1. Introduction
Cancer continues to be a major cause of death worldwide. Recently, large-scale genetic profiling in cancer has been utilized to identify additional therapeutic agents through genetic profiling.[
1] Consequently, prospective molecular profiling has emerged as an essential instrument in the diagnosis and treatment of cancer. Genomic alterations of the MET proto-oncogene receptor tyrosine kinase have been well studied in NSCLC. MET tyrosine kinase inhibitors (TKIs), which target MET exon 14 mutations, have been approved by the FDA.
TKIs are classified into three types based on their mechanism of action. Type I MET TKIs act as ATP competitors and target the ATP binding pocket of the active form of the MET receptor. There are two subtypes of Type I inhibitors: Ia inhibitors, such as crizotinib, which target the amino acid residue known as G1163, and Ib inhibitors, including capmatinib, tepotinib, and savolitinib, which strongly bind to the amino acid residue Y1230. Type II MET TKIs are also ATP competitors but bind to the inactive state of the MET receptor, inhibiting ATP-dependent activation by targeting the receptor's inactive form. We will focus on discussing savolitinib, which belongs to the Type Ib MET TKI class. Savolitinib specifically interacts with the Y1230 residue and effectively inhibits the activity of the MET receptor. By targeting this specific region, savolitinib successfully hinders the signaling pathway associated with MET activation.
2. HGF-MET signaling
2.1. HGF-MET signaling
Mesenchymal epithelial transition factor (MET), also known as c-MET or the Hepatocyte Growth Factor (HGF) receptor, is a tyrosine kinase receptor that is typically expressed in various cell types, including epithelial cells, endothelial cells, neurons, hepatocytes, and hematopoietic cells [
1,
2,
3,
4,
5]. MET, along with its ligand HGF, plays a crucial role in multiple cellular processes, including cell proliferation, motility, morphogenesis, angiogenesis, tissue regeneration, and the transition from epithelial to mesenchymal cells. It is also involved in wound healing, normal liver development, embryonic placental development, as well as the development of neurons and muscles [
6,
7,
8].
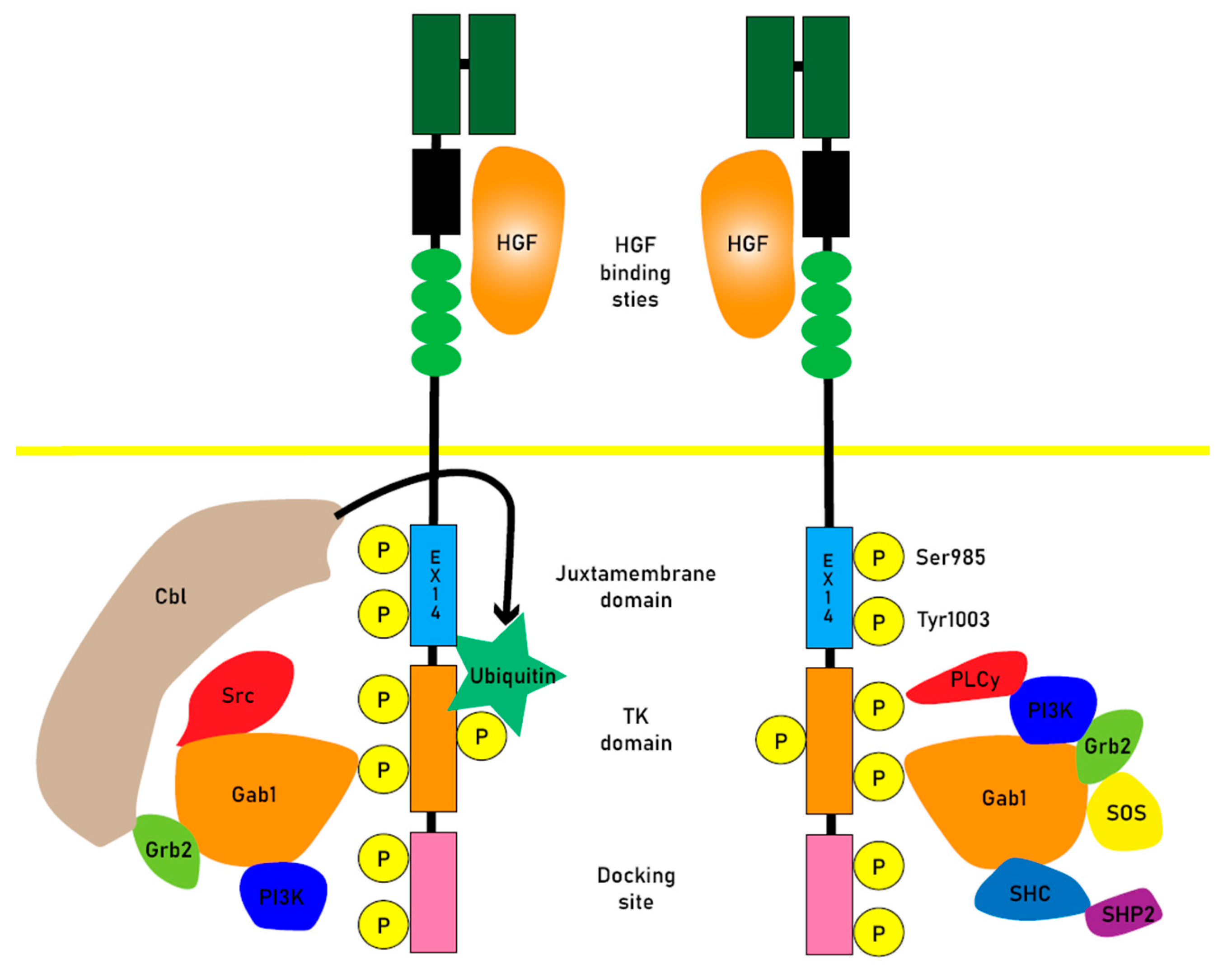
HGF is the ligand for MET, and its binding induces the dimerization of MET receptors and the subsequent phosphorylation of specific tyrosine residues, called Tyr1230, Tyr1234, and Tyr1235, within the kinase domain. This phosphorylation creates a docking site for proteins involved in RTK-mediated signal transduction, leading to the activation of downstream signaling pathways[
9,
10]. One of the downstream signaling pathways activated by MET involves the attachment of phosphorylated GAB1, which further recruits docking molecules and enzymes, including PI3K, CRK-like protein (CRKL), and SRC homology 2 domain-containing phosphatase 2 (SHP2). These activated signaling pathways include phosphoinositide 3-kinase (PI3K)/AKT (protein kinase B), mitogen-activated protein kinase (MAPK), and NF-kB[
11,
12,
13].
HGF-bound MET receptors are normally ubiquitination and they are internalized via endocytosis, they are either degraded or recycled back to the plasma membrane. Consequently, dysregulation of the MET signaling system is associated with various malignancies. Aberrant receptor trafficking, degradation, or disrupted recycling can result in sustained signaling, contributing to cellular transformation, oncogenesis, and metastasis.
