Submitted:
01 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Skin cancers
3. Phenolic compounds
4. Overview of selected spices
Allspice
Alpinia galanga
Black cumin
Black pepper
Cinnamon
Coriander
Fenugreek
Ginger
Oregano
Nutmeg
Red chili
Rosemary
Saffron
Sichuan pepper
Star anise
Sumac
Thyme
Turmeric
5. Effect of spices-derived phenolic compounds against melanoma and non-melanoma skin cancer
5.1. Phenolic acids
5.2. Flavonoids
5.3. Other phenolic compounds
6. Conclusion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shabir Ahmad, R., Imran, M., Kamran Khan, M., Haseeb Ahmad, M., Sajid Arshad, M., Ateeq, H.,Abdul Rahim, M., Introductory Chapter: Herbs and Spices - An Overview. In Herbs and Spices - New Processing Technologies; R. Shabir Ahmad; IntechOpen Limited: London, UK, 2021; Vol. 1, p. 276. [CrossRef]
- Bi, X., Lim, J.,Henry, C.J. Spices in the management of diabetes mellitus. Food Chem 2017, 217: p. 281-293. [CrossRef]
- Tapsell, L.C., Hemphill, I., Cobiac, L., Patch, C.S., Sullivan, D.R., Fenech, M., Roodenrys, S., Keogh, J.B., Clifton, P.M., Williams, P.G., Fazio, V.A.,Inge, K.E. Health benefits of herbs and spices: the past, the present, the future. Med J Aust 2006, 185(S4): p. S1-S24. [CrossRef]
- Essa, M.M., Akbar, M.,Guillemin, G., In The Benefits of Natural Products for Neurodegenerative Diseases; M.M. Essa, M. Akbar, and G. Guillemin; Springer Cham: Switzerland, 2016; Vol. 12, p. 496. [CrossRef]
- Singh, N.,Yadav, S.S. A review on health benefits of phenolics derived from dietary spices. Curr Res Food Sci 2022, 5: p. 1508-1523. [CrossRef]
- Avila-Galvez, M.A., Gimenez-Bastida, J.A., Espin, J.C.,Gonzalez-Sarrias, A. Dietary Phenolics against Breast Cancer. A Critical Evidence-Based Review and Future Perspectives. Int J Mol Sci 2020, 21(16). [CrossRef]
- Muller, A.G., Sarker, S.D., Saleem, I.Y.,Hutcheon, G.A. Delivery of natural phenolic compounds for the potential treatment of lung cancer. Daru 2019, 27(1): p. 433-449. [CrossRef]
- De, S., Paul, S., Manna, A., Majumder, C., Pal, K., Casarcia, N., Mondal, A., Banerjee, S., Nelson, V.K., Ghosh, S., Hazra, J., Bhattacharjee, A., Mandal, S.C., Pal, M.,Bishayee, A. Phenolic Phytochemicals for Prevention and Treatment of Colorectal Cancer: A Critical Evaluation of In Vivo Studies. Cancers (Basel) 2023, 15(3). [CrossRef]
- Kountouri, A.M., Kaliora, A.C., Koumbi, L.,Andrikopoulos, N.K. In-vitro gastric cancer prevention by a polyphenol-rich extract from olives through induction of apoptosis. Eur J Cancer Prev 2009, 18(1): p. 33-9. [CrossRef]
- Spagnuolo, C., Flores, G., Russo, G.L.,Ruiz Del Castillo, M.L. A Phenolic Extract Obtained from Methyl Jasmonate-Treated Strawberries Enhances Apoptosis in a Human Cervical Cancer Cell Line. Nutr Cancer 2016, 68(7): p. 1140-50. [CrossRef]
- Ji, X., Usman, A., Razalli, N.H., Sambanthamurthi, R.,Gupta, S.V. Oil palm phenolics (OPP) inhibit pancreatic cancer cell proliferation via suppression of NF-kappaB pathway. Anticancer Res 2015, 35(1): p. 97-106.
- Leiter, U., Keim, U.,Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv Exp Med Biol 2020, 1268: p. 123-139. [CrossRef]
- O'Neill, C.H.,Scoggins, C.R. Melanoma. J Surg Oncol 2019, 120(5): p. 873-881. [CrossRef]
- Garbe, C., Keim, U., Gandini, S., Amaral, T., Katalinic, A., Hollezcek, B., Martus, P., Flatz, L., Leiter, U.,Whiteman, D. Epidemiology of cutaneous melanoma and keratinocyte cancer in white populations 1943-2036. Eur J Cancer 2021, 152: p. 18-25. [CrossRef]
- Krynitz, B., Olsson, H., Lundh Rozell, B., Lindelof, B., Edgren, G.,Smedby, K.E. Risk of basal cell carcinoma in Swedish organ transplant recipients: a population-based study. Br J Dermatol 2016, 174(1): p. 95-103. [CrossRef]
- Peris, K., Fargnoli, M.C., Garbe, C., Kaufmann, R., Bastholt, L., Seguin, N.B., Bataille, V., Marmol, V.D., Dummer, R., Harwood, C.A., Hauschild, A., Holler, C., Haedersdal, M., Malvehy, J., Middleton, M.R., Morton, C.A., Nagore, E., Stratigos, A.J., Szeimies, R.M., Tagliaferri, L., Trakatelli, M., Zalaudek, I., Eggermont, A., Grob, J.J., European Dermatology Forum, t.E.A.o.D.-O., the European Organization for, R.,Treatment of, C. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer 2019, 118: p. 10-34. [CrossRef]
- Tanese, K. Diagnosis and Management of Basal Cell Carcinoma. Curr Treat Options Oncol 2019, 20(2): p. 13. [CrossRef]
- Roewert-Huber, J., Stockfleth, E.,Kerl, H. Pathology and pathobiology of actinic (solar) keratosis - an update. Br J Dermatol 2007, 157 Suppl 2: p. 18-20. [CrossRef]
- Eisen, D.B., Asgari, M.M., Bennett, D.D., Connolly, S.M., Dellavalle, R.P., Freeman, E.E., Goldenberg, G., Leffell, D.J., Peschin, S., Sligh, J.E., Wu, P.A., Frazer-Green, L., Malik, S.,Schlesinger, T.E. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol 2021, 85(4): p. e209-e233. [CrossRef]
- Kallini, J.R., Hamed, N.,Khachemoune, A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol 2015, 54(2): p. 130-40. [CrossRef]
- Mogensen, M.,Jemec, G.B. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: a review of diagnostic accuracy of nonmelanoma skin cancer diagnostic tests and technologies. Dermatol Surg 2007, 33(10): p. 1158-74. [CrossRef]
- Waldman, A.,Schmults, C. Cutaneous Squamous Cell Carcinoma. Hematol Oncol Clin North Am 2019, 33(1): p. 1-12. [CrossRef]
- Neale, H., Michelon, M., Jacob, S., Pinkston, M., Ukaegbu, R., Zamor, W., Morrison, E., Deng, A.,Levin, N.A. Topical 5% 5-fluorouracil versus procedural modalities for squamous cell carcinoma in situ and superficial basal cell carcinoma: A retrospective cohort analysis. J Am Acad Dermatol 2022, 87(2): p. 423-425. [CrossRef]
- Dirschka, T., Bierhoff, E., Pflugfelder, A.,Garbe, C. Topical 3.0% diclofenac in 2.5% hyaluronic acid gel induces regression of cancerous transformation in actinic keratoses. J Eur Acad Dermatol Venereol 2010, 24(3): p. 258-63. [CrossRef]
- Piquero-Casals, J., Morgado-Carrasco, D., Gilaberte, Y., Del Rio, R., Macaya-Pascual, A., Granger, C.,Lopez-Estebaranz, J.L. Management Pearls on the Treatment of Actinic Keratoses and Field Cancerization. Dermatol Ther (Heidelb) 2020, 10(5): p. 903-915. [CrossRef]
- Garbe, C., Amaral, T., Peris, K., Hauschild, A., Arenberger, P., Basset-Seguin, N., Bastholt, L., Bataille, V., Del Marmol, V., Dreno, B., Fargnoli, M.C., Forsea, A.M., Grob, J.J., Holler, C., Kaufmann, R., Kelleners-Smeets, N., Lallas, A., Lebbe, C., Lytvynenko, B., Malvehy, J., Moreno-Ramirez, D., Nathan, P., Pellacani, G., Saiag, P., Stratigos, A.J., Van Akkooi, A.C.J., Vieira, R., Zalaudek, I., Lorigan, P., European Dermatology Forum, t.E.A.o.D.-O., the European Organization for, R.,Treatment of, C. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur J Cancer 2022, 170: p. 236-255. [CrossRef]
- Erdei, E.,Torres, S.M. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther 2010, 10(11): p. 1811-23. [CrossRef]
- Rastrelli, M., Tropea, S., Rossi, C.R.,Alaibac, M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28(6): p. 1005-11.
- Leonardi, G.C., Falzone, L., Salemi, R., Zanghi, A., Spandidos, D.A., McCubrey, J.A., Candido, S.,Libra, M. Cutaneous melanoma: From pathogenesis to therapy (Review). Int J Oncol 2018, 52(4): p. 1071-1080. [CrossRef]
- Rashid, S., Shaughnessy, M.,Tsao, H. Melanoma classification and management in the era of molecular medicine. Dermatol Clin 2023, 41(1): p. 49-63. [CrossRef]
- Gong, H.Z., Zheng, H.Y.,Li, J. Amelanotic melanoma. Melanoma Res 2019, 29(3): p. 221-230. [CrossRef]
- Garbe, C., Peris, K., Hauschild, A., Saiag, P., Middleton, M., Bastholt, L., Grob, J.J., Malvehy, J., Newton-Bishop, J., Stratigos, A.J., Pehamberger, H.,Eggermont, A.M. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline - Update 2016. Eur J Cancer 2016, 63: p. 201-17. [CrossRef]
- Dummer, R., Hauschild, A., Lindenblatt, N., Pentheroudakis, G.,Keilholz, U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015, 26 Suppl 5: p. v126-32. [CrossRef]
- Swetter, S.M., Thompson, J.A., Albertini, M.R., Barker, C.A., Baumgartner, J., Boland, G., Chmielowski, B., DiMaio, D., Durham, A., Fields, R.C., Fleming, M.D., Galan, A., Gastman, B., Grossmann, K., Guild, S., Holder, A., Johnson, D., Joseph, R.W., Karakousis, G., Kendra, K., Lange, J.R., Lanning, R., Margolin, K., Olszanski, A.J., Ott, P.A., Ross, M.I., Salama, A.K., Sharma, R., Skitzki, J., Sosman, J., Wuthrick, E., McMillian, N.R.,Engh, A.M. NCCN Guidelines(R) Insights: Melanoma: Cutaneous, Version 2.2021. J Natl Compr Canc Netw 2021, 19(4): p. 364-376. [CrossRef]
- Alu'datt, M.H., Rababah, T., Alhamad, M.N., Al-Mahasneh, M.A., Almajwal, A., Gammoh, S., Ereifej, K., Johargy, A.,Alli, I. A review of phenolic compounds in oil-bearing plants: Distribution, identification and occurrence of phenolic compounds. Food Chem 2017, 218: p. 99-106. [CrossRef]
- Robbins, R.J. Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem 2003, 51(10): p. 2866-87. [CrossRef]
- Zhang, Y., Cai, P., Cheng, G.,Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Natural Product Communications 2022, 17(1): p. 1934578X211069721. [CrossRef]
- Rodriguez De Luna, S.L., Ramirez-Garza, R.E.,Serna Saldivar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. ScientificWorldJournal 2020, 2020: p. 6792069. [CrossRef]
- Dias, M.C., Pinto, D.,Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26(17). [CrossRef]
- Serrano, J., Puupponen-Pimia, R., Dauer, A., Aura, A.M.,Saura-Calixto, F. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res 2009, 53 Suppl 2: p. S310-29. [CrossRef]
- Pecyna, P., Wargula, J., Murias, M.,Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10(8). [CrossRef]
- Stringlis, I.A., de Jonge, R.,Pieterse, C.M.J. The Age of Coumarins in Plant-Microbe Interactions. Plant Cell Physiol 2019, 60(7): p. 1405-1419. [CrossRef]
- Saleem, M., Kim, H.J., Ali, M.S.,Lee, Y.S. An update on bioactive plant lignans. Nat Prod Rep 2005, 22(6): p. 696-716. [CrossRef]
- Ginwala, R., Bhavsar, R., Chigbu, D.I., Jain, P.,Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants (Basel) 2019, 8(2). [CrossRef]
- Song, B., Guan, S., Lu, J., Chen, Z., Huang, G., Li, G., Xiong, Y., Zhang, S., Yue, Z.,Deng, X. Suppressive effects of fisetin on mice T lymphocytes in vitro and in vivo. J Surg Res 2013, 185(1): p. 399-409. [CrossRef]
- Kamisah, Y., Jalil, J., Yunos, N.M.,Zainalabidin, S. Cardioprotective Properties of Kaempferol: A Review. Plants (Basel) 2023, 12(11). [CrossRef]
- Shen, N., Wang, T., Gan, Q., Liu, S., Wang, L.,Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem 2022, 383: p. 132531. [CrossRef]
- Dourado, N.S., Souza, C.D.S., de Almeida, M.M.A., Bispo da Silva, A., Dos Santos, B.L., Silva, V.D.A., De Assis, A.M., da Silva, J.S., Souza, D.O., Costa, M.F.D., Butt, A.M.,Costa, S.L. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer's Disease. Front Aging Neurosci 2020, 12: p. 119. [CrossRef]
- Górniak, I., Bartoszewski, R.,Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 2019, 18(1): p. 241-272. [CrossRef]
- Fernandez, J., Silvan, B., Entrialgo-Cadierno, R., Villar, C.J., Capasso, R., Uranga, J.A., Lombo, F.,Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed Pharmacother 2021, 143: p. 112241. [CrossRef]
- Abdullah, H., Ismail, I., Suppian, R.,Zakaria, N.M. Natural Gallic Acid and Methyl Gallate Induces Apoptosis in Hela Cells through Regulation of Intrinsic and Extrinsic Protein Expression. Int J Mol Sci 2023, 24(10). [CrossRef]
- Zhang, L.C., Liu, Y.N., La, X.Q., Li, S.T., Wen, L.N., Liu, T., Li, H.Q., Li, A.P., Wu, H., Wu, C.X.,Li, Z.Y. The bound polyphenols of foxtail millet (Setaria italica) inner shell inhibit breast cancer by promoting lipid accumulation-induced autophagic death. Food Chem Toxicol 2023, 177: p. 113855. [CrossRef]
- Lin, W.,Tongyi, S. Role of Bax/Bcl-2 family members in green tea polyphenol induced necroptosis of p53-deficient Hep3B cells. Tumour Biol 2014, 35(8): p. 8065-75. [CrossRef]
- Michalkova, R., Kello, M., Kudlickova, Z., Gazdova, M., Mirossay, L., Mojzisova, G.,Mojzis, J. Programmed Cell Death Alterations Mediated by Synthetic Indole Chalcone Resulted in Cell Cycle Arrest, DNA Damage, Apoptosis and Signaling Pathway Modulations in Breast Cancer Model. Pharmaceutics 2022, 14(3). [CrossRef]
- Barreca, M.M., Alessandro, R.,Corrado, C. Effects of Flavonoids on Cancer, Cardiovascular and Neurodegenerative Diseases: Role of NF-kappaB Signaling Pathway. Int J Mol Sci 2023, 24(11). [CrossRef]
- Russo, M., Moccia, S., Luongo, D.,Russo, G.L. Senolytic Flavonoids Enhance Type-I and Type-II Cell Death in Human Radioresistant Colon Cancer Cells through AMPK/MAPK Pathway. Cancers (Basel) 2023, 15(9). [CrossRef]
- Mirossay, L., Varinska, L.,Mojzis, J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. Int J Mol Sci 2017, 19(1). [CrossRef]
- Varinska, L., van Wijhe, M., Belleri, M., Mitola, S., Perjesi, P., Presta, M., Koolwijk, P., Ivanova, L.,Mojzis, J. Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur J Pharmacol 2012, 691(1-3): p. 125-33. [CrossRef]
- Khater, M., Greco, F.,Osborn, H.M.I. Antiangiogenic Activity of Flavonoids: A Systematic Review and Meta-Analysis. Molecules 2020, 25(20). [CrossRef]
- Zhang, L.,Lokeshwar, B.L. Medicinal properties of the Jamaican pepper plant Pimenta dioica and Allspice. Curr Drug Targets 2012, 13(14): p. 1900-6. [CrossRef]
- Al-Rehaily, A., Al-Said, M., Al-Yahya, M., Jaber, S.,Rafatullah, S. Ethnopharmacological Studies on Allspice (Pimenta dioica) in Laboratory Animals. Pharm Biol 2002, 40: p. 200-205. [CrossRef]
- Miyajima, Y., Kikuzaki, H., Hisamoto, M.,Nikatani, N. Antioxidative polyphenols from berries of Pimenta dioica. Biofactors 2004, 21(1-4): p. 301-3. [CrossRef]
- El Gizawy, H.A., Boshra, S.A., Mostafa, A., Mahmoud, S.H., Ismail, M.I., Alsfouk, A.A., Taher, A.T.,Al-Karmalawy, A.A. Pimenta dioica (L.) Merr. Bioactive Constituents Exert Anti-SARS-CoV-2 and Anti-Inflammatory Activities: Molecular Docking and Dynamics, In Vitro, and In Vivo Studies. Molecules 2021, 26(19). [CrossRef]
- Marzouk, M.S., Moharram, F.A., Mohamed, M.A., Gamal-Eldeen, A.M.,Aboutabl, E.A. Anticancer and antioxidant tannins from Pimenta dioica leaves. Z Naturforsch C J Biosci 2007, 62(7-8): p. 526-36. [CrossRef]
- Milenkovic, A., Stanojević, J., Stojanović-Radić, Z., Pejčić, M., Cvetkovic, D., Zvezdanović, J.,Stanojević, L. Chemical composition, antioxidative and antimicrobial activity of allspice (Pimenta dioica (L.) Merr.) essential oil and extract. Advanced Technologies 2020, 9: p. 27-36. [CrossRef]
- Rao, P.S., Navinchandra, S.,Jayaveera, K.N. An important spice, Pimenta dioica (Linn.) Merill: A Review. Int Curr Pharm J 2012, 1(8): p. 221-225. [CrossRef]
- Rema, J.,Krishnamoorthy, B., Allspice. 2nd Edition ed. Handbook of Herbs and Spices. 2012: Woodhead Publishing. 166-192.
