1. Introduction
Benzoxazine resins are a relatively new class of thermoset phenolic resins developed in the last decade, which combine the superior high temperature resistance and acid and alkali resistance of phenolic resins with the remarkable mechanical properties and manufacturability of epoxy resins [
1,
2,
3]. They are characterized by the mechanism of ring cleavage and curing crosslinking during the curing process, which does not produce small molecules and results in low curing shrinkage, enabling the production of products with improved heat resistance and mechanical properties compared to conventional phenolic resins [
4]. Due to its various advantageous properties, it holds enormous potential for applications under high-temperature conditions, such as high-temperature resistant shells for rocket engines and flame retardant materials for aircraft, as well as other fields.
Benzoxazine resins was first synthesized by Cope and Holly [
5] in 1944 by condensation reactions between primary amines and formaldehyde with phenol, but the potential of polybenzoxazine was not fully realized until much later. Riess et al. [
6] demonstrated the regioselectivity of the preferential reaction of the oxazine ring with phenolic compounds. Ishida and colleagues further advanced the monomeric benzoxazine by inventing a solvent-free synthesis. These studies showed that various structures of benzoxazine monomers can be achieved by precisely designing the benzoxazine monomers with different functionalities in combination with various phenols and amines. This provides a theoretically sound basis for the modification of benzoxazine resins to obtain polymeric materials with desired properties.
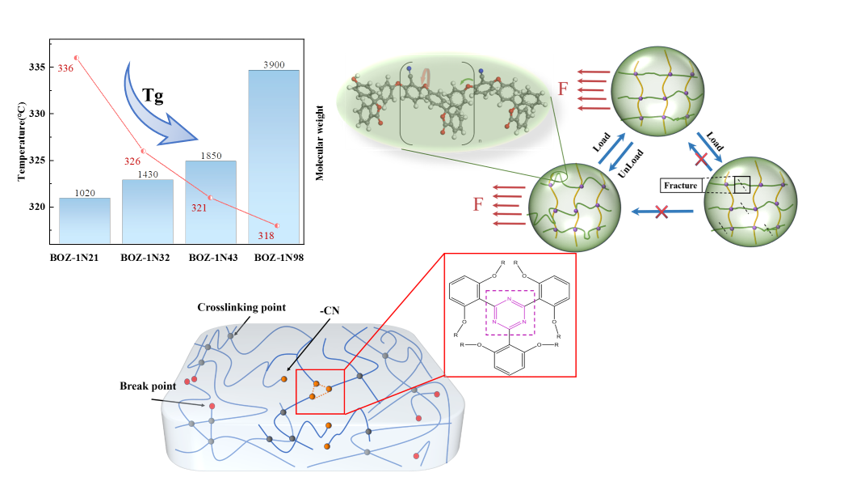
The main obstacle to the development and application of benzoxazine resins is a common problem with other high-temperature resins, namely the tendency to become easily brittle and to exhibit low toughness of the cured products. The primary approach to overcoming this problem is to develop a novel molecular structure, whichi includes increase the molecular weight of the monomer, thus reduces the density of curing crosslinks, improves the toughness of the resin without affecting high temperature resistance to the greatest extent possible. To achieve this, there is an urgent need to develop high-performance benzoxazine resins. In the last two decades, research on benzoxazine resins has focused particularly on (i) chemical modification/molecular design, such as the incorporation of phenylacetylene groups, cyano groups [
7,
8], allyl [
9], propargylether [
10] ect; (ii) modification by blending with other resins (including epoxy resins, bimatic resins [
11], etc.; (iii) cure kinetics and mechanistic analysis [
12]. It was found that the addition of alkyne groups can increase the number of functional groups in the benzoxazine molecule that can be crosslinked [
13]. In addition, the alkyne groups formed a six-membered ring structure during curing and crosslinking, increasing the crosslinking density of the resin and thus its Tg, which gave the resin higher heat resistance [
14,
15]. This excellent thermal property is of great advantage in the field of heat resistance and ablation [
16]. The characteristics that ensure excellent thermal and mechanical properties of the resin can be provided by the rigid and stable triazine rings formed by cyano groups. The Tg of high-performance resins prepared from benzoxazine monomers, cyano and alkyne groups in Zhang.S et al studies is 292.7°C [
17,
18,
19,
20,
21]. The presence of phenolphthalein structure can effectively reduce the proportion of crystal regions in the resin structure, thereby increasing toughness. At the same time, the cyclic structure of phenolphthalein also makes an important contribution to the heat resistance of the resin. R. M. Ghetiya et al. [
22] synthesized two benzoxazines containing bisphenol C (BCO) and phenolphthalein (PHO) using bisphenol C/phenolphthalein, formaldehyde and aniline. The residual percentage of the phenolphthalein samples (56-67%) was significantly higher than that of the bisphenol C samples (20-25%) at 550°C. Above all, the above work is still difficult to meet the usage needs in the high-end field. Therefore, according to the concept of molecular structure design and based on the previous three, it is of great significance to prepare alkynyl-functionalized benzoxazine containing phthalide side group and cyano group.
In this study, the curing behaviors of BOZ-1N were investigated by DSC and FT-IR, which suggested that the cyclization reaction of alkynyl group and the ring cleavage reaction of benzoxazine ring occur simultaneously, and the conversion rate of alkynyl group is significantly lower than benzoxazine ring that of during curing process. Thermal properties and structures of BOZ-1N system were investigated systematically. The results showed that the cured resins possessed high glass transition temperatures and excellent thermal. Meanwhile, the cyano group absorption of the polymer network was observed in IR spectra, indicating the cyano group almost unconsumed in the curing process. Interestingly, the storage modulus showed an upward trend around 325°C in DMA testing. This may be because triazine has been formed in the polymer network, the curing mechanism accord with the traditional curing system with lewis acids/bases as agent [
23]. The molecular weight of monomers also has a certain impact on their heat resistance performance.
2. Materials and Methods
2.1. Raw materials
Phenolphthalein(PP), 3-ethynylaniline, 2,6-Dichlorobenzonitrile, formaldehyde, acetone, anhydrous potassium carbonate, deionized water, and anhydrous ethanol were purchased from Shenyang Xinhua Reagent Factory, while diphenylmethane bismaleimide and o,o'-diallyl bisphenol A were purchased from Honghu Shuangma New Material Technology Co. All reagents were of analytically pure quality and were used as received. Finally, T700 carbon fiber was obtained from Toray Corporation, Japan.
2.2. Synthesis of BOZ-1N series resin
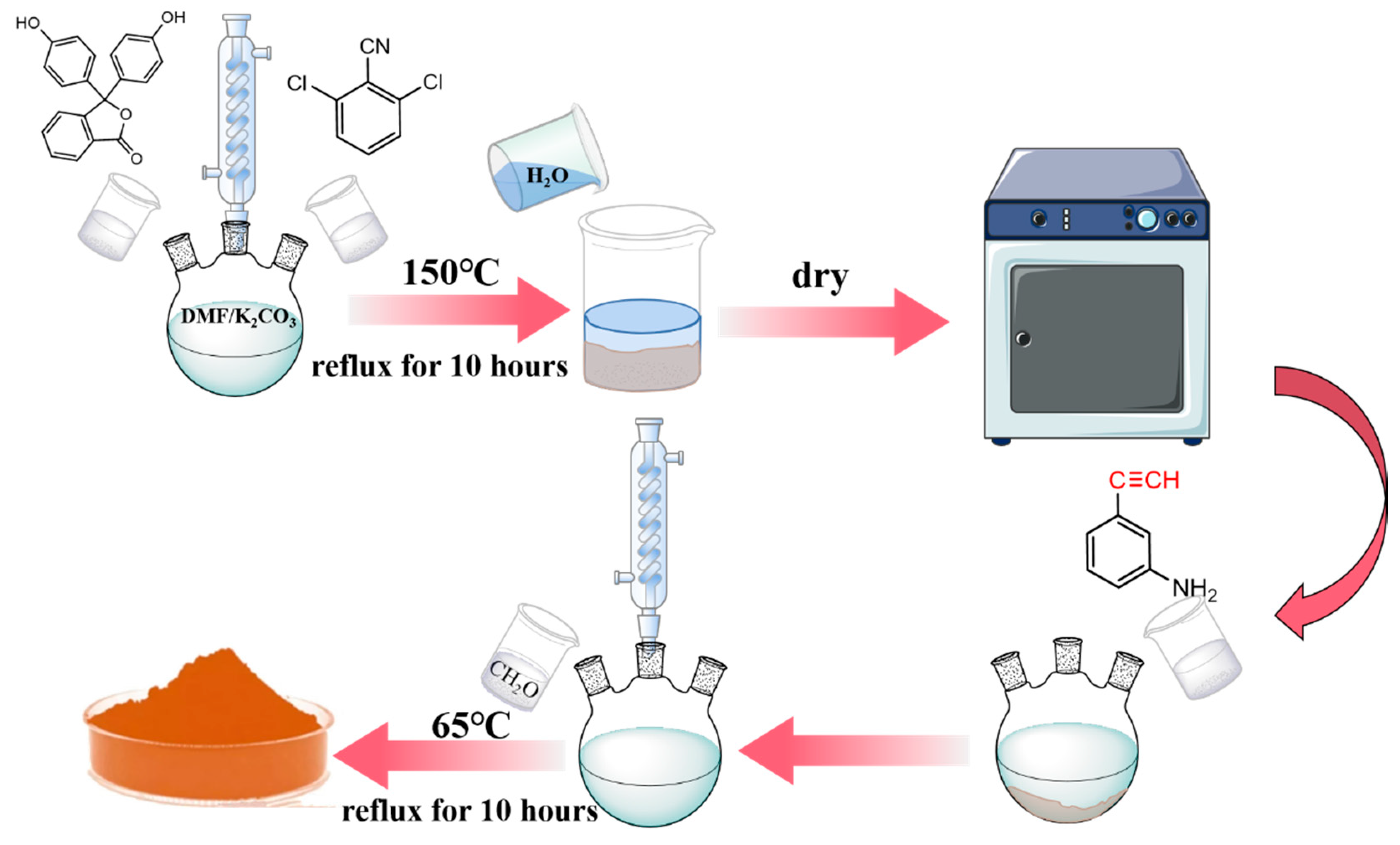
According to the dosage given in
Table 1, the required amounts of phenolphthalein, 2,6-Dichlorobenzonitrile, and anhydrous potassium carbonate were weighed and then added to a three-neck flask containing DMF as solvent. Then the stirrer was activated and the reaction solution was heated to 150°C and kept at reflux for 10 hours. The reaction solution was filtered off while it was still hot, and deionized water was added to the filtrate until the precipitate began to stratify. The filter residue was then washed and dried to obtain a phenol hydroxyl sealing polyarylether-cyano oligomers.
