1. Introduction
The phylum Chordata presents three subphyla: Vertebrata, Cephalochordata and Urochordata [
1]. Marine invertebrates represent two of these three subphyla, Cephalochordata and Urochordata. The urochordates are animals that live in the marine environment from coastal areas to regions with great depths. The classes Thaliacea, Larvacea (Appendicularia) and Ascidiacea are part of this group, the latter being the one comprising ascidians [
2,
3]. Members belonging to the class Ascidiacea are the most common, diverse and well-known, comprising more than 2,000 species. The traditional classification of the class Ascidiacea includes three orders, Aplousobranchia, Stolidobranchia and Phlebobranchia. These animals are also known as tunicates because they have a resistant outer coat made of cellulose - synthesized by cellulose synthase in the epidermis and incorporated into the coat - which covers and supports their body [
3,
4,
5].
These animals are filter feeders and can be found arranged in their habitats in two different arrangements. Some live as individual ascidians and others live in colonies. While all orders that integrate the Ascidiacea family include colonial forms, the solitary species are only present in Stolidobranchia and Phlebobranchia [
5,
6,
7]. In addition to the inhalant (or oral) and exhalant (or atrial) siphons, and the tunic, ascidians have a mantle, which is an inner membrane that covers the tunic [
7].
Ascidians are hermaphrodite animals that have developed remarkable reproductive strategies, combining sexual and asexual modes of reproduction, thus allowing rapid expansion of the individual population [
3,
6,
7,
8].
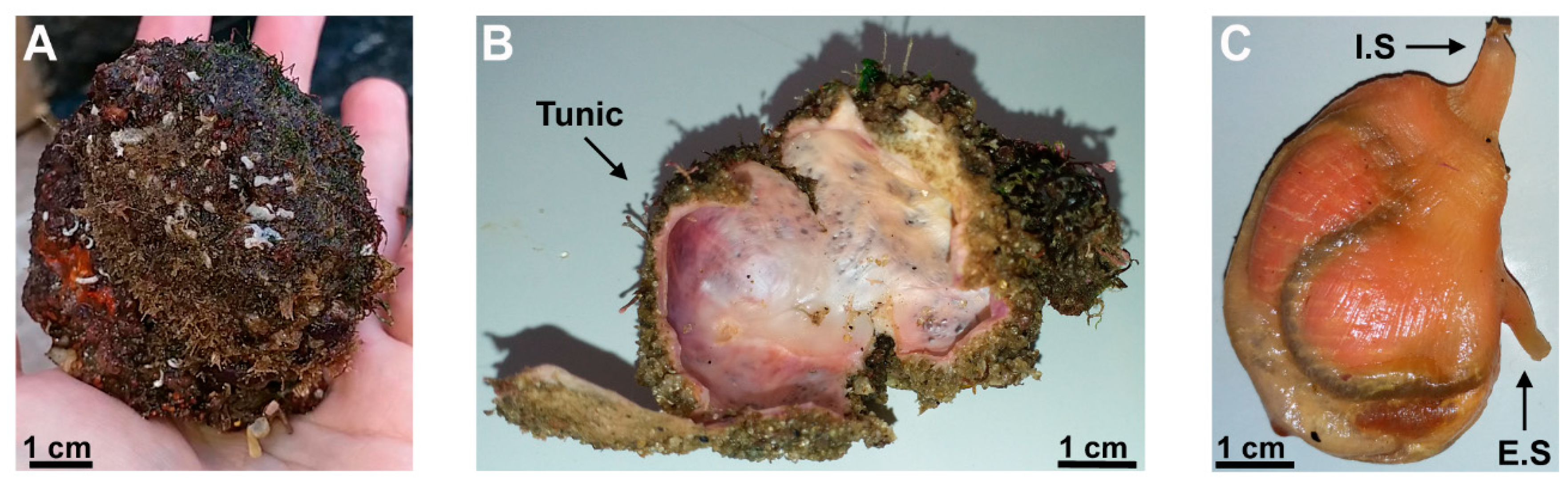
On the coast of the South Zone of Rio de Janeiro, more specifically at Praia Vermelha, Urca, a species of ascidian that has not been much studied so far can be found,
Microcosmus exasperatus (Stolidobranchia; Pyuridae). This species has a wide global distribution and is very common in tropical and subtropical waters.
M. exasperatus is found in shallow and medium depths and occurs disposed in colonies in Rio de Janeiro, and as solitary ascidians in the Mediterranean. On the Mediterranean coast, it is known to reproduce all year round, except for the winter months, and can be found in both natural and artificial substrates. These animals are externally cohabited by other microorganisms, a characteristic associated with the name of this gender,
Microcosmus. They have an orange color in their viscera, and in their mantle, it is possible to observe an intense purple color. This species has also been described in the literature and used in studies as marine biological indicators for monitoring stress and environmental pollution [
9,
10,
11].
Studies have also shown that this species has its tunic predominantly constituted by sulfated polysaccharides, more specifically, high molecular weight sulfated L-galactans that present anticoagulant activity in a dose-dependent manner by inhibiting the coagulation intrinsic pathway. This galactan has a similar structure when compared to galactans obtained from other ascidian species such as
Styela Plicata,
Herdmania monus,
Phallusia nigra and
Clavelina oblonga [
12,
13]. However, the types, structures and biological activity of glycosaminoglycans (GAGs) present in the
Microcosmus exasperatus viscera had not been evaluated until then. Therefore, these points are one of the goals of the present work.
Many species of ascidians, such as
Styela plicata,
Phallusia nigra and
Ciona intestinalis are known and studied for synthesizing GAGs in their viscera, and having other types of sulfated polysaccharides in their tunic, such as galactans and fucans [
14]. These ascidian-derived GAGs are considered analogous to mammalian GAGs, playing essential roles in different biological processes such as tissue integrity, fertilization, infection, inflammation, cell growth and others [
15]. Therefore, ascidians are seen today as a promising natural source for obtaining these compounds with natural bioactive properties, with antitumor, anticoagulant, antimicrobial and anti-inflammatory action [
16,
17].
