1. Introduction
Microplastics (MPs) are non-uniform plastic particles less than 5 mm in diameter that enter the ecosystem through various pathways. Industrial production, personal care products, and the decomposition of large plastic debris are the main sources of MPs [
1]. The widespread use of plastic products and poor waste management have led to the ubiquity of MPs in aquatic water bodies, including rivers, lakes, estuaries, shorelines, and marine ecosystems. Previous research has estimated that a large number of MPs from domestic producers enter wastewater treatment plants (WWTPs) through the drainage system [
2]. Therefore, WWTPs could represent the last barrier before the discharge of MPs into an aquatic ecosystem. A recent survey of 95 WTTPs in 12 countries found that the concentrations of microplastics in the influent and effluent were 0.28–18285 particles/L and 0–447 particles/L [
3]. Due to the high hydrophobicity of activated sludge in biological treatment units [
4], most of the MPs in raw wastewater can be captured by sludge. However, the accumulation of MPs in activated sludge has the potential to impact sludge characteristics, extracellular polymeric substances (EPS) fractions, microbial activities and compositions, and eventually system treatment performance.
The most common MPs in activated sludge are polyethylene (PE), polyethylene terephthalate (PET), polyvinyl chloride (PVC), polystyrene (PS), and polypropylene (PP) [
5]. These MPs and smaller nanoplastics (NPs) may not only physically harm microorganisms through ingestion, but also release or adsorb toxic and harmful pollutants, inhibiting microbial activity in wastewater treatment systems. Wei et al. [
6] investigated the effect of PS NPs on anaerobic granular sludge and found that higher concentrations of PS NPs caused changes in EPS composition of sludge, while microorganisms produced more proteins to defend against potential harm [
7]. He et al. [
8] showed that when the particle size of PS MPs was 150–300 μm and the concentration was 0.01–0.10 g/L, the nitrifying activity in activated sludge was significantly inhibited and the ammonia oxidation rate decreased to 71%–92% compared to the control. Zhang et al. [
9] found that PVC MPs (50 μm diameter) at concentrations of 20–100 mg/L significantly inhibited the denitrification process of aerobic granular sludge (AGS) and reduced the total nitrogen (TN) removal by 3.8%–17.8%.
However, most studies on the effects of various MPs on activated sludge systems have focused on traditional non-degradable plastics such as PVC, PE, and PP, while there are few and insufficient studies on biodegradable plastics. To alleviate plastic pollution, biodegradable plastics are widely used as environmentally friendly products [
10] in medical, food packaging, agriculture and other industries [
11]. The global production capacity of biodegradable bio-based plastics was 0.732 million tons in 2018, and is expected to increase to 1.3 million tons per year by 2030 [
12]. Biodegradable plastics are usually classified by product type as polyhydroxyalkanoate (PHA), polylactic acid (PLA), polybutylene succinate (PBS), and polycaprolactone (PCL) [
12]. PLA is one of the current focuses in the field of biodegradable plastics. The enzymatic polymerization or azeotropic dehydration of monomeric lactic acid is used to form PLA [
13], and lactic acid can be produced by fermenting renewable resources such as bagasse, corn, starch, and food waste [
14]. With its high mechanical strength, good biocompatibility and solvent-carrying properties, and low prices, PLA is widely used in food packaging, automotive industry, textile industry, medical industry and agriculture, and the global market has been expanding steadily with increasing demand [
15,
16]. The huge future consumption of PLA means that a large number of PLA MPs will be produced and enter the WWTPs, and PLA plastics may produce more MPs and oligomer NPs than traditional plastics with potential risk [
10], while there is still a lack of research on whether they and their degradation products have an impact on the activated sludge system.
In this study, the impact of different concentrations of PLA MPs on a typical lab-scale activated sludge system was investigated by analyzing effluent water quality, EPS, metabolic activity, and community structure. It will help to understand the potential risk of PLA MPs in the wastewater treatment process and take appropriate regulatory measures in the future.
2. Materials and Methods
2.1. Reactor setup
With an inoculation of activated sludge from the Xi'an No. 4 WTTP, Xi'an, Shaanxi, China, four lab-scale anaerobic-aerobic activated sludge sequencing batch reactors (SBR) (
Figure S1) were constructed with a working volume of 1.4 L and used to simulate a typical secondary treatment process. The operating cycle of the SBR reactor was 6 h, including 5 min for filling, 90 min for anaerobic phase, 210 min for aerobic phase, 45 min for settling, and 10 min for draw and idle phases. The four reactors were operated for 36 days to achieve a steady state before the formal experiment.
2.2. Operational Conditions
Synthetic wastewater was used in this study. A mixture of acid-hydrolyzed casein, acetate (HAc) and propionate (HPr) were used as carbon sources with a COD ratio of 2:7:7 and a total COD of ~280 mg/L. Ammonium chloride (NH
4Cl) and potassium dihydrogen phosphate (KH
2PO
4) were used as inorganic N and P sources, respectively, resulting in 20 mg N/L of NH
4+-N and 4.5 mg P/L of PO
43--P. Trace elements were added as previously described [
17], and the detailed concentrations are listed in
Table S1.
The SBR reactor was operated at a temperature of 20 ± 5°C, a pH range of 7.0–8.5, a hydraulic retention time (HRT) of 12 h, and a sludge retention time (SRT) of 15 d. The mixed liquor suspended solids (MLSS) were maintained at approximately 3500 mg/L. After stabilizing reactor performance, different concentrations of PLA MPs were added to the four reactors for comparison analysis, with an experiment duration of 30 d. Mirka et al. [
18] investigated the particle size distribution range of MPs in several full-scale WTTPs worldwide and found that MPs with a diameter greater than 500 μm could account for over 70% of the influent. Liu et al. [
19] found that the MP concentrations in sludge ranged from 4.4 ×10
3 to 2.4×10
5 particles/kg in 38 WWTPs in 11 countries worldwide. A similar study pointed out that the average MP concentration in sludge from WTTPs in 12 countries was 12.8×10
3 particles/kg [
20]. Accordingly, the particle size of PLA MPs chosen in this study was as ~580 μm, and the dosages in the four reactors were 0, 50, 100, and 200 particles/(g TS), respectively. The reactors were then named R0, R1, R2, and R3, respectively.
