1. Introduction
Vaccinations are the most effective approach to preventing the spread of infectious diseases. The influence of vaccination on the healthcare system is significant, as it reduces the expenses associated with treating infectious diseases. Vaccines have been instrumental in averting, managing, and even eliminating numerous illnesses globally. The vaccine work by stimulating the body's immune system to produce antibodies against specific pathogens, and protect individuals from getting sick or help to reduce the spread of diseases in communities (Stanley et al., 2021). Vaccination campaigns have been used to prevent, control, and eradicate diseases like smallpox, polio, measles, human papillomavirus (HPV), many other bacterial disease, and COVID-19 (Stanley et al., 2014; Stanley et al., 2021).
Smallpox, a contagious disease that is caused by the variola virus and it was transmitted through respiratory droplets and close contact. The disease had a high mortality rate, and survivors were often left with permanent scars or blindness. In 1967, the World Health Organization (WHO) launched an intensive global smallpox eradication campaign, aiming to wipe out the disease within a decade (Mascola and Anthony, 2020). The smallpox vaccine, developed in the late 18th century by Edward Jenner, is a live virus vaccine that uses the related cowpox virus to stimulate immunity against smallpox. Vaccination played a crucial component in the smallpox eradication campaign, in reducing the transmission and impact of the disease. During the eradication campaign, vaccination was used as a preventive measure, actively searching for cases of smallpox and vaccinating individuals who had come into contact with infected individuals or who were at high risk of exposure (Stanley and Neal, 2021). This approach, known as ring vaccination, involved creating a protective ring of vaccinated individuals around each case of smallpox, thereby containing the spread of the disease which finally resulted in the eradication.
Polio was once a devastating disease that paralyzed thousands of children every year, but as a result of widespread vaccination efforts, the number of polio cases has decreased by more than 99% since 1988, and the disease has been eradicated in most countries (Paula et al., 2021). Similarly, a highly contagious measles viral disease that can cause severe health complications including pneumonia and brain damage have a vaccine since the 1960s and widespread vaccination efforts have led to a 73% decrease in measles deaths globally between 2000 and 2018 (Mascola and Anthony, 2020). Conversely, human papillomavirus (HPV) is a prevalent sexually transmitted infection that has been linked to the development of several types of cancer, such as cervical, anal, and throat cancers (Stanley et al., 2014). Vaccines against HPV have been available since 2006 and be highly effective in preventing HPV infections and related cancers.
The COVID-19 pandemic of the recent past has had a profound impact on the global health landscape, economies, and societies at large. The emergence of efficacious vaccines against the SARS-CoV-2 virus, which is responsible for causing COVID-19, represents a significant milestone in the ongoing efforts to curb the spread of the pandemic (Paula et al., 2021; Yingxiang et al., 2021). During the pandemic, vaccination efforts have been shown to reduce hospitalizations, severe illness, and death from COVID-19 (Xu et al., 2021; August et al., 2022).
The need for new vaccine development arises as new and emerging infectious diseases continually pose a threat to global health, as demonstrated by recent outbreaks of Ebola, Zika, and COVID-19 (Maruggi et al., 2019; Kumar et el., 2022). These diseases can spread rapidly and have significant morbidity and mortality, stressing the need for effective vaccines to prevent their transmission and reduce their impact. On the other hand, existing vaccines may have limitations in terms of their effectiveness, safety, and accessibility (John and Anthony, 2020; Knezevic et al., 2021; Rosa et al., 2021). Some vaccines may not provide long-term immunity or may be less effective against certain strains of a pathogen. similarly, some vaccines may be associated with adverse effects, and access to vaccines may be limited in low-resource settings (Nitesh et al., 2021; Niqash et al., 2021). In addition, advances in technology, the need to avail effective vaccines rapidly and current ways of understanding pathogen molecular structure and possible manipulations are driving the development of new and innovative vaccines (Nitesh et al., 2021; Poula et al., 2021). Approaches such as mRNA vaccines and viral vector vaccines offer advantages in terms of their speed of development, scalability, and potential for inducing strong and long-lasting immune responses (Ali et al., 2021; Xu et al., 2020). Thus, the need for new vaccine development is driven by the ongoing threat of infectious diseases, limitations of existing vaccines, advances in technology, and the imperative to prepare for potential bioterrorism events.
2. Approaches in Vaccine Development
2.1. The Path of Vaccine Development
There are several types of approaches to developing vaccines, each with its advantages and limitations. For example, live attenuated vaccines use live viruses or bacteria that have been weakened so that they cannot cause disease in humans. They stimulate a strong and long-lasting immune response, but can be unsafe for people with weakened immune systems (Stanley et al., 2014; Pardi et al., 2018; John et al., 2020). On the other hand, inactivated or killed vaccines use viruses or bacteria that have been killed or inactivated, so they cannot cause disease. Although they are considered safe for individuals with compromised immune systems, in comparison to live attenuated vaccines, they may not elicit as robust of an immune response (Pardi et al., 2018). In the other manner; subunit, recombinant, or conjugate type of vaccines use specific proteins or parts of the pathogen to stimulate an immune response. These types of vaccines are often safer than live attenuated vaccines but may require booster shots to maintain a protective immunity level (Stanley et al., 2014; Stanly et al., 2021).
Another approach called vector vaccines uses viruses or bacteria as a vector to carry a specific antigen from the pathogen of interest. This type of approaches is often used for diseases that are difficult to prevent with other vaccine types. Inversely, the new promising vaccine development approach called virus-like particle (VLP) vaccines use non-infectious virus-like particles that mimic the structure of the virus but do not contain the genetic material necessary for replication and pathogenesis but can initiate an immune response (Nitesh et al., 2021). The nucleic acid vaccine development approach, which utilizes genetic material such as DNA or RNA to elicit an immune response, represents a more contemporary and sophisticated technological advancement. They are relatively new and have been developed for diseases such as COVID-19 (Liu et al., 2019; Knezevic et al., 2021; Barbier et al., 2022).
2.2. Recent Advances in Vaccine Development
Recent advanced vaccine development and manufacturing technologies have revolutionized the way vaccines are developed, tested, and produced. For example, recombinant DNA technology involves the manipulation of genes to create proteins that can be used as vaccines. This technology has been utilized to develop vaccines for various diseases, including hepatitis B, human papillomavirus (HPV), and others (Ali et al., 2021). Adopting a comparable methodology, the technology of virus-like particles entails the fabrication of non-infectious particles that imitate the structural characteristics of a virus. VLPs can be used as vaccines to stimulate an immune response without causing disease. This type of vaccine development technology has been used to develop vaccines against HPV and hepatitis B (Ali et al., 2021; Yingxiang et al., 2021). The latest and groundbreaking technology in vaccine development and production is the mRNA vaccine technology, which utilizes genetic material from the virus to elicit an immune response. This technology has been used to develop vaccines against COVID-19 and is being explored for use against other infectious and non-infectious diseases (Pardi et al., 2018; Pardi et al., 2020).
3. mRNA Vaccines
The messenger RNA (ribonucleic acid) vaccine development is based on DNA and uses the gene in the cell as a template, and after being transcribed and generated based on the principle of complementary base pairing (August
et al., 2022). This describes the presence of base sequences in DNA molecules that correspond to functional fragments. These fragments can serve as a direct template for protein biosynthesis. While mRNA only accounts for a small percentage of total RNA, it is highly diverse and metabolized rapidly, with a very short half-life. In fact, it can break down within minutes of synthesis (Jackson
et al., 2020). The mRNA that encodes the disease-specific antigen is delivered to the body, where it is used by antigen-presenting cells to synthesize the antigen. This antigen is then expressed and recognized by the immune system, ultimately leading to the prevention and treatment of the disease.
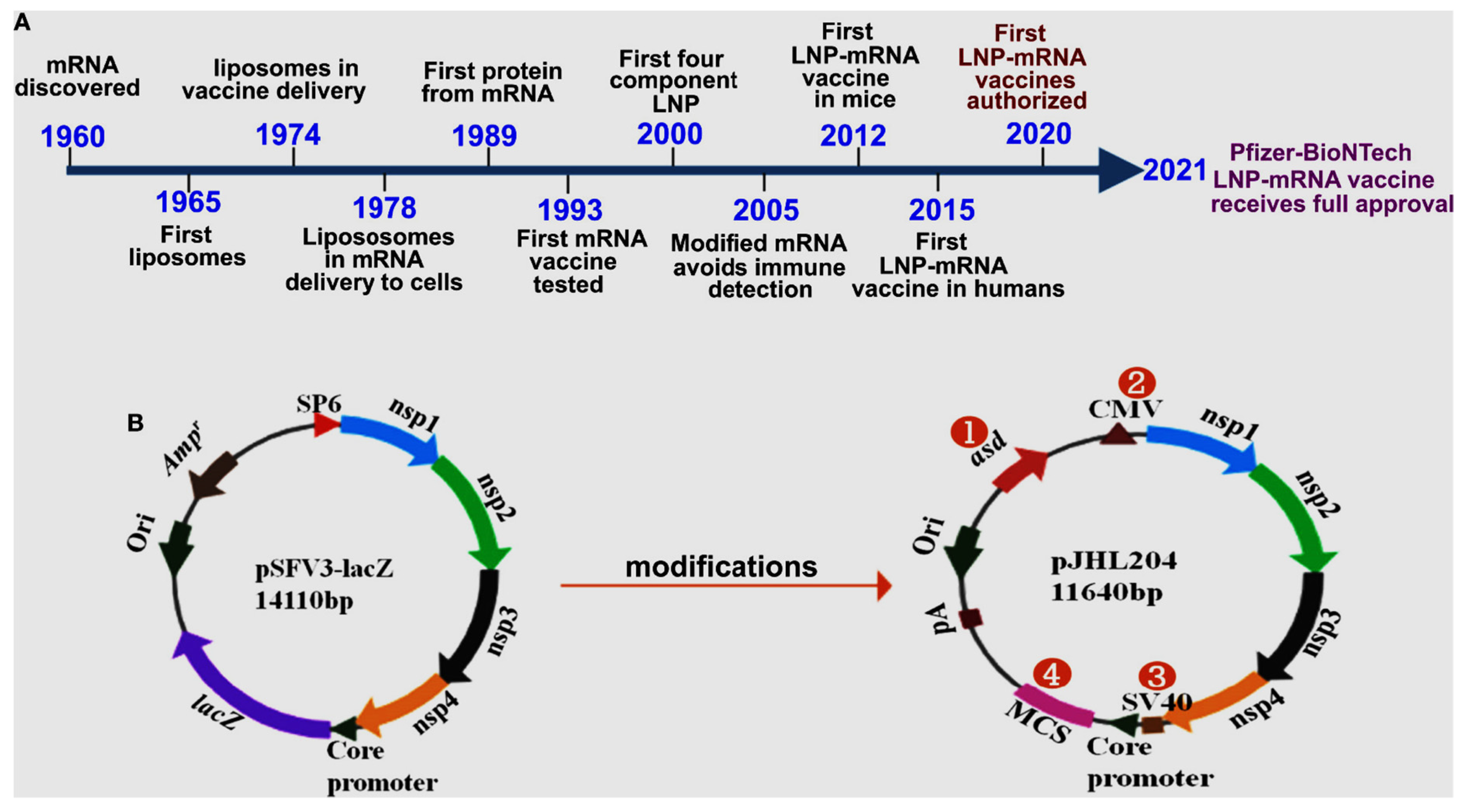
Figure 1 below illustrates advances in mRNA vaccine development.