The phenol hydroxyl sealing polyarylether-cyano oligomers synthesized in the previous step were dissolved in acetone containing 3-ethynylaniline (molar ratio 1:2), then formaldehyde was added and the reaction was carried out at 65℃ under reflux for 10 hours. At the end of the reaction, the reaction solution was poured into a rotary evaporator to evaporate and dry. Four BOZ-1N resins with different molecular weight (BOZ-1N21, BOZ-1N32, BOZ-1N43, BOZ-1N98) were obtained, which appeared as brown powder solids.
2.3. Preparation of T700/ BOZ-1N composite laminate
The BOZ-1N resin was dissolved in ethanol and configured into a resin solution with a mass fraction of 30%. This solution was then used for the impregnation process to produce a T700/BOZ-1N prepreg, followed by a molding process to produce a unidirectional composite plate. The curing process of CF /BOZ-1N composite is 180°C/2h, 208°C/3h, 240°C/3h and 260°C/2h.
2.4. Representation
DSC profiles were recorded using a PerkinElmer Diamond DSC instrument at various heating rates under a nitrogen atmosphere.
FT- IR spectra were recorded using a PerkinElmer Spectrum 100 FT-IR spectrometer with the average signal of 4 scans and collected in the wavenumber range 4000 to 450 cm-1.
Varian Unity INOVA 400 NMR was used to test the 1H-NMR of BOZ series resin using deuterium chloroform (CDCl3) as solvent and tetramethylsilane (TMS) as internal reference. The observed frequency is 400 MHz and chemical shifts are expressed in ppm.
DMA was performed using a TA Q800 single cantilever beam model with a drive frequency of 1 Hz and a heating rate of 5℃/min ranging from 30 to 400℃.
The gelation time (tgel) was measured by isothermal DMA in the single cantilever model with T700/BOZ-1N prepregs wrapped in tin foil paper at temperatures of 208°C and 240°C, respectively.
3. Results
3.1. Chemical structural characterization of BOZ series resins
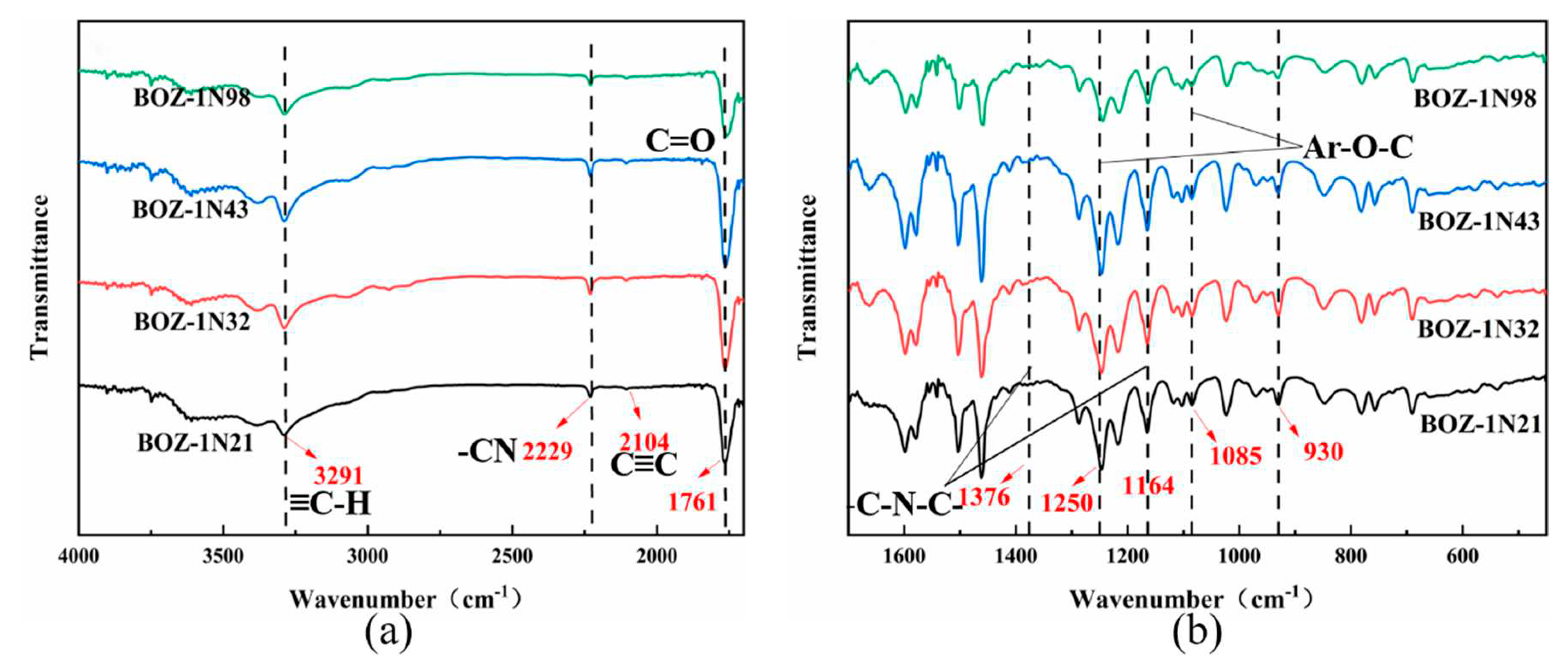
FTIR appears to be an efficient method for quantitative as well as qualitative curing investigation and structural characterisation of resins. The FTIR spectra of BOZ-1N resin can be seen in
Figure 2. The -C≡C and ≡C-H stretching vibrations of the alkynyl groups are responsible for the observed weak peak at 2104 cm
-1 and strong peak at 3291 cm
-1 on four curves, respectively. The vibrational absorption peak of lactone carbonyl (C=O) in the Cardo ring structure is 1761 cm
-1. Both 1376cm-1 and 1164 cm
-1 stretching vibrations of -C-N-C in the imide ring are recorded. The absorption at 1250 cm
-1 and 1085 cm
-1 might be attributed to the Ar-O-C stretching mode of the oxazine ring. Furthermore, the absorption peak at 930 cm
-1 is widely utilized to identify oxazine ring [
24,
25] formation. The -CN groups' characteristic absorption peaks occur at 2229 cm
-1, demonstrating the effective production of BOZ-1N resin. Furthermore, no influence of the varied molar ratios of 2,6-Dichlorobenzonitrile and phenolphthalein on the structure of the resin products was found, and their FT-IR spectra were almost comparable.
