1. Introduction
Enterococcus faecalis is a commensal bacterium of the human gastrointestinal and biliary tracts whose pathogenic action is one of the main causes of infections developing in surgical sites, the urinary tract, and the bloodstream.[
1,
2,
3] Likewise, Group D streptococci (eg,
Streptococcus gallolyticus,
Streptococcus bovis), which have been reclassified as enterococci spp, are known to promote infective endocarditis (IE) associated with gastrointestinal and urogenital tract disorders, which utilize the portal venous system as a gateway. [
4,
5,
6] Molecular biology studies and clinical therapeutics supporting enterococcal infective endocarditis have suggested that the role of the pili is pertinent to higher bacterial aggressiveness due to biogenesis, host immunity response, and resistance to antimicrobial therapy. [
7,
8,
9,
10,
11,
12] Evidence has pointed out that F pili play at least three roles in bacterial mating: first, initiating contacts between mating pairs; second, facilitating the transfer of genetic material, and lastly drawing the mating cells into close contact which increases the fertility of the bacterial union. [
13,
14] The latter phenomenon could at least partly explain the role of
E. faecalis among the causative pathogens of bacterial infective endocarditis responsible for the high mortality advocated by severe complications, including the worsening of congestive heart failure, glomerulonephritis, and septic embolism. [
3,
4,
5]
Current international guidelines recommend medical management of the infection by administering a combination of antimicrobials in the immediate post-diagnosis of enterococcal endocarditis. However, concerns related to the emergence of strains of the bacterium with resistance to multiple antibiotics, including the emergence of strains of enterococcus resistant to vancomycin, have raised a veil of uncertainty about optimal guideline-directed medical therapy (GDMT) by opening an ever-increasing chasm. [
15,
16,
17,
18] Several epidemiological studies are available to support the evidence suggesting a rate of greater than 20% of enterococci isolated from infections of intensive care unit (ICU) patients demonstrate resistance to vancomycin. [
8,
9,
16]
The incidence of IE sustained by
E. faecalis involves 1/3 of patients older than 70 years, [
19,
20,
21,
22,
23] with further increments expected in the future due to increased life expectancy. [
22,
23] Worldwide, the greatest relative increase in the incidence of IE is recorded in the elderly population, where elderly patients have a 4.6 times higher risk of IE than the general population. [
19,
23] Causes being considered in promoting IE are the high prevalence of undiagnosed degenerative valvular disease and the increased use of invasive procedures and implanted medical devices. [
17,
18] These factors could also influence the outcome of IE in elderly patients considering that they have substantially higher morbidity and mortality than younger adults. However, there is a lack of recommendations in the international guidelines for the treatment of IE in this category of patients, due to the the limited evaluation of IE in the elderly which does not allow for precise guidance for their management. [
24] The recurrence of infection after valve replacement for IE remains concerning, and accordingly, the ideal valvular substitute in this setting has been debated for decades. (2,6,17,18,24) Surgical dogma suggests that autologous or allogeneic tissue is preferable to synthetic material in an infected field colonized by 60% gram-positive cocci (GPC). Given the reluctance to use foreign artificial materials, such as with mechanical xenograft valve prosthetics or conventional stents, the use of allogenic tissue has been preferred among cardiac surgeons, with benefits reported particularly in cases of prosthetic valve endocarditis (PVE) and other cardiac injuries complex and aggressive, such as evolving root abscess and invasion of the intervalvular fibrosa causing bivalvular involvement (aortic and mitral valve) and higher risk of fistulization in the left atrium. (2,6,24)
The implementation of a common effort involving necessary multidisciplinary research that has the potential to detect new therapies or preventive measures capable of disrupting the pathogenetic mechanisms underlying enterococcal endocarditis is urgently needed. Therefore, this review aims to broadly highlight the pathogenetic mechanisms that support infective endocarditis caused by E. faecalis which, due to a deficient effect of antimicrobial therapies ominously evolves towards the surgical option.
The review continues after a case study involving the Endocarditis multidisciplinary team, which considered a complicated clinical case of E. faecalis-induced IE to drive clinical therapeutics. We believe that the data presented in this overview may provide a basis for understanding the development of infective endocarditis and biofilm-associated pili in the colonization of cardiac structures. Furthermore, we trust that the emerging evidence will assist microbiologists and healthcare professionals (family doctors, internists, cardiologists, and cardiac surgeons) in the doctor-patient discussion on the risks and expectations of cardiac involvement from infection promoted by enterococcal spp.
1.1. Case description
An 86-year-old man with a holosystolic murmur was admitted to our emergency department with dyspnea, fever up to 39°C, decreased level of consciousness, and dysarthria. Following a medical consultation performed a month earlier by the general practitioner for the onset of fever, a non-specific "inflammatory syndrome" was diagnosed and was diagnosed with suspected pneumonia. The use of an empirical therapy with antibiotic administration improved pyrexia. The patient was scheduled for routine checkups which he performed regularly due to aortic valve stenosis associated with non-insulin dependent diabetes and hypertension. Four months before hospital admission, a self-expandable transcatheter aortic valve implantation (TAVI) by means of a transfemoral approach was successfully implanted for severe symptomatic aortic stenosis.
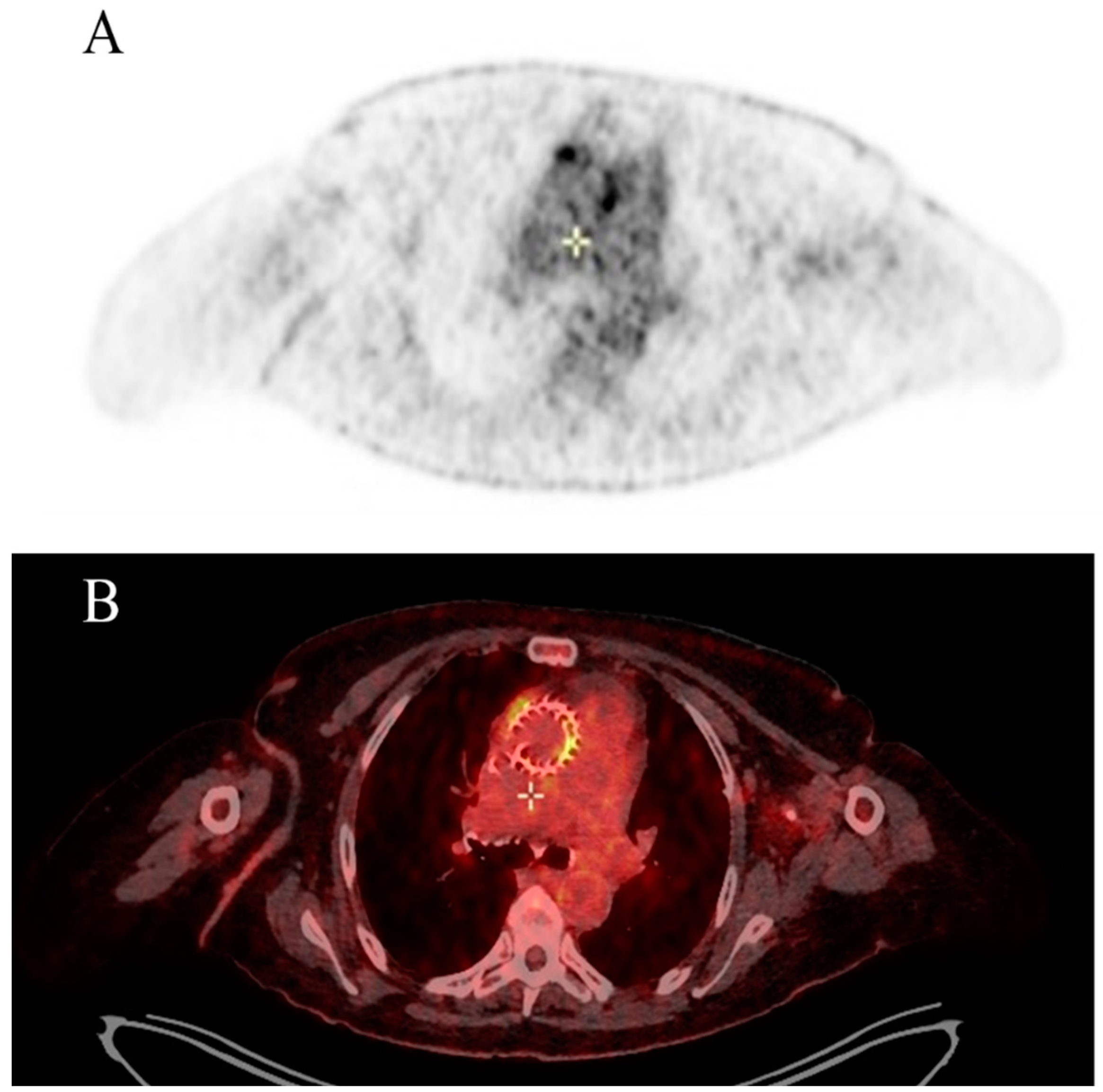
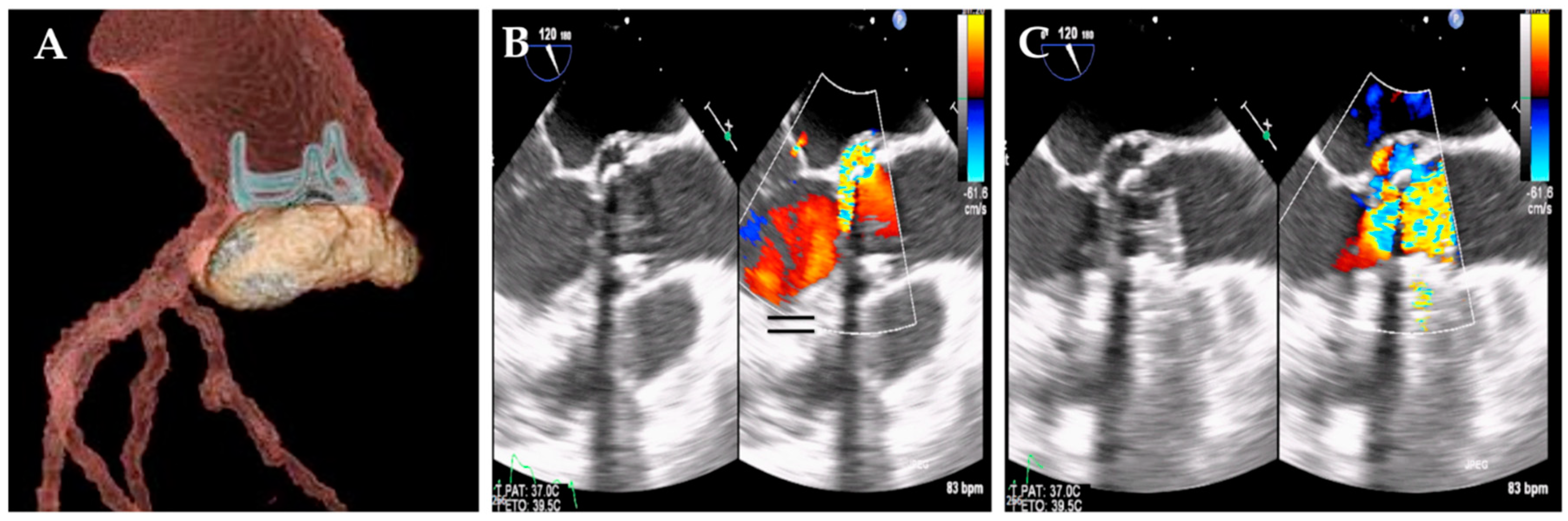
During hospitalization, transthoracic echocardiogram (TTE) was negative for vegetations and para-valvular leak; however, the presence of TAVI leaflet thickening and augmented transvalvular gradient were observed. Brain computed tomography (CT) scan showed two recent ischemic lesions which evoked a possible embolic aetiology. Transoesophageal echocardiogram (TEE) showed a mobile vegetation attached to the frame of the transcatheter prosthetic valve and an abscess in the intervalvular fibrosa. Positive blood cultures for
Enterococcus faecalis completed the diagnosis of IE. A Fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) also confirmed multifocal uptake of the prosthetic.
Figure 1 The patient is referred to a cardiac surgeon for consideration of aortic-valve replacement.
Figure 2
2. The Clinical Problem
Heart valve endocarditis (HVE) is defined as an infectious disease of one or more heart valve leaflets, which can involve contiguous heart structures such as the valvular annulus, aortic root, left atrium, and trigones. The estimated annual prevalence of HVE was 3 to 9 cases per 100,000 persons, and 60% of those affect the aortic valve structure resulting in valve failure.[
5,
6,
17,
18] In high-income countries, it is the one of most common causes of acute valve regurgitation revealing a discrepancy between the trends toward earlier diagnosis and surgical intervention with respect to the 1-year mortality that has not improved in over 2 decades. This indicates that HVE persists as a primal concern even if it occurs in a new form. This reason is because 80–90% of HVE is sustained by the gram-positive cocci of the staphylococcus, streptococcus, and enterococcus species. However, in the recent era, the characteristic of HVE as an acquired disease has been associated with the molecular biology investigation necessary to study antibiotic resistance and the worrying selection of methicillin-resistant strains of Staphylococcus aureus and enterococcus spp. [
20,
21,
22,
23]
S aureus is recognized as the most routinely isolated causative bacteria associated with IE in high-income countries reaching up to 30% of cases of infection. (5,20,23) Enterococci, are broadly noted as normal intestinal commensals, have emerged as opportunistic pathogens deserving particular consideration, (7) thus gaining the primacy of being among the top 3 causes of nosocomial infections of the bloodstream, surgical site, and urinary tract (3). Among enterococci, E. faecalis is the most clinically relevant causative pathogen, approaching approximately 5%–8% of hospital-associated bacteremia and approximately 5%– 20% of all cases of endocarditis (15). Endocarditis due to E. Faecalis evolves as an infection of the heart’s valves or inner lining that leads to valvular destruction and death without effective antibiotic therapy. The acquisition of resistance to multiple antibiotics has revealed the importance of achieving new insights into enterococcal endocarditis which represents a life-threatening clinical challenge, thus highlighting the need for alternatives to current antibiotic strategies (8,9,25). In this regard, the alternative options to be undertaken could be aimed at the acquisition of based therapies including immunoprophylaxis or immunotherapy targeting proteins expressed in vivo and important for virulence.
