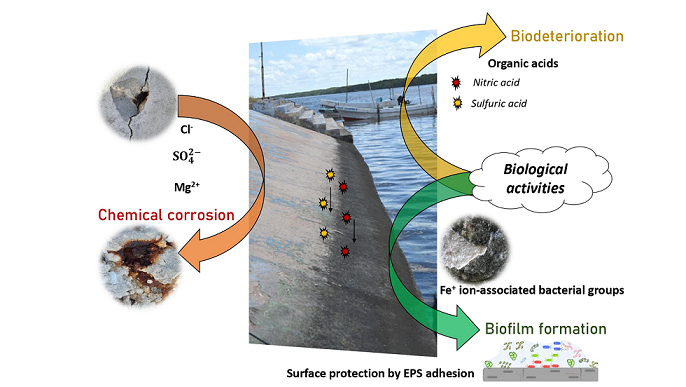
1. Introduction
1.1. History and functions of concrete
Concrete is an artificial (consolidated) material,
with properties similar to natural stone. It is a constructional material made
from a mixture of cement, fine aggregates (usually sand), coarse aggregates
(gravel or crushed rock) and water. The mixture hardens (cures) with time to
produce a strong, durable, reflective and versatile material that is employed
in huge quantities worldwide for construction. A form of concrete has been in
use for thousands of years. The first proven concrete structure was the floor
of a hut in what is modern-day Israel, made about 7000 BC by burning limestone
to produce quicklime that was then mixed with stones and water before leaving
to solidify (The Irish Concrete Society,
https://concrete.ie.about >
concrete, accessed 2nd May, 2023). Portland cement was invented in England in
1824; it consists of four main compounds, tricalcium silicate, dicalcium
silicate, tricalcium aluminate, and a tetra-calcium aluminoferrite. This
innovation led to the subsequent developments that have made concrete, without
doubt, the most important building material worldwide [1]. A full and fascinating history can be found in
the book by [2].
The internal structure of concrete, with its
complicated pore structure, is determined by the cement hydration reaction,
which is complex. Studies to determine the structures of the many varieties of
concrete have involved the use of, for example, synchrotron X-rays [3] and neutron scattering [4]. Pore structure has generally been determined by
mercury intrusion porosimetry (e.g.,[5]).
Recent developments for improving some of the properties of concrete (such as
drying shrinkage, crack resistance and freeze-thaw durability) include the
addition of fly ash [6], glass fibre [7], and nanoparticles [8].
Most recently, the use of old concrete, as recycled aggregate, has come to the
fore [9], reducing the need for natural
aggregates, whose use is becoming unsustainable [10].
Concrete, then, is employed all over the world in
many different situations. This article concentrates on concrete used for
marine structures. The development of the North Sea oil industry in the 1970s
led to the construction of around 20 concrete platforms, containing
approximately 2 million m3 of high quality concrete [11]. In addition, there are pipes, tunnels,
underwater foundations (such as those of the famous Norwegian underwater
restaurant “Under”), seawalls, storm barriers, breakwaters, revetments and
other deep sea and shoreline structures. Although the aim of this article is to
review the negative effects of seawater chemistry and microorganisms on the
structure of concrete, artificial reefs will also be considered briefly. These
are structures where biological colonization is deemed desirable in order to
encourage biodiversity; hence there has been more interest in their
biocolonization.
2. The marine environment and concrete deterioration
The deterioration of concrete is still referred to,
especially by engineers and materials scientists, as “corrosion”, although this
term is, in fact, defined by Collins English Dictionary, Wikipedia and other
sources as a deterioration of metal, associated with an electrochemical
reaction with the environment. In this article we shall use the alternative
accepted definition of corrosion that includes materials other than metals.
The seawater environment is one of the most
aggressive for concrete, because of its chloride, sulfate and magnesium levels [12]. The aggressive nature of seawater is increased
by biofouling, the growth of marine micro and macro organisms on the surface
and within the concrete [13];[14]. There is an extensive literature on biofouling
of concrete structures in the marine environment [15];
[16]; [17]; [18]; [19] and
macrofouling (growth of higher organisms such as bivalves, algae and
seagrasses) will not be covered in the current article; they are without the
remit of this special issue. In the case of reinforced concrete, only
deterioration of the concrete component, and not the metal, will be reviewed.
We will examine the interactions between built concrete structures and
seawater under vertical (tidal) zonation and consider the associated marine
microorganisms that initiate biodeterioration, which occurs together with
non-biological activities such as carbonation and chloride and sulfate attack.
Carbonation by carbon dioxide is necessary to reduce the initially high surface
pH of new concrete to around 9, enabling further chemical reactions, as well as
adhesion of microorganisms.
2.1. Chemical corrosion
Following carbonation, severe chemical attack
occurs in the presence of the surrounding chloride and sulfate ions in
seawater. Chlorides can be incorporated in calcium chloroaluminate hydrates
attached to calcium-silicate hydrate, blocking pores [20].
Sulfate ions react with cement hydration products to produce gypsum and
ettringite, which damage the concrete matrix and increase chloride penetration [21]. The high chloride content of seawater,
however, can suppress the formation of ettringite [22].
Magnesium is not corrosive at the normal low pH of seawater, nor on the
carbonated surface, but becomes more aggressive at the high pH levels (above
pH12) encountered within the concrete pores, when brucite precipitates (12].
This ion, however, shows limited penetration into concrete, affecting only the
surface layers [22].
The depth below the sea surface, governing oxygen
and particulates levels in the water, influences the overall reaction rate, as
does the exact exposure regime of the concrete to seawater. Vertical zonation
defines intertidal and subtidal ecosystems. Subtidal ecosystems are always
submerged, whereas intertidal ecosystems are found between the high and low
(flood and ebb) tides, experiencing fluctuating influences of land, open air
and sea (triple interfaces).This contrasting exposure to the seawater column
and atmosphere influences a differential chemical and physical regime in terms
of salinity, oxygen, water stress (desiccation) etc. These chemical differences
influence physicochemistry and atmospheric exposure, directly affecting the
rate of concrete deterioration. Salinity, which varies according to climate and
geographic conditions, obviously affects chloride ingress into the concrete,
with resulting loss of compressive strength, but other ions, such as sulfate
and magnesium, can suppress the reaction with chloride [22].
Durability of concrete in the marine environment
differs depending on the seawater exposure regime, atmospheric, splash, tidal,
or submerged [23]. The most damaging
conditions are found in the splash zone ([24];
[25]), where chloride transport is greater,
oxygen is readily available [26] and sulfate
attack is more important [27].The splashing
action can remove previously formed, non-adhesive corrosion products, allowing
them to re-form when the area is once again covered by the sea [22]. In addition, this region suffers from wave
impact, freezing and thawing in certain geographical locations, and wetting and
drying cycles [22]. Where concrete has dried
and possibly cracked during temporary open air exposure in hot environments for
example, the subsequent reimmersion can cause hydration of products, leading to
formation of ettringite in the pores and cracks, with resulting loss of
strength [27]. This tidal zone exposure is
less aggressive than the splash zone, but generally more inducive of corrosion
than the submerged zone with its relatively low oxygen levels. In the submerged
zone, oxygen levels are limited and thus corrosion reactions reduced [28]. Salt weathering is the only type of attack and
there is no alleviation of the chemically-induced corrosion products. [29] confirmed the relative aggressivity of
atmospheric, immersion and splash marine zones for ordinary Portland cement
(OPC) and 2 types of cements blended with OPC (PPC and PSC), exposed over 10
years. They reported that blended cements showed reduced resistance to chloride
penetration and increased biofilm formation, but no explanation was given for
these results.
Chloride is regarded as the most important abiotic
influence on marine concrete corrosion; although there are areas in which
chloride content of marine waters is low (e.g., the Baltic and Black Seas, at 3
000 and 8 500mg.l-1, respectively), the main constituents of
seawater around the Earth are basically the same. Impinging chloride ions
rapidly produce a so-called “skin effect” at the immersed concrete surface,
where chloride concentrations are at their highest level [30]. There have been many attempts to model the
ingress of aggressive ions and subsequent deterioration reactions in concrete
subjected to the marine environment [28]; [31]; [32]; [33]. Results differ considerably; this is a highly
complex area.
