1. Introduction
The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has dramatically altered the global landscape, leaving no corner of the world untouched. Since its emergence in late 2019, the virus has infected millions of individuals and claimed countless lives, highlighting the urgent need for effective containment strategies. As the scientific community races to understand and combat this relentless virus, monitoring SARS-CoV-2 variants has emerged as a crucial aspect of the ongoing battle.
All viruses, including SARS-CoV-2 change via mutations over time. Most changes have little to no impact on the virus’s properties. However, some changes may affect the virus’s fitness, such as transmissibility, virulence or susceptibility to vaccines and therapeutics. Certain mutations can also negatively impact the detectability of the viral pathogen with conventional diagnostic tests and so derail public health and social measures.
In June 2020, the WHO Virus Evolution Working Group was established with a specific focus on SARS-CoV-2 variants, their phenotype and their impact on countermeasures. In late 2020, the emergence of variants that posed an increased risk to global public health prompted WHO to characterize some as “variants of interest” (VOIs) and “variants of concern” (VOCs) in order to prioritize global monitoring and research, required to inform and adjust the COVID-19 response. From May 2021 onwards, WHO began assigning simple, easy-to-say labels for key variants.
Considerable progress has been made in establishing and strengthening a global system to detect signals of potential VOIs or VOCs and rapidly assess the risk posed by SARS-CoV-2 variants to public health. It remains critical that these systems are maintained, and data are shared, according to good principles and in a timely fashion, as SARS-CoV-2 continues to circulate at high levels around the world. While monitoring the circulation of SARS-CoV-2 globally, it also remains essential to monitor their spread in animal populations and chronically infected individuals, which are crucial aspects of the global strategy to reduce the occurrence of mutations that have negative public health implications. In March 2023, WHO updated its tracking system and working definitions for variants of concern and variants of interest [
1].
Phylogenetic analysis is a common tool used for monitoring the evolution of viruses. It involves studying the genetic relationships and evolutionary history of viral strains or variants by analyzing their genetic and protein sequences. Homology-based phylogenetic analysis is a commonly used method to infer evolutionary relationships among organisms based on similarities in their DNA or protein sequences. However, when dealing with highly homologous sequences that differ by only a small number of mutations, this approach can have some weaknesses: (i) saturation of substitutions which can lead to erroneous inferences about the relationships between sequences, (ii) insufficient phylogenetic signal to confidently resolve the relationships between closely related sequences, (iii) multiple sequence alignment ambiguities, which can introduce errors in the phylogenetic analysis and lead to incorrect interpretations of the evolutionary relationships, (iv) long branch attraction which can distort the inferred evolutionary relationships, leading to incorrect branching patterns and inaccurate phylogenetic reconstructions, and (v) lack of independent mutational events which can make it difficult to resolve the true evolutionary relationships and can lead to ambiguous or conflicting results.
To overcome this challenge and enhance functional sequence analysis, we propose an additional, novel distance measure based on the Informational Spectrum Method (ISM) [
2]. The ISM-based phylogenetic approach has been successfully employed in the analysis of various viruses, including influenza [
3,
4,
5] and Ebola [
6]. Furthermore, we previously utilized the ISM-based phylogenetic approach to evaluate the impact of mutations in the spike protein of SARS-CoV-2 on the efficacy of the COVID-19 vaccine [
7]. This approach enables the assessment of the biological implications of mutations, thereby advancing our understanding of viral evolution and vaccine effectiveness.
Here, a novel phylogenetic algorithm utilizing entropy as a protein distance measure has been introduced. The effectiveness of this innovative entropy-based method in discriminating between VOCs and VOIs, surpassing traditional homology-based and ISM-based phylogenetic approaches, has been demonstrated. It has the potential to serve as a valuable tool for monitoring the evolutionary progression of SARS-CoV-2 viruses.
2. Materials and Methods
2.2. Protein Sequence Entropy
Protein sequence entropy, or amino acid (AA) based entropy, is defined as the Shannon entropy [
8]:
where the aa(i) is the probability of given amino acid i.e. the number of the given amino acid in the sequence divided by N, and N is the length of the protein sequence.
