1. Introduction
There are several inherited disorders that are correlated to hemoglobin. Thalassemia is one of the major disorders of hemoglobin related to unusual hemoglobin formation [
1]. Thalassemia can be of different types such as α-thalassemia, β-thalassemia, Sickle thalassemia, etc [
2,
3]. Among them, β-thalassemia is one of the most prominent types of thalassemia caused by single gene alteration. Approximately, throughout the world, 7% of the population bears hemoglobinopathies as carriers of which 1.5% (~80-90 million) are carriers or heterozygotes of β-thalassemia [
4,
5]. The highest frequencies of β-thalassemia were observed in the Mediterranean and European regions, especially in countries such as Cyprus (15%), Sardinia (10-12%), Greece (8%), Italy (4-5%), Spain (1-2%), UK (0.4%) as well as in eastern and central Europe (0.2-5%) among other continents such as Africa, America, and Asia. In North Africa, the frequency of β-thalassemia was higher in Egypt (2-9%) followed by Morocco (6-7%), Tunisia (5%), and Libya (4-5%). The Maldives displayed the highest rate (18%) of this genetic disease followed by Indonesia (3-10%), India (1-10%), Thailand (3-9%), Pakistan (1-8%), and China-Hong Kong (3-6%) in the South-East region of Asia whereas Iran, Syria, and Oman showed similar rate (4-5%) which was the highest among the other countries of Middle-East and West region of Asia [
4]. This prevalence rate clearly presents the current global burden of thalassemia.
As mentioned earlier, thalassemia can be mainly of two different types- α-thalassemia and β-thalassemia, based on the globin chain that is affected. β-thalassemia occurs when the β-globin gene is mutated whereas α-thalassemia occurs due to α-globin gene mutation. Sickle cell anemia on the other hand results due to the heterozygosity of the β-thalassemia and sickle cell genes [
2,
3]. Again, thalassemia major (TM) refers to the severity of the clinical condition or phenotype which implies the red blood cell (RBC) transfusion more than eight times in a year, and thalassemia intermedia (TI) refers to the condition where infrequent or absolutely no transfusion is required. Thalassemia minor refers to the condition of being an asymptomatic carrier who might have mild anemia [
2,
6]. The clinical symptoms such as diarrhea, fever, irritability, abdominal distention, nutritional complications, and enlargement of the liver and spleen are usually expressed within 6-24 months of birth in thalassemia major. In thalassemia intermedia, symptoms might appear between 2-6 years of age with milder anemia where irregular blood transfusions may require depending on the physical condition [
6].
Glomerular filtration is a physiological process by which impure blood carrying nitrogenous and other waste materials converts to ultrafiltrate blood by flowing through the glomerular capillaries of the kidney [
7]. Estimating the glomerular filtration rate (GFR) is a crucial practice for diagnosis, prognosis, monitoring, and drug dose management especially for kidney diseases [
7,
8]. Theoretically, the GFR is the outcome of the total number of nephrons times the average single-nephron GFR [
7]. Although GFR is a primary index of kidney disease, the changes of GFR were also found to be associated with other diseases such as coronary heart disease (CHD), cardiovascular disease (CVD), fatty liver, UTI and so on [
9,
10,
11,
12]. Interestingly, GFR was also found to have a correlation with thalassemia, however, in several investigations, a positive correlation between GFR and thalassemia was identified and in some other cases GFR was detected to have an inverse relation with thalassemia [
13,
14]. Therefore, in this meta-analysis, we tried to evaluate the exact association between GFR and thalassemia.
2. Materials and Methods
2.1. Search Strategy
Primarily, three different databases such as Google Scholar, PubMed, and ScienceDirect were searched respectively with specific keywords which include “glomerular”, “GFR”, “thalassemia”, “randomized control trial”, “RCT”, “case-control” and “healthy”. “Advanced” search mood was used to search articles in PubMed and ScienceDirect. “Title and abstract” and “Title, abstract, keywords” were used in the “advanced” mood for PubMed and ScienceDirect respectively. However, “allintitle” was used during the search in Google Scholar. The search was adjusted with Boolean operators where necessary. The detailed search strategy has been illuminated in the supplementary
Table 1.
2.2. Eligibility Criteria
Narrative or Systematic reviews, Documents, correspondences, book chapters, letter to editor, editorials, or articles that do not focus on Thalassemia and GFR were not considered eligible. Duplicate articles assessed from different databases were removed carefully. Only English-language written articles were considered for this study. However, no year restriction was applied.
2.3. Quality Assessment
To investigate the quality of all the included studies, a number of questions were acquired from the Study Quality Assessment Tools, NIH, and Systematic Reviews: Step 6: Assess Quality of Included Studies, UNC [
15,
16]. The score was given as a percentage depending on different answers (i.e. Yes= 1, No=0, Unclear=0, NR=no score, NA=no score) with specific scores. The total score ≤50, 60-70, and ≥80 was classified as low-scored study (high risk of bias), moderate-scored study (moderate risk of bias), and high-scored study (low risk of bias) respectively.
