1. Introduction
Multidrug resistance (MDR) is a great, and rapidly increasing challenge for scientists, in latest 8 decades. The different forms of MDR appearing in pathogen organisms of clinical and veterinary, (as well as of agricultural) importance partly due to the consequence of the astonishingly unprofessional applications in inaccurate doses providing optimal selective conditions and selective advantages for MDR organisms to original (wild-type) ones of the same species, [
1] (Fodor et al., 2020). One attempt to overcome resistance problems is to test the general and specific antimicrobial potential to apply antimicrobial peptides (AMPs) [
2] (Ötvös and Wade, 2014). AMPs are of great potential to combat MDR prokaryotes because of several reasons. First, there is an option of taking advantage from the option of using the remarkable toolkit called quantitative structure-activity relation (QSAR) [
3] (Karelson, Lobanov, Katriczki) 1996); [
4] (Gini, 2022) technology in designing novel antimicrobial-active sister molecules of a newly discovered natural drug-candidate AMP molecule. The drug designer has a powerful way to produce efficient, or even-more powerful (but physiologically less harmful analogs of AMPs which may overcome resistance problems in such a way. Furthermore, according to recent data, antibiotic-resistant bacteria tend to perform collateral sensitivity to AMPs, [
5] (Lázár et al., 2018), and the mobility patterns of traditional AMP-resistance genes are different from that of the antibiotics-resistance genes, [
6] (Kintses et al., 2019), [
1] (Fodor et al., 2020).
We are interested in testing the antipathogenic potential of AMPs in controlling MDR eukaryotic pathogens. As for the two selected targets: they are
Leishmania. donovani [
7] (Chaoulitch, 1954); a mammalian pathogen, is transmittable by insects like cave-dwelling sand flies [
8] (Dutra-Rêgo et al., 2022), and
Histomonas. meleagridis is the causative factor (or etiological agent) of the life-threatening histomoniasis (or blackhead) disease) of birds (turkeys, chicken pheasants, first described in 1893 [
9] (McDougald, 1993), [
10] (McDougald, 2005), and have been studied in detail in the laboratory of [
11] (Hess et al., 2015).
When planning experiments aiming at applying AMPs against eukaryotic pathogens, we have to take into consideration that the strategy of designing antimicrobials to control eukaryotic parasites (pathogens) must be different from that of designing antimicrobials to control prokaryotic pathogens, because targettable intracellular structures of a eukaryotic parasite (pathogen) biochemically might be similar to some intracellular structure of vital function in the eukaryote host to be protected.
We aim to learn whether any of those biosynthetic NR_AMPs (like fabclavine, [
12], (Fuchs et al., 2012), [
13] (Fuchs et al., 2014), phenazine, ˙[
14] (Shi et al., 2019, or something else), produced by those entomopathogenic-nematode symbiont bacteria (EPB) we have been working on [
15] Lengyel et al., et al., 2005), [
16] (Fodor et al., 2022), were suitable for applying anti-protist drug candidates either against the intracellular parasite
Leishmania, or the extracellular parasite
Histononas. The arguments for choosing in vitro EPB liquid cultures as appropriate sources of efficient AMPs for this purpose are as follows: Obligate bacterial symbionts of entomopathogenic nematode species synthesize and release several non-ribosomal hybrid peptides [
17] (Akhurst, 1982), of large target specificities which mainly serve to provide well-balanced pathobiome conditions for this symbiosis in polyxenic soil,- and cadaver environments [
18] (Ogier, et al., 2020). These peptides are also considered potential sources of potent natural anti-microbial compounds, [
17] (Akhurst, 1982). The motivation for choosing in vitro EPB liquid cultures as a source of efficient AMPs for this purpose are as follows: proven active on Gram-positive [19 (Furgani), [
20] (Fodor et al., 2018), Gram-negative [
22] (Böszörményi, et al., 2009) pathogenic bacteria, and oomycetes and different other plant pathogens, [
23], (Fodor et al., 2010), [
24] (Vozik et a., 2015) in our previous studies. We have reasons to believe that some of the autoclaved
Xenohabdus cultures may not be harmful or toxic for toxic when added as a food supplement while some antimicrobial ingredients are still active, [
21] (Fodor et al., 2023), we may not give up the option of considering potential probiotic applications, at least at experimental level.