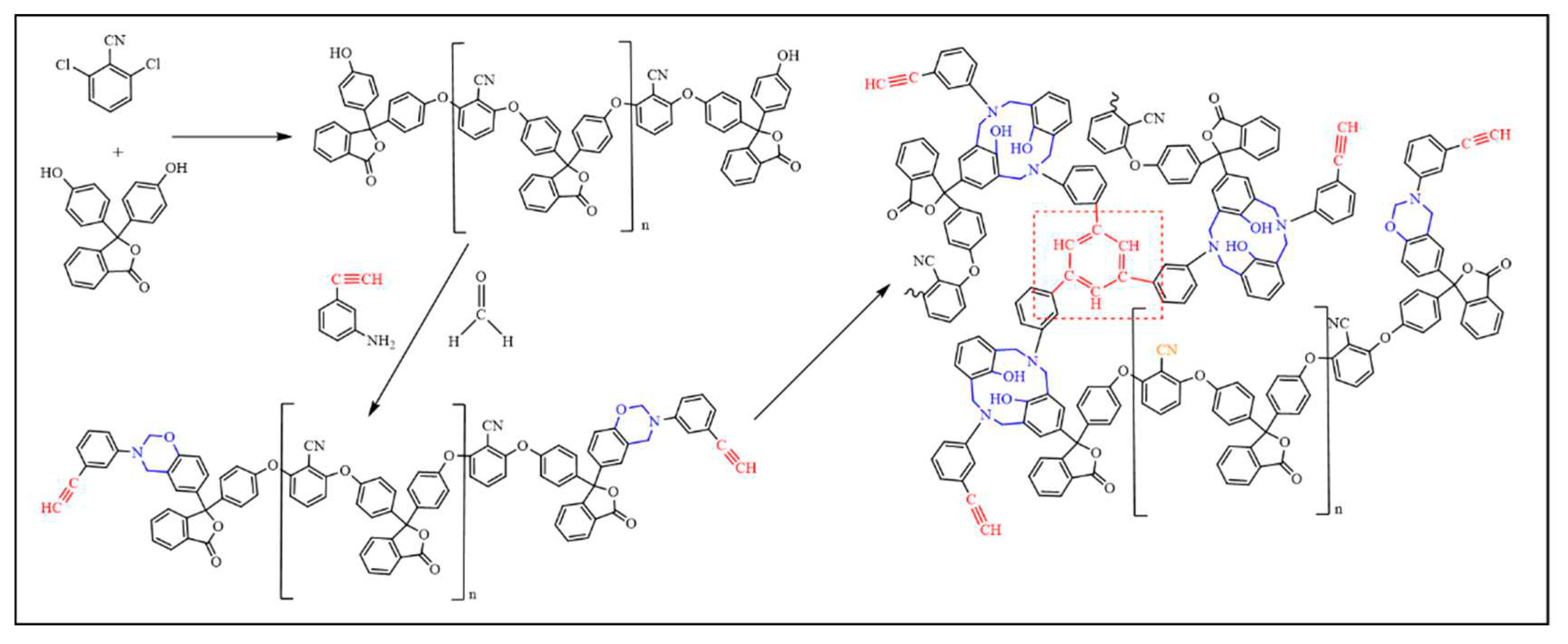
Figure 1.
Synthesis flow diagram.
Figure 1.
Synthesis flow diagram.
The
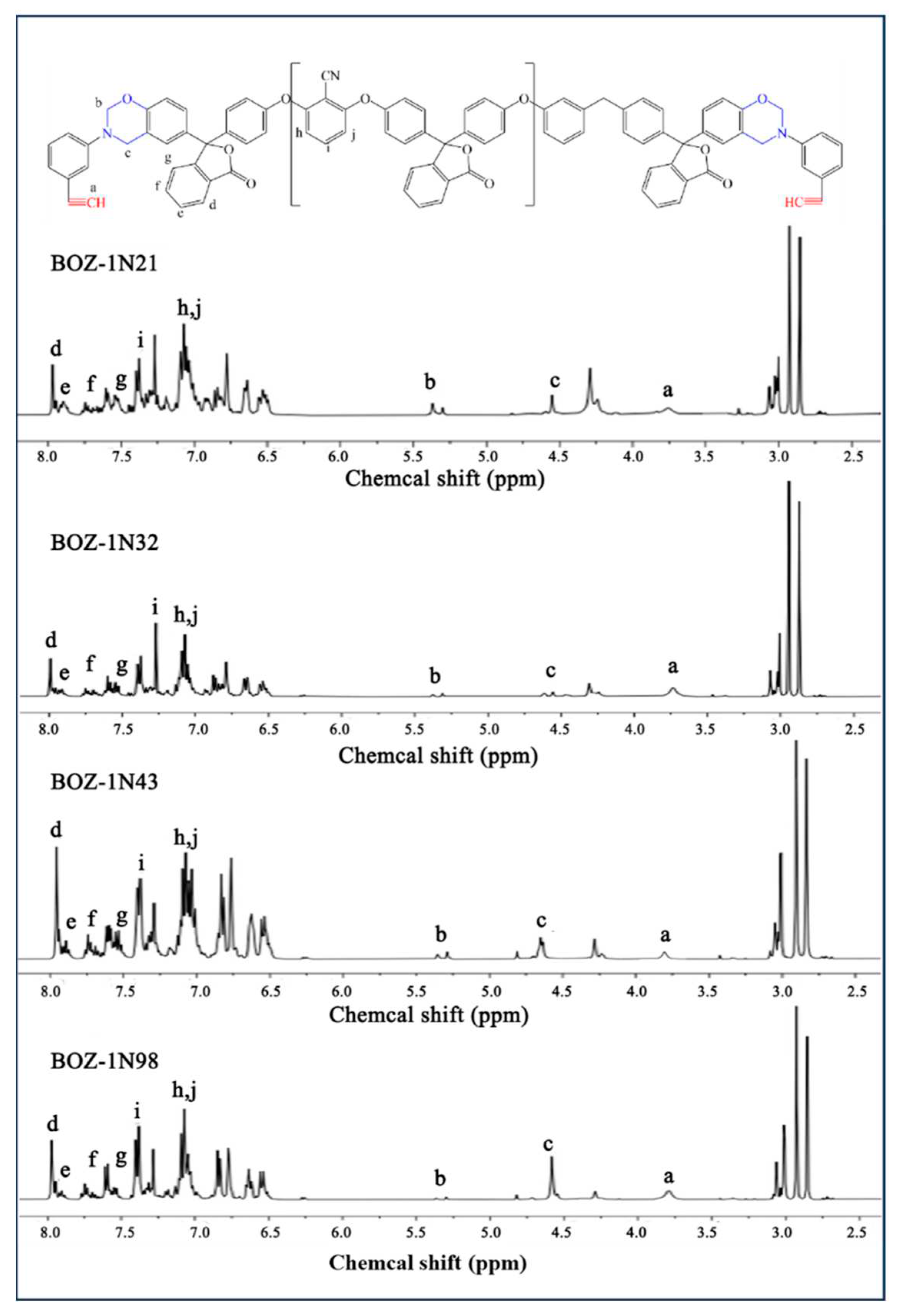
1H-NMR spectra of four distinct molecular weights of BOZ-1N are shown in
Figure 3. Because of the introduction of the characteristic functional groups ether bond, acetylene groups, and cyano groups into the structure, as well as the different molecular weights, the content of each functional group in the resin molecule varies, resulting in more complicated
1H-NMR spectra of the four BOZ-1N, but the positions of each peak are essentially the same.
Figure 3 reveals that the methylene of the oxazine ring has resonance peaks at 4.61 ppm and 5.39 ppm, respectively, while the proton of the acetylene groups [
26,
27] has a resonance peak at 3.74 ppm The resonance peaks at 7.11 ppm and 7.42 ppm in benzonitrile groups are caused by protons on the benzene ring, while the resonance peaks at 7.64 ppm, 7.81 ppm, and 7.96 ppm demonstrate the presence of the phenolphthalein structure. Combining the FTIR and
1H-NMR spectra, it is possible to conclude that the target product was successfully made, and the molar ratio of Phenolphthalein and 2,6-Dichlorobenzonitrile merely alters the size of the molecular weight, with no discernible influence on the product structure.
