Submitted:
02 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
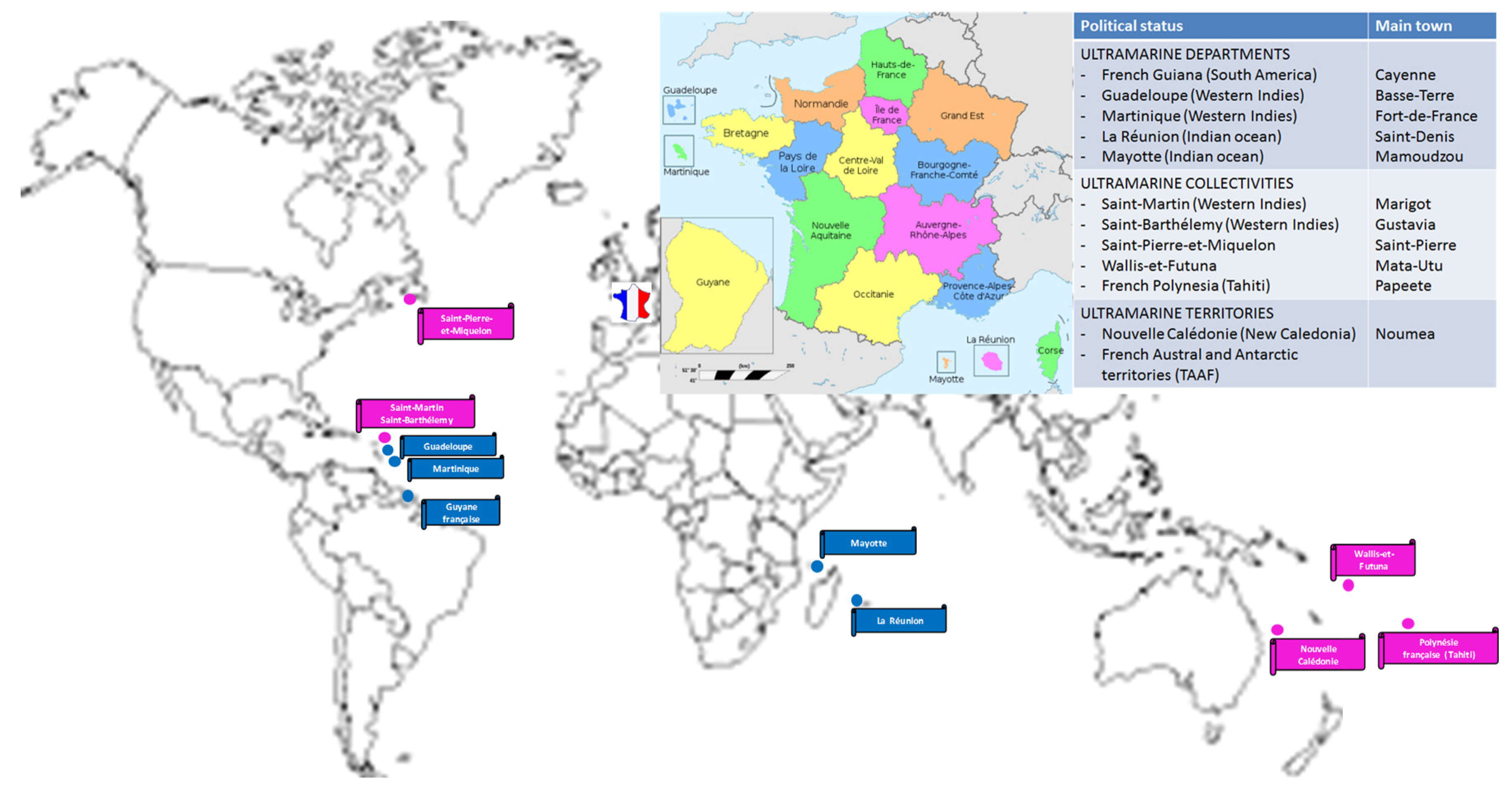
1. Overall Presentation of the Main Risks of Arboviral Diseases in Graft Recipients with Special Reference to the French Context
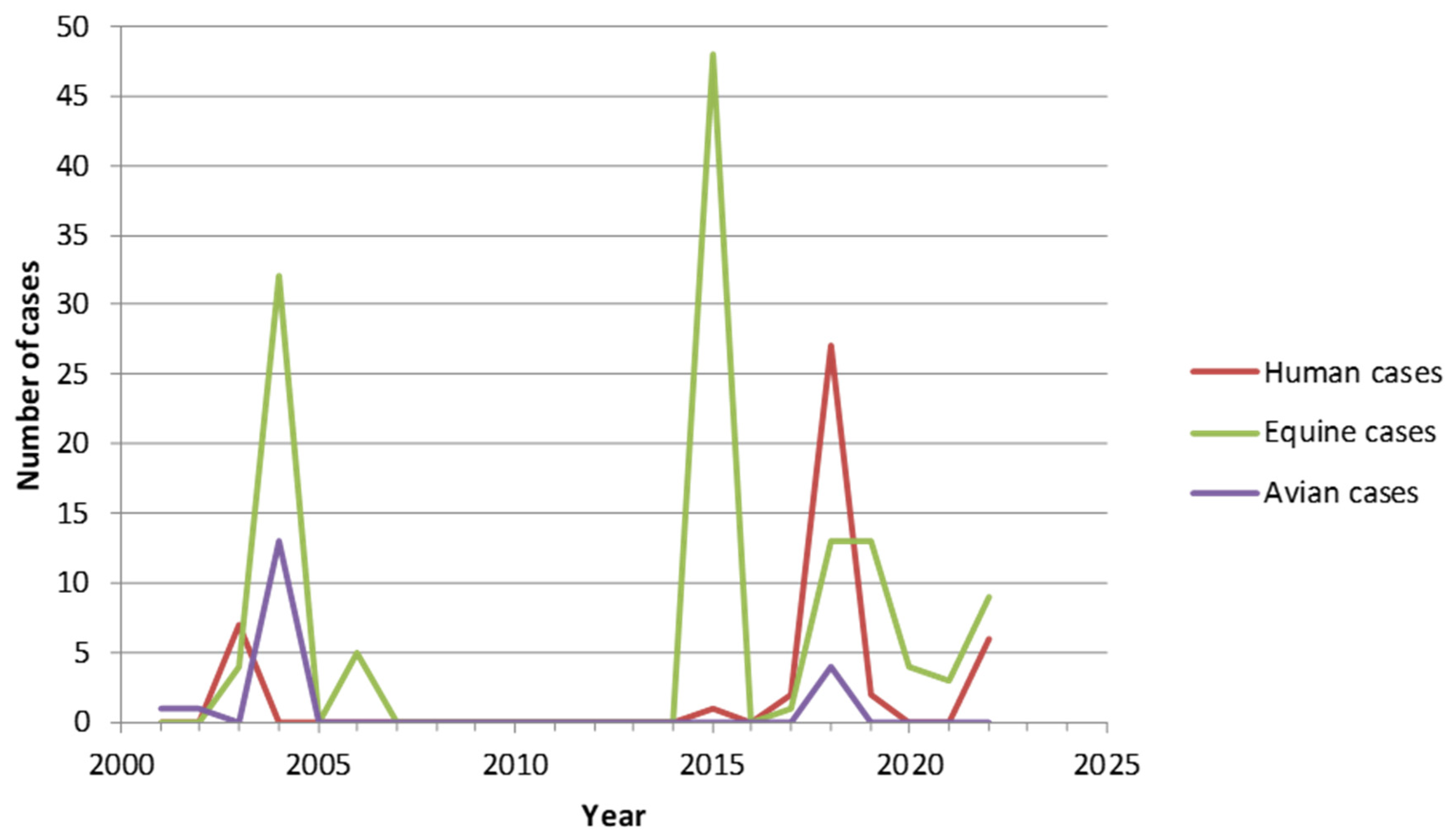
2. West Nile Virus (WNV)
- –
- regarding blood products, all platelet concentrates and a part of the fresh frozen plasma must be secured by using a pathogen reduction method (Intercept blood System®, amotosalen/UVA); in case of identification of a new confirmed autochthonous case, an individual NAT screening (ID-NAT) must be implemented without delay for all blood donations collected in the impacted Department ; the blood products already collected in this area must be tested by ID-NAT if blood samples are still available with the exception of those treated by the Intercept® process. Blood donors having stayed (at least one night) in an affected area must be deferred until 28 days after their return or tested by ID-NAT. Candidate for donation reporting diagnosis of WNV infection must be deferred 122 days after the end of symptoms;
- –
- as for SOT and HSCT, in case of identification of a new autochthonous case, donors living or having stayed in the Region or Department concerned must be tested by WNV NAT and IgM/IgG serology must be performed, ideally before transplantation. The decision to use or not the organs or cells from donors tested positive either by NAT or IgM serology is submitted to the benefit-risk balance, with the recommendation of delaying the grafts that are not urgently needed;
- –
- a list of countries at risk for WNV during the period of circulation of the virus (namely June to November) is updated each year [20]. Candidates for blood, organ or cell donations originating from or having traveled in these countries must be either tested for WNV (NAT for blood and NAT + serology for grafts) or their donation postponed for 28 days after returning from the risk area;
- –
- in parallel, a surveillance of the circulation of WNV is recommended in horses and birds, notably when an epidemic episode is identified at the European level.
3. Dengue Virus
- –
- When a case or a cluster of dengue fever is identified in an endemic area, ID-NAT screening must be performed for blood donations at the Department level. When positive, the products must be discarded and the donor is deferred 28 days from the date of the end of the symptoms. For donors of solid organs and bone marrow present in the cluster areas or having stayed there, PCR and IgM/IgG serology must be performed close to the time of the donation. In case of positivity of one of these markers, it is recommended for living donors to postpone the graft or to use another donor if available; in deceased donors, it is recommended to discard the organs, except in case of vital emergency for the recipient for which a benefit-risk evaluation must be performed; if organs are transplanted, a specific follow-up of the recipient is required [44].