As for the eukaryotic targets used in this study, the genus
Leishmania belongs to the Order Trypanosomatida, the Class Kinetoplastea, and the Phylum: Euglenozoa, [
25] (Fernández-Arévalo et al., 2020), and is considered a blood parasite. It is the causative agent of leishmaniasis in humans, [
26] (McGwire and Satoskar, 2014), and other mammals like dogs, [
27] (Alvar et al., 2004); [
28] (Dantas-Torres, et al., 2019].
The
Leishmania pathogens act as intracellular parasites [
29] (Walker et al., 2014). They retained the ability to produce extracellular vesicles. Both human,- and canine (caused by
Leishmania infantum) can appear in a severe form called visceral leishmaniasis (VL), or kala-azar, [
30] (Burza et al., 2018), [
31] (Safavi et al, 2021). It has become to-endemic diseases have become global due to human migration, climate change, and anthropogenic disturbance, causing significant worldwide health and economic burden [
32] (Altamura et al., 2022). As for the existing defense strategies: despite promising research activities, no clinically usable vaccine is available. In the absence of an effective vaccine, [
33] (Mauël, 2002), the control of leishmaniasis relies exclusively on chemotherapy, [
34] (Ibarra-Meneses et al., 2022). Each used clinical chemotherapy is based on very few chemotherapeutic agents, mainly antimonial derivatives (Sb). [
35] Douanne et al, 2020]. As for available chemotherapies: Amphotericin B (AmB), a polyene macrolide antibiotic derived from actinomycetes, [
36] (Golenser and Domb, 2006) has routinely been used in cases of visceral and canine leishmaniasis It had been selected from 200 known polyene agents, because of its moderate toxicity. It has been permitted for intravenous administration, [
36] (Golenser and Domb, 2006). AmB acts in a membrane-destroying manner, [
37] (Ellis, 2004), and has not provoked clinical resistance up to 1956, [
36] (Golenser, and Domb, 2006). Since then severe drug resistance to both AmB and the other routinely used drug, miltefosine, (especially in
L. infantum strains), appeared and continues spreading at alarming rates [
34] (Ibarra-Meneses et al., 2022).
Leishmania has always become an exceptional t model for studying microbial virulence, [38 [Chang, 2003). To outline the fascinating resistance mechanisms developed by
Leishmania would require a long detailed review, so we want to refer to some publications instead ([
35] Douanne et al, 2020], [
20] (Golenser and Domb, 2006; [
34] (Ibarra-Meneses et al., 2022). [
39] (Segovia, 1994),
The other chosen target,
H. meleagridis belongs to the genus
Histomonas, a member of the family Monocercomonadidae within the class Trichomonada, in the class Trichomonadida of Phylum Metamonda. Infections in turkeys may cause nearly 100% mortality. Outbreaks in chickens are more often marked by morbidity and subsequent recovery. The outbreaks in broiler breeder pullets at that time caused excessive losses from mortality (5 to 20%) culling, and overall poor flock performance.
Histomonas parasites are carried by the eggs of the cecal worm
Heterakis gallinarum enabling them to survive for long periods in the soil as a source of infection, [
9] (McDougald, 1998), [
10]] (McDougald, 1998), [
11] (Hess et al., 2015). In the U.S. there were never any drug products available for the treatment of blackhead disease, [
9] (McDougald, 1998), [
10]] (McDougald, 1998), [
11] (Hess et al., 2015). As for molecular taxonomy, and identification, the 5.8S, ITS-1, and ITS-2 rRNA regions were first sequenced [
40] (Lollis et al. 2011). The most preferable remarkable reference gene to investigate gene expression as a symptom of the infection in selected organs from turkeys and chickens (called 60S ribosomal protein L13) has been identified [
41] (Mitra et al., 2016), and used different infection models related to immunization, and vaccination, [
42,
43] (Kidane et al, 2016; (Mitra et al., 2018). The complete, annotated Histomonas reference genome sequence has been available, [
44] (Palmieri et al., 2021). Histomoniasis is a fastidious disease in turkeys, with pathological lesions in the caeca and liver, sometimes with high mortality. In chickens, the disease is less fatal, and lesions are often confined to the caeca. In Europe, the disease was well controlled by applying nitroimidazoles and nitrofurans for therapy or prophylaxis, [
11] (Hess et al., 2015). It infects a wide range of birds including chickens, turkeys, peafowl, quail, and pheasants, causing infectious enterohepatic lesions. Since their introduction into the market in the middle of the previous century, further research nearly ceased as outbreaks of histomoniasis occurred only very rarely.