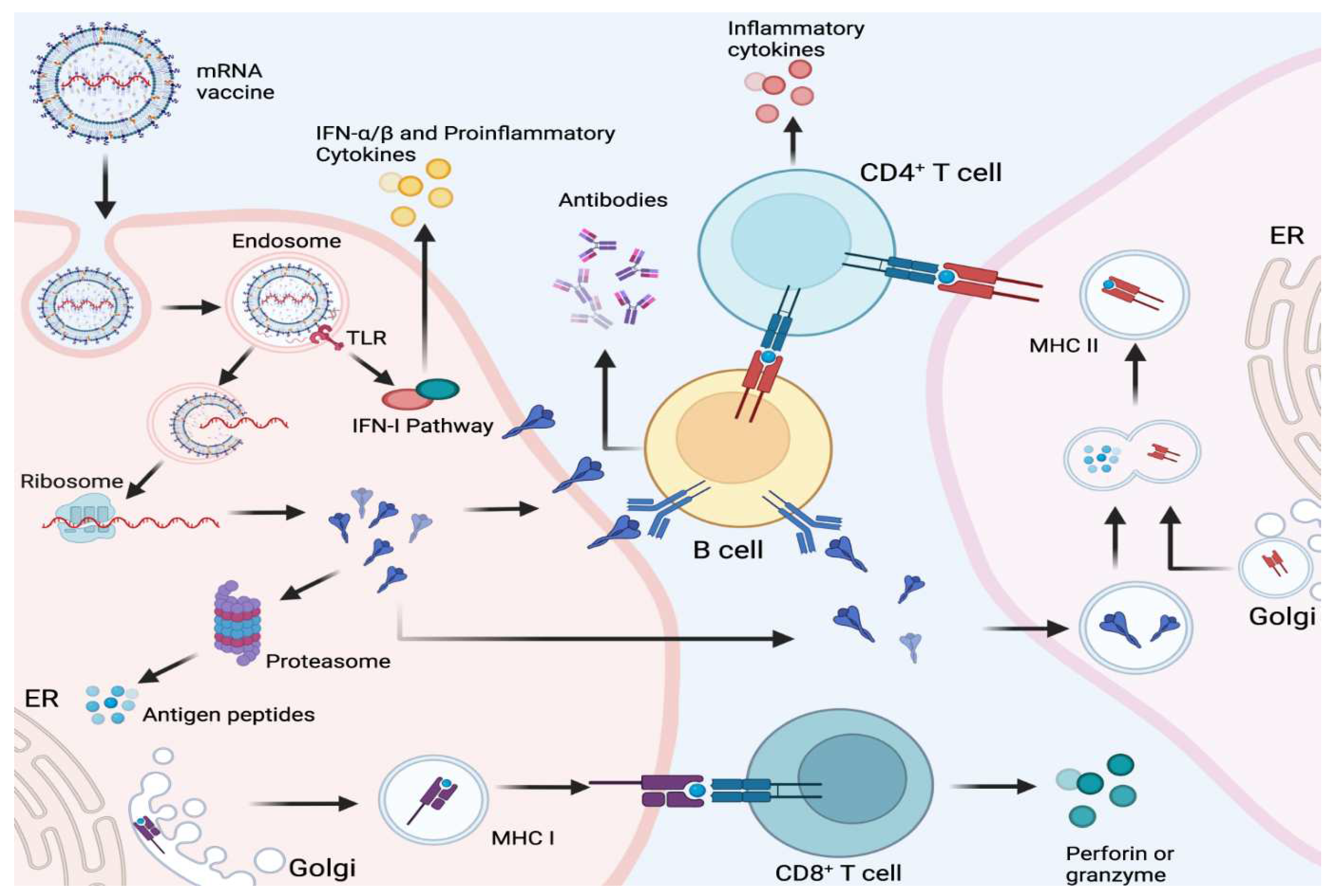
When a person receives an mRNA vaccine, the lipid nanoparticles containing the mRNA are injected into the muscle. Once inside the cells, the mRNA instructs the cells to produce the viral or bacterial protein. This protein is then displayed on the surface of the cell, triggering an immune response. The immune system recognizes the displayed protein as foreign and mounts a defense by producing antibodies and activating immune cells to target and eliminate the protein. These immune responses create a memory of the protein, enabling the immune system to quickly respond if it encounters the actual virus or bacteria in the future (Zeng et al., 2020).
3.1. Approaches in mRNA Vaccine Development
3.1.1. mRNA Structure and Vaccine Development Target
The process of translating the genetic sequence of DNA into proteins by ribosomes in the cytoplasm of cells is facilitated by mRNA molecules. Currently, two types of mRNAs, namely non-replicating and self-amplifying, are being investigated as potential vaccines for antigens. Conventional, non-replicating mRNA-based vaccines encode antigens for the immunogenic reaction, which contain the 50 and 30 untranslated regions (UTRs) and open reading frame (ORF). Conversely, self-amplifying mRNA contains components with an additional coding region in their ORF, which codes for viral replication machinery, enabling continuous intracellular RNA amplification followed by amplified antigen expression (Brito et al., 2015; Gote et al., 2023). The 50 end of the mRNA contains a 7-methylguanosine moiety, trailed by a triphosphate moiety to the first nucleotide (m7GpppN), which is a protective structure known to safeguard RNA from exonuclease cleavage, regulate pre-mRNA splicing, and initiate mRNA translation and nuclear export (Ramanathan et al., 2016). The 50 cap is also crucial in the recognition of non-self mRNA or exogenous mRNA from self mRNA or the endogenous mRNA by the innate immune system (Daffis et al., 2010).
In order to enhance the efficacy and stability of mRNA, post-transcriptional modifications can be introduced to its structure. Modification of the 50-cap structure not only improves mRNA translation efficiency, but also prevents the activation of endosomal and cytosolic receptors, such as RIG-I and MDA5, which act as defensive mechanisms against viral mRNA (Daffis et al., 2010). Therefore, the 20-O-methylation of the 50-cap structure is highly desirable in increasing protein production from mRNA following transcription and blocking any undesirable immune responses from the host immune system. Co-transcriptional reactions can be utilized to create Cap 1 analogs, such as m7GpppNm containing any nucleotide with a 20 O-methylation and trinucleotide cap analogs. The use of m7GpppAG analogs for capping IVT mRNA has been shown to permit mRNA to have the m7G moiety at the 50-ends with no reverse-capped 50 end mRNA products (Sikorski et al., 2020; Zeng et al., 2020). The m7Gpppm6AmG cap has been found to result in the maximum luciferase expression in vitro transfection. The effects of changing transcribed nucleotide with or without the 20-O-methylation in mRNA IVT reaction have also been studied (Pardi et al., 2015). The 50-capping structure is crucial for efficiently targeting dendritic cells in generating a desired immune response, as mRNA translation in a dendritic cell (DC) has an 8-fold value difference between m6A and m6Am 50-caps (Sikorski et al., 2020).
3.1.2. Modified Nucleotides
During the process of post-transcriptional modification of natural mRNA molecules, which consist of ATP, CTP, GTP, and UTP as the four fundamental nucleotides, specific nucleotides undergo modification, such as pseudouridine and 5-methylcytidine, which can be utilized in the in vitro transcription (IVT) of mRNA (Kariko et al., 2007). Modified nucleotides offer advantages as they can prevent the recognition of IVT mRNA by the innate immune system, thereby avoiding undesirable immune responses and enhancing the translation efficiency of mRNA to the desired antigen (Anderson et al., 2010). A recent study revealed that mRNA containing the N(1)-methyl-pseudo-uridine modification outperformed the pseudo-uridine modified mRNA platform by providing 44-fold higher and 13-fold higher reporter gene expression upon transfection into cell lines or mice, respectively (Firdessa et al., 2020). This report demonstrated that (m5C/) modified mRNA resulted in a reduction in intracellular innate immunogenicity upon in vitro transfection. The modification results in controlled activation of the toll-like receptor 3 (TLR3) and initiates the downregulation of innate immune signaling, which is a desired characteristic of an mRNA vaccine (Cullis et al., 2017).
3.2. The Mechanism by which mRNA Vaccines Work
To develop an mRNA vaccine, scientists first need to understand the structure of the virus and its genetic material. Once proteins have been identified, designing a small piece of mRNA that codes for that protein will follow. The mRNA is essentially a set of instructions that tells the body's cells how to produce the protein. The mRNA molecule is fragile and can be quickly degraded by the body's enzymes. To protect the mRNA and ensure it gets into cells, scientists encapsulate it in a lipid nanoparticle. The lipid nanoparticle is essentially a tiny ball of fat that acts as a protective coating around the mRNA (Chaudhary
et al., 2021). The vaccine is then injected into the patient's arm, usually in the form of two doses given several weeks apart. Once injected, the lipid nanoparticle delivers the mRNA to cells in the body. Immunological action can be illustrated in below
Figure 2 (Source: Fang
et al., 2022).
The identification of single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA) by immune cells within the host is aided by a range of innate receptors located in both the endosomal and cytosolic compartments. These receptors are essential components of the human innate immune response to foreign antigens. The Toll-like receptor immune cells, TLR3 and TLR7, have the ability to bind to exogenous ssRNA within the endosome. Conversely, inflammation signaling receptors, including RIG-I, MDA5, NOD2, and PKR, bind to both ssRNA and dsRNA within the cytosol (Chaudhary et al., 2021; Fang et al., 2022). This binding process results in cellular activation and the production of type I interferon, which inhibits cellular translation and reduces the amount of antigen produced by mRNA vaccines. The current mRNA vaccines available in the market contain purified IVT mRNA, which is single-stranded and contains modified nucleotides (Firdessa et al., 2020). The aforementioned alteration results in a decrease in the affinity towards TLR3 and TLR7, along with immune sensors, thereby restricting the overproduction of type I interferon and its hindrance on the cellular translation of mRNA (Raeven et al., 209). mRNA vaccines possess the ability to transfect immune cells that are present in the tissue, including antigen-presenting cells like dendritic cells and macrophages (Lindsay et al., 2019).
The mRNA vaccines can be developed and manufactured more quickly than traditional vaccines, as the mRNA sequence can be synthesized in a lab using readily available materials. Additionally, mRNA vaccines do not contain live viruses which means there is no risk of getting the disease from the vaccine. The mRNA vaccines can also be easily modified to target new strains of a virus or even different viruses altogether. However, mRNA vaccines are relatively new, and long-term safety data is still being collected. Like all vaccines, mRNA vaccines can cause side effects, including fever, fatigue, and soreness at the injection site (Gote et al., 2023).