2.3. Biological Activity Batch Tests
To further evaluate the effect of different concentrations of PLA MPs on functionally relevant microbial activities for nitrogen and phosphorus removal, 800 mL of activated sludge was taken from each reactor at the end of the aerobic phase, washed using synthetic wastewater without carbon, nitrogen and phosphorus sources [
21,
22], and transferred to a reactor (1 L) for batch tests.
2.3.1. Enhanced Biological Phosphorus Removal (EBPR) Activity Batch Tests
Firstly, nitrogen gas was continuously introduced into the sludge to achieve anaerobic conditions with dissolved oxygen (DO) <0.2 mg/L. Subsequently, HAc and KH
2PO
4 were added to achieve COD and PO
43--P concentrations of 300 mg/L and 10 mg P/L, respectively. The anaerobic condition was maintained for 1 h. Then, air was continuously introduced to ensure aerobic conditions (DO > 2 mg/L) for 3 h. During batch tests, pH was maintained at 7.0 ± 0.1 by adding NaOH or HCl. Samples were periodically collected for determination of COD, PO
43--P, MLSS and mixed liquor volatile suspended solids (MLVSS) The contents of intracellular storage polymers, such as polyhydroxyalkanoates (PHAs) and glycogen, were also analyzed accordingly [
17,
23].
2.3.2. Nitrification Activity Batch Tests
Air was continuously introduced into the sludge to maintain aerobic conditions for 3 h. NH
4Cl solution was added to achieve NH
4+-N concentration of 30 mg N/L. During the batch test, samples were periodically collected for determination of NH
4+-N, NO
3--N, NO
2--N, MLSS and MLVSS. In addition, the specific ammonia oxidation rate (AOR) and nitrite oxidation rate (NOR) were calculated accordingly [
8].
2.4. Microbial Community Analysis
During the experiment, activated sludge samples were collected from the four reactors for DNA extraction and 16S rRNA gene amplicon sequencing. Genomic DNA was extracted using DNeasy PowerSoil Kit (QIAGEN, Inc., Netherlands). The primers, 341F (CCTACGGGAGGCAGCAG) and 806R(GGACTACHVGGGTWTCTAAT), were used for PCR amplification of the bacterial 16S rRNA gene. The amplification products were purified by Agencourt AMPure Beads (Beckman Coulter, Brea, CA, USA) and quantified by PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA), and sequenced using the Illumina MiSeq platform (Shanghai Paisano Biotechnology Co., Ltd.). Finally, QIIME (v1.8.0) software was used for bioinformatics analysis of the sequencing data. The microorganisms identified in the activated sludge system were linked to their functional information using the Activated Sludge Microbial Database (MiDAS:
https://www.midasfieldguide.org/guide/search).
2.5. Chemical Analysis
To evaluate the pollutant removal performance during the experiment, the concentrations of COD, PO
43—P, NH
4+-N, NO
3--N, NO
2--N, TN, MLSS and MLVSS were analyzed using the Standard Methods [
24]. EPS is extracted by thermal extraction [
25]. The protein and humic acid contents were determined using the modified Lowry method [
26], and the polysaccharide content was determined by anthrone-sulfuric acid method [
27]. PHAs were determined using Shimadzu gas chromatograph coupled to triple quadrupole mass spectrometer (GCMS-TQ8040) [
28]. The glycogen content was analyzed using the glucose content kit (BC2505, Beijing Solarbio Science & Technology Co., Ltd.).
3. Results and Discussion
3.1. Effects of PLA MPs on Pollutant Removal Performance
3.1.1. COD Removal Performance
As shown in
Figure 1a, there was no significant difference (
p > 0.05) in the COD removal performance among the four SBRs (R0, R1, R2 and R3), with average removal efficiencies of 80.1%, 79.3%, 80.2% and 78.4%, respectively. Dai et al. [
29] showed that PVC MPs (50 mg/L) had no negative effect on COD removal performance in an AGS system, probably due to a better tolerance of most heterotrophic microorganisms in AGS to MPs. Similarly, a study by Zhang et al. [
9] found that PS MPs (particle size 50–100 μm) had no significant effect on the organic matter removal performance in AGS systems. Moreover, compared to non-biodegradable MPs, PLA MPs can be readily degraded by microorganisms through the secretion of proteases and lipases [
30]. The resulting small molecule organic matters can potentially serve as supplementary carbon sources for heterotrophic microorganisms. Therefore, PLA MPs do not have a significant impact on COD removal. The average effluent COD concentration in R3 was 57.2 mg/L, slightly higher than in other SBRs, possibly due to the release of smaller PLA particles (e.g., nanoparticles (NPs)) and their degradation products during biodegradation of PLA MPs.
3.1.2. Phosphorus Removal Performance
As shown in
Figure 1b, the effluent PO
43--P concentration decreased with the increase in PLA MP dosage (
p<0.05), from 0.42 ± 0.35 mg/L in R0 to 0.32 ± 0.24 mg/L in R1 and 0.22 ± 0.16 mg/L in R3. Consequently, the range of effluent PO
43--P concentration in R3 (0.05–0.61 mg/L) was lower than in other SBRs (0.10–1.10 mg/L). Previous studies have shown that PE, PVC, and polyethersulfone (PES) MPs (50–10000 particles/L) have no significant effect on PO
43--P removal performance and activity of polyphosphate (polyP) accumulating organisms (PAOs) in activated sludge systems [
31]. He et al. [
8] reported that PS MPs with a size range of 0–300 μm did not affect PAO activity. The results of this study showed that the dosing of PLA MPs may lead to improved biological P removal, while the causes of this phenomenon need further study.