2.3. EIIP Entropy
The EIIP entropy is the numerical property of the protein sequence. It is based on the electron-ion interaction potential (EIIP) values of the amino acid sequence (
Table 1). The E(2)IIP entropy of the protein sequence X is defined as:
where the x(i) is the EIIP value of the i-th amino acid of the sequence X and N is the length of the sequence X.
The EIIP is a descriptor of long-range interaction properties, and its definition and properties can be found in previous research [
9].
2.4. New Protein Distances Based on AA and EIIP Entropies
Let X and Y be two sequences. Then the AA entropy based distance between X and Y can be defined as:
where the SE(X) and SE(Y) are the AA entropy properties of the X and Y sequences respectfully.
In the similar manner, the EIIP entropy based distance between X and Y can be defined as:
where the EE(X) and EE(Y) are the EIIP entropy properties of the X and Y sequences respectfully.
2.5. Algorithm of Generating Entropy Based Phylogenetic Trees
The AA entropy based phylogenetic trees can be generated using the following algorithm:
In the similar manner, the EIIP entropy based phylogenetic trees can be generated using the following algorithm:
-
For each sequence calculate its EIIP entropy:
Calculate the distance matrix with the distance measure defined in (4).
Construct the tree using the UPGMA method.
2.6. Properties of the EIIP Entropy Distance
EIIP entropy property is not based on the multiple sequence alignment (MSA) and does not use any of the substitution model. Therefore EIIP entropy based phylogenetic analysis escapes the drawbacks of the MSA based phylogenetic approaches: insensitivity to single mutation and position, failure to consider deletion within sequence, time complexity, limited number of sequence, ambiguity of the alignment cost criteria, etc. [
11].
The EIIP entropy based distance d is sensitive to a single mutation, the type of the substitution and deletion.
2.7. Evolutionary Analyses
The ISM based phylogenetic tree was generated using the distance measure defined previously in [
2] on the characteristic frequencies F1= 0.257 and F2= 0.4795.
The traditional homology-based tree was inferred using the UPGMA method [
10], where the evolutionary distances were computed using the Poisson correction method [
12]
All trees were generated using MEGA X software [
13].
3. Results
The World Health Organization (WHO) has suggested the categorization of COVID-19 viruses into "Variants of Concern" (VOC) and "Variants of Interest" (VOI) to enhance surveillance and respond effectively to the dynamic SARS-CoV-2 virus and its effects on public health. Initiatives are underway to devise standardized and universally acknowledged bioinformatics criteria for variant categorization. These efforts comprise the monitoring of viral evolution, the exchange of data and insights, and the refinement of classification criteria as new data emerges. International cooperatives spearheaded by the WHO and other global health institutions aim to standardize variant classification methodologies and establish agreed-upon guidelines. Despite these endeavors, a definitive bioinformatics criterion that distinguishes VOCs from VOIs remains elusive.
We conducted a phylogenetic analysis of the spike protein SP1, a key antigenic component of the SARS-CoV-2 virus, utilizing various protein distance measures.