- Mandal, D., Sarkar, T.,Chakraborty, R. Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Appl Biochem Biotechnol 2023, 195(2): p. 1319-1513. [CrossRef]
- Chudiwal, A.K., Jain, D.P.,Rahul Somani, R. Alpinia galanga Willd.- An overview on phyto-pharmacological properties. Indian J Nat Prod Resour 2010, 1(2): p. 143-149.
- Eram, S., Mujahid, M., Bagga, P., Ansari, V., Ahmad, M., Kumar, A., Ahsan, F.,Akhter, M. A Review on Phytopharmacological Activity of Alpinia Galanga. Int J Pharm Pharm Sci 2019: p. 6-11. [CrossRef]
- Chouni, A.,Paul, S. A Review on Phytochemical and Pharmacological Potential of Alpinia galanga. Pharmacogn J 2018, 10(1): p. 09-15. [CrossRef]
- Namdeo, A.G.,Kale, V.M. Comparative pharmacognostic and phytochemical investigation of two Alpinia species from Zingiberaceae Family. World J Pharm Res 2015, 4(5): p. 1417-32.
- Tungmunnithum, D., Tanaka, N., Uehara, A.,Iwashina, T. Flavonoids Profile, Taxonomic Data, History of Cosmetic Uses, Anti-Oxidant and Anti-Aging Potential of Alpinia galanga (L.) Willd. Cosmetics 2020, 7(4): p. 89. [CrossRef]
- Ramanunny, A.K., Wadhwa, S., Gulati, M., Vishwas, S., Khursheed, R., Paudel, K.R., Gupta, S., Porwal, O., Alshahrani, S.M., Jha, N.K., Chellappan, D.K., Prasher, P., Gupta, G., Adams, J., Dua, K., Tewari, D.,Singh, S.K. Journey of Alpinia galanga from kitchen spice to nutraceutical to folk medicine to nanomedicine. J Ethnopharmacol 2022, 291: p. 115144. [CrossRef]
- Zhou, Y.-Q., Liu, H., He, M.-X., Wang, R., Zeng, Q.-Q., Wang, Y., Ye, W.-C.,Zhang, Q.-W., Chapter 11 - A Review of the Botany, Phytochemical, and Pharmacological Properties of Galangal. In Natural and Artificial Flavoring Agents and Food Dyes; A.M. Grumezescu and A.M. Holban; Academic Press, 2018, p. 351-396. [CrossRef]
- Nampoothiri, S.V., Esakkidurai, T.,Pitchumani, K. Identification and Quantification of Phenolic Compounds in Alpinia galanga and Alpinia calcarata and its Relation to Free Radical Quenching Properties: A Comparative Study. J Herbs Spices Med Plants 2015, 21(2): p. 140-147. [CrossRef]
- Suja, S.,Chinnaswamy, P. Inhibition of in vitro cytotoxic effect evoked by Alpinia galanga and Alpinia officinarum on PC - 3 cell line. Anc Sci Life 2008, 27(4): p. 33-40.
- Choi, J.Y., Lee, N.K., Wang, Y.Y., Hong, J.P., Son, S.R., Gu, D.H., Jang, D.S.,Choi, J.H. 1'-Acetoxyeugenol Acetate Isolated from Thai Ginger Induces Apoptosis in Human Ovarian Cancer Cells by ROS Production via NADPH Oxidase. Antioxidants (Basel) 2022, 11(2). [CrossRef]
- Suciati, A.,Maryati. Systematic Review: Anticancer Potential of Active Compounds from Galangal (Alpinia galanga). In Proceedings of the 4th International Conference Current Breakthrough in Pharmacy (ICB-Pharma 2022), Sukoharjo, Indonesia, 14-15 January 2022. [CrossRef]
- Ali, B.H.,Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res 2003, 17(4): p. 299-305. [CrossRef]
- Kooti, W., Hasanzadeh-Noohi, Z., Sharafi-Ahvazi, N., Asadi-Samani, M.,Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin J Nat Med 2016, 14(10): p. 732-745. [CrossRef]
- Ismail, N., Abd Ghafar, S.A.,Abu Bakar, M.Z., Chapter Fifteen - Antioxidant activity and phenolic content of black cumin seeds. In Biochemistry, Nutrition, and Therapeutics of Black Cumin Seed; A.A. Mariod; Academic Press, 2023, p. 169-188. [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: traditional uses, chemical constituents, and nutraceutical effects. Food Qual Saf 2018, 2(1): p. 1-16. [CrossRef]
- Milenkovic, A.,Stanojević, L. Black pepper: Chemical composition and biological activities. Advanced Technologies 2021, 10: p. 40-50. [CrossRef]
- Al-Khayri, J.M., Upadhya, V., Pai, S.R., Naik, P.M., Al-Mssallem, M.Q.,Alessa, F.M. Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules 2022, 27(18). [CrossRef]
- Ashokkumar, K., Murugan, M., Dhanya, M.K., Pandian, A.,Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: a review. Clin Phytoscience 2021, 7(1): p. 52. [CrossRef]
- Butt, M.S., Pasha, I., Sultan, M.T., Randhawa, M.A., Saeed, F.,Ahmed, W. Black pepper and health claims: a comprehensive treatise. Crit Rev Food Sci Nutr 2013, 53(9): p. 875-86. [CrossRef]
- Zhang, M., Qiu, B., Sun, M., Wang, Y., Wei, M., Gong, Y.,Yan, M. Preparation of Black pepper (Piper nigrum L.) essential oil nanoparticles and its antitumor activity on triple negative breast cancer in vitro. J Food Biochem 2022, 46(12): p. e14406. [CrossRef]
- Cardoso, L.P., de Sousa, S.O., Gusson-Zanetoni, J.P., de Melo Moreira Silva, L.L., Frigieri, B.M., Henrique, T., Tajara, E.H., Oliani, S.M.,Rodrigues-Lisoni, F.C. Piperine Reduces Neoplastic Progression in Cervical Cancer Cells by Downregulating the Cyclooxygenase 2 Pathway. Pharmaceuticals (Basel) 2023, 16(1). [CrossRef]
- Manayi, A., Nabavi, S.M., Setzer, W.N.,Jafari, S. Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies. Curr Med Chem 2018, 25(37): p. 4918-4928. [CrossRef]
- Rao, P.V.,Gan, S.H. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Alternat Med 2014, 2014: p. 642942. [CrossRef]
- Sadeghi, S., Davoodvandi, A., Pourhanifeh, M.H., Sharifi, N., ArefNezhad, R., Sahebnasagh, R., Moghadam, S.A., Sahebkar, A.,Mirzaei, H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur J Med Chem 2019, 178: p. 131-140. [CrossRef]
- Sahib, A.S. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J Intercult Ethnopharmacol 2016, 5(2): p. 108-13. [CrossRef]
- Schink, A., Naumoska, K., Kitanovski, Z., Kampf, C.J., Frohlich-Nowoisky, J., Thines, E., Poschl, U., Schuppan, D.,Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct 2018, 9(11): p. 5950-5964. [CrossRef]
- Raeisi, M., Tajik, H., Yarahmadi, A.,Sanginabadi, S. Antimicrobial Effect of Cinnamon Essential Oil Against Escherichia Coli and Staphylococcus aureus. Health Scope 2015, 4(4): p. e21808. [CrossRef]
- Pagliari, S., Forcella, M., Lonati, E., Sacco, G., Romaniello, F., Rovellini, P., Fusi, P., Palestini, P., Campone, L., Labra, M., Bulbarelli, A.,Bruni, I. Antioxidant and Anti-Inflammatory Effect of Cinnamon (Cinnamomum verum J. Presl) Bark Extract after In Vitro Digestion Simulation. Foods 2023, 12(3): p. 452. [CrossRef]
- Shahidi, F.,Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J Food Bioact 2018, 3: p. 8-75. [CrossRef]
- Cardoso-Ugarte, G.A., López-Malo, A.,Sosa-Morales, M.E., Chapter 38 - Cinnamon (Cinnamomum zeylanicum) Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; V.R. Preedy; Academic Press: San Diego, 2016, p. 339-347. [CrossRef]
- Vallverdu-Queralt, A., Regueiro, J., Martinez-Huelamo, M., Rinaldi Alvarenga, J.F., Leal, L.N.,Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem 2014, 154: p. 299-307. [CrossRef]
- Singh, N., Rao, A.S., Nandal, A., Kumar, S., Yadav, S.S., Ganaie, S.A.,Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem 2021, 338: p. 127773. [CrossRef]
- Baliga, M.S., Mane, P.P., Timothy Nallemgera, J., Thilakchand, K.R.,Kalekhan, F., Chapter 5 - Dietary Spices in the Prevention of Rheumatoid Arthritis: Past, Present, and Future. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; R.R. Watson; Academic Press: San Diego, 2015, p. 41-49. [CrossRef]
- Kumar, V., Marković, T., Mila, E.,Dey, A., Herbs: Composition and Dietary Importance. In Encyclopedia of Food and Health; B. Caballero, P.M. Finglas, and F. Toldrá; Elsevier: Kidlington, Oxford, 2015; Vol. 3, p. 332-337. [CrossRef]
- Laribi, B., Kouki, K., M'Hamdi, M.,Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103: p. 9-26. [CrossRef]
- Msaada, K., Jemia, M.B., Salem, N., Bachrouch, O., Sriti, J., Tammar, S., Bettaieb, I., Jabri, I., Kefi, S., Limam, F.,Marzouk, B. Antioxidant activity of methanolic extracts from three coriander (Coriandrum sativum L.) fruit varieties. Arab J Chem 2017, 10: p. S3176-S3183. [CrossRef]
- Barros, L., Dueñas, M., Dias, M.I., Sousa, M.J., Santos-Buelga, C.,Ferreira, I.C.F.R. Phenolic profiles of in vivo and in vitro grown Coriandrum sativum L. Food Chem 2012, 132(2): p. 841-848. [CrossRef]
- Oganesyan, E.T., Nersesyan, Z.M.,Parkhomenko, A.Y. Chemical composition of the above-ground part of Coriandrum sativum. Pharm Chem J 2007, 41(3): p. 149-153. [CrossRef]
- Nambiar, V., Daniel, M.,Guin, P. Characterization of polyphenols from coriander leaves (Coriandrum sativum), red amaranthus (A paniculatus) and green amaranthus (A frumentaceus) using paper chromatography and their health implications. J Herb Med Toxicol 2010, 4: p. 173-177.