Mammalian heparin is a GAG that has been used for decades in the clinic as an effective anticoagulant and in the treatment of thrombosis and related diseases. This molecule has also been studied as an interesting alternative for cancer treatment and tumor progression. However, the occurrence of molecules such as heparin is not restricted to mammals, and the search for analogous compounds derived from marine invertebrates such as ascidians, with similar biological activities, but possibly devoid of unwanted side effects, offers a promising alternative in this area. This search is motivated by questions under discussion about the use of mammal’s heparin, such as the risk of contamination by pathogens due to evolutionary proximity, such as prion proteins with transmissivity potential for spongiform encephalopathies of bovine heparins. This reason has already led the United States of America and Japan to commercialize heparins obtained only from porcine origin, but contamination by prion proteins or even viruses, remains present in this model yet. Furthermore, its production process generates a great environment impact and its adverse effects, such as the risk of bleeding, are always present [
14,
16,
17,
18].
Different types of sulfated polysaccharides derived from ascidians have already been described in the literature regarding their structure and also some biological activities.
Ciona intestinalis,
Phallusia nigra and
Styela plicata were the first ascidian species that had their GAGs extensively studied. High molecular weight galactans are exclusively present in the tunica, whereas analogues of mammalian GAGs are present in the viscera, such as heparan sulfate with low sulfate degree and dermatan sulfate with high sulfate degree. Heparin is also present as an intracellular product of test cells and hemocytes, which circulate in the hemolymph. In general, marine invertebrate GAGs occur in high concentrations in tissues (about 0.5% of the dry weight, compared to 0.022% of the porcine intestinal mucosa), and can be easily isolated by procedures similar to those already used in the pharmaceutical industry for the preparation of mammalian heparin [
17,
19].
A heparin that has a similar structure to vertebrate heparin has already been identified in the ascidian
Styela plicata. Activated partial thromboplastin time (aPTT) assays showed that
S. plicata heparin has low anticoagulant activity when compared to mammalian heparin, being twenty times less potent in stimulating thrombin inhibition by antithrombin. However, heparin from the species
S. plicata activates heparin cofactor II with approximately the same potency as vertebrate heparin. In studies to compare the hemorrhagic effect between ascidian and mammalian heparin, it was seen that at the necessary doses for the thrombus formation prevention in an animal model, ascidian heparin did not increase blood loss when compared to the control, while mammalian heparin increased blood loss by almost two times [
17].
In addition to heparin, dermatan sulfate molecules analogous to those of mammals have also been identified in ascidians. The sulfation pattern of ascidian dermatan sulfate is order-specific. Dermatan sulfates of the most primitive order species, Stolidobranchia, the primary disaccharide repeat unit is composed of iduronic acid sulfated at position 2 and N-acetyl-galactosamine sulfated at position 4 (IdoA2S-GalNAc4S), while the disaccharides present in ascidians most recent order dermatan sulfate, Phlebobranchia, are formed primarily by disaccharide units of iduronic acid sulfated at position 2 and N-acetyl-galactosamine sulfated at position 6 (IdoA2S-GalNAc6S). The difference in N-acetyl-galactosamine sulfation position of different ascidian dermatan sulfates confers the difference in heparin cofactor II-mediated anticoagulant activity. Therefore, IdoA2S-GalNAc4S has high anticoagulant activity, while IdoA2S-GalNAc6S has no anticoagulant activity. The anticoagulant activity occurs through thrombin inhibition by heparin cofactor II. The difference in the anticoagulant activity of these dermatan sulfates suggests that binding of GAGs to heparin cofactor II requires a specific sulfate pattern, composed of enriched sequences of [α-L-IdoA(2SO4)-1→3β-D-GalNac(4SO4)]. It was also seen that the mechanism mediated by heparin cofactor II is also associated with antithrombotic effects of these dermatan sulfates, since intravascular administration of dermatan sulfate from the ascidian
Styela plicata (IdoA2S-GalNAc4S), prevented thrombus formation in veins, and the administration of dermatan sulfate from the ascidian
Phallusia nigra (IdoA2S-GalNAc6S), had no antithrombotic effect in the same model of venous thrombosis and dose [
17,
19].
As mentioned above, due to the clinical potential of GAGs derived from marine invertebrates and because they are analogous to mammalian heparin, it is important to establish a specific protocol for the collection, isolation, extraction and purification of sulfated polysaccharides present in the viscera of these animals, specific for this ascidian specie. Understanding the identity of the molecules produced by Microcosmus exasperatus, which is an ascidian species that has not been much studied so far, and thus increase the library of compounds produced by these animals. It is also important to understand the role of these molecules within the ascidian organism, the composition and structure of these molecules, how they maintain their bioactivity after the extraction and purification processes and explore their clinical applications.
3. Discussion
Since ascidians are sources of GAGs and other types of sulfated polysaccharides, and since these molecules are analogous to mammalian GAGs, it has been proven that these diverse polysaccharides also play essential roles in different biological processes. These animals have been widely studied as an alternative and promising natural source for obtaining these compounds with natural bioactive properties. Many of them have already been described, such as antitumor, anticoagulant, antimicrobial and anti-inflammatory action [
14,
16,
17,
18].
Many ascidians species have already had their GAGs and biological activities described in the literature [
17]. However, each species is a unique organism, presenting diversity in the variety, structure and biological activity of these molecules. Therefore, it is very important to study new species and uncover the diversity of GAGs and sulfated polysaccharides to be considered mammalian analogues and the specific biological activities presented by them. From this, a new species commonly found on the Brazilian coast, identified as
Microcosmus exasperatus, was studied. Moreover, it showed to have sulfated polysaccharides such as fucans and galactans in its tunic. In addition, it has also been shown that these sulfated galactans have an anticoagulant action linked to heparin cofactor II [
12,
13]. However, the GAGs and/or sulfated polysaccharides present in its viscera, in addition to their biological activities, had not yet been studied. Based on these discoveries, the abundant occurrence of this species at Praia Vermelha, Rio de Janeiro, the ease of specimen collection and the good yield in the sulfated polysaccharides extraction, we began to investigate the presence of these molecules in these animals’ viscera as a promising alternative to mammalian GAGs with antitumoral properties.
Three collections were carried out, each taking place in different periods of the year so that we could observe a possible seasonality of this species. According to the literature, ascidians’ reproduction period coincides with the maximum food production period, i.e. when plankton production is high. This occurs in warmer temperatures (summer), however, when the summer temperature peaks, water layer stratification occurs and, consequentely, the depletion of food for ascidians. In contrast, when seawater cools (autumn) there is a decrease in the thermocline, causing mixing in the water and bringing in these deep-sea food sources [
21].
