1. Introduction
To create medical devices (polymeric matrices, implants, etc.) characterized by high biodegradation rate in living organisms (bioresorption), it is necessary to develop materials with low density and high specific surface area [
1].
The most optimal solution is nonwoven fibrous materials composed of nanoscale and micron-scale fibers obtained through the electrospinning method [
2]. Electrospinning (ES) of ultrafine polymeric fibers represents a complex multiparametric process, influenced by numerous characteristics that affect both the geometry and structure of individual filaments and the performance of the resulting nonwoven fibrous material [
3]. The key characteristics of electrospinning that determine the phase and physicochemical state of the polymer solution are electrical conductivity and viscosity, which are influenced by the concentration and molecular weight of the polymer [
4]. The electrospinning process and the morphology of the nonwoven material are also affected by environmental factors such as temperature, humidity, pressure, oxygen concentration, etc. [
5], as well as technological parameters of the process, including production rate, electrode distance, electrode shape, capillary diameter, flow rate of the polymer solution, and others [
6].
Therefore, in this study, it was necessary to create highly porous biopolymeric materials based on ultrafine fibers (diameter 0.5 – 3 μm), obtained through the modern technological process of electrospinning a polymer solution. This method allows the formation of fibrous nonwoven material layers with specific thickness, density, and specific surface area. The incorporation of additional functional components into the polymer solution can also influence the structural organization of the fibers. It is essential to investigate how the characteristics of the structural hierarchy impact the performance properties of the materials, as this will play a crucial role in the kinetics of diffusion transport and degradation processes (oxidative, photo-oxidative, biodegradation, etc.).
To achieve the desired set of properties for polymeric materials, various types of chemical, physical, or physicochemical modifications are applied at the raw material preparation stage or during the product forming process, leading to radical changes in the polymer material's structure at different levels of structural organization.
Hence, it becomes necessary to establish a correlation between external factors and changes in the parameters of the polymer material's structure. This correlation allows for targeted adjustment of the properties of the polymer system based on the intended application of the product.
One of the progressive polymers used in medicine is poly(3-hydroxybutyrate) (PHB). This bacterial biopolymer exhibits high crystallinity [
7]. By modifying the polymer's crystallinity during the film or fiber formation process, their transport properties can be regulated to a great extent [
8].
Usually, elevated temperatures negatively affect the structure of most biopolymers, which is why forming products from their solutions is preferred. This is particularly crucial for medical devices, such as matrices for controlled drug release since only a limited number of drugs can withstand relatively high temperatures.
This study will investigate the structural organization of nonwoven fibrous PHB materials depending on the technological parameters of the electrospinning process, as well as the influence of polar low-molecular-weight substances and nanoparticles that impart functional properties to the materials.
3. Results
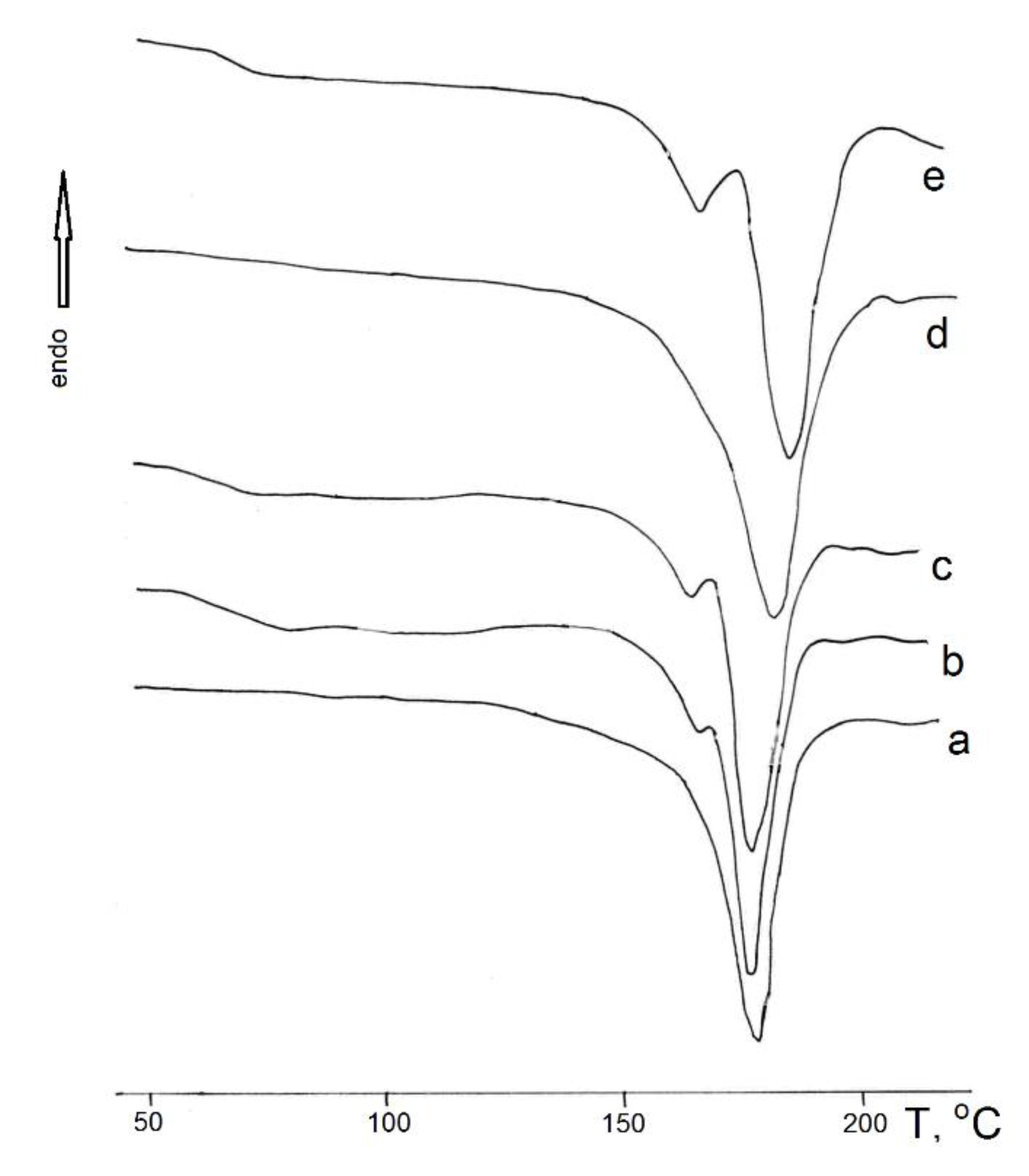
One of the important technological aspects of forming film or fibrous materials is the choice of solvent for the base polymer. Solvents differ not only in boiling point and molecular weight but also in their degree of polarity. Let's consider the influence of solvent polarity on the supramolecular structure of polyhydroxybutyrate (PHB) matrices. For this purpose, we used the method of differential calorimetric analysis.
Figure 1 shows typical heating thermograms of PHB films.