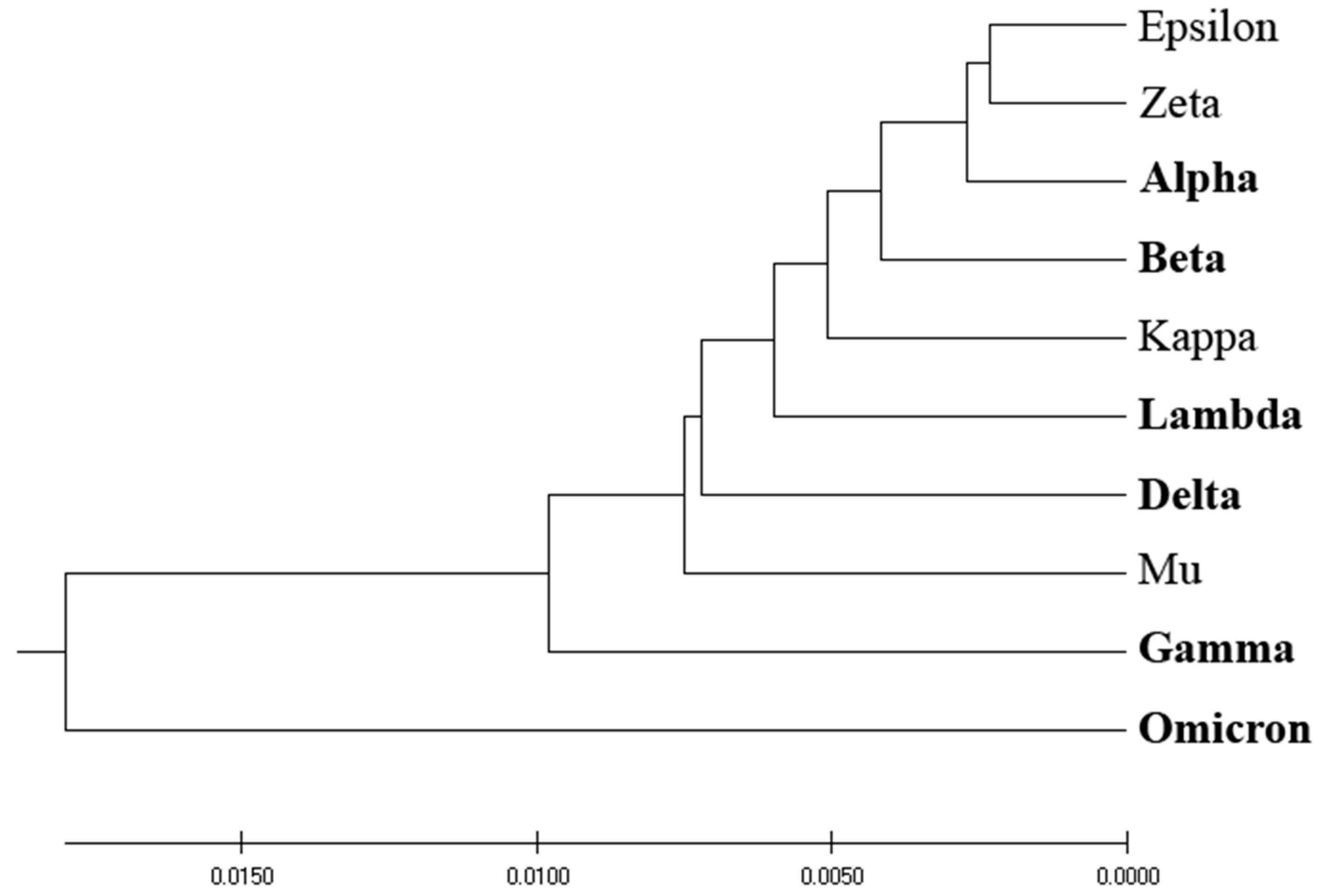
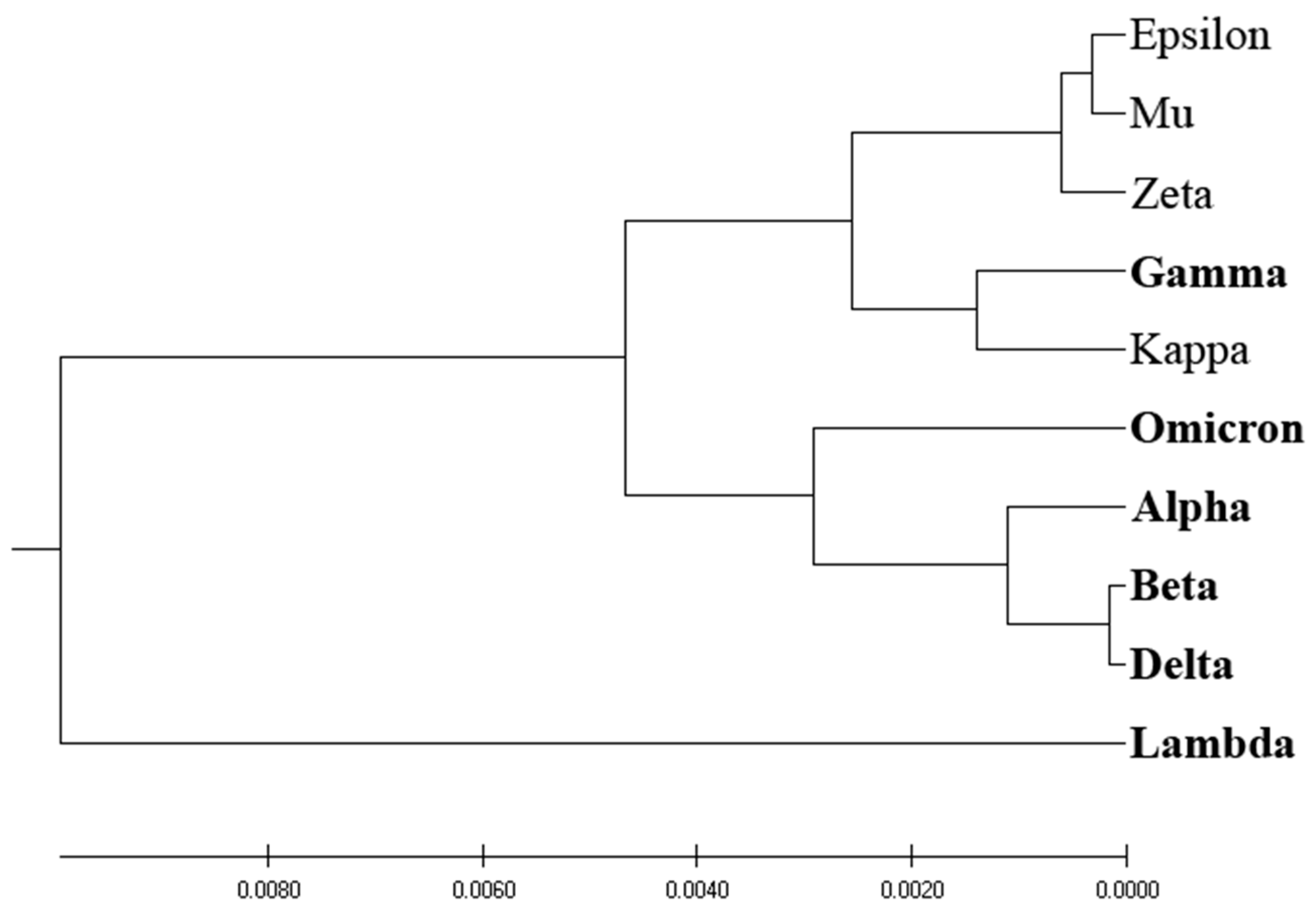
Figure 1 depicts the homology-based phylogenetic analysis of SP1 from VOCs and VOIs. The derived phylogenetic tree shows that VOCs and VOIs are not distinctly separable due to the high homology of these viruses and the small number of mutations distinguishing them.
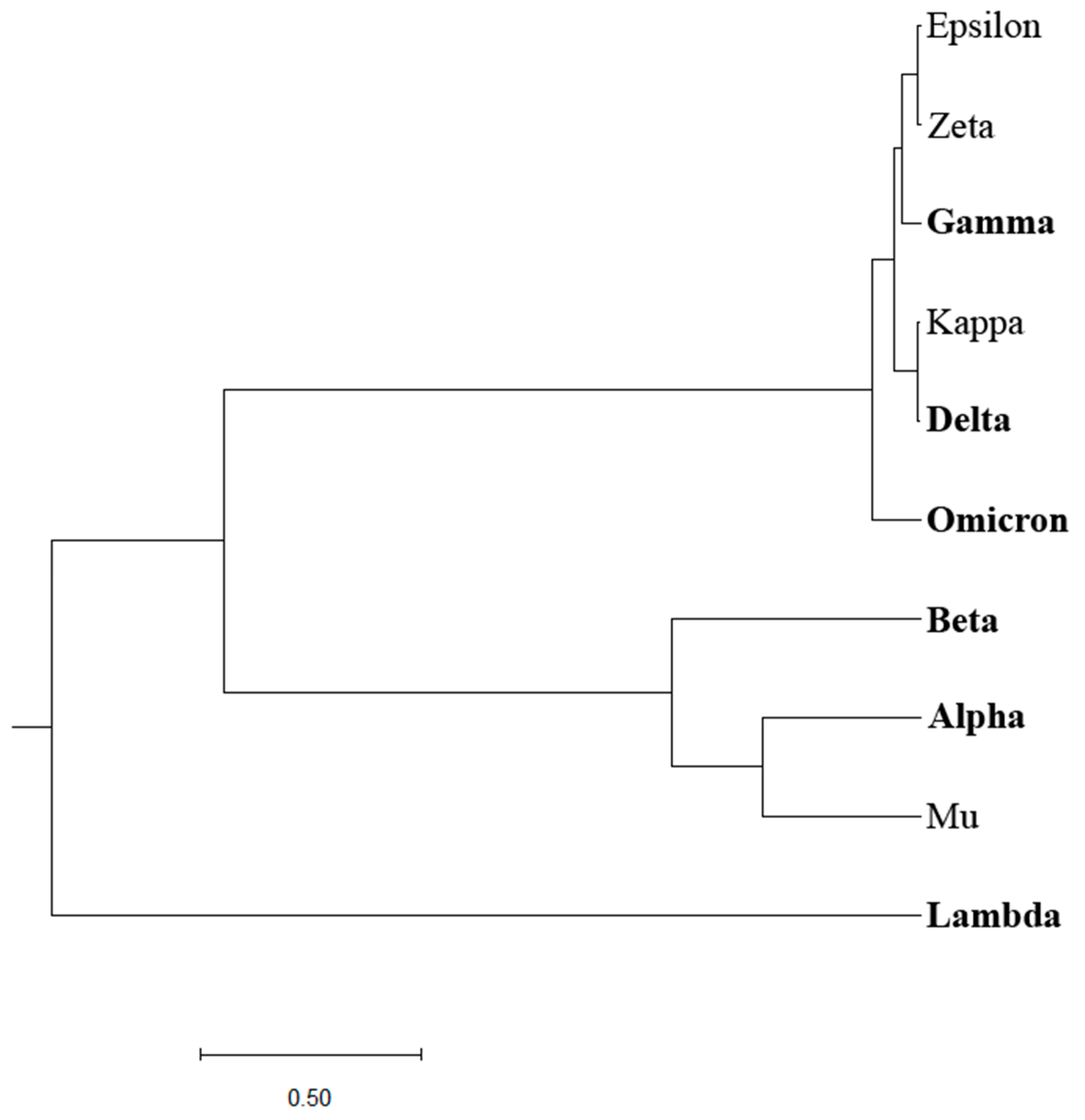
Previously, we designed an Information Spectrum Method (ISM)-based phylogenetic approach that allows the assessment of the biological effects of single mutations and their combinations in proteins [
2]. The ISM-based phylogenetic tree is illustrated in
Figure 2. Although this approach somewhat better distinguishes VOCs and VOIs compared to the homology-based phylogenetic approach, a clear separation between the two groups of COVID-19 viruses is still lacking.
Entropy calculations for biological macromolecules yield valuable insights into their structural stability, folding processes, ligand binding, thermodynamics, and molecular interactions. Comprehending entropy aids in demystifying the intricacies of these macromolecules and their roles in biological systems. We implemented entropy as a unique distance measure in the phylogenetic analysis of proteins.
