1. Introduction
Up to January 2023, more than 670 million people worldwide have been affected from SARS-CoV-2 that is responsible for COVID-19. As per 2nd of May 2023 report, the outbreak of COVID-19 has spread to almost every country in the world, and more than 6.86 million deaths (Table 1
) have been attributed to COVID-19 with increasing evidence supporting the existence of long COVID-19 syndrome [
1]. COVID-19, caused by SARS-CoV-2 commonly presents as a pulmonary illness but may also affect extrapulmonary organs including the heart [
2]. Higher risk of adverse cardiovascular outcomes post COVID-19 exposure, even after recovery from the acute infection have been described in several studies [
3,
4]. The myocardial injury in patients with COVID-19 has been attributed to coronary spasm, microthrombi formation, plaque rupture, hypoxic injury or cytokine storm disposing the same pathophysiology with the three clinical variants of Kounis syndrome [
5]. One of the main proposed mechanisms for development of cardiovascular complications and new onset hypertension is the ACE2 and its interactions with RAAS and KKS [
6]. The ACE2 receptors are expressed throughout the human body located mainly in heart, blood vessels’ endothelium, lungs, intestines, testes and neurons. The SARS-CoV-2 directly invades the endothelial cells that contain ACE2 receptors and constitute the main pathway via which the virus enters the cardiovascular system [
7]. The S1 subunit of the SARS-CoV-2 spike protein binds to the ACE2 receptors on the cell surface and allows the virus to enter the cell membrane. This leads to downregulation of the ACE2 receptors and causes angiotensin II accumulation leading to prothrombotic effects such as hemostatic imbalance via activation of the coagulation cascade, impaired fibrinolysis, thrombin generation, vasoconstriction and hypertension, endothelial and platelet activation, and pro-inflammatory cytokine release [
8]. Indeed, angiotensin I has no direct biological function except that its high levels can stimulate catecholamine production. Angiotensin I exerts important effects completely different and opposite to those of angiotensin II, such as vasodilation induction , inflammation and thrombosis inhibition. However, it is metabolized to its biologically active byproduct angiotensin II, a potent vasoconstrictor, via cleavage of the two terminal amino acids. Moreover, SARS-CoV-2 infection prevents the counterbalancing action of the KKS system that normally causes vasodilation and regulates tissue repair, inflammation, cell proliferation, and platelet aggregation [
9,
10].
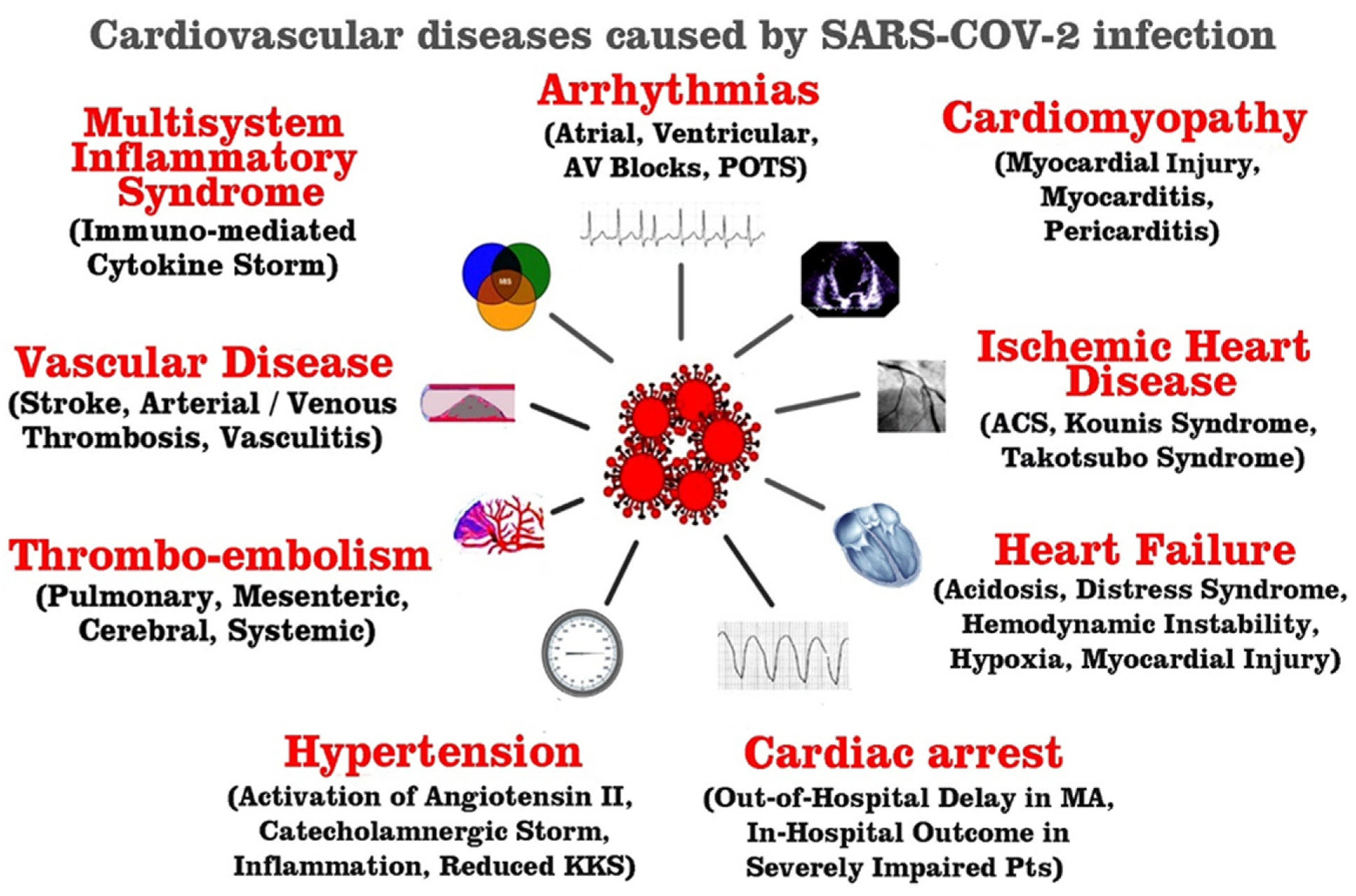
Since cardiovascular complications are a major risk factor for COVID-19 mortality, the aim of this narrative review is to search the published literature up to June 2023, in order to elucidate the risk factors, putative causes, diagnosis, and pathophysiology of cardiovascular complications of COVID-19 (
Figure 1).
2. Methods
A literature search was conducted on the PubMed, MedLine, Embase databases and Google and updated on 30 June 2023 with the keywords “COVID-19”, “cytokine storm”, “SARS-CoV-2”, “SARS-CoV”, “COVID-19 and the heart”, “Cardiovascular complications of COVID-19 infection”, “ischemic myocardial injury”, “coronaviruses”, “mast cells” and Kounis syndrome. Bibliographic search was also undertaken. Articles in this review needed to be published up to the end of June 2023, available as full text in English, categorized as original research, reviews, meta-analyses or letters to the editor. Database screening was closed on 30 June 2023. Titles and abstracts were reviewed to verify these criteria. The articles were reviewed in full if all inclusion requirements were present or if this remained unclear. Searching references included in the manuscripts was an additional literature screening tool. The abstracts were scanned to assess their appropriateness to be included in this narrative review
3. Results
3.1. Arrhythmias and COVID-19
Arrhythmias in COVID-19 might be triggered either from hypoxia due to direct viral lung tissue injury, myocarditis or from secondary causes as a consequence of myocardial ischemia, myocardial strain, electrolyte abnormalities, changes in intravascular volume, drug adverse effects and in the context of systemic illness [
11,
12].
In one retrospective series of COVID-19 admitted patients, atrial arrhythmias were demonstrated in 7.1% while ventricular arrhythmias in 0.3 % of mechanically ventilated individuals [
13]. In another study, atrial arrhythmias were reported in 16,5% of intensive care unit (ICU) admissions [
14]. Whereas, He et al [
15] revealed 1 of the 2 COVID-19 patients with transient complete heart block accompanied by transient
S wave in lead 1, Q wave in lead 3, inverted T wave in lead 3 (S1Q3T3), supporting the diagnosis of temporary pulmonary artery hypertension secondary to
acute respiratory distress syndrome (ARDS). Beyond complete heart block and brady-arrhythmias, supraventricular tachyarrhythmias, atrial fibrillation, atrial flutter as well as ventricular arrhythmias such as polymorphic ventricular tachycardia (VT), monomorphic VT, and multifocal VT have been demonstrated in COVID-19 patients, suggesting various mechanisms for arrhythmogenesis [
11].
Hypoxia plays a major role in arrhythmogenesis as hypoxia induced cellular damage can activate anaerobic glycolysis and increase cytosolic calcium levels further facilitating early and late depolarizations, inducing changes in action potential duration [
16]. In addition, hypoxia can increase extracellular potassium levels resulting in enhancement of electrical conduction, while it can decrease electrical coupling and tissue anisotropy via connexin dephosphorylation in the gap junctions [
17].
SARS-COV-2 myocarditis pathophysiology mechanism can be attributed to direct viral myocardial tissue injury, migration /accumulation of alveolar macrophages or via lymphocyte cell mediated cytotoxicity resulting to myocardial inflammation and further injury [
16,
18,
19]. As a consequence myocarditis can trigger arrhythmias due to direct myocardial cell damage and further altered expression of myocardial connexins leading to gap junction impairment or due to ion channel dysfunction especially in individuals with coexisting channelopathies [
11]. Viral myocarditis contributes on structural and electrophysiological remodeling causing impaired calcium handling and down regulation of potassium channels that further can lead to prolonged repolarization and conduction abnormalities, such as decreased conduction velocity and reduced refractoriness. Triggered activity can be induced by prolonged repolarization while conduction abnormalities can cause circus type- or phase 2- re-entry [
20]. Interestingly, arrhythmias can also be observed in post myocardial inflammation phase due to re-entry in case of myocardial scar [
21]. Finally, inflammatory cytokines such as interleukins -6 (IL-6) and -1 (IL-1) and tumor necrosis factor-a can further modify the expression and function of calcium and potassium channels, contributing to inflammatory cardiac channelopathies and alteration in ventricular action potential [
22,
33]. Consequently, inflammatory cytokines due to enhanced sympathetic system activation can cause QT prolongation and arrhythmias in patients with long QT syndrome.
Another contributing factor of arrhythmogenesis in COVID -19 can be the myocardial ischemia stemmed from microvascular dysfunction in conjunction with enhanced inflammatory state, where activation of inflammatory cells can induce vasoconstriction due to impaired vascular endothelial function in individuals with pre-existing atherosclerotic lesions. It has been reported that IL-6 and tumor necrosis factor-a can significantly impair coagulation and fibrinolysis, promoting bleeding and thrombosis [
24]. Microvascular dysfunction and myocardial ischemia can also be induced via viral mediated vasculitis due to SARS-CoV-2 attachment to ACE receptors in arterial endothelial cells [
25]. Myocardial injury with ST segment elevation has been revealed in COVID patients, while cytokine storm can induce acute coronary syndromes in cases of pre-existing atherosclerotic plaque due to inflammatory cell activation and impaired endothelial vascular function [
26].
Arrhythmias can be triggered by right ventricular myocardial strain in the context of pulmonary embolism, a common thrombotic complication in COVID -19, or due to pulmonary hypertension that can be induced in cases of elevated right heart pressures due to ARDS, sepsis or co-existing heart failure [
27]. Atrial arrhythmias are the most common arrhythmias in cases of increased atrial pressures and enhanced sympathetic activity in individuals with pulmonary hypertension [
28].
Finally, fluid imbalance and electrolyte abnormalities can trigger various arrhythmias. Especially, intravascular volume alterations in critically ill patients attributed either to sepsis caused by ARDS or secondary due to heart failure can predispose to atrial fibrillation [
29]. Electrolyte abnormalities can trigger de novo or potentiate pre-existing arrhythmias. Based on previous retrospective studies, electrolyte abnormalities can be caused either by acute or chronic renal dysfunction or COVID associated diarrhea [
30]. Atrial arrhythmias are the most common encountered ones in electrolyte disturbances.
Moreover, drugs such hydroxylchloroquine and azithromycin used as ‘off label’ therapies in COVID-19 patients can cause QT prolongation, increasing the risk of Torsades de Pointes [
31]. Specifically, these agents via inhibition of the human Ether-à-go-go-Related Gene (hERG-K) + channel can induce action potential prolongation and trigger early after depolarizations due to enhanced inward Na+ and Ca2+ currents that can lead to Torsades de Pointes.
3.2. Cardiac Arrest
Cardiac arrest (CA) is a mostly fatal condition and its annual incidence, before the COVID-19 pandemic, ranged from 40 to 80 per 100,000, with an average survival rate of 9% [
32]. During COVID-19 pandemic, an increase in incidence of both out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA) has been observed together with decline in survival. [
33,
34]. During the first weeks of the COVID-19 pandemic, the OHCA was worse and this was observed not only in areas with high case-fatality rates but also in the areas with lower rates [
35]. According to a recent study from the United States, the age-standardized annual CA incidence increased from 39 per 100, 000 pre-pandemic to 54 per 100,000 during the pandemic. Among Hispanics, the incidence increased by 77%, from 38 per 100,000 to 68 per 100,000 with disproportionate increases in overall cardiovascular mortality [
36].
This increase was attributed to malignant tachyarrhythmias, delays in seeking care among individuals with cardiac symptoms [
37], and delayed Emergency Medical Services (EMS) activation/response during COVID-19 waves [
38]. In a retrospective study of 136 patients with COVID-19 [
39], the respiratory causes for CA had affected 119 (87.5%) patients, the initial rhythm was asystole in 89.7%, pulseless electrical activity was present in 4.4%, and shockable rhythm was present in 5.9%. The return-of-spontaneous-circulation rate was 13.2% and 30-day survival rate was only 2.9% [
40]. In a multicenter study of more than 5000 critically ill patients with COVID-19, 14% suffered IHCA [
40]. In another study of 54 patients with COVID-19, after cardiopulmonary resuscitation the mortality rate was even worse (100%). Although the initial rhythm was non shockable for 52 patients (96.3%), pulseless electrical activity was the most common (81.5%) clinical finding. The return-of-spontaneous-circulation rate was achieved in 29 patients (53.7%), but none survived to be discharged home [
41,
42].
The presence of the underlying illness during the CA, mechanical ventilation, kidney replacement therapy, vasopressor support, high percentage of non-shockable rhythms, lack of therapies to treat the underlying disease process, and potential delays in response time due to isolation procedures, need to use personal protective equipment, and restricted access to COVID-19 units seem to represent some of the causes for the low survival rates of IHCA [
43].
Moreover, the incidence of OHCA significantly increased during the COVID-19 pandemic [
44], in different cities by 21% (Los Angeles County, CA, USA) [
45], by 62% (Detroit, MI, USA) [
46] and in various geographic regions throughout the world with similar trends. Lim and colleagues [
47] in a meta-analysis of 10 studies with more than 35,000 OHCA events in various geographic regions, found a 120% increase in OHCA. Common and uncommon causes and mechanisms [
43] during COVID-19 leading to cardiac arrest are shown in
Table 2.
Table 2.
Causes and mechanisms predisposing for cardiac arrest during COVID-2019.
Table 2.