3.2. Intrinsic viscosity of BOZ-1N benzoxazine resin
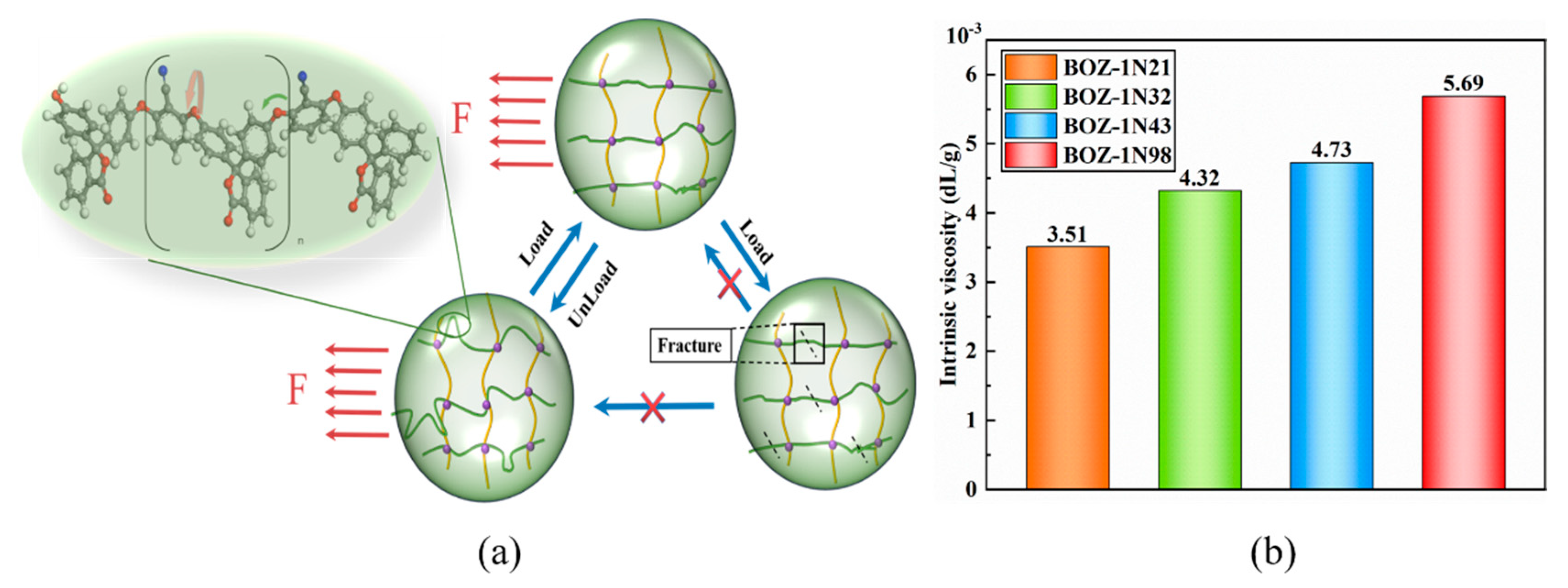
The toughness of the resin is connected to the crosslinking density and the length of the molecular chain, and the length of the molecular chain will affect the impact strength of the resin to some extent, as seen in
Figure 4a. When the molecular chain with curled conformation in the monomer is affected by an external force, the force will not directly act on the weakest point in the molecular structure, but will force the molecular chain to stretch and consume part of the force. When the load is unloaded, the molecular chain tends to curl to achieve the purpose of toughening due to the entropy increase principle.
The resin was evaluated for viscosity using an Ubbelohde viscometer in this study.
Figure 4b depicts the inherent viscosity data for four distinct molecular weights. And, as expected, as the concentration of 2,6-Dichlorobenzonitrile increased, the characteristic viscosity of BOZ-1N resin increased significantly, because 2,6-Dichlorobenzonitrile expands the structure of the molecular chain in the system, causing greater friction between the molecular chains and increasing viscosity. Because of the curled molecular conformation and asymmetric structure, the crystal area of the molecular chain is reduced and the entropy value is increased; when subjected to external forces, the energy can be converted into heat generated by the relative motion of the molecular chain, thereby increasing toughness.
3.3. Curing behavior and gelation point analysis of BOZ-1N21 resins
DSC is a broad approach for investigating curing kinetics [
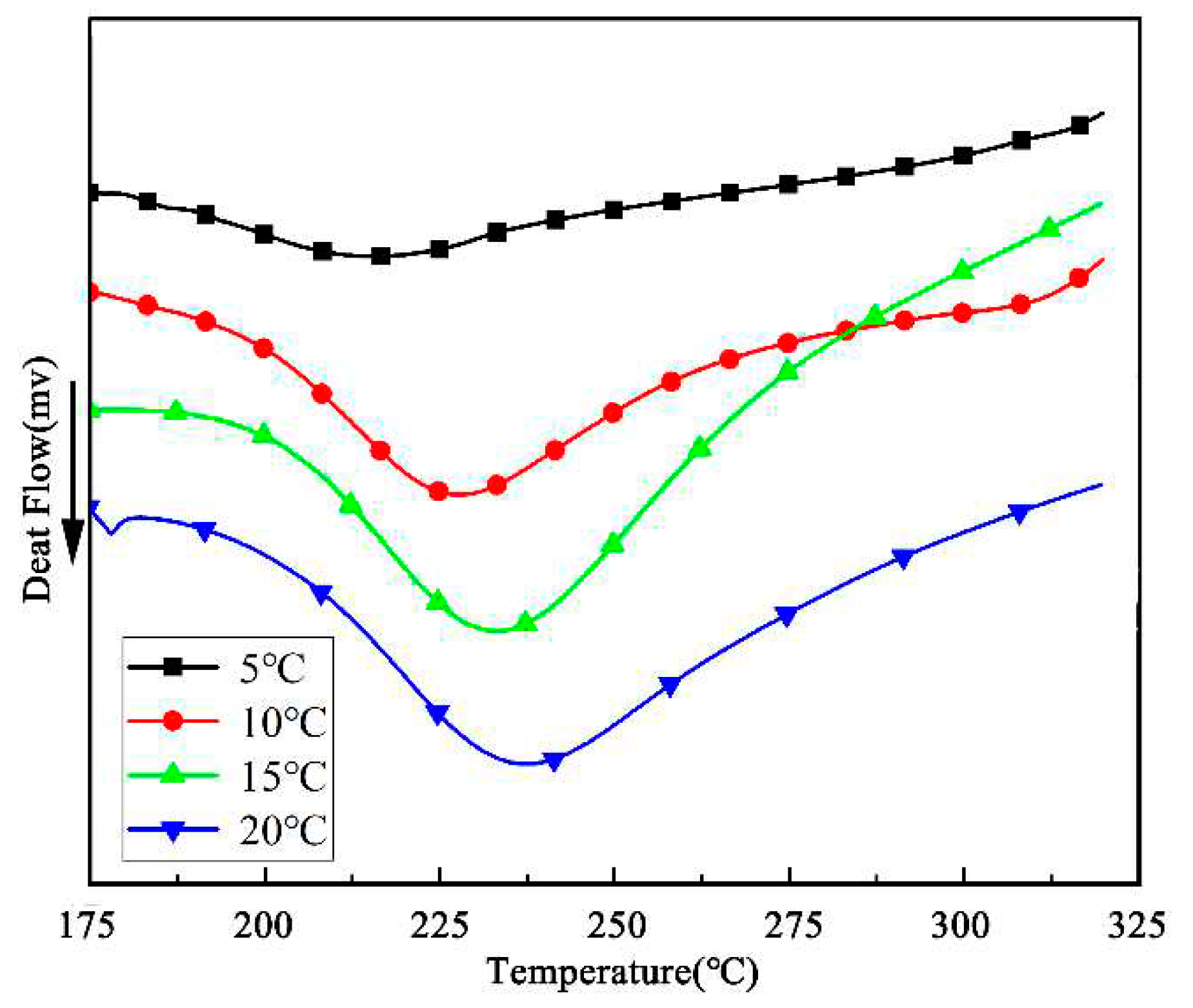
28]. Non-isothermal approaches can be used to investigate the unknown cure mechanism. The curing kinetics of BOZ-1N21 resin are described as an example since the curing behaviors of four distinct BOZ-1N resins with varied molecular weights are comparable at different heating rates and the primary exothermic peak is in the high temperature zone.
Figure 5 depicts the exothermic curves of BOZ-1N21 resin at various heating rates of 5°C/min, 10°C/min, 15°C/min, and 20°C/min. Due to the thermal hysteresis effect, these curves exhibit substantially wider exothermic peaks as the heating rate increases into the high temperature region. The curing properties are listed in
Table 2.
The apparent activation energy (Ea) is a measure of the smallest amount of energy necessary for a resin to undergo a curing reaction, and its magnitude is one of the most critical parameters in determining the pace of the curing process. The Kissinger and Ozawa method [
29,
30,
31] was applied to calculate the kinetic parameters and build a kinetic model, and it may be derived by combining the non-isothermal DSC curves (
Figure 5) with the Kissinger (Equation (1)) and Ozawa (Equation (2)) methods.
Ozawa method:
where: the rate of temperature rise (°C/min); the peak temperature (°C); R the molar gas constant 8.314 J/(mol·K); Ea the apparent activation energy (J/mol).