Therefore, the first collection was carried out during the summer, and it was possible to observe a great occurrence of
M. exasperatus specimens in Praia Vermelha, Rio de Janeiro. We obtained a greater number of specimens collected and, consequently, a higher amount in dry weight (81.98 g). The second collection, carried out in the winter period, also showed a high occurrence of these specimens, where 61.87 g of dry weight were obtained, but even so, lower than in the summer period. On the other hand, in the third collection during autumn, it was possible to observe a more discreet occurrence of these specimens in the same place, and, therefore, we obtained the lowest value in dry weight (18.86 g). Thus, our hypothesis is that
M. exasperatus reproduces mainly in the summer, as observed by the abundance of young and adult specimens found during these collections, also corroborating with what is described in the literature regarding the short period necessary for ascidians development, with the larval stage lasting only a few minutes or, at most, a few hours. The differentiation to an adult individual with functional organs, occurring from a few days to a few weeks [
22,
23,
24]. However, since in most areas Brazil do not present well-defined seasons, such as in temperate climates, and there is no sudden change in temperature and consequent seawater cooling, it was also possible to observe a high occurrence of
M. exasperatus specimens during the winter. Because of this, even with the seasons not being well defined, the late-summer peak still occurs [
21], which may explain the low occurrence of ascidians during the third collection, together with a high reproduction rate during the summer (first collection). Our hypothesis is that this phenomenon led to a high ascidians population density, causing a decrease in plankton and suspended organic matter available, which led to a decrease in the ascidians population density in that particular location.
It is also important to emphasize that no apparent difference was seen in the number of organisms that inhabit the tunic of these ascidians in the three collections carried out. Suggesting that the ecological relationship between epibiont organisms and ascidians is always present, regardless of seasonality, and may serve as a kind of camouflage or barrier to predators throughout the non-larval phase of ascidians [
21].
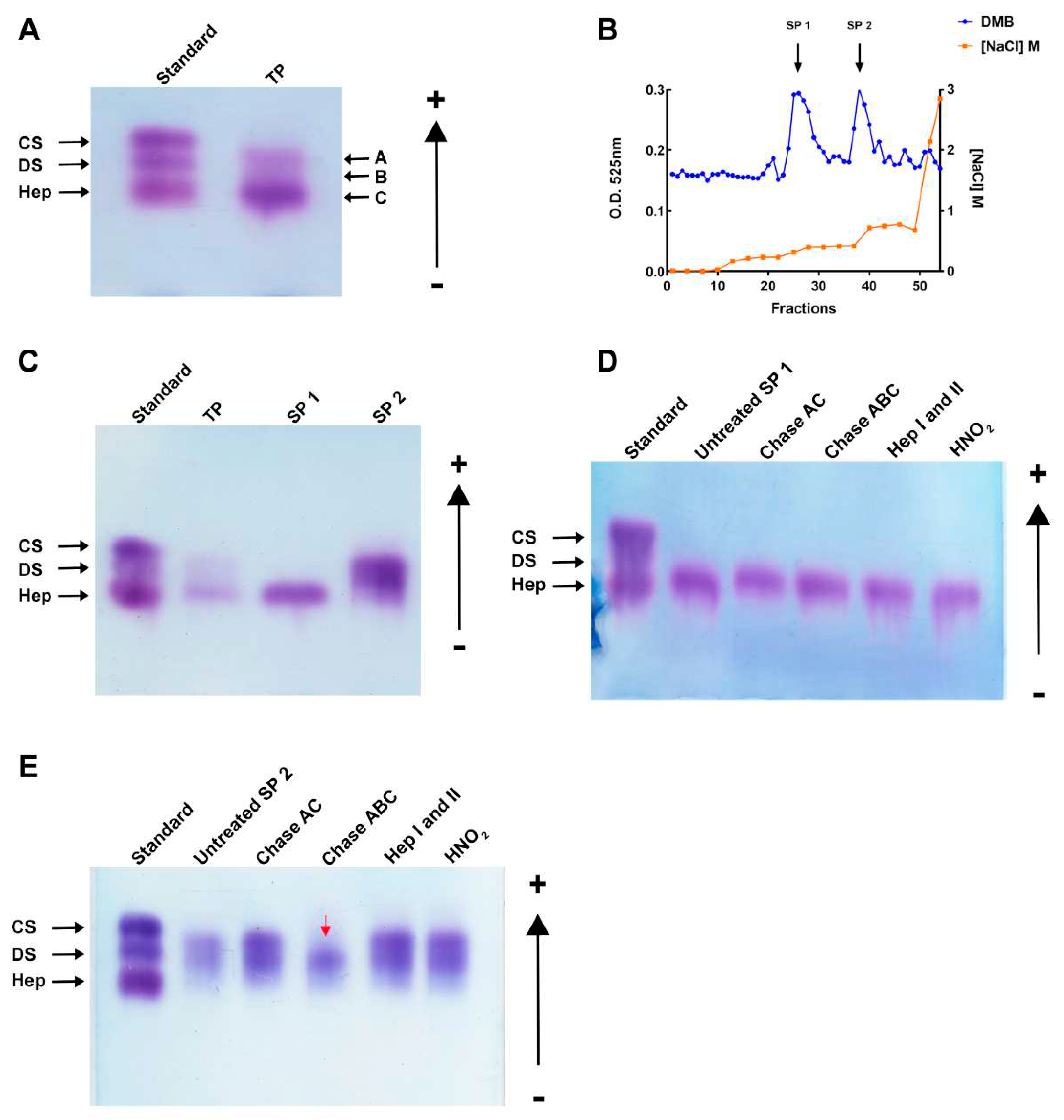
After specimens’ collection, viscera dissection and sulfated polysaccharides extraction were performed, a solution containing total polysaccharides, called TP, was obtained. Through agarose gel electrophoresis, it was possible to compare the migration pattern of the molecules found with commercially known GAG patterns, such as porcine heparin, mammalian dermatan sulfate and shark chondroitin sulfate, and then it was possible to observe three types of sulfated polysaccharides present in this TP. One migrating at the level of dermatan sulfate, another migrating at the level of heparin, with greater intensity than the others, and a third showing a migration pattern between dermatan sulfate and heparin. This result demonstrated that we successfully established the specific extraction protocol for these molecules for this species of ascidian. And, like the other species of marine invertebrates already studied and described in the literature [
16], we can say that
Microcosmus exasperatus actually presents sulfated polysaccharides in its viscera, having three distinct patterns of these uronic acid-containing molecules.
