Submitted:
02 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Early embryonic development in mice
2.1. Preimplantation embryonic development
2.2. Peri- and post-implantation embryonic development
2.3. Gastrulation
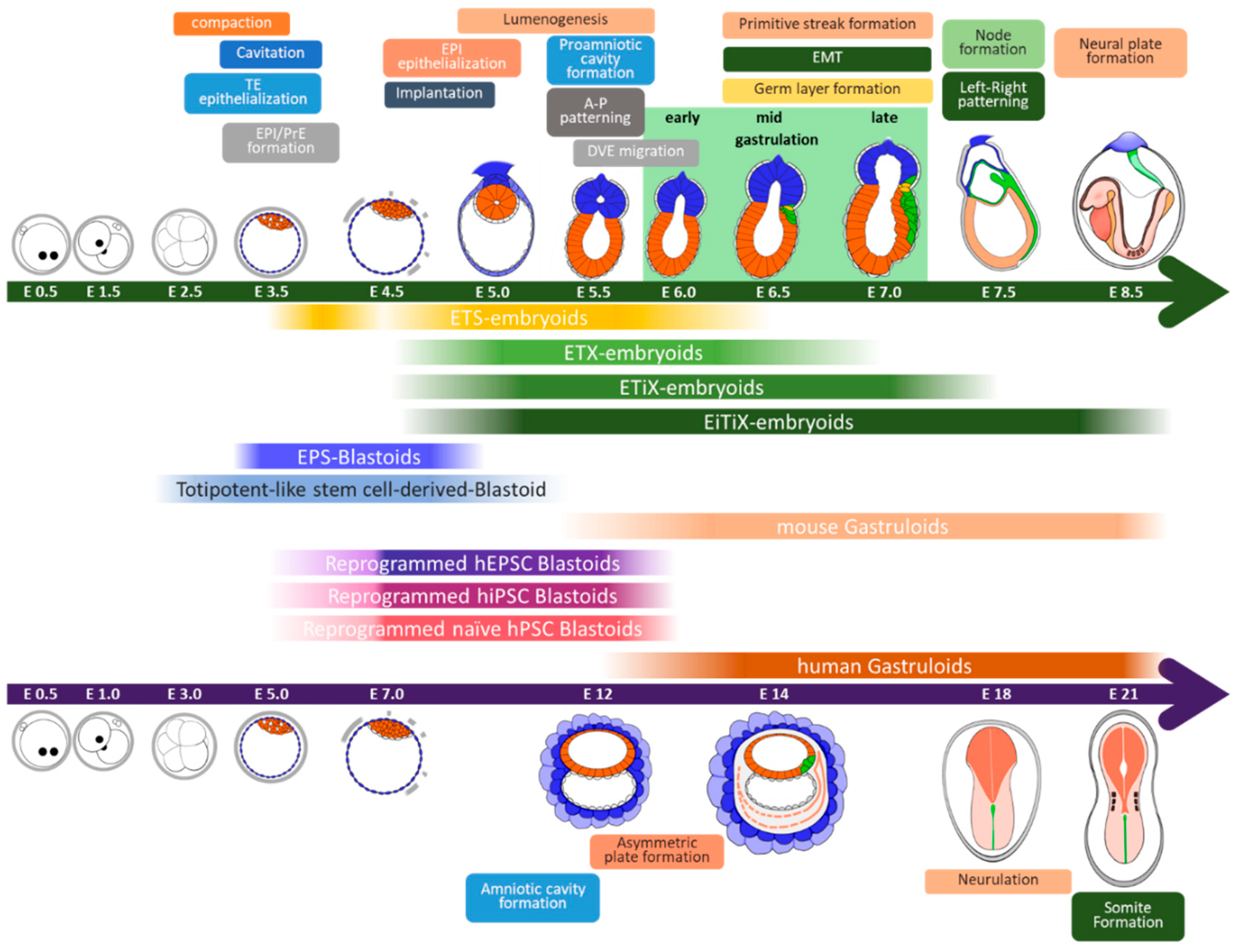
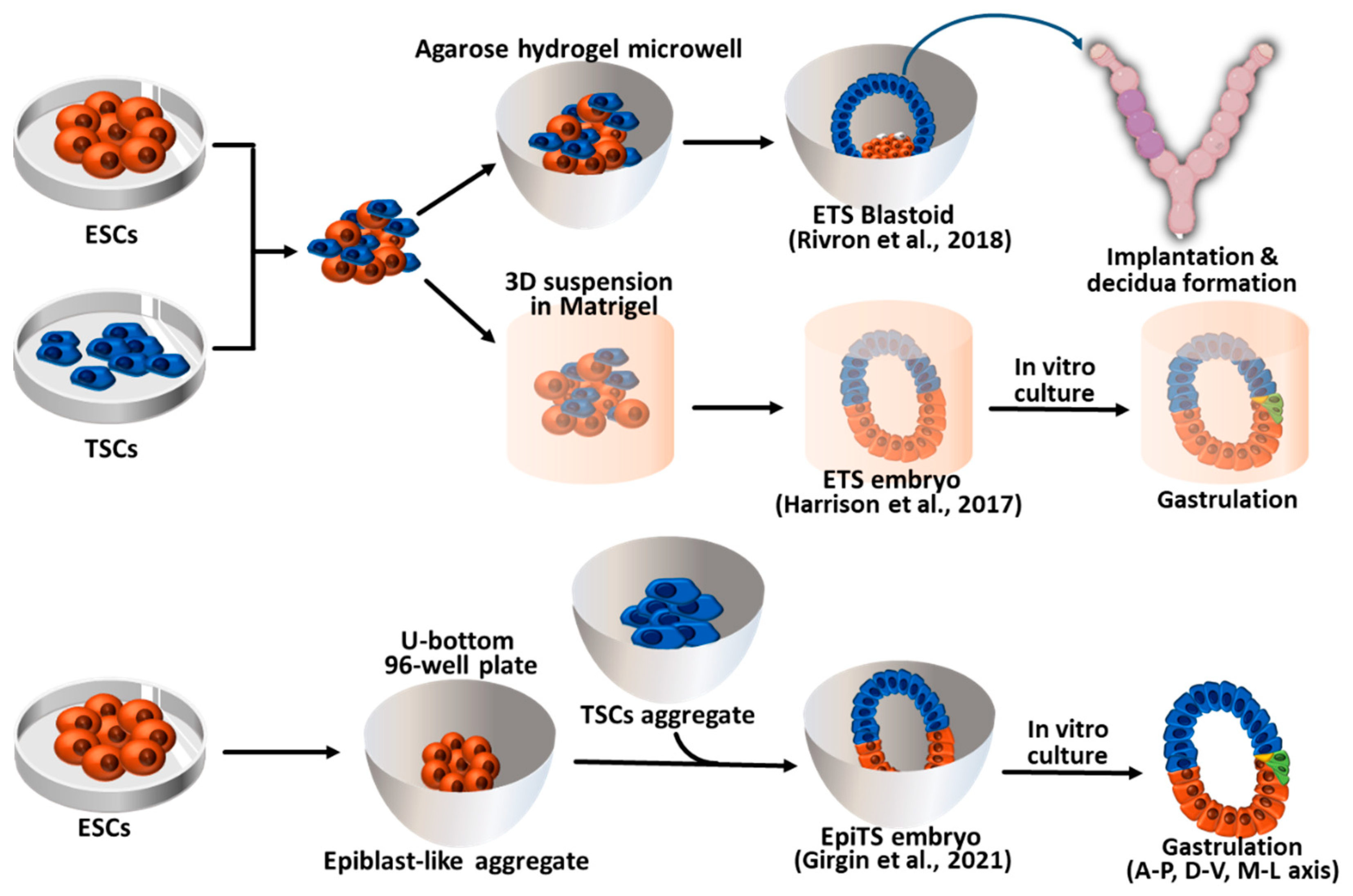
3. Synthetic embryos constructed with mouse ESCs and TSCs (ETS-embryoids)
4. Synthetic embryos constructed with ESCs, TSCs, and XENCs
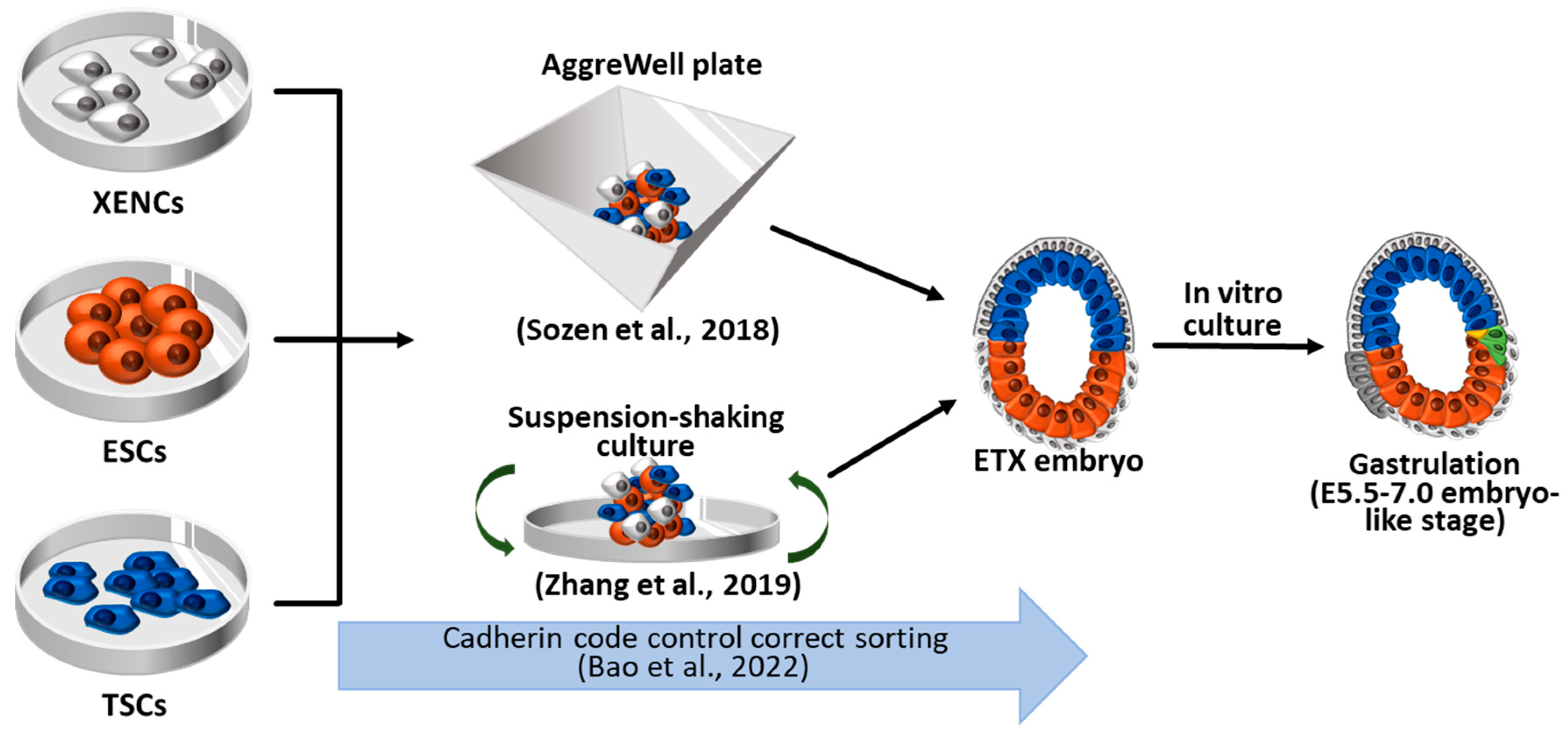
4.1. ETX embryos using wild-type ESCs, TSCs, and XENCs
4.2. ETiX embryos using ESCs facilitating PrE-lineage differentiation
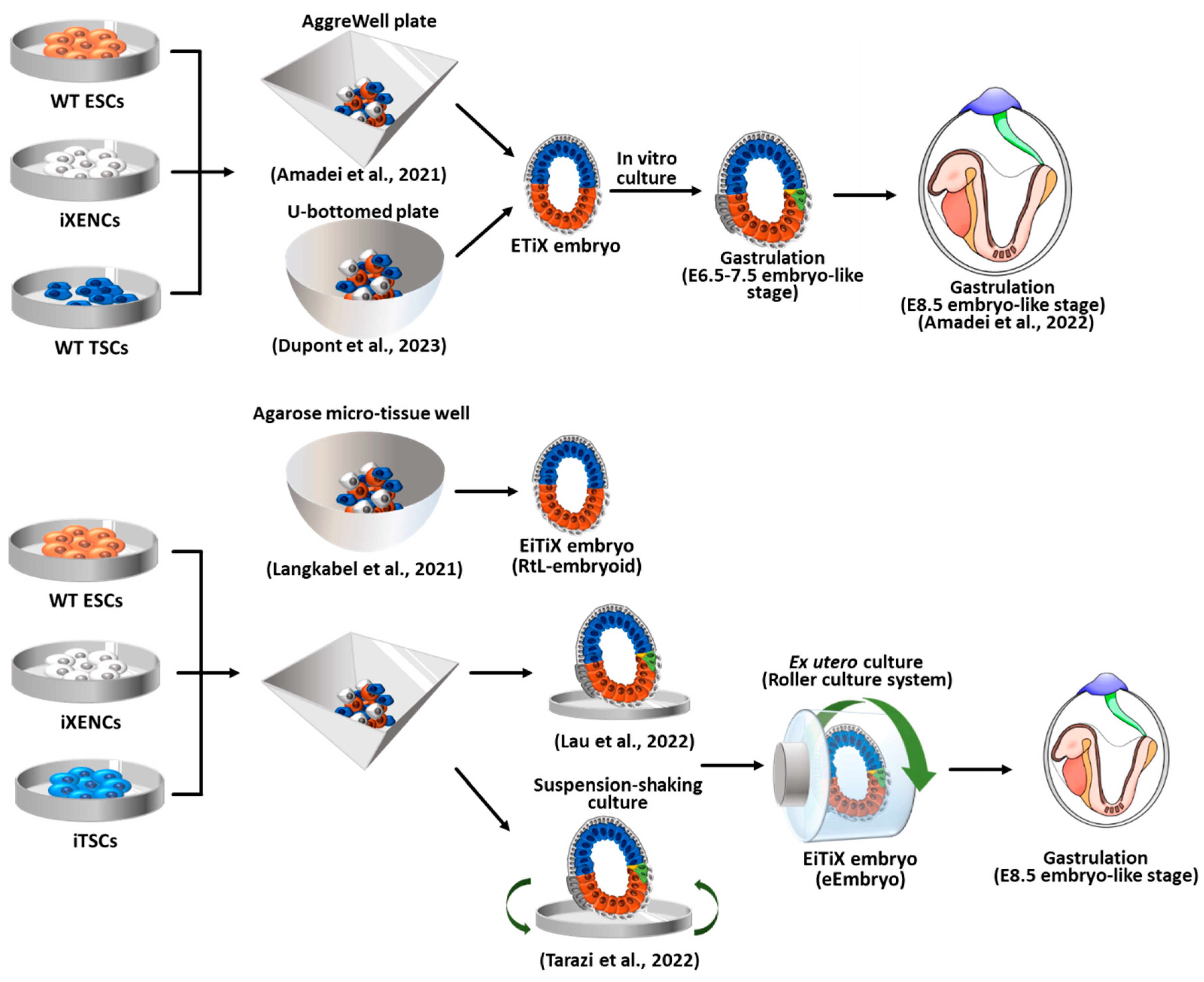
4.3. EiTiX embryos constructed with ESCs and induced TSCs (iTSCs) and induced XENCs (iXENCs)
5. Blastoid formation using totipotent-like stem cells
5.1. Blastoid formation from expanded potential stem cells (EPSCs), EPS- and EPST-blastoids
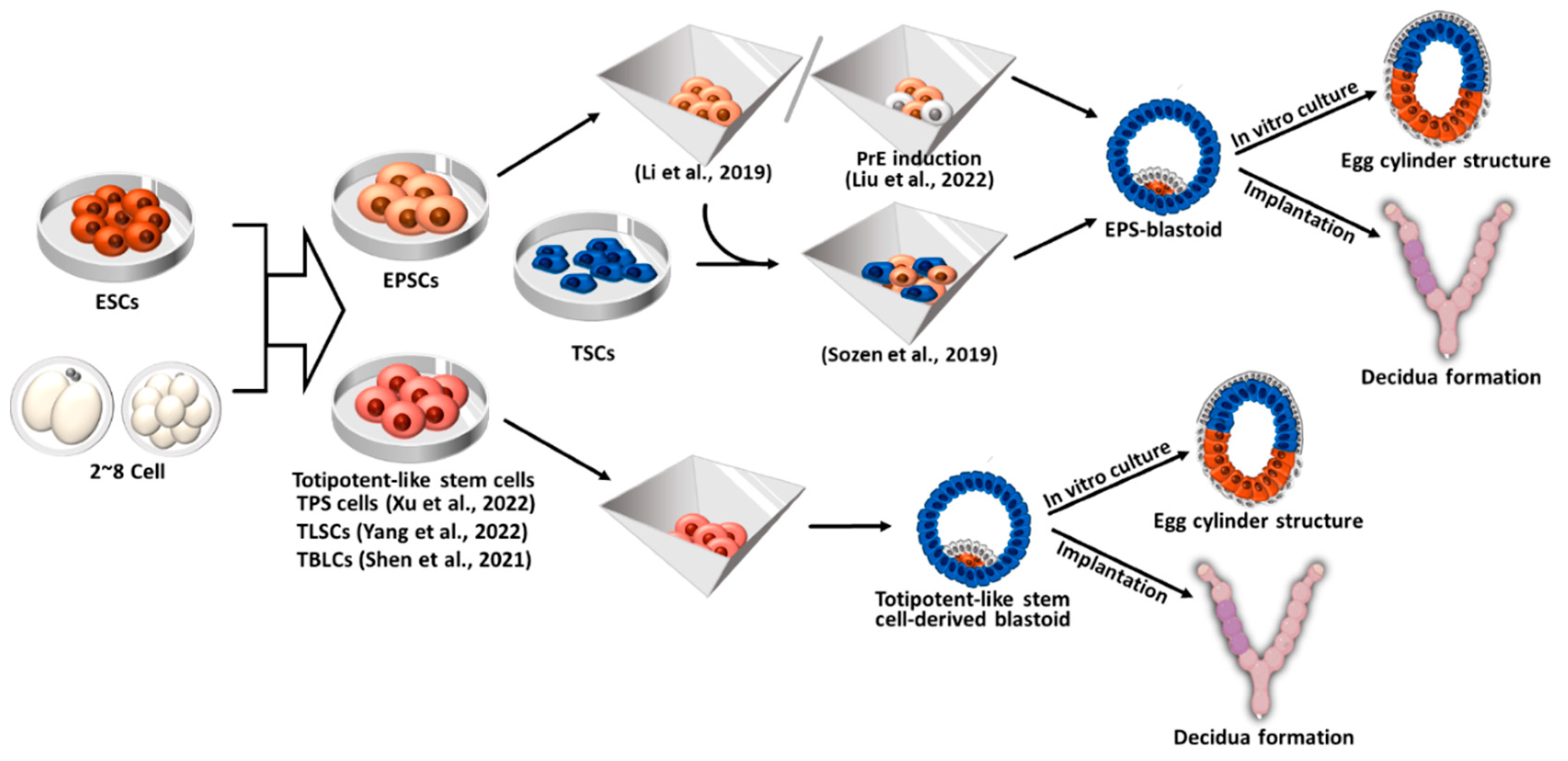
5.2. Blastoid formation from totipotent-like stem cells other than EPSCs
6. Human blastoid formation and in vitro implantation development
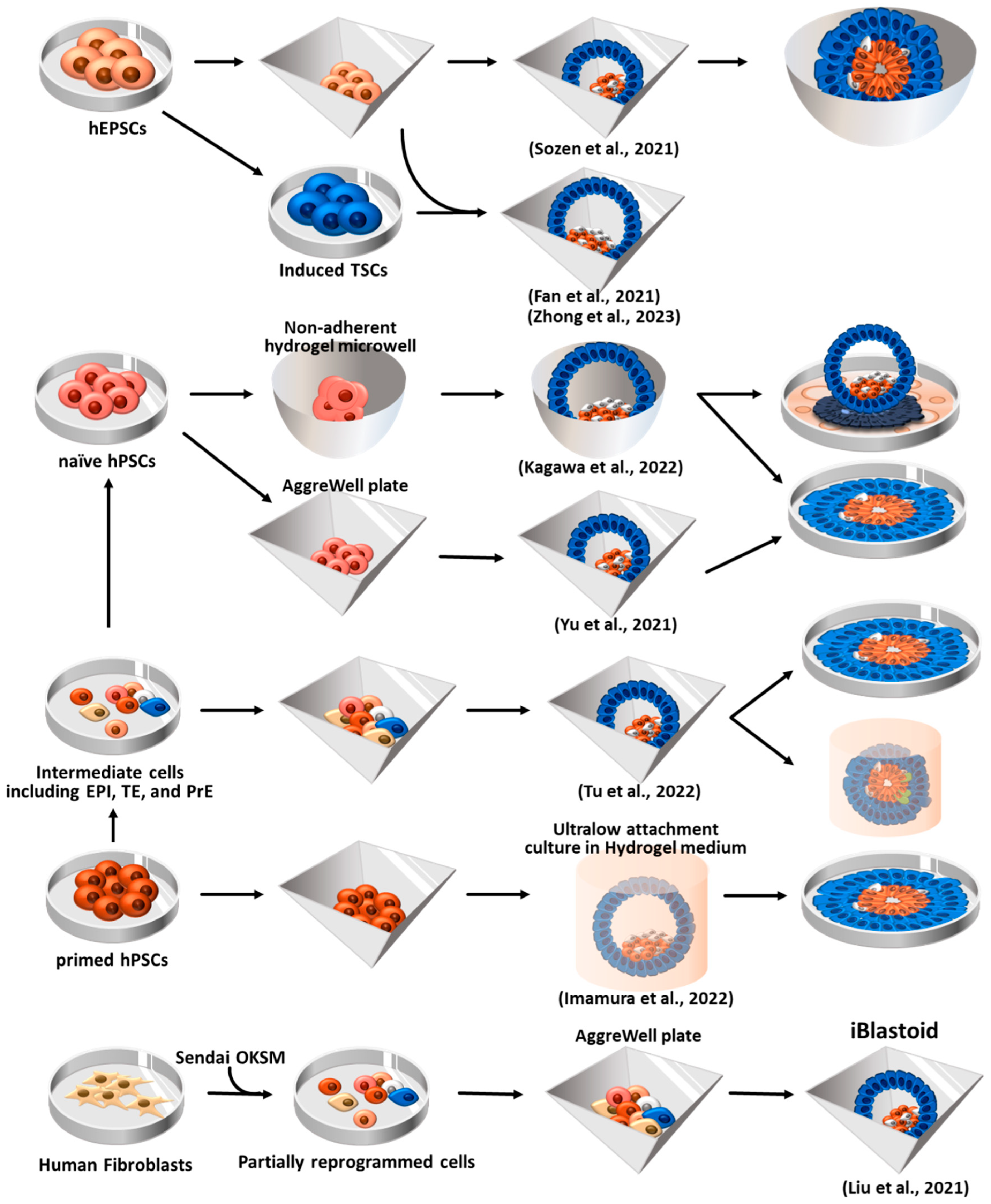
6.1. Blastoid formation using human EPSCs (hEPSCs)
6.2. Human blastoid induction via reprogramming of fibroblasts
6.3. Human blastoid formation from primed and naïve hPSCs
7. Gastruloids
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, Y.; Tam, O.H.; Tam, P.P. Timing of developmental events in the early mouse embryo. Semin Cell Dev Biol 2014, 34, 65–75. [Google Scholar] [CrossRef]
- ten Berge, D.; Koole, W.; Fuerer, C.; Fish, M.; Eroglu, E.; Nusse, R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 2008, 3, 508–518. [Google Scholar] [CrossRef]
- Fuchs, C.; Scheinast, M.; Pasteiner, W.; Lagger, S.; Hofner, M.; Hoellrigl, A.; Schultheis, M.; Weitzer, G. Self-organization phenomena in embryonic stem cell-derived embryoid bodies: axis formation and breaking of symmetry during cardiomyogenesis. Cells Tissues Organs 2012, 195, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, S.C.; Baillie-Johnson, P.; Balayo, T.; Hadjantonakis, A.-K.; Nowotschin, S.; Turner, D.A.; Martinez Arias, A.J.D. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. development 2014, 141, 4231–4242. [Google Scholar] [CrossRef]
- Warmflash, A.; Sorre, B.; Etoc, F.; Siggia, E.D.; Brivanlou, A.H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 2014, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, T.; Chen, N.; Gao, D.; Shi, B.; Kong, S.; West, R.C.; Yuan, Y.; Zhi, M.; Wei, Q.; et al. Implantation initiation of self-assembled embryo-like structures generated using three types of mouse blastocyst-derived stem cells. Nat Commun 2019, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.E.; Sozen, B.; Christodoulou, N.; Kyprianou, C.; Zernicka-Goetz, M.J.S. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 2017, 356, eaal1810. [Google Scholar] [CrossRef]
- Sozen, B.; Amadei, G.; Cox, A.; Wang, R.; Na, E.; Czukiewska, S.; Chappell, L.; Voet, T.; Michel, G.; Jing, N.; et al. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat Cell Biol 2018, 20, 979–989. [Google Scholar] [CrossRef]
- Langkabel, J.; Horne, A.; Bonaguro, L.; Holsten, L.; Hesse, T.; Knaus, A.; Riedel, Y.; Becker, M.; Handler, K.; Elmzzahi, T.; et al. Induction of Rosette-to-Lumen stage embryoids using reprogramming paradigms in ESCs. Nat Commun 2021, 12, 7322. [Google Scholar] [CrossRef]
- Girgin, M.U.; Broguiere, N.; Hoehnel, S.; Brandenberg, N.; Mercier, B.; Arias, A.M.; Lutolf, M.P. Bioengineered embryoids mimic post-implantation development in vitro. Nat Commun 2021, 12, 5140. [Google Scholar] [CrossRef]