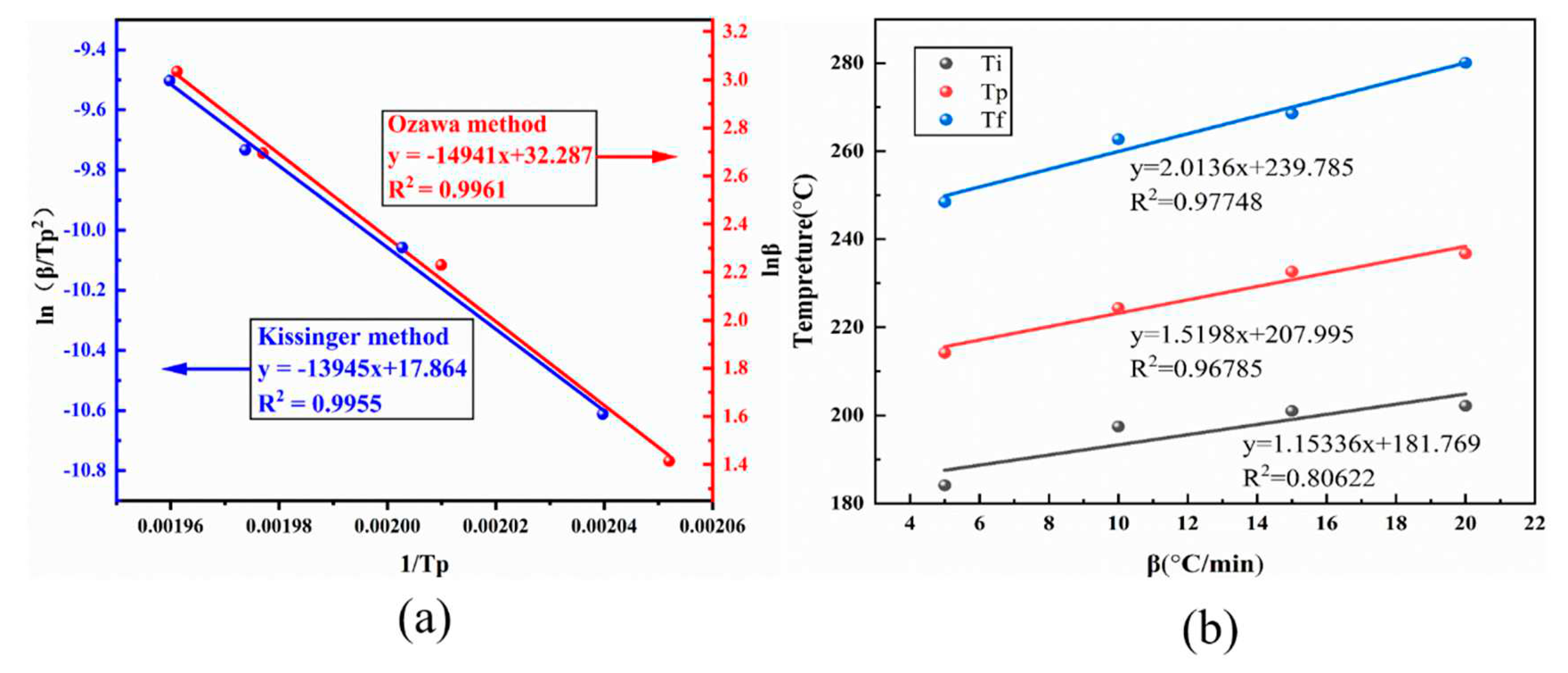
Scatter plots were constructed with ln(β/T
p2) for 1/Tp and ln(β) for 1/Tp, respectively, based on the peak temperature Tp of DSC at varied heating rates of BOZ-1N21 resin, and the fitted curve computed by the Kissinger and Ozawa techniques similarly exhibited fairly linear connections.
Figure 6a depicts the fitted pictures. The slopes of the pictures produced from the linear fits were entered into Equations (1) and (2) to calculate activation energies of 115.86 J/mol for the Kissinger equation and 117.94 J/mol for the Ozawa equation, respectively, with an average value of 116.90 J/mol for both equations. The gradient temperatures of 180°C, 208°C, and 240°C for the curing cycle of the lower BOZ-1N21 resin at a temperature increase rate of 0°C/min were determined using the linear extrapolation method (as shown in
Figure 6b).
The most significant features of polymers are their thermodynamic properties [
32]. Dynamic mechanical analysis (DMA) was used to evaluate the thermodynamic behavior of cured resins throughout various curing cycles. The gelation point (Pc) of a thermoset resin is an essential parameter that indicates the transition between the two phases during isothermal curing. DMA is a more accurate and adaptable instrument for measuring gelation time according to Tung's theory and has been used to numerous resin systems [
33,
34,
35]. When the curing system achieves the gelation state, the gelationation point (Pc) is the junction of the storage modulus (G') and loss modulus (G") curves.
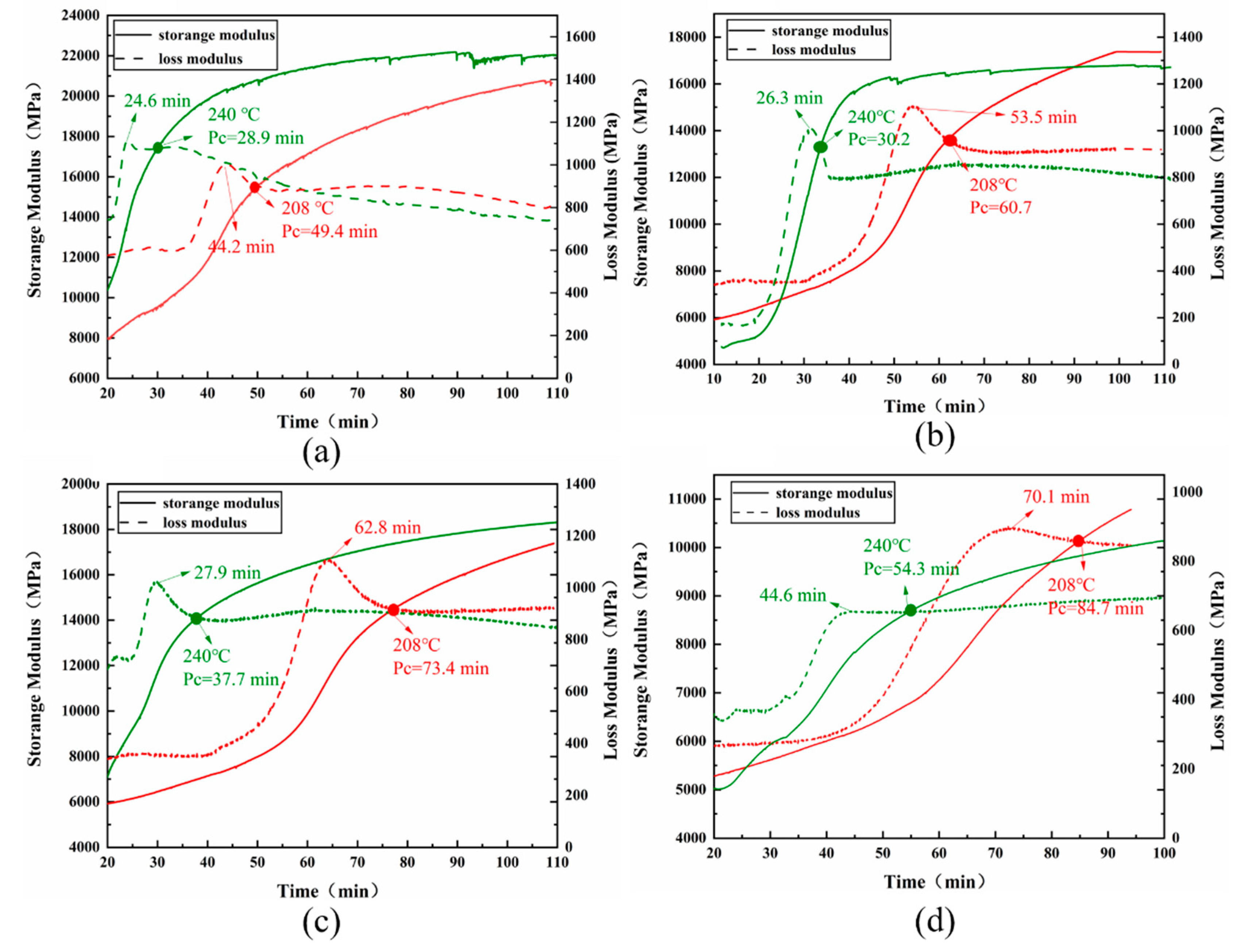
Figure 7 depicts the temperature dependency of G' and G" The tgelation of four T700/BOZ-1N prepregs coated in thin tin foil was measured using a single cantilever beam model at 208°C and 240°C, respectively.
Figure 7 illustrates that increasing the molecular weight of BOZ-1N resin delays the gelation point at the same curing temperature. The discrepancies in the cured network architectures induced by differing molecular weights are attributable to the aforementioned events. The cyano groups and phenolphthalein introduced into the molecular chain of BOZ-1N resin extend the length of the molecular chain; the higher the proportion of addition, the longer the molecular chain, which leads to a greater internal consumption of the molecular chain when the gelation occurs, delaying the occurrence of the gelation; and the larger the proportion of the first two additions, the corresponding reduction of the points where crosslinking can occur, delaying the occurrence of the gelation. The storage modulus of the BOZ-1N98 resin system is lower than at the first three, as shown in
Figure 7 (d). This might be attributed to a drop in the proportion of alkyne groups, resulting in a decrease in crosslinking density and hence a change in storage modulus. As a result, the molecular chain length should be stretched to the greatest degree feasible, and the number of crosslinking points should be assured, resulting in a bigger solidified 3D molecular network system, which enhances the stiffness of the composite material.