2.2. Microbiological corrosion (biodeterioration)
The study of microbial deterioration of concrete
involves materials science, mineralogy and microbial ecology. Many authors have
considered the corrosion of concrete in sewer pipes, and biodeterioration in
these structures has been thoroughly investigated (see, for example, reviews by
[34]; [35]; [36]), but there is relatively scarce information on
marine biocorrosion of concrete. Seawater composition and environmental
parameters such as temperature, pH and pollution influence not only chemical
corrosion of concrete, but also adhering microorganisms. Alternating conditions
of wetting and drying have been shown to increase chemical deterioration of
poor quality concrete in seawater [37];
whether microbially induced deterioration is also affected is unknown. The
microbial species attaching to concrete structures that are permanently or
intermittently submerged in seawater might be expected to differ, with
intermittently submerged biofilms drying out periodically, favoring the more
dehydration resistant organisms. Conversely, the presence of a biofilm could
maintain a degree of humidity on the concrete surface, reducing the drying
effects of the exposed time period. Controlled experiments to determine the
effects of biofilm on the physicochemical corrosion of concrete have never been
performed. Indeed, there is, as yet, no information on the microbiology of
concrete subjected to the different sea immersion zones, splash, tidal and
submerged.
The microbial content and abundance of the oceans
differs enormously around the globe. Does the attached microbial population on
concrete differ equally, or is there a specific concrete biofilm on marine
structures? The recent enormous interest in marine pollution by microplastics
has led to the identification of a plastic-specific biofilm (the
“plastisphere”,[38]); whether the equivalent
exists for concrete (a concretosphere ?) is unknown, although there is no
evidence for this from studies on the better studied sewer pipe corrosion.
Recently, however, there have been publications indicating that the initial
microbial population attaching to concrete in the marine environment changes
with time to produce a common “generic” concrete biofilm with time [39]; [40]; [41] marine corrosion. Earlier results had indicated
this possibility. [42] used DGGE analysis to
identify the dominance of Thiobacillus thiooxidans and
Acidithiobacillus sp. on corroded areas of concrete sewer pipes and [43], using metagenomic techniques on 175 samples
from various surfaces of 9 piers along the Hong Kong coast, identified
considerable differences between microbiomes on concrete and metal structures.
Interestingly, they found that metal surfaces contained more functional genes
involved in iron uptake, while those involved in iron regulation and storage
were more common on concrete. Relatively few iron-oxidizing bacteria were found
on either type of surface, however. The pier floors yielded higher Cyanobacteria-dominated
microbiomes than the walls, consistent with floors wetted by wave action
remaining damp for longer. Cyanobacteria rely on liquid water more than
chlorophytes, which can use water vapor for growth. Table 1 shows the microorganisms that we have
identified in the literature as attaching to concrete under continuous and
intermittent seawater exposure conditions. The processes involved in microbial
biodeterioration of concrete structures in the marine environment are discussed
in the following sections.
Table 1.
Some of the microorganisms detected in biofilms on permanently or intermittently immersed concrete.
Table 1.
Some of the microorganisms detected in biofilms on permanently or intermittently immersed concrete.
| Microorganism |
Permanent |
Intermittent |
Reference |
Comments |
| Lyngbya |
|
+ |
[94] |
Cyanobacteria Yangtze river reservoir |
| Leptolyngbya |
|
+ |
[94] |
Cyanobacteria Yangtze river reservoir |
| Cyanobacteria |
+ |
+
+ |
[83]
[41]
[44] |
Breakwater
AR
In situ concrete test |
| Proteobacteria |
|
+ |
[83] |
Breakwater |
| Bacteroidetes |
|
+
+ |
[83]
[44] |
Breakwater
In situ concrete test |
| Anaerolinea |
|
+ |
[94] |
Yangtze river reservoir |
| Polynucleobacter |
|
+ |
[94] |
Yangtze river reservoir |
| Sulfate-reducers |
|
+ |
[94] |
Yangtze river reservoir |
Ammonia-oxidizers
Nitrosopumilus sp |
|
+
+ |
[94]
[86] |
Yangtze river reservoir
Ammonia-oxidizing archaea. Oslofjord undersea tunnel |
Desulfobacteria
Desulfobacterales |
|
+
+
+ |
[114]
[44]
[86] |
Sulfate reducers
In situ concrete test
Sulfate reducers. Oslofjord undersea tunnel |
| Firmicutes |
|
+
+ |
[114]
[44] |
Tidal areas. Phylum includes sulfur bacteria. May produce endospores
In situ concrete test |
| Acidobacteria |
|
+ |
[44] |
In situ concrete test. Acid-producers |
| Chloroflexi |
|
+ |
[44] |
In situ concrete test
Heterophototrophic filamentous bacteria |
| Nitrospina, Nitrospira |
|
+ |
[86] |
Nitrite-oxidizing bacteria. Oslofjord undersea tunnel
|
| Nitrosomonas |
|
+ |
[86] |
Anoxic ammonia oxidisers. Oslofjord undersea tunnel |
| Scalindua |
|
+ |
[86] |
Anammox bacteria. Oslofjord undersea tunnel
|
| Mariprofundus |
|
+ |
[86] |
Stalked iron-oxidising bacteria. Oslofjord undersea tunnel |
| Ponticaulus |
+ |
|
[40] |
Archaea
In vitro study |
| Hyphomonas |
+ |
|
[40] |
Stalked bacteria. In vitro study |
| Planctomycetales |
+
+ |
+ |
[40]
[41]
[44]
|
In vitro study. Budding bacteria
AR
In situ concrete test |
| Rhodobacterales |
+ |
|
[40] |
Primary marine surface colonizers. In vitro study |
| Caulobacteriales |
+ |
|
[40] |
In vitro study. Stalked bacteria |
| Portibacter |
+ |
|
[40] |
In vitro study. Bacteroidetes. |
|
Bacillus (Firmicutes) |
+ |
|
[115] |
Bridge |
| Brachybacterium |
+ |
|
[115] |
Bridge |
| Flavobacterium |
+ |
|
[115] |
Bridge |
| Lysinibacillus |
+ |
|
[115] |
Bridge |
| Thiomonas perometabolis |
+ |
|
[115] |
Bridge. Sulfur oxidizer |
| Propiogenium |
+ |
|
[41] |
AR
Anaerobe |
| Vibrio |
+ |
|
[41] |
AR |
| Clostridium |
* |
|
[41] |
AR
Anaerobe |
| Fusobacteria |
+ |
|
[41] |
AR
Anaerobes |
| Actinobacteria |
+ |
+ |
[41]
1[44] |
AR
In situ concrete test |
2.2.1. Microbial adhesion and biofilm development
Biodeterioration of concrete in the marine environment begins with microorganisms adhering to its surface, forming a biofilm. The alkaline nature of new concrete inhibits the activity of microorganisms [
44], but the pH falls rapidly under the wetting and leaching influence of the marine environment, whose pH is generally between 7.5 and 9 [
45], enabling microbial adhesion and metabolic activity. [
46], in laboratory experiments, clearly demonstrated the effect of reducing the surface pH of concrete samples on the rate of microbial adhesion from seawater. The first colonizers are bacteria, which can themselves produce acid metabolites that further reduce pH and increase susceptibility of the concrete surface to microbial attack. The earliest studies concentrated on concrete corrosion in sewers by acid producing bacteria [
47], but more recent studies have determined the effects of concrete structure and composition on general bacterial adhesion (e.g., [
48]; [
49]; [
46]; [
40]). The effect of surface roughness of the substratum on biofilm formation has been shown to influence the types of bacteria initially adhering to immersed materials [
49], although it has been suggested that this may not be very important for particles as small as bacteria adhering to concrete [
50]. The presence of reinforcing rods within the concrete has a much greater effect than rugosity [
40]. The last cited authors found that bacterial groups with increased adherence on reinforced concrete included members of the family
Magnetospiraceae and the genera
Portibacter,
Rubripirellula, and
Rhodopirellula; these are not particularly associated with a requirement for iron, although
Magnetospiraceae have been found in Fe-Mn deposits [
51] and
Portibacter in iron slags in marine situations [
52]. It is likely that metal ions from within the corroding concrete can reach the surface and become available to bacteria in the surrounding seawater. Iron is an important nutrient for bacterial cells and, as a component of certain natural stones, may act as an attractant for adhering microorganisms [
53].