- Amadei, G.; Lau, K.Y.; De Jonghe, J.; Gantner, C.W.; Sozen, B.; Chan, C.; Zhu, M.; Kyprianou, C.; Hollfelder, F.; Zernicka-Goetz, M.J.D.c. Inducible stem-cell-derived embryos capture mouse morphogenetic events in vitro. Developmental cell 2021, 56, 366–382. e369. [Google Scholar] [CrossRef]
- Amadei, G.; Handford, C.E.; Qiu, C.; De Jonghe, J.; Greenfeld, H.; Tran, M.; Martin, B.K.; Chen, D.Y.; Aguilera-Castrejon, A.; Hanna, J.H.; et al. Embryo model completes gastrulation to neurulation and organogenesis. Nature 2022, 610, 143–153. [Google Scholar] [CrossRef]
- Dupont, C.; Schäffers, O.J.; Tan, B.F.; Merzouk, S.; Bindels, E.M.; Zwijsen, A.; Huylebroeck, D.; Gribnau, J.J.S.A. Efficient generation of ETX embryoids that recapitulate the entire window of murine egg cylinder development. Science Advances 2023, 9, eadd2913. [Google Scholar] [CrossRef]
- Aguilera-Castrejon, A.; Oldak, B.; Shani, T.; Ghanem, N.; Itzkovich, C.; Slomovich, S.; Tarazi, S.; Bayerl, J.; Chugaeva, V.; Ayyash, M.; et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature 2021, 593, 119–124. [Google Scholar] [CrossRef]
- Lau, K.Y.C.; Rubinstein, H.; Gantner, C.W.; Hadas, R.; Amadei, G.; Stelzer, Y.; Zernicka-Goetz, M. Mouse embryo model derived exclusively from embryonic stem cells undergoes neurulation and heart development. Cell Stem Cell 2022, 29, 1445–1458. [Google Scholar] [CrossRef] [PubMed]
- Tarazi, S.; Aguilera-Castrejon, A.; Joubran, C.; Ghanem, N.; Ashouokhi, S.; Roncato, F.; Wildschutz, E.; Haddad, M.; Oldak, B.; Gomez-Cesar, E.; et al. Post-gastrulation synthetic embryos generated ex utero from mouse naive ESCs. Cell 2022, 185, 3290–3306. [Google Scholar] [CrossRef] [PubMed]
- Bedzhov, I.; Leung, C.Y.; Bialecka, M.; Zernicka-Goetz, M. In vitro culture of mouse blastocysts beyond the implantation stages. Nat Protoc 2014, 9, 2732–2739. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Ren, Y.; Wang, X.; Lyu, Y.; Xie, B.; Sun, Y.; Yuan, X.; Liu, H.; Yang, W.; et al. Derivation of totipotent-like stem cells with blastocyst-like structure forming potential. Cell Res 2022, 32, 513–529. [Google Scholar] [CrossRef]
- Yang, M.; Yu, H.; Yu, X.; Liang, S.; Hu, Y.; Luo, Y.; Izsvak, Z.; Sun, C.; Wang, J. Chemical-induced chromatin remodeling reprograms mouse ESCs to totipotent-like stem cells. Cell Stem Cell 2022, 29, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yang, M.; Li, S.; Zhang, J.; Peng, B.; Wang, C.; Chang, Z.; Ong, J.; Du, P. Mouse totipotent stem cells captured and maintained through spliceosomal repression. Cell 2021, 184, 2843–2859.e2820. [Google Scholar] [CrossRef]
- Zhang, P.; Zhai, X.; Huang, B.; Sun, S.; Wang, W.; Zhang, M. Highly efficient generation of blastocyst-like structures from spliceosomes-repressed mouse totipotent blastomere-like cells. Science China Life Sciences 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cossec, J.-C.; Traboulsi, T.; Sart, S.; Loe-Mie, Y.; Guthmann, M.; Hendriks, I.A.; Theurillat, I.; Nielsen, M.L.; Torres-Padilla, M.-E.; Baroud, C.N. Transient suppression of SUMOylation in embryonic stem cells generates embryo-like structures. Cell Reports 2023, 42. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, E.; Wilson, I.J.N. In vitro support system for the study of blastocyst differentiation in the mouse. Nature 1970, 228, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, J.P.; Schroder, J.; Aberkane, A.; Ouyang, J.F.; Mohenska, M.; Lim, S.M.; Sun, Y.B.Y.; Chen, J.; Sun, G.; et al. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 2021, 591, 627–632. [Google Scholar] [CrossRef]
