1. Introduction
Video capsule endoscopy (VCE) has been identified as a prospective substitute for the traditional endoscopy in recent times [
1] owing to the noninvasive nature of the species and its comparatively diminutive size [2-4]. The VCE device is designed to include a camera, a miniature lighting system, and an electronic box within its compact form factor, which is comparable in size to a tablet [5-7]. Despite its small size, VCE has the ability to generate high-quality images at a significantly higher frame rate [8, 9]. Despite the numerous advantages of VCE, a significant drawback of this technology is its lack of narrow band imaging (NBI) functionality, which is commonly present in conventional endoscopes [
10].
NBI is a modality of medical imaging that employs a specialized filter to selectively permit the transmission of a specific wavelength of light [11, 12]. The aforementioned wavelengths have the ability to enhance the contrast, thereby accentuating the features of the mucosal layer [13, 14]. Typically, the central wavelength of NBI is either 415 or 540 nm, which correspond to the blue and green spectral bands, respectively [15, 16]. The absorption of light by hemoglobin in the bloodstream is heightened at these particular wavelengths to provide enhanced precision, resulting in a darkened appearance of the blood vessels [17, 18]. Consequently, the distinguishing features of the mucosa can be readily discerned from the neighboring milieu [
19]. NBI bands are predominantly utilized in endoscopy for diagnosing diverse types of cancer [
20].
Specific cancers, such as esophageal EC, which does not have any particular biomarker in early stages, can be diagnosed early with the use of NBI [20, 21]. Many previous studies have proven that the accuracy, sensitivity, and specificity of early detection of various types of cancer increased significantly with the use of NBI [22-24]. Tsai et al. used single-shot detector and hyperspectral imaging to detect early EC and revealed that the overall accuracy of the NBI images was 91% and the RGB image’s accuracy was only 88% [
25]. Lee et al. examined EC by using the conventional white light imaging (WLI) and NBI and concluded that NBI was much more effective than the conventional WLI in detecting dysplasia [
26]. Yosidha et al. demonstrated that the NBI system improved the accuracy of magnifying endoscopy [
27]. Hence, the use of NBI is critical in VCE to detect early EC.
Therefore, in this study, the usability of NBI images simulated from decorrelated color spaces for VCE imaging of the esophagus was evaluated. The simulated NBI images were compared with three distinct parameters, namely, PSNR, SSIM, and entropy. The narrow band conversion technology and the dataset employed in this study were discussed in depth. Subsequently, the outcomes obtained from the image comparison using various parameters were elucidated. Finally, a summary of the findings, the potential avenues for future research, and the limitations of this study were provided.
2. Materials and Methods
2.1. Dataset
Obtaining the necessary dataset for identifying and categorizing the esophagus can often be a challenging undertaking [
28]. Moreover, a huge amount of pertinent information can be found in the VCE of the esophagus. In the present study, a series of esophageal images was obtained from two collaborating hospitals. The dataset consisted of a comprehensive collection of 3415 WLI VCE images of the esophagus. WLI images were obtained using VCE (InsightEyes EGD System, Insight Medical Solutions Inc). VCE images of 640 × 480 pixels in dimension were acquired from Taipei Veterans General Hospital. A supplementary set of 2000 WLI images obtained via a conventional endoscope (CV-290, Olympus) was used for analysis. The dataset containing Olympus images was obtained from Chung-Ho Memorial Hospital at Kaohsiung Medical University. The dimension of these images was 640 × 480 pixels.
2.2. NBI
The lack of NBI functionality is one of the most significant drawbacks associated with the use of VCE. Increasing the size of the VCE device is not possible because of the convenience factor. Therefore, the NBI features can be added only after the processing is completed. Given that NBI performs far more effectively with computer-aided detection machine learning techniques than WLI, this step is essential for early EC detection. Thus, in this study, a color space that replicated the NBI image was chosen to have decorrelated axes because it has been shown to be an effective tool for manipulating color images. The method developed by Reinhard et al. was applied [
29]. Simply imposing the mean and standard deviation (SD) over the data points is a straightforward operation that, when combined with credible input images, results in the production of convincing output images. Only the mean and standard deviation in tandem with any of the three dimensions were needed in this investigation. Therefore, these metrics were calculated for the original image and the target image. A notable detail that the average and standard deviation for each axis in one space were determined on an individual basis. First, a method that should be considered reasonable for converting RGB signals to l was demonstrated, the perception-based color space developed by Ruderman et al. Considering l is a transform of LMS cone space, the image was converted to LMS space by using the LMS transform in two steps. First, the RGB tristimulus values were converted to XYZ ones. By using the standard matrix provided by the International Telecommunications Union, a vector that can be applied to multiply the columns was obtained, resulting in the RGB-to-XYZ conversion. The image was converted for it to be in LMS space by using the traditional conversion matrix, as shown in Equation 1.
The information in this color space exhibited a significant amount of skew, which was reduced to a significant degree after transforming the data to the logarithmic space, as shown in Equation 2.
First, the data points were taken, and the mean was subtracted from them. Then, the data points that made up the synthetic image were adjusted using the factors determined by the standard deviations of each of the individual data points, as shown in Equation 3.