Causes and mechanisms predisposing for cardiac arrest during COVID-2019.
| Common causes |
|---|
| Acute coronary syndrome |
| -Arrhythmias |
| -Cytokine storm |
| -Drug treatment causing risk for arrhythmias |
| -Exaggerated immune response |
| -Hypoxia from Respiratory disease |
| -Inflammatory Endothelial illness |
| -Myocarditis |
| -Pericarditis |
| -Prothrombotic state triggers |
| -pulmonary embolism |
| -Vascular thrombosis |
|
Uncommon causes
|
| -At-risk patients alone more often |
| -Delay in patient care |
| -Neglect of hygienic care |
| -Overwhelmed emergency medical service and hospital systems |
| -Reduction in hospital work force |
| -Reduction in preventive measures |
| -Reduction of emergent diagnostic testing and procedures |
| -Reorganization order to stay-at-home |
| -Strict lockdown measures - Use of personal protective equipment |
3.3. COVID-17 Multisystem Inflammatory Syndrome-Induced Cardiac Manifestations in Children and Adults
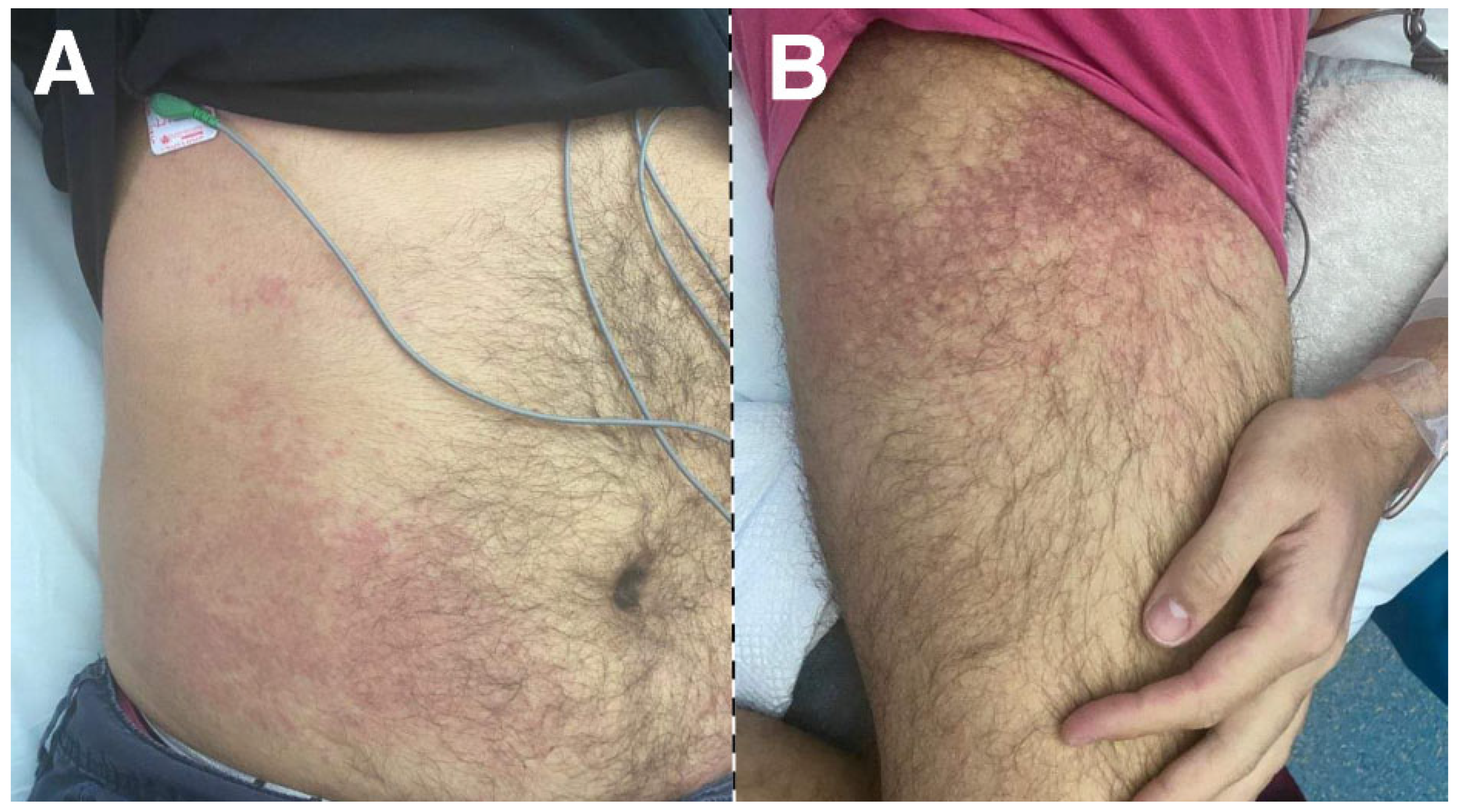
Multisystem inflammatory syndrome (MIS) represents an extreme manifestation after SARS-CoV-2 infection. The true incidence of MIS is unknown, but it seems to be an under-diagnosed entity. The syndrome usually occurs within 1–6 weeks following COVID-19 infection, affecting both children (MIS-C) and adults (MIS-A) [
48,
49].
Primary classical signs of syndrome include, fever, mucocutaneous manifestations (e.g., strawberry tongue, malar rash, periorbital erythema, conjunctivitis), lymphadenopathy, peripheral edema, raised inflammatory biomarkers and multiorgan involvement (
Figure 2).
The systemic involvement in MIS varies, with cardiovascular, gastrointestinal, neurological and hematological systems, being commonly affected. The cardiovascular system is the most commonly affected. The main cardiac manifestations of MIS include Left ventricular systolic dysfunction, coronary artery aneurysms (CAAs), arrhythmias, conduction abnormalities, and pericardial involvement TABLE 3 [
50].
Table 3.
Cardiovascular manifestations of Multisystem Inflammatory Syndrome in Children (MIS-C) and Adults (MIS-A).
Table 3.
Cardiovascular manifestations of Multisystem Inflammatory Syndrome in Children (MIS-C) and Adults (MIS-A).
| Pericardial involvement (pericardial effusion, pericarditis, cardiac tamponade) |
| Coronary involvement (dilation, aneurysms, thrombosis) |
| Myocarditis |
| Shock (vasodilatory, cardiogenic) |
| Left ventricular myocardial dysfunction (systolic, diastolic) |
| Valve regurgitation (mitral, tricuspid) |
| Left ventricular apical thrombus |
| Pulmonary embolism |
| Arterial and venous thrombosis |
|
Conduction disturbances (1nd/2nd/3rd degree A-V block, LBBB, RBBB, sinus bradycardia)
|
| Brugada pattern |
| Prolonged QTc interval |
| ST-T wave segment abnormalities |
| Elevated cardiovascular biomarkers (BNP, NT-pro-BNP, troponin, D-Dimers) |
| Arrhythmias (ventricular, supraventricular) |
Patients with MIS-C may present with some features consistent with toxic shock syndrome and Kawasaki’s disease. Patients with MIS compared with Kawasaki
’s disease are usually more critically ill and ventricular dysfunction is more frequent. Nevertheless, CAAs occur less frequent in patients with MIS [
51,
52].
The exact pathophysiology remains unclear. However, evidence suggests that MIS is characterized by post-infectious cytokine storm triggered by a dysfunctional immune response leading to systemic inflammation, endothelial dysfunction and procoagulant state [
53,
54].
Management of MIS includes supportive care, immunomodulatory therapy, glucocorticoids and intravenous immunoglobulin, anakinra or Tocilizumab, and low dose of aspirin. Goals for treatment are patient stabilization, and CAAs and myocardial fibrosis prevention. In patients with coronary artery z-score (coronary arterial diameter) greater>10.0 and/or EF<35% [
55], anti-coagulation may be considered.
Capone et al. studied the early and midterm outcomes of 50 children recovering from MIS-C. Although some of them developed myocardial fibrosis on MRI, coronary aneurysm and myocardial dysfunction complete resolution was observed in the majority of them at follow-up [
56]. In a study of 95 MIS-C patients, troponin was found in 71%, myocarditis in 53%, 80% were admitted to intensive care unit, and 2 died [
57]. In another cohort from 55 centers, a high incidence of myocardial injury (93%), shock (40%), and arrhythmia (35%) was demonstrated. Coronary artery involvement was seen in 24% and mechanical ventilation was required in 15.3% [
58].
3.4. Cardiomyopathy
Cardiomyopathy is a Greek word ('cardio’ means heart, ‘myo’ means muscle and ‘pathy’ means disease) and refers to a group of diseases that affect the structure of the heart muscle. Large database studies have demonstrated that cardiomyopathy constitutes a serious complication of SARS-Cov-2 induced acute COVID-19 infection [
59,
60,
61].
Other studies have demonstrated that the risk of ischemic and nonischemic cardiomyopathy, in vaccinated and nonvaccinated patients who tested positive for COVID-19 was higher in SARS-CoV-2- positive individuals than in noninfected control participants [
59,
60,
61]. Ischemic cardiomyopathy starts to manifest 1-12 months after a positive COVID-19 test [
60]. In these studies, ischemic cardiomyopathy was ranked among the two greatest risks following COVID-19 infection for all ages and preferably in the female gender [
59,
60,
61].
Several mechanisms have been proposed to explain how COVID-19 infection can lead to cardiomyopathy. It has been demonstrated that COVID-19 can cause myocardial injury as a consequent to LV systolic dysfunction; however, the exact pathophysiology is not fully elucidated [
62].
Cytokine storm induced inflammatory cascade is the most commonly proposed hypothesis for the ensuing myocardial injury [
63]. This mechanism derives from the parallel rise of the inflammatory mediators such as interleukin-1, C-reactive protein and other inflammatory biomarkers along with the rise in cardiac troponins [
64]. Other suggested mechanisms include acute inflammatory triggering of atheromatous plaque destabilization leading to acute coronary syndrome, microvascular injury secondary to disseminated intravascular coagulation and thrombosis. Moreover, the oxygen supply and demand mismatch can lead to myocardial injury similar to type 1 and type 2 myocardial infarctions [
62,
63,
64]. The direct invasion of the myocardium by the virus itself resulting in acute cardiomyopathy is another mechanism that has been hypothesized [
62]. The only diagnostic technique able to establish an etiological diagnosis of myocarditis or inflammatory cardiomyopathy is the endomyocardial biopsy [
65]. However, autopsy series have failed to demonstrate myocardial necrosis as a result of COVID-19 [
66]. Furthermore, considering the contagious spread risk, the required expertise and the false negative rates in performance of endomyocardial biopsies in all suspected cases, renders this technique questionable [
67]. Despite the fact that myocardial damage, in the setting of COVID-19 infection, is likely related to an exaggerated inflammatory response, direct myocardial invasion cannot be excluded.3.
3.5. Cytokine Storm
Severe COVID-19 is dictated by an over-exuberant activation of the immune system associated with a hyper-inflammatory state. Cytokines are considered the “lingua franca” of the immune system, mediating the efficient dialogue between immune and non-immune cells. Cytokine storm is a hallmark of critical COVID-19 and a major determinant of unfavorable outcomes. In particular, SARS-CoV-2 infection is associated with an exaggerated production of cytokines and chemoattractant molecules, including interleukin (IL)-1β, IL-2, IL-6, IL-8, IL-10, IL-18, IL-33, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, monocyte chemoattractant protein 1 (MIP-1), granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Of note, the levels of circulating cytokines exhibit a strong correlation with the severity of the disease, and they often serve as valuable prognostic indicators or potential therapeutic targets [
68].
The underlying mechanisms precipitating cytokine storm during SARS-CoV-2 infection are not yet fully clarified, but several hypotheses exist that collectively contribute to our current understanding. SARS-CoV-2 inflicts direct cytopathic damage to ACE-2-expressing alveolar epithelial cells and resident macrophages. Subsequently, tissue injury and the ensuing release of damage-associated molecular patterns trigger the production of pro-inflammatory mediators that orchestrate the recruitment and activation of additional innate and adaptive immune cells, thereby amplifying the inflammatory cascade [
69]. At the early stages of infection, SARS-CoV-2 is capable of hijacking the innate immune system, primarily by interfering with interferon responses. This prevents efficient clearance of the virus, leading to the development of paradoxical hyper-inflammation [
69]. SARS-CoV-2 also manipulates programmed cell death mechanisms to facilitate its replication and promote pyroptosis-mediated inflammation [
70]. Defective adaptive immune responses have also been implicated in the development of the cytokine storm during COVID-19. In fact, antibody-dependent enhancement is a phenomenon in which preexisting antibodies bind to SARS-CoV-2, but instead of neutralizing the virus, they can facilitate its propagation by interacting with fragment crystalizable (Fc) receptors on immune cells [
71]. In parallel, the virus can induce direct injury to intestinal epithelial cells that express the ACE-2 receptor or impair gut barrier integrity by disrupting the expression of tight junction proteins. Furthermore, COVID-19 is accompanied by profound alterations in intestinal microflora, i.e., dysbiosis, and dysregulation of mucosal immune responses. This multifaceted disruption of the gut barrier integrity allows an aberrant influx of microbes, fungi, and other pathogen-associated molecular patterns in the systemic circulation and contributes, at least to a certain degree, to immune system overactivation during COVID-19 [
72]. Finally, hypoxia and oxidative stress act as additional driving mechanisms for the cytokine storm, further fueling this vicious cycle [
73].
Cytokine storm is a complex and dynamic inflammatory process that engenders detrimental complications, including capillary leak, thrombo-inflammation, ARDS, and multiorgan failure, contributing to increased mortality during COVID-19 [
69]. Systemic inflammation plays a pivotal role in the development of cardiovascular complications, and hyper-cytokinemia is strongly implicated in various heart-related conditions, such as myocarditis, unstable plaque rupture, acute myocardial injury, stress-induced cardiomyopathy, arrhythmias, and heart failure [
74]. Patients with a pre-existing inflammatory state or impaired gut barrier function, such as obesity, diabetes mellitus, or chronic coronary syndromes, may instinctively face an increased risk of experiencing more fierce inflammatory responses and deteriorating disease progression [
75]. The cardiovascular complications of cytokine storm in general, and in viral infections in particular, have been described as arrhythmias, myocarditis, myocardial ischemia, pericarditis, and type 1 (atherosclerotic plaque rupture) and type 2 (secondary to an ischemic imbalance) myocardial infarction and heart failure [
76].
3.6. Heart Failure
COVID-19 myocardial injury leading to heart failure is not rare. According to a retrospective study of 191 COVID patients in Wuhan, China, heart failure (HF) was found to be the 4th most common outcome of the disease [
77]. Moreover, in another study including 131 patients who have died of COVID-19, 49% of all deaths were due to HF despite that these patients had no previous history of cardiovascular diseases [
78].
It seems that, after sepsis, the most common complications of COVID-19 are respiratory failure including ARDS, cardiac injury, and HF [
79].
In a recent study, the risk of HF in patients with SARS-CoV-2 infection was reported to be increased beyond the first 30 days [
80]. According to this study, the risks and burdens were evident even among individuals who were not hospitalized and increased in a graded fashion according to the care setting during the acute phase e. g. non-hospitalized, hospitalized and admitted to intensive care [
81].
