Submitted:
01 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Queiroz, H. M.; Nóbrega, G. N.; Ferreira, T. O.; Almeida, L.S.; Romero, T. B.; Santaella, S. T.; Bernardino, A. F.; Otero, X. L. The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Science of the Total Environment 2018, 637, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.; de Oliveira, B. C.; Cordeiro, M. C.; Masi, B. P.; Rangel, T. P.; Paz, P.; Freitas, T.; Lopes, G.; Silva, B. S.; Cabral, A. S. Severe impacts of the Brumadinho dam failure (Minas Gerais, Brazil) on the water quality of the Paraopeba River. Science of the Total Environment 2020, 705, 135914. [Google Scholar] [CrossRef] [PubMed]
- National Mining Agency – ANM. National dam safety information system. Available on-line: https://www.gov.br/anm/pt-br. (Accessed on May 2023). (In Portuguese). 20 May.
- Porto, T. B.; Torres, A. C. A.; Gomes, R. C. Behavior of reinjectable and prestressed anchors in soil masses: construction case study in Congonhas - Brazil (Case Study). Soil & Rocks 2017 v. 40, p. 177-186. [CrossRef]
- Apaza, F. R.; Guimarães, A. C. R.; Vivoni, A. M.; Schroder, R. Evaluation of the performance of iron ore waste as potential recycled aggregate for micro-surfacing type cold asphalt mixtures. Construction and Building Materials 2021, 266-B, 121020. [CrossRef]
- Lima, L. M. K. 2006. Retroanálise da formação de um depósito de rejeitos finos de mineração construído pelo método subaéreo Master’s dissertation, Federal University of Ouro Preto, Ouro Preto, MG, Brazil (In Portuguese). Retrieved on May 15, 2023, from http://www.repositorio.ufop.br/handle/123456789/2246. 15 May.
- Fan, X.; Li, Z.; Zhang, W.; Jin, H.; Liu, J.; Xing, F.; Tang, L. New applications of municipal solid waste incineration bottom ash (MSWIBA) and calcined clay in construction: Preparation and use of an eco-friendly artificial aggregate. Construction and Building Materials 2023, 387, 131629. [Google Scholar] [CrossRef]
- Ghazali, N.; Muthusamy, K.; Abdullah, N. A.; Asri, M. I. M.; Jamaludin, N. F. A. Effect of coal bottom ash as partial sand replacement for lightweight aggregate concrete. Key Engineering Materials 2022, 912, 119–125. [Google Scholar] [CrossRef]
- Cabral, E. M.; Sá, R. J.; Vieira, R. K.; Vasconcelos, R. P. Utilization of ceramic masses in the production of synthetic calcined clay aggregate for use in concrete. Ceramics 2008, 54, 404–410. (In Portuguese) [Google Scholar] [CrossRef]
- Cabral, G. L. L. 2011. Utilização do Agregado Artificial de Argila Calcinada em Obras de Pavimentação e Aperfeiçoamento da Tecnologia. Ph.D. thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Rio de Janeiro, Brazil (In Portuguese). Received in june 13, 2023, from http://www.coc.ufrj.br/pt/teses-de-doutorado/155-2011/1251-gustavo-da-luz-lima-cabral.
- Batista, F. G. S. 2004. Caracterização física e mecanística dos agregados de argila calcinada produzidos com solos finos da BR-163/PA. Master’s dissertation, Military Institute of Engineering, Rio de Janeiro, Rio de Janeiro, Brazil (In Portuguese).
- Silva, C. L.; Frota, H. O.; Frota; C. A. Sintered calcined clay as an alternative coarse aggregate for asphalt pavement construction. Open Journal of Civil Engineering 2015, 05, 281–288. [Google Scholar] [CrossRef]
- Santos, M. G. R. 2007. Study of synthetic calcinated clay aggregates behavior for use in pavement asphalt for Manaus. Master’s dissertation, University of Brasília, Brasília/DF, Brazil (In Portuguese). Received in , 2023, from https://repositorio.unb.br/handle/10482/2919. 25 June.
- Campelo, N. S.; Campos, A. M. L. S.; Aragão, A. F. Comparative analysis of asphalt concrete mixtures employing pebbles and synthetic coarse aggregate of calcined clay in the Amazon region. International Journal of Pavement Engineering 2019, 20:5, 507-518. [CrossRef]
- Barbosa, V. H. R.; Marques, M. E. S.; Guimarães, A. C. R.; Silveira, V. L. Study of two soils from Acre to produce calcined clay aggregates and mixtures for pavement bases. In 32nd Congress of Research and Teaching in Transportation of ANPET, Gramado, Brazil, 10--14 November 2018. (In Portuguese). 14 November.
- Cabral, G. L. L. 2005. Metodologia de produção e emprego de agregados de argila calcinada para pavimentação. Master’s dissertation, Military Institute of Engineering, Rio de Janeiro, Rio de Janeiro, Brazil (In Portuguese).
- Yinfei, D.; Pusheng, L.; Jiacheng, W.; Hancheng, D.; Hao, W.; Yingtao, L. Effect of lightweight aggregate gradation on latent heat storage capacity of asphalt mixture for cooling asphalt pavement. Construction and Building Materials 2020, 250, 118849. [Google Scholar] [CrossRef]
- Khan, A.; Mrawira, D.; Hildebrand, E. E. Use of lightweight aggregate to mitigate frost damage in flexible pavements. International Journal of Pavement Engineering 2009, 10:5,329-339. [CrossRef]
- Agrawal, Y.; Gupta, T.; Sharma, R.; Panwar, N. L.; Siddique, S. A comprehensive review on the performance of structural lightweight aggregate concrete for sustainable construction. Construction Materials 2021, 1:1, 39-62. :1. [CrossRef]
- Pracidelli, S.; Melchiades, F.G. Importance of the granulometric composition of clay masses for red ceramics. Industrial Ceramics 1997, 2, 31-35. (In Portuguese).