2.2. MET mutation
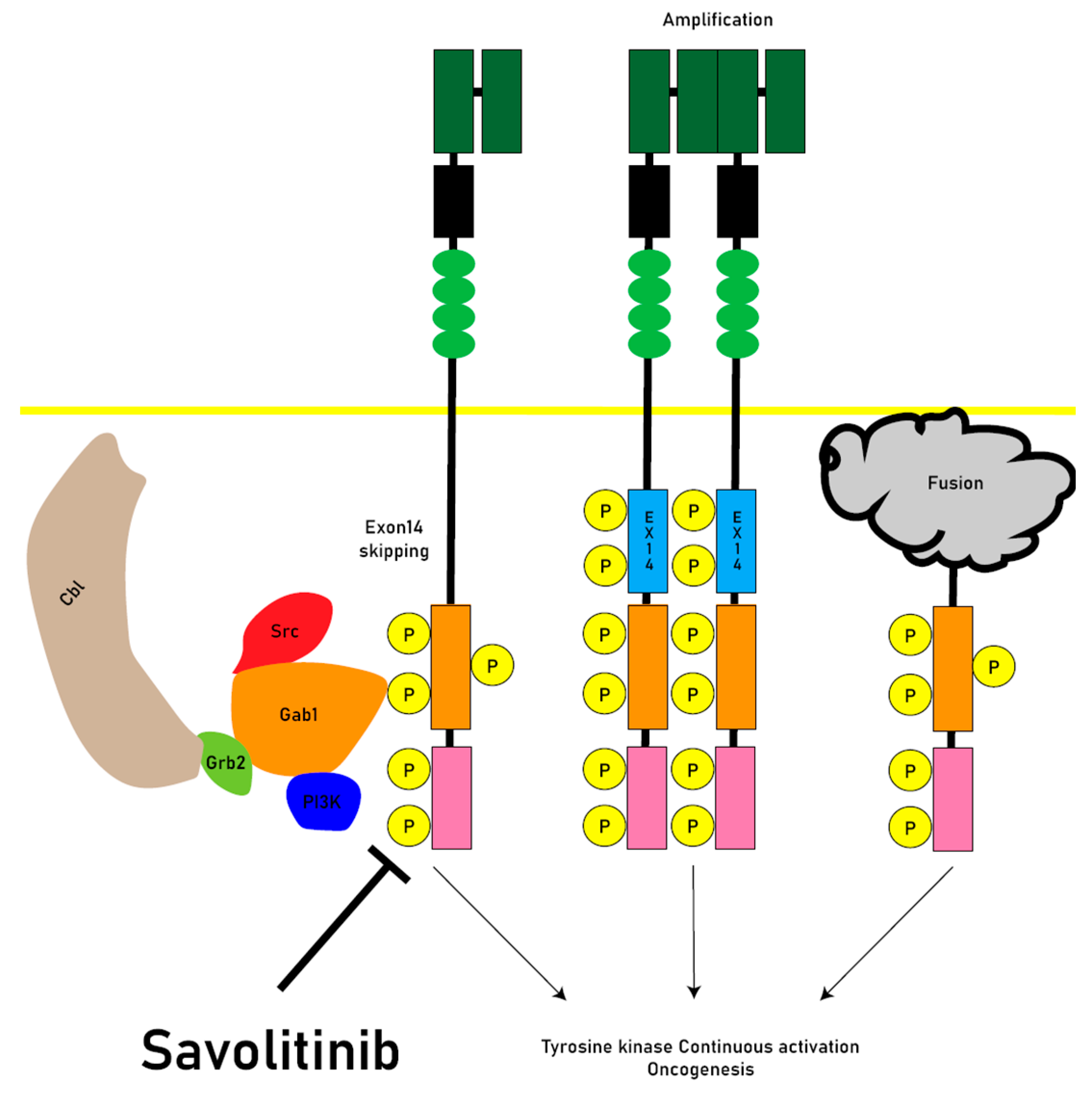
Abnormalities in the MET signaling system can arise from different mechanisms, including gene amplification, receptor protein overexpression, exon 14 junctional membrane skipping mutations (METex14) and MET gene chromosomal fusions[
14]. The association between MET mutations and cancer development was initially observed in hereditary and sporadic forms of papillary renal cell carcinoma. There were somatic missense mutations in the tyrosine kinase (TK) domain of the MET gene. These mutations are typically gain-of-function mutations[
15].
Juxtamembrane domain (JMD)-deleted MET was generated by exon 14 skipping (METex14) due to intronic mutations. The expression of MET-exon14 variants in cells disrupts the association with CBL E3 ubiquitin ligase, reducing ubiquitination and prolonging the activation of signaling molecules. The phosphorylation of MET-Y1003 in the juxtamembrane domain is typically involved in CBL binding for ubiquitination. Therefore, MET-exon14 variants may have increased protein stability and enhanced signal transduction, contributing to oncogenesis[
16,
17](
Figure 1).
Abnormal activation of the MET pathway in tumor tissue, including MET gene overexpression, gene amplification, exon14 skipping, and other activating mutations (
Figure 2), is associated to shorter survival and poor prognosis. MET amplification (METAmp) and overexpression are frequently observed genomic abnormalities in solid tumors, occurring in advanced stages of tumorigenesis and exacerbating the malignant properties of transformed cells. MET participates in crucial cellular processes such as proliferation, differentiation, and the formation of distant metastases[
18].
MET exon14 skipping has been recognized as a true oncogenic driver and MET tyrosine kinase inhibitors (TKIs) targeting METex14 skipping have shown significant improvements in clinical outcomes, including response rates and progression-free survival. Studies investigating the HGF/MET pathway have been conducted in various cancer types, including NSCLC, breast cancer, head and neck cancer, colorectal cancer, gastric cancer, pancreatic cancer, and other gastrointestinal cancers. Genetic alterations in the MET gene and pathway are common in solid tumors [
18].
MET overexpression is indeed believed to be one of the early dysregulations in the process of carcinogenesis. In the presence of hypoxia and inflammation, MET receptors are transcriptionally upregulated, leading to their overexpression. This overexpression contributes to tumorigenesis by promoting cell proliferation, inhibiting apoptosis, and enhancing cell migration. MET overexpression has been observed in various types of cancer, including epithelial, mesenchymal, and hematologic cancers. Additionally, MET can also be overexpressed in cancers with an activated genomic signature, including those with primary and/or secondary MET amplifications or METex14 skipping mutations.
2.3. Oncogenesis
During the characterization of HGF as a fibroblast-secreted protein that promotes motility and matrix invasion of epithelial cells, the induction of invasiveness into collagen by HGF was observed. HGF as a factor derived from fibroblasts, plays a role in facilitating the aggressive invasion of cancer cells. The microenvironment of metastatic tumors plays a crucial role in metastatic colonization and growth. Various stromal cells, including inflammatory cells, endothelial cells, and fibroblasts, contribute to the formation of the metastatic microenvironment. The function of HGF as a stromal cell-derived factor influences cancer cell invasiveness within the tumor microenvironment. Inhibiting HGF activity has been shown to prevent invasion induced by stromal fibroblasts.
Additionally, MET receptors present in exosomes have been found to promote the formation of a metastatic microenvironment in metastatic melanoma[
19]. Exosomes derived from highly metastatic mouse and human melanoma cells contain high levels of MET receptors. These circulating exosomes localize to metastatic tissue sites and increase vascular permeability, thereby promoting tumor cell migration. Moreover, exosomes contribute to the activation of MET in bone marrow-derived cells, leading to their reprogramming into an angiogenic phenotype. These bone marrow-derived cells migrate to the lungs and can contribute to angiogenesis, invasion, and metastasis. Administration of exosomes with prominent levels of MET receptors promotes metastasis of melanoma cells that originally had low metastatic capacity[
20].
3. Savolitinib
3.1. Introducing savolitinib
Savolitinib is an oral medication classified as a selective MET inhibitor. It has been specifically developed for the treatment of several types of cancers, including non-small cell lung cancer (NSCLC), breast cancer, head and neck cancer, colorectal cancer, gastric cancer, pancreatic cancer, and other gastrointestinal cancers. preclinical studies have shown that savolitinib exhibits superior efficacy in these cancer types [
21].
