1. Introduction
Vulcanization or curing is very important process in rubber technology. During this process plastics rubber compound changes into highly deformable and elastic material – vulcanizate by formation of three-dimensional cross-linked structure within the compound matrix. This structure is generated by chemical reactions between curing agents and functional groups of rubbers. A lot of vulcanization additives have been used for cross-linking of rubber compounds, as sulfur curing systems, phenolic resins, organic peroxides, quinones, ureas, diamines, metal oxides, etc. The choice of vulcanization system is dependent mainly on the type of rubber or rubbers in the formulations and on the requirements of the final product properties. Vulcanization with sulfur-based systems and organic peroxides is still the most frequently used.
Sulfur vulcanization is generally used for general purpose rubbers as well as specialty type elastomers containing double bonds. Sulfur is always used together with accelerators and activators. During sulfur vulcanization, sulfidic cross-links with different lengths (monosulfidic -S-, disulfdidic -S2- and polysulfidic cross-links -Sx-, in which x = 3-6) are formed between polymer chain segments. Even though sulfur vulcanization process has been known for more than 160 years, the mechanism and chemical pathways are very complex and are still matter of ongoing research. Several mechanisms have been proposed having ionic or radical character [
1,
2,
3,
4]. The main attributes of sulfur based vulcanizates are good physical-mechanical properties, good tensile and tear strength, or good elastic and dynamic properties. The drawbacks are poor heat ageing stability and low resistance to thermo-oxidative ageing [
5,
6].
Organic peroxides are used to vulcanize both, saturated and unsaturated rubbers. Peroxide vulcanization is well defined process proceeding via radical pathways. The first step is homolytic cleavage of organic peroxide at a high temperature with formation of primary radicals. The primary radicals can be split into secondary intermediates [
7,
8,
9]. The formed radicals react with polymer chain segments to form carbon-carbon bonds between them [
10,
11]. C-C cross-links have higher bonding energy when compared to sulfidic bridges and thus the main features of peroxide cured materials are good resistance to elevated temperature and ageing [
2,
12]. On the contrary, they exhibit worse tensile behavior and weaker dynamic characteristics.
Cross-linking of rubber compounds with organic peroxides can be efficiently enhanced by application of co-agents. Co-agents are organic substances with activated double bonds, which can react with rubber chains by addition and abstraction of hydrogen or by addition reactions only [
13,
14]. By grafting onto rubber chains they can form extra cross-links, which are dependent on the type of the co-agent used [
15]. Several benefits have been described for vulcanizates cured with peroxide and co-agent compared to those treated only with organic peroxide, as higher tensile and tear stress, higher hardness, higher modulus, enhanced dynamic properties or improved compression set, etc. [
16,
17,
18,
19].
Ethylene-propylene-diene-monomer rubber (EPDM) is a terpolymer comprising of ethylene and propylene structural units with randomly distributed non-conjugated diene, as 5-vinyl-2-norbornene, dicyclopentadiene or 5-ethylidene-2-norbornene. It has saturated polymer backbone and pendant unsaturation in diene monomer unit. EPDM belongs to the most versatile specialty type rubbers. Its production has been continually increasing as it can be used in applications for special as well as general utilizations. EPDM has very good electro-insulating properties, very good resistance to polar agents, water, and vapor. It has also very good resistance to oxygen, ozone, elevated temperature, and several chemicals. EPDM exhibits very good elastic properties at low temperatures and low compression set. However, tensile strength is rather low. EPDM can be effectively cured with peroxide as well as sulfur vulcanization systems [
8,
20,
21,
22].
In this work, peroxide vulcanization system, sulfur vulcanization system and combined vulcanization systems have been used for cross-linking of virgin EPDM and EDPM filled with carbon black. As each curing system has benefits but also drawbacks, the main point was to combine vulcanization system to combine the patterns of the cross-links provided by each vulcanization system to suppress the negatives and possibly to highlight the positives.
2. Experimental
2.1. Materials
Ethylene-propylene-diene-monomer rubber (EPDM, type KEP 570F) was provided by Kumho Polychem Co. Ltd., South Korea. The rubber consisted of 70 wt.% ethylene units, 4.5 wt.% of 5-ethylidene-2-norbornene monomer units (ENB) and the rest were propylene structural units. Carbon black (CB, type Continex N330) was provided by Continental Carbon Co., Belgium. Sulfur curing system consisted of activators—zinc oxide and stearic acid (Slovlak, Košeca, Slovakia), accelerator—N-cyclohexyl-2-benzothiazole sulfenamide (CBS, Duslo, Šaľa, Slovakia) and curing agent - sulfur (Siarkopol, Tarnobrzeg, Poland). Peroxide curing system consisted of dicumyl peroxide (DCP) in combination with trimethylolpropane trimethacrylate (TMPTMA) as a co-agent. They were supplied from Sigma-Aldrich, USA.
2.2. Methods
2.2.1. Preparation and curing of rubber compounds
Five types of rubber compounds based on virgin EPDM were fabricated and tested in this study. The first rubber compound was fabricated only by application of peroxide vulcanization system, while in the last one, only sulfur vulcanization system was introduced. In the rest three types of rubber formulations, the mutual ratio of peroxide and sulfur vulcanization system was changed. The next five types of rubber formulations were fabricated with the same composition of curing systems, but carbon black in the constant amount 25 phr was used as a filler in each rubber compound. The carbon black rubber masterbatch was pre-compounded in kneading machine Buzuluk (Komárov, Czech Republic) in Vipo. a.s, Partizánske. The composition of unfilled and filled rubber compounds is summarized in
Table 1 and
Table 2.
The rubber compounds were fabricated using laboratory kneading machine Brabender (Brabender GmbH & Co. KG, Duisburg, Germany) in two-step mixing process. The temperature of kneading chamber was 80 °C and the speed of the rotor was set up to 55 rpm. First, rubber or rubber masterbatch was plasticated for 1 min. Zinc oxide and stearic acid were then added with next 3 min compounding. The mixed materials were subsequently cooled down in calender roll mill and put back in the kneading chamber. In the second step, the additives of sulfur curing (sulfur, accelerator) and/or peroxide curing system (peroxide, co-agent) were added. The second step mixing took 4 min at 55 rpm and 80 °C. In the final step, the compounds were cooled down and sheeted in calender.
The vulcanization process of compounded formulations was performed at 180 ºC and pressure of 15 MPa by employing of a hydraulic heated platens press Fontijne (Fontijne Presses b.v., Vlaardingen, Holland) based on previously determined optimum cure time.
2.2.2. Determination of curing characteristics
The curing parameters of rubber formulations were evaluated from corresponding vulcanization curves, which were obtained by performing oscillatory measurements in moving die rheometer MDR 2000 (Alpha Technologies, Akron, Ohio, USA).
The investigated characteristics were:
∆M (dN.m) - torque difference, the difference between the maximum and minimum torque (∆M = MH - ML).
tc90 (min) – optimum curing time
ts1 (min) – scorch time
Rv (min
-1) – curing rate index, which is defined as:
Rrev (min
-1) – reversion rate index, which is defined as:
trev – time at which Mrev = MH - 0.02.ΔM
tH – time at which M = MH
2.2.3. Determination of cross-link density
To calculate the cross-link density
ν, small specimens of vulcanizates were fully immersed in xylene for 30 hours at a laboratory temperature. During time, xylene diffuses into the samples and disrupts almost all physical bonds and interactions within the compound matrix, while chemical cross-links remain intact. The weight of samples was regularly detected every hour until the equilibrium swelling was reached. This means that no physical bonds were present in the rubber compound and free space was occupied by solvent. The chemical cross-link density, i.e. the concentration of chemical cross-links is then possible to calculate. The Flory-Rehner equation for unfilled vulcanizates and Krause modified Flory-Rehner equation for carbon black filled vulcanizates [
23] was used to determine the cross-link density based on previously achieved equilibrium swelling state.
2.2.4. Investigation of physical-mechanical characteristics
Zwick Roell/Z 2.5 (Zwick GmbH & Co. KG, Ulm, Germany) was employed to investigate the tensile behavior of vulcanizates. Dumbbell-shaped test specimens having width 6.4 mm, length 80 mm and thickness 2 mm were used for measurements while cross-head speed of the appliance was set up to 500 mm.min-1. Durometer was used to detect the hardness of vulcanizates in Shore A.
2.2.5. Determination of dynamical-mechanical properties
Visco-elastic properties of vulcanizates were investigated by using a dynamic-mechanical thermal analyzer DMTA MkIII (Mettler-Toledo GmbH, Greifensee, Switzerland) The samples were analyzed in tensile mode in temperature range from -60 ºC to 80 ºC, at a frequency 10 Hz, amplitude of dynamic deformation 64 μm and static force 0.2 N.