- Department of National Roads and Highways – DNIT. Coarse synthetic aggregates of calcined clay: EM 230/94, Rio de Janeiro, 1994. (In Portuguese).
- Department of National Roads and Highways – DNIT. Aggregates in loose state - determination of bulk density: ME 152/95, Rio de Janeiro, 1995. (In Portuguese).
- Department of National Roads and Highways – DNIT. Synthetic aggregate of calcined clay - determination of mass loss after boiling: ME 225/94, Rio de Janeiro, 1994. (In Portuguese).
- Department of National Roads and Highways – DNIT. Synthetic aggregate manufactured with clay - abrasion test: ME 222/94, Rio de Janeiro, 1994. (In Portuguese).
- Department of National Roads and Highways – DNIT. Aggregates - determination of shock loss using the Treton apparatus: ME 399/99, Rio de Janeiro, 1999. (In Portuguese).
- Department of National Roads and Highways – DNIT. Soils - determination of real density: ME 093/94, Rio de Janeiro, 1994. (In Portuguese).
- Department of National Roads and Highways – DNIT. Aggregates - Determination of absorption and bulk density of coarse aggregate: ME 081/98, Rio de Janeiro, 1998. (In Portuguese).
- Ledbetter, W. B.; Moore, W. M.; Gallaway, B. M. A synthetic coarse aggregate classification system-final report. Texas Highway Department – THD. Research Report No 81-15. Synthetic Aggregate Research. Study 2-8-65-81. Coley Station: Texas Transportation Institute, 1971.
- Baucia Jr., J. A.; Koshimizu, L.; Gibertoni, C; Morelli, M. R. Study of alternative fluxes for use in porcelain formulations. Ceramics 2010, 56, 262–272. [Google Scholar] [CrossRef]