Here we deal with the case of a bacterial infection supported by enterococcus faecalis. To encourage wider knowledge on bacterial endocarditis induced by enterococcus species we herein discuss the current evidence basis on the pathophysiology of enterococcus faecalis-induced IE and propose an evidence-based algorithm for the choice of the best treatment option.
3. Search Strategy and Selection Criteria
We searched MEDLINE, Embase, and the Cochrane Library using the search terms “endocarditis” or “infective endocarditis” together with “Enterococcus endocarditis”, “pathogenesis”, “manifestations”, “imaging”, “treatment”, “surgery” or “TAVI”. We primarily chose publications from the past 10 years, but did not exclude widely referenced and highly regarded older publications. We also retrieved the reference lists of articles identified by this search strategy and selected articles that we judged relevant. Recommended review articles are included in tables and references alongside the text, providing readers with more details and background references.
4. Pathophysiology
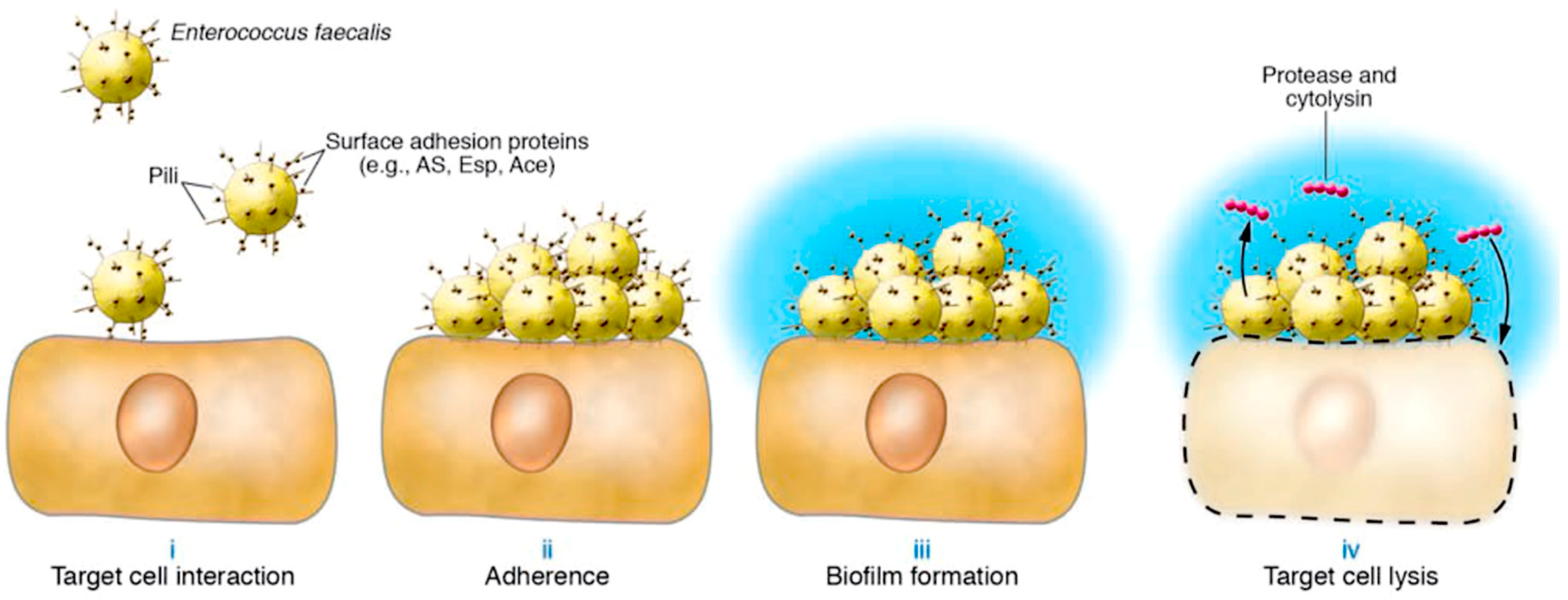
The first question to address is what makes E. faecalis a pathogen. The answer lies in the pathogenic action of E. faecalis which is peculiar in its deployment. Indeed, unlike group A streptococci or Staphylococcus aureus, which are virulent pathogens whose specificity is linked to the secretion of a plethora of hemolysins and toxins to powerfully counteract innate immune responses (26, 27), the pathogenesis of E faecalis uses fewer virulence factors (11). Emerging evidence has pointed out that the first step in the E. faecalis infection process is essentially induced by the attachment and colonization of host tissue surfaces. (7,11) In support of this hypothesis we identified mainly gram-positive pathogens suggesting the crucial role offered by proteins of the family of adhesive matrix molecules (MSCRAMM) that may serve as potential antigenic candidates for the development of new promising immunotherapies.(28) Sillanpaa and colleagues (29) recently detected 17 proteins from the E. faecalis V583 genome (12) with cell wall anchoring motifs and with MSCRAMM-like structural features. (12,29) Interestingly, these proteins incorporate 1 or more regions of 150 to 500 aa segments containing Ig-like folds characteristic of the Staphylococcus aureus Ig family MSCRAMM (29, 30). The presence of antibodies in the sera of patients with E. faecalis endocarditis that interact with at least some of these proteins offers a clear explanation that these antibodies are effectively expressed in vivo during infection. The investigators noted that the majority of sera from E. faecalis patients had particularly high titers against 3 of these proteins, namely EbpA, EbpB, and EbpC. (29)
There is evidence to advocate the contribution of two protein adhesins, enterococcus surface protein (Esp) and collagen adhesion protein of
E. faecalis (Ace), in effectively promoting the attachment of enterococcus to tissues of the host (31, 32). In convergence with the work offered by these two proteins, there is the action of the aggregation substance (AS) which favors the aggregation of the replicating bacteria (33). During the enterococcus infection, the intervention of a virulence factors secreted by the bacterium and involved in tissue degradation is of substantial importance. Enterococci regulate protease expression through the quorum-sensing locus of
E. faecalis regulators (fsr), which comprise of
fsrC sensory kinase,
fsrA response regulator, and
fsrB-derived autoinducer (34). Carniol and colleagues (34) investigated the pathogenetic and biomolecular mechanisms after the establishment of an infectious nest that accommodates a threshold number of enterococci where non-latent
Fsr signaling leads to the activation of protease secretion. The next step involves the formation of a biofilm which is mediated by Esp, Ace, Fsr, and proteases. The authors observed that in the formed bacterial community, attached to the surface enclosed in an extracellular matrix, enterococci assume a slower growth rate, higher resistance to antibiotics, and a high frequency of lateral gene transfer (34). In this phase, the action of cytolysin is expressed. Cytolysin is a toxin secreted by enterococci, whose cytolytic activity results from 2 peptides possessing modifications characteristic of lantibiotics. The latter is composed of thioether amino acids which derive by post-translational modification from precursors synthesized at the ribosomal level (35, 36). Together, these peptides lyse several host cells, including polymorphonuclear leukocytes, a critical immune defense against E. faecalis infection (
Figure 3) (14).
Microbiologists recently confirmed the presence of pili on the surface of gram-positive bacteria for different strains of Streptococcus spp., Actinomyces spp., and Corynebacterium spp (38-40). Investigators have proven the existence of the adhesins on the tip of the pilus and the major and minor subunits of the pilus using antibodies as detection reagents (41). Analysis performed on the genome identified the structural genes coding for the pilina subunits in C. diphtheriae and A. naeslundii located close to the genes coding for sortase-like enzymes (41-43). Sortases work as transpeptidases in the cell wall envelope of gram-positive bacteria such as staphylococci, listeria, or bacilli. The crucial function is to cleave the selection signals of surface proteins to block their C-terminal carboxyl group through the formation of amide bonds (44, 45). Mutations that remove sortase or pilin subunit gene expression remove pili formation (41). Ton That and colleagues (41) reported that a pilin motif sequence with a preserved lysine residue was common in all major pilin subunit genes, and this lysine residue was also essential for pili formation. Again, Ton That and colleagues (46) through biochemical tests have shown that gram-positive pili cannot be dissolved with SDS or organic solvents, and the cleavage was mediated by protease. Together these data suggested the elaboration of a sortase-linked model as highlighted in a further report by Ton-That and colleagues. (47) They accentuated the role of sortases in promoting the assembly of the pilus through the genesis of transpeptide bonds between the C-terminal carboxyl group of cleaved pilin precursors and the amino group of the side chain of lysine residues in the pilina motif sequences. Several predictions can be derived from this model which substantially supports the greater aggressiveness of the pathogens that are capable of processing it. Gram-positive pathogens are primarily involved in which their genomic sequences harbor clusters of genes encoding sortase as well as surface proteins with sorting signals and pilin motif sequences which must produce pili (47). This prediction has been corroborated in several studies focusing on the role of pili in promoting the pathogenicity of enterococci (48) but also in the identification of pili on the surface of group B streptococci or pneumococci (49, 50).
4.1. Enterococcal pili and Infective Endocarditis
Sillanpää and colleagues (29) observed three open reading frames, ef1091-ef1093 which displayed sorting signals and pilin motif sequences. Antibodies against these proteins were found more frequently in sera from patients infected with E. faecalis than in sera from uninfected control patients (29). Nallapareddy and colleagues (48) furthered the work with f1091-ef1093 and their associated Sortase C (srtC), thus revealing that these 3 open reading frames and srtC are part of an operon encoding endocarditis and biofilm-associated pili (ebp). With the use of immunofluorescence electron microscopy, the investigators demonstrated the presence of pili composed of EbpA, EbpB, and EbpC on the surface of E. faecalis and that mutants without pili were unable to form biofilms through the use of biofilm assays. A rat endovascular infection model was developed to recover microbes from aortic vegetations and study the mechanisms of IE determination. It was observed that pilus mutants could not adequately compete with wild-type enterococci, suggesting that pili are required for the establishment of enterococcal infections. Importantly, using the use of specific probes, 408 E. faecalis isolates from 4 continents revealed ebpA, ebpB and ebpC genes leading the authors to conclude that pili formation may be a ubiquitous trait of E. faecalis. (48) Likewise, Tendolkar and colleagues (51) identified a group of surface protein and sortase genes involved in biofilm formation that also included the biofilm enhancer in Enterococcus [bee]), although this report did not prove the ability of enterococci to form pili (51). Results from two recent studies have emerged corroborating this hypothesis and demonstrated that immunization of animals with the pilin subunit prevents both neonatal infectious meningitis caused by group B streptococcus and infection caused by group A streptococcus (52, 53). Thus, the discovery of pili and pili assembly in gram-positive bacteria not only promises new technologies but may also initiate promising public health measures affecting humans.
In concert with the MSCRAMM-mediated colonization process, several studies have suggested that another key factor promoting E. faecalis infection is the ability of strains to form biofilms (34,54,55). Mohamed and colleagues analyzed biofilm formation by a large number of E. faecalis isolates and reported that endocarditis isolates produced biofilms significantly more often and even to a greater extent than non-endocarditis isolates (55). The discovery of 7 genes or gene clusters that have been implicated in E. faecalis biofilm formation has been corroborated by several studies (51,55-59).
4.2. Lesion Development and Progression of Infective Endocarditis in Heart Structure
Enterococci are involved as causative pathogens with the specificity of constituting infectious foci with aggressive colonization due to their peculiar biogenesis. (1,7,10,11) The most highlighted strain is Enterococcus faecalis, which causes both native valvular endocarditis (NVE) and prosthetic valvular endocarditis (PVE) in elderly patients or with chronic diseases. The observed lesions are frequently characterized by a progressive evolution with the formation of large abscess cavities involving one or more valves and with the destruction of extensive portions of the heart (aortic root, intervalvular fibrosa, and heart trigones) in the most aggressive forms of IE. (60-65) We also know that the evolution of Enterococcus faecalis infection is due to the growing resistance to vancomycin, aminoglycosides, and ampicillin that these pathogens may frequently develop. (8,9,12,51, 54-59)
The healthy cardiac endothelium is generally invulnerable to bacteraemia which however may occur in the course of
E. faecalis-induced IE related to an underlying colon tumor. After the endothelial injury, the release of inflammatory cytokines and tissue factors with associated fibronectin expression leads to the formation of a platelet-fibrin thrombus that facilitates bacterial adhesion. (66) Endothelial damage is caused by the presence of valvular sclerosis, rheumatic valvulitis, or by direct bacterial activity, essentially in the course of IE sustained by
