Submitted:
02 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
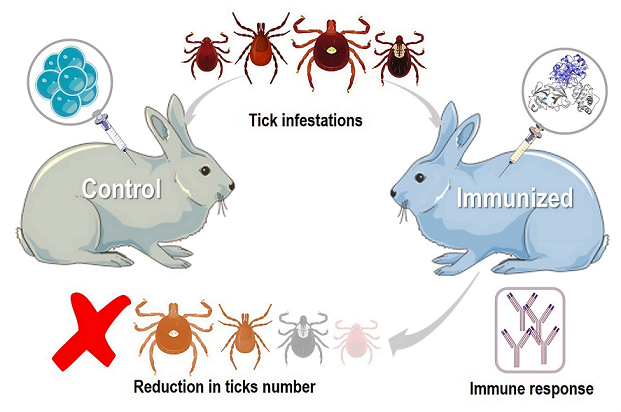
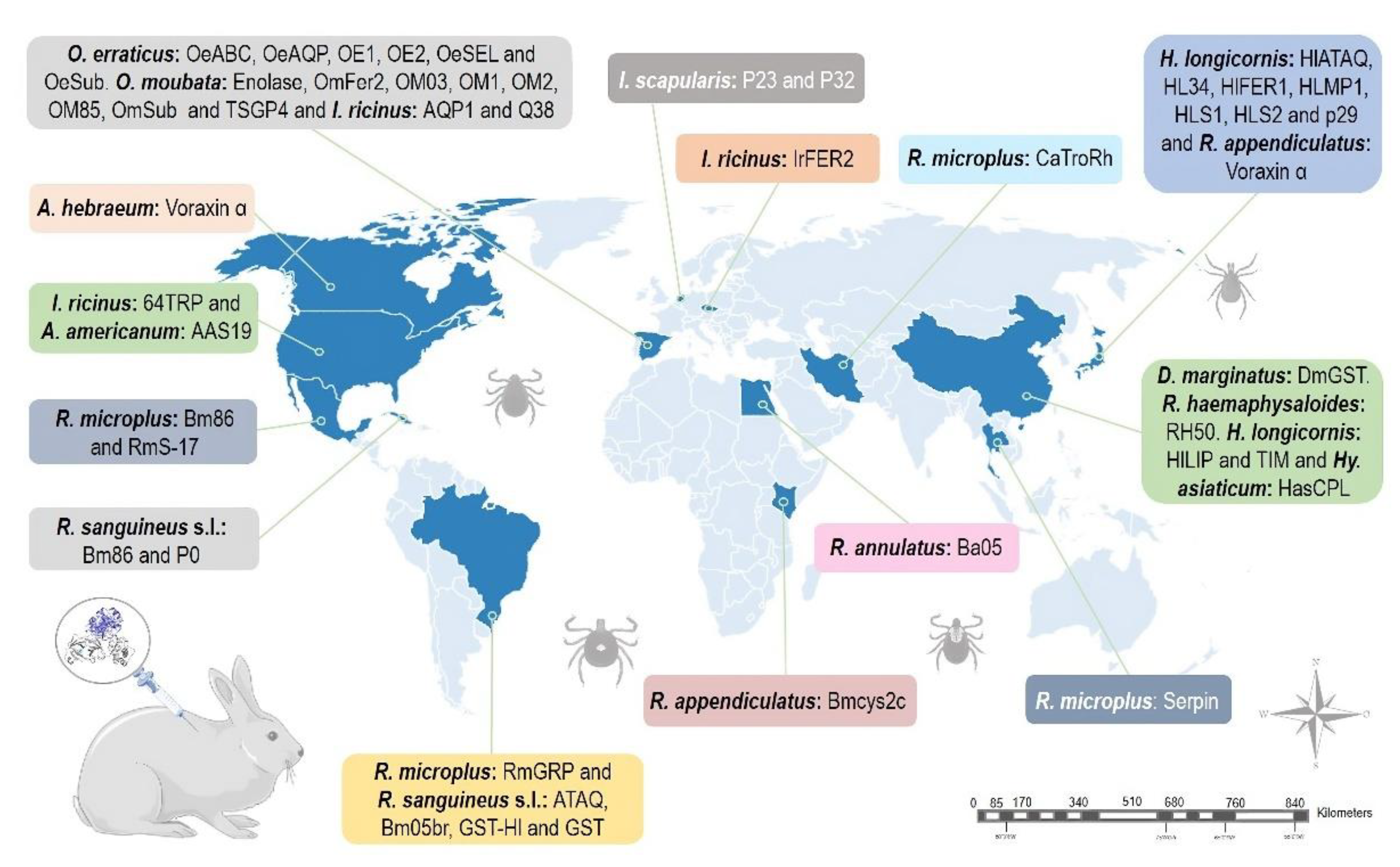
1. Introduction
2. Vaccination in Rabbits
2.1. Haemaphysalis spp.
2.2. Ornithodoros spp.
2.3. Rhipicephalus spp.
2.4. Ixodes spp.
2.5. Dermacentor spp.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brisola, C. Mites (ticks and others). In Medical and veterinary entomology; Publishing Athens, 2011; pp. 263–315. [Google Scholar]
- Alcantara, E.; Ferreira da Silva, C.; Ávila, R.; Pacheco, R.; Muñoz, L.; Honorio, D. Ticks (Acari: Argasidae and Ixodidae) infesting amphibians and reptiles in northeastern Brazil. Syst. Appl. Acarol. 2018, 23, 1497. [Google Scholar] [CrossRef]
- Santos, M.; Bahiense, T.; Silva, A.; Onofrio, V.; Barral, T.; Souza, B.; Lira-da-Silva, R.; Biondi, I.; Meyer, R.; Portela, R. Ticks and associated pathogens from rescued wild animals in rainforest fragments of northeastern Brazil. Front. Vet. Sci. 2020, 7, 177. [Google Scholar] [CrossRef]
- Cortés-Vecino, J. Changes in the distribution and abundance of ticks and their relationship with global warming. J. Vet. Med. Zoot. 2010, 57, 65–75. [Google Scholar]
- Guglielmone, A.; Nava, S.; Robbins, R. Geographic distribution of the hard ticks (Acari: Ixodida: Ixodidae) of the world by countries and territories. Zootaxa 2023, 5251, 1–274. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of tick. Parasitology 2004, 129, 3–14. [Google Scholar] [CrossRef]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.; Kocan, K.; Sonenshine, D. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2007, 13, 6938–6946. [Google Scholar] [CrossRef]
- Abbas, R.; Zaman, M.; Colwell, D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Expert consultation on the sustainable management of parasites in livestock challenged by the global emergence of resistance - Part 1: Current status and management of acaricide resistance in livestock ticks. FAO Animal Production and Health Report No. 17; Rome, 2022; pp. 9–10. [Google Scholar] [CrossRef]
- Obaid, M.; Islam, N.; Alouffi, A.; Zeb, A.; da Silva Vaz, I.; Tetsuya, T.; Abid, A. Acaricides resistance in ticks: selection, diagnosis, mechanisms, and mitigation. Front. Cell. Infect. Microbiol. 2022, 12, 941831. [Google Scholar] [CrossRef]
- Willadsen, P. Tick control: thoughts on a research agenda. Vet. Parasitol. 2006, 138, 161–168. [Google Scholar] [CrossRef]
- Mapholi, N.; Maiwashe, A.; Matika, O.; Riggio, V.; Banga, C.; MacNeil, M.; Dzama, K. Genetic parameters for tick counts across months for different tick species and anatomical locations in South African Nguni cattle. Trop. Anim. Health Prod. 2017, 49, 1201–1210. [Google Scholar] [CrossRef]
- Porto-Neto, L.; Reverter, A.; Prayaga, K.; Barendse, W. The genetic architecture of climatic adaptation of tropical cattle. PloS One 2014, 9, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Chi, M.; Rodriguez-Vivas, R.; Galindo-Velasco, E.; Lezama-Gutierrez, R. Laboratory and field evaluation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for the control of Rhipicephalus microplus (Acari: Ixodidae) in the Mexican tropics. Vet. Parasitol. 2010, 170, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, L.; Mesquita, E.; Almeida, T.; de Oliveira, R.; Oliveira, J.; Fernandes, F.; Guedes, M.; Pinheiro, E. Encapsulation of entomopathogenic fungal conidia: evaluation of stability and control potential of Rhipicephalus microplus. Ticks Tick Borne Dis. 2023, 14, 102184. [Google Scholar] [CrossRef]
- Freitas-Ribeiro, G.; Furlong, J.; Vasconcelos, V.; Dolinski, C.; Ribeiro, A. Analysis of biological parameters of Boophilus microplus Canestrini, 1887 exposed to entomopathogenic nematodes Steinernema carpocapsae Santa Rosa and ALL strains (Steinernema: Rhabditidae). Braz. Arch. Biol. Technol. 2005, 48, 911–919. [Google Scholar] [CrossRef]
- de Oliveira, C.; da Silva Matos, R.; Xavier, L.; de Souza, W.; Rita, V.; Pinheiro, E.; Dolinski, C.; de Azevedo, C. First report of pathogenicity of entomopathogenic nematodes of the genus Heterorhabditis on partially engorged females of Dermacentor nitens (Acari: Ixodidae). Biol. Control 2014, 69, 78–81. [Google Scholar] [CrossRef]
- Zingg, S.; Dolle, P.; Voordouw, M.; Kern, M. The negative effect of wood ant presence on tick abundance. Parasit. Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Platts-Mills, T.; Retterer, M.; Workman, L.; Wilson, J. A consistent “shortage” of cases of the alpha-gal syndrome (AS) on the gulf coast: possible relevance of fire ants as a predator of lone star ticks. J. Allergy Clin. Immunol. 2019, 143, AB278. [Google Scholar] [CrossRef]
- Adenubi, O.; Ahmed, A.; Fasina, F.; McGaw, L.; Eloff, J.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crops. Prod. 2018, 123, 779–806. [Google Scholar] [CrossRef]
- Charlie-Silva, I.; Giglioti, R.; Magalhaes, P.; Sousa, I.; AnnFoglio, M.; Oliveira, M.; Chagas, A. Lack of impact of dietary inclusión of dried Artemisia annua leaves for cattle on infestation by Rhipicephalus (Boophilus) microplus tick. Ticks Tick Borne Dis. 2018, 9, 1115–1119. [Google Scholar] [CrossRef]
- de la Fuente, J.; Contreras, M. Tick vaccines: current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376. [Google Scholar] [CrossRef]
- Parizi, L.; Konrdörfer, C.; Alves, G.; Fagundes, B.; Kiio, I.; Amaral, M.; da Silva, R.; Camargo-Mathias, M.; Seixas, A.; Konnai, S.; Ohashi, K.; Wangombe, N.; da Silva Vas, I. Rhipicephalus microplus cystatin as a potential cross-protective tick vaccine against Rhipicephalus appendiculatus. Ticks Tick Borne Dis. 2020, 11, 101378. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lihong, L.; Pinxing, W.; Hongmeng, D.; Shuwen, X.; Jingze, L.; Yonghong, H. Gene cloning, analysis and effect of a new lipocalin homologue from Haemaphysalis longicornis as a protective antigen for an anti-tick vaccine. Vet. Parasitol. 2021, 290, 109–358. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmad, S.; de Albuquerque, P.; Kamil, A.; Alshammari, F.; Alouffi, A.; da Silva Vaz, I. Prediction of novel drug targets and vaccine candidates against human lice (Insecta), Acari (Arachnida), and their associated pathogens. Vaccines 2022, 10, 8. [Google Scholar] [CrossRef]