3.1.3. Nitrogen Removal Performance
As shown in
Figure 1c, with the increase in PLA MP dosage, the average effluent NH
4+-N concentration gradually increased from 0.25 mg/L in R0 to 1.35 mg/L in R2 (
p < 0.05). It has been shown that PVC and PS MPs could reduce N removal performance in activated sludge systems by inhibiting the activities of ammonia oxidizing bacteria (AOB) and nitrite oxidizing bacteria (NOB) [
32]. The average NH
4+-N removal efficiencies in SBRs were above 97% except in R2, which may be partially related to the declined biological N removal activities (see
Section 3.2.2).
As shown in
Figure 1d, the average effluent TN concentration increased gradually (
p < 0.05) with the increase in PLA MP dosage. The effluent TN concentration in R3 was 7.11 ± 1.68 mg/L, with an average removal efficiency of 66.1%. Previous study has shown that 0.05 g/L of PS MPs with a diameter of 150–300 μm could reduce the NO
3--N reduction rate to 69%–94% as compared with the blank [
8]. Caruso et al. [
33] reported that tiny plastic debris resulting from weathering and fragmentation can cellular structures and/or release hazardous chemicals, such as plastic additives, ultimately leading to reduced enzymatic activity and cell inactivation. It is possible that high concentrations of PLA MPs and their degradation products inhibited the activity and abundance of denitrifying bacteria (DNB), thus affecting denitrification performance.
3.2. Effects of PLA MPs on Metabolic Activity
3.2.1. EBPR Activity
To evaluate the effect of biodegradable PLA MPs on P removal activity in the four SBRs, EBPR batch tests were conducted, and changes in COD and PO
43--P concentrations during the tests are shown in
Figure S2. As shown in
Table 1, the specific average anaerobic P release rate (P
rel) was 37.7 mg P/(g VSS·h) after the introduction of low concentrations (50 particles/(g TS)) of PLA MPs, which was higher than that in R0 (29.1 mg P/(g VSS·h)). Specific average aerobic P uptake rate (P
up) in SBRs generally remained consistent at ~7.0 mg P/(g VSS·h). After the exposure to high concentrations (200 particles/(g TS)) of PLA MPs, P
rel and P
up decreased to 23.1 and 6.3 mg P/(g VSS·h), respectively. This indicates that excessive accumulation of PLA MPs would potentially affect the P release/uptake capacity of PAOs, while it did not lead to deteriorated P removal performance in SBRs at the relatively low influent PO
43--P concentration (4.5 mg/L).
The P release to acetate uptake ratio (P/HAc) is commonly used as an indicator of changes in the activity and abundance of PAOs and their competitors, glycogen accumulating organisms (GAOs) [
34]. As shown in
Table 1, the P/HAc ratio increased to 0.60 P-mol/C-mol in R1, while decreased to 0.49 and 0.48 P-mol/C-mol in R2 and R3, respectively. Compared to R0, the P removal performance in all SBRs dosed with PLA MPs increased to varying degrees. This may be because PLA MPs can act as not only supplementary carbon sources but also biofilm carriers for the attachment and growth of heterotrophic microorganisms, such as PAOs, which would promote the formation of biofilm and further enhance PAO activity by providing competitive advantages [
35]. When the density of PLA MPs increased to 200 particles/(g TS), the biofilm would fall off due to collision and friction between particles, which reduces biofilm formation efficiency, thus reducing the PAO activity. However, the P/HAc value (0.48 P-mol/C-mol) remains high, indicating robust PAO activity and abundance, which is consistent with microbial community results (see Section 3.3.2).
PAOs take up carbon sources such as acetate and store them in the form of PHAs during the anaerobic phase, and subsequently use PHAs as the main energy source for excessive P uptake and polyP synthesis during the aerobic phase [
36]. The amount of PHAs stored by PAOs will directly affect their P uptake capacity in the aerobic phase [
17]. However, GAOs exhibit a metabolism similar to PAOs that compete with PAOs for VFA uptake and PHA synthesis without performing anaerobic P release or aerobic P uptake. Therefore, a higher PHA production to acetate uptake ratio (PHA/HAc) implies either a higher involvement of GAOs or a higher utilization of the glycolysis pathway by PAOs [
37]. In this study, the PHA/HAc ratio was 0.83 C-mol/C-mol in R0, which was higher than that in R2 and R3 (0.54 and 0.68 C-mol/C-mol, respectively). Considering the low GAO abundance as detected by microbial community analysis, the result indicates that the large number of PLA MPs may prompt PAOs to use the TCA cycle over glycolysis pathway to produce reducing equivalents. During the PHA synthesis process in the anaerobic phase, intracellularly stored glycogen could provide a significant portion of the energy for PAOs/GAOs through glycolysis [
38]. Therefore, higher glycogen utilization to acetate uptake ratio (Glyc/HAc) would be related to increased relative abundance of GAOs or increased use of glycolytic pathways [
39]. In this study, Glyc/HAc ratio decreased from 0.21 C-mol/C-mol in R0 to 0.14 C-mol/C-mol in R3, indicating that excessive PLA MPs may reduce glycogen utilization by PAOs, which is correlated with PHA/HAc ratio results, while the causes need further study.
Table 1.
Comparison of EBPR activities during the batch tests with the sludge collected from different SBRs.
Table 1.