From the analysis of the thermograms, it can be observed that the original structure of PHB is disrupted after treatment with solvents: an amorphous phase appears, characterized by a distinct glass transition, and a fine crystalline modification or regions with disrupted crystallinity emerge. This is evidenced by the appearance of a low-temperature melting peak.
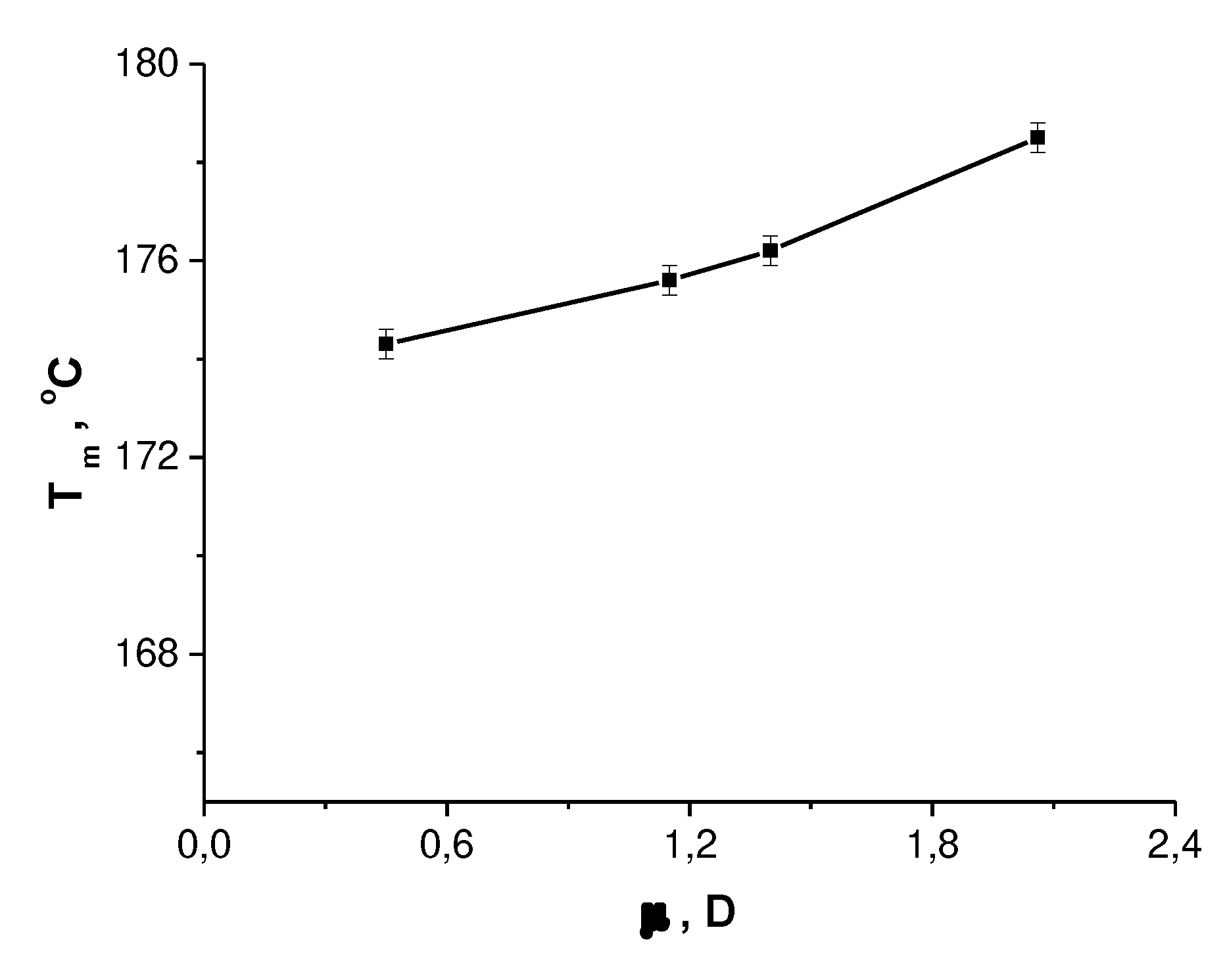
Table 1 presents the thermophysical properties of PHB films from different solvents and solvent parameters. From the table, it can be seen that with an increase in the solvent's dipole moment, the crystalline phase of PHB improves (becomes more ordered). This is evidenced by the increase in the polymer's melting temperature (Tm), as shown in
Figure 2.
Where: DGm, J/g - Gibbs melting energy; Tm, °C - melting temperature; Tg, °C - glass transition temperature; DCp, J/g·K - specific heat capacity; e 1/2 - cohesion energy density; m, D - dipole moment.
Concurrently, the specific heat capacity (DCp) of PHB decreases. This may be related to a decrease in the proportion of the amorphous phase in the polymer.
The PHB molecule contains a complex ester group in its main chain capable of interacting with polar solvent molecules. As a result, the conformation of macromolecules in both crystalline and amorphous regions of the polymer can change under the influence of solvent molecules. The structure of crystallites is stabilized, and macromolecules in the amorphous phase lose their flexibility. These effects have been observed by authors [
12], that studied the influence of water molecules on PHB films. It was found that the interaction with polar water molecules leads to the formation of hydrogen bonds between neighboring chains, resulting in the stabilization of the polymer's crystalline structure. The hydrogen bonding network formed at the crystallite boundaries enhances the orientation of crystallites in the polymer matrix.
In the present study, among the considered solvents, which have dipole moments lower or higher (dichloroethane) than that of water (1.84 D) [
13], these effects may occur to a lesser extent due to the presence of PHB films with defective crystalline structures, which exhibit lower melting temperatures, as shown in
Figure 1.
In conclusion, an increase in solvent polarity corresponds to an increased ability of the solvent to interact with polar groups of PHB. As a result of this interaction, the crystallites are improved, and the amorphous phase of the polymer is densified during film formation.
The study also revealed that the changes in the Gibbs melting energy of the PHB crystalline phase and the cohesion energy density of the solvent molecules are well correlated in a certain sequence, as shown in
Table 1.
The cohesion energy density is known to characterize the degree of intermolecular interaction in a substance [
14]. From
Table 1, it follows that a decrease in the intermolecular interaction in the solvent leads to an increase in disorder in the crystalline phase of PHB films, characterized by a decrease in the Gibbs melting energy. In other words, the lower the intermolecular interaction in the solvent, the higher the mobility of its molecules, and consequently, the higher their rate of transition to the vapor phase (evaporation). Due to the different rates of phase transition of molecules in the considered solvents, the formation of PHB films proceeds differently. This is reflected in the degree of completion of the crystallization process and the parameters of the polymer's crystalline structure.