- Yadav, N.; Upadhyay, R. European journal of biological research tick saliva antigen-based vaccines, disease protection and prophylaxis. Eur. J. Biol. Res. 2020, 12, 77–101. [Google Scholar] [CrossRef]
- de la Fuente, J.; Kopáček, P.; Lew-Tabor, A.; Maritz -Olivier, C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016, 38, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Ndawula, C.; Alves, G.; Parizi, L.; da Silva Vaz, I. Constituting a glutathione S-transferase-cocktail vaccine against tick infestation. Vaccine 2019, 37, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, B.; Han, Q. Understanding tick biology and its implications in anti-tick and transmission blocking vaccines against tick-borne pathogens. Front. Vet. Sci. 2020, 7, 319–456. [Google Scholar] [CrossRef] [PubMed]
- Huercha, R.; Min, L.; Xinli, F.; Zhengxiang, H.; Lijiang, W.; Yongchang, L.; Wei, Z.; Yang, Z.; Yuhui, M.; Chahan, B. Characterization of glutathione S-transferase of Dermacantor marginatus and effect of the recombinant antigen as a potential anti tick vaccine. Vet. Parasitol. 2020, 279, 109043. [Google Scholar] [CrossRef]
- Willadsen, P.; Riding, G.; McKenna, R.; Kemp, D.; Tellam, R.; Nielsen, J.; Gough, J. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol. 1989, 143, 1346–1351. [Google Scholar] [CrossRef]
- Johnston, L.; Kemp, D.; Pearson, R. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: Effects of induced immunity on tick populations. Int. J. Parasitol. 1986, 16, 27–34. [Google Scholar] [CrossRef]
- Willadsen, P.; Smith, D.; Cobon, G.; McKenna, R. Comparative vaccination of cattle against Boophilus microplus with recombinant antigen Bm86 alone or in combination with recombinant Bm91. Parasite Immunol. 1999, 18, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.; Moraes, J.; Githaka, N.; Martins, R.; Isezaki, M.; da Silva Vaz, I.; Logullo, C.; Konnai, C.; Ohashi, K. Vaccination with cyclin-dependent kinase tick antigen confers protection against Ixodes infestation. Vet. Parasitol. 2015, 211, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Manzano-Román, R.; Obolo-Mvoulouga, P.; Oleaga, A. Function-guided selection of midgut antigens from Ornithodoros erraticus ticks and an evaluation of their protective efficacy in rabbits. Vet. Parasitol. 2019, 272, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.; Černý, L.; Kurokawa, C.; Diktaş, H.; Matias, J.; Sajid, A.; Arora, G.; DePonte, K.; Narasimhan, S.; Fikrig, E. Immunization of guinea pigs with cement extract induces resistance against Ixodes scapularis ticks. Ticks Tick Borne Dis. 2022, 13, 10201. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, Z.; Liu, A.; Ren, Q.; Liu, J.; Liu, Z.; Li, Y.; Yin, H.; Guan, G.; Luo, J. Biological parameters of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) fed on rabbits, sheep, and cattle. Korean. J. Parasitol. 2016, 54, 301–305. [Google Scholar] [CrossRef]
- Colby, L.; Quenee, L.; Zitzow, L. Considerations for infectious disease research studies using animals. Comp Medi. 2017, 67, 222–231. [Google Scholar]
- Contreras, M.; de la Fuente, J. Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 subolesin/akirin chimera. Vaccine 2016, 34, 3010–3013. [Google Scholar] [CrossRef]
- Stokes, J.; Walker, D.; Varela-Stokes, A. The guinea pig model for tick-borne spotted fever rickettsioses: A second look. Ticks Tick Borne Dis 2020, 11, 101538. [Google Scholar] [CrossRef]
- Burkholder, T.; Linton, G.; Hoyt, J.; Young, R. The laboratory rabbit, guinea pig, hamster, and other rodents. In The rabbit as an experimental model; Suckow, M., Stevens, K., Wilson, R., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 529–560. [Google Scholar]
- Esteves, P.; Abrantes, J.; Baldauf, H.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; Keppler, O.; Knight, K.; Kong, X.; Lanning, D.; Pendu, J.; Lemos, A.; Liu, J.; Liu, S.; Lopes, A.; Lu, S.; Lukehart, S.; Manabe, Y.; Neves, F.; McFadden, G.; Mage, R. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef]
- Soares, J.; Pinheiro, A.; Esteves, P. The rabbit as an animal model to study innate immunity genes: Is it better than mice? Front. Immunol. 2022, 13, 981815. [Google Scholar] [CrossRef]
- Valentine, H.; Daugherity, E.; Singh, B.; Maurer, K. The experimental use of Syrian hamsters. In American College of Laboratory Animal Medicine. The laboratory rabbit, guinea pig, hamster, and other rodents; Suckow, M., Stevens, K., Wilson, R., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 875–906. [Google Scholar] [CrossRef]
- Miedel, E.; Hankenson, F. Biology and diseases of hámsters. In American College of Laboratory Animal Medicine. Laboratory Animal Medicine, 3rd ed.; Fox, J., Anderson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 209–245. [Google Scholar] [CrossRef]
- Shomer, N.; Holcombe, H.; Harkness, J. Biology and diseases of guinea pigs. In American College of Laboratory Animal Medicine. Laboratory Animal Medicine, 3rd ed.; Fox, J., Anderson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 247–283. [Google Scholar] [CrossRef]
- Lagunes-Quintanilla, R.; Valdez-Espinoza, U.; Hernández-Ortiz, R.; Castro-Saines, E.; Merino, O.; Mendoza-Martínez, N. Experimental vaccination in rabbits using the peptide RmS-17 antigen reduces the performance of a Mexican Rhipicephalus microplus tick strain. Ticks Tick Borne Dis. 2022, 13, 102044. [Google Scholar] [CrossRef] [PubMed]