3. Results and discussion
3.1. Curing process
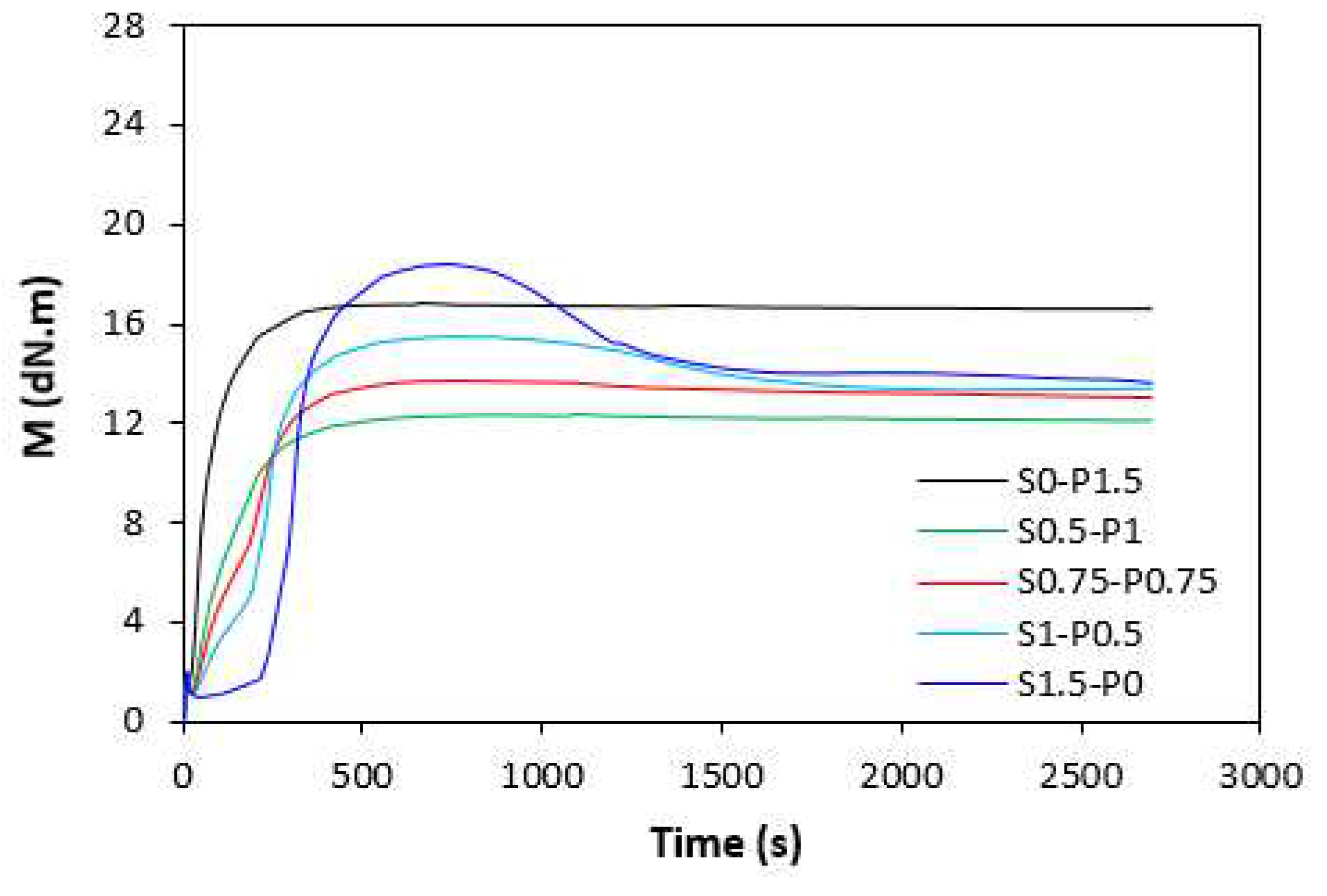
The influence of curing systems on vulcanization process of rubber compounds was evaluated based on determination of vulcanization characteristics, which were calculated from vulcanization isotherms (
Figure 1 and
Figure 2). From
Figure 1 it becomes apparent that the rubber compounds cured with peroxide system (S0-P1.5) exhibited the lowest scorch time
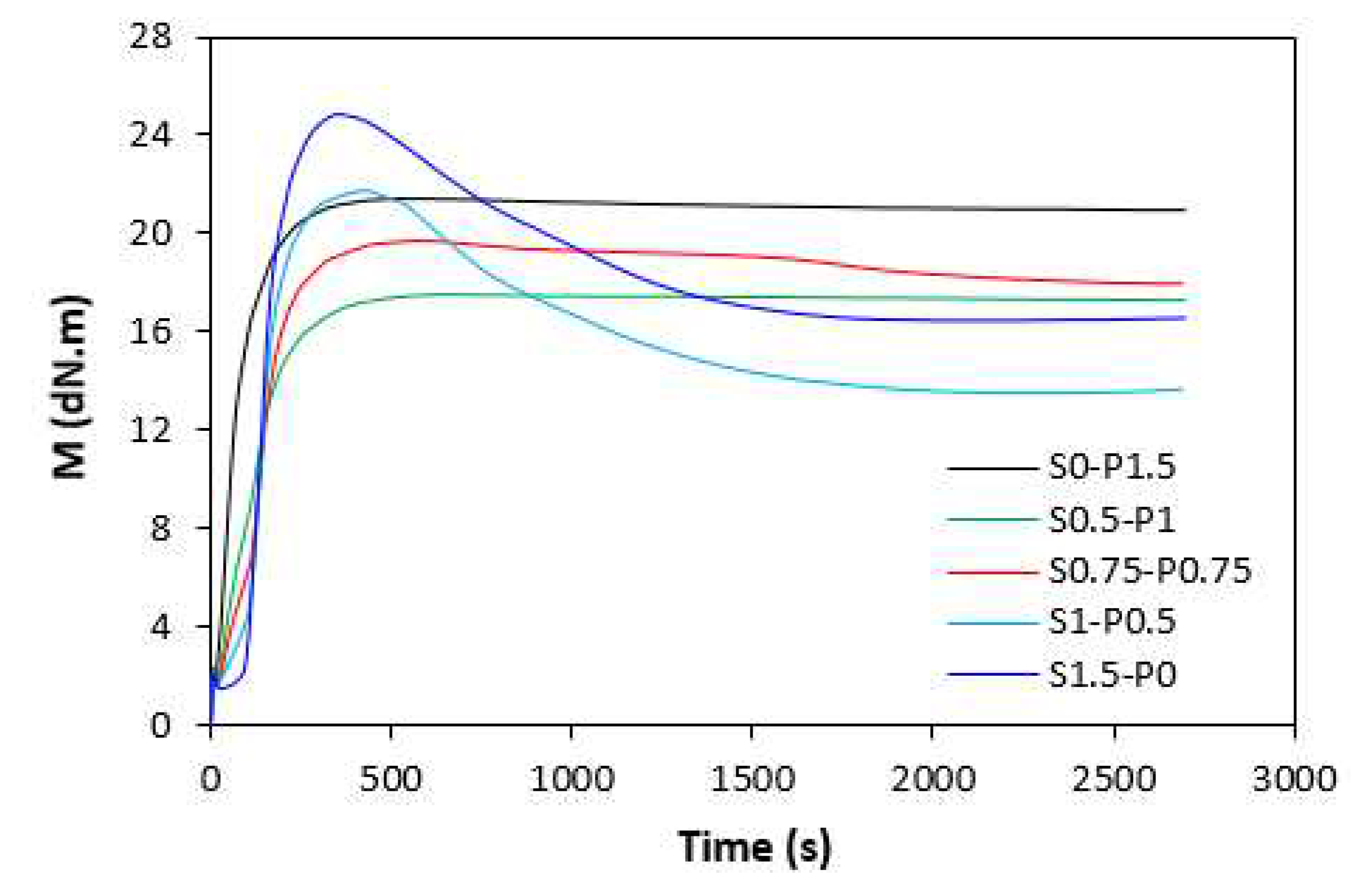
ts1 and the torque stabilized at constant value after reaching the maximum. The samples with higher proportion of peroxide system (S0.5-P1) and equivalent ratio of sulfur to peroxide (S0.75-P0.75) were found to have low scorch time and keeping ,,plato,, after reaching maximum torque. On the other hand, rubber compounds with higher ratio of sulfur system (S1-P0.5 and S1.5-P0) demonstrated longer scorch time, but after reaching the maximum torque, the reversion occurred. Similar behavior was possible to observe from curing isotherms of filled rubber compounds (
Figure 2). From
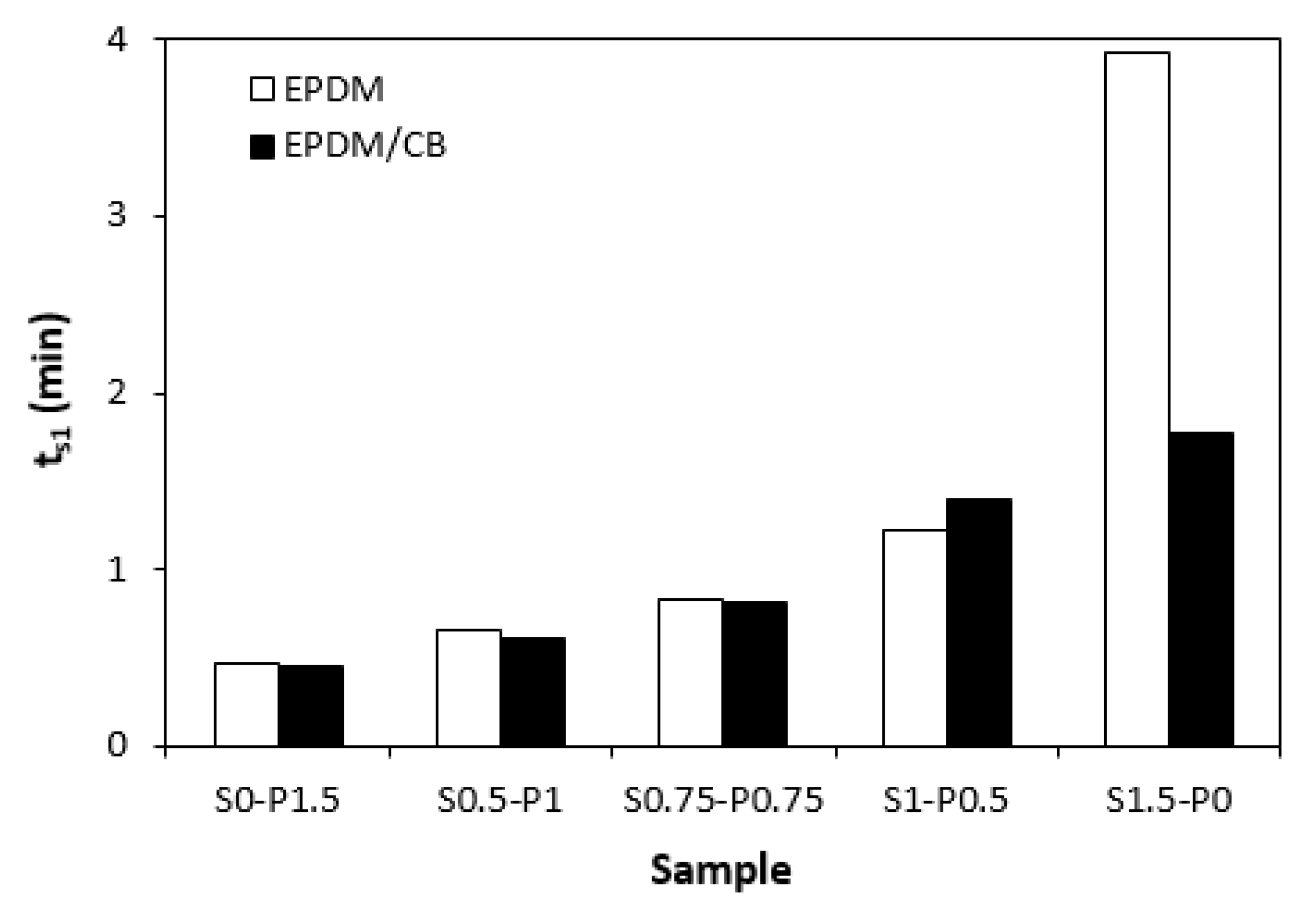
Figure 3 it becomes apparent that the lowest scorch time exhibited both unfilled and filled rubber compounds cured only with peroxide system (S0-P1.5). The higher was the ratio of sulfur in combined vulcanization systems, the longer was the scorch time. The highest
ts1 exhibited the unfilled rubber compound cured only with sulfur system (S1.5-P0).
Peroxide cross-linking of rubber compounds is a relatively simple radical process, during which organic peroxide undergoes homolytic cleavage at a vulcanization temperature. Peroxide derived radicals immediately react with rubber functional groups to form cross-links between rubber chain segments [
11,
18,
24,
25]. Thus, the regulation of scorch time during peroxide curing process can only be done by the type of peroxide and its dissociation rate at a curing temperature. On the other hand, sulfur vulcanization is very intricate process running in several stages. The scorch time as well as the whole vulcanization process can be well controlled by the composition of sulfur vulcanization system, mainly by the amount and also the type of accelerator [
2,
26]. N-cyclohexyl-2-benzothiazole sulfonamide (CBS) belongs to the class called ,,delayed action,, accelerators. During the curing process, it prolongs the induction period, which is good for processing of rubber compounds to the desired shapes without premature cross-linking. The main cross-linking phase is then very fast, which is more pronounced from economical and time aspects [
27]. To that corresponds prolongation of scorch time with increasing ratio of sulfur in both, unfilled and filled rubber formulations.