Long-term effects of SARS-CoV-2 on cardiac function, residual adverse effects of cardiac involvement in the acute COVID-phase, or the worsening of previous cardiovascular disease post-infection are some predisposing factors of HF. Other studies have shown that there is no difference, in long-term mortality, in patients with ST-elevation myocardial infarction with or without a COVID-19 infection [
82]. However, there are higher rates of major cardiovascular and cerebrovascular events and hospitalization with HF in patients who tested positive for COVID-19 vs those who did not [
82]. Other studies have shown that there is no difference, in structural and functional cardiac characteristics on echocardiography, in patients who experienced severe and mild acute COVID-19 illness and to matched control participants at a median of 41 days from COVID-19 diagnosis [
83,
84]. However, later changes in cardiac function like diastolic dysfunction can appear. Indeed, echocardiography changes often present in 3-6 months [
84]. Both left and right ventricular dysfunction have been described after acute COVID-19 infection.
a. Left ventricular dysfunction (LVD). There are several studies which have demonstrated statistically significant reductions in LV ejection fraction (LVEF) compared with controls, 2-9 months post acute COVID-19 infection. These studies included patients with acute disease ranging from asymptomatic to severe illness [
86]. Indeed, congestive heart failure and a prominent reduction in LVEF (mean LVEF 28%) has been observed in patients with biopsy-proven post-COVID-19 myocarditis at an average of 5.5 months after COVID-19 infection [
87]. Moreover, there are reports of subclinical low voltage direct LVD following COVID-19 infection, in asymptomatic patients with low cardiac risk who recovered from acute symptoms at home [
88]
The echocardiographic LVEF, LV diameter, LV mass, or left atrial volumes on at a median of 4.3 months after discharge were similar in patients with COVID-19 with elevated cardiovascular biomarkers on admission compared with those without elevated markers.
[
59]. Perhaps, this method might not be sensitive enough to detect cases of minor LV impairment [
89]. It has been suggested that LV longitudinal strain (LVLS) has a greater sensitivity for detecting minor LV myocardial function impairment compared with measurement of LVEF [
90]. In patients with COVID-19 infection, concomitant conditions such as peripartum cardiomyopathy may severely reduce left ventricular systolic function with an ejection fraction (EF) reduction to 10-15% [
91].
Both, LV systolic and diastolic dysfunction can complicate COVID-19 infection. However, significant cardiac diastolic abnormalities without systolic involvement 6 months after hospitalization have been also reported [
92].
b. Right ventricular dysfunction (RVD). The majority of SARS-CoV-2-related deaths have been associated with cardiovascular events and ARDS. Indeed, much higher death rate was found in COVID-19 cases with myocardial injury than those without (59.6% vs. 8.9%) [
93]. RVD can be evident in almost 1 out of 5 patients, whereas the severity of COVID-19 disease plays an important role. It seems that RVD may represent one crucial marker for prognostic stratification in COVID-19 patients [
94].
According to a recent study, the decrease in RV global longitudinal strain and RV free wall longitudinal strain at 3 months was negatively correlated with the levels of C-reactive protein, neutrophil to lymphocyte ratio, d-dimer, ferritin, and platelet to lymphocyte ratio. Since these markers are indicators of inflammation and thrombosis, the possible pathology behind RVD, is the combination of reduced contractility due to myocardial damage and increased right ventricular afterload. Indeed, this study showed both an increase in RV diameter and systolic pulmonary artery pressure at 3 months after COVID infection, suggesting the same mechanism [
95]. However, other studies have shown that subclinical RV dysfunction by abnormal RV longitudinal strain was associated with PH or increased RV afterload in 42% of patients 2-3 months post COVID infection [
96].
However, RV dysfunction without increased afterload might point to reduced contractility as the main potential mechanism according to Parhizgar et al [
59].
3.7. Ischemic Myocardial Injury and Coronary Thrombosis
There is an established link between the incidence of acute myocardial infarction (AMI) and respiratory infections. For example, patients who are affected by influenza virus or other respiratory infections have an increased hazard of AMI [
97]. Respiratory illness can lead to prothrombotic states, myocardial supply–demand mismatch, platelet activation, coronary vasoconstriction and endothelial cell dysfunction [
98]. Indeed, platelet surface expresses specific low affinity FcγRIIa receptors and additional high affinity for IgE FcεRI and low affinity for IgE FcεRII/CD32 receptors that constitute potential targets for many antigens and can further promote thrombosis [
99]. These factors can lead to plaque rupture and thrombosis, leading to myocardial injury. According to recent reports, ST segment-elevation myocardial infarction (STEMI), may represent the first clinical manifestation of COVID-19. Indeed, STEMI represented the first clinical manifestation of COVID-19 in 85.7% of patients who did not have a COVID-19 test result at the time of coronary angiography [
100]. Therefore, further elucidation of the myocardial injury pathophysiology in patients with COVID-19 seems of paramount importance.
A multi-center observational study assessing clinical and prognosis differences in patients with COVID-19 STEMI demonstrated a significant increase in in-hospital mortality, stent thrombosis and cardiogenic shock after percutaneous coronary intervention in patients with STEMI and COVID-19 in comparison with contemporaneous non-COVID-19 STEMI patients [
101]. The North American COVID-19 STEMI registry [
102], investigated the outcome of 230 patients with confirmed COVID-19 infection compared to 995 controls. Patients with COVID-19 and STEMI were more likely to present cardiogenic shock or cardiac arrest and were less likely to have primary PCI as compared to age and sex-matched control patients. The door-to-balloon time was longer in patients with COVID-19 infection who were more likely to have no culprit lesions identified on invasive angiography. COVID-19 positive patients had longer intensive care and hospital lengths of stay, and the primary outcome of in-hospital death, stroke, recurrent MI or unplanned revascularization occurred in 36%, compared to only 4% in control patients, which was driven primarily by in-hospital mortality.
These results were expected as COVID-19 positive patients presenting with STEMI, had greater infarct size, reduced left ventricular function, greater intracoronary thrombus burden, higher rates of cardiogenic shock, requirement for hemodynamic support and life-threatening arrhythmias [
103]. A meta-analysis by Gharilbzadeh et al. who summarized all these studies [
104] demonstrated that the pooled prevalence of mortality in COVID-19 patients with STEMI was just over 25%.
Although myocardial infarction has been observed in the acute phase of COVID-19 infection, fewer studies have focused on myocardial ischemic disease in the post COVID-19 period [
59]. It has been estimated that approximately 20%-30% of patients with COVID-19 infection experience chest pain, and angina-like chest discomfort, as post COVID-19 symptoms [
105]. However, post-COVID-19 chest pain might be caused in part by noncardiac causes including anxiety, physical and emotional stress, musculoskeletal, and pulmonary factors and physicians should be careful for the correct diagnosis. In a study of patients who were hospitalized with severe SARS-CoV-2 infection and presented with elevated troponin levels on admission, 26% showed ischemic-pattern findings on a follow-up cardiac MRI, 2 months after hospitalization [
106]. In patients who admitted to ICU and received mechanical ventilation during their acute COVID-19 illness, 19% had newly diagnosed coronary artery disease 6 months post ICU admission [
107]. In another study, patients without history of cardiovascular disease who experienced chest pain during their COVID-19 illness, the adenosine stress perfusion cardiac magnetic resonance imaging (MRI) showed significant circumferential subendocardial perfusion defect in 50% of cases over 8 months post SARS-CoV-2 infection [
108]. Therefore, post COVID-19 atypical chest pain needs detailed attention.
3.8. Kounis Syndrome and COVID-19
Kounis syndrome is a disease manifesting as coronary spasm, myocardial infarction and stent thrombosis. It is related to mast cell activation and inflammatory cell interactions including T-lymphocytes and macrophages, and associated with allergic, hypersensitivity, or anaphylactic insults [
109]. In COVID-19 infection, mast cells constitute a key source of proinflammatory cytokines [
110].
Moreover, mast cell derived proteases and eosinophil-associated mediators are elevated in COVID-19 patient sera and lung tissues [
111]
. Whereas these cells are typically activated by allergic triggers, they can also be triggered by virus-associated molecular patterns [
111]. This is achieved via activation of toll-like receptors (TLRs), including SARS-CoV-2, in order to release a variety of proinflammatory mediators such as IL-6 and IL-1β, thus potentially contributing to COVID-19 pathology [
112]. These cells enter the circulation from bone marrow as mononuclear cell precursors and circulate as mast cell precursors, disposing in their surface KIT receptors (cytokine receptors) for stem cell factor (SCF). SCF is essential for mast cell growth, survival, differentiation, proliferation, adhesion, and homing. Mast cells can adhere to all human tissues, even to the brain tissue that does not suffer from allergic reactions because IgE antibodies cannot cross the blood–brain barrier. In these tissues, mast cells differentiate and mature during several days or even weeks. In the coronary arteries, mast cells, mature under the influence of local micro-environmental factors, resulting in different phenotypes [
112]. The followings indicate and support the view of an association between COVID-19 and Kounis syndrome:
a. COVID-19 affects the coronary and peripheral arterial vasculature as it can induce coronary spasm, direct endothelial or vascular injury, plaque rupture and microthrombi, hypoxic injury, cytokine storm, and, a higher than expected, incidence of stent thrombosis. This is attributed to the underlying hypercoagulable state that clinically coincides with the 3 main Kounis syndrome types namely coronary spasm, acute myocardial infarction, and stent thrombosis [
113]
b. Antihistamines (famotidine, rupatadine, ebastine) that block histamine receptors, and corticosteroids (dexamethasone) which are potent anti-inflammatory and immunomodulating agents, are the drugs of choice for treating both COVID-19 and Kounis syndrome [
114].
c. In asymptomatic subjects, the COVID-19-induced activation of the immune system could increase the risk of conversion from asymptomatic, subclinical, or atherosclerotic disease into an unstable state with vulnerable plaques prone to thrombosis, as in Kounis syndrome [
115]
d. COVID-19-induced cytokine storm and hyper-coagulopathy can present with large cerebral vessel occlusion and Kounis syndrome as a result of increased risk of fatal arterial thrombosis [
116].
e. COVID-19 cardiac arrest due to Prinzmetal’s angina resembling Kounis syndrome in a previously normal heart has been described recently [
117].
f. COVID-19 is associated with an increase in effector cells including IgEs and eosinophils [
118].
g. A female patient with erythematous lesions, mild itching, nausea, diaphoresis and weakness, was diagnosed to suffer from scombroid syndrome after a meal with canned tuna. She was found positive for SARS–CoV2 infection and developed acute coronary syndrome of Kounis type I [
119].
This case demonstrated a linkage of Kounis syndrome with SARS–CoV–2 infection and histamine fish poisoning [
119].
3.9. Long COVID-19 and the Heart
Long COVID-19 is characterized as symptoms lasting four weeks or more since the follow-up index date and include symptom onset, admission to a hospital, and hospital discharge. This condition is attributed to numerous risk factors that include ethnic minority, chronic obstructive pulmonary disease, female gender, obesity, psychiatric conditions, smoking and socio-economic deprivation [
120].
General symptoms of long COVID included general pain, muscle or joint pain, and mobility dysfunction, fatigue, fever, hair fall, skin rash, and weight loss. Cardiopulmonary symptoms have reported six months after recovery and include chest pain which was reported to be 5%, palpitations 9%, sore throat, dyspnea, dyspnea and cough [
121].
In a study of 534 patients who were submitted to cardiac magnetic resonance imaging at baseline, 6 months and 12 months after the onset of long COVID symptoms, findings such as ventricular dilatation, systolic dysfunction, reduced global strain and elevated native T1 signals were present in approximately 20% of patients [
122]. New onset of atrial fibrillation (AF) in patients with acute COVID-19 and in patients with any other systemic illness have been also reported [
123]. Clinical experience suggests that many of these patients may not necessarily develop recurrent AF after recovery, which obviates the need for rate, rhythm, and antithrombotic therapy [
124]. The intensity and timespan of long COVID-19 cardiac symptoms differ from patient to patient. Detecting long COVID-19 risk factors constitutes an important and active area of research [
125].
3.10. Myocardial Fibrosis
Myocardial fibrosis after recovery from acute SARS-CoV-2 infection is the result of acute myocardial injury, including ischemic myocardial involvement and myocarditis [59, 126]. Other studies have reported that approximately 20%-30% of the patients experience myocardial fibrosis after recovery from acute COVID-19 infection [
59,
127]. In a prospective study of 159 patients hospitalized with COVID-19, it was found that 1 in 5 patients had evidence of myocardial fibrosis 28-60 days post-discharge [
128]. Moreover, 21% of patients in another investigation, using late gadolinium enhancement imaging, were found to have myocardial fibrosis 6 months after ICU admission [
129]. Two additional studies using also cardiac magnetic resonance imaging have demonstrated that the incidence of myocardial fibrosis was 31% and 32% respectively with a similar distribution of injury [
130,
131]. All patients reported cardiac symptoms at follow-up and all had been hospitalized with COVID-19 infection. In the study by Puntmann et al [
129], one third of patients required hospitalization, and 19% required ventilatory support.
3.11. Myocarditis Related to SARS-CoV-2 Infection
Myocarditis is an inflammatory disease of the myocardium with myocardial damage but without an underlying ischemic etiology. Viral infection is a frequent cause of myocarditis leading to hospitalizations, heart failure, and can be followed by sudden cardiac death. Confusion still exists on the proper definition and differentiation of myocarditis caused by vaccines, drugs, or substances. The types of myocarditis [
132] are classified by causative, histological, and clinicopathological criteria are represented in
Table 4.
Histological evidence of an inflammatory cell infiltrate with or without myocardial damage is the gold standard for diagnosing myocarditis. However, due to its mild initial clinical course, the pathogenesis of COVID-19-associated myocarditis is poorly understood as myocardial biopsies are not routinely performed.
Despite that myocarditis is considered as an infrequent cardiovascular complication of COVID-19, several cases of acute myocarditis in patients with this viral infection have been published. According to a recent consensus statement, in patients admitted to the hospital with COVID-19, the average prevalence of definitive/probable myocarditis was estimated to be 2.4 cases/ 1000 hospitalized patients and there may have been elevated mortality (20.4%). Moreover, the risk of myocarditis was increased by about 10 times in the month after a positive SARS-CoV-2 test, and its development was more frequent in men (60%) [
133]. Several hypotheses have been proposed for the pathophysiological mechanisms of COVID-19 associated myocarditis. They include cytotoxicity mediated by a hyperimmune response triggered by mRNA molecules, excessive release of inflammatory mediators triggering lymphocyte dysregulation that causes T lymphocyte-mediated necrosis, direct myocardial damage and autoimmune disorders mediated by molecular mimicry. The incidence of myocarditis in hospitalized COVID-19-patients in Germany was 1.28 cases per 1000 hospitalizations in 2020. Risk factors for myocarditis in COVID-19 were young age, male sex, pneumonia, and multisystemic inflammatory COVID-19-infection. Myocarditis was independently associated with increased case-fatality [
134]. Another study using cardiac magnetic resonance imaging in COVID-19 patients with increased troponin levels has demonstrated that the 50% of patients had pathological findings of myocarditis (27%), ischemic heart disease (22%), and the rest were nonspecific [
135]. According to a
Propensity Matched Analysis of National Inpatient Sample in the USA myocarditis as part of long COVID has also been reported in several studies [
136]. In a large study, an extra 40 myocarditis events per million were noted 1–28 days after a positive SARS-COV-2 test in people 16 years and older [
137]. Moreover, another study indicated that males aged 12–17 were likely to have 450 cases per million infections within three months of COVID-19 infection [
138,
139]. Clinically, in patients with persistent chest pain, exertional dyspnea or asthenia, palpitations, or syncope, in the absence of myocardial damage, the possibility of myocarditis should always be considered. The management of COVID-19-related myocarditis is primarily supportive while treating the underlying COVID-19 infection. Drugs such as steroids, antivirals, and comfort medications are typically given to COVID-19 patients. Further management can include treatment of complications and tertiary comorbidities such as heart failure, which should be treated based on current standards of care with goal-directed medical therapy [
140].