With the ban of these drugs in the last two decades in North America, the European Union, and elsewhere, in combination with the changes in animal husbandry, the disease re-emerged, [
11] (Hess et al., 2015). But at that time the previously applied drugs were banned. Consequently, research programs were set up in various places focusing on different features of the parasite and the disease. For the first time studies were performed to elucidate the molecular repertoire of the parasite. In addition, research has been started to investigate the parasite's interaction with its host, [
11] (Hess et al., 2015). New diagnostic methods and tools were developed and tested with samples obtained from field outbreaks or experimental infections. Some of these studies aimed to clarify the introduction of the protozoan parasite into a flock and the transmission between birds, [
45] (Hauck et al., 2010). Finally, a strong focus was placed on research concentrated on the development of new treatments and prophylactic strategies, urgently needed to combat the disease, [
11] (Hess et al., 2015).
As for vaccination, studies on the immune response against histomoniasis focus mainly on different traits of the adaptive immune system. Activation of toll-like receptors (TLR) leads to the interplay between cells of innate and adaptive immunity with consequences on B and T cell clonal expansion. Efforts for successful vaccinations are on the way for years, [
46] (Lagler et al., 2021), but various challenges need to be addressed until the first ever developed vaccine based upon live flagellates in human or bird medicine can be marketed. Summarizing: the poultry industry works without approved prophylactics, therapeutics, or vaccines to combat histomonosis, [
47] (Beer et al., 2022).
Meanwhile, numerous chemical compounds were also tested for their efficacy against
H. meleagridis with varying outcomes. One of the explanations for these half-failures / half-successes may be the absence of reliable and reproducible in vitro
Histomonas culturing techniques.
Escherichia coli strongly supports the growth
of H. meleagridis in a monoxenic culture, without influencing its pathogenicity, [
48] (Ganas et al., 2012). Based on clonal cultures of
H. meleagridis, monoxenic cultures have, to our knowledge for the first time, been established in a liquid medium. The fecal flora was exchanged for defined bacterial strains by selective destruction of the initial bacteria with a variety of antibiotics, keeping the flagellate alive. The growth of the protozoan parasite was found to depend on the bacteria, especially on their energy metabolism.
E. coli was found to strongly support the growth of the parasite, whereas
Salmonella enterica serovar Typhimurium and
Pseudomonas aeruginosa were less efficient. Confocal laser microscopy showed that
H. meleagridis could take up green fluorescent protein-tagged
E. coli DH5α, suggesting that bacteria served as a food supply for the protozoa. By exchanging the bacterial flora for
E. coli strain DH5α in
H. meleagridis cultures that underwent continuous in vitro passages, it was possible to show that the in vivo attenuation process was independent of the bacteria. Furthermore, the gut flora in infected turkeys had no negative effect on the protozoan's virulence. Consequently, attenuation depends not on the bacteria in the culture but on the in vitro passages. Finally, the experiments provided evidence that the infection of turkeys with
H. meleagridis enabled infection of the liver with
E. coli, [
48] (Ganas et al., 2012)..
In the following study identical monoxenic settings for cultures of the same
H. meleagridis clonal strain in its virulent low passage and attenuated high passage form enabled a comparative analysis of parasite characteristics. For the first time, it could be shown that long-term in vitro cultivation led to a severe shift in cell morphology, with the occurrence of a very distinct phenotype expressing a flagellated and highly amoebic cell morphology. Furthermore, the attenuated parasites showed better growth rates and a higher tenacity when confronted with adverse conditions. During these experiments up to 100% of the parasites, both virulent and attenuated, assumed a completely rounded morphology elucidated by electron microscopy. The findings indicated that such previously reported cyst-like stages were a defense strategy of
H. meleagridis, independent of the passage level in vitro and pathogenicity
in vivo. In conclusion, long-term in vitro passaging of
H. meleagridis led not only to an attenuation of the parasite, as previously demonstrated but also to a shift in the parasite's phenotype regarding morphology, growth behavior, and a higher level of tenacity, [
49] (Gruber et al., 2017).