- Mechchate, H., Costa de Oliveira, R., Es-Safi, I., Vasconcelos Mourao, E.M., Bouhrim, M., Kyrylchuk, A., Soares Pontes, G., Bousta, D.,Grafov, A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals (Basel) 2021, 14(8). [CrossRef]
- Elmas, L., Secme, M., Mammadov, R., Fahrioglu, U.,Dodurga, Y. The determination of the potential anticancer effects of Coriandrum sativum in PC-3 and LNCaP prostate cancer cell lines. J Cell Biochem 2019, 120(3): p. 3506-3513. [CrossRef]
- Eroglu, C., Secme, M., Bagci, G.,Dodurga, Y. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumour Biol 2015, 36(12): p. 9437-46. [CrossRef]
- Kim, S.J., Pham, T.H., Bak, Y., Ryu, H.W., Oh, S.R.,Yoon, D.Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCalpha/ ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 2018, 50: p. 35-42. [CrossRef]
- Huang, H., Nakamura, T., Yasuzawa, T.,Ueshima, S. Effects of Coriandrum sativum on Migration and Invasion Abilities of Cancer Cells. J Nutr Sci Vitaminol (Tokyo) 2020, 66(5): p. 468-477. [CrossRef]
- Wani, S.A.,Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci 2018, 17(2): p. 97-106. [CrossRef]
- Sun, W., Shahrajabian, M.H.,Cheng, Q. Fenugreek Cultivation with Emphasis on Historical Aspects and its uses in Traditional Medicine and Modern Pharmaceutical Science. Mini Rev Med Chem 2021, 21(6): p. 724-730. [CrossRef]
- Visuvanathan, T., Than, L.T.L., Stanslas, J., Chew, S.Y.,Vellasamy, S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants (Basel) 2022, 11(11). [CrossRef]
- Bahmani, M., Shirzad, H., Mirhosseini, M., Mesripour, A.,Rafieian-Kopaei, M. A Review on Ethnobotanical and Therapeutic Uses of Fenugreek (Trigonella foenum-graceum L). J Evid Based Complementary Altern Med 2016, 21(1): p. 53-62. [CrossRef]
- Nagulapalli Venkata, K.C., Swaroop, A., Bagchi, D.,Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol Nutr Food Res 2017, 61(6). [CrossRef]
- Skaltsa, H., Fenugreek - The Genus Trigonella. 1st Edition ed. 2002, London: CRC Press. 226. [CrossRef]
- Benayad, Z., Gomez-Cordoves, C.,Es-Safi, N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC-DAD-ESI/MS analysis. Int J Mol Sci 2014, 15(11): p. 20668-85. [CrossRef]
- Niknam, R., Kiani, H., Mousavi, Z.,Mousavi, M., Extraction, Detection, and Characterization of Various Chemical Components of Trigonella foenum-graecum L. (Fenugreek) Known as a Valuable Seed in Agriculture. 2021, p. 189-217. [CrossRef]
- Shawky, E., Sobhy, A.A., Ghareeb, D.A., Shams Eldin, S.M.,Selim, D.A. Comparative metabolomics analysis of bioactive constituents of the leaves of different Trigonella species: Correlation study to α-amylase and α-glycosidase inhibitory effects. Ind Crops Prod 2022, 182: p. 114947. [CrossRef]
- Al-Timimi, L.A.N. Antibacterial and Anticancer Activities of Fenugreek Seed Extract. Asian Pac J Cancer Prev 2019, 20(12): p. 3771-3776. [CrossRef]
- Alsemari, A., Alkhodairy, F., Aldakan, A., Al-Mohanna, M., Bahoush, E., Shinwari, Z.,Alaiya, A. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella Foenum-Graecum. BMC Complement Altern Med 2014, 14: p. 114. [CrossRef]
- Ajmal, S., Shafqat, M., Ajmal, L., Younas, H., Tasadduq, R.,Mahamood, N. Evaluation of Anti-cancer and Anti-proliferative Activity of Medicinal Plant Extracts (Saffron, Green Tea, Clove, Fenugreek) on Toll Like Receptors Pathway. Nat Prod Sci 2022, 28(3): p. 121-129. [CrossRef]
- Alrumaihi, F.A., Khan, M.A., Allemailem, K.S., Alsahli, M.A., Almatroudi, A., Younus, H., Alsuhaibani, S.A., Algahtani, M.,Khan, A. Methanolic Fenugreek Seed Extract Induces p53-Dependent Mitotic Catastrophe in Breast Cancer Cells, Leading to Apoptosis. J Inflamm Res 2021, 14: p. 1511-1535. [CrossRef]
- Singletary, K. Ginger: An Overview of Health Benefits. Nutr Today 2010, 45: p. 171-183. [CrossRef]
- Khodaie, L.,Sadeghpoor, O. Ginger from ancient times to the new outlook. Jundishapur J Nat Pharm Prod 2015, 10(1): p. e18402. [CrossRef]
- Kiyama, R. Nutritional implications of ginger: chemistry, biological activities and signaling pathways. J Nutr Biochem 2020, 86: p. 108486. [CrossRef]
- Semwal, R.B., Semwal, D.K., Combrinck, S.,Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117: p. 554-568. [CrossRef]
- Schadich, E., Hlavac, J., Volna, T., Varanasi, L., Hajduch, M.,Dzubak, P. Effects of Ginger Phenylpropanoids and Quercetin on Nrf2-ARE Pathway in Human BJ Fibroblasts and HaCaT Keratinocytes. Biomed Res Int 2016, 2016: p. 2173275. [CrossRef]
- Ozkur, M., Benlier, N., Takan, I., Vasileiou, C., Georgakilas, A.G., Pavlopoulou, A., Cetin, Z.,Saygili, E.I. Ginger for Healthy Ageing: A Systematic Review on Current Evidence of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Oxid Med Cell Longev 2022, 2022: p. 4748447. [CrossRef]
- Kundu, J.K., Na, H.K.,Surh, Y.J. Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum Nutr 2009, 61: p. 182-192. [CrossRef]
- Bae, W.Y., Choi, J.S., Kim, J.E., Park, C.,Jeong, J.W. Zingerone suppresses angiogenesis via inhibition of matrix metalloproteinases during tumor development. Oncotarget 2016, 7(30): p. 47232-47241. [CrossRef]
- Gird, C.E., Costea, T.,Mitran, V. Evaluation of cytotoxic activity and anticancer potential of indigenous Rosemary (Rosmarinus officinalis L.) and Oregano (Origanum vulgare L.) dry extracts on MG-63 bone osteosarcoma human cell line. Rom J Morphol Embryol 2021, 62(2): p. 525-535. [CrossRef]
- Bouyahya, A., Chamkhi, I., Benali, T., Guaouguaou, F.E., Balahbib, A., El Omari, N., Taha, D., Belmehdi, O., Ghokhan, Z.,El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J Ethnopharmacol 2021, 265: p. 113318. [CrossRef]
- Ozkan, G., Baydar, H.,Erbas, S. The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of Turkish oregano (Origanum onites L.). J Sci Food Agric 2010, 90(2): p. 205-9. [CrossRef]
- Teixeira, B., Marques, A., Ramos, C., Serrano, C., Matos, O., Neng, N.R., Nogueira, J.M., Saraiva, J.A.,Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J Sci Food Agric 2013, 93(11): p. 2707-14. [CrossRef]
- Geetha, P.P.S.R.P.G.L.P.G.S.S.R.S.B.S. Pharmacology and chemistry of Myristica fragrans Houtt. - a review. J Spices Aromat 2005, 14(2): p. 94-101.
- Kuete, V., Chapter 23 - Myristica fragrans: A Review. In Medicinal Spices and Vegetables from Africa; V. Kuete; Academic Press, 2017, p. 497-512. [CrossRef]
- Zhang, W.K., Tao, S.S., Li, T.T., Li, Y.S., Li, X.J., Tang, H.B., Cong, R.H., Ma, F.L.,Wan, C.J. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res 2016, 60: p. 30849. [CrossRef]
- Hayfaa, A.A., Sahar, A.M.,Awatif, M.A. Evaluation of analgesic activity and toxicity of alkaloids in Myristica fragrans seeds in mice. J Pain Res 2013, 6: p. 611-5. [CrossRef]
- Shafiei, Z., Shuhairi, N.N., Md Fazly Shah Yap, N., Harry Sibungkil, C.A.,Latip, J. Antibacterial Activity of Myristica fragrans against Oral Pathogens. Evid Based Complement Alternat Med 2012, 2012: p. 825362. [CrossRef]
- Narasimhan, B.,Dhake, A.S. Antibacterial principles from Myristica fragrans seeds. J Med Food 2006, 9(3): p. 395-9. [CrossRef]
- Morita, T., Jinno, K., Kawagishi, H., Arimoto, Y., Suganuma, H., Inakuma, T.,Sugiyama, K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J Agric Food Chem 2003, 51(6): p. 1560-5. [CrossRef]
- Rezende, D., Oliveira, C.D., Batista, L.R., Ferreira, V.R.F., Brandao, R.M., Caetano, A.R.S., Alves, M.V.P.,Cardoso, M.G. Bactericidal and antioxidant effects of essential oils from Satureja montana L., Myristica fragrans H. and Cymbopogon flexuosus. Lett Appl Microbiol 2022, 74(5): p. 741-751. [CrossRef]
- Bandyopadhyay, P.S.V.C. Estimation of phenolic acids in cinnamon, clove, cardamom, nutmeg and mace by high performance liquid chromatography. J Spices Aromat 1995, 4(2): p. 129-134.
- Odubanjo, O., Olasehinde, T., Oyeleye, I., Oboh, G.,Boligon, A. Seed extracts from Myristica fragrans (Nutmeg) and Moringa oleifera (Drumstick tree) inhibits enzymes relevant to erectile dysfunction and metal-induced oxidative damage in rats' penile tissues. J Food Biochem 2017, 42: p. e12452. [CrossRef]
- Kraft, K.H., Brown, C.H., Nabhan, G.P., Luedeling, E., Luna Ruiz Jde, J., Coppens d'Eeckenbrugge, G., Hijmans, R.J.,Gepts, P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc Natl Acad Sci U S A 2014, 111(17): p. 6165-70. [CrossRef]
- Reyes-Escogido Mde, L., Gonzalez-Mondragon, E.G.,Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16(2): p. 1253-70. [CrossRef]
- Pugliese, A., O'Callaghan, Y., Tundis, R., Galvin, K., Menichini, F., O'Brien, N.,Loizzo, M.R. In vitro investigation of the bioaccessibility of carotenoids from raw, frozen and boiled red chili peppers (Capsicum annuum). Eur J Nutr 2014, 53(2): p. 501-10. [CrossRef]
- Antonio, A.S., Wiedemann, L.S.M.,Veiga Junior, V.F. The genus Capsicum: a phytochemical review of bioactive secondary metabolites. Rsc Adv 2018, 8(45): p. 25767-25784. [CrossRef]
- Zhang, D., Sun, X., Battino, M., Wei, X., Shi, J., Zhao, L., Liu, S., Xiao, J., Shi, B.,Zou, X. A comparative overview on chili pepper (capsicum genus) and sichuan pepper (zanthoxylum genus): From pungent spices to pharma-foods. Trends Food Sci 2021, 117: p. 148-162. [CrossRef]
- Ayob, O., Hussain, P.R., Suradkar, P., Naqash, F., Rather, S.A., Joshi, S.,Ahmad Azad, Z.R.A. Evaluation of chemical composition and antioxidant activity of Himalayan Red chilli varieties. LWT 2021, 146: p. 111413. [CrossRef]
- Batiha, G.E., Alqahtani, A., Ojo, O.A., Shaheen, H.M., Wasef, L., Elzeiny, M., Ismail, M., Shalaby, M., Murata, T., Zaragoza-Bastida, A., Rivero-Perez, N., Magdy Beshbishy, A., Kasozi, K.I., Jeandet, P.,Hetta, H.F. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int J Mol Sci 2020, 21(15). [CrossRef]
- Clark, R.,Lee, S.H. Anticancer Properties of Capsaicin Against Human Cancer. Anticancer Res 2016, 36(3): p. 837-43.
- Heinrich, M., Kufer, J., Leonti, M.,Pardo-de-Santayana, M. Ethnobotany and ethnopharmacology--interdisciplinary links with the historical sciences. J Ethnopharmacol 2006, 107(2): p. 157-60. [CrossRef]
- de Oliveira, J.R., Camargo, S.E.A.,de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J Biomed Sci 2019, 26(1): p. 5. [CrossRef]
- Hussain, A.I., Anwar, F., Chatha, S.A., Jabbar, A., Mahboob, S.,Nigam, P.S. Rosmarinus officinalis essential oil: antiproliferative, antioxidant and antibacterial activities. Braz J Microbiol 2010, 41(4): p. 1070-8. [CrossRef]
- Hcini, K., Abidi, M., Quílez, M., Jordán Maria, J.,Sadok, B., Total Phenolic Content and Polyphenolic Profile of Tunisian Rosemary (<em>Rosmarinus officinalis</em> L.) Residues. In Natural Drugs from Plants; A.E.-S. Hany; IntechOpen: Rijeka, 2021, p. Ch. 9. [CrossRef]
- Moore, J., Yousef, M.,Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8(11). [CrossRef]
- Gonzalez-Vallinas, M., Reglero, G.,Ramirez de Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr Cancer 2015, 67(8): p. 1221-9. [CrossRef]
- Saxena, R.B. Botany, Taxonomy and Cytology of Crocus sativus series. Ayu 2010, 31(3): p. 374-81. [CrossRef]
- Melnyk, J.P., Wang, S.,Marcone, M.F. Chemical and biological properties of the world's most expensive spice: Saffron. Food Res Int 2010, 43(8): p. 1981-1989. [CrossRef]
- Xing, B., Li, S., Yang, J., Lin, D., Feng, Y., Lu, J.,Shao, Q. Phytochemistry, pharmacology, and potential clinical applications of saffron: A review. J Ethnopharmacol 2021, 281: p. 114555. [CrossRef]
- Groppo, M.,Pirani, J.R. A new species of Zanthoxylum (Rutaceae) with a key to the species from Northeastern Brazil. Phytotaxa 2017, 314: p. 259. [CrossRef]
- Okagu, I.U., Ndefo, J.C., Aham, E.C.,Udenigwe, C.C. Zanthoxylum Species: A Comprehensive Review of Traditional Uses, Phytochemistry, Pharmacological and Nutraceutical Applications. Molecules 2021, 26(13). [CrossRef]
- Ekka, G., Jadhav, S.,Quraishi, A., An Overview of Genus Zanthoxylum with Special Reference to Its Herbal Significance and Application. 2020, p. [CrossRef]
- Tanoh, E.A., Boue, G.B., Nea, F., Genva, M., Wognin, E.L., Ledoux, A., Martin, H., Tonzibo, Z.F., Frederich, M.,Fauconnier, M.L. Seasonal Effect on the Chemical Composition, Insecticidal Properties and Other Biological Activities of Zanthoxylum leprieurii Guill. & Perr. Essential oils. Foods 2020, 9(5). [CrossRef]
- Ji, Y., Li, S.,Ho, C.-T. Chemical composition, sensory properties and application of Sichuan pepper (Zanthoxylum genus). Food Sci Hum Wellness 2019, 8(2): p. 115-125. [CrossRef]
- Wang, G.W., Hu, W.T., Huang, B.K.,Qin, L.P. Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. J Ethnopharmacol 2011, 136(1): p. 10-20. [CrossRef]
- Sharafan, M., Jafernik, K., Ekiert, H., Kubica, P., Kocjan, R., Blicharska, E.,Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27(3). [CrossRef]
- Ghosh, S., Chisti, Y.,Banerjee, U.C. Production of shikimic acid. Biotechnol Adv 2012, 30(6): p. 1425-31. [CrossRef]
- Li, W., Wu, Z., Xia, Y., Tan, J., Zhao, H., Chen, S., Li, Y., Tang, H., Wang, G.,Zhang, Y. Antiviral and Antioxidant Components from the Fruits of Illicium verum Hook.f. (Chinese Star Anise). J Agric Food Chem 2022, 70(12): p. 3697-3707. [CrossRef]
- De, M., De, A.K., Sen, P.,Banerjee, A.B. Antimicrobial properties of star anise (Illicium verum Hook f). Phytother Res 2002, 16(1): p. 94-5. [CrossRef]
- Majali, I.S. Antioxidant and Anti-Inflammatory Activity of Star Anise (Illicium Verum) in Murine Model. Biomed Pharmacol J 2022, 15(2): p. 1097-1108. [CrossRef]
- Ibrahim, F., Ibrahim, A., E, E.L., Hussein, M.,Ahmed, K. Illicium verum Extracts Anti-Gastro Ulcerogenic Potential on Experimentally Rat Models. Int J PharmTech Res 2016, 9: p. 65-80.
- Patra, J.K., Das, G., Bose, S., Banerjee, S., Vishnuprasad, C.N., Del Pilar Rodriguez-Torres, M.,Shin, H.S. Star anise (Illicium verum): Chemical compounds, antiviral properties, and clinical relevance. Phytother Res 2020, 34(6): p. 1248-1267. [CrossRef]
- Asif, M., Yehya, A.H.S., Al-Mansoub, M.A., Revadigar, V., Ezzat, M.O., Khadeer Ahamed, M.B., Oon, C.E., Murugaiyah, V., Abdul Majid, A.S.,Abdul Majid, A.M.S. Anticancer attributes of Illicium verum essential oils against colon cancer. S Afr J Bot 2016, 103: p. 156-161. [CrossRef]
- Pahore, A.K., Khan, S.,Karim, N. Anticancer effect of Illicium verum (star anise fruit) against human breast cancer MCF-7 cell line. Pak J Med Sci 2023, 39(1): p. 70-74. [CrossRef]
- Chen, C.H.,deGraffenried, L.A. Anethole suppressed cell survival and induced apoptosis in human breast cancer cells independent of estrogen receptor status. Phytomedicine 2012, 19(8-9): p. 763-7. [CrossRef]
- Khan, M.,Shamim, S. Anisi Stellati Fructus, a Significant Traditional Chinese Medicine (TCM) Herb and Its Bioactivity against Gastric Cancer. Evid Based Complement Alternat Med 2022, 2022: p. 4071489. [CrossRef]
- Najar, B., Shortrede, J.E., Pistelli, L.,Buhagiar, J. Chemical Composition and in Vitro Cytotoxic Screening of Sixteen Commercial Essential Oils on Five Cancer Cell Lines. Chem Biodivers 2020, 17(1): p. e1900478. [CrossRef]
- Kim, A., Im, M.,Ma, J.Y. Anisi stellati fructus extract attenuates the in vitro and in vivo metastatic and angiogenic potential of malignant cancer cells by downregulating proteolytic activity and pro-angiogenic factors. Int J Oncol 2014, 45(5): p. 1937-48. [CrossRef]
- Batiha, G.E., Ogunyemi, O.M., Shaheen, H.M., Kutu, F.R., Olaiya, C.O., Sabatier, J.M.,De Waard, M. Rhus coriaria L. (Sumac), a Versatile and Resourceful Food Spice with Cornucopia of Polyphenols. Molecules 2022, 27(16). [CrossRef]
- Alsamri, H., Athamneh, K., Pintus, G., Eid, A.H.,Iratni, R. Pharmacological and Antioxidant Activities of Rhus coriaria L. (Sumac). Antioxidants (Basel) 2021, 10(1).