Staphylococcus aureus, by strains of enterococcus spp (eg,
E. faecalis, Group D streptococci S.
gallolyticus, S. bovis) or by
Streptococcus viridans (S. mutans, S. salivarius, S. anginosus, S. mitis, and S. sanguinis). Bacterial colonisation triggers additional cycles of endothelial injury and thrombus deposition, eventually generating infected vegetation. Production of a biofilm, characterized by a multilayered bacterial aggregate containing a polysaccharide and proteinaceous matrix, supports bacterial persistence and confers antibiotic tolerance. (67) In this context, it appears suggestive that pili anchored to sortase, already found in
Corynebacterium spp.,
Actinomyces spp.,
group A and group B streptococci,
pneumococci, may also play a crucial role in enterococcal infectious disease. Nallapareddy and colleagues (48) observed that
E. faecalis pili play an important role in biofilm formation as well as in the mediation of endovascular infection that ultimately advocates a thriving colonization of the valve endothelium. The Spread of
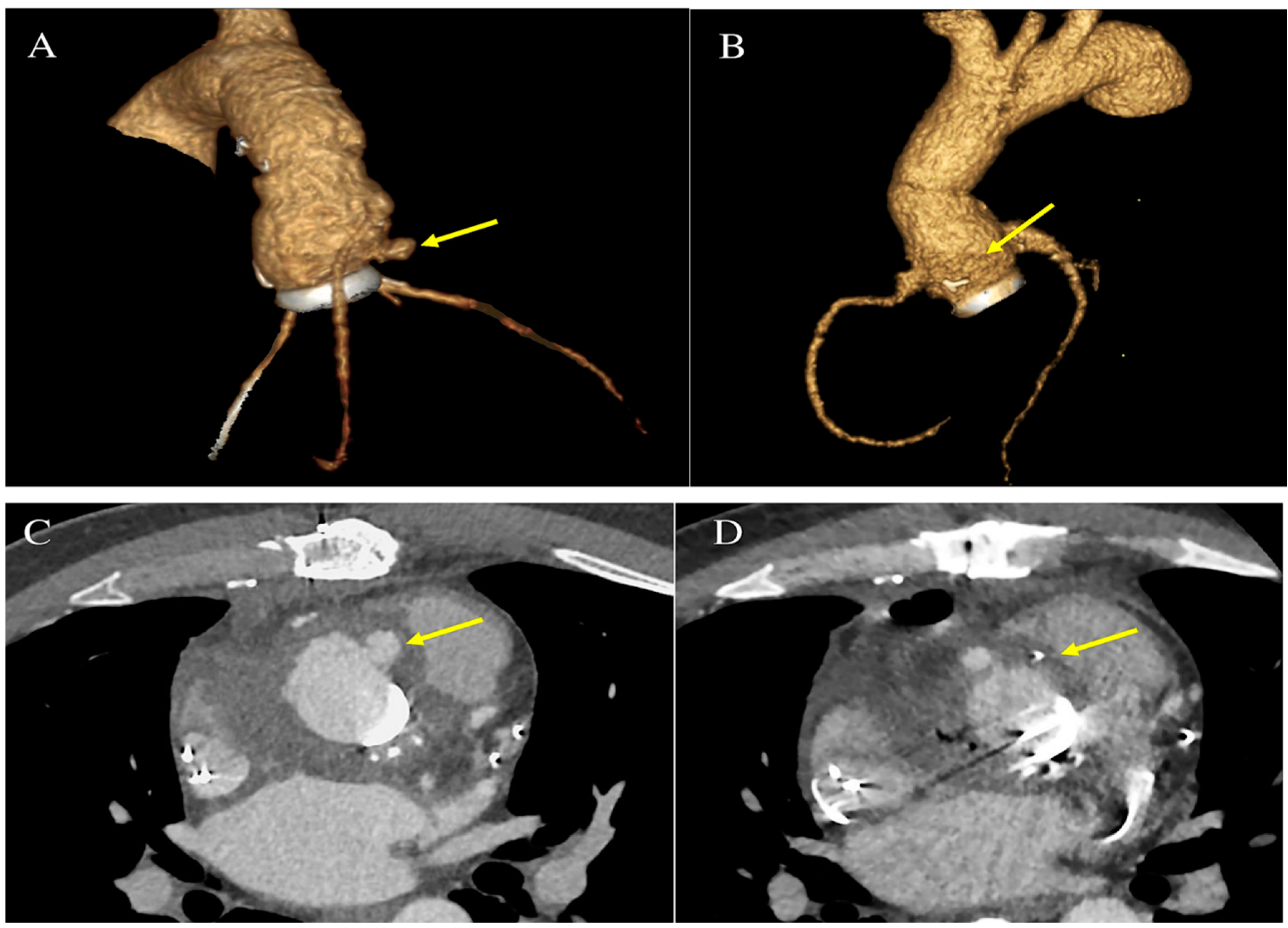
Enterococcus faecalis infection beyond the valve annulus could cause an abscess, pseudoaneurysm, fistula, or atrioventricular block. The genesis of a pseudoaneurysm can take the form of a perivalvular cavity that communicates with the cardiovascular lumen, as shown by color Doppler flow with echocardiography (
Figure 4), or as an abscess in which the thickened, pus-filled perivalvular cavity has no such communication (
Figure 5). (68,69)
In the most serious forms, fistulisation leads to the extreme condition of progressive perivalvular infection (aorto-cavitary fistula) and involves a mortality rate higher than 40%, even with surgery considered.(70,71)
The findings on the presence of E. faecalis pili and sortase-anchored pili are crucial because these ubiquitously encoded surface proteins are antigenic in humans during endocarditis infection. (48) Therefore, the possibility of potential new preventive measures that may disrupt the pathogenesis of enterococcal endocarditis constitutes an open door for future prevention and treatment strategies
5. Clinical Use
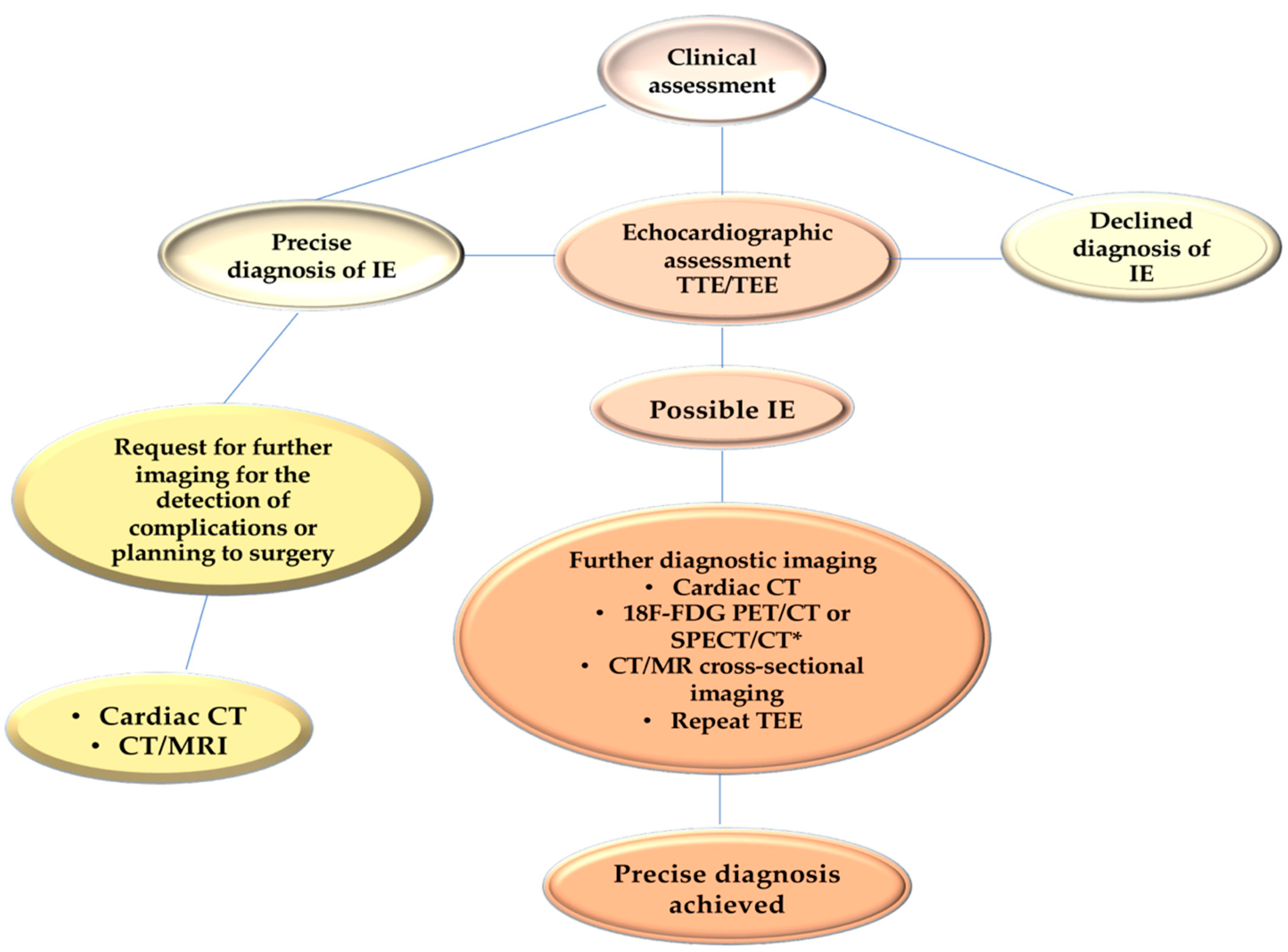
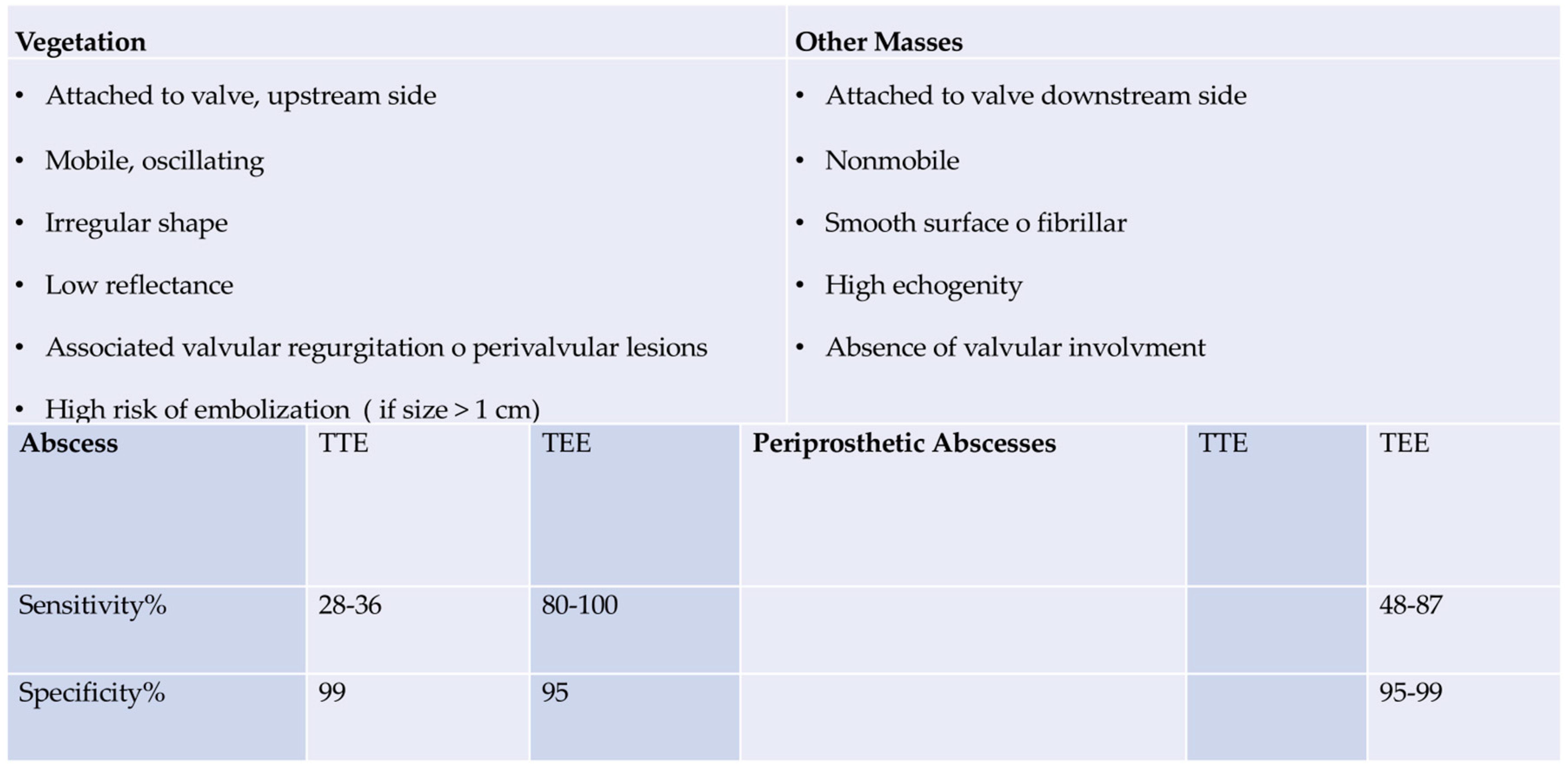
Infective endocarditis is a complex pathology with significant morbidity and high in-hospital mortality. A multidisciplinary approach with a dedicated Endocarditis Team, prompt access to advanced imaging modalities, and early surgical intervention when indicated is useful in the management of IE with a fall in-hospital mortality. (24,71) Patients with IE caused by
E. faecalis should have a careful assessment of symptoms and should undergo transthoracic echocardiography (TTE), mainly to evaluate the development of vegetations involving one or more leaflets, the extent of infectious in the various components of the heart and aorta (leaflet, annulus, trigones, intervalvular fibrous, left atrium and aortic root) as well as size and function of the left ventricle. Transesophageal echocardiography (TEE) is designed to evaluate abscess formation and progression, as well as the mechanism and severity of the valve(s) regurgitation. (68,69) Of note, moderate sensitivity (75%) and specificity (more than 90%) of transthoracic echocardiography (TTE), which demonstrates the detection of vegetation confirming suspected native valvular endocarditis, have been observed (68,69). Patients with equivocal or negative evidence of infection on TTE, but with a high clinical probability of infective endocarditis,should undergo transesophageal echocardiography (TEE) which has a sensitivity greater than 90%. A negative TEE to the presence of vegetations is strongly predictive of the absence of disease, but if the clinical suspicion is high, the examination must be repeated 7-10 days later to confirm the diagnostic negativity. If this test is still negative, it is possible to exclude the diagnosis of infective endocarditis and further echocardiography would not add any other useful information. Since the specificity of TEE does not reach 100%, a differential diagnosis excluding false positives such as cardiac tumors, thrombi, and fibrous filaments on the aortic valve is recommended. Today it is strongly recommended to complete the diagnostic procedure with a CT scan, an 18F-FDG-PET/CT, and a cardiac MRI.