3.12. New Onset Hypertension and COVID-19
Arterial hypertension has been recognized as one of the most common comorbidities in COVID-19 patients and as an independent predictor of short-term mortality and severe disease [
141]. New onset hypertension has been also observed after acute COVID-19 illness. A recent analysis [
142] of 19,293,346 patients (mean age 54.6 years, 54.6% males), including all studies published at any time up to February 11, 2023, and reporting the long-term risk of new-onset hypertension in COVID-19 survivors, demonstrated the following: During a mean follow-up of 6.8 months, new-onset hypertension observed to 12.7 [95% CI 11.4–13.5] out of 1000 patients survived from COVID-19 infection compared to 8.17 [95% CI 7.34–8.53] out of 1000 control subjects. Moreover, of 543 patients who were hospitalized or discharged from an emergency department for COVID-19, 12 (2.2%) had onset of high blood pressure in the following year [
143]. This study had no control group and the authors felt that this finding might relate to pandemic stress rather than COVID-19 infection. Another study showed that 1.3% of 538 patients suffering from COVID- 19 developed hypertension by approximately 3 months after hospital discharge [
144]. New onset hypertension has been observed even among young and previously healthy patients. One study of young adults (mean age: 21 [SD, 20-22] years) demonstrated a prolonged effect on systolic and mean arterial blood pressure after acute COVID-19 illness, with gradual improvement 6 months post-infection [
145]. Healthy adolescences have also developed new onset hypertension several months after mild COVID-19 illness [
146]. A possible explanation for the new onset hypertension is the action of ACE2 receptors. When the SARS-CoV-2 virus binds to ACE2, it prevents ACE2 from performing its normal function to regulate angiotensin II (ANG II) signaling. Thus, ACE2 action is “inhibited,” and making more ANG II available to injure tissues. This surge of ANG II likely contributes to vasoconstriction and new onset hypertension in COVID-19 patients,
3.13. Pericarditis and Pericardial Involvement
The association between pericardial diseases and infections was first reported by Bing in 1933 [
147]. Parvovirus B19, Echovirus, Coxsackie and Herpesviruses are considered as the most common infecting agents. It is plausible that coronaviruses, similar to other infections, may lead to pericardial involvement. In the past, cases of pericarditis in patients infected with other members of the coronavirus family have also been reported [
148]. Pericardial involvement represents a frequently under-diagnosed entity, associated with significantly higher all-cause mortality in patients with COVID-19 [
149]. Clinically, presents as pericardial effusion, acute pericarditis, as well as more rarely constrictive pericarditis or as life-threatening cardiac tamponade. Usually, the pericarditis is accompanied with myocarditis and the frequency of pericardial involvement alone is estimated to be much lower [
150].
Although the main pathophysiology mechanism of pericardial diseases in COVID-19 is not yet fully understood, it is hypothesized that it occurs secondary to the systemic cytokine storm and the subsequent cytotoxic and immune-mediated effects related to SARS-CoV-2 [
151]. Proinflammatory cytokines (IL-6, TGF-β) and vascular endothelial growth factor released via the associated angiotensin II pathway could promote inflammation and fibrosis of the pericardium in COVID-19 infection [
152]. In addition, in a case with cardiac tamponade, the viral presence was detected in the pericardial fluid, providing a new piece in the complex puzzle of pathophysiology mechanism [
153]. Many epidemiological studies have been demonstrated an increase in cases of pericarditis in infected patients with COVID-19. Furthermore, it seems to be a marker of worse outcome [
154]. Pericarditis induced by COVID-19 resembles to other forms of viral pericarditis. There is no specific strategy regarding the medical management of pericarditis in patients with COVID-19 and pre-COVID pericarditis [
155].
Usually, pericardial involvement can present as an asymptomatic pericardial effusion. Nevertheless, despite several studies the exact prevalence of pericardial effusion in patients with COVID-19 is unclear. In a systematic review and meta-analysis pericardial effusion was seen in approximately 5% of patients with COVID-19 on chest computed tomography [
156]. In contrast, Liu et al. detected pericardial effusion in approximately 90% of critically ill patients [
157]. In a retrospective cohort study of 718.365 patients with COVID-19, 5.0% of them, developed new-onset pericarditis. Six-month all-cause mortality was 15.5% for pericarditis and 6.7% in matched controls. Furthermore, patients with pericarditis seemed to be associated with more new-onset cardiovascular sequelae than those with myocarditis [
158]. In a observational cohort study on 100 patients with severe COVID-19, pericardial effusion had a high prevalence (27%) and only in 30% of them developed pericarditis. Additionally, the overall mortality was higher in patients with pericardial effusion [
159]. In a large prospective cohort of 530 hospitalized patients with COVID-19, pericardial effusion was found in 75 (14%), but only 17 patients (3.2%) fulfilled the criteria for acute pericarditis. Additionally, pericardial effusion was associated with COVID-19 severity, myocardial dysfunction and excess mortality [
160]. Hence, these several studies suggesting that pericardial fluid could be used as a marker for several disease and a poor outcome.
Cardiac magnetic resonance (CMR) is the most sensitive imaging modality for identifying pericardial involvement and may be helpful in some cases. It has been used in several studies to evaluate symptomatic and asymptomatic individuals with COVID-19 [
161]. In a cohort study of 100 patients recently recovered from COVID-19 infection, CMR revealed pericardial involvement (22%), even in the absence of cardiac symptoms [
162].
Despite cardiac tamponade is thought to be a rare presentation of pericardial diseases, in a systematic review was found a higher proportion (35%) of cardiac tamponade in patients with COVID-19 compared with other viral infections. After reviewing the literature, we found several case reports and case series of cardiac tamponade secondary to COVID-19 [
163,
164,
165]. Although this could be overestimated as the information is derived from case reports, it is possible that the large inflammatory response presented in COVID-19 patients leads to the development of this complication [
166]. Constrictive pericarditis rarely occurs in these patients and only few cases in the literature have been reported [
167].
In conclusion, pericardial involvement in patients with COVID-19, represents a frequently under-diagnosed entity, associated with significantly higher all-cause mortality.
3.14. Postural Orthostatic Tachycardia Syndrome (POTS)
Postural orthostatic tachycardia syndrome is a heterogenous multidisciplinary and multisystem disorder
characterized by orthostatic intolerance and tachycardia, which significantly impairs quality of life [
168].
The syndrome is thought to be the most prevalent cardiovascular dysautonomia and is considered to be a major phenotype/ sub-syndrome in the post-acute COVID-19 syndrome that can develop after SARS-CoV-2 infection [
169]. The majority of patients are predominately female (4:1) and relatively young. Patients usually present with varied symptoms, such as chest discomfort, fatigue, muscle weakness, gastrointestinal symptoms, sleep disturbances, dyspnea, palpitations and pre-syncope [
170]. Diagnostic criteria are defined as the presence of chronic symptoms of orthostatic intolerance accompanied by an increased heart rate≥30 bpm within 10min assuming upright posture without significant hypotension. Additionally, the orthostatic tachycardia must occur in the absence of other overt cause of orthostatic symptoms or tachycardia (eg, hypovolemia, hyperthyroidism, medications) [
171].
There is strong evidence that SARS-CoV-2 may trigger POTS in patients with long COVID-19 syndrome [
172]. Although the exact pathophysiology mechanism is unclear, the combination between autoimmunity, relative central hypovolemia, small fiber neuropathy, hyperadrenergic stimulation and pro-inflammatory cytokine production (ie. cytokine storm) is thought to be a critical aspect between POTS and COVID-19 infection [
173]. Of particular interest is that both sympathetic and parasympathetic nervous system receptors are immune-mediated targets. Gunning et al. demonstrated that POTS is associated with elevated G-Protein coupled receptor autoantibodies [
174]. Other proposed mechanisms included those against ACE-2 and muscarinic receptors [175.] Additionally, have been identified associated polymorphisms in several candidate genes [
176]. Recently, a number of case reports and case series have been published describing patients who developed POTS after SARS-CoV-2 infection [177, 178]. From the current literature, the exact prevalence of POTS in COVID-19 survivors varies due to heterogeneity of examined populations and the lack of applying rigorous diagnostic criteria [
179]. In a retrospective analysis from Mayo clinic, where autonomic testing was conducted a median of 119 days following acute COVID-19 infection, 22% of patients fulfilled the criteria for POTS [
180]. In a prospective study, 29 patients with long-haul COVID symptoms, underwent a tilt table test with cerebral blood flow measurement for quantification of their orthostatic intolerance. In the first 12 months after the infection, 71% of them developed POTS and none of them after 24 months during follow-up. The authors concluded that the incidence of POTS decreased over time after the onset of the long COVID-19 disease, with none of them after 24 months [
181]. An online survey from 56 countries, analyzed 3672 participants with long-COVID symptoms. Of the 2308 patients who reported tachycardia, 72.8% reported being able to measure their heart rate in standing versus sitting posture. Of those, 30.65% reported an increase in heart rate, suggesting the possibility of POTS [
182]. In contrast with other studies, Monaghan et al. showed that POTS was not associated with orthostatic intolerance in 85 patients with long- COVID symptoms, presenting only in 1 of them. However, this study had several important limitations, such as a median delay to testing of 302 days [
183].
The knowledge about the management of POTS induced by COVID-19 is limited, so further controlled studies are warranted. Thus, the current guidelines for POTS should be used for patients presenting with post–COVID-19 POTS. As first line therapy, include increasing intake of fluids and salt, physical counter-pressure maneuvers, and aerobic exercise. In cases where non-pharmacological management is insufficient a variety of medications can be used, including beta-blockers, fludrocortisone, midodrine and ivabradine [
184]. In several cases, with promising effects, can be used the intravenous immunoglobulin [
185]. A small Indian study, demonstrated that ivabradine was more effective than carvedilol for symptomatic control of tachycardia in post-COVID-19 survivors [
186]. In a prospective observational study, a total of 55 young patients with post-COVID-19 POTS, were treated with ivabradine. Within 7 days of ivabradine treatment, reported improvement of their symptoms (78%), with significant reduction of 24-hour average heart rate, and improvement of heart rate variability time domains [
187]. In conclusion, recent literature highlights the importance of recognizing POTS as a possible complication induced by SARS-CoV-2. Further prospective studies are also necessary to understand the possible mechanisms, therapies and future directions.
3.15. Pulmonary Hypertension
Pulmonary hypertension (PH) is a pulmonary vascular disease characterized by vasoconstriction and pulmonary arterial remodeling leading to elevated pulmonary artery pressure and, ultimately, right heart failure. Some recent studies have reported PH after acute COVID-19 illness, although evidence remains limited. Indeed, few cases of COVID-19 disease in patients with PH have been reported.
A current retrospective cohort study, which included elderly critically ill patients with severe COVID-19 pneumonia without previous history of heart failure demonstrated increased pulmonary artery systolic pressure. The increased pulmonary artery systolic pressure predicted admission to intensive care unit and the hospital mortality [
188]. Another recent cross-sectional study which was conducted at a large tertiary center for PH patients demonstrated that COVID-19 infection in patients with increased PH was associated with high mortality and morbidity. The authors concluded that more scientific proof is needed to clarify different aspect of COVID-19 infection in this population [
189]. Some other studies have demonstrated that patients suffering from COVID-19 infection developed PH after their illness. Tudoran and colleagues reported that of 91 patients hospitalized for moderate COVID-19, 7 patients (7.69%) were diagnosed with PH 2 months after discharge [
59,
190]. These patients were younger than 55 years of age, did not require mechanical ventilation during hospitalization and they did not have any previous history of cardiovascular pathology. Oher studies have demonstrated development of PH 6-12 weeks after relatively severe COVID-19 illness [
59,
191].
An incidence of 2.9 cases of COVID-19 infection per 1000 patients was demonstrated in a survey of 58 PH centers in the United States [
192,
193]. Hospitalization was markedly higher than for the general population at 30% of cases, and mortality occurred in 12% of cases [
193,
194]. This study included 7 pediatric centers, and there was not a breakdown of the pediatric patients with PH and COVID-19. Pulmonary hypertension has been categorized as an underlying condition that confers higher risk for serious SARS-CoV-2 infection. There are several mechanisms by which COVID-19 can worsen underlying PH [
190,
193,
194]:
a. Hypoxia due to pneumonia and hypercapnic vasoconstriction is a potential mechanism for worsening PH.
b. Cytokine release due to accumulation of inflammatory cells in the endothelium results in further cell death and inflammation, potentially worsening underlying PH. The endothelin upregulation has been proposed as a promoter of PH development.
c. Thickening of pulmonary arterial walls has been observed histologically in patients who died of COVID-19.
d. Pulmonary damage and vascular remodeling has also been linked to post COVID PH and PH shares similar molecular features including endothelial and mitochondrial dysfunction.
3.16. Stroke and Cerebrovascular Disease
The patients who suffer from COVID-19 and having additional comorbidities are at risk for cerebrovascular complications and stroke. Several studies have demonstrated that stroke and cerebrovascular diseases are not rare in these patients [
195].
In a retrospective study of 214 hospitalized patients conducted in Wuhan, China, 78 (36.4%) had manifestations associated with the nervous system including stroke, dizziness, consciousness-level alterations, ataxia and convulsions [
196]. Stroke has also been reported in younger patients of aged 33-49 years who suffer from COVID-19 [
197].
In a survey of 125 COVID-19 patients carried out in the United Kingdom, the cerebrovascular events were present in 77 (62%), of which stroke was the main associated complication, with 57 (74%) cases [
198]. Other studies have demonstrated that cerebrovascular disease was more frequent in deceased patients in comparison to recovered (4% vs.0%) [
199] and was higher in non-survivors in comparison to survivor groups (22 vs. 0%) [
200].
In another study, cerebrovascular disease was observed in 6.8% of the patients included, with a statistically significant difference between severe and non-severe forms of COVID-19 (20% versus 2.4%, P < 0.001) [
201]. Moreover, it was statistically significant different in patients with cardiac injury in comparison with patients with no cardiac injury (15.9% versus 2.7%, P = 0.001) [
202] and in chronic heart failure there was statistically significant difference in the group of non-survivors in comparison with the survivor group (12.9% versus 2.3%, P < 0.001) [
203].
3.17. Takotsubo Syndrome
In this pandemic, one would expect a rise in the incidence of potentially life-threatening Takotsubo Syndrome (TTS) due to enhanced stress and anxiety. However, this remains to be proven as current literature of COVID-19-related cardiac effects usually do not even introduce TTS. Nevertheless, the incidence of TTS ranges from 4–8% during the pandemic, higher than the anticipated 1.7–2.0% before the pandemic in patients with acute coronary syndrome [
204]. Clinical diagnosis is based mainly on the precordial chest pain (in 80–90% of cases), electrocardiographic ST and T wave acute ischemic changes (95%), and the pathognomonic large symmetrical areas of apical and/or midventricular akinesia or dyskinesia on echocardiography [
205], which is reversible within 30 days. While serum testing in COVID-19 patients was not routinely performed, the catecholamine surge alone is frequently claimed to cause TTS without definite evidence; however, if that is the case, the use of catecholamines to manage cardiogenic shock would be contraindicated. Hence, the surge is more likely secondary to TTS event, suggesting the complex pathophysiology of TTS. TTS associated with COVID-19 has been reported to occur as either direct infectious complication or indirect psychological consequence of social isolation due to quarantine. Because some COVID-19 patients have cutaneous microvascular changes and pulmonary vascular endothelialitis in histological studies of the lungs [
206], similar changes could be systemic or cardiac, leading to luminal thrombosis due to endothelial dysfunction in both the lungs and other organs [206-208]. Therefore, the interplay of coronary endothelial dysfunction, septic state, inflammatory storm, hypercoagulability, endothelial necrosis, and small-vessel clotting, have been hypothesized as a link between COVID-19 and TTS. Angelini et al. postulated a theory of “coronary endothelial dysfunction-coronary artery spasm (CAS)-myocardial stunning” [
209]. According to this theory, a patient becomes infected with COVID-19, and about one week the coronary endothelium develops hyperactive immune or inflammatory reaction, leading to acute TTS in the presence of stress and/or catecholamine surge (either naturally produced or administered for shock). The initial CAS can be suppressed by nitroglycerin administration within a few minutes of onset in spontaneous TTS or at CAS provocative testing. Because, for unknown cause, the enhanced CAS related to endothelial dysfunction gradually dissolves, TTS spontaneously resolves within a few days in survivors [
210], and after about 1 week, TTS usually is not reproducible by CAS provocative testing, whereas recurrent TTS is rare. However, delayed spontaneous recurrence of TTS may appear in more than a week, when recurrent CAS can be reproduced by provocative testing [
211]. Of note, while coronary endothelial dysfunction is reportedly necessary for the development of CAS and CAS-related TTS in COVID-19 patients [
205], microvascular dysfunction has not been demonstrated.