- Sakhr, K.,El Khatib, S. Physiochemical properties and medicinal, nutritional and industrial applications of Lebanese Sumac (Syrian Sumac - Rhus coriaria): A review. Heliyon 2020, 6(1): p. e03207. [CrossRef]
- Kubatka, P., Kello, M., Kajo, K., Samec, M., Liskova, A., Jasek, K., Koklesova, L., Kuruc, T., Adamkov, M., Smejkal, K., Svajdlenka, E., Solar, P., Pec, M., Busselberg, D., Sadlonova, V.,Mojzis, J. Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma. Int J Mol Sci 2020, 22(1). [CrossRef]
- Athamneh, K., Hasasna, H.E., Samri, H.A., Attoub, S., Arafat, K., Benhalilou, N., Rashedi, A.A., Dhaheri, Y.A., AbuQamar, S., Eid, A.,Iratni, R. Rhus coriaria increases protein ubiquitination, proteasomal degradation and triggers non-canonical Beclin-1-independent autophagy and apoptotic cell death in colon cancer cells. Scientific Reports 2017, 7(1): p. 11633. [CrossRef]
- Gabr, S.A.,Alghadir, H.A. Potential anticancer activities of Rhus coriaria (sumac) extract against human cancer cell lines. Biosci Rep 2021, 41(5). [CrossRef]
- Romeo, F.V., Ballistreri, G., Fabroni, S., Pangallo, S., Nicosia, M.G., Schena, L.,Rapisarda, P. Chemical Characterization of Different Sumac and Pomegranate Extracts Effective against Botrytis cinerea Rots. Molecules 2015, 20(7): p. 11941-58. [CrossRef]
- Bozan, B., Koşar, M., Z.Tunalier, Öztürk, N.,Baser, K.H.C. Antioxidant and Free Radical Scavenging Activities of Rhus coriaria and Cinnamomum cassia Extracts. Acta Alimentaria 2003, 32: p. 53-61. [CrossRef]
- Abu-Reidah, I.M., Ali-Shtayeh, M.S., Jamous, R.M., Arraez-Roman, D.,Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem 2015, 166: p. 179-191. [CrossRef]
- Patil, S.M., Ramu, R., Shirahatti, P.S., Shivamallu, C.,Amachawadi, R.G. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon 2021, 7(5): p. e07054. [CrossRef]
- Hammoudi Halat, D., Krayem, M., Khaled, S.,Younes, S. A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb. Nutrients 2022, 14(10). [CrossRef]
- Ahmad, A., Saeed, M.,Ansari, I.A. Molecular insights on chemopreventive and anticancer potential of carvacrol: Implications from solid carcinomas. J Food Biochem 2021, 45(12): p. e14010. [CrossRef]
- Niksic, H., Becic, F., Koric, E., Gusic, I., Omeragic, E., Muratovic, S., Miladinovic, B.,Duric, K. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci Rep 2021, 11(1): p. 13178. [CrossRef]
- Kowalczyk, A., Przychodna, M., Sopata, S., Bodalska, A.,Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25(18). [CrossRef]
- Roby, M.H.H., Sarhan, M.A., Selim, K.A.-H.,Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crops Prod 2013, 43: p. 827-831. [CrossRef]
- Teixeira, B., Marques, A., Ramos, C., Neng, N.R., Nogueira, J.M.F., Saraiva, J.A.,Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crops Prod 2013, 43: p. 587-595. [CrossRef]
- Kotha, R.R.,Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24(16). [CrossRef]
- Meng, F.-C., Zhou, Y.-Q., Ren, D., Wang, R., Wang, C., Lin, L.-G., Zhang, X.-Q., Ye, W.-C.,Zhang, Q.-W., Chapter 10 - Turmeric: A Review of Its Chemical Composition, Quality Control, Bioactivity, and Pharmaceutical Application. In Natural and Artificial Flavoring Agents and Food Dyes; A.M. Grumezescu and A.M. Holban; Academic Press, 2018, p. 299-350. [CrossRef]
- Qiong-Qiong, Y. Phenolic profiles, antioxidant, and antiproliferative activities of turmeric (Curcuma longa). Ind Crops Prod 2020, v. 152: p. 112561. [CrossRef]
- Fan, Y., Zhang, X., Tong, Y., Chen, S.,Liang, J. Curcumin against gastrointestinal cancer: A review of the pharmacological mechanisms underlying its antitumor activity. Front Pharmacol 2022, 13: p. 990475. [CrossRef]
- Shahrajabian, M.H.,Sun, W. The Golden Spice for Life: Turmeric with the Pharmacological Benefits of Curcuminoids Components, including Curcumin, Bisdemethoxycurcumin, and Demethoxycurcumin. Curr Org Synth 2023. [CrossRef]
- Tang, Y.,Cao, Y. Curcumin Inhibits the Growth and Metastasis of Melanoma via miR-222-3p/SOX10/Notch Axis. Dis Markers 2022, 2022: p. 3129781. [CrossRef]
- Rocha Silva, L., Alves Nunes, J., Zhan, P., K, Z.L., Helena Cardoso, S.,Ferreira da Silva-Junior, E. Natural Coumarin Derivatives Targeting Melanoma. Curr Med Chem 2023. [CrossRef]
- Venza, I., Visalli, M., Oteri, R., Beninati, C., Teti, D.,Venza, M. Genistein reduces proliferation of EP3-expressing melanoma cells through inhibition of PGE2-induced IL-8 expression. Int Immunopharmacol 2018, 62: p. 86-95. [CrossRef]
- Danciu, C., Borcan, F., Bojin, F., Zupko, I.,Dehelean, C. Effect of the isoflavone genistein on tumor size, metastasis potential and melanization in a B16 mouse model of murine melanoma. Nat Prod Commun 2013, 8(3): p. 343-6. [CrossRef]
- Yang, G., Fu, Y., Malakhova, M., Kurinov, I., Zhu, F., Yao, K., Li, H., Chen, H., Li, W., Lim, D.Y., Sheng, Y., Bode, A.M., Dong, Z.,Dong, Z. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev Res (Phila) 2014, 7(10): p. 1056-66. [CrossRef]
- Kang, N.J., Lee, K.W., Shin, B.J., Jung, S.K., Hwang, M.K., Bode, A.M., Heo, Y.S., Lee, H.J.,Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30(2): p. 321-30. [CrossRef]
- Balupillai, A., Nagarajan, R.P., Ramasamy, K., Govindasamy, K.,Muthusamy, G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol Appl Pharmacol 2018, 352: p. 87-96. [CrossRef]
- Pelinson, L.P., Assmann, C.E., Palma, T.V., da Cruz, I.B.M., Pillat, M.M., Mânica, A., Stefanello, N., Weis, G.C.C., de Oliveira Alves, A., de Andrade, C.M., Ulrich, H., Morsch, V.M.M., Schetinger, M.R.C.,Bagatini, M.D. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol Biol Rep 2019, 46(2): p. 2085-2092. [CrossRef]
- Kimsa-Dudek, M., Synowiec-Wojtarowicz, A., Krawczyk, A., Kosowska, A., Kimsa-Furdzik, M.,Francuz, T. The Apoptotic Effect of Caffeic or Chlorogenic Acid on the C32 Cells That Have Simultaneously Been Exposed to a Static Magnetic Field. Int J Mol Sci 2022, 23(7). [CrossRef]
- Kimsa-Dudek, M., Synowiec-Wojtarowicz, A.,Krawczyk, A. Phenolic acids and a static magnetic field change the expression of transforming growth factor beta isoforms in amelanotic melanoma cells. Mol Biol Rep 2023, 50(5): p. 4207-4216. [CrossRef]
- Kimsa-Dudek, M., Krawczyk, A., Synowiec-Wojtarowicz, A., Dudek, S.,Pawlowska-Goral, K. The impact of the co-exposure of melanoma cells to chlorogenic acid and a moderate-strength static magnetic field. J Food Biochem 2020, 44(12): p. e13512. [CrossRef]
- Cui, K., Zhang, L., La, X., Wu, H., Yang, R., Li, H.,Li, Z. Ferulic Acid and P-Coumaric Acid Synergistically Attenuate Non-Alcoholic Fatty Liver Disease through HDAC1/PPARG-Mediated Free Fatty Acid Uptake. Int J Mol Sci 2022, 23(23). [CrossRef]
- Ghaderi, S., Gholipour, P., Komaki, A., Salehi, I., Rashidi, K., Esmaeil Khoshnam, S.,Rashno, M. p-Coumaric acid ameliorates cognitive and non-cognitive disturbances in a rat model of Alzheimer's disease: The role of oxidative stress and inflammation. Int Immunopharmacol 2022, 112: p. 109295. [CrossRef]
- Moradi, M., Farbood, Y., Mard, S.A., Dianat, M., Goudarzi, G., Khorsandi, L.,Seyedian, S.S. p-Coumaric acid has pure anti-inflammatory characteristics against hepatopathy caused by ischemia-reperfusion in the liver and dust exposure. Iran J Basic Med Sci 2023, 26(2): p. 164-175. [CrossRef]
- Venkatesan, A., Roy, A., Kulandaivel, S., Natesan, V.,Kim, S.J. p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats. Metabolites 2022, 12(12). [CrossRef]
- Tehami, W., Nani, A., Khan, N.A.,Hichami, A. New Insights Into the Anticancer Effects of p-Coumaric Acid: Focus on Colorectal Cancer. Dose Response 2023, 21(1): p. 15593258221150704. [CrossRef]
- Saremi, S., Kolahi, M., Tabandeh, M.R.,Hashemitabar, M. Induction of apoptosis and suppression of Ras gene expression in MCF human breast cancer cells. J Cancer Res Ther 2022, 18(4): p. 1052-1060. [CrossRef]
- Hu, X., Yang, Z., Liu, W., Pan, Z., Zhang, X., Li, M., Liu, X., Zheng, Q.,Li, D. The Anti-tumor Effects of p-Coumaric Acid on Melanoma A375 and B16 Cells. Front Oncol 2020, 10: p. 558414. [CrossRef]
- Gastaldello, G.H., Cazeloto, A.C.V., Ferreira, J.C., Rodrigues, D.M., Bastos, J.K., Campo, V.L., Zoccal, K.F.,Tefe-Silva, C. Green Propolis Compounds (Baccarin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10. Medicines (Basel) 2021, 8(5). [CrossRef]
- Ciazynska, M., Olejniczak-Staruch, I., Sobolewska-Sztychny, D., Narbutt, J., Skibinska, M.,Lesiak, A. Ultraviolet Radiation and Chronic Inflammation-Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life (Basel) 2021, 11(4). [CrossRef]
- Staniforth, V., Huang, W.C., Aravindaram, K.,Yang, N.S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J Nutr Biochem 2012, 23(5): p. 443-51. [CrossRef]
- Ambothi, K., Prasad, N.R.,Balupillai, A. Ferulic acid inhibits UVB-radiation induced photocarcinogenesis through modulating inflammatory and apoptotic signaling in Swiss albino mice. Food Chem Toxicol 2015, 82: p. 72-8. [CrossRef]
- Wianowska, D.,Olszowy-Tomczyk, M. A Concise Profile of Gallic Acid-From Its Natural Sources through Biological Properties and Chemical Methods of Determination. Molecules 2023, 28(3). [CrossRef]
- Lo, C., Lai, T.Y., Yang, J.H., Yang, J.S., Ma, Y.S., Weng, S.W., Chen, Y.Y., Lin, J.G.,Chung, J.G. Gallic acid induces apoptosis in A375.S2 human melanoma cells through caspase-dependent and -independent pathways. Int J Oncol 2010, 37(2): p. 377-85. [CrossRef]
- Lo, C., Lai, T.Y., Yang, J.S., Yang, J.H., Ma, Y.S., Weng, S.W., Lin, H.Y., Chen, H.Y., Lin, J.G.,Chung, J.G. Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and Ras. Melanoma Res 2011, 21(4): p. 267-73. [CrossRef]
- Liu, C., Lin, J.J., Yang, Z.Y., Tsai, C.C., Hsu, J.L.,Wu, Y.J. Proteomic study reveals a co-occurrence of gallic acid-induced apoptosis and glycolysis in B16F10 melanoma cells. J Agric Food Chem 2014, 62(48): p. 11672-80. [CrossRef]
- Tseng, T.H., Hsu, J.D., Lo, M.H., Chu, C.Y., Chou, F.P., Huang, C.L.,Wang, C.J. Inhibitory effect of Hibiscus protocatechuic acid on tumor promotion in mouse skin. Cancer Lett 1998, 126(2): p. 199-207. [CrossRef]
- Nakamura, Y., Torikai, K., Ohto, Y., Murakami, A., Tanaka, T.,Ohigashi, H. A simple phenolic antioxidant protocatechuic acid enhances tumor promotion and oxidative stress in female ICR mouse skin: dose-and timing-dependent enhancement and involvement of bioactivation by tyrosinase. Carcinogenesis 2000, 21(10): p. 1899-907. [CrossRef]
- Nakamura, Y., Torikai, K.,Ohigashi, H. A catechol antioxidant protocatechuic acid potentiates inflammatory leukocyte-derived oxidative stress in mouse skin via a tyrosinase bioactivation pathway. Free Radic Biol Med 2001, 30(9): p. 967-78. [CrossRef]
- Lin, H.H., Chen, J.H., Chou, F.P.,Wang, C.J. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-kappaB pathway and MMP-2 production by targeting RhoB activation. Br J Pharmacol 2011, 162(1): p. 237-54. [CrossRef]
- da Silva, G.B., Manica, D., da Silva, A.P., Marafon, F., Moreno, M.,Bagatini, M.D. Rosmarinic acid decreases viability, inhibits migration and modulates expression of apoptosis-related CASP8/CASP3/NLRP3 genes in human metastatic melanoma cells. Chem Biol Interact 2023, 375: p. 110427. [CrossRef]
- Huang, L., Chen, J., Quan, J.,Xiang, D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3beta axis. Bioengineered 2021, 12(1): p. 3065-3076. [CrossRef]
- Olivares, A., Alcaraz-Saura, M., Achel, D.G.,Alcaraz, M. Effect of Rosmarinic Acid and Ionizing Radiation on Glutathione in Melanoma B16F10 Cells: A Translational Opportunity. Antioxidants (Basel) 2020, 9(12). [CrossRef]
- Alcaraz, M., Alcaraz-Saura, M., Achel, D.G., Olivares, A., López-Morata, J.A.,Castillo, J. Radiosensitizing effect of rosmarinic acid in metastatic melanoma B16F10 cells. Anticancer Res 2014, 34(4): p. 1913-21.