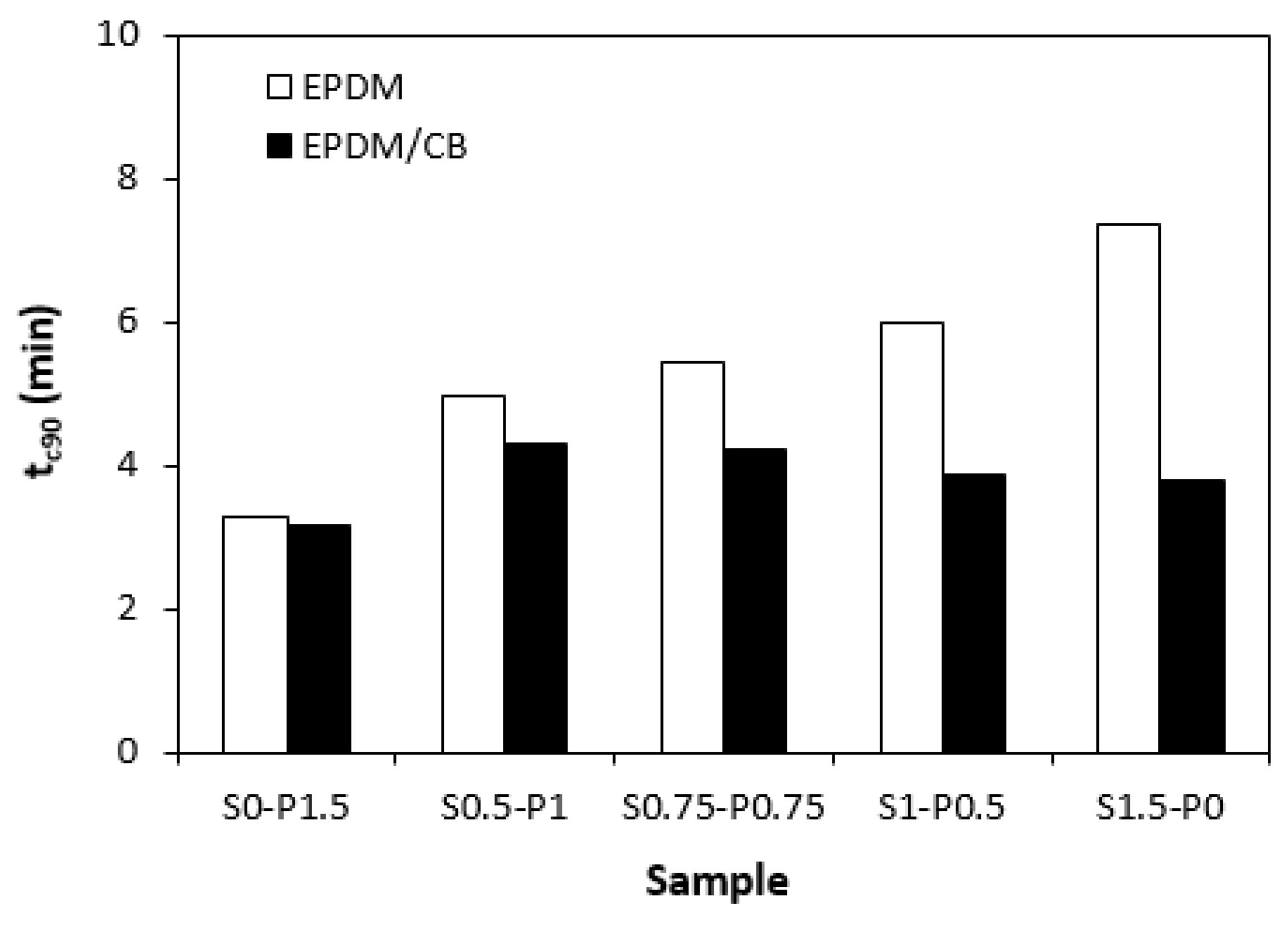
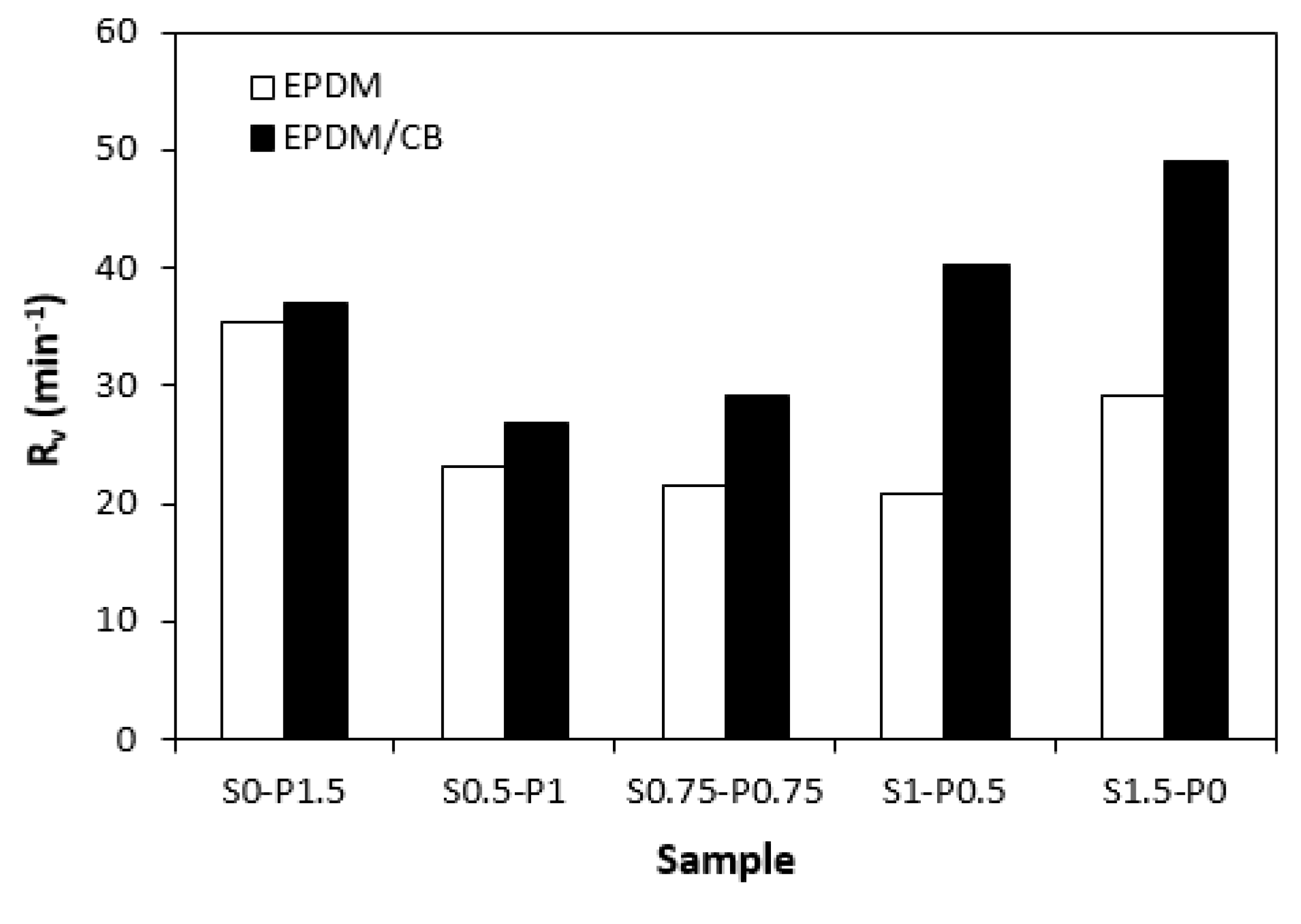
When comparing vulcanization isotherms of unfilled and filled rubber compounds it becomes apparent that curing process of rubber compounds with incorporated carbon black was faster, which indicates steeper slope of curing isotherms in the main cross-linking phase. The calculated values of optimum cure time
tc90 (
Figure 4) and curing rate index
Rv (
Figure 5) clearly pointed out to faster curing kinetics of filled rubber compounds. This can be attributed to carbon black that enhanced heat transfer within the rubber compounds, which resulted in acceleration of the curing process. The optimum cure time of unfilled rubber compounds increased with increasing ratio of sulfur in combined vulcanization systems. The sample cured with sulfur system (S1.5-P0) required more than 4 min longer time needed for optimum cross-linking when compared to the rubber compound cured with peroxide system (S0-P1.5). As also seen from
Figure 4, the
tc90 of filled rubber compounds was shorter than that of unfilled formulations. There were recorded no significant changes of the optimum cure time in dependence on curing system composition and the filled rubber compounds cured either with peroxide (S0-P1.5) or sulfur (S1.5-P0) system were found to have very similar
tc90. It can be stated that the influence of curing system composition on scorch time and optimum cure time of filled rubber systems was less visible in comparison with the unfilled equivalents. The highest curing rate index
Rv exhibited the filled rubber compound cured with sulfur system (S1.5-P0), followed by the sample cured with peroxide system (S0-P1.5). From
Figure 5 it also becomes apparent that both, filled as well as unfilled rubber compounds cured with combined vulcanization systems (S0.5-P1, S0.75-P0.75, S1-P0.5) demonstrated lower
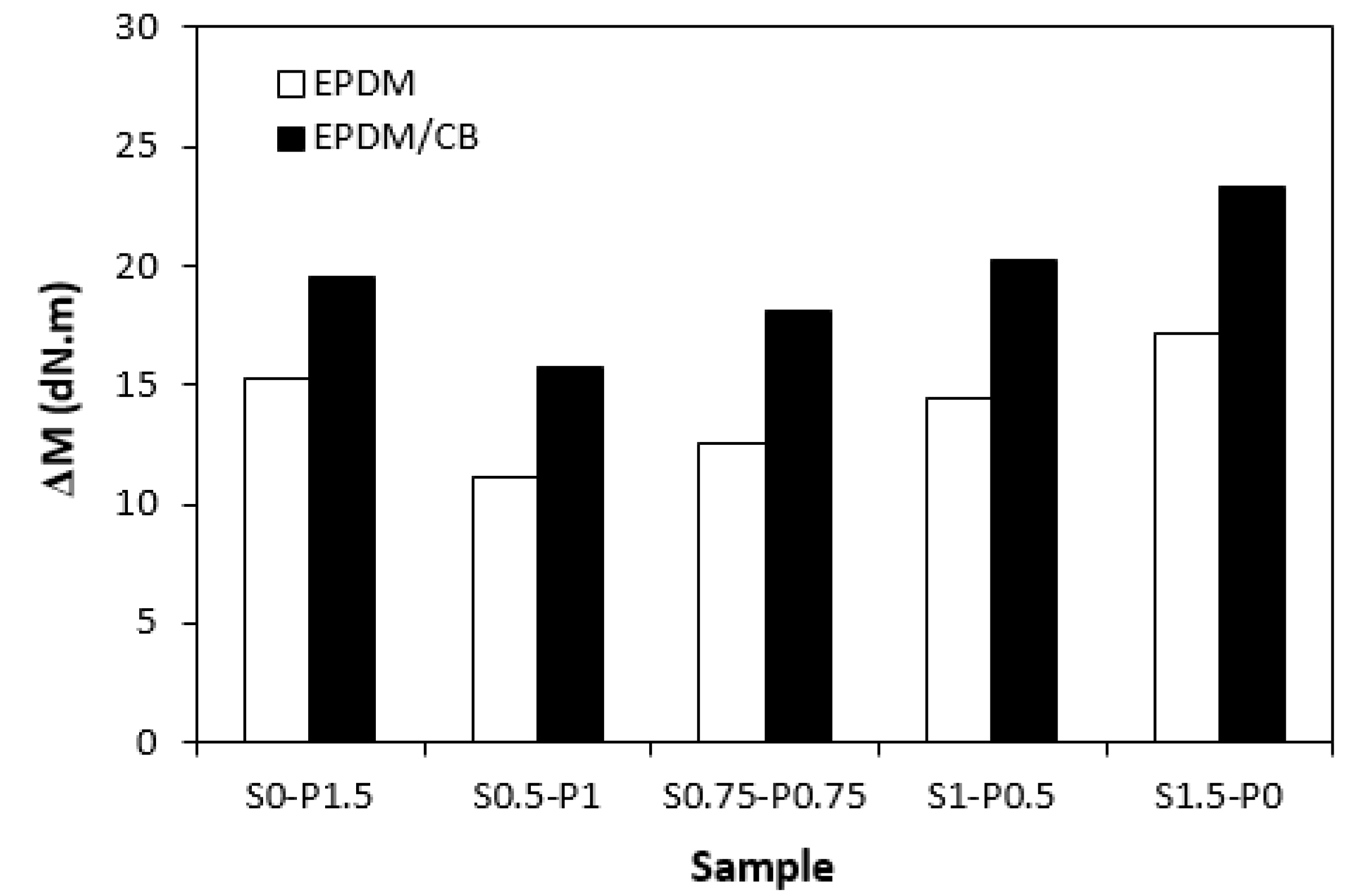
Rv when compared to their equivalents cured with sulfur or peroxide system. Similarly, both types of rubber compounds cured with sulfur or peroxide curing system exhibited the highest difference between the maximum and minimum torque. Application of combined vulcanization systems caused the decrease in
ΔM, although the differences in dependence on curing system composition were not very significant. As also seen in
Figure 6, higher
ΔM values exhibited filled rubber compounds, which can be again attributed to the reinforcing filler. Carbon black stiffens and reinforces the rubber matrix and increases the viscosity of the compounds, which was reflected in the increase of minimum and maximum torque as well as in the increase of torque difference.