Comparison of EBPR activities during the batch tests with the sludge collected from different SBRs.
| Reactor |
Prel
(mg P/(g VSS·h))
|
Pup
(mg P/(g VSS·h))
|
Pup/Prel |
P/HAc
(P-mol/C-mol)
|
PHA/HAc
(C-mol/C-mol)
|
Glyc/HAc
(C-mol/C-mol)
|
References |
| R0 |
29.1 |
7.5 |
0.26 |
0.41 |
0.83 |
0.21 |
This study |
| R1 |
37.7 |
7.4 |
0.20 |
0.60 |
0.71 |
0.18 |
| R2 |
28.2 |
7.2 |
0.26 |
0.49 |
0.54 |
0.26 |
| R3 |
23.1 |
6.3 |
0.27 |
0.48 |
0.68 |
0.14 |
| Other systems |
2.8–31.9 |
1.9–11.0 |
0.2–0.7 |
0.11–0.66 |
0.67–2.10 |
0.02–0.82 |
[17,40,41,42] |
3.2.2. Nitrification Activity
To reveal the effect of PLA MPs on nitrifying bacteria, the nitrification activity batch tests were conducted with sludge collected from the four SBRs, and changes in NH
4+-N, NO
2--N, and NO
3--N concentrations during the tests are shown in
Figure S3. As shown in
Table 2, both AOR and NOR showed a decreasing trend with the increase in PLA MP dosage. Compared to R0, AOR in R1 decreased from 1.04 to 0.67 mg N/(g VSS·h), while NOR in R1 (1.06 mg N/(g VSS·h)) was comparable to R0 (1.15 mg N/(g VSS·h)). When the PLA MP dosage increased to 100 particles/(g TS), AOR decreased significantly to 0.31 mg N/(g VSS·h), indicating that AOB was more sensitive to PLA MP exposure. When the dosage of PLA MPs continued to increase to 200 particles/g TS, NOR decreased to 0.98 mg N/(g VSS·h), indicating that the addition of PLA MPs also had an impact on NOB activity. Similarly, Li et al. [
32] found that 5000 particles/L of different MPs (PP, PE, PS, PES, and PVC) inhibited the activated sludge nitrification. In addition, a recent study found that lactate dehydrogenase (LDH) release increased by 40% after exposure to 10 mg/L of polytetrafluoroethylene (PTFE) NPs, which further affected microbial activity due to destroyed cell integrity [
44]. The NPs produced during MP degradation could block cell channels and induce microorganisms to produce large amounts of reactive oxygen species (ROS), potentially causing damage to protein, cell structure, and DNA [
45]. Although no distinct changes in NH
4+-N removal performance were detected in this study, nitrification activity tests showed that high concentrations (100-200 particles/(g TS)) of biodegradable PLA MPs would negatively affect the activities of autotrophic AOB and NOB, especially AOB.
3.3. Effects of PLA MPs on EPS
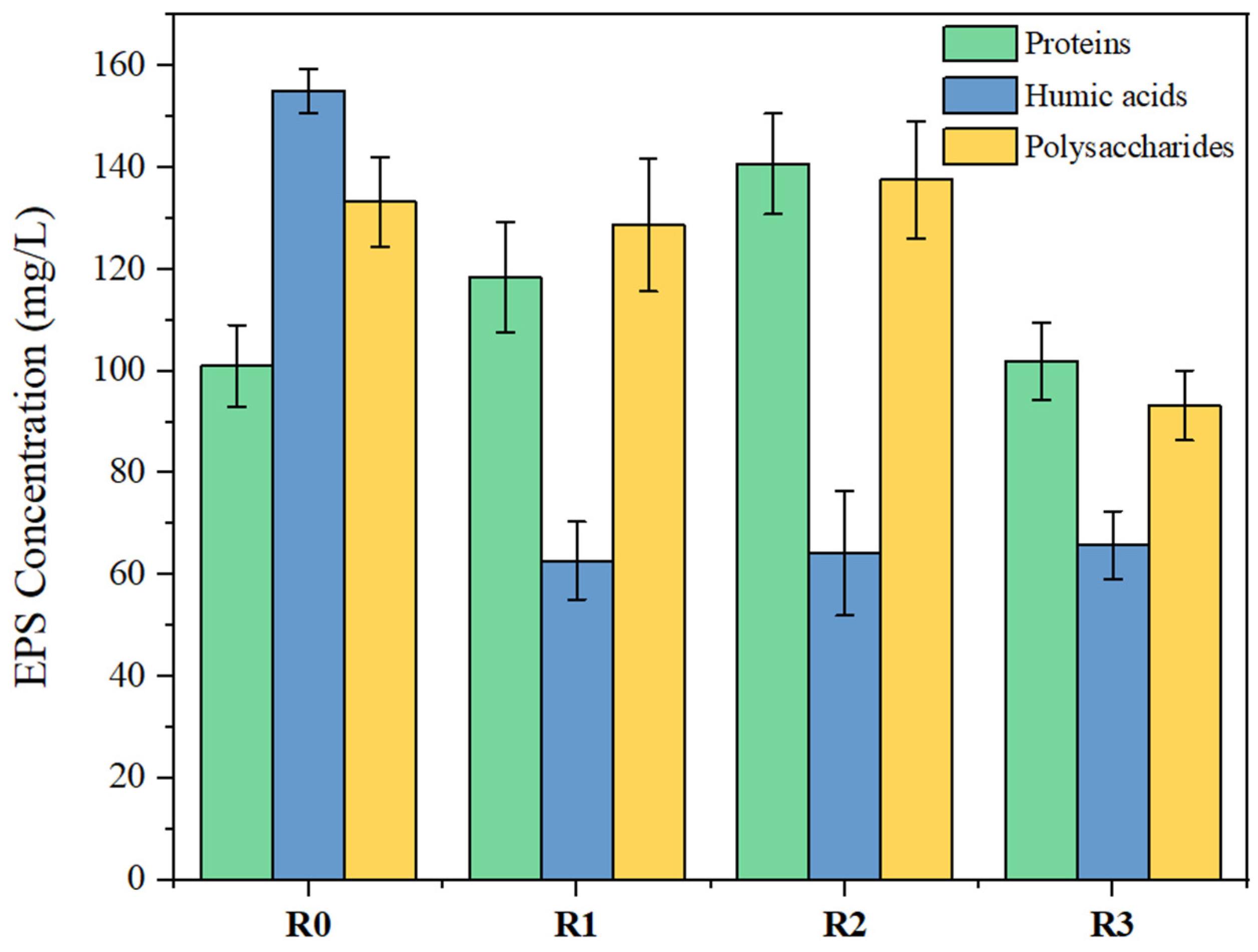
After 20 days of PLA MPs exposure, EPS was extracted from sludge flocs in the four SBRs to analyze the contents of protein, humic acid, and polysaccharide. As shown in
Figure 2, with the increase in PLA MP dosage, total EPS content showed a decreasing trend. Similarly, Wei et al. [
49] found that high concentrations of PS MPs caused a reduction of cell viability, and the total EPS content was lower than that of the control group. In this study, humic acid content in EPS decreased significantly from 155.1 mg/L in R0 to 65.8 mg/L in R3. Polysaccharide content generally remained consistent at ~130 mg/L but decreased to 92.42 mg/L in R3, which may be related to the relatively larger particle size of MPs that are more likely to form biofilms on the surface. The high metabolic activity of microorganisms in the biofilms would then consume the biodegradable components of EPS [
50,
51]. In addition, it was observed that protein content increased slightly with PLA MPs at concentrations of 50 and 100 particles/(g TS). Recent studies have revealed that EPS in biofilm systems tend to be enriched in protein instead of carbohydrates, which has carboxylate and amine groups that can complex with NPs [
52], and microbial aggregates would secrete more proteins to protect them from toxic chemicals [
53]. However, the EPS extraction method used in this study could not efficiently extract EPS from biofilms attached to the MP surface, and the components and changes of EPS in biofilms need further study.
