Submitted:
01 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction; scope and importance
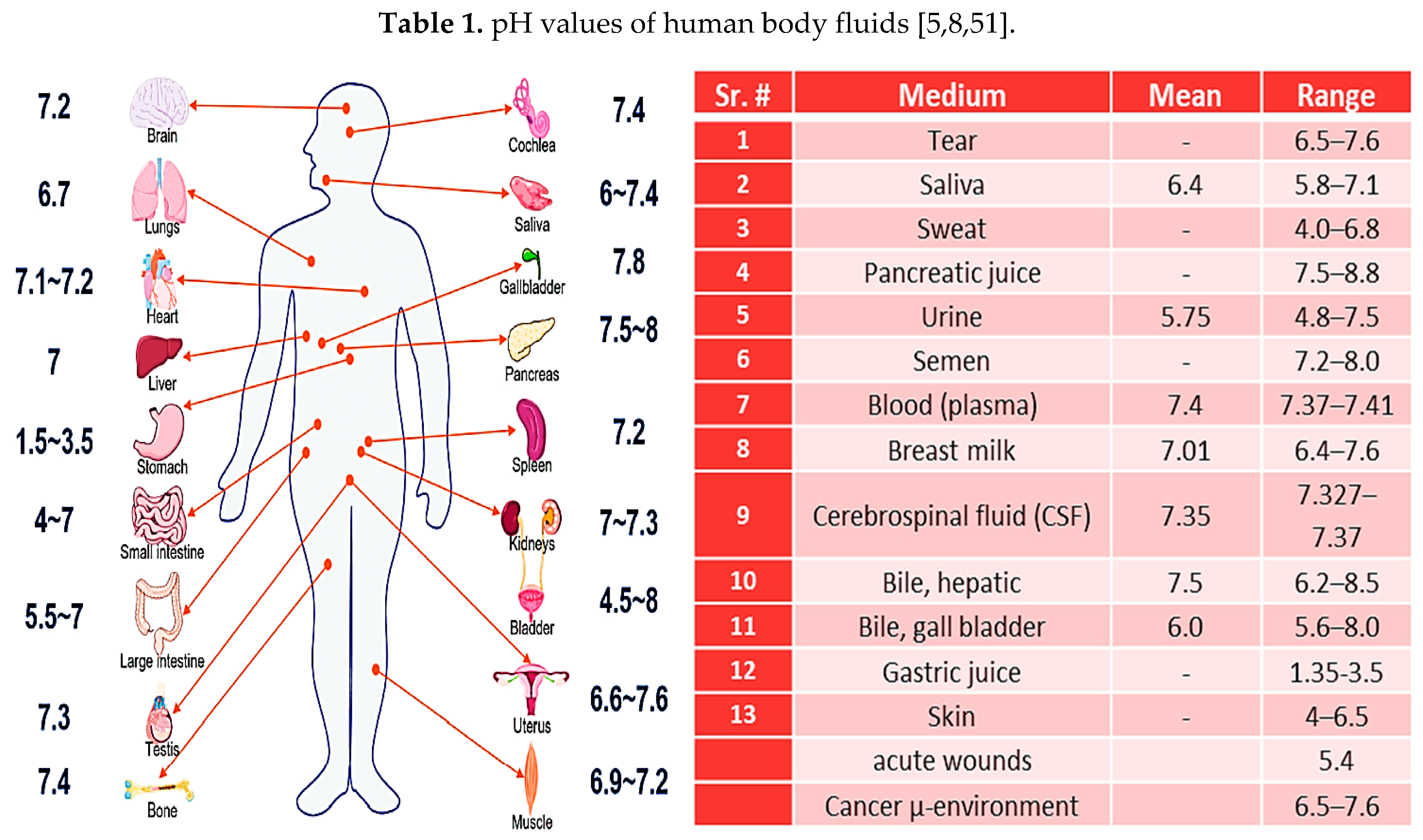
2. pH regulation in human body
2.1. pH in cancerous environment
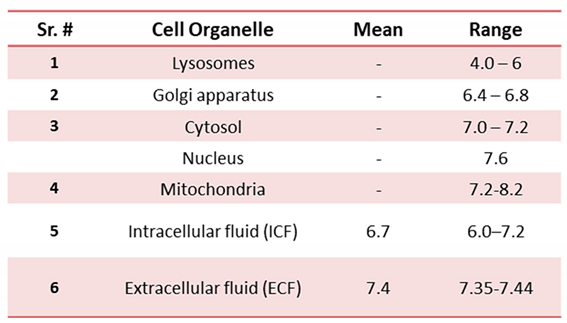
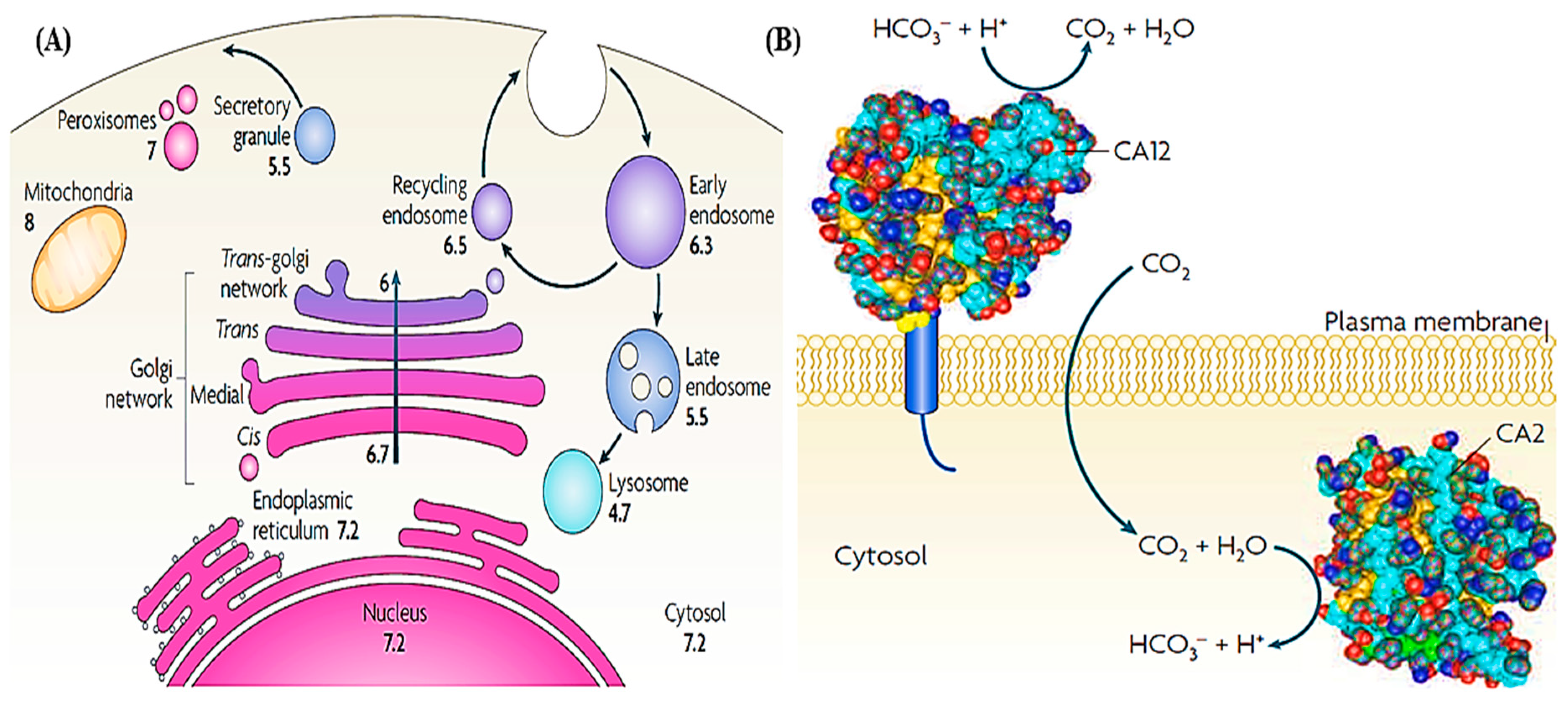
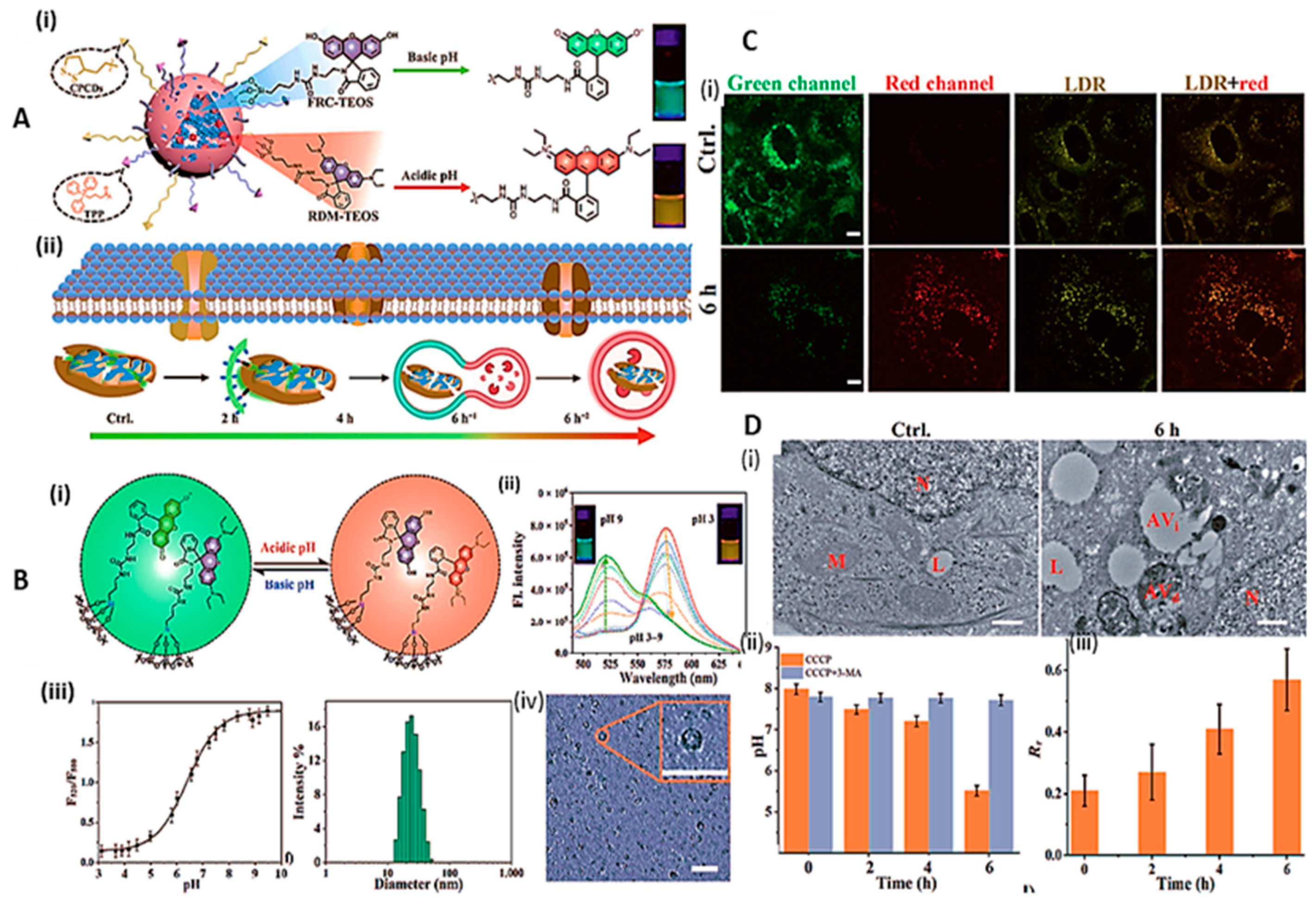
2.2. Intracellular/ extracellular pH sensing
3. An overview of pH sensing techniques
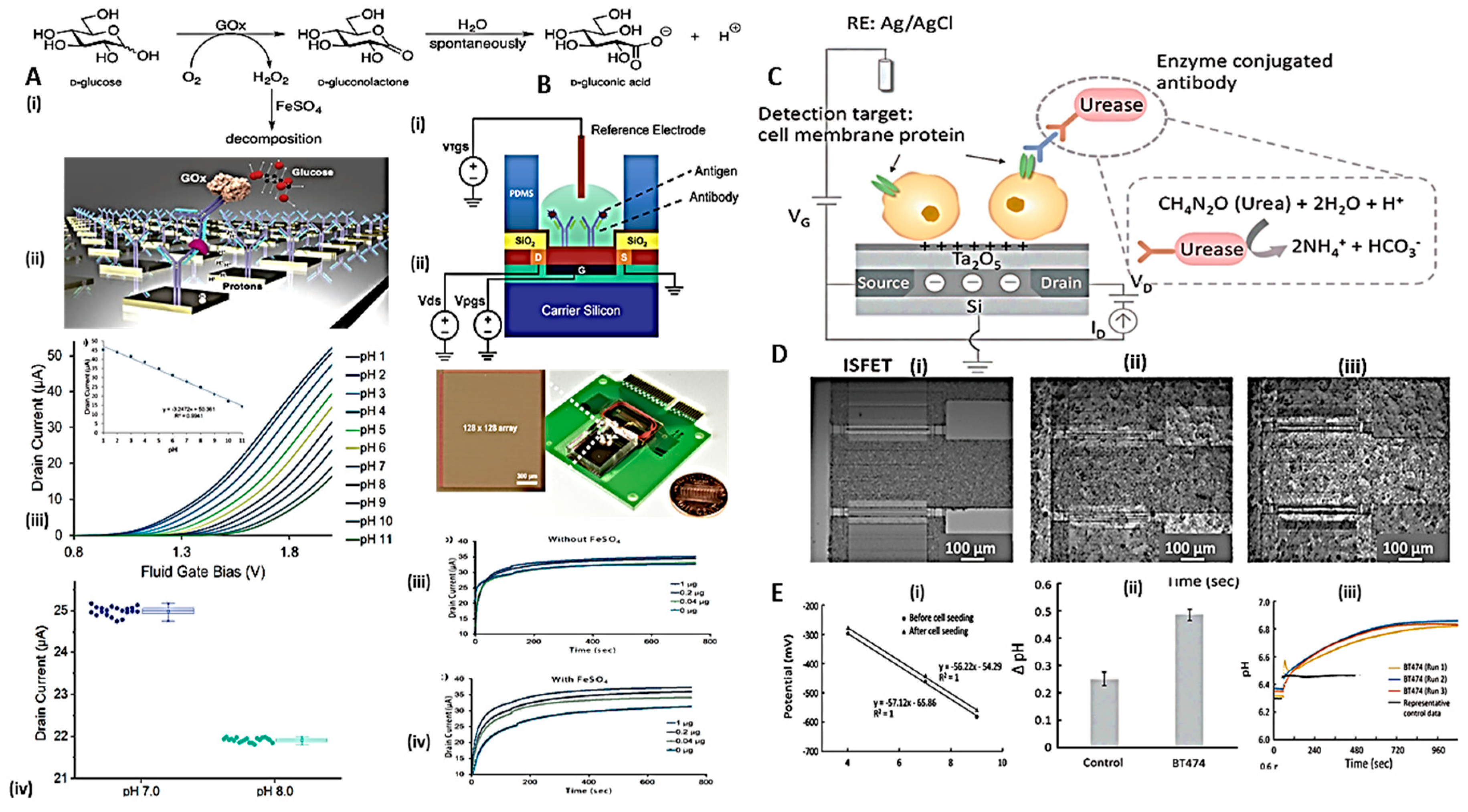
3.1. Electrochemical sensors
3.2. Optical-based pH sensors
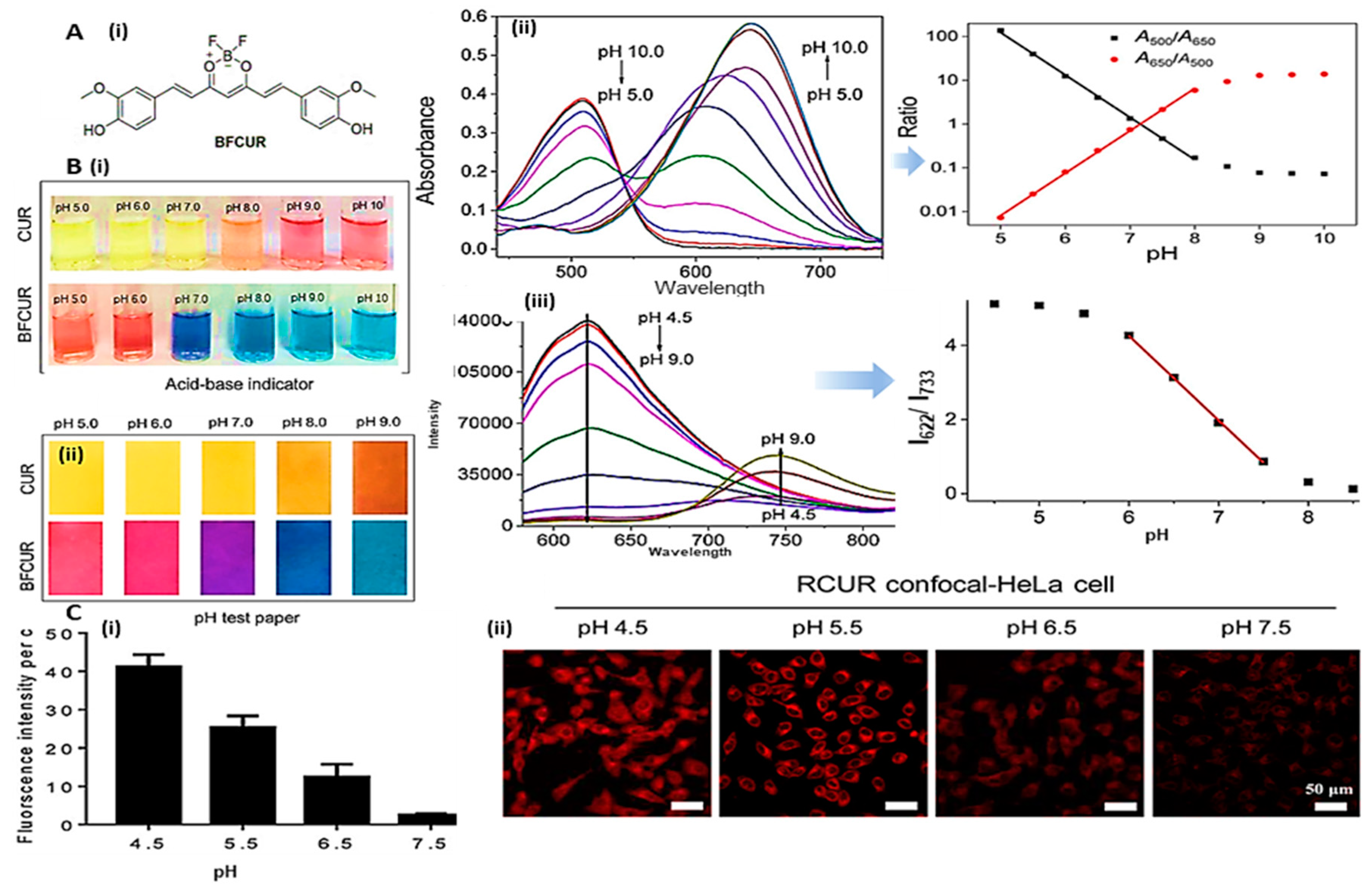
3.2.1. Absorption/Visual-based pH sensors
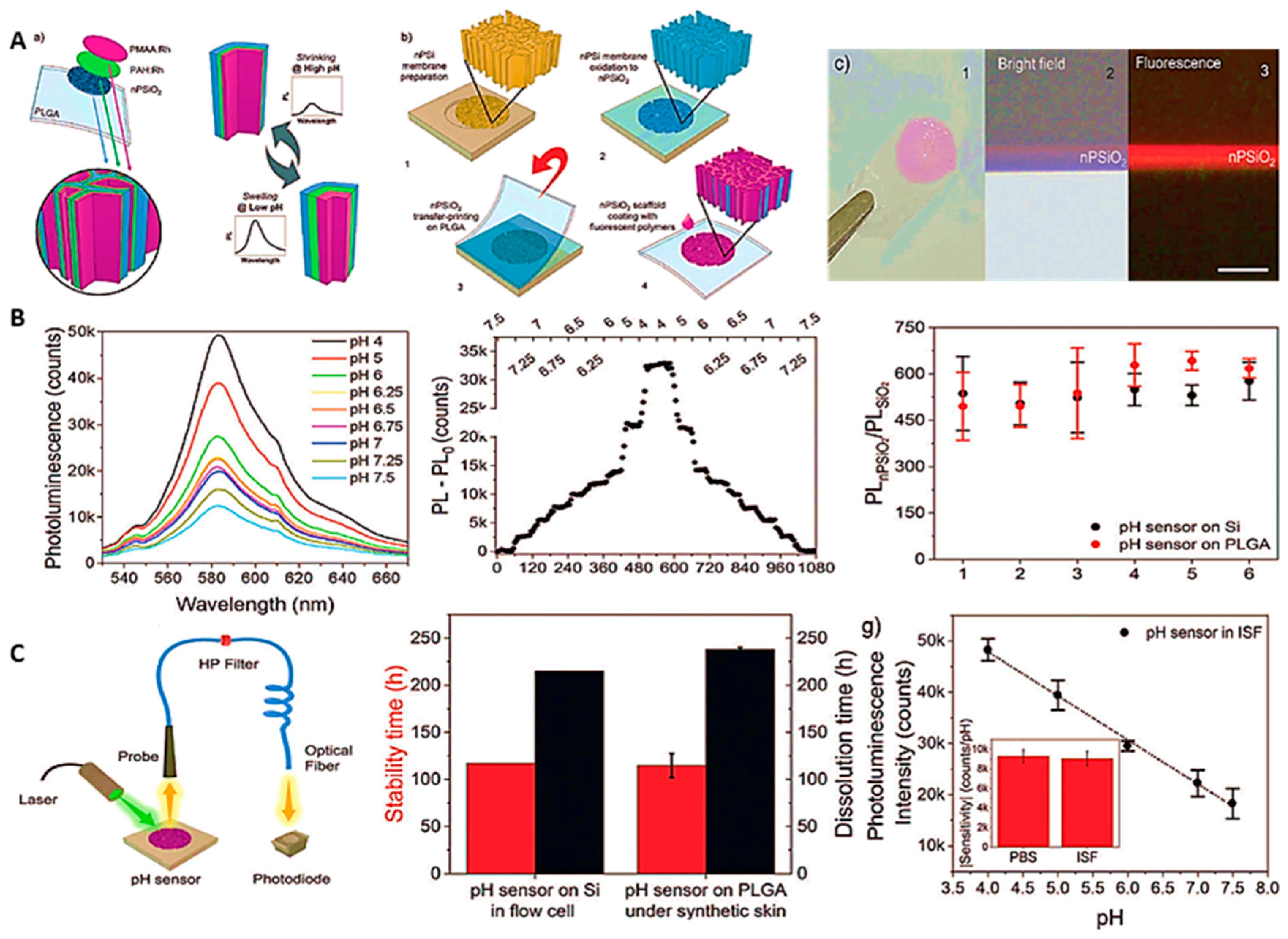
4. Cutting-Edge Technology; Wearable pH Sensors
- Wearable electrochemical pH sensors and
- Wearable optical pH sensors
4.1. Wearable pH sensors in real life applications
- Wound Management
- Wearable sweat pH sensing
5. Conclusion, challenges and future perspectives
Challenges
Future perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghoneim, M.; Nguyen, A.; Dereje, N.; Huang, J.; Moore, G.; Murzynowski, P.; Dagdeviren, C. Recent progress in electrochemical pH-sensing materials and configurations for biomedical applications. Chemical Reviews 2019, 119, 5248–5297. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsystem Technologies 2017, 23, 4391–4404. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Metabolic acidosis: pathophysiology, diagnosis and management. Nature Reviews Nephrology 2010, 6, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, J.W.; Theparambil, S.M.; Ruminot, I.; Noor, S.I.; Becker, H.M. Energy dynamics in the brain: contributions of astrocytes to metabolism and pH homeostasis. Frontiers in Neuroscience 2019, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P. What is pH regulation, and why do cancer cells need it? Cancer Metastasis Reviews 2019, 38, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, E., Sanvictores, T., Sharma, S.. Physiology, acid base balance. 2022.