2.4. Data Extraction and Analyses
Based on the eligibility criteria data extraction was done carefully from the included studies. Several data regarding the general characteristics of the included studies were extracted along with the data of mean ± standard deviation (SD) of the GFR and the number of total participants of case and control for meta-analysis. Endnote (version X8) software was operated for data extraction.
A random-effects model of mean difference (MD) with 95% confidence intervals (CIs) was preferred to analyze the difference of GFR level between case and control among the included study. Heterogeneity of the included studies was determined using I2 statistics (I2>75% implies substantial heterogeneity). RevMan (version-5.4) software was used for the Meta-analysis.
2.5. Publication Bias and Sensitivity Analyses
Publication bias and sensitivity analyses were done using different methods of analysis to investigate the strength of the result following previous study with slight modifications [
17,
18]. Primarily, funnel plot was created to identify the publication bias. Followed by Galbraith’s plot was constructed to visualize any outlier studies. Further, forest plot was constructed using the random effect model considering the non-outlier homogenous studies. Again, forest plot was constructed using fixed effect model with all the included studies to reconfirm the main outcome. To construct the funnel and Galbraith’s plots RStudio software (version 4.3.0), and the ‘metafor’ package (version 4.2-0) of R was used.
3. Results
3.1. Inclusion of the Study
After the initial search, a total of 96 articles were identified from three online databases (
i.e. PubMed, ScienceDirect and Google Scholar). From those articles, 36 articles were immediately excluded due to the ineligibility as they were case reports, review articles, correspondence, letter, editorial or original articles worked with animal model,
in vitro,
in silico experiments only other than full length research article worked with human case-control model. From the rest 60 articles 24 articles were excluded due to study duplication. From the remaining 36 articles 21 articles were eliminated for not matching with our focus and study criteria (
i.e. including different types of control, had lack of data, assessed other diseases etc.). Finally, 15 articles were included for this meta-analysis (
Figure 1).
Figure 1.
A simplified flow diagram of methodology.
Figure 1.
A simplified flow diagram of methodology.
3.2. Assessment of Quality
The quality assessment of the study determined that all the included studies were of moderate (moderate risk of bias) or high quality (low risk of bias) (
Table 1). No study had a score of ≤50 with high risk of bias.
Table 1.
Quality assessment of the included studies.
Table 1.
Quality assessment of the included studies.
| Study ID |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
Overall score (%) |
| Ahmed 2022 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
| Ali 2013 |
Y |
Y |
Y |
Y |
Y |
N |
NA |
Y |
Y |
87.5 |
| Bilir 2020 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
| Behairy 2017 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
| Bekhit 2017 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
| Capolongo 2020 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
| Cetinkaya 2020 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
| Ghobrial 2015 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
| Hamed 2010 |
Y |
Y |
Y |
Y |
Y |
N |
Y |
Y |
Y |
88.8 |
| Katopodis 1997 |
Y |
Y |
Y |
NR |
NR |
N |
U |
Y |
Y |
71.4 |
| Mahmoud 2012 |
Y |
Y |
Y |
Y |
Y |
N |
NA |
Y |
Y |
87.5 |
| Mahmoud 2021 |
Y |
Y |
Y |
Y |
Y |
N |
NA |
Y |
Y |
87.5 |
| Saghir 2020 |
Y |
Y |
Y |
Y |
Y |
N |
NA |
Y |
Y |
87.5 |
| Sen 2015 |
Y |
Y |
Y |
U |
Y |
N |
U |
Y |
Y |
66.6 |
| Uzun 2015 |
Y |
Y |
Y |
Y |
Y |
N |
U |
Y |
Y |
77.7 |
3.3. Study Characteristics
The major characteristics such as study location, study type, demographics of the study participants (i.e. male, female ratio in study, BMI, age, case and control type etc.) and the GFR measuring method were extracted from each of the included study (
Table 2). Not reported (NR) was mentioned if any information was not provided in any of the study. Among the studies only the data of participant’s BMI were not reported in Ahmed 2022, Ali 2013, Bekhit 2017, Centinkaya 2020, Katopodis 1997, Mahmoud 2021, Saghir 2020, Sen 2015, Uzun 2015 [
19,
20,
21,
22,
23,
24,
25,
26,
27]. Percentages of male and female participants were missing in Capolongo 2020 [
28]. Every single data was reported in Bilir 2020, Behairy 2017, Ghobrial 2015, Hamed 2010, Mahmoud 2012 [
13,
14,
29,
30,
31].
Table 2.
Characteristics of the included studies.
Table 2.