- Trimnell, A.; Davies, G.; Lissina, O.; Hails, R.; Nuttall, P. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005, 23, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, C.; Narasimhan, S.; Vidyarthi, A.; Sameet, C.; Meister, L.; Diktas, H.; Strank, N.; Lynn, G.; DePonte, K.; Craft, J.; Fikrig, E. Repeat tick exposure elicits distinct immune responses in guinea pigs and mice. Ticks Tick Borne Dis. 2020, 11, 101529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Santos-Burgoa, C. Treatment against human rabies: a bit of its history. Public Health. 1994, 28, 454–63. [Google Scholar]
- Russell, R.; Schilling, P. Selected topics on laboratory medicine: The rabbit. In Series of scientific and technical monographs. WHO 1976, 4, 12–86. [Google Scholar]
- Kirkland, W. Ultrastructural changes in the nymphal salivary glands of the rabbit tick, Haemaphysalis leporispalustris, during feeding. J. Insect Physio. 1971, 17, 1933–1946. [Google Scholar] [CrossRef]
- McGowan, M.; Homer, T.; Odell, G.; McNew, R.; Barker, R. Performance of ticks fed on rabbits inoculated with extracts derived from homogenized tick Amblyomma maculatum Koch (Acari: Ixodidae). J. Parasitol. 1980, 66, 42–48. [Google Scholar] [CrossRef]
- Walker, A.; Fletcher, J. Histological study of the attachment sites of adult Rhipicephalus appendiculatus on rabbits and cattle. Int. J. Parasitol. 1986, 16, 399–413. [Google Scholar] [CrossRef]
- Fox, R. The biology of the laboratory rabbit. In Taxonomy and genetics; Weisbroth, S., Kraus, A., Eds.; Academic Press: New York, 1974; p. 22. [Google Scholar]
- ARBA. American Rabbit Breeders Association. Available online: https://www.arba.net/breeds.htm (accessed on 30 October 2018).
- Rodríguez-Mallon, A.; Fernández, E.; Encinosa, P.; Bello, Y.; Méndez-Pérez, L.; Cepero, L.; Pérez, D.; González, M.; Garay, H.; Reyes, O.; Méndez, L.; Estrada, M. A novel tick antigen shows high vaccine efficacy against the dog tick, Rhipicephalus sanguineus. Vaccine 2012, 30, 1782–1789. [Google Scholar] [CrossRef]
- Graur, D.; Duret, L.; Gouy, M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 1996, 379, 333–335. [Google Scholar] [CrossRef]
- Bryda, E. The mighty mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013, 110, 207–211. [Google Scholar]
- Mullane, K.; Williams, M. Animal models of asthma: reprise or reboot? Biochem Pharmacol. 2014, 87, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Trager, W. Acquired immunity to ticks. J. Parasitol. 1939, 25, 57–81. [Google Scholar] [CrossRef]
- Suckow, M.; Stevens, K.; Wilson, R. The laboratory rabbit, guinea pig, hamster, and other rodents; Suckow, M., Stevens, K., Wilson, R., Eds.; A volume in American College of Laboratory Animal Medicine, 2012; pp. 572–689. [Google Scholar] [CrossRef]
- Mulenga, A.; Sugimoto, Y.; Sako, K.; Musoke, A.; Mozaria, S.; Onuma, M. Molecular characterisation of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect. Immun. 1999, 40, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Sugino, M.; Imamura, S.; Mulenga, A.; Nakajima, M.; Tsuda, A.; Ohashi, K.; Onuma, M. A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine 2003, 21, 2844–2851. [Google Scholar] [CrossRef]
- Galay, R.; Umemiya-Shirafuji, R.; Bacolod, E.; Maeda, H.; Kusakisako, K.; Koyama, J. Two kinds of ferritin protect ixodid ticks from iron overload and consequent oxidative stress. PLoS ONE 2014, 9, e90661. [Google Scholar] [CrossRef]
- Liang, N.; Hong-Meng, D.; Xiang-Yuan, F.; Ya-Xue, W.; Feng, Y.; Xiao-Ya, L.; Yong-Hong, H. Characterization and evaluation of a new triosephosphate isomerase homologue from Haemaphysalis longicornis as a candidate vaccine against tick infection. Ticks Tick Borne Dis. 2022, 13, 101–968. [Google Scholar] [CrossRef]
- Egizi, A.; Bulaga-Seraphin, L.; Alt, E.; Bajwa, W.; Bernick, J.; Bickerton, M.; Fonseca, D. First glimpse into the origin and spread of the Asian longhorned tick Haemaphysalis longicornis, in the United States. Zoonoses Public Health 2020, 67, 637–650. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X.; Yin, C.; Pan, Y.; Wen, H.; Wang, Q.; Fuzhong, X.; Sun, Y.; Jiang, J.; Li, S.; Cao, W. Distribution of Haemaphysalis longicornis and associated pathogens: analysis of pooled data from a China field survey and global published data. Lancet Planet. Health 2020, 4, e320–e329. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, L.; Wen, H.; Zhang, Z.; Liu, J.; Fang, L.; Yu, X. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. J. Emerg. Infect. Dis. 2015, 21, 1770. [Google Scholar] [CrossRef]
- Tufts, D.; Sameroff, S.; Tagliafierro, T.; Jain, K.; Oleynik, A.; VanAcker, M.; Tokarz, R. A metagenomic examination of the pathobiome of the invasive tick species, Haemaphysalis longicornis, collected from a New York City borough, USA. Ticks Tick Borne Dis. 2020, 11, 101516. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, A.; Mulenga, A.; Sugimoto, C.; Nakajima, M.; Ohashi, K.; Onuma, M. cDNA cloning, characterization and vaccine effect analysis of Haemaphysalis longicornis tick saliva proteins. Vaccine 2001, 19, 4287–4296. [Google Scholar] [CrossRef] [PubMed]
- Pospelova-Shtrom, M. On the system of classification of ticks of the family Argasidae CAN. Acarologia 1969, 11, 1–22. [Google Scholar] [PubMed]
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R. African swine fever: how can global spread be prevented? Philos Trans R Soc Lond B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef]
- ECDC. European Centre for Disease Prevention and Control and European Food Safety Authority. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data (accessed on 12 June 2022).