The option of the monogenic culturing technique opens the door to, preliminary attempts to protect birds with cultured attenuated histomonads forecasting the possibility of vaccination, (50] (Liebhart et al., 2017). Whether the special feature of the parasite's intricate interplay with bacteria in vitro and
in vivo, can be considered mutualistic or a predator-prey one is a philosophical question, [
51] (Bilic, and Hess, 2020). As for drug resistance, the first documented example of drug-resistant
H. meleagridis appeared in the literature, and the antimicrobial potential of alkaloids (harmaline and harmalol) in crude extracts of the medical plant
Peganum harmala, was tested against it, ˙[
52] (Arshad et al., 2008).
After banning all previously used prophylactic and therapeutic drugs against
H. meleagridis in the European Union, some benzimidazoles were only antiparasitic drugs, licensed for use in food-producing animals. [
33] (Hauck and, Hafez, 2009). Benzimidazoles act on beta-tubulin, and the beta-tubulin sequence allows predictions about the efficacy of benzimidazoles. When sequenced and analyzed a part of the beta-tubulin gene of five
H. meleagridis strains was found. all histomonal amino acid sequences predicted susceptibility to benzimidazoles. However, when the efficacy of five benzimidazoles, (albendazole, fenbendazole, flubendazole, mebendazole, and nocodazole), were tested on the 5
H. meleagridis strains
in vitro, all tested drugs showed no efficacy, [
38] (Hauck and, Hafez, 2009). Reduced sensitivity of
H. meleagridis to nitarsone in vitro and
in vivo. [
54] [Abraham et al., 2014). Parasites are highly prevalent in poultry; thus, the management of parasites is a key component in the profitable production of poultry. The most common nematode parasite of poultry,
Heterakis gallinarum, typically causes no direct pathology but is the vector of
Histomonas meleagridis, a highly pathogenic protozoan parasite that causes blackhead disease. There are no approved treatments for
H. meleagridis, making control reliant on controlling the helminth vector, [
55] (Collins et al., 2022).
Herein we compared the spectrum of in vitro antimicrobial activity of cell-free conditioned culture media (CFCM) from three EPB species together with testing against Leishmania donovanii, Trypanosoma cruzi, Histomonas meleagridis, and as well as against panel of clinical bacterial and fungal isolates, as positive controls. Considering the roles of the bacterial symbiont in the EPN/EPB symbiosis, we suppose that ani-protozoal compounds must be coexisting with other antimicrobial compounds in the CFCM of EPBs. Therefore for reliable bioassays, we should use appropriate controls. Two main aspects ought to be taken into consideration: (A) To be able to distinguish between the specified antiprotist activity and general cytotoxicity of the future drug-candidate antimicrobial compounds in the CFCM. (B) To be able to identify the special anti-protist compounds. In this paper, we described the very first steps toward d these goals
As for (A), one has to take into consideration that the lack of uniformity regarding the choice of cell types for cytotoxicity assays may lead to incomparable and inconclusive data. In vitro assays relying solely on non-phagocytic cell models may not represent a realistic result as the effect of an antileishmanial agent should ideally be presented based on its cytotoxicity profile against reticuloendothelial system cells. [
56] (Brioschi et al., 2022). In the
Leishmania studies, we used macrophage cell line J774A.1 [
57] (Tominaga et al., 1998), [
58] (Andreu et al., 2017), [
59]. (Geroldinger et al., 2019), as cytopathogenecity control test organism, (see Materials and Methods).
In the
Histomonas studies, we used permanent chicken liver cells (LMH (leghorn male hepatoma, LMH) cell line [
60] (Kawaguchi et al., 1987), [
61] (Amin et al., 2012), [
21] (Fodor et al., 2023), as cytopathogenecity control test-organism
, (see Materials and Methods).
As for (
B), one has to take into consideration the following points: each NR_AMP-producing EPB cell is in the so-called primary (1
0) phase, or Phase 1, [
62] (Forst and Nealson, 1996). In other terms, the phenotypic precondition for the antibiotic production (as well as for the capability of symbiosis) of the EPB species is the primary phase, [
63] (Akhurst, 1980), [
64] (Akhurst, 1982), [
65]( Boemare and Akhurst., 1994). From the aspect of NR_AMP production, it means, that in a liquid culture of the primary (1
0) cells (of each of the studied EPB species, strain, isolate) more than one biosynthetic AMPs are present at the same time. Each expresses antimicrobial (maybe antiprotist) activity, so the antimicrobial (antiprotist) activity of a CFCM sample represents a cumulative activity. But in an optimal test system, one would prefer to determine the antimicrobial activity of each of the single antimicrobial compounds one by one.
