.; Wu, B.H.; Hao, S.Q.; Zhang, Q.H.; Gu, L.; Zhou, Q.; Liu, Z.; et al. Surface coordination layer passivates oxidation of copper. Nature 2020, 586, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.X.; Hong, L.; Chen, B.H.; Ko, T.M. A study on the interfacial composition of the electroless-copper-plated BPDA-PDA polyimide sheet. J. Mater. Chem. 2003, 13, 818–824. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