- Baizheng, W.; Xin, L.; Jingze, L.; Rong, B. Predicting the potential habitat for Ornithodoros tick species in China. Vet. Parasitol. 2022, 311, 109793. [Google Scholar] [CrossRef]
- De Morais, J.; Lopes, I.; Nuncio, M. Spanish-African recurrent fever in Portugal: historical and clinical-epidemic escorco. Int. Med. 2007, 14, 170–178. [Google Scholar]
- Assous, M.; Wilamowski, A. Relapsing fever borreliosis in Eurasia-forgotten, but certainly not gone! Clin. Microbiol. Infect. 2009, 15, 407–414. [Google Scholar] [CrossRef]
- Oleaga, A.; González-Pérez, S.; Pérez-Sánchez, R. First molecular and functional characterisation of ferritin 2 proteins from Ornithodoros argasid ticks. Vet. Parasitol. 2022, 304, 109–684. [Google Scholar] [CrossRef]
- Manzano-Román, R.; Díaz-Martín, V.; Oleaga, A.; Siles-Lucas, M.; Pérez-Sánchez, R. Subolesin/akirin orthologs from Ornithodoros spp. soft ticks: cloning, RNAi gene silencing and protective effect of the recombinant proteins. Vet. Parasitol. 2012, 185, 248–259. [Google Scholar] [CrossRef]
- Manzano-Román, R.; Díaz-Martín, A.; Pérez-Sánchez, R. Identification of protective linear B-cell epitopes on the subolesin/akirin orthologues of Ornithodoros spp. soft ticks. Vaccine 2015, 33, 1046–1055. [Google Scholar] [CrossRef]
- Guglielmone, A.; Petney, T.; Robbins, R. Ixodidae (Acari: Ixodoidea): descriptions and redescriptions of all known species from 1758 to December 31, 2019. Zootaxa 2020, 4871, 1–322. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Keirans, J.; Horak, I. The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world, 1st ed.; Cambridge University Press: Cambridge, 2000; pp. 79–104. [Google Scholar]
- Makwarela, T.; Nyangiwe, N.; Masebe, T.; Mbizeni, S.; Nesengani, L.; Djikeng, A.; Mapholi, N. Tick diversity and distribution of hard (Ixodidae) cattle ticks in South Africa. Microbiol. Res. 2023, 14, 42–59. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vectors 2010, 3, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Brophy, M.; Riehle, M.; Mastrud, N.; Ravenscraft, A.; Adamson, J.; Walker, K. Genetic variation in Rhipicephalus sanguineus s.l. ticks across Arizona. Int. J. Environ. Res. Public Health 2022, 19, 4223. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Konnai, S.; Imamura, S.; Ito, T.; Onuma, M.; Ohashi, K. Cloning and characterization of Rhipicephalus appendiculatus voraxinα and its effect as anti-tick vaccine. Vaccine 2009, 27, 5989–5997. [Google Scholar] [CrossRef]
- Leal, B.; Alzugaray, M.; Seixas, A.; da Silva Vaz, I.; Ferreira, C. Characterization of a glycine-rich protein from Rhipicephalus microplus: Tissue expression, gene silencing and immune recognition. Parasitolgy 2018, 145, 927–938. [Google Scholar] [CrossRef]
- Jittapalapong, S.; Kaewhom, P.; Pumhom, P.; Canales, M.; de la Fuente, J.; Stich, R. Immunization of rabbits with recombinant serine protease inhibitor reduces the performance of adult female Rhipicephalus microplus. Transbound. Emerg. Dis. 2010, 57, 103–106. [Google Scholar] [CrossRef]
- Zhou, J.; Gong, H.; Zhou, Y.; Xuan, X.; Fujisaki, K. Identification of a glycine-rich protein from the tick Rhipicephalus haemaphysaloides and evaluation of its vaccine potential against tick feeding. Parasitol. Res. 2006, 100, 77–84. [Google Scholar] [CrossRef]
- Gray, J. The ecology of ticks transmitting Lyme borreliosis. Exp. Appl. Acarol. 1998, 22, 249–258. [Google Scholar] [CrossRef]
- Gilbert, L.; Maffey, G.; Ramsay, S.; Hester, A. The effect of deer management on the abundance of Ixodes ricinus in Scotland. Ecol Appl 2012, 22, 658–667. [Google Scholar] [CrossRef]
- Hofmeester, T.; Sprong, H.; Jansen, P.; Prins, H.; Van, S. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in dutch forests. Parasit. Vectors 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Ostfeld, R.; Canham, C.; Oggenfuss, K.; Winchcombe, R.; Keesing, F. Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk. PLoS ONE 2006, 4, e145. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, A.; Palli, S. Mapping distributions of the Lyme disease vector, Ixodes scapularis, and spirochete, Borrelia burgdorferi, in Kentucky using passive and active surveillance. Ticks Tick Borne Dis. 2022, 13, 101885. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.; Narasimhan, S.; Daffre, S.; de Ponte, K.; Hovius, J.; Veer, V. Identification and characterization of Ixodes scapularis antigens that elicit immunity to ticks by visualizing the yeast surface. PLoS ONE 2011, 6, e15926. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328. [Google Scholar] [CrossRef]