The initial management of TTS includes dual antiplatelet therapy, anticoagulants, beta-blockers, diuretics, angiotensin-converting enzyme inhibitors or aldosterone receptor blockers and levosimendan [
212]. While animal immobilization stress-induced TTS models have shown a protective effect of beta-blockers and alpha-blockers [
213], their combination effects in humans at the acute stage of TTS do not persevere long term [
214]. The use of sublingual or intravenous nitroglycerin could provide further symptomatic relief. Although the pathophysiology of TTS is complex and still not fully understood, early TTS diagnosis within 15–60 min of onset is potentially life-saving [
209] and can be subsequently confirmed by CAS provocative testing. Awareness of COVID-19-related TTS among physicians is therefore necessary.
3.18. Thromboembolism and COVID-19
COVID-19 infection may be associated with a hyper-coagulable state, which leads to microvascular and macrovascular arterial and venous thromboembolism. According to 35 observational studies reported from around the world, the incidence of thromboembolism complications in COVID-19 patients ranged from 1.7 to 16.5% in (total N = 9249) [
215,
216]. In England and Wales, amongst 1.4 million patients who were found positive for COVID-19, when adjusted for age, sex and region, the hazard ratio (HR) for developing deep vein thrombosis was 12 in the first week after COVID-19 diagnosis, and reduced to 2.6 at 27–49 weeks. The same findings were observed for pulmonary embolism (adjusted HR 39 at 1 week, declining to 2.2 at 27–49 weeks) and arterial thrombosis (HR 27 at 1 week, and 1.9 at 27–49 weeks) [
215,
217].
Cytokine storm, complement activation, and endotheliosis have been postulated as potential causes of COVID-19 induced thromboembolism. [
218]. Several risk factors associated with higher severity of COVID-19 and higher mortality such as inflammatory markers including interleukin-6, D-dimer, ferritin, and lactate dehydrogenase have been also proposed for these thrombotic events [
219]. Moreover, autopsies studies in patients succumbed during COVID-19 illness have demonstrated microthrombi [
220].
In addition, a hypercoagulable state consisting of several elevated circulating prothrombotic factors such as elevated von Willebrand factor, factor VIII, D-dimer, fibrinogen, neutrophil extracellular traps, prothrombotic microparticles, and anionic phospholipids has been observed to correlate with illness severity and mortality present in severe cases of COVID-19. This hyper-coagulant state together with abnormal blood flow and endothelial injury constitute the Virchow's trial of thrombosis [
221]. Indeed, the myocardial injury is clinically manifesting as arterial and venous thromboembolism. Moreover, there is a medical paradox about COVID-19 associated with thrombosis and hormonal contraception. Indeed, whereas the higher estrogen levels may be protective against severe COVID-19 disease [
222], the hormonal contraception use during the COVID-19 pandemic, is an independent risk factor for thrombosis, particularly with estrogen-containing formulations [
223].
a. Venous Thromboembolism: Deep-vein thrombosis (DVT) usually affects the venous system of the lower extremities, with clot formation originating in the deep veins of the femur and spreading proximally [
224]. The incidence of DVT in patients suffering from COVID-19 infection, has been reported at 14.8% [
225]. A history of previous or active cancer, the elevated D-dimer levels, the length of stay in the hospital and the need for a high-flow nasal cannula or noninvasive ventilation were significantly associated with the development of DVT in patients with a COVID-19 infection. Indeed, the prevalence of DVT on days 7, 14, and 21 was 16%, 33%, and 42%, respectively [
225]. Moreover, an increased incidence of DVT has been observed in critically ill COVID-19 patients with high D-dimer [
226].
The thrombus formation during DVT may spread to general circulation and induce pulmonary embolism. Pulmonary embolism can be predicted with a sensitivity of 100% and a specificity of 62% [
224] when the D-dimer levels are higher of 1600 ng/ml.
Thrombus formation during COVID-19 infection can affect the cerebral venous vasculature and induce cerebral venous thrombosis (CVT). The percentage of CVT among all cardiovascular events associated with COVID-19 was approximately 4% [
226]. COVID-19 associated CVT affects the transverse sinus (65%) the sigmoid sinus and superior sagittal sinus which are affected in approximately 45%. Similar to COVID-19 associated PE and DVT, elevated D-dimer and
C-reactive protein levels in most COVID-19 associated CVT cases. Lymphopenia was another commonly reported laboratory finding.
b. Arterial Thromboembolism (ATE): Apart from coronary arteries, the COVID-19 infection can affect the mesenteric, splenic and renal arteries [
227]. However, the ATE of these arteries is rare and mostly only case reports and small case series have been published so far. The reported rates, of COVID-19 associated mesenteric ischemia, range from 15 to 38%, suggesting a predominant small vessel affection [
227,
228] Abdominal pain, vomiting and nausea together with abdominal distention are common signs and symptoms during the physical examination. The diagnostic biomarkers are the same in arterial and venous mesenteric ischemia. The reported mortality rates of COVID-19 associated mesenteric ischemia is high ranging from 34 to 54% [
229].
4. Conclusion
This narrative review highlights evolving evidence on the potential cardiovascular complications during and after the course of acute COVID-19 infection together with proposals on underlying mechanisms. Indeed, a variety of cardiovascular complications seem to occur frequently in COVID-19 which may worsen the clinical course. Damage associated with the acute COVID-19 illness, inflammation inducing injury, and exacerbation of preexisting conditions might be the cause of cardiovascular complications [
59]. Vascular ischemia causing myocardial injury, cardiac dysfunction, arrhythmias, myocarditis, POTS, dysautonomia are commonly published outcomes of COVID-19 illness. Some of these complications have been observed in mild cases fact that needs vigilance especially in the young healthy sufferers. In athletes, for example, recovering from COVID-19, is recommended 2-week convalescence period followed by no diagnostic cardiac testing if asymptomatic, and an electrocardiogram and transthoracic echocardiogram in mildly symptomatic [
230].
Despite that some studies have shown improvement over the time, the continued vigilance for long COVID complications and even for years after will be needed to better characterize long-term outcomes. We must not forget that myocarditis can cause dilated cardiomyopathy resulting in end-stage heart failure requiring advanced therapies including orthotopic heart transplantation [
231]. Therefore, further clinical research is required for specific diagnostic and therapeutic options and to reveal robust outcome and long-term sequelae data in COVID-19 associated myocarditis.
The current evidence remains limited in quantity and quality, therefore we, as other colleagues, hope that such reviews will encourage clinicians to be aware of possible cardiovascular risks that can prompt future investigations.
Author Contributions
Conceptualization, S.P., S.N.K., E.P.T., V.M., P.P., G.S., M.Y.H., I.K., M.A.M., P.D., N.G.K.; writing-original draft preparation, N.G.K., C.d.G., S.A., E.P.T., I.K., M.Y.H., C.G. drafting the figure, revision, N.G.K., C.d.G., I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Abbreviations
| AMI |
Acute Myocardial Infarction |
| ANG II |
Angiotensin II |
| ARDS |
Acute Respiratory Distress Syndrome |
| AF |
Atrial Fibrillation |
| ACE2 |
Angiotensin Converting Enzyme 2 |
| ATE |
Arterial Thromboembolism |
| CA |
Cardiac arrest |
| CVT |
Cerebral Venous Thrombosis |
| CMR |
Cardiac magnetic resonance |
| COVID-19 |
Coronavirus Disease-2019 |
| CAS |
Coronary Artery Spasm |
| CAAs |
Coronary Artery Aneurysms |
| DVT |
Deep-vein thrombosis |
| EF |
Ejection Fraction |
| Fc |
Fragment crystallizable |
| FcγRIIa |
Fragment crystallizable Γαμα (gama) region IIa |
| HF |
Heart Failure |
| FcεRI |
Fragment crystallizable epsinon region I |
| HR |
Hazard Ratio |
| hERG-K |
human Ether-à-go-go-Related Gene |
| G-CSF |
Granulocyte Colony-Stimulating Factor |
| GM-CSF |
Granulocyte-Macrophage Colony-Stimulating Factor |
| IgE |
Immunoglobulin Epsilon |
| ICU |
Intensive Care Unit |
| IHCA |
In-Hospital Cardiac Arrest |
| IL |
Interleukin |
| KKS |
Kinin-Kallikrein System |
| LVEF |
Left Ventricular Ejection Fraction |
| LVLS |
Left Ventricular Longitudinal Strain |
| LVD |
Left Ventricular Dysfunction |
| MA |
Medical assistance |
| MRI |
Medical Resonance Imaging |
| MIP-1 |
Monocyte Chemoattractant Protein 1 |
| MIS |
Multisystem inflammatory syndrome |
| OHCA |
Out-of-hospital Cardiac Arrest |
| POTS |
Postural Orthostatic Tachycardia Syndrome |
| PH |
Pulmonary Hypertension |
| RAAS |
Renin-Aldosterone System |
| RV |
Right Ventricular |
| RVD |
Right Ventricular Dysfunction |
| STEMI |
ST segment-elevation myocardial infarction |
| SCF |
Stem Cell Factor |
| SARS-Co-2 |
Severe Acute Respiratory Coronavirus-2 |
| S1Q3T3 |
wave in lead 1, Q wave in lead 3, inverted T wave in lead 3 |
| TTS |
Takotsubo Syndrome |
| TNF |
Tumor Necrosis Factor |
| TLRs |
Toll-like Receptors |
| VT |
|
References
- Vosko, I.; Zirlik, A.; Bugger, H. Impact of COVID-19 on Cardiovasculardisease. Viruses 2023, 15, 508. [Google Scholar] [PubMed]
- Condurache, D.-G.; Shanmuganathan, M.; Raisi-Estabragh, Z.; Raman, B. Editorial: Post-COVID-19 cardiovascular sequelae. Front. Cardiovasc. Med. 2023, 10, 1191953. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Mathur, S.; Zhang, R.; Yan, V.K.C.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: a prospective cohort in UK Biobank. Cardiovasc Res 2023, cvac195. [Google Scholar]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Assimakopoulos, S.F.; Velissaris, D.; Hung, M.-Y.; Mplani, V.; Saba, L.; Brinia, A.; Kouni, S.N.; et al. COVID-19 Disease, Women’s Predominant Non-Heparin Vaccine-Induced Thrombotic Thrombocytopenia and Kounis Syndrome: A Passepartout Cytokine Storm Interplay. Biomedicines 2021, 9, 959. [Google Scholar] [CrossRef]
- Cooper, S.L.; Boyle, E.; Jefferson, S.R.; Heslop, C.R.A.; Mohan, P.; Mohanraj, G.G.J.; Sidow, H.A.; Tan, R.C.P.; Hill, S.J.; Woolard, J. Role of the Renin-Angiotensin-Aldosterone and Kinin-Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. Int J Mol Sci 2021, 22, 8255. [Google Scholar] [CrossRef]
- Barthe, M.; Hertereau, L.; Lamghari, N.; Osman-Ponchet, H.; Braud, V.M. Receptors and Cofactors That Contribute to SARS-CoV-2 Entry: Can Skin Be an Alternative Route of Entry? Int J Mol Sci 2023, 24, 6253. [Google Scholar]
- Bellavite, P.; Ferraresi, A.; Isidoro, C. Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines. Biomedicines 2023, 11, 451. [Google Scholar]
- Martyniak, A.; Tomasik, P.J. A New Perspective on the Renin-Angiotensin System. Diagnostics 2022, 13, 16. [Google Scholar] [CrossRef]
- Cooper, S.L.; Boyle, E.; Jefferson, S.R.; Heslop, C.R.A.; Mohan, P.; Mohanraj, G.G.J.; Sidow, H.A.; Tan, R.C.P.; Hill, S.J.; Woolard, J. Role of the Renin-Angiotensin-Aldosterone and Kinin-Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. Int J Mol Sci 2021, 2, 8255. [Google Scholar]
- Dherange, P.; Lang, J.; Qian, P.; Oberfeld, B.; Sauer, W.H.; Koplan, B.; Tedrow, U. Arrhythmias and COVID-19: A Review. JACC Clin Electrophysiol 2020, 6, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020, 17, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Colon, C.M.; Barrios, J.G.; Chiles, J.W.; McElwee, S.K.; Russell, D.W.; Maddox, W.R.; Kay, G.N. Atrial Arrhythmias in COVID-19 Patients. JACC: Clin. Electrophysiol. 2020, 6, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, B.; Chen, Y.; Tang, J.; Liu, Q.; Zhou, S.; Chen, C.; Qin, Q.; Huang, K.; Lv, J.; et al. Characteristic Electrocardiographic Manifestations in Patients With COVID-19. Can. J. Cardiol. 2020, 36, 966.e1–966.e4. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Boutjdir, M.; Capecchi, P.L. COVID- 19, arrhythmic risk and inflammation: mind the gap! Circulation 2020, 142, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Kolettis, T.M. Coronary artery disease and ventricular tachyarrhythmia: pathophysiology and treatment. Curr. Opin. Pharmacol. 2013, 13, 210–217. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Hear. Rhythm. 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Tse, G.; Yeo, J.M.; Chan, Y.W.; Lai, E.T.H.L.; Yan, B.P. What Is the Arrhythmic Substrate in Viral Myocarditis? Insights from Clinical and Animal Studies. Front. Physiol. 2016, 7, 308. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Rizzo, S.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Benedetti, G.; Palmisano, A.; Esposito, A.; Tresoldi, M.; et al. Arrhythmias in myocarditis: State of the art. Hear. Rhythm. 2018, 16, 793–801. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front. Cardiovasc. Med. 2015, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat. Rev. Immunol. 2018, 19, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.J. Bleeding and coagulopathies in critical care. N Engl J Med 2014, 370, 847–59. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.; Montani, D.; Savale, L.; et al. Endothelial cell dysfunction: a major player in SARS- CoV-2 infection (COVID-19)? Eur Respir J 2020, 56, 2001634. [Google Scholar] [CrossRef]
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute Infection and Myocardial Infarction. N. Engl. J. Med. 2019, 380, 171–176. [Google Scholar] [CrossRef]
- Ñamendys-Silva, S.; Santos-Martínez, L.; Pulido, T.; Rivero-Sigarroa, E.; Baltazar-Torres, J.; Domínguez-Cherit, G.; Sandoval, J. Pulmonary hypertension due to acute respiratory distress syndrome. Braz. J. Med Biol. Res. 2014, 47, 904–910. [Google Scholar] [CrossRef]