3.3. Unique Characteristics of mRNA Vaccines
In late 2020, the mRNA vaccines based on SARS COV-2 have been approved for emergency use to protect against COVID-19 disease in many countries, and there has been a push to expand their use to other diseases as well. As an example, Moderna is developing mRNA vaccines for influenza and cytomegalovirus, and BioNTech is working on mRNA vaccines for multiple sclerosis and cancer (Zhang et al., 2020). The effectiveness of mRNA vaccines against emerging variants of COVID-19 has been a concern, leading to the development of booster shots. These booster shots contain a modified version of the original vaccine that targets the new variants and currently Pfizer-BioNTech and Moderna have both developed booster shots for COVID-19 (Buschmann et al., 2021; Bernal et al., 2021).
The possibilities of combining mRNA vaccines with other types of vaccines to create "multivalent" vaccines that protect against multiple diseases. For example, the mRNA vaccine for COVID-19 could be safely and effectively administered alongside the influenza vaccine (Walsh et al., 2020). One of the challenges of mRNA vaccines is that they need to be kept at very low temperatures to remain stable. However, recent advances in storage and distribution technology have made it easier to transport and store mRNA vaccines. For example, Moderna's COVID-19 vaccine can now be stored at normal freezer temperatures, making it easier to distribute in areas with limited refrigeration capacity (Schoenmaker et al., 2021). Thus, the success of mRNA vaccines in protecting against COVID-19 has generated significant interest and investment in this technology, and we will likely see further advances and applications shortly soon.
3.4. Manufacturing of mRNA Vaccine
The mRNA vaccines have demonstrated a multitude of advantages over traditional vaccines, such as their simplified development process, scalability, structural adaptability, and expedited manufacturing. As with other vaccines, the production of mRNA vaccine products follows a standard three-stage process, which includes upstream production, downstream purification, and final formulation of the mRNA vaccine substance.
3.4.1. Upstream Manufacturing Process
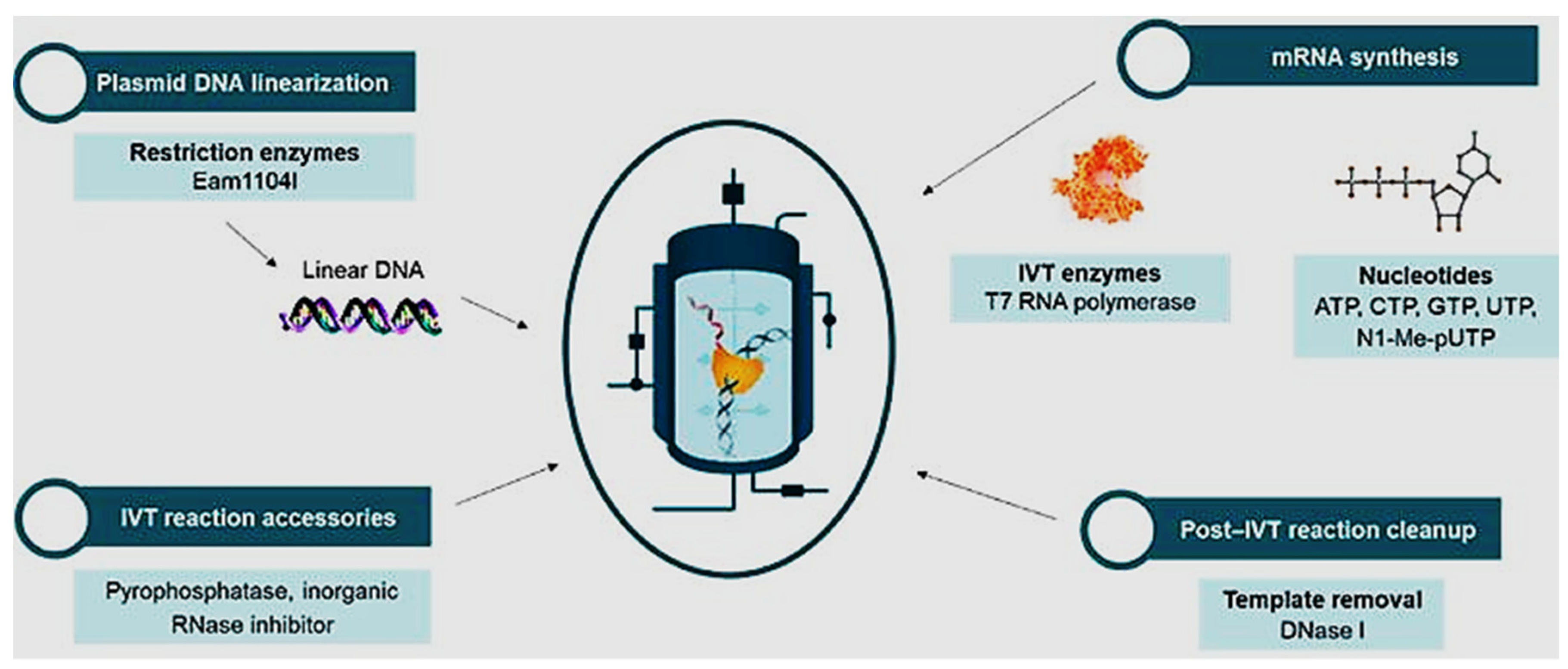
The bioprocessing steps involved in the manufacture of mRNA vaccines vary depending on the specific vaccine and the manufacturing process used. The initial stage of bioprocessing mRNA vaccines involves the production of the mRNA sequence responsible for encoding the target antigen. This is achieved through the generation of a DNA template containing the gene of interest, which can be accomplished by either cleaving a plasmid containing the gene of interest using restriction endonucleases enzymes or amplifying the gene of interest through PCR. Following mRNA synthesis, purification is necessary to eliminate any impurities that may have been introduced during the IVT process. Chromatography techniques, such as reverse-phase high-performance liquid chromatography (RP-HPLC) or ion-exchange chromatography (IEX), are utilized to achieve this purification. The diagrammatic approach in the production process can be seen in
Figure 3. (Source: Bioscience online).
The initial stage of mRNA vaccine production involves the generation of mRNA transcripts from plasmids containing the desired gene. This process is commonly achieved through in vitro transcription (IVT) technology, which utilizes enzymes to transcribe the target gene sequence into mRNA. The purified mRNA is mixed with a lipid nanoparticle (LNP) formulation. The LNP is typically composed of a lipid bilayer that surrounds the mRNA and helps to protect it from degradation and facilitate its delivery to cells. The LNP formulation can be optimized to improve the stability and efficacy of the vaccine. Throughout the bioprocessing steps, the vaccine undergoes extensive quality control testing to ensure that it meets safety and efficacy standards. This includes testing the vaccine for purity, potency, and sterility. The important point to note is that the bioprocessing of mRNA vaccines is a complex and highly specialized process that requires specialized equipment and expertise. Additionally, the quality control process and regulatory requirements for mRNA vaccines are rigorous, and strict adherence to these requirements is essential to ensure the safety and efficacy of the vaccine. The mRNA vaccine will accommodate these all in availing the required vaccine at a fast development process compared to the traditional vaccine development process (Hong et al., 2021).
3.4.2. Downstream Process
In the upstream production process, the mRNA bulk product generated by the IVT reaction undergoes multiple purification steps in downstream processing to ensure its purity. The IVT reaction mixture contains various impurities, such as enzymes, residual NTPs, incorrect structure mRNAs, and DNA plasmid templates, which must be removed to obtain a pure mRNA product with the intended efficacy and safety profile. DNase enzyme digestion and lithium chloride (LiCl) precipitation are employed to remove DNA and other impurities from the IVT mRNA. The downstream purification technique is critical to achieving high translation efficiency and avoiding an unwanted immunostimulatory profile in the mRNA vaccine product. Reverse-phase HPLC has been shown to increase mRNA transfection and related protein production when used to purify modified mRNA before delivery to dendritic cells (Trenkenschuh et al., 2021). Chromatography is a widely accepted purification process in the biopharmaceutical industry for vaccines and biological drug products.
Size exclusion chromatography (SEC) is widely utilized as the primary purification process for large-scale RNA oligonucleotide nucleic acid purification. This technique offers numerous benefits, including selectivity, scalability, versatility, cost-effectiveness, and high purity and yields for nucleic acid products. Conversely, ion-pair reverse-phase chromatography (IEC) has been shown to be an exceptional purification method for mRNA vaccines (Weissman et al., 2013).
Recent advances in mRNA vaccine purification have focused on improving the yield and purity of the mRNA from the reaction mixture. One such approach is the use of chromatography, a process that separates molecules based on their physical and chemical properties. Chromatography techniques such as ion exchange chromatography, size exclusion chromatography, and affinity chromatography have been used to purify mRNA from the reaction mixture (Polizzotto et al., 2021). Another approach to improve mRNA purification is the use of magnetic bead-based isolation, which allows for rapid and efficient purification of mRNA from the reaction mixture. This technique involves binding mRNA to magnetic beads that are then separated using a magnetic field. These advances in mRNA vaccine purification have improved the yield and purity of mRNA, making it easier and more efficient to produce large quantities of high-quality mRNA for vaccine production (Hagan et al., 2018; Polizzotto et al., 2021; Lazzarotto et al., 2021). This has been paying crucial in the rapid development and production of mRNA vaccines for COVID-19 and other diseases.
3.5. mRNA Vaccine Delivery Technologies
The dimensions of mRNA vaccine molecules are significant, ranging from 104 to 106 Da, and they possess a negative charge that impedes their ability to penetrate the lipid bilayer of cell membranes. When administered in their unencapsulated form, these molecules are vulnerable to degradation by nucleases present in the bloodstream, and they are also susceptible to being engulfed by immune cells in the tissue and serum (Heine et al., 2021). To surmount these obstacles, various techniques have been developed to facilitate the delivery of mRNA molecules into cells, including gene guns, electroporation, and ex-vivo transfection. In vivo methods of mRNA delivery involve the transfection of immune or non-immune cells using lipids or transfecting agents, and there are several approaches to mRNA delivery that will be discussed below.
3.5.1. Lipid Nanoparticles (LNPs)
Lipid nanoparticles (LNP) are nanoparticles designed using phospholipids and structured as vesicles consisting of phospholipid bilayers. By loading nucleic acid containing vaccine substance into LNPs, the encapsulated nucleic acid substance is protected from degradation and clearance, and their transport across the cell membrane to the target site is facilitated (Gote et al., 2023). The LNP vaccine delivery method is a strategy employed for the administration of mRNA vaccines utilized by different companies including the Pfizer-BioNTech and Moderna COVID-19 vaccines. LNPs are minute particles composed of a lipid bilayer enveloping a hydrophobic nucleus. The mRNA is encapsulated within the core of the LNP, and the lipid bilayer serves as a protective barrier that helps the mRNA evade degradation by enzymes in the body (Pilkington et al., 2021). The LNP vaccine delivery approach has several advantages, including the ability to rapidly produce vaccines using mRNA technology and the potential for rapid adaptation to new variants of viruses or bacteria. However, there are also challenges associated with LNP vaccine delivery, such as the potential for adverse reactions and the need for specialized cold storage and transportation to maintain the stability of the mRNA-LNP formulation.