- Hajdusek, O.; Almazán, C.; Loosova, G.; Villar, M.; Canales, M.; Grubhoffer, L.; Kopacek, P.; de la Fuente, J. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010, 28, 2993–2998. [Google Scholar] [CrossRef]
- Yunker, C.; Keirans, J.; Cliffornd, C.; Easton, E. Dermacentor ticks (Acari: Ixodoidea: Ixodidae) of the new world: a scanning electron microscope atlas. Proc. Entomol. Soc. Wash. 1986, 88, 609–627. [Google Scholar]
- Eisen, R.; Kugeler, K.; Eisen, J.; Beard, C.; Paddock, C. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J 2017, 58, 319–335. [Google Scholar] [CrossRef]
- Dergousoff, S.; Galloway, T.; Lindsay, L.; Curry, P.; Chilton, N. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 2013, 50, 510–520. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, D.; Tian, Y.; Li, S. A dataset of distribution and diversity of ticks in China. Sci. Data 2019, 6, 105. [Google Scholar] [CrossRef]
- Martin, J.; Fischhoff, I.; Castellanos, A.; Han, B. Ecological predictors of zoonotic vector status among Dermacentor ticks (Acari: Ixodidae): A trait-based approach. J. Med. Entomol. 2022, 59, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; da Silva Vaz, I.; Sugino, M.; Ohashi, K.; Onuma, M. A serine protease inhibitor (Serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 2005, 23, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Chinzei, Y.; Minoura, H. Reduced oviposition in Ornithodoros moubata (Acari: Argasidae) fed on tick-sensitized and vitellin-immunized rabbits. J. Med. Entomol. 1988, 25, 26–31. [Google Scholar] [CrossRef]
- Weiss, B.; Kaufman, W. Two feeding-induced proteins from the male gonad trigger engorgement of the female tick, Amblyomma hebraeum. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 5874–5879. [Google Scholar] [CrossRef] [PubMed]
- Shahein, Y. Molecular cloning and expression of a larval immunogenic protein from the cattle tick Boophilus annulatus. Vet. Immunol. Immunopathol. 2008, 121, 281–289. [Google Scholar] [CrossRef]
- Canales, C.; Labruna, M.; Soares, J.; Prudencio, C.; de la Fuente, J. Protective efficacy of bacterial membranes containing surface-exposed BM95 antigenic peptides for the control of cattle tick infestations. Vaccine 2009, 27, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martín, V.; Manzano-Román, R.; Valero, L.; Oleaga, A.; Encinas-Grandes, A.; Pérez-Sánchez, R. An insight into the proteome of the saliva of the argasid tick Ornithodoros moubata reveals important differences in saliva protein composition between the sexes. J Proteomics 2013, 80, 216–235. [Google Scholar] [CrossRef]
- Manzano-Román, R.; Díaz-Martín, V.; Oleaga, V.; Obolo-Mvoulouga, P.; Pérez-Sánchez, R. TSGP4 from Ornithodoros moubata: molecular cloning, phylogenetic analysis and vaccine efficacy of a new member of the lipocalin clade of cysteinyl leukotriene scavengers. Vet. Parasitol. 2016, 227, 130–137. [Google Scholar] [CrossRef]
- Kim, T.; Radulovic, Z.; Mulenga, A. Target validation of highly conserved Amblyomma americanum tick saliva serine protease inhibitor 19. Ticks Tick Borne Dis. 2016, 7, 405–414. [Google Scholar] [CrossRef]
- Rangel, A.; Pereira, F.; Casquero, R.; Valério, M.; Andreotti, R. Design of the ATAQ peptide and its evaluation as an immunogen to develop a Rhipicephalus vaccine. Vet. Parasitol. 2016, 221, 30–38. [Google Scholar] [CrossRef]
- Alzugaray, M.; Parizi, L.; Seixas, A.; Benavides, U.; da Silva Vaz, I. Molecular and functional characterization of Bm05br antigen from Rhipicephalus microplus. Ticks Tick Borne Dis. 2017, 8, 320–329. [Google Scholar] [CrossRef]
- Sabadin, G.; Parizi, F.; Kiio, I.; Amaral, M.; da Silva, R.; Camargo-Mathias, M.; Wangombe, N.; Nene, V.; da Silva Vaz, I. Effect of recombinant glutathione S-transferase as vaccine antigen against Rhipicephalus appendiculatus and Rhipicephalus sanguineus infestation. Vaccine 2017, 35, 6649–6656. [Google Scholar] [CrossRef]
- Obolo-Mvoulouga, P.; Oleaga, A.; Manzano-Román, R.; Pérez-Sánchez, R. Evaluation of the protective efficacy of Ornithodoros moubata midgut membrane antigens selected using omics and in silico prediction algorithms. Ticks Tick Borne Dis 2018, 9, 1158–1172. [Google Scholar] [CrossRef]