Based on the conducted research, it can be concluded that with an increase in the polarity of the solvent, there is an increase and improvement in the crystalline structure, and the proportion of the amorphous phase of PHB decreases. With a decrease in the degree of intermolecular interaction in the solvent, the proportion of defective crystalline phase in PHB films increases. It was found that the highest quality films are obtained from chloroform. Films obtained from dioxane have a non-homogeneous heterogeneous structure. In the course of experimental work on solvent combination, it was found that high-quality films can be obtained from dioxane by re-dissolving the films from chloroform. Films from dichloroethane were characterized by increased brittleness and heterogeneity. Combining solvents did not improve the quality of the films. Films based on formic acid were not cohesive and disintegrated into fragments after desorption of the solvent. Additionally, formic acid accelerates the hydrolysis processes of PHB. Therefore, based on the analysis of the film morphology, chloroform was chosen as a good solvent for obtaining homogeneous film materials for the study of water sorption-diffusion properties. Besides solvent characteristics, important factors for fiber structure formation include polymer solution parameters. The fiber morphology primarily determines the geometric parameters of the monofilament nonwoven fibrous material. Further in the study, we attempted to summarize the influence of the main technological characteristics of the polymer solution on electroforming and the structure of polyhydroxybutyrate (PHB) fibers. As a large number of factors are responsible for various stages of electroforming, researchers and technologists are faced with the task of identifying the most significant and dominating characteristics that have the greatest impact on the fiber's structure, morphology, porosity, and geometry. In this regard, we will now consider some key physicochemical characteristics of polymer solutions of PHB intended for electroforming.
Geometric parameters of monofilaments primarily determine the morphology of the nonwoven fibrous material (packing density, filament orientation, defectiveness, etc.). At the same time, it affects a complex of performance properties, including diffusion, physical-mechanical, biomedical, etc. The geometry of the fiber mainly depends on the polymer's molecular weight, viscosity, electrical conductivity, and solution flow rate (productivity).
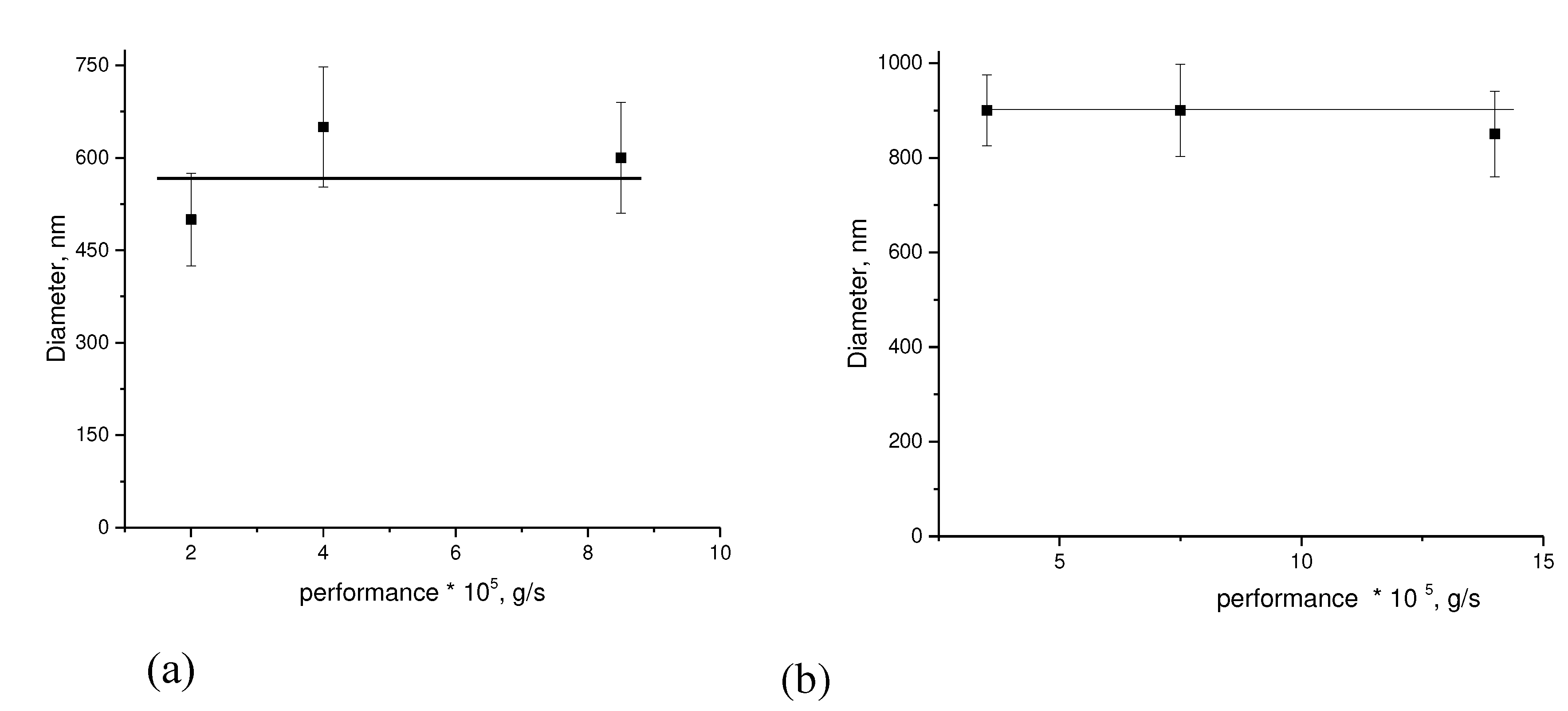
In
Figure 3 (a, b), the dependencies of the PHB fiber diameter on the electroforming process productivity are presented for 5% and 7% polymer solutions in chloroform.
As can be seen in
Figure 3, the fiber diameter does not depend on the performance of the electrospinning process at different concentrations of PHB in solution. Apparently, as a result of the presence of strong intermolecular interaction in the PHB, as evidenced by a high degree of crystallinity (60-80%), an increase in the productivity fiber formation does not lead to a change in the degree of crystallinity and free intermolecular volume, respectively.