3.5.2. Liposomes
The utilization of liposome-based mRNA presents a broad spectrum of delivery solutions, encompassing tailored liposome production and characterization, as well as various applications. The encapsulation of mRNA within liposomes can provide a secure and efficacious delivery mechanism for mRNA-based vaccine products. Studies have demonstrated that cationic liposome formulations can significantly enhance the functional delivery of negatively charged nucleic acids to cells. Liposome vaccine delivery is a technique that employs small liposomes, which are spherical vesicles composed of a lipid bilayer capable of encapsulating a diverse array of biomolecules, such as antigens, adjuvants, and immunomodulators (Hong et al., 2021). The liposome delivery system is being explored for a variety of vaccine applications, including COVID-19 vaccines. The liposome vaccine delivery approach has several advantages, including the ability to encapsulate multiple vaccine components, such as antigens and adjuvants, in a single particle, and the potential for enhanced immune responses. However, there are also challenges associated with liposome vaccine delivery, such as the potential for adverse reactions and the need for specialized manufacturing and storage conditions to maintain the stability of the liposomes.
3.5.3. Polymer Complexes
The polymerization of mRNA with polymeric materials such as polymethyl glycol (PEGs), polyamines, dendrimers, and copolymers is a valuable technique for the delivery of mRNA vaccines. These materials offer protection against RNase-mediated degradation and facilitate intracellular delivery. The coupling of mRNA with polymers is a service that is available with a variety of materials and modifications, including the incorporation of lipid chains, hyperbranched groups, and biodegradable subunits (Iqbal et al., 2020). In this approach, the vaccine components, such as antigens and adjuvants, are encapsulated in a polymeric nanoparticle or microparticle that can protect the vaccine from degradation and enhance its delivery to immune cells. The polymer complex vaccine delivery approach has several advantages, including the ability to encapsulate multiple vaccine components in a single particle, and the potential for enhanced immune responses. Additionally, the polymeric particles can be engineered to have controlled release properties, which can prolong the duration of the immune response. However, there are also challenges associated with polymer complex vaccine delivery, such as the potential for adverse reactions and the need for specialized manufacturing and storage conditions to maintain the stability of the polymeric particles.
3.5.4. Cationic Polypeptides
Protamine is a cationic peptide that is being used in many early studies to deliver mRNA vaccines by coupling target mRNA sequences to protamine to aid in the efficient delivery of mRNA vaccines. Cationic polypeptides are positively charged peptides that have been studied as potential vaccine delivery vehicles (Zhuang et al., 2020). In this methodology, the constituents of the vaccine, namely antigens and adjuvants, are combined with cationic polypeptides to generate nanoparticles. These nanoparticles serve the dual purpose of safeguarding the vaccine from degradation and augmenting its transportation to immune cells. The cationic polypeptide vaccine-delivery approach has several advantages, including the ability to encapsulate multiple vaccine components in a single particle and the potential for enhanced immune responses. The cationic polypeptides can also be synthesized and modified to have specific properties, such as improved stability and targeting capabilities. But there are also challenges associated with cationic polypeptide vaccine delivery, such as the potential for adverse reactions and the need for further optimization of the delivery system to enhance its efficacy.
3.6. Current Status of mRNA Vaccines
3.6.1. mRNA Vaccines in Clinical Trials
As per clinical trial data from Global Data, the industry has sponsored 85% of mRNA vaccine trials in 2023, marking a significant increase from the 34% of trials initiated in 2021. Clinical development is a crucial stage for any vaccine candidate, following successful preclinical studies and preceding market launch. The clinical development of an mRNA vaccine involves a series of clinical trials that assess the safety, immunogenicity, and efficacy of the vaccine in humans. These trials are categorized into Phases 1, 2, 3, and 4, based on the patient population and trial objectives. Phase 1 clinical studies are conducted on a small group of individuals to primarily determine the safety and pharmacokinetics of the vaccine candidate. Phase 2 trials are proof-of-concept studies that aim to confirm the results obtained in Phase 1 clinical studies and evaluate efficacy in a slightly larger group of participants. Phase 3 studies are confirmatory studies conducted across multiple centers and a diverse population to confirm the safety and efficacy of the vaccine candidate (Retzer et al., 2022).
Several mRNA vaccines in various stages of clinical trials since the authorization of COVID-19 vaccines from Pfizer-BioNTech and Moderna. Thus, the landscape of clinical trials is continuously evolving, and there are several new developments. Moderna initiated Respiratory Syncytial Virus (RSV) Vaccine, a Phase 1/2 clinical trial in September 2021 to evaluate an mRNA vaccine candidate for the respiratory syncytial virus (Aliprantis et al., 2021), a common respiratory virus that can cause severe illness, particularly in infants and older adults. Similarly, CureVac, a German biopharmaceutical company, was developing an mRNA-based COVID-19 vaccine and started a Phase 3 clinical trial in September 2021, to assess the safety and efficacy of the vaccine candidate (Uddin & Rone, 2021).
Similarly, Sanofi and Translate Bio collaborated to develop an mRNA-based COVID-19 vaccine candidate and was conducting a Phase 1/2 clinical trial since September 2021 to evaluate the safety and immune response generated by the candidate vaccine (Morais et al., 2021). The company behind the Pfizer-BioNTech COVID-19 vaccine was also exploring the use of mRNA technology for a potential vaccine against multiple sclerosis (MS) and initiated a Phase 1 clinical trial in April 2021 to evaluate the safety and immunogenicity of mRNA-based MS vaccine (Tallantyre et al., 2022).
The rapid progress in mRNA vaccine design and delivery technology has expedited the advancement and clinical implementation of mRNA-based cancer vaccines. The nucleic acid-based vaccine platform holds great promise and appeal owing to its capacity to administer multiple antigens with ease and elicit robust MHC I-mediated CD8+ T cell responses (Qin et al., 2021). In contrast to conventional vaccines, nucleic acid vaccines for cancer confer several benefits, such as safety, specificity in inducing immune response for the antigen of interest, stimulation of both humoral and cellular immune responses, relatively low production cost, and facile manufacturing (Vishweshwaraiah & Dokholyan, 2022). BioNTech's BNT111, an mRNA-based cancer vaccine, is currently undergoing clinical trials for the treatment of various solid tumors, including melanoma and head and neck cancer.
It aims to elicit an immune response against specific tumor antigens. mRNA-4157 is an mRNA-based vaccine developed by Moderna, which is being studied in clinical trials for the treatment of solid tumors, including ovarian cancer, colorectal cancer, and pancreatic cancer. It is designed to induce an immune response against tumor-associated antigens. RO7198457 is an mRNA-based vaccine developed through a collaboration between Merck being evaluated in clinical trials for the treatment of various types of cancers, including colorectal cancer, ovarian cancer, and lung cancer (Miao et al., 2021).
3.7. mRNA Vaccines on the Market
3.7.1. Pfizer-BioNTech COVID-19 Vaccine
The vaccine developed jointly by Pfizer and BioNTech, was the first mRNA vaccine authorized for emergency use by the FDA in December 2020. The vaccine contains a small piece of mRNA that encodes for the spike protein found on the surface of the SARS-CoV-2 virus that causes the disease COVID-19. The vaccine is administered in two doses, given three weeks apart, and is highly effective in preventing COVID-19 (Mahase, 2020).
The Pfizer-BioNTech COVID-19 vaccine, which utilizes messenger RNA (mRNA) technology, was developed through a series of crucial steps. Initially, scientists identified the SARS-CoV-2 virus responsible for COVID-19 in early 2020 and proceeded to sequence its genetic material. Subsequently, they analyzed its structure to gain insight into its mechanism of infecting human cells (Thorn et al., 2022). Following, the researchers identified the spike protein on the surface of the virus as a target for the vaccine which helps the virus attach to and enter human cells, so targeting this protein can prevent infection. After target identification, design a synthetic mRNA sequence that codes for the spike protein. Thus, mRNA is designed to be delivered to human cells, where it will instruct the cells to produce the spike protein (Thorn et al., 2022).
The mRNA needs to be delivered to human cells in a way that protects them from degradation and allows them to enter the cells. The Pfizer-BioNTech vaccine uses lipid nanoparticles as a delivery system, which protect the mRNA and facilitate its entry into human cells (Wilson et al., 2022). After several evaluation in pre-clinical and clinical trials, the vaccine is now being manufactured and distributed globally to help control the spread of COVID-19. Generally, the development of the mRNA Pfizer-BioNTech COVID-19 vaccine involved a combination of cutting-edge technology, extensive testing, and collaboration between scientists, regulators, and manufacturers.
3.7.2. Moderna COVID-19 Vaccine
Moderna has developed an additional COVID-19 vaccine that is based on mRNA technology and incorporates a fragment of mRNA that encodes the spike protein present on the SARS-CoV-2 virus. Similar to the Pfizer-BioNTech vaccine, this vaccine is administered in two doses, with a four-week interval between doses. The FDA granted emergency use authorization for this vaccine in December 2020, and it has demonstrated significant efficacy in preventing COVID-19 (Livingston, 2021). This vaccine also targets the spike protein on the surface of the virus as an antigenic target for the vaccine. The Moderna vaccine also uses lipid nanoparticles as a delivery system, which protect the mRNA and facilitate its entry into human cells. The vaccine was granted emergency use authorization by regulatory agencies based on the results of the clinical trials. Generally, the development of the Moderna mRNA COVID-19 vaccine involved a combination of cutting-edge technology, extensive testing, and collaboration between different stakeholders.
The Moderna and Pfizer-BioNTech mRNA COVID-19 vaccines have a lot in common, as they are both based on the same mRNA technology and are highly effective at preventing COVID-19. However, there are a few key differences between the two vaccines. The Moderna vaccine requires two doses, given four weeks apart, while the Pfizer-BioNTech vaccine requires two doses, given three weeks apart. The Moderna vaccine can be stored at -20°C (-4°F) for up to six months, and at standard refrigeration temperatures (2-8°C or 36-46°F) for up to 30 days, while the Pfizer-BioNTech vaccine must be stored at ultra-cold temperatures (-80°C/-112°F to -60°C/-76°F) for up to six months, and can be stored at standard refrigeration temperatures for only up to five days (Meo et al., 2021). During clinical trials at different conditions, the Moderna vaccine was found to be 94.1% effective at preventing COVID-19, while the Pfizer-BioNTech vaccine was found to be 95% effective at preventing COVID-19. Concerning age restrictions, the Moderna vaccine is authorized for use in people aged 18 and older, while the Pfizer-BioNTech vaccine is authorized for use in people aged 12 and older (Rudrapal et al., 2020).
3.7.3. CureVac COVID-19 Vaccine
CureVac is an mRNA COVID-19 vaccine developed by the German biopharmaceutical company, and authorized for emergency use by the European Medicines Agency (EMA) in November 2021 (Verbeke et al., 2021). This vaccine is authorized for use in European countries. The CureVac COVID-19 vaccine, akin to other mRNA vaccines, possesses several distinctive attributes that differentiate it from conventional vaccines. The vaccine employs messenger RNA (mRNA) technology to direct cells to generate a protein present on the exterior of the SARS-CoV-2 virus. Furthermore, the CureVac vaccine is formulated to be dispensed in a reduced dosage in comparison to other COVID-19 vaccines (Barbier et al., 2022). This is because the mRNA technology used in the vaccine is highly efficient at stimulating an immune response, so less vaccine is needed to achieve protection.