Characteristics of the included studies.
| Study ID |
Location |
Study type |
Participants demographics |
GFR measurement method |
References |
| Male (%) |
Female (%) |
BMI |
Age |
Case type |
Control type |
| Ahmed 2022 |
Egypt |
Case-control |
64 |
36 |
NR |
9.35 ± 6.14 |
β-TMa
|
Healthy control |
Simple height-independent formula |
[19] |
| Ali 2013 |
Egypt |
Cross-sectional |
63.2* |
36.8* |
NR |
9.80 ± 1.70* |
β-TM |
Healthy control |
Schwartz formula |
[20] |
| Bilir 2020 |
Turkey |
Cross-sectional |
58.23 |
41.77 |
18.57 ± 2.32 |
12.03 ± 4.47 |
β-TM |
Healthy control |
Schwartz formula |
[13] |
| Behairy 2017 |
Egypt |
Case-control |
64.5 |
35.5 |
21.9 ± 5.35 |
9.5 ± 3.66 |
β-TM |
Healthy control |
Schwartz formula |
[14] |
| Bekhit 2017 |
Egypt |
Case-control |
46.66* |
53.34* |
NR |
8.30 ± 3.50* |
β-TM |
Healthy control |
Schwartz formula |
[21] |
| Capolongo 2020 |
Italy |
Case-control |
NR |
NR |
23.35 ± 2.35 |
33.5 ± 13 |
β-TM |
Healthy control |
Schwartz formula |
[28] |
| Cetinkaya 2020 |
Turkey |
Case-control |
60 |
40 |
NR |
21.5 ± 23 |
β-TM |
Healthy control |
Schwartz formula |
[22] |
| Ghobrial 2015 |
Egypt |
Cross-sectional |
61.11 |
38.89 |
16.36 ± 0.7 |
7.96 ± 4.38 |
Sickle thalassemia |
Control |
Schwartz formula |
[29] |
| Hamed 2010 |
Egypt |
Case-control |
66.67 |
33.33 |
16.69 ± 2.75 |
8.56 ± 3.9 |
TDβ-TM |
Healthy control |
Schwartz formula |
[30] |
| Katopodis 1997 |
Greece |
Case-control |
47* |
53* |
NR |
37.5* |
S-βT |
Healthy control |
99mTC DPTA |
[23] |
| Mahmoud 2012 |
Egypt |
Cross-sectional |
61.33 |
38.67 |
17.75 ± 2.6 |
9.7 ± 1.4 |
β-TM |
Healthy control |
Schwartz formula |
[31] |
| Mahmoud 2021 |
Egypt |
Case-control |
59 |
41 |
NR |
9 ± 9.75 |
β-TM |
Healthy control |
Schwartz formula |
[24] |
| Saghir 2020 |
Pakistan |
Cross-sectional |
100 |
0 |