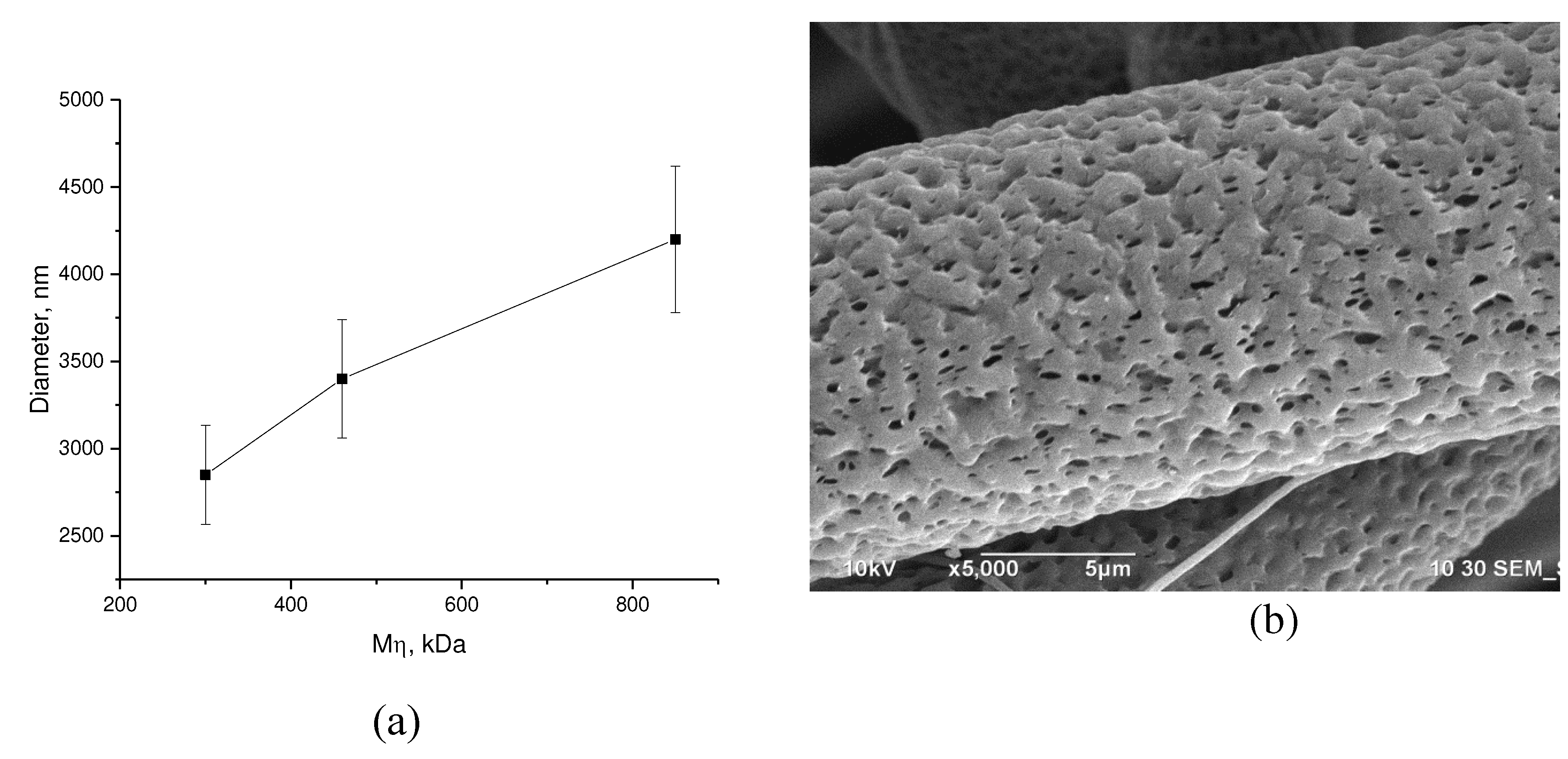
Figure 4 shows the dependence of the fiber diameter on the molecular weight of PHB for 7% solutions in chloroform.
As shown in
Figure 4, there is an increase in the diameter of the fiber with an increase in the medium-viscosity molecular weight of PHB. The increase in diameter with an increase in the length of the macromolecular chains of PHB is quite natural and is explained by an increase in the number of intermolecular engagements in the polymer solution, which prevent the stretching of the jet during electroforming. The mesh of physical meshes formed in the solution interferes with orientation processes and increases the viscoelastic properties of the polymer system.
In the process of electrospinning, the electric field creates free ionic charges that migrate along the polymer solution jet from one electrode to another. In organic nonionic polymer solutions with low dielectric permittivity, the concentration of such ions is extremely low, which determines the low values of the electrical conductivity of the molding system. Such systems include solutions of PHB, which is known to be highly soluble in chloroform, dichloroethane and some other nonionic organic solvents [
15].
The flow of the primary jet of the polymer solution between two oppositely charged electrodes is accompanied by two processes: the interaction of an electric field with the polymer solution and the evaporation of the solvent, resulting in the formation of an ultrathin fiber. The first process is largely determined by the electrical conductivity of the polymer solution. In this case, the electromechanical effect on the formed polymer jet sets the conditions for changing the diameter and degree of defect of the resulting ultra-thin fiber [
16]. The second process is determined by the intermolecular interaction between the polymer and the solvent, as well as the cohesion energy of the solvent. When the solvent diffuses from the fiber, a micro- and nanoporous systems are usually formed (
Figure 4b).
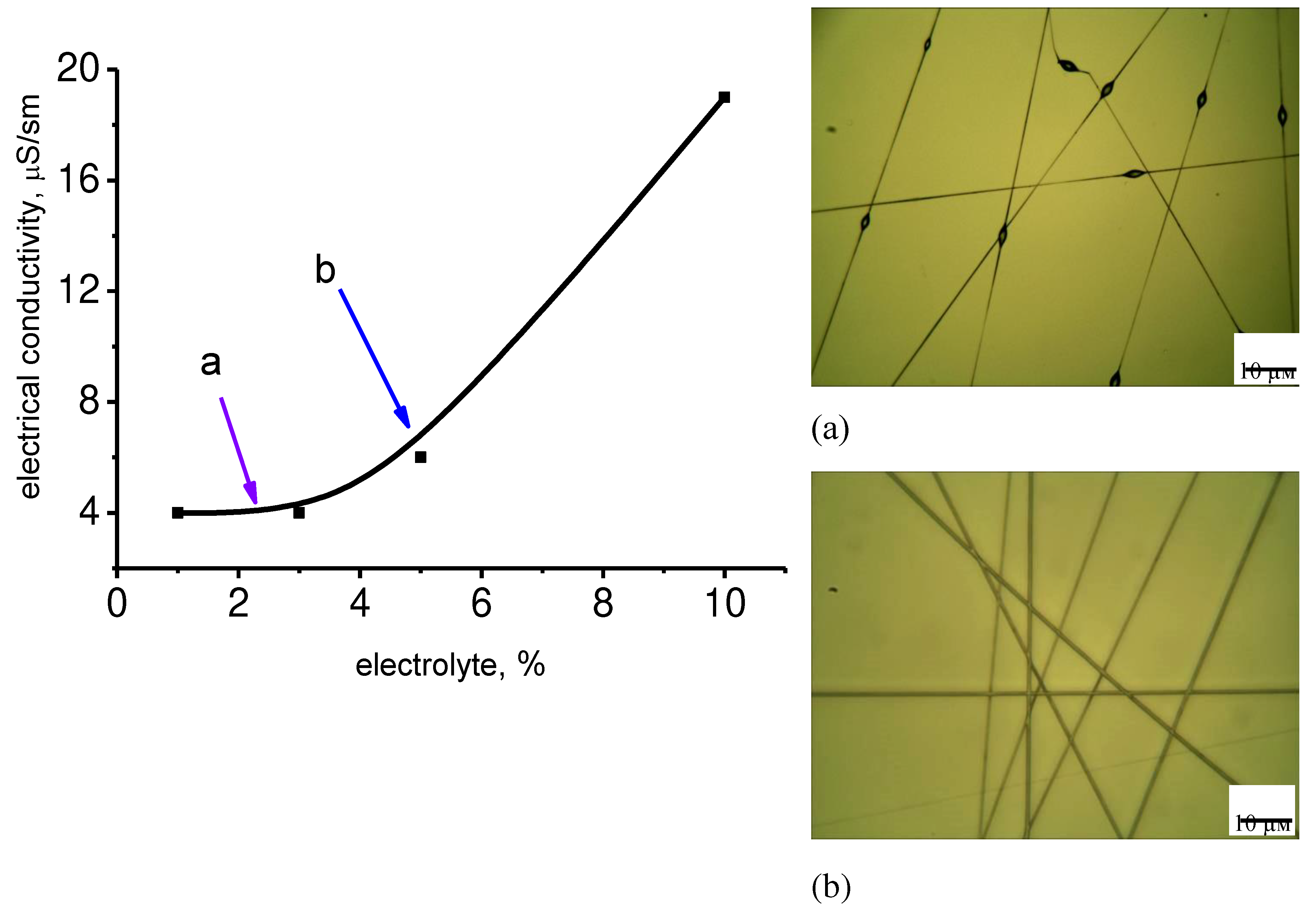
The effect of electrical conductivity and viscosity on the electrospinning process of a binary solution of PHB in chloroform was studied (
Figure 5). In particular, it was found that the low electrical conductivity of the PHB solution prevents the formation of fibers of uniform thickness, which is demonstrated in
Figure 5a.