3.4. Effect of PLA MPs on Microbial Community
3.4.1. Microbial Community Diversity
The effect of different concentrations of PLA MPs on the microbial community structure was analyzed by 16S rRNA gene amplicon sequencing. The amplicon sequence variants (ASVs) were obtained by clustering sequences with 100% similarity, and the Alpha diversity index was calculated for each sample based on the ASVs (
Table S2). Good's coverage of the four samples was all above 0.99, indicating that the sequencing depth occupied more than 99% of the entire sequence and the sequencing depth was sufficient. The Shannon and Chao1 indices collectively reflect community richness, while the Gini-Simpson index reflects community diversity [
54]. The diversity results showed a generally upward trend in diversity indices for samples with different concentrations of PLA MPs. Compared to R0, Shannon and Chao1 indices in R3 increased by 70% and 67%, respectively. The Gini-Simpson index in R3 (0.961) is also higher than in R0 (0.860), indicating that the presence of PLA MPs can potentially increase the richness and diversity of microbial communities. Non-degradable MPs may affect the microbial community diversity by releasing toxic additives used in the production process or their own toxic degradation products. For example, PET MPs release the toxic di-n-butyl phthalate (DBP) polymer that induces microbial ROS production, leading to inactivity and even death of microbial cells such as methanogenic bacteria [
55]. As PLA MPs in this study have relatively good biocompatibility and biodegradability and can act as carriers, it would allow specific microorganisms to attach to the surface to form a stable biofilm community structure, thereby increasing community diversity [
56]. Through the analysis of ASV data in
Figure S4, it was found that 18%–25% of the unique ASV existed in samples with PLA MP dosage, indicating that the community composition in SBRs gradually changed to one that was more adapted to the exposure of PLA MPs [
57].
3.4.2. Microbial Composition
The microbial composition at the phylum level is shown in
Figure 3a. Proteobacteria was the dominant phylum in R0, with relative abundance of 97.79%. After the addition of PLA MPs, the relative abundance of Proteobacteria decreased to 90.16%–94.24%, but still dominated. Previous studies have shown that Proteobacteria are the main dominant phylum commonly found in activated sludge systems, containing a variety of microorganisms that degrade organic pollutants and remove nutrients such as nitrogen (e.g., DNB, etc.) [
58,
59]. Therefore, the decrease in TN removal capacity with the addition of PLA MPs may be related to the decrease in the relative abundance of Proteobacteria. The presence of PLA MPs promoted the growth of Bacteroidetes, with relative abundance of 4.63%–7.80%. Some members of the phylum Bacteroidetes are capable of degrading complex organic matter [
60], and potentially have the ability to denitrification [
61]. The microbial composition at the genus level is shown in
Figure 3b. The dominant genus in R0 was
Pseudomonas, with a relative abundance of 95.43%.
Pseudomonas exhibited high sensitivity to PLA MPs, and its relative abundance gradually decreased to 58.98% in R3. Correspondingly,
Acinetobacter,
Acidovorax and
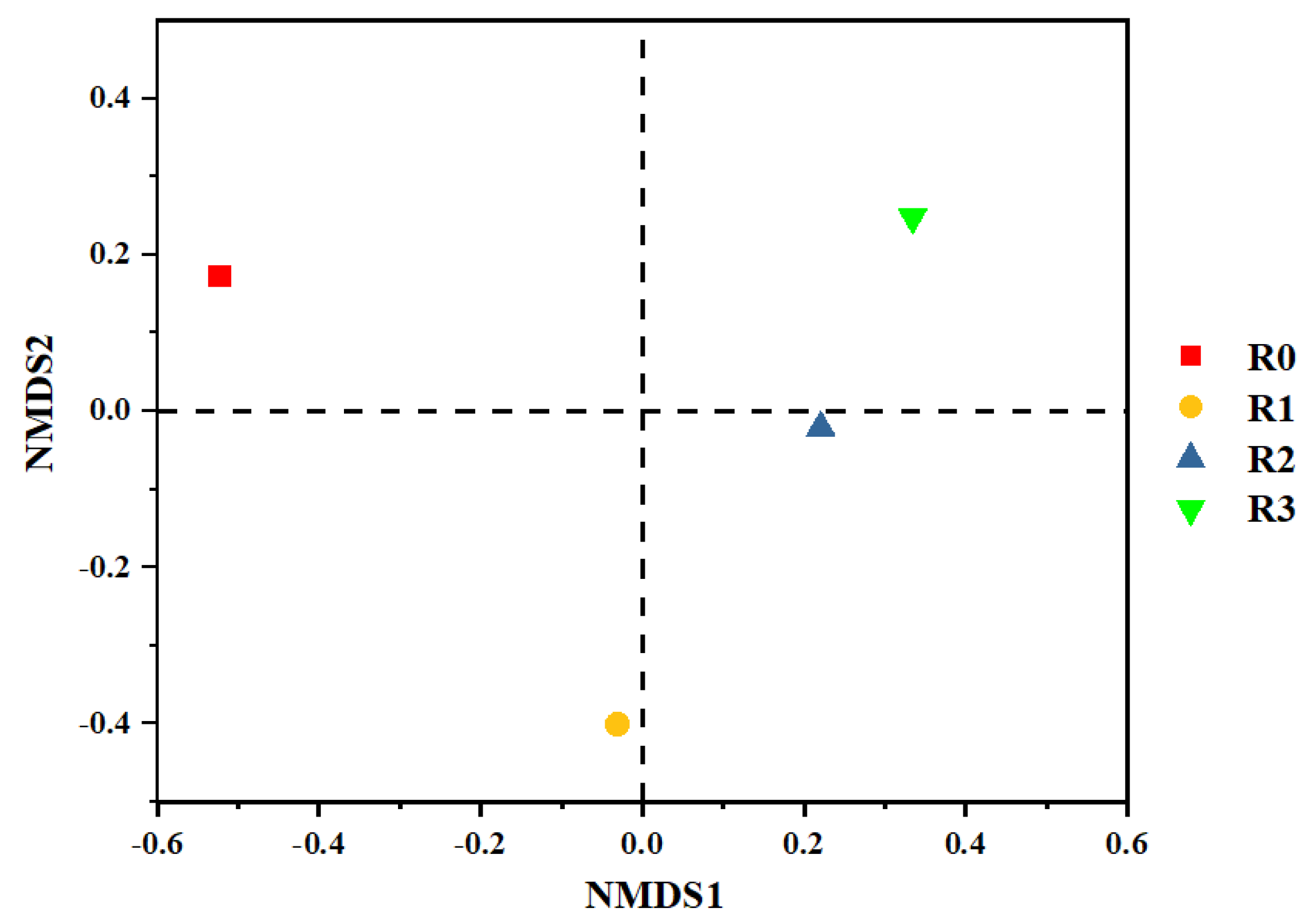
Azoarcus gradually gained advantages in community competition, with relative abundance increasing from less than 0.3% in R0 to 12.57%, 8.47%, and 2.86% in R3, respectively. The results of non-metric multidimensional scanning (NMDS) analysis showed that the microbial community structure evolved significantly after the addition of PLA MPs for better adaptation (