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. Journal of Cell Science 2017, 130, 663–669. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nature Reviews Molecular Cell Biology 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Magder, S.; Magder, A.; Samoukovic, G. Intracellular pH regulation and the acid delusion. Canadian Journal of Physiology and Pharmacology 2021, 99, 561–576. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Frontiers in Physiology 2013, 4, 370. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical sensing and imaging of pH values: Spectroscopies, materials, and applications. Chemical Reviews 2020, 120, 12357–12489. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, Y.; Li, D.; Sun, C.; Lei, Z.; Lu, L.; Wang, T.; Zhang, F. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nature Communications 2019, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Wanas, W.; Abd El-Kaream, S.A.; Ebrahim, S.; Soliman, M.; Karim, M. Cancer bioimaging using dual mode luminescence of graphene/FA-ZnO nanocomposite based on novel green technique. Scientific Reports 2023, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.-Q.; Kim, J.S. Fluorescent bioimaging of pH: from design to applications. Chemical Society Reviews 2017, 46, 2076–2090. [Google Scholar] [CrossRef]
- Bakker, E. Wearable sensors. ACS Sensors 2023, 8, 1368–1370. [Google Scholar] [CrossRef]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.-C.; Ngo, Q.P. Roll-to-roll gravure printed electrochemical sensors for wearable and medical devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef]
- Zhao, H.; Su, R.; Teng, L.; Tian, Q.; Han, F.; Li, H.; Cao, Z.; Xie, R.; Li, G.; Liu, X. Recent advances in flexible and wearable sensors for monitoring chemical molecules. Nanoscale 2022, 14, 1653–1669. [Google Scholar] [CrossRef]
- Min, J.; Tu, J.; Xu, C.; Lukas, H.; Shin, S.; Yang, Y.; Solomon, S.A.; Mukasa, D.; Gao, W. Skin-interfaced wearable sweat sensors for precision medicine. Chemical Reviews 2023, 123, 5049–5138. [Google Scholar] [CrossRef]
- Di Costanzo, L.; Panunzi, B. Visual pH sensors: from a chemical perspective to new bioengineered materials. Molecules 2021, 26, 2952. [Google Scholar] [CrossRef]
- Venketeswaran, A.; Lalam, N.; Wuenschell, J.; Ohodnicki Jr, P.R.; Badar, M.; Chen, K.P.; Lu, P.; Duan, Y.; Chorpening, B.; Buric, M. Recent advances in machine learning for fiber optic sensor applications. Advanced Intelligent Systems 2022, 4, 2100067. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.; Lei, Z.; Lu, L.; Wang, S.; Zhang, H.; Li, B.; Zhang, F. NIR-II pH sensor with a FRET adjustable transition point for in situ dynamic tumor microenvironment visualization. Angewandte Chemie International Edition 2021, 60, 5091–5095. [Google Scholar] [CrossRef]
- Gond, S.; Yadav, P.; Chauhan, B.S.; Srikrishna, S.; Singh, V.P. Development of an ‘OFF-ON-OFF’colorimetric and fluorometric pH sensor for the study of physiological pH and its bioimaging application. Journal of Molecular Structure 2022, 1252, 132147. [Google Scholar] [CrossRef]
- Werner, J.; Belz, M.; Klein, K.-F.; Sun, T.; Grattan, K. Characterization of a fast response fiber-optic pH sensor and illustration in a biological application. Analyst 2021, 146, 4811–4821. [Google Scholar] [CrossRef]
- Krivenkov, V.; Samokhvalov, P.; Nabiev, I.; Rakovich, Y.P. pH-Sensing platform based on light–matter coupling in colloidal complexes of silver nanoplates and J-aggregates. The Journal of Physical Chemistry C 2021, 125, 1972–1979. [Google Scholar] [CrossRef]
- Wencel, D.; Kaworek, A.; Abel, T.; Efremov, V.; Bradford, A.; Carthy, D.; Coady, G.; McMorrow, R.C.; McDonagh, C. Optical sensor for real-time pH monitoring in human tissue. Small 2018, 14, 1803627. [Google Scholar] [CrossRef]
- Kurniawan, D.; Chiang, W.-H. Microplasma-enabled colloidal nitrogen-doped graphene quantum dots for broad-range fluorescent pH sensors. Carbon 2020, 167, 675–684. [Google Scholar] [CrossRef]
- Laysandra, L.; Kurniawan, D.; Wang, C.-L.; Chiang, W.-H.; Chiu, Y.-C. Synergistic effect in a graphene quantum dot-enabled luminescent skinlike copolymer for long-term pH detection. ACS Applied Materials Interfaces 2021, 13, 60413–60424. [Google Scholar] [CrossRef] [PubMed]
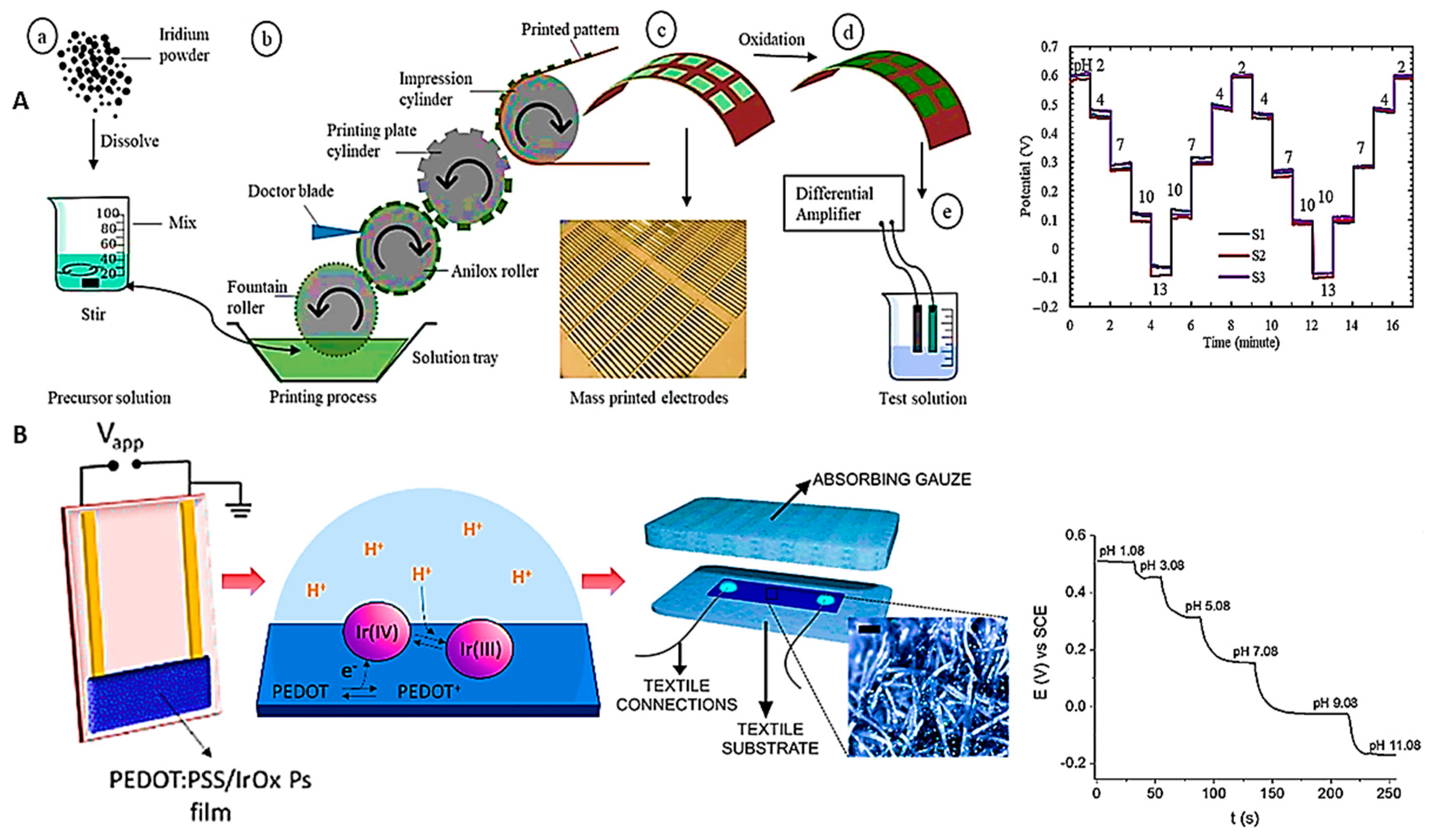
- Chou, S.-C.; Hsieh, Y.-C.; Cheang, W.-H.; Sun, B.-Y.; Chu, C.-Y.; Chen, S.-Y.; Chiao, J.-C.; Wu, P.-W. A flexible IrO2 membrane for pH sensing. Scientific Reports 2022, 12, 11712. [Google Scholar] [CrossRef]
- Li, S.; Ferrer-Ruiz, A.; Dai, J.; Ramos-Soriano, J.; Du, X.; Zhu, M.; Zhang, W.; Wang, Y.; Herranz, M.Á.; Jing, L. A pH-independent electrochemical aptamer-based biosensor supports quantitative, real-time measurement in vivo. Chemical Science 2022, 13, 8813–8820. [Google Scholar] [CrossRef]