Choosing a source image and a target image that do not go together very well was possible because this investigation aimed to copy the appearance of one image onto another. The compositional similarities between the images can determine the quality of the final product. For instance, if the synthetic image has a significant amount of grass, whereas the photograph has a great amount of sky, then the transformation of statistics could be assumed to be unsuccessful.
2.3. Parameters for Comparision
2.3.1. SSIM
SSIM is a widely recognized quality measurement that is utilized in the process of determining how similar two images are to one another [
30]. It was introduced by Wang et al., and it has been proposed to be associated with the human visual system’s quality perception [
31]. SSIM is developed by predicting every image distortion as an amalgamation of three factors, which are loss of correlation, contrast distortion, and luminance distortion, rather than using traditional error summation methods [32, 33]. SSIM is used as a measure to compare the similarity between WLI Images and NBI images [
34]. SSIM metric has gained significant popularity in the field of digital image analysis due to its ease of use, widespread application, and established validity through rigorous testing [35, 36]. SSIM values usually ranges between 0 and 100%, and SSIM values of more than 90% are considered as better results [
37].
2.3.2. Entropy
The second criterion employed to assess the algorithm formulated in this investigation was entropy [
38]. The calculation of entropy was similar to that of SSIM. The entropy discrepancy between the WLI images acquired through the Olympus endoscope and the simulated NBI images was compared. Entropy can be utilized in image processing for the purpose of texture classification. A specific texture may exhibit a distinct entropy value as certain patterns tend to recur in a relatively consistent manner [