Figure 4).
3.4.3. Functionally Relevant Microbial Populations
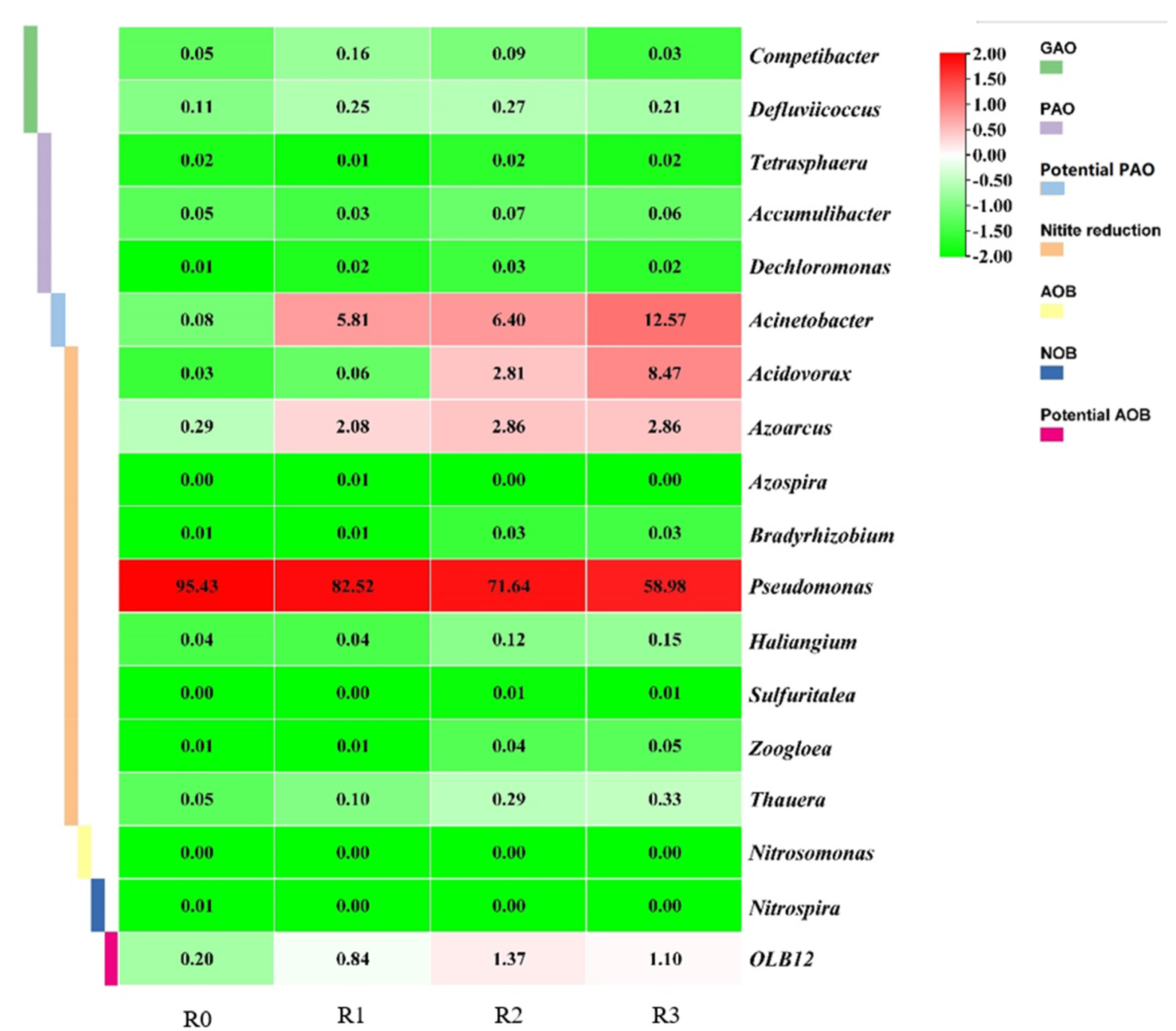
To better investigate the driving factors of N and P removal performance in SBRs at different concentrations of PLA MPs, the MiDAS database [
62] was used to further screen the functionally relevant microbial populations associated with EBPR, nitrification, and denitrification (
Figure 5). Known PAOs (e.g.,
Accumulibacter,
Dechloromonas and
Tetrasphaera) and GAOs (e.g.,
Competibacter and
Defluviicoccus) involved in EBPR processes were all present at very low abundance (<0.5%) in the four SBRs. Notably, the relative abundance of potential PAO (i.e.,
Acinetobacter) increased from 0.08% to 5.81%–12.57% with the addition of PLA MPs. Although
Acinetobacter's contribution to EBPR remains controversial, many studies have shown that
Acinetobacter has the ability to accumulate polyP and PHA [
63,
64,
65],and it has previously been proposed to behave as PAOs in EBPR systems [
66,
67]. The relative abundance of
Competibacter increased from 0.05% in R0 to 0.16% in R1, but decreased to 0.03% in R3 as PLA MPs concentration continued to increase. The relative abundance of
Defluviicoccus is 0.11% in R0, and the presence of PLA MPs increases it to 0.21%–0.27%. Previous studies have shown that heterotrophic organisms such as PAOs are more tolerant to MPs and engineered NPs [
31,
68,
69]. Qi et al. [
70] noted that PLA MPs could act as biocarriers while releasing small-molecule organic matter as a slow-release carbon source. Therefore, the addition of PLA MPs may have changed the competition between PAOs and GAOs, so that the potential PAOs (e.g.,
Acinetobacter) gradually gain advantages, which may be related to their specific carbon usage and metabolic characteristics.
The relative abundance of known AOB (i.e.,
Nitrosomonas) and NOB (i.e.,
Nitrospira) was less than 0.1% in all SBRs. The relative abundance of
OLB12, which has been reported to have nitrification capacity [
71], increased from 0.2% in R0 to 1.1% in R3, potentially contributing to nitrification performance in this study. Zhang et al. [
72] found that a large number of heterotrophic DNB belong to the genus
Pseudomonas. The relative abundance of
Pseudomonas was 95.4% in R0, while it gradually decreased in SBRs with the addition of PLA MPs, reaching 59.0% in R3. The relative abundance of
Acidovorax and
Azoarcus, which also possess denitrification capacity [
73,
74], largely increased from 0.03% and 0.29% in R0, respectively, to 8.47% and 2.86% in R3, respectively. However, the increased involvement and contribution of
Acidovorax and
Azoarcus was not sufficient to compensate for the decrease in
Pseudomonas abundance, ultimately leading to depressed denitrification performance in the SBRs with PLA MP exposure. Further studies are warranted to better understand the underlying inhibition mechanisms.
5. Conclusions
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
(1) The low concentration of PLA MPs (50 particles/(g TS)) had little effect on the pollutant removal performance in the activated sludge system. High concentration of PLA MPs (200 particles/(g TS)) reduced TN removal performance by 5.5%, while improved P removal performance by 3.9%.
(2) The addition of PLA MPs caused various effects on the activity of specific functional microorganisms such as AOB, NOB and PAOs. AOB was more sensitive to PLA MP exposure than NOB. Increased PLA MP dosage also resulted in declined total EPS content in sludge flocs.