3.2. Metabolism
Savolitinib is rapidly absorbed. Absolute oral bioavailability was 69%, median maximum observed concentration was 3.5 hours, and mean terminal half-life was 6.1 hours. 56% was found in urine and 38% in feces. Approximately 3% of the administered dose was excreted as unmetabolized savolitinib in the urine. savolitinib has moderate tissue distribution, low to moderate clearance, and low accumulation. Most of the elimination of savolitinib occurs through metabolism via multiple pathways. Hepatic oxidative metabolism followed by urinary and biliary excretion were the major elimination pathways. The concentration at which half of the maximal Hs746t tumor reduction by savolitinib was achieved and the IC50 for cMet inhibition were equal to 12.5 and 3.7 nM (free drug), respectively [
22]. There was a drug-drug interaction reported that co-administration of rifampicin significantly reduced exposure to savolitinib compared to savolitinib alone [
23].
3.3. Side effects and safety
Savolitinib demonstrated a well-tolerated safety profile consistent with previous clinical trials. Most adverse events were of grade 1 or 2 and resolved with dose adjustment or discontinuation. The most common treatment-related adverse events (TRAEs) were peripheral edema (56%), nausea (46%), and aminotransferase elevations (38%). The highest treatment-related grade 3 adverse event was AST elevation (13%)[
24]. Common serious adverse events (SAEs) reported included hepatic dysfunction (4.3%), drug hypersensitivity reactions (2.9%), and pyrexia (2.9%). There was one treatment-related fatal SAE reported, specifically tumor lysis syndrome. It is worth noting that pulmonary interstitial pneumonia and interstitial lung disease (ILD) did not occur with savolitinib, while ILD was observed with other drugs such as tepotinib and capmatinib.
Savolitinib had a well-tolerated safety profile consistent with previous clinical trials, with most adverse events being grade 1-2 and resolved with dose adjustment and discontinuation. The most common treatment-related adverse events (TRAEs) were Common SAEs reported were hepatic dysfunction (4.3%), drug hypersensitivity reactions (2.9%), and pyrexia (2.9%). A treatment-related fatal SAE, tumor lysis syndrome, was reported in one patient. Pulmonary interstitial pneumonia and interstitial lung disease (ILD) did not occur with savolitinib, whereas ILD was observed with tepotinib and capmatinib.
Savolitinib has been associated with the side effect of QTc prolongation, which refers to a lengthening of the QT interval on an electrocardiogram (ECG). In a study specifically examining QT/QTc, a single dose of 600 mg of savolitinib resulted in the highest root mean square ΔΔQTcF (change in QTc interval corrected for heart rate) of 12 milliseconds, observed 5 hours after administration. However, savolitinib did not have any significant effects on other ECG intervals such as PR, QRS, QT, or RR intervals. The study found that QTcF prolongation occurred with a single dose of 600 mg of savolitinib, and a lesser increase of 5 milliseconds was noted with a dose of 300 mg. As a result, ongoing and future clinical trials involving savolitinib will include ECG monitoring to assess the clinical relevance of these observed QT changes [
25,
26].
One case reported septic shock-like symptoms when savolitinib was used in an HIV-1 patient with NSCLC [
27].
3.4. In vivo and xenograft study
Savolitinib has been shown to inhibit the growth of gastric cancer cell lines in vitro studies. Additionally, in vivo studies, it has demonstrated anti-tumor activity in models of gastric cancer with MET amplification and papillary renal cell carcinoma (PRCC)[
28]. A pharmacokinetic-pharmacodynamic (PK-PD) model conducted by Jones et al. found that savolitinib effectively inhibited the activity of phospho-MET, a protein associated with cancer in a xenograft mouse model using human lung and gastric cancer cells lines[
22].
3.5. First in human phase I trial
A phase 1 clinical trial was conducted in Australia (NCT01773018), involving 48 patients with locally advanced solid tumors. Savolitinib was administered at various doses, with a maximum tolerated dose of 800 mg. The trial showed preliminary effectiveness of savolitinib in patients with papillary renal cell carcinoma who had MET gene copy number alterations. The most common adverse events reported were nausea (63%), vomiting (42%), fatigue (35%), and peripheral edema (27%). Savolitinib was considered tolerable, and the recommended phase 2 dose was established at 600 mg daily[
29,
30].
In another phase 1 clinical trial conducted in China (NCT019855), involving patients with progressive tumors, and MET mutations, savolitinib demonstrated a manageable safety profile and promising anti-tumor activity. Although no partial response was achieved, there was a significant reduction in tumor size in some lesions (55% and 27%). The most common treatment-related side effects included nausea (29.4%), vomiting (27.1%), and peripheral edema (21.2%). The recommended phase 2 dose of savolitinib in this trial was set at 600 mg once daily or 500 mg twice daily. There were similarities between the patients enrolled in the phase 1 clinical studies conducted in Australia, allowing for comparative analysis of the results[
29,
30].
3.6. Pivotal phase 2 trial
A pivotal Phase 2 trial (NCT02897479) was conducted in China to assess the efficacy and safety of savolitinib in patients with non-resectable or metastatic non-small cell lung cancer (NSCLC) carrying the METex14 mutation. The trial included 70 patients who received savolitinib monotherapy until disease progression or unacceptable toxicity. The daily dosage ranged from 400 mg (less than 50kg) to 600 mg(more than 50kg) based on patient weight. Most enrolled patients were elderly and had advanced NSCLC with prior systemic treatment.
The trial analyzed the data using the Independent Review Committee (IRC) and investigator assessments in the full analysis set (FAS) and tumor response evaluable set (TRES). Among the TRES patients evaluated by the IRC, there were 62 patients. Most patients in the FAS had an Eastern Cooperative Oncology Group (ECOG) performance status of 1. Subset analyses were performed comparing different NSCLC subtypes and treatment experiences.
With a median follow-up duration of 28.4 months, the trial demonstrated encouraging efficacy results. The median overall survival (OS) was 12.5 months, with 18-month and 24-month OS rates of 42.1% and 31.5%, respectively. Pretreated patients had a median OS of 19.4 months, while treatment-naive patients had a median OS of 10.9 months. Patients with primary squamous cell carcinoma (PSC) had a median OS of 10.6 months, while other NSCLC subtypes had a median OS of 17.3 months. Patients with brain metastases had a median OS of 17.7 months. No new safety concerns were observed with prolonged follow-up and exposure, indicating that savolitinib had an acceptable safety profile [
31,
32,
33].
3.7. Phase 3 trials on the way
Currently, there are four phase 3 clinical trials investigating the efficacy and safety of savolitinib in various treatment settings for NSCLC patients.
Phase 3b Study (CTR20211151) aims to evaluate the efficacy and safety of savolitinib in two cohorts of locally progressive or metastatic NSCLC patients with METex14 mutations in China. One cohort includes patients who have previously received platinum-based chemotherapy but experienced disease progression or intolerable toxicity, while the other cohort consists of patients who have not received systemic chemotherapy for advanced disease. Patients in both cohorts are treated with savolitinib until disease progression or intolerable toxicity.
Phase 3 Clinical Trial SACHI (CTR20211441) that is randomized, two-arm, open-label, multi-organ study is being conducted in China. It aims to evaluate the efficacy and safety of savolitinib in combination with osimertinib (an EGFR-TKI) compared to chemotherapy in Chinese patients with MET-amplified NSCLC who developed the disease after treatment with 1st to 3rd generation EGFR-TKIs. This trial has already started recruiting patients at multiple centers.