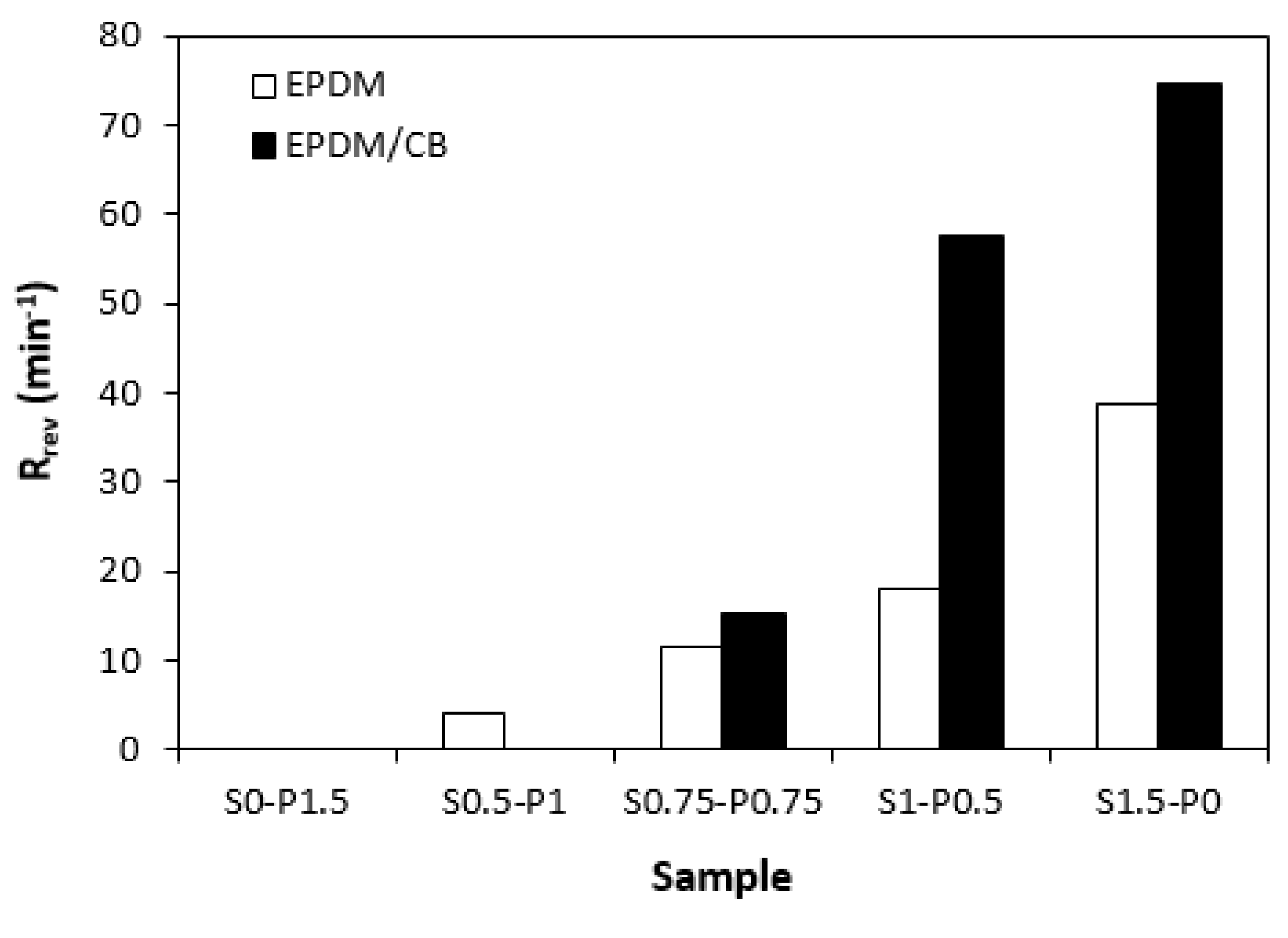
From curing isotherms one can also see that some rubber compounds undergo reversion after reaching the maximum torque. Reversion is undesired phenomenon during which, the destruction of the cross-links formed in the main vulcanization phase, occurs. From
Figure 7 it becomes obvious that reversion rate index
Rrev showed increasing trend with increasing ratio of sulfur curing system. The reason is ascribed to the structure of the formed cross-links. Application of peroxide curing systems leads to the formation of carbon-carbon and multifunctional cross-links between rubber chains, which have high bonding energy and high thermal stability. On the other hand, sulfidic cross-links with different length (monosulfidic, disulfic, polysulfidic cross-links) are generated within the rubber compounds when they are cured in the presence of sulfur curing systems. Sulfidic bonds have lower dissociation energy and are less thermally stable [
28,
29]. Therefore, thermal decomposition of sulfidic cross-links occurs with increase in cure time. This leads to the decrease in cross-link density and viscosity of cured rubber compounds, which is indicated as the decrease in torque in curing isotherms. As also seen in
Figure 7, higher reversion rate index exhibited rubber compounds with incorporated carbon black, which is more evident for samples with designations S1-P0.5 and S1.5-P0. Thus, it can be concluded that carbon black leads to faster curing process of rubber compounds on one hand. On the other hand, the CB filled rubber compounds are more prone to reversion, probably due to increased heat transfer trough the samples.
3.2. Cross-link density
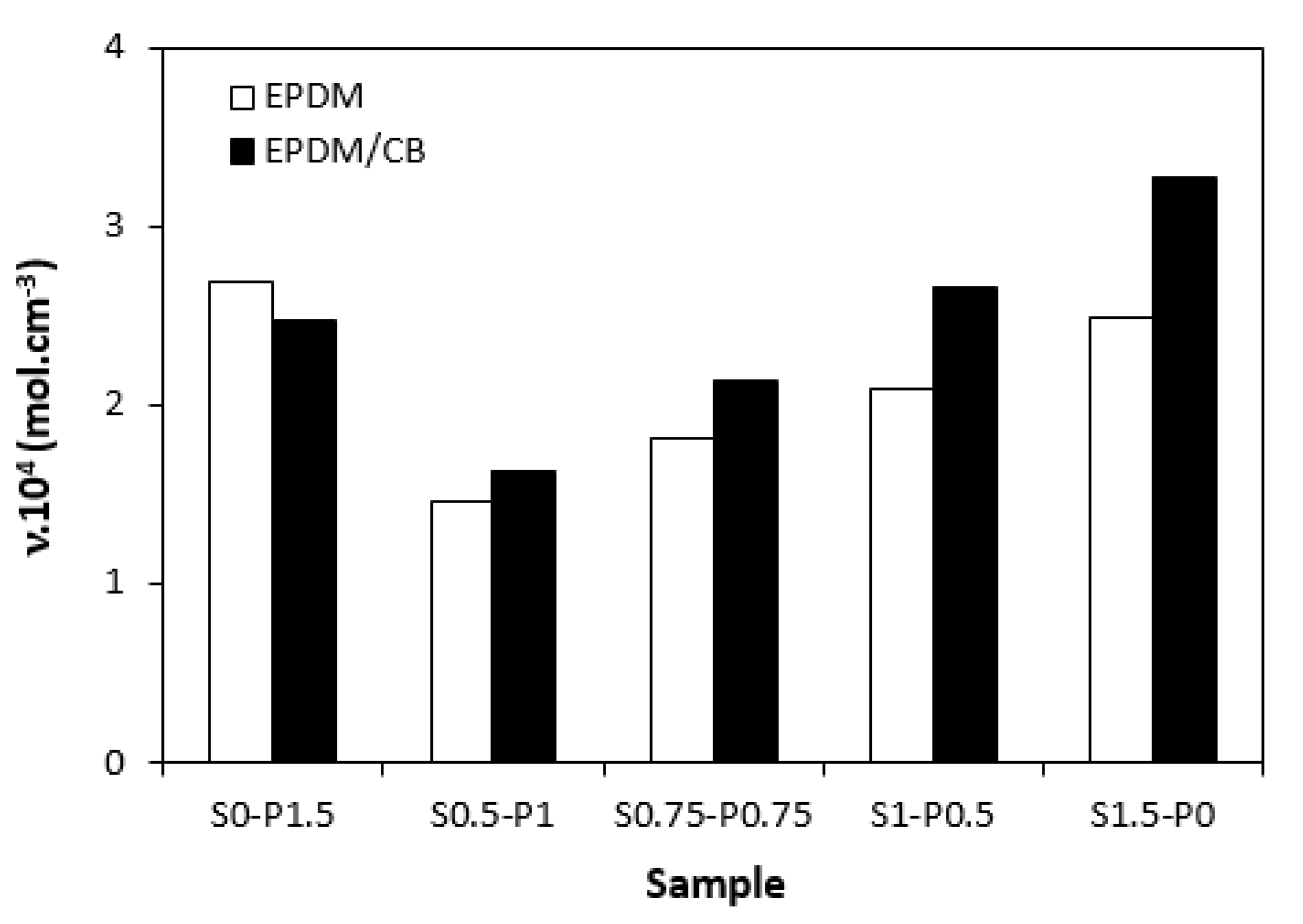
The dependences of cross-link density (
Figure 8) on curing system composition are in close correlation with the dependences of torque difference (
Figure 6), clearly confirming close relation between both characteristics. When considering unfilled vulcanizates, the highest cross-link density demonstrated the sample cured with peroxide system (S0-P1.5), followed by the vulcanizate cured sulfur system (S1.5-P0). The cross-link density of vulcanizates cured with combined vulcanization systems (S0.5-P1, S0.75-P0.75, S1-P0.5) was found to increase with increasing ratio of sulfur to peroxide. The similar results were also recorded for filled vulcanizates, but the highest cross-link density was determined in the structure of the sample cured with sulfur system. The unfilled vulcanizate cured with peroxide system exhibited higher cross-link density when compared to its filled equivalent. When applying sulfur curing system and combined peroxide and sulfur curing systems, higher cross-linking degree exhibited vulcanizates with incorporated carbon black. It also becomes apparent that differences between cross-link densities of unfilled and filled vulcanizates became higher with increasing amount of sulfur in vulcanization systems. In general, carbon black filled rubber systems exhibit higher cross-link density when compared to their unfilled equivalents. This is because rubber chains in the proximity of CB particles are strongly physically adsorbed or chemisorbed on the filler and they behave like a polymer in glassy state. The strongly bonded rubber-filler layers are insoluble in the solvents used for determination of cross-link density and thus contribute to the apparent increase of cross-linking degree. As seen in
Figure 8, with the increase of peroxide to sulfur ratio, the differences in cross-link density between both vulcanizate types became lower, while the unfilled vulcanizate with designation S0-P1.5 was found to have higher cross-linking degree when compared to the filled counterpart. The reason might be attributed to the fact that acidic substances, like some types of carbon black can cause heterolytic decomposition of the peroxide with formation of ions and not radicals, which are not effective in the cross-link formation. The results might suggest that for unfilled EPDM based vulcanizates are more convenient peroxide curing systems, while for CB filled counterparts seems to be more suitable sulfur curing systems. However, it must be remarked that the values of cross-link density are not a determining step for the selection of vulcanization system. Instead, the structure of the formed cross-links related to the properties of vulcanizates and their thermo-oxidative stability should be under consideration.