- Sozen, B.; Jorgensen, V.; Weatherbee, B.A.T.; Chen, S.; Zhu, M.; Zernicka-Goetz, M. Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat Commun 2021, 12, 5550. [Google Scholar] [CrossRef]
- Zhong, K.; Luo, Y.-X.; Li, D.; Min, Z.-Y.; Fan, Y.; Yu, Y. Generation of blastoids from human parthenogenetic stem cells. Life Medicine 2023, 2, lnad006. [Google Scholar] [CrossRef]
- Yu, L.; Wei, Y.; Duan, J.; Schmitz, D.A.; Sakurai, M.; Wang, L.; Wang, K.; Zhao, S.; Hon, G.C.; Wu, J. Blastocyst-like structures generated from human pluripotent stem cells. Nature 2021, 591, 620–626. [Google Scholar] [CrossRef]
- Fan, Y.; Min, Z.; Alsolami, S.; Ma, Z.; Zhang, E.; Chen, W.; Zhong, K.; Pei, W.; Kang, X.; Zhang, P. Generation of human blastocyst-like structures from pluripotent stem cells. Cell Discovery 2021, 7, 81. [Google Scholar] [CrossRef]
- Appleby, J.B.; Bredenoord, A.L. Should the 14-day rule for embryo research become the 28-day rule? EMBO molecular medicine 2018, 10, e9437. [Google Scholar] [CrossRef]
- Condic, M.L. Totipotency: what it is and what it is not. Stem Cells Dev 2014, 23, 796–812. [Google Scholar] [CrossRef]
- Maemura, M.; Taketsuru, H.; Nakajima, Y.; Shao, R.; Kakihara, A.; Nogami, J.; Ohkawa, Y.; Tsukada, Y.I. Totipotency of mouse zygotes extends to single blastomeres of embryos at the four-cell stage. Sci Rep 2021, 11, 11167. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.H.; Loskutoff, N.M.; Plante, Y.; Betteridge, K.J. Production of four identical calves by the separation of blastomeres from an in vitro derived four-cell embryo. Vet Rec 1995, 137, 15–16. [Google Scholar] [CrossRef]
- Calarco, P.G.; Brown, E.H. An ultrastructural and cytological study of preimplantation development of the mouse. J Exp Zool 1969, 171, 253–283. [Google Scholar] [CrossRef]
- Ducibella, T.; Ukena, T.; Karnovsky, M.; Anderson, E. Changes in cell surface and cortical cytoplasmic organization during early embryogenesis in the preimplantation mouse embryo. J Cell Biol 1977, 74, 153–167. [Google Scholar] [CrossRef]
- White, M.D.; Bissiere, S.; Alvarez, Y.D.; Plachta, N. Mouse Embryo Compaction. Curr Top Dev Biol 2016, 120, 235–258. [Google Scholar] [CrossRef]
- Arnold, S.J.; Robertson, E.J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol 2009, 10, 91–103. [Google Scholar] [CrossRef]
- Chazaud, C.; Yamanaka, Y.; Pawson, T.; Rossant, J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell 2006, 10, 615–624. [Google Scholar] [CrossRef]
- Niwa, H.; Toyooka, Y.; Shimosato, D.; Strumpf, D.; Takahashi, K.; Yagi, R.; Rossant, J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 2005, 123, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Varelas, X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014, 141, 1614–1626. [Google Scholar] [CrossRef]
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 2009, 16, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Bedzhov, I.; Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 2014, 156, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Varlet, I.; Collignon, J.; Robertson, E.J. nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development 1997, 124, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Papanayotou, C.; Benhaddou, A.; Camus, A.; Perea-Gomez, A.; Jouneau, A.; Mezger, V.; Langa, F.; Ott, S.; Sabéran-Djoneidi, D.; Collignon, J. A novel nodal enhancer dependent on pluripotency factors and smad2/3 signaling conditions a regulatory switch during epiblast maturation. PLoS biology 2014, 12, e1001890. [Google Scholar] [CrossRef]