From this, we developed a purification and fractionation method for these sulfated polysaccharides based on ion exchange liquid chromatography, using an FPLC device. As these molecules can present negative charges due to the presence of sulfations, it was possible to elute these molecules in a DEAE cationic column, with an anion exchange being performed. First, a method based on NaCl linear gradient concentrations was set up, where it was possible to observe at which concentrations these sulfated polysaccharides were eluted. Once these concentrations were previously determined, it was possible to set up a purification method based on a discontinuous NaCl gradient, seen in
Figure 2b, where it is possible to optimize the purification and reduce the running time. With this result, we observed that it was possible to fractionate these sulfated polysaccharides into two distinct fractions, called SP1 and SP2. Furthermore, observing this chromatogram, we saw that these sulfated polysaccharides are eluted from the cationic column at low NaCl concentrations when compared to sulfated polysaccharides present in the viscera of other ascidian species already described in the literature [
5,
17,
19]. This data suggests that
M. exasperatus sulfated polysaccharides have a lower sulfation degree, since they need low NaCl concentrations to be displaced from the DEAE column. Thus, in
Figure 2c we can observe the fraction present in the first part of the chromatogram, called SP1, which was completely purified and migrated with standard heparin. SP2 contained a band migrating with standard dermatan sulfate and an intermediate band migrating between standard heparin and dermatan sulfate. This indicates that these three types of sulfated polysaccharides are eluted from the DEAE cationic column at very close NaCl concentrations and may present similar charge densities.
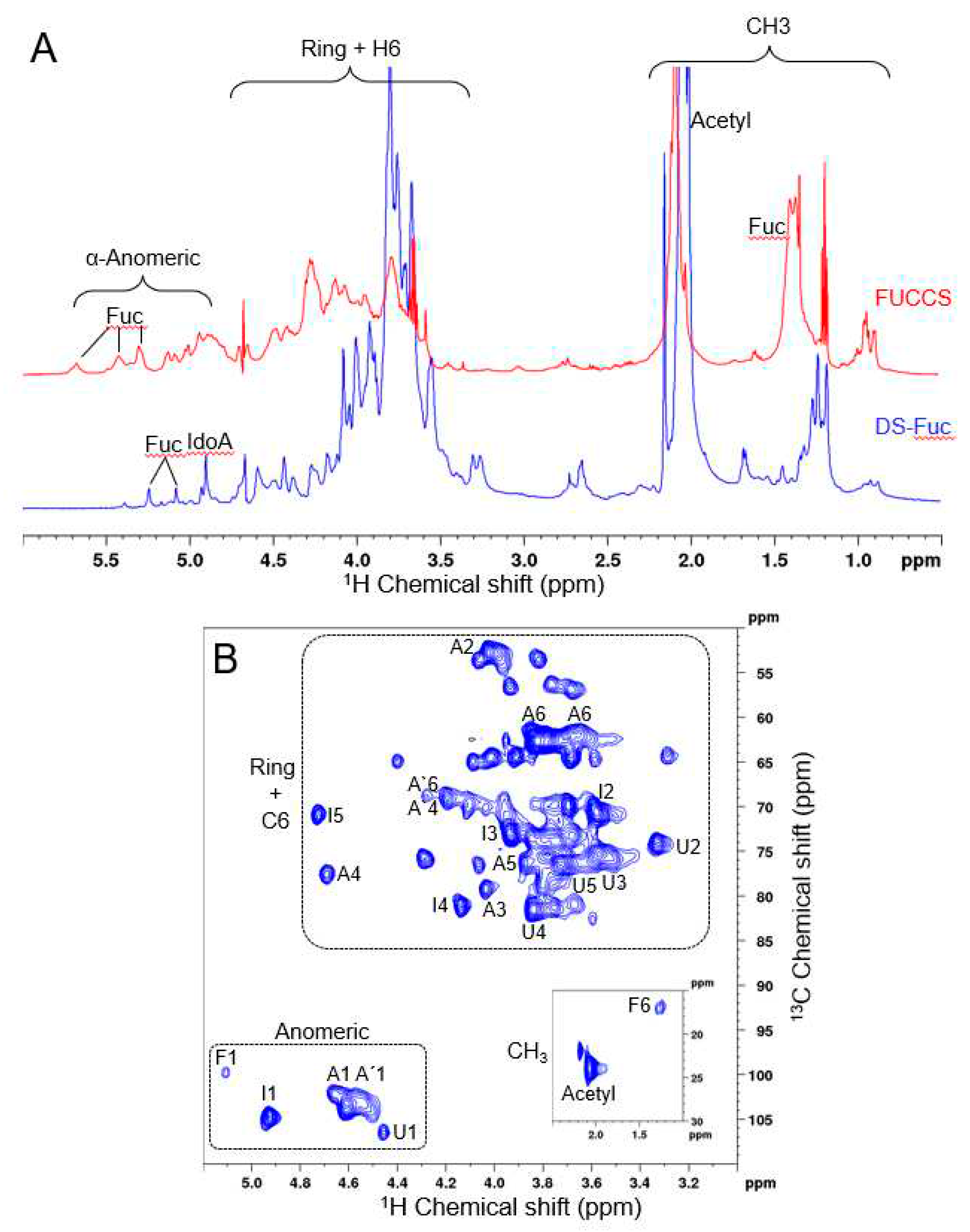
To identify the species of previously fractionated sulfated polysaccharides, digestion assays were carried out from the incubation of these molecules with specific lyases and the deamination with nitrous acid. Therefore, it was possible to observe that the molecule that migrates with standard heparin present in the SP1 fraction did not undergo degradation with any of the treatments. Combining this information with our NMR data on SP1, we hypothesize that this fraction is composed of a fucosylated-dermatan sulfate that is resistant to chondroitinases due to fucose residues attached to the polysaccharide. To our knowledge, this is the first description of a fucosylated dermatan sulfate.
Furthermore, the treatment with lyases on the SP2 fraction made it possible to visualize its effect on the compounds present in this fraction. Thus, we observed that the treatment with chondroitinase ABC degraded the molecule that migrates with standard dermatan sulfate. These enzymes degrade both dermatan sulfate and chondroitin sulfate. Dermatan sulfate is composed of repeats of N-acetyl-galactosamine and glucuronic or iduronic acid, since the epimerase enzyme causes a chain of chondroitin sulfate to contain residues of iduronic acid, which makes it a dermatan sulfate [
25,
26,
27]. In order to be able to confirm that this molecule is in fact a dermatan sulfate and not a chondroitin sulfate, the treatment with chondroitinase AC was also performed, since these enzymes degrade only chondroitin sulfate chains. Therefore, we determined that SP2 fraction presents a dermatan sulfate, since the molecule did not undergo degradation when it was incubated with the AC chondroitinase enzyme.