- Booth, M.A.; Gowers, S.A.; Hersey, M.; Samper, I.C.; Park, S.; Anikeeva, P.; Hashemi, P.; Stevens, M.M.; Boutelle, M.G. Fiber-based electrochemical biosensors for monitoring pH and transient neurometabolic lactate. Analytical Chemistry 2021, 93, 6646–6655. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, S.-M.; Park, H.J.; Kim, Y.K.; Oh, D.X.; Cho, H.-W.; Lee, K.G.; Hwang, S.Y.; Park, J.; Choi, B.G. Highly self-healable and flexible cable-type pH sensors for real-time monitoring of human fluids. Biosensors and Bioelectronics 2020, 150, 111946. [Google Scholar] [CrossRef]
- Zamora, M.L.; Domínguez, J.M.; Trujillo, R.M.; Goy, C.B.; Sanchez, M.A.; Madrid, R.E. Potentiometric textile-based pH sensor. Sensors Actuators B: Chemical 2018, 260, 601–608. [Google Scholar] [CrossRef]
- Zea, M.; Texidó, R.; Villa, R.; Borrós, S.; Gabriel, G. Specially designed polyaniline/polypyrrole ink for a fully printed highly sensitive pH microsensor. ACS Applied Materials Interfaces 2021, 13, 33524–33535. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, J.J.; Pérez-Ràfols, C.; Cuartero, M.; Crespo, G.A. Toward in vivo transdermal pH sensing with a validated microneedle membrane electrode. ACS Sensors 2021, 6, 1129–1137. [Google Scholar] [CrossRef]
- Han, S.T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.S.; Lau, S.C.; Zhou, Y.; Roy, V. An overview of the development of flexible sensors. Advanced Materials 2017, 29, 1700375. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wen, F.; Yang, Y.; Le, X.; Liu, W.; Lee, C. Emerging wearable chemical sensors enabling advanced integrated systems toward personalized and preventive medicine. Analytical Chemistry 2023, 95, 490–514. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable chemical sensors: emerging systems for on-body analytical chemistry. Analytical Chemistry 2019, 92, 378–396. [Google Scholar] [CrossRef]
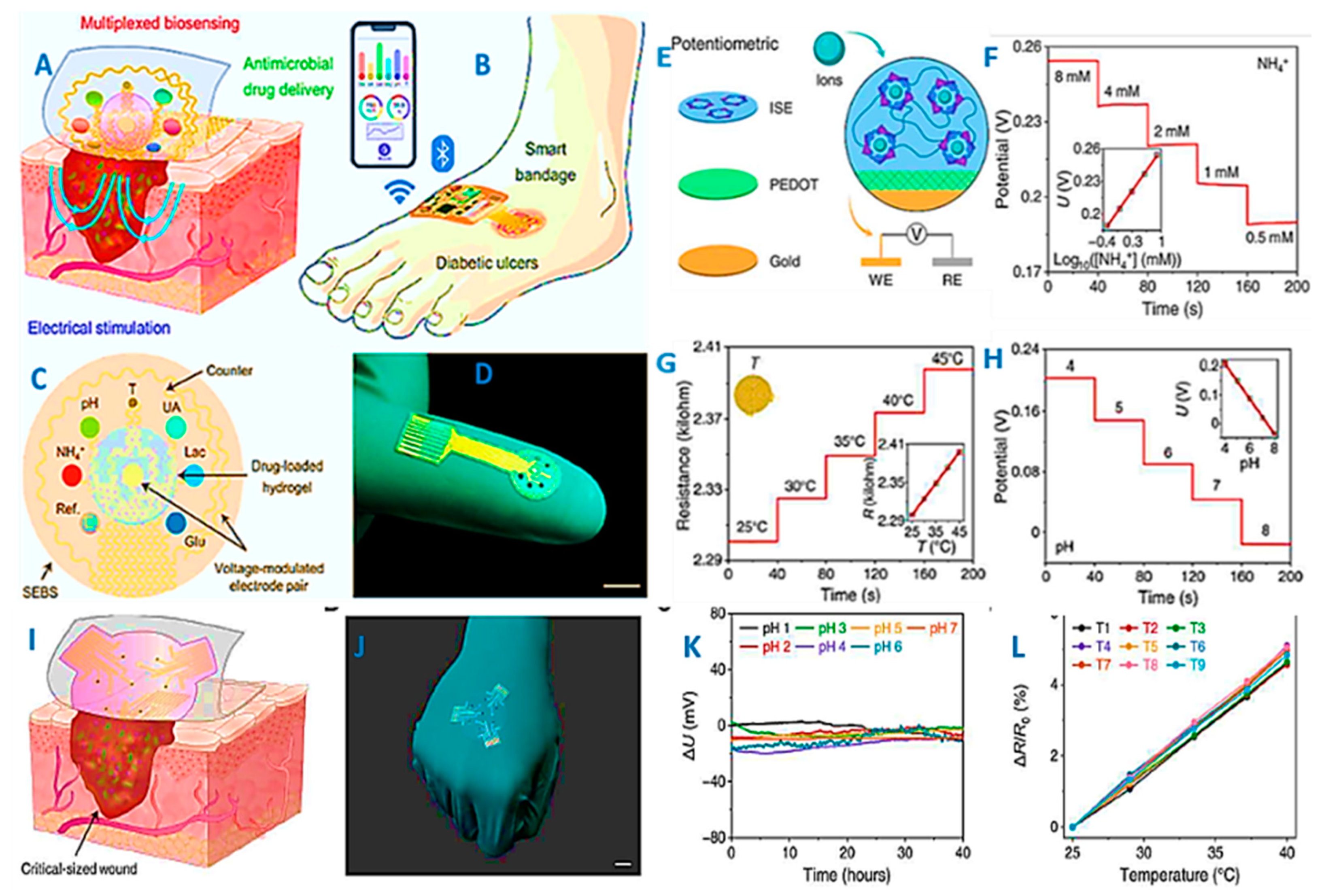
- Bi, Y.; Sun, M.; Wang, J.; Zhu, Z.; Bai, J.; Emran, M.Y.; Kotb, A.; Bo, X.; Zhou, M. Universal fully integrated wearable sensor arrays for the multiple electrolyte and metabolite monitoring in raw sweat, saliva, or urine. Analytical Chemistry 2023, 95, 6690–6699. [Google Scholar] [CrossRef]
- Lin, K.; Xie, J.; Bao, Y.; Ma, Y.; Chen, L.; Wang, H.; Xu, L.; Tang, Y.; Liu, Z.; Sun, Z. Self-adhesive and printable tannin–graphene supramolecular aggregates for wearable potentiometric pH sensing. Electrochemistry Communications 2022, 137, 107261. [Google Scholar] [CrossRef]
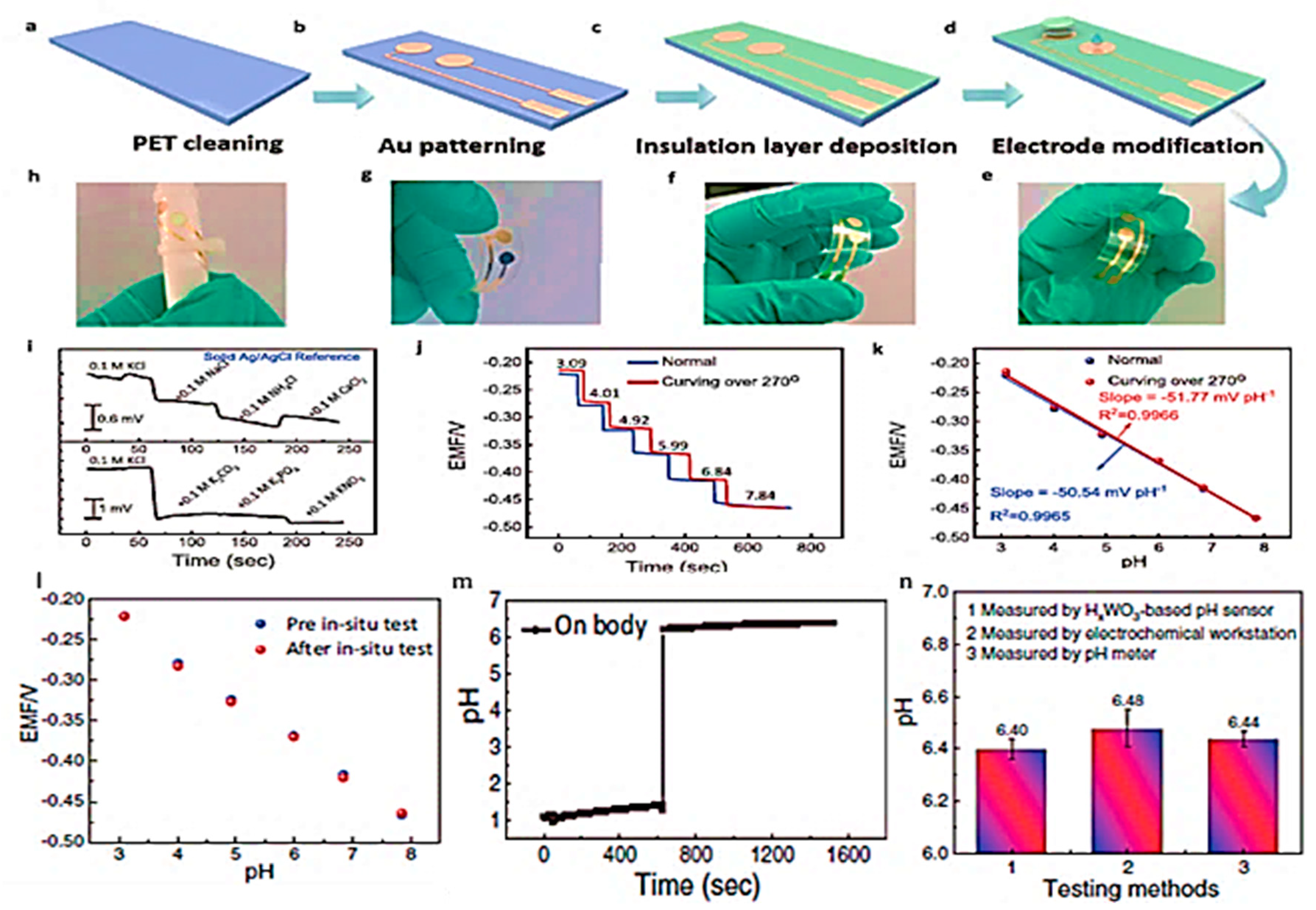
- Tang, Y.; Gan, S.; Zhong, L.; Sun, Z.; Xu, L.; Liao, C.; Lin, K.; Cui, X.; He, D.; Ma, Y. Lattice proton intercalation to regulate WO3-based solid-contact wearable pH sensor for sweat analysis. Advanced Functional Materials 2022, 32, 2107653. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nature Reviews Materials 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Liu, W.; Xie, R.; Zhu, J.; Wu, J.; Hui, J.; Zheng, X.; Huo, F.; Fan, D. A temperature responsive adhesive hydrogel for fabrication of flexible electronic sensors. npj Flexible Electronics 2022, 6, 68. [Google Scholar] [CrossRef]
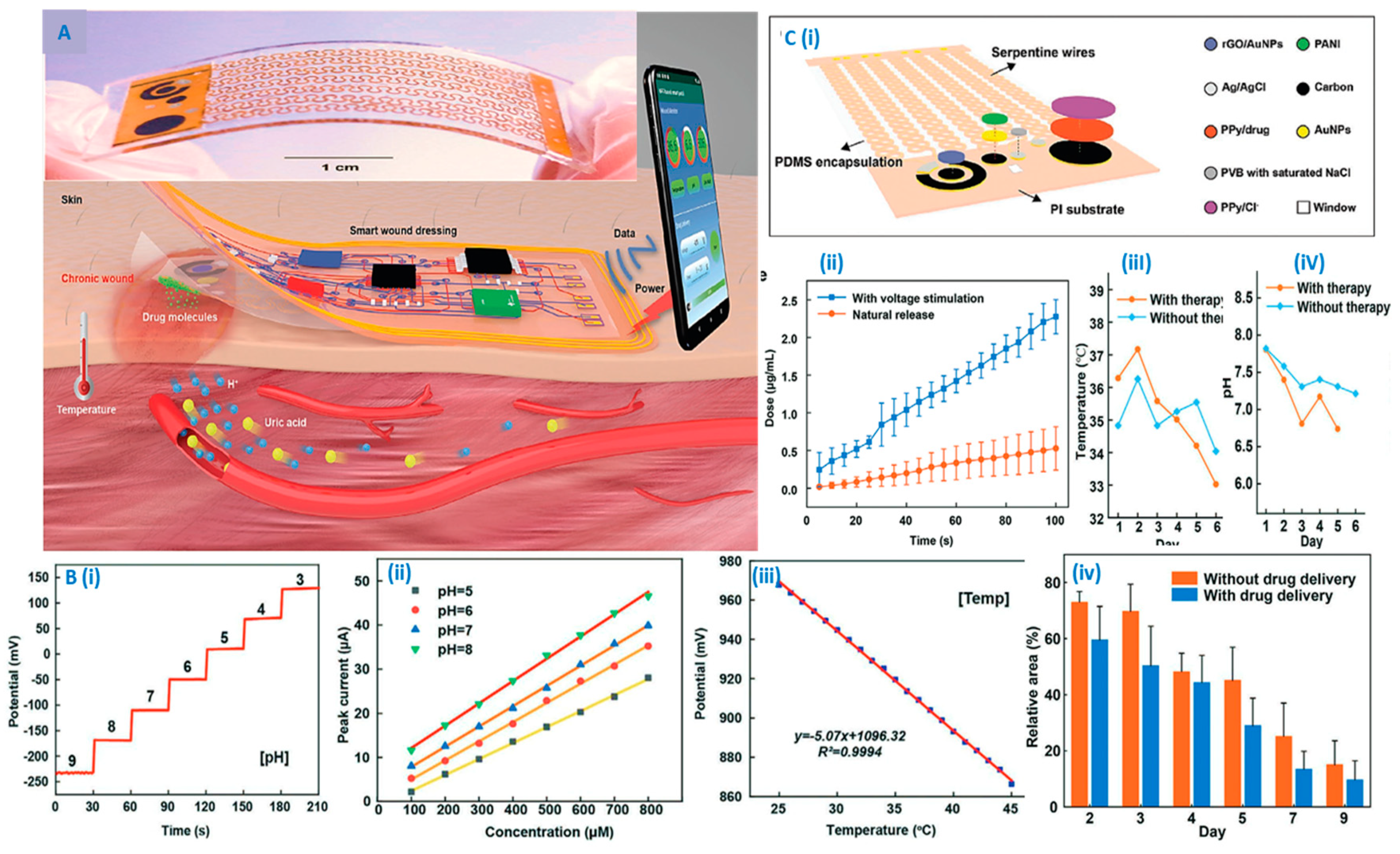
- Xu, G.; Lu, Y.; Cheng, C.; Li, X.; Xu, J.; Liu, Z.; Liu, J.; Liu, G.; Shi, Z.; Chen, Z. Battery-free and wireless smart wound dressing for wound infection monitoring and electrically controlled on-demand drug delivery. Advanced Functional Materials 2021, 31, 2100852. [Google Scholar] [CrossRef]
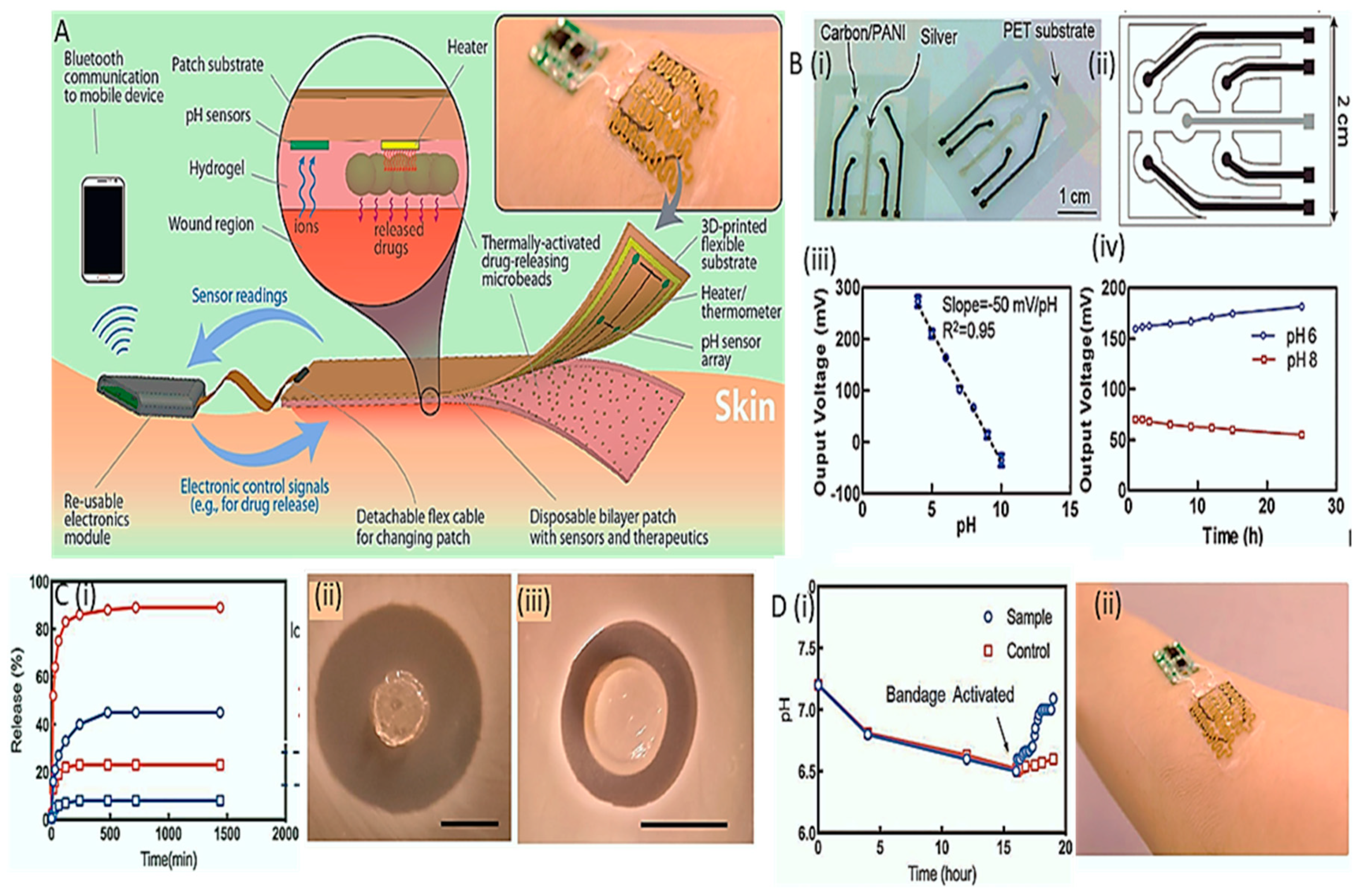
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B. Smart bandage for monitoring and treatment of chronic wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Xu, C.; Wang, C.; Song, Y.; Min, J.; Tu, J.; Solomon, S.A.; Li, J.; Banks, J.L.; Armstrong, D.G. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Science Advances 2023, 9, eadf7388. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, M.; Zahed, M.A.; Reza, M.S.; Asaduzzaman, M.; Jeong, S.; Song, H.; Kim, D.K.; Zhang, S.; Park, J.Y. MXene/fluoropolymer-derived laser-carbonaceous all-fibrous nanohybrid patch for soft wearable bioelectronics. Advanced Functional Materials 2023, 2208894. [Google Scholar] [CrossRef]
- Jung, H.S.; Jung, W.-B.; Wang, J.; Abbott, J.; Horgan, A.; Fournier, M.; Hinton, H.; Hwang, Y.-H.; Godron, X.; Nicol, R. CMOS electrochemical pH localizer-imager. Science Advances 2022, 8, eabm6815. [Google Scholar] [CrossRef]
- Saladin, K.S.; Porth, C. Anatomy & physiology: the unity of form and function; McGraw-Hill: New York, 2010; Volume 5. [Google Scholar]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of physiological pH and chemical pKa under the umbrella of physiologically based pharmacokinetic modeling of drug absorption, distribution, metabolism, excretion, and toxicity. Expert Opinion on Drug Metabolism Toxicology 2021, 17, 1103–1124. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. The alkaline diet: is there evidence that an alkaline pH diet benefits health? Journal of Environmental Public Health 2012, 2012. [Google Scholar] [CrossRef]
