Submitted:
01 August 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
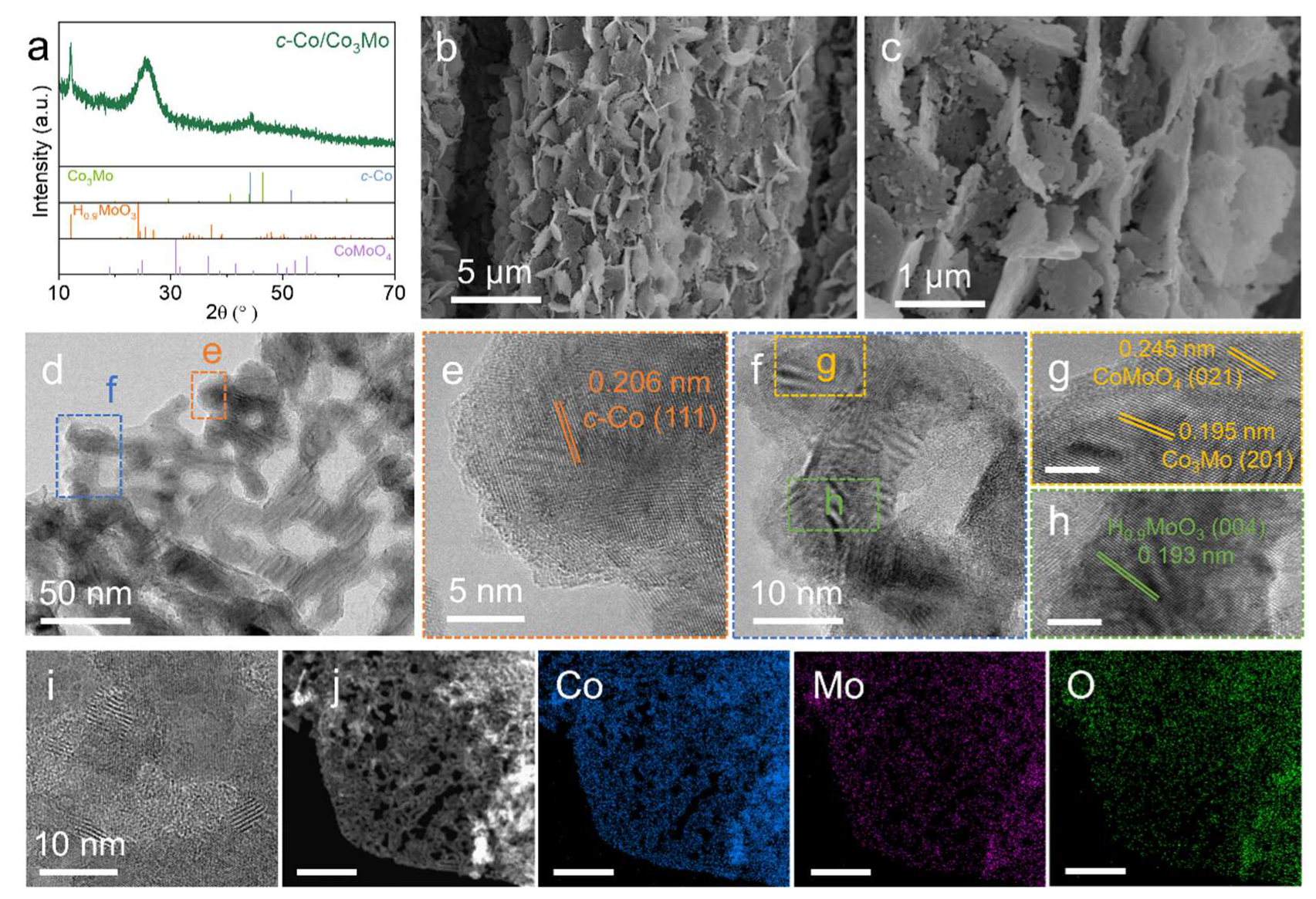
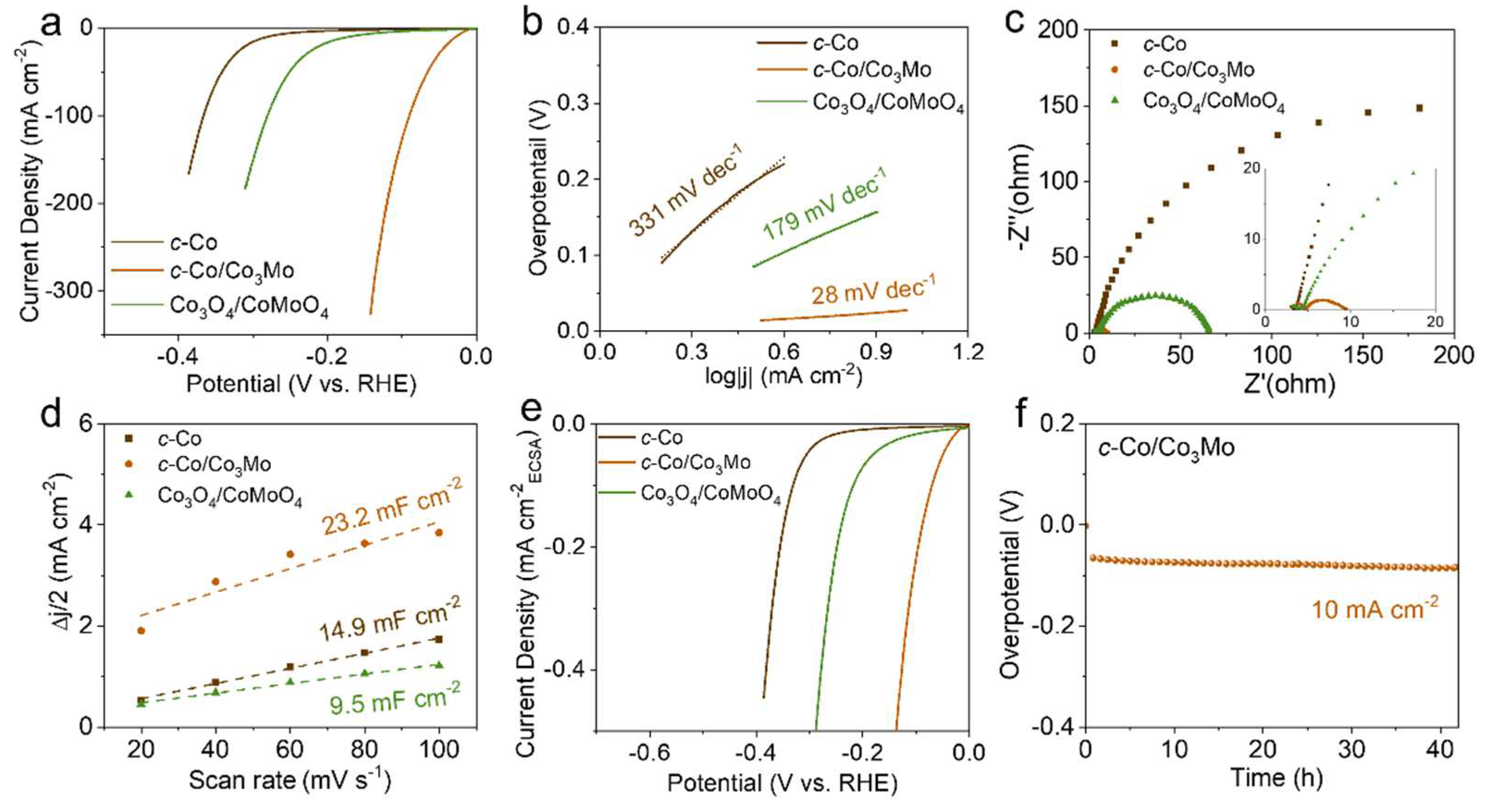
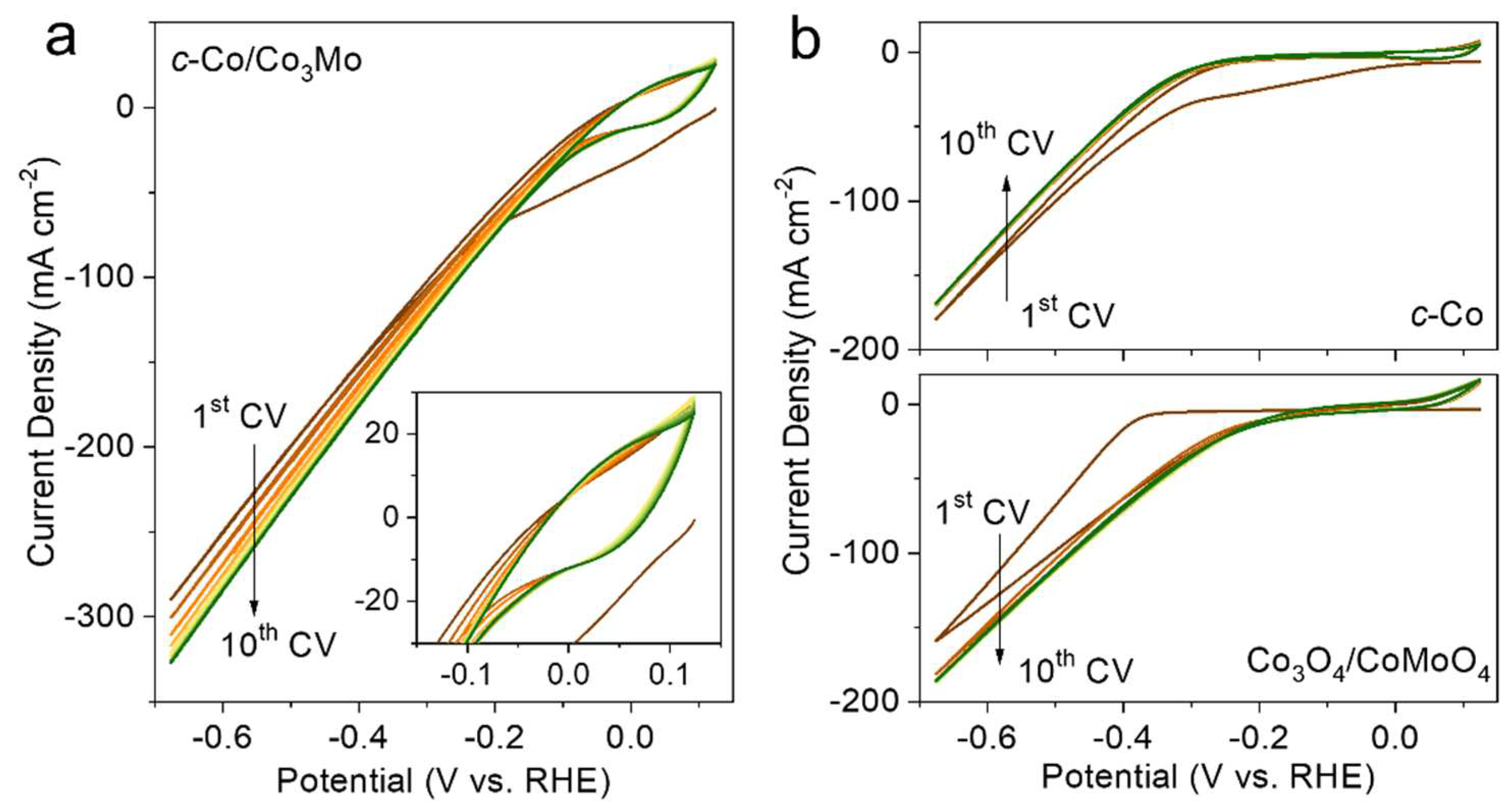
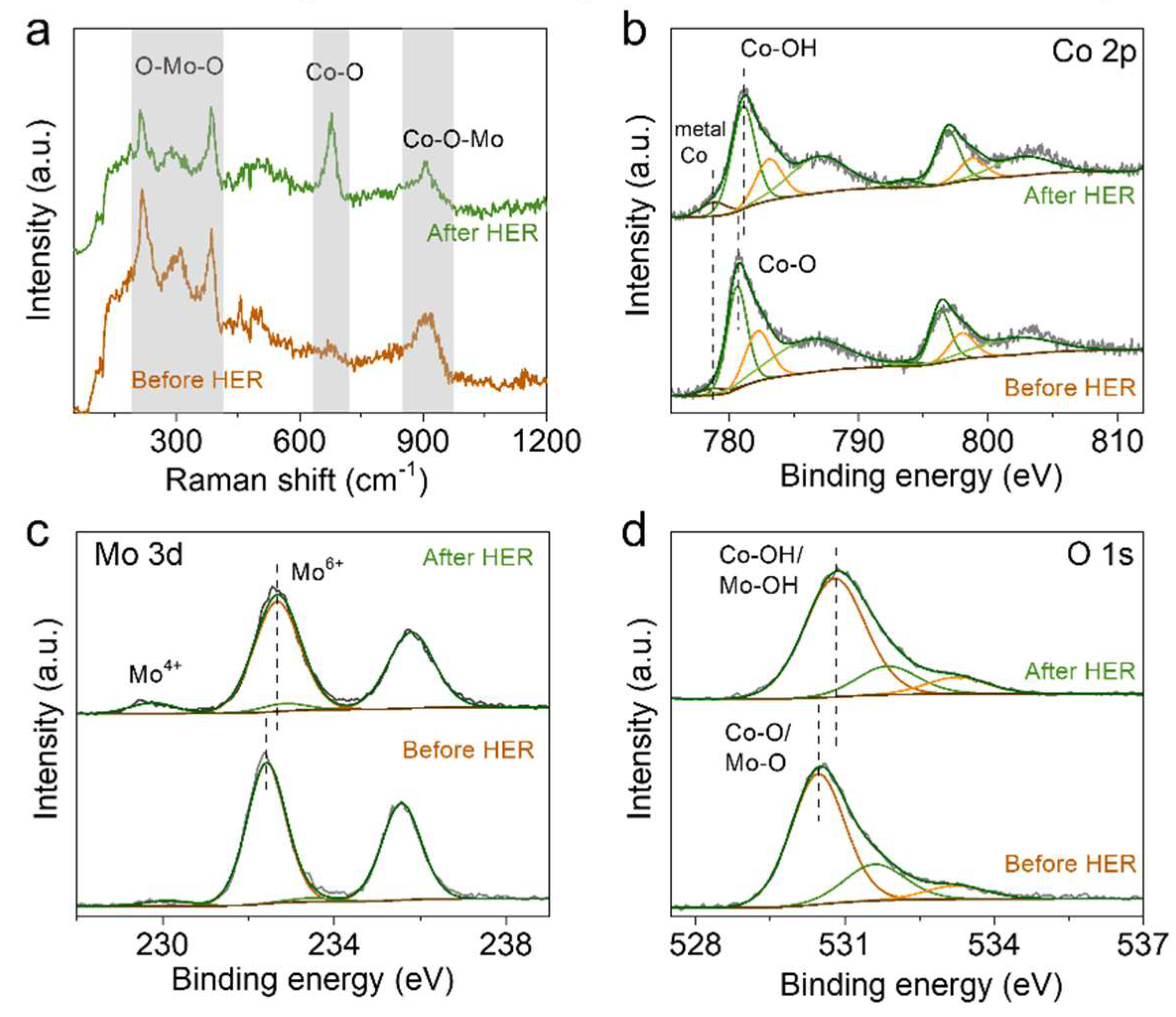
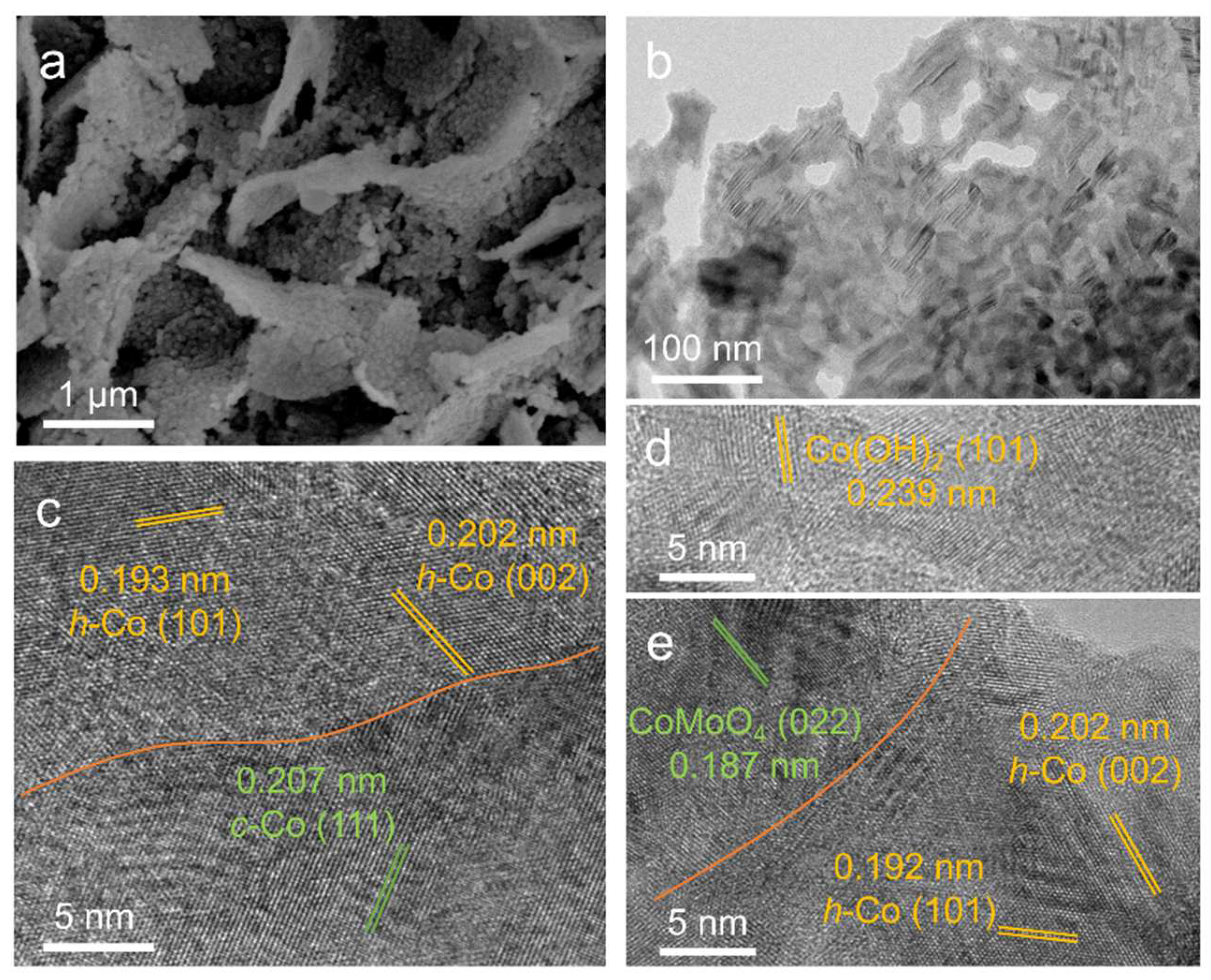
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of Co(OH)2/CoMoO4 Precursor on Carbon Cloth
4.3. Synthesis of c-Co/Co3Mo on Carbon Cloth
4.4. Synthesis of c-Co on Carbon Cloth
4.5. Synthesis of Co3O4/CoMoO4 on Carbon Cloth
4.6. Characterizations
4.6.1. Scanning Electron Microscopy (SEM)
4.6.2. Transmission Electron Microscopy (TEM) with Energy Dispersive X-ray Spectroscopy (EDX)
4.6.3. Powder X-ray Diffraction (XRD)
4.6.4. X-ray Photoelectron Spectroscopy (XPS)
4.6.5. Raman Spectroscopy
4.7. Electrochemical Measurements
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, L.; Shi, L.; Chen, H.; Liang, X.; Tian, B.; Zhang, K.; Zou, Y.; Zou, X. A Highly Active, Long-Lived Oxygen Evolution Electrocatalyst Derived from Open-Framework Iridates. Adv. Mater. 2023, 35, 2208539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Zhu, S.S.; Liu, Y.Y.; Zhang, X.; Wang, J.J.; Braun, A. Covalent S-O Bonding Enables Enhanced Photoelectrochemical Performance of Cu2S/Fe2O3 Heterojunction for Water Splitting. Small, 2021, 17, 2100320. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Shi, R.; Li, Z.H.; Zhao, J.Q.; Su, C.L.; Zhang, T.R. Triphase Photocatalytic CO2 Reduction over Silver-Decorated Titanium Oxide at a Gas–Water Boundary. Angew. Chem. Int. Ed. 2022, 61, e202200802. [Google Scholar] [CrossRef]
- Miao, Z.Y.; Huang, Y.; Xin, J.P.; Su, X.W.; Sang, Y.H.; Liu, H.; Wang, J.J. High Performance Symmetric Supercapacitor Constructed Using Carbon Cloth Boosted by Engineering Oxygen-containing Functional Groups, ACS Appl. Mater. Interfaces, 2019, 11, 18044–18050. [Google Scholar] [CrossRef]
- Yi, P.; Song, Y.Y.; Li, C.Y.; Liu, R.Z.; Sun, J.K. Heterostructured Mn-doped NiSx/NiO/Ni3N Nanoplate Arrays as Bifunctional Electrocatalysts for Energy-Saving Hydrogen Production and Urea Degradation. Appl. Surf. Sci. 2023, 619, 156789. [Google Scholar] [CrossRef]
- Jiao, S.; Fu, X.; Wang, S.; Zhao, Y. Perfecting the Electrocatalysts via Imperfections: Towards Large-Scale Deployment of Water Electrolysis Technology. Energy Environ. Sci. 2021, 14, 1722–1770. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, L.W.; Liu, X.L.; Tan, T.; Liu, H.; Wang, J.J. Precisely engineering the electronic structure of active sites boosts the activity of iron-nickel selenide on nickel foam for highly efficient and stable overall water splitting. Appl. Catal. B 2021, 299, 120678. [Google Scholar] [CrossRef]
