Submitted:
02 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mites’ distribution and their economic impact on the poultry production system
3.2. Chemical Control
3.3. Non-chemical measures against blood-sucking mites
3.3.1. Plant-derived compounds
3.3.2. Entomopathogenic fungi
3.3.3. Diatomaceous earth and synthetic silica-based products
| Product | Mite | Test environment | Mortality* | Action | Reference |
|---|---|---|---|---|---|
| Neutral detergent 10%1, diatomaceous earth 10%2 | D. gallinae | Laboratory | 100%1, and 97%2 | Intoxication | [53] |
| Diatomaceous earth 10%1, diatomaceous earth 10% + mechanical cleaning2 | D. gallinae | Laboratory | 93.4%1, and 90%2 | Intoxication and paralysis | [3] |
| Natural diatomaceous earth | D. gallinae | Laboratory | 100% | Intoxication | [104] |
3.3.4. Semiochemicals
3.4.5. Vaccines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Ghaffar, F.; Sobhy, H.M.; Al-Quraishy, S.; Semmler, M. Field study on the efficacy of an extract of neem seed (Mite -Stop®) against the red mite Dermanyssus gallinae naturally infecting poultry in Egypt. Parasitology Research 2008, 103, 481–485. [Google Scholar] [CrossRef]
- Acquah, F.K.; Adjah, J.; Williamson, K.C.; Amoah, L.E. Trasmission-Blocking Vaccines: Old Friends and New Prospects. Infection and Immunity 2019, 87, e00775-18. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.F.A.; Oliveira, D.G.P.; Pares, R.B.; Sparagano, O.A.E.; Godinho, R.P. Association of mechanical cleaning and a liquid preparation of diatomaceous earth in the management of poultry red mite, Dermanyssus gallinae (Mesostigmata: Dermanyssidae). Experimental and Applied Acarology 2020, 81, 215–222. [Google Scholar] [CrossRef]
- Baran, A.I.; Jahanghiri, F.; Hajipour, N.; Sparagano, O.A.E.; Norouzi, R.N.; Moharramnejad, S. In vitro acaricidal activity of essential oil and alcoholic extract of Trachyspermum ammi against Dermanyssus gallinae. Veterinary Parasitology 2020, 278, 109030. [Google Scholar] [CrossRef] [PubMed]
- Bartley, K.; Turnbull, F.; Wright, H.W.; Huntley, J.F.; Palarea-Albaladejo, J.; Nath, M.; Nisbet, A.J. Field evaluation of poultry red mite (Dermanyssus gallinae) native and recombinant prototype vaccines. Veterinary Parasitology 2017, 15, 25–34. [Google Scholar] [CrossRef]
- Bartley, K.; Wright, H.W.; Huntley, J.F.; Manson, E.D.T.; Inglis, N.F.; Mclean, K.; Nath, M.; Batley, Y.; Nisbet, A.J. Identification and evaluation of vaccine candidate antigens from the poultry red mite (Dermanyssus gallinae). International Journal for Parasitology 2015, 45, 819–830. [Google Scholar] [CrossRef]
- Bassani-Silva, R.; Castro-Santiago, A.C.; Calchi, A.C.; Perles, L.; Takatsu, J.C.; Alencar, I.D.C.C.; Ochoa, R.; Dowling, A.P.G.; Werther, K.; André, M.R.; Barros-Battesti, D.M.; Jacinavicius, F.C. Sleeping with the enemy: case reports of Ornithonyssus bursa (Berlese, 1888) (Mesostigmata: Macronyssidae) causing human dermatitis in Brazil. Parasitology Research 2022, 121, 2641–2649. [Google Scholar] [CrossRef]
- Bhowmick, B.; Han, Q. Understanding Tick Biology and its implications in anti-tick and transmission blocking vaccines against Tick-Borne Pathogens. Frontiers in Veterinary Science 2020, 7, 319. [Google Scholar] [CrossRef]
- Billingsley, P.F.; Foy, B.; Rasgon, J.L. Mosquitocidal vaccines: a neglected addition to malaria and dengue control strategies. Trends in Parasitology 2008, 24, 396–400. [Google Scholar] [CrossRef]
- Brannstrom, S.; Hansson, I.; Chirico, J. Experimental study on possible transmission of the bacterium Erysipelothrix rhusiopathiae to chickens by the poultry red mite, Dermanyssus gallinae. Experimental and Applied Acarology 2010, 50, 299–307. [Google Scholar] [CrossRef]
- Brauneis, M.D.; Zoller, H.; Williams, H.; Zschiesche, E.; Heckeroth, R. The acaricidal speed of kill of orally administered fluralaner against poultry red mites (Dermanyssus gallinae) on laying hens and its impact on mite reproduction. Parasites e Vectors 2017, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Vazquez, F.; Schumm, Y.R.; Bentele, A.; Quillfeldt, P.; Merino, S. Experimental manipulation of cavity temperature produces differential effects on parasite abundances in blue tit nests at two different latitudes. International Journal for Parasitology: Parasites and Wildlife 2021, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Catão, R.C. Avaliação da qualidade de ovos de galinhas caipiras, criadas em sistem Cage Free armazenados em tempertaura ambinete e refrigerados. Rural Federal University of Pernambuco: Garanhuns, Brazil, 2019. [Google Scholar]
- Chirico, J.; Tauson, R. Traps containing acaricides for the control of Dermanyssus gallinae. Veterinary Parasitology 2002, 110, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Cocciolo, G.; Circella, E.; Pugliese, N.; Lupini, C.; Mescolini, G.; Catelli, E.; Borchert-Stuhltrager, M.; Zoller, H.; Thomas, E.; Camarda, A. Evidence of vector borne transmission of Salmonella enterica enterica serovar Gallinarum and fowl typhoid mediated by the poultry red mite, Dermanyssus gallinae (De Geer, 1778). Parasites and Vectors 2020, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.H.; Rappuoli, R.; Hotez, P.J.; Duffy, P.E. Transmission-Blocking Vaccines for Malaria: Time to Talk about Vaccine Introduction. Trends in Parasitology 2019, 35, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.M. Dermanyssus gallinae (Acari: Dermanyssidae) (De Geer, 1778): colonização e resposta de protoninfas alimentadas a correntes de ar e a odores de extratos de ácaros co-específicos em olfatômetro discriminante; Federal University of Minas Gerais: Belo Horizonte, Brazil, 2008. [Google Scholar]
- Di-Palma, A.; Giangaspero, A.; Cafiero, M.A.; Germinara, G.S. A gallery of the key characters to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites & Vectors 2012, 5, 104. [Google Scholar] [CrossRef]
- Dinglasan, R.R.; Jacobs-Lorena, M. Flipping the paradigm on malaria transmission-blocking vaccines. Trends in Parasitology 2008, 24, 364–370. [Google Scholar] [CrossRef]
- EMBRAPA. Embrapa Suínos e Aves. Brasília-DF. 2022.