- –
- In clusters of autochthonous dengue in mainland France, blood collections must be postponed in the area, donors living or having stayed in this area must be discarded from blood donation for 28 days and blood products already collected in this area and not treated by the Intercept® process must be placed in quarantine in order to be tested by ID-NAT; by contrast, due to the very low risk of selecting a positive donor of solid organ or bone marrow, no specific information is done in this context [45].
4. Tick-Borne Encephalitis Virus
- –
- for blood products, donors having experienced a tick bite in the 28 days preceding the donation in an area known to be at risk for TBEV (in or out of France) during the period of virus circulation (March to November) must be excluded for 28 days after the tick bite’s date. In addition, blood collection is interrupted in areas where a source of foodborne outbreak of TBEV is recognized, with quarantine of blood products already collected and not secured by the Intercept® process, until tested negative by TBEV NAT;
- –
- for SOT and HSCT, living donors staying or traveling in at-risk areas for TBEV must be made aware of the risks of tick bites and of the consumption of unpasteurized milk and milk products from March to November to avoid contamination. All living donors should be questioned for a possible recent tick bite in a zone at risk for TBEV when completing the pre-donation check-up list. In case of positive answer to this question, living donors must be tested for TBEV (NAT and IgM/IgG serology) prior to the gift; if at least one of these tests is positive, it is recommended to postpone the graft or to select another donor if available. For deceased donors recently exposed to a tick bite, when this information can be recorded from his/her relatives or after skin inspection, as it may be difficult to obtain virological tests prior to the transplantation, it is recommended to inform the recipient(s) and their medical team of the potential risk of TBEV infection and to perform specific virological tests in case of fever or neurological symptoms in the two months following the transplantation.
5. Other Arboviruses Circulating in France
5.1. Usutu Virus
5.2. Chikungunya Virus
- –
- –
- The French Territories in the Americas experienced also an important CHIKV outbreak in 2013-2014 with respectively 72500, 81200 and 15000 cases in Martinique, Guadeloupe, and French Guiana. No significant re-emergence of the virus was further observed.
- –
- In French Polynesia, a CHIKV outbreak developed in 2014-2015.
- –
5.3. Zika Virus
6. Concludings Remarks
Acknowledgments
Conflicts of Interest
References
- Devillers J, David JP, Barrès B; et al. Integrated plan of insecticide resistance surveillance in mosquito vectors in France. Insects. 2023, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Robert MA, Stewart-Ibarra AM, Estallo EL. Climate change and viral emergence: Evidence from Aedes-borne arboviruses. Curr. Opin. Virol. 2020, 40, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chancey C, Grinev A, Volkova E, Rios M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- Petersen LR, Brault AC, Nasci RS. West Nile virus: Review of the literature. JAMA. 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Nash D, Mostashari F, Fine A; et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef]
- Pealer LN, Marfin AA, Petersen LR; et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003, 349, 1236. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Update: Investigations of West Nile virus infections in recipients of organ transplantation and blood transfusion–Michigan, 2002. Morb Mortal Wkly Rep. 2002, 51, 879. [Google Scholar]
- Iwamoto M, Jernigan DB, Guasch A; et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N. Engl. J. Med. 2003, 348, 2196. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Fatal West Nile virus infection after probable transfusion-associated transmission--Colorado, 2012. Morb Mortal Wkly Rep. 2013, 62, 622–624. [Google Scholar]
- Pervanidou D, Detsis M, Danis K; et al. West Nile virus outbreak in humans, Greece, 2012: Third consecutive year of local transmission. Euro Surveill. 2014, 19, 20758. [Google Scholar] [CrossRef]
- Winston DJ, Vikram HR, Rabe IB; et al. Donor-derived West Nile virus infection in solid organ transplant recipients: Report of four additional cases and review of clinical, diagnostic, and therapeutic features. Transplantation. 2014, 97, 881–889. [Google Scholar] [CrossRef]
- Anesi JA, Silveira FP; AST Infectious Diseases Community of Practice. Arenaviruses and West Nile Virus in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13576. [Google Scholar] [CrossRef]
- Morelli MC, Sambri V, Grazi GL; et al. Absence of neuroinvasive disease in a liver transplant recipient who acquired West Nile virus (WNV) infection from the organ donor and who received WNV antibodies prophylactically. Clin. Infect. Dis. 2010, 51, e34–7. [Google Scholar] [CrossRef]
- Inojosa WO, Scotton PG, Fuser R; et al. West Nile virus transmission through organ transplantation in north-eastern Italy: A case report and implications for pre-procurement screening. Infection. 2012, 40, 557–562. [Google Scholar] [CrossRef]
- Barzon L, Montarsi F, Quaranta E; et al. Early start of seasonal transmission and co-circulation of West Nile virus lineage 2 and a newly introduced lineage 1 strain, northern Italy, June 2022. Euro Surveill. 2022, 27, 2200548. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Avis du 7 novembre 2022 sur la sécurisation des produits du corps humain dans un contexte de circulation du virus West Nile en France métropolitaine. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=1259.