The other SP2 band that migrates between standard dermatan sulfate and heparin is a uronic acid-containing sulfated polysaccharide, which, in addition to presenting a unique migration pattern, is not degraded by any of the lyases used or by nitrous acid deamination.
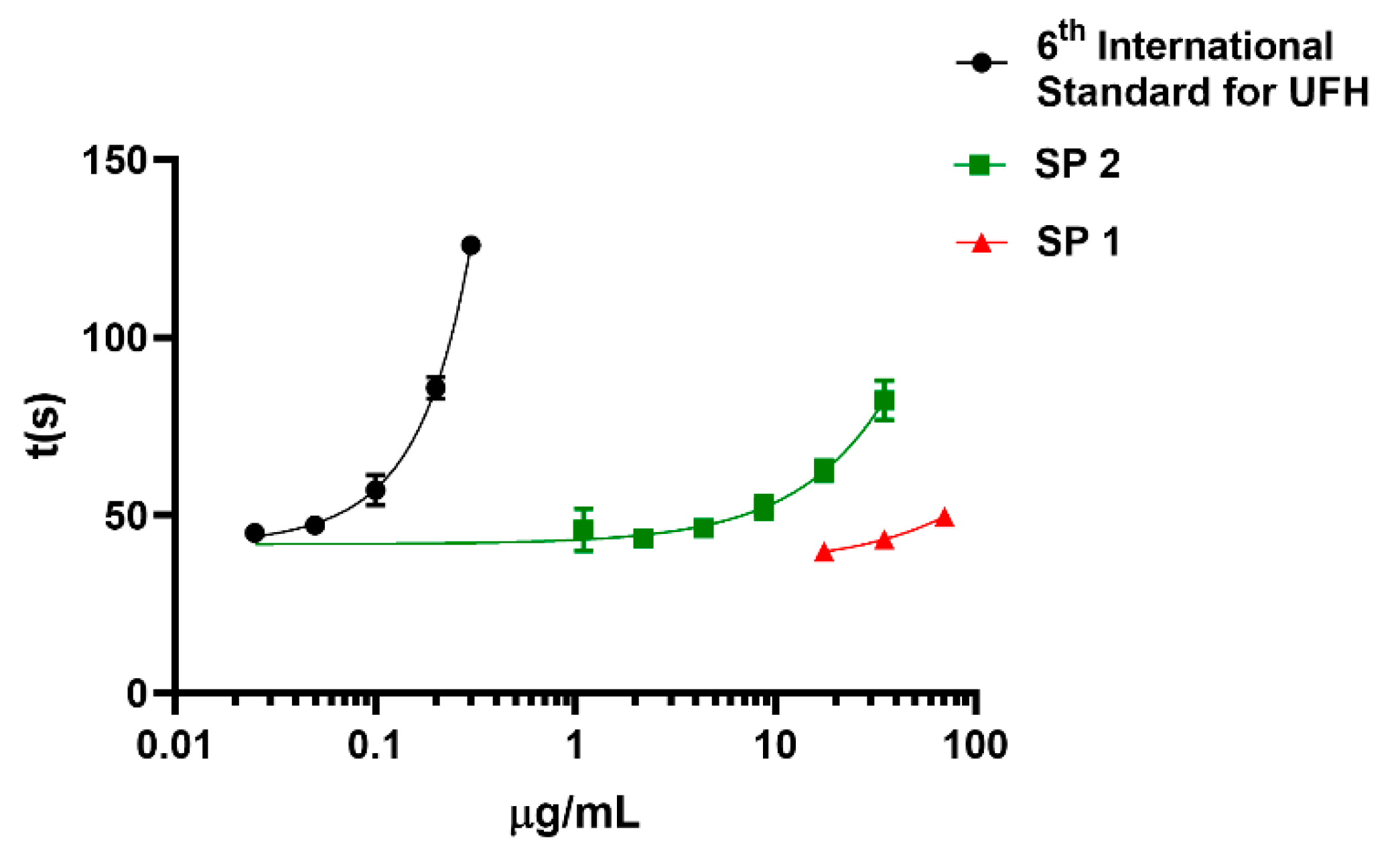
After identifying the sulfated polysaccharides contained in SP1 and SP2 fractions, we proceeded to evaluate their biological activities. In view of what has already been demonstrated in relation to dermatan sulfates derived from other ascidian species, which may present anticoagulant activity similar to mammalian heparin, however, through different mechanisms of action, this approach was chosen [
18]. After carrying out the aPTT assay with the SP2 fraction it was seen that this fraction presents anticoagulant activity approximately one hundred times lower than unfractionated bovine heparin. As already seen in the literature, the anticoagulant action of dermatan sulfate from ascidians is related to the sulfated chain pattern, showing that 2-4 dermatan sulfates have anticoagulant activity, while 2-6 dermatan sulfates do not present such activity [
5,
17,
19]. Thus, we can suggest that, considering that
M. exasperatus belongs to the Stolidobranchia order, its low anticoagulant activity results from the combination of a low degree of sulfation arranged possibly as a 2-4 dermatan sulfate. The anticoagulant activity of the fucosylated dermatan sulfate (SP1 fraction) was also evaluated and it was seen that this sulfated polysaccharide does not have anticoagulant activity at the tested concentrations.
Considering the growing interest in combination therapy to enhance cancer treatment efficacy, we sought to investigate if M. exasperatus polysaccharides would be a suitable alternative to the use of mammalian heparin. M. exasperatus may be considered a good alternative to mammalians, because, as a marine invertebrate, its large-scale production could be directed towards a more sustainable approach, particularly, requiring less dry land to occur. Additionally, the evolutionary distance between ascidians and mammalians adds to the safety against cross-species contamination, therefore, we consider M. exasperatus, a particularly interesting organism to be considered for antitumoral polysaccharide production.