SAFPRON Phase 3 Clinical Trial focuses on advanced NSCLC patients worldwide with advanced MET amplification or c-MET overexpression after treatment with osimertinib. It aims to evaluate the efficacy and safety of the combination therapy of savolitinib plus osimertinib compared to chemotherapy.
SANOVO Phase 3 Clinical Study (NCT05009836) is evaluating the efficacy and safety of the combination therapy of savolitinib and osimertinib in patients with EGFR mutation-positive NSCLC and c-MET overexpression who have not received prior treatment.
3.8. Pivotal phase 2 trial
There have been studies in which MET TKI (especially in NSCLC with MET exon 14 skip mutation) can be seen as a combination therapy of epidermal growth factor receptor (EGFR) TKI [
34].
3.8.1. Savolitinib + Osimertinib
The combination of savolitinib and osimertinib has shown promising results in preclinical studies and clinical trials for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutation, MET amplification, or c-MET overexpression.
In preclinical models, the combination of savolitinib and erlotinib (a first-generation EGFR-TKI) demonstrated significant tumor suppressive effects in NSCLC cell line models with MET amplification. The combination of savolitinib and osimertinib showed superior anti-tumor activity compared to monotherapy in NSCLC models with EGFR mutation, MET amplification, and activated MET[
35].
Phase 1 Study (TATTON, Part A): The combination of savolitinib and osimertinib was evaluated in patients with advanced NSCLC who had previously progressed on EGFR-TKI treatment. The study demonstrated safety and resistance of Osimertinib and Savolitinib in patients with advanced NSCLC (n = 18) who had previously advanced disease after EGFR-TKI treatment[
36,
37]. The dose of savolitinib was increased from OD 600 mg to 800 mg with Osimertinib 80 mg as a fixed dose. The study demonstrated promising anti-tumor activity with an objective response rate (ORR) of 44%. Extended cohorts of the TATTON study evaluated the combination of savolitinib and osimertinib in patients inclduing MET-amplified and EGFR mutant-positive NSCLC. The ORR was 49% in patients with MET amplification and EGFR mutation. The combination therapy showed a manageable safety profile[
38].
Phase 2 Study (SAVANNAH) evaluated the efficacy of savolitinib in combination with osimertinib in patients with EGFR mutation and MET amplification and/or c-MET overexpression. The overall response rate (ORR) was 32%, and the median duration of response was 8.3 months. Patients with high MET amplification or high threshold c-MET overexpression showed better efficacy [
39].
Ongoing Trials: The FLOWERS trial is investigating the efficacy and safety of osimertinib in patients with novel MET amplification and/or c-MET overexpression, with or without savolitinib. The SAFFRON trial is comparing the combination of savolitinib and osimertinib with platinum-based chemotherapy in patients with NSCLC after osimertinib treatment[
40].
A phase 3 trial SAFFRON trial(NCT05261399) is underway to compare savolitinib and Osimertinib combination therapy with platinum-based double chemotherapy in patients with disease-induced NSCLC (EGFR mutation, c-MET overexpression, and/or MET gene amplification) after Osimertinib treatment.
Overall, the combination of savolitinib and osimertinib has shown promising anti-tumor activity and manageable safety profiles in patients with EGFR mutation, MET amplification, or c-MET overexpression NSCLC.
3.8.2. Savolitinib + Gefatinib
A phase 1 clinical study (NCT02374645) conducted in China evaluated the combination of savolitinib and gefitinib (a first-generation EGFR-TKI) in patients with advanced disease EGFRm and MET amplification NSCLC. The study demonstrated promising anti-tumor activity. Two different doses of savolitinib, 600 milligrams and 800 milligrams, were administered along with 250 milligrams of gefitinib. The objective response rate (ORR) for patients with EGFR T790M negative and positive status was 52% and 9% respectively. Overall, the daily combination of savolitinib 600 mg and gefitinib 250 mg showed acceptable safety profiles and promising anti-tumor activity in patients with advanced disease EGFRm and MET amplification NSCLC who had received previous EGFR-TKI treatment[
41].
3.8.3. Savolitinib + Docetaxel
In a phase I trial, it was suggested that the combination of savolitinib at a dose of 600 mg once daily with docetaxel at a dose of 60 mg/m2 would be the recommended phase II dose. This combination therapy showed highly encouraging anti-tumor effects and resulted in sustained responses among patients with MET-amplified Gastric cancer in the subsequent phase II clinical trial [
42].
3.8.4. Savolitinib + Durvalumab
The combination therapy of savolitinib and durvalumab showed remarkable effectiveness in overcoming drug resistance and demonstrated a high clinical response rate in exploratory MET-based metastatic papillary kidney cancer. Durvalumab has side effects such as rash (48%), vomiting (43%), and diarrhea (39%) [
43].
The ongoing SOUND Clinical Trial (NCT05374603) is an open-label, multicenter, exploratory clinical trial that aims to investigate the combination therapy of savolitinib and durvalumab in Chinese patients with EGFR wild type topical or metastatic NSCLC who have MET mutations. The trial will include 30 patients with MET amplification and 30 patients with METex14 mutation. These patients will receive treatment with 1500 mg of durvalumab and 300 to 600 mg of savolitinib (once daily) for a 28-day treatment cycle. The treatment will continue until disease progression, death, or occurrence of toxicity. The trial is currently ongoing.
4. Resistance
Resistance to MET-tyrosine kinase inhibitors (TKIs) is inevitable. Previous studies have suggested that savolitinib resistance in NSCLC is driven in part by MYC overexpression in H1993 cells. [
44].
According to Melanie M. et al, the patient with gastric cancer who received savolitinib maintained a partial response (PR) based on radiologic and clinical assessments. Initially, the patient's ctDNA analysis at baseline revealed the presence of the TP53 P190L mutation with an allele frequency of 44%, along with MET copy number of 3.0 and MYC copy number of 5.6. In addition, new observations detected low frequencies of MET D1228H (5%), MET D1228N (5%), MET D1228V (35%), and MET Y1230C (3%). However, after 3.5 months of treatment with savolitinib, the patient experienced rapid disease progression. The patient's ctDNA analysis revealed persistently low MET and MYC copy numbers compared to baseline. During the PR phase, the allele frequencies of MET D1228H increased to 31%, while MET D1228N increased to 12% compared to the ctDNA samples collected at that time. In the progression phase, the presence of MET D1228V (1%) and MET Y1230C (1%) mutations was also observed[
45].
A 74-year-old woman with gastric cancer and extensive liver metastases experienced rapid disease progression following TS-1 chemotherapy. She was then treated with savolitinib. Initially, ctDNA analysis during the partial response (PR) phase showed no MET amplification. However, after four cycles of savolitinib, her PR was confirmed radiologically, accompanied by a slight increase in MET copy number (2.7) and the emergence of a TP53 G245D variant with a 2% allele frequency in ctDNA. The tumor size decreased by 83.5% compared to baseline. Unfortunately, after six months of treatment, radiologic progression occurred. The patient exhibited high levels of MET amplification (13 copies) and a CDK6 copy number of 3.9 at the time of disease progression. Notably, there was strong concordance between the ctDNA and tumor tissue DNA derived from liver metastasis. Both samples displayed a resurgence of MET and CDK6 amplification, which was not detected during the PR phase. Additionally, the ctDNA sample obtained at the time of progression indicated the expansion of a clone containing the TP53 G245D substitution, evidenced by an increase in allele fraction from 2% (at the third follow-up) to 22% (at progression)[
45].