- Wanamaker, B.; Cascino, T.; McLaughlin, V.; Oral, H.; Latchamsetty, R.; Siontis, K.C. Atrial Arrhythmias in Pulmonary Hypertension: Pathogenesis, Prognosis and Management. Arrhythmia Electrophysiol. Rev. 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Bosch, N.A.; Cimini, J.; Walkey, A.J. Atrial Fibrillation in the ICU. Chest 2018, 154, 1424–1434. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin Proc 2020, 95, 1213–21. [Google Scholar] [CrossRef] [PubMed]
- Bharmal, M.; DiGrande, K.; Patel, A.; Shavelle, D.M.; Bosson, N. Impact of Coronavirus Disease 2019 Pandemic on Cardiac Arrest and Emergency Care. Hear. Fail. Clin. 2023, 19, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Girotra, S.; Tang, Y.; Al-Araji, R.; Nallamothu, B.K.; McNally, B. Outcomes for Out-of-Hospital Cardiac Arrest in the United States During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2021, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Girotra, S.; Tang, Y.; et al. Outcomes for outof-hospital cardiac arrest in the United States during the coronavirus disease 2019 pandemic. JAMA Cardiol 2021, 6, 296–303. [Google Scholar] [CrossRef]
- Chugh, H.S.; Sargsyan, A.; Nakamura, K.; Uy-Evanado, A.; Dizon, B.; Norby, F.L.; Young, C.; Hadduck, K.; Jui, J.; Shepherd, D.; et al. Sudden cardiac arrest during the COVID-19 pandemic: A two-year prospective evaluation in a North American community. Hear. Rhythm. 2023, 20, 947–955. [Google Scholar] [CrossRef]
- Garcia, S.; Albaghdadi, M.S.; Meraj, P.M.; Schmidt, C.; Garberich, R.; Jaffer, F.A.; Dixon, S.; Rade, J.J.; Tannenbaum, M.; Chambers, J.; et al. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2871–2872. [Google Scholar] [CrossRef]
- Uy-Evanado, A.; Chugh, H.S.; Sargsyan, A.; Nakamura, K.; Mariani, R.; Hadduck, K.; Salvucci, A.; Jui, J.; Chugh, S.S.; Reinier, K. Out-of-Hospital Cardiac Arrest Response and Outcomes During the COVID-19 Pandemic. JACC: Clin. Electrophysiol. 2020, 7, 6–11. [Google Scholar] [CrossRef]
- Lo, Y.A.; Jok, C.; Tse, H. Cardiovascular complications of COVID-19. Hong Kong Med J. 2022, 28, 249–256. [Google Scholar] [CrossRef]
- Hayek, S.S.; Brenner, S.K.; Azam, T.U.; Shadid, H.R.; Anderson, E.; Berlin, H.; Pan, M.; Meloche, C.; Feroz, R.; O’hayer, P.; et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ 2020, 371. [Google Scholar] [CrossRef]
- Shao, F.; Xu, S.; Ma, X.; Xu, Z.; Lyu, J.; Ng, M.; Cui, H.; Yu, C.; Zhang, Q.; Sun, P.; et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation 2020, 151, 18–23. [Google Scholar] [CrossRef]
- Thapa, S.B.; Kakar, T.S.; Mayer, C.; Khanal, D. Clinical Outcomes of In-Hospital Cardiac Arrest in COVID-19. JAMA Intern. Med. 2021, 181, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Bharmal, M.; DiGrande, K.; Patel, A.; Shavelle, D.M.; Bosson, N. Impact of Coronavirus Disease 2019 Pandemic on Cardiac Arrest and Emergency Care. Cardiol. Clin. 2022, 40, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Alqudah, Z.; Stub, D.; Anderson, D.; Nehme, Z. The effect of the COVID-19 pandemic on the incidence and survival outcomes of EMS-witnessed out-of-hospital cardiac arrest. Resuscitation 2023, 187, 109770. [Google Scholar] [CrossRef]
- Rollman, J.E.; Kloner, R.A.; Bosson, N.; Niemann, J.T.; Gausche-Hill, M.; Williams, M.; Clare, C.; Tan, W.; Wang, X.; Shavelle, D.M.; et al. Emergency medical services responses to out-of-hospital cardiac arrest and suspected ST-segment-elevation myocardial infarction during the COVID-19 pandemic in Los Angeles county. J Am Heart Assoc 2021, 10, e019635. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Harrison, N.; Chalek, A.D.; Gorelick, D.; Brennan, E.; Wise, S.; Gandolfo, L.; O'Neil, B.; Dunne, R. Effects of the COVID-19 pandemic on out-of-hospital cardiac arrest care in Detroit. Am. J. Emerg. Med. 2021, 46, 90–96. [Google Scholar] [CrossRef]
- Lim, S.L.; Smith, K.; Dyson, K.; Chan, S.P.; Earnest, A.; Nair, R.; Bernard, S.; Leong, B.S.; Arulanandam, S.; Ng, Y.Y.; et al. Incidence and Outcomes of Out-of-Hospital Cardiac Arrest in Singapore and Victoria: A Collaborative Study. J. Am. Hear. Assoc. 2020, 9, e015981. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.J.; Reddy, M.P.; Afroz, A.; Billah, B.; Shekar, K.; Subramaniam, A. Incidence and outcome of out-of-hospital cardiac arrests in the COVID-19 era: A systematic review and meta-analysis. Resuscitation 2020, 157, 248–258. [Google Scholar] [CrossRef]
- Wu, E.Y.; Campbell, M.J. Cardiac Manifestations of Multisystem Inflammatory Syndrome in Children (MIS-C) Following COVID-19. Curr. Cardiol. Rep. 2021, 23, 168. [Google Scholar] [CrossRef]
- Filippatos, F.; Tatsi, E.-B.; Michos, A. Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 5711. [Google Scholar] [CrossRef]
- Alsaied, T.; Tremoulet, A.H.; Burns, J.C.; Saidi, A.; Dionne, A.; Lang, S.M.; Newburger, J.W.; de Ferranti, S.; Friedman, K.G. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021, 143, 78–88. [Google Scholar] [CrossRef]
- Ahmed, M.; Advani, S.; Moreira, A.; Zoretic, S.; Martinez, J.; Chorath, K.; Acosta, S.; Naqvi, R.; Burmeister-Morton, F.; Burmeister, F.; et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine 2020, 26, 100527–100527. [Google Scholar] [CrossRef] [PubMed]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Kunal, S.; Ish, P.; Sakthivel, P.; Malhotra, N.; Gupta, K. The emerging threat of multisystem inflammatory syndrome in adults (MIS-A) in COVID-19: A systematic review. Hear. Lung 2022, 54, 7–18. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Kernan, K.F.; Schulert, G.S.; Seo, P.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022, 74, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- Capone, C.A.; Misra, N.; Ganigara, M.; Epstein, S.; Rajan, S.; Acharya, S.S.; Hayes, D.A.; Kearney, M.B.; Romano, A.; Friedman, R.A.; et al. Six Month Follow-up of Patients With Multi-System Inflammatory Syndrome in Children. PEDIATRICS 2021, 148, e2021050973. [Google Scholar] [CrossRef]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med 2020, 383, 347–358. [Google Scholar] [CrossRef]
- Valverde, I.; Singh, Y.; Sanchez-De-Toledo, J.; Theocharis, P.; Chikermane, A.; Di Filippo, S.; Kuciñska, B.; Mannarino, S.; Tamariz-Martel, A.; Gutierrez-Larraya, F.; et al. Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation 2021, 143, 21–32. [Google Scholar] [CrossRef]
- Parhizgar, P.; Yazdankhah, N.; Rzepka, A.M.; Chung, K.Y.C.; Ali, I.; Fur, R.L.F.; Russell, V.; Cheung, A.M. Beyond Acute COVID-19: A Review of Long-term Cardiovascular Outcomes. Can. J. Cardiol. 2023, 39, 726–740. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.Y.; Wang, S.I.; Wei, J.C.C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinical Medicine 2022, 53, 101619. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Elango, K.; Sossou, C.; Fakhra, S.; Asad, S.; Ahsan, C. COVID-19 Induced Cardiomyopathy Successfully Treated with Tocilizumab. Case Rep. Cardiol. 2022, 2022, 994393. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Sondhi, S.; Sharma, R.; Mahajan, K. COVID-19 associated viral myocarditis: does it exist? Monaldi Arch Chest Dis 2020, 90, 1382. [Google Scholar] [CrossRef]
- Mahajan, K.; Chandra, K. Cardiovascular comorbidities and complications associated with coronavirus disease 2019. Med J. Armed Forces India 2020, 76, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pilati, M.; Rebonato, M.; Formigari, R.; Butera, G. Endomyocardial Biopsy in Pediatric Myocarditis and Dilated Cardiomyopathy: A Tool in Search for a Role. J. Cardiovasc. Dev. Dis. 2022, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.B.; Botelho, B.G.; de Hollanda, J.V.G.; Ferreira, L.V.L.; de Andrade, L.Z.J.; Oei, S.S.M.L.; Mello, T.d.S.; Muxfeldt, E.S. Covid-19 and the cardiovascular system: a comprehensive review. J. Hum. Hypertens. 2020, 35, 4–11. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Hear. Rhythm. 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Hsu, R.-J.; Yu, W.-C.; Peng, G.-R.; Ye, C.-H.; Hu, S.; Chong, P.C.T.; Yap, K.Y.; Lee, J.Y.C.; Lin, W.-C.; Yu, S.-H. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front. Immunol. 2022, 13, 832394. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The Signal Pathways and Treatment of Cytokine Storm in Covid-19. Signal Transduct Target Ther 2021, 6, 255. [Google Scholar] [CrossRef]
- Wang, M.; Chang, W.; Zhang, L.; Zhang, Y. Pyroptotic Cell Death in Sars-Cov-2 Infection: Revealing Its Roles During the Immunopathogenesis of Covid-19. Int J Biol Sci 2022, 18, 5827. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine Storm Induced by Sars-Cov-2. Clin Chim Acta 2020, 509, 280. [Google Scholar] [CrossRef]
- Tsounis, E.P.; Triantos, C.; Konstantakis, C.; Marangos, M.; Assimakopoulos, S.F. Intestinal barrier dysfunction as a key driver of severe COVID-19. World J. Virol. 2023, 12, 68–90. [Google Scholar] [CrossRef] [PubMed]
- Petrushevska, M.; Zendelovska, D.; Atanasovska, E.; Eftimov, A.; Spasovska, K. Presentation of cytokine profile in relation to oxidative stress parameters in patients with severe COVID-19: a case-control pilot study. F1000Research 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Buicu, A.-L.; Cernea, S.; Benedek, I.; Buicu, C.-F.; Benedek, T. Systemic Inflammation and COVID-19 Mortality in Patients with Major Noncommunicable Diseases: Chronic Coronary Syndromes, Diabetes and Obesity. J. Clin. Med. 2021, 10, 1545. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Triantos, C.; Maroulis, I.; Gogos, C. The Role of the Gut Barrier Function in Health and Disease. Gastroenterol. Res. 2018, 11, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Assimakopoulos, S.F.; Velissaris, D.; Hung, M.-Y.; Mplani, V.; Saba, L.; Brinia, A.; Kouni, S.N.; et al. COVID-19 Disease, Women’s Predominant Non-Heparin Vaccine-Induced Thrombotic Thrombocytopenia and Kounis Syndrome: A Passepartout Cytokine Storm Interplay. Biomedicines 2021, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.A.; Eid, N.; Singh, J. Mechanisms of COVID-19-induced heart failure: a short review. Hear. Fail. Rev. 2020, 26, 363–369. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Kiris, T.; Avci, E.; Ekin, T.; et al. Comparison of long-term outcome of patients with ST-segment elevation myocardial infarction between preCOVID-19 and COVID-19 era. Eur J Clin Invest 2022, 52, e13834. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Colussi, G.; Bulfone, L.; Brosolo, G.; Da Porto, A.; Peghin, M.; Patruno, V.; Tascini, C.; Catena, C. Short-term cardiac outcome in survivors of COVID-19: a systematic study after hospital discharge. Clin. Res. Cardiol. 2021, 110, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.; Antoni, M.; ter Kuile, M.; Arbous, M.; Duinisveld, A.; Feltkamp, M.; Groeneveld, G.; Hinnen, S.; Janssen, V.; Lijfering, W.; et al. Short-term outpatient follow-up of COVID-19 patients: A multidisciplinary approach. EClinicalMedicine 2021, 32, 100731. [Google Scholar] [CrossRef]
- Ramadan, M.S.; Bertolino, L.; Zampino, R.; Durante-Mangoni, E.; Iossa, D.; Ursi, M.P.; D'Amico, F.; Karruli, A.; Andini, R.; Bernardo, M.; et al. Cardiac sequelae after coronavirus disease 2019 recovery: a systematic review. Clin. Microbiol. Infect. 2021, 27, 1250–1261. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef]
- Petersen, E.L.; Goßling, A.; Adam, G.; et al. Multiorgan assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: the Hamburg City Health Study COVID programme. Eur Heart J 2022, 43, 1124–37. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Lutokhina, Y.; Kogan, E.; Kukleva, A.; Ainetdinova, D.; Novosadov, V.; Rud', R.; Savina, P.; Zaitsev, A.; Fomin, V. Chronic biopsy proven post-COVID myoendocarditis with SARS-Cov-2 persistence and high level of antiheart antibodies. Clin. Cardiol. 2022, 45, 952–959. [Google Scholar] [CrossRef]
- Turan, T.; Özderya, A.; S¸ahin, S.; et al. Left ventricular global longitudinal strain in low cardiac risk outpatients who recently recovered from coronavirus disease 2019. Int J Cardiovasc Imaging 2021, 37, 2979–89. [Google Scholar] [CrossRef]
- Ródenas-Alesina, E.; Rodríguez-Palomares, J.; Bach-Oller, M.; Jordán, P.; Badia, C.; Herrador, L.; García-De-Acilu, M.; Clau-Terré, F.; González-Del-Hoyo, M.; Fernández-Galera, R.; et al. Echocardiographic assessment of COVID19 sequelae in survivors with elevated cardiac biomarkers. Int. J. Cardiol. 2022, 360, 104–110. [Google Scholar] [CrossRef]
- Karagodin, I.; Singulane, C.C.; Descamps, T.; Woodward, G.M.; Xie, M.; Tucay, E.S.; Sarwar, R.; Vasquez-Ortiz, Z.Y.; Alizadehasl, A.; Monaghan, M.J.; et al. Ventricular Changes in Patients with Acute COVID-19 Infection: Follow-up of the World Alliance Societies of Echocardiography (WASE-COVID) Study. J. Am. Soc. Echocardiogr. 2022, 35, 295–304. [Google Scholar] [CrossRef]
- Lim, R.; Johnson, R.R.; Denney, B.; Zalikha, L. Severe Heart Failure After Fetal Loss and COVID-19: A Diagnostically Challenging Case With Complicated Timing. Cureus 2023, 15, e36866. [Google Scholar] [CrossRef] [PubMed]
- Fayol, A.; Livrozet, M.; Boutouyrie, P.; Khettab, H.; Betton, M.; Tea, V.; Blanchard, A.; Bruno, R.; Hulot, J. ; French COVID cohort study group Cardiac performance in patients hospitalized with COVID-19: a 6 month follow-up study. ESC Hear. Fail. 2021, 8, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Corica, B.; Marra, A.M.; Basili, S.; Cangemi, R.; Cittadini, A.; Proietti, M.; Romiti, G.F. Prevalence of right ventricular dysfunction and impact on all-cause death in hospitalized patients with COVID-19: a systematic review and meta-analysis. Sci. Rep. 2021, 11, 17774. [Google Scholar] [CrossRef]
- Akkaya, F.; Yenerçag, F.N.T.; Kaya, A.; S¸ener, Y.Z.; Bagcı, A. Long term effects of mild severity COVID-19 on right ventricular functions. Int J Cardiovasc Imaging 2021, 3451–3457. [Google Scholar]