As already outlined, during peroxide vulcanization, dicumyl peroxide first undergoes homolytic heat induced cleavage with formation of primary cumyloxy radicals that are dissociated into secondary methyl radicals [
30]. Acetophenone is released as a byproduct from thermal decomposition of the peroxide. Both, cumyloxy and methyl radicals are supposed to be efficient during peroxide cross-linking of rubbers [
18,
31]. EPDM has saturated main polymer chain with randomly distributed non-conjugated diene monomer having pendant unsaturation. Peroxide cross-linking of EPDM is relatively efficient. Radical fragments from peroxide can abstract hydrogen atoms from the main elastomer backbone yielding alkyl macroradicals, but also from diene units, in which H-atom is situated in allylic position (allyl macromolecular radicals). Cross-linking occurs trough two pathways. Recombination of alkyl and allyl macroradicals leads to the formation of allyl/allyl, alkyl/alkyl and alkyl/allyl cross-link combinations. EPDM alkyl and allyl macroradicals can also add to the double bonds in diene monomer units. For steric reason, the macromolecular radical thus formed would not propagate with another diene unit, but probably terminates via hydrogen transfer with formation of allyl/alkene and alkyl/alkene cross-links [
32,
33,
34].
Dicumyl peroxide was used in combination with trimethylolpropane trimethacrylate. During co-agent assisted peroxide vulcanization, not only carbon-carbon bonds within the rubber matrix are formed, but also multifunctional cross-links trough grafting of co-agents between rubber chains segments are generated [
2,
19,
35,
36]. In general, co-agents are low molecular weight organic molecules having high reactivity with free radicals. They boost the cross-linking efficiency of the curing process with organic peroxides and they tend to increase the cross-link density. Most of them have been reported to homopolymerize and graft to macroradicals and thus form co-agent bridges between the polymer chain segments as extra cross-links [
13,
14,
37,
38,
39]. This subsequently leads to the property improvements of the final products.
Chemism and mechanism of sulfur vulcanization is very intricate and still not comprehensibly clarified. It involves a series and complex parallel and consecutive reactions, which may have additive, substitutive or even elimination character. Not only the input materials, but also products of their transformations and intermediates can take part in the process. Sulfur cross-linking mechanism for EPDM has been reported to be similar to that, which is generally accepted for diene rubbers [
2,
40]. In the first stage, accelerator together with activators form transition complex, which react with sulfur to form active sulfurating agent. The complex thus formed substitutes the hydrogen atom in allylic position of ENB and it is attached to the rubber via a sulfur bridge, forming cross-link precursors [
41]. In the second stage, primary vulcanizate network is formed with dominance of polysulfidic bonds. The last stage relates to the restructuralization of the primary network by lowering of the number of sulfur atoms in sulfidic bridges and modification of rubber chains and final cross-linked network is generated.
As seen in
Figure 8, cross-link density of vulcanizates cured with combined vulcanization systems was lower when compared to the samples cured either with peroxide or sulfur curing system. For both, unfilled and filled vulcanizates, the lowest cross-link degree demonstrated the sample with designation S0.5-P1. Probably, interactions between the additives of both curing systems occurred that resulted in the decrease of effective concentration of peroxide and sulfur fragments, which were then not efficient during the curing process.
3.3. Physical-mechanical properties
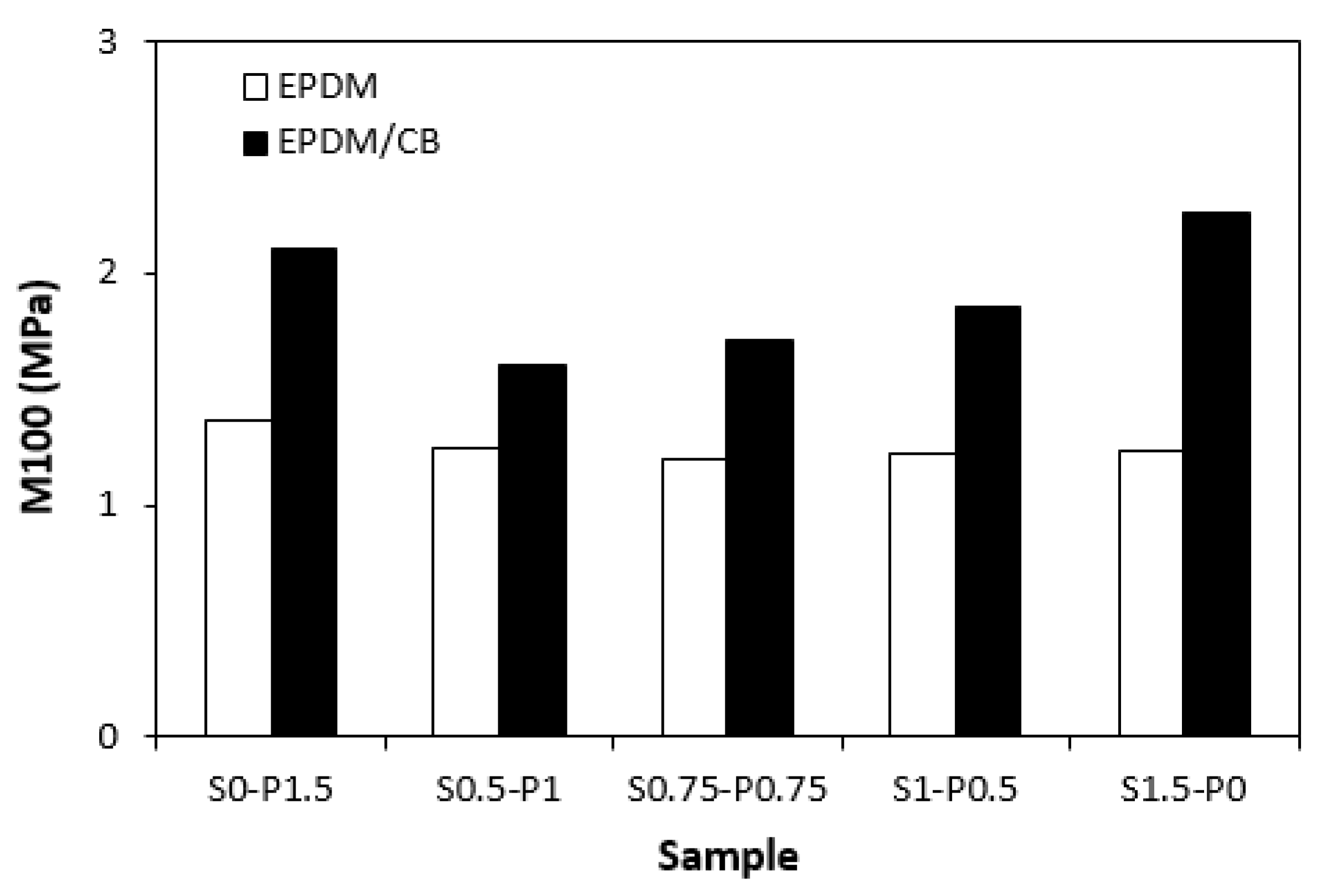
The dependences modulus M100 (
Figure 9) on curing systems correlates with the dependences of cross-link density (
Figure 8). The highest modulus M100 exhibited the filled vulcanizate cured with sulfur system (S1.5-P0) with the highest cross-link density, followed by the vulcanizate cured in the presence of peroxide system (S0-P1.5). The modulus of the filled vulcanizates cured with combined vulcanization systems (S0.5-P1, S0.75-P0.75, S1-P0.5) showed increasing tendency with increasing sulfur to peroxide ratio. The influence of curing system composition on modulus of unfilled vulcanizates was much less visible as the values of M100 were very similar for each tested cured rubber compound. Higher modulus of filled vulcanizates can be ascribed to their higher cross-linking degree (with exception of the sample S0-P1.5) and to reinforcing effect of carbon black. The highest cross-link density of the filled vulcanizate cured with sulfur system (S1.5-P0) was also reflected in the highest hardness of the corresponding vulcanizate (
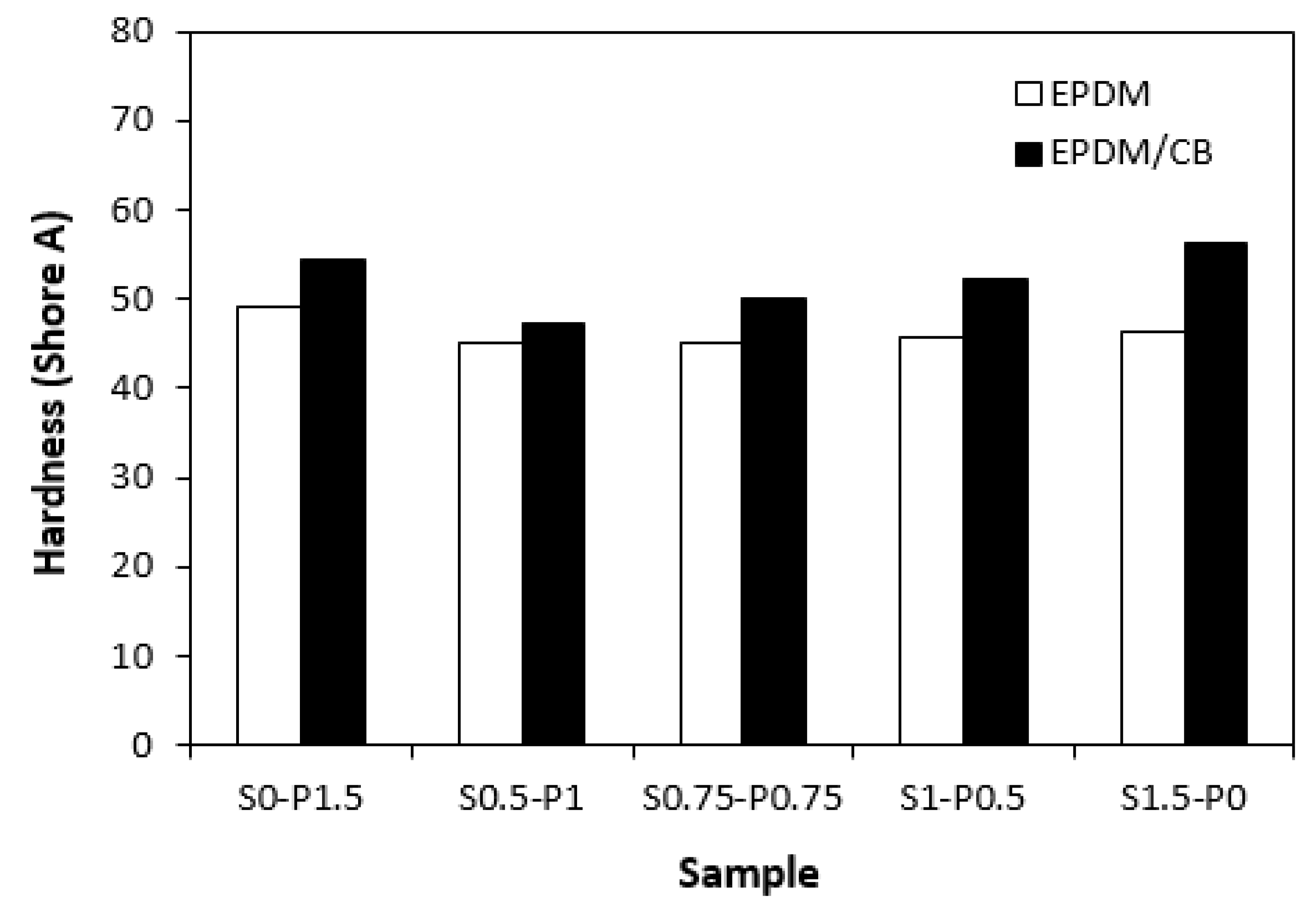
Figure 10). The vulcanizates cured with combined vulcanization systems were found to have lower hardness when compared to equivalent rubber compounds cured only with sulfur or peroxide system. Again, the influence of curing system composition on hardness of unfilled vulcanizates was less pronounced.