5. Biomarker
Several studies have demonstrated the predictive value of MET biomarkers in identifying patients who will benefit most from HGF/MET-targeted therapies administered as monotherapy or in combination[
46]. Inflammatory mediators, including interleukin-1-alpha (IL-1alpha), IL-1b, tumor necrosis factor-alpha, and prostaglandin E2, increase the gene expression of HGF in stromal cells. It is likely that these inflammatory mediators are involved in the upregulation of HGF in tumors because they are increased in the tumor microenvironment and contribute to a drug-resistant and/or metastatic tumor microenvironment [
47]. In addition, MET gene amplification and/or protein overexpression is frequent in cancer, accelerating research on intratumoral MET gene copy number or circulating soluble DNA, intratumoral MET protein content and phosphorylation (activation) status[
48].
The VIKTORY (targeted agent eValuation In gastric cancer basket KORea) trial is the first and largest platform study in gastric cancer, supporting both the validity and clinical utility of tumor profiling. The study was designed to stratify patients with metastatic gastric cancer based on clinical sequencing and assign patients to one of 10 second-line treatment-related clinical trials, focusing on eight biomarker groups (RAS aberrations, TP53 mutation, PIK3CA mutation/amplification, MET amplification, MET overexpression, all negative, TSC2 deficiency, and RICTOR amplification). The study showed that treatment cohorts assigned to biomarkers had encouraging response rates and survival compared to conventional second-line chemotherapy. Analysis of circulating tumor (ctDNA) showed a good correlation between high MET copy number by ctDNA and response to savolitinib [
49].
ctDNA biomarkers also allow longitudinal monitoring of clinical outcomes with savolitinib in patients with METex14-positive NSCLC and other NSCLC subtypes. Specifically, undetectable baseline METex14 or post-treatment clearance may predict favorable clinical outcomes, and secondary MET mutations and other acquired genetic alterations may explain resistance to savolitinib[
50].
6. Non-Small Cell Lung Cancer (NSCLC)
6.1. Non-Small Cell Lung Cancer
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of all lung cancers, with considerable heterogeneity and may be associated with some known and/or unknown causative gene changes. difficult. NSCLC has a very poor prognosis, with significantly lower overall survival (OS) and 5-year survival rates compared to other types of lung cancer.
NSCLC is increasingly treated with targeted therapies. savolitinib is a highly selective mesenchymal epithelial transition (MET)-tyrosine kinase inhibitor (TKI) for advanced NSCLC with MET exon 14 skipping mutation (METex14). MET exon 14 skipping mutation is most common in NSCLC. Mutations resulting in the loss of exon14 in the MET gene lead to dysregulation and inappropriate signaling, which is associated with increased responsiveness to MET TKIs [
51,
52].
MET genomic amplification is indeed more common in METex14 non-small cell lung cancer (NSCLC), with reported frequencies of 15-21%. MET amplification occurs about 4% in lung adenocarcinoma and 1% in squamous cell lung cancer. The overall incidence of METex14 mutation in NSCLC is 3% to 4%. with untreated NSCLC or previously treated molecularly driven NSCLC, such as epidermal growth factor receptor (EGFR), and acts as a mechanism of acquired resistance. Adenocarcinoma has a relatively higher incidence than squamous cell lung cancer (1.5% - 2.0%). Pulmonary sarcomatoid carcinoma has a 20-30% METex14 alteration[
53]. The METex14 mutation itself occurs with higher frequency in Caucasian patients compared to Asian patients, and it is more frequently observed in smokers or ex-smokers. However, it is worth noting that approximately one-third of patients with METex14 NSCLC are never smokers. Furthermore, the METex14 mutation is more commonly found in women than in men. In general, METex14 NSCLC tends to occur in older individuals, with a median age of 74, which is higher than NSCLC cases with other molecular drivers [
54]. Data from retrospective studies have suggested attenuated activity of immune checkpoint inhibitors in patients with METex14 skipping mutation, independent of expression of programmed death ligand-1 (PD-L1) [
55].
Savolitinib has demonstrated efficacy in first- and second-line settings, including patients with NSCLC and aggressive pulmonary sarcomatoid carcinoma, and has an acceptable safety profile. It can also cross the blood-brain barrier and has demonstrated some activity against central nervous system metastases[
33]. Patients with exon 14 skipping NSCLC had higher response rates than patients with overexpressed or amplified MET protein and had higher rates of intracranial disease control. Therefore, savolitinib may become a new standard of care to address MET dysregulation in patients with advanced or metastatic NSCLC, even those with brain metastases[
56,
57].
6.2. Neoadjuvant therapy in NSCLC
Savolitinib has been shown to be effective in neoadjuvant chemotherapy for lung cancer, specifically in cases of METex14m-positive locally advanced primary lung adenocarcinoma. In one case, a patient received 5 weeks of neoadjuvant treatment with savolitinib and experienced a significant reduction in tumor burden and lymph node size. Subsequently, a successful lobectomy and lymph node dissection was performed, resulting in a pathological response of 50% and a post-operative pathological staging of pT1cN0M0, IA3[
58].
In another study, three patients with locally advanced, unresectable non-small cell lung cancer (NSCLC) received induction therapy with savolitinib as a first or second line treatment after their disease had progressed following pre-operative chemotherapy. All three patients experienced a significant reduction in tumor size, with previously unresectable tumors becoming resectable after treatment with savolitinib[73]. In a separate case, a 76-year-old male patient with resectable stage IIIB lung adenocarcinoma harbouring the METex14 mutation was successfully treated with savolitinib. After neoadjuvant savolitinib, the primary tumor shrank by 82% and only 5% of the tumor remained viable at the time of subsequent radical surgery[
59,
60].
Another case report described a patient with METΔex14 mutant NSCLC who was initially unsure of intrapulmonary metastases and had recently undergone percutaneous coronary intervention for acute myocardial infarction. The patient received savolitinib at a dose of 600 mg once daily and experienced significant tumor shrinkage. After six months, no metastatic lesions were found and the patient was diagnosed with early stage lung cancer. A radical tumor resection was performed and the patient recovered successfully[
61].
Based on these cases, savolitinib appears to be a valuable treatment strategy for patients with METΔex14 mutant NSCLC, particularly those who are not suitable candidates for surgery. It has demonstrated the potential to reduce tumor size, make previously unresectable tumors resectable and improve pathological responses in the neoadjuvant setting.
6.3. Lung sarcomatoid carcinoma
Pulmonary sarcomatoid carcinoma (PSC) is a rare and aggressive subtype of non-small cell lung cancer (NSCLC) that accounts for a small percentage of primary lung cancers. PSC is characterised by poor differentiation and a more aggressive behaviour compared to other types of lung cancer. Survival rates for PSC are generally low, and patients with advanced disease have poor outcomes, including survival rates of less than 5% and shorter survival times.[
53,
62].
Recent studies have focused on gene mutations associated with PSC, particularly in the MET proto-oncogene. The most common and well-studied mutation is the exon 14 skipping mutation (METex14), which is found in a significant proportion of PSC cases. In addition, amplification and overexpression of the MET gene have been observed in a smaller percentage of patients. These molecular alterations have important implications for targeted therapies in PSC[
53].
A Phase 2 clinical trial is underway to evaluate a treatment specifically targeting the METex14 mutation in patients with locally advanced or metastatic NSCLC, including those with sarcomatoid histology. Preliminary data from the trial, involving 70 patients, demonstrated promising results. The treatment showed a notable objective response rate (tumor reduction) of 47.5% and a median progression-free survival of 6.8 months. Importantly, these positive outcomes were observed in patients who had not received prior MET inhibitor therapy.