- EPA. United States Environmental Protection Agency. Climate change indicators: weather and climate. United States. 2022.
- Erikson, H.; Brannstrom, S.; Skarin, H.; Chirico, J. Characterization of Erysipelothrix rhusiopathiae isolates from laying hens and poultry red mites (Dermanyssus gallinae) from an outbreak of erysipelas. Avian Pathology 2010, 39, 505–509. [Google Scholar] [CrossRef]
- Faleiro, D.C.C. Ácaros associados a ninhos abandonados por pássaros e a aves de postura de ovos comerciais, no Vale do Taquari, Rio Grande do Sul. Univates University Center: Lajeado, Brazil, 2012.
- FAO-Food and Agriculture Organization of the United Nations. Agricultural production statistics 2000-2020. Rome, Italy, 2022.
- FAO-Food and Agriculture Organization of the United Nations. Gateway to poultry production and products: Production.
- Filho, E.B.; Macedo, L.P.M. Fundamentos de controle biológico de inseto-praga. Federal Institute of Rio Grande do Norte: Natal, Brazil, 2010.
- Fujisawa, S.; Murata, S.; Takehara, M.; Aoyama, J.; Morita, A.; Isezaki, M.; Win, S.Y.; Ariizumi, T.; Sato, T.; Oishi, E.; Taneno, A.; Maekawa, N.; Okagawa, T.; Ichii, O.; Konnai, S.; Ohashi, K. In vitro characterization of adipocyte plasma membrane-associated protein from poultry red mites, Dermanyssus gallinae, as a vaccine antigen for chickens. Vaccine 2021, 39, 6057–6066. [Google Scholar] [CrossRef]
- Gabriel, M.H. Diversidade e taxonomia de ácaros de pena (arachnida: acari: astigmata) em aves no campus da UNESP de Rio Claro. São Paulo State University Júlio de Mesquita Filho: Rio Claro, Brazil, 2019.
- George, D.R. Effect of plant essential oils as acaricides against the poultry red mite, Dermanyssus gallinae, with special focus on exposure time. Veterinary Parasitology 2010, 169, 222–225. [Google Scholar] [CrossRef]
- George, D.R.; Finn, R.D.; Graham, K.M.; Mul, M.F.; Maurer, V.; Moro, C.V. Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasites & Vectors 2015, 178, 1–10. [Google Scholar]
- George, D.R.; Masic, D.; Sparagano, O.A.E.; Guy, J.H. Variation in chemical composition and acaricidal activity against Dermanyssus gallinae of four eucalyptus essential oils. Experimental and Applied Acarology 2008, 48, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.; Canales, M.; Fuente, J.L.; Luna, C.; Robinson, K.; Guy, J.; Sparagano, O. Immunisation with recombinant proteins subolesin and Bm86 for the control of Dermanyssus gallinae in poultry. Vacine. 2009, 27, 4056–4063. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.; El Din, H.M.; Guy, J.; Robinson, K.; Spagarano, O. Characterization of the immune response of domestic fowl following immunization with proteins extracted from Dermanyssus gallinae. Veterinary Parasitology 2008, 160, 285–294. [Google Scholar] [CrossRef]
- Hartcher, K.M.H.; Jones, B. The welfare of layer hens in cage and cage-free housing systems. World’s Poulty Science Journal 2017, 73, 767–782. [Google Scholar] [CrossRef]
- Hinkle, N.C.; Jirjis, F.; Szewczyk, E.; Sun, F.; Flochlay-Sigognault, A. Efficacy and safety assessment of a water-soluble formulation of fluralaner for treatment of natural Ornithonyssus sylviarum infestations in laying hens. Parasites and Vectors 2018, 11. [Google Scholar] [CrossRef]
- Horn, T.B.; Rocha, M.S.; Granich, J.; Korbes, J.H.; Alves, L.F.A.; Ferla, N.J. Ectoparasitism of commercial laying hen by Megninia ginglymura (Mégnin) (Acari): population dynamic and distribution on the body regions. Poultry Science 2017, 96, 4253–4260. [Google Scholar] [CrossRef]
- Jarrett, R.A.; Erasmus, M.A.; Murillo, A.C.; Scoles, K.L.; Robison, C.I.; Jones, D.R.; Karcher, D.M. Laying hen production and welfare in a cage-free setting is impacted by the northern fowl mite. Journal of Applied Poultry Research 2022, 31, 100290. [Google Scholar] [CrossRef]
- Junior, E.C.G. Demanda por ovos produzidos em sistemas livres de gaiolas: motivação, estratégias e estruturas de governança. Federal University of Uberlândia: Uberlândia, Brazil, 2018.
- Kaoud, H.A.; El-Dahshan, R.A. Effect of Red Mite (Dermanyssus gallinae) Infestation on the Performance and Immune Profile in Vaccinated broiler breeder flocks. Journal of American Science 2010, 6, 72–79. [Google Scholar]
- Kasburg, C.R. Seleção e caracterização de isolados de fungos entomopatogênicos visando ao controle do ácaro vermelho Dermanyssus gallinae (Acari: Dermanyssidae). State University of Western Paraná: Cascavel, Brazil, 2016.