- Srinivasulu, C., Ramgopal, M., Ramanjaneyulu, G., Anuradha, C.M.,Suresh Kumar, C. Syringic acid (SA) ‒ A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed Pharmacother 2018, 108: p. 547-557. [CrossRef]
- Cikman, O., Soylemez, O., Ozkan, O.F., Kiraz, H.A., Sayar, I., Ademoglu, S., Taysi, S.,Karaayvaz, M. Antioxidant Activity of Syringic Acid Prevents Oxidative Stress in l-arginine-Induced Acute Pancreatitis: An Experimental Study on Rats. Int Surg 2015, 100(5): p. 891-6. [CrossRef]
- Mihanfar, A., Darband, S.G., Sadighparvar, S., Kaviani, M., Mirza-Aghazadeh-Attari, M., Yousefi, B.,Majidinia, M. In vitro and in vivo anticancer effects of syringic acid on colorectal cancer: Possible mechanistic view. Chem Biol Interact 2021, 337: p. 109337. [CrossRef]
- Ha, S.J., Lee, J., Park, J., Kim, Y.H., Lee, N.H., Kim, Y.E., Song, K.M., Chang, P.S., Jeong, C.H.,Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem Pharmacol 2018, 154: p. 435-445. [CrossRef]
- Ghitu, A., Schwiebs, A., Radeke, H.H., Avram, S., Zupko, I., Bor, A., Pavel, I.Z., Dehelean, C.A., Oprean, C., Bojin, F., Farcas, C., Soica, C., Duicu, O.,Danciu, C. A Comprehensive Assessment of Apigenin as an Antiproliferative, Proapoptotic, Antiangiogenic and Immunomodulatory Phytocompound. Nutrients 2019, 11(4). [CrossRef]
- Madunic, J., Madunic, I.V., Gajski, G., Popic, J.,Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett 2018, 413: p. 11-22. [CrossRef]
- Zhao, G., Han, X., Cheng, W., Ni, J., Zhang, Y., Lin, J.,Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol Rep 2017, 37(4): p. 2277-2285. [CrossRef]
- Cao, H.H., Chu, J.H., Kwan, H.Y., Su, T., Yu, H., Cheng, C.Y., Fu, X.Q., Guo, H., Li, T., Tse, A.K., Chou, G.X., Mo, H.B.,Yu, Z.L. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci Rep 2016, 6: p. 21731. [CrossRef]
- Woo, J.S., Choo, G.S., Yoo, E.S., Kim, S.H., Lee, J.H., Han, S.H., Kim, H.J., Jung, S.H., Park, Y.S., Kim, B.S., Kim, S.K., Park, B.K., Cho, S.D., Nam, J.S., Choi, C.S., Che, J.H.,Jung, J.Y. Apigenin induces apoptosis by regulating Akt and MAPK pathways in human melanoma cell A375SM. Mol Med Rep 2020, 22(6): p. 4877-4889. [CrossRef]
- Xu, L., Zhang, Y., Tian, K., Chen, X., Zhang, R., Mu, X., Wu, Y., Wang, D., Wang, S., Liu, F., Wang, T., Zhang, J., Liu, S., Zhang, Y., Tu, C.,Liu, H. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J Exp Clin Cancer Res 2018, 37(1): p. 261. [CrossRef]
- Mirzoeva, S., Tong, X., Bridgeman, B.B., Plebanek, M.P.,Volpert, O.V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20(9): p. 930-942. [CrossRef]
- Bridgeman, B.B., Wang, P., Ye, B., Pelling, J.C., Volpert, O.V.,Tong, X. Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention. Cell Signal 2016, 28(5): p. 460-468. [CrossRef]
- Byun, S., Park, J., Lee, E., Lim, S., Yu, J.G., Lee, S.J., Chen, H., Dong, Z., Lee, K.W.,Lee, H.J. Src kinase is a direct target of apigenin against UVB-induced skin inflammation. Carcinogenesis 2013, 34(2): p. 397-405. [CrossRef]
- Van Dross, R.T., Hong, X., Essengue, S., Fischer, S.M.,Pelling, J.C. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol Carcinog 2007, 46(4): p. 303-14. [CrossRef]
- Caltagirone, S., Rossi, C., Poggi, A., Ranelletti, F.O., Natali, P.G., Brunetti, M., Aiello, F.B.,Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer 2000, 87(4): p. 595-600. [CrossRef]
- Riaz, A., Rasul, A., Hussain, G., Zahoor, M.K., Jabeen, F., Subhani, Z., Younis, T., Ali, M., Sarfraz, I.,Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv Pharmacol Sci 2018, 2018: p. 9794625. [CrossRef]
- You, O.H., Shin, E.A., Lee, H., Kim, J.H., Sim, D.Y., Kim, J.H., Kim, Y., Khil, J.H., Baek, N.I.,Kim, S.H. Apoptotic Effect of Astragalin in Melanoma Skin Cancers via Activation of Caspases and Inhibition of Sry-related HMg-Box Gene 10. Phytother Res 2017, 31(10): p. 1614-1620. [CrossRef]
- Rosenbaum, S.R., Tiago, M., Caksa, S., Capparelli, C., Purwin, T.J., Kumar, G., Glasheen, M., Pomante, D., Kotas, D., Chervoneva, I.,Aplin, A.E. SOX10 requirement for melanoma tumor growth is due, in part, to immune-mediated effects. Cell Rep 2021, 37(10): p. 110085. [CrossRef]
- Min, K.J.,Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr Med Res 2014, 3(1): p. 16-24. [CrossRef]
- Ravindranath, M.H., Ramasamy, V., Moon, S., Ruiz, C.,Muthugounder, S. Differential growth suppression of human melanoma cells by tea (Camellia sinensis) epicatechins (ECG, EGC and EGCG). Evid Based Complement Alternat Med 2009, 6(4): p. 523-30. [CrossRef]
- Sudha, T., Salaheldin, T.A., Darwish, N.H.,Mousa, S.A. Antitumor/anti-angiogenesis efficacy of epigallocatechin gallate nanoformulated with antioxidant in melanoma. Nanomedicine (Lond) 2022, 17(15): p. 1039-1053. [CrossRef]
- Du, B.X., Lin, P.,Lin, J. EGCG and ECG induce apoptosis and decrease autophagy via the AMPK/mTOR and PI3K/AKT/mTOR pathway in human melanoma cells. Chin J Nat Med 2022, 20(4): p. 290-300. [CrossRef]
- Ravindran Menon, D., Li, Y., Yamauchi, T., Osborne, D.G., Vaddi, P.K., Wempe, M.F., Zhai, Z.,Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals (Basel) 2021, 14(11). [CrossRef]
- Chen, H.Y., Jiang, Y.W., Kuo, C.L., Way, T.D., Chou, Y.C., Chang, Y.S.,Chung, J.G. Chrysin inhibit human melanoma A375.S2 cell migration and invasion via affecting MAPK signaling and NF-kappaB signaling pathway in vitro. Environ Toxicol 2019, 34(4): p. 434-442. [CrossRef]
- Sassi, A., Maatouk, M., El Gueder, D., Bzeouich, I.M., Abdelkefi-Ben Hatira, S., Jemni-Yacoub, S., Ghedira, K.,Chekir-Ghedira, L. Chrysin, a natural and biologically active flavonoid suppresses tumor growth of mouse B16F10 melanoma cells: In vitro and In vivo study. Chem Biol Interact 2018, 283: p. 10-19. [CrossRef]
- Pichichero, E., Cicconi, R., Mattei, M.,Canini, A. Chrysin-induced apoptosis is mediated through p38 and Bax activation in B16-F1 and A375 melanoma cells. Int J Oncol 2011, 38(2): p. 473-83. [CrossRef]
- Alvarez, N., Vicente, V.,Martínez, C. Synergistic effect of diosmin and interferon-alpha on metastatic pulmonary melanoma. Cancer Biother Radiopharm 2009, 24(3): p. 347-52. [CrossRef]
- Buddhan, R.,Manoharan, S. Diosmin reduces cell viability of A431 skin cancer cells through apoptotic induction. J Cancer Res Ther 2017, 13(3): p. 471-476. [CrossRef]
- Martínez Conesa, C., Vicente Ortega, V., Yáñez Gascón, M.J., Alcaraz Baños, M., Canteras Jordana, M., Benavente-García, O.,Castillo, J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J Agric Food Chem 2005, 53(17): p. 6791-7. [CrossRef]
- Martinez, C., Vicente, V., Yanez, J., Alcaraz, M., Castells, M.T., Canteras, M., Benavente-Garcia, O.,Castillo, J. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol Histopathol 2005, 20(4): p. 1121-9. [CrossRef]
- Zhang, W., Tang, B., Huang, Q.,Hua, Z. Galangin inhibits tumor growth and metastasis of B16F10 melanoma. J Cell Biochem 2013, 114(1): p. 152-61. [CrossRef]
- Benguedouar, L., Lahouel, M., Gangloff, S.C., Durlach, A., Grange, F., Bernard, P.,Antonicelli, F. Ethanolic Extract of Algerian Propolis and Galangin Decreased Murine Melanoma Tumor Progression in Mice. Anticancer Agents Med Chem 2016, 16(9): p. 1172-83. [CrossRef]
- Madduma Hewage, S.R., Piao, M.J., Kim, K.C., Cha, J.W., Han, X., Choi, Y.H., Chae, S.,Hyun, J.W. Galangin (3,5,7-trihydroxyflavone) shields human keratinocytes from ultraviolet B-induced oxidative stress. Biomol Ther (Seoul) 2015, 23(2): p. 165-73. [CrossRef]
- Madduma Hewage, S.R.K., Piao, M.J., Kang, K.A., Ryu, Y.S., Fernando, P., Oh, M.C., Park, J.E., Shilnikova, K., Moon, Y.J., Shin, D.O.,Hyun, J.W. Galangin Activates the ERK/AKT-Driven Nrf2 Signaling Pathway to Increase the Level of Reduced Glutathione in Human Keratinocytes. Biomol Ther (Seoul) 2017, 25(4): p. 427-433. [CrossRef]
- Jaiswal, N., Akhtar, J., Singh, S.P., Badruddeen,Ahsan, F. An Overview on Genistein and its Various Formulations. Drug Res (Stuttg) 2019, 69(6): p. 305-313. [CrossRef]
- Hou, S. Genistein: Therapeutic and Preventive Effects, Mechanisms, and Clinical Application in Digestive Tract Tumor. Evid Based Complement Alternat Med 2022, 2022: p. 5957378. [CrossRef]
- Bhat, S.S., Prasad, S.K., Shivamallu, C., Prasad, K.S., Syed, A., Reddy, P., Cull, C.A.,Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr Issues Mol Biol 2021, 43(3): p. 1502-1517. [CrossRef]
- Wang, H.Z., Zhang, Y., Xie, L.P., Yu, X.Y.,Zhang, R.Q. Effects of genistein and daidzein on the cell growth, cell cycle, and differentiation of human and murine melanoma cells(1). J Nutr Biochem 2002, 13(7): p. 421-426. [CrossRef]
- Darbon, J.M., Penary, M., Escalas, N., Casagrande, F., Goubin-Gramatica, F., Baudouin, C.,Ducommun, B. Distinct Chk2 activation pathways are triggered by genistein and DNA-damaging agents in human melanoma cells. J Biol Chem 2000, 275(20): p. 15363-9. [CrossRef]
- Cui, S., Wang, J., Wu, Q., Qian, J., Yang, C.,Bo, P. Genistein inhibits the growth and regulates the migration and invasion abilities of melanoma cells via the FAK/paxillin and MAPK pathways. Oncotarget 2017, 8(13): p. 21674-21691. [CrossRef]
- Heo, J.R., Lee, G.A., Kim, G.S., Hwang, K.A.,Choi, K.C. Phytochemical-induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis and differentiation in malignant melanoma cells. Phytomedicine 2018, 39: p. 100-110. [CrossRef]
- Tamura, S., Bito, T., Ichihashi, M.,Ueda, M. Genistein enhances the cisplatin-induced inhibition of cell growth and apoptosis in human malignant melanoma cells. Pigment Cell Res 2003, 16(5): p. 470-6. [CrossRef]
- Alhasan, S.A., Ensley, J.F.,Sarkar, F.H. Genistein induced molecular changes in a squamous cell carcinoma of the head and neck cell line. Int J Oncol 2000, 16(2): p. 333-341. [CrossRef]
- Record, I.R., Broadbent, J.L., King, R.A., Dreosti, I.E., Head, R.J.,Tonkin, A.L. Genistein inhibits growth of B16 melanoma cells in vivo and in vitro and promotes differentiation in vitro. Int J Cancer 1997, 72(5): p. 860-864. [CrossRef]
- Menon, L.G., Kuttan, R., Nair, M.G., Chang, Y.C.,Kuttan, G. Effect of isoflavones genistein and daidzein in the inhibition of lung metastasis in mice induced by B16F-10 melanoma cells. Nutr Cancer 1998, 30(1): p. 74-7. [CrossRef]
- Farina, H.G., Pomies, M., Alonso, D.F.,Gomez, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep 2006, 16(4): p. 885-91. [CrossRef]
- Wei, H., Bowen, R., Zhang, X.,Lebwohl, M. Isoflavone genistein inhibits the initiation and promotion of two-stage skin carcinogenesis in mice. Carcinogenesis 1998, 19(8): p. 1509-14. [CrossRef]
- Wei, H., Saladi, R., Lu, Y., Wang, Y., Palep, S.R., Moore, J., Phelps, R., Shyong, E.,Lebwohl, M.G. Isoflavone genistein: photoprotection and clinical implications in dermatology. J Nutr 2003, 133(11 Suppl 1): p. 3811S-3819S. [CrossRef]
- Wei, H., Zhang, X., Wang, Y.,Lebwohl, M. Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by isoflavone genistein. Cancer Lett 2002, 185(1): p. 21-9. [CrossRef]
- Valentova, K., Vrba, J., Bancirova, M., Ulrichova, J.,Kren, V. Isoquercitrin: pharmacology, toxicology, and metabolism. Food Chem Toxicol 2014, 68: p. 267-82. [CrossRef]
- Wullschleger, S., Loewith, R.,Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124(3): p. 471-84. [CrossRef]
- Won, Y.S., Kim, J.H., Lizardo, R.C.M., Min, H.J., Cho, H.D., Hong, S.M.,Seo, K.I. The Flavonol Isoquercitrin Promotes Mitochondrial-Dependent Apoptosis in SK-Mel-2 Melanoma Cell via the PI3K/AKT/mTOR Pathway. Nutrients 2020, 12(12). [CrossRef]
- Gong, G., Guan, Y.Y., Zhang, Z.L., Rahman, K., Wang, S.J., Zhou, S., Luan, X.,Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed Pharmacother 2020, 128: p. 110301. [CrossRef]
- Duan, R., Liang, X., Chai, B., Zhou, Y., Du, H., Suo, Y., Chen, Z., Li, Q.,Huang, X. Isorhamnetin Induces Melanoma Cell Apoptosis via the PI3K/Akt and NF-kappaB Pathways. Biomed Res Int 2020, 2020: p. 1057943. [CrossRef]
- Kim, J.E., Lee, D.E., Lee, K.W., Son, J.E., Seo, S.K., Li, J., Jung, S.K., Heo, Y.S., Mottamal, M., Bode, A.M., Dong, Z.,Lee, H.J. Isorhamnetin suppresses skin cancer through direct inhibition of MEK1 and PI3-K. Cancer Prev Res (Phila) 2011, 4(4): p. 582-91. [CrossRef]
- Li, J., Mottamal, M., Li, H., Liu, K., Zhu, F., Cho, Y.Y., Sosa, C.P., Zhou, K., Bowden, G.T., Bode, A.M.,Dong, Z. Quercetin-3-methyl ether suppresses proliferation of mouse epidermal JB6 P+ cells by targeting ERKs. Carcinogenesis 2012, 33(2): p. 459-65. [CrossRef]
- Qiang, D., Ci, C., Liu, W., Wang, J., He, C., Ji, B.,Shao, X. Inhibitory effect of kaempferol on mouse melanoma cell line B16 in vivo and in vitro. Postepy Dermatol Alergol 2021, 38(3): p. 498-504. [CrossRef]
- Yang, J., Xiao, P., Sun, J.,Guo, L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J buon 2018, 23(1): p. 218-223. [PubMed]
- Zheng, X., Pan, Y., Yang, G., Liu, Y., Zou, J., Zhao, H., Yin, G., Wu, Y., Li, X., Wei, Z., Yu, S., Zhao, Y., Wang, A., Chen, W.,Lu, Y. Kaempferol impairs aerobic glycolysis against melanoma metastasis via inhibiting the mitochondrial binding of HK2 and VDAC1. Eur J Pharmacol 2022, 931: p. 175226. [CrossRef]
- Yao, K., Chen, H., Liu, K., Langfald, A., Yang, G., Zhang, Y., Yu, D.H., Kim, M.O., Lee, M.H., Li, H., Bae, K.B., Kim, H.G., Ma, W.Y., Bode, A.M., Dong, Z.,Dong, Z. Kaempferol targets RSK2 and MSK1 to suppress UV radiation-induced skin cancer. Cancer Prev Res (Phila) 2014, 7(9): p. 958-967. [CrossRef]
- Lee, K.M., Lee, K.W., Jung, S.K., Lee, E.J., Heo, Y.S., Bode, A.M., Lubet, R.A., Lee, H.J.,Dong, Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem Pharmacol 2010, 80(12): p. 2042-9. [CrossRef]
- Schomberg, J., Wang, Z., Farhat, A., Guo, K.L., Xie, J., Zhou, Z., Liu, J., Kovacs, B.,Liu-Smith, F. Luteolin inhibits melanoma growth in vitro and in vivo via regulating ECM and oncogenic pathways but not ROS. Biochem Pharmacol 2020, 177: p. 114025. [CrossRef]
- Yao, X., Jiang, W., Yu, D.,Yan, Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct 2019, 10(2): p. 703-712. [CrossRef]
- Lamouille, S., Xu, J.,Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014, 15(3): p. 178-96. [CrossRef]
- Li, C., Wang, Q., Shen, S., Wei, X.,Li, G. HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells. Phytother Res 2019, 33(3): p. 798-807. [CrossRef]
- Ruan, J.S., Liu, Y.P., Zhang, L., Yan, L.G., Fan, F.T., Shen, C.S., Wang, A.Y., Zheng, S.Z., Wang, S.M.,Lu, Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting beta3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol Sin 2012, 33(10): p. 1325-31. [CrossRef]
- Kim, J.K., Kang, K.A., Ryu, Y.S., Piao, M.J., Han, X., Oh, M.C., Boo, S.J., Jeong, S.U., Jeong, Y.J., Chae, S., Na, S.Y.,Hyun, J.W. Induction of Endoplasmic Reticulum Stress via Reactive Oxygen Species Mediated by Luteolin in Melanoma Cells. Anticancer Res 2016, 36(5): p. 2281-9.