The electrical conductivity can be regulated by the introduction of an electrolyte into the solution for electrospinning.
Figure 5 shows the dependences of the electrical conductivity on the concentration of the electrolyte and the diameter of the fibers on the electrical conductivity of the PHB solution in chloroform. Conentration of the electrolyte has a great impact on the electrical conductivity of the solution which leads to the changes in the diameter of fibers (
Figure 6). This dependencies are exponential. The initial section is characterized by a low level of indicators, which is reflected in the formation of a defective fiber structure (thickening). In the case of low electrical conductivity, the fiber is not formed at all, but droplet formation occurs. When the optimal level of indicators is reached, smooth fibers are formed. These dependencies are quite correlated with the dependence of the viscosity of the PHB solution on the concentration of the polymer (
Figure 7). This correlation is primarily related to the properties of the polymer solution at the polymer-air interface. The surface tension determines the conditions for the extraction of the jet and its disintegration into separate fragments (droplets).
The dependence of the average diameter on the electrical conductivity is characterized by an exponential descending curve (
Figure 6). With increasing electrical conductivity, the average diameter decreases to a certain value, and then there is a tendency to some growth. This growth is determined by an increase in the viscosity of the solution due to the high concentration of the electrolyte
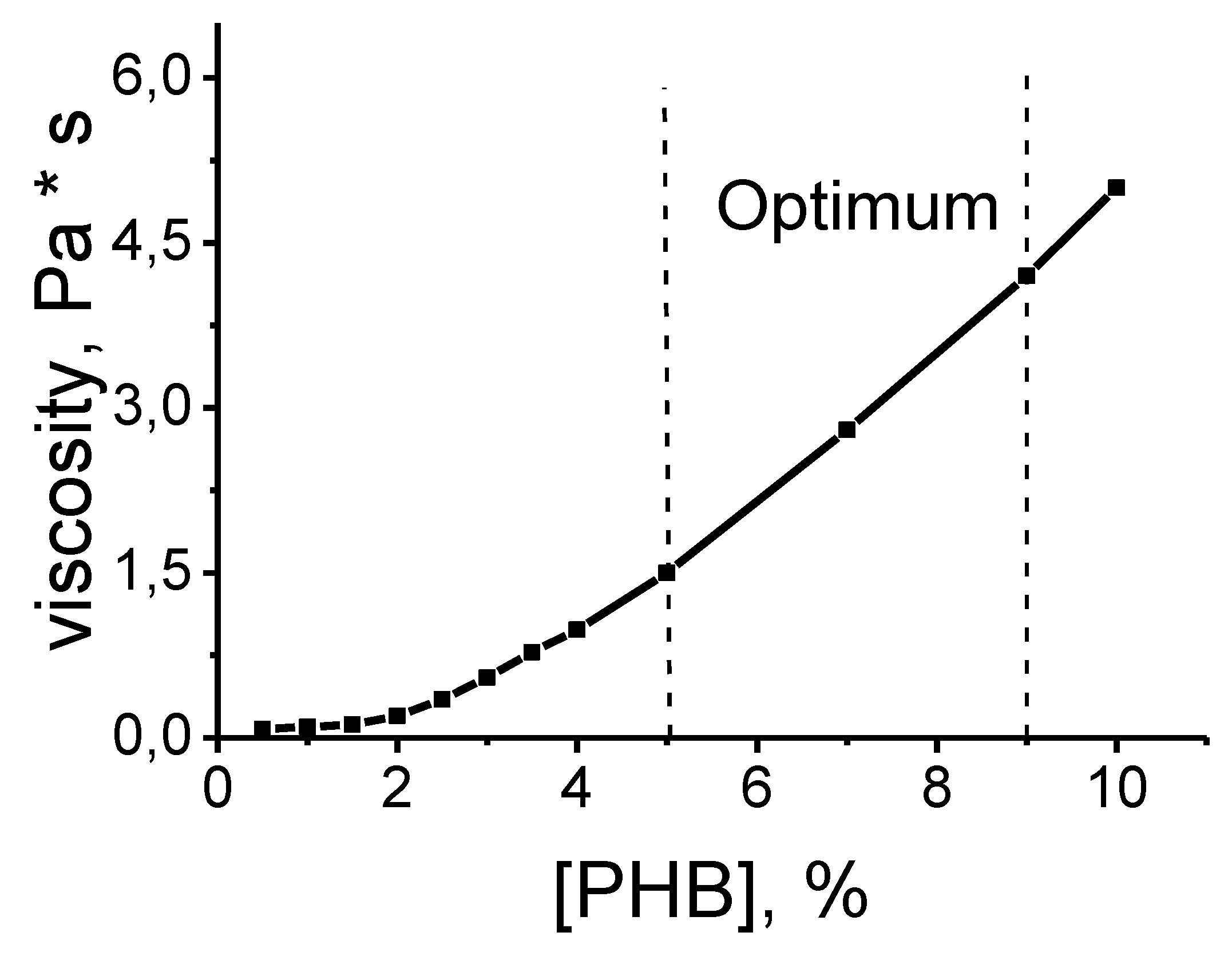
A gradual increase in the concentration of PHB in the solution leads to the emergence and increase in the number of intermolecular knots of engagement and interlacing of polymer chains, which allows the polymer solution at an appropriate concentration to stretch into a thin jet and form a fiber (
Figure 7). At a low polymer concentration, intermolecular entanglements are not enough to form a stable jet and it breaks up into individual droplets. As shown in
Figure 8, this occurs in the concentration range of 1-3 wt.% PHB. In the transition region of concentrations of 3-6 wt.% PHB, the lack of molecular meshing does not lead to the disintegration of the jet, but to the formation of fibers of complex geometry with the alternation of cylindrical sections and teardrop-shaped thickenings. At these concentrations, the polymer solution exhibits a sufficiently high value of surface tension, which does not allow the jet to disintegrate into separate fragments. At an optimal concentration of PHB in solution over 6 wt. %. The number of molecular meshes is large enough to form smooth cylindrical fibers. It should be noted that with an increase or decrease in the molecular weight of the polymer, this dependence shifts to the region of lower or higher concentrations.