- Senft, A.D.; Bikoff, E.K.; Robertson, E.J.; Costello, I. Genetic dissection of Nodal and Bmp signalling requirements during primordial germ cell development in mouse. Nat Commun 2019, 10, 1089. [Google Scholar] [CrossRef]
- Brennan, J.; Lu, C.C.; Norris, D.P.; Rodriguez, T.A.; Beddington, R.S.P.; Robertson, E.J. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 2001, 411, 965–969. [Google Scholar] [CrossRef]
- Ciruna, B.; Rossant, J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell 2001, 1, 37–49. [Google Scholar] [CrossRef]
- Ciruna, B.G.; Schwartz, L.; Harpal, K.; Yamaguchi, T.P.; Rossant, J. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development 1997, 124, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Vogel, R.; Brinkmann, V.; Erdmann, B.; Birchmeier, C.; Birchmeier, W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol 2000, 148, 567–578. [Google Scholar] [CrossRef]
- Mishina, Y.; Suzuki, A.; Ueno, N.; Behringer, R.R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes & development 1995, 9, 3027–3037. [Google Scholar] [CrossRef]
- Liu, P.; Wakamiya, M.; Shea, M.J.; Albrecht, U.; Behringer, R.R.; Bradley, A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet 1999, 22, 361–365. [Google Scholar] [CrossRef]
- Kinder, S.J.; Tsang, T.E.; Wakamiya, M.; Sasaki, H.; Behringer, R.R.; Nagy, A.; Tam, P.P. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development 2001, 128, 3623–3634. [Google Scholar] [CrossRef]
- Sulik, K.; Dehart, D.B.; Iangaki, T.; Carson, J.L.; Vrablic, T.; Gesteland, K.; Schoenwolf, G.C. Morphogenesis of the murine node and notochordal plate. Developmental dynamics : an official publication of the American Association of Anatomists 1994, 201, 260–278. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Tamplin, O.J.; Beckers, A.; Gossler, A.; Rossant, J. Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev Cell 2007, 13, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Pituello, F. Neuronal specification: generating diversity in the spinal cord. Current biology : CB 1997, 7, R701–R704. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A. Fate mapping the mouse embryo. The International journal of developmental biology 1999, 43, 773–775. [Google Scholar] [PubMed]
- Di-Gregorio, A.; Sancho, M.; Stuckey, D.W.; Crompton, L.A.; Godwin, J.; Mishina, Y.; Rodriguez, T.A. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development 2007, 134, 3359–3369. [Google Scholar] [CrossRef]
- Camus, A.; Perea-Gomez, A.; Moreau, A.; Collignon, J. Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol 2006, 295, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Yamaji, M. Primordial germ cells in mice. Cold Spring Harbor perspectives in biology 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A.; Dunn, N.R.; Roelen, B.A.; Zeinstra, L.M.; Davis, A.M.; Wright, C.V.; Korving, J.P.; Hogan, B.L. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes & development 1999, 13, 424–436. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, X.M.; Marble, A.; Lawson, K.A.; Zhao, G.Q. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Molecular endocrinology (Baltimore, Md.) 2000, 14, 1053–1063. [Google Scholar] [CrossRef]
- Tremblay, K.D.; Dunn, N.R.; Robertson, E.J. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001, 128, 3609–3621. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Matzuk, M.M. Smad5 is required for mouse primordial germ cell development. Mechanisms of development 2001, 104, 61–67. [Google Scholar] [CrossRef]