- Santos, H. S.; Kiyohara, P.; Coelho, A. C. V.; Santos, P. S. Study by electron microscopy of transformations during firing of highly alumina Brazilian clays. Ceramics 2006, 52, 125–137. [Google Scholar] [CrossRef]
- Silva, F. A. N. G. 2007. Estudos de caracterização tecnológica e beneficiamento do caulim da região Borborema-Sericó (RN). Master’s dissertation, COPPE/University of Rio de Janeiro, Rio de Janeiro, Rio de Janeiro, Brazil (In Portuguese).
- Peroni, R. Mineralogy - Study of Minerals. Department of Mining Engineering, Federal University of Rio Grande do Sul, Porto Alegre, 2003.
- National Department of Highways and Roads – DNER. Coarse Synthetic Aggregates of Calcined Clay - Use in Road Works: ES 227/89, Rio de Janeiro, 1989.
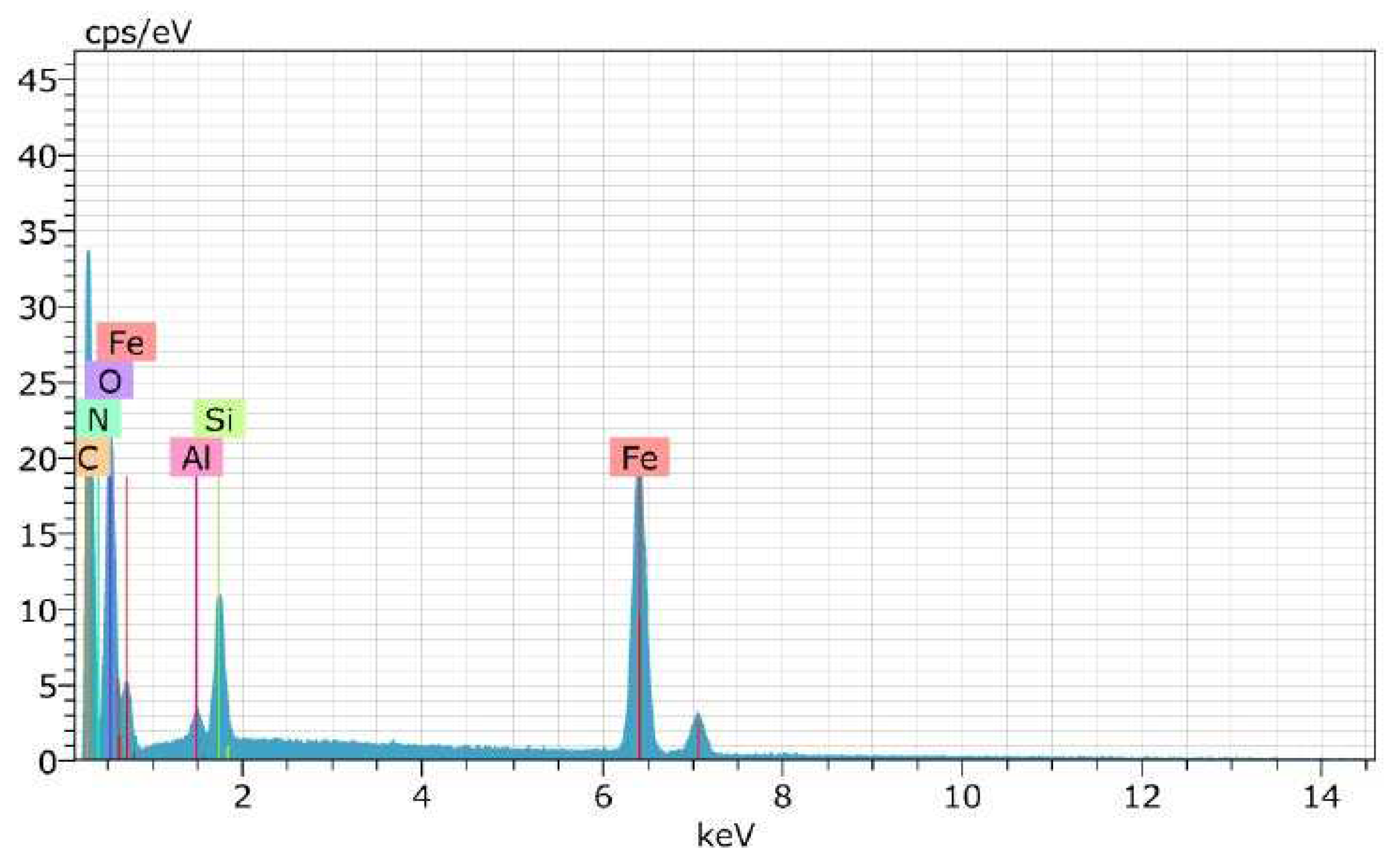
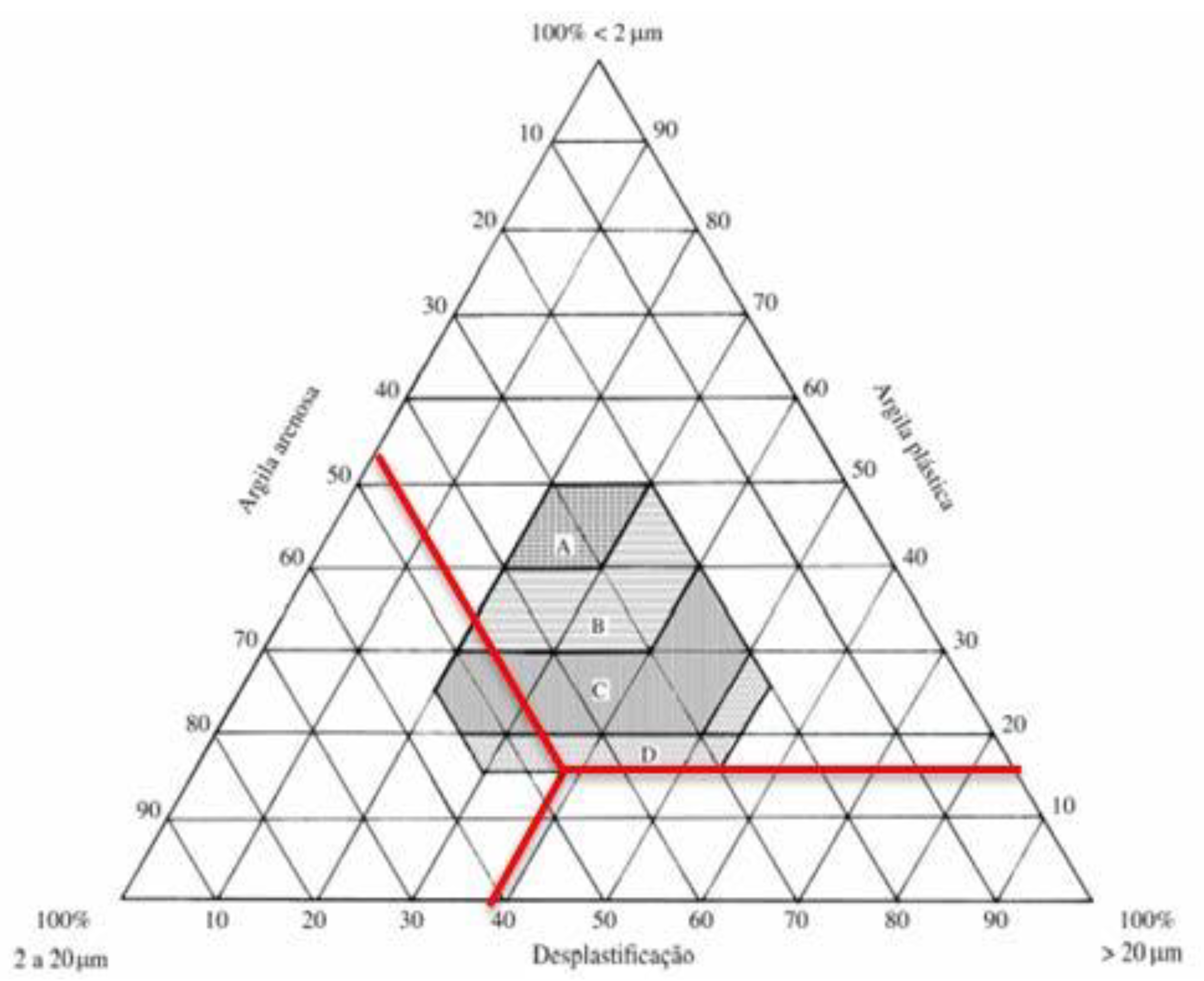
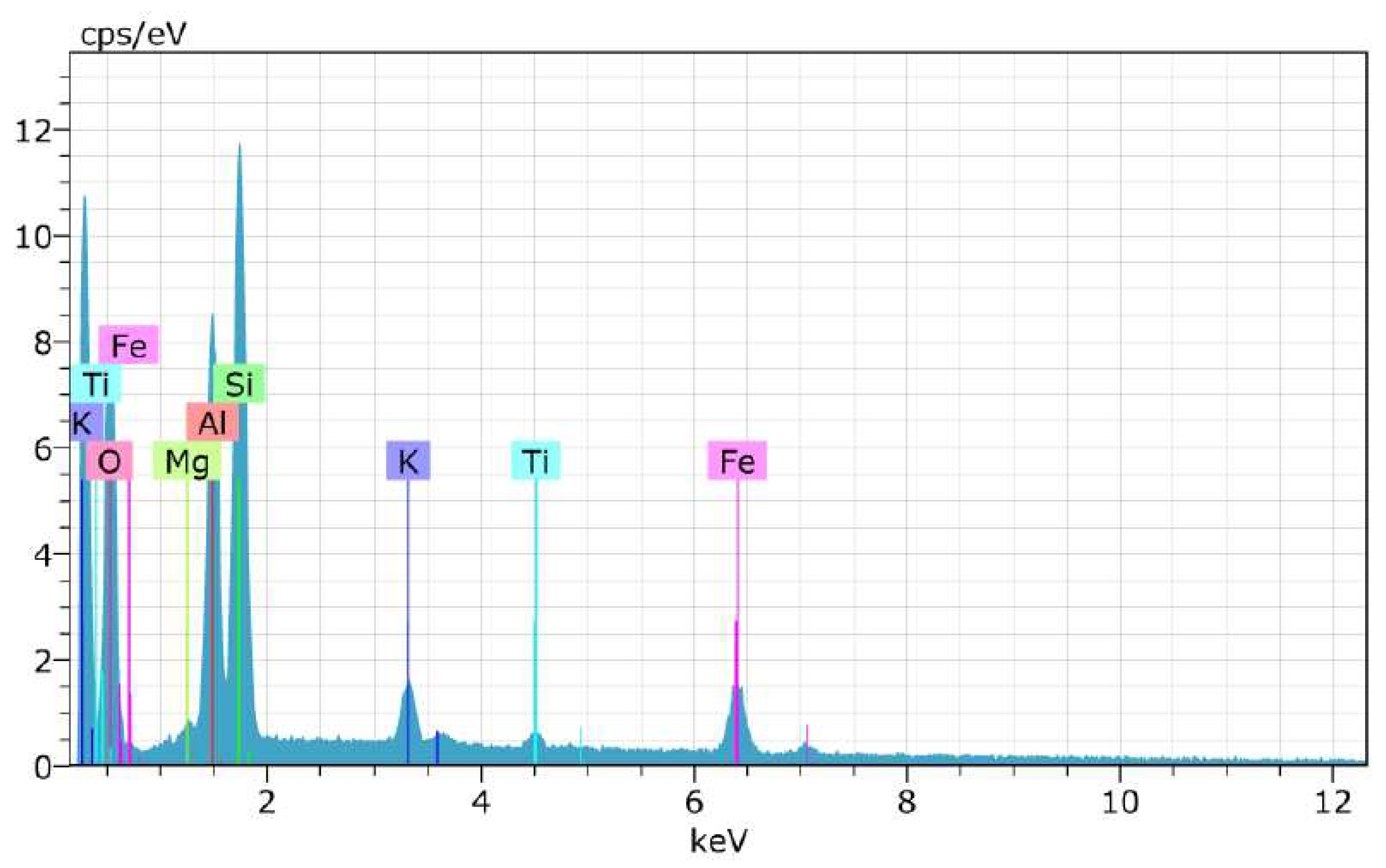
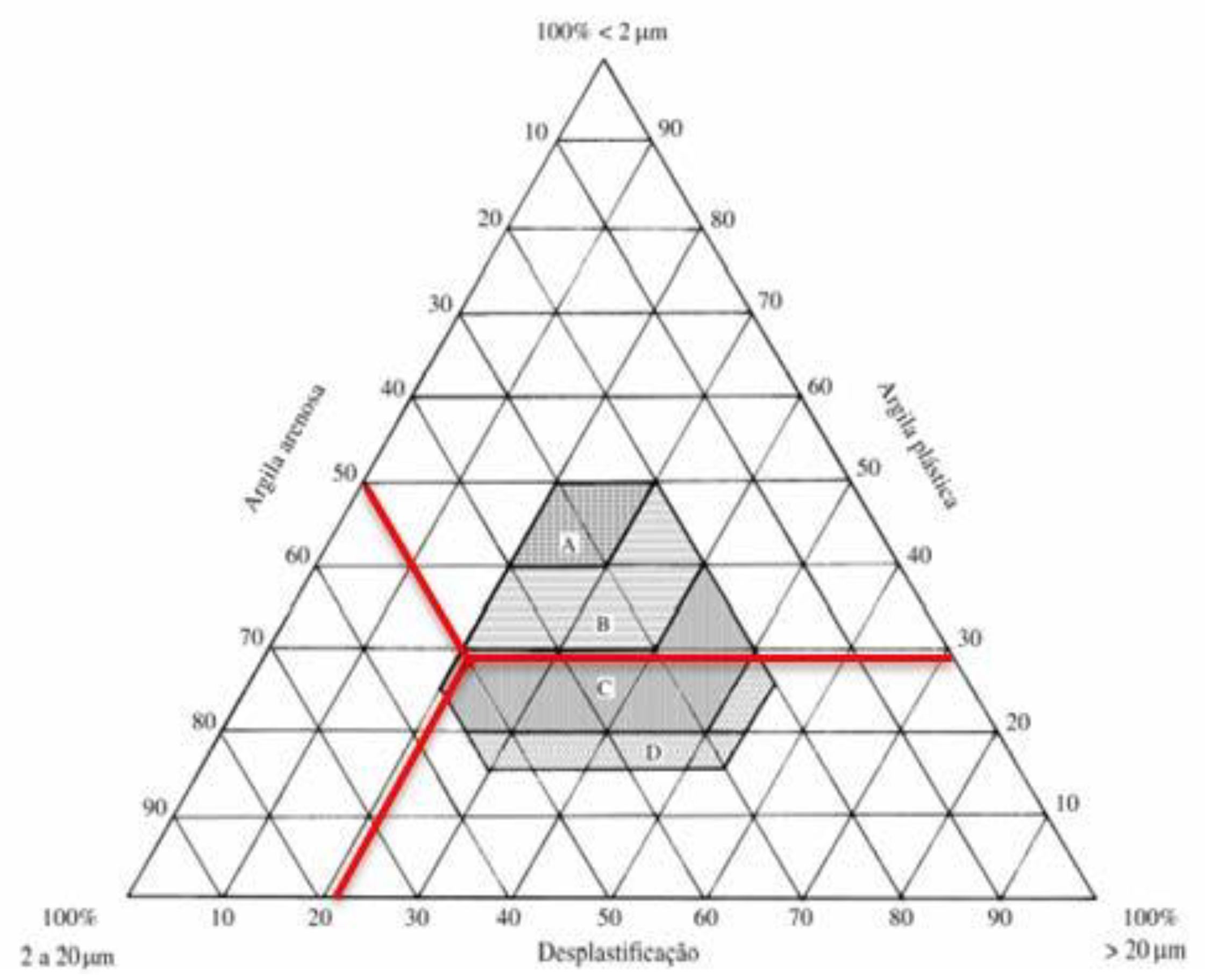



| Test | Result |
|---|---|
| Liquid Limit (%) | 20 |
| Plastic Limit (%) | 14 |
| Plasticity Index (%) | 6 |
| Real Density (g/cm3) | 3,93 |
| Test | Result |
|---|---|
| Liquid Limit (%) | 39 |
| Plastic Limit (%) | 22 |
| Plasticity Index (%) | 17 |
| Real Density (g/cm3) | 2,60 |
| Group | Sample | Present Elements | Crystal structure |
|---|---|---|---|
| A | A800 | * Quartz (Si O2) | * Hexagonal |
| * Hematite (Fe2 O3) | * Rhombohedral H. Axes | ||
| * Halloysite (OH8 Al2 Si2 O3) | * Monoclinic | ||
| * Goethite (Fe O OH) | * Orthorhombic | ||