NR |
8 ± 3 |
Thalassemia |
Healthy control |
99mTC DPTA & 25I labeled iothalamate |
[25] |
| Sen 2015 |
Turkey |
Cross-sectional |
55.55 |
44.45 |
NR |
8.95 ± 4.2 |
β-TM |
Healthy control |
Schwartz formula |
[26] |
| Uzun 2015 |
Turkey |
Case-control |
52 |
48 |
NR |
9.95 ± 4.1 |
β-TM |
Healthy control |
Schwartz formula |
[27] |
3.4. Main outcome
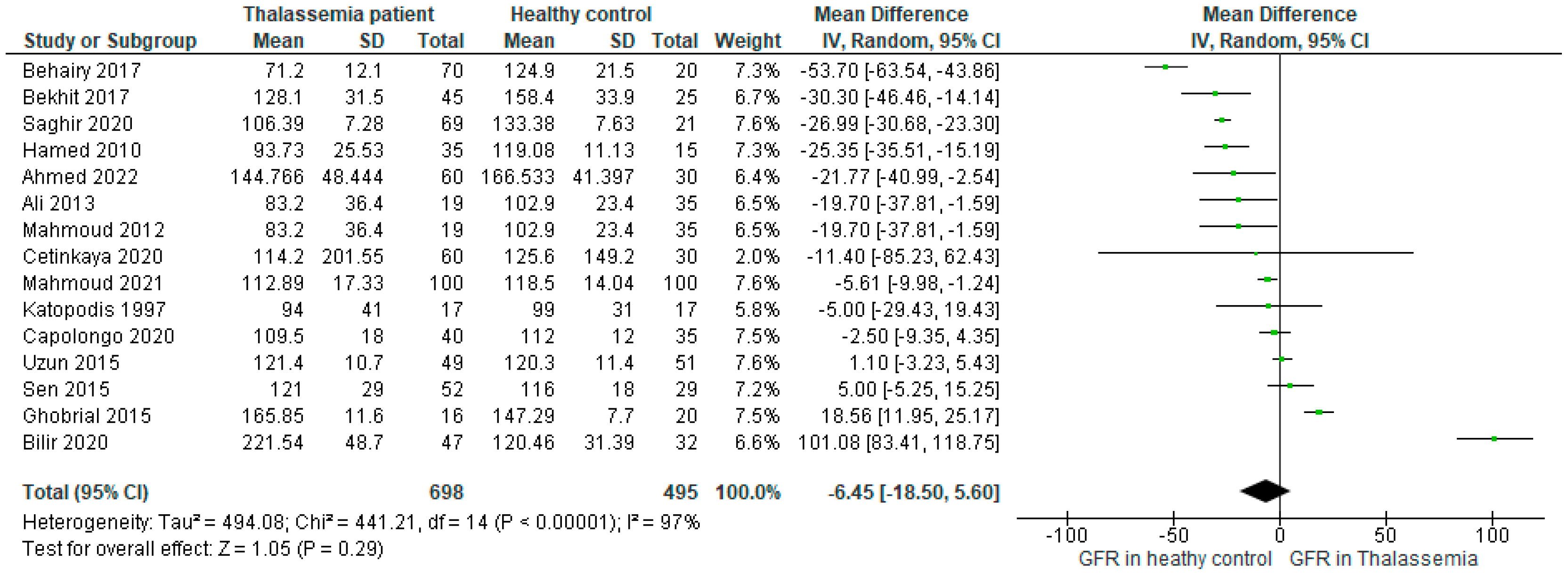
The forest plot including all the studies (n=15) (
Figure 2) investigated that the mean difference (MD) of GFR level was -6.45, 95%CI: -18.50, 5.60 (P<0.00001) favoring the healthy control as compared to the Thalassemia patient. The determined I2>97% for the analysis implied that the heterogeneity of the included studies was significantly high.
Figure 2.
Forest plot showing the mean difference (MD) of GFR in patients with Thalassemia as compared to healthy controls in the random effect model.
Figure 2.
Forest plot showing the mean difference (MD) of GFR in patients with Thalassemia as compared to healthy controls in the random effect model.
3.5. Assessment of Publication Bias and Sensitivity Analysis
Funnel plot was constructed based on all the included studies confirmed to be no high asymmetry in the plot (
Figure 3).
Figure 3.
Funnel plot investigating the publication bias reporting GFR in Thalassemia patients in comparison to healthy controls.
Figure 3.
Funnel plot investigating the publication bias reporting GFR in Thalassemia patients in comparison to healthy controls.
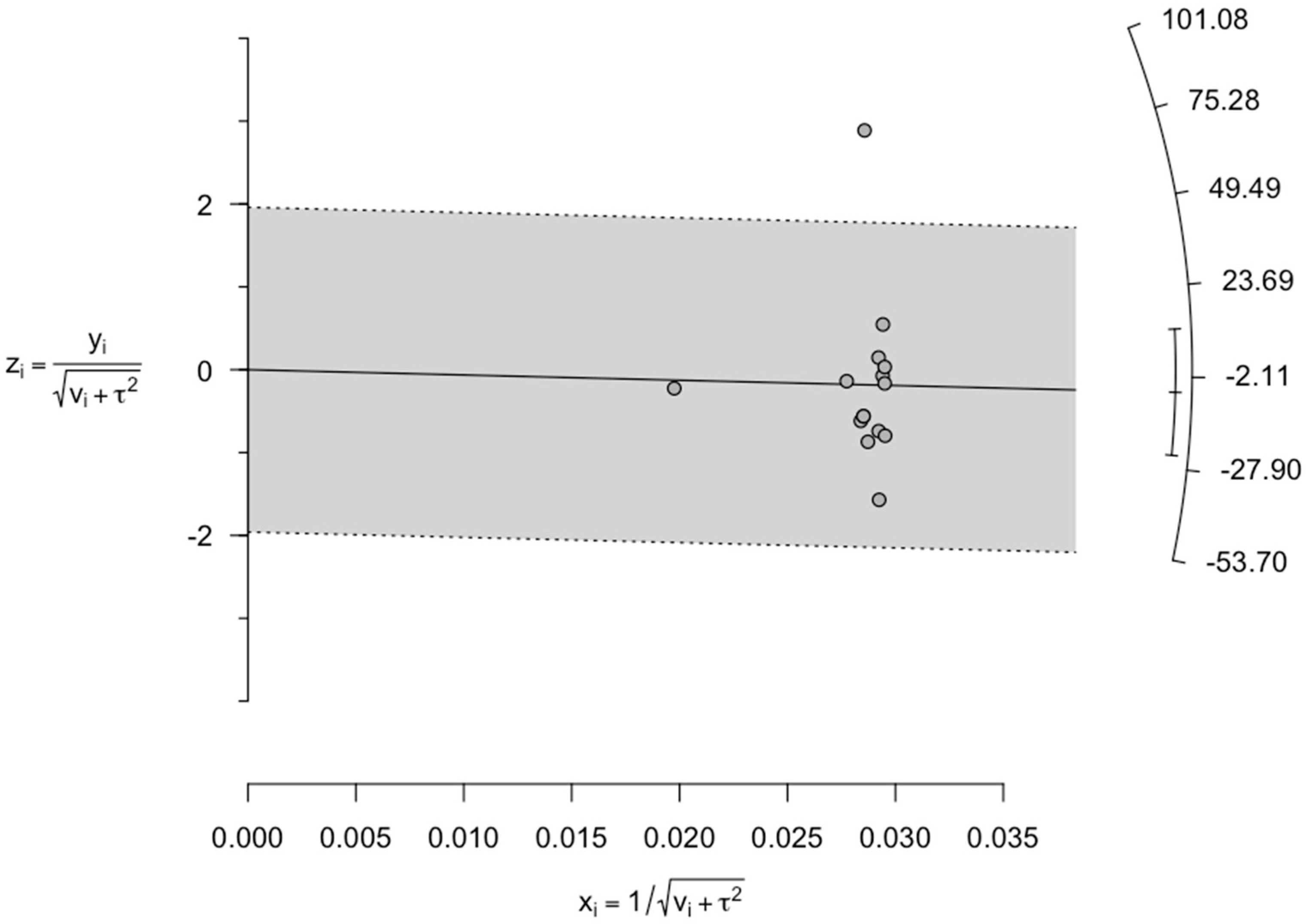
This confirmed the absence of any publication bias. Again, due to high heterogeneity, we further considered the data for sensitivity analysis. For sensitivity analyses we initially constructed Galbraith’s plot. From Galbraith’s plot, Bilir 2020 was considered as a single outlier study (
Figure 4).