- Gillies, R.J. Cancer heterogeneity and metastasis: Life at the edge. Clinical Experimental Metastasis 2022, 39, 15–19. [Google Scholar] [CrossRef]
- Czowski, B.J.; Romero-Moreno, R.; Trull, K.J.; White, K.A. Cancer and pH dynamics: Transcriptional regulation, proteostasis, and the need for new molecular tools. Cancers 2020, 12, 2760. [Google Scholar] [CrossRef]
- Qi, Q.; Fox, M.S.; Lim, H.; Sullivan, R.; Li, A.; Bellyou, M.; Desjardins, L.; McClennan, A.; Bartha, R.; Hoffman, L. Glucose infusion induced change in intracellular pH and its relationship with tumor glycolysis in a C6 rat model of glioblastoma. Molecular Imaging Biology 2023, 25, 271–282. [Google Scholar] [CrossRef]
- Kovács, A.; Kis, N.; Budai-Szűcs, M.; Gácsi, A.; Csányi, E.; Csóka, I.; Berkó, S. QbD-based investigation of dermal semisolid in situ film-forming systems for local anaesthesia. Drug Design, Development Therapy 2020, 5059–5076. [Google Scholar] [CrossRef]
- Osei-Owusu, J.; Kots, E.; Ruan, Z.; Mihaljević, L.; Chen, K.H.; Tamhaney, A.; Ye, X.; Lü, W.; Weinstein, H.; Qiu, Z. Molecular determinants of pH sensing in the proton-activated chloride channel. Proceedings of the National Academy of Sciences 2022, 119, e2200727119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Z.; Xie, L.; Shang, Z.-J. Burden of oral cancer on the 10 most populous countries from 1990 to 2019: Estimates from the Global Burden of Disease study 2019. International Journal of Environmental Research Public Health 2022, 19, 875. [Google Scholar] [CrossRef]
- Albatany, M.; Ostapchenko, V.G.; Meakin, S.; Bartha, R. Brain tumor acidification using drugs simultaneously targeting multiple pH regulatory mechanisms. Journal of Neuro-Oncology 2019, 144, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Hashim, A.; Estrella, V. Acidosis and cancer: from mechanism to neutralization. Cancer Metastasis Reviews 2019, 38, 149–155. [Google Scholar] [CrossRef]
- Ye, X.; Xiang, Y.; Wang, Q.; Li, Z.; Liu, Z. A red emissive two-photon fluorescence probe based on carbon dots for intracellular pH detection. Small 2019, 15, 1901673. [Google Scholar] [CrossRef]
- Chen, Q.; Zhai, J.; Li, J.; Wang, Y.; Xie, X. Exploring ratiometric endolysosomal pH nanosensors with hydrophobic indicators responding at the nanoscale interface and multiple fluorescence resonance energy transfers. Nano Research 2022, 1–8. [Google Scholar] [CrossRef]
- Shen, J.; Zeng, Y.; Zhuang, X.; Sun, L.; Yao, X.; Pimpl, P.; Jiang, L. Organelle pH in the arabidopsis endomembrane system. Molecular plant 2013, 6, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Donahue, C.E.; Siroky, M.D.; White, K.A. An optogenetic tool to raise intracellular pH in single cells and drive localized membrane dynamics. Journal of the American Chemical Society 2021, 143, 18877–18887. [Google Scholar] [CrossRef]
- Loiselle, F.B.; Casey, J.R. Measurement of intracellular pH; 2010; pp. 311-331.
- Dong, B.; Du, S.; Wang, C.; Fu, H.; Li, Q.; Xiao, N.; Yang, J.; Xue, X.; Cai, W.; Liu, D. Reversible self-assembly of nanoprobes in live cells for dynamic intracellular pH imaging. ACS Nano 2019, 13, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Kim, H.; Sun, P.Z. Detection of tissue pH with quantitative chemical exchange saturation transfer magnetic resonance imaging. NMR in Biomedicine 2023, 36, e4711. [Google Scholar] [CrossRef]
- Boyd, P.S.; Breitling, J.; Korzowski, A.; Zaiss, M.; Franke, V.L.; Mueller-Decker, K.; Glinka, A.; Ladd, M.E.; Bachert, P.; Goerke, S. Mapping intracellular pH in tumors using amide and guanidyl CEST-MRI at 9. 4 T. Magnetic Resonance in Medicine 2022, 87, 2436–2452. [Google Scholar] [CrossRef]
- Singh, M. Synthesis of two-dimensional metal, metal oxide and metal hydroxide nanomaterials for biosensing. In Nanozymes in Medicine; Springer, 2023; pp. 161–185. [Google Scholar]
- Gu, B.; Zhang, Q. Recent advances on functionalized upconversion nanoparticles for detection of small molecules and ions in biosystems. Advanced Science 2018, 5, 1700609. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Hu, J.; Du Rietz, A.; Wu, X.; Zhang, R.; Zhang, Z.; Uvdal, K.; Hu, Z. Single-wavelength-excited fluorogenic nanoprobe for accurate realtime ratiometric analysis of broad pH fluctuations in mitophagy. Nano Research 2022, 15, 6515–6521. [Google Scholar] [CrossRef]
- Wu, Q.; Fu, S.; Xiao, H.; Du, J.; Cheng, F.; Wan, S.; Zhu, H.; Li, D.; Peng, F.; Ding, X. Advances in extracellular vesicle nanotechnology for precision theranostics. Advanced Science 2023, 10, 2204814. [Google Scholar] [CrossRef]