7. Renal cell carcinoma
Papillary renal cell carcinoma (PRCC) is the most common subtype of non-clear cell renal cell carcinoma and has a poor prognosis, particularly in advanced stages [
28]. ince some cases of PRCC are driven by the MET gene, targeting MET may be a promising therapeutic approach. Savolitinib, a highly selective MET-tyrosine kinase inhibitor, has demonstrated anti-tumor activity in patients with PRCC. In previous studies, MET-driven PRCC has shown better treatment response compared to MET-independent PRCC [
63,
64].
The SAVOIR phase 3 clinical trial, a multicenter study, evaluated the efficacy of savolitinib compared to sunitinib, a standard treatment for advanced PRCC. The trial enrolled 60 patients, with most having chromosome 7 gain and no prior therapy. However, due to the availability of external data on progression-free survival (PFS) with sunitinib in patients with MET-driven disease, enrollment in the study was closed prematurely. Preliminary results showed that savolitinib had numerically greater median PFS, overall survival (OS), and objective response rate (ORR) compared to sunitinib. However, the difference in median PFS between the two groups (7.0 months for savolitinib and 5.6 months for sunitinib) was not statistically significant. Grade 3 or higher adverse events (AEs) were less frequent in the savolitinib group compared to the sunitinib group, and fewer dose modifications related to AEs were required. After discontinuation of treatment, a higher proportion of patients in the savolitinib group received subsequent anticancer therapy. Although the study had limited patient numbers and follow-up, savolitinib demonstrated promising efficacy and a more favorable safety profile compared to sunitinib in MET-driven PRCC. Further investigation is needed to determine the potential of savolitinib as a treatment option for MET-driven PRCC [
64].
8. Gastric cancer
The phase 2 VIKTORY umbrella trial showed that in patients with metastatic and/or recurrent gastric adenocarcinoma, savolitinib monotherapy had an ORR of 50% (10/20) in the subset of gastric cancer patients with MET amplification. Further genomic analysis showed that patients with a MET GCN >10 by tissue next-generation sequencing had an ORR of 70% for savolitinib, inferring that the subset of patients with MET amplification experienced a greater absolute reduction in tumor burden [
49].
A case was reported of a 35-year-old man with advanced gastric cancer and bone, adrenal, and lumbar-2 vertebral metastases. The patient was resistant to chemotherapy, was in poor general condition, and had thrombocytopenia and anemia. NGS analysis revealed MET gene amplification in the tumor. After 39 days of daily treatment with 400 mg savolitinib, the patient achieved a partial response (PR) and both anemia and thrombocytopenia improved. No significant side effects were observed, and the patient remained progression free for 14 weeks [
65].
Another case showed that 31-year-old woman underwent total gastrectomy in 2013 for stage pT4N3M0 gastric cancer. The tumor was poorly differentiated tubular adenocarcinoma, and the patient was microsatellite stable and HER2-negative. Oophorectomy revealed MET IHC3+ and MET amplification confirmed by FISH. With savolitinib treatment, the patient experienced a significant reduction in tumor volume, achieving a partial response (PR) lasting six months. The maximum tumor diameter decreased by 47.7% compared to the baseline measurement. Genomic sequencing of ctDNA samples indicated that the patient had a non-shedding tumor, as no variants, including MET amplification or mutations, were detected across a 100-gene panel[
45].
The other case presented that a case study of a 47-year-old male with advanced gastric cancer, bone marrow invasion, and extensive metastases. The patient experienced severe pain, thrombocytopenia, and hemorrhagic anemia. Due to chemotherapy resistance, the patient underwent monotherapy with savolitinib. Savolitinib was administered based on the presence of MET gene amplification and rearrangement in the tumor. Following savolitinib treatment, the patient's condition improved significantly, achieving partial remission. At the time of reporting, the patient remained alive and free of disease progression for 15 weeks without any notable adverse reactions. Additionally, another case of a female gastric cancer patient with MET amplification who received savolitinib monotherapy as a third-line treatment. This patient also showed no disease progression for 12 weeks [
66].
9. Hepatocellular carcinoma
Systemic treatment of hepatocellular carcinoma (HCC) includes immune checkpoint inhibitors, bevacizumab, and other TKIs. It is not the primary option of choice due to poor overall response and median progression-free survival. A case has been reported of a patient with MET amplified HCC progressing after 3 months on bevacizumab and sintilimab and maintaining PR for more than 8 months with manageable adverse events with savolitinib. This suggests that savolitinib may be a therapeutic option in MET amplified HCC[
67].
10. Colorectal cancer
The phase II study (NCT03592641) is currently underway to determine how well savolitinib works in treating patients with MET-amplified colorectal cancer that cannot be removed by surgery (inoperable) or has metastasis. They have RAS wild type mCRC who have previously been treated with standard therapies. MET amp will be detected using a blood-based genomic profiling assay. Savolitinib may inhibit the growth of tumor cells by blocking some of the enzymes needed for cell growth. Patients receive oral savolitinib 600mg daily on days 1-28. Cycles are repeated every 28 days in the absence of disease progression or unacceptable toxicity. The study aims to estimate the objective response rate (ORR) of savolitinib in this patient population. Secondary objectives include evaluating progression-free survival, duration of response, safety, and tolerability. The study will also explore the correlation between tissue and blood-based biomarkers and clinical outcomes. Blood samples will be collected at baseline and during restaging to determine if savolitinib eliminates MET amp in circulating cfDNA[
68].
11. Pancreatic cancer
Pancreatic cancer, specifically pancreatic ductal adenocarcinoma (PDAC), is a highly aggressive illness known for its tendency to spread early. It is characterized by a dense and collagen-rich supportive tissue called desmoplasia or stroma, which is primarily generated by pancreatic stellate cells (PSCs). PSCs have a role in communicating with cancer cells and other stromal cells, thereby facilitating the advancement of the disease. One specific pathway involving growth factors that potentially enables this interaction is the hepatocyte growth factor (HGF)/MET pathway. HGF is produced by PSCs, while its receptor MET is present on pancreatic cancer cells and endothelial cells[
69]. The activation of the MET/HGF pathway is a consequence of the tumor microenvironment (TME) that supports tumor growth. The TME is a significant source of HGF and MET/HGF signaling influences the TME by directly affecting stromal cells expressing MET. Therefore, targeting the MET/HGF pathway could be an option for adjuvant therapy in pancreatic cancer. One of the reasons for chemotherapy resistance in PDAC is the extensive desmoplastic reaction surrounding the tumor, which creates a physical barrier that hinders drug penetration. In this context, reducing the metastatic potential of cancer cells and reprogramming the dysfunctional tumor microenvironment could have potential benefits for the treatment of pancreatic cancer.
There were studies of other MET TKI like as cabozantinib, crizotinib, capamatinib for pancreatic cancer has shown that studies suggest that TKIs such as savolitinib may be effective in pancreatic cancer. In 2013, Hage et al. examined the therapeutic potential of cabozatinib through in vitro studies. They observed that the effectiveness of gemcitabine, a commonly used chemotherapy drug, was enhanced even in pancreatic cancer (PC) cells that had developed resistance to high levels of gemcitabine. Additionally, they investigated patient-derived primary spheroidal cultures that were enriched in cancer stem cell markers and found that the agent demonstrated increased efficacy in these cultures as well[
70].
In vitro experiments showed a synergistic interaction between crizotinib and gemcitabine, as evidenced by reduced growth of primary PDAC cells. Similarly, in vivo experiments revealed a synergistic effect on primary tumor growth. However, the impact of this combination on metastatic spread remains unclear and requires further investigation[
71].
An in vivo study utilizing mouse models of PC, treatment with capamatinib demonstrated a reduction in the movement of PC cells. The treatment group exhibited a 30% lymph node involvement, whereas the control group had a higher 60% involvement, indicating a potential suppression of metastasis. Furthermore, the researchers investigated various PC cell lines (human and murine) to confirm MET expression and evaluate the cells' response to capamatinib future. They observed that inhibiting MET decreased the proliferation and migration of PC cells induced by hepatocyte growth factor (HGF)[
72].