[
40] found that
Ponticaulus sp. and
Hyphomonas sp. were two of the most abundant bacterial genera in early biofilms on concrete in seawater, and suggested that they are pioneer organisms, which are later outgrown by other genera. These two genera have been found to play important roles in biofilm formation on steel in the marine environment [
54]. [
39], after a series of experiments to assess the effects of different stone substrata (including concrete) on marine biofilm formation, similarly concluded that the biofilms converged over time to a generic marine type, and that the underlying substrata did not play a significant role in community composition. Where the substrata are basically similar, as in this case where they were all types of natural or artificial stone, this may be true, but there are certainly differences between the biofilm-formers on very different substrates in seawater, such as concrete and wood, for example [
55].
The initial attachment of cells to the concrete surface leads to the up-regulation of bacterial genes that code for the production of extracellular polymeric substances (EPS), which strengthen the attachment and encourage the adhesion of circulating cells and other materials. EPS are diverse, including not only polysaccharides, the principal component, but also proteins, nucleic acids, and lipids [
56]; their potential effects on concrete are varied and complex. Cactus polymer, for example, has been used in Mexico to improve the properties of Portland cement [
57] and, along with bacterial EPS, to consolidate limestone surfaces [
58]. Bacterial EPS can be used to improve the viscosity and cohesion of concrete [
59]. On the other hand, the EPS-containing biofilm is a stable environment for microbial cells, enabling them to remain active and attached to the concrete surface and minimizing the loss of corrosive metabolites [
60]. There is considerable information on EPS, their make-up, production and effects [
61]; [
62]; [
63]; [
64]; [
65]; [
66]; [
56]) and this will not be further detailed here. Their production plays a part in the development of a complex biofilm, which eventually leads to the adherence of higher organisms, the readily visible “marine biofouling”, sometimes called “hard fouling” because of the presence of shell-bearing animals such as barnacles and oysters; this final stage in the marine fouling process is not within the remit of the current article.
2.2.2. Concrete corroding microorganisms and mechanisms
Although the initial bacterial biofilm on seawater-immersed concrete may have little immediate effect on its structure, it encourages adhesion and growth of further microorganisms, many of which may have corrosive activity.
2.2.2.1. Organic acid producing microorganisms.
Many heterotrophic bacteria, such as
Vibrio, Acidobacteria,
Bacillus, produce organic acids during their metabolism. Organic acids attack the concrete, causing decalcification of hydration products, which leads to higher inherent porosity and cracking [
67]. They are also produced by fungi, although these filamentous microorganisms are less common in the marine environment. Indeed, [
68] discuss whether fungi are, in fact, metabolically active in seawater, pointing out that many of them are parasitic on other living creatures. They emphasize that DNA sequencing techniques for fungi in general are underdeveloped, explaining, at least in part, why so few marine fungi have been described. [
69] state that most marine fungi are saprobes that rely on the high levels of organic matter generally found in coastal environments. Whether fungi in the open sea are potentially corrosive is debatable. Nevertheless, many terrestrial fungi produce organic acids and, if active in saltwater and sediments, will be capable of concrete deterioration.
Direct evidence for the fungal decay of concrete, then, is missing, although there is no doubt that these microorganisms have the metabolic capacity to produce corrosive compounds [
70] and recent genomic and metabolomic analyses have demonstrated that at least one marine fungal species (
Emericellopsis cladophorae, associated with marine algae) has the genetic capacity to produce carbohydrate-active enzymes under saline conditions [
71]. [
72] suggested that a
Fusarium species isolated from degraded concrete along with
Thiobacillus could be responsible for more rapid degradation than the bacteria. [
73] reported a fungus (
Fusarium oxysporum) as being responsible for the corrosion of 3 concrete bridges over the River Nile, but only the presence of the fungus was reported and there was no direct evidence of its involvement in the corrosion process. Other workers have reported the isolation of microorganisms from corroded concrete that were deemed unlikely to be involved in biodeterioration (e.g., [
74]). Nevertheless, there is some evidence that fungi, simply by their acid producing metabolism, may be able to corrode concrete under certain conditions [
75]. [
76] selected 3 fungi previously shown to grow on concrete surfaces, although not underwater, to test a nanosilica coating as a protective layer on concrete. When incubated with control uncoated concrete blocks in a fungal growth medium, all caused weight loss after 3 months. The most destructive fungus was
Aspergillus tamarii, a fungus isolated originally from historic buildings in Havana. [
70] discuss in detail the potential of fungi to cause concrete corrosion, although they do not cite any definitive cases of such corrosion in the marine environment.
2.2.2.2. Inorganic acid producing bacteria
Autotrophs can produce inorganic (mineral) acids, which are more corrosive than organic acids. These include nitrifying bacteria, such as
Nitrosomonas and
Nitrobacter, which produce nitric acid [
77]; [
78], and sulfur oxidizing bacteria (SOB)
Thiobacillus,
Thiothrix,
Thiomicrospira,
Beggiatoa, etc [
78]; [
79]. The latter group produces sulfuric acid that reacts with concrete to form gypsum, which has poor structural properties. This is considered to be the main concrete corrosion process in sewers, often in conjunction with the anaerobic, heterotrophic sulfate-reducing bacteria (SRB), such as the genera
Desulfovibrio and
Desulfomicrobium, which, under low oxygen conditions, reduce the sulfate in seawater to sulfides that can then be converted to corrosive sulfuric acids by the SOB [
80]. SRB are mainly, but not exclusively, anaerobic bacteria and would be active in the marine environment either in sediments or beneath biofilms. [
81] give a detailed description of SRB corrosion in concrete sewers.
Heterotrophic (organic carbon-utilizing) bacteria produce a variety of organic acids during metabolism and growth. A very wide variety of bacterial species with this ability are found in seawater and their concrete corroding ability cannot be ignored, although there is little published evidence relating to specific genera and species.
The potential, specifically corrosive nature of the microorganisms detected in immersed concrete biofilms is noted, where relevant, in
Table 1.
2.3. Influence of seawater exposure regime on concrete biofilm formation
Oxygen is a critical component in determining the growth of microorganisms. Those found in well oxygenated waters are very different from the facultative or obligate anaerobes that can adsorb to surfaces at lower depths. Our review of the literature has not yielded well constructed experiments that allow direct comparisons of permanently and intermittently immersed concrete. The perfect set-up would be similar to that employed by [
82], who studied the corrosion of a concrete beam exposed for 7 years to atmospheric, splash, tidal and submerged zones around Qingdao Wheat Island, China. Unfortunately, they did not include biofilm formation in their study, although they did report that barnacles were only found in the tidal zone and oysters in the submerged zone. Comparison between these types of concrete immersion in seawater must rely, therefore, on published case histories, with no possibilities of citing controlled experiments, or even paired results.
2.3.1. Concrete in the submerged zone
Permanently immersed concrete may avoid strong corrosion if the seawater is sufficiently low in oxygen, although microorganisms active at low oxygen levels may still produce aggressive compounds [
83]; [
84]; [
85]; [
86]. In less deep waters, sufficient oxygen may still be present to allow chemical corrosion and certainly biological activity will be present, with its associated dangers.
Figure 1 shows a concrete slope in a small dock; the black biofilm in the splash zone is very different from the green, submerged zone film containing photosynthetic cyanobacteria and algae.
Apart from concrete structures such as oil storage tanks associated with offshore oil platforms, underwater tunnels and foundations, this section also includes laboratory and
in situ simulations aimed at determining parameters involved in concrete biocorrosion, testing protective processes and evaluating new types of concrete. Even when such experimental pieces are subjected to the real marine environment
in situ, they are normally kept completely immersed for the required time (e.g., [
87]; [
88]). The laboratory tests almost always involve complete immersion of test pieces in natural or artificial seawater; they are often inoculated with single microorganisms and in this case do not represent real conditions. Nevertheless, we are including in this article references to studies where conditions are sufficiently similar to those
in vivo, as examples of biodeterioration of permanently immersed concrete.