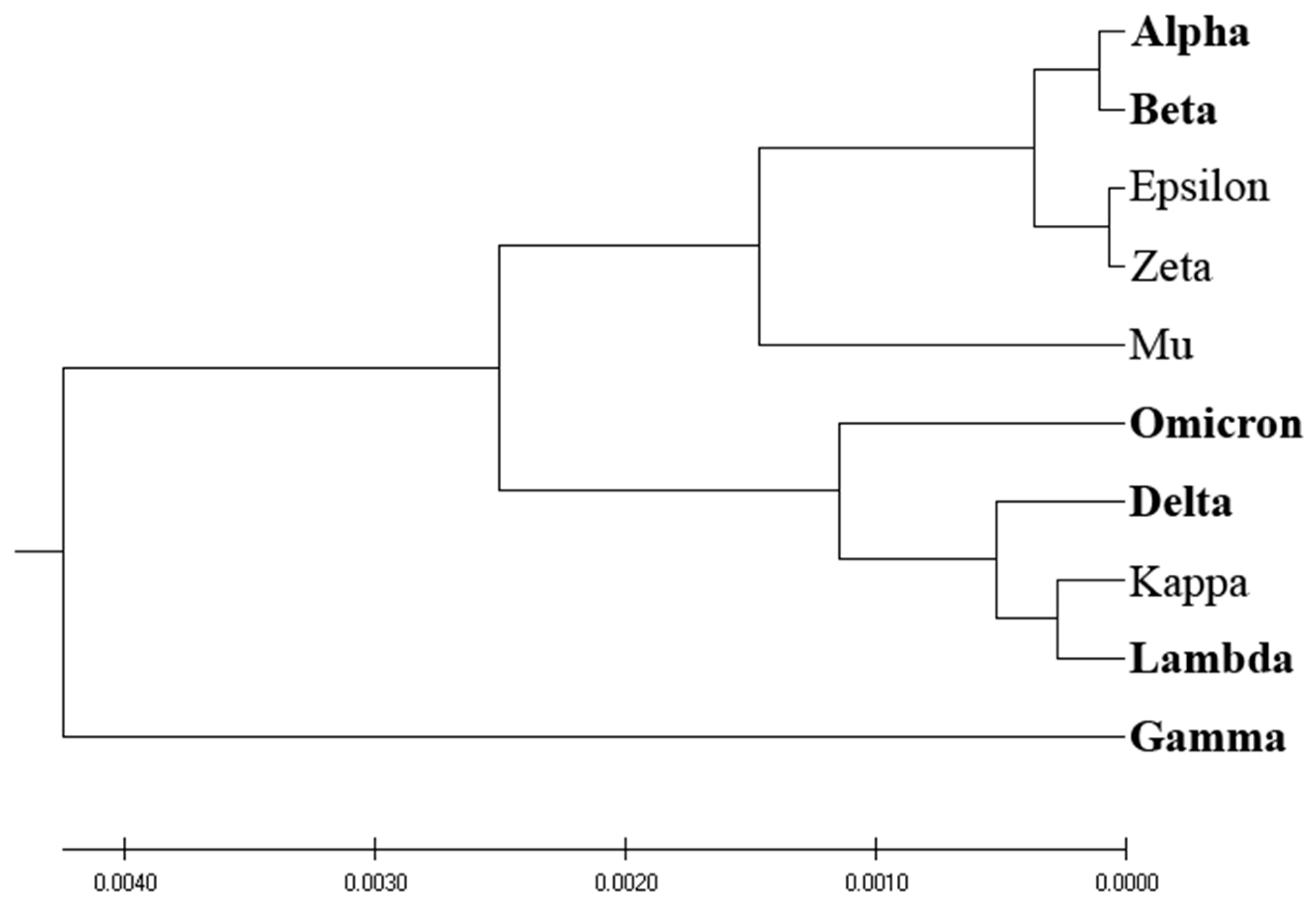
Figure 3 presents the phylogenetic analysis of VOCs and VOIs derived by the entropy calculated by the distribution of amino acids (AA-entropy) in SP1 proteins. However, the segregation between VOCs and VOIs is insubstantial and mirrors that derived by homology-based and ISM-based phylogenetic analyses, as depicted in
Figure 1 and
Figure 2.
Figure 4 showcases the phylogenetic tree obtained via EIIP-entropy as the distance measure, calculated based on the distribution of Electron-Ion Interaction Potential (EIIP) values of amino acids in SP1 proteins. Interestingly, the EIIP-entropy-based phylogenetic approach effectively separates VOCs and VOIs, with the exception of the VOC variant Gamma, which is classified with VOIs.
Our analysis reveals that the EIIP-entropy-based phylogenetic approach outperforms the traditional homology-based, ISM-based, and AA-entropy-based phylogenetic methodologies in separating SP1 from VOCs and VOIs. This outcome suggests that employing EIIP-entropy as the distance measure in a novel phylogenetic approach could serve as a valuable tool for classifying emerging SARS-CoV-2 variants as potential VOCs or VOIs.