Since the studied fibrous materials are recommended for use in medicine as matrices for prolonged delivery of drugs, antiseptics, and tissue engineering, next we will consider the effect of these substances on the fiber structure. For the model experiment, we selected substances with terminal hydroxyl groups and a complex containing a metal halide. Intermolecular interaction of these components with the polar groups of PHB in the process of fiber formation should be expected.
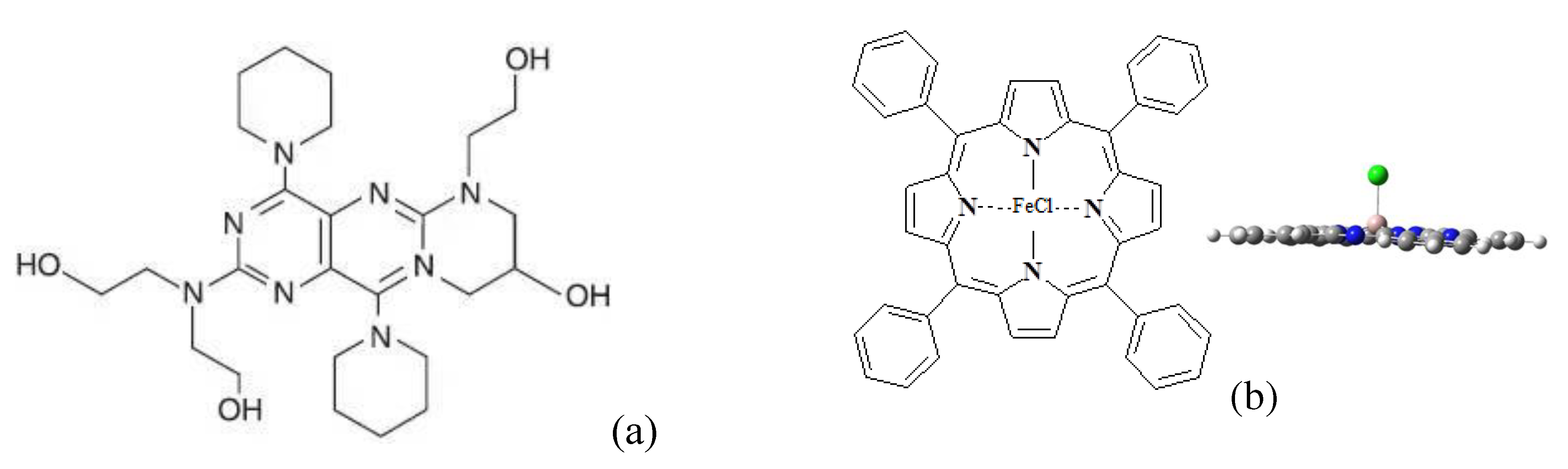
The presence of polar functional groups or complexes with metal in a chemical compound (medicinal substance, biologically active substance, antiseptic) should affect not only the technological characteristics of the molding solution, but also the processes of fiber structure formation. As an illustration, we chose two different substances - dipiidamol (DPD), containing terminal -OH groups, and the iron (III) chlorine terrafenylporphyrin (FeClTPP) containing ferric chloride (3+) as part of the complex.
Figure 9 shows the structural formulas of these compounds.
Since the presence of foreign substances is not allowed in biomedical materials, the electrolyte was not introduced into the molding solution. However, as our research has shown, the introduction of a medicinal substance, antiseptic or other ingredient containing polar groups of atoms into the molding solution can improve the technological parameters of the solution. It was demonstrated on the PHB-DPD and PHB-FeClTPP fibrous matrices.
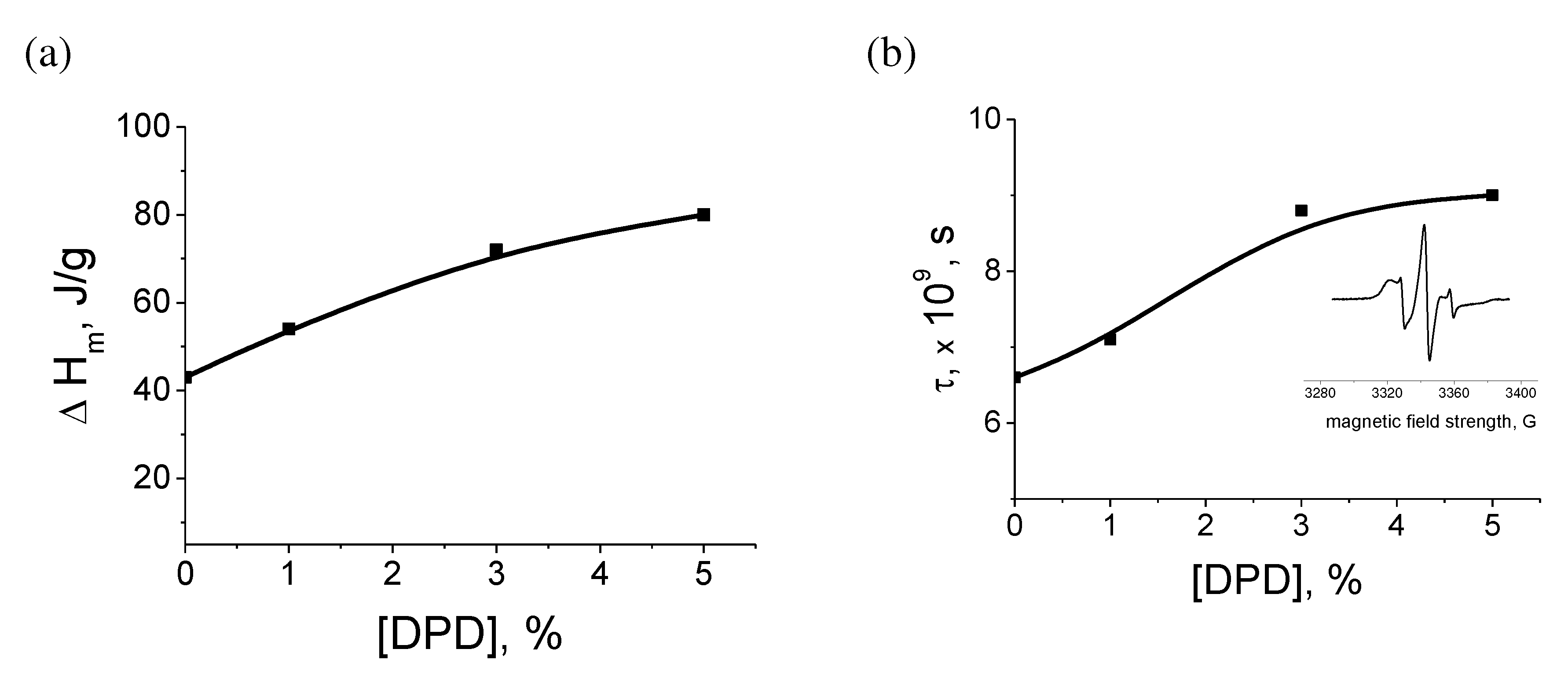
Figure 10 shows that with an increase in the concentration of DPD, the geometry of the fibers changes from transitional (cylinders-drops) to smooth cylindrical. At the same time, the fibers themselves have a porous structure. The average diameter of the fiber is 2-5 microns, and the pore size ranges from 0.05-0.2 microns.