- Yao, Z.C.; Tang, T.; Jiang, Z.; Wang, L.; Hu, J.S.; Wan, L.J. Electrocatalytic Hydrogen Oxidation in Alkaline Media: From Mechanistic Insights to Catalyst Design. ACS Nano 2022, 16, 5153–5183. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Zhai, W.; Ma, Y.; Chen, D.; Ho, J.C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMat 2022, 4, e12357. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhao, P.; Sheng, W. Hydrogen Evolution and Oxidation: Mechanistic Studies and Material Advances. Adv. Mater. 2019, 31, 1808066. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Du, W.; Shi, Y.; Zhou, W.; Yu, Y.; Zhang, B. Unveiling the In Situ Dissolution and Polymerization of Mo in Ni4Mo Alloy for Promoting Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 7051–7055. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Liu, J.; Zhong, C.; Tu, Y.; Li, P.; Du, L.; Chen, S.; Cui, Z. OH spectator at IrMo intermetallic narrowing activity gap between alkaline and acidic hydrogen evolution reaction. Nat. Commun. 2022, 13, 5497. [Google Scholar] [CrossRef]
- Jiang, L.W.; Huang, Y.; Zou, Y.; Meng, C.; Xiao, Y.; Liu, H.; Wang, J.J. Boosting the Stability of Oxygen Vacancies in α-Co(OH)2 Nanosheets with Coordination Polyhedrons as Rivets for High-Performance Alkaline Hydrogen Evolution Electrocatalyst. Adv. Energy Mater. 2022, 12, 2202351. [Google Scholar] [CrossRef]