Since the discovery that the activity (that is, the „switch-on” / „switch-off” states) of those group genes (operons, biosynthetic gene complexes, BGCs) which are responsible for the primary- secondary phenotypic phase shift in of each known EPB strain, not only work under the control of the versatile regulator hexA, [
66] (Joyce and Clarke, 2003), [
67] (Langer et al., 2017) but also are coordinately regulated at a higher level (via
hfq-gene controlled sRNS/ HexA-mRNA base pairing, more precizely, the Hfq-dependent sRNA, ArcZ, directly base-pairs with the HexA-encoding mRNA, [
68] (Tobias et al., 2016), [
69]´(Tobias et al., 2017), [
70] (Neubacher, 2020)) it is possible to construct double-mutant strains from each EPB species (or isolate) following the genuine strategy called easyPACId (easy Promoter Activated Compound Identification) approach, [
71] (Bode et al., 2019), each of which biosynthesizes and releases only one single NR_AMP molecule into the culture media.
This discovery may be a starting point of the renaissance of
Xenorabdus antimicrobial peptide research, [
72] (Wenski et al., 2019), [
73] (Wenski et al., 2020), [
74] (Cimen et al., [
75] 2022), (Gulsen et al., 2022).
We also managed to reconstruct the “easyPACId”
hfq deletion mutant strains from EMA and EMC (
Boros et al., in preparation) and publish here some information ab including data about their antimicrobial potential). In this paper, we give an account of the detail of constructing our
hfq-del mutants from our EMA and EMC [
16] (Fodor et al., 2022) strains, and provide phenotypic descriptions of them. Our easyPACId strains will be available for cooperation with fellow scientists. all over the world.
4. Discussion
This paper is about the anti-protist potential of antimicrobial compounds produced by entomopathogenic bacterium species (EMA, EMC, EMK
Photorhabdus luminexscens TT01 [
15] (Lengyel et al.,2005; [
16] Fodor et al., 2022); Fodor et al.,2023, [
85] (Waterfield, 2005) the obligate symbionts of certain entomopathogenic nematodes, (EPN), into their conditioned culture media (CFCM)
in vitro, As for their chemical nature, of these antcrovials, they are non-ribosomal antimicrobial peptides, (NR-AMPs) which are enzymatically synthetized through more than one steps, in their respective biosynthetic pathways. Those genes encoding for the enzymes forming the respective biosynthetic pathway of a given NR-AMP are clustered in a single operon [
92] (Jacob et al., 1960), also coalled as biosynthetic gene complex (BGC), [
93] (Gualtieri et al., 2022), .One of the natural role of the procryiote partner in the EPN/EPB symbiosis is to protect of the given monoxenic symbitic association in a given polyxenic milieu, producing a chemical toolkit (e.g. a particular set of NR-AMPs) providing competitiveness against the prokaryotic and eukaryotic competitors, including protists. The biosynthesis of the given sets set of these effective antimicrobials are synchronized by unique, genuine hierarcchical genetic regulation mechanism called primary-secondary phase shift [
62] (Nealson and Forst, 1996) with the
hfq gene on the top, [
66,
67,
68,
69,
70]. In laboratory liquid culture conditions (as well is in the active symbiotic stage) these compounds are produced abundantly providing an unexhausstible “golden mine” to the antimicrobial searching scientist, The bioassays of the CFCMs are the tools find e best sources and optimize the culture conditions, We had found that those strains (EMA and EMC) which were discovered and further developed not only by our international team of a laboratory without wallbot in those famous laboratories as those of Maxime Guatier in France and Helge Bode in Germany proving that these two strains are probably best what have eves found so far. and other laboartories are probably really excellent sources. These modest data presented here on an anti-Leishmanial and anti-Histonal potentials are seem to confirm this conclusion. The next step is to find a way to get mutant (not “genetically manipulated”, but mutant strains which are produce only single NR_AMP molecules instead of the banch of them. Te method to do that was recently discovered in the Bode laboratory [
71,
72,
73,
74,
75] (Bode et al., 2019). We demonstrated heret hat we have strarted this project as well by constructing and characterizing hfq-del mutants from both EPB species EMA, and EMC). Our goals is to targeting to pathogens of veterinary and agricultural significance.