Figure 4.
Galbraith plot displaying the plausible sources of heterogeneity. Studies within the limits are interpreted as homogeneous. Studies outside the limits may be outliers. According to the plot, one study (Bilir 2020) was found to be an outlier.
Figure 4.
Galbraith plot displaying the plausible sources of heterogeneity. Studies within the limits are interpreted as homogeneous. Studies outside the limits may be outliers. According to the plot, one study (Bilir 2020) was found to be an outlier.
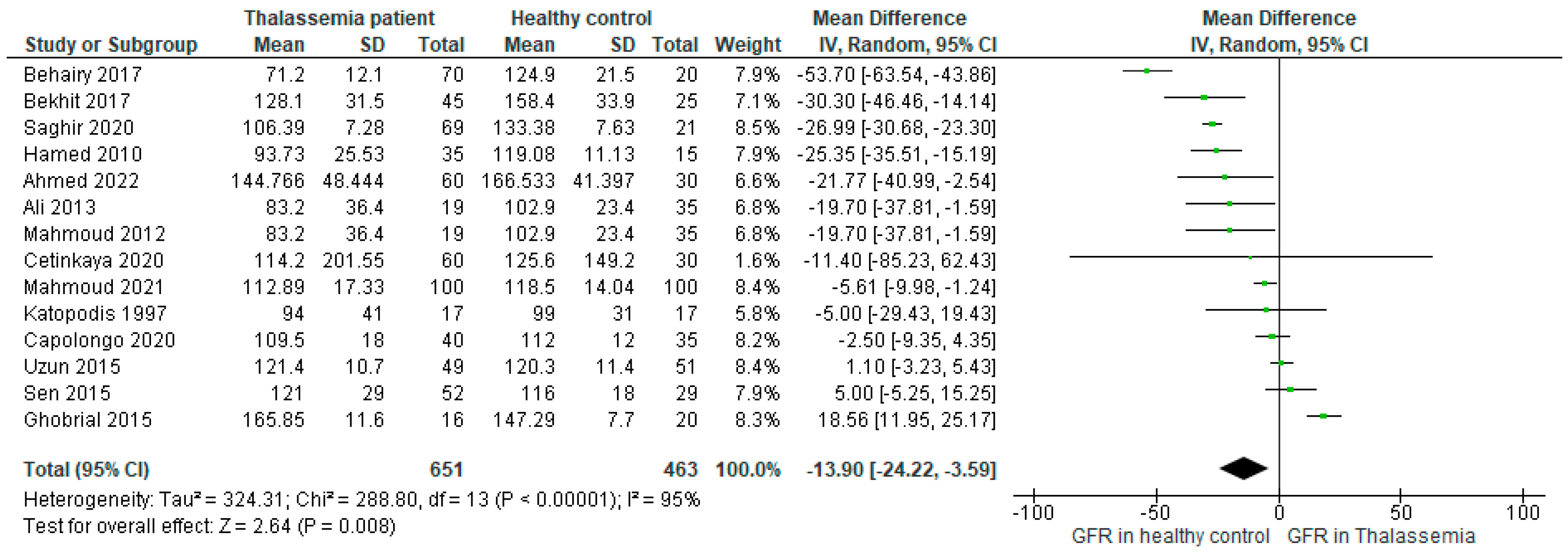
Therefore, excluding Bilir 2020 the forest plot was constructed further using 14 studies (
Figure 5), where the mean difference (MD: -13.90, 95%CI: -24.22, -3.59 (P<0.00001)) was found to be two times higher than the main outcome (-6.45, 95%CI: -18.50, 5.60 (P<0.00001), (
Figure 2)) favoring the higher GFR in healthy control. Besides, the heterogeneity reduced (I2=95%) in comparison to the main result as well.