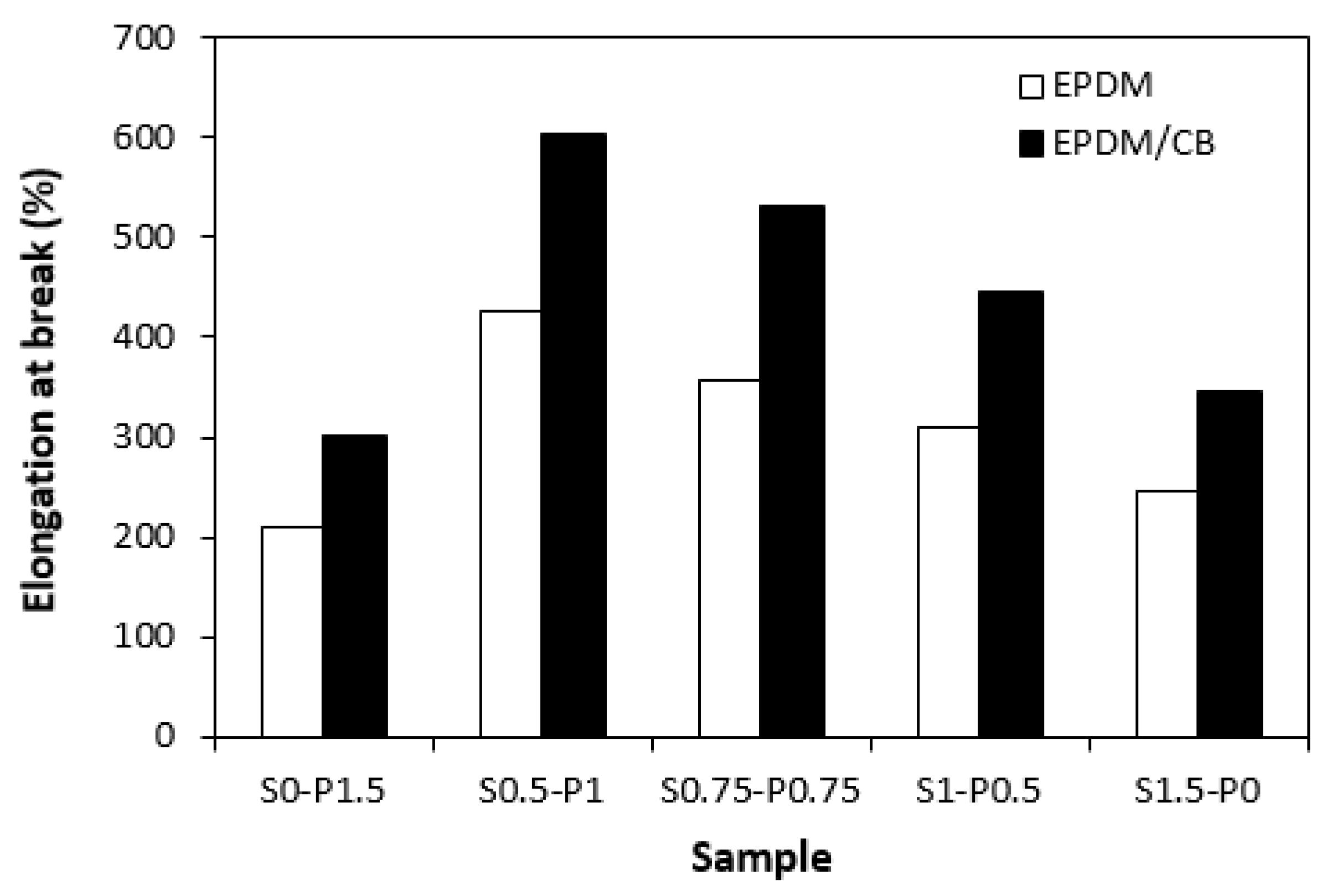
One can also observe certain correlation between the dependences of cross-link density and elongation at break of vulcanizates. From
Figure 11 it becomes obvious that the highest elongation at break demonstrated both, unfilled and filled vulcanizates with designation S0.5-P1 with the lowest cross-linking degree. The higher was the cross-link density, the more was restricted the elasticity and mobility of rubber chain segments, which had negative impact on elongation at break. Thus, it becomes apparent that the lowest elongation at break reached the vulcanizates cured with sulfur (S1.5-P0) or peroxide (S0-P1.5) system with the highest cross-link density. Higher elongation at break exhibited vulcanizates with incorporated carbon black despite their higher cross-link density.
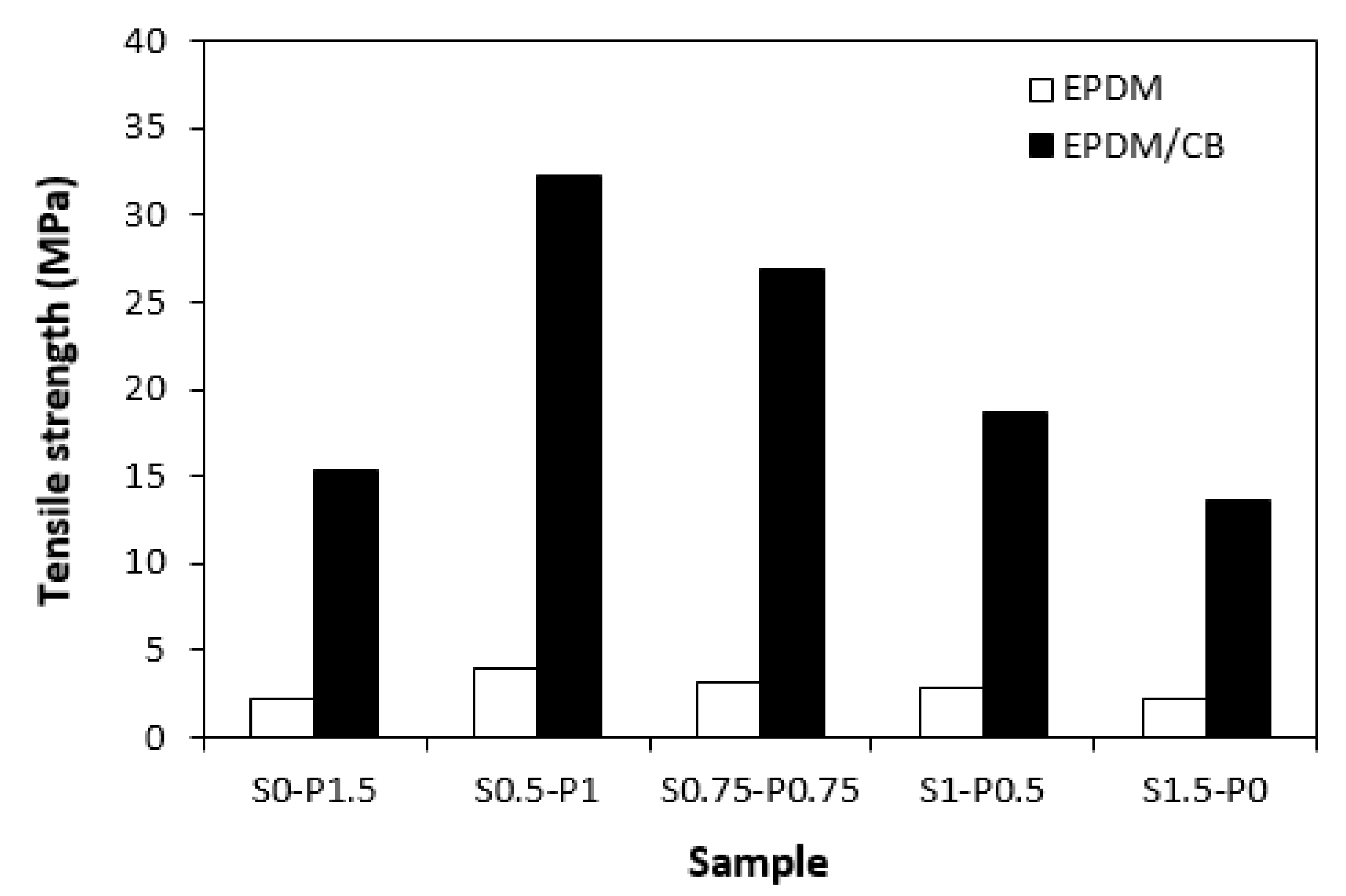
When looking at graphical dependence of curing system composition on tensile strength (
Figure 12), one can see that the highest values exhibited unfilled and filled vulcanizates cured in the presence of 1 phr DCP and 0.5 phr sulfur (S0.5-P1). On the other hand, the lowest tensile properties demonstrated the vulcanizates cured only with sulfur or peroxide system. It could seem that the tensile behavior of vulcanizates is also proportional to the cross-link density. However, the tensile strength is a complex property, which is dependent not only on the cross-linking degree, but mainly on the type and structure of the formed cross-links and on the type and amount of the filler as well [
42]. It becomes apparent that CB filled vulcanizates cured with combined vulcanization systems (S0.5-P1, S0.75-P0.75, S1-P0.5) exhibited much higher tensile strength in comparison with their counterparts cured only in the presence of peroxide (S0-P1.5) or sulfur (S1.5-P0) system. The tensile strength of the vulcanizate with designation S0.5-P1 (over than 32 MPa) was more than two times higher in comparison with the vulcanizate S0-P1.5 (over 15 MPa) and with the vulcanizate S1.5-P0 (13.5 MPa). The unfilled vulcanizates cured with combined vulcanization systems also demonstrated higher tensile strength when compared to vulcanizates with designations S0-P1.5 and S1.5-P0. However, the differences in their values were not so pronounced as in the case of filled vulcanizates. The vulcanizates cured with sulfur (S1.5-P0) or peroxide (S0-P1.5) system reached almost the same tensile strength (2.2 MPa). When peroxide system was combined with sulfur system in the ratio S0.5-P1, the tensile strength increased up to 4 MPa. It also becomes apparent that much higher tensile strength demonstrated filled vulcanizates due to the reinforcing carbon black.
The obtained results pointed out to some synergic effect of both curing systems. By mutual combination of both systems, the pattern of sulfidic cross-links, carbon-carbon cross-links and multifunctional cross-links from co-agent is combined, which results in modification of physical-mechanical characteristics of the final products. Based upon the achieved experimental outputs it can also be stated that the influence of vulcanization systems is more evident for filled vulcanizates. Thus, also the type of the filler must be considered when applying vulcanization systems for cross-linking of rubber compounds.