[
89], for example, used a laboratory system to demonstrate the effect of dissolved oxygen on concrete corrosion by sulfur-oxidizing bacteria added to seawater; lower dissolved oxygen levels resulted in reduced bacterial growth and less corrosion.
[
90], similarly
in vitro, showed that the marine benthic diatom,
Cylindrotheca closterium, was able to liberate and utilize silicon from a cementitious mortar. The diatom utilized the Si to produce new frustule material during cell division. Diatoms have often been shown to deposit on immersed concrete; indeed, [
91] suggested that diatoms adhered to a recycled concrete surface could offer increased durability and demonstrated increased water resistance in laboratory experiments.
[
40] reported controlled experiments to determine the formation of biofilms on concrete of similar composition to that of the Oslofjord subsea tunnel, which had shown deterioration at sites where saline groundwater had intruded in previous investigations [
92]. At this earlier time, the group had demonstrated the presence of ammonia- and nitrite-oxidizing microorganisms, in particular
Nitrosopumilus sp., and iron-oxidizing bacteria within the
Mariprofundus sp., as well as various heterotrophic bacteria and archaea at these tunnel sites. However, this microbial population will have included organisms from groundwater, as well as marine bacteria. In their later investigations [
40], they incubated in water from the fjord concretes of similar composition to those in the tunnel. The fjord water was filtered (50um), kept in the dark and recirculated to ensure oxygen saturation during incubation with the concrete samples, which were removed at intervals over 65 weeks. Bacteria and archaea in the concrete and seawater samples were analyzed using DNA analysis. The microbial populations in the thin biofilms on the concrete were significantly different from those in the seawater, even though both populations were composed of typical marine genera, and changed with time. Different concrete compositions showed different colonization patterns during the first weeks, but these differences tended to even out, suggesting a replacement of initially adhering microorganisms with more generic concrete colonizers, as also suggested by [
39]. No special acid-producing genera were detected in the sessile populations, although calcium was removed from the concrete surfaces, indicating corrosion. However, metabolomic studies indicated that the mixed acid fermentation pathway was common in the biofilm.
[
40] on microorganisms present in the biofilms, along with results from other articles on seawater and rare, but interesting, freshwater situations, are included in
Table 1.
2.3.2. Concrete in the splash and tidal zones
Artificial shoreline structures such as piers, reefs, seawalls, embankments and jetties are common in developed coastal regions and serve as examples of intermittently immersed concrete which are also, incidentally, often steeply inclined. Obviously, the more steeply inclined the concrete, the less time it will spend covered in water. As noted in the section on chemical concrete corrosion, the splash zone is most affected by the corrosive action of seawater constituents plus oxygen from the atmosphere. However, simple observation indicates that this is not the zone most affected by biodeterioration (
Figure 2 and
Figure 3).
Figure 2 demonstrates intense corrosion and biofilm formation on a vertical concrete structure in Campeche, Mexico.
Figure 2.
Intertidal concrete structures such as the base of this fisherman’s house, built over a rocky shore, exhibit a heterogeneous coverage of microorganisms. Lerma village, Campeche, Mexico.
Figure 2.
Intertidal concrete structures such as the base of this fisherman’s house, built over a rocky shore, exhibit a heterogeneous coverage of microorganisms. Lerma village, Campeche, Mexico.
The intertidal zone shows deep pits and cracks in the concrete, associated with dark biofilms, while the splash zone above demonstrates black/green biofilms, but without the intense corrosion of the intertidal zone. It is possible that the microbiological colonization has protected this area from the normally more intense chemical corrosion that occurs in the splash zone. Alternatively, it may be the greater wave impact at the wall base that is at least partially responsible for greater physical degradation.
A similar, but less intense, situation can be seen in
Figure 3. Here, corrosion (surface spalling) of the concrete in the more inclined splash zone can be seen, but the intertidal zone is covered by a dark gray/black biofilm, with associated, relatively light, biodegradation.
Figure 3.
Biofilms covering concrete surfaces often exhibit a black phenotype indicating the likely presence of scytonemin-producing cyanobacteria as a functional adaptation for water stress and excessive insolation. San Francisco de Campeche city, Campeche, Mexico. .
Figure 3.
Biofilms covering concrete surfaces often exhibit a black phenotype indicating the likely presence of scytonemin-producing cyanobacteria as a functional adaptation for water stress and excessive insolation. San Francisco de Campeche city, Campeche, Mexico. .
[
93] found that bacterial biofilms colonizing breakwaters along an island coast were dominated by Cyanobacteria, Proteobacteria and Bacteroidetes.They were different from the planktonic bacteria at the same locations, as shown by [
40].
Although not normally in contact with seawater, reservoirs are associated with various concrete structures, such as walls, gate piers and slopes, that are only intermittently immersed in water and could give an indication of concrete colonizers on intermittently-immersed structures. [
94] studied the bacterial communities at various points on 4 reservoirs in the Yangtze River basin, using Miseq DNA sequencing. Proteobacteria, Cyanobacteria and Chloroflexi were the major colonizers, with most common groups being
Leptolyngbya, Anaerolineaceae, and Polynuceobacter. The concrete gate piers had the highest proportion of sulfate-reducing bacteria and were considered at highest risk, although ammonia-oxidisers were also predominant.
A recent report of the presence of metagenomes associated with anammox (ammonium oxidizing) bacteria in water seepages on the concrete inner surface of the Subsea Oslofjord tunnel [
95] is the first indication that these as yet unisolated anaerobes could be involved in deepsea concrete corrosion. Anammox bacteria are associated with biofilms that can lead to localized acidification [
96]. Nitrifying bacterial markers (
Nitrosomonas,
Nitrosopumilus,
Nitrospirales and
Nitrospirota) were also detected at the site. Once leaked into the tunnel, (the equivalent of a splash zone) the higher oxygen environment would allow the production of inorganic nitrogen acids, encouraging concrete corrosion.
3. Interactions of chemistry and microbiology in concrete degradation - some speculations
Although difficult to model, it is still fairly clear that certain ions in seawater lead to a reduction in the durability of concrete. These ions can be both utilized and produced by microorganisms and hence it is impossible to ignore their influence on so-called “abiotic” concrete corrosion in the oceans, although this will vary enormously,of course, depending on the microorganisms present and the surrounding physicochemical conditions. The effects of microbial adhesion and biofilm formation are themselves extremely variable, following the almost limitless variability of microorganisms. It is, however, possible to speculate on the possible interactions between chemical corrosion and microbial growth and activity.
The bacterial biofilm, with its accompanying EPS formed on the concrete surface, can confer a degree of protection on the substratum. EPS can maintain the hydration of the underlying concrete, even when seawater is not covering it. Hence the desiccation associated with tidal and splash zones would not occur and abiotic corrosion would be reduced. It has also been suggested that biofilm can block the entry of aggressive sulfate and chloride ions into the concrete structure, thus enhancing its durability [
97]. Biofilm can physically impair the leaching of calcium hydroxide from the concrete [
98], reducing ongoing corrosion. Nevertheless, there is no doubt that microbial activities can adversely affect concrete durability [
99]; the infiltration of bacteria and fungi into concrete pores can lead not only to their blocking, but also to chemical concrete deterioration by microbial activities such as acid production.
The analysis of both the chemical and biological components of concrete biofilms from the marine environment would enable further potential interactions to be determined. Both qualitative and quantitative changes in cells and chemical compounds occur over the formation of the biofilm (e.g., [
100]; [
101]; [
48]; [
102]; [
103]), but no detailed analyses of such changes in biofilm formation on concrete in the marine environment have been published.
4. Artificial reefs: a special case.
Although in use by mankind for thousands of years, artificial reefs are now being increasingly considered as possible answers to the diminishing biodiversity in coastal ecosystems [
104], [
105]; [
106]; [
107]; [
108]; [
109]. They are not always made of concrete, natural rocks and other materials often being used. However, [
110] showed that there was no significant difference between the facultative marine fungi colonizing artificial reefs made of concrete or limestone sampled over 23 months in the Mississippi Gulf, although occasional phylotypes occurred on only one type of substrate. Overall, the concrete reef showed the highest fungal diversity. A wide variety of fungi, belonging to basidiomycetes, zygomycetes and, principally, ascomycetes, was identified.