Figure 5.
Forest plot showing the mean difference (MD) of GFR in patients with Thalassemia as compared to healthy controls in the random effect model excluding the outlier study (n=1).
Figure 5.
Forest plot showing the mean difference (MD) of GFR in patients with Thalassemia as compared to healthy controls in the random effect model excluding the outlier study (n=1).
Similarly, reconstruction of the forest plot including all the included studies using fixed effect model was found to be favoring healthy control as compared to Thalassemia patients (MD: -9.54, 95%CI: -11.47, -7.62) (
Figure 6) which also supported the main outcome. These sensitivity analyses further supported the strength of our primary result.
Figure 6.
Forest plot showing the mean difference (MD) of GFR in patients with Thalassemia as compared to healthy controls in the fixed effect model. .
Figure 6.
Forest plot showing the mean difference (MD) of GFR in patients with Thalassemia as compared to healthy controls in the fixed effect model. .
4. Discussion
To the best of our knowledge, this is the single meta-analysis that focused on the association between thalassemia and GFR, compiling the research data from all over the world. Research says thalassemia can alter GFR through mechanisms such as renal hypoxia, iron overload, oxidative stress, and renal tubular dysfunction [
25,
32].
Thalassemia results in chronic anemia from the decreased synthesis of functional hemoglobin. Anemia lowers the blood’s ability to transport oxygen, which causes tissue hypoxia, notably in the kidneys and thus causes renal hypoxia. Renal hypoxia may result in diminished renal performance, including a drop in GFR. Again, to treat their anemia, people with thalassemia frequently need blood transfusions. Iron overload in the body may result from these transfusions. The kidneys, among other organs, can become overloaded with iron, which can cause tissue damage and malfunction. Damaged kidneys brought on by iron excess may lower GFR [
25].
The upsurge of oxidative stress by creating an imbalance between the antioxidant defense mechanism and the generation of reactive oxygen species (ROS) inside the body may occur because of thalassemia. The renal vasculature and glomeruli may be harmed through the oxidative stress, ultimately impairing the GFR. Thalassemia can also interfere with the renal tubules, which are crucial for reabsorbing filtered chemicals and preserving fluid and electrolyte balance. Electrolyte imbalances and poor water absorption carried on by renal tubule dysfunction can eventually have an negative impact on GFR [
32].
Through the MD value of our main outcome (MD: -6.45, 95%CI: -18.50, 5.60) (
Figure 2) we determined that the GFR was higher in healthy population as compared to the people with thalassemia disease. This finding ultimately indicates that the GFR is generally disrupted in thalassemia patients whether an individual may or may not have a current or have previous history of any types of kidney disease which is further supported by previous researches [
25,
32].
From our analysis we found exceptional results where the MD value of GFR was higher in thalassemia patients in comparison to healthy control (
Figure 2). Among the included studies MD value of GFR was found the highest in Thalassemia patients in Bilir 2020 (MD: 101.08, 95%CI: 83.41, 118.75) followed by Ghobrial 2015 (MD: 18.56, 95%CI: 11.95, 25.17), Sen 2015 (MD: 5, 95%CI: -5.25,15.25) and Uzun 2015 (MD: 1.1, 95%CI: -3.23, 5.46).
Billir et al. mentioned that the small sample size of their study could be one of the reasons for such unusual positive correlation between thalassemia and GFR. Besides, age, prolonged anemia and medicinal side effects or toxicity were supposed to have an association with this incident as well [
13,
33]. Deferoxamine is a popular medicine which is used in thalassemia as a chelating agent. This was used in thalassemia in both the studies Bilir 2020 and Ghobrial 2015 [
13,
29]. Research observed the effect of subcutaneous deferoxamine on 27 patients with thalassemia major, where interestingly it was identified that after the deferoxamine treatment, 41% of the patients’ GFR values were above the normal range [
34]. Therefore, the argument that the deferoxamine toxicity plays a role in increasing GFR might be possible but need to be confirmed through further rigorous research. Again, increased proteinuria in thalassemia patients was investigated in both the studies of Bilir 2020 and Ghobrial 2015. Besides, Ghobrial et al. also stated that among the sickle thalassemia patients muscle mass and diet were not maintained accordingly, which lead to increased serum protein which translated into enhanced proteinuria [
13,
29]. A previous study claimed the high prevalence of microalbuminuria in patients with sickle cell leads to macroproteinuria, and ultimately chronic renal failure, where glomerular hyperfiltration is the early step [
35]. Therefore, enhanced proteinuria might be another plausible cause of increased GFR in thalassemia patients.