Figure 6
For anatomical assessments of developing lesions, TEE is better than TTE for the detection of major cardiac complications such as abscess, flap perforation, and pseudoaneurysm. TEE should therefore be performed in most cases, even if TTE has provided sufficient indications for a conclusive diagnosis. (73,74) In cases of suspected endocarditis in patients with prosthetic valves, the sensitivity of the TTE is poor and does not reach 36-69%, therefore proceeding with a TEE is usually necessary. The choice of the TEE is also a priority in the presence of a heart device infection. (69,75-78) Again, TTE should be replicated if a complication is suspected, and at the fulfillment of therapy as a baseline for follow-up.
Table 1
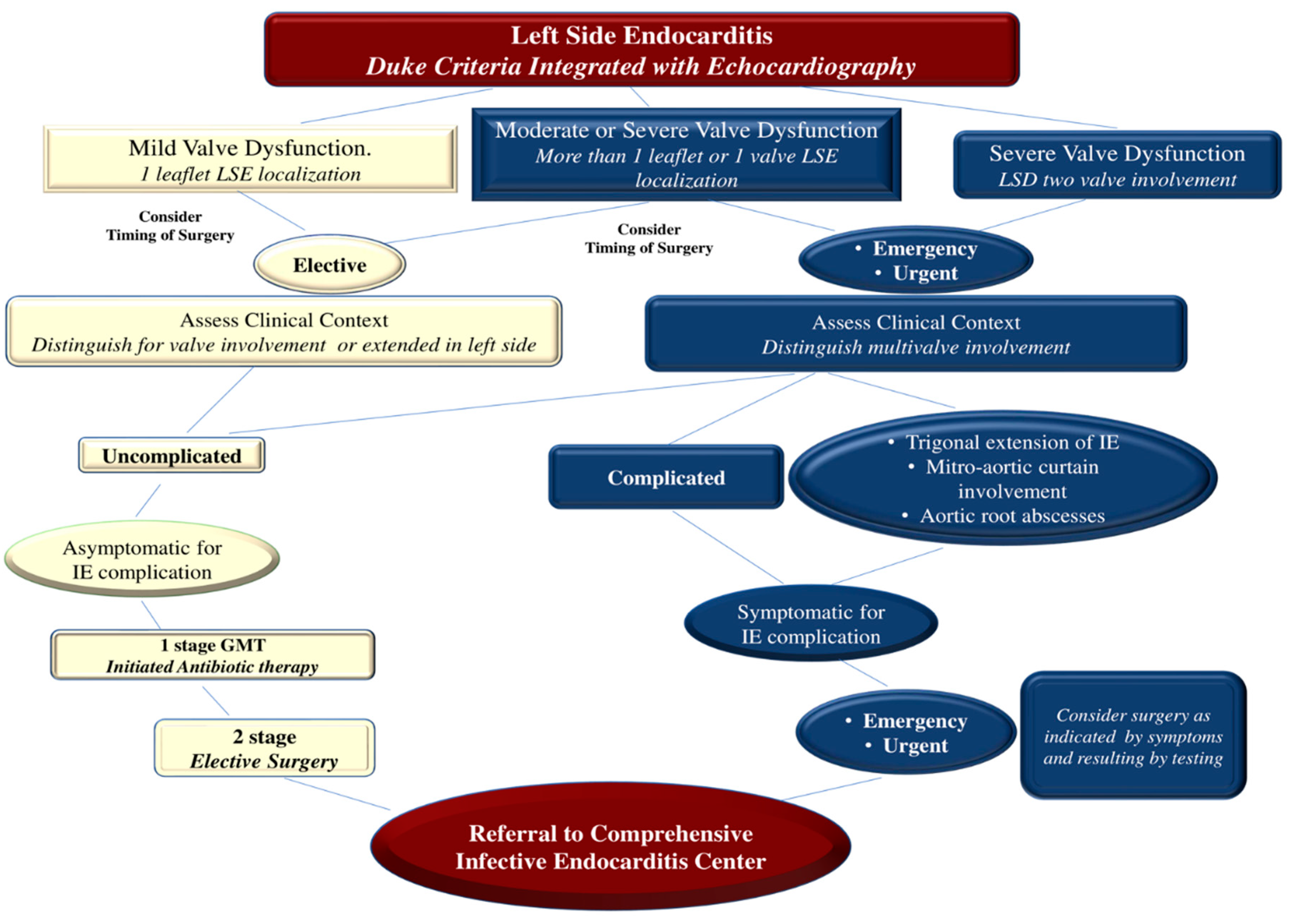
Patients demonstrating severe valvular regurgitation with symptoms or left ventricular ejection fraction (LVEF) <60%, dilatation with left ventricular end-systolic dimension (LVEDD) >40 mm, or both should be referred to a heart center for consideration of surgery. Similarly, asymptomatic patients without left ventricular dysfunction or dilatation but with valvular vegetation greater than 10 mm should be considered for surgery. Asymptomatic persons with mild to moderate valve regurgitation and no evidence of left ventricular dysfunction or dilatation should be observed until symptoms or severe valve regurgitation develop. (17,18,62,65,71,79-81)
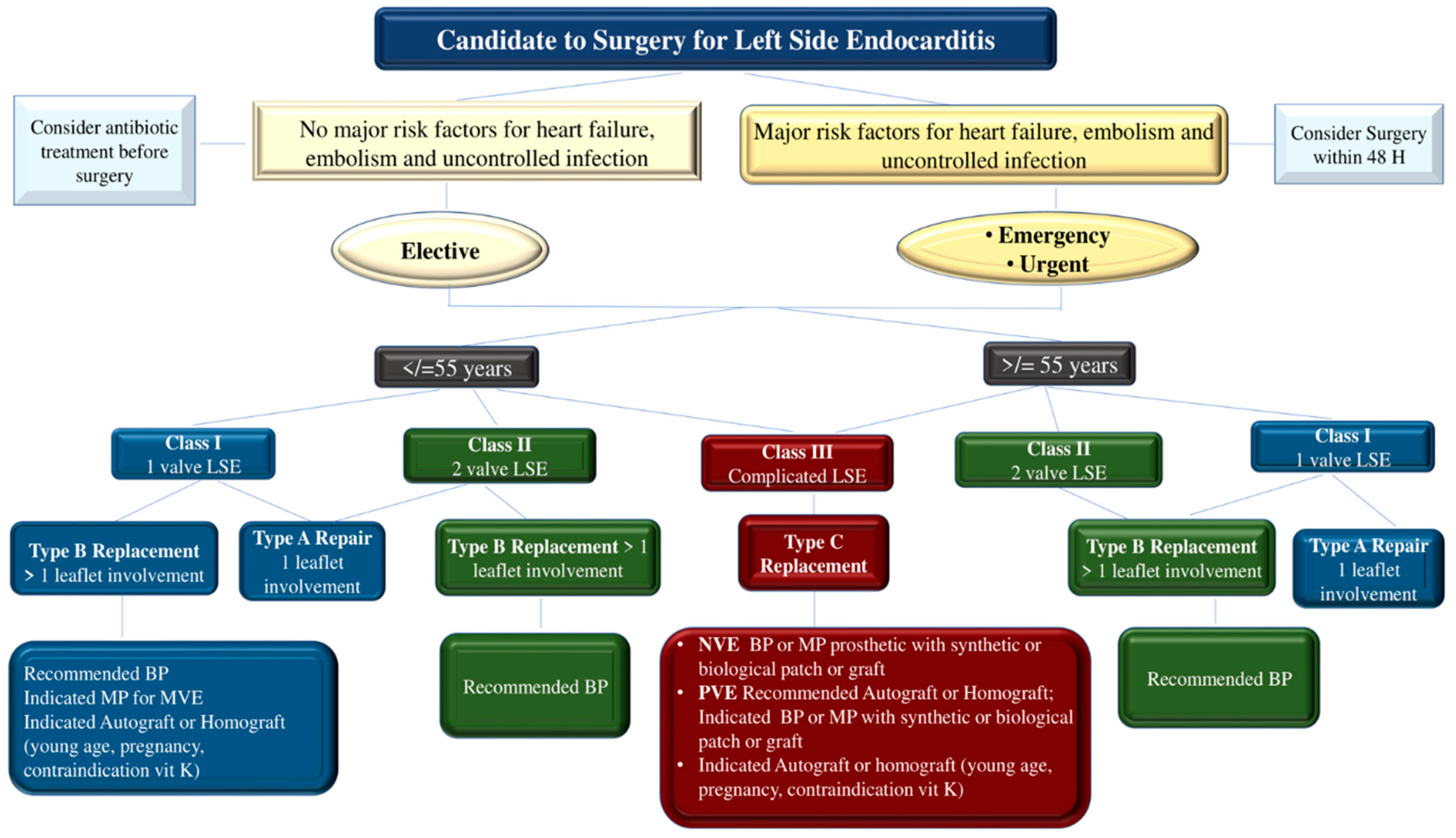
Surgery is dictated in 40–50% of patients with IE. (82) Before the advent of valve repair procedures, valve replacement was the preferred choice for severe regurgitation of the valve(s) due to the recurrence of IE. (71,83) Valve replacement may still be preferred in certain situations, such as in patients with advanced age or requirement for a combined or complex surgical procedure involving complete tissue debridement in cases of PVE, or in the extensive and noxious NVE where infectious fields include a large portion of the damaged heart. (24,71,82, 84-90). The most advanced therapies involve transcatheter valve therapy (TVT) for the treatment of structural heart disease which has proven to be a safe and effective approach for a large number of patients, although indications are preferably restricted to elderly individuals with different comorbidities. Patients are referred to centers specialized in the use of TVT because this cohort of subjects are potentially exposed to a high risk of mortality and complications after performing the standard surgical approach. (91)
Individual and institutional experience is decisive in determining the likelihood of success of all these procedures because high-volume centers have the lowest mortality rates and the highest proportion of patients undergoing successful treatment after valve surgery. When advising the patient or the patient's family, the surgeon should accurately assess the likelihood of successful valve surgery in light of the patient's clinical condition, characteristics and extent of the infectious process, and his or her own experience in the context of surgical treatment of IE. (83-90) The success of valve surgery for IE includes four general principles. First, surgery must ensure the complete removal of infectious vegetations followed by the repair or replacement of one or more heart valves. Secondly, the complete integrity of the cardiac structures should be restored. Third, to prevent the relapse of infection, complete debridement of the infected tissue should be performed. Valve replacement with allogeneic or autologous tissue is recommended. Finally, if the surgeon has performed a valve repair procedure, they should ensure that no more than a trace to mild valve regurgitation is present upon completion of the repair. (65,71,83-90)
Figure 7
E. faecalis-induced endocarditis is often associated with a very serious clinical picture that requires prompt hospital admission to a referral center for the treatment of endocarditis and consideration for undertaking a surgical procedure should not be postponed. Patients with IE due to E. faecalis may present with heart failure caused by regurgitation or valve obstruction which is the most common indication for surgery. Reported evidence from historical cohorts has revealed that the outcome is disastrous if surgery is not performed within 24 hours of hospitalization. These patients may develop refractory pulmonary edema or cardiogenic shock that rapidly worsens clinical conditions. (92,93) Patients with well-tolerated severe valvular regurgitation due to localization of extensive E. faecalis vegetations may be appropriate candidates for deferred surgery, after a period of stabilization with antibiotic therapy, although recent evidence recommends surgical treatment no later than 48 hours. (71,89,94,95)
The second concern due to E. faecalis IE driving the indication for surgery is the prevention of embolism. This devastating complication affects 25-50% of patients. (71,89,96) Stroke is the most frequent neurological complication, but embolic complications in any vascular bed results in infarction of the end organs (limbs, spleen, kidney, and coronary arteries). Furthermore, vegetation embolism may be responsible for a secondary evolution of the infection localized in the vascular wall, leading to the creation of a mycotic aneurysm. These aneurysms have most often been recorded in cerebral vessels and are diagnosed by brain imaging techniques in 3-5% of subjects experiencing an IE caused by E. faecalis, although most of these aneurysms remain clinically silent. (97-101)
The development of E. faecalis endocarditis in the right side of the heart may place patients at high risk of embolization to the lungs, or systemic circulation through a patent foramen ovale. Most emboli occur in the first 2 weeks after diagnosis and the risk decreases quickly after administration of antibiotics therapy. (100,101) As for embolism secondary to IE caused by Staphylococcus aureus, also for E. faecalis the vegetations that promote the embolic process are large more than 10 mm in length, highly mobile, and most frequently localized on the mitral valve. (71,102) Surgery is dictated by the recurrence of emboli and is indicated in patients with persistent threatening vegetations as evidenced by echocardiography. Surgical intervention is not contraindicated after ischemic stroke, and the delay of at least 1 month recommended to avoid cerebral hemorrhage has now been reconsidered and the intervention times have been significantly reduced. (71,89)
These considerations about the management of E. faecalis-induced IE are even more effective after TAVI due to the complexity of diagnosis and decision-making in this patient group. Indeed TAVI-IE is a new emerging entity whereby the clinical features, microbiology, risk factors, and outcomes, that have been well-documented, are markedly different from SAVR infective endocarditis. (103) The overall increased risk of bacteremia, as a result of higher exposure to healthcare procedures, older age, higher rates of comorbidities, and technical factors linked to the procedure may contribute to increasing the risk of TAVI-IE. (104-107)
Current guidelines do not include any specific provisions for this condition, which remains challenging from a diagnostic and management perspective. The modified Duke criteria has a lower diagnostic value in TAVI-IE because transthoracic and transesophageal echocardiography have lower sensitivity due to artifacts caused by the metallic frame. Supplementary imaging techniques including CT and FDG-PET/CT can provide complementary information but their role is not yet well established. Delayed diagnosis and the higher amount of prosthesis material, may explain the higher rate of peri-annular complications. (71,89)
Although indication for early surgery is recommended, the majority of patients are managed with intravenous antibiotics alone, due to older age and high operative risk(105,106). This may partly explain the high in-hospital mortality (36 % to 63.6%). These results suggest the need for a more aggressive approach and rapid diagnosis in these patients to improve clinical outcomes.