3.4. Curing mechanism of BOZ-1N resin
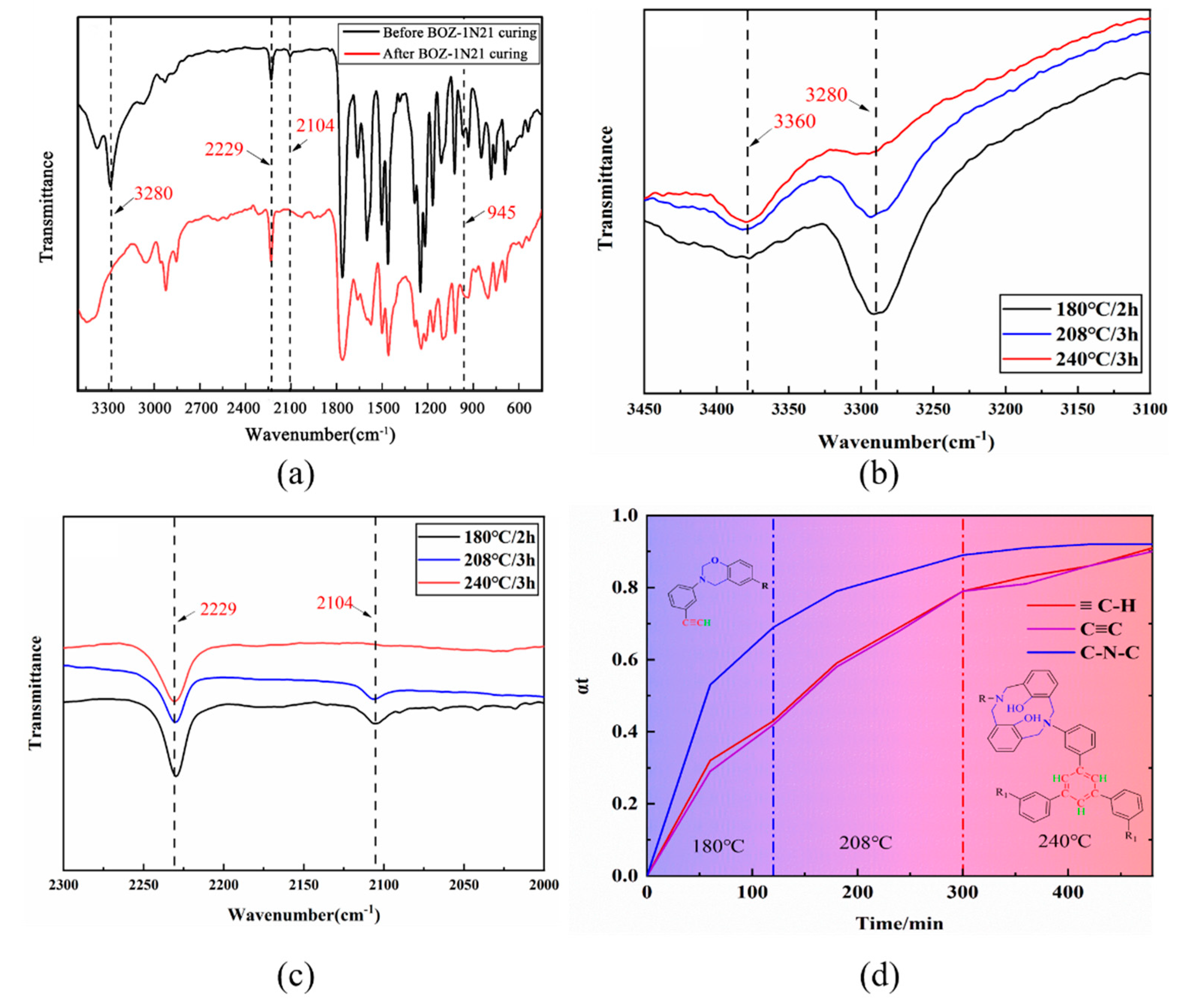
The biggest variation between the four resins is obviously their molecular weight, however this has no effect on the curing procedure. In conjunction with Figgure 5, all DSC curves of BOZ-1N21 resin at various heating rates contain just an exothermic peak. This suggests that the trigger conditions for oxazine ring cleavage polymerization and alkynyl reaction coincide. In order to elucidate the complex curing mechanism of BOZ-1N resin,the in-situ isothermal infrared method was used to determine the curing process of resins. as shown in
Figure 9.
Figure 9a depicts the changes in the distinctive peaks of BOZ-1N resin before and after curing. As the BOZ-1N resin cures, the alkyne groups eventually change into crosslinking or trimeric cyclization, causing the distinctive peaks at 3280 cm
-1 and 2104 cm
-1 to fade. Meanwhile, a new peak about 3360 cm
-1 is seen, which is caused by Ar-OH formed by oxazine ring cleavage. In
Figure 9b
,c, the strength of the distinctive peak signals of the aforementioned characteristic functional groups gradually increases or decreases with the degree of cure. These observations suggest that the terminal alkyne groups undergo simultaneous branching or trimerization cyclization events during oxazine ring cleavage polymerization. The results provided in
Figure 9a also reveal that
Figure 8 is the major structure after curing is complete. The cyano groups in BOZ-1N21 resin did not engage in the curing process and had no effect on the ring cleavage polymerization of the benzoxazine ring or the branching, crosslinking, and cyclic reactions of alkyne groups at the present temperature.
).
To compare the reaction rate of above-mentioned two types of polymerization, the conversion rates (αt) of the functional groups at specific temperature based on the change of the height of their characteristic peaks. The absorption peak (1761 cm
-1) of the phthalide carbonyl group, which did not participate any curing reaction, was chosen as reference peak. αt was calculated respectively by formulas (3), which could eliminate the effect of overlap peaks on the characteristic absorption peak.
Figure 9d depicts the conversion rate of the functional groups as a function of curing time at Tc=180°C, Tc=208°C, and Tc=240°C. The distinctive peaks of the alkyne groups -C-C- (2104 cm
-1) and C-H (3280cm
-1) rise virtually concurrently, showing that the estimated result is correct. After curing the BOZ-1N21 resin at 180°C for 2 hours, the alkynyl-C-C and C-H values reached 0.43 and 0.42, respectively; after curing at 200°C for 3 hours, they rose by 0.36 and 0.37, respectively; and after curing at 240°C for 3 hours, they increased by 0.12 and 0.10. After curing at 180°C for 2 hours, the C-N-C value (1374 cm
-1) associated with the benzoxazine ring reached 0.69, which is greater than that of the alkyne groups, indicating that the reactivity of the oxazine ring cleavage reaction is greater than that of the alkyne groups' branched or trimerized cyclization reaction. In comparison to Xiong et al's [
36] preliminary study, the cyano groups in BOZ-1N resin had no effect on the ring cleavage polymerization of benzoxazine and the cyclization process of alkynyl groups, corroborating the earlier findings.