Based on this, we believe that savolitinib, a MET-TKI targeting the MET/HGF pathway, may be beneficial in the treatment of pancreatic cancer and further studies may be conducted in the future.
12. Conclusions
With the development of NGS technology, MET-TKIs are being actively studied in addition to traditional targeting agents. Particularly, savolitinib as a small molecule, highly selective type Ib mesenchymal epithelial transition factor (MET) tyrosine kinase inhibitor, is developed for the treatment of non-small cell lung cancer with MET mutations. It has been developed for the treatment of non-small cell lung cancer, breast cancer, head and neck cancer, colorectal cancer, gastric cancer, pancreatic cancer, and other gastrointestinal cancers and has demonstrated good efficacy in preclinical and clinical studies.
In non-small cell lung cancer, savolitinib has shown promising anti-cancer activity in chemotherapy-resistant patients with single or combination therapy, and has also shown promise in kidney, gastric, liver, and pancreatic cancers. Interactions of the HGF/MET signaling pathway in the tumor microenvironment, we can expect savolitinb to be particularly effective in pancreatic cancer. The results of these preclinical studies are encouraging for further research, and savolitinib is expected to be a promising treatment in other types of cancer, including gastrointestinal cancers, where MET mutations are found.
Author Contributions
Conceptualization, T.S.L. and S.H.L.; validation, T.S.L. and S.H.L.; formal analysis, T.S.L.; investigation, T.S.L.; resources, T.S.L.; data curation, T.S.L.; writing—original draft preparation, T.S.L.; writing—review and editing, T.S.L.; visualization, T.S.L.; supervision, S.H.L.; project administration, T.S.L.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by AstraZeneca.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
AstraZeneca.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hammerman, P.S. , et al., Comprehensive genomic characterization of squamous cell lung cancers. Nature, 2012. 489(7417): p. 519-525.
- Collisson, E.A. , et al., Comprehensive molecular profiling of lung adenocarcinoma. Nature, 2014. 511(7511): p. 543-550.
- Govindan, R. , et al., Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell, 2012. 150(6): p. 1121-34.
- Rosell, R. , et al., Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol, 2012. 13(3): p. 239-46.
- Yang, J.C. , et al., Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol, 2015. 16(2): p. 141-51.
- Weidner, K.M. , et al., Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol, 1990. 111(5 Pt 1): p. 2097-108.
- Montesano, R. , et al., Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell, 1991. 67(5): p. 901-8.
- Bladt, F. , et al., Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature, 1995. 376(6543): p. 768-71.
- Weidner, K.M. , et al., Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature, 1996. 384(6605): p. 173-176.
- Birchmeier, C. , et al., Met, metastasis, motility and more. Nature Reviews Molecular Cell Biology, 2003. 4(12): p. 915-925.
- Sipeki, S. , et al., Phosphatidylinositol 3-kinase Contributes to Erk1/Erk2 MAP Kinase Activation Associated with Hepatocyte Growth Factor-induced Cell Scattering. Cellular Signalling, 1999. 11(12): p. 885-890.
- Zhang, Y.-W. , et al., Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene, 2002. 21(2): p. 217-226.
- Van Der Steen, N. , et al., cMET in NSCLC: Can We Cut off the Head of the Hydra? From the Pathway to the Resistance. Cancers, 2015. 7(2): p. 556-573.
- Zaborowska-Szmi, M. , et al., Savolitinib for non-small cell lung cancer. Drugs Today (Barc), 2023. 59(1): p. 17-36.
- Schmidt, L. , et al., Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet, 1997. 16(1): p. 68-73.
- Coleman, N. , et al., Targeting un-MET needs in advanced non-small cell lung cancer. Lung Cancer, 2022. 164: p. 56-68.
- Tong, J.H. , et al., MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non–Small Cell Lung Carcinoma with Poor Prognosis. Clinical Cancer Research, 2016. 22(12): p. 3048-3056.
- Safi, D., T. Abu Hejleh, and M. Furqan, Narrative review: mesenchymal-epithelial transition inhibitors-meeting their target. Transl Lung Cancer Res, 2021. 10(1): p. 462-474.
- Peinado, H. , et al., Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med, 2012. 18(6): p. 883-91.
- Matsumoto, K. , et al., Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci, 2017. 108(3): p. 296-307.
- Markham, A. , Savolitinib: First Approval. Drugs, 2021. 81(14): p. 1665-1670.
- Gu, Y. , et al., Preclinical pharmacokinetics, disposition, and translational pharmacokinetic/pharmacodynamic modeling of savolitinib, a novel selective cMet inhibitor. Eur J Pharm Sci, 2019. 136: p. 104938.
- Ren, S. , et al., Clinical evaluation of the potential drug-drug interactions of savolitinib: Interaction with rifampicin, itraconazole, famotidine or midazolam. Br J Clin Pharmacol, 2022. 88(2): p. 655-668.
- Cortot, A. , et al., Safety of MET Tyrosine Kinase Inhibitors in Patients With MET Exon 14 Skipping Non-small Cell Lung Cancer: A Clinical Review. Clin Lung Cancer, 2022. 23(3): p. 195-207.
- Sahota, T. , et al., A Randomized, Double-Blind, Placebo- and Positive-Controlled, Three-Way Crossover Study in Healthy Participants to Investigate the Effect of Savolitinib on the QTc Interval. Clin Pharmacol Drug Dev, 2021. 10(5): p. 521-534.
- Schalkwijk, S. , et al., Parent and Metabolite Concentration-QT Modeling to Evaluate QT-Interval Prolongation at Savolitinib Therapeutic Doses. AAPS J, 2021. 23(3): p. 46.
- Xiong, Y. , et al., Case report: Savolitinib induced severe adverse reactions resembling septic shock in an HIV-1-positive patient with advanced non-small cell lung cancer. Front Pharmacol, 2023. 14: p. 1089184.
- Schuller, A.G. , et al., The MET Inhibitor AZD6094 (Savolitinib, HMPL-504) Induces Regression in Papillary Renal Cell Carcinoma Patient-Derived Xenograft Models. Clin Cancer Res, 2015. 21(12): p. 2811-9.
- Gan, H.K. , et al., First-in-Human Phase I Study of the Selective MET Inhibitor, Savolitinib, in Patients with Advanced Solid Tumors: Safety, Pharmacokinetics, and Antitumor Activity. Clin Cancer Res, 2019. 25(16): p. 4924-4932.
- Wang, Y. , et al., Phase Ia/Ib Study of the Selective MET Inhibitor, Savolitinib, in Patients with Advanced Solid Tumors:
Safety, Efficacy, and Biomarkers. Oncologist, 2022. 27(5): p. 342-e383.
- Lu, S. , et al., Long-Term Efficacy, Safety, and Subgroup Analysis of Savolitinib in Chinese Patients With NSCLCs Harboring MET Exon 14 Skipping Alterations. JTO Clin Res Rep, 2022. 3(10): p. 100407.
- Lu, S. , et al., 2MO Final OS results and subgroup analysis of savolitinib in patients with MET exon 14 skipping mutations (METex14+) NSCLC. Volume : 33: p. Copyright © 2022 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
- Lu, S. , et al., Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med, 2021. 9(10): p. 1154-1164.
- Hong, L. , et al., Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Therapeutic Advances in Medical Oncology, 2021. 13: p. 1758835921992976.