| * Albite (Na Al Si3 O8) | * Triclinic | ||
| A900 | * Quartz (Si O2) | * Hexagonal | |
| * Hematite (Fe2 O3) | * Rhombohedral H. Axes | ||
| * Halloysite (OH4 Al2 Si2 O5) | * Hexagonal | ||
| * Orthoclase (K0,58 NaO0,42 Al Si3 O8) | * Triclinic | ||
| A1000 | * Quartz (Si O2) | * Hexagonal | |
| * Orthoclase (K Al Si3 O8) | * Monoclinic | ||
| A1100 | * Quartz (Si O2) | ||
| * Mullite (Al(Al69 Si1.220) | |||
| M | M800 | * Quartz (Si O2) | * Hexagonal |
| * Pargasitic hornblende ((Na,K)0,72 (Ca,Fe)2 (Mg,Fe,Al)5 (Si,Al)8 O22 OH2) | * Monoclinic | ||
| * Halloysite (OH8 Al2 Si2 O3) | * Hexagonal | ||
| * Microcline ((K0,95Na0,05) Al Si3 O8) | * Triclinic | ||
| M900 | * Quartz (Si O2) | * Hexagonal | |
| * Pargasitic hornblende ((Na,K)0,72 (Ca,Fe)2 (Mg,Fe,Al)5 (Si,Al)8 O22 OH2) | * Monoclinic | ||