Unlike the Pfizer/BioNTech vaccine, which requires ultra-cold storage temperatures, and the Moderna vaccine, which requires freezing temperatures for long-term storage, the CureVac vaccine can be stored at standard refrigeration temperatures (2-8°C), making it easier to distribute and administer (Kon et al., 2022). CureVac's mRNA technology is designed to be easily scalable, which could allow for large-scale production and distribution of the vaccine (Bown et al., 2022). This could be particularly important for increasing access to vaccines in low- and middle-income countries. In addition, the mRNA technology used in the CureVac vaccine can be quickly and easily modified to target new variants of the SARS-CoV-2 virus, if necessary, by changing the genetic sequence of the mRNA which helps the efforts to combat COVID-19 and its variants. It's worth noting that the efficacy and safety of the CureVac.
3.8. Advantages of mRNA Vaccines
The technology utilized in the development of mRNA vaccines has played a pivotal role in the worldwide response to the COVID-19 pandemic. These vaccines were expeditiously developed and authorized for emergency use, and have demonstrated remarkable efficacy in preventing both COVID-19 infection and severe illness (Knezevic et al., 2021). mRNA vaccines can be developed rapidly compared to traditional vaccine platforms. Once the genetic sequence of a pathogen is known, the mRNA can be synthesized in the laboratory without the need for growing and inactivating the actual pathogen. This accelerated development process allows for a quicker response to emerging infectious diseases or rapidly mutating viruses. This is important for responding to future pandemics, as well as for developing vaccines against diseases that are currently difficult to develop vaccines. In other way, mRNA vaccines do not contain live viruses or bacteria and are not able to cause disease or alter a person's DNA, as the mRNA is quickly broken down by the body after it has been used to stimulate an immune response (Abbasi, 2020). Clinical trials have also shown that mRNA vaccines, those developed by Pfizer/BioNTech and Moderna, are highly effective at preventing COVID-19 infection and severe disease. The mRNA vaccines are highly adaptable and can be designed to target various pathogens by simply changing the mRNA sequence. The adaptability of mRNA technology facilitates the creation of vaccines for a diverse array of infectious diseases, encompassing viral infections as well as potentially bacterial or parasitic infections. Furthermore, mRNA vaccines are deemed safe due to their lack of live or inactivated pathogens, thereby eliminating the possibility of inducing the targeted disease. Moreover, mRNA is a transient molecule that undergoes rapid degradation and elimination by the body, thereby augmenting its safety profile. Additionally, mRNA vaccines can be produced with greater ease and speed than conventional vaccines, which necessitate laborious procedures such as cultivating substantial quantities of live viruses or bacteria (Bown et al., 2022). This means mRNA vaccines can be produced on a larger scale, which is especially important during a pandemic response.
3.9. Shortcomings of mRNA Vaccines
Antigens produced subsequent to mRNA vaccination are taken up by antigen-presenting cells (APCs) and conveyed to lymph nodes. The interaction among B cells, APCs, and follicular helper T cells stimulates the establishment of a germinal center, where B cells undergo amplification and differentiation to generate high-affinity neutralizing antibodies against the pathogen. This sequence of immunological biochemical reactions is of paramount importance for antibody persistence, which corresponds to an extended duration of efficacy against the infectious pathogen (Goel et al., 2021). Preclinical investigations have exhibited that mRNA vaccines elicit robust germinal center immunogenic reactions and TFH cell induction against HIV-1, SARS-CoV-2, Zika virus, and influenza virus (Cagigi & Lore, 2021). Nevertheless, the duration of the antibody response is a multifaceted phenomenon that varies considerably from antigen to antigen. There have been certain isolated safety incidents that require further refinement of mRNA vaccines and all their constituents. Analogous to most medicinal treatments, unfavorable reactions to mRNA vaccines have frequently intensified and escalated with dosage. During the recent COVID-19 vaccination campaign, a mild anaphylactic reaction was observed in 4.7 per million vaccinations. The Moderna vaccine had 2.5 per million vaccinations, while the Pfizer-BioNTech vaccine had 2.2 per million vaccinations (Wolfson et al., 2021). Scientists have suggested that this allergic response may be attributed to pre-existing antibodies that patients have against the PEGylated lipids used in LNPs. These lipids can be formed in the body in response to the presence of PEG in many consumer products, such as toothpaste and shampoos. Studies have indicated that PEG accomplishes this by directly cross-linking the B cell receptor and introducing IgM production. Anti-PEG antibodies are reported in 40% of the population during vaccination, which can accelerate and intensify the risk of allergic reactions and hinder vaccine efficacy (Guerrini et al., 2022).
During pregnancy and the neonatal period of infants, the immune system experiences notable alterations, which increase the vulnerability of individuals to infectious diseases that are not presently targeted by mRNA vaccines. The Zika virus has the capacity to invade cortical neurons and glial cells in the developing fetus, resulting in cellular demise, neuroinflammation, and grave congenital anomalies (Nir et al., 2022).
The mRNA vaccines need to be stored at ultra-low temperatures, typically around -70 0C for long-term storage and transportation (Meo et al., 2021). This poses logistical challenges, particularly in resource-limited settings or regions with inadequate cold chain infrastructure. The other is that mRNA vaccines have a relatively short shelf life compared to traditional vaccines and once thawed, they must be used within a specific timeframe which can be challenging for areas with difficult distribution limiting the effort of vaccination.
The production of mRNA vaccines is more intricate and time-consuming compared to traditional vaccine production methods. Scaling up production and achieving mass distribution can be a significant challenge. Currently, available mRNA vaccines require intramuscular injection, which necessitates trained healthcare professionals for administration (Rosa et al., 2021). This may limit accessibility in areas with a shortage of healthcare workers or remote regions with limited access to medical facilities. In addition, like other new medical technology, mRNA vaccines have faced public hesitancy and misinformation. Concerns about the newness of the technology, long-term effects, and safety have led to vaccine skepticism in some individuals, which can impact vaccine uptake and population-level protection (Danchin & Buttery, 2021). Ongoing efforts are focused on improving vaccine stability, simplifying manufacturing processes, exploring alternative delivery methods, and addressing concerns through research and development.
3.10. Next-Generation mRNA Vaccine Studies
Currently, efforts are underway to develop next-generation mRNA vaccines with a primary objective of enhancing safety, efficacy, storage, and handling while addressing inefficiencies. These improvements entail stabilizing the vaccines at ambient temperature and reducing the cold chain requirement for storage and transportation, while maintaining the same level of efficacy and safety. Moreover, ongoing research is focused on identifying more potent and ligand-targeted nanocarriers that can offer a superior safety and mRNA delivery efficacy profile. Additionally, extensive research is being conducted to explore the potential of various RNA-based molecules as vaccines.
3.10.1. Lipid Nanoparticle Improvements
The lipid nanoparticles (LNP) are carriers that encapsulate and protect the mRNA molecule facilitating its delivery into target cells. Lyophilized mRNA vaccines have the ability to maintain stability and efficacy when stored at sub-zero temperatures. The long-term stability of the vaccine is a significant concern in the development of lipid nanoparticles (LNPs), which aim to address physical and chemical instabilities observed when LNPs are stored as an aqueous suspension (Gote et al., 2023). Chemical degradation involves the alteration of bonds in the mRNA molecule, while physical degradation encompasses the loss of secondary and tertiary molecular structure, denaturation/aggregation, fusion, and leakage of encapsulated mRNA. Researchers are currently working on optimizing LNPs to enhance their stability, improve cellular uptake, and minimize potential side effects (Hameed et al., 2022). LNPs facilitate the cellular uptake of mRNA and are engineered to possess a positively charged surface, which aids in binding to the negatively charged cell membranes. LNPs can also be engineered to release the mRNA payload in a controlled manner, optimizing its availability for translation into protein. This controlled release ensures efficient and sustained production of the target protein, maximizing the immune response and efficacy of the vaccine. These advancements can increase the efficiency of mRNA delivery and improve the immune response.
3.10.2. Self-Amplifying mRNA
Self-amplifying mRNA vaccines are designed to produce more copies of the encoded antigen within the body (Callaway, 2023). They utilize a modified form of mRNA that includes additional genetic material, allowing for the replication and amplification of the mRNA once it enters the host cells. This amplification leads to higher expression levels of the target antigen, which can potentially result in a more robust immune response. By incorporating additional genetic elements, saRNA vaccines can replicate and produce more copies of the encoded antigen within the cells. This amplification process leads to increased antigen expression, which can stimulate a stronger immune response compared to conventional mRNA vaccines (Rangachayra
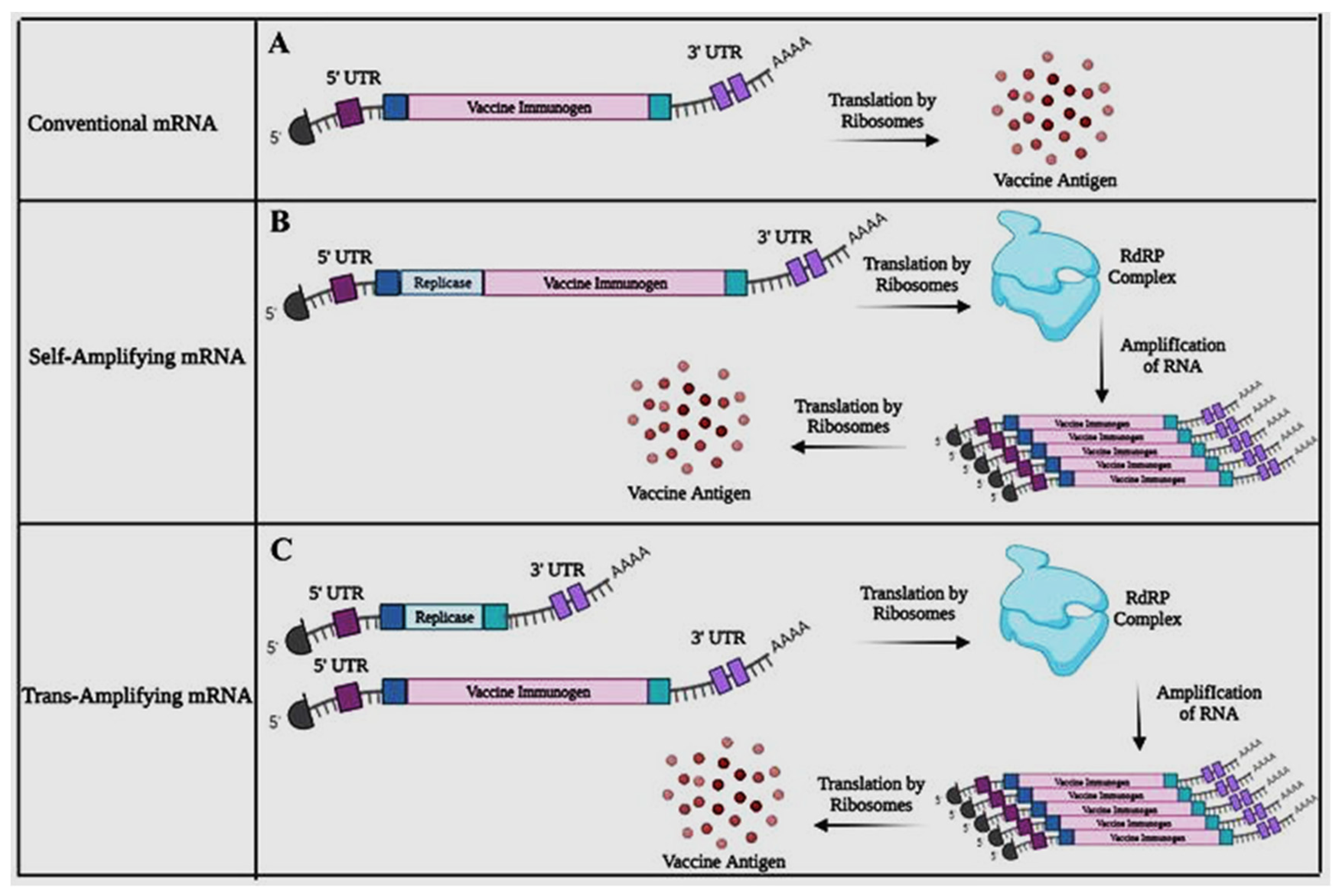
et al., 2023). Different approaches in designing mRNA can be seen in
Figure 4 below, (Source: Bloom
et al., 2021).