[
55] showed that permanently immersed concrete blocks had a much higher microbial diversity than wood, both being common artificial reef materials. These two substrates were suspended approximately 10m below the surface, close to the sea floor in Shuangdao Bay, China, for up to 5 months, with weekly sampling when possible. Proteobacteria and Bacteroidetes were the major OTUs identified on both substrates. Cyanobacteria were dominant on concrete in the first 4 weeks, thereafter diminishing in prevalence.
[
111] used metagenomics to determine the bacteria present in marine benthic samples taken from beneath one and ten year old concrete reefs in the Beibu Gulf, China. They found that Rickettsiales, Moraxellaceae,and
Acinetobacter were enriched in 10-year samples and
Francisella in the one-year reef sediments. Unfortunately, the bacteria adhered to the concrete reefs were not sampled, although it might be assumed that some of these microorganisms would attach to buried parts of the reefs.
[
41] used metagenomics to study the bacterial colonization of 3 types of cement for use in artificial reefs around the coral reefs off Weizhou Island, China, sampling over 3 concurrent seasons. Initially, Cyanobacteria dominated all the concrete samples, with Proteobacteria also common. The latter then began to overtake the phototrophic bacteria, especially in the standard concrete samples, where Fusobacteriota also began to appear. The initial but non-permanent dominance of Cyanobacteria on immersed concrete has also been reported by[
55]. [
41] found that the standard, unmodified cement in artificial reefs showed lower biodiversity than the other two cements, which contained bioactive materials. The lower microbial diversity on its surface indicates the slightly toxic nature of this cement to marine bacteria, as previously shown for bacteria in groundwater stored in concrete or earthen ponds, the former being lower in diversity and abundance [
112]. Indeed, concrete has been described as having especially deleterious consequences on biodiversity in all aquatic ecosystems [
113]; this concords with research suggesting that a relatively mature microbial biofilm on concrete in the marine environment is somewhat generic, differing little with normal structural concrete composition [
39]; [
40]; [
41].
5. Conclusions and perspectives
The chemical corrosion of concrete in the marine environment is well understood in terms of the aggressive seawater ions and their interaction with concrete components. The long-term durability of coastal or deep-sea concrete structures, however, depends on a host of factors and it can be impossible to predict useful life simply based on the structural components. More detailed studies of different types of concrete and varied seawater constituents are necessary to enable useful prediction of concrete durability.
Although, in principle, it is possible to understand the theoretical basis of chemical concrete corrosion, its complexity has, so far, prevented us from efficiently predicting this process in the different types and zones of seawater around the world.
How much more difficult, then, is the understanding and prediction of microbial biodeterioration of concrete and the potential interactions between abiotic and microbial corrosion. Studies on “simple” colonization of concrete surfaces by marine microorganisms are hampered by lack of suitable detection techniques. Nevertheless, new methods of study such as metagenomics and metabolomics are being developed and employed and will surely lead to a vast increase in our knowledge and understanding of the interactions between marine microorganisms and concrete, At the same time, surface protection methods, although not discussed in this article, are continually being developed. It is not necessary to understand how microbial cells attach to and attack concrete if effective methods of preventing this are available, although the purist will understand that a lack of knowledge of the reasons for any effective treatment can prejudice such treatment and its control.The acknowledgement that there is is need to conserve some concrete raw materials (such as sand) and that concrete itself is prejudicing the world’s biodiversity is already leading to changes in the way that we produce and monitor marine long-term structures. Concrete scientists and biologists will doubtless find themselves participating more frequently in future collaborations.
Author Contributions
Conceptualization, development of the ideas, discussion of and contribution to final manuscript, C.C.G.; Contribution to the concept and the ideas; discussion of results and contribution to final manuscript, B.O.O.M.
Funding
This article received no external funding.
Acknowledgments
The authors would thank M.Sc. Pedro Alberto Camacho Chab for the design of the graphical abstract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scrivener, K.L.; Snellings, R. The rise of Portland cements. Elements. 2022, 18, 308-313.
- Jahren, P.; Sui, T. History of Concrete: A Very Old and Modern Material. World Scientific, Singapore, 2017. [CrossRef]
- Monteiro, P.J.; Kirchheim, A.P.; Chae, S.; Fischer, P.; MacDowell, A.A.; Schaible, E.; Wenk, H.R. Characterizing the nano and micro structure of concrete to improve its durability. Cem Concr Comp. 2009. 31, 577-584. [CrossRef]
- Wenk, H.R.; Lutterotti, L.; Vogel, S. Texture analysis with the new HIPPO TOF diffractometer. Nucl Instrum Methods Phys Res. 2003, 515, 575-588. [CrossRef]
- Han, X.; Wang, B.; Feng, J. Relationship between fractal feature and compressive strength of concrete based on MIP. Construct Build Mats. 2022, 322, 126504. [CrossRef]
- Wang, L.; Yu, Z.; Liu, B.; Zhao, F.; Tang, S.; Jin, M. Effects of fly ash dosage on shrinkage, crack resistance and fractal characteristics of face slab concrete. Fractal Fract. 2022, 6, 335. [CrossRef]
- Ahmad, J.; González-Lezcano, R.A., Majdi, A.; Ben Kahla, N.; Deifalla, A.F.; El-Shorbagy, M.A. Glass fibers reinforced concrete: overview on mechanical, durability and microstructure analysis. Materials. 2022, 15, 5111. [CrossRef]
- Wang, R.; Hu, Z.; Li, Y.; Wang, K.; Zhang, H., 2022b. Review on the deterioration and approaches to enhance the durability of concrete in the freeze–thaw environment. Construct Build Mats. 2022, 321, 126371. [CrossRef]
- Xiao, J.; Zhang, K.; Ding, T.; Zhang, Q.; Xiao, X. Fundamental issues towards unified design theory of recycled and natural aggregate concrete components. Eng J. 2023, . [CrossRef]
- Torres, A.; Simoni, M.U.; Keiding, J.K.; Müller, D.B.; zu Ermgassen, S.O.; Liu, J.; Jaeger, J.A.; Winter, M.; Lambin, E.F. Sustainability of the global sand system in the Anthropocene. One Earth. 2021, 4, 639-650. [CrossRef]
- Mehta PK. Concrete in the marine environment. CRC Press. 1991. [CrossRef]
- Qu, F.; Li, W.; Dong, W.; Tam, V.W.; Yu, T. Durability deterioration of concrete under marine environment from material to structure: A critical review. J Build Eng. 2021, 35, 102074. [CrossRef]
- Becker, L.R.; Ehrenberg, A.; Feldrappe, V.; Kröncke, I.; Bischof, K. The role of artificial material for benthic communities–establishing different concrete materials as hard bottom environments. Mar Environ Res. 2020, 161, 105081. [CrossRef]
- Bone, J.R.; Stafford, R.; Hall, A.E.; Herbert, R.J. Biodeterioration and bioprotection of concrete assets in the coastal environment. Int Biodeter Biodegr, 2022, 175, 105507. [CrossRef]
- Scott, P.J.B.; Moser, K.A.; Risk, M.J. Bioerosion of concrete and limestone by marine organisms: A 13-year experiment from Jamaica. Mar Poll Bull. 1988, 19, 219-222. [CrossRef]
- Richmond, M.D.; Seed, R., 1991. A review of marine macrofouling communities with special reference to animal fouling. Biofouling. 1991, 3, 151-168. [CrossRef]
- Hughes, P.; Fairhurst, D.; Sherrington, I.; Renevier, N.; Morton, L.H.G.; Robery, P.C.; Cunningham, L. Microscopic study into biodeterioration of marine concrete. Int Biodeter Biodegr. 2013, 79,14-19. [CrossRef]
- Hopkins, G.; Davidson, I.; Georgiades, E.; Floerl, O.; Morrisey, D.; Cahill, P. Managing biofouling on submerged static artificial structures in the marine environment–assessment of current and emerging approaches. Front Mar Sci. 2021, 8, 759194. [CrossRef]
- Vuong, P.; McKinley, A.; Kaur, P. Understanding biofouling and contaminant accretion on submerged marine structures. npj Mater Degrad. 2023, 7, 50. [CrossRef]