3.5. Dynamical mechanical analysis of BOZ-1N composite material
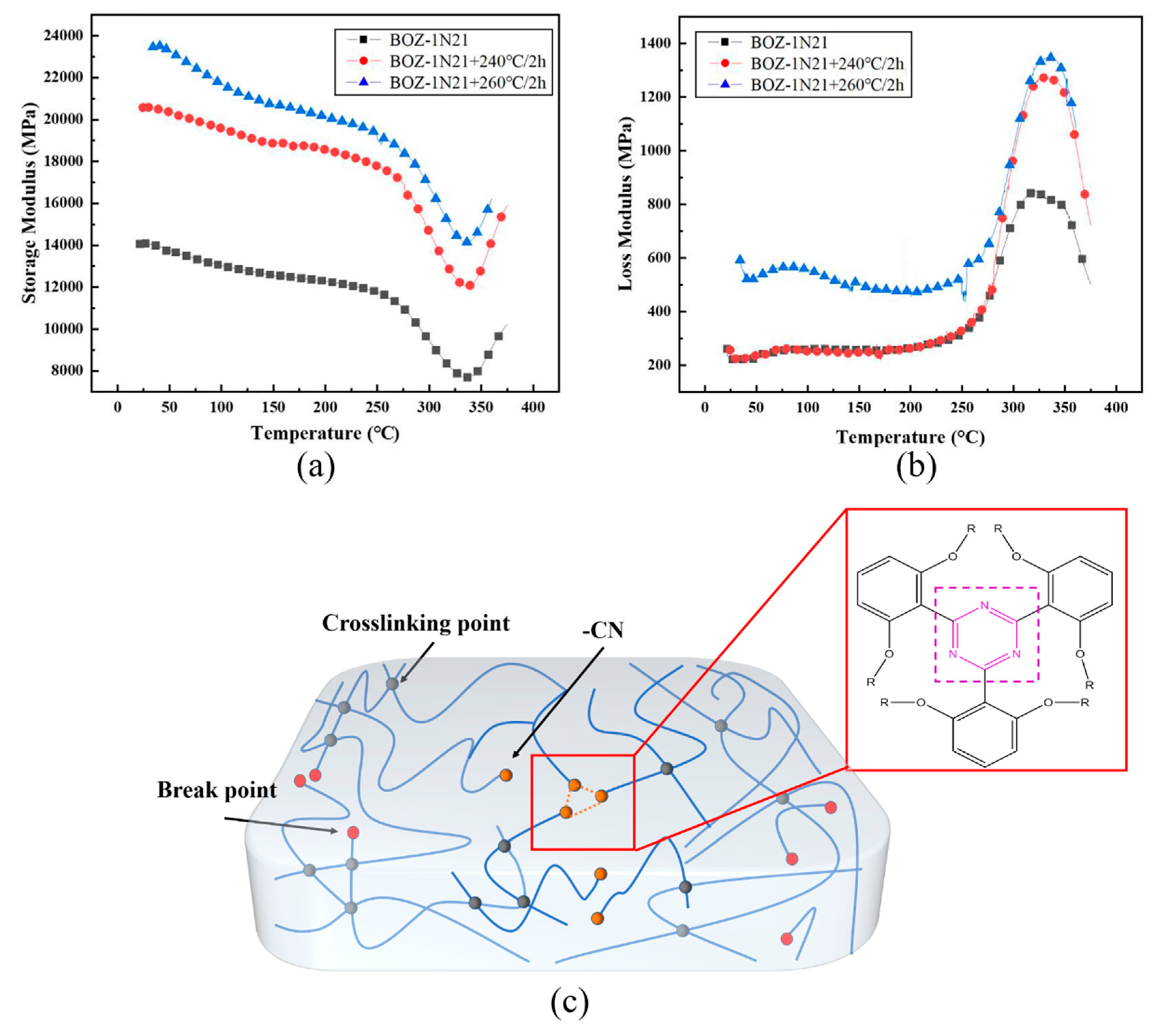
Based on the above-mentioned study of the curing temperature derived by DSC extrapolation, gelationation time determined by isothermal DMA, and curing mechanism determined by in-situ isothermal FT-IR, the curing cycle is as follows: 180°C/2h, 208°C/3h, 240°C/3h, and two post-treatment procedures, 240°C/2h and 260°C/2h, were designed to explore the rationality of the curing process of CF/BOZ-1N composites, and the findings are given in
Figure 10a. The storage modulus rises with post-curing time and temperature, as can be shown. The storage modulus, in instance, can reach 24 GPa at room temperature following the 260°C/2h processing procedure. At 200°C, the cured BOZ-1N composite material retained a high storage modulus of roughly 20 GPa. This means that some flexibility and mobility within the polymer network. However, this the mobility dose not result in a complete loss of mechanical stability. Continued heating to 275℃ resulted in an abrupt decrease in storage modulus, meanwhile the tand started to rise but not peaking as the temperature approached 275℃ indicating a slight softening of the sample. It is worth noting that at a temperature of 330℃, the storage modulus of the resin showed an increasing trend with the three curing processes rather than the usual downward trend. The storage modulus of BOZ-1N-21 composite in this temperature range of 30400°C demonstrates that cured BOZ-1N21 has outstanding stiffness throughout a wide temperature range, which is advantageous for high temperature applications. The declining pattern of storage modulus of the three distinct curing procedures is congruent; as the temperature exceeds 250°C, the rate of decline of storage modulus increases, most likely due to a decrease in the contact force between molecular chains as temperature rises. At the same time, it demonstrates that the curing period has no major effect on the curing process and curing structure of the resin system.
When the curing temperature approaches 200°C, a huge number of exothermic processes are thermally triggered, resulting in a substantially greater temperature in the system than 200°C. When the reaction of the reactive groups is complete, the exterior temperature of the resin falls faster than the inside temperature, causing a large residual thermal stress in the system. The explanation for the rise in storage modulus after the 260°C/2h processing program is obvious: the high temperature reduces stress within the resin system, and the molecular chain arrangement is more ordered, resulting in an increase in energy storage modulus at the macro level. At temperatures over 325°C, the cyano groups in the molecular structure form triazines structure with thermal stability, increasing the system's crosslinking density and hence the energy storage modulus somewhat. Finally, a complete investigation indicated that the curing procedure of CF/BOZ-1N composite was 180°C/2h, 208°C/3h, 240°C/3h, and 260°C/2h.
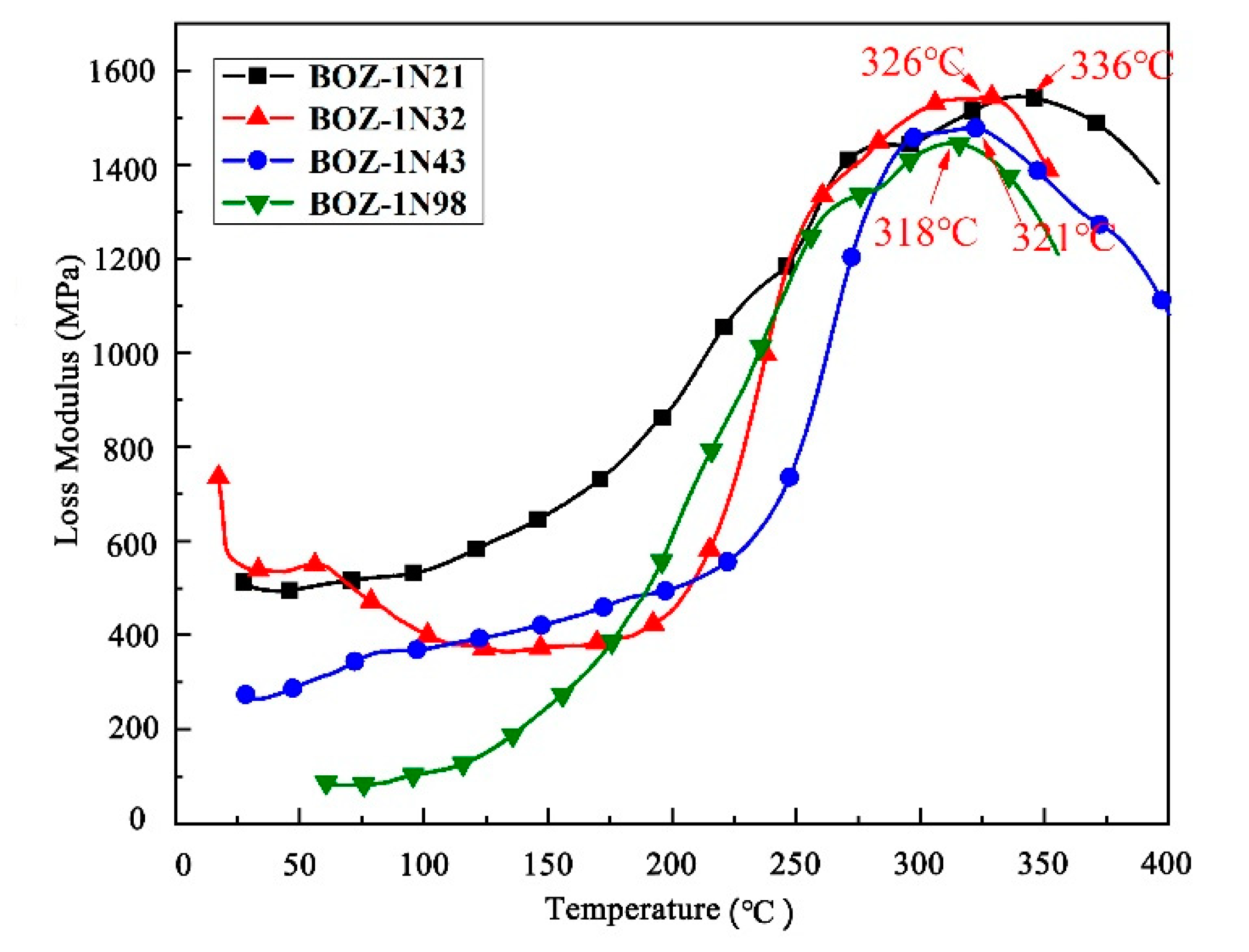
Figure 11 depicts the trend of loss modulus with increasing temperature for four different molecular weight CF/BOZ-1N composites cured at 180°C/2h, 208°C/3h, 240°C/3h, and 260°C/2h using the procedure described above. According to
Figure 11, the glass transition temperature Tg of the BOZ-1N resin decreases with increasing molecular weight. The higher the molecular weight, the more flexible group groups the BOZ-1N monomer contains and the lower the proportion of terminal alkyne groups, resulting in the growth of the flexible segment in the molecular chain, which decreases the crosslink density of the resin and thus leads to a decrease in Tg with molecular chain growth.
4. Conclusions
A novel alkynyl functionalized benzoxazine with phthalide and cyano side groups has been created. The cyano group, as a latent crosslinking site, has no effect on the curing reaction of alkynyl and amphiphilic rings. When the temperature hits about 325 °C, the triazine ring generated by cyano crosslinking can improve the resin system's energy storage modulus. The storage modulus of the monomer increases as its molecular weight grows, yet the toughness may theoretically be enhanced, and the influence on high-temperature resistance is less than 6%. These findings result in a great thermal stability, high Tg, and energy storage modulus for the produced resin, which is likely to be used in high-temperature and impact-resistant composite material architectures.