In conventional mRNA molecules, the vaccine immunogen is typically encoded along with flanking 5′ and 3′ UTRs, as well as a 5′ cap (m7G) and poly A tail that are common to all RNA transcripts. Translation of the nonreplicating transcript results in the production of the antigen. However, self-amplifying RNA incorporates 5′ and 3′ CSE sequences, the nsP1-4 genes, a sub-genomic promoter, and the vaccine immunogen to achieve self-amplification within host cells (Bloom et al., 2021). Self-amplifying mRNA (SAM) vaccines utilize specific genetic elements derived from alphaviruses, such as Semliki Forest virus (SFV), Sindbis virus (SINV), and Venezuelan equine encephalitis virus (VEEV), to encode replicase genes responsible for replicating the mRNA within the cells. The alphavirus replicase proteins enable the amplification of the mRNA within the host cells, leading to increased production of both replicase proteins and the target antigen (Blankney & Geall, 2021). Upon undergoing in situ translation, the nsP1-4 proteins assemble into the RdRP complex, which identifies flanking CSE sequences and amplifies transcripts encoding the vaccine, leading to an accumulation of the antigen within the cell (Kunzil et al., 2022).
In order to achieve a comparable outcome to self-amplifying mRNAs, trans-amplifying mRNAs employ two distinct transcripts. The first transcript, which is already known, encodes the nsP1-4 genes and is flanked by 5′ and 3′ UTRs. The second transcript encodes the sub-genomic promoter, the viral CSE sequences, and the vaccine immunogen. These two transcripts are co-delivered, and in situ translation of the conventional mRNA results in the formation of the RdRP complex, which subsequently amplifies the vaccine-encoding transcript, leading to the accumulation of the intended antigen. This approach has the potential to enhance vaccine potency and reduce the required vaccine dose.
3.10.3. Nucleosides Modification
The process of nucleoside modification in mRNA vaccines entails the modification of the constituent elements of mRNA, namely nucleotides, with the aim of enhancing the stability, translational efficacy, and immunogenicity of the mRNA molecule. Such modifications have the potential to significantly augment the performance of mRNA vaccines across multiple dimensions. Pseudouridine is a modified nucleoside that can replace uridine (U) in mRNA as it is incorporated into mRNA to increase stability and reduce immune activation. Pseudouridine modifications can enhance the half-life of the mRNA molecule within cells, prolonging the duration of protein production and potentially improving the immune response (Tavakoli
et al., 2023). In another way, 5-Methylcytidine is a modified form of cytidine (C) in mRNA incorporation that may enhance mRNA stability, protect the mRNA from degradation by cellular enzymes, and improve translational efficiency (Higuchi
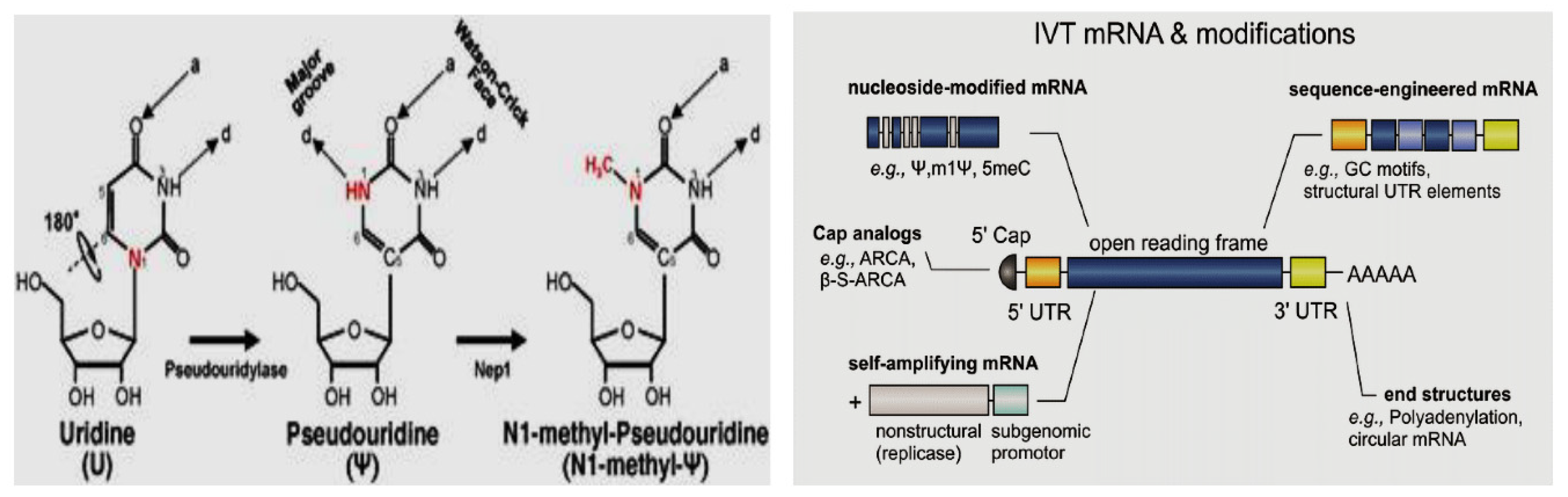
et al., 2023). The nucleoside modification approach can be seen in
Figure 5, (Source: Science Direct).
This modification can contribute to increased protein expression levels. Generally, nucleoside modifications help address some of the limitations of mRNA vaccines, such as susceptibility to degradation and potential immune activation. These modifications can improve the stability and translational efficiency of mRNA, leading to increased protein production and potentially stronger immune responses. The specific nucleoside modifications used can vary depending on the mRNA vaccine platform and target disease.
Pseudouridine can enhance stability and translation while lowering the immunological response in mRNA vaccines. Toll-like receptors 7 and 8 (TLR7 and TLR8), the cellular detectors of foreign RNA, are less able to recognize pseudouridine-modified mRNA, which lowers the innate immune response (Higuchi et al., 2023). The stability and translational effectiveness of pseudouridine are further improved by 1-methylpseudouridine (m1). According to studies, mRNA vaccines containing m1 inhibit the innate immune system's ability to respond as strongly as those containing unaltered nucleotides. Additionally, mammalian cells translate m1-modified mRNA more effectively (Pardi et al., 2018). A combination of 5-methylcytidine (m5C) and 2-thiouridine (s2U) can also be used to stabilize mRNA. m5C is a naturally occurring RNA modification, and s2U, a sulfur-containing derivative of uridine, is commonly found in tRNA.
3.10.4. Intranasal Delivery
Current mRNA vaccines are administered via injection, but intranasal delivery is being explored as an alternative route. Intranasal vaccines can target the respiratory mucosa, which is a primary site of infection for many respiratory viruses. Intranasal administration involves delivering the mRNA vaccine through the nasal route, typically in the form of a nasal spray. The nasal mucosa has a large surface area and is rich in blood vessels, facilitating rapid absorption and immune activation. By optimizing the size, surface charge, and composition of LNPs, the vaccine can be designed to enhance its penetration and interaction with the nasal epithelium. Additionally, to improve the residence time of the vaccine in the nasal cavity, mucoadhesive agents can be included in the formulation. These agents help the vaccine adhere to the mucus layer, allowing for prolonged contact with the underlying cells and facilitating absorption (Shahzamani et al., 2021). This could potentially result in a quicker immune response compared to intramuscular injection. This approach may induce both systemic and mucosal immune responses, providing broader protection (Marks et al., 2023). Intranasal delivery allows the vaccine to directly target the respiratory tract, which is the primary site of respiratory infections which is particularly beneficial for diseases transmitted through the respiratory route, such as influenza and respiratory syncytial virus (RSV) (Morens et al., 2023).
3.10.5. Thermal Stability
Ongoing research endeavors are currently focused on enhancing the thermostability of existing mRNA vaccines. The objective is to create formulations that can endure a broader range of temperatures, thereby reducing or eliminating the need for ultra-cold storage requirements. One area of research is centered on improving the stability of mRNA vaccines, particularly with regards to temperature sensitivity. Enhancing thermostability would simplify storage and distribution, particularly in regions with limited access to cold chain infrastructure. One approach involves exploring various formulation strategies to improve the stability of mRNA vaccines, including optimizing the composition of lipid nanoparticles that safeguard and deliver the mRNA (Huguchi et al., 2023). By fine-tuning the lipid composition, researchers aim to enhance the stability of the mRNA at higher temperaturesThe untranslated regions (UTR) located at the 5' and 3' ends of messenger RNAs (mRNAs) have been shown to not only impact thermal stability, but also play a crucial role in regulating transcript translation (Cao et al., 2021). Incorporating internal ribosomal entry sites or the Kozak sequence within the 5'-UTR can facilitate ribosomal loading and translation initiation. The 3'-UTR, on the other hand, can incorporate either a stabilization motif, such as the b-globulin 3'-UTR, to prolong transcript half-life, or a regulatory motif, such as the miRNA-122 binding site, to achieve tissue-specific expression and minimize systemic toxicity by reducing off-target transgene expression (Xu et al., 2023). Furthermore, ongoing research is exploring the feasibility of lyophilizing mRNA vaccines to extend their shelf life and reduce cold storage requirements. In general, the aim of such research efforts is to develop mRNA vaccine formulations that remain stable at higher temperatures, allowing for easier storage, transportation, and distribution in various settings, including resource-limited areas. By improving the thermostability of mRNA vaccines, it becomes more feasible to reach populations with limited access to ultra-cold storage infrastructure, expanding the potential impact of mRNA vaccination campaigns.