- Zhu, X., Y. Lu, and S. Lu, Landscape of Savolitinib Development for the Treatment of Non-Small Cell Lung Cancer with MET Alteration-A Narrative Review. Cancers (Basel), 2022. 14(24).
- Sequist, L.V. , et al., Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol, 2020. 21(3): p. 373-386.
- Oxnard, G.R. , et al., TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol, 2020. 31(4): p. 507-516.
- Hartmaier, R.J. , et al., Osimertinib + Savolitinib to Overcome Acquired MET-Mediated Resistance in Epidermal Growth Factor Receptor-Mutated, MET-Amplified Non-Small Cell Lung Cancer: TATTON. Cancer Discov, 2023. 13(1): p. 98-113.
- Oxnard, G.R. , et al., SAVANNAH: A Phase II trial of osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-driven (MET+), locally advanced or metastatic non-small cell lung cancer (NSCLC), following disease progression on osimertinib. Journal of Clinical Oncology, 2019. 37(15_suppl): p. TPS9119-TPS9119.
- Li, A., H. J. Chen, and J.J. Yang, Design and Rationale for a Phase II, Randomized, Open-Label, Two-Cohort Multicenter Interventional Study of Osimertinib with or Without Savolitinib in De Novo MET Aberrant, EGFR-Mutant Patients with Advanced Non-Small-Cell Lung Cancer: The FLOWERS Trial. Clin Lung Cancer, 2023. 24(1): p. 82-88.
- Yang, J.J. , et al., A phase Ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Invest New Drugs, 2021. 39(2): p. 477-487.
- Kim, S.T. , et al., Combination of Docetaxel Plus Savolitinib in Refractory Cancer Patients: A Report on Phase I Trial. Transl Oncol, 2019. 12(4): p. 597-601.
- Suárez, C. , et al., Phase II Study Investigating the Safety and Efficacy of Savolitinib and Durvalumab in Metastatic Papillary Renal Cancer (CALYPSO). J Clin Oncol, 2023. 41(14): p. 2493-2502.
- Henry, R.E. , et al., Acquired savolitinib resistance in non-small cell lung cancer arises via multiple mechanisms that converge on MET-independent mTOR and MYC activation. Oncotarget, 2016. 7(36): p. 57651-57670.
- Frigault, M.M. , et al., Mechanisms of Acquired Resistance to Savolitinib, a Selective MET Inhibitor in MET-Amplified Gastric Cancer. JCO Precis Oncol, 2020. 4.
- Zhang, Y., Z. Du, and M. Zhang, Biomarker development in MET-targeted therapy. Oncotarget, 2016. 7(24): p. 37370-37389.
- Matsumoto, K. and T. Nakamura, Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int, 2001. 59(6): p. 2023-38.
- Srivastava, A.K. , et al., Pharmacodynamic Response of the MET/HGF Receptor to Small-Molecule Tyrosine Kinase Inhibitors Examined with Validated, Fit-for-Clinic Immunoassays. Clinical Cancer Research, 2016. 22(14): p. 3683-3694.
- Lee, J. , et al., Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov, 2019. 9(10): p. 1388-1405.
- Yu, Y. , et al., Circulating tumour DNA biomarkers in savolitinib-treated patients with non-small cell lung cancer harbouring MET exon 14 skipping alterations: a post hoc analysis of a pivotal phase 2 study. Ther Adv Med Oncol, 2022. 14: p. 17588359221133546.
- Subramanian, J. and O. Tawfik, Detection of MET exon 14 skipping mutations in non-small cell lung cancer: overview and community perspective. Expert Rev Anticancer Ther, 2021. 21(8): p. 877-886.
- Drusbosky, L.M. , et al., Therapeutic strategies in METex14 skipping mutated non-small cell lung cancer. J Hematol Oncol, 2021. 14(1): p. 129.
- Gong, C. , et al., MET alterations in advanced pulmonary sarcomatoid carcinoma. Front Oncol, 2022. 12: p. 1017026.
- Xu, Z. , et al., Incidence and PD-L1 Expression of MET 14 Skipping in Chinese Population: A Non-Selective NSCLC Cohort Study Using RNA-Based Sequencing. Onco Targets Ther, 2020. 13: p. 6245-6253.
- Sabari, J.K. , et al., PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol, 2018. 29(10): p. 2085-2091.
- Xu, L., F. Wang, and F. Luo, MET-targeted therapies for the treatment of non-small-cell lung cancer: A systematic review and meta-analysis. Front Oncol, 2022. 12: p. 1013299.
- Gu, L. , et al., A durable response to savolitinib in a patient with lung adenocarcinoma harboring two novel MET exon 14 skipping sites. Anticancer Drugs, 2023.
- Fu, M. , et al., Neoadjuvant Savolitinib targeted therapy stage IIIA-N2 primary lung adenocarcinoma harboring MET Exon 14 skipping mutation: A case report. Front Oncol, 2022. 12: p. 954886.
- Deng, H.Y. , et al., The safety and feasibility of preoperative induction therapy of Savolitinib in non-small cell lung cancer patients with MET exon 14 skipping mutation. J Cancer Res Clin Oncol, 2022.
- Tian, J. , et al., Dramatic response to neoadjuvant savolitinib in marginally resectable lung adenocarcinoma with MET exon 14 skipping mutation: A case report and literature review. Front Oncol, 2022. 12: p. 1006634.
- Yang, F. and Q.F. Chen, A case of lung adenocarcinoma with MET∆ex14 mutation regressed after preoperative treatment with savolitinib, and successfully underwent radical resection. Anticancer Drugs, 2023. 34(2): p. 302-305.
- Zhang, L. , et al., Multimodality Treatment of Pulmonary Sarcomatoid Carcinoma: A Review of Current State of Art. J Oncol, 2022. 2022: p. 8541157.
- Choueiri, T.K. , et al., Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. Journal of Clinical Oncology, 2017. 35(26): p. 2993-3001.
- Choueiri, T.K. , et al., Efficacy of Savolitinib vs Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Randomized Clinical Trial. JAMA Oncol, 2020. 6(8): p. 1247-1255.
- He, X. and G. An, Significant role of savolitinib in a case of advanced gastric cancer with abnormal mesenchymal-epithelial transition factor (MET): A case report. Medicine (Baltimore), 2022. 101(48): p. e32072.
- Ye, W. , et al., Case Report: Prompt Response to Savolitinib in a Case of Advanced Gastric Cancer With Bone Marrow Invasion and MET Abnormalities. Front Oncol, 2022. 12: p. 868654.
- Yan, N. , et al., Advanced HCC with amplified mesenchymal epithelial transition factor receptor responds well to savolitinib: a case report. Front Med (Lausanne), 2023. 10: p. 1130012.
- Jia, J. , et al., A phase II study of savolitinib (volitinib, AZD6094, HMPL-504) in subjects with MET amplified metastatic colorectal cancer (mCRC) detected by cell-free (cf)DNA. Journal of Clinical Oncology, 2020. 38(4_suppl): p. TPS270-TPS270.
- Pothula, S.P. , et al., Targeting HGF/c-MET Axis in Pancreatic Cancer. Int J Mol Sci, 2020. 21(23).
- Hage, C. , et al., The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis, 2013. 4(5): p. e627.
- Avan, A. , et al., Crizotinib Inhibits Metabolic Inactivation of Gemcitabine in c-Met–driven Pancreatic Carcinoma. Cancer Research, 2013. 73(22): p. 6745-6756.
- Brandes, F. , et al., Targeting cMET with INC280 impairs tumour growth and improves efficacy of gemcitabine in a pancreatic cancer model. BMC Cancer, 2015. 15(1): p. 71.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).