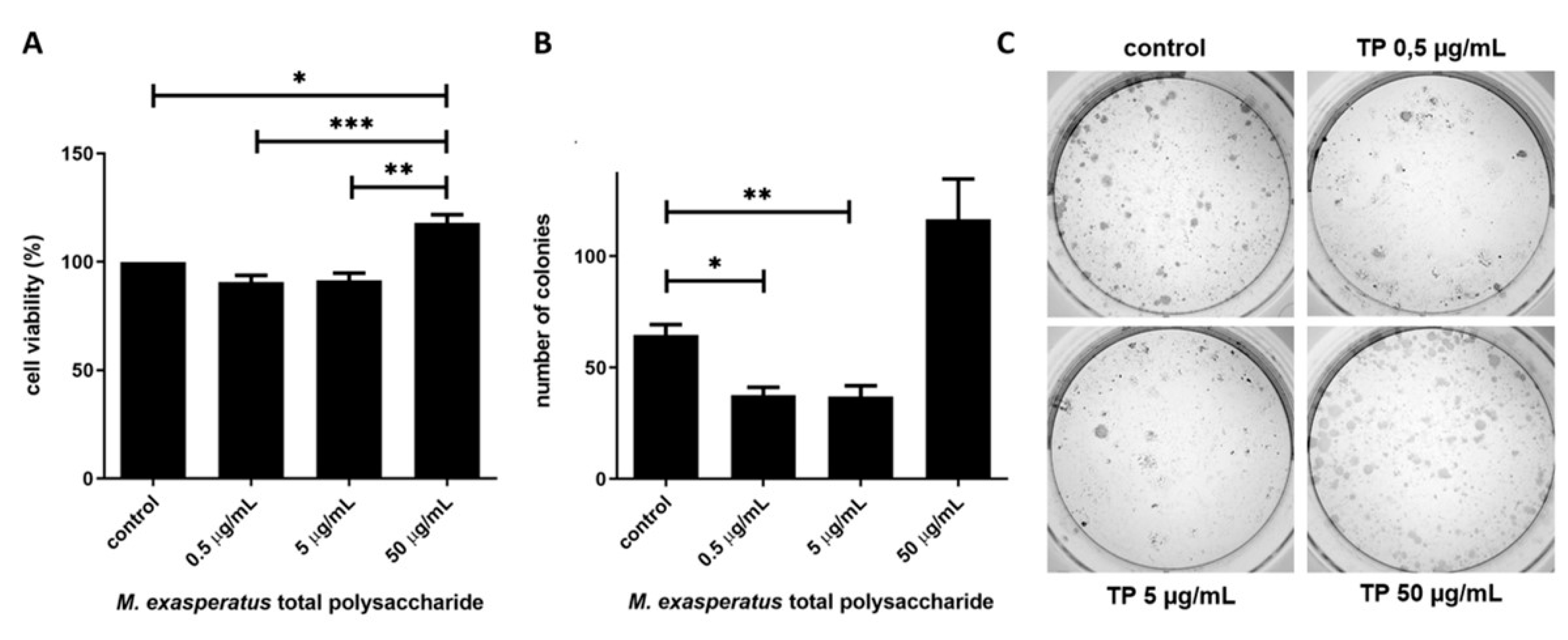
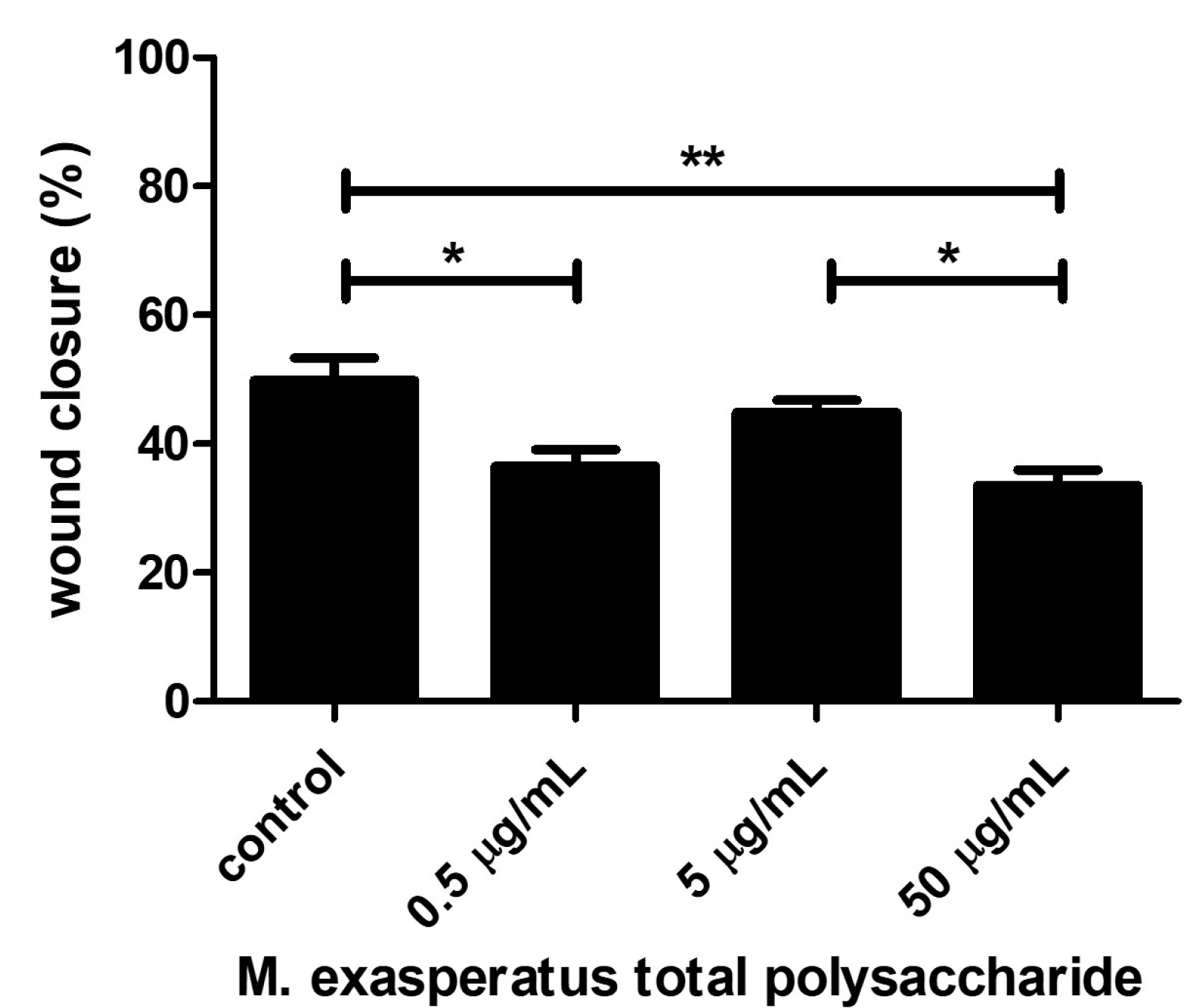
Antitumoral activity was evaluated using three different approaches in a cellular model using the LLC cell line. We found that M. exasperatus total polysaccharides exerts different effects on tumor cells from short- to long-term treatments, as well as, from low (0.5µg/mL) to high (50µg/mL) concentrations. We found that the high-TP dose acted as a tumor cell stimulant, enhancing viability and colony growth, while cell migration was reduced compared to controls. The low-TP dose, on the other hand, presented antitumoral effects regarding colony growth and cell migration. While the short-term treatment did not present differences from the control condition.