- Li, T., Fu, X., Liu, B., Wang, X., Li, J., Zhu, P., Niu, X., Bai, J., Liu, Y., Lu, X.,Yu, Z.L. Luteolin binds Src, promotes STAT3 protein ubiquitination and exerts anti-melanoma effects in cell and mouse models. Biochem Pharmacol 2022, 200: p. 115044. [CrossRef]
- George, V.C., Naveen Kumar, D.R., Suresh, P.K., Kumar, S.,Kumar, R.A. Comparative studies to evaluate relative in vitro potency of luteolin in inducing cell cycle arrest and apoptosis in HaCaT and A375 cells. Asian Pac J Cancer Prev 2013, 14(2): p. 631-7. [CrossRef]
- Kang, N.J., Jung, S.K., Lee, K.W.,Lee, H.J. Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann N Y Acad Sci 2011, 1229: p. 124-32. [CrossRef]
- Afroze, N., Pramodh, S., Hussain, A., Waleed, M.,Vakharia, K. A review on myricetin as a potential therapeutic candidate for cancer prevention. 3 Biotech 2020, 10(5): p. 211. [CrossRef]
- Jung, S.K., Lee, K.W., Byun, S., Kang, N.J., Lim, S.H., Heo, Y.S., Bode, A.M., Bowden, G.T., Lee, H.J.,Dong, Z. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res 2008, 68(14): p. 6021-9. [CrossRef]
- Sun, W., Tao, Y., Yu, D., Zhao, T., Wu, L., Yu, W.,Han, W. Myricetin exerts potent anticancer effects on human skin tumor cells. Trop J Pharm 2018, 17: p. 1067-1072. [CrossRef]
- Lee, K.M., Kang, N.J., Han, J.H., Lee, K.W.,Lee, H.J. Myricetin down-regulates phorbol ester-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking activation of nuclear factor kappa B. J Agric Food Chem 2007, 55(23): p. 9678-84. [CrossRef]
- Lee, K.W., Kang, N.J., Rogozin, E.A., Kim, H.G., Cho, Y.Y., Bode, A.M., Lee, H.J., Surh, Y.J., Bowden, G.T.,Dong, Z. Myricetin is a novel natural inhibitor of neoplastic cell transformation and MEK1. Carcinogenesis 2007, 28(9): p. 1918-27. [CrossRef]
- Kumamoto, T., Fujii, M.,Hou, D.X. Myricetin directly targets JAK1 to inhibit cell transformation. Cancer Lett 2009, 275(1): p. 17-26. [CrossRef]
- Jung, S.K., Lee, K.W., Byun, S., Lee, E.J., Kim, J.E., Bode, A.M., Dong, Z.,Lee, H.J. Myricetin inhibits UVB-induced angiogenesis by regulating PI-3 kinase in vivo. Carcinogenesis 2010, 31(5): p. 911-7. [CrossRef]
- Choi, J., Lee, D.H., Jang, H., Park, S.Y.,Seol, J.W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int J Med Sci 2020, 17(18): p. 3049-3057. [CrossRef]
- Pafumi, I., Festa, M., Papacci, F., Lagostena, L., Giunta, C., Gutla, V., Cornara, L., Favia, A., Palombi, F., Gambale, F., Filippini, A.,Carpaneto, A. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Sci Rep 2017, 7(1): p. 5121. [CrossRef]
- Skelding, K.A., Barry, D.L., Theron, D.Z.,Lincz, L.F. Targeting the two-pore channel 2 in cancer progression and metastasis. Explor Target Antitumor Ther 2022, 3(1): p. 62-89. [CrossRef]
- Lentini, A., Forni, C., Provenzano, B.,Beninati, S. Enhancement of transglutaminase activity and polyamine depletion in B16-F10 melanoma cells by flavonoids naringenin and hesperitin correlate to reduction of the in vivo metastatic potential. Amino Acids 2007, 32(1): p. 95-100. [CrossRef]
- Ahamad, M.S., Siddiqui, S., Jafri, A., Ahmad, S., Afzal, M.,Arshad, M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS One 2014, 9(10): p. e110003. [CrossRef]
- Anand David, A.V., Arulmoli, R.,Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev 2016, 10(20): p. 84-89. [CrossRef]
- Soll, F., Ternent, C., Berry, I.M., Kumari, D.,Moore, T.C. Quercetin Inhibits Proliferation and Induces Apoptosis of B16 Melanoma Cells In Vitro. Assay Drug Dev Technol 2020, 18(6): p. 261-268. [CrossRef]
- Zhang, X.M., Chen, J., Xia, Y.G.,Xu, Q. Apoptosis of murine melanoma B16-BL6 cells induced by quercetin targeting mitochondria, inhibiting expression of PKC-alpha and translocating PKC-delta. Cancer Chemother Pharmacol 2005, 55(3): p. 251-62. [CrossRef]
- Zhang, X., Xu, Q.,Saiki, I. Quercetin inhibits the invasion and mobility of murine melanoma B16-BL6 cells through inducing apoptosis via decreasing Bcl-2 expression. Clin Exp Metastasis 2000, 18(5): p. 415-21. [CrossRef]
- Cao, H.H., Tse, A.K., Kwan, H.Y., Yu, H., Cheng, C.Y., Su, T., Fong, W.F.,Yu, Z.L. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem Pharmacol 2014, 87(3): p. 424-34. [CrossRef]
- Kim, S.H., Yoo, E.S., Woo, J.S., Han, S.H., Lee, J.H., Jung, S.H., Kim, H.J.,Jung, J.Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur J Pharmacol 2019, 860: p. 172568. [CrossRef]
- Zhang, X.M., Huang, S.P.,Xu, Q. Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother Pharmacol 2004, 53(1): p. 82-8. [CrossRef]
- Lin, Y.S., Tsai, P.H., Kandaswami, C.C., Cheng, C.H., Ke, F.C., Lee, P.P., Hwang, J.J.,Lee, M.T. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci 2011, 102(10): p. 1829-39. [CrossRef]
- Patel, D.H.,Sharma, N. Inhibitory effect of quercetin on epithelial to mesenchymal transition in SK-MEL-28 human melanoma cells defined by in vitro analysis on 3D collagen gels. Onco Targets Ther 2016, 9: p. 6445-6459. [CrossRef]
- Fan, J.J., Hsu, W.H., Lee, K.H., Chen, K.C., Lin, C.W., Lee, Y.A., Ko, T.P., Lee, L.T., Lee, M.T., Chang, M.S.,Cheng, C.H. Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling. Antioxidants (Basel) 2019, 8(11). [CrossRef]
- Cao, H.H., Cheng, C.Y., Su, T., Fu, X.Q., Guo, H., Li, T., Tse, A.K., Kwan, H.Y., Yu, H.,Yu, Z.L. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer 2015, 14: p. 103. [CrossRef]
- Huang, Y.T., Hwang, J.J., Lee, P.P., Ke, F.C., Huang, J.H., Huang, C.J., Kandaswami, C., Middleton, E., Jr.,Lee, M.T. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol 1999, 128(5): p. 999-1010. [CrossRef]
- Rafiq, R.A., Quadri, A., Nazir, L.A., Peerzada, K., Ganai, B.A.,Tasduq, S.A. A Potent Inhibitor of Phosphoinositide 3-Kinase (PI3K) and Mitogen Activated Protein (MAP) Kinase Signalling, Quercetin (3, 3', 4', 5, 7-Pentahydroxyflavone) Promotes Cell Death in Ultraviolet (UV)-B-Irradiated B16F10 Melanoma Cells. PLoS One 2015, 10(7): p. e0131253. [CrossRef]
- Peng, D., Chen, L., Sun, Y., Sun, L., Yin, Q., Deng, S., Niu, L., Lou, F., Wang, Z., Xu, Z., Wang, C., Fan, L., Wang, H.,Wang, H. Melanoma suppression by quercein is correlated with RIG-I and type I interferon signaling. Biomed Pharmacother 2020, 125: p. 109984. [CrossRef]
- Ganeshpurkar, A.,Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm J 2017, 25(2): p. 149-164. [CrossRef]
- Pinzaru, I., Chioibas, R., Marcovici, I., Coricovac, D., Susan, R., Predut, D., Georgescu, D.,Dehelean, C. Rutin Exerts Cytotoxic and Senescence-Inducing Properties in Human Melanoma Cells. Toxics 2021, 9(9). [CrossRef]
- Menon, L.G., Kuttan, R.,Kuttan, G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Letters 1995, 95(1): p. 221-225. [CrossRef]
- Choi, K.S., Kundu, J.K., Chun, K.S., Na, H.K.,Surh, Y.J. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch Biochem Biophys 2014, 559: p. 38-45. [CrossRef]
- Liu, N., Wang, K.S., Qi, M., Zhou, Y.J., Zeng, G.Y., Tao, J., Zhou, J.D., Zhang, J.L., Chen, X.,Peng, C. Vitexin compound 1, a novel extraction from a Chinese herb, suppresses melanoma cell growth through DNA damage by increasing ROS levels. J Exp Clin Cancer Res 2018, 37(1): p. 269. [CrossRef]
- Zhang, W., LiPing, Z.,Guo, L. Vitexin inhibited the invasion, metastasis, and progression of human melanoma cells by targeting STAT3 signaling pathway. bioRxiv 2020: p. 2020.09.24.311233. [CrossRef]
- Murakami, A., Ohura, S., Nakamura, Y., Koshimizu, K.,Ohigashi, H. 1'-Acetoxychavicol acetate, a superoxide anion generation inhibitor, potently inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in ICR mouse skin. Oncology 1996, 53(5): p. 386-91. [CrossRef]
- Batra, V., Syed, Z., Gill, J.N., Coburn, M.A., Adegboyega, P., DiGiovanni, J., Mathis, J.M., Shi, R., Clifford, J.L.,Kleiner-Hancock, H.E. Effects of the tropical ginger compound,1'-acetoxychavicol acetate, against tumor promotion in K5.Stat3C transgenic mice. J Exp Clin Cancer Res 2012, 31(1): p. 57. [CrossRef]
- Li, J., Mao, Y.,Li, W. Anethole suppresses the growth of human skin cancer cells by targeting microRNA498/STAT4 axis. Trop J Pharm 2022, 21(11): p. 2391-2396. [CrossRef]
- Wang, T., Ma, L., Li, W., Ding, L.,Gao, H. MicroRNA-498 reduces the proliferation and invasion of colorectal cancer cells via targeting Bcl-2. FEBS Open Bio 2020, 10(1): p. 168-175. [CrossRef]
- Zhang, X., Xu, X., Ge, G., Zang, X., Shao, M., Zou, S., Zhang, Y., Mao, Z., Zhang, J., Mao, F., Qian, H.,Xu, W. miR-498 inhibits the growth and metastasis of liver cancer by targeting ZEB2. Oncol Rep 2019, 41(3): p. 1638-1648. [CrossRef]
- Lombardi, V.R., Carrera, I.,Cacabelos, R. In Vitro Screening for Cytotoxic Activity of Herbal Extracts. Evid Based Complement Alternat Med 2017, 2017: p. 2675631. [CrossRef]
- Nahar, L., Al-Groshi, A., Kumar, A.,Sarker, S.D. Arbutin: Occurrence in Plants, and Its Potential as an Anticancer Agent. Molecules 2022, 27(24). [CrossRef]
- Jiang, L., Wang, D., Zhang, Y., Li, J., Wu, Z., Wang, Z.,Wang, D. Investigation of the pro-apoptotic effects of arbutin and its acetylated derivative on murine melanoma cells. Int J Mol Med 2018, 41(2): p. 1048-1054. [CrossRef]
- Nawarak, J., Huang-Liu, R., Kao, S.H., Liao, H.H., Sinchaikul, S., Chen, S.T.,Cheng, S.L. Proteomics analysis of A375 human malignant melanoma cells in response to arbutin treatment. Biochim Biophys Acta 2009, 1794(2): p. 159-67. [CrossRef]
- Caterina, M.J., Schumacher, M.A., Tominaga, M., Rosen, T.A., Levine, J.D.,Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389(6653): p. 816-24. [CrossRef]
- Chen, Z.Y., Huang, H.H., Li, Q.C., Zhan, F.B., Wang, L.B., He, T., Yang, C.H., Wang, Y., Zhang, Y.,Quan, Z.X. Capsaicin Reduces Cancer Stemness and Inhibits Metastasis by Downregulating SOX2 and EZH2 in Osteosarcoma. Am J Chin Med 2023, 51(4): p. 1041-1066. [CrossRef]
- Kim, M.Y. Nitric oxide triggers apoptosis in A375 human melanoma cells treated with capsaicin and resveratrol. Mol Med Rep 2012, 5(2): p. 585-91. [CrossRef]
- Shin, D.H., Kim, O.H., Jun, H.S.,Kang, M.K. Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp Mol Med 2008, 40(5): p. 486-94. [CrossRef]
- Jun, H.S., Park, T., Lee, C.K., Kang, M.K., Park, M.S., Kang, H.I., Surh, Y.J.,Kim, O.H. Capsaicin induced apoptosis of B16-F10 melanoma cells through down-regulation of Bcl-2. Food Chem Toxicol 2007, 45(5): p. 708-15. [CrossRef]
- Chu, H., Li, M.,Wang, X. Capsaicin induces apoptosis and autophagy in human melanoma cells. Oncol Lett 2019, 17(6): p. 4827-4834. [CrossRef]
- Islam, A., Hsieh, P.F., Liu, P.F., Chou, J.C., Liao, J.W., Hsieh, M.K.,Chueh, P.J. Capsaicin exerts therapeutic effects by targeting tNOX-SIRT1 axis and augmenting ROS-dependent autophagy in melanoma cancer cells. Am J Cancer Res 2021, 11(9): p. 4199-4219. [PubMed]
- Bahri, S., Jameleddine, S.,Shlyonsky, V. Relevance of carnosic acid to the treatment of several health disorders: Molecular targets and mechanisms. Biomed Pharmacother 2016, 84: p. 569-582. [CrossRef]
- Lin, K.I., Lin, C.C., Kuo, S.M., Lai, J.C., Wang, Y.Q., You, H.L., Hsu, M.L., Chen, C.H.,Shiu, L.Y. Carnosic acid impedes cell growth and enhances anticancer effects of carmustine and lomustine in melanoma. Biosci Rep 2018, 38(4). [CrossRef]
- Park, S.Y., Song, H., Sung, M.K., Kang, Y.H., Lee, K.W.,Park, J.H. Carnosic acid inhibits the epithelial-mesenchymal transition in B16F10 melanoma cells: a possible mechanism for the inhibition of cell migration. Int J Mol Sci 2014, 15(7): p. 12698-713. [CrossRef]