- Haut Conseil de la Santé Publique. Statement on measures of security for human body products owing to a human case report of West Nile virus (WNV) infection in mainland France outside the seasonal alert period. 13th January 2023. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=1270.
- Haut Conseil de la Santé Publique. Avis du 28 mars 2023 sur la sécurisation des éléments et produits issus du corps humain en prévision de la circulation du virus West Nile en France métropolitaine. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=1284.
- Haut Conseil de la Santé Publique. Avis du 7 février 2020 relatif à l’inscription à la liste des maladies à déclaration obligatoire de l’infection due au virus West-Nile. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=843.
- Haut Conseil de la Santé Publique. Liste des pays à risque de transmission du virus West Nile (WNV) pour les produits du corps humain, saison 2023. Avis du 24 mai 2023. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=1307.
- Bhatt S, Gething PW, Brady OJ; et al. The global distribution and burden of dengue. Nature. 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Gwee XWS, Chua PEY, Pang J. Global dengue importation: A systematic review. BMC Infect. Dis. 2021, 21, 1078. [Google Scholar] [CrossRef]
- Pozzetto B, Memmi M, Garraud O. Is. transfusion-transmitted dengue fever a potential public health threat? World J. Virol. 2015, 4, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Chuang VWM, Wong TY, Leung YH; et al. Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Med J. 2008, 14, 170–177. [Google Scholar]
- Tambyah PA, Koay ESC, Poon MLM, Lin RVTP, Ong BKC, Transfusion-Transmitted Dengue Infection Study Group. Dengue hemorrhagic fever transmitted by blood transfusion. N. Engl. J. Med. 2008, 359, 1526–1527. [Google Scholar] [CrossRef]
- Stramer SL, Linnen JM, Carrick JM; et al. Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion. 2012, 52, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Matos D, Tomashek KM, Perez-Padilla J; et al. Probable and possible transfusion-transmitted dengue associated with NS1 antigen-negative but RNA confirmed-positive red blood cells. Transfusion. 2016, 56, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Oh HB, Muthu V, Daruwalla ZJ, Lee SY, Koay ES, Tambyah PA. Bitten by a bug or a bag? Transfusion-transmitted dengue: A rare complication in the bleeding surgical patient. Transfusion. 2015, 55, 1655–1661. [Google Scholar] [CrossRef]
- Sabino EC, Loureiro P, Lopes ME; et al. Transfusion-transmitted dengue and associated clinical symptoms during the 2012 epidemic in Brazil. J Infect Dis. 2016, 213, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Levi JE, Nishiya A, Félix AC, Salles NA; et al. Real-time symptomatic case of transfusion-transmitted dengue. Transfusion. 2015, 55, 961–964. [Google Scholar] [CrossRef]
- Rigau-Pérez JG, Vorndam AV, Clark GG. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994-1995. Am J Trop Med Hyg. 2001, 64, 67–74. [Google Scholar] [CrossRef]
- Tan FL-S, Loh DLSK, Prabhakaran K, Tambyah PA, Yap H-K. Dengue haemorrhagic fever after living donor renal transplantation. Nephrol. Dial. Transplant. 2005, 20, 447–448. [Google Scholar] [CrossRef]
- Tangnararatchakit K, Tirapanich W, Tapaneya-Olarn W; et al. Severe nonfebrile dengue infection in an adolescent after postoperative kidney transplantation: A case report. Transplant. Proc. 2012, 44, 303–306. [Google Scholar] [CrossRef]
- Saigal S, Choudhary NS, Saraf N, Kataria S, Mohanka R, Soin AS. Transmission of dengue virus from a donor to a recipient after living donor liver transplantation. Liver Transpl. 2013, 19, 1413–1414. [Google Scholar] [CrossRef]
- Punzel M, Korukluoğlu G, Caglayik DY; et al. Dengue virus transmission by blood stem cell donor after travel to Sri Lanka; Germany, 2013. Emerg Infect Dis. 2014, 20, 1366–1369. [Google Scholar] [CrossRef]
- Gupta RK, Gupta G, Chorasiya VK; et al. Dengue virus transmission from living donor to recipient in liver transplantation: A case report. J. Clin. Exp. Hepatol. 2016, 6, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Rosso F, Pineda JC, Sanz AM, Cedano JA, Caicedo LA. Transmission of dengue virus from deceased donors to solid organ transplant recipients: Case report and literature review. Braz. J. Infect. 2018, 22, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shaji Mathew J, Menon VP, Menon VP; et al. Dengue virus transmission from live donor liver graft. Am. J. Transplant. 2019, 19, 1838–1846. [Google Scholar] [CrossRef]
- Lecadieu A, Teysseyre L, Larsen K; et al. Case report: Transmission of dengue virus from a deceased donor to a kidney transplant recipient previously infected by dengue virus. Am. J. Trop. Med. Hyg. 2021, 104, 2199–2201. [Google Scholar] [CrossRef] [PubMed]
- Janani MK, Durgadevi P, Padmapriya J, Malathi J, Kulandai LT, Rao Madhavan HN. First report on detection of dengue virus in the donor cornea. Cornea. 2018, 37, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- L’Azou M, Jean-Marie J, Bessaud M; et al. Dengue seroprevalence in the French West Indies: A prospective study in adult blood donors. Am. J. Trop. Med. Hyg. 2015, 92, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Richard V, Cao-Lormeau VM. Mosquito vectors of arboviruses in French Polynesia. New Microbes New Infect. 2019, 31, 100569. [Google Scholar] [CrossRef]
- La Ruche G, Souarès Y, Armengaud A; et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010, 15, 19676. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Avis du 28 novembre 2020 relatif à l’actualisation des mesures de prévention vis-à-vis du virus de la dengue à appliquer aux produits issus du corps humain dans les Antilles françaises. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=953.