We have been interested in the drug potential of the NR-AMP of EMA and EMC against eukaryotic parasites and pathogens of eukaryotic (mammal and bird) hosts. We wonder whether a chemotherapeutic approach based on applying
Xenorhabdus NR-AMPs to prevent and controlling of flagellate parasitic pathogen protozoan species like the intracellular mammalian (and human) pathogenic
Leishmania donovani (Laveran & Mesnil, 1903) species complex, [
25] (Fernández-Arévalo et al., 2020) and the extracellular avian pathogenic
Histomonas meleagridis, (Smith, 1895), [
89] (Gerbod et al., 2001), are a realistic possibility. Both target species are flagellated protozoon, however,
Leishmania is intracellular, while
Histomonas is an extracellular parasite, and both own strong, unique, and efficient resistance mechanisms. The strategy for targeting antimicrobials to the prokaryotic or eukaryotic parasite is entirely different because of the similarities between the intracellular structures of eukaryotic hosts and the eukaryotic parasites (pathogens). Our research conception is that we suppose that some
Xenorhabdus NR_AMP (phenazine, fabclavine, or something else) may be capable of dysregulating mitochondrial function and stimulating apoptosis, [
90] (Rochford et al., 2020).
Leishmania ssp. is one of the most problem-causing pathogens considering its sophisticated resistance mechanisms. Still, at the same time, it is an excellent model for analyzing the mechanisms of drug resistance in eukaryotes [
39] (Segovia 1994) since 1956 when the first cases of clinical resistance have been appeared in [
91] (Ponte-Sucre et al., 2017). From an application point of view, the medical or veterinary perspectives of natural compounds are at stake several aspects have to be taken into consideration: A) the antimicrobial potential; (B) the durability, thermotolerance, and bioavailability; (C) cytotoxicity, and unwanted side effects, especially if one group of target organisms are of eukaryotic (protist) pathogens.
We have demonstrated that
Xenorhabdus CFCM has broad-spectrum antimicrobial activity against several - human microbial pathogens. However, it is unclear what the precise nature of the antimicrobial effects is. In other terms, the question is whether or if the same active molecules are responsible for the killing of each organism or just a special group of organisms. The CFCMs of all three
Xenorhabdus species proved active against
Leishmania promastigotes in our experiments, but neither of them was efficient against not
Trypanosoma cruzi epimastigotes. Other researchers [
75] (Gulsen et al., 2022) found that EMA and EMK CFCMs were equally efficient against both
Leishmania and Trypanosoma. They stated that
X. budapestensis, X. cabanillasii, X. hominickii, X. indica, X. innexii, and
X. stockiae supernatants caused 100% mortality at the highest tested centration (10%) against the promastigote form of
L. tropica. No differences occurred between this treatment group and positive control (P > 0.05). (N-methyl meglumine was used as positive control). They did not publish data on EMC in the quoted article.
The number of alternative explanations of these discrepancies between the data of the Hazír and those of our team, maybe more than one. One of the explanations may be related to the differences in the surface compositions between the two parasites that either confer resistance to the active compounds or the inactivated penetration intracellularly to act on specific molecular targets.
The identification of the antimicrobial component(s) present in the CFCMs is important to understand, as well as their mechanisms of action. Another possible alternative explanation is that the CFCM of the hfq-del mutants used in the experiments in Ankara exerted some antagonistic effects on Trypanosoma.
We decided to compare the antimicrobial potential of each of the used hfq-del mutants before using it as the source of double mutants producing single NR-AMPs. We focus only on EMA and EMC species. and Our data in Tables 35 suggest to beware of the potential basic antimicrobial activities of the hfq-del mutants because they may have unexpected antimicrobial potential. It would not be a surprise considering that several previous studies of different labs have revealed that approximately 7.5% of the total genes in Xenorhabdus bacteria are dedicated to secondary metabolite biosynthesis, and probably that majority of these products are NR-AMPs.
Calculating with about an average of 2.4Mb-size Xenorhabdus genomes, 1 kb locus-size, and about 10 ORF per operon, then we have to think about 2,400 genes and 240 respective operons (BGS) per genome. It compares with the average genome of Staphylococcus, we may count on about 225, operons. In EMA we are interested in 3, and in EMC and EMC in 12 operons only, and we cannot rule out that are several other operons encoding for enzymes to synthesize compounds with antimicrobial potential. We intend to establish and analyze RNA-seq from both the wild-type and the hfq-strains.