- Asadollahi, Z.; Nabian, S.; Taheri, M.; Ebrahimzadeh, E. Introducing a new anti-Rhipicephalus (Boophilus) microplus tick recombinant vaccine candidate using cathepsin and tropomyosin multi-epitope gene. Vet Res Forum 2021, 12, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ge, T.; Hu, E.; Fan, X.; Zhang, Y.; Zhai, X.; Li, M.; Zhang, W.; Wu, L.; Ka, A.; Cheung, L.; Chahan, B. Recombinant cysteine proteinase as anti-tick targeting Hyalomma asiaticum infestation. Exp. Parasitol. 2022, 235, 108234. [Google Scholar] [CrossRef]
- Adjou, P.; Naomasa, S.; Tuvshintulga, B.; Sato, N.; Okado, K.; Zheng, W.; Lee, S.; Mosqueda, J.; Suzuki, H.; Xuan, X.; Umemiya-Shirafuji, R. Identification and characterization of Rhipicephalus microplus ATAQ homolog from Haemaphysalis longicornis ticks and its immunogenic potential as an anti-tick vaccine candidate molecule. Microorganisms 2023, 11, 822. [Google Scholar] [CrossRef]
- Duo, D.; Ru, L.; Ya-Xue, W.; Xiang-Yuan, F.; Xiao-Ya, L.; Feng, Y.; Tian-Tian, Z.; Jing-Yi, M. Molecular characterization of hexokinase (HK) in Haemaphysalis longicornis and evaluation of HK protein- and DNA-based vaccines against adult ticks. Pest Manag. Sci. 2023, 79, 1721–1730. [Google Scholar] [CrossRef]
- Carnero-Morán, A.; Oleaga, A.; Cano-Argüelles, A.; Pérez-Sánchez, R. Function-guided selection of salivary antigens from Ornithodoros erraticus argasid ticks and assessment of their protective efficacy in rabbits. Ticks Tick Borne Dis. 2023, 14, 102218. [Google Scholar] [CrossRef]
- Kemp, D.; Pearson, R.; Gough, J.; Willadsen, P. Vaccination against Boophilus microplus: localization of antigens on the tick gut cells and their interaction with the host immune system. Exp. Appl. Acarol. 1989, 7, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Namangala, B.; Tajima, T.; Tembo, M.; Yasuda, J.; Ohashi, K.; Onuma, M. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine 2006, 24, 2230–2237. [Google Scholar] [CrossRef]
- Vargas-Hernandez, V.; Montero-Espinosa, C.; Sánchez-Villaurrutia, D.; Duarte, C.; Henrique, G.; Fuentes-Castillo, A.; Ancisar, J.; Suárez-Alba, J.; Mosqueda-Lobaina, O.; Suárez-Pedroso, M. Infestation of rabbits with just-molted adults of the cattle tick Rhipicephalus microplus: biological parameters and efficiency. Rev. Bras. DE Parasitol. Vet. 2023, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Daemon, E.; Prata, M.; Faccini, J. Goats as alternative hosts of Boophilus microplus (Acari: ixodidae). Rev. Bras. DE Parasitol. Vet. 1998, 7, 123–128. [Google Scholar]
- Franque, M.; Santos, H.; Silva, G.; Tajiri, J.; Massard, C. Biological characteristics of Boophilus microplus (Acari: Ixodidae) on dog under experimental infestation. Rev. Bras. DE Parasitol. Vet. 2007, 16, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Franque, M.; Santos, H.; Linarez, F.; Massard, C. Experimental infestation of horses by Rhipicephalus (Boophilus) microplus. Cienc Rural 2009, 39, 2117–2122. [Google Scholar] [CrossRef]
- Guimarães, C.; Wilwerth, D.; Daemon, E.; Faccini, J. Effect of the number of Boophilus microplus (Can., 1887) (Acari: lxodidae) larvae on the recovery of engorged females in rabbits. Rev. Bras. DE Parasitol. Vet. 1999, 3, 59–61. [Google Scholar] [CrossRef]
- Hitcheock, L. Studies on the parasitic stages of the cattle tick Boophilus microplus (Canestrini) (Acarina: Ixodidae). Aust. J. Zool. 1955a, 3, 145–155. [Google Scholar] [CrossRef]
- Nuttall, P.; Labuda, M. Tick-host interactions: saliva-activated transmission. Parasitology 2004, 129, S177–S189. [Google Scholar] [CrossRef]
- Silva, C.; Cunha, D.; Daemon, E.; Faccini, J. Effect of the number of Boophilus microplus (Can., 1887) larvae (Acari: lxodidae) on the recovery of engorged females in rabbits. Rev. Bras. DE Parasitol. Vet. 1996, 3, 59–61. [Google Scholar] [CrossRef]
- Zacarias, M.; Azevedo, M.; Daemon, E.; Furlong, J. Biological parameters of cattle ticks fed on rabbits. Rev. Bras. DE Parasitol. Vet. 2012, 21, 22–27. [Google Scholar]
- Francischetti, I. The role of saliva in tick feeding. Front. Biosci. 2009, 14, 2051–2088. [Google Scholar] [CrossRef]
- Mudenda, L.; Pierlé, S.; Turse, J.; Scoles, G.; Purvine, S.; Nicora, C.; Clauss, T.; Ueti, M.; Brown, W.; Brayton, K. Proteomics informed by transcriptomics identifies novel secreted proteins in Dermacentor andersoni saliva. Int. J. Parasitol. 2014, 44, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, L.; Reck, J.; Terra, R.; Martins, J.; Mulenga, A.; Sherman, N.; Fox, J.; Yates, J.; Termignoni, C.; Pinto, A.; da Silva Vaz, I. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: A comparison between partially and fully engorged females. PLoS ONE 2014, 9, e94831. [Google Scholar] [CrossRef]