3.4. Dynamical-mechanical analysis
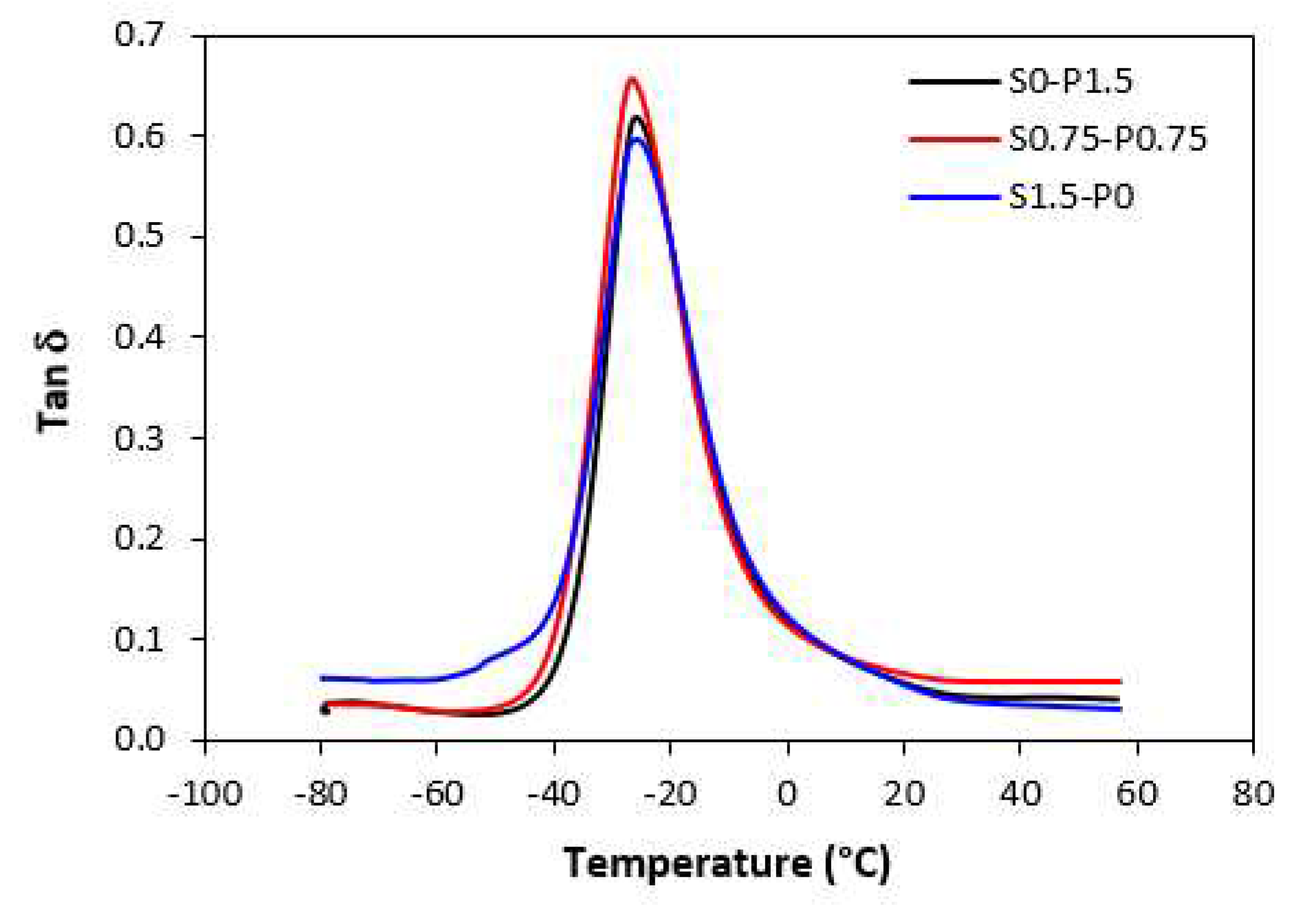
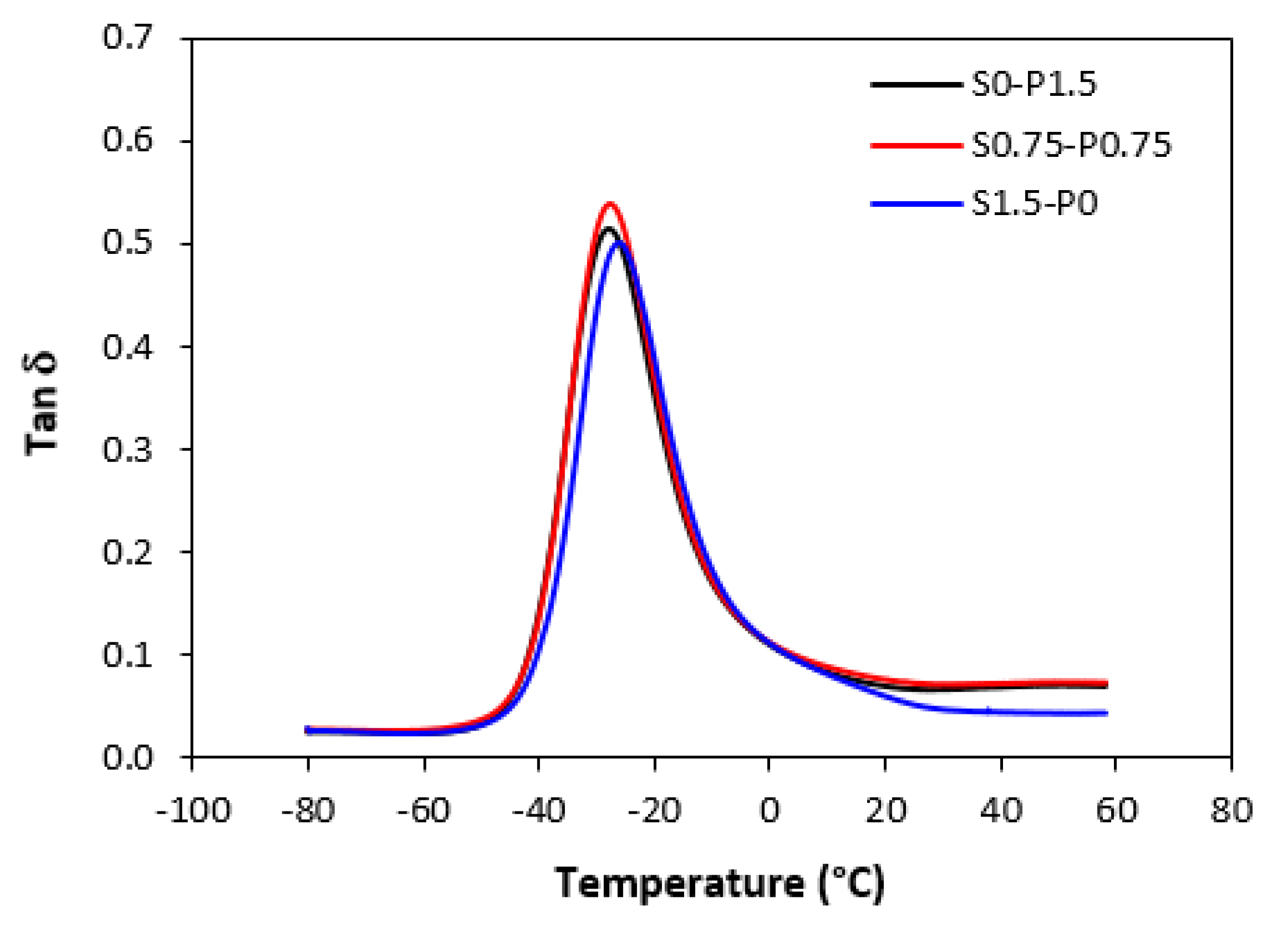
Dynamic-mechanical analysis was carried out to evaluate the influence of the composition of curing systems on viscoelastic properties of vulcanizates. Samples cured only with sulfur, peroxide, and combined sulfur and peroxide system in their equivalent ratio were chosen for the measurement.
The results revealed that storage
G’ as well as loss
G” modulus were not significantly influenced by the composition of the curing systems. Thus, also no meaningful change in loss factor
tan δ was recorded in dependence on curing system composition. The temperature dependences of
tan δ for unfilled and filled vulcanizates are graphically illustrated in
Figure 13 and
Figure 14, while the calculated values at specific temperatures are summarized in Tabs. 3 and 4. As shown, almost no change of loss factor was recorded in dependence on curing system composition for both types of vulcanizates. The higher was temperature, the lower was
tan δ. At very low temperatures, higher loss factor exhibited unfilled vulcanizates. With increase in temperature, the differences in
tan δ for both vulcanizate types became smaller. The peak maximum in loss factor temperature dependences corresponds to glass transition temperature
Tg. In general, the
Tg is dependent mainly on the type of rubber, or rubbers in the formulations, but can also be influenced by other factors, as presence of additives, mostly plasticizers or fillers, cross-link density, etc. The type and structure of the formed cross-links might have an influence on glass transition temperature, too [
43]. From
Figure 13 and
Table 3 it becomes apparent that
Tg of unfilled rubber systems was almost the same regardless of the composition of curing system. The same statement can also be applied for filled vulcanizates. As seen in
Figure 14 and
Table 4, the glass transition temperature for filled vulcanizates moved only in low range of experimental values. It can also be stated that neither the presence of carbon black in rubber formulations influenced the
Tg. Although, the
Tg of filled vulcanizates was in about 1 - 1.5 °C lower when compared to unfilled equivalents, the decline can be considered as negligible.
4. Conclusion
The results revealed the higher was the amount sulfur in mixed curing systems the longer was the scorch time. The optimum cure time of unfilled rubber compounds showed increasing tendency with increasing ratio of sulfur, while the optimum cure time of filled rubber formulations seems not to be so dependent on curing system composition. Rubber formulations with higher amount of sulfur system were more prone to reversion after reaching maximum torque. Faster vulcanization was observed for fille d rubber compounds due to carbon black that enhanced heat transfer trough cured samples. The highest cross-link density exhibited both, unfilled and filled vulcanizates cured only with sulfur or peroxide system. The cross-link density of vulcanizates cured with combined sulfur/peroxide systems showed increasing trend with increasing sulfur to peroxide ratio. The dependences of hardness, modulus and elongation at break were in close correlation with the dependences of cross-link density. The tensile strength of vulcanizates cured in the presence of combined vulcanization systems was higher when compared their equivalents cured only with sulfur or peroxide system. This points out to the fact that by mutual combinations of curing systems, the pattern of sulfidic cross-links, carbon-carbon bonds and multifunctional cross-links from co-agents are suitably combined, which resulted in improvement of tensile behavior of vulcanizates. On the other hand, no considerable effect of curing system compositions on dynamic-mechanical characteristics was recorded.
Ackowledgement
This work was supported by the Slovak Research and Development Agency under the contract No. APVV-19-0091 and APVV-22-0011.
References
- Akiba M, Hashim AS: Vulcanization and crosslinking in elastomers. Prog Polym Sci. 1997, 22, 475–521. [CrossRef]
- Kruželák J, Sýkora R, Hudec I: Sulphur and peroxide vulcanisation of rubber compounds – overview. Chem Pap. 2016, 70, 1533–1555.
- Lian Q, Li Y, Li K, Cheng J, Zhang J: Insights into the vulcanization mechanism through a simple and facile approach to the sulfur cleavage behavior. Macromolecules. 2017, 50, 803–810. [CrossRef]
- Dondi D, Buttafava A, Zeffiro A, Palamini C, Lostritto A, Giannini L, Faucitano A: The mechanisms of the sulphur-only and catalytic vulcanization of polybutadiene: An EPR and DFT study. Eur Polym J. 2015, 62, 222–235. [CrossRef]
- Milani G, Milani F: Fast and reliable meta-data model for the mechanistic analysis of NR vulcanized with sulphur. Polym Test. 2014, 33, 64–78. [CrossRef]
- Shahrampour H, Motavalizadehkakhky A: The Effects of sulfur curing systems (insoluble-rhombic) on physical and thermal properties of the matrix polymeric of styrene butadiene rubber. Pet Chem. 2017, 57, 700–704. [CrossRef]
- Gao Y, Xue Y, Lü ZG, Wang Z, Chen Q, Shi N, Sun F: Self-accelerating decomposition temperature and quantitative structure-property relationship of organic peroxides. Process Saf Environ Prot. 2015, 94, 322–328. [CrossRef]
- Radosavljević J, Nikolić L: The effect of organic peroxides on the curing behavior of EPDM isolation medium voltage cables. Adv Technol. 2018, 71, 56–63.
- Kruželák J, Sýkora R, Hudec I: Peroxide vulcanization of natural rubber. Part I: effect of temperature and peroxide concentration. J Polym Eng. 2014, 34, 617–624.
- Rodríguez Garraza AL, Mansilla MA, Depaoli EL, Macchi C, Cerveny S, Marzocca AJ, Somoza A: Comparative study of thermal, mechanical and structural properties of polybutadiene rubber isomers vulcanized using peroxide. Polym Test. 2016, 52, 117–123. [CrossRef]
- Wang H, Zhuang T, Shi X, Van Duin M, Zhao S: Peroxide cross-linking of EPDM using moving die rheometer measurements. II: Effects of the process oils. Rubber Chem Technol. 2018, 91, 561–576.
- Cong C, Liu Q, Li J, Meng X, Zhou Q: The effect of peroxide crosslinking on the synergistic crosslink of double bond and nitrile group of nitrile rubber in H2S environment. Polym Test. 2019, 76, 298–304. [CrossRef]
- Kruželák J, Kvasničáková, Hložeková K, Hudec I: Influence of dicumyl peroxide and Type I and II co-agents on cross-linking and physical–mechanical properties of rubber compounds based on NBR. Plast Rubber Compos. 2020, 49, 307–320. [CrossRef]
- Laing B, De Keyzer J, Seveno D, Van Bael A: Effect of co-agents on adhesion between peroxide cured ethylene–propylene–diene monomer and thermoplastics in two-component injection molding. J Appl Polym Sci. 2020, 137, 48414. [CrossRef]
- Kruželák J, Sýkora R, Hudec I: Peroxide vulcanization of natural rubber. Part II: effect of peroxides and co-agents. J Polym Eng. 2015, 35, 21–29.