- Alcaraz, M., Olivares, A., Andreu-Galvez, M., Achel, D.G., Mercado, A.M.,Alcaraz-Saura, M. Paradoxical Radiosensitizing Effect of Carnosic Acid on B16F10 Metastatic Melanoma Cells: A New Treatment Strategy. Antioxidants (Basel) 2022, 11(11). [CrossRef]
- Arakawa, N., Okubo, A., Yasuhira, S., Takahashi, K., Amano, H., Akasaka, T., Masuda, T., Shibazaki, M.,Maesawa, C. Carnosic acid, an inducer of NAD(P)H quinone oxidoreductase 1, enhances the cytotoxicity of beta-lapachone in melanoma cell lines. Oncol Lett 2018, 15(2): p. 2393-2400. [CrossRef]
- Johnson, J.J. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett 2011, 305(1): p. 1-7. [CrossRef]
- O'Neill, E.J., Hartogh, D.J.D., Azizi, K.,Tsiani, E. Anticancer Properties of Carnosol: A Summary of in Vitro and In Vivo Evidence. Antioxidants (Basel) 2020, 9(10). [CrossRef]
- Huang, S.C., Ho, C.T., Lin-Shiau, S.Y.,Lin, J.K. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol 2005, 69(2): p. 221-32. [CrossRef]
- Choi, S.M., Kim, D.-H., Chun, K.-S.,Choi, J.-S. Carnosol induces apoptotic cell death through ROS-dependent inactivation of STAT3 in human melanoma G361 cells. Appl Biol Chem 2019, 62(1): p. 55. [CrossRef]
- Tong, L.,Wu, S. The Mechanisms of Carnosol in Chemoprevention of Ultraviolet B-Light-Induced Non-Melanoma Skin Cancer Formation. Sci Rep 2018, 8(1): p. 3574. [CrossRef]
- Huang, M.T., Ho, C.T., Wang, Z.Y., Ferraro, T., Lou, Y.R., Stauber, K., Ma, W., Georgiadis, C., Laskin, J.D.,Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res 1994, 54(3): p. 701-8. [PubMed]
- Yeo, I.J., Park, J.H., Jang, J.S., Lee, D.Y., Park, J.E., Choi, Y.E., Joo, J.H., Song, J.K., Jeon, H.O.,Hong, J.T. Inhibitory effect of Carnosol on UVB-induced inflammation via inhibition of STAT3. Arch Pharm Res 2019, 42(3): p. 274-283. [CrossRef]
- Maczka, W., Twardawska, M., Grabarczyk, M.,Winska, K. Carvacrol-A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics (Basel) 2023, 12(5). [CrossRef]
- Joshi, S., Kundu, S., Priya, V.V., Kulhari, U., Mugale, M.N.,Sahu, B.D. Anti-inflammatory activity of carvacrol protects the heart from lipopolysaccharide-induced cardiac dysfunction by inhibiting pyroptosis via NLRP3/Caspase1/Gasdermin D signaling axis. Life Sci 2023, 324: p. 121743. [CrossRef]
- Cerrah, S., Ozcicek, F., Gundogdu, B., Cicek, B., Coban, T.A., Suleyman, B., Altuner, D., Bulut, S.,Suleyman, H. Carvacrol prevents acrylamide-induced oxidative and inflammatory liver damage and dysfunction in rats. Front Pharmacol 2023, 14: p. 1161448. [CrossRef]
- Singh, J., Luqman, S.,Meena, A. Carvacrol as a Prospective Regulator of Cancer Targets/Signalling Pathways. Curr Mol Pharmacol 2023, 16(5): p. 542-558. [CrossRef]
- Govindaraju, S.,Arulselvi, P.I. Characterization of Coleus aromaticus essential oil and its major constituent carvacrol for in vitro antidiabetic and antiproliferative activities. J Herbs Spices Med 2018, 24(1): p. 37-51. [CrossRef]
- Osanloo, M., Alipanah, H., Farjam, M., Taheri, A.,Zarenezhad, E. Anticancer Activity of Chitosan Nanoparticles Containing Satureja khuzistanica Essential Oil, and Carvacrol against Human Melanoma and Breast Cancer. Russ J Bioorg 2023, 49(3): p. 594-601. [CrossRef]
- Nanni, V., Di Marco, G., Sacchetti, G., Canini, A.,Gismondi, A. Oregano Phytocomplex Induces Programmed Cell Death in Melanoma Lines via Mitochondria and DNA Damage. Foods 2020, 9(10). [CrossRef]
- de Oliveira, I.C.V., Galvao-Moreira, L.V., Vilela, J.L., Duarte-Silva, M., Aguiar-da-Silva, L.D., Pereira, C.A.A., Pereira, D.M.S., Pinheiro, A., Lima-Neto, L.G., Fernandes, E.S., Cardoso, C.R.B.,Branco-de-Almeida, L.S. Cinnamaldehyde modulates host immunoinflammatory responses in rat ligature-induced periodontitis and peripheral blood mononuclear cell models. Int Immunopharmacol 2023, 115: p. 109669. [CrossRef]
- Qureshi, K.A., Mohammed, S.A.A., Khan, O., Ali, H.M., El-Readi, M.Z.,Mohammed, H.A. Cinnamaldehyde-Based Self-Nanoemulsion (CA-SNEDDS) Accelerates Wound Healing and Exerts Antimicrobial, Antioxidant, and Anti-Inflammatory Effects in Rats' Skin Burn Model. Molecules 2022, 27(16). [CrossRef]
- Zhang, G., Li, T., Liu, J., Wu, X.,Yi, H. Cinnamaldehyde-Contained Polymers and Their Biomedical Applications. Polymers (Basel) 2023, 15(6). [CrossRef]
- Cabello, C.M., Bair, W.B., 3rd, Lamore, S.D., Ley, S., Bause, A.S., Azimian, S.,Wondrak, G.T. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med 2009, 46(2): p. 220-31. [CrossRef]
- Zhou, L., Lu, Y., Yang, G.,Wu, J. Research on tumorigenicity of cinnamaldehyde in melanoma cell lines and its mechanism. Tumour Biol 2014, 35(6): p. 5717-22. [CrossRef]
- Patra, K., Jana, S., Sarkar, A., Mandal, D.P.,Bhattacharjee, S. The inhibition of hypoxia-induced angiogenesis and metastasis by cinnamaldehyde is mediated by decreasing HIF-1alpha protein synthesis via PI3K/Akt pathway. Biofactors 2019, 45(3): p. 401-415. [CrossRef]
- Zhang, Y.P., Li, Y.Q., Lv, Y.T.,Wang, J.M. Effect of curcumin on the proliferation, apoptosis, migration, and invasion of human melanoma A375 cells. Genet Mol Res 2015, 14(1): p. 1056-67. [CrossRef]
- Marin, Y.E., Wall, B.A., Wang, S., Namkoong, J., Martino, J.J., Suh, J., Lee, H.J., Rabson, A.B., Yang, C.S., Chen, S.,Ryu, J.H. Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res 2007, 17(5): p. 274-83. [CrossRef]
- Siwak, D.R., Shishodia, S., Aggarwal, B.B.,Kurzrock, R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer 2005, 104(4): p. 879-90. [CrossRef]
- Zheng, M., Ekmekcioglu, S., Walch, E.T., Tang, C.H.,Grimm, E.A. Inhibition of nuclear factor-kappaB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res 2004, 14(3): p. 165-71. [CrossRef]
- Jiang, A.J., Jiang, G., Li, L.T.,Zheng, J.N. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol Biol Rep 2015, 42(1): p. 267-75. [CrossRef]
- Bush, J.A., Cheung, K.J., Jr.,Li, G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res 2001, 271(2): p. 305-14. [CrossRef]
- Liao, W., Xiang, W., Wang, F.F., Wang, R.,Ding, Y. Curcumin inhibited growth of human melanoma A375 cells via inciting oxidative stress. Biomed Pharmacother 2017, 95: p. 1177-1186. [CrossRef]
- Kocyigit, A.,Guler, E.M. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell Mol Biol (Noisy-le-grand) 2017, 63(11): p. 97-105. [CrossRef]
- Bakhshi, J., Weinstein, L., Poksay, K.S., Nishinaga, B., Bredesen, D.E.,Rao, R.V. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis 2008, 13(7): p. 904-14. [CrossRef]
- Zhao, G., Han, X., Zheng, S., Li, Z., Sha, Y., Ni, J., Sun, Z., Qiao, S.,Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol Rep 2016, 35(2): p. 1065-74. [CrossRef]
- Chiu, Y.J., Yang, J.S., Tsai, F.J., Chiu, H.Y., Juan, Y.N., Lo, Y.H.,Chiang, J.H. Curcumin suppresses cell proliferation and triggers apoptosis in vemurafenib-resistant melanoma cells by downregulating the EGFR signaling pathway. Environ Toxicol 2022, 37(4): p. 868-879. [CrossRef]
- Yu, T., Ji, J.,Guo, Y.L. MST1 activation by curcumin mediates JNK activation, Foxo3a nuclear translocation and apoptosis in melanoma cells. Biochem Biophys Res Commun 2013, 441(1): p. 53-8. [CrossRef]
- Shimada, K., Ushijima, K., Suzuki, C., Horiguchi, M., Ando, H., Akita, T., Shimamura, M., Fujii, J., Yamashita, C.,Fujimura, A. Pulmonary administration of curcumin inhibits B16F10 melanoma lung metastasis and invasion in mice. Cancer Chemother Pharmacol 2018, 82(2): p. 265-273. [CrossRef]
- Sonavane, K., Phillips, J., Ekshyyan, O., Moore-Medlin, T., Roberts Gill, J., Rong, X., Lakshmaiah, R.R., Abreo, F., Boudreaux, D., Clifford, J.L.,Nathan, C.A. Topical curcumin-based cream is equivalent to dietary curcumin in a skin cancer model. J Skin Cancer 2012, 2012: p. 147863. [CrossRef]
- Phillips, J., Moore-Medlin, T., Sonavane, K., Ekshyyan, O., McLarty, J.,Nathan, C.A. Curcumin inhibits UV radiation-induced skin cancer in SKH-1 mice. Otolaryngol Head Neck Surg 2013, 148(5): p. 797-803. [CrossRef]
- Tsai, K.D., Lin, J.C., Yang, S.M., Tseng, M.J., Hsu, J.D., Lee, Y.J.,Cherng, J.M. Curcumin Protects against UVB-Induced Skin Cancers in SKH-1 Hairless Mouse: Analysis of Early Molecular Markers in Carcinogenesis. Evid Based Complement Alternat Med 2012, 2012: p. 593952. [CrossRef]
- Jensen, J.D., Dunn, J.H., Luo, Y., Liu, W., Fujita, M.,Dellavalle, R.P. Ellagic acid inhibits melanoma growth in vitro. Dermatol Reports 2011, 3(3): p. e36. [CrossRef]
- Wang, F., Chen, J., Xiang, D., Lian, X., Wu, C.,Quan, J. Ellagic acid inhibits cell proliferation, migration, and invasion in melanoma via EGFR pathway. Am J Transl Res 2020, 12(5): p. 2295-2304.
- Hseu, Y.C., Chou, C.W., Senthil Kumar, K.J., Fu, K.T., Wang, H.M., Hsu, L.S., Kuo, Y.H., Wu, C.R., Chen, S.C.,Yang, H.L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol 2012, 50(5): p. 1245-55. [CrossRef]
- Lembo, S., Balato, A., Di Caprio, R., Cirillo, T., Giannini, V., Gasparri, F.,Monfrecola, G. The modulatory effect of ellagic acid and rosmarinic acid on ultraviolet-B-induced cytokine/chemokine gene expression in skin keratinocyte (HaCaT) cells. Biomed Res Int 2014, 2014: p. 346793. [CrossRef]
- Islam, S.U., Ahmed, M.B., Ahsan, H., Islam, M., Shehzad, A., Sonn, J.K.,Lee, Y.S. An Update on the Role of Dietary Phytochemicals in Human Skin Cancer: New Insights into Molecular Mechanisms. Antioxidants (Basel) 2020, 9(10). [CrossRef]
- Zari, A.T., Zari, T.A.,Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26(23). [CrossRef]
- Padhy, I., Paul, P., Sharma, T., Banerjee, S.,Mondal, A. Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life (Basel) 2022, 12(11). [CrossRef]
- Jaganathan, S.K.,Supriyanto, E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules 2012, 17(6): p. 6290-304. [CrossRef]
- Kim, G.-C., Choe, D.S., Im, J.-S., Jeong, H., Kim, I., Lee, M.-H.,Park, B.-S. Caspases-dependent Apoptosis in Human Melanoma Cell by Eugenol. Anat Cell Biol 2006, 39: p. 245-253.
- Ghosh, R., Nadiminty, N., Fitzpatrick, J.E., Alworth, W.L., Slaga, T.J.,Kumar, A.P. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem 2005, 280(7): p. 5812-9. [CrossRef]
- Ra Choi, B.-B., Shin, S.-H., Kim, U.-K., Hong, J.-W.,Kim, G.-C. S Phase Cell Cycle Arrest and Apoptosis is Induced by Eugenol in G361 Human Melanoma Cells. Int J Oral Biol 2011, 36(3): p. 129-134.
- Júnior, P.L., Câmara, D.A., Costa, A.S., Ruiz, J.L., Levy, D., Azevedo, R.A., Pasqualoto, K.F., de Oliveira, C.F., de Melo, T.C., Pessoa, N.D., Fonseca, P.M., Pereira, A., Araldi, R.P.,Ferreira, A.K. Apoptotic effect of eugenol envolves G2/M phase abrogation accompanied by mitochondrial damage and clastogenic effect on cancer cell in vitro. Phytomedicine 2016, 23(7): p. 725-35. [CrossRef]
- Valizadeh, A., Khaleghi, A.A., Alipanah, H., Zarenezhad, E.,Osanloo, M. Anticarcinogenic Effect of Chitosan Nanoparticles Containing Syzygium aromaticum Essential Oil or Eugenol Toward Breast and Skin Cancer Cell Lines. BioNanoScience 2021, 11(3): p. 678-686. [CrossRef]
- Pal, D., Banerjee, S., Mukherjee, S., Roy, A., Panda, C.K.,Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J Dermatol Sci 2010, 59(1): p. 31-9. [CrossRef]
- Sukumaran, K., Unnikrishnan, M.C.,Kuttan, R. Inhibition of tumour promotion in mice by eugenol. Indian J Physiol Pharmacol 1994, 38(4): p. 306-8.