- Kilpinen, O. Activation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae), by increasing temperatures. Experimental and Applied Acarology 2001, 25, 859–867. [Google Scholar] [CrossRef]
- Kim, S.; Yi, J.H.; Tak, J.H.; Ahn, Y.J. Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari: Dermanyssidae. Veterinary Parasitology 2004, 120, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, H.K.; Kim, G.H. Toxicity and efects of essential oils and their components on Dermanyssus gallinae (Acari: Dermanyssidae). Experimental and Applied Acarology 2019, 78, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Leite, R.G.; Harada, E.S.; Salgado, D.D.; Neto, M.M.; Bueno, L.G.F. Behavior of laying hens in cage free system at different ages and egg quality. Research, Society and Development 2021, 10, e6010413833. [Google Scholar] [CrossRef]
- Lima-Barrero, J.F.; Contreras, M.; Bartley, K.; Price, D.R.G.; Nunn, F.; Sanchez-Sanchez, M.; Prado, E.; Hofle, U.; Villar, M.; Nisbet, A.J.; Fuente, J. Reduction in Oviposition of Poultry Red Mite (Dermanyssus gallinae) in Hens Vaccinated with Recombinant Akirin. Vaccines 2019, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Lima-Barbero, J.F.; Sánchez, M.S.; Cabezas-Cruz, A.; Mateos-Hernández, L.; Contreras, M.; e Mera, I.G.F.; Villar, M.; Fuente, J. Clinical gamasoidosis and antibody response in two patients infested with Ornithonyssus bursa (Acari: Gamasida: Macronyssidae). Experimental and Applied Acarology 2019, 78, 555–564. [Google Scholar] [CrossRef]
- Lima-Barbero, J.F.; Villar, M.; Hofle, U.; Fuente, J. Challenges for the Control of Poultry Red Mite (Dermanyssus gallinae). Parasitology and Microbiology Research 2020. [Google Scholar]
- Lundh, J.; Wiktelius, D.; Chirico, J. Azadirachtin-impregnated traps for the control of Dermanyssus gallinae. Veterinary Parasitology 2005, 130, 337–342. [Google Scholar] [CrossRef]
- Makert, G.R.; Vorbruggen, S.; Krautwald-Junghanns, M.E.; Voss, M.; Sohn, K.; Buschmann, T.; Ulbert, S. A method to identify protein antigens of Dermanyssus gallinae for the protection of birds from poultry mites. Parasitology Research 2016, 115, 2705–2713. [Google Scholar] [CrossRef]
- Mariano, R.M.S.; Gonçalves, A.A.M.; Oliveira, D.S.; Ribeiro, H.S.; Pereira, D.F.S.; Santos, I.S.; Lair, D.F.; Silva, A.V.; Galdino, A.S.; Chávez-Fumagalli, A.A.; Silveira-Lemos, D.; Dutra, W.O.; Giunchetti, R.C. A Review of Major Patents on Potential Malaria Vaccine Targets. Pathogens 2023, 12, 247. [Google Scholar] [CrossRef]
- Morrone, F.; Mayworm, M.A.S. Acaricidal action of foliar extracts of Coffea species (Rubiaceae) on Dermanyssus Gallinae (De Gerr, 1778) (Acari, Dermanyssidae). Archives of the Biological Institute 2001, 68, 43–47. [Google Scholar]
- Meyer-Kuhling, B.; Pfister, K.; Muller-Lindloff, J.; Heine, J. Field efficacy of phoxim 50% (ByeMite1) against the poultry red mite Dermanyssus gallinae in battery cages stocked with laying hens. Veterinary Parasitology 2007, 147, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Miguel, R.F. Eficiência de métodos não convencionais para o controle do ácaro vermelho Dermanyssus gallinae (De Geer, 1778) (Mesostigmata: Dermanyssidae). Federal Technological University of Paraná: Santa Helena, Brazil, 2019.
- Mironov, S.V. Allopsoroptoides galli n. g., n. sp., a new genus and species of feather mites (Acari: Analgoidea: Psoroptoididae) causing mange in commercially raised domestic chicken in Brazil. Cyst Parasitol 2013, 85, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.V.; Chauve, C.; Zenner, L. Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae. Veterinary Parasitology 2007, 146, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.V.; Thioulouse, J.; Chauve, C.; Zenner, L. Diversity, geographic distribution, and habitat-specific variations of microbiota in natural populations of the chicken mite, Dermanyssus gallinae. Journal of Medical Entomology 2011, 48, 788–796. [Google Scholar] [CrossRef]
- Moya, C.A.G.; Flechtmann, C.H.W.; Bermúdez, S.E.C. Primer reporte de Ornithonyssus bursa (Berlese, 1888) (Acari: Mesostigmata: Macronyssidae) en República Dominicana. Novitates Caribaea 2022, 21, 76–83. [Google Scholar]
- Mullens, B.A.; Murillo, A.C.; Zoller, H.; Heckeroth, A.R.; Jirjis, F.; Sigognault, A.F. Comparative in vitro evaluation of contact activity of fluralaner, spinosad, phoxim, propoxur, permethrin and deltamethrin against the northern fowl mite, Ornithonyssus sylviarum. Parasites and Vectors 2017, 10. [Google Scholar] [CrossRef]
- Mullens, B.A.; Owen, J.P.; Kuney, D.R.; Szijj, C.E.; Klinger, K.A. Temporal changes in distribution, prevalence and intensity of northern fowl mite (Ornithonyssus sylviarum) parasitism in commercial caged laying hens, with a comprehensive economic analysis of parasite impact. Veterinary Parasitology 2009, 160, 116–133. [Google Scholar] [CrossRef]
- Murata, S.; Taniguchi, A.; Isezaki, M.; Fujisawa, S.; Sakai, E.; Taneno, A.; Ichii, O.; Ito, T.; Maekawa, N.; Okagawa, T.; Konnai, S.; Ohashi, K. Characterisation of a cysteine protease from poultry red mites and its potential use as a vaccine for chickens. Parasite 2021, 28. [Google Scholar] [CrossRef]
- Murillo, A.C.; Abdoli, A.; Blatchford, R.A.; Keogh, E.J.; Gerry, A.C. Parasitic mites alter chicken behaviour and negatively impact animal welfare. Scientific Reports 2020, 10, 8236. [Google Scholar] [CrossRef]
- Murillo, A.C.; Chappell, M.A.; Owen, J.P.; Mullens, B.A. Northern fowl mite (Ornithonyssus sylviarum) effects on metabolism, body temperatures, skin condition, and egg production as a function of hen MHC haplotype. Poultry Science 2016, 95, 2536–2546. [Google Scholar] [CrossRef]
- Murillo, A.C.; Mullens, B.A. A review of the biology, ecology, and control of the northern fowl mite, Ornithonyssus sylviarum (Acari: Macronyssidae). Veterinary Parasitology 2017, 246, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.C.; Mullens, B.A. Collecting and Monitoring for Northern Fowl Mite (Acari: Macronyssidae) and Poultry Red Mite (Acari: Dermanyssidae) in Poultry Systems. Journal of Insect Science 2020, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M. Armadilhas impregnadas com Beauveria bassiana (Bals.) Vuill. Visando ao controle de Dermanyssus gallinae (De Geer) (Acari: Dermanyssidae). State University of Western Paraná: Cascavel, Brazil, 2019.