- Inobeme, A.; Mathew, J.T.; Adetunji, C.O.; Ajai, A.I.; Inobeme, J.; Maliki, M.; Okonkwo, S.; Adekoya, M.A.; Bamigboye, M.O.; Jacob, J.O. Recent advances in nanotechnology for remediation of heavy metals. Environmental Monitoring Assessment 2023, 195, 111. [Google Scholar] [CrossRef]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current applications and future scope in food. Food Frontiers 2021, 2, 3–22. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomedical Reports 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Lee, S.-E.; Kim, D.-H.; Pyo, Y.-C.; Park, J.-S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. Journal of Pharmaceutical Investigation 2020, 50, 261–270. [Google Scholar] [CrossRef]
- Zhang, G.; Han, W.; Zhao, P.; Wang, Z.; Li, M.; Sui, X.; Liu, Y.; Tian, B.; He, Z.; Fu, Q. Programmed pH-responsive core–shell nanoparticles for precisely targeted therapy of ulcerative colitis. Nanoscale 2023, 15, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Alam, A.U.; Howlader, M.M.; Hu, N.X.; Deen, M.J. Inkjet printing of a highly loaded palladium ink for integrated, low-cost pH sensors. Advanced Functional Materials 2016, 26, 4923–4933. [Google Scholar] [CrossRef]
- Erdem, Ö.; Derin, E.; Zeibi Shirejini, S.; Sagdic, K.; Yilmaz, E.G.; Yildiz, S.; Akceoglu, G.A.; Inci, F. Carbon-based nanomaterials and sensing tools for wearable health monitoring devices. Advanced Materials Technologies 2022, 7, 2100572. [Google Scholar] [CrossRef]
- Wang, F.-T.; Wang, L.-N.; Xu, J.; Huang, K.-J.; Wu, X. Synthesis and modification of carbon dots for advanced biosensing application. Analyst 2021, 146, 4418–4435. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, F.; Zhao, J.; Fu, L.; Gu, Y.; Qu, L.; Zhu, C.; Zhu, J.-J.; Lin, Y. MXenes-based nanomaterials for biosensing and biomedicine. Coordination Chemistry Reviews 2023, 479, 215002. [Google Scholar] [CrossRef]
- NajafiKhoshnoo, S.; Kim, T.; Tavares-Negrete, J.A.; Pei, X.; Das, P.; Lee, S.W.; Rajendran, J.; Esfandyarpour, R. A 3D Nanomaterials-Printed Wearable, Battery-Free, Biocompatible, Flexible, and Wireless pH Sensor System for Real-Time Health Monitoring. Advanced Materials Technologies 2023, 8, 2201655. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Zhao, Z.; Li, L.; Tong, J.; Shang, Q.; Liu, Y.; Zhang, Z. Carbon nanotube field-effect transistor based pH sensors. Carbon 2023, 205, 540–545. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, J.; Zhao, G.; Zhou, L.; Kong, F.; Jiang, T.; Jiang, H. Tetrazine bioorthogonal chemistry makes nanotechnology a powerful toolbox for biological applications. Nanoscale 2023, 15, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Khademi, R.; Mohammadi, Z.; Khademi, R.; Saghazadeh, A.; Rezaei, N. Nanotechnology-based diagnostics and therapeutics in acute lymphoblastic leukemia: a systematic review of preclinical studies. Nanoscale Advances 2023. [CrossRef]
- Zhao, C.; Kang, J.; Li, Y.; Wang, Y.; Tang, X.; Jiang, Z. Carbon-based stimuli-responsive nanomaterials: classification and application. Cyborg Bionic Systems 2023, 4, 0022. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Sun, C.; Wei, X.; Liu, S.; Jiang, Y. Gastrointestinal pH-Sensitive Pickering Emulsions Stabilized by Zein Nanoparticles Coated with Bioactive Glycyrrhizic Acid for Improving Oral Bioaccessibility of Curcumin. ACS Applied Materials Interfaces 2023, 15, 14678–14689. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Itoo, A.M.; Paul, M.; Kondapaneni, L.P.; Ghosh, B.; Biswas, S. Block HPMA-based pH-sensitive gemcitabine pro-drug nanoaggregates for cancer treatment. European Polymer Journal 2023, 186, 111843. [Google Scholar] [CrossRef]
- Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible potentiometric pH sensors for wearable systems. RSC Advances 2020, 10, 8594–8617. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Peng, R.; Mao, W.; Lin, Y.; Yu, H. Recent advances in ion-sensitive field-effect transistors for biosensing applications. Electrochemical Science Advances 2022, e2100163. [Google Scholar] [CrossRef]
- Kim, Y.-U.; Cho, W.-J. Self-sensitivity amplifiable dual-gate ion-sensitive field-effect transistor based on a high-k engineered dielectric layer. Japanese Journal of Applied Physics 2023, 62, SC1056. [Google Scholar] [CrossRef]
- Chen, H.J.; Lee, T.N.; Tseng, S.-L.; Chen, S.-Z.; Chiu, P.-W. Characterizations of ion-sensitive field-effect transistors with silicon wire array channels and stack-sensing membrane. Journal of The Electrochemical Society 2022, 169, 037511. [Google Scholar] [CrossRef]
- Ren, H.; Liang, K.; Li, D.; Chen, Y.; Tang, Y.; Wang, Y.; Li, F.; Liu, G.; Zhu, B. Field-effect transistor-based biosensor for pH sensing and mapping. Advanced Sensor Research 2023, 2200098. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Huang, N.-T. A proton-selective membrane (PSM)-deposited dual-gate ion-sensitive field-effect transistor (DG-ISFET) integrating a microchamber-embedded filter membrane for bacterial enrichment and antimicrobial susceptibility test. Sensors Actuators B: Chemical 2022, 359, 131580. [Google Scholar] [CrossRef]