- Hayashi, K.; Kobayashi, T.; Umino, T.; Goitsuka, R.; Matsui, Y.; Kitamura, D. SMAD1 signaling is critical for initial commitment of germ cell lineage from mouse epiblast. Mechanisms of development 2002, 118, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.J.F.i.B.-L. Specification of the germ cell lineage in mice. Front Biosci 2009, 14, 1068–1087. [Google Scholar] [CrossRef]
- Tanaka, S.; Kunath, T.; Hadjantonakis, A.K.; Nagy, A.; Rossant, J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998, 282, 2072–2075. [Google Scholar] [CrossRef]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.-L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.G.; Bhatt, S.; Herrmann, B.G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 1990, 343, 657–659. [Google Scholar] [CrossRef]
- Rivera-Pérez, J.A.; Magnuson, T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol 2005, 288, 363–371. [Google Scholar] [CrossRef]
- Yamaji, M.; Seki, Y.; Kurimoto, K.; Yabuta, Y.; Yuasa, M.; Shigeta, M.; Yamanaka, K.; Ohinata, Y.; Saitou, M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nature Genetics 2008, 40, 1016–1022. [Google Scholar] [CrossRef]
- Auman, H.J.; Nottoli, T.; Lakiza, O.; Winger, Q.; Donaldson, S.; Williams, T. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development 2002, 129, 2733–2747. [Google Scholar] [CrossRef]
- Rivron, N.C.; Frias-Aldeguer, J.; Vrij, E.J.; Boisset, J.C.; Korving, J.; Vivie, J.; Truckenmuller, R.K.; van Oudenaarden, A.; van Blitterswijk, C.A.; Geijsen, N. Blastocyst-like structures generated solely from stem cells. Nature 2018, 557, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Rivron, N.; Rivron, N. Formation of blastoids from mouse embryonic and trophoblast stem cells. Protocol Exchange 2018. [Google Scholar] [CrossRef]
- Frias-Aldeguer, J.; Kip, M.; Vivié, J.; Li, L.; Alemany, A.; Korving, J.; Darmis, F.; Oudenaarden, A.v.; Geijsen, N.; Rivron, N.C. Embryonic signals perpetuate polar-like trophoblast stem cells and pattern the blastocyst axis. 2020. [Google Scholar] [CrossRef]
- Kemp, C.; Willems, E.; Abdo, S.; Lambiv, L.; Leyns, L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Developmental dynamics : an official publication of the American Association of Anatomists 2005, 233, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Kunath, T.; Arnaud, D.; Uy, G.D.; Okamoto, I.; Chureau, C.; Yamanaka, Y.; Heard, E.; Gardner, R.L.; Avner, P.; Rossant, J. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 2005, 132, 1649–1661. [Google Scholar] [CrossRef]
- Enders, A.C.; Given, R.L.; Schlafke, S. Differentiation and migration of endoderm in the rat and mouse at implantation. The Anatomical record 1978, 190, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.P.; Beddington, R.S. Establishment and organization of germ layers in the gastrulating mouse embryo. Ciba Foundation symposium 1992, 165, 27–41, discussion 42-29. [Google Scholar] [CrossRef]
- Viotti, M.; Nowotschin, S.; Hadjantonakis, A.K.J.g. Afp:: mCherry, a red fluorescent transgenic reporter of the mouse visceral endoderm. genesis 2011, 49, 124–133. [Google Scholar] [CrossRef]
- Perea-Gomez, A.; Meilhac, S.M.; Piotrowska-Nitsche, K.; Gray, D.; Collignon, J.; Zernicka-Goetz, M. Regionalisation of the mouse visceral endoderm as the blastocyst transforms into the egg cylinder. BMC Developmental Biology 2007, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Edgar, D.; Fässler, R.; Wadsworth, W.; Yurchenco, P.D.J.D.c. The role of laminin in embryonic cell polarization and tissue organization. 2003, 4, 613–624.