- Jin, Q.; Ren, B.; Li, D.; Cui, H.; Wang, C. In situ promoting water dissociation kinetic of Co based electrocatalyst for unprecedentedly enhanced hydrogen evolution reaction in alkaline media. Nano Energy 2018, 49, 14–22. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 4998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xing, Y.; Xu, S.; Lu, Y.; Sun, S.; Jiang, D. Interfacing Co3Mo with CoMoOx for synergistically boosting electrocatalytic hydrogen and oxygen evolution reactions. Chem. Eng. J. 2022, 431, 133240. [Google Scholar] [CrossRef]
- Chen, J.; Ge, Y.; Feng, Q.; Zhuang, P.; Chu, H.; Cao, Y.; Smith, W.R.; Dong, P.; Ye, M.; Shen, J. Nesting Co3Mo Binary Alloy Nanoparticles onto Molybdenum Oxide Nanosheet Arrays for Superior Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 9002–9010. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2016, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, H.; Sun, L.; Jia, F.; Liu, B. Ordered Mesoporous Intermetallic Trimetals for Efficient and pH-Universal Hydrogen Evolution Electrocatalysis. Adv. Energy Mater. 2022, 12, 2201478. [Google Scholar] [CrossRef]
- Shah, A.H.; Zhang, Z.; Huang, Z.; Wang, S.; Zhong, G.; Wan, C.; Alexandrova, A.N.; Huang, Y.; Duan, X. The role of alkali metal cations and platinum-surface hydroxyl in the alkaline hydrogen evolution reaction. Nat. Catal. 2022, 5, 923–933. [Google Scholar] [CrossRef]
- Wang, J.; Xin, S.; Xiao, Y.; Zhang, Z.; Li, Z.; Zhang, W.; Li, C.; Bao, R.; Peng, J.; Yi, J.; Chou, S. Manipulating the Water Dissociation Electrocatalytic Sites of Bimetallic Nickel-Based Alloys for Highly Efficient Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202202518. [Google Scholar] [CrossRef]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.I.; Lee, K. Morphology-Controlled Metal Sulfides and Phosphides for Electrochemical Water Splitting. Adv. Mater. 2019, 31, e1806682. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Shi, Z.; Yang, L.; Tang, Y. Structural Design and Electronic Modulation of Transition-Metal-Carbide Electrocatalysts toward Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1802880. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Niu, X.; Zhang, K.; Wang, Z.; Cui, Y.; Wang, R. Chemical Interactions and Mechanisms of Different pH Regulators on Copper and Cobalt Removal Rate of Copper Film CMP for GLSI. ECS J. Solid State Sci. Technol. 2019, 8, P99–P105. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, Z.; Ren, L.; Qi, X.; Wei, X.; Zhong, J. Facile hydrothermal synthesis of NiMoO4@CoMoO4 hierarchical nanospheres for supercapacitor applications. Phys. Chem. Chem. Phys. 2015, 17, 20795–20804. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Chen, J.; Yang, R.; Mao, S.; Qin, M.; Wang, Y. Multi-hierarchical cobalt-based electrocatalyst towards high rate H2 production. Appl. Catal. B 2022, 316, 121666. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO–Co3O4 crossover using Raman spectroscopy in magnetic octahedron-shaped nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, L.W.; Liu, H.; Wang, J.J. Electronic structure regulation and polysulfide bonding of Co-doped (Ni, Fe)1+xS enable highly efficient and stable electrocatalytic overall water splitting. Chem. Eng. J. 2022, 441, 136121. [Google Scholar] [CrossRef]
- Men, Y.N.; Jia, S.F.; Li, P.; Tan, Y.; Wang, J.B.; Zhao, P.P.; Cheng, G.Z.; Chen, S.L.; Luo, W. Boosting alkaline hydrogen evolution electrocatalysis through electronic communicating vessels on Co2P/Co4N heterostructure catalyst. Chem. Eng. J. 2022, 433, 133831. [Google Scholar] [CrossRef]
- Jiang, L.W.; Huang, Y.; Chen, B.B.; Zhou, J.Q.; Liu, H.; Wang, J.J. Electrochemically induced in-situ generated Co(OH)2 nanoplates to promote the Volmer process toward efficient alkaline hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 8497–8506. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Xu, S.; Yang, X.; Bian, J.; Lv, C.; Yu, Z.; He, T.; Huang, Z.; Boukhvalov, D.W.; Cheng, C.; Huang, Y.; Zhang, C. Modulation of Volmer step for efficient alkaline water splitting implemented by titanium oxide promoting surface reconstruction of cobalt carbonate hydroxide. Nano Energy 2021, 82, 105732. [Google Scholar] [CrossRef]
- Yin, Z.H.; Huang, Y.; Jiang, L.W.; Meng, C.; Wu, Y.Z.; Liu, H.; Wang, J.J. Revealing the In Situ Evolution of Tetrahedral NiMoO4 Micropillar Array for Energy-Efficient Alkaline Hydrogen Production Assisted by Urea Electrolysis. Small Struct. 2023, 2300028. [Google Scholar] [CrossRef]
- Gao, W.Q.; Zou, Y.; Zang, Y.M.; Zhao, X.L.; Zhou, W.J.; Dai, Y.; Liu, H.; Wang, J.J.; Ma, Y.D.; Sang, Y.H. Magnetic-field-regulated Ni-Fe-Mo ternary alloy electrocatalysts with enduring spin polarization enhanced oxygen evolution reaction, Chem. Eng. J. 2023, 455, 140821. [Google Scholar]
- Yang, C.; Zhong, W.; Shen, K.; Zhang, Q.; Zhao, R.; Xiang, H.; Wu, J.; Li, X.; Yang, N. Electrochemically Reconstructed Cu-FeOOH/Fe3O4 Catalyst for Efficient Hydrogen Evolution in Alkaline Media. Adv. Energy Mater. 2022, 12, 2200077. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