(3) PLA MPs can be used as biocarriers and slow-release carbon sources to enrich or screen functional microorganisms. With the increase in PLA MP dosage, the relative abundance of potential heterotrophic PAO (Acinetobacter) increased while the relative abundance of denitrifying bacteria (Pseudomonas) decreased, which in turn affected the P and N removal performance.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Figure S1: SBRs used in this study; Figure S2: Profiles of PO
43--P and COD during EBPR activity batch tests with the sludge collected from (a) R0, (b) R1, (c) R2, and (d) R3; Figure S3: Profiles of NH
4+-N, NO
2--N and NO
3--N during nitrification activity batch tests with the sludge collected from (a) R0, (b) R1, (c) R2, and (d) R3; Figure S4: Common and unique ASVs in different samples. Table S1: Composition and concentration of trace elements in synthetic wastewater; Table S2: Alpha diversity indices.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, D.W., M.F.; methodology, M.H., D.W., S.Z. and K.L.; software, M.H., S.Z. and K.L.; validation, S.Z., M.H., K.L., and Y.W.; formal analysis, S.Z., M.H., K.L., and Y.W.; investigation, S.Z., M.H., K.L., and Y.W.; resources, M.F. and J.L.; data curation, M.H., S.Z.; writing—original draft preparation, M.H.; writing—review and editing, D.W.; visualization, M.H.; supervision, D.W.; project administration, D.W.; funding acquisition, D.W. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52070156), the Open Research Project of State Key Laboratory of Eco-Hydraulics in Arid Northwest China (2019KFKT-10), the Scientific Research Project for Returned Overseas Scholars in Shaanxi Province of China, and "Scientists+Engineers" Team Construction Based on QinChuangYuan Platform, Shaanxi Province (No. 2022KXJ-115).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mengjie, Z.; Yanxiao, C.; Tiantian, C.; Honghu, L.; Yifei, T.; Wenbo, F.; Yuwei, X.; Ye, T.; Jingcheng, Z. Characteristics and source-pathway of microplastics in freshwater system of China: A review. Chemosphere 2022, 297. [Google Scholar] [CrossRef]
- Cristina, C.M.; Marco, C.M.; Maria, C.F.; Chiara, M. Microplastics in Sewage Sludge: A Known but Underrated Pathway in Wastewater Treatment Plants. Sustainability 2021, 13. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, L.; Cizdziel, J.; Huang, Y. Research progress on microplastics in wastewater treatment plants: A holistic review. Journal of Environmental Management 2023, 325, 116411. [Google Scholar] [CrossRef] [PubMed]
- Chang-Bum, J.; Eun-Ji, W.; Hye-Min, K.; Min-Chul, L.; Dae-Sik, H.; Un-Ki, H.; Bingsheng, Z.; Sami, S.; Su-Jae, L.; Jae-Seong, L. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environmental science & technology 2016, 50. [Google Scholar] [CrossRef]
- M, R.C.; Eunha, H.; T, H.B.; Shawn, K. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environmental science & technology 2013, 47. [Google Scholar] [CrossRef]
- Wei, W.; Qi-Su, H.; Jing, S.; Jun-Yue, W.; Shu-Lin, W.; Bing-Jie, N. Polyvinyl Chloride Microplastics Affect Methane Production from the Anaerobic Digestion of Waste Activated Sludge through Leaching Toxic Bisphenol-A. Environmental science & technology 2019, 53. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnology Advances 2010, 28. [Google Scholar] [CrossRef]
- Yanjiao, H.; Lu, L.; Kang, S.; Qun, L.; Zhouyang, L.; Fazhi, X.; Xiaoli, Z. Effect of microplastic particle size to the nutrients removal in activated sludge system. Marine Pollution Bulletin 2021, 163. [Google Scholar] [CrossRef]
- Bing, Z.; Shuchang, H.; Lian, W.; Yuan, G.; Wenxin, S.; N.L., L.P. Micro(nano)plastic size and concentration co-differentiate the treatment performance and toxicity mechanism in aerobic granular sludge systems. Chemical Engineering Journal 2023, 457. [Google Scholar] [CrossRef]