4. Discussion
The WHO introduced the terms VOC and VOI to classify different strains of the SARS-CoV-2 virus, which causes COVID-19 disease. This classification was necessary to distinguish between variants that might pose different levels of risk to global public health. A VOC is a variant of the virus that has shown to be more contagious, more deadly, or more resistant to current treatments and vaccines. The introduction of this term was crucial in guiding public health actions and policies, as well as focusing scientific research on these potentially dangerous strains. A VOI, on the other hand, is a variant that has genetic changes that may affect virus characteristics such as transmissibility and disease severity and may pose a future risk. These are monitored and studied closely to prevent their possible escalation into VOC. Classifying new SARS-CoV-2 strains as VOCs or VOIs is essential for monitoring the virus's evolution, guiding research and public health policies, and prioritizing resources. It helps to understand if a new variant is more transmissible, more dangerous, or resistant to treatments and vaccines. Early identification and understanding of such variants can facilitate appropriate public health actions and interventions to mitigate the spread and impact of the virus.
Developing bioinformatic criteria for discriminating between VOCs and VOIs is crucial for a few key reasons: (i) Bioinformatics tools can quickly analyze large volumes of genomic data, allowing for faster detection and classification of new variants, (ii) These tools can provide a more precise and detailed understanding of the genomic changes in new variants, informing their classification, (iii) Bioinformatics allows for the analysis of vast amounts of data, crucial given the global scale of the pandemic, (iv) Sophisticated bioinformatics models can potentially predict the impact of specific mutations on viral characteristics like transmissibility and virulence, aiding in identifying potential VOCs or VOIs early. By providing quick, detailed, and scalable analysis, bioinformatics criteria can help streamline the process of variant classification, improving the global response to emerging variants.
As of now, there isn't a standardized bioinformatics criterion for distinguishing Variants of Concern (VOCs) and Variants of Interest (VOIs) because the impact of specific mutations on virus behavior is not always predictable and often relies on clinical and epidemiological data. While bioinformatics can help identify and analyze genomic changes in new variants, linking these changes to real-world impacts like increased transmissibility, disease severity, or vaccine resistance, still requires extensive in vitro and in vivo studies and observational data. Therefore, although bioinformatics plays a crucial role in initial identification and surveillance, comprehensive classification requires additional data and analysis.
The spike protein SP1 of the SARS-CoV-2 virus, which causes COVID-19, is a good target for discriminating VOCs and VOIs due to a few key reasons: (i) S1 protein enables the virus to enter host cells. Changes in the spike protein can affect how easily the virus infects cells, which can influence transmissibility and virulence; (ii) S1 protein is the primary target of the immune response, including the response elicited by vaccines. Variations in the SP1can potentially affect vaccine effectiveness; (iii) Many significant mutations identified in VOCs and VOIs so far have occurred in SP1 protein, suggesting it's an area of the virus genome where mutations can have meaningful effects on virus behavior. Monitoring changes in SP1protein through genomic sequencing can therefore provide important clues about how a variant might behave and its potential to become a VOC or VOI.
Here we developed a novel phylogenetic algorithm for analysis of SP1 proteins from SARS-CoV-2. In this algorithm as distance measure for separation proteins the EIIP-entropy was used. This entropy was calculated based on distribution of EIIP of amino acids in SP1. It was showed (
Figure 1,
Figure 2,
Figure 3 and
Figure 4) that the novel EIIP-entropy-based phylogenetic approach outperforms the traditional homology-based, ISM-based, and AA-entropy-based phylogenetic methodologies in separating SP1 from VOCs and VOIs.
In this study, we have pioneered an innovative phylogenetic algorithm for the analysis of SARS-CoV-2's spike protein SP1. This algorithm leverages the calculation of EIIP-entropy, a measure of distance calculated as the distribution of the EIIP of amino acids within the SP1 protein. A comparative analysis conducted, as visualized in
Figure 1,
Figure 2,
Figure 3 and
Figure 4, corroborates that our novel EIIP-entropy-based phylogenetic method exhibits superior performance in distinguishing SP1 proteins from VOCs and VOIs. This comparison was made against traditional homology-based, ISM-based, and AA-entropy-based phylogenetic methodologies.