- Nuzzi, V.; Castrichini, M.; Collini, V.; Roman-Pognuz, E.; Di Bella, S.; Luzzati, R.; Berlot, G.; Confalonieri, M.; Merlo, M.; Stolfo, D.; et al. Impaired Right Ventricular Longitudinal Strain Without Pulmonary Hypertension in Patients Who Have Recovered From COVID-19. Circ. Cardiovasc. Imaging 2021, 14, e012166. [Google Scholar] [CrossRef]
- Lee, S.; Randhawa, S.; Alsamarrai, A.; Somaratne, J. Cardiac Complications of COVID-19 Infection. Hear. Lung Circ. 2023, 32, S271–S272. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Soufras, G.D.; Chourdakis, E.; Despotopoulos, S.; Davlouros, P.; Hahalis, G. Respiratory Infections as Predictors of Hospital Admission for Myocardial Infarction and Stroke: Pathophysiologic and Therapeutic Considerations. Clin. Infect. Dis. 2018, 68, 533–533. [Google Scholar] [CrossRef]
- Hasegawa, S.; Tashiro, N.; Matsubara, T.; Furukawa, S.; Ra, C. A comparison of Fcepsilon RI-mediated RANTES release from human platelets between allergic patients and healthy individuals. Int Arch Allergy Immunol. 2001, 125 (Suppl. 1), 42–47. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G.; et al. ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation 2020, 141, 2113–2116. [Google Scholar] [CrossRef]
- Rodriguez-Leor, O.; Cid Alvarez, A.B.; Pérez de Prado, A.; Rossello, X.; Ojeda, S.; Serrador, A.; López-Palop, R.; Martin-Moreiras, J.; Rumoroso, J.R.; Cequier, A.; et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention 2021, 16, 1426–1433. [Google Scholar] [CrossRef]
- Garcia, S.; Dehghani, P.; Grines, C.; Davidson, L.; Nayak, K.R.; Saw, J.; Waksman, R.; Blair, J.; Akshay, B.; Garberich, R.; et al. Society for Cardiac Angiography and Interventions, the Canadian Association of Interventional Cardiology, and the American College of Cardiology Interventional Council. Initial Findings from the North American COVID-19 Myocardial Infarction Registry. J. Am. Coll. Cardiol. 2021, 77, 1994–2003. [Google Scholar] [CrossRef]
- Toscano, O.; Cosentino, N.; Campodonico, J.; Bartorelli, A.L.; Marenzi, G. Acute Myocardial Infarction During the COVID-19 Pandemic: An Update on Clinical Characteristics and Outcomes. Front. Cardiovasc. Med. 2021, 8, 648290. [Google Scholar] [CrossRef] [PubMed]
- Gharibzadeh, A.; Shahsanaei, F.; Petrudi, N.R. Clinical and Cardiovascular Characteristics of Patients Suffering ST-Segment Elevation Myocardial Infarction After Covid-19: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101045–101045. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2020, 27, 258–263. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Raafs, A.G.; Ghossein, M.A.; Brandt, Y.; Henkens, M.T.; Kooi, M.E.; Vernooy, K.; Spaanderman, M.E.; Gerretsen, S.; van Santen, S.; Driessen, R.G.; et al. Cardiovascular outcome 6 months after severe coronavirus disease 2019 infection. J. Hypertens. 2022, 40, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Vallejo Camazón, N.; Teis, A.; Martínez Membrive, M.J.; Llibre, C.; Bayes-Genís, A.; Mateu, L. Long COVID-19 and microvascular disease-related angina. Rev Esp Cardiol (Engl Ed) 2022, 75, 444–446. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Velissaris, D.; Tzanis, G.; Hahalis, G. Kounis Syndrome—not a Single-organ Arterial Disorder but a Multisystem and Multidisciplinary Disease. Balk. Med J. 2019, 36, 212–221. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C. COVID-19 and Kounis Syndrome: Deciphering Their Relationship. Balk. Med J. 2021, 38, 145–149. [Google Scholar] [CrossRef]
- Theoharides, T.C. Potential association of mast cells with coronavirus disease 2019. Ann. Allergy, Asthma Immunol. 2020, 126, 217–218. [Google Scholar] [CrossRef]
- Prieto-Lobato, A.; Ramos-Martínez, R.; Vallejo-Calcerrada, N.; Corbí-Pascual, M.; Córdoba-Soriano, J.G. A case series of stent thrombosis During the COVID-19 pandemic. JACC Case Rep 2020, 2, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, A. Triggered by Covid-19? Large vascular occlusion resulting in cytokine Storm syndrome and Kounis syndrome: A case report. J Biomed Res Environ Sci 2021, 2, 030–033. [Google Scholar]
- Wang, M.; Talon, A.; Saririan, M. COVID-19 cardiac arrest due to Prinzmetal's angina in a previously normal heart. Clin Case Rep 2021, 9, e04205. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Orlando, L.; Trapani, G.; Vatrano, M.; Giovanni Versace, A.; Imbalzanoa, E. Kounis syndrome associated with COVID-19 infection: cause or coincidence? Cor Vasa 2021, 63, 592–596. [Google Scholar] [CrossRef]
- Randhawa, S.; Alsamarrai, A.J.; Lee, S.; Somaratne, J.B. Cardiac complications of COVID-19 infection. N Z Med J 2023, 136, 73–82. [Google Scholar] [CrossRef]
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q.; et al. A systematic review and meta-analysis of long COVID symptoms. Syst Rev 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Roca-Fernandez, A.; Wamil, M.; Telford, A.; Carapella, V.; Borlotti, A.; Monteiro, D.; Thomaides-Brears, H.; Kelly, M.; Dennis, A.; Banerjee, R.; et al. Cardiac abnormalities in Long COVID 1-year post-SARS-CoV-2 infection. Open Heart 2023, 10, e002241. [Google Scholar] [CrossRef]
- Siripanthong, B.; Asatryan, B.; Hanff, T.C.; Chatha, S.R.; Khanji, M.Y.; Ricci, F.; Muser, D.; Ferrari, V.A.; Nazarian, S.; Santangeli, P.; et al. The Pathogenesis and Long-Term Consequences of COVID-19 Cardiac Injury. JACC Basic Transl Sci 2022, 7, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhao, C.; He, M.Z.; Senter, C.; Zhou, Z.; Peng, J.; Li, S.; Fitzpatrick, A.L.; Lindström, S.; Stebbins, R.C.; Noppert, G.A.; Li, C. Long-term cardiac symptoms following COVID-19: a systematic review and meta-analysis. medRxiv [Preprint]. 2023 Jan 17:2023.01.16.23284620.
- Morello, R.; Mariani, F.; Mastrantoni, L.; De Rose, C.; Zampino, G.; Munblit, D.; Sigfrid, L.; Valentini, P.; Buonsenso, D. Risk factors for post-COVID-19 condition (Long Covid) in children: a prospective cohort study. EClinicalMedicine 2023, 59, 101961. [Google Scholar] [CrossRef]
- Morrow, A.J.; Sykes, R.; McIntosh, A.; Kamdar, A.; Bagot, C.; Bayes, H.K.; Blyth, K.G.; Briscoe, M.; Bulluck, H.; Carrick, D.; et al. A multisystem, cardio-renal investigation of post-COVID-19 illness. Nat. Med. 2022, 28, 1303–1313. [Google Scholar] [CrossRef]
- Gorecka, M.; Jex, N.; Thirunavukarasu, S.; Chowdhary, A.; Corrado, J.; Davison, J.; Tarrant, R.; Poenar, A.-M.; Sharrack, N.; Parkin, A.; et al. Cardiovascular magnetic resonance imaging and spectroscopy in clinical long-COVID-19 syndrome: a prospective case–control study. J. Cardiovasc. Magn. Reson. 2022, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Raafs, A.G.; Ghossein, M.A.; Brandt, Y.; Henkens, M.T.; Kooi, M.E.; Vernooy, K.; Spaanderman, M.E.; Gerretsen, S.; van Santen, S.; Driessen, R.G.; et al. Cardiovascular outcome 6 months after severe coronavirus disease 2019 infection. J. Hypertens. 2022, 40, 1278–1287. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC: Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Krishnan, A.; Ellenberger, K.; Phetsouphanh, C.; Kelleher, A.; Matthews, G.; Darley, D.; Holloway, C. Myocardial fibrosis occurs in non-hospitalised patients with chronic symptoms after COVID-19. IJC Hear. Vasc. 2022, 39, 100964. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Mplani, V.; Plotas, P.; Velissaris, D. Hypersensitivity Myocarditis and the Pathogenetic Conundrum of COVID-19 Vaccine-Related Myocarditis. Cardiology 2022, 147, 413–415. [Google Scholar] [CrossRef]
- Barreiro-Pérez, M.; Pastor Pueyo, P.; Raposeiras-Roubín, S.; Montero Corominas, D.; Uribarri, A.; Eiros Bachiller, R.; Rozado Castaño, J.; García-Cuenllas Álvarez, L.; Serratosa Fernández, L.; Domínguez, F.; et al. Myocarditis related SARS-CoV-2 infection or vaccination: an expert consensus statement on its diagnosis and management. Rev Esp Cardiol (Engl Ed) 2023, 76, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Sagoschen, I.; Konstantinides, S.; Gori, T.; Münzel, T.; Hobohm, L. Incidence and risk factors of myocarditis in hospitalized patients with COVID-19. J. Med Virol. 2023, 95, e28646. [Google Scholar] [CrossRef]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC: Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.G.; Bobba, A.; Chourasia, P.; Gangu, K.; Shuja, H.; Dandachi, D.; Farooq, A.; Avula, S.R.; Shekhar, R.; Sheikh, A.B. COVID-19 Associated Myocarditis Clinical Outcomes among Hospitalized Patients in the United States: A Propensity Matched Analysis of National Inpatient Sample. Viruses 2022, 14, 2791. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Wilson, C. Myocarditis more likely after infection than vaccination. New Sci. 2021, 251, 14. [Google Scholar] [CrossRef]
- Singer, M.E.; Taub, I.B.; Kaelber, D.C. Risk of Myocarditis from COVID-19 Infection in People Under Age 20: A Population-Based Analysis. medRxiv [Preprint]. 2022 Mar 21:2021.07.23.21260998.
- Nagai, T.; Inomata, T.; Kohno, T.; Sato, T.; Tada, A.; Kubo, T.; Nakamura, K.; Oyama-Manabe, N.; Ikeda, Y.; Fujino, T.; et al. Japanese Circulation Society Joint Working Group. JCS 2023 Guideline on the Diagnosis and Treatment of Myocarditis. Circ. J. 2023, 87, 674–754. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Zuliani, G.; Rigatelli, A.; Mazza, A.; Roncon, L. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J. Infect. 2020, 81, e84–e86. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Pasquetto, G.; Mazza, A. Risk of Incident New-Onset Arterial Hypertension After COVID-19 Recovery: A Systematic Review and Meta-analysis. High Blood Press. Cardiovasc. Prev. 2023, 30, 227–233. [Google Scholar] [CrossRef]
- Maestre-Muñiz, M.M.; Arias, Á.; Mata-Vázquez, E.; Martín-Toledano, M.; López-Larramona, G.; Ruiz-Chicote, A.M.; Nieto-Sandoval, B.; Lucendo, A.J. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med 2021, 10, 2945. [Google Scholar] [CrossRef]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin. Microbiol. Infect. 2020, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Szeghy, R.E.; Stute, N.L.; Province, V.M.; Augenreich, M.A.; Stickford, J.L.; Stickford, A.S.L.; Ratchford, S.M. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J Appl Physiol (1985) 2022, 132, 1297–309. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.; Akça, T.; Akacı, O.; Uysal, F. The Prevalence of Post-COVID-19 Hypertension in Children. Clin. Pediatr. 2022, 61, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Bing, H.I. Epidemical pericarditis. Acta Medica Scandinavica. 1933, 80, 29–33. [Google Scholar] [CrossRef]
- Al-Abdallat, M.M.; Payne, D.C.; Alqasrawi, S.; Rha, B.; Tohme, R.A.; Abedi, G.R.; Al Nsour, M.; Iblan, I.; Jarour, N.; Farag, N.H.; et al. Hospital-Associated Outbreak of Middle East Respiratory Syndrome Coronavirus: A Serologic, Epidemiologic, and Clinical Description. Clin. Infect. Dis. 2014, 59, 1225–1233. [Google Scholar] [CrossRef]
- Tung-Chen, Y. Acute pericarditis due to COVID-19 infection: An underdiagnosed disease? Med Clin (Engl Ed). 2020, 155, 44–45. [Google Scholar] [PubMed]
- Furqan, M.M.; Verma, B.R.; Cremer, P.C.; Imazio, M.; Klein, A.L. Pericardial Diseases in COVID19: a Contemporary Review. Curr. Cardiol. Rep. 2021, 23, 90. [Google Scholar] [CrossRef]
- Kermani-Alghoraishi, M.; Pouramini, A.; Kafi, F.; Khosravi, A. Coronavirus Disease 2019 (COVID-19) and Severe Pericardial Effusion: From Pathogenesis to Management: A Case Report Based Systematic Review. Curr. Probl. Cardiol. 2021, 47, 100933. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Farina, A.; Uccello, G.; Spreafico, M.; Bassanelli, G.; Savonitto, S. SARS-CoV-2 detection in the pericardial fluid of a patient with cardiac tamponade. Eur J Intern Med. 2020, 76, 100–101. [Google Scholar] [CrossRef]
- Parato, V.M.; Galieni, M.C.; Marcelli, S.; La Carruba, S. 669 COVID-19-related acute pericarditis. A retrospective observational study. Eur Heart J Suppl. 2021, 23 (Suppl. G), suab135.034. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Di Florio, D.N.; Musigk, N.; Heidecker, B.; Cooper, L.T., Jr. COVID-19, Myocarditis and Pericarditis. Circ Res. 2023, 132, 1302–1319. [Google Scholar] [CrossRef]
- Bao, C.; Liu, X.; Zhang, H.; Li, Y.; Liu, J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J. Am. Coll. Radiol. 2020, 17, 701–709. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Gao, P.; Tian, R.; Qian, H.; Guo, F.; Yan, X.; Song, Y.; Chen, W.; Fang, L.; et al. Swollen heart in COVID-19 patients who progress to critical illness: a perspective from echo-cardiologists. ESC Hear. Fail. 2020, 7, 3621–3632. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.J.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13679. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.; Barbu, E.C.; Chitu, C.E.; Anghel, A.-M.; Niculae, C.-M.; Manea, E.-D.; Damalan, A.-C.; Bel, A.-A.; Patrascu, R.-E.; Hristea, A.; et al. Pericardial Involvement in Severe COVID-19 Patients. Medicina 2022, 58, 1093. [Google Scholar] [CrossRef]
- Ghantous, E.; Szekely, Y.; Lichter, Y.; Levi, E.; Taieb, P.; Banai, A.; Sapir, O.; Granot, Y.; Lupu, L.; Hochstadt, A.; et al. Pericardial Involvement in Patients Hospitalized With COVID-19: Prevalence, Associates, and Clinical Implications. J Am Heart Assoc. 2022, 11, e024363. [Google Scholar] [CrossRef]
- Brito, D.; Meester, S.; Yanamala, N.; Patel, H.B.; Balcik, B.J.; Casaclang-Verzosa, G.; Seetharam, K.; Riveros, D.; Beto, R.J.; Balla, S.; et al. High Prevalence of Pericardial Involvement in College Student Athletes Recovering From COVID-19. JACC: Cardiovasc. Imaging 2021, 14, 541–555. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Diaz-Arocutipa, C.; Saucedo-Chinchay, J.; Imazio, M. Pericarditis in patients with COVID-19: a systematic review. J. Cardiovasc. Med. 2021, 22, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, M.F.; Aurora, L.; D’souza, P.; Weinmann, A.J.; Bhargava, P.; Basir, M.B. Cardiac Tamponade Secondary to COVID-19. JACC: Case Rep. 2020, 2, 1326–1330. [Google Scholar] [CrossRef]