In summary, our data points towards the use of long-term, very low doses of M. exasperatus TP, ideally well below anticoagulant concentration, when considering combination therapy. Also, M. exasperatus TP extraction, and possibly fractionation, may easily be adapted to a large-scale regimen, as all steps may be executed in bulk. And finally, considering that this ascidian species can be found in many parts of the world, we highlight M. exasperatus as a promising organism for extraction of unique sulfated polysaccharides, dermatan sulfate and fucosylated-dermatan sulfate, with therapeutical properties as modulators of cell behavior. Marine invertebrates’ glycans represent a relevant and growing area of research, both from the clinical and environmental aspects of science and further investigations in this area should be stimulated.
4. Materials and Methods
4.1. Specimens collection and dissection
Microcosmus exasperatus ascidians species were collected (SISBIO permit: 66457-1) by free diving in Praia Vermelha, Rio de Janeiro, placed in collection buckets with sea water and transported to the laboratory. At the laboratory, the viscera were dissected using scalpel and scissors, cutting the tunic for viscera removal. Viscera were fixed in 100% ethanol, while tunics were frozen (for use in other projects) or discarded.
4.2. GAGs extraction from the Microcosmus exasperatus viscera
After
M. exasperatus viscera dissection, GAGs extraction was performed as described in [
19] to obtain a final solution containing only the sulfated polysaccharides (named total polysaccharides – TP), and then perform the fractioning.
4.2.1. Delipidation and depigmentation
To ensure that the extracted material was free of any fatty tissue and pigments, viscera were incubated in 92.8% ethanol at room temperature. Ethanol was replaced daily for a total of five days for the efficient removal of lipids and pigments. Then, the viscera were placed to dry in an incubator at 60°C for 24 hours. Once dry, the material was weighted.
4.2.2. Proteolytic digestion
In the next step, the material was digested with papain in order to release polysaccharides from tissue. The dry material was rehydrated for 2hs in digestion buffer (100 mM sodium acetate; 5 mM EDTA; 5 mM cysteine; pH 5.0) at 5mL per gram of dry material. Digestion was started by adding papain at a final concentration of 0.5 mg/mL and incubated at 60°C for 24 hours. After that, the material was mechanically homogenized using a spatula, additional papain was added, and samples were incubated at 60oC. This process was repeated three times, in order to complete the homogenization of the solid samples. The final digested suspension was centrifuged for 30 minutes at 3200 rpm, and then the supernatant (solubilized fraction containing the free polysaccharides) was collected, and the pellet (undigested fraction) discarded.
4.2.3. GAGs precipitation
Polysaccharides precipitation in the supernatant after proteolytic digestion was performed by adding cetylpyridinium chloride (CPC) until reaching a final concentration of 0.5% m/v. After CPC addition, the final mixture was homogenized and incubated at room temperature for 24 hours. Subsequently, the suspension was centrifuged at 3200 rpm for 30 minutes. The supernatant was then discarded, and the pellet was completely dissolved in a 2 M sodium chloride (NaCl) solution containing 15% ethanol. Then, polysaccharides free of CPC were precipitated by adding ethanol to 70% in solution, incubating at -20°C for 24 hours. Next, the suspension was centrifuged for 30 minutes (3200 rpm), the supernatant was discarded, and the pellet was further rinsed in 80% ethanol, centrifuged again for 30 minutes (3200 rpm) and the resulting pellet was air dried. The pelleted TP was rehydrated in DNAse buffer (20 mM Tris-HCl pH 7.5; 5 mM NaCl; 3 mM MgCl2; 5 mM CaCl2) (1:1) and treated with 510 U Kunitz/mL of DNAse (Sigma) at 37°C for 24 hours.
4.3. Sulfated polysaccharides electrophoretic profile analysis
After obtaining the TP, an agarose gel electrophoresis was performed to identify the polysaccharides electrophoretic profile found in M. exasperatus viscera, based on the comparison to commercially available GAGs standards: porcine heparin, shark chondroitin sulfate and mammalian dermatan sulfate. Samples were applied to a 0.5% agarose gel in 1,3-diaminopropane / 50 mM acetic acid buffer, pH 9.0 and ran for, approximately, 2 hours, at 100 V. Polysaccharides were, then, precipitated by immersion of the gel in a cetyltrimethylammonium bromide (cetavlon 0.1%) solution for 24 hours. Next, the gel was dried under lamp heat (Ourolux-250 watts-Infrared) and the resulting dried gel sheet stained with 0.1% toluidine blue in a acetic acid:ethanol:water (0.1:5 :5, v/v) solution.
4.4. Sulfated polysaccharides purification and fractionation by ion exchange liquid chromatography
TP was fractionated by ion exchange liquid chromatography. Initially, 200 µg of material were applied to a diethylaminoethyl cellulose anion exchange column (DEAE sepharose) (GE Healthcare) coupled to the FPLC (fast protein liquid chromatography) Äkta Prime equipment (Amersham Biosciences, United Kingdom, SN: 1102380), equilibrated in 0.02 M Tris-HCl buffer, pH 8.0. The GAGs were eluted from the column in a linear NaCl gradient (0-3 M), using a 0.02 M Tris-HCl buffer containing 3 M NaCl, pH 8.0, at a flow rate of 3 mL per minute, with fractions of 3 mL per tube being collected. After that, conductivity was measured to assess NaCl concentration. Thereby, 240, 370 and 700 mM NaCl concentrations were determined as concentrations used in a stepwise NaCl gradient for bulk sulfated polysaccharides fractionation. Sulfated polysaccharides elution pattern was assessed by metachromasia properties using 100 µL of DMB and 20 µL of sample in a 96-well plate, and then read in a Versamax spectrophotometer with microplate reader (Molecular Devices, USA) at 525 nm.