- Lambert, P.; Page, C.L.; Short, N.R. Pore solution chemistry of the hydrated system tricalcium silicate/sodium chloride/water. Cement Concr Res. 1985, 15, 675-680. [CrossRef]
- Yu, L.; Chu, H.; Zhu, Z.; Jiang, L.; Dong, H. Determination of the chloride ion content in concrete under simultaneous chloride and sulphate ion attack. J Build Eng. 2023, 72, 106579. [CrossRef]
- Yi, Y.; Zhu, D.; Guo, S.; Zhang, Z.; Shi, C. A review on the deterioration and approaches to enhance the durability of concrete in the marine environment. Cement Concrete Comp. 2020, 113, 103695. [CrossRef]
- Balestra, C.E.T.; Reichert, T.A.; Savaris, G. Contribution for durability studies based on chloride profiles analysis of real marine structures in different marine aggressive zones. Construct Build Mats. 2019, 206, 140-150. [CrossRef]
- Islam, M.S.; Mondal, B.C.; Islam, M.M. Effect of sea salts on structural concrete in a tidal environment. Austral J Struct Eng. 2010, 10, 237-252, . [CrossRef]
- Wu, L.; Li, W; Yu, X. Time-dependent chloride penetration in concrete in marine environments. Construct Build Mats. 2017, 152, 406-413. [CrossRef]
- Wang, Y.; Guo, S.; Yan, B.; Liu, Z.; Wang, Y.; Yuan, C. Experimental and analytical investigation on chloride ions transport in concrete considering the effect of dry-exposure ratio under diurnal tidal environment. Construct Build Mats. 2022, 328,127138. [CrossRef]
- Rahman, R.O.A.; Ojovan, M.I. Sustainability of cementitious structures, systems, and components (SSC’s): Long-term environmental stressors. In Sustainability of life cycle management for nuclear cementation-based technologies, 1st ed; Rehab O. Abdel Rahman, Michael I. Ojovan, Eds.; Woodhead Publishing, 2021, pp. 181-232. [CrossRef]
- Iqbal, P.O.N.; Ishida, T. Modeling of chloride transport coupled with enhanced moisture conductivity in concrete exposed to marine environment. Cement Concr Res. 2009, 39, pp. 329-339. [CrossRef]
- Kwon, S.J.; Lee, H.S.; Karthick, S.; Saraswathy, V.; Yang, H.M. Long-term corrosion performance of blended cement concrete in the marine environment–A real-time study. Constr Build Mats. 2017, 154, 349-360. [CrossRef]
- Andrade, C.; Dı́ez, J.M.; Alonso, C. Mathematical modeling of a concrete surface “skin effect” on diffusion in chloride contaminated media. Adv Cement Based Mats. 1997, 6, 39-44. [CrossRef]
- Qiao, X.; Chen, J. Correlation of propagation rate of corrosive crack in concrete under sulfate attack and growth rate of delayed ettringite. Eng Fract Mech. 2019, 209, 333-343. [CrossRef]
- Lv, H.; Chen, J.; Lu, C. A Statistical Evolution Model of Concrete Damage Induced by Seawater Corrosion. Materials. 2021, 14, 1007. [CrossRef]
- Ting, M.Z.Y.; Yi, Y. Durability of cementitious materials in seawater environment: A review on chemical interactions, hardened-state properties and environmental factors. Construct Build Mats. 2023, 367,130224. [CrossRef]
- Chaudhari, B.; Panda, B.; Šavija, B.; Chandra-Paul, S. Microbiologically Induced Concrete Corrosion: A Concise Review of Assessment Methods, Effects, and Corrosion-Resistant Coating Materials. Materials. 2022, 15, 4279. [CrossRef]
- Anwar, A.; Liu, X.; Zhang, L. Biogenic corrosion of cementitious composite in wastewater sewerage system-A review. Proc Safety Environ Protect. 2022. [CrossRef]
- Li, X.; Jiang, G.; Grengg, C.; Mittermayr, F. Mechanisms and Processes of Concrete Corrosion in Sewers. In Microbiologically Influenced Corrosion of Concrete Sewers, 1st ed.; Jiang, G.; Ed; Springer, Cham, 2023; pp. 21-34. [CrossRef]
- Ragab, A.M.; Elgammal, M.A.; Hodhod, O.A.; Ahmed, T.E. Evaluation of field concrete deterioration under real conditions of seawater attack. Construct Build Mats. 2016, 119, 130-144. [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol. 2013, 47, 7137-7146. [CrossRef]
- Summers, S.; Pek, Y.S.; Vinod, D.P.; McDougald, D.; Todd, P.A.; Birch, W.R.; Rice, S.A. Bacterial biofilm colonization and succession in tropical marine waters are similar across different types of stone materials used in seawall construction. Front Microbiol. 2022, 13, 928877. [CrossRef]
- Karačić, S.; Modin, O.; Hagelia, P.; Persson, F.; Wilén, B.M. The effect of time and surface type on the composition of biofilm communities on concrete exposed to seawater. Int Biodeter Biodegr. 2022, 173, 105458. [CrossRef]
- Mohamed, H.F.; Abd-Elgawad, A.; Cai, R.; Luo, Z.; Pie, L.; Xu, C. Microbial community shift on artificial biological reef structures (ABRs) deployed in the South China Sea. Sci Rep. 2023, 13, 3456. [CrossRef]
- Vincke, E.; Boon, N.; Verstraete, W. Analysis of the microbial communities on corroded concrete sewer pipes – a case study. Appl Microbiol Biotechnol. 2001, 57, 776–785. [CrossRef]
- Tong, X.; Leung, M.H.; Shen, Z.; Lee, J.Y.; Mason, C.E.; Lee, P.K. Metagenomic insights into the microbial communities of inert and oligotrophic outdoor pier surfaces of a coastal city. Microbiome. 2021, 9, 1-15. [CrossRef]
- Li, X.; Li, S.; Huang, X.; Chen, Y.; Cheng, J.; Zhan, A. Protein-mediated bioadhesion in marine organisms: a review. Mar Environ Res. 2021, 170, 105409. [CrossRef]
- Halevy, I.; Bachan, A. The geologic history of seawater pH. Science. 2017, 355, 1069–1071, . [CrossRef]
- Hayek, M.; Salgues, M.; Habouzit, F.; Bayle, S.; Souche, J.C.; De Weerdt, K.; Pioch, S. In vitro and in situ tests to evaluate the bacterial colonization of cementitious materials in the marine environment. Cement Concrete Comp. 2020, 113, 103748. [CrossRef]
- Parker, C.D. Species of sulphur bacteria associated with the corrosion of concrete. Nature, 1947, 159, pp.439-440. [CrossRef]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol Molec Biol Revs. 2016, 80, 91-138. [CrossRef]
- Caruso, G. Microbial colonization in marine environments: overview of current knowledge and emerging research topics. J Mar Sci Eng. 2020, 8, 78. [CrossRef]
- Voegel, C.; Durban, N.; Bertron, A.; Landon, Y.; Erable, B. Evaluation of microbial proliferation on cementitious materials exposed to biogas systems. Env Technol. 2020, 41, 2439-2449. [CrossRef]
- Bergo, N.M.; Torres-Ballesteros, A.; Signori, C.N.; Benites, M.; Jovane, L.; Murton, B.J.; da Rocha, U.N.; Pellizari, V.H. Spatial patterns of microbial diversity in Fe-Mn deposits and associated sediments in the Atlantic and Pacific oceans. Sci Total Environ. 2022, 837, 155792. [CrossRef]
- Ogawa, A.; Tanaka, R.; Hirai, N.; Ochiai, T.; Ohashi, R.; Fujimoto, K.; Akatsuka, Y.; Suzuki, M. Investigation of biofilms formed on steelmaking slags in marine environments for water depuration. Int J Mol Sci. 2020, 21, 6945. [CrossRef]
- Gaylarde, C.; Little, B. Biodeterioration of stone and metal—Fundamental microbial cycling processes with spatial and temporal scale differences. Sci Total Environ. 2022, 153193. [CrossRef]
- Procópio, L. Microbial community profiles grown on 1020 carbon steel surfaces in seawater-isolated microcosm. Ann Microbiol. 2020, 70, 13. [CrossRef]
- Guo, Z.; Wang, L.; Cong, W.; Jiang, Z.; Liang, Z. Comparative analysis of the ecological succession of microbial communities on two artificial reef materials. Microorganisms. 2021, 9, 120. [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nature Revs Microbiol. 2023, 21, 70-86. [CrossRef]
- Chandra, S.; Eklund, L.; Villarreal, R.R. Use of cactus in mortars and concrete. Cement Concr Res. 1998, 28, 41-51. [CrossRef]
- Camacho-Chab, J.C.; Pereañez-Sacarías, J.E.; Camacho-Chab, P.A.; Gaylarde, C.; Arena-Ortiz, M.L.; Ortiz-Alcántara, J.M.; Chan-Bacab, M.J.; Quintana-Owen, P.; Ortega-Morales, B.O. Influence of bacterial biopolymers on physical properties of experimental limestone blocks. World J Microbiol Biotechnol. 2022, 38, 254. [CrossRef]
- Cano-Barrita, P.D.J.; León-Martínez, F.M. Biopolymers with viscosity-enhancing properties for concrete. In Biopolymers and biotech admixtures for eco-efficient construction materials, 1st ed.; Fernando Pacheco-Torgal, Volodymyr Ivanov, Niranjan Karak, Henk Jonkers, Eds; Woodhead Publishing, 2016; pp. 221-252. [CrossRef]
- Rong, H.; Zhang, S.; Ma, G.; Zheng, X.; Qian, C.; Zhang, L.; Zhang, Y.; Xu, R. Formation, growth and corrosion effect of sulfur oxidizing bacteria biofilm on mortar. Constr Build Mats. 2021, 268, 121218. [CrossRef]
- Rodrigues, C.; Bhosle, N.B. Exopolysaccharide production by Vibrio fischeri, a fouling marine bacterium, Biofouling. 1991, 4, 301-308, . [CrossRef]
- Callow, M.E.; Callow, J.A. Marine biofouling: a sticky problem. Biologist. 2002, 49, pp. 1-5.