Author Contributions
Nianjun Kang; Formal analysis,Shuai Yang; Writing—original draft,Xuhai Xiong; Writing—review & editing,Anchang Han and Rong Ren; methodology,Jing Wang; data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 51873109); Xingliao Talents Program of Liaoning Province (XLYC1907198, XLYC1907019); Science and Technology Major Project of Liaoning Province (2019JH1/10100028); Scientific Research Fund of Liaoning Provincial Education Department (JYT2020022).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Supplemental data can be provided upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
-
Dumas.L, Bonnaud.L, Olivier.M, Poorteman.M, Dubois.P, Chavicol benzoxazine: Ultrahigh Tg biobased thermoset with tunable extended network. European Polymer Journal,2016, 81, 337-346.
- Cao.G.P, Chen.W.J, Liu.X.B, Synthesis and thermal properties of the thermosetting resin based on cyano functionalized benzoxazine[J]. Polymer degradation and Stability, 2008, 93(3): 739-744.
- Xiang.H, Ling.H, Wang.J, Song. L,Gu.Y, A novel high performance RTM resin based on benzoxazine[J]. Polymer composites, 2005, 26(5): 563-571.
- Yu.Liping, Yan.Chun, Zhang.Xiaoqing, Li.HongZhou, Zhu Yingdan, Liu Junlong, Liao Dong, Research on toughening of polybenzoxazine resin by epoxy methylphenyl silicone oil [J]. Material Guide, 2012,26 (22): 71-75.
- Holly.F.W, Cope.A.C, Condensation products of aldehydes and ketones with o-aminobenzyl alcohol and o-hydroxybenzylamine[J]. Journal of the American Chemical Society, 1944, 66(11): 1875-1879.
- Riess.G, Schwob.J.M, Guth.G, Roche.M, Laude.B, Ring opening polymerization of benzoxazines—a new route to phenolic resins[J]. Advances in polymer synthesis, 1985: 27-49.
- Brunovska.Z, Lyon.R, Ishida.H, Thermal properties of phthalocyano functional polybenzoxazines[J]. Thermochimica acta, 2000, 357: 195-203.
- Liu.J, Agag.T, Ishida.H, Main-chain benzoxazine oligomers: a new approach for resin transfer moldable neat benzoxazines for high performance applications[J]. Polymer, 2010, 51(24): 5688-5694.
- Agag.T, Takeichi.T, Synthesis and characterization of novel benzoxazine monomers containing allyl groupss and their high performance thermosets[J]. Macromolecules, 2003, 36(16): 6010-6017.
- Agag.T, Takeichi.T, Novel benzoxazine monomers containing p-phenyl propargyl ether: polymerization of monomers and properties of polybenzoxazines[J]. Macromolecules, 2001, 34(21): 7257-7263.
- Wang.Z, Gu.Y, Polybenzoxazine/Bismaleimide Alloys[M]//Advanced and Emerging Polybenzoxazine Science and Technology. Elsevier, 2017: 301-318.
- Jubsilp.C, Damrongsakkul.S, Takeichi T, Rimdusit S, Curing kinetics of arylamine-based polyfunctional benzoxazine resins by dynamic differential scanning calorimetry[J]. Thermochimica Acta, 2006, 447(2): 131-140.
- Kim.H.J, Brunovska.Z, Ishida.H, Dynamic mechanical analysis on highly thermally stable polybenzoxazines with an acetylene functional groups[J]. Journal of applied polymer science, 1999, 73(6): 857-862.
- Zhong.Z.X, Geng.L.Y, Zhu.J.J, Yu.Z.M, Cheng Shuaifeng, Liu Li, and Huang Yudong. Progress of thermostable resin containing alkynyl and cyanogen [J]. Chemistry and bonding, 2018,40 (05): 357-362.
- Sha.X.L, Yuan.L, Liang.G, Gu.A, Heat-resistant and robust biobased benzoxazine resins developed with a green synthesis strategy[J]. Polymer Chemistry, 2021, 12(3): 432-438.
- Li.P, Dai.J, Xu.Y, Ran.Q, Gu.Y, A conjugated alkyne functional bicyclic polybenzoxazine with superior heat resistance[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2019, 57(14): 1587-1592.
- Wang.Y, You.S, Hu.J, Zhang.K, Synthesis and properties of benzoxazine monomers bearing both 3-methyltetrahydrophtalimide and cyano groupss: para-para vs. ortho-ortho[J]. Macromolecular Research, 2020, 28(1): 74-81.
- Yu.X, Zhang.K, Studies on the isomeric effect of cyano functionality on the polymerization and thermal properties of ortho-norbornene-based benzoxazine resins[J]. Journal of Polymer Research, 2020, 27: 1-8.
- Chaisuwan.T, Ishida.H, High-performance maleimide and cyano-functionalized benzoxazines with good processibility for advanced composites applications[J]. Journal of applied polymer science, 2006, 101(1): 548-558.
- Zhang.K, Liu.Y, Han.L, Wang.J,Ishida.H, Synthesis and thermally induced structural transformation of phthalimide and cyano-functionalized benzoxazine: Toward smart ortho-benzoxazine chemistry for low thermosets[J]. RSC advances, 2019, 9(3): 1526-1535.
- Zhang S, Li Q, Ye J, Sun.H,Liu.X, Probing the copolymerization of alkynyl and cyano groupss using monocyclic benzoxazine as model compound[J]. Polymer, 2022, 252: 124932.
- Ghetiya.R.M, Kundariya.D.S, Parsania.P.H, Patel.V. A, Synthesis and characterization of cardo bisbenzoxazines and their thermal polymerization[J]. Polymer-Plastics Technology and Engineering, 2008, 47(8): 836-841.
- Laskoski, Matthew, et al. "Improved synthesis and properties of aryl ether-based oligomeric phthalocyano resins and polymers." Polymer 67 (2015): 185-191.
- Wang.Z, Gu.Y, Polybenzoxazine/Bismaleimide Alloys[M]//Advanced and Emerging Polybenzoxazine Science and Technology. Elsevier, 2017: 301-318.
- Jubsilp.C, Damrongsakkul.S, Takeichi.T, Rimdusit.S, Curing kinetics of arylamine-based polyfunctional benzoxazine resins by dynamic differential scanning calorimetry[J]. Thermochimica Acta, 2006, 447(2): 131-140.
- Zhang.Yingqiang, Xu.Wei, Wu.Haixia, Mei.Liwen, Chen.Jie, Qiao.Jiang, Xi.Minjie, Wu.Wei, Preparation and analysis of alkynyl benzoxazines [J]. FRP / composite material, 2009 (05): 69-71.
- Li.Jianchuan, Ran.Qichao, Gu.Yi, Synthesis study of 3-acetylene aniline / phenol type benzoxazine [J]. Thermosetting resin, 2017,32 (3): 13-18.
- Catalani.A, Bonicelli.M.G, Kinetics of the curing reaction of a diglycidyl ether of bisphenol A with a modified polyamine[J]. Thermochimica acta, 2005, 438(1-2): 126-129.
- Kissinger.H.E, Reaction kinetics in differential thermal analysis[J]. Analytical chemistry, 1957, 29(11): 1702-1706.
- Crane.L.W, Dynes.P.J, Kaelble D H,Analysis of curing kinetics in polymer composites[J]. Journal of Polymer Science: Polymer Letters Edition, 1973, 11(8): 533-540.
- Bai.Y, Yang.P, Zhang.S, Li.Y,Gu.Y, Curing kinetics of phenolphthalein–aniline-based benzoxazine investigated by non-isothermal differential scanning calorimetry[J]. Journal of Thermal Analysis and Calorimetry, 2015, 120: 1755-1764.
- Liu.X, Li.L, Chen.Z,Duan.X,Yu.Y,Sun.L, Curing behavior, thermal, and mechanical properties of N, N′-(4, 4′-diphenylmethane) bismaleimide/2, 2′-diallylbisphenol A/3-allyl-5, 5-dimethylhydantoin resin system[J]. High Performance Polymers, 2020, 32(6): 631-644.
- Babayevsky.P.G, Gillham.J.K, Epoxy thermosetting systems: dynamic mechanical analysis of the reactions of aromatic diamines with the diglycidyl ether of bisphenol A[J]. Journal of Applied Polymer Science, 1973, 17(7): 2067-2088.
- Wolff.F, Kugler.C, Münstedt.H, Time-and temperature-dependent crosslinking behaviour of a silicone resin[J]. Rheologica acta, 2012, 51: 71-80.
- Tung.C.Y.M, Dynes.P.J, Relationship between viscoelastic properties and gelationation in thermosetting systems[J]. Journal of Applied Polymer Science, 1982, 27(2): 569-574.
- Xiong.X, Ren.R, Cui.X, Chen P, Alkynyl-functionalized benzoxazine containing phthalide side groups: Synthesis, characterization and curing mechanism[J]. Polymer Testing, 2018, 72: 232-237.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).