- Na, Y.E.; Kim, S.I.; Bang, H.S.; Kim, B.S.; Ahn, Y.J. Fumigant toxicity of cassia and cinnamon oils and cinnamaldehyde and structurally related compounds to Dermanyssus gallinae (Acari: Dermanyssidae). Veterinary Parasitology 2011, 178, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Nechita, I.S.; Poirel, M.T.; Cozma, V.; Zenner, L. The repellent and persistent toxic effects of essential oils against the poultry red mite, Dermanyssus gallinae. Veterinary Parasitology 2015, 214, 348–352. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Tonin, A.A.; Monteiro, S.G. Ornithonyssus bursa mite parasitism in humans in southern Brazil. Acta Scientiae Veterinariae 2012, 40, 1–3. [Google Scholar]
- Oliveira, T.M. Bioeconomia das infestações de moscas sinantrópicas e ácaros hematófagos em galpão de granja de postura do estado de Minas Gerais. Federal University of Minas Gerais, Belo Horizonte, Brazil, 2021.
- Oliveira, T.M.; Teixeira, C.M.; Araújo, I.L.; Rezende, L.C.; Cunha, L.M.; Diniz, S.A.; Silva, M.X. Epidemiology and risk assessment associated with the presence of hematophagous mites in poultry laying sheds. Brazilian Archive of Veterinary Medicine and Animal Science 2020, 72, 2148–2156. [Google Scholar]
- Patz, J.A.; Reisen, W.K. Immunology, climate change and vector-borne diseases. Trends in Immunology 2001, 22, 171–172. [Google Scholar] [CrossRef]
- Pageat, P. Allomone repulsive and kairomone attractive compositions for controlling arachnds. Patent Application Publication 2014, 23. [Google Scholar]
- Pavan, A.M.; Schussler, M.; Silva, F.R.; Ferla, N.J.; Joham, L.; Silva, G.L. Influence of laying hen-systems and ecologic variables on mites of medical and veterinary importance. Veterinary Parasitology 2022, 304, 109682. [Google Scholar] [CrossRef]
- Percilio, C.S.P.; Oliveira, W.D.; Lages, L.S.; Araújo, K.K.C.; Bezerra, D.C.; Bezerra, N.P.C.; Fonseca, L.S.; Coimbra, V.C.S. Socioeconomic, productive and sanitary profile of commercial poultry in the sertão of Maranhão. Zootechnics: Research and Contemporary Practices 2022, 3, 136–150. [Google Scholar]
- Pereira, D.F.S.; Ribeiro, H.S.; Gonçalves, A.A.M.; Silva, A.V.; Lair, D.F.; Oliveira, D.S.; Vilas Boas, D.F.; Conrado, I.S.S.; Leite, J.C.; Barata, L.M.; Reis, P.C.C.; Mariano, R.M.S.; Santos, T.A.P.; Coutinho, D.C.O.; Gontijo, N.F.; Araujo, R.N.; Galdinho, A.S.; Paes, P.R.O.; Melo, M.M.; Nagem, R.A.P.; Dutra, W.O.; Silveria-Lemos, D.; Rodrigues, D.S.; Giunchetti, R.C. Rhipicephalus microplus: An overview of vaccine antigens against the cattle tick. Ticks and Tick-borne Diseases. 2022, 13, 101828. [Google Scholar] [CrossRef] [PubMed]
- Price, D.R.G.; Kuster, T.; Pines, O.; Oliver, E.M.; Bartley, K.; Nunn, F.; Barbero, J.F.L.; Pritchard, J.; Karp-Tatham, E.; Hauge, H.; Blake, D.P.; Tomley, F.M.; Nisbet, A.J. Evaluation of vaccine delivery systems for inducing long-lived antibody responses to Dermanyssus gallinae antigen in laying hens. Avian Pathology 2019, 48, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.; Kuster, T.; Sparagano, O.; Tomley, F. Understanding the biology and control of the poultry red mite Dermanyssus gallinae: a review. Avian Pathology. 2014, 44, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Raele, D.A.; Galante, D.; Pugliesi, N.; La Salandra, G.; Lomuto, M.; Cafiero, M.A. First report of Coxiella burnetii and Borrelia burgdorferi sensu lato in poultry red mites, Dermanyssus gallinae (Mesostigmata, Acari), related to urban outbreaks of dermatitis in Italy. New Microbes and New Infections 2018, 23, 103–109. [Google Scholar] [CrossRef]
- Reis, T.L.; Quintero, J.C.P.; Luchese, R.H.; Adler, G.H.; Freitas Júnior, C.V.; Silva, L.G.; Calixto, L.F.L. Influence of floor rearing system on bed and cage on bone characteristics and physical-chemical and microbiological quality of chicken eggs. Brazilian Archive of Veterinary Medicine and Animal Science 2019, 71, 1623–1630. [Google Scholar]
- Rezende, L.C.; Cunha, L.M.; Martins, N.R.S.; Teixeira, C.M.; Oliveira, P.R. Epidemiology of Megninia spp. in laying establishments in the State of Minas Gerais, Brazil. Brazilian Journal of Veterinary Parasitology 2015, 24, 198–203. [Google Scholar]
- Rezende, L.C.; Cunha, L.M.; Teixeira, C.M.; Oliveira, P.R.; Martins, N.R.S. Mites affecting hen egg production: some considerations for Brazilian farms. Rural Sciense 2014, 43, 1230–1237. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Pereira, D.F.S.; Melo-Junior, O.; Mariano, R.M.S.; Leite, J.C.; Silva, A.V.; Oliveira, D.S.; Gonçalves, A.A.M.; Lair, D.F.; Soares, I.S.; Santos, T.A.P.; Galdinho, A.S.; Silveria-Lemos, D.; Paes, P.R.O.; Melo, M.M.; Dutra, W.O.; Araujo, R.N.; Giunchetti, R.C. Vaccine approaches applied to controlling dog ticks. Ticks and Tick-borne Diseases 2021, 12, 101631. [Google Scholar] [CrossRef]
- Santos, A.M.; Jurkevicz, R.M.B.; Romani, I.; Simone, M.R.C.; Antonucci, A.M. Use of Eborellia sp. (Dermaptera: forficulidae) for biological control of Mesostigmatid mites in laying hen farms. Brazilian Journal of Development 2022, 8, 55412–55429. [Google Scholar] [CrossRef]
- Schulz, J.; Berk, J.; Suhl, J.; Schrader, L.; Kaufhold, S.; Mewis, I.; Hafez, H.M.; Ulrichs, C. Characterization, mode of action, and efficacy of twelve silica-based acaricides against poultry red mite (Dermanyssus gallinae) in vitro. Parasitology Research 2014, 113, 3167–3175. [Google Scholar] [CrossRef]
- Shimp, R.L., Jr.; Rowe, C.; Reiter, K.; Chen, B.; Nguyen, V.; Aebig, J.; Rausch, K.M.; Kumar, K.; Wu, Y.; Jin, A.J.; Jones, D.S.; Narum, D.L. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 2013, 31, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Sillos, P.P. Sazonalidade e testes de eficácia com drogas contra o Dermanyssus gallinae (DE GEER, 1778) e Ornithonyssus sylviarum (Canestrini and Fanzago, 1877) nas granjas de postura da região metropolitana de Curitiba. Federal University of Paraná: Curitiba, 2002.