However, we suppose that the situation is probably somewhat simpler since the
hfq probably regulates a well-defined group of operons providing the genetic background of the so-called “primary-secondary phase shift” [
63] (Akhurst, 1980), (“phenotypic phase variation”, the term of Professor S. A. Forst) scenario. That is, only a relatively small set of natural product (NP) genes are involved, and most of the antimicrobial-synthetic genes are among them.
Toxicity studies using J774 macrophages [
57] (Tominaga, 1998), [
58] (Andreu et al., 2017) indicated that only the CFCM of EMA was able to cause significant killing of these cells. In contrast, the CFCMs of EMC or EMK had no effect. This experiment confirmed our previous unpublished observations that EMA exerts stronger cytotoxicity on a range of eukaryotic cells, which may cause limitations concerning future medical applications. But this selective cytotoxicity may provide an option to select for and find specific CFCMs against different eukaryotic parasites of different eukaryotic (animal, plant) hosts.
To put the right place the significance of our anti-leishmanial results, we have to realize that the current chemotherapies used to handle these dangerous diseases have rather limited efficacy and Sb- resistance [
94] ( Kazemi-Rad et al., 2013) has ben spreading. As for the future, the main trend may be based upon the complete genome sequencing of multiple strains, taking advantage of the application of CRISPR/Cas9 technology, and
in vivo, bioluminescence-based imaging has set the stage for advancing target-based drug discovery, [
32] (Altamura et al., 2022). But, new drugs are urgently needed anyway.
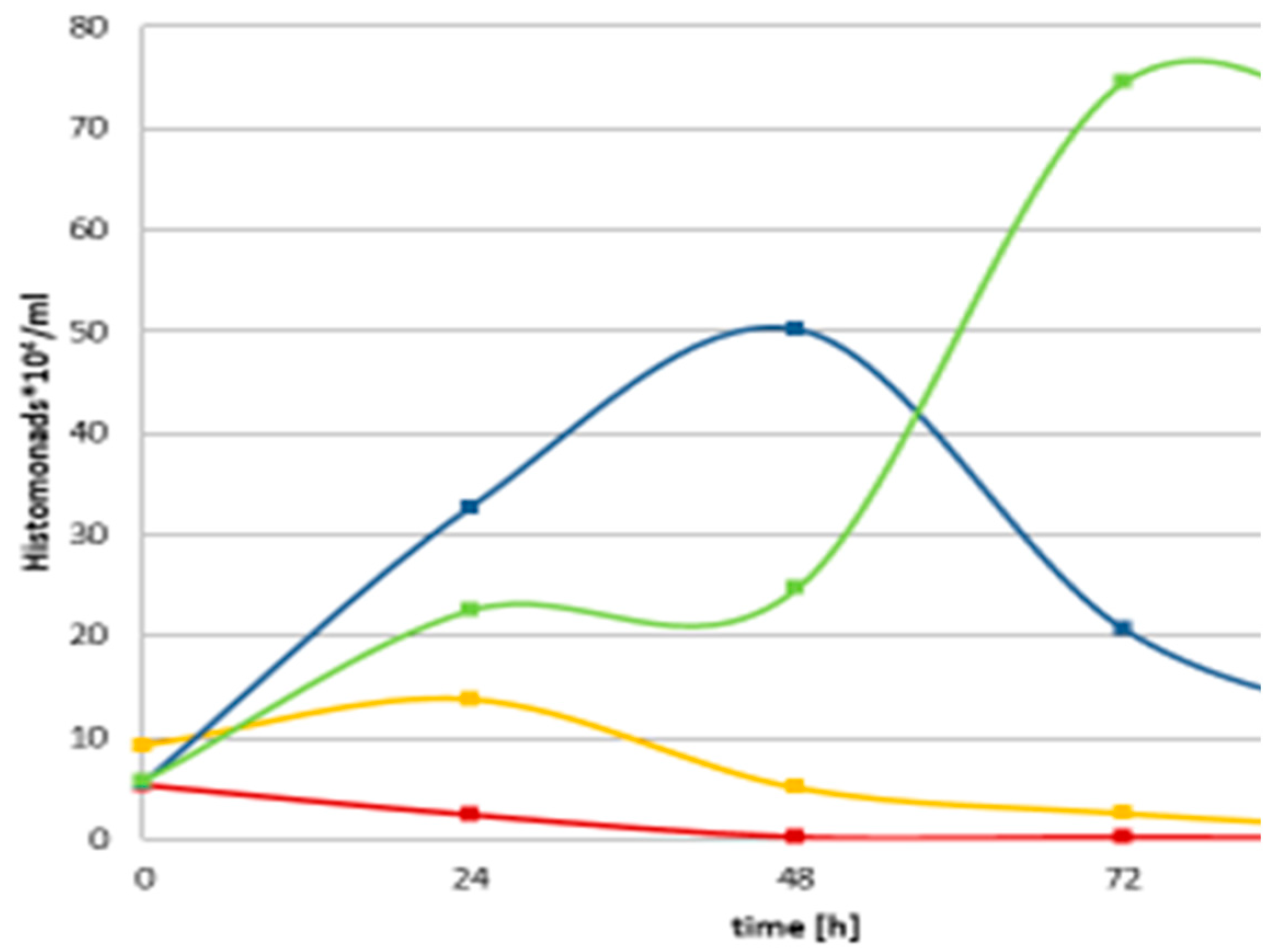
As for
Histomonas experiments, we concluded that in different degrees, but each studied EPB CFCM exerted rather strong cytopathogenic effects on the monoxenic culture of
H. meleagridis Turkey/Austria/ 2922-C6/04 cells co-cultured eukaryotic targets as well, similarly to the
Eimeria (data not shown). Considering the cytopathogenic effects were also unambiguously detected on a confluent monolayer of LMH cells (permanent chicken hepatocarcinoma liver cell line) [