| * Halloysite (OH8 Al2 Si2 O3) | * Monoclinic | ||
| * Orthoclase (K0,59 Ba 0,19 Na0,22 (Al1,18 Si2,82 O8) | * Monoclinic | ||
| M1000 | * Quartz (Si O2) | * Hexagonal | |
| * Pargasitic hornblende ((Na,K)0,72 (Ca,Fe)2 (Mg,Fe,Al)5 (Si,Al)8 O22 OH2) | * Monoclinic | ||
| * Hematite (Fe2 O3) | * Rhombohedral H. Axes | ||
| * Orthoclase (K0,59 Ba 0,19 Na0,22 (Al1,18 Si2,82 O8) | * Monoclinic | ||
| M1100 | * Quartz (Si O2) | ||
| * Mullite (Al (Al69 Si1.220) |
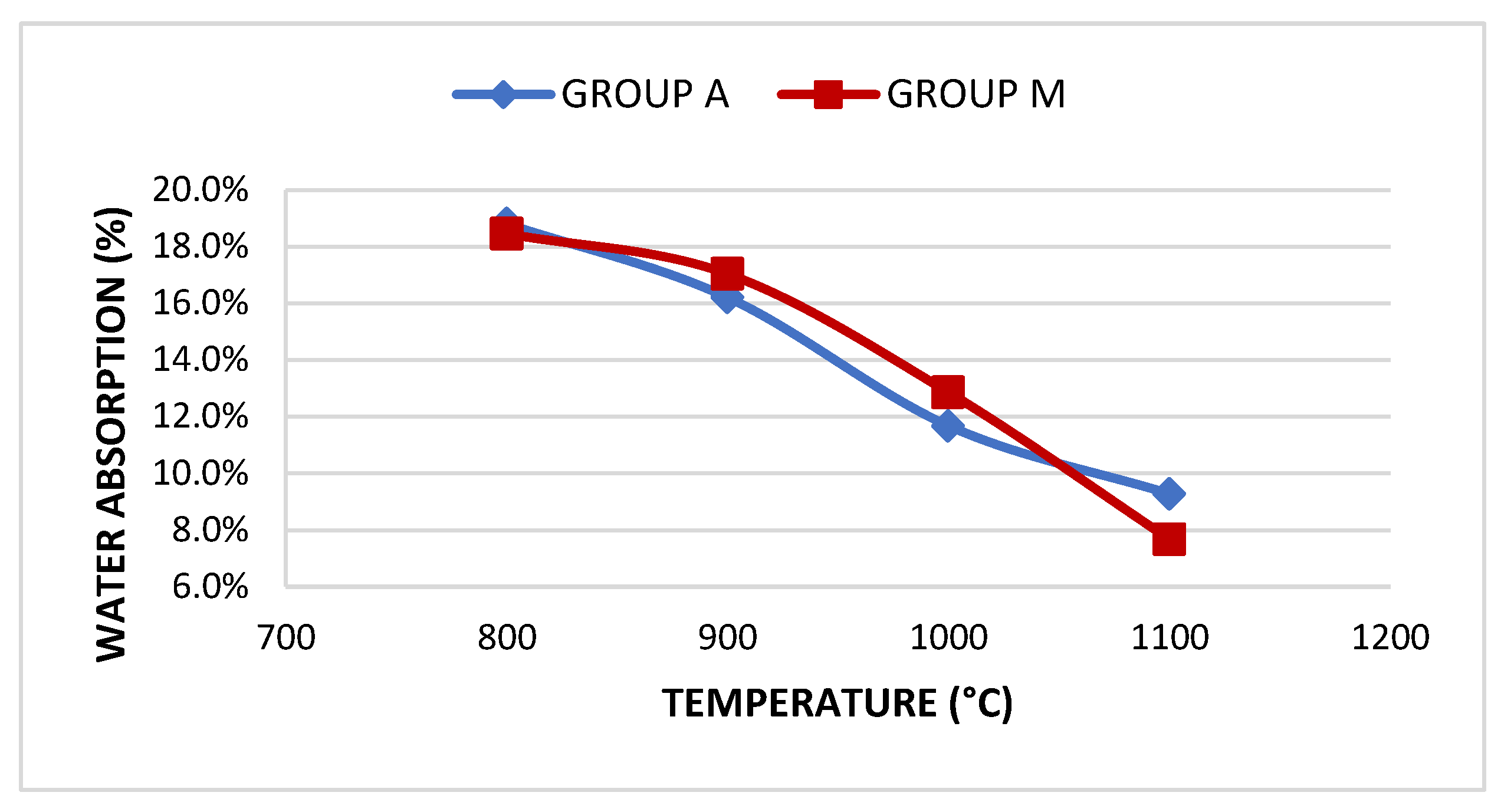
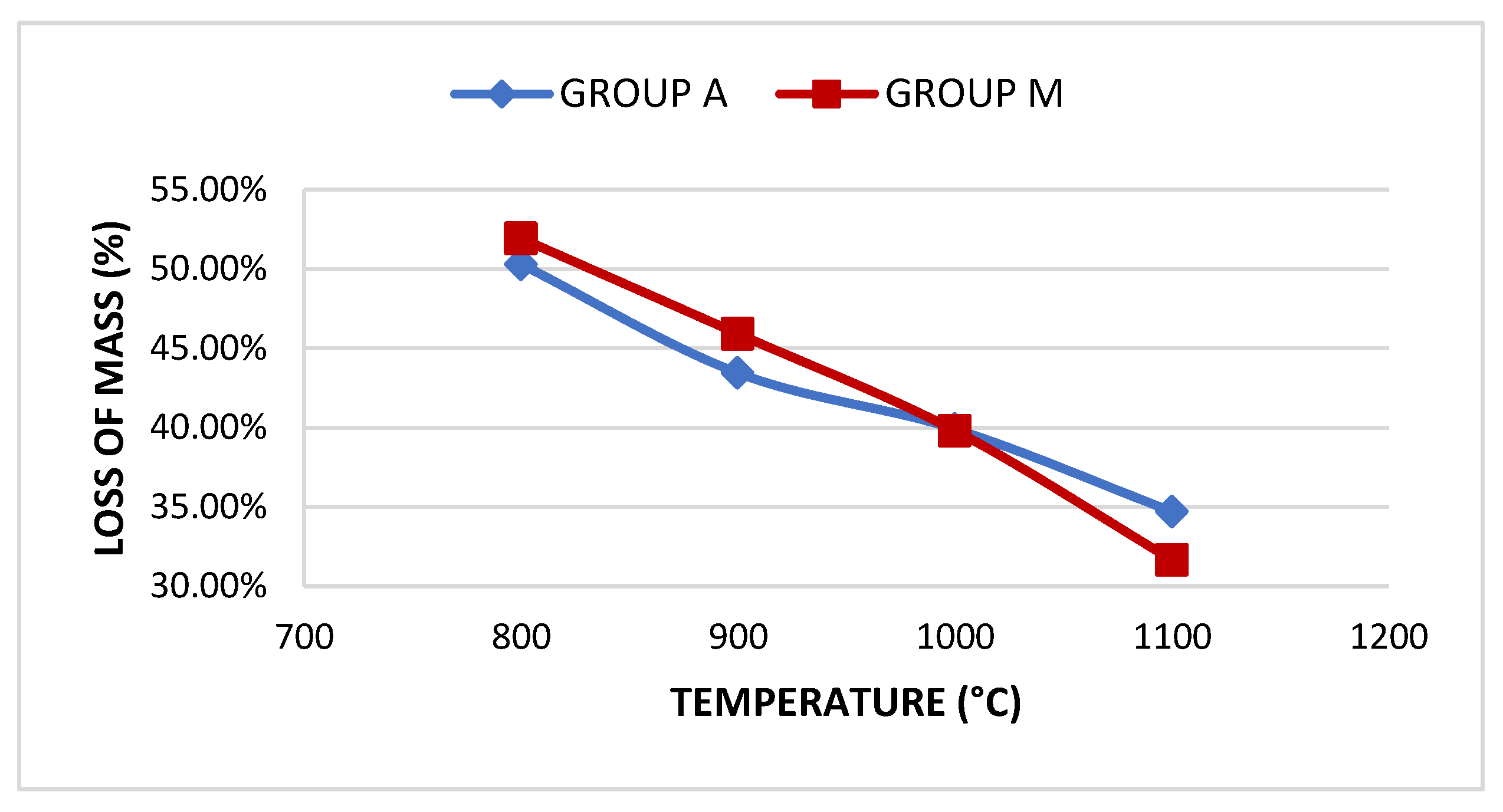
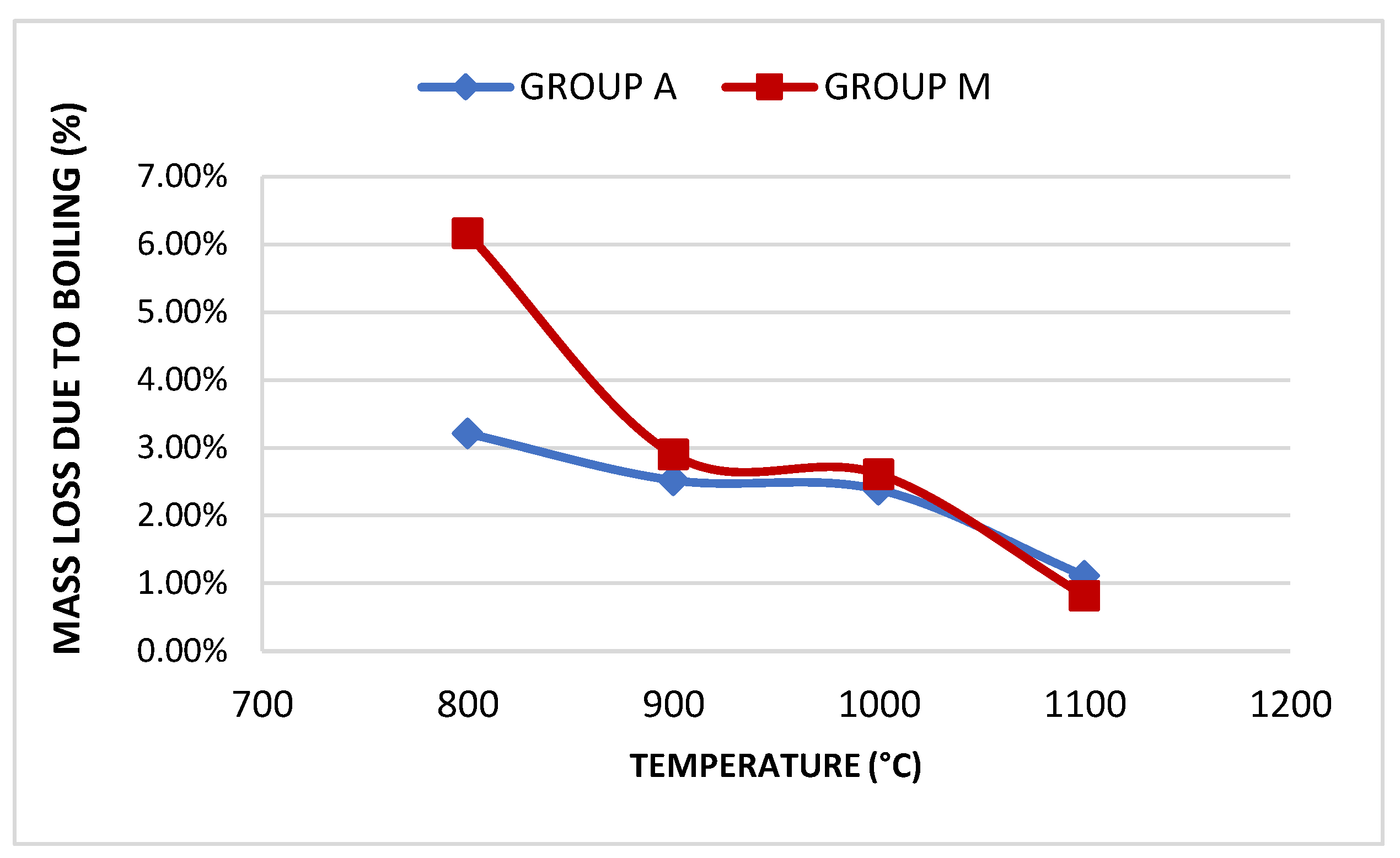
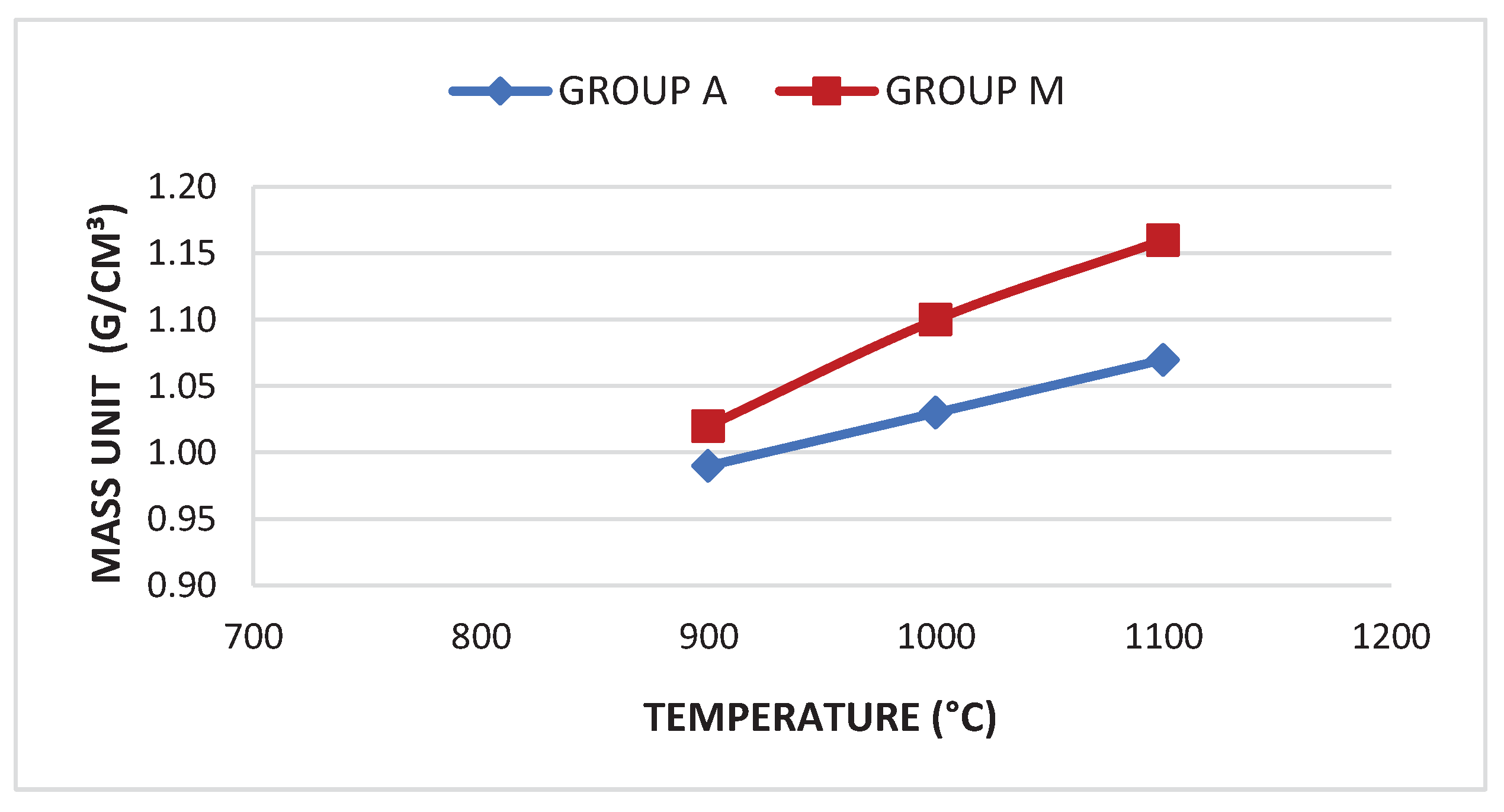
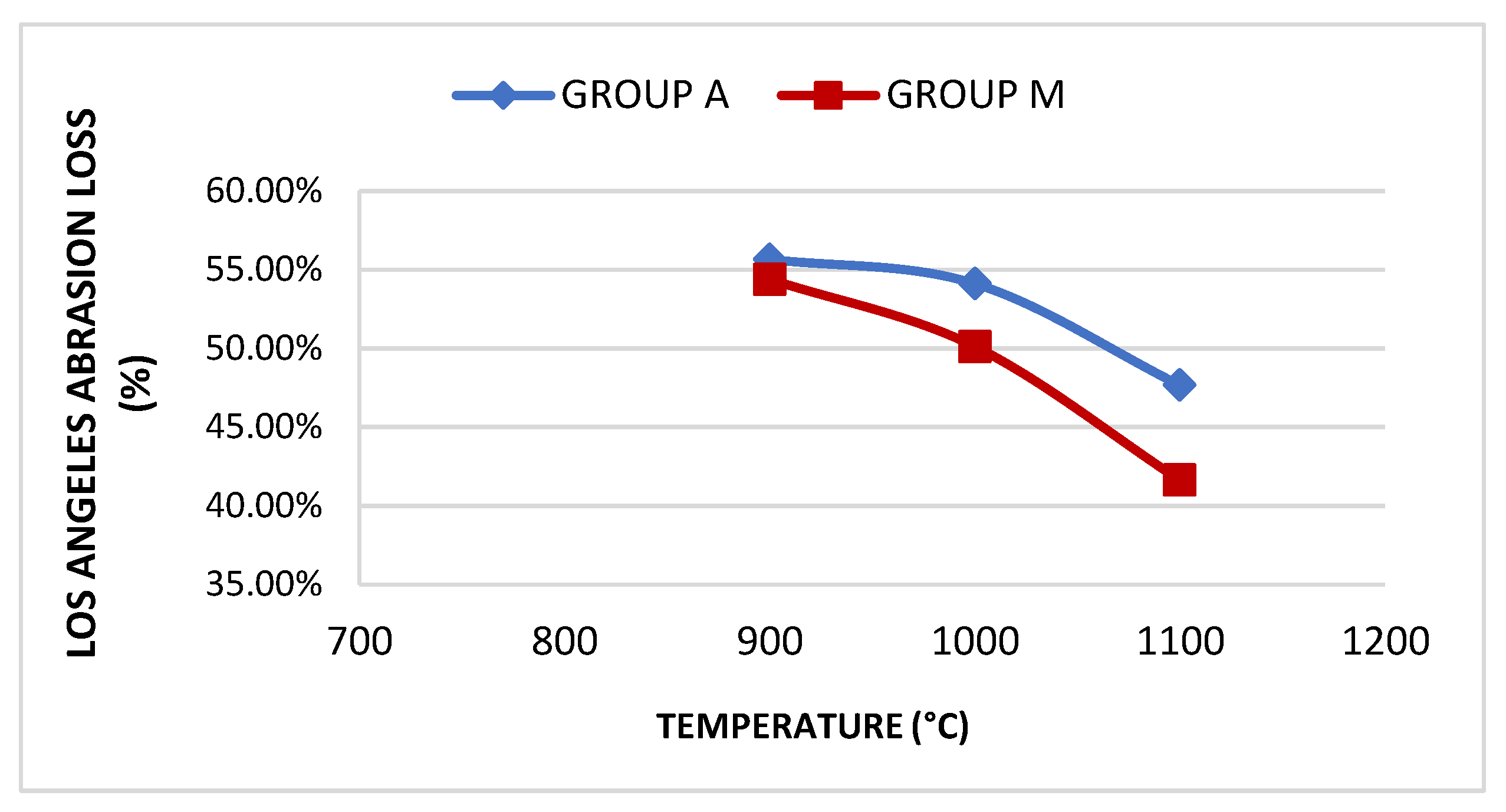
| Group | Sample | Absorption | Treton shock loss | Boiling Loss | Mass Unit. (g/cm³) | Los Angeles Abrasion (LA) Wear | |||
|---|---|---|---|---|---|---|---|---|---|
| Res. | Standard | Res. | Standard | Res. | Standard | ||||
| A | A800 | 18.81% | 50.3% | 3.21% | <10% | - | - | - | - |
| A900 | 16.21% | 43.44% | 2.52% | <10% | 0.99 | >0.88 | 55.66% | <45% | |
| A1000 | 11.68% | 39.91% | 2.38% | <10% | 1.03 | >0.88 | 54.11% | <45% | |
| A1100 | 9.28% | 34.71% | 1.1% | <10% | 1.07 | >0.88 | 47.66% | <45% | |
| M | M800 | 18.46% | 51.93% | 6.16% | <10% | - | - | - | |
| M900 | 17.04% | 45.91% | 2.9% | <10% | 1.02 | >0.88 | 54.4% | <45% | |
| M1000 | 12.86% | 39.81% | 2.61% | <10% | 1.1 | >0.88 | 50.12% | <45% | |
| M1100 | 7.68% | 31.67% | 0.82% | <10% | 1.16 | >0.88 | 41.63% | <45% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