The precise role and optimal timing of surgery remain controversial. At present, surgical intervention is established after careful case-by-case analysis and which group of patients may benefit from surgery remains unclear. TAVI-IE is also technically challenging due to the difficulties of removing the stent frame adherent to the aorta (particularly for self-expandable TAVI) and mitral-aortic fibrosa, especially in inflamed tissues. It is accepted that no difference in terms of TAVI-IE occurrence subsists between the two types of TAVI prosthesis. Only two studies showed a higher frequency of TAVI-IE6 and a higher frequency of vegetations attached to the stent frame (102) in patients receiving self-expandable prostheses. Although there is no clear explanation for these findings, we agree that the much larger stent frame of self-expandable devices and the larger contact surface between the frame and the native tissues can act as potential anchoring and disseminating points during bacteremia. (105-107) We think that the risk of occurrence of peri-annular complications is the same between the two types of valves but in self-expandable prosthesis implantation, the risk of an aortic tear in the context of inflammatory tissues is higher.
6. The use of Biological Substitutes in the Context of E. faecalis Infective Endocarditis
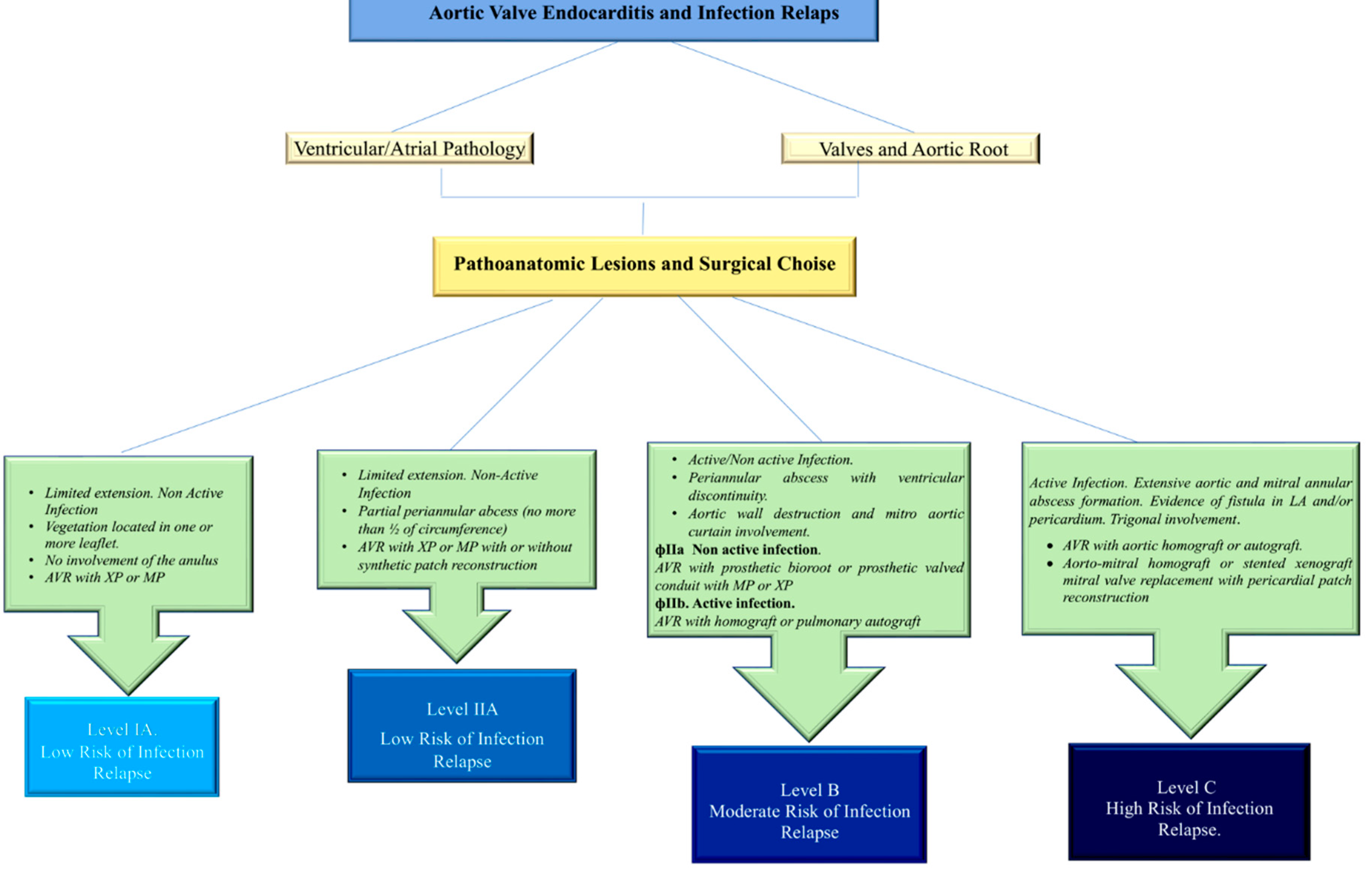
Surgery is particularly challenging in cases of abscess developed into aortic annulus due to the colonization of E. faecalis which mostly promote the detachment of the aorto-mitral curtain, involvement of the aortic root, fibrous trigones, and left atrial fistula formation.(71,84,108,109) The pili on the surface of E. faecalis attract mating cells into close contact, which increases the fertility of bacterial union leading to the formation of abscess cavities with a potential tendency to invade large portions of the heart. Moreover, the growth and expansion of raised pili-dependent infectious fields in E. faecalis-induced IE lead to lesions greater than 10 mm involving more than one valve leaflet, which is often associated with a severe inflammatory response, also involving the perivalvular tissues, which become extremely friable. (63,71,84,109) The consistency of the annulus in this case as well as of the adjacent regions is not reliable for suturing. (63,84,109) These anatomical-pathological features are appreciated of E. faecalis-induced IE are appreciated both in NVE and in PVE prosthetic valve endocarditis occurring in 3-4% of patients within 5 years of index surgery and can affect either mechanical or biologic bioprostheses alike. (71,79,84,108) More than a third of cases are acquired in the healthcare setting.(110) Early E. faecalis-induced IE occurring in prosthetic valves with a latency of less than 1 year after initial surgery has recently increased. Previous reports highlighted the role predominantly exerted by colonization of coagulase-negative S. Aureus in the first 2 months after surgery, (111) although this trend has recently been questioned in the cases of endocarditis and biofilm-associated pili of Enterococcus faecalis infection. (48)
Over 1 year, the range of causative pathogens responsible for PVE is the same as for NVE with enterococcal spp largely represented. As we described in the case report, the clinical presentation is often sustained by atypical and negative imaging results as well as the Duke criteria that are less evident. (112) Root abscess formation and evolution to valve dehiscence are common with the occurrence rate reaching up to 60% of patients. Surgery is usually required and performed before the patient's clinical condition deteriorates within 48 hours of diagnosis. The surgical procedure is often technically demanding and high-risk, with rates of recurrent PVE ranging from 6% to 15%. (82) The mortality of PVE is very high, particularly with S aureus infection, compared with that sustained from enterococcal spp, with 1-year mortality that can reach 50%. (84,108,110,115)
In these patients, to counteract the recurrence of the infection due to the persistence of active colonies especially of S. aureus and E. faecalis the use of a biological substitute such us cryopreserved aortic homograft (CAH) (as in our case) or another full root xenograft is recommended. However, whether the use of CAH in the field of infective endocarditis can improve clinical outcomes remains questionable because of the lack of RCTs (103,108, 114-121) However, the latest report from the Cleveland Clinic's Endocarditis Study Group suggested that allogeneic tissue reduces the risk of infection relapse. (122) The use of a homograft in first-time aortic valve replacement for IE decreased over time (9,4% to 5,6%) and in reoperation (37,5% to 28,5%) in a report from the STS database between 2005-2011. (103) Nevertheless, the cryopreserved aortic homograft (CAH) is used more often in patients who require reoperations than in primary interventions (32.2% vs 7.0%, p < 0.0001) in both valve replacements (14,6%) and for root replacements (53,2%). (103)
Of note that no significant differences in overall mortality and recurrence of infection have been described in comparison to conventional mechanical versus biological substitutes in IE. (108,115,116) Klieverik and colleagues (119) reported a similar rate of recurrent endocarditis in patients who received homografts compared to those who had mechanical valves with a lower freedom from reoperation (76% vs 93% respectively). Sabik and colleagues (120) described 103 patients with prosthetic IE, including 78% with periannular and root abscesses who were managed with aortic homografts and were associated with freedom from recurrent infection at more than 2 years of 95% and operative mortality of 3.9%. Fukushima and colleagues (117) disclosed a low rate (0,2%) of reinfection at 30 days and 5,5% of late infection with a median time of 5 years (4 months to 16 years) post-allograft implantation. Arabkhani and colleagues (118) revealed excellent results at 27 years postoperatively using aortic homografts, with a low incidence of reoperation for relapsing infections (2,2%) . The use of allogenic tissues revealed favorable responses to antibiotic treatment (effective in 21% to 25% of cases) (103,108)
Musci and colleagues (123) and Yanka and colleagues (124) first independently reported systematic use of the homograft aortic root replacement for active endocarditis and peri-annular abscess. The former used allografts in 221 of 1163 patients reporting a lower rate of recurrence of infection (5,4%) both in native valve endocarditis (NVE) and in prosthetic valve endocarditis (PVE) with a 10-year freedom from reoperation rate of 92.9%±3.2% and 92.1%±2.5%, respectively. Early mortality was 16% for NVE and 25% for PVE with improved 10-year survival in NVE (47% vs 35%). Of note, more than 25% of the deaths were intraoperative, suggesting the complexity of this operation among critically ill patients. Likewise, Yanha and colleagues (124) revealed excellent clinical performance and durability with a low rate of reinfection and a late mortality of 7.9%. Patient survival at 1 year was 97% and 91% at 10 years, respectively.
Very few IE candidates have contraindications to the use of allograft; the allogenic substitute has a low incidence of reinfection within 1-year and good hemodynamic performance. (114,116) The most favorable target to implant an allogenic tissue is the periannular abscess with the involvement of intervalvular fibrosa in PVE, but the allograft can be used also in the setting of NVE. (84,109,114,116120,125-127)
We described the use of cryopreserved aortic or mitral homograft to replace diseased valves in 56,2% and 21% of patients with abscess formation. Again, a double homograft was used in the field of aggressive IE with extension to the aorto-mitral junction and mitral valve. (63,64,71,109,114,125,126) Two-thirds of recipients received monobloc implants, (109,114,125,126) while the remaining were managed with separate blocs using partial mitral homograft insertion. (84,109,114,125-127) The technique of implant has provided good results even in the presence of fragile tissue. (109)
The use of allogeneic tissues in extensive infection of the heart structure, either in native or prosthetic valves, is supported by Steffen and colleagues.(128) After implantation, the allogenic tissue revealed marked antibacterial activity despite the 5-year storage period. Combinations of antibiotics applied during the treatment of allograft suggest a significant influence on their resistance to infection. Tests performed on ascending aortic homograft tissue have significantly shown an improvement in bacterial resistance against staphylococcal and enterococcal bacteria (
Enterococcus faecalis and
S aureus) with lower bacterial contamination compared to homograft aortic valves. (128) Kuhen et colleagues (129) suggested that the application of antibiotics after the thawing of allograft led to a crucial decrease in the recurrence of infection that has not been proven for conventional prostheses or dacron grafts, although the risk of vascular graft infection is reduced by pretreating the prosthesis with antibiotics. (129) However, we are unaware whether this favorable action may be related to an interaction with pili function in
E. faecalis-induced IE
Figure 8
7. Points and Counterpoints
E. faecalis as a nosocomial opportunist has become a very common pathogen in the promotion of bacterial endocarditis, underlining the need for alternative therapeutic approaches such as immunotherapy or immunoprophylaxis. On an empirical basis, antibiotics should be started as soon as blood cultures have been acquired, but if the patient is clinically stable therapy directed by culture results are often mandated by clinicians. (16) Despite the use of empiric antibiotic regimens for native valve endocarditis and prosthetic valve endocarditis are based on definitive guidelines (16) produced by the British Society for Antimicrobial Chemotherapy, however, the resistance demonstrated by E. faecalis to antibiotic treatment has recommended undertaking future directions by acquiring new knowledge on the biogenesis and resistance of E. faecalis. Knowledge of molecular biology associated with that of microbiology is strongly recommended in the shared decision-making process with the involvement of microbiological experts. The concern is related to the presence of selected layers of methicillin-resistant bacteria which significantly complicate the outcome of antibiotic therapy and accelerate the decision-making process towards surgical treatment. Although the antimicrobial regimen can be modified relatively on culture results, resistance patterns, severity of infection, and the presence or absence of prosthetic material; however, this is often not enough to avoid the progression of IE. In general, intravenous combination therapy is preferred over monotherapy to reduce the occurrence of resistance and provide synergistic antimicrobial activity. The exceptions are S aureus and E. faecalis methicillin-sensitive, for which flucloxacillin monotherapy is sufficient and the addition of gentamicin increases nephrotoxicity. (130)
More recently a study investigated the detection of antibiotic resistance genes (CTX-M, Van A, and Van B) of Enterococcus faecalis isolated from children with bacteremia by RT-PCR. Sulainam and colleagues observed that 91.67% of E. faecalis isolates were sensitive to levofloxacin, 83.33% to amoxiclav, 66.67% to erythromycin, 58.33% to amikacin, 50% to ampicillin, 33.33% to cefotaxime and ceftriaxone, and 25% to vancomycin. For 9 vancomycin-resistant isolates, the study suggested that 88.89% of them were associated with Van A gene production as detected by real-time PCR (P < 0.001). Two points are to be emphasized. In the first, 77.78% revealed Van B gene production as noted by real-time PCR (P < 0.001). In the second, all isolates of E. faecalis resistant to cefotaxime and ceftriaxone were characterized by the production of the CTX gene detected by real-time PCR (P<0.001). (131)
Given the increase in antibiotic resistance, the interest in microbiological research directed toward the use of bacterial factors as immunotherapeutic targets has consequently increased. This choice is dictated by the fact that bacterial factors are of substantial importance for the ability of an organism to colonize, infect and ultimately cause disease. (28) Among these factors, the central role played by MSCRAMMs, which promote the initiation of infections including endocarditis (33), has recently attracted considerable interest which is linked to their widespread nature and their peculiar action in traditional and opportunistic pathogens ( 28, 133). Unfortunately, criticalities have been observed in the isolation and characterization of MSCRAMM from E. faecalis which have shown limited success, since this microorganism does not easily adhere to ECM proteins under laboratory growth conditions (34, 35), unlike its relatives such as staphylococci and streptococci that endowed with increased aggressiveness.