The supramolecular structure of PHB changes with increasing of DPD concentration.
Figure 11a shows the dependence of the enthalpy of melting of PHB on the concentration of DPD (DSC method). With an increase in the content of DPD, an increase in the enthalpy of melting of PHB is observed, which indicates an increase in the degree of crystallinity. DPD particles are effective nuclei of crystallization. At the same time, the average size of the crystallites practically does not change, as indicated by the invariance of the melting temperature (168-169 ° C).
The analysis of the amorphous phase of PHB fibers by the EPR method using a probe (TEMPO) (
Figure 11b) showed the presence of a superposition of the spectra of the probe rotation speeds, showing the disequilibrium of the structure of the amorphous phase in the fibers: the presence of regions with different packing densities of macromolecule segments. The dependence of the average correlation time on the concentration of DPD is shown in
Figure 11b. It can be seen that with an increase in the concentration of DPD, the correlation time increases. This allows us to assert about the compaction of the amorphous phase in the fibers. This dependence correlates with the DSC data, which show an increase in crystallinity (
Figure 11a).
For comparison, the structure of heterogeneous fibrous matrices based on PHB and FeClTPP with high antibacterial properties. This compound is a derivative of chlorophyll and has a high antibacterial effect due to a specific reaction with oxygen [
17].
Figure 9b shows the structural formulas of one of FeClTPP.
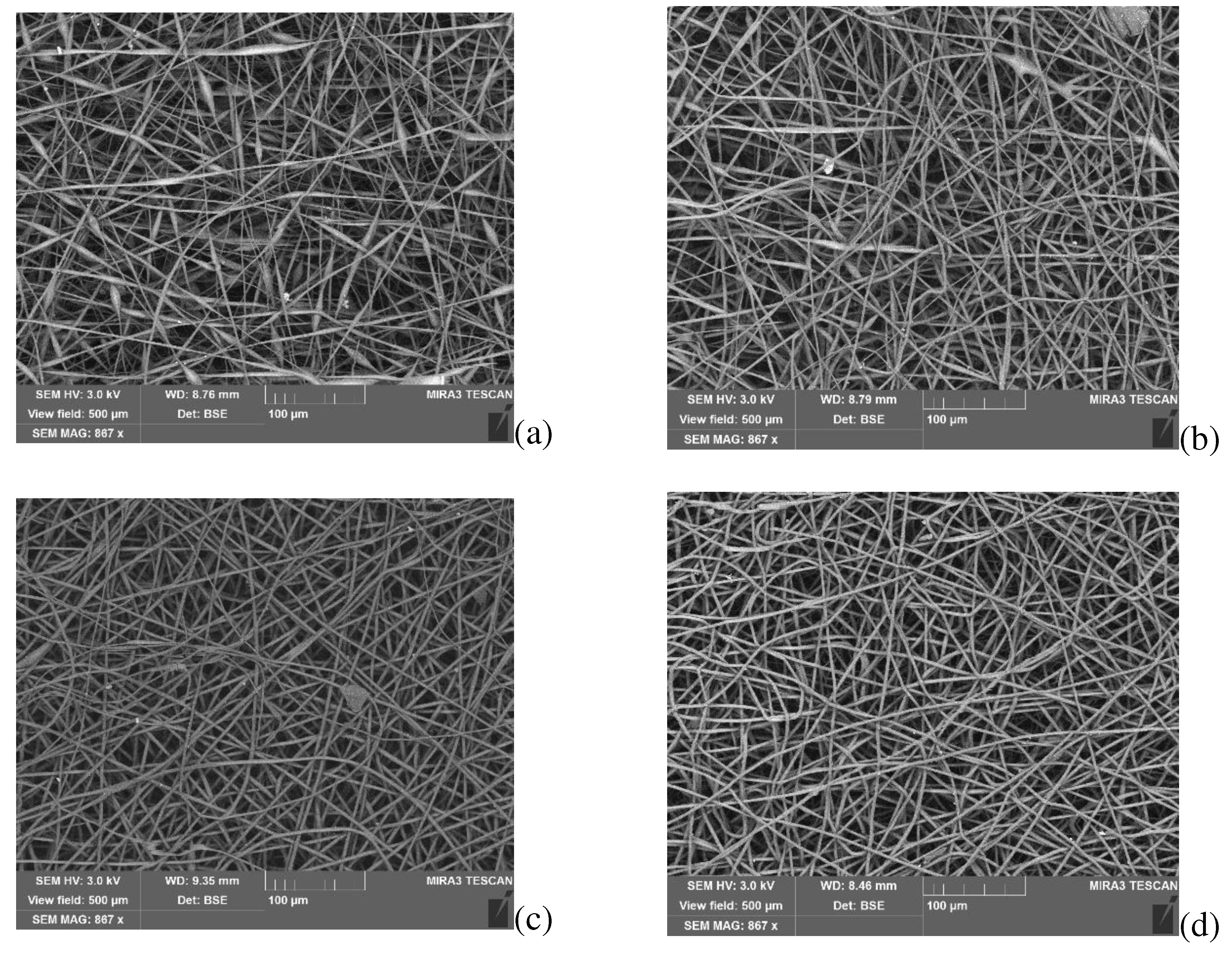
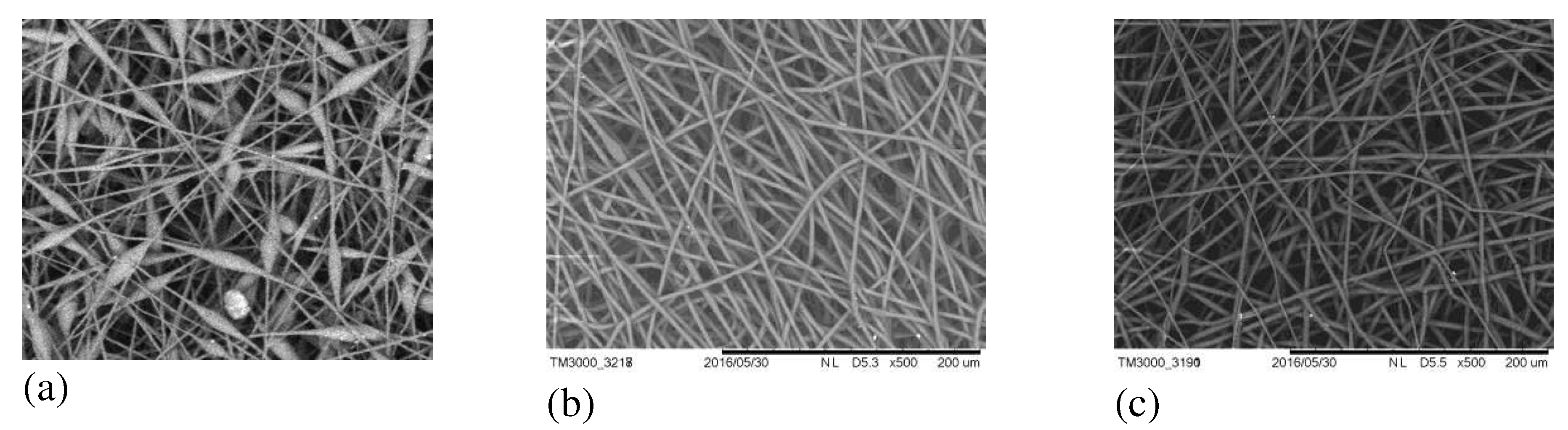
Figure 12 shows SEM images of PHB- FeClTPP electrospun materials.