- Pan, T.-M.; Lin, C.-H.; Pang, S.-T.J.J.o.A.; Compounds. Structural properties and sensing performance of TaOx/Ta stacked sensing films for extended-gate field-effect transistor pH sensors. Journal of Alloys Compounds 2022, 903, 163955. [Google Scholar] [CrossRef]
- Juang, D.S.; Lin, C.-H.; Huo, Y.-R.; Tang, C.-Y.; Cheng, C.-R.; Wu, H.-S.; Huang, S.-F.; Kalnitsky, A.; Lin, C.-C. Proton-ELISA: Electrochemical immunoassay on a dual-gated ISFET array. Biosensors and Bioelectronics 2018, 117, 175–182. [Google Scholar] [CrossRef]
- Tabata, M.; Khamhanglit, C.; Kotaki, S.; Miyahara, Y. Detection of cell membrane proteins using ion-sensitive field effect transistors combined with chemical signal amplification. Chemical Communications 2022, 58, 7368–7371. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Ding, F.; Tang, L.-N.; Li, T.; Li, Y.-H.; Zhang, Y.-J.; Gong, H.-Y.; Li, Y.-T.; Zhang, G.-J. Monitoring of pH changes in a live rat brain with MoS2/PAN functionalized microneedles. Analyst 2018, 143, 4469–4475. [Google Scholar] [CrossRef]
- Du, W.; Shen, Z.; Liang, Y.; Gong, S.; Meng, Z.; Li, M.; Wang, Z.; Wang, S. A highly effective “naked eye” colorimetric and fluorimetric curcumin-based fluorescent sensor for specific and sensitive detection of H2 O2 in vivo and in vitro. Analyst 2023, 148, 1824–1837. [Google Scholar] [CrossRef]
- Ye, X.; Wang, A.; Zhang, D.; Zhou, P.; Zhu, P. Light and pH dual-responsive spiropyran-based cellulose nanocrystals. RSC Advances 2023, 13, 11495–11502. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M.; Boyce, M.W.; Whitman, N.A.; Kromhout, B.P.; Lockett, M.R. A pH-sensing optode for mapping spatiotemporal gradients in 3D paper-based cell cultures. Analytical Chemistry 2018, 90, 2376–2383. [Google Scholar] [CrossRef]
- Petrunina, N.A.; Shtork, A.S.; Lukina, M.M.; Tsvetkov, V.B.; Khodarovich, Y.M.; Feofanov, A.V.; Moysenovich, A.M.; Maksimov, E.G.; Shipunova, V.O.; Zatsepin, T.S. Ratiometric i-motif-based sensor for precise long-term monitoring of pH micro alterations in the nucleoplasm and interchromatin granules. ACS Sensors 2023, 8, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Lesani, P.; Singh, G.; Lu, Z.; Mirkhalaf, M.; New, E.J.; Zreiqat, H. Two-photon ratiometric carbon dot-based probe for real-time intracellular pH monitoring in 3D environment. Chemical Engineering Journal 2022, 433, 133668. [Google Scholar] [CrossRef]
- Koide, Y.; Kojima, R.; Hanaoka, K.; Numasawa, K.; Komatsu, T.; Nagano, T.; Kobayashi, H.; Urano, Y. Design strategy for germanium-rhodamine based pH-activatable near-infrared fluorescence probes suitable for biological applications. Communications Chemistry 2019, 2, 94. [Google Scholar] [CrossRef]
- Kulkarni, C.A.; Fink, B.D.; Gibbs, B.E.; Chheda, P.R.; Wu, M.; Sivitz, W.I.; Kerns, R.J. A novel triphenylphosphonium carrier to target mitochondria without uncoupling oxidative phosphorylation. Journal of Medicinal Chemistry 2021, 64, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chemical Reviews 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, Y.; Zhang, Y.; Yu, G.-Y.; Liao, Z.-P.; Wu, H.-F.; Shi, C.-G. Ratiometric intracellular pH sensors based on nitrogen-doped graphene oxide quantum dots. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Xia, S.; Yu, S.; Duan, Y.; Wang, L.; Zhu, H.; He, H. Design of a cellulose nanocrystal-based upconversion ratiometric fluorescent nanoprobe for pH monitoring and imaging. Chemical Engineering Journal 2023, 454, 140456. [Google Scholar] [CrossRef]
- Xue, X.-L.; Wang, Y.; Chen, S.; Wang, K.-P.; Niu, S.-Y.; Zong, Q.-S.; Jiang, Y.; Hu, Z.-Q. Monitoring intracellular pH using a hemicyanine-based ratiometric fluorescent probe. Spectrochimica Acta Part A: Molecular Biomolecular Spectroscopy 2023, 284, 121778. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Jiang, D.; Wang, L.; Sun, X.; Liu, H.; Tian, Y.; Chen, M. A series of simple curcumin-derived colorimetric and fluorescent probes for ratiometric-pH sensing and cell imaging. Chinese Chemical Letters 2022, 33, 339–343. [Google Scholar] [CrossRef]
- Wang, X.-d.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Analytical Chemistry 2019, 92, 397–430. [Google Scholar] [CrossRef]
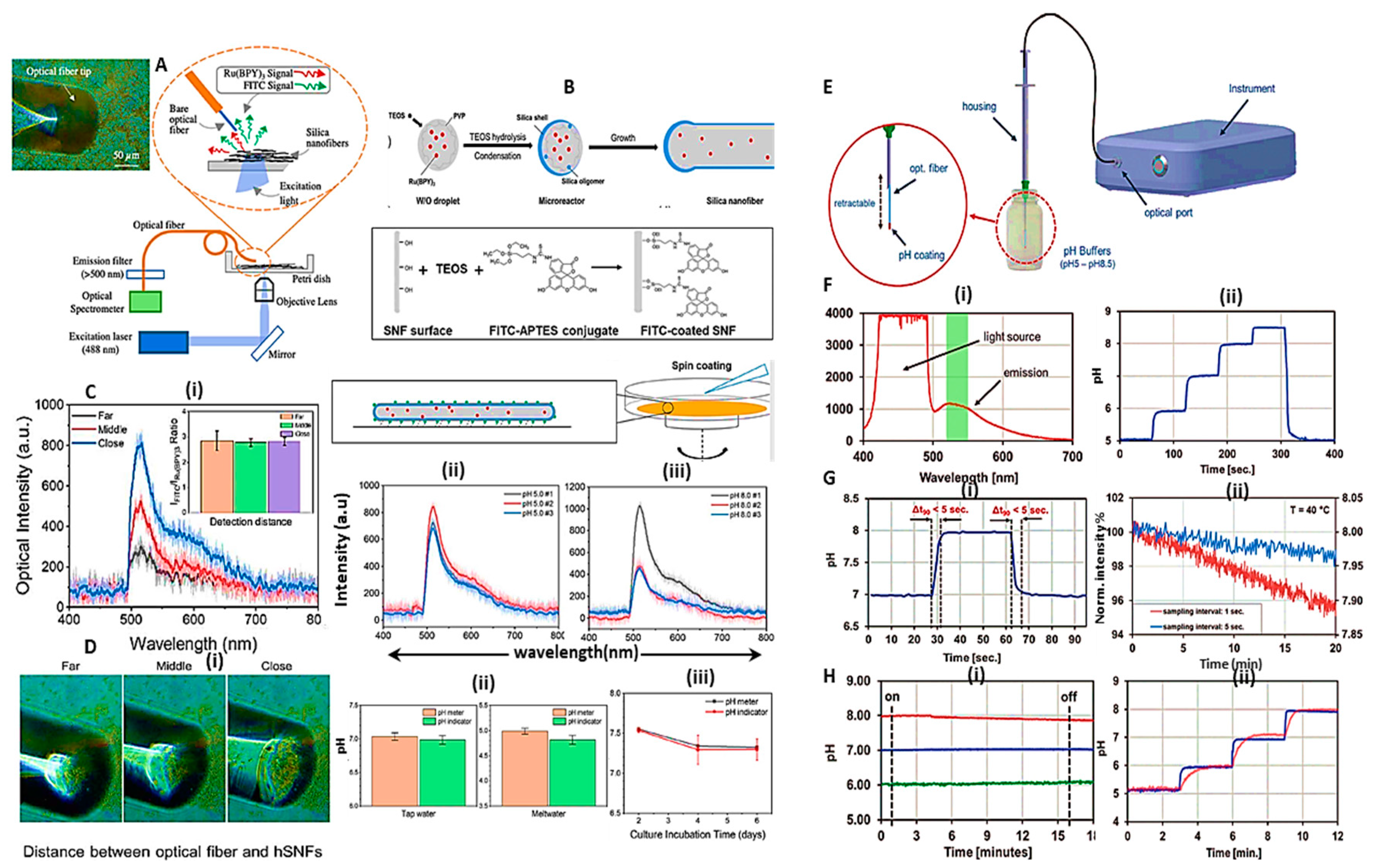
- Zhou, J.; Ren, Y.; Nie, Y.; Jin, C.; Park, J.; Zhang, J.X. Dual fluorescent hollow silica nanofibers for in situ pH monitoring using an optical fiber. Nanoscale Advances 2023, 5, 2180–2189. [Google Scholar] [CrossRef]