- Tirloni, L.; Islam, M.; Kim, T.; Diedrich, J.; Yates, J.; Pinto, A.; Mulenga, A.; You, M.; da Silva, I. Saliva from nymph and adult females of Haemaphysalis longicornis: a proteomic study. Parasit. Vectors 2015, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Tirloni, L.; Pinto, A.; Moresco, J.; Yates, J.; da Silva Vaz, I.; Mulenga, A. Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS. Negl. Trop. Dis. 2016b, 10, e0004323. [Google Scholar] [CrossRef]
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Leboulle, G.; Rochez, C.; Louahed, J.; Ruti, B.; Brossard, M. Isolation of Ixodes ricinus salivary gland mRNA encoding factors induced during blood feeding. Am. J. Trop. Med. Hyg. 2022, 66, 225–233. [Google Scholar] [CrossRef]
- Francischetti, I.; Sa-Nunes, A.; Mans, B.; Santos, I.; Ribeiro, J. The role of saliva in tick feeding. Front. Biosci. 2009, 14, 2051–2088. [Google Scholar] [CrossRef]
- Mans, B. Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J Innate Immun. 2011, 3, 41–51. [Google Scholar] [CrossRef]
- Ribeiro, J.; Francischetti, I. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef]
- Wikel, S. Host immunity to ticks. Annu. Rev. Entomol. 1996, 41, 1–22. [Google Scholar] [CrossRef]
- Tirloni, L.; Kim, T.; Pinto, A.; Yates, J.; da Silva Vaz, I.; Mulenga, A. Tick-Host range adaptation: Changes in protein profiles in unfed adult Ixodes scapularis and Amblyomma americanum saliva stimulated to feed on different hosts. Front. Cell. Infect. Microbiol. 2017, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Mulenga, A.; Blandon, M.; Khumthong, R. The molecular basis of the Amblyomma americanum tick attachment phase. Exp. Appl. Acarol. 2007, 41, 267–287. [Google Scholar] [CrossRef] [PubMed]
- Lew-Tabor, A.; Moolhuijzen, P.; Vance, M.; Kurscheid, S.; Valle, M.; Jarrett, S.; Minchin, C.; Jackson, L.; Jonsson, N.; Bellgard, M.; Guerrero, F. Suppressive subtractive hybridization analysis of Rhipicephalus (Boophilus) microplus larval and adult transcript expression during attachment and feeding. Vet. Parasitol. 2010, 167, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Tirloni, L.; Pinto, A.; Diedrich, J.; Moresco, J.; Yates, J.; da Silva Vaz, I.; Mulenga, A. Time resolved proteomic profile of Amblyomma americanum tick saliva during feeding. PLoS. Negl. Trop. Dis. 2020, 14, e0007758. [Google Scholar] [CrossRef]
- Schwarz, A.; von Reumont, B.; Erhart, J.; Chagas, A.; Ribeiro, J.; Kotsyfakis, M. De novo Ixodes ricinus salivary gland transcriptome analysis using two nextgeneration sequencing methodologies. FASEB J. 2013, 27, 4745–4756. [Google Scholar] [CrossRef]
- Imamura, S.; da Silva Vaz, I.; Konnai, S.; Yamada, Y.; Nakajima, C.; Onuma, M.; Ohashi, K. Effect of vaccination with a recombinant metalloprotease from Haemaphysalis longicornis. Exp. Appl. Acarol. 2009, 48, 345–358. [Google Scholar] [CrossRef]
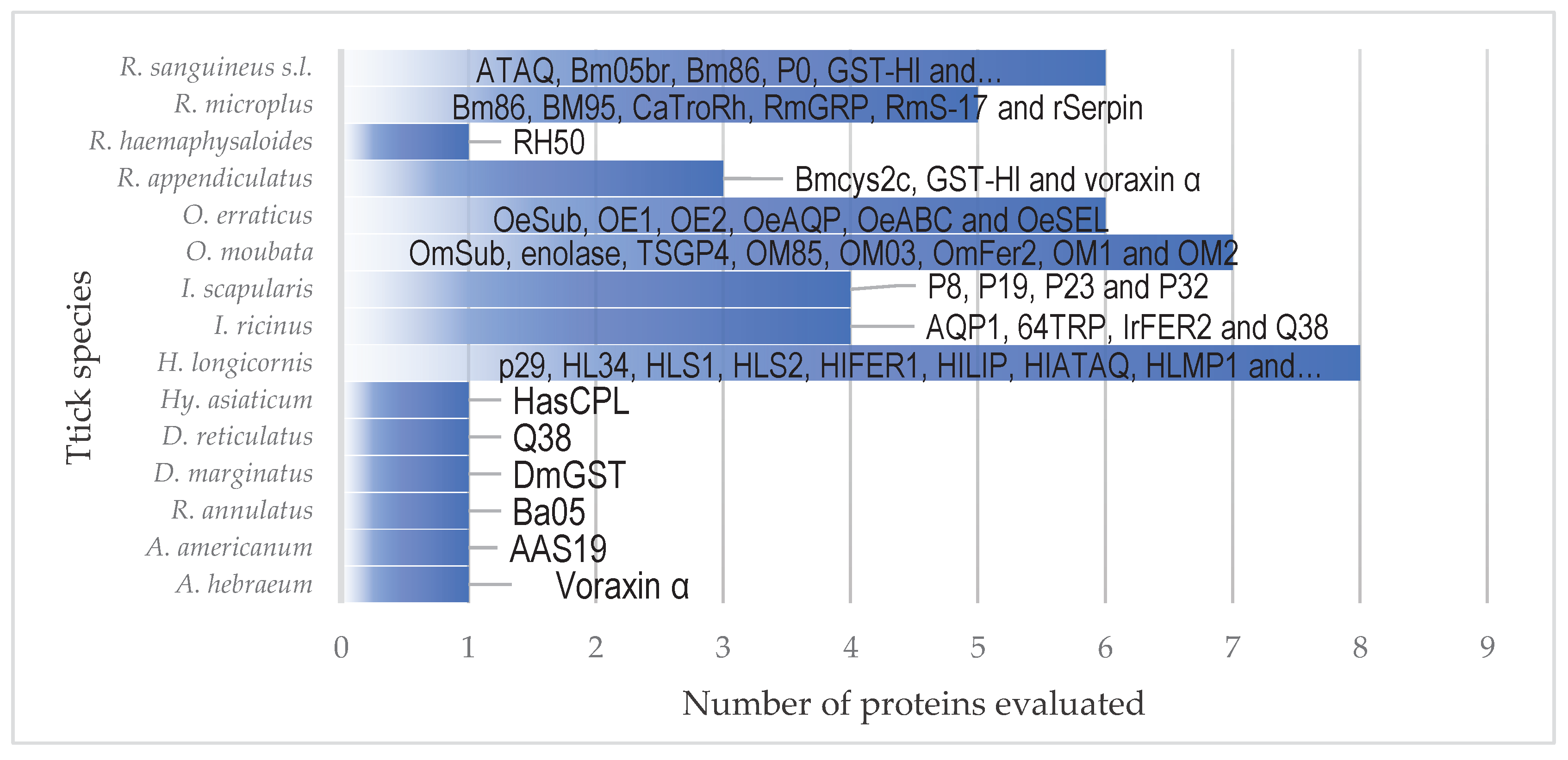
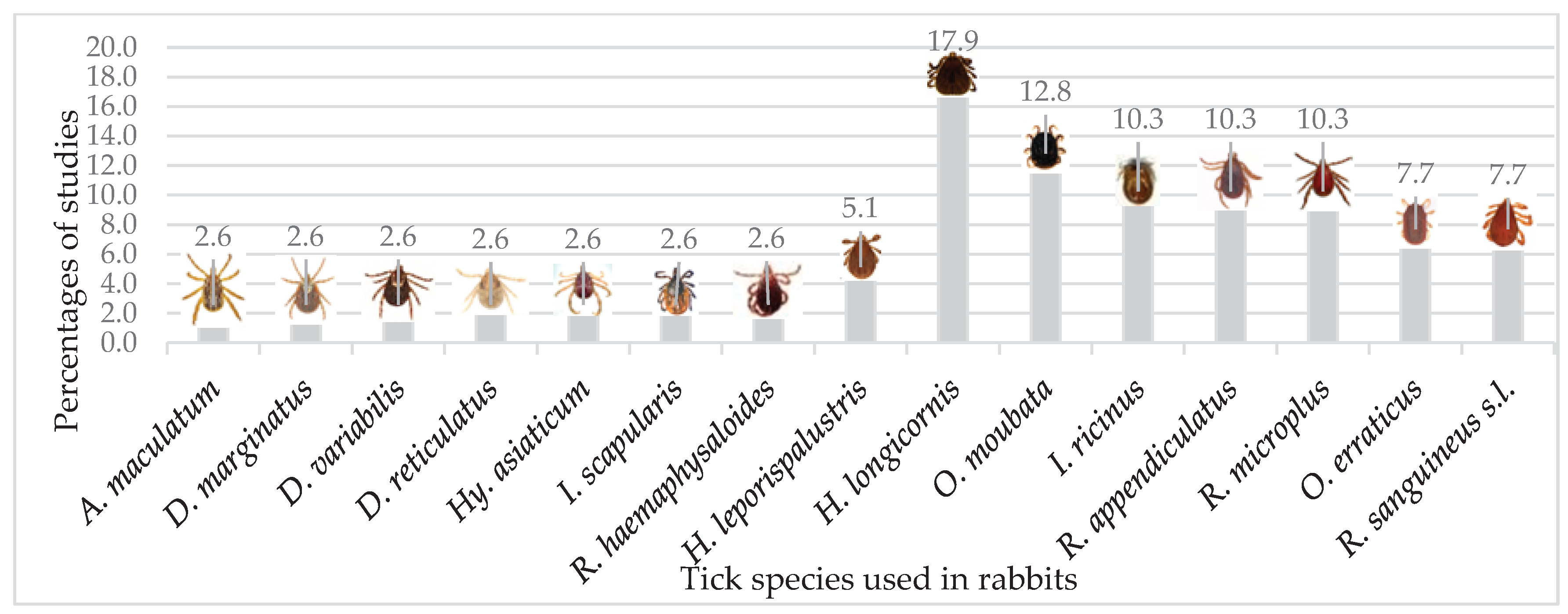
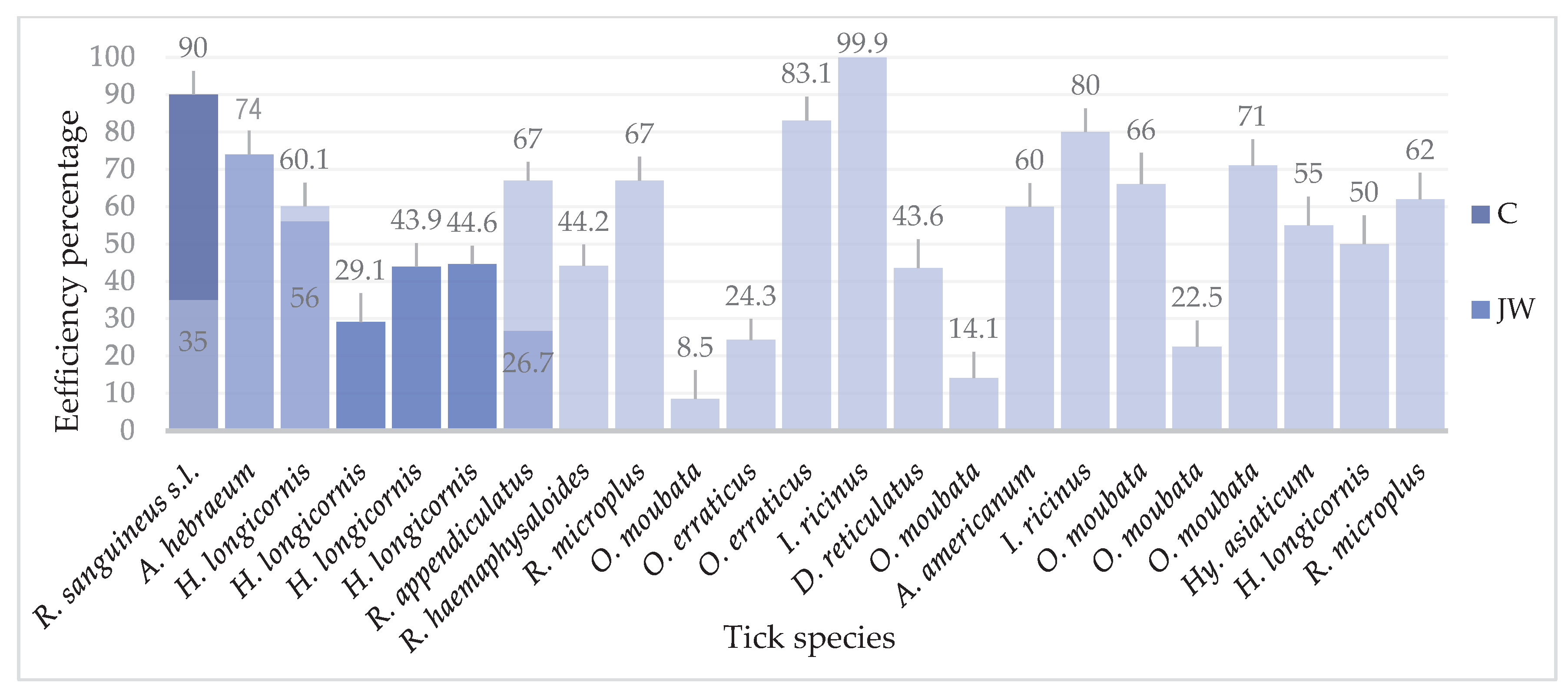
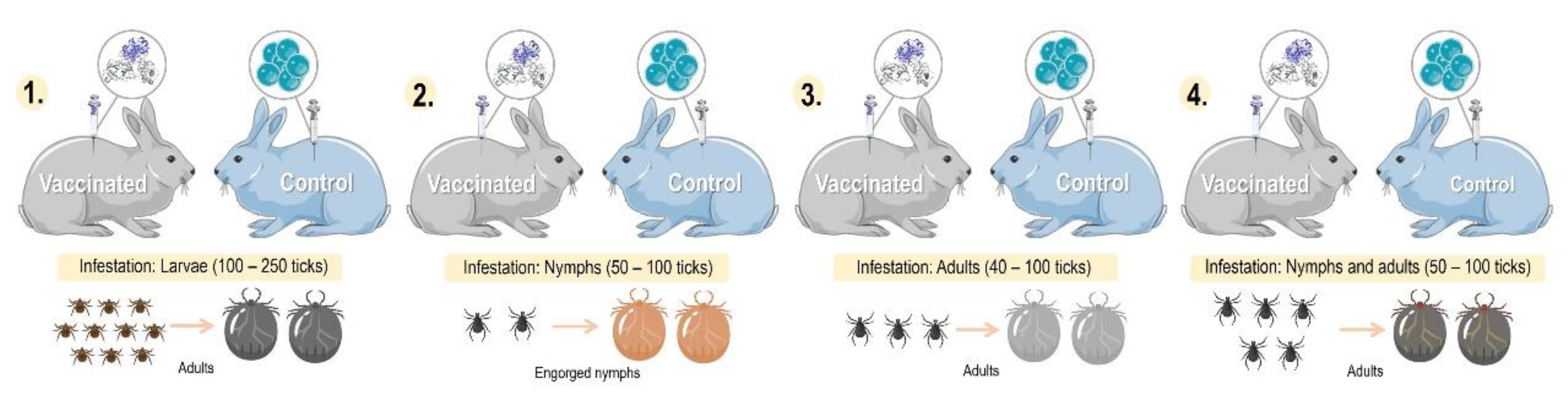
| No | Experiment/Molecule Name | # of Rabbits | Rabbitsbreed | Tick Species | Tick Stages | Immunization | Tick per Rabbit | % Reduction | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Evaluation of the immune response | 6 | NR | D. variabilis and H. leporispalustris | Larvae | 1 | 159 | NA | [61] |
| 2 | Evaluation of the immune response | NR | NZ | H. leporispalustris | Nymphs | 1 | NR | NA | [52] |
| 3 | Whole tick tissues collected from Amblyomma maculatum | 8 | NZ | A. maculatum | Nymphs and adults | 2 | 75 | NA | [53] |
| 4 | Attachment sites of Rhipicephalus appendiculatus | 5 | NZ | R. appendiculatus | Adults | 3 | 80 | NA | [54] |
| 5 | Tick egg yolk protein (vitellin) | 4 | JW | O. moubata | Nymphs and adults | 4 | 112 | NA | [104] |
| 6 | Native protein (p29) | 10 | JW | H. longicornis | Nymphs and adults | 3 | 2110 | 56 | [63] |
| 7 | Haemaphysalis longicornis protein 34 (HL34) | 4 | JW | H. longicornis | Nymphs and adults | 2 | 115 | 29,1 | [71] |
| 8 | Haemaphysalis longicornis serpin 1 (HLS1) | 4 | JW | H. longicornis | Nymphs and adults | 2 | 120 | 43,9 | [64] |
| 9 | Voraxin of Amblyomma hebraeum | 2 | FL | A. hebraeum | Adults | 3 | 62 | 74 | [105] |