- Wang, M.J.; Li, Q.Q.; Shi, C.Z.; Lv, J.; Xu, Y.D.; Yang, J.J.; Chua, S.L.; Jia, L.R.; Chen, H.W.; Liu, Q.; et al. Oligomer nanoparticle release from polylactic acid plastics catalysed by gut enzymes triggers acute inflammation. NATURE NANOTECHNOLOGY 2023. [Google Scholar] [CrossRef]
- Havstad, M.R. Chapter 5 - Biodegradable plastics. In Plastic Waste and Recycling, Letcher, T.M., Ed.; Academic Press: 2020; pp. 97-129. [CrossRef]
- Döhler, N.; Wellenreuther, C.; Wolf, A. Market dynamics of biodegradable bio-based plastics: Projections and linkages to European policies. EFB Bioeconomy Journal 2022, 2, 100028. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K.; Kowalczuk, M. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials 2016, 9. [Google Scholar] [CrossRef]
- A., A.R.; Tak, L.L.; M., S.S.E.; Hideto, T. Poly(Lactic Acid):Synthesis, Structures, Properties, Processing, Applications, and End of Life; John Wiley & Sons, Inc.; p. 2022.
- V., A.; Abhispa, B.; Nallathambi, S.; Kumar, P.; Govarthanan, M.; A., A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste - Its applications and degradation. International Journal of Biological Macromolecules 2023, 234. [Google Scholar] [CrossRef]
- Vieira, T.L.; Vitor, B.J.; Almeida, O.F.d.; Andrade, C.P.L.d. The Diffusion of Bioplastics: What Can We Learn from Poly(Lactic Acid)? Sustainability 2023, 15. [Google Scholar] [CrossRef]
- Wang, D.; Tooker, N.B.; Srinivasan, V.; Li, G.; Fernandez, L.A.; Schauer, P.; Menniti, A.; Maher, C.; Bott, C.B.; Dombrowski, P.; et al. Side-stream enhanced biological phosphorus removal (S2EBPR) process improves system performance - A full-scale comparative study. Water Research 2019, 167. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Research 2018, 133. [Google Scholar] [CrossRef]
- Weiyi, L.; Jinlan, Z.; Hang, L.; Xiaonan, G.; Xiyue, Z.; Xiaolong, Y.; Zhiguo, C.; Tingting, Z. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environment International 2021, 146. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Current Opinion in Environmental Science & Health 2020, 14. [Google Scholar] [CrossRef]
- Lv, T.; Wang, D.; Hui, J.; Cheng, W.; Ai, H.; Qin, L.; Huang, M.; Feng, M.; Wu, Y. Effect of return activated sludge diversion ratio on phosphorus removal performance in side-stream enhanced biological phosphorus removal (S2EBPR) process. Environmental research 2023, 116546–116546. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, J.; Xu, P.; Liu, Y.; Jing, X.; Liu, L.; Zhang, Z.; Qin, L.; Chai, G.; Lv, T.; et al. Performance and microbial dynamics of side-stream activated sludge hydrolysis process at different influent food-to-microorganism (F/M) ratios. INTERNATIONAL JOURNAL OF ENVIRONMENTAL SCIENCE AND TECHNOLOGY 2022. [Google Scholar] [CrossRef]
- Xiaojun, F.; Yishi, Q.; Peng, X.; Rui, C.; Lu, Q.; Shengwei, Z.; Guodong, C.; Mengbo, H.; Kailong, L.; Yi, X.; et al. Partial Nitrification and Enhanced Biological Phosphorus Removal in a Sequencing Batch Reactor Treating High-Strength Wastewater. International Journal of Environmental Research and Public Health 2022, 19. [Google Scholar] [CrossRef]
- Standard methods for the examination of water and wastewater. Choice Reviews Online 2012, 49.
- Li, H.; Wen, Y.; Cao, A.; Huang, J.; Zhou, Q. The influence of multivalent cations on the flocculation of activated sludge with different sludge retention times. Water Research 2014, 55. [Google Scholar] [CrossRef] [PubMed]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Research 1996, 30. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 2002, 28. [Google Scholar] [CrossRef