- Corsi, M.; Paghi, A.; Mariani, S.; Golinelli, G.; Debrassi, A.; Egri, G.; Leo, G.; Vandini, E.; Vilella, A.; Dähne, L. Bioresorbable nanostructured chemical sensor for monitoring of pH level In vivo. Advanced Science 2022, 9, 2202062. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nature Nanotechnology 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Science Advances 2017, 3, e1601314. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-based wearable biosensor system for high-performance in vitro perspiration analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, S.; Sun, Y.; Zhou, N.; Yu, H.; Zhang, H.; Jia, D. Wearable optical sensing in the medical internet of things (MIoT) for pervasive medicine: Opportunities and challenges. ACS Photonics 2022, 9, 2579–2599. [Google Scholar] [CrossRef]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J. Technology roadmap for flexible sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef]
- Chandra Kishore, S.; Samikannu, K.; Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Alagan, M.; Sundramoorthy, A.K.; Lee, Y.R. Smartphone-operated wireless chemical sensors: A review. Chemosensors 2022, 10, 55. [Google Scholar] [CrossRef]
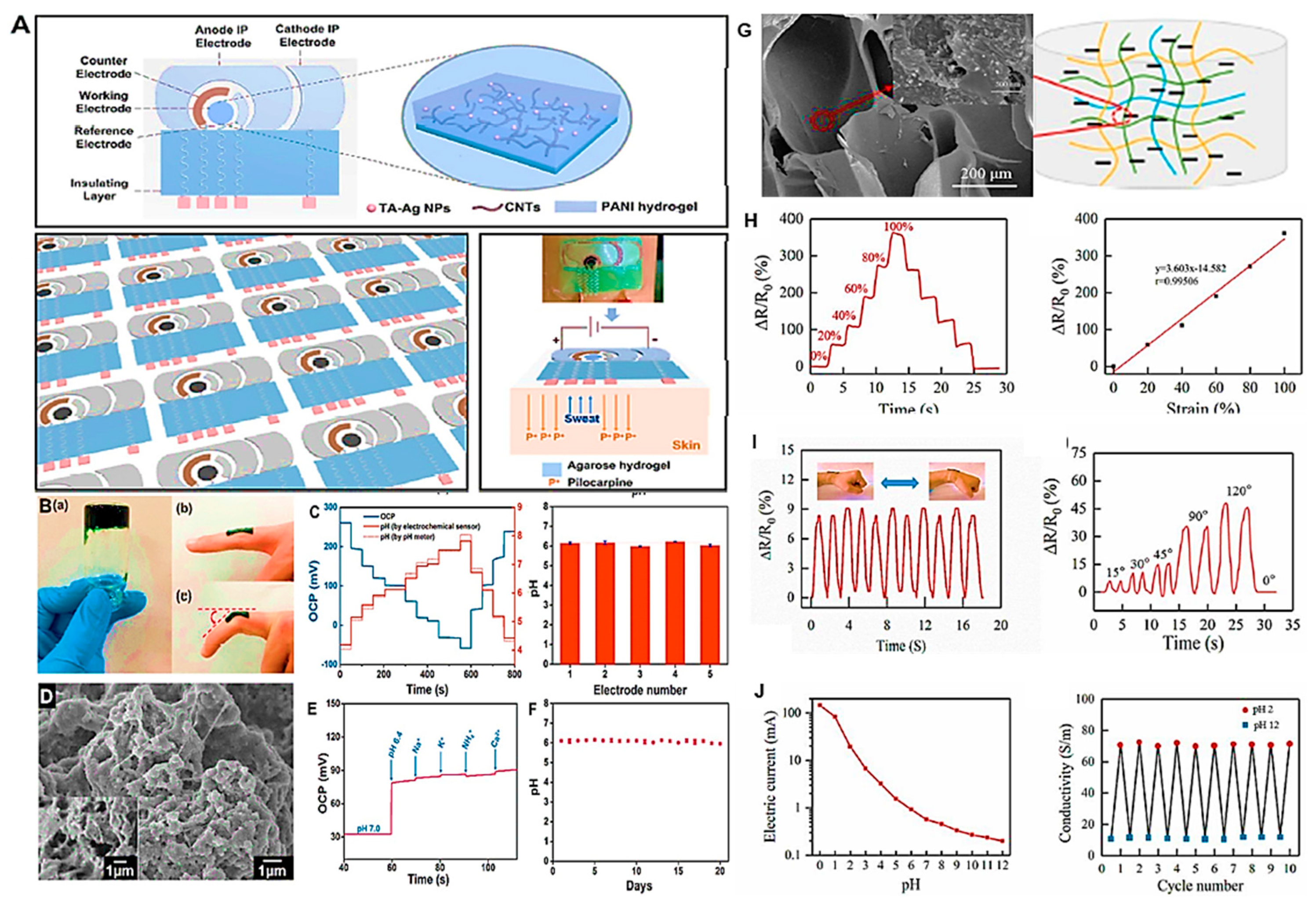
- Tang, Y.; Zhong, L.; Wang, W.; He, Y.; Han, T.; Xu, L.; Mo, X.; Liu, Z.; Ma, Y.; Bao, Y. Recent advances in wearable potentiometric pH sensors. Membranes 2022, 12, 504. [Google Scholar] [CrossRef]
- Yin, J.; Li, J.; Reddy, V.S.; Ji, D.; Ramakrishna, S.; Xu, L. Flexible textile-based sweat sensors for wearable applications. Biosensors 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced wound dressing for real-time pH monitoring. ACS Sensors 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Han, Z.; Yuan, M.; Liu, L.; Zhang, K.; Zhao, B.; He, B.; Liang, Y.; Li, F. pH-Responsive wound dressings: advances and prospects. Nanoscale Horizons 2023, 8, 422–440. [Google Scholar] [CrossRef]
- Chawang, K.; Bing, S.; Chiao, J.-C. Printable and flexible iridium oxide-based pH sensor by a roll-to-roll process. Chemosensors 2023, 11, 267. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Tai, L.-C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M. A wearable microfluidic sensing patch for dynamic sweat secretion analysis. ACS Sensors 2018, 3, 944–952. [Google Scholar] [CrossRef]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. A wearable sensor based on multifunctional conductive hydrogel for simultaneous accurate pH and tyrosine monitoring in sweat. Biosensors and Bioelectronics 2023, 234, 115360. [Google Scholar] [CrossRef]
- Zhan, T.; Xie, H.; Mao, J.; Wang, S.; Hu, Y.; Guo, Z. Conductive PNIPAM/CMCS/MWCNT/PANI hydrogel with temperature, pressure and pH sensitivity. ChemistrySelect 2021, 6, 4229–4237. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Li, Z.; Hu, M.; Bian, C.; Lin, S. All fabric and flexible wearable sensors for simultaneous sweat metabolite detection and high-efficiency collection. Talanta 2023, 260, 124610. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, F.; Liu, G.; Lin, H.; Bao, Y.; Han, D.; Wang, W.; Ma, Y.; Zhang, B.; Niu, L. Superhydrophobic functionalized Ti3C2T x MXene-based skin-attachable and wearable electrochemical pH sensor for real-time sweat detection. Analytical Chemistry 2022, 94, 7319–7328. [Google Scholar] [CrossRef]
- Demuru, S.; Kunnel, B.P.; Briand, D. Thin film organic electrochemical transistors based on hybrid PANI/PEDOT: PSS active layers for enhanced pH sensing. Biosensors and Bioelectronics: X 2021, 7, 100065. [Google Scholar]
- Fan, J.; Montemagno, C.; Gupta, M. 3D printed high transconductance organic electrochemical transistors on flexible substrates. Organic Electronics 2019, 73, 122–129. [Google Scholar] [CrossRef]
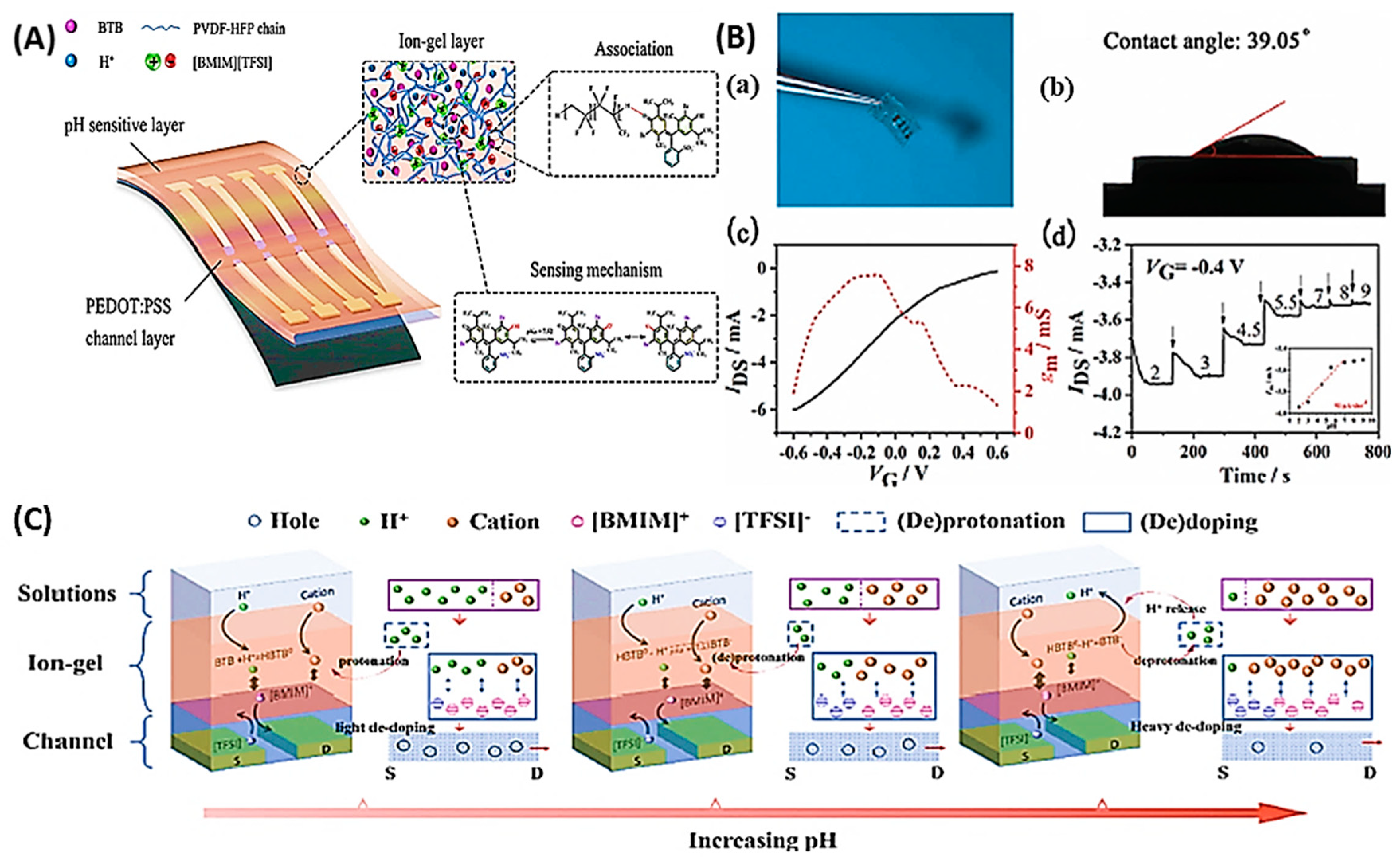
- Chen, X.; Ji, J.; Peng, Y.; Gao, Z.; Zhao, M.; Tang, B.; Liu, Y. Flexible pH sensors based on OECTs with a BTB dye-embedded ion-gel gate dielectric. Journal of Materials Chemistry C 2023, 11, 7722–7731. [Google Scholar] [CrossRef]
- Mariani, F.; Gualandi, I.; Tessarolo, M.; Fraboni, B.; Scavetta, E. PEDOT: dye-based, flexible organic electrochemical transistor for highly sensitive pH monitoring. ACS applied Materials Interfaces 2018, 10, 22474–22484. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Mastronardi, V.; Guido, F.; Algieri, L.; Qualtieri, A.; Fiammengo, R.; Rizzi, F.; De Vittorio, M. Wearable piezoelectric mass sensor based on pH sensitive hydrogels for sweat pH monitoring. Scientific Reports 2020, 10, 10854. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Fomina, N.; Johnson, C.; Rocznik, T.; Ahmad, H.; Staley, R.P.-A.; Weller, J.; Lang, C. Toward Rapid and automated immunoassays: using a localized electrochemical pH modulation platform to perform a single-step immunoassay. Analytical Chemistry 2022, 94, 13171–13180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).