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Marine Drugs. 2010, 8, 1779-1802. [CrossRef]
- Rajitha, K.; Nancharaiah, Y.V.; Venugopalan, V.P. Role of bacterial biofilms and their EPS on settlement of barnacle (Amphibalanus reticulatus) larvae. Int Biodeter Biodegr. 2020, 150, 104958. [CrossRef]
- Wang, Y.; Zhang, R.; Duan, J.; Shi, X.; Zhang, Y.; Guan, F.; Sand, W.; Hou, B. Extracellular polymeric substances and biocorrosion/biofouling: recent advances and future perspectives. Int J Molec Sci. 2022, 23, 5566. [CrossRef]
- Ma, W.; Wang, X.; Zhang, W.; Hu, X.; Yang, J.L.; Liang, X. Two-Component system response regulator ompR regulates mussel settlement through exopolysaccharides. Int J Molec Sci. 2023, 24, 7474. [CrossRef]
- Ninan, C.M.; Ajay, A.; Ramaswamy, K.P.; Thomas, A.V.; Bertron, A. A critical review on the effect of organic acids on cement-based materials. In IOP Conference Series: Earth Environ Sci. 2020, 491, 012045. [CrossRef]
- Gladfelter, A.S.; James, T.Y.; Amend, A.S. Marine fungi. Curr Biol. 2019, 29, R191-R195. [CrossRef]
- Jones, E.G.; Ramakrishna, S.; Vikineswary, S.; Das, D.; Bahkali, A.H.; Guo, S.Y.; Pang, K.L. How do fungi survive in the sea and respond to climate change? Journal of Fungi. 2022, 8, 291. [CrossRef]
- Jiang, L.; Pettitt, T.R.; Buenfeld, N.; Smith, S.R. A critical review of the physiological, ecological, physical and chemical factors influencing the microbial degradation of concrete by fungi. Build Environ. 2022, 108925. [CrossRef]
- Gonçalves, M.F.; Hilário, S.; Van de Peer, Y.; Esteves, A.C.; Alves, A. Genomic and metabolomic analyses of the marine fungus Emericellopsis cladophorae: insights into saltwater adaptability mechanisms and its biosynthetic potential. J Fungi, 2022, 8, 31. [CrossRef]
- Gu, J.D.; Ford, T.E.; Berke, N.S.; Mitchell, R. Biodeterioration of concrete by the fungus Fusarium. Int Biodeterior Biodeg. 1998, 41, 101-109. [CrossRef]
- Geweely, N.S.I. Evaluation of ozone for preventing fungal influenced corrosion of reinforced concrete bridges over the River Nile, Egypt. Biodegradation. 2011, 22, 243–252. [CrossRef]
- Nica, D.; Davis, J.L.; Kirby, L.; Zuo, G.; Roberts, D.J. Isolation and characterization of microorganisms involved in the biodeterioration of concrete in sewers. Int Biodeter Biodeg. 2000, 46, 61-68. [CrossRef]
- De Windt, L.; Devillers, P. Modeling the degradation of Portland cement pastes by biogenic organic acids. Cem Concr Res. 2010, 40, 1165-1174. [CrossRef]
- 66. Bhattacharyya, S.; Akhtar, S.; Chaudhuri, A.; Mahanty, S.; Chaudhuri, P.; Sudarshan, M. Affirmative nanosilica mediated approach against fungal biodeterioration of concrete materials. Case Stud Construc Mats. 2022, 17, e01258. [CrossRef]
- Diercks, M.; Sand, W.; Bock, E. Microbial corrosion of concrete. Experientia. 1991, 47, 514–516. [CrossRef]
- Sand, W.; Bock, E., 1990. Biodeterioration of concrete by thiobacilli and nitrifying bacteria. Matériaux & Techniques. 1990, 78, 70-72. [CrossRef]
- Yousefi, A., Allahverdi, A. and Hejazi, P. Accelerated biodegradation of cured cement paste by Thiobacillus species under simulation condition. Int Biodeter Biodegr, 2014, 86, 317-326. [CrossRef]
- Zhou, J.; Yin, S.; Fu, Q.; Wang, Q.; Huang, Q.; Wang, J. Microbial-induced concrete corrosion under high-salt conditions: Microbial community composition and environmental multivariate association analysis. Int Biodeter Biodegr. 2021, 164, 105287. [CrossRef]
- Rooyackers, F.A.; Bosco, E.; Suiker, A.S.; Clemens, F.H. A chemo-mechanical model for biogenic sulphide corrosion of concrete. Cement Concr Res, 2022, 160, 106809. [CrossRef]
- Li, S.; Jin, Z.; Pang, B.; Li, J. Durability performance of an RC beam under real marine all corrosion zones exposure for 7 years. Case Studies in Constr Mats. 2022, 17, e01516. [CrossRef]
- Huang, S.P.; Chen, T.Y.; Chen, J.S.; Wang, L.T.; Huang, L.; Lin, S.T.; Wei, C.L.; Lin, S.; Wang, P.L.; Chen, Y.M.; Shieh, W.Y. Dongshaea marina gen. nov., sp. nov., a facultatively anaerobic marine bacterium that ferments glucose with gas production. Int J Systemat Evolut Microbiol. 2019, 69, 3318-3325. [CrossRef]
- Pelikan, C.; Wasmund, K.; Glombitza, C.; Hausmann, B.; Herbold, C.W.; Flieder, M.; Loy, A. Anaerobic bacterial degradation of protein and lipid macromolecules in subarctic marine sediment. The ISME J. 2021, 15, 833-847. [CrossRef]
- Fincker, M.; Huber, J.A.; Orphan, V.J.; Rappé, M.S.; Teske, A.; Spormann, A.M. Metabolic strategies of marine subseafloor Chloroflexi inferred from genome reconstructions. Environ Microbiol. 2020, 22, 3188-3204. [CrossRef]
- Ding, W.; Wang, S.; Qin, P.; Fan, S.; Su, X.; Cai, P.; Lu, J.; Cui, H.; Wang, M.; Shu, Y.; Wang, Y. Anaerobic thiosulfate oxidation by the Roseobacter group is prevalent in marine biofilms. Nature Commun. 2023, 14, 2033. [CrossRef]
- Georges, M.; Bourguiba, A.; Chateigner, D.; Sebaibi, N.; Boutouil, M. The study of long-term durability and bio-colonization of concrete in marine environment. Environ Sustain Indicators. 2021, 10, 100120. [CrossRef]
- Rong, H.; Chen, X.; Feng, Y.; Zhang, Y.; Zhang, J. Adhesion of biofilm to mortar surface with protective coating in seawater environment and the influence on the mortar performance. Journal of Mats Civil Eng. 2023, 35, 04023016. [CrossRef]
- Sun, M.; Xu, W.; Rong, H.; Chen, J.; Yu, C. Effects of dissolved oxygen (DO) in seawater on microbial corrosion of concrete: Morphology, composition, compression analysis and transportation evaluation. Construct Build Mats. 2023, 367, 130290. [CrossRef]
- Georges, M.; Bourguiba, A.; Boutouil, M.; Chateigner, D.; Jolly, O.; Claquin, P. Interaction between the diatom Cylindrotheca closterium and a siliceous mortar in a silica-limited environment. Const Build Mats. 2022, 321, 126277. [CrossRef]