- Williams, M.; Burdsal, C.; Periasamy, A.; Lewandoski, M.; Sutherland, A. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Developmental Dynamics 2012, 241, 270–283. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.J.C.s.c. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell stem cell 2016, 118, 330–340. [Google Scholar] [CrossRef]
- Ohinata, Y.; Sano, M.; Shigeta, M.; Yamanaka, K.; Saitou, M.J.R. A comprehensive, non-invasive visualization of primordial germ cell development in mice by thePrdm1-mVenus andDppa3-ECFP double transgenic reporter. Reproduction 2008, 136, 503–514. [Google Scholar] [CrossRef]
- Zernicka-Goetz, M. Patterning of the embryo: the first spatial decisions in the life of a mouse. 2002. [Google Scholar]
- Saitou, M.J.C.o.i.g. ; development. Germ cell specification in mice. 2009, 19, 386–395. [Google Scholar]
- Bao, M.; Cornwall-Scoones, J.; Sanchez-Vasquez, E.; Cox, A.L.; Chen, D.-Y.; De Jonghe, J.; Shadkhoo, S.; Hollfelder, F.; Thomson, M.; Glover, D.M.; et al. Stem cell-derived synthetic embryos self-assemble by exploiting cadherin codes and cortical tension. Nature Cell Biology 2022, 24, 1341–1349. [Google Scholar] [CrossRef]
- Toda, S.; Blauch, L.R.; Tang, S.K.Y.; Morsut, L.; Lim, W.A. Programming self-organizing multicellular structures with synthetic cell-cell signaling. Science 2018, 361, 156–162. [Google Scholar] [CrossRef]
- Paca, A.; Séguin, C.A.; Clements, M.; Ryczko, M.; Rossant, J.; Rodriguez, T.A.; Kunath, T.J.D.b. BMP signaling induces visceral endoderm differentiation of XEN cells and parietal endoderm. Developmental biology 2012, 361, 90–102. [Google Scholar] [CrossRef]
- Moerkamp, A.T.; Paca, A.; Goumans, M.J.; Kunath, T.; Kruithof, B.P.; Kruithof-de Julio, M.J.D. , growth; differentiation. Extraembryonic endoderm cells as a model of endoderm development. 2013, 55, 301–308. [Google Scholar]
- Shimosato, D.; Shiki, M.; Niwa, H.J.B.d.b. Extra-embryonic endoderm cells derived from ES cells induced by GATA factors acquire the character of XEN cells. BMC developmental biology 2007, 7, 1–12. [Google Scholar] [CrossRef]
- Schröter, C.; Rué, P.; Mackenzie, J.P.; Martinez Arias, A.J.D. FGF/MAPK signaling sets the switching threshold of a bistable circuit controlling cell fate decisions in embryonic stem cells. Development 2015, 142, 4205–4216. [Google Scholar]
- Tosic, J.; Kim, G.-J.; Pavlovic, M.; Schröder, C.M.; Mersiowsky, S.-L.; Barg, M.; Hofherr, A.; Probst, S.; Köttgen, M.; Hein, L.J.N.c.b. Eomes and Brachyury control pluripotency exit and germ-layer segregation by changing the chromatin state. Nature cell biology 2019, 21, 1518–1531. [Google Scholar] [CrossRef]
- Ramkumar, N.; Omelchenko, T.; Silva-Gagliardi, N.F.; McGlade, C.J.; Wijnholds, J.; Anderson, K.V. Crumbs2 promotes cell ingression during the epithelial-to-mesenchymal transition at gastrulation. Nature Cell Biology 2016, 18, 1281–1291. [Google Scholar] [CrossRef]
- Nowotschin, S.; Setty, M.; Kuo, Y.-Y.; Liu, V.; Garg, V.; Sharma, R.; Simon, C.S.; Saiz, N.; Gardner, R.; Boutet, S.C.J.N. The emergent landscape of the mouse gut endoderm at single-cell resolution. Nature 2019, 569, 361–367. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hayashi, M.; Kubota, Y.; Nagai, H.; Sheng, G.; Nishikawa, S.-I.; Samokhvalov, I.M.J.P.o.t.N.A.o.S. Early ontogenic origin of the hematopoietic stem cell lineage. Proceedings of the National Academy of Sciences 2012, 109, 4515–4520. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sanchez, V.; Takata, N.; Yokomizo, T.; Yamanaka, Y.; Kataoka, H.; Hoppe, P.S.; Schroeder, T.; Nishikawa, S.-I.J.C.r. Circulation-independent differentiation pathway from extraembryonic mesoderm toward hematopoietic stem cells via hemogenic angioblasts. Cell reports 2014, 8, 31–39. [Google Scholar] [CrossRef]
- José-Edwards, D.S.; Oda-Ishii, I.; Kugler, J.E.; Passamaneck, Y.J.; Katikala, L.; Nibu, Y.; Di Gregorio, A.J.P.g. Brachyury, Foxa2 and the cis-Regulatory Origins of the Notochord. PLoS genetics 2015, 11, e1005730. [Google Scholar] [CrossRef]
- Ybot-Gonzalez, P.; Gaston-Massuet, C.; Girdler, G.; Klingensmith, J.; Arkell, R.; Greene, N.D.; Copp, A.J. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development 2007, 134, 3203–3211. [Google Scholar] [CrossRef]
- Balmer, S.; Nowotschin, S.; Hadjantonakis, A.K.J.D.D. Notochord morphogenesis in mice: Current understanding & open questions. Developmental Dynamics 2016, 245, 547–557. [Google Scholar]
- Kahane, N.; Kalcheim, C.J.D. Neural tube development depends on notochord-derived sonic hedgehog released into the sclerotome. Development 2020, 147, dev183996. [Google Scholar] [CrossRef] [PubMed]
- Pevny, L.H.; Sockanathan, S.; Placzek, M.; Lovell-Badge, R.J.D. A role for SOX1 in neural determination. Development 1998, 125, 1967–1978. [Google Scholar] [CrossRef]
- Ericson, J.; Rashbass, P.; Schedl, A.; Brenner-Morton, S.; Kawakami, A.; Van Heyningen, V.; Jessell, T.; Briscoe, J.J.C. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 1997, 90, 169–180. [Google Scholar] [CrossRef]
- Novitch, B.G.; Chen, A.I.; Jessell, T.M.J.N. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron 2001, 31, 773–789. [Google Scholar] [CrossRef]
- Sasaki, H.; Hogan, B.L.J.C. HNF-3β as a regulator of floor plate development. Cell 1994, 76, 103–115. [Google Scholar] [CrossRef]
- Kelsh, R.N. Sorting out Sox10 functions in neural crest development. BioEssays : news and reviews in molecular, cellular and developmental biology 2006, 28, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, D.; Rigby, P.W.J. 8 Transcriptional Regulation during Somitogenesis. In Current Topics in Developmental Biology; Ordahl, C.P., Ed.; Academic Press, 1999; Volume 48, pp. 301–318. [Google Scholar]
- Heikinheimo, M.; Scandrett, J.M.; Wilson, D.B. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev Biol 1994, 164, 361–373. [Google Scholar] [CrossRef]
- England, J.; Loughna, S. Heavy and light roles: myosin in the morphogenesis of the heart. Cellular and molecular life sciences : CMLS 2013, 70, 1221–1239. [Google Scholar] [CrossRef]
- de Boer, B.A.; van den Berg, G.; de Boer, P.A.J.; Moorman, A.F.M.; Ruijter, J.M. Growth of the developing mouse heart: An interactive qualitative and quantitative 3D atlas. Developmental Biology 2012, 368, 203–213. [Google Scholar] [CrossRef]
- Ross, C.; Boroviak, T.E. Origin and function of the yolk sac in primate embryogenesis. Nat Commun 2020, 11, 3760. [Google Scholar] [CrossRef]
- Frankenberg, S.; Gerbe, F.; Bessonnard, S.; Belville, C.; Pouchin, P.; Bardot, O.; Chazaud, C.J.D.c. Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Developmental cell 2011, 21, 1005–1013. [Google Scholar] [CrossRef]
- Saitou, M.; Yamaji, M.J.R. Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences. Reproduction 2010, 139, 931. [Google Scholar] [CrossRef]
- Pereira, P.N.; Dobreva, M.P.; Graham, L.; Huylebroeck, D.; Lawson, K.A.; Zwijsen, A.J.B.d.b. Amnion formation in the mouse embryo: the single amniochorionic fold model. BMC developmental biology 2011, 11, 1–13. [Google Scholar] [CrossRef]
- Huber, T.L.; Kouskoff, V.; Fehling, H.J.; Palis, J.; Keller, G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 2004, 432, 625–630. [Google Scholar] [CrossRef]
- Bergiers, I.; Andrews, T.; Vargel Bölükbaşı, Ö.; Buness, A.; Janosz, E.; Lopez-Anguita, N.; Ganter, K.; Kosim, K.; Celen, C.; Itır Perçin, G.J.E. Single-cell transcriptomics reveals a new dynamical function of transcription factors during embryonic hematopoiesis. Elife 2018, 7, e29312. [Google Scholar] [CrossRef]