- Silva, I.J.O.; Abreu, P.G.; Mazucco, H. Embrapa Suínos e Aves. Manual de boas práticas para o bem-estar de galinhas poedeiras criadas livres de gaiola. Concórdia, Suínos e Aves, 2020.
- Sioutas, G.; Petridou, E.; Minoudi, S.; Papadopoulos, K.V.; Symeonidou, I.; Giantsis, I.A.; Triantafyllidis, A.; Papadopoulos, E. Isolation of Listeria monocytogenes from poultry red mite (Dermanyssus gallinae) infesting a backyard chicken farm in Greece. Scientific Reports 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.R.; Ximenes, L.F. Egg Production. Northeast Economic Studies Technical Office, 2022. [Google Scholar]
- Soares, N.M.; Tucci, E.C.; Guastalli, E.A.L.; Yajima, H. Control of infestation by Onithornyssus sylviarum (Canestrini and Fanzago, 1877 (Acari: Macronyssidae) in commercial laying hens using Azadirachta indica extract. Brazilian Journal of Veterinary Parasitology 2008, 17, 175–178. [Google Scholar]
- Soares, S.S. Ectoparasitismo de aves em um fragmento de Mata Atlântica no Distrito de Cacaria (Piraí, RJ). Federal Rural University of Rio de Janeiro: Seropédica, Brazil, 2018.
- Sparagano, O.A.E.; George, D.R.; Harrington, D.W.J.; Giangaspero, A. Significance and control of the poultry red mite, Dermanyssus gallinae. Annual Review of Entomology 2014, 59, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Sparagano, O.A.E.; Ho, J. Parasitic Mite Fauna in Asian Poultry Farming Systems. Frontiers in Veterinary Science 2020, 7. [Google Scholar] [CrossRef]
- Sulzbach, A.; Mallmann, D.; Silva, F.R.; Ferla, N.J.; Silva, G.L.; Johann, L. In vitro evaluation of the Dermanyssus gallinae to productsin aqueous suspension. Experimental and AppliedAcarology 2022, 86, 201–2009. [Google Scholar]
- Tabari, M.A.; Jafari, A.; Jafari, M.; Youssefi, M.R. Laboratory and field efficacy of terpene combinations (carvacrol, thymol and menthol) against the poultry red mite (Dermanyssus gallinae). Veterinary Parasitology 2023, 313, 109842. [Google Scholar] [CrossRef]
- Tabari, M.A.; Khodashenas, A.; Jafari, M.; Petrelli, R.; Cappellacci, L.; Nabissi, M.; Maggi, F.; Pavela, R.; Youssefi, M.R. Acaricidal properties of hemp (Cannabis sativa L.) essential oil against Dermanyssus gallinae and Hyalomma dromedarii. Industrial Crops and Products 2020, 147, 112238. [Google Scholar] [CrossRef]
- Taborda, J.V.S. Viabilidade econômica do sistema Cage-free para poederias comerciais. Brazil University: Descalvado, Brazil, 2018.
- Takken, W.; Knols, B.G.J. Olfaction in vector – host interactions. In Ecology and control of vector-borne diseases, 2nd ed.; Wageningen Academic Publishers: The Netherlands, 2010. [Google Scholar]
- Teixeira, C.M.; Mendonça de Oliveira, T.; Soriano-Araújo, A.; Rezende, L.C.; Roberto de Oliveira, P.; Cunha, L.C.; Martins, N.R.S. Ornithonyssus sylviarum (Acari: Macronyssidae) parasitism among poultry farm workers in Minas Gerais state, Brazil. Parasitology 2020, 50, e20190358. [Google Scholar] [CrossRef]
- Teixeira, C.M. Population dynamics and strategic control of Ornithonyssus sylviarum (Acari: Macronyssidae) in commercial laying farms in Minas Gerais, Brazil. Federal University of Minas Gerais: Belo Horizonte, Brazil, 2016.