39]. Within the framework of the paper, low entropy is indicative of reduced disorder and diminished variance among the constituent elements. Thus, reducing the entropy results in an improved reproduction of the image. The entropy disparity between the WLI images acquired through VCE and the simulated NBI images was compared.
2.3.3. PSNR
PSNR is a byte-by-byte comparison of the quality of two images [40, 41]. It is one of the simplest methods to compare the source and the reproduced image [
42]. PSNR is analyzed similar to SSIM. The corresponding WLI images from VCE and the Olympus endoscope were separately compared with the simulated NBI images. PSRN values range between 20 and 60 dB, and the higher the value is, the better the result. For an 8-bit data representation, the accepted PSNR value is about 25 dB [
43].
3. Results
In this study, three important parameters were considered for analyzing the effectiveness of the algorithm; PSNR, SSIM, and entropy. Comparing the results of these parameters can help understand the limitations and advantages of the proposed method.
3.1. SSIM
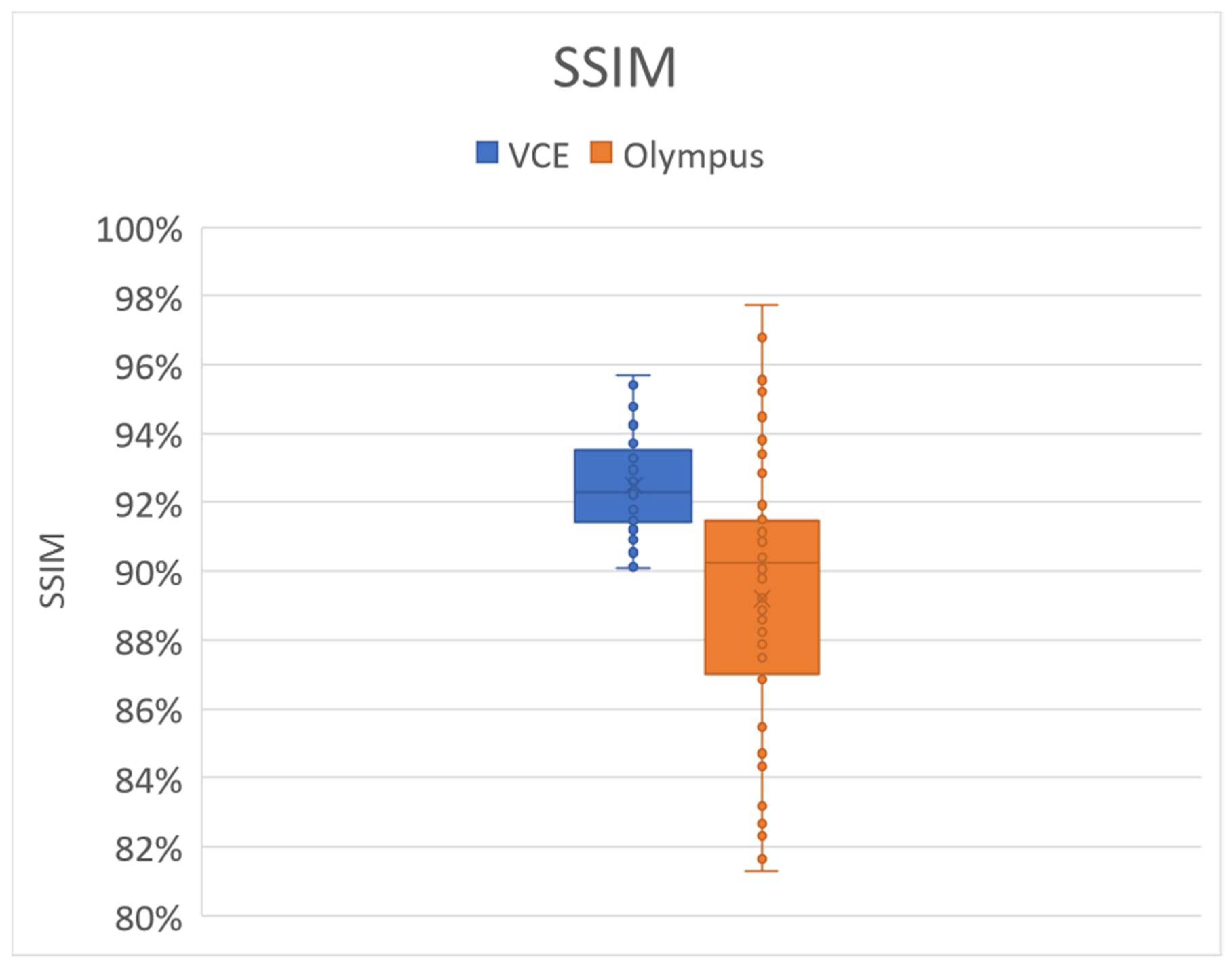
Figure 1 shows the results of the SSIM analysis. The SSIM value lies in the range of 0 and 100%, with a higher value indicating a better result. In this study, 50 random WLI images and their corresponding simulated NBI images were compared to compare the SSIM between the WLI images and the simulated NBI images. The average SSIM values for the Olympus and VCE images were 90% and 92.49%, respectively. However, out of the 50 images, 21 VCE images had a high SSIM of more than 93%. Out of the 50 randomly selected images, 19 had an SSIM of 91%, and only nine images had less than 84% (Supplement 1