| 10 | 64TRP | 14 | NR | I. ricinus | Adults | 3 | 30 | NA | [48] |
| 11 | Haemaphysalis longicornis serpin-2 (HLS2) | 4 | JW | H. longicornis | Nymphs and adults | 2 | 160 | 44,6 | [103] |
| 12 | RH50 | 6 | NZ | R. haemaphysaloides | Nymphs and adults | 3 | 120 | 74,7 | [89] |
| 13 | Protein 05 from Boophilus annulatus (Ba05) | 1 | NR | B. annulatus | Larvae | NA | NA | NA | [106] |
| 14 | Recombinant BM95-MSP1a fusion protein and Bm86 | 16 | NZ | R. microplus | Adults | 1 | 50 | 65.5 and 55.9 | [107] |
| 15 | rVoraxin from Rhipicephalus appendiculatus | 3 | JW | R. appendiculatus | Adults | 3 | 60 | 26,7 | [86] |
| 16 | IrFER2 | 4 | NR | I. ricinus | Nymphs | 3 | 50 | 98 | [97] |
| 17 | Haemaphysalis longicornis metalloprotease (HLMP1) | 3 | NR | H. longicornis | Nymphs and adults | 3 | 120 | 15,6 and 14,6 | [147] |
| 18 | Serpin | 6 | NZ | R. microplus | Larvae | 3 | 500 | 67 | [88] |
| 19 | Salivary antigens P8, P19, P23 and P32 | 3 | NZ | I. scapularis | Nymphs | 3 | 50 | NA | [95] |
| 20 | P0 protein and Bm86 | 10 | C | R. sanguineus s.l. | Nymphs and adults | 4 | 400 | 90 | [57] |
| 21 | Subolesin Ornithodoros erraticus and Ornithodoros moubata (rOeSub and rOmSub) | 9 | NZ | O. erraticus and O. moubata | Nymphs and adults | 3 | 90 | 8,5 and 24,3 | [79] |
| 22 | REnolase | 3 | NZ | O. moubata | Adults | 3 | 90 | NA | [108] |
| 23 | Haemaphysalis longicornis ferretin 1 (HlFER1) | 3 | JW | H. longicornis | Adults | 1 | 50 | NA | [65] |
| 24 | Subolesin/akirin orthologues of Ornithodoros erraticus (OE1, OE2 and OM1) | 3 | NZ | O. erraticus | Adults and nymphs | 3 | 200 | 48,6, 83,1 and 50,3 | [80] |
| 25 | Q38 | 3 | NZ | I. ricinus and D. reticulatus | Larvae | 2 | 200 | 99,9 and 43,6 | [39] |
| 26 | Ornithodoros moubata salivary lipocalin (TSGP4) | 6 | NZ | O. moubata | Adults and nymphs | 3 | 100 | 14,1 | [109] |
| 27 | Amblyomma americanum serine protease inhibitor 19 (AAS19) | 2 | NZ | Amblyomma americanum | Adults | 2 | 40 | 60 | [110] |
| 28 | ATAQ protein from Rhipicephalus microplus | 9 | NZ | R. sanguineus s.l. | Adults | 3 | NR | 47 | [111] |
| 29 | Rhipicephalus microplus ticks from Brazil (Bm05br) | 1 | NZ | R. sanguineus s.l. | Adults | 3 | NR | NA | [112] |
| 30 | CoAQP | 6 | NZ | I. ricinus | Larvae | 2 | 200 | 32 and 80 | [96] |
| 31 | Glutathione S-transferase from Haemaphysalis longicornis (GST-Hl) | 14 | NZ | R. sanguineus s.l. and R. appendiculatus | Nymphs and adults | 3 | 190 | 67 | [113] |
| 32 | RmGRP | N/A | NZ | R. microplus | N/A | 9 | N/A | NA | [87] |
| 33 | OM85 and OM03 | 6 | NZ | O. moubata | Nymphs and adults | 3 | 40 | 20,7 and 66,1 | [114] |
| 34 | Aquaporin of Ornithodoros erraticus (OeAQP) and selenoprotein T of Ornithodoros moubata (OeSEL) | 9 | NZ | O. erraticus and O. moubata | Nymphs and adults | 3 | 180 | 47,5 and 22,5 | [35] |
| 35 | Glutathione S-transferase GST-cocktail | 6 | NZ | R. sanguineus s.l. | Adults | 3 | 60 | 35 | [28] |
| 36 | Bmcys2c | 6 | NZ | R. appendiculatus | Nymphs and adults | 3 | 250 | 11,5 | [23] |
| 37 | Dermacentor marginatus S-transferase (DmGST) | 6 | NZ | D. marginatus | Nymphs and adults | 3 | 110 | 43,6 | [30] |
| 38 | Haemaphysalis longicornis lipocalin (HlLIP) | 6 | NZ | H. longicornis | Adults | 3 | 46 | 60,1 | [24] |
| 39 | Cathepsin L and tropomyosin proteins derived from Rhipicephalus microplus (CaTroRh) | 6 | NZ | R. microplus | NA | 3 | NA | NA | [115] |
| 40 | Ferritin 2 in Ornithodoros moubata (OmFer2) | 6 | NZ | O. moubata | Nymphs and adults | 3 | 95 | 71 | [78] |
| 41 | athepsin L rom Hyalomma asiaticum (HasCPL) | 6 | NZ | Hy. asiaticum | Larvae | 3 | 250 | 55 | [116] |
| 42 | Triosephosphate isomerase homologue from Haemaphysalis longicornis (HlTIM) | 27 | NZ | H. longicornis | Adults | 1 | 92 | 50 | [66] |
| 43 | RmS-17, and Bm86 | 6 | NZ | R. microplus | Adults | 3 | 120 | 79 and 62 | [47] |
| 44 | ATAQ in Haemaphysalis longicornis (HlATAQ) | 2 | JW | H. longicornis | Adults | 2 | 30 | NA | [117] |
| 45 | Hexokinase of Haemaphysalis longicornis (HlHK) | 12 | NZ | H. longicornis | Adults | 3 | 46 | 65.6 | [118] |
| 46 | Acid tail salivary protein (OeATSP), multiple coagulation factor deficiency protein 2 homolog (OeMCFD2), Cu/Zn-superoxide dismutase (OeSOD) and sulfotransferase (OeSULT) of Ornithodoros erraticus | 6 | NZ | O. erraticus | Nymphs and adults | 3 | 95 | 58.3 | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