- Haut Conseil de la Santé Publique. Avis du 19 octobre 2022 sur les mesures de prévention pour la sécurité infectieuse transfusionnelle et de la greffe à la suite de cas de dengue autochtones dans le sud de la France. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=1258.
- Ličková M, Fumačová Havlíková S, Sláviková M, Slovák M, Drexler JF, Klempa B. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick. Borne Dis. 2020, 11, 101414. [Google Scholar] [CrossRef]
- Kunze M, Banović P, Bogovič P; et al. Recommendations to improve tick-borne encephalitis surveillance and vaccine uptake in Europe. Microorganisms. 2022, 10, 1283. [Google Scholar] [CrossRef]
- Michelitsch A, Wernike K, Klaus C, Dobler G, Beer M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses. 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Ličková M, Fumačová Havlíková S, Sláviková M, Klempa B. Alimentary infections by tick-borne encephalitis virus. Viruses. 2021, 14, 56. [Google Scholar] [CrossRef]
- Martello E, Gillingham EL, Phalkey R; et al. Systematic review on the non-vectorial transmission of tick-borne encephalitis virus (TBEv). Ticks Tick. Borne Dis. 2022, 13, 102028. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg P, Saikku P, Brummer-Korvenkontio M. Tick-borne viral encephalitis in Finland. The clinical features of Kumlinge disease during 1959-1987. J Intern Med. 1989, 225, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Lipowski D, Popiel M, Perlejewski K; et al. A cluster of fatal tick-borne encephalitis virus infection in organ transplant setting. J. Infect. Dis. 2017, 215, 896–901. [Google Scholar] [CrossRef]
- Gonzalez G, Bournez L, Moraes RA; et al. A One-health approach to investigating an outbreak of alimentary tick-borne encephalitis in a non-endemic area in France (Ain, Eastern France): A longitudinal serological study in livestock, detection in ticks, and the first tick-borne encephalitis virus isolation and molecular characterisation. Front. Microbiol. 2022, 13, 863725. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Inscription de l’encéphalite à tiques sur la liste des maladies à déclaration obligatoire. 5 juin 2020. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=856.
- Saegerman C, Humblet MF, Leandri M; et al. First Expert Elicitation of knowledge on possible drivers of observed increasing human cases of tick-borne encephalitis in Europe. Viruses. 2023, 15, 791. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Avis du 23 juillet 2020 relatif aux risques transfusionnels et de contamination des greffons induits par le virus de l’encéphalite à tiques (TBEV) ; précautions à prendre lors de foyers infectieux. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=895.
- Vilibic-Cavlek T, Petrovic T, Savic V; et al. Epidemiology of Usutu virus: The European scenario. Pathogens. 2020, 9, 699. [Google Scholar] [CrossRef]
- Lecollinet S, Blanchard Y, Manson C; et al. Dual emergence of Usutu virus in common blackbirds, Eastern France, 2015. Emerg Infect Dis. 2016, 22, 2225. [Google Scholar] [CrossRef]
- Simonin Y, Sillam, O, Carles MJ; et al. Human Usutu virus infection with atypical neurologic presentation, Montpellier, France, 2016. Emerg Infect Dis. 2018, 24, 875–878. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Avis du 28 avril 2023 sur la sécurisation des produits issus du corps humain en prévision de cas d’infection à virus West Nile. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=1284.