4. Conclusion
Years of extensive research in mRNA design and its delivery technology have rendered mRNA vaccines a highly promising tool in the fight against emerging pandemics and existing infectious diseases. The development of the first two mRNA vaccines to combat SARS-CoV-2 was achieved at an unprecedented pace, setting a new record. The progress made in the development of mRNA-based vaccines has surpassed expectations, establishing a robust foundation and indispensable groundwork for the future of mRNA vaccines. In addition to COVID-19, mRNA vaccines are currently under development for other infectious diseases, including influenza, Zika virus, and cytomegalovirus (CMV). Preclinical and early clinical studies have shown promising results for mRNA vaccines targeting these diseases, and further research is ongoing. Moreover, researchers are also exploring the potential of mRNA vaccines in other areas, such as cancer immunotherapy and genetic diseases. Early-stage clinical trials have shown that mRNA vaccines can induce immune responses against cancer cells and potentially offer a new treatment option for cancer patients. Furthermore, mRNA vaccines are being investigated as a possible treatment for genetic diseases, such as cystic fibrosis.
Presently, ongoing research and development endeavors are concentrated on enhancing various aspects of mRNA vaccine production and technology with the objective of improving the stability of mRNA vaccines and reducing temperature sensitivity to facilitate storage and distribution. In certain instances, scientists are exploring self-amplifying mRNA vaccines that generate multiple copies of mRNA within cells, potentially leading to a stronger immune response. Additionally, combination vaccine development is being pursued as an approach that could provide protection against multiple diseases or target different strains or variants of a pathogen. Novel delivery systems such as nanoparticles or liposomes are being investigated to improve the specificity and efficiency of mRNA vaccine delivery. Overall, mRNA vaccine technology has emerged as a highly effective approach for developing vaccines against a wide range of diseases.
References
- Abbasi, J. (2020). COVID-19 and mRNA vaccines—first large test for a new approach. Jama, 324(12), 1125-1127.
- Aliprantis, A. O. , Shaw, C. A., Griffin, P., Farinola, N., Railkar, R. A., Cao, X.,... & Panther, L. (2021). A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Human vaccines & immunotherapeutics, 17(5), 1248-1261.
- August, A. , Brito, L., Paris, R., & Zaks, T. (2022). Clinical development of mRNA vaccines: challenges and opportunities. mRNA Vaccines, 167-186.
- Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R., & Anderson, D. G. (2022). The clinical progress of mRNA vaccines and immunotherapies. Nature biotechnology, 40(6), 840-854.
- Bernal, J. L. , Andrews, N., Gower, C., Gallagher, E., Simmons, R., Thelwall, S.,... & Ramsay, M. (2021). Effectiveness of Covid-19 Vaccines against the B.1.617. 2 (Delta) Variant. New England Journal of Medicine, 385(7), 585-594.
- Blakney, A. K. , Ip, S., & Geall, A. J. (2021). An update on self-amplifying mRNA vaccine development. Vaccines, 9(2), 97.
- Bloom, K. , van den Berg, F., & Arbuthnot, P. (2021). Self-amplifying RNA vaccines for infectious diseases. Gene therapy, 28(3-4), 117-129.
- Bown, C. P. , & Bollyky, T. J. (2022). How COVID-19 vaccine supply chains emerged in the midst of a pandemic. The World Economy, 45(2), 468-522.
- Brito, L. A. , Kommareddy, S., Maione, D., Uematsu, Y., Giovani, C., Scorza, F. B.,... & Geall, A. J. (2015). Self-amplifying mRNA vaccines. Advances in genetics, 89, 179-233.
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for MRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Cagigi, A., & Lore, K. (2021). Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines, 9(1), 61.
- Callaway, E. (2023). The next generation of coronavirus vaccines. Nature, 614, 22-25.
- Cao, J. , Novoa, E. M., Zhang, Z., Chen, W. C., Liu, D., Choi, G. C.,... & Lu, T. K. (2021). High-throughput 5′ UTR engineering for enhanced protein production in non-viral gene therapies. Nature communications, 12(1), 4138.
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Daffis, S.; Szretter, K.J.; Schriewer, J.; Li, J.; Youn, S.; Errett, J.; Lin, T.Y.; Schneller, S.; Zust, R.; Dong, H.; et al. 2’-O Methylation of the Viral MRNA Cap Evades Host Restriction by IFIT Family Members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Danchin, M. , & Buttery, J. (2021). COVID-19 vaccine hesitancy: a unique set of challenges. Internal Medicine Journal, 51(12), 1987.
- Fang, E. , Liu, X., Li, M., Zhang, Z., Song, L., Zhu, B.,... & Li, Y. (2022). Advances in COVID-19 mRNA vaccine development. Signal Transduction and Targeted Therapy, 7(1), 94.
- FDA. (2021). Moderna COVID-19 Vaccine.
- FDA. (2021). Pfizer-BioNTech COVID-19 Vaccine.
- Firdessa-Fite, R. , & Creusot, R. J. (2020). Nanoparticles versus dendritic cells as vehicles to deliver mRNA encoding multiple epitopes for immunotherapy. Molecular Therapy-Methods & Clinical Development, 16, 50-62.
- Gebre, M. S. , Brito, L. A., Tostanoski, L. H., Edwards, D. K., Carfi, A., & Barouch, D. H. (2021). Novel approaches for vaccine development. Cell, 184(6), 1589-1603.
- Goel, R. R. , Apostolidis, S. A., Painter, M. M., Mathew, D., Pattekar, A., Kuthuru, O.,... & Wherry, E. J. (2021). Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Science immunology, 6(58), eabi6950.
- Gote, V. , Bolla, P. K., Kommineni, N., Butreddy, A., Nukala, P. K., Palakurthi, S. S., & Khan, W. (2023). A Comprehensive Review of mRNA Vaccines. International Journal of Molecular Sciences, 24(3), 2700.
- Guerrini, G. , Gioria, S., Sauer, A. V., Lucchesi, S., Montagnani, F., Pastore, G.,... & Calzolai, L. (2022). Monitoring anti-PEG antibodies level upon repeated lipid nanoparticle-based COVID-19 vaccine administration. International Journal of Molecular Sciences, 23(16), 8838.
- Guo, X. , Liu, D., Huang, Y., Deng, Y., Wang, Y., Mao, J.,... & Gao, X. (2023). Revolutionizing viral disease vaccination: the promising clinical advancements of non-replicating mRNA vaccines. Virology Journal, 20(1), 1-17.
- Hagan T, Nakaya HI, Subramaniam S, et al. (2018). Magnetic Bead-Based Isolation of RNA and DNA from Human Plasma for Disease Diagnosis. ACS Nano.;12(4):3726-3734.
- Hare, J. I. , Lammers, T., Ashford, M. B., & Puri, S. (2017). Storming the castle: Nanoparticle-mediated delivery of payloads to the cytosol. Journal of Controlled Release, 261, 52-56.
- Higuchi, A. , Sung, T. C., Wang, T., Ling, Q. D., Kumar, S. S., Hsu, S. T., & Umezawa, A. (2023). Material Design for Next-Generation mRNA Vaccines Using Lipid Nanoparticles. Polymer Reviews, 63(2), 394-436.
- Hong, H.C.; Kim, K.S.; Park, S.A.; Chun, M.J.; Hong, E.Y.; Chung, S.W.; Kim, H.J.; Shin, B.G.; Braka, A.; Thanappan, J.; et al. An MRNA Vaccine against SARS-CoV-2: Lyophilized, Liposome-Based Vaccine Candidate EG-COVID Induces High Levels of Virus Neutralizing Antibodies. bioRxiv 2021. [Google Scholar]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for Therapeutic Delivery of Protein and Nucleic Acid Macromolecules: From Design to Therapeutic Applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L. A. , Anderson, E. J., Rouphael, N. G., Roberts, P. C., Makhene, M., Coler, R. N.,... & Hill, C. (2020). An mRNA Vaccine against SARS-CoV-2—Preliminary Report. New England Journal of Medicine, 383(20), 1920–1931.
- Jackson, N. A. , Kester, K. E., Casimiro, D., Gurunathan, S., & DeRosa, F. (2020). The promise of mRNA vaccines: a biotech and industrial perspective. npj Vaccines, 5(1), 11.
- John, R. Mascola and Anthony S. Fauci (2020). "Vaccine Development, Testing, and Regulation". The New England Journal of Medicine; 383: 2200-2211.
- Kariko, K.; Weissman, D. Naturally Occurring Nucleoside Modifications Suppress the Immunostimulatory Activity of RNA: Implication for Therapeutic RNA Development. Curr. Opin. Drug Discov. Dev. 2007, 10, 523–532. [Google Scholar]
- Knezevic, I. , Liu, M. A., Peden, K., Zhou, T., & Kang, H. N. (2021). Development of mRNA vaccines: scientific and regulatory issues. Vaccines, 9(2), 81.
- Kon, E., Elia, U., & Peer, D. (2022). Principles for designing an optimal mRNA lipid nanoparticle vaccine. Current opinion in Biotechnology, 73, 329-336.
- Kowalzik, F. , Schreiner, D., Jensen, C., Teschner, D., Gehring, S., & Zepp, F. (2021). mRNA-based vaccines. Vaccines, 9(4), 390.
- Krammer, F. (2020). SARS-CoV-2 vaccines in development. Nature, 586(7830), 516–527.
- Kulkarni, J. A., Darjuan, M. M., Mercer, J. E., Chen, S., Van Der Meel, R., & Thewalt, J. L. (2018). On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano, 12(5), 4787-4795.
- Kumar, A., Blum, J., Le, T. T., Havelange, N., Magini, D., & Yoon, I. K. (2022). The mRNA vaccine development landscape for infectious diseases. Nat Rev Drug Discov., 21(5), 333-334.
- Kunzli, M. , O’Flanagan, S. D., LaRue, M., Talukder, P., Dileepan, T., Stolley, J. M.,... & Masopust, D. (2022). Route of self-amplifying mRNA vaccination modulates the establishment of pulmonary resident memory CD8 and CD4 T cells. Science Immunology, 7(78), eadd3075.
- Kwon, H.; Kim, M.; Seo, Y.; Moon, Y.S.; Lee, H.J.; Lee, K.; Lee, H. Emergence of Synthetic MRNA: In Vitro Synthesis of MRNA and Its Applications in Regenerative Medicine. Biomaterials 2018, 156, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Labrunie, M. L. , Penna, C. C., & Kupfer, D. (2021). The resurgence of industrial policies in the age of advanced manufacturing: an international comparison of industrial policy documents. Revista Brasileira de Inovação, 19.
- Lazzarotto CR, Boffo EF, Oliveira SC, et al. (2021). mRNA vaccines: An overview. Int J Gen Med.; 14:2741-2757.
- Le, T. T. , Andreadakis, Z., Kumar, A., Román, R. G., Tollefsen, S., Saville, M., & Mayhew, S. (2020). The COVID-19 vaccine development landscape. Nat Rev Drug Discov, 19(5), 305-306.
- Li, M. , Wang, Z., Xie, C., & Xia, X. (2022). Advances in mRNA vaccines. International Review of Cell and Molecular Biology.