- Dalen, H.; Holte, E.; Guldal, A.U.; Hegvik, J.-A.; Stensaeth, K.H.; Braaten, A.T.; Mjølstad, O.C.; Rossvoll, O.; Wiseth, R. Acute perimyocarditis with cardiac tamponade in COVID-19 infection without respiratory disease. BMJ Case Rep. 2020, 13, e236218. [Google Scholar] [CrossRef] [PubMed]
- Biong, K.E.; Lapitan, L.P.; Lapitan, R.L. Abstract 10594: Cardiac Tamponade in a Covid-19 Positive Patient With Idiopathic Capillary Leak Syndrome: A Perilous Combination. Circulation 2022, 146, A10594. [Google Scholar] [CrossRef]
- Diaconu, R.; Popescu, L.; Voicu, A.; Donoiu, I. Subacute effusive-constrictive pericarditis in a patient with COVID-19. BMJ Case Rep. 2021, 14, e242443. [Google Scholar] [CrossRef]
- Benrud-Larson, L.M.; Dewar, M.S.; Sandroni, P.; Rummans, T.A.; Haythornthwaite, J.A.; Low, P.A. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002, 77, 531–537. [Google Scholar] [CrossRef]
- Ståhlberg, M.; Reistam, U.; Fedorowski, A.; Villacorta, H.; Horiuchi, Y.; Bax, J.; Pitt, B.; Matskeplishvili, S.; Lüscher, T.F.; Weichert, I.; et al. Post-COVID-19 Tachycardia Syndrome: A Distinct Phenotype of Post-Acute COVID-19 Syndrome. Am. J. Med. 2021, 134, 1451–1456. [Google Scholar] [CrossRef]
- Carew, S.; Connor, M.O.; Cooke, J.; Conway, R.; Sheehy, C.; Costelloe, A.; Lyons, D. A review of postural orthostatic tachycardia syndrome. Eur. 2008, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.R. Postural tachycardia syndrome (POTS). Circulation. 2013, 127, 2336–2342. [Google Scholar] [CrossRef]
- Narasimhan, B.; Calambur, A.; Moras, E.; Wu, L.; Aronow, W. Postural Orthostatic Tachycardia Syndrome in COVID-19: A Contemporary Review of Mechanisms, Clinical Course and Management. Vasc. Heal. Risk Manag. 2023, ume 19, 303–316. [Google Scholar] [CrossRef]
- Raj, S.R.; Guzman, J.C.; Harvey, P.; Richer, L.; Schondorf, R.; Seifer, C.; Thibodeau-Jarry, N.; Sheldon, R.S. Canadian Cardiovascular Society position statement on Postural Orthostatic Tachycardia Syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol 2020, 36, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Gunning, W.T., 3rd; Kvale, H.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Postural Orthostatic Tachycardia Syndrome Is Associated With Elevated G-Protein Coupled Receptor Autoantibodies. J Am Heart Assoc. 2019, 8, e013602. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Calambur, A.; Moras, E.; Wu, L.; Aronow, W. Postural Orthostatic Tachycardia Syndrome in COVID-19: A Contemporary Review of Mechanisms, Clinical Course and Management. Vasc. Heal. Risk Manag. 2023, ume 19, 303–316. [Google Scholar] [CrossRef]
- Jacob, G.; Garland, E.M.; Costa, F.; Stein, C.M.; Xie, H.G.; Robertson, R.M.; Biaggioni, I.; Robertson, D. Beta2-adrenoceptor genotype and function affect hemodynamic profile heterogeneity in postural tachycardia syndrome. Hypertension. 2006, 47, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gall, N.P.; James, S.; Kavi, L. Observational case series of postural tachycardia syndrome (PoTS) in post-COVID-19 patients. Br. J. Cardiol. 2022, 29, 3. [Google Scholar] [CrossRef]
- Johansson, M.; Stahlberg, M.; Runold, M.; Nygren-Bonnier, M.; Nilsson, J.; Olshansky, B.; Bruchfeld, J.; Fedorowski, A. Long-haul post-COVID-19 symptoms presenting as a variant of a postural orthostatic tachycardia syndrome: the Swedish experience. J Am Coll Cardiol Case Rep. 2021, 3, 573–580. [Google Scholar] [CrossRef]
- Ormiston, C.K.; Świątkiewicz, I.; Taub, P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Hear. Rhythm. 2022, 19, 1880–1889. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef]
- Campen, C.L.M.C.V.; Visser, F.C. Long-Haul COVID Patients: Prevalence of POTS Are Reduced but Cerebral Blood Flow Abnormalities Remain Abnormal with Longer Disease Duration. Healthcare 2022, 10, 2105. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re'Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Monaghan, A.; Jennings, G.; Xue, F.; Byrne, L.; Duggan, E.; Romero-Ortuno, R. Orthostatic Intolerance in Adults Reporting Long COVID Symptoms Was Not Associated With Postural Orthostatic Tachycardia Syndrome. Front. Physiol. 2022, 13, 833650. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. The possible association between COVID-19 and postural tachycardia syndrome. Hear. Rhythm. 2020, 18, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.; Hoepner, R.; Salmen, A.; Kamber, N.; Z’graggen, W.J. Immunomodulatory treatment in postural tachycardia syndrome: A case series. Eur. J. Neurol. 2020, 28, 1692–1697. [Google Scholar] [CrossRef]
- Jadhav, K.; Jariwala, P. ‘Ivabradin’ versus ‘Carvedilol’ in the management of Post-COVID-19 palpitation with sinus tachycardia. Indian Heart J. 2020, 72, S33. [Google Scholar] [CrossRef]
- Abdelnabi, M.; Ahmed, A.; Benjanuwattra, J.; Saleh, Y.; Leelaviwat, N.; Almaghraby, A. IVABRADINE EFFECTS ON COVID-19 INDUCED POSTURAL ORTHOSTATIC TACHYCARDIA SYNDROME. J. Am. Coll. Cardiol. 2023, 81, 56. [Google Scholar] [CrossRef]
- Kurnik, M.; Božič, H.; Vindišar, A.; Kolar, P.; Podbregar, M. Pulmonary hypertension at admission predicts ICU mortality in elderly critically ill with severe COVID-19 pneumonia: retrospective cohort study. Cardiovasc. Ultrasound 2023, 21, 1. [Google Scholar] [CrossRef]
- Serati, A.; Keshmiri, M.S.; Shafaghi, S.; Mohammad, M.M.; Kashani, B.S.; Naghashzadeh, F.; Mohamadifar, A.; Shafaghi, M.; Noorali, S.; Hajimoradi, M.; et al. The Outcome of Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension during the COVID-19 Pandemic. Crit. Pathways Cardiol. 2023, 22, 60–64. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Lazureanu, V.E.; Marinescu, A.R.; Pop, G.N.; Pescariu, A.S.; Enache, A.; Cut, T.G. Evidence of Pulmonary Hypertension after SARS-CoV-2 Infection in Subjects without Previous Significant Cardiovascular Pathology. J Clin Med 2021, 10, 199. [Google Scholar] [CrossRef]
- Khan, A.W.; Ullah, I.; Khan, K.S.; Tahir, M.J.; Masyeni, S.; Harapan, H. Pulmonary arterial hypertension post COVID-19: a sequala of SARSCoV-2 infection? Respir Med Case Rep 2021, 33, 101429. [Google Scholar] [CrossRef]
- Jone, P.N.; John, A.; Oster, M.E.; Allen, K.; Tremoulet, A.H.; Saarel, E.V.; Lambert, L.M.; Miyamoto, S.D.; de Ferranti, S.D.; American Heart Association Leadership Committee and Congenital Cardiac Defects Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Hypertension, and Council on Peripheral Vascular Disease. SARS-CoV-2 Infection and Associated Cardiovascular Manifestations and Complications in Children and Young Adults: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e1037–e1052. [Google Scholar] [CrossRef]
- Lee, J.D.; Burger, C.D.; Delossantos, G.B.; Grinnan, D.; Ralph, D.D.; Rayner, S.G.; Ryan, J.J.; Safdar, Z.; Ventetuolo, C.E.; Zamanian, R.T.; et al. A Survey-based Estimate of COVID-19 Incidence and Outcomes among Patients with Pulmonary Arterial Hypertension or Chronic Thromboembolic Pulmonary Hypertension and Impact on the Process of Care. Ann. Am. Thorac. Soc. 2020, 17, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B. COVID-19 and pulmonary hypertension in children: what do we know so far? Medicina (Kaunas) 2020, 56, E716. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.A.; Jok, C.; Tse, H. Cardiovascular complications of COVID-19. Hong Kong Med J. 2022, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. New Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Sousa Rêgo, L.O.; Alves Braga, L.L.; Vilas-Boas, G.S.; Oliveira Cardoso, M.S.; Duraes, A.R. Cardiovascular and Neurological Complications of COVID-19: A Narrative Review. J Clin Med 2023, 12, 2819. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, C.; Luo, L.; Fang, F.; Chen, Y.; Li, J.; Peng, Z.; Pan, H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020, 127, 104364–104364. [Google Scholar] [CrossRef]
- Toloui, A.; Moshrefiaraghi, D.; Neishaboori, A.M.; Yousefifard, M.; Aghajani, M.H. Cardiac Complications and Pertaining Mortality Rate in COVID-19 Patients; a Systematic Review and Meta-Analysis. Arch Acad Emerg Med 2021, 9, e18. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Cai, Y.; Liu, T.; Shen, B.; Yang, F.; Cao, S.; Liu, X.; Xiang, Y.; Zhao, Q.; et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Hear. J. 2020, 41, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Jabri, A.; Kalra, A.; Kumar, A.; Alameh, A.; Adroja, S.; Bashir, H.; Nowacki, A.S.; Shah, R.; Khubber, S.; Kanaa’n, A.; et al. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw. Open 2020, 3, e2014780. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Uribe, C. Is Transient Takotsubo Syndrome Associated with Cancer? Why, and With What Implications for Oncocardiology? J Am Heart Assoc. 2019, 8, e013201. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Pellegrini, D.; Kawakami, R.; Guagliumi, G.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Nasr, A.; Kutys, R.; Guo, L.; Cornelissen, A.; et al. Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study. Circulation. 2021, 143, 1031–1042. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Angelini, P.; Postalian, A.; Hernandez-Vila, E.; Uribe, C.; Costello, B. COVID-19 and the Heart: Could Transient Takotsubo Cardiomyopathy Be Related to the Pandemic by Incidence and Mechanisms? Front Cardiovasc Med 2022, 9, 919715. [Google Scholar] [CrossRef]
- Hegde, S.; Khan, R.; Zordok, M.; Maysky, M. Characteristics and outcome of patients with COVID-19 complicated by Takotsubo cardiomyopathy: case series with literature review. Open Hear. 2020, 7, e001360. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Q.; Guo, X. Alternate recurrent coronary artery spasm and stress cardiomyopathy: a case report. BMC Cardiovasc. Disord. 2020, 20, 476. [Google Scholar] [CrossRef]
- Madias, J.E. Takotsubo Cardiomyopathy: Current Treatment. J. Clin. Med. 2021, 10, 3440. [Google Scholar] [CrossRef]
- Ueyama, T. Emotional Stress-Induced Tako-tsubo Cardiomyopathy: Animal Model and Molecular Mechanism. Ann. New York Acad. Sci. 2004, 1018, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Laukkanen, J.A. Incidence of venous and arterial thrombo embolic complications in COVID-19: a systematic review and meta-analysis. Thromb Res 2020, 196, 27–30. [Google Scholar] [CrossRef]
- Lee, S.; Randhawa, S.; Alsamarrai, A.; Somaratne, J. Cardiac Complications of COVID-19 Infection. Hear. Lung Circ. 2023, 32, S271–S272. [Google Scholar] [CrossRef]
- Knight, R.; Walker, V.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Denholm, R.; Akbari, A.; Abbasizanjani, H.; Torabi, F.; et al. Association of COVID-19 With Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales. Circulation 2022, 146, 892–906. [Google Scholar] [CrossRef]
- Lee, Y.; Jehangir, Q.; Li, P.; Gudimella, D.; Mahale, P.; Lin, C.-H.; Apala, D.R.; Krishnamoorthy, G.; Halabi, A.R.; Patel, K.; et al. Venous thromboembolism in COVID-19 patients and prediction model: a multicenter cohort study. BMC Infect. Dis. 2022, 22, 462. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lutgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schroder, A.S.; et al. Autopsy fndings and venous thromboembolism in patients with COVID-19. Ann Internal Med 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Gogos, C.; Assimakopoulos, S.F. Hypercoagulation and myocardial injury as risk factors for mortality in patients with COVID-19 pneumonia. Am. J. Emerg. Med. 2021, 47, 313–314. [Google Scholar] [CrossRef]
- Dambha-Miller, H.; Hinton, W.; Wilcox, C.R.; Joy, M.; Feher, M.; de Lusignan, S. Mortality in COVID-19 among women on hormone replacement therapy: a retrospective cohort study. Fam. Pr. 2022, 39, 1049–1055. [Google Scholar] [CrossRef]
- Ramanadhan, S.; Hansen, K.; Henderson, J.T.; Cohen, M.A.; Paynter, R.; Edelman, A. Risk of thromboembolism in patients with COVID-19 who are using hormonal contraception. Cochrane Database Syst Rev 2023, 5, CD014908. [Google Scholar]
- Sutanto, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 Infection: Pathogenesis, Diagnostic Approach, and Management. Hematol Rep 2023, 15, 225–243. [Google Scholar] [PubMed]
- Riyahi, S.; Dev, H.; Behzadi, A.; Kim, J.; Attari, H.; Raza, S.I.; Margolis, D.J.; Jonisch, A.; Megahed, A.; Bamashmos, A.; et al. Pulmonary Embolism in Hospitalized Patients with COVID-19: A Multicenter Study. Radiology 2021, 301, E426–E433. [Google Scholar] [CrossRef] [PubMed]
- Goswami, J.; MacArthur, T.A.; Sridharan, M.; Pruthi, R.K.; McBane RD 2nd Witzig, T.E.; Park, M.S. A Review of Pathophysiology, Clinical Features, and Management Options of COVID-19 Associated Coagulopathy. Shock 2021, 55, 700–716. [Google Scholar] [PubMed]
- Greistorfer, T.; Jud, P. Clinical characteristics of COVID-19 associated vasculopathic diseases. Thromb. J. 2023, 21, 1–13. [Google Scholar] [CrossRef]
- Abdalkader, M.; Shaikh, S.P.; Siegler, J.E.; Cervantes-Arslanian, A.M.; Tiu, C.; Radu, R.A.; Tiu, V.E.; Jillella, D.V.; Mansour, O.Y.; Vera, V.; et al. Cerebral Venous Sinus Thrombosis in COVID-19 Patients: A Multicenter Study and Review of Literature. J. Stroke Cerebrovasc. Dis. 2021, 30, 105733–105733. [Google Scholar] [CrossRef]
- Ojha, V.; Mani, A.; Mukherjee, A.; Kumar, S.; Jagia, P. Mesenteric ischemia in patients with COVID-19: an updated systematic review of abdominal CT findings in 75 patients. Abdom. Imaging 2021, 47, 1565–1602. [Google Scholar] [CrossRef]
- Ojha, V.; Mani, A.; Mukherjee, A.; Kumar, S.; Jagia, P. Mesenteric ischemia in patients with COVID-19: an updated systematic review of abdominal CT findings in 75 patients. Abdom. Imaging 2021, 47, 1565–1602. [Google Scholar] [CrossRef]
- Phelan, D.; Kim, J.H.; Chung, E.H. A game plan for theresumption of sport and exercise after coronavirus disease2019 (COVID-19) infection. JAMA Cardiol 2020, 5, 1085–1086. [Google Scholar]
- ElAmm, C.A.; Al-Kindi, S.G.; Oliveira, G.H. Characteristics and Outcomes of Patients With Myocarditis Listed for Heart Transplantation. Circ. Hear. Fail. 2016, 9, e003259. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).