- Merino-Maldonado, D.; Antolín-Rodríguez, A.; Serrano-González, L.; Blanco, S.; Juan-Valdés, A.; Morán-del Pozo, J.M.; García-González, J. Innovative approach for the protection of recycled concrete by biogenic silica biodeposition. Constr Build Mats. 2023, 368, 130475. [CrossRef]
- Karačić, S.; Wilén, B.M.; Suarez, C.; Hagelia, P.; Persson, F. Subsea tunnel reinforced sprayed concrete subjected to deterioration harbours distinct microbial communities. Biofouling. 2018, 34, 1161-1174. [CrossRef]
- Li, K.; Guan, W.; He, P.; Li, K.J. Comparison of bacterial communities on the surface of concrete breakwater structures and ambient bacterioplankton. Letts Appl Microbiol. 2022, 75, 1193-1202. [CrossRef]
- Cai, W.; Li, Y.; Niu, L.; Zhang, W.; Wang, C.; Wang, P.; Meng, F. New insights into the spatial variability of biofilm communities and potentially negative bacterial groups in hydraulic concrete structures. Water Research. 2017, 123, 495-504. [CrossRef]
- Suarez, C.; Dalcin Martins, P.; Jetten, M.S.; Karačić, S.; Wilén, B.M.; Modin, O.; Hagelia, P.; Hermansson, M.; Persson, F. Metagenomic evidence of a novel family of anammox bacteria in a subsea environment. Environ Microbiol. 2022, 24, pp.2348-2360. [CrossRef]
- Karačić, S. Microbial biofilm communities associated with degradation of sprayed concrete in subsea tunnels, Doctoral thesis, Chalmers Tekniska Hogskola (Sweden), 2021. https://www.researchgate.net/profile/Sabina-Suceska-Karacic/publication/356732212_Microbial_biofilm_communities_associated_with_degradation_of_sprayed_concrete_in_subsea_tunnels/links/61a9561aca2d401f27be3899/Microbial-biofilm-communities-associated-with-degradation-of-sprayed-concrete-in-subsea-tunnels.pdf Accessed on 27th June, 2023.
- Yu, C.; Yuan, Q.; Rong, H.; Liu, D.; Feng, Y. Effects of dissolved oxygen on marine biofilm formation and its on microstructure and chloride ion permeability of concrete. Journal of Building Engineering. 2023, 75, 107074. [CrossRef]
- Ertelt, M.J.; Raith, M.; Eisinger, J.; Grosse, C.U.; Lieleg, O. Bacterial Additives Improve the Water Resistance of Mortar. ACS Sustain Chem Eng. 2020, 8, 5704-5715. [CrossRef]
- Wang, J.; Yin, S.; Lu, L.; Zhou, J.; Fu, Q. Characterization of microbial-induced concrete corrosion by combining morphology observation and fluorescence staining. Case Stud Constr Mater. 2022, 17, e01586. [CrossRef]
- Chung, H.; Lee, O.; Huang, Y.L.; Mok, S.Y.; Qian, P.Y. Bacterial community succession and chemical profiles of subtidal biofilms in relation to larval settlement of the polychaete Hydroides elegans. ISME J. 2010, 4, 817–828. [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.F. Marine biofilms on artificial surfaces: structure and dynamics. Environ microbial. 2013, 15, 2879-2893. [CrossRef]
- Qian, P.Y.; Cheng, A.; Wang, R.; Zhang, R. Marine biofilms: diversity, interactions and biofouling. Nat Rev Microbiol. 2022, 20, 671-684. [CrossRef]
- Kundukad, B.; Ho, J.C.; Mugunthan, S.; Wong, L.L.; Rice, S.A.; Parikh, A.N.; Seviour, T.; Hinks, J.; Kjelleberg, S. Viewing biofilm formation through a multifocal lens of physics and biology. Microbiology Aust. 2023, 44, 69-74. [CrossRef]
- Davis, J.; Levin, L.; Walther, S. Artificial armored shorelines: sites for open-coast species in a southern California bay. Mar Biol. 2002, 140, 1249-1262. [CrossRef]
- Miller, M.W. Using ecological processes to advance artificial reef goals. ICES J Mar Sci, 2002, 59, S27-S31. [CrossRef]
- Ramm, L.A.; Florisson, J.H.; Watts, S.L.; Becker, A.; Tweedley, J.R. Artificial reefs in the Anthropocene: a review of geographical and historical trends in their design, purpose, and monitoring. Bull Mar Sci, 2021, 97, 699-728. [CrossRef]
- Ly, O.; Yoris-Nobile, A.I.; Sebaibi, N.; Blanco-Fernandez, E.; Boutouil, M.; Castro-Fresno, D.; Hall, A.E.; Herbert, R.J.; Deboucha, W.; Reis, B.; Franco, J.N. Optimisation of 3D printed concrete for artificial reefs: Biofouling and mechanical analysis. Constr Build Mats. 2021, 272, 121649. [CrossRef]
- Kong, J.; Cong, G.; Ni, S.; Sun, J.; Guo, C.; Chen, M.; Quan, H. Recycling of waste oyster shell and recycled aggregate in the porous ecological concrete used for artificial reefs. Constr Build Mats, 2022, 323, 126447. [CrossRef]
- Berman, O.; Weizman, M.; Oren, A.; Neri, R.; Parnas, H.; Shashar, N.; Tarazi, E. Design and application of a novel 3D printing method for bio-inspired artificial reefs. Ecol Eng. 2023, 188, 106892. [CrossRef]
- Salamone, A.L.; Robicheau, B.M.; Walker, A.K. Fungal diversity of marine biofilms on artificial reefs in the north-central Gulf of Mexico. Botan Mar. 2016, 59, 291-305. [CrossRef]
- Tong, F.; Chen, G.; Feng, X.; Liu, Y.; Chen, P. The effect of the artificial reef on the structure and function of sediment bacterial community. Sustain. 2022, 14, 14728. [CrossRef]
- Xiong, Y.; Tang, L.; Jia, H.; Shao, C.; Tang, J.; Xu, Y.; Yan, L.; Zhang, D. Microbial networks reveal the structure of water microbial communities in Kalamaili Mountain Ungulate Nature Reserve. Water, 2022, 14, 2188. [CrossRef]
- Cooke, S.J.; Bergman, J.N.; Nyboer, E.A.; Reid, A.J.; Gallagher, A.J.; Hammerschlag, N.; Van de Riet, K.; Vermaire, J.C. Overcoming the concrete conquest of aquatic ecosystems. Biol Conserv. 2020, 247, 108589. [CrossRef]
- Li, S.; Liu, J.; Geng, Y.; Liu, A.; Xu, A.; Hou, D.; Lang, X. Efficacy and mechanism of GO/IBTS coating against microbial fouling of concrete surfaces in marine tidal areas. JCTR. 2022, 19, 875-885. [CrossRef]
- Trejo, D.; de Figueiredo, P.; Sanchez, M.; Gonzalez, C.; Wei, S.; Li, L. Analysis and assessment of microbial biofilm-mediated concrete deterioration. Technical Report, U.S. Department of Transportation, 2008.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).