- Home, P.; Ray, S.; Dutta, D.; Bronshteyn, I.; Larson, M.; Paul, S.J.J.o.B.C. GATA3 is selectively expressed in the trophectoderm of peri-implantation embryo and directly regulates Cdx2 gene expression. Journal of Biological Chemistry 2009, 284, 28729–28737. [Google Scholar] [CrossRef] [PubMed]
- Kuales, G.; Weiss, M.; Sedelmeier, O.; Pfeifer, D.; Arnold, S.J. A Resource for the Transcriptional Signature of Bona Fide Trophoblast Stem Cells and Analysis of Their Embryonic Persistence. Stem cells international 2015, 2015, 218518. [Google Scholar] [CrossRef]
- Kuckenberg, P.; Buhl, S.; Woynecki, T.; van Fürden, B.; Tolkunova, E.; Seiffe, F.; Moser, M.; Tomilin, A.; Winterhager, E.; Schorle, H.J.M.; et al. The transcription factor TCFAP2C/AP-2γ cooperates with CDX2 to maintain trophectoderm formation. Molecular and cellular biology 2010, 30, 3310–3320. [Google Scholar] [CrossRef]
- Shimozaki, K. Sox2 transcription network acts as a molecular switch to regulate properties of neural stem cells. World journal of stem cells 2014, 6, 485–490. [Google Scholar] [CrossRef]
- Scott, I.C.; Anson-Cartwright, L.; Riley, P.; Reda, D.; Cross, J.C. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Molecular and cellular biology 2000, 20, 530–541. [Google Scholar] [CrossRef]
- Kröger, C.; Vijayaraj, P.; Reuter, U.; Windoffer, R.; Simmons, D.; Heukamp, L.; Leube, R.; Magin, T.M. Placental vasculogenesis is regulated by keratin-mediated hyperoxia in murine decidual tissues. The American journal of pathology 2011, 178, 1578–1590. [Google Scholar] [CrossRef]
- Kang, J.; Nathan, E.; Xu, S.M.; Tzahor, E.; Black, B.L. Isl1 is a direct transcriptional target of Forkhead transcription factors in second-heart-field-derived mesoderm. Dev Biol 2009, 334, 513–522. [Google Scholar] [CrossRef]
- Kresoja-Rakic, J.; Santoro, R. Nucleolus and rRNA gene chromatin in early embryo development. Trends in Genetics 2019, 35, 868–879. [Google Scholar] [CrossRef]
- Baker, C.L.; Pera, M.F. Capturing totipotent stem cells. Cell stem cell 2018, 22, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhang, Y. Cell totipotency: molecular features, induction, and maintenance. National science review 2015, 2, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Genet, M.; Torres-Padilla, M.-E. The molecular and cellular features of 2-cell-like cells: a reference guide. Development 2020, 147, dev189688. [Google Scholar] [CrossRef]
- Yang, J.; Ryan, D.J.; Wang, W.; Tsang, J.C.-H.; Lan, G.; Masaki, H.; Gao, X.; Antunes, L.; Yu, Y.; Zhu, Z.; et al. Establishment of mouse expanded potential stem cells. Nature 2017, 550, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhong, C.; Yu, Y.; Liu, H.; Sakurai, M.; Yu, L.; Min, Z.; Shi, L.; Wei, Y.; Takahashi, Y.; et al. Generation of Blastocyst-like Structures from Mouse Embryonic and Adult Cell Cultures. Cell 2019, 179, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Posfai, E.; Schell, J.P.; Janiszewski, A.; Rovic, I.; Murray, A.; Bradshaw, B.; Yamakawa, T.; Pardon, T.; El Bakkali, M.; Talon, I. Evaluating totipotency using criteria of increasing stringency. Nature cell biology 2021, 23, 49–60. [Google Scholar] [CrossRef]
- Wang, H.; Ding, T.; Brown, N.; Yamamoto, Y.; Prince, L.S.; Reese, J.; Paria, B. Zonula occludens-1 (ZO-1) is involved in morula to blastocyst transformation in the mouse. Developmental biology 2008, 318, 112–125. [Google Scholar] [CrossRef]
- Liu, K.; Xu, X.; Bai, D.; Li, Y.; Zhang, Y.; Jia, Y.; Guo, M.; Han, X.; Liu, Y.; Sheng, Y.; et al. Bilineage embryo-like structure from EPS cells can produce live mice with tetraploid trophectoderm. Protein & Cell 2022. [Google Scholar] [CrossRef]
- Vrij, E.J.; Reimer, Y.S.S.o.; Aldeguer, J.F.; Guerreiro, I.M.; Kind, J.; Koo, B.-K.; Blitterswijk, C.A.v.; Rivron, N.C. Chemically-defined induction of a primitive endoderm and epiblast-like niche supports post-implantation progression from blastoids. BioRxiv 2019, 120, 510396. [Google Scholar] [CrossRef]
- Sozen, B.; Cox, A.L.; De Jonghe, J.; Bao, M.; Hollfelder, F.; Glover, D.M.; Zernicka-Goetz, M. Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid. Dev Cell 2019, 51, 698–712. [Google Scholar] [CrossRef]
- Williamson, R.A.; Henry, M.D.; Daniels, K.J.; Hrstka, R.F.; Lee, J.C.; Sunada, Y.; Ibraghimov-Beskrovnaya, O.; Campbell, K.P. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Human molecular genetics 1997, 6, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Suwińska, A. Preimplantation mouse embryo: developmental fate and potency of blastomeres. Mouse Development: From Oocyte to Stem Cells 2012, 141–163. [Google Scholar]
- Chai, N.; Patel, Y.; Jacobson, K.; McMahon, J.; McMahon, A.; Rappolee, D.A. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Developmental biology 1998, 198, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Goldin, S.N.; Papaioannou, V.E. Paracrine action of FGF4 during periimplantation development maintains trophectoderm and primitive endoderm. genesis 2003, 36, 40–47. [Google Scholar] [CrossRef]
- Graham, S.J.; Wicher, K.B.; Jedrusik, A.; Guo, G.; Herath, W.; Robson, P.; Zernicka-Goetz, M. BMP signalling regulates the pre-implantation development of extra-embryonic cell lineages in the mouse embryo. Nature communications 2014, 5, 5667. [Google Scholar] [CrossRef]
- Sankar, A.; Lerdrup, M.; Manaf, A.; Johansen, J.V.; Gonzalez, J.M.; Borup, R.; Blanshard, R.; Klungland, A.; Hansen, K.; Andersen, C.Y. KDM4A regulates the maternal-to-zygotic transition by protecting broad H3K4me3 domains from H3K9me3 invasion in oocytes. Nature Cell Biology 2020, 22, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Kime, C.; Kiyonari, H.; Ohtsuka, S.; Kohbayashi, E.; Asahi, M.; Yamanaka, S.; Takahashi, M.; Tomoda, K. Induced 2C Expression and Implantation-Competent Blastocyst-like Cysts from Primed Pluripotent Stem Cells. Stem Cell Reports 2019, 13, 485–498. [Google Scholar] [CrossRef]
- Wahl, M.C.; Will, C.L.; Lührmann, R. The spliceosome: design principles of a dynamic RNP machine. cell 2009, 136, 701–718. [Google Scholar] [CrossRef]
- Will, C.L.; Lührmann, R. Spliceosome structure and function. Cold Spring Harbor perspectives in biology 2011, 3, a003707. [Google Scholar] [CrossRef]
- Rodriguez-Terrones, D.; Gaume, X.; Ishiuchi, T.; Weiss, A.; Kopp, A.; Kruse, K.; Penning, A.; Vaquerizas, J.M.; Brino, L.; Torres-Padilla, M.-E. A molecular roadmap for the emergence of early-embryonic-like cells in culture. Nature genetics 2018, 50, 106–119. [Google Scholar] [CrossRef]
- Kotake, Y.; Sagane, K.; Owa, T.; Mimori-Kiyosue, Y.; Shimizu, H.; Uesugi, M.; Ishihama, Y.; Iwata, M.; Mizui, Y. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nature chemical biology 2007, 3, 570–575. [Google Scholar] [CrossRef]
- Fu, J.; Warmflash, A.; Lutolf, M.P. Stem-cell-based embryo models for fundamental research and translation. Nature materials 2021, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.N.M.; Campbell, A.; Devito, L.; Ilic, D.; et al. Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol 2016, 18, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Yin, Y.; Zheng, Y.; Ma, Y.; Li, Y.; Zhao, Z.; Guo, J.; Ai, Z.; Niu, Y.; Duan, K. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 2020, 577, 537–542. [Google Scholar] [CrossRef]