As in the case of DPD, the addition of 1-5 wt.% FeClTPP improves the quality of the polymeric solution for electrospinning process, which leads to the formation of smooth fibers with an average diameter of 2-4 microns. Strong polarity of this complex may cause its active interaction with oxygen-containing groups of PHB during the formation of fibers.
Table 2 shows the results of the study of fibers by the DSC method.
Where: (Χ) is the degree of crystallinity; melting point (Tm) and crystallization (Tcr).
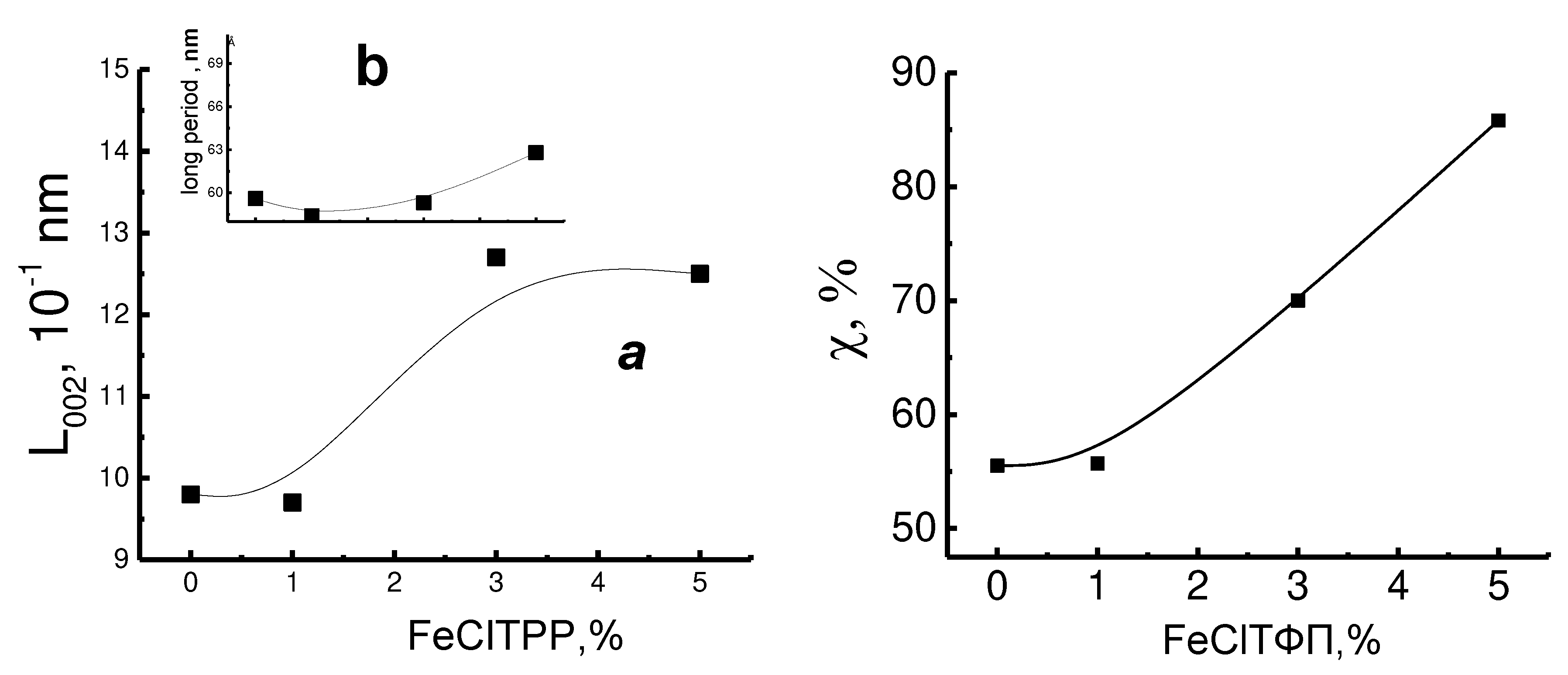
Table 2 shows that the increasing of concentration of the FeClTPP leads to an increase the degree of crystallinity of PHB. In this case, a typical mechanism of nucleation of the crystalline phase is observed. Upon cooling of the material, the inhibition of crystallization processes in PHB is visible, which is associated with strong intermolecular interaction and high viscosity of PHB in the melted state.
Sstudying the crystalline structure of PHB by the X-ray method showed that with an increase in the concentration of FeClTPP the longitudinal size of the crystallites and the long period of the crystalline phase of the PHB increased. This observation is consistent with the overall increase in the degree of crystallinity measured by the DSC method (
Table 2). This is in good agreement with the patterns obtained earlier when adding the DPA.
Results of EPR studying of PHB- FeClTPP fibers is shown in
Figure 15.
Introduction of FeClTPP in PHB leads to the compaction of the amorphous phase of the polymer during the formation of the fiber. The proportion of dense regions increases with an increase in the concentration of FeClTPP. Apparently, the disequilibrium of the fiber is caused by a large number of mesomorphic structures (unfinished crystals), the formation of which occurs as a result of intermolecular interactions of polar groups of PHB with strongly polar FeClTPP complexes. Adhesive bonds prevent the realization and complete passage of the crystallization process of the polymer matrix.
Both DPD and FeClTPP are highly soluble in chloroform and are distributed evenly in the fiber at the molecular level. In the case of polar nanoparticles, a different structure formation of PHB can be expected due to the insolubility of the particles in chloroform and their agglomeration in the fiber during the formation of the supramolecular structure. Our task was to study the effect of titanium and silicon oxide nanoparticles on the formation of the crystal structure of the fiber.
It is known that nanoparticles in crystallizing polymers can act as crystallite nuclei [
18]. However, how ultra-low concentrations of polar nanoparticles will behave in a moderately hydrophobic and weakly polar biopolymer of PHB [
19] requires clarification. The data of the DSC studies are presented in
Table 3.
It can be seen from the data (
Table 3) that polar nanoparticles in the PHB matrix contribute to an increase in the number and size of crystallites according to the known mechanism of crystallization, which is indicated by an increase in the melting temperature and the degree of crystallinity. At the same time, we observe a decrease in the crystallization temperature and the size of the average diameter of the fibers. This effect can be explained by the intermolecular interaction between the polar carbonyl and terminal hydroxyl groups of PHB with the polar surface of nanoparticles as a result of electrostatic forces of attraction. At the same time, during the cooling of the material, the crystallization processes are inhibited and the amorphized plastic fiber under the action of tensile stresses is capable of additional extraction.