- Thomas, E.; Zoller, H.; Liebisch, G.; Alves, L.F.A.; Vettorato, L.; Chiummo, R.M.; Sigognault-Flochay, A. In vitro activity of fluralaner and commonly used acaricides against Dermanyssus gallinae isolates from Europe and Brazil. Parasites e Vectors 2018, 11, 361. [Google Scholar] [CrossRef]
- Tucci, E.C.; Guastali, E.A.L.; Rebouças, M.M.; Mendes, M.C.; Gama, N.M.S.Q. Megninia infestation app. in industrial breeding of poultry producing eggs for consumption. Biological Institute Archives 2005, 72, 121–124. [Google Scholar] [CrossRef]
- Tucci, E.D.; Soares, N.M.; Faccini, J.L.H.; Boas, D.V. Additional information about a mange outbreak by Allopsoroptoides galli (Acari: Psoroptoididae) in commercial laying hens in the state of São Paulo. Brazilian Veterinary Research 2014, 34, 760–762. [Google Scholar] [CrossRef]
- Ulrichs, C.; Han, Y.J.; Abdelhamid, M.T.; Mewis, I. Management of the poultry red mite, Dermanyssus gallinae, using silica-based acaricides. Experimental and Applied Acarology 2020, 82, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Venu, R.; Rao, P.V.; Surya, U.N.S. Severe infestation of Ornithonyssus bursa in a commercial poultry layer farm and its successful management. Journal of Entomology and Zoology Studies 2020, 8, 1490–1492. [Google Scholar] [CrossRef]
- Vezzoli, G.; King, A.J.; Mench, J.A. The effect of northern fowl mite (Ornithonyssus sylviarum) infestation on hen physiology, physical condition, and egg quality. Poultry Science 2016, 95, 1042–1049. [Google Scholar] [CrossRef]
- Waap, H.; Aguin-Pombo, D.; Maia, M. Case Report: Human Dermatitis Linked to Ornithonyssus bursa (Dermanyssoidea: Macronyssidae) Infestation in Portugal. Frontiers in Veterinary Science 2020, 7. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.; Huang, Y.; Su, S.; Wang, L.; Sun, Y.; Wan, Q.; Li, H.; Zhang, S.; Øines, Ø.; Pan, B. Darkness increases the population growth rate of the poultry red mite Dermanyssus gallinae. Parasites Vectors 2019, 12. [Google Scholar] [CrossRef]
- Wright, H.W.; Bartley, K.; Huntley, J.F.; Nisbet, A.J. Characterisation of tropomyosin and paramyosin as vaccine candidate molecules for the poultry red mite, Dermanyssus gallinae. Parasites e Vectors 2016, 09. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Zhang, S.; Huang, Y.; Pan, T.; Wang, B.; Pan, B. Acaricidal efcacy of orally administered macrocyclic lactones against poultry red mites (Dermanyssus gallinae) on chicks and their impacts on mite reproduction and blood-meal digestion. Parasites and Vectors 2019, 12. [Google Scholar]
- Xu, X.; Wang, C.; Zhang, S.; Huang, Y.; Pan, T.; Wang, B.; Pan, B. Evaluation of the vaccine efficacy of three digestive protease antigens from Dermanyssus gallinae using an in vivo rearing system. Vaccine 2020, 17, 7842–7849. [Google Scholar] [CrossRef]
- Zamora-Vilchis, I.; Williams, S.E.; Johnson, C.N. Environmental temperature affects prevalence of blood parasites of birds on an elevation gradient: implications for disease in a warming climate. Plos One 2012, 7, e39208. [Google Scholar] [CrossRef] [PubMed]
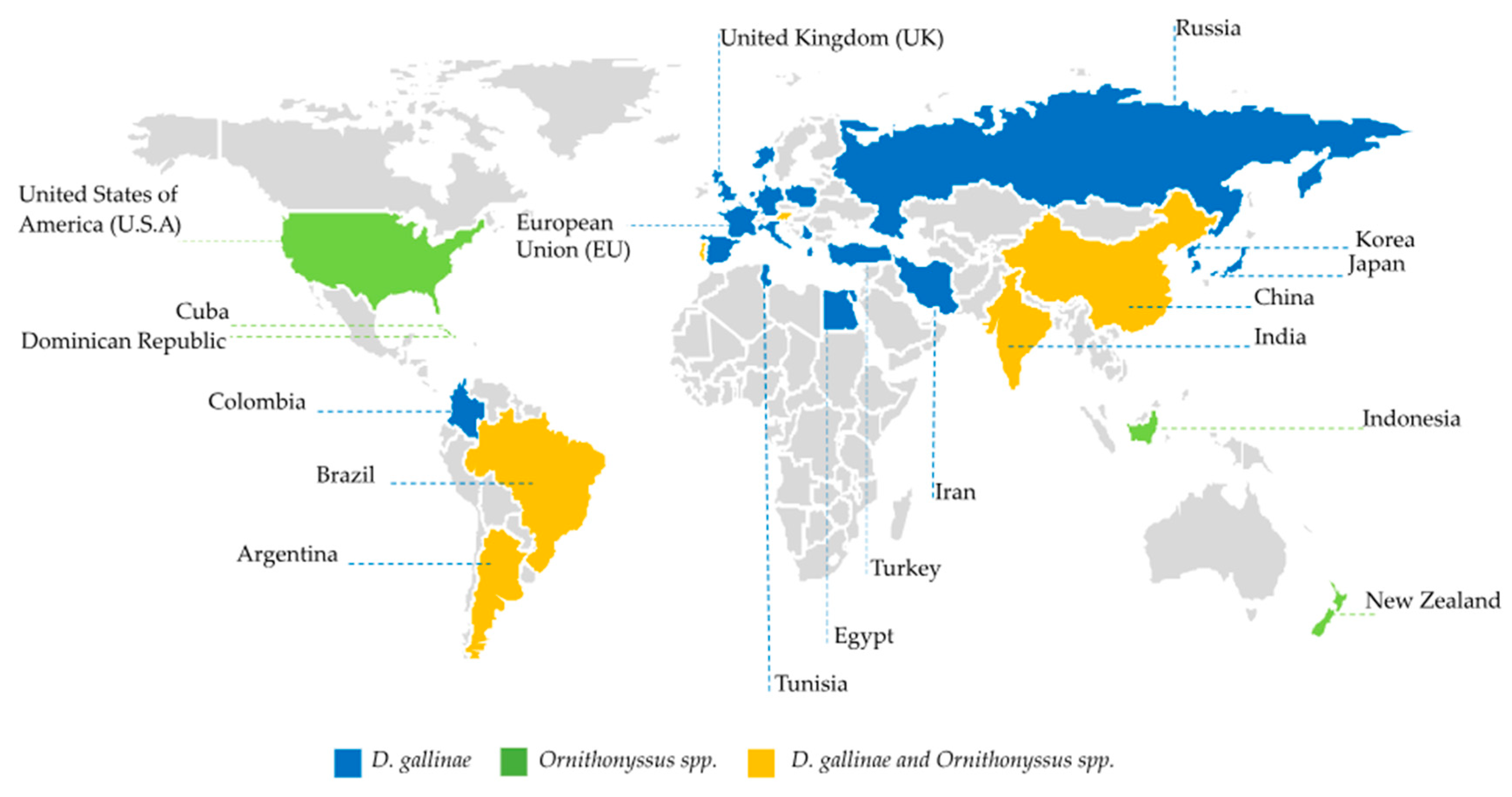
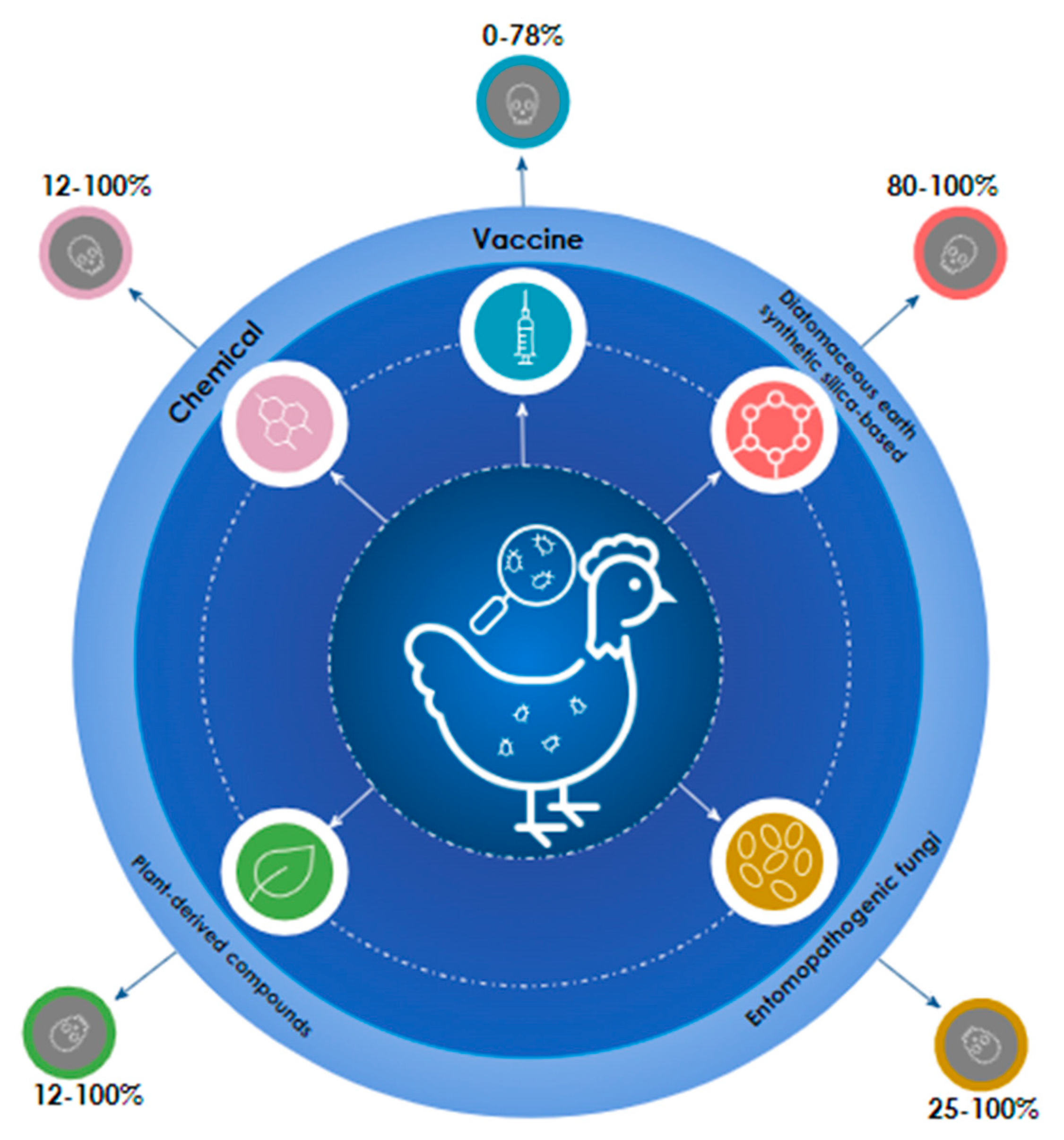
| Product | Chemical class | Mite | Test environment | Mortality* | Action | Reference |
|---|---|---|---|---|---|---|
| Metrifonate (trichlorfon) | Organophosphate | D. gallinae | Field | 99% | Paralysis and death | [14] |
| D.D.V.P (Dichlorvos) diluted in water1,D.D.V.P (Dichlorvos) diluted in oil 2, deltamethrin3, amitraz4 | Organophosphate1,organophosphate2, pyrethroid3 and formadin4 | D. gallinae, O. sylviarum | Laboratory | DL50=513.34 ppm1, DL50=314.15 ppm2, DL50=389.57 ppm3, and DL50=347.24 ppm4# | Paralysis and death | [86] |