5. Conclusions
To summarize, the data presented herein strongly suggest that the innovative EIIP-entropy-based phylogenetic approach we propose offers a compelling foundation for the development of an instrumental tool for classifying emergent SARS-CoV-2 variants into potential VOCs or VOIs. The algorithm's potency lies in its enhanced discriminatory capacity for variant classification, which can potentially streamline surveillance efforts and augment our understanding of variant characteristics. Furthermore, given the universality of the principles of protein structure and function, this novel phylogenetic methodology may well hold promise for the analysis and classification of other emerging viral pathogens. This approach's wider application could potentially enhance our global readiness and response strategies in the face of evolving viral diseases. Moreover, this method could become very useful for rapid detection of mutations leading to “functional changes” of any protein(s) and so enhance our research capabilities in protein research.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Dataset S1: The list of analyzed sequences of subunit 1 of S proteins (SP1) from human SARS-CoV-2 viruses deposited in the GISAID (
https://www.epicov.org/epi3/cfrontend#18c7c7).
Author Contributions
Conceptualization, V.V. and V.P.; methodology, V.P and V.V.; software, V.P.; formal analysis, S.G. and M.V.; investigation, S.P.; resources, M.V.; data curation, S.G.; writing—original draft preparation, V.V., S.P. and V.P.; writing—review and editing, S.P.; visualization, V.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- https://www.who.int/news/item/16-03-2023-statement-on-the-update-of-who-s-working-definitions-and-tracking-system-for-sars-cov-2-variants-of-concern-and-variants-of-interest.
- Perovic, V.R.; Muller, C.P.; Niman, H.L.; Veljkovic, N.; Dietrich, U.; Tosic, D.D.; Glisic, S.; Veljkovic, V. Novel Phylogenetic Algorithm to Monitor Human Tropism in Egyptian H5N1-HPAIV Reveals Evolution toward Efficient Human-to-Human Transmission. PLOS ONE 2013, 8, e61572. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.; Veljkovic, N.; Muller, C.P.; Müller, S.; Glisic, S.; Perovic, V.; Köhler, H. Characterization of conserved properties of hemagglutinin of H5N1 and human influenza viruses: possible consequences for therapy and infection control. BMC Struct. Biol. 2009, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.; Niman, H.L.; Glisic, S.; Veljkovic, N.; Perovic, V.; Muller, C.P. Identification of hemagglutinin structural domain and polymorphisms which may modulate swine H1N1 interactions with human receptor. BMC Struct. Biol. 2009, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.; Veljkovic, N.; Paessler, S.; Goeijenbier, M.; Perovic, V.; Glisic, S.; Muller, C.P. Predicted Enhanced Human Propensity of Current Avian-Like H1N1 Swine Influenza Virus from China. PLOS ONE 2016, 11, e0165451. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.; Glisic, S.; Muller, C.P.; Scotch, M.; Branch, D.R.; Perovic, V.R.; Sencanski, M.; Veljkovic, N.; Colombatti, A. In silico analysis suggests interaction between Ebola virus and the extracellular matrix. Front. Microbiol. 2015, 6, 135–135. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic V, Perovic V, Chambers I, Paessler S. Evolution of SARS-CoV-2 virus and assessment of the effectiveness of COVID-19 vaccine. F1000Research 2021, 10, 28. [CrossRef] [PubMed]
- Sen, S.; Dey, A.; Chowdhury, S.; Maulik, U.; Chattopadhyay, K. Understanding the evolutionary trend of intrinsically structural disorders in cancer relevant proteins as probed by Shannon entropy scoring and structure network analysis. BMC Bioinform. 2019, 19, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Veljković, V.; Slavić, I. Simple General-Model Pseudopotential. Phys. Rev. Lett. 1972, 29, 105–107. [Google Scholar] [CrossRef]
- Sneath, P.H.A. and Sokal R.R. (1973). Numerical Taxonomy. Freeman, San Francisco.
- Perovic, V.R. Novel algorithm for phylogenetic analysis of proteins: application to analysis of the evolution of H5N1 influenza viruses. J. Math. Chem. 2013, 51, 2238–2255. [Google Scholar] [CrossRef]
- Zuckerkandl, E. and Pauling L. (1965). Evolutionary divergence and convergence in proteins. Edited in Evolving Genes and Proteins by V. Bryson and H.J. Vogel, pp. 97-166. Academic Press, New York.
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).