Table S3). These findings revealed that the average SSIM decreased because of these nine images. These images were either blurred, had too much light reflected on them, or had some flare. Therefore, the SSIM value decreased below 91%. If the dataset was filtered and had a clearer WLI, then the NBI reproduction of the images could be better. Similar to the Olympus images, the eight WLI images in VCE had considerable reflection that made the SSIM value less than 90%. However, in all the different conditions, the SSIM values did not reduce to below 90% in VCE. Therefore, regardless of the errors present in the image, the NBI reproduction can be profound.
3.2. Entropy
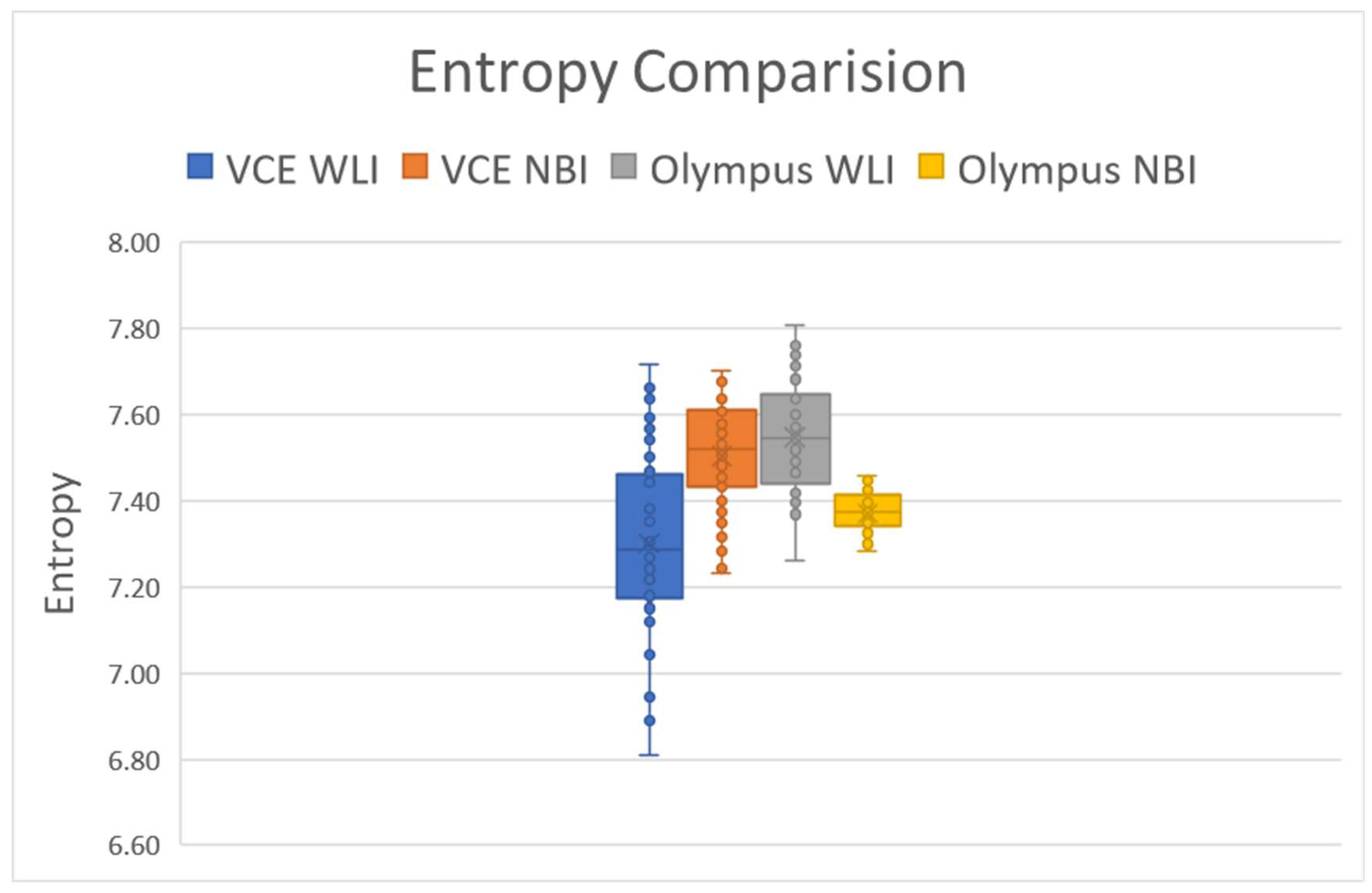
The entropy difference between the Olympus endoscope and VCE is illustrated in
Figure 2. The average entropy difference between the WLI and simulated NBI images in VCE was around 2.6942%, and that between the WLI and simulated images from the Olympus endoscope was 2.3457% (Supplement 1
Table S2). The results showed that the entropy in the Olympus endoscope and VCE followed a similar pattern. When the entropy increased in the WLI images, the entropy of the NBI images also increased and vice versa. In VCE and the Olympus endoscope, the entropy in the NBI images increased because of three images (image numbers 20, 25, and 46). This finding can be attributed to the excessive reflection seen in the WLI image, indicating the successful utilization of the algorithm in this study.
3.3. PSNR
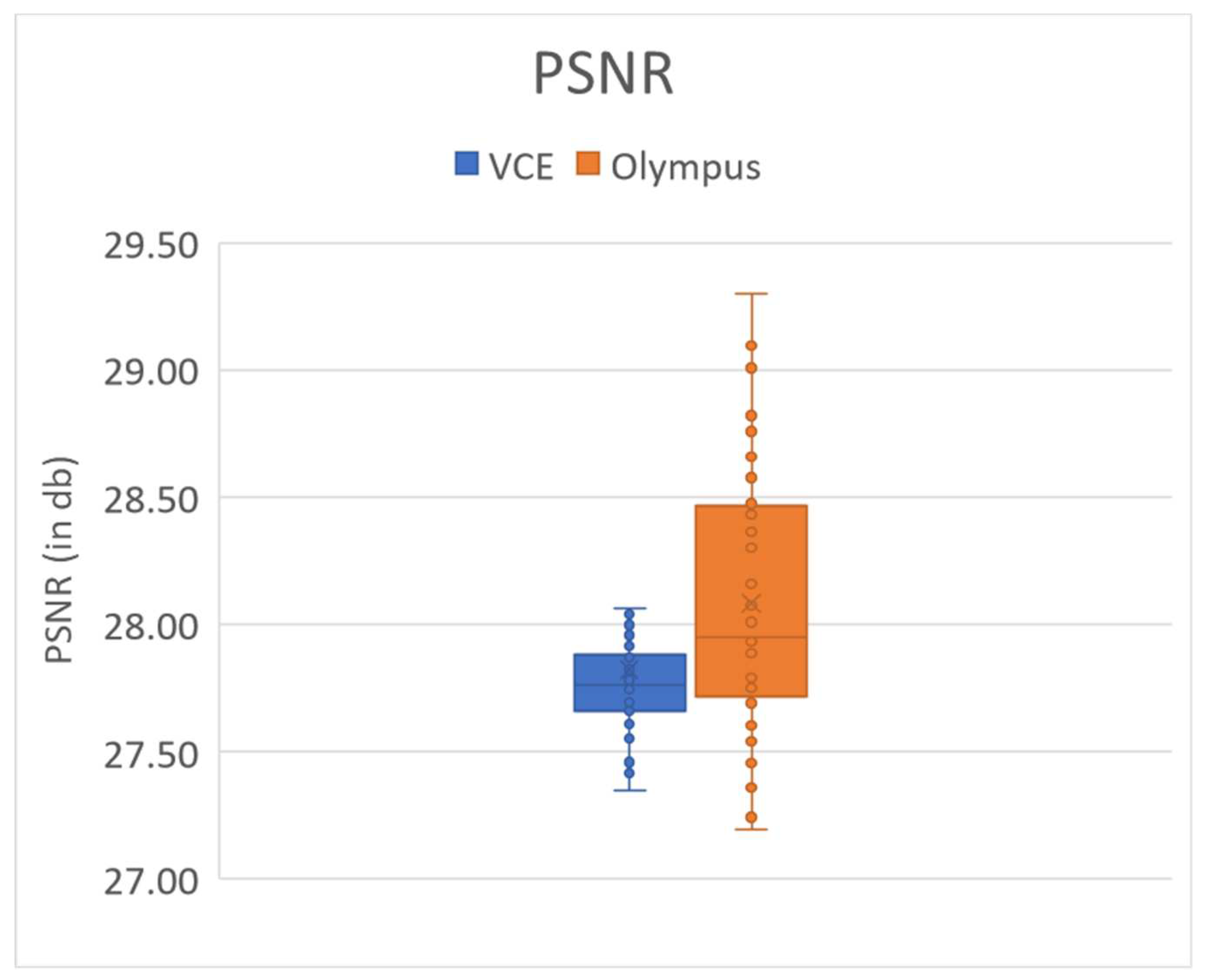
Figure 3 shows the PSRN value of the 50 randomly chosen VCE and Olympus endoscopic images. The average PSNR values of the VCE and Olympus endoscopic images were 27.8212 and 28.0813 dB, respectively (Supplement 1
Table S1). The results of SSIM, entropy, and PSNR showed that the proposed algorithm performed better.