- Desdouits M, Kamgang B, Berthet N; et al. Genetic characterization of chikungunya virus in the Central African Republic. Infect. Genet. Evol. 2015, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sharif N, Sarkar MK, Ferdous RN; et al. Molecular epidemiology, evolution and reemergence of Chikungunya virus in South Asia. Front. Microbiol. 2021, 12, 689979. [Google Scholar] [CrossRef]
- Fritel X, Rollot O, Gerardin P; et al. Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg Infect Dis. 2010, 16, 418–425. [Google Scholar] [CrossRef]
- Josseran L, Paquet C, Zehgnoun A; et al. Chikungunya disease outbreak, Reunion Island. Emerg. Infect. Dis. 2006, 12, 1994–1995. [Google Scholar] [CrossRef] [PubMed]
- Borgherini G, Poubeau P, Staikowsky F; et al. Outbreak of chikungunya on Reunion Island: Early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis. 2007, 44, 1401–1407. [Google Scholar] [CrossRef]
- Grandadam M, Caro V, Plumet S; et al. Chikungunya virus, southeastern France. Emerg. Infect. Dis. 2011, 17, 910–913. [Google Scholar] [CrossRef]
- Rezza G, Nicoletti L, Angelini R; et al. Infection with Chikungunya virus in Italy: An outbreak in a temperate region. Lancet. 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Watson, R. Chikungunya fever is transmitted locally in Europe for first time. Br. Med. J. 2007, 335, 532–533. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Dengue et chikungunya : Mesures pour la sécurité transfusionnelle et des greffes. 14 juin 2019. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=722.
- Pierrotti LC, Lopes MIBF, Nascimento APD; et al. Chikungunya in kidney transplant recipients: A series of cases. Int. J. Infect. Dis. 2017, 64, 96–99. [Google Scholar] [CrossRef]
- Rosso F, Rodríguez S, Cedano JA, Mora BL, Moncada PA, Velez JD. Chikungunya in solid organ transplant recipients, a case series and literature review. Transpl. Infect. Dis. 2018, 20, e12978. [Google Scholar] [CrossRef]
- Smithburn, KC. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. Immunol. 1952, 69, 22334. [Google Scholar] [CrossRef]
- Duffy MR, Chen TH, Hancock WT; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Eng. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. lnfect. 2014, 20, O595–O596. [Google Scholar] [CrossRef]
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus. French Polynesia. December 2013 and February 2014. Euro Surveil. 2014, 9, 20751. [Google Scholar] [CrossRef]
- Petersen LR, Jamieson DJ, Honein MA. Zika virus. N. Engl. J. Med. 2016, 375, 294–295. [Google Scholar] [CrossRef]
- Cao-Lormeau VM, Blake A, Mons S; et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Campos GS, Bandeira AC, Sardi Sl. Zika vírus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1005–1006. [Google Scholar]
- Faria NR, Azevedo R, Kraemer MUG; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Foy BD, Kobylinski KC. Chilson Foy JL; et al. Probable non-vector- borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef]
- Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. 2017, 23, 296–305. [Google Scholar] [CrossRef]
- Funk AL, Hoen B, Vingdassalom I; et al. Reassessment of the risk of birth defects due to Zika virus in Guadeloupe, 2016. PLoS Negl Trop Dis. 2021, 15, e0009048. [Google Scholar] [CrossRef]
- Moore CA, Staples JE, Dobyns WB; et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Hoen B, Schaub B, Funk AL; et al. Pregnancy outcomes after ZIKV infection in French Territories in the Americas. N. Engl. J. Med. 2018, 378, 985–94. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Saines K, Brasil P, Kerin T; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 2019, 25, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Simonin Y, van Riel D, van de Perre P, Rockx B, Salinas S. Differential virulence between Asian and African lineages of Zika virus. PloS Negl. Trop. Dis. 2017, 11, e0005821. [CrossRef]
- Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Euro Surveill 2016, 21, 30269. [Google Scholar] [CrossRef]
- Herriman, R. Transfusion-associated Zika virus reported in Brazil. Outbreak news today. December 18, 2015. http://outbreaknewstoday.com/transfusion-associated-zika-virus-reported-in-brazil-76935/.
- Barjas-Castro ML, Angerami RN, Cunha MS; et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 2016, 56, 1684–1688. [Google Scholar] [CrossRef]
- Motta IJ, Spencer BR, Cordeiro da Silva SG; et al. Evidence for transmission of Zika virus by platelet transfusion. N. Engl. J. Med. 2016, 375, 1101–1103. [Google Scholar] [CrossRef]
- Beau F, Lastère S, Mallet HP, Mauguin S, Broult J, Laperche S. Impact on blood safety of the last arboviruses outbreaks in French Polynesia (2012-2018). Transfus Clin Biol. 2020, 27, 4–9. [Google Scholar] [CrossRef]
- Giron S, Franke F, Decoppet A; et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Euro Surveill. 2019, 24, 1900655. [Google Scholar] [CrossRef]
- Brady OG, Hay S. The first local cases of Zika virus in Europe. Lancet. 2019, 394, 1991–1992. [Google Scholar] [CrossRef] [PubMed]
- Haut Conseil de la Santé Publique. Infection par le virus Zika : Inscription sur la liste des maladies à déclaration obligatoire. 2 février 2016. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=539.