To circumvent this obstacle Sillanpa and colleagues have recently used a bioinformatics approach through which they have been able to identify several proteins that predict MSCRAMM-like structures (29). Based on reactivity with sera from E. faecalis-infected patients, the authors deduced that some of these predicted proteins are indeed expressed by E. faecalis during infection. In particular, the work of Sillanpa and colleagues (29) testing the presence of antibodies in the sera of patients with E. faecalis endocarditis identified recombinant forms of 9 proteins anchored to the cell wall of E. faecalis. The authors characterized an in vivo expressed locus of 3 genes and a sortase-associated gene encoding Sortase C (SrtC). Therefore, by demonstrating the existence of antibodies against 3 of these proteins, with significantly higher levels in the sera of the majority of infected patients (29), the authors thus paved the way for further investigations.
Several studies have demonstrated that the virulence of Enterococcus faecalis is promoted by cell wall-associated proteins, including sortase-assembled endocarditis and biofilm-associated pilus (Ebp), important for biofilm formation in vitro and in vivo. Likewise, a large body of recent literature has demonstrated an increase in multi-resistance to drugs that fight Enterococcus faecalis infection. As for biofilm formation, it is considered crucial in various infections including IE caused by enterococcal spp. The genesis of biofilms is of particular concern not only because of its potential to further protect already drug-resistant organisms from antibiotics and opsonophagocytosis but also because it allows for increased horizontal gene transfer (34, 134). Previously published studies of E. faecalis have shown that several factors can lead to a significant reduction in biofilm density. (55-59) The latter is promoted by disruption of esp which encodes the surface protein of enterococci of some strains, by the fsr 2-component system, by gelE encoding for gelatinase, by the Epa gene cluster encoding for the polysaccharide Epa and finally by atn encoding an autolysin or bopD sugar-binding transcriptional regulator (55-59). A recent study that identified the traits involved in the high biofilm-producing strain E99 by transposon mutagenesis highlighted a gene cluster called bee that showed similar organization to that of Ebp (51). However, it was observed that this gene cluster was rare in E. faecalis and instead appears to be located in a conjugative plasmid. Likewise, the esp, gelE, and fsr loci are also restricted to a subpopulation of E. faecalis. Furthermore, gene association studies using clinical isolates have demonstrated that neither esp (55) nor fsr/gelE (135) is required for E. faecalis biofilm formation. This variation observed in the presence or absence of multiple biofilm-related genes may partially explain the variable capacity for biofilm formation (i.e., strong producers to non-producers) observed within this species.
Recently Nallapareddy and colleagues (48) focused their research on mutation and complementation analysis demonstrating that both the ebp operon, encoding endocarditis and biofilm-associated pili, and srtC were important for biofilm production of E. faecalis strain OG1RF. Furthermore, with the use of immunogold electron microscopy using antisera against EbpA-EbpC proteins and patient serum E. faecalis was observed to produce pleomorphic superficial pili. Of note that the assembly mechanism of pili and their attachment to the cell wall appeared to be favored by a cross-linking mechanism of Ebp proteins by the designated SrtC. Importantly, a non-piliated allelic replacement mutant was substantially mitigated in a model of IE. These biologically important surface pili, which were antigenic in humans during endocarditis and encoded by a ubiquitous E. faecalis operon, may be a useful immunological target for studies aimed at the prevention and/or treatment of this pathogen.
Clinical practice has taught us that infective endocarditis caused by E. faecalis is very aggressive because of its multi-drug resistance. Our experience proves that E. faecalis-induced IE is the most severe and clinically challenging of all E. faecalis-related infections. Endocardial vegetations appear to be the result of a biofilm formation process on the heart valves in patients not responding favorably to antibiotic treatment. (62-65,71,80,81,109,114) Evidence suggested by Nallapareddy and colleagues who tested an unpiliated ebpA deletion mutant in the endocarditis model confirms our experience. (49) The authors observed that rats treated with a mixture of equal numbers of wild-type E. faecalis OG1RF and its isogenic deletion mutant ebpA showed different levels of ebp of the vegetations. The percentages of ebpA were markedly lower in unpilated mutants recovered from vegetation and kidneys 24 hours after inoculation than in the percentages of wild-type mutants. These results suggest that Ebp pili play an important role in this endovascular infection, paralleling the role played by biofilm in vitro. The precise mechanism of action in causing endocarditis has yet to be elucidated. Interestingly, while Ebp pili are produced in a fraction of cells (at least in vitro), they contribute significantly to the pathogenicity of E. faecalis, which underscores the possibility of using Ebp proteins as an immunotarget. (49)
How the evidence of microbiology and molecular biology translate into clinical practice is an important challenge that can dictate the choice of the ideal substitute for the infected valve/s. Recently a study by Witten and colleagues addressed the risk of allogenic tissue infection in patients with biological allografts implanted for indications other than endocarditis and in patients experiencing infectious endocarditis. The researchers undertook a time-varying instantaneous risk of allograft infection by a parametric multiphase temporal decomposition nonproportional hazards and machine learning method analysis of patients undergoing aortic valve replacement with allogenic tissue from 1987 to 2017. A large cohort of patients (n=2042) received 2110 allografts for diseased aortic valve replacement with non-endocarditis indications (53%) and endocarditis indications (47%). 68% included prosthetic valve endocarditis (PVE), and the mean age of allograft recipients was 52±14 years in the endocarditis-free group and 57±15 years in endocarditis patients. The most common pathogens were gram-positive cocci (GPC) such as
Streptococcus viridans (22 %),
Staphylococcus aureus (20%),
Enterococcus faecalis (10%), and group D streptococci (11%) which are now classified as enterococcal spp. Among those
S. aureus were responsible for IE in 73% of injecting drug users. 63% of pathogens causative an IE were GPC biofilm-associated pili. At 20 years, the probability of allograft infection was 14% in patients with IE and 5.6% in the cohort without IE. Additionally, during follow-up, 42 infected allografts were explanted in the endocarditis cohort while 26 were retrieved in patients without endocarditis. Importantly, risk factors for allograft infection in patients with endocarditis were observed to be previous implants, intravenous drug use, and younger age. In patients with endocarditis and 18% of allograft infections, the causative pathogen was the same as the original organism and Enterococcus faecalis was present in 10% of the cases, 7% in patients with IE receiving an allograft, and 3% in those who had reinfection of the implanted allograft. In summary, long-term reinfection-free survival improved significantly after the use of allogeneic tissue. It is of further significance that the majority of patients received an allograft for an unattributable cause ad IE did not manifest a relapse of infection. The low infection rates reported in the study by Witten and colleagues in both patients with and without endocarditis support the continued use of allografts in the modern era, particularly for the treatment of invasive endocarditis of the aortic root supported by gram-positive cocci biofilm-associated pili confirming that biogenesis, multi-drug resistance, and infectious aggressiveness are closely linked.
Figure 9
Conclusion
Infective Endocarditis after TAVI is an emergent pathology that is difficult from a diagnostic and management perspective alongside challenging surgically. The precise role and optimal timing of surgery remains controversial but a multidisciplinary approach, prompt diagnosis, and early surgical intervention may improve outcomes as in our case. The rising cases of multidrug-resistant strains of enterococci such as Enterococcus faecalis, often require a multimodal management plan. The use of an allogenic valve substitute may be considered especially in an infective setting after a TAVI valve. The antimicrobial resistance of allogenic valves have been shown in multiple studies with favourable long-term outcomes.
Author Contributions
Conceptualization, F.N.; methodology, F.N. and S.S.A.S.; software, S.S.A.S. and V.J.; validation, F.N , S.S.A.S. and VJ; formal analysis, F.N. and S.S.A.S.; investigation, F.N.; data curation, F.N. and S.S.A.S.; writing—original draft preparation, F.N.; writing—review and editing, F.N.; S.S.A.S.; V.J.; A.F. visualization, F.N.; S.S.A.S. and A.F. supervision, F.N.;S.S.A.S. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murray, B.E.; Weinstock, G.M. Enterococci: New Aspects of an Old Organism. Proc. Assoc. Am. Physicians 1999, 111, 328–334. [CrossRef]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P.; National Nosocomial Infections Surveillance System. Nosocomial Infections in Combined Medical-Surgical Intensive Care Units in the United States. Infect. Control. Hosp. Epidemiol. 2000, 21, 510–515. [CrossRef]
- Megran, D.W. Enterococcal endocarditis. Clin. Infect. Dis. 1992, 15, 63–71.
- Chirouze, C.; Athan, E.; Alla, F.; Chu, V.; Corey, G.R.; Selton-Suty, C.; Erpelding, M.-L.; Miro, J.; Olaison, L.; Hoen, B. Enterococcal endocarditis in the beginning of the 21st century: analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Clin. Microbiol. Infect. 2013, 19, 1140–1147. [CrossRef]
- Murdoch DR, Corey GR, Hoen B, et al, and the International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009, 169, 463–73. [CrossRef]
- Nappi, F.; Martuscelli, G.; Bellomo, F.; Singh, S.S.A.; Moon, M.R. Infective Endocarditis in High-Income Countries. Metabolites 2022, 12, 682. [CrossRef]
- Gilmore, M.S. 2002. The Enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press. Washington, DC, USA. 439 pp.
- Fridkin, S.K.; Gaynes, R.P. Antimicrobial resistance in intensive care units. Clin. Chest Med. 1999, 20, 303–316. [CrossRef]
- Ammerlaan, H.S.M.; Harbarth, S.; Buiting, A.G.M.; Crook, D.W.; Fitzpatrick, F.; Hanberger, H.; Herwaldt, L.A.; van Keulen, P.H.J.; Kluytmans, J.A.J.W.; Kola, A.; et al. Secular Trends in Nosocomial Bloodstream Infections: Antibiotic-Resistant Bacteria Increase the Total Burden of Infection. Clin. Infect. Dis. 2012, 56, 798–805. [CrossRef]
- Koch, S.; Hufnagel, M.; Theilacker, C.; Huebner, J. Enterococcal infections: host response, therapeutic, and prophylactic possibilities. Vaccine 2004, 22, 822–830. [CrossRef]
- Pillar, C.M., and Gilmore, M.S. Enterococcal virulence--pathogenicity island of E. faecalis. Front. Biosci. 2004, 9, 2335–2346.
- Paulsen, I.T.; Banerjei, L.; Myers, G.S.A.; Nelson, K.E.; Seshadri, R.; Read, T.D.; Fouts, D.E.; Eisen, J.A.; Gill, S.R.; Heidelberg, J.F.; et al. Role of Mobile DNA in the Evolution of Vancomycin-Resistant Enterococcus faecalis. Science 2003, 299, 2071–2074. [CrossRef]
- Wang, Y.A.; Yu, X.; Ng, S.Y.; Jarrell, K.F.; Egelman, E.H. The Structure of an Archaeal Pilus. J. Mol. Biol. 2008, 381, 456–466. [CrossRef]
- Wang, Y.A.; Yu, X.; Silverman, P.M.; Harris, R.L.; Egelman, E.H. The Structure of F-Pili. J. Mol. Biol. 2009, 385, 22–29. [CrossRef]
- E Giessel, B.; Koenig, C.J.; Blake, R.L. Management of bacterial endocarditis.. Am. Fam. Physician 2000, 61, 1725–1732.
- Gould, F.K.; Denning, D.W.; Elliott, T.S.J.; Foweraker, J.; Perry, J.D.; Prendergast, B.D.; Sandoe, J.A.T.; Spry, M.J.; Watkin, R.W. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2011, 67, 269–289. [CrossRef]
- Cahill, T.J.; Baddour, L.M.; Habib, G.; Hoen, B.; Salaun, E.; Pettersson, G.B.; Schäfers, H.J.; Prendergast, B.D. Challenges in Infective Endocarditis. J. Am. Coll. Cardiol. 2017, 69, 325–344. [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2015, 387, 882–893. [CrossRef]
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al, and the International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008,168, 2095–2103.
- Correa de Sa DD, Tleyjeh IM, Anavekar NS, Schultz JC, Thomas JM, Lahr BD, et al. Epidemiological trends of infective endocarditis: a population-based studyin Olmsted County, Minnesota. Mayo Clin Proc. 2010, 85, 422–426.
- Chen H, Zhan Y, Zhang K, et al. The Global, Regional, and National Burden and Trends of Infective Endocarditis From 1990 to 2019: Results From the GlobalBurden of Disease Study 2019.Front Med (Lausanne). 2022, 9, 774224.