4. Discussion
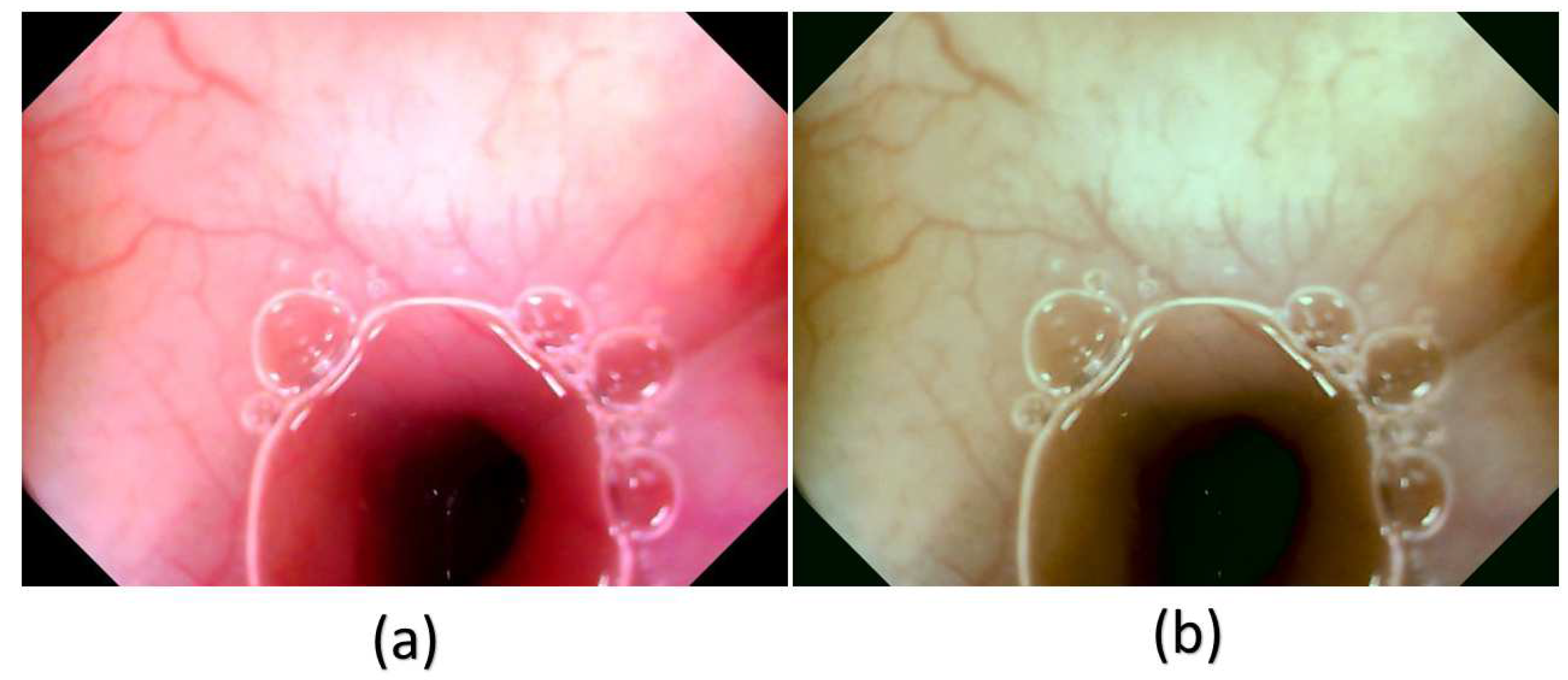
In this study, a decolored axis color-matching function was used to simulate NBI images from the VCE WLI images by using the NBI image from an Olympus endoscope as a reference. Given that VCE is more preferred than the traditional endoscopy, incorporating NBI capability onto VCE is an important requisite because VCE does not currently have NBI functionality. Such functionality has been proven to be more effective in detecting early cancer cells, specifically EC, which does not have any early biomarkers. This incorporation could increase the 5-year survival rate of EC drastically. The results of this study showed that the reproduced NBI images had better comparison metric values. First, the average SSIM values of the Olympus and VCE endoscopic images were 89.1995% and 92.4919%, respectively. Second, the average entropy values of the randomly chosen WLI images and their corresponding NBI images in VCE and the Olympus endoscope were 2.6942% and 2.3457%, respectively. Finally, the PSNRs of the WLI images and their corresponding NBI images in VCE and the Olympus endoscope were 27.8212 and 28.0813 dB, respectively. The future scope of this study is to test the reproduced NBI images with a YOLOv5 deep learning model with a dataset of EC to detect and classify cancers on the basis of stage severity. Then, the same model could be used to compare the accuracy, sensitivity, and specificity of WLI those of NBI. However, one of the limitations of this method is that it did not consider the lighting spectrum of the WLI and NBI images. Therefore, if the WLI image is blurred or has light reflections on it, the NBI image could not be a perfect simulation. The simulated NBI image with the corresponding WLI of the VCE endoscope are shown in
Figure 4 (Supplement 1
Figure S1 shows 50 randomly chosen images of WLI in VCE, and
Figure S2 shows 50 randomly chosen images of the simulated NBI in VCE). The simulated NBI image with the corresponding WLI and a similar NBI image from the Olympus endoscope are shown in
Figure 5 (Supplement 1
Figure S3 shows six randomly chosen images of WLI in VCE, and
Figure S5 shows another 12 randomly chosen images of simulated NBI in VCE).