Finally, the fractions containing metachromatic material correlated to the peaks in the graph (SP1 and SP2) were grouped, precipitated in 70% ethanol at -20°C for 24 hours. The suspension was centrifuged (3200 rpm for 30 minutes), the pellet was rinsed in 80% ethanol, centrifuged again (3200 rpm for 30 minutes) and let to air dry.
4.5. Lyases specific degradation and nitrous acid deamination
For the sulfated polysaccharides identification after fractionation by ion exchange liquid chromatography, enzymatic treatments were performed on SP1 and SP2 with chondroitinases AC and ABC (Seikagaku, Japan), a mixture containing heparinases I and II and deamination with nitrous acid (HNO2) to investigate the identity of the sulfated polysaccharides species present in the samples. Each sample was incubated for approximately 24 hours with 0.05 units of chondroitinases AC and ABC, and 0.2 units of heparinases I and II in 2x digestion buffer (100 mM Tris–HCl, 30 mM sodium acetate, 1.0 mM EDTA, pH 8.0) for chondroitinases, and (40 mM Tris–HCl, 100 mM NaCl, 8 mM CaCl2, pH 7.5) for heparinases at 37°C, using sample, enzyme and 2x buffer ratio of 1 :1:1. After 24h, a new addition of each enzyme was made.
Deamination was also performed, and the nitrous acid was produced from the reaction of sulfuric acid (H2SO4) at a final concentration of 0.25 M with sodium nitrite (NaNO2) at a concentration of 0.25 M for 20 minutes. Afterwards, the samples were mixed with the same volume of nitrous acid (HNO2) (1:1, v/v) and incubated at room temperature for one hour.
Enzymatic degradation and deamination were evaluated by agarose gel electrophoresis for the comparison of intact and digested material, in addition to commercial standards for reference.
4.6. Uronic acid analysis
The polysaccharides concentration contained in the TP solution, SP1 and SP2 was estimated by its uronic acid content through the carbazole method [
28].
Briefly, the method consists of incubating 10 μL of the polysaccharide solution with 190 μL of distilled water and 1 mL of sulfuric acid with 0.1% borate, homogenized and incubated for 12 minutes at 100°C. After cooling, 40 μL of carbazole (0.2% in ethanol) are added to the solution, which is again homogenized and incubated at 100°C for 10 minutes, then cooled once more and the absorbance measured at 525 nm. The samples concentration was estimated from a glucuronolactone standard curve.
4.7. Nuclear Magnetic Resonance (NMR) analyses
Nuclear magnetic resonance (NMR, 1D and 2D spectra) was performed for SP1 fraction (2mg) using a triple resonance probe (900 MHz Bruker, Karlsruhe, Germany). 1H NMR 1D spectra was recorded using 32 scans, 1,5 second inter-scan delay; 35 °C. 1H/13C HSQC (heteronuclear single quantum coherence) spectrum using time proportion phase incrementation-TPPI for quadrature detection in indirect dimension. A total of 64 scans and 1048x512 points were recorded.
4.8. Activated partial thromboplastin time (APTT)
Anticoagulant activity was measured by the activated partial thromboplastin time (APTT) assay, as described by Eggleton [
29] Thomas [
30].
Briefly, 100 µL of human plasma mixture was incubated with 10 µL of solution containing different polysaccharides concentrations (SP1: 17.5; 35; 70 µg/mL; SP2: 1.1; 2.19; 4.38; 8.75; 17.5; 35 µg/mL; UFH: 0.025; 0.05; 0.1; 0.2; 0.3 µg/mL) for 1 minute at 37ºC. Then, 100 µL of cephalin (Biolab-Merieux AS, Rio de Janeiro, Brazil) were added. After 2 minutes of incubation at 37ºC, 100 µL of 25 mM CaCl2 was added to the mixture, and the time required for coagulation was measured through the formation time of a stable clot capable of retaining the metallic sphere present in the well, with the aid of a coagulometer (KC4 Delta).
Activity is expressed in polysaccharides micrograms per milliliter (µg/mL). The data obtained resulted in a polynomial function, according to the equation: [T/T0] = a + b1.[UI] + b2.[UI]2, using a standard curve based on the 6th International Standard for Unfractionated Heparin (6thUFH) (2145 units per vial), obtained from NIBSC (Potters Bar, UK), diluted in order to obtain a UFH solution with anticoagulant activity equivalent to 10 IU/mL.
4.9. Cell viability assays (MTT)
In order to assess cell viability exposed to M. exasperatus TP, 5*103 cells were plated per well in 96-well plates. Then, 24hs after plating, cells were treated with vehicle (PBS) or M. exasperatus TP at different concentrations (0.5µg/mL, 5µg/mL and 50µg/mL) for additional 24hs. Next, cells were incubated with tetrazolium salt (MTT, Roche) 0.5 mg/mL in cell medium for 2 h. After incubation, cell medium was discarded, and formazan crystals were dissolved in 200 µL dimethyl sulfoxide (DMSO, Sigma-Aldrich) per well. Absorbance at 560 nm was acquired using SpectraMax Plus microplate reader (Molecular Devices) and analyzed using Softmax Pro software (Molecular Devices). Viability was calculated as percentage of absorbance using control cells as reference.
4.10. Clonogenic assay
In order to evaluate LLC cells’ ability to form colonies, a clonogenic assay was performed. Regularly cultured cells were trypsinized, quantified and plated at 500 cells/well in 6-plate wells. M. exasperatus TP was added to culture medium at 0.5µg/mL, 5µg/mL and 50µg/mL, or vehicle (control condition) during the low-density plating. Cells were maintained in regular culture conditions for 72hs, then, culture medium (control and TP medium) was replaced by regular medium and cells were cultured for additional 7 days, with medium changes every 48hs. After a total of 10 days, cells were rinsed with PBS, fixed in 100% ethanol for 1h and stained for 15 minutes with 0.4% trypan blue. Wells were imaged in a stereomicroscope and analyzed using ImageJ software for colony quantification.
4.11. Wound healing – cell migration – assay
Cells were plated in 6-well plates, at 1.5*105cells/well, and cultured until 80% confluence. After reaching adequate confluence, cells were mechanically removed from the plate in a cross-shaped pattern using a sterile P1000 tip. Wells were rinsed for removal of cell debris and incubated in control (regular culture medium) or TP-medium at 0.5µg/mL, 5µg/mL and 50µg/mL for 24hs. In order to evaluate cell migration and wound closure, cells were imaged, using the cross center as a reference at 0h and 12hs. Migration was quantified using ImageJ software and expressed as the percentage of migrated distance relative to the original wound width.