- Kaur, G., Athar, M.,Alam, M.S. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol Carcinog 2010, 49(3): p. 290-301. [CrossRef]
- Kalmes, M., Hennen, J.,Blömeke, B. Eugenol and isoeugenol as antiproliferative agents in skin cancer cells. Toxicol Lett 2009, 189. [CrossRef]
- Chrubasik, S., Pittler, M.H.,Roufogalis, B.D. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12(9): p. 684-701. [CrossRef]
- Ganjikunta, V.S., Maddula, R.R., Bhasha, S., Sahukari, R., Kondeti Ramudu, S., Chenji, V., Kesireddy, S.R., Zheng, Z.,Korivi, M. Cardioprotective Effects of 6-Gingerol against Alcohol-Induced ROS-Mediated Tissue Injury and Apoptosis in Rats. Molecules 2022, 27(23). [CrossRef]
- de Lima, R.M.T., Dos Reis, A.C., de Menezes, A.P.M., Santos, J.V.O., Filho, J., Ferreira, J.R.O., de Alencar, M., da Mata, A., Khan, I.N., Islam, A., Uddin, S.J., Ali, E.S., Islam, M.T., Tripathi, S., Mishra, S.K., Mubarak, M.S.,Melo-Cavalcante, A.A.C. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother Res 2018, 32(10): p. 1885-1907. [CrossRef]
- Aloliqi, A.A. Therapeutic Potential of 6-Gingerol in Prevention of Colon Cancer Induced by Azoxymethane through the Modulation of Antioxidant Potential and Inflammation. Curr Issues Mol Biol 2022, 44(12): p. 6218-6228. [CrossRef]
- Kim, E.C., Min, J.K., Kim, T.Y., Lee, S.J., Yang, H.O., Han, S., Kim, Y.M.,Kwon, Y.G. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun 2005, 335(2): p. 300-8. [CrossRef]
- Nigam, N., Bhui, K., Prasad, S., George, J.,Shukla, Y. [6]-Gingerol induces reactive oxygen species regulated mitochondrial cell death pathway in human epidermoid carcinoma A431 cells. Chem Biol Interact 2009, 181(1): p. 77-84. [CrossRef]
- Kim, S.O., Kundu, J.K., Shin, Y.K., Park, J.H., Cho, M.H., Kim, T.Y.,Surh, Y.J. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene 2005, 24(15): p. 2558-67. [CrossRef]
- Kim, J.K., Kim, Y., Na, K.M., Surh, Y.J.,Kim, T.Y. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic Res 2007, 41(5): p. 603-14. [CrossRef]
- Ju, S.A., Park, S.M., Lee, Y.S., Bae, J.H., Yu, R., An, W.G., Suh, J.H.,Kim, B.S. Administration of 6-gingerol greatly enhances the number of tumor-infiltrating lymphocytes in murine tumors. Int J Cancer 2012, 130(11): p. 2618-28. [CrossRef]
- Park, K.K., Chun, K.S., Lee, J.M., Lee, S.S.,Surh, Y.J. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett 1998, 129(2): p. 139-44. [CrossRef]
- Bischoff-Kont, I.,Furst, R. Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals (Basel) 2021, 14(6). [CrossRef]
- Wu, H., Hsieh, M.C., Lo, C.Y., Liu, C.B., Sang, S., Ho, C.T.,Pan, M.H. 6-Shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Mol Nutr Food Res 2010, 54(9): p. 1296-306. [CrossRef]
- Chen, F., Tang, Y., Sun, Y., Veeraraghavan, V.P., Mohan, S.K.,Cui, C. 6-shogaol, a active constiuents of ginger prevents UVB radiation mediated inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes (HaCaT cells). J Photochem Photobiol B 2019, 197: p. 111518. [CrossRef]
- Krause, S.T., Liao, P., Crocoll, C., Boachon, B., Forster, C., Leidecker, F., Wiese, N., Zhao, D., Wood, J.C., Buell, C.R., Gershenzon, J., Dudareva, N.,Degenhardt, J. The biosynthesis of thymol, carvacrol, and thymohydroquinone in Lamiaceae proceeds via cytochrome P450s and a short-chain dehydrogenase. Proc Natl Acad Sci U S A 2021, 118(52). [CrossRef]
- Rathod, N.B., Kulawik, P., Ozogul, F., Regenstein, J.M.,Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci 2021, 116: p. 733-748. [CrossRef]
- Satooka, H.,Kubo, I. Effects of thymol on B16-F10 melanoma cells. J Agric Food Chem 2012, 60(10): p. 2746-52. [CrossRef]
- Calo, R., Visone, C.M.,Marabini, L. Thymol and Thymus Vulgaris L. activity against UVA- and UVB-induced damage in NCTC 2544 cell line. Mutat Res Genet Toxicol Environ Mutagen 2015, 791: p. 30-7. [CrossRef]
- Mapelli, M., Calò, R.,Marabini, L. Thymol and Thymus vulgaris extract protects human keratinocyte cell line (HaCaT) from UVA and UVB damage. Oxid Antioxid Med Sci 2016, 5: p. 39. [CrossRef]
- Cornaghi, L., Arnaboldi, F., Calo, R., Landoni, F., Baruffaldi Preis, W.F., Marabini, L.,Donetti, E. Effects of UV Rays and Thymol/Thymus vulgaris L. Extract in an ex vivo Human Skin Model: Morphological and Genotoxicological Assessment. Cells Tissues Organs 2016, 201(3): p. 180-92. [CrossRef]
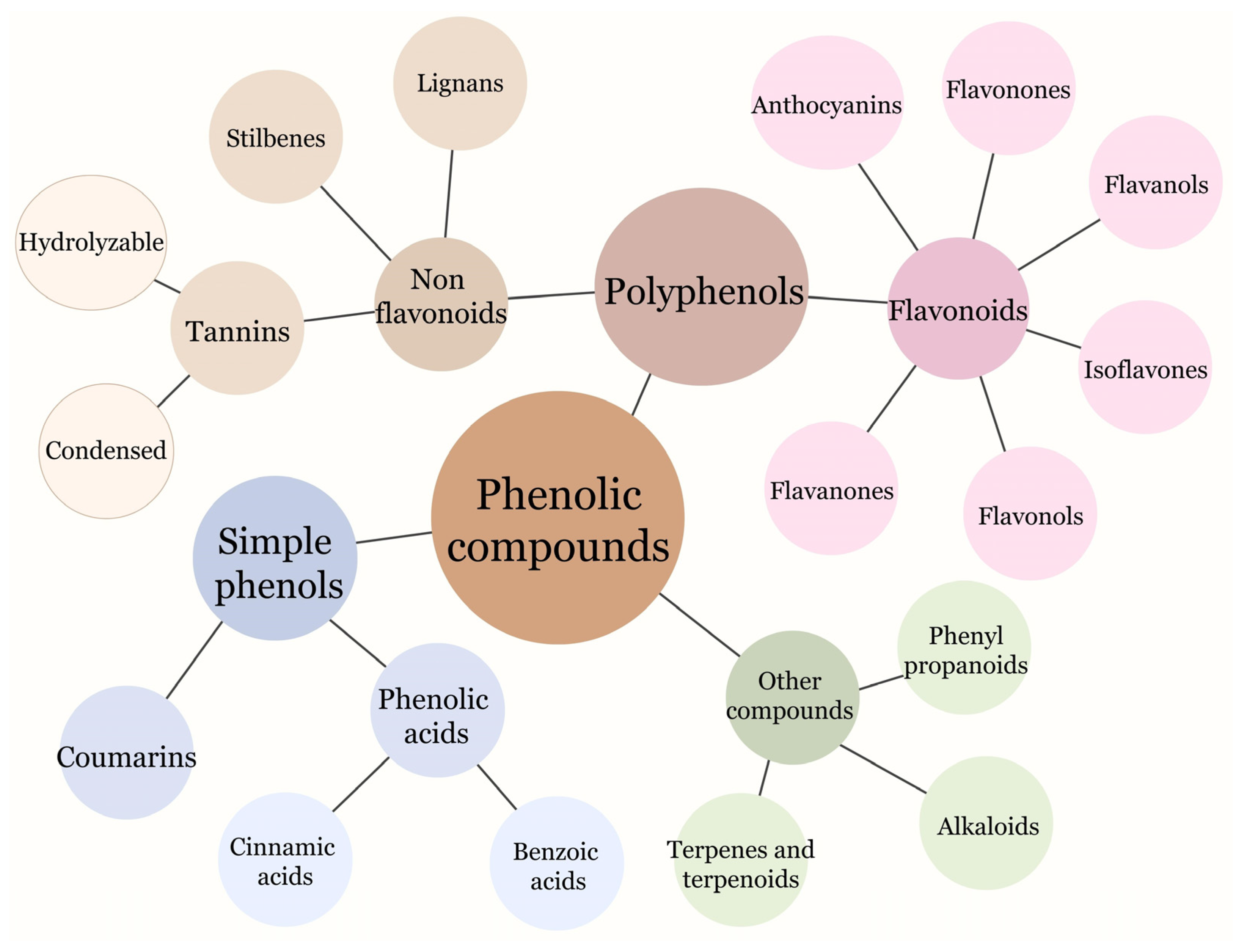
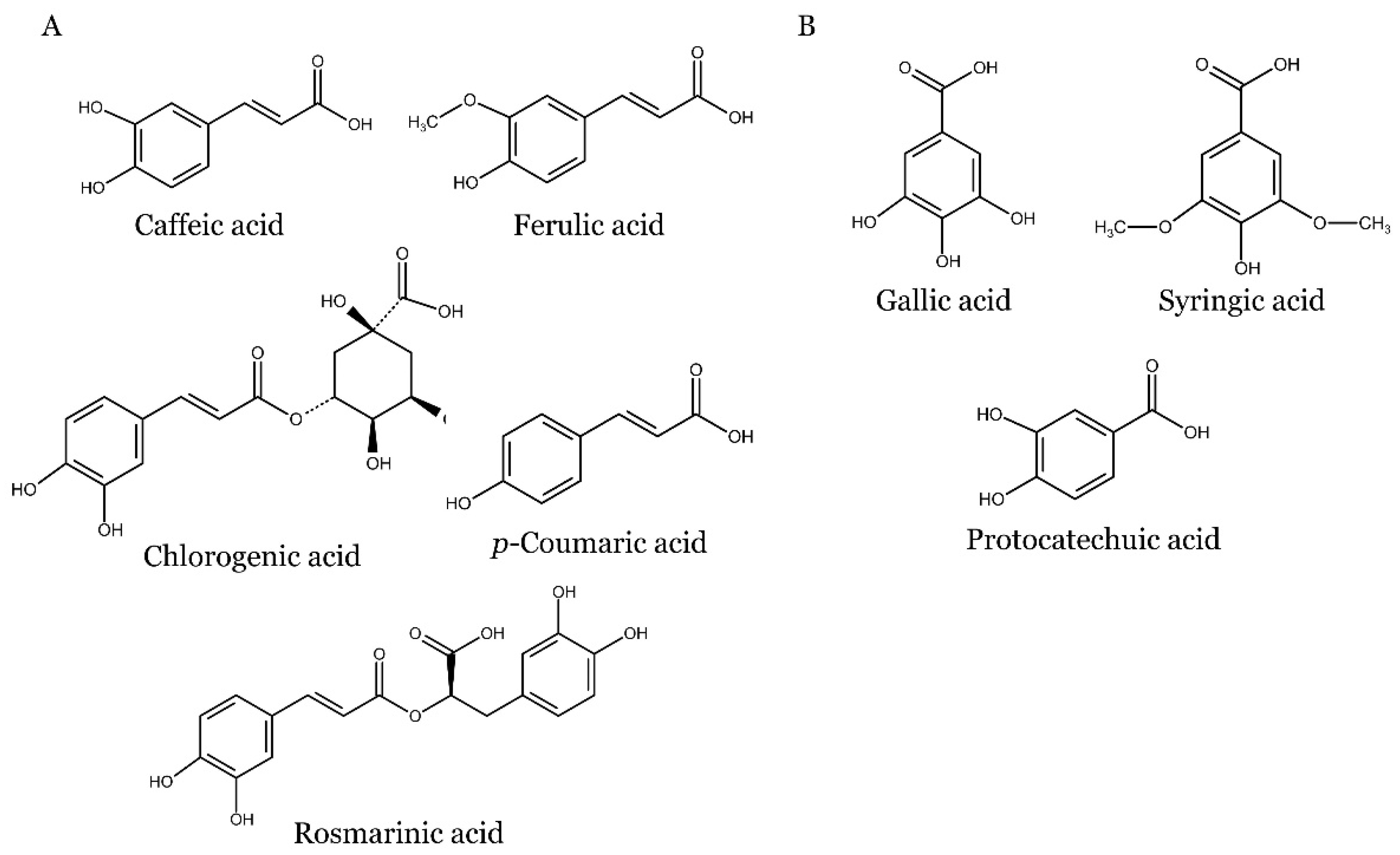
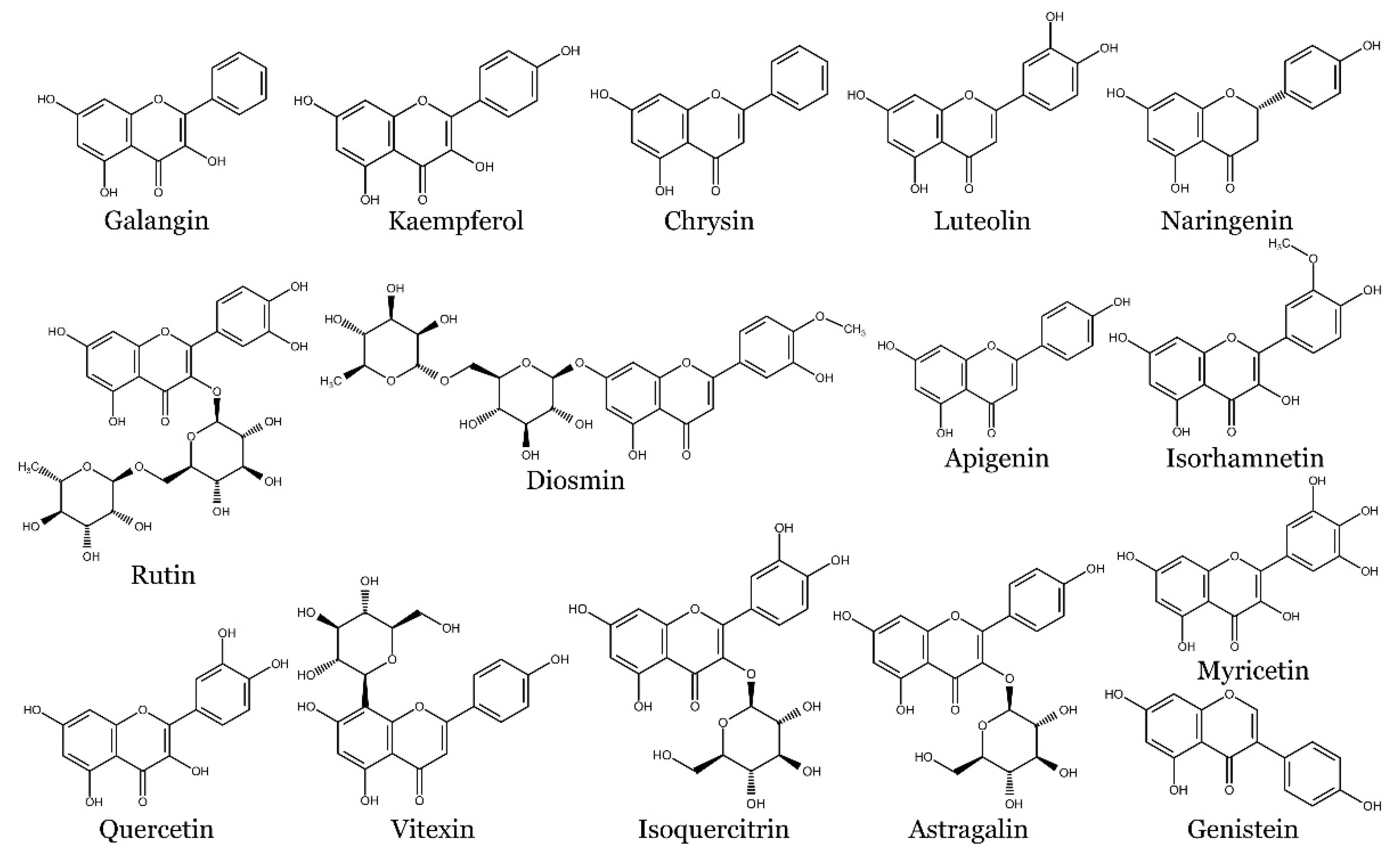
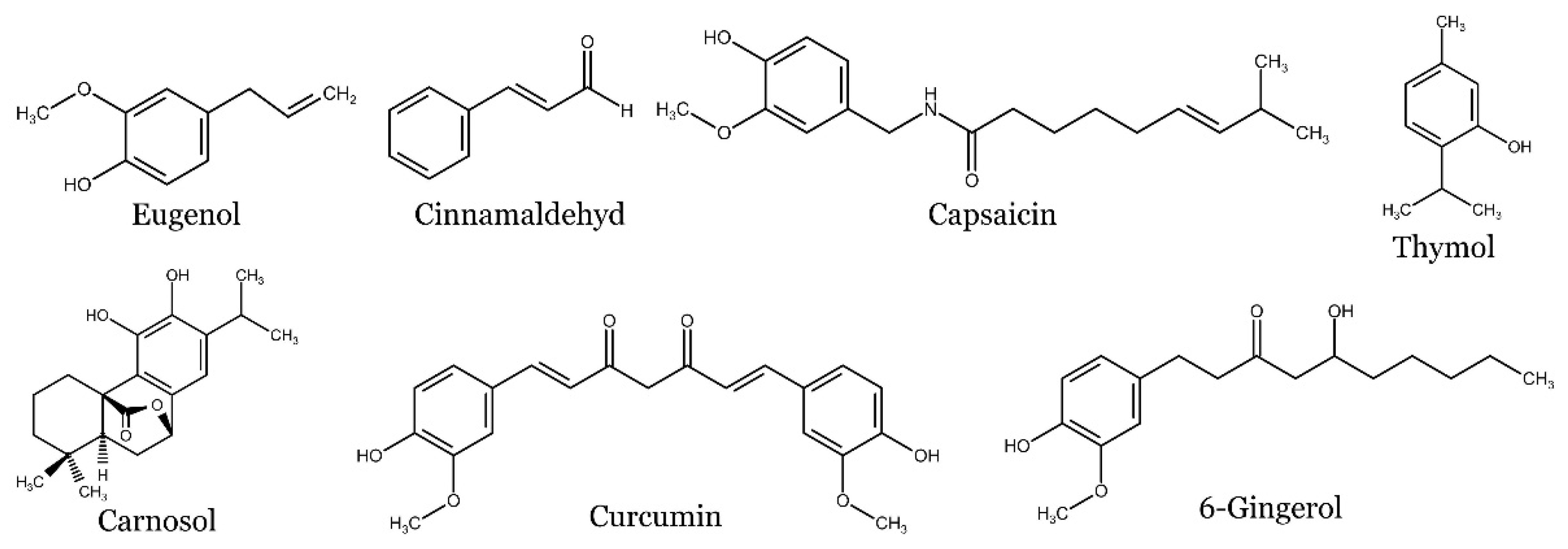
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