- Lee YS, Ha K: Effects of co-agent type and content on curing characteristics and mechanical properties of HNBR composite. Elastomers Compos. 2020, 55, 95–102.
- Rim K, Park J: Organic co-agents for maintaining mechanical properties of rubber elastomers at high processing temperatures. Korean J Chem Eng. 2023. [CrossRef]
- Kruželák J, Sýkora R, Hudec I: Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem Technol. 2017, 90, 60–88. [CrossRef]
- Zhao X, Cornish K, Vodovotz Y: Synergistic mechanisms underlie the peroxide and coagent improvement of natural-rubber-toughened poly(3-hydroxybutyrate-co-3-hydroxyvalerate) mechanical performance. Polymers. 2019, 11, 565. [CrossRef]
- Twigg C, Dees M, van Duin M: Hot-air ageing characteristics of peroxide-cured EPDM rubber with 5-vinyl-2-norbornene as third monomer. Kautsch Gummi Kunstst. 2010, 63, 436–445.
- Milani G, Milani F: EPDM accelerated sulfur vulcanization: a kinetic model based on a genetic algorithm. J Math Chem. 2011, 49, 1357–1383. [CrossRef]
- Butuc SG, van Leerdam K, Rossenar B, Swart J, Geurts F, Bossinga-Geurts B, Verwer P, Talma A, Blume A: Elucidation of the role of ZnO in sulfur cure in novel EPDM-CTS blends. Polym Test. 2023, 117, 107843. [CrossRef]
- Kraus G: Swelling of filler-reinforced vulcanizates. J Appl Polym Sci. 1963, 7, 861–871. [CrossRef]
- Raffaele E, Dusserre G, del Confetto S, Eberling-Fux N, Descamps C, Cutard T: Effect of dicumyl peroxide concentration on the polymerization kinetics of a polysilazane system. Polym Eng Sci. 2018, 58, 859–869. [CrossRef]
- Rodríguez Garraza AL, Mansilla MA, Depaoli EL, Macchi C, Cerveny S, Marzocca AJ, Somoza: Comparative study of thermal, mechanical and structural properties of polybutadiene rubber isomers vulcanized using peroxide. Polym Test. 2016, 52, 117–123. [CrossRef]
- Ghosh J, Ghorai S, Jalan AK, Roy M, De D: Manifestation of accelerator type and vulcanization system on the properties of silica-reinforced SBR/devulcanize SBR blend vulcanizates. Adv Polym Technol. 2018, 37, 2636–2650. [CrossRef]
- Charoeythornkhajhornchai P, Samthong Ch, Somwangthanaroj A: Influence of sulfenamide accelerators on cure kinetics and properties of natural rubber foam. J Appl Polym Sci. 2017, 134, 44822.
- Boonkerd K, Limphirat W: Investigation of crosslink structure of natural rubber during vulcanization using X-ray absorption near edge spectroscopy. J Met Mater Miner. 2020, 30, 119–123.
- Hayeemasae N, Masa A: Relationship between stress relaxation behavior and thermal stability of natural rubber vulcanizates. Polimeros. 2020, 30, e2020016. [CrossRef]
- Azevedo M, Monks AM, Kerschbaumer RC, Schlögl S, Holzer C: Peroxide-based crosslinking of solid silicone rubber, part I: insights into the influence of dicumyl peroxide concentration on the curing kinetics and thermodynamics determined by a rheological approach. Polymers. 2022, 14, 4404.
- Przybysz M, Hejna A, Haponiuk J, Formela K: Structural and thermo-mechanical properties of poly(ε-caprolactone) modified by various peroxide initiators. Polymers. 2019, 11, 1101. [CrossRef] [PubMed]
- Orza RA, Magusin PCMM, Litvinov VM, Van Duin M, Michels MAJ: Mechanism for peroxide cross-Linking of EPDM rubber from MAS 13C NMR spectroscopy. Macromolecules. 2009, 42, 8914–8924. [CrossRef]
- Saleesung T, Reichert D, Saalwächter K, Sirisinha Ch: Correlation of crosslink densities using solid state NMR and conventional techniques in peroxide-crosslinked EPDM rubber. Polymer. 2015, 56, 309–317. [CrossRef]
- Kruželák J, Hakošová S, Kvasničáková A, Hudec I: Dicumyl peroxide used as curing agent for different types of rubber matrices. Part I: effect of temperature. Kautsch Gummi Kunstst. 2020, 10, 36–42.
- Strohmeier L, Balasooriya W, Schrittesser B, van Duin M, Schlögl S: Hybrid in situ reinforcement of EPDM rubber compounds based on phenolic novolac resin and ionic coagent. Appl Sci. 2022, 12, 2432. [CrossRef]
- Çakır NY, ˙Inan Ö, Ergün M, Kodal M, Özkoç G: Unlocking the potential use of reactive POSS as a coagent for EPDM/PP-based TPV. Polymers. 2023, 15, 2267. [CrossRef] [PubMed]
- Lin Y, Amornkitbamrung L, Mora P, Jubsilp Ch, Hemvichian K, Soottitantawat A, Ekgasit S, Rimdusit S: Effects of coagent functionalities on properties of ultrafine fully vulcanized powdered natural rubber prepared as toughening filler in rigid PVC. Polymers. 2021, 13, 289. [CrossRef]
- Shimizu T, Inagaki S: Development of a novel cross-linking agent with excellent resistance to high-temperature vapour. Seal Technol. 2017, 5, 7–11.
- Sun Y, Fan Ch, Zhao Y, Jia L: Peroxide-cured isobutylene-isoprene rubber composite: methacrylate coagent and enhanced mechanical properties by in situ formed methacrylate domains. Ind Eng Chem Res. 2021, 60, 2728–2735. [CrossRef]
- Heideman G, Datta RN, Noordermeer JWM, van Baarle B: Influence of zinc oxide during different stages of sulfur vulcanization. Elucidated by model compound studies. J Appl Polym Sci. 2005, 95, 1388–1404.
- van Duin M: Chemistry of EPDM cross-linking. Kautsch Gummi Kunstst. 2002, 55, 150–156.
- Ravindran A, Kamaraj M, Vasanthmurali N, Meghavarshini V, Balachandran M: Nanosilica reinforced EPDM silicone rubber blends: Experimental and theoretical evaluation of mechanical and solvent sorption properties. Mater Today Proc. 2021, 46, 4381–4386. [CrossRef]
- Bandzierz K, Reuvekamp L, Dryzek J, Dierkes W, Blume A, Bielinski D: Influence of network structure on glass transition temperature of elastomers. Materials. 2016, 9, 607. [CrossRef] [PubMed]
Figure 1.
Vulcanization isotherms for unfilled rubber compounds.
Figure 1.
Vulcanization isotherms for unfilled rubber compounds.
Figure 2.
Vulcanization isotherms for filled rubber compounds.
Figure 2.
Vulcanization isotherms for filled rubber compounds.
Figure 3.
Influence of curing system composition on scorch time ts1 of rubber compounds.
Figure 3.
Influence of curing system composition on scorch time ts1 of rubber compounds.
Figure 4.
Influence of curing system composition on optimum cure time tc90 of rubber compounds.
Figure 4.
Influence of curing system composition on optimum cure time tc90 of rubber compounds.
Figure 5.
Influence of curing system composition on curing rate index Rv of rubber compounds.
Figure 5.
Influence of curing system composition on curing rate index Rv of rubber compounds.
Figure 6.
Influence of curing system composition on torque difference ΔM of rubber compounds.
Figure 6.
Influence of curing system composition on torque difference ΔM of rubber compounds.
Figure 7.
Influence of curing system composition on reversion rate index Rrev of rubber compounds.
Figure 7.
Influence of curing system composition on reversion rate index Rrev of rubber compounds.
Figure 8.
Influence of curing system composition on cross-link density ν of vulcanizates.
Figure 8.
Influence of curing system composition on cross-link density ν of vulcanizates.
Figure 9.
Influence of curing system composition on modulus M100 of vulcanizates.
Figure 9.
Influence of curing system composition on modulus M100 of vulcanizates.
Figure 10.
Influence of curing system composition on hardness of vulcanizates.
Figure 10.
Influence of curing system composition on hardness of vulcanizates.
Figure 11.
Influence of curing system composition on elongation at break of vulcanizates.
Figure 11.
Influence of curing system composition on elongation at break of vulcanizates.
Figure 12.
Influence of glycerol content on tensile strength of vulcanizates.
Figure 12.
Influence of glycerol content on tensile strength of vulcanizates.
Figure 13.
Temperature dependences of loss factor tan δ for unfilled vulcanizates.
Figure 13.
Temperature dependences of loss factor tan δ for unfilled vulcanizates.
Figure 14.
Temperature dependences of loss factor tan δ for filled vulcanizates.
Figure 14.
Temperature dependences of loss factor tan δ for filled vulcanizates.
Table 1.
Composition of unfilled rubber compounds in phr and their designation.
Table 1.
Composition of unfilled rubber compounds in phr and their designation.
| |
S0 – P1.5 |
S0.5 – P1 |
S0.75 – P0.75 |
S1 – P0.5 |
S1.5 – P0 |
| EPDM |
100 |
100 |
100 |
100 |
100 |
| ZnO |
0 |
1 |
2 |
3 |
4 |
| Stearic acid |
0 |
0.5 |
1 |
1.5 |
2 |
| Sulfur |
0 |
0.5 |
0.75 |
1 |
1.5 |
| CBS |
0 |
1 |
2 |
3 |
4 |
| DCP |
1.5 |
1 |
0.75 |
0.5 |
0 |
| TMPTMA |
4 |
3 |
2 |
1 |
0 |
Table 2.
Composition of filled rubber compounds in phr and their designation.
Table 2.
Composition of filled rubber compounds in phr and their designation.
| |
S0 – P1.5 |
S0.5 – P1 |
S0.75 – P0.75 |
S1 – P0.5 |
S1.5 – P0 |
| EPDM |
100 |
100 |
100 |
100 |
100 |
| CB |
25 |
25 |
25 |
25 |
25 |
| ZnO |
0 |
1 |
2 |
3 |
4 |
| Stearic acid |
0 |
0.5 |
1 |
1.5 |
2 |
| Sulfur |
0 |
0.5 |
0.75 |
1 |
1.5 |
| CBS |
0 |
1 |
2 |
3 |
4 |
| DCP |
1.5 |
1 |
0.75 |
0.5 |
0 |
| TMPTMA |
4 |
3 |
2 |
1 |
0 |
Table 3.
Glass transition temperature Tg and loss factor tan δ for unfilled vulcanizates.
Table 3.
Glass transition temperature Tg and loss factor tan δ for unfilled vulcanizates.
| Sample |
Tg (°C) |
tan δ (-20 °C) |
tan δ (0 °C) |
tan δ (20 °C) |
tan δ (50 °C) |
| S0-P1.5 |
-26 |
0.50 |
0.12 |
0.06 |
0.04 |
| S0.75-P0.75 |
-26.6 |
0.50 |
0.12 |
0.07 |
0.06 |
| S1.5-P0 |
-25.8 |
0.50 |
0.12 |
0.06 |
0.03 |
Table 4.
Glass transition temperature Tg and loss factor tan δ for filled vulcanizates.
Table 4.
Glass transition temperature Tg and loss factor tan δ for filled vulcanizates.
| Sample |
Tg (°C) |
tan δ (-20 °C) |
tan δ (0 °C) |
tan δ (20 °C) |
tan δ (50 °C) |
| S0-P1.5 |
-27.9 |
0.35 |
0.11 |
0.07 |
0.07 |
| S0.75-P0.75 |
-27.6 |
0.38 |
0.11 |
0.08 |
0.07 |
| S1.5-P0 |
-26.3 |
0.39 |
0.11 |
0.06 |
0.04 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).