| Phoxim 50% | Organophosphate | D. gallinae | Field | 99% | Paralysis and death | [52] |
| Cypermethrin and Cypermethrin1 + Chlorpyrifos2 | Pyrethroid1 and Pyrethroid2 | O. sylviarum | Laboratory | > 95% | Paralysis and death | [100] |
| Fluralaner | Isoxazoline | D. gallinae | Laboratory | 100% | Paralysis and death | [11] |
| Fluralaner1, Spinosad2, Phoxim3, Propoxur4, Permethrin5 and Deltamethrin6 | Isoxazoline1, Macrocyclic lactone2, Organophosphate3, Carbamate4, Pyrethroid5 and Pyrethroid6 | O. sylviarum | Laboratory | 100%1, 98% 2, 100%3, 100%4, 12%5, and 14%6 | Paralysis and death | [58] |
| Fluralaner | Isoxazoline | O. sylviarum | Laboratory | >90% | Paralysis and death | [35] |
| Fluralaner | Isoxazoline | D. gallinae | Field1 and laboratory2 | 190,6%, and 2100% | Paralysis and death | [101] |
| Phoxim | Organophosphate | D. gallinae | Field1 and laboratory2 | 100%1, and 100%2 | Paralysis and death | [101] |
| Cypermethrin | Pyrethroid | D. gallinae | 1Field | 15.6% | Paralysis and death | [101] |
| Moxidectin1, ivermectin2 and eprinomectin3 | Macrocyclic lactone | D. gallinae | Laboratory | 45.60%1, 71.32%2, and 100%3 | Paralysis and death | [110] |
| Cypermethrin + Chlorpyrifos + Piperonyl Butoxide1, Alkyl Benzyl Dimethyl Ammonium, Chloride + Glutaraldehyde + Deltamethrin2, Dichlorvos3, and Fluralaner4 | Pyrethroid + organophosphosphateus1 + organic compound, pyrethroid2, organophospha te3 and isoxazoline4 | D. gallinae | Laboratory | 197.5%, 2100%, 3100%, and 4100% | Paralysis and death | [94] |
| Product | Mite | Type of assay | Mortality (M)/Repellency (R) | Action | Reference |
|---|---|---|---|---|---|
| Coffea aqueous extract1 and Coffea chloroform extract 2 | D. gallinae | Laboratory | M =25%1, and 100%2 | Intoxication* | [51] |
| Neem Oil1, Assist2 | D. gallinae, O. sylviarum | Laboratory | M = 42.86%1, and 15% 2 | Intoxication | [86] |
| Oil (individual) of bay, cade, cumin seed, ceylon cardamin, cedarwood, cinnamon, clove bud, clover leaf, coriander, eucalyptus, fir needle, ginger, horseradish, juniper berry, lavender, lemon 10, lemongrass, limedis 5F, mandarin orange, marjoram, mustard, oregano, palmarosa, pennyroyal, peppermint, pimento berry, rosemary, rosemary, peppermint, tea tree, thyme, haiti vetiver and absinthe | D. gallinae | Laboratory | M = 100% | Intoxication | [42] |
| Basil1 oil or extract, java citronella2, clary sage3, geranium4, nutmeg5 and sage6 | D. gallinae | Laboratory | M = 56%1, 96%2, 92%3, 93%4, 51%5, and 89% 6 | Intoxication | [42] |
| Neem oil | D. gallinae | Field | M = 92% | Intoxication | [48] |
| Neem seed extract | D. gallinae | Field | M = 80% | Intoxication | [1] |
| Eucalyptus essential oil: Eucalyptus citriodora1, E. staigeriana2, E. globulus3 and E. radiata4. | D. gallinae | Laboratory | M = 85%1,>65%2, 11%3, and 19% 4 | Intoxication | [31] |
| 2% liquid neem leaf extract + mineral oil + 0.1% degerming agent | O. sylviarum | Laboratory | M = > 50% | Intoxication | [90] |
| Thyme oil | D. gallinae | Laboratory | M = 50% | Intoxication | [29] |
| Lavender oil1, thyme oil 2, oregano oil3, juniper oil4 | D. gallinae | Laboratory | M =>97%1, 84%2, 50%3, and 50%4 | Intoxication | [67] |
| Acerola cherry oil (individual), bergamot peel, caraway, cinnamon bark, cinnamon leaf, java citronella, clary sage, clove bud, garlic, gurjan balm, hyssop, lavender, lemon peel, lemongrass, lime, marjoram, mint avensis, mustard, onion, pennyroyal, peppermint, pine, rosemary, white thyme | D. gallinae | Laboratory | M = 100% | Intoxication | [43] |