- Haut Conseil de la Santé Publique. Mesures de prévention pour la sécurité infectieuse transfusionnelle et de la greffe résultant de la circulation de virus Zika à la suite de cas autochtones en France métropolitaine. 22 novembre 2019. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=752.
|
Virological Data Family / Genus |
Vectors | Usual Vertebrate Hosts | Geographic Distribution |
Incubation (days) |
Percent of Asymptomatic Forms |
Main Clinical Symptoms |
Current Vaccine Prophylaxis |
Documented Transmission by Blood or Grafts |
|
Flaviviridae / Flavivirus (ssRNA, Enveloped) |
||||||||
| - West Nile virus (WNV) - Usutu virus (USUV) - Saint-Louis encephalitis virus (SLEV) - Tick-borne encephalitis virus (TBEV) - Dengue virus (DENV, serotypes 1 to 4) |
Mosquitoes (genus Culex but also Aedes albopictus) Mosquitoes (genus Culex but also Aedes) Mosquitoes (genus Culex) Ticks (genus Ixodes) Mosquitoes (Aedes aegypti and Aedes albopictus) |
Birds Birds Birds Rodents Dears Humans Non-human primates |
Asia, Africa, Europe, Americas Africa, Israel, Europe Americas Europe, Asia World (mainly intertropical regions) |
2-14 3-6 4-21 2-28 2-14 |
80 Frequent > 99 80 50-75 |
Fever, Encephalitis Fever, Rash, Encephalitis, Meningo-encephalitis Fever, Encephalitis Fever, Encephalitis Fever, Hemorrhagic dengue, Shock |
No No No Yes Yes |
Yes (numerous cases) No No Yes Yes (numerous cases) |
| - Zika virus (ZIKV) – Yellow fever virus (YFV) Togaviridae / Alphavirus (ssRNA, enveloped) - Chikungunya virus (CHIKV) - Ross River virus (RRV) |
Different mosquitoes (genus Aedes, Anopheles, Mansonia) Mosquitoes (Aedes aegypti) Mosquitoes (Aedes aegypti and Aedes albopictus) Different mosquitoes (genus Culex, Aedes, Anopheles, Mansonia) |
Humans Non-human primates Humans Non-human primates Humans Non-human primates Kangaroos and wallabies |
Africa, Oceania, India, South-East Asia, Western Indies, Central and South America, Europe Africa Central and South America Africa, Asia, Indian and Pacific oceans, Europe, Western Indies, Americas Oceania, South Pacific |
3-12 3-6 1-12 5-15 |
30-80 55 15 50-75 |
Fever, Rash, Conjunctivitis, Arthralgia, Myalgia, Guillain-Barre syndrome, Microcephaly Fever, Jaundice Hemorrhagic fever Shock Fever, Fatigue, Arthralgia Fever, Rash, Arthralgia |
No Yes No No |
Yes No No Yes |
|
+Reoviridae / Coltivirus (dsRNA, naked) - Colorado tick fever virus (CTFV) |
Ticks (Dermacentor andersoni) |
Humans |
Western USA and Canada |
3-6 |
Low |
Fever, Encephalitis |
No |
Yes |
|
Year/Location |
Donor | Recipient(s) | |||||||
| Infection route | Serum testing | Organ | OSPT1 | Serum testing | CSF testing | Treatment | Symptoms | Outcome | |
| 2002 / USA | Blood transfusion | NAT2+ | Kidney Kidney Heart Liver |
13 17 8 7 |
IgM+ IgM- NAT+ IgM+ |
IgM+ IgM- IgM+ Non tested |
None None None None |
Neuroinvasive disease Neuroinvasive disease Neuroinvasive disease Fever |
Survived Died3 Survived Survived |
| 2005 / USA | Probable mosquito bite | NAT – IgM+ IgG+ |
Liver Lung Kidney Kidney |
13 17 AS4 AS |
IgM+ IgM+;IgG+ NAT+;IgM-;IgG+ NAT-; IgM-;IgG- |
NAT+;IgM+ NAT+;IgM+ Not tested Not tested |
Immunotherapy Immunotherapy Immunotherapy Immunotherapy |
Neuroinvasive disease Neuroinvasive disease Asymptomatic Not infected |
Coma Coma Survived Survived |
| 2008 / USA | Blood transfusion | NAT-;IgM+ | Heart | 8 | IgM+ | IgM+ | Supportive care | Neuroinvasive disease | Survived |
| 2009 / USA | Probable mosquito bite | NAT+;IgM- | Liver | 15 | NAT-; IgM+;IgG- |
IgM+ | Immunotherapy |
Neuroinvasive disease | Survived |
|
2009 / USA |
Mosquito bite |
NAT+;IgM+;IgG equivocal |
Kidney Kidney Liver |
NA5 AS AS |
NA NA NA |
NA NA NA |
NA NA NA |
Neuroinvasive disease Asymptomatic Asymptomatic |