5. Conclusions
In this study, a decolored axis color-matching function was implemented to simulate NBI images from VCE WLI images by making use of the NBI image obtained from an Olympus endoscope as a reference. VCE does not currently have NBI functionality, which has been proven to be more effective in detecting early cancer cells, particularly early EC, which does not have any early biomarkers. Given that VCE is more preferred than the traditional endoscopy, incorporating NBI capability into VCE is an important requisite that could result in a significant increase in the 5-year survival rate of patients with EC. The results showed that the reproduced NBI images had higher comparison metric values. The images from the Olympus endoscope and VCE had average SSIM values of 98.415% and 93.215%, respectively. The random WLI images and their corresponding NBI images obtained from VCE and the Olympus endoscopes showed average entropy values of 3.45% and 4.36%, respectively. The PSNRs of the WLI images with their corresponding NBI images in VCE and the Olympus endoscope were 28.06 and 28.15%, respectively.
Supplementary Materials
The following supporting information can be downloaded at
www.mdpi.com/xxx/s1, Table S1. Results of PSNR comparison of each image in Olympus and VCE; Table S2. Results of Entropy comparison of each image in Olympus and VCE; Table S3. Results of SSIM comparison of each image in Olympus and VCE; Figure S1. 50 Randomly chosen images of WLI in VCE; Figure S2. 50 Randomly chosen images of NBI in VCE; Figure S3. 6 Randomly choses WLI images, simulated NBI images and a similar original NBI in Olympus endoscope. (a) Olympus WLI images. (b) simulated NBI image and (c) a similar NBI image from Olympus; Figure S4. 6 Randomly choses WLI images, simulated NBI images and a similar original NBI in Olympus endoscope. (a) Olympus WLI images. (b) simulated NBI image and (c) a similar NBI image from Olympus.
Author Contributions
Conceptualization, K.-Y.Y, H.-C.W and A.M.; data curation, K.-Y.Y, Y.-M.T and A.M.; formal analysis, K.-Y.Y, R.K and A.M.; funding acquisition, C.-W.H, Y.-J.F and H.-C.W.; investigation, R.K, Y.-M.T and A.M.; methodology, Y.-J.F, H.-C.W and A.M.; project administration, Y.-M.T, A.M. and H.-C.W.; resources, C.-W.H, H.-C.W and A.M.; software, Y.-M.T and A.M.; supervision, C.-W.H, Y.-M.T and H.-C.W.; validation, Y.-J.F, Y.-M.T and A.M.; writing—original draft, R.K and A.M.; writing—review and editing, R.K, A.M. and H.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Science and Technology Council, the Republic of China under grants NSTC 112-2221-E-194-036. This work was financially or partially supported by the National Chung Cheng University-National Taiwan University Hospital Yunlin Branch Joint Research Program (CCU-NTUHYB-112-NC002), National Taiwan University Hospital Yunlin Branch (112-C001) and Kaohsiung Armed Forces General Hospital research project 112-010 in Taiwan.
Institutional Review Board Statement
The study was conducted by the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of National Taiwan University Hospital (NTUH) (NTUH-202009096RIND) and the Institutional Review Board of Kaohsiung Armed Forces General Hospital (KAFGHIRB 112-018).
Informed Consent Statement
Written informed consent was waived in this study because of the retrospective, anonymized nature of study design.