The disease thalassemia has a wide range of morbidities and affects practically all organ systems. Among thalassemia patients who depend on blood transfusions, renal illness is one of the leading causes of death. Early detection of kidney illness is crucial to avoid impairment since it might arise from progressive renal tubular and glomerular damage [
36]. The results indicate a substantial relationship between thalassemia and GFR. The GFR can be helpful for the early detection and prevention of renal failure in thalassemia patients since this study demonstrated a notable difference between GFR in healthy control and thalassemia patients. Hence, it is important for thalassemia patients to periodically monitor their GFR rate. Thus, the above finding can help patients with thalassemia major in identifying renal diseases, lowering the fatality rates.
It is necessary to evaluate GFR using a more precise approach. An approach like this could make it possible to identify GFR decline early in TM patients and stop or slow its progression to acute kidney injury (AKI), chronic kidney disease (CKD), any other kidney related complications and the requirement for renal replacement therapy (RRT) [
37]. GFR can be determined through the identification of the clearance rate of different endogenous or exogenous biomarkers such as inulin, creatinine, Cystatin C, iohexol etc. and all are clinically accepted [
24,
38,
39]. However, the measurement methods and biomarkers need to be more specified to get highly accurate results, which is crucial for the proper diagnosis, prognosis, medication, and treatment of patients. The adoption of a highly precise and specified technique could enable the early diagnosis of decreased GFR in TM and delay the steady decline towards kidney dysfunction.
5. Conclusions
In conclusion, thalassemia patients are more susceptible to renal glomerular and tubular damage, which is rarely detected in conventional renal testing. GFR in thalassemia patients is a reliable indicator for evaluating glomerular thus kidney dysfunction. In thalassemia patients, the GFR can be useful for the early identification or even prevention of renal failure since it varies between healthy controls and cases.
Author Contributions
Conceptualization, S.S.K. and E.S.; methodology, N.J., D.S., A.H.P., I.A.H; S.S.K., software, S.S.K., J.Z., E.S.; formal analysis, S.S.K., J.Z., E.S.; investigation, S.S.K; N.J., D.S., A.H.P., I.A.H., resources, E.S.; data curation, E.S., M.N.U.; writing—original draft preparation, S.S.K., E.S.; writing—review and editing, S.S.K., E.S., visualization, S.S.K., J.Z., E.S.; supervision, E.S.; project administration, E.S. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data relevant to this review are included in the text, Supplementary Materials, and references.
Conflicts of Interest
The authors declare no conflict of interest
References
- Helmi, N., et al., Thalassemia review: features, dental considerations and management. Electronic physician, 2017. 9(3): p. 4003. [CrossRef]
- Cunningham, M.J., Update on thalassemia: clinical care and complications. Hematology/oncology clinics of North America, 2010. 24(1): p. 215-227. [CrossRef]
- Aessopos, A., et al., Endothelial function and arterial stiffness in sickle-thalassemia patients. Atherosclerosis, 2007. 191(2): p. 427-432. [CrossRef]
- Colah, R., A. Gorakshakar, and A. Nadkarni, Global burden, distribution and prevention of β-thalassemias and hemoglobin E disorders. Expert Review of Hematology, 2010. 3(1): p. 103-117. [CrossRef]
- Weatherall, D., et al., Inherited disorders of hemoglobin. Disease Control Priorities in Developing Countries. 2nd edition, 2006.
- Tari, K., et al., Thalassemia an update: molecular basis, clinical features and treatment. International journal of biomedicine and public health, 2018. 1(1): p. 48-58. [CrossRef]
- Levey, A.S., L.A. Inker, and J. Coresh, GFR estimation: from physiology to public health. American Journal of Kidney Diseases, 2014. 63(5): p. 820-834. [CrossRef]
- Stevens, L.A. and A.S. Levey, Measured GFR as a confirmatory test for estimated GFR. Journal of the American society of nephrology, 2009. 20(11): p. 2305-2313. [CrossRef]
- Matsushita, K., et al., Change in estimated GFR associates with coronary heart disease and mortality. Journal of the American Society of Nephrology: JASN, 2009. 20(12): p. 2617. [CrossRef]
- Mathisen, U.D., et al., Estimated GFR associates with cardiovascular risk factors independently of measured GFR. Journal of the American Society of Nephrology: JASN, 2011. 22(5): p. 927. [CrossRef]
- Di Bonito, P., et al., High uric acid, reduced glomerular filtration rate and non-alcoholic fatty liver in young people with obesity. Journal of Endocrinological Investigation, 2020. 43: p. 461-468. [CrossRef]
- Arnello, F., et al., Evolution of single kidney glomerular filtration rate in urinary tract infection. Pediatric nephrology, 1999. 13: p. 121-124. [CrossRef]
- Bilir, Ö.A., et al., Renal function and the oxidative status among children with thalassemia major and healthy controls: A cross-sectional study. Transfusion and Apheresis Science, 2020. 59(4): p. 102746. [CrossRef]
- Behairy, O.G., et al., Role of serum cystatin-C and beta-2 microglobulin as early markers of renal dysfunction in children with beta thalassemia major. International journal of nephrology and renovascular disease, 2017: p. 261-268. [CrossRef]
- NIH. Study Quality Assessment Tools. 2021; Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- UNC. Systematic Reviews: Step 6: Assess Quality of Included Studies. 2023; Available from: https://guides.lib.unc.edu/systematic-reviews/assess-quality.