- Resende, P.; Fortes, C.Q.; Nascimento, E.M.D.; Sousa, C.; Fortes, N.R.Q.; Thomaz, D.C.; Pereira, B.d.B.; Pinto, F.J.; de Oliveira, G.M.M. In-hospital Outcomes of Infective Endocarditis from 1978 to 2015: Analysis Through Machine-Learning Techniques. CJC Open 2021, 4, 164–172. [CrossRef]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011, 377, 228–241. [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. College Cardiol. 2021, 77, 450–500. [CrossRef]
- Molton, J.S.; Tambyah, P.A.; Ang, B.S.P.; Ling, M.L.; Fisher, D.A. The Global Spread of Healthcare-Associated Multidrug-Resistant Bacteria: A Perspective From Asia. Clin. Infect. Dis. 2013, 56, 1310–1318. [CrossRef]
- Novick, R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003, 48, 1429–1449. [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511.
- Rivas, J.M., Speziale, P., Patti, J.M., and Hook, M. MSCRAMM-targeted vaccines and immu- notherapy for staphylococcal infection. Curr. Opin. Drug Discov. Devel. 2004, 7, 223–227.
- Sillanpaa, J., Xu, Y., Nallapareddy, S.R., Murray, B.E., and Hook, M. A family of putative MSCRAMMs from Enterococcusfaecalis. Microbiology. 2004, 150, 2069 2078.
- Deivanayagam, C.C., et al. A novel variant of the immunoglobulin fold in surface adhesins of Staphylococcus aureus: crystal structure of the fibrin- ogen binding MSCRAMM, clumping factor A. EMBO J. 2002, 21, 6660–6672.
- Rich, R.L.; Kreikemeyer, B.; Owens, R.T.; LaBrenz, S.; Narayana, S.V.L.; Weinstock, G.M.; Murray, B.E.; Höök, M. Ace Is a Collagen-binding MSCRAMM from Enterococcus faecalis. J. Biol. Chem. 1999, 274, 26939–26945. [CrossRef]
- Shankar, V., Baghdayan, A.S., Huycke, M.M., Lin- dahl, G., and Gilmore, M.S. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 1999, 67, 193–200.
- Galli, D., and Wirth, R. Comparative analy- sis of Enterococcus faecalis sex pheromone plasmids identifies a single homologous DNA region which codes for aggregation substance. J. Bacteriol. 1991, 173, 3029–3033.
- Carniol, K., and Gilmore, M.S. Signal transduction, quorum-sensing, and extracellular protease activity in Enterococcus faecalis biofilm for- mation. J. Bacteriol. 2004, 186, 8161–8163.
- Coburn, P.S., and Gilmore, M.S. The Entero- coccus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell. Microbiol. 2003, 5, 661–669.
- Sahl, H.-G.; Bierbaum, G. LANTIBIOTICS: Biosynthesis and Biological Activities of Uniquely Modified Peptides from Gram-Positive Bacteria. Annu. Rev. Microbiol. 1998, 52, 41–79. [CrossRef]
- Coburn, P.S., Pillar, C.M., Jett, B.D., Haas, W., and Gilmore, M.S. Enterococcus faecalis senses tar- get cells and in response expresses cytolysin. Science. 2004, 306, 2270–2272.
- Yanagawa, R., and Honda, E. Presence of pili in species of human and animal parasites and pathogens of the genus Corynebacterium. Infect. Immun. 1976, 13, 1293–1295.
- Yeung, M.K. Actinomyces: surface macromol- ecules and bacteria–host interactions. In Gram- positive pathogens. V.A. Fischetti, R.P. Novick, J.J. Fer- retti, D.A. Portnoy, and J.I. Rood, editors. American Society for Microbiology. Washington, DC, USA. 2000; 583–593.
- Wu, H.; Fives-Taylor, P.M. Molecular Strategies for Fimbrial Expression and Assembly. Crit. Rev. Oral Biol. Med. 2001, 12, 101–115. [CrossRef]
- Ton-That, H., and Schneewind, O. Assembly of pili on the surface of C. diphtheriae. Mol. Microbiol. 2003, 50, 1429–1438.
- Yeung, M.K.; Donkersloot, J.A.; Cisar, J.O.; Ragsdale, P.A. Identification of a Gene Involved in Assembly of Actinomyces naeslundii T14V Type 2 Fimbriae. Infect. Immun. 1998, 66, 1482–1491. [CrossRef]
- Li, T., Khah, M.K., Slavnic, S., Johansson, I., and Stromberg, N. Different type I fimbrial genes and tropisms of commensal and potentially patho- genic Actinomyces spp. with different salivary acidic proline-rich protein and statherin ligand specifici- ties. Infect. Immun. 2001, 69, 7224–7233.
- Mazmanian, S.K., Liu, G., Ton-That, H., and Sch- neewind, O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999, 285, 760–763.
- Ton-That, H., Liu, G., Mazmanian, S.K., Faull, K.F., and Schneewind, O. Purification and char- acterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 12424–12429.
- Ton-That, H.; Marraffini, L.A.; Schneewind, O. Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol. Microbiol. 2004, 53, 251–261. [CrossRef]
- Ton-That, H.; Schneewind, O. Assembly of pili in Gram-positive bacteria. Trends Microbiol. 2004, 12, 228–234. [CrossRef]
- Nallapareddy, S.R.; Singh, K.V.; Sillanpää, J.; Garsin, D.A.; Höök, M.; Erlandsen, S.L.; Murray, B.E. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Investig. 2006, 116, 2799–2807. [CrossRef]
- Lauer, P.; Rinaudo, C.D.; Soriani, M.; Margarit, I.; Maione, D.; Rosini, R.; Taddei, A.R.; Mora, M.; Rappuoli, R.; Grandi, G.; et al. Genome Analysis Reveals Pili in Group B Streptococcus. Science 2005, 309, 105–105. [CrossRef]
- Barocchi, M.A.; Ries, J.; Zogaj, X.; Hemsley, C.; Albiger, B.; Kanth, A.; Dahlberg, S.; Fernebro, J.; Moschioni, M.; Masignani, V.; et al. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. UAS 2006, 103, 2857–2862. [CrossRef]
- Tendolkar, P.M.; Baghdayan, A.S.; Shankar, N. Putative Surface Proteins Encoded within a Novel Transferable Locus Confer a High-Biofilm Phenotype to Enterococcus faecalis. J. Bacteriol. 2006, 188, 2063–2072. [CrossRef]
- Maione, D.; Margarit, I.; Rinaudo, C.D.; Masignani, V.; Mora, M.; Scarselli, M.; Tettelin, H.; Brettoni, C.; Iacobini, E.T.; Rosini, R.; et al. Identification of a Universal Group B Streptococcus Vaccine by Multiple Genome Screen. Science 2005, 309, 148–150. [CrossRef]
- Mora, M.; Bensi, G.; Capo, S.; Falugi, F.; Zingaretti, C.; Manetti, A.G.O.; Maggi, T.; Taddei, A.R.; Grandi, G.; Telford, J.L. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. 2005, 102, 15641–15646. [CrossRef]
- O’Toole, G., Kaplan, H.B., and Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49–79. Donlan, R.M., and Costerton, J.W. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2000, 15, 167–193.
- Mohamed, J.A., Huang, W., Nallapareddy, S.R., Teng, F., and Murray, B.E. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 3658–3663.
- Toledo-Arana, A., et al. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 2001, 67, 4538–4545.
- Hancock, L.E.; Perego, M. The Enterococcus faecalis fsr Two-Component System Controls Biofilm Development through Production of Gelatinase. J. Bacteriol. 2004, 186, 5629–5639. [CrossRef]
- Hufnagel, M.; Koch, S.; Creti, R.; Baldassarri, L.; Huebner, J. A Putative Sugar-Binding Transcriptional Regulator in a Novel Gene Locus inEnterococcus faecalisContributes to Production of Biofilm and Prolonged Bacteremia in Mice. J. Infect. Dis. 2004, 189, 420–430. [CrossRef]
- Kristich, C.J.; Li, Y.-H.; Cvitkovitch, D.G.; Dunny, G.M. Esp-Independent Biofilm Formation by Enterococcus faecalis. J. Bacteriol. 2004, 186, 154–163. [CrossRef]
- López, J.; Revilla, A.; Vilacosta, I.; Villacorta, E.; González-Juanatey, C.; Gómez, I.; Rollán, M.J.; Román, J.A.S. Definition, clinical profile, microbiological spectrum, and prognostic factors of early-onset prosthetic valve endocarditis. Eur. Hear. J. 2007, 28, 760–765. [CrossRef]
- Alonso-Valle, H.; Fariñas-Álvarez, C.; García-Palomo, J.D.; Bernal, J.M.; Martín-Durán, R.; Díez, J.F.G.; Revuelta, J.M.; Fariñas, M.C. Clinical course and predictors of death in prosthetic valve endocarditis over a 20-year period. J. Thorac. Cardiovasc. Surg. 2010, 139, 887–893. [CrossRef]
- Nappi, F.; Singh, S.S.A.; Nappi, P.; Spadaccio, C.; Nenna, A.; Gentile, F.; Chello, M. Heart Valve Endocarditis.. Surg. Technol. Online 2020, 37.
- Nappi, F.; Spadaccio, C. Simplest solutions are not always the cleverest: Can we stitch in an infected annulus? Should we rethink the current guidelines?. J. Thorac. Cardiovasc. Surg. 2017, 154, 1899–1900. [CrossRef]
- Nappi, F.; Spadaccio, C. keep fumbling around in the dark when it comes to infective endocarditis, or produce new, reliable data to redesign the guidelines?. J. Thorac. Cardiovasc. Surg. 2018, 155, 75–76. [CrossRef]
- Nappi, F.; Singh, S.S.A.; Timofeeva, I. Learning From Controversy: Contemporary Surgical Management of Aortic Valve Endocarditis. Clin. Med. Insights: Cardiol. 2020, 14. [CrossRef]
- Widmer, E.; Que, Y.-A.; Entenza, J.M.; Moreillon, P. New concepts in the pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 2006, 8, 271–279. [CrossRef]
- Flemming H-C, Wingender J. The biofilm matrix. Nat Rev Microbiol 2010, 8, 623–633.
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.-U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [CrossRef]
- Singh, S.S.A.; Costantino, M.F.; D’addeo, G.; Cardinale, D.; Fiorilli, R.; Nappi, F. A narrative review of diagnosis of infective endocarditis—imaging methods and comparison. Ann. Transl. Med. 2020, 8, 1621–1621. [CrossRef]
- Anguera, I.; Miro, J.M.; Vilacosta, I.; Almirante, B.; Anguita, M.; Muñoz, P.; Roman, J.A.S.; de Alarcon, A.; Ripoll, T.; Navas, E.; et al. Aorto-cavitary fistulous tract formation in infective endocarditis: clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur. Hear. J. 2004, 26, 288–297. [CrossRef]
- Nappi, F.; Spadaccio, C.; Dreyfus, J.; Attias, D.; Acar, C.; Bando, K. Mitral endocarditis: A new management framework. J. Thorac. Cardiovasc. Surg. 2018, 156, 1486–1495.e4. [CrossRef]
- Vieira, M.L.C.; Grinberg, M.; A Pomerantzeff, P.M.; Andrade, J.L.; Mansur, A.J. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Hear. 2004, 90, 1020–1024. [CrossRef]
- De Castro, S.; Cartoni, D.; D'Amati, G.; Beni, S.; Yao, J.; Fiorelli, M.; Gallo, P.; Fedele, F.; Pandian, N.G. Diagnostic Accuracy of Transthoracic and Multiplane Transesophageal Echocardiography for Valvular Perforation in Acute Infective Endocarditis: Correlation with Anatomic Findings. Clin. Infect. Dis. 2000, 30, 825–826. [CrossRef]
- Daniel, W.G.; Mügge, A.; Martin, R.P.; Lindert, O.; Hausmann, D.; Nonnast-Daniel, B.; Laas, J.; Lichtlen, P.R. Improvement in the Diagnosis of Abscesses Associated with Endocarditis by Transesophageal Echocardiography. New Engl. J. Med. 1991, 324, 795–800. [CrossRef]
- Victor, F.; De Place, C.; Camus, C.; Le Breton, H.; Leclercq, C.; Pavin, D.; Mabo, P.; Daubert, C. Pacemaker lead infection: echocardiographic features, management, and outcome. Hear. 1999, 81, 82–87. [CrossRef]
- Dundar, C.; Tigen, K.; Tanalp, C.; Izgi, A.; Karaahmet, T.; Cevik, C.; Erkol, A.; Oduncu, V.; Kirma, C. The Prevalence of Echocardiographic Accretions on the Leads of Patients with Permanent Pacemakers. J. Am. Soc. Echocardiogr. 2011, 24, 803–807. [CrossRef]
- Mihos, C.G.; Nappi, F. A narrative review of echocardiography in infective endocarditis of the right heart. Ann. Transl. Med. 2020, 8, 1622–1622. [CrossRef]
- Nappi, F.; Iervolino, A.; Singh, S.S.A. The New Challenge for Heart Endocarditis: From Conventional Prosthesis to New Devices and Platforms for the Treatment of Structural Heart Disease. BioMed Res. Int. 2021, 2021, 1–17. [CrossRef]
- Nappi, F.; Spadaccio, C.; Moon, M.R. A management framework for left sided endocarditis: a narrative review. Ann. Transl. Med. 2020, 8, 1627–1627. [CrossRef]
- Benedetto, U.; Spadaccio, C.; Gentile, F.; Moon, M.R.; Nappi, F. A narrative review of early surgery versus conventional treatment for infective endocarditis: do we have an answer?. Ann. Transl. Med. 2020, 8, 1626–1626. [CrossRef]
- Nappi, F.; Singh, S.S.A.; Spadaccio, C.; Acar, C. Revisiting the guidelines and choice the ideal substitute for aortic valve endocarditis. Ann. Transl. Med. 2020, 8, 952–952. [CrossRef]
- Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation 2010, 121, 1141–1152.