Data Availability Statement
The data presented in this study are available in this article upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sahafi, A.; Wang, Y.; Rasmussen, C.L.M.; Bollen, P.; Baatrup, G.; Blanes-Vidal, V.; Herp, J.; Nadimi, E.S. Edge artificial intelligence wireless video capsule endoscopy. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, W.; Zhang, K.; Ming, F.; Yangdai, T.; Xu, T.; Shi, H.; Bao, Y.; Yao, H.; Peng, H.; et al. Novel scheme for non-invasive gut bioinformation acquisition with a magnetically controlled sampling capsule endoscope. Gut 2021, 70, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Hosoe, N.; Takabayashi, K.; Ogata, H.; Kanai, T. Capsule endoscopy for small-intestinal disorders: Current status. Dig. Endosc. 2019, 31, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Vasilakakis, M.; Koulaouzidis, A.; Marlicz, W.; Iakovidis, D. The future of capsule endoscopy in clinical practice: from diagnostic to therapeutic experimental prototype capsules. Gastroenterol. Rev. 2020, 15, 179–193. [Google Scholar] [CrossRef]
- Noormohammadi, R. Khaleghi, and I. Balasingham. Battery-free wireless communication for video capsule endoscopy. in 2019 13th International Symposium on Medical Information and Communication Technology (ISMICT). 2019. IEEE.
- Vedaei, S.S.; Wahid, K.A. A localization method for wireless capsule endoscopy using side wall cameras and IMU sensor. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, L.-J.; Gao, S.; Geyi, W. Integrated Design of Wideband Omnidirectional Antenna and Electronic Components for Wireless Capsule Endoscopy Systems. IEEE Access 2018, 6, 29626–29636. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.; Lee, K.-R.; Lee, J.; Kim, M.; Lee, Y.; Bae, J.; Yoo, H.-J. 4-Camera VGA-resolution capsule endoscope with 80Mb/s body-channel communication transceiver and Sub-cm range capsule localization. 2018, 282–284. [Google Scholar] [CrossRef]
- Almalioglu, Y.; Ozyoruk, K.B.; Gokce, A.; Incetan, K.; Gokceler, G.I.; Simsek, M.A.; Ararat, K.; Chen, R.J.; Durr, N.J.; Mahmood, F.; et al. EndoL2H: Deep Super-Resolution for Capsule Endoscopy. IEEE Trans. Med Imaging 2020, 39, 4297–4309. [Google Scholar] [CrossRef]
- Yen, C.-T.; Lai, Z.-W.; Lin, Y.-T.; Cheng, H.-C. Optical Design with Narrow-Band Imaging for a Capsule Endoscope. J. Heal. Eng. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Sano, Y.; Tanaka, S.; Kudo, S.-E.; Saito, S.; Matsuda, T.; Wada, Y.; Fujii, T.; Ikematsu, H.; Uraoka, T.; Kobayashi, N.; et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig. Endosc. 2016, 28, 526–533. [Google Scholar] [CrossRef]
- Sun, C.; Han, X.; Li, X.; Zhang, Y.; Du, X. Diagnostic Performance of Narrow Band Imaging for Laryngeal Cancer: A Systematic Review and Meta-analysis. Otolaryngol. Neck Surg. 2017, 156, 589–597. [Google Scholar] [CrossRef]
- Vu, A.; Farah, C.S. Narrow band imaging: clinical applications in oral and oropharyngeal cancer. Oral Dis. 2016, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.; Sonoda, Y.; Gardner, G.J.; Park, K.J.; Elliott, S.L.; Zhou, Q.C.; Iasonos, A.; Abu-Rustum, N.R. Prospective Comparative Study of Laparoscopic Narrow Band Imaging (NBI) Versus Standard Imaging in Gynecologic Oncology. Ann. Surg. Oncol. 2018, 25, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Rodrigues, J.A.; Ghaderi, M.; Goncalves, L.M.; de Graaf, G.; Wolffenbuttel, R.F.; Correia, J.H. NBI Optical Filters in Minimally Invasive Medical Devices. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 1–7. [Google Scholar] [CrossRef]
- Kimza, H.; Jackowska, J.; Wierzbicka, M. The usefulness of the NBI – narrow band imaging for the larynx assessment. Otolaryngol. Polska 2018, 72, 1–5. [Google Scholar] [CrossRef]
- White, J.R.; Sami, S.S.; Reddiar, D.; Mannath, J.; Ortiz-Fernández-Sordo, J.; Beg, S.; Scott, R.; Thiagarajan, P.; Ahmad, S.; Parra-Blanco, A.; et al. Narrow band imaging and serology in the assessment of premalignant gastric pathology. Scand. J. Gastroenterol. 2018, 53, 1611–1618. [Google Scholar] [CrossRef]
- Wisotzky, E.L.; Uecker, F.C.; Arens, P.; Dommerich, S.; Hilsmann, A.; Eisert, P. Intraoperative hyperspectral determination of human tissue properties. J. Biomed. Opt. 2018, 23, 091409–8. [Google Scholar] [CrossRef]
- Zhou, F. , et al., The accuracy of magnifying narrow band imaging (ME-NBI) in distinguishing between cancerous and noncancerous gastric lesions: A meta-analysis. Medicine, 2018. 97(9).