Survived Survived Survived |
|
2009 / Italy |
Mosquito bite |
NAT+ |
Liver |
AS |
NAT+; IgM+ |
Not tested |
Immunotherapy |
Asymptomatic |
Survived |
| 2010 / USA | Mosquito bite | NAT+;IgM-;IgG+ | Kidney Kidney Liver |
8 AS AS |
IgM+;IgG+ NAT+;IgM+;IgG+ NAT-; IgM-;IgG+ |
NAT-;IgM+ Not tested Not tested |
Supportive care None None |
Neuroinvasive disease Asymptomatic Asymptomatic |
Died Survived Survived |
| 2011 / Italy | Mosquito bite | NAT-;IgM+;IgG+ | Kidney Kidney Liver Lung Heart |
10 10 AS AS AS |
NAT+;IgM+;IgG+ NAT+;IgM+;IgG+ NAT-;IgM+;IgG+ NAT+;IgM+;IgG+ NAT-; IgM-;IgG- |
NAT+;IgM+;IgG+ NAT+;IgM+;IgG+ Not tested Not tested Not tested |
Immunotherapy None None None None |
Neuroinvasive disease Neuroinvasive disease Asymptomatic Asymptomatic Not infected |
Coma Survived Survived Survived Survived |
| 2011 / USA | Increased WNV activity in the donor region | NAT-;IgM+;IgG+ (NAT+ in lymph nodes and spleen) |
Kidney Kidney Lungs Liver |
10 17 20 AS7 |
NAT+ NAT+;IgM+ NAT+ NAT-;IgM-;IgG+ |
NAT+;IgM- NAT+;IgM- NAT+;IgM+ NAT+;IgM- |
Immunotherapy + IFNα2b6 Immunotherapy + IFNα2b Immunotherapy + IFNα2b Immunotherapy + oral ribavirin |
Neuroinvasive disease Neuroinvasive disease Neuroinvasive disease No sign of WNV infection |
Died Survived Survived Survived |
|
Country (reference) |
Period | Donor | Recipient(s) | Accountability | ||||||
| Sex/Age/Status | Viral diagnosis | Clinical picture | Graft | Sex/Age | Viral diagnosis | Clinical picture | Evolution | |||
| (31) | 1994 | ?/?/Alive | Not tested | Fever 2 days after gift | Bone marrow | ?/6 | Not tested | Not reported | Deceased | Low |
| Singapore
(32) |
Not reported | F/ ?/Alive | Not tested | Fever | Kidney | M/23 | PCR+ (DENV-1) | Hemorrhagic dengue | Survived | Intermediate |
| Thailand
(33) |
Not reported | F/ ?/Alive | Positive serology without details | Fever one month before gift | Kidney | F/16 | NS1 Ag + PCR+ (DENV-1) Culture + |
Hemorrhagic dengue | Survived | Low |
| India
(34) |
Not reported | M/19/Alive | NS1 Ag + PCR+ (type?) |
Not reported | Liver | M/38 | NS1Ag + PCR+ (type?) |
Fever + hepatitis | Survived | Strong |
| Germany
(35) |
2013 | F/24/Alive | IgM -/IgG - NS1 Ag + PCR+ (DENV-1) |
Fever one day before gift, 8 days after return from Sri Lanka | Bone marrow | M/51 | IgM +/IgG + NS1 Ag + PCR+ (DENV-1) |
Leukemia worsening Enterocolitis Veno-occlusive disease |
Death not dependent of dengue | Very strong (same sequence) |
| India
(36) |
Not reported | M/29/Alive | NS1 Ag + | Fever 3 days after gift | Liver | M/40 | NS1 Ag + | Fever | Survived | Strong |
| Columbia
(37) |
2007 | M/40/Deceased | IgM +/IgG + | Fever | Liver | M/53 | IgM +/IgG - PCR + (DENV-3) |
Fever + transient encephalopathy + hepatitis | Survived | Strong |
| Heart | M/41 | IgM -/IgG - PCR + (DENV-3) |
Hemorrhagic dengue + shock | Survived | Strong | |||||
| 2010 | M/32/Deceased | IgM -/IgG + NS1+ |
Asymptomatic | Kidney | F/31 | IgM -/IgG - NS1 Ag + PCR + (DENV-4) |
Hemorrhagic dengue | Survived | Strong | |
| Kidney | F/48 | IgM +/IgG - NS1 Ag -, PCR - |
Fever | Survived | Strong | |||||
| India
(38) |
2016 | 58/M/Alive | IgM +/IgG + NS1 Ag - PCR + (DENV-1) |
Fever 6 days after gift Encephalopathy |
Liver | M/58 | IgM -/IgG - NS1 Ag + PCR+ (DENV-1) |
Fever + encephalopathy + liver and kidney failure | Deceased | Very strong (same sequence) |
| La Reunion,
France (39) |
2020 | 62/M/Deceased | IgM +/IgG + PCR - |
Asymptomatic | Kidney Kidney |
M/58 M/61 |
PCR+ (DENV-1) IgM +/IgG + PCR+ (DENV-1) IgM +/IgG + |
Pancytopenia + hepatic cytolysis + hemorrhagic shock Intraabdominal collection operated infected by S. epidermidis Thrombopenia + hepatic cytolysis |
Survived Survived |
Strong Strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