- Lindsay, K.E.; Bhosle, S.M.; Zurla, C.; Beyersdorf, J.; Rogers, K.A.; Vanover, D.; Xiao, P.; Araínga, M.; Shirreff, L.M.; Pitard, B.; et al. Visualization of Early Events in MRNA Vaccine Delivery in Non-Human Primates via PET–CT and near-Infrared Imaging. Nat. Biomed. Eng. 2019, 3, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. A. (2019). A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines, 7(2), 37.
- Livingston, E. H. (2021). Necessity of 2 doses of the Pfizer and Moderna COVID-19 vaccines. Jama, 325(9), 898-898.
- Lukavsky, P.J.; Puglisi, J.D. (2004). Large-Scale Preparation and Purification of Polyacrylamide-Free RNA Oligonucleotides. RNA, 10, 889–893.
- Lutz, J., Lazzaro, S., Habbeddine, M., Schmidt, K. E., Baumhof, P., Mui, B. L., ... & Fotin-Mleczek, M. (2017). Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ vaccines, 2(1), 29.
- Mahase, E. (2020). Covid-19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows.
- Marks, P. W. , Gruppuso, P. A., & Adashi, E. Y. (2023). Urgent need for next-generation COVID-19 vaccines. JAMA, 329(1), 19-20.
- Maruggi, G. , Ulmer, J. B., Rappuoli, R., & Yu, D. (2021). Self-amplifying mRNA-based vaccine technology and its mode of action.
- Maruggi, G., Zhang, C., Li, J., Ulmer, J. B., & Yu, D. (2019). mRNA as a transformative technology for vaccine development to control infectious diseases. Molecular Therapy, 27(4), 757-772.
- Mbunge, E. , Akinnuwesi, B., Fashoto, S. G., Metfula, A. S., & Mashwama, P. (2021). A critical review of emerging technologies for tackling COVID-19 pandemic. Human behavior and emerging technologies, 3(1), 25-39.
- Meo, S. A. , Bukhari, I. A., Akram, J., Meo, A. S., & Klonoff, D. C. (2021). COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci, 1663-1669.
- Miao, L., Zhang, Y., & Huang, L. (2021). mRNA vaccine for cancer immunotherapy. Molecular Cancer, 20(1), 1-23.
- Moon, J. J. , & Irvine, D. J. (2012). Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nature Reviews Immunology, 12(8), 592-603.
- Morais, P. , Adachi, H., & Yu, Y. T. (2021). The critical contribution of pseudouridine to mRNA COVID-19 vaccines. Frontiers in cell and developmental biology, 9, 789427.
- Morens, D. M. , Taubenberger, J. K., & Fauci, A. S. (2023). Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host & Microbe, 31(1), 146-157.
- Newland, M. Newland, M., Durham, D., Asher, J., Treanor, J. J., Seals, J., Donis, R. O., & Johnson, R. A. (2021). Improving pandemic preparedness through better, faster influenza vaccines. Expert Review of Vaccines, 20(3), 235-242.
- Nir, O. , Schwartz, A., Toussia-Cohen, S., Leibovitch, L., Strauss, T., Asraf, K.,... & Yinon, Y. (2022). Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. American journal of obstetrics & gynecology MFM, 4(1), 100492.
- Okay, S. , Özcan, Ö., & Karahan, M. (2020). Nanoparticle-based delivery platforms for mRNA vaccine development. AIMS Biophysics, 7.
- Oussoren, C. , & Zuidema, J. (1997). Polymeric micelles as drug carriers. Journal of Controlled Release, 52(1-2), 141-153.
- Pardi, N. , Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261-279.
- Pardi, N., Hogan, M. J., & Weissman, D. (2020). Recent advances in mRNA vaccine technology. Current opinion in immunology, 65, 14-20.
- Pardi, N. , Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261–279.
- Pascolo, S. (2004). Messenger RNA-Based Vaccines. Expert Opin. Biol. Ther., 4, 1285–1294.
- Pilkington, E.H.; Suys, E.J.A.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From Influenza to COVID-19: Lipid Nanoparticle MRNA Vaccines at the Frontiers of Infectious Diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef] [PubMed]
- Polizzotto MN, Uthamanthil R, Tangudom Y, et al. (2021). mRNA vaccine production using a scalable membrane chromatography purification platform. Vaccine.; 39(6):974-977.
- Qin, F. , Xia, F., Chen, H., Cui, B., Feng, Y., Zhang, P.,... & Luo, M. (2021). A guide to nucleic acid vaccines in the prevention and treatment of infectious diseases and cancers: from basic principles to current applications. Frontiers in cell and developmental biology, 830.
- Raeven, R.H.M.; van Riet, E.; Meiring, H.D.; Metz, B.; Kersten, G.F.A. Systems Vaccinology and Big Data in the Vaccine Development Chain. Immunology 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Ramanathan, A. , Robb, G. B., & Chan, S. H. (2016). mRNA capping: biological functions and applications. Nucleic acids research, 44(16), 7511-7526.
- Rangacharya, O. , Parab, A., Adkine, S., & Nagargoje, R. (2023). A study on the design of an in silico self-amplifying mRNA vaccine against Nipah virus using immunoinformatics. Journal of Biomolecular Structure and Dynamics, 1-12.
- Retzer, A. , Aiyegbusi, O. L., Rowe, A., Newsome, P. N., Douglas-Pugh, J., Khan, S.,... & Calvert, M. (2022). The value of patient-reported outcomes in early-phase clinical trials. Nature medicine, 28(1), 18-20.
- Rosa, S. S. , Prazeres, D. M., Azevedo, A. M., & Marques, M. P. (2021). mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine, 39(16), 2190-2200.
- Rudrapal, M. , Khairnar, S. J., Borse, L. B., & Jadhav, A. G. (2020). Coronavirus disease-2019 (COVID-19): an updated review. Drug research, 70(09), 389-400.
- Schlake, T. , Thess, A., Fotin-Mleczek, M., & Kallen, K. J. (2012). Developing mRNA-vaccine technologies. RNA biology, 9(11), 1319-1330.
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Shahzamani, K. , Mahmoudian, F., Ahangarzadeh, S., Ranjbar, M. M., Beikmohammadi, L., Bahrami, S.,... & Javanmard, S. H. (2021). Vaccine design and delivery approaches for COVID-19. International Immunopharmacology, 100, 108086.
- Sikorski, P.J.; Warminski, M.; Kubacka, D.; Ratajczak, T.; Nowis, D.; Kowalska, J.; Jemielity, J. The Identity and Methylation Status of the First Transcribed Nucleotide in Eukaryotic MRNA 50 Cap Modulates Protein Expression in Living Cells. Nucleic Acids Res. 2020, 48, 1607–1626. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A. Plotkin and Neal A. Halsey. A brief history of vaccines and vaccination. Pediatric Research. 2021; 89(5): 920-927.
- Stanley, A. Plotkin, Walter A. Orenstein, and Paul A. Offit. Health Affairs. 2014"Vaccines: Past, Present, and Future; 33(4): 581-587.
- Tallantyre, E. C. , Vickaryous, N., Anderson, V., Asardag, A. N., Baker, D., Bestwick, J.,... & Dobson, R. (2022). COVID-19 vaccine response in people with multiple sclerosis. Annals of neurology, 91(1), 89-100.
- Tavakoli, S. , Nabizadeh, M., Makhamreh, A., Gamper, H., McCormick, C. A., Rezapour, N. K.,... & Rouhanifard, S. H. (2023). Semi-quantitative detection of pseudouridine modifications and type I/II hypermodifications in human mRNAs using direct long-read sequencing. Nature Communications, 14(1), 334.
- Thorn, C. R. , Sharma, D., Combs, R., Bhujbal, S., Romine, J., Zheng, X.,... & Badkar, A. (2022). THE JOURNEY OF A LIFETIME–DEVELOPMENT OF PFIZER’S COVID-19 VACCINE. Current Opinion in Biotechnology, 102803.
- Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145-160.
- Trenkenschuh, E.; Friess, W. Freeze-Drying of Nanoparticles: How to Overcome Colloidal Instability by Formulation and Process Optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M. N. , & Roni, M. A. (2021). Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines, 9(9), 1033.
- Verbeke, R. , Lentacker, I., De Smedt, S. C., & Dewitte, H. (2021). The dawn of mRNA vaccines: The COVID-19 case. Journal of Controlled Release, 333, 511-520.
- Vishweshwaraiah, Y. L., & Dokholyan, N. V. (2022). Toward rational vaccine engineering. Advanced Drug Delivery Reviews, 114142.
- Wang, H. , Yuan, Q., Sun, L., & Han, X. (2021). Cationic polypeptide-based vaccine delivery systems: Recent advances and future perspectives. Journal of Biomaterials Science, Polymer Edition, 32(1), 1-19.
- Wolfson, A. R. , Robinson, L. B., Li, L., McMahon, A. E., Cogan, A. S., Fu, X.,... & Banerji, A. (2021). First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. The Journal of Allergy and Clinical Immunology: In Practice, 9(9), 3308-3320.
- Xu, S. , Yang, K., Li, R., & Zhang, L. (2020). mRNA vaccine era—mechanisms, drug platform and clinical prospection. International journal of molecular sciences, 21(18), 6582.
- Xu, Y. , Liu, L., Yang, F., Feng, X., & Yan, H. (2020). Cationic polypeptide-based vaccine delivery systems. Journal of Controlled Release, 326, 554-569.
- Xu, Z. , & Fisher, D. (2023). mRNA melanoma vaccine revolution spurred by the COVID-19 pandemic. Frontiers in Immunology, 14, 1660.
- You, H., Jones, M. K., Gordon, C. A., Arganda, A. E., Cai, P., Al-Wassiti, H., ... & McManus, D. P. (2023). The mRNA Vaccine Technology Era and the Future Control of Parasitic Infections. Clinical Microbiology Reviews, e00241-21.
- Zeng, C. , Hou, X., Yan, J., Zhang, C., Li, W., Zhao, W.,... & Dong, Y. (2020). Leveraging mRNA sequences and nanoparticles to deliver SARS-CoV-2 antigens in vivo. Advanced Materials, 32(40), 2004452.
- Zeng, C. , Zhang, C., Walker, P. G., & Dong, Y. (2020). Formulation and delivery technologies for mRNA vaccines.
- Zeng, C.; Hou, X.; Yan, J.; Zhang, C.; Li, W.; Zhao, W.; Du, S.; Dong, Y. Leveraging MRNA Sequences and Nanoparticles to Deliver SARS-CoV-2 Antigens In Vivo. Adv. Mater. 2020, 32, 2004452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C., Maruggi, G., Shan, H., & Li, J. (2019). Advances in mRNA vaccines for infectious diseases. Frontiers in immunology, 594.
- Zhang, N.N.; Li, X.F.; Deng, Y.Q.; Zhao, H.; Huang, Y.J.; Yang, G.; Huang, W.J.; Gao, P.; Zhou, C.; Zhang, R.R.; et al. A Thermostable MRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283e16. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Qi, Y.; Wang, M.; Yu, N.; Nan, F.; Zhang, H.; Tian, M.; Li, C.; Lu, H.; Jin, N. MRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).