- Tsai, T.-J.; Mukundan, A.; Chi, Y.-S.; Tsao, Y.-M.; Wang, Y.-K.; Chen, T.-H.; Wu, I.-C.; Huang, C.-W.; Wang, H.-C. Intelligent Identification of Early Esophageal Cancer by Band-Selective Hyperspectral Imaging. Cancers 2022, 14, 4292. [Google Scholar] [CrossRef]
- Fang, Y.-J.; Mukundan, A.; Tsao, Y.-M.; Huang, C.-W.; Wang, H.-C. Identification of Early Esophageal Cancer by Semantic Segmentation. J. Pers. Med. 2022, 12, 1204. [Google Scholar] [CrossRef]
- Puvanakrishnan, P.; Diagaradjane, P.; Kazmi, S.S.; Dunn, A.K.; Krishnan, S.; Tunnell, J.W. Narrow band imaging of squamous cell carcinoma tumors using topically delivered anti-EGFR antibody conjugated gold nanorods. Lasers Surg. Med. 2012, 44, 310–317. [Google Scholar] [CrossRef]
- Tan, N.C.-W.; Herd, M.K.; Brennan, P.A.; Puxeddu, R. The role of narrow band imaging in early detection of head and neck cancer. Br. J. Oral Maxillofac. Surg. 2012, 50, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Taniguchi, M.; Tsujie, H.; Hosokawa, M.; Fujita, M.; Sasaki, S. The value of narrow band imaging for early detection of laryngeal cancer. Eur. Arch. Oto-Rhino-Laryngology 2008, 266, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Mukundan, A.; Chung, C.-S.; Chen, Y.-H.; Wang, Y.-K.; Chen, T.-H.; Tseng, Y.-S.; Huang, C.-W.; Wu, I.-C.; Wang, H.-C. Hyperspectral Imaging Combined with Artificial Intelligence in the Early Detection of Esophageal Cancer. Cancers 2021, 13, 4593. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Chang, C.Y.; Lee, Y.C.; Tai, C.M.; Wang, W.L.; Tseng, P.H.; Hwang, J.C.; Hwang, T.Z.; Wang, C.C.; Lin, J.T. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy 2010, 42, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Inoue, H.; Usui, S.; Satodate, H.; Fukami, N.; Kudo, S.-E. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest. Endosc. 2004, 59, 288–295. [Google Scholar] [CrossRef]
- Thamir, N.N.; Mohammed, F.G. Early Esophageal Cancer detection using Deep learning Techniques. (Review Article). J. Physics: Conf. Ser. 2021, 1963. [Google Scholar] [CrossRef]
- Reinhard, E. , et al., Color transfer between images. IEEE Computer graphics and applications 2001, 21, 34–41. [Google Scholar] [CrossRef]
- Brunet, D.; Vrscay, E.R.; Wang, Z. On the Mathematical Properties of the Structural Similarity Index. IEEE Trans. Image Process. 2011, 21, 1488–1499. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image Quality Assessment: From Error Visibility to Structural Similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef]
- Hore, A. and D. Ziou. Image quality metrics: PSNR vs. SSIM. in 2010 20th international conference on pattern recognition. 2010. IEEE.
- Dosselmann, R.; Yang, X.D. A comprehensive assessment of the structural similarity index. Signal, Image Video Process. 2009, 5, 81–91. [Google Scholar] [CrossRef]
- Sara, U., M. Akter, and M.S. Uddin, Image quality assessment through FSIM, SSIM, MSE and PSNR—a comparative study. J. Comput. Commun. 2019, 7, 8–18. [Google Scholar] [CrossRef]
- Setiadi, D.R.I.M. PSNR vs SSIM: imperceptibility quality assessment for image steganography. Multimedia Tools Appl. 2020, 80, 8423–8444. [Google Scholar] [CrossRef]
- Ndajah, P. , et al. SSIM image quality metric for denoised images. in Proc. 3rd WSEAS Int. Conf. on Visualization, Imaging and Simulation. 2010.
- Nilsson, J. and T. Akenine-Möller, Understanding ssim. arXiv:2006.13846, 2020.
- Tsai, D.-Y.; Lee, Y.; Matsuyama, E. Information Entropy Measure for Evaluation of Image Quality. J. Digit. Imaging 2007, 21, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Pal, S. Entropy: a new definition and its applications. IEEE Trans. Syst. Man, Cybern. 1991, 21, 1260–1270. [Google Scholar] [CrossRef]
- Winkler, S.; Mohandas, P. The Evolution of Video Quality Measurement: From PSNR to Hybrid Metrics. IEEE Trans. Broadcast. 2008, 54, 660–668. [Google Scholar] [CrossRef]
- Huynh-Thu, Q.; Ghanbari, M. The accuracy of PSNR in predicting video quality for different video scenes and frame rates. Telecommun. Syst. 2010, 49, 35–48. [Google Scholar] [CrossRef]
- Tanchenko, A. , Visual-PSNR measure of image quality. J. Vis. Commun. Image Represent. 2014, 25, 874–878. [Google Scholar] [CrossRef]
- Deshpande, R.G.; Ragha, L.L.; Sharma, S.K. Video Quality Assessment through PSNR Estimation for Different Compression Standards. Indones. J. Electr. Eng. Comput. Sci. 2018, 11, 918–924. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).