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254. [Google Scholar] [CrossRef]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of human trophoblast stem cells. Cell stem cell 2018, 22, 50–63. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, J.F.; Rossello, F.J.; Tan, J.P.; Davidson, K.C.; Valdes, D.S.; Schröder, J.; Sun, Y.B.; Chen, J.; Knaupp, A.S. Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature 2020, 586, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Beltcheva, M.; Gontarz, P.; Zhang, B.; Popli, P.; Fischer, L.A.; Khan, S.A.; Park, K.-m.; Yoon, E.-J.; Xing, X. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. elife 2020, 9, e52504. [Google Scholar] [CrossRef]
- Linneberg-Agerholm, M.; Wong, Y.F.; Romero Herrera, J.A.; Monteiro, R.S.; Anderson, K.G.; Brickman, J.M. Naïve human pluripotent stem cells respond to Wnt, Nodal and LIF signalling to produce expandable naïve extra-embryonic endoderm. Development 2019, 146, dev180620. [Google Scholar] [CrossRef]
- Guo, G.; Stirparo, G.G.; Strawbridge, S.E.; Spindlow, D.; Yang, J.; Clarke, J.; Dattani, A.; Yanagida, A.; Li, M.A.; Myers, S. Human naive epiblast cells possess unrestricted lineage potential. Cell stem cell 2021, 28, 1040–1056.e1046. [Google Scholar] [CrossRef]
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Ganz, K.; Shi, L. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell stem cell 2014, 15, 471–487. [Google Scholar] [CrossRef]
- Kagawa, H.; Javali, A.; Khoei, H.H.; Sommer, T.M.; Sestini, G.; Novatchkova, M.; Scholte Op Reimer, Y.; Castel, G.; Bruneau, A.; Maenhoudt, N.; et al. Human blastoids model blastocyst development and implantation. Nature 2022, 601, 600–605. [Google Scholar] [CrossRef]
- Guo, G.; von Meyenn, F.; Santos, F.; Chen, Y.; Reik, W.; Bertone, P.; Smith, A.; Nichols, J. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem cell reports 2016, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; von Meyenn, F.; Rostovskaya, M.; Clarke, J.; Dietmann, S.; Baker, D.; Sahakyan, A.; Myers, S.; Bertone, P.; Reik, W. Epigenetic resetting of human pluripotency. Development 2017, 144, 2748–2763. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, A.; Spindlow, D.; Nichols, J.; Dattani, A.; Smith, A.; Guo, G. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 2021, 28, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Tu, Z.; Zhou, J.; Zhu, X.; Wang, H.; Gao, S.; Wang, Y. Cell fate roadmap of human primed-to-naive transition reveals preimplantation cell lineage signatures. Nature Communications 2022, 13, 3147. [Google Scholar] [CrossRef]
- Chang, P.-H.; Chao, H.-M.; Chern, E.; Hsu, S.-h. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: A novel tool to sustain pluripotency and facilitate differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef]
- Imamura, S.; Wen, X.; Terada, S.; Yamamoto, A.; Mutsuda-Zapater, K.; Sawada, K.; Yoshimoto, K.; Tanaka, M.; Kamei, K.-i. Human blastoid from primed human embryonic stem cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tu, Z.; Bi, Y.; Zhu, X.; Liu, W.; Hu, J.; Wu, L.; Mao, T.; Zhou, J.; Wang, H.; Wang, H. Modeling human pregastrulation development by 3D culture of blastoids generated from primed-to-naïve transitioning intermediates. Protein & Cell 2023, 14, 337–349. [Google Scholar]
- Williams, K.; Johnson, M.H. Adapting the 14-day rule for embryo research to encompass evolving technologies. Reproductive Biomedicine & Society Online 2020, 10, 1–9. [Google Scholar]
- Lovell-Badge, R.; Anthony, E.; Barker, R.A.; Bubela, T.; Brivanlou, A.H.; Carpenter, M.; Charo, R.A.; Clark, A.; Clayton, E.; Cong, Y. ISSCR guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Reports 2021, 16, 1398–1408. [Google Scholar] [CrossRef]
- Yui, H.; Muto, K.; Yashiro, Y.; Watanabe, S.; Kiya, Y.; Kamisato, A.; Inoue, Y.; Yamagata, Z. Comparison of the 2021 International Society for Stem Cell Research (ISSCR) guidelines for “laboratory-based human stem cell research, embryo research, and related research activities” and the corresponding Japanese regulations. Regenerative Therapy 2022, 21, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.R.; Moralí, D. National human embryo and embryoid research policies: a survey of 22 top research-intensive countries. Regenerative Medicine 2020, 15, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Sawai, T.; Akatsuka, K.; Okui, G.; Minakawa, T. The regulation of human blastoid research: A bioethical discussion of the limits of regulation. EMBO reports 2022, 23, e56045. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Girgin, M.; Alonso-Crisostomo, L.; Trivedi, V.; Baillie-Johnson, P.; Glodowski, C.R.; Hayward, P.C.; Collignon, J.; Gustavsen, C.; Serup, P.; et al. Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development 2017, 144, 3894–3906. [Google Scholar] [CrossRef] [PubMed]
- Beccari, L.; Moris, N.; Girgin, M.; Turner, D.A.; Baillie-Johnson, P.; Cossy, A.C.; Lutolf, M.P.; Duboule, D.; Arias, A.M. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 2018, 562, 272–276. [Google Scholar] [CrossRef]
- van den Brink, S.C.; Alemany, A.; van Batenburg, V.; Moris, N.; Blotenburg, M.; Vivié, J.; Baillie-Johnson, P.; Nichols, J.; Sonnen, K.F.; Martinez Arias, A.; et al. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 2020, 582, 405–409. [Google Scholar] [CrossRef]
- Veenvliet, J.V.; Bolondi, A.; Kretzmer, H.; Haut, L.; Scholze-Wittler, M.; Schifferl, D.; Koch, F.; Guignard, L.; Kumar, A.S.; Pustet, M.J.S. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science 2020, 370, eaba4937. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Broguiere, N.; Miyamoto, M.; Boni, A.; Guiet, R.; Girgin, M.; Kelly, R.G.; Kwon, C.; Lutolf, M.P. Capturing Cardiogenesis in Gastruloids. Cell Stem Cell 2021, 28, 230–240.e236. [Google Scholar] [CrossRef]
- Xu, P.-F.; Borges, R.M.; Fillatre, J.; de Oliveira-Melo, M.; Cheng, T.; Thisse, B.; Thisse, C.J.N.C. Construction of a mammalian embryo model from stem cells organized by a morphogen signalling centre. Nature Communications 2021, 12, 3277. [Google Scholar] [CrossRef]
- Cossec, J.-C.; Theurillat, I.; Chica, C.; Aguín, S.B.; Gaume, X.; Andrieux, A.; Iturbide, A.; Jouvion, G.; Li, H.; Bossis, G. SUMO safeguards somatic and pluripotent cell identities by enforcing distinct chromatin states. Cell stem cell 2018, 23, 742–757.e748. [Google Scholar] [CrossRef] [PubMed]
- Borkent, M.; Bennett, B.D.; Lackford, B.; Bar-Nur, O.; Brumbaugh, J.; Wang, L.; Du, Y.; Fargo, D.C.; Apostolou, E.; Cheloufi, S. A serial shRNA screen for roadblocks to reprogramming identifies the protein modifier SUMO2. Stem cell reports 2016, 6, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Baik, H.; Boulanger, M.; Hosseini, M.; Kowalczyk, J.; Zaghdoudi, S.; Salem, T.; Sarry, J.-E.; Hicheri, Y.; Cartron, G.; Piechaczyk, M. Targeting the SUMO Pathway Primes All-trans Retinoic Acid–Induced Differentiation of Nonpromyelocytic Acute Myeloid LeukemiasSUMO Represses ATRA-Induced AML Differentiation. Cancer research 2018, 78, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Moris, N.; Anlas, K.; van den Brink, S.C.; Alemany, A.; Schroder, J.; Ghimire, S.; Balayo, T.; van Oudenaarden, A.; Martinez Arias, A. An in vitro model of early anteroposterior organization during human development. Nature 2020, 582, 410–415. [Google Scholar] [CrossRef]
- Olmsted, Z.T.; Paluh, J.L. Co-development of central and peripheral neurons with trunk mesendoderm in human elongating multi-lineage organized gastruloids. Nat Commun 2021, 12, 3020. [Google Scholar] [CrossRef]
- Olmsted, Z.T.; Paluh, J.L. A combined human gastruloid model of cardiogenesis and neurogenesis. Iscience 2022, 25, 104486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