21] (Fodor et al, 2023), we have to work on finding a CFCM with strong antihistomonal, and low cytotoxic activity. To do that we intended to compare the anti-histomonal, and cytotoxic potential of the wild, the hfq-delated, and different single antibiotics.-producer double mutants (hfq-del, and reactivated induced promoter-carrying single antibiotic-producer EMA and EMC strains. Considering the estimated selective cytotoxic potential of different strains we intend to use not only manganous but normal cell lines in the cytotoxicity studies. As mentioned in the Introduction, in vitro assays relying solely on non-phagocytic cell models may not represent a realistic result as the effect of an antileishmanial agent should ideally be presented based on its cytotoxicity profile against reticuloendothelial system cells. [
59]. (Brioschi et al., 2022).
As for the results in the control (bacterium, fungi and other strains), previous studies have identified several anti-microbial molecules in the CFCM of
Xenorhabdus, including fabclavine, [
12] (Fuchs et al., 2012). [
13] (Fuchs et al., 2014) a specialized metabolite polypeptide/polyketide hybrid molecule that is synthesized via non-ribosomal peptide synthesis. Many different-sized derivatives of fabclavines can be produced by the
X. szentirmaii species [
72] (Wenski et al., 2019), and can vary between entomopathogenic nematode-symbiont (
Xenorhabdus, Photorhabdus) bacterium species [
72] (Wenski et al., 2019). These have a wide-ranging anti-microbial activity against various microbes [
16] (Fodor, et al., 2022).
Multiple other antimicrobial molecules can also be produced in CFCMs, including xenofuranone A and B, [
95] (Brachmann, et al., 2006) cabanillasin [
96] (Houard, et al 2013), PAX peptides [97 (Fuchs et al., 2011), odilorhabdins, [
98] (Racine, and Gualtieri, 2019), [
93] (Lanois-Nouri et al., 2022) xenematides [
99] (Xi et al, 2019), anti-oomycete peptides [
100] (Xiao et al., 2012)¸ xenortide [
101] (Reimer, 2014), rhabdoopeptided/xenortide-like peptides, [
102] (Zhao, 2018), cyclic depsipeptide xenematide F, and G rhabdopeptides [
103] (Bi et l., 2018), szentiamids [
104] (Ohlendorf, et al., 2011), [
105] (Nollmann, et al, 2012). These latter peptides have earlier been published to be active against
Trypanosoma brucei, but we cannot confirm that.
Whether any of them, can be considered as a potential antihistomonal drug candidate will be learned if we had a chance to screen both the single mutant hfq-del stains of EMA and EMC and those double mutants (3 from EMA and 11 of EMC) in which the hfq is deleted end only one single operon is activated