- Islam, M.A., et al., Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmunity reviews, 2019. 18(11): p. 102392. [CrossRef]
- Islam, M.A., et al., Prevalence of antiphospholipid antibodies in Behçet’s disease: a systematic review and meta-analysis. PLoS One, 2020. 15(1): p. e0227836. [CrossRef]
- Ahmed, H.M., et al., The association between plasma microRNA-451 expression levels and chronic kidney disease in children with β-thalassemia major. Iranian Journal of Kidney Diseases, 2022. 16(3): p. 188.
- Ali, B.A. and A.M. Mahmoud, Frequency of glomerular dysfunction in children with beta thalassaemia major. Sultan Qaboos University Medical Journal, 2014. 14(1): p. e88. [CrossRef]
- Bekhit, O.E., H.H. El Dash, and M.S. Ahmed, Early detection of kidney dysfunction in Egyptian patients with beta-thalassemia major. Egyptian Pediatric Association Gazette, 2017. 65(3): p. 85-89. [CrossRef]
- Cetinkaya, P.U., et al., β2-microglobulin, neutrophil gelatinase-associated lipocalin, and endocan values in evaluating renal functions in patients with β-thalassemia major. Hemoglobin, 2020. 44(3): p. 147-152. [CrossRef]
- Katopodis, K.P., et al., Renal abnormalities in patients with sickle cell-beta thalassemia. Journal of Nephrology, 1997. 10(3-1997): p. 163-167.
- Mahmoud, A.A., et al., Assessment of subclinical renal glomerular and tubular dysfunction in children with beta thalassemia major. Children, 2021. 8(2): p. 100. [CrossRef]
- Saghir, S., et al., Cystatin C an early marker of Glomerular dysfunction in thalassemia major. The professional medical journal, 2020. 27(02): p. 300-308. [CrossRef]
- Şen, V., et al., Urinary early kidney injury molecules in children with beta-thalassemia major. Renal failure, 2015. 37(4): p. 607-613. [CrossRef]
- Uzun, E., et al., Glomerular and tubular functions in children with different forms of beta thalassemia. Renal failure, 2015. 37(9): p. 1414-1418. [CrossRef]
- Capolongo, G., et al., Urinary metabolic profile of patients with transfusion-dependent β-thalassemia major undergoing deferasirox therapy. Kidney and Blood Pressure Research, 2020. 45(3): p. 455-466. [CrossRef]
- Ghobrial, E.E., et al., Urinary transforming growth factor β-1 as a marker of renal dysfunction in sickle cell disease. Pediatrics & Neonatology, 2016. 57(3): p. 174-180. [CrossRef]
- Hamed, E.A. and N.T. ElMelegy, Renal functions in pediatric patients with beta-thalassemia major: relation to chelation therapy: original prospective study. Italian journal of pediatrics, 2010. 36: p. 1-10. [CrossRef]
- Mahmoud, A. and B. Ali, Cystatin C as an Early Marker of Glomerular Dysfunction in Children with Beta Thalassemia Major. Bulletin of Egyptian Society for Physiological Sciences, 2012. 32(2): p. 265-278. [CrossRef]
- Cai, Q., et al., Dietary patterns based on estimated glomerular filtration rate and kidney function decline in the general population: The lifelines cohort study. Nutrients, 2020. 12(4): p. 1099. [CrossRef]
- Jalali, A., et al., Renal function in transfusion-dependent pediatric beta-thalassemia major patients. Hematology, 2011. 16(4): p. 249-254. [CrossRef]
- Koren, G., et al., The effects of subcutaneous deferoxamine administration on renal function in thalassemia major. International Journal of Hematology, 1991. 54(5): p. 371-375.
- Haymann, J.-P., et al., Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clinical journal of the American Society of Nephrology: CJASN, 2010. 5(5): p. 756. [CrossRef]
- Sleiman, J., A. Tarhini, and A.T. Taher, Renal complications in thalassemia. Thalassemia Reports, 2018. 8(1): p. 7481. [CrossRef]
- de Dreuzy, E., et al., Current and future alternative therapies for beta-thalassemia major. Biomedical journal, 2016. 39(1): p. 24-38. [CrossRef]
- Milo, G., et al., GFR in Patients with β-Thalassemia Major. Clinical journal of the American Society of Nephrology: CJASN, 2015. 10(8): p. 1350. [CrossRef]
- Schwartz, G.J. and S.L. Furth, Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatric nephrology, 2007. 22: p. 1839-1848. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).