- David, T.E. Aortic valve repair for active infective endocarditis. Eur. J. Cardio-Thoracic Surg. 2012, 42, 127–128. [CrossRef]
- Navia, J.L.; Elgharably, H.; Hakim, A.H.; Witten, J.C.; Haupt, M.J.; Germano, E.; Houghtaling, P.L.; Bakaeen, F.G.; Pettersson, G.B.; Lytle, B.W.; et al. Long-term Outcomes of Surgery for Invasive Valvular Endocarditis Involving the Aortomitral Fibrosa. Ann. Thorac. Surg. 2019, 108, 1314–1323. [CrossRef]
- Paul, G.; Ochs, L.; Hohmann, C.; Baldus, S.; Michels, G.; Meyer-Schwickerath, C.; Fätkenheuer, G.; Mader, N.; Wahlers, T.; Weber, C.; et al. Surgical Procedure Time and Mortality in Patients with Infective Endocarditis Caused by Staphylococcus aureus or Streptococcus Species. J. Clin. Med. 2022, 11, 2538. [CrossRef]
- Grubitzsch, H.; Schaefer, A.; Melzer, C.; Wernecke, K.-D.; Gabbieri, D.; Konertz, W. Outcome after surgery for prosthetic valve endocarditis and the impact of preoperative treatment. J. Thorac. Cardiovasc. Surg. 2014, 148, 2052–2059. [CrossRef]
- Hussain, S.T.; Shrestha, N.K.; Gordon, S.M.; Houghtaling, P.L.; Blackstone, E.H.; Pettersson, G.B. Residual patient, anatomic, and surgical obstacles in treating active left-sided infective endocarditis. J. Thorac. Cardiovasc. Surg. 2014, 148, 981–988.e4. [CrossRef]
- Manne, M.B.; Shrestha, N.K.; Lytle, B.W.; Nowicki, E.R.; Blackstone, E.; Gordon, S.M.; Pettersson, G.; Fraser, T.G. Outcomes After Surgical Treatment of Native and Prosthetic Valve Infective Endocarditis. Ann. Thorac. Surg. 2012, 93, 489–493. [CrossRef]
- Samura, T.; Yoshioka, D.; Toda, K.; Sakaniwa, R.; Yokoyama, J.; Suzuki, K.; Miyagawa, S.; Yoshikawa, Y.; Hata, H.; Takano, H.; et al. Emergency valve surgery improves clinical results in patients with infective endocarditis complicated with acute cerebral infarction: analysis using propensity score matching†.. Eur. J. Cardio-Thoracic Surg. 2019, 56, 942–949. [CrossRef]
- Eranki, A.; Wilson-Smith, A.R.; Ali, U.; Saxena, A.; Slimani, E. Outcomes of surgically treated infective endocarditis in a Western Australian population. J. Cardiothorac. Surg. 2021, 16, 1–9. [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [CrossRef]
- Richardson, J.V.; Karp, R.B.; Kirklin, J.W.; E Dismukes, W.; R, B.; M, G.; L, B.; L, B.; M, J.; P, L.; et al. Treatment of infective endocarditis: a 10-year comparative analysis.. Circulation 1978, 58, 589–597. [CrossRef]
- Croft, C.H.; Woodward, W.; Elliott, A.; Commerford, P.J.; Barnard, C.N.; Beck, W. Analysis of surgical versus medical therapy in active complicated native valve infective endocarditis. Am. J. Cardiol. 1983, 51, 1650–1655. [CrossRef]
- Kang, D.-H.; Kim, Y.-J.; Kim, S.-H.; Sun, B.J.; Kim, D.-H.; Yun, S.-C.; Song, J.-M.; Choo, S.J.; Chung, C.-H.; Song, J.-K.; et al. Early Surgery versus Conventional Treatment for Infective Endocarditis. New Engl. J. Med. 2012, 366, 2466–2473. [CrossRef]
- Malhotra, A.; Rayner, J.; Williams, T.M.; Prendergast, B. Infective Endocarditis: Therapeutic Options and Indications for Surgery. Curr. Cardiol. Rep. 2014, 16. [CrossRef]
- Thuny, F.; DiSalvo, G.; Belliard, O. Risk of Embolism and Death in Infective Endocarditis: Prognostic Value of Echocardiography. A Prospective Multicenter Study. ACC Curr. J. Rev. 2005, 14, 31. [CrossRef]
- Peters, P.J.; Harrison, T.; Lennox, J.L. A dangerous dilemma: management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect. Dis. 2006, 6, 742–748. [CrossRef]
- Duval X, Iung B, Klein I, et al, and the IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Faculty Opinions recommendation of Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study.. 2015, 152. [CrossRef]
- Hess, A.; Klein, I.; Iung, B.; Lavallée, P.; Ilic-Habensus, E.; Dornic, Q.; Arnoult, F.; Mimoun, L.; Wolff, M.; Duval, X.; et al. Brain MRI Findings in Neurologically Asymptomatic Patients with Infective Endocarditis. Am. J. Neuroradiol. 2013, 34, 1579–1584. [CrossRef]
- Vilacosta I, Graupner C, San Román JA, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol 2002, 39, 1489–1495.
- Dickerman, S.A.; Abrutyn, E.; Barsic, B.; Bouza, E.; Cecchi, E.; Moreno, A.; Doco-Lecompte, T.; Eisen, D.P.; Fortes, C.Q.; Fowler, V.G.; et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: An analysis from the ICE Prospective Cohort Study (ICE-PCS). Am. Hear. J. 2007, 154, 1086–1094. [CrossRef]
- Chambers, H.F.; Bayer, A.S. Native-Valve Infective Endocarditis. New Engl. J. Med. 2020, 383, 567–576. [CrossRef]
- Savage, E.B.; Saha-Chaudhuri, P.; Asher, C.R.; Brennan, J.M.; Gammie, J.S. Outcomes and Prosthesis Choice for Active Aortic Valve Infective Endocarditis: Analysis of The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 2014, 98, 806–814. [CrossRef]
- Harding, D.; Cahill, T.J.; Redwood, S.R.; Prendergast, B.D. Infective endocarditis complicating transcatheter aortic valve implantation. Hear. 2020, 106, 493–498. [CrossRef]
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; Cheema, A.N.; et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016, 316, 1083–1092. [CrossRef]
- Mangner, N.; Woitek, F.; Haussig, S.; Schlotter, F.; Stachel, G.; Höllriegel, R.; Wilde, J.; Lindner, A.; Holzhey, D.; Leontyev, S.; et al. Incidence, Predictors, and Outcome of Patients Developing Infective Endocarditis Following Transfemoral Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2907–2908. [CrossRef]
- Amat-Santos IJ, Messika-Zeitoun D, Eltchaninoff H, et al. Faculty Opinions recommendation of Infective endocarditis after transcatheter aortic valve implantation: results from a large multicenter registry. Circulation 2015, 131. [CrossRef]
- David, T.E.; Gavra, G.; Feindel, C.M.; Regesta, T.; Armstrong, S.; Maganti, M.D. Surgical treatment of active infective endocarditis: A continued challenge. J. Thorac. Cardiovasc. Surg. 2007, 133, 144–149. [CrossRef]
- Nappi, F.; Acar, C. Monobloc or Separate Aortic and Mitral Homografts for Endocarditis of the Intervalvular Fibrosa?. Ann. Thorac. Surg. 2021, 112, 1382–1383. [CrossRef]
- Wang A, Athan E, Pappas PA, et al, and the International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361.
- Habib, G.; Thuny, F.; Avierinos, J.-F. Prosthetic Valve Endocarditis: Current Approach and Therapeutic Options. Prog. Cardiovasc. Dis. 2008, 50, 274–281. [CrossRef]
- Pérez-Vázquez A, Fariñas MC, García-Palomo JD, Bernal JM, Revuelta JM, González-Macías J. Evaluation of the Duke criteria in 93 episodes of prostheticvalve endocarditis: could sensitivity be improved? Arch Intern Med 2000, 160, 1185–1191.
- Chirouze C, Alla F, Fowler VG Jr, et al, and the ICE Prospective Investigators. Impact of early valve surgery on outcome of Staphylococcus aureus prostheticvalve infective endocarditis: analysis in the International Collaboration of Endocarditis- Prospective Cohort Study. Clin Infect Dis 2015, 60, 741–749.
- Nappi, F.; Nenna, A.; Petitti, T.; Spadaccio, C.; Gambardella, I.; Lusini, M.; Chello, M.; Acar, C. Long-term outcome of cryopreserved allograft for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1357–1365.e6. [CrossRef]
- Moon, M.R.; Miller, D.C.; Moore, K.A.; Oyer, P.E.; Mitchell, R.S.; Robbins, R.C.; Stinson, E.B.; Shumway, N.E.; Reitz, B.A. Treatment of endocarditis with valve replacement: the question of tissue versus mechanical prosthesis. Ann. Thorac. Surg. 2001, 71, 1164–1171. [CrossRef]
- Kim JB, Ejiofor JI, Yammine M, et al. Are homografts superior to conventional prosthetic valves in the setting of infective endocarditis involving the aortic valve? J Thorac Cardiovasc Surg 2016, 151, 1239–1246, 1248.e1231-1232.
- Fukushima, S.; Tesar, P.J.; Pearse, B.; Jalali, H.; Sparks, L.; Fraser, J.F.; Pohlner, P.G. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. J. Thorac. Cardiovasc. Surg. 2014, 148, 65–72.e2. [CrossRef]
- Arabkhani, B.; Bekkers, J.A.; Andrinopoulou, E.-R.; Roos-Hesselink, J.W.; Takkenberg, J.J.; Bogers, A.J. Allografts in aortic position: Insights from a 27-year, single-center prospective study. J. Thorac. Cardiovasc. Surg. 2016, 152, 1572–1579.e3. [CrossRef]
- Klieverik, L.M.; Yacoub, M.H.; Edwards, S.; Bekkers, J.A.; Roos-Hesselink, J.W.; Kappetein, A.P.; Takkenberg, J.J.; Bogers, A.J. Surgical Treatment of Active Native Aortic Valve Endocarditis With Allografts and Mechanical Prostheses. Ann. Thorac. Surg. 2009, 88, 1814–1821. [CrossRef]
- Sabik, J.F.; Lytle, B.W.; Blackstone, E.H.; Marullo, A.G.; Pettersson, G.B.; Cosgrove, D.M. Aortic root replacement with cryopreserved allograft for prosthetic valve endocarditis. Ann. Thorac. Surg. 2002, 74, 650–659. [CrossRef]
- Jassar AS, Bavaria JE, Szeto WY, et al. Graft selection for aortic root replacement in complex active endocarditis : does it matter ? Ann Thorac Surg 2012, 93, 480–487.
- Witten JC, Houghtaling PL, Shrestha NK, Gordon SM, Jaber W, Blackstone EH, et al; on behalf of the Infectious Endocarditis Working Group. Aorticallograft infection risk. J Thorac Cardiovasc Surg. 2023, 165, 1316–1317. e9.
- Musci, M.; Weng, Y.; Hübler, M.; Amiri, A.; Pasic, M.; Kosky, S.; Stein, J.; Siniawski, H.; Hetzer, R. Homograft aortic root replacement in native or prosthetic active infective endocarditis: Twenty-year single-center experience. J. Thorac. Cardiovasc. Surg. 2010, 139, 665–673. [CrossRef]
- Yankah, A.; Klose, H.; Petzina, R.; Musci, M.; Siniawski, H.; Hetzer, R. Surgical management of acute aortic root endocarditis with viable homograft: 13-year experience. Eur. J. Cardio-Thoracic Surg. 2002, 21, 260–267. [CrossRef]
- Olivito, S.; Lalande, S.; Nappi, F.; Hammoudi, N.; D’alessandro, C.; Fouret, P.; Acar, C. Structural deterioration of the cryopreserved mitral homograft valve. J. Thorac. Cardiovasc. Surg. 2012, 144, 313–320.e1. [CrossRef]
- Nappi F, Spadaccio C, Acar C. Use of allogeneic tissue to treat infective valvular disease: Has everything been said?J Thorac Cardiovasc Surg. 2017, 153, 824–828.
- Elgharably, H.; Pettersson, G.B.; Navia, J.L. Aortic allograft for endocarditis of the intervalvular fibrosa. Ann. Thorac. Surg. 2021, 112, 1383–1384. [CrossRef]
- Steffen, V.; Marsch, G.; Burgwitz, K.; Kuehn, C.; Teebken, O.E. Resistance to infection of long-term cryopreserved human aortic valve allografts. J. Thorac. Cardiovasc. Surg. 2015, 151, 1251–1259. [CrossRef]
- Kuehn, C.; Graf, K.; Mashaqi, B.; Pichlmaier, M.; Heuer, W.; Hilfiker, A.; Stiesch, M.; Chaberny, I.F.; Haverich, A. Prevention of Early Vascular Graft Infection Using Regional Antibiotic Release. J. Surg. Res. 2010, 164, e185–e191. [CrossRef]
- Cosgrove, S.E.; Vigliani, G.A.; Campion, M.; Fowler, J.V.G.; Abrutyn, E.; Corey, G.R.; Levine, D.P.; Rupp, M.E.; Chambers, H.F.; Karchmer, A.W.; et al. Initial Low-Dose Gentamicin forStaphylococcus aureusBacteremia and Endocarditis Is Nephrotoxic. Clin. Infect. Dis. 2009, 48, 713–721. [CrossRef]
- Sulaiman, A.M.; A Hussein, S.; I Husain, V. Detection of Antibiotic Resistance Genes (CTX-M, Van A and Van B) of Enterococcus faecalis Isolated from Children with Bacteremia by RT-PCR.. 2023, 78, 73–77. [CrossRef]
- Moreillon, P.; A Que, Y.; Bayer, A.S. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. North Am. 2002, 16, 297–318. [CrossRef]
- Foster, T.J.; Höök, M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998, 6, 484–488. [CrossRef]
- Parsek, M.R., and Singh, P.K. Bacterial bio- films: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701.
- Mohamed, J.A., and Murray, B.E. Lack of correlation of gelatinase production and biofilm for- mation in a large collection of Enterococcus faecalis isolates. J. Clin. Microbiol. 2005, 43, 5405–5407.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).