| Cedarwood oil1, redhead oil 2, grapefruit oil3, lemon oil4, peanut 5 oil, sandalwood oil6 | D. gallinae | Laboratory | M = 48.9%1, 42;2%2, 8;9%3, 33.3%4, 8.9% 5, and 20%6 | Intoxication | [43] |
| Clove bud and leaf oil1, steamed lychee oil2, hemp essential oil3 | D. gallinae | Laboratory | M = 100%1, 80%2, and 79.26%3 | Intoxication | [96] |
| Diatomaceous earth 10%1, Diatomaceous earth 10% + mechanical cleaning2 | D. gallinae | Laboratory | M = 93.4%1, and 90%2 | Intoxication and paralysis | [3] |
| Ajowan essential oil and ajowan alcoholic extract | D. gallinae | Laboratory | >90% | Intoxication | [4] |
| Product | Mite | Test environment | Mortality* | Reference |
|---|---|---|---|---|
| Entomopathogenic fungi: Beauveria bassiana1 and Metarhizium anisopliae2 | D. gallinae | Laboratory | 78%1, and 44%2 | [40] |
| Solution of entomopathogenic fungi: Beauveria bassiana + Metarhizium anisopliae | D. gallinae | Field | 61.7% | [40] |
| Fungus Trap: Trichoderma album | D. gallinae | Field and laboratory | 100% | [65] |
| Fungus Trap: Beauveria bassiana | D. gallinae | Field1 andlaboratory2 | 80%1, and 100%2 | [65] |
| Formulated with entomopathogenic fungi: Beauveria bassiana | D. gallinae | Laboratory | 98% | [53] |
| Entomopathogenic fungus: Aspergillus oryzae | D. gallinae | Laboratory | 24.83% | [108] |
| Antigen | Presentation | IgY levels* | Feeding challenge/model | Efficiency/mortality | Action | Reference |
|---|---|---|---|---|---|---|
| DGE | Brute | ↑ (p ≤ 0.05) | in vitro/laboratory | 50.60% | Tissue paralysis | [33] |
| Bm86 | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 23.03% | Interference with the digestive system | [32] |
| Subolesin | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 35.10% | Interference in the expression of gene regulation of transcription | [32] |
| Tropomyosin D. gallinae (Der g 10) | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 19% | Interference with muscle movement and structural integrity of tissue | [109] |
| Paramyosin (Der g 11) | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 23% | interference with muscle movement and structural integrity of tissue | [109] |
| SME | Brute | ↑ (p ≤ 0.05) | in vitro/laboratory | 78.00% | − | [5] |
| (Deg-VIT-1) +(Deg-SRP-1) +(Deg-PUF -1) | Recombinant | ↑ (p ≤ 0.05) | In vivo/Field | 0% | − | [5] |
| PRM | Brute | ↑ (p ≤ 0.05) | in vitro/laboratory | 58.30% | − | [5] |
| Deg-AKR | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 42%* | − | [45] |
| CatD-1 in Montanide™ ISA 71 VG adjuvant | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 50%* | − | [76] |
| Dg-CatD-1 DNA | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 0% | − | [76] |
| Dg-CatD-1 E. tenella | Transgenic | ↑ (p ≤ 0.05) | in vitro/laboratory | 0% | − | [76] |
| rDg-CatD-1 (Cathepsin D, CatD) | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 63.40% | Interference in the digestive process | [111] |
| rDg-CatL-1(Cathepsin L, CatL) | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 48.01% | Interference in the digestive process | [111] |
| rDg-Lgm (legumain, Lgm) | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 18.37% | Interference in the digestive process | [111] |
| Dg-APMAP | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | 61.88% | Plasma membrane interference | [27] |
| Deg-CPR-1 | Recombinant | ↑ (p ≤ 0.05) | in vitro/laboratory | >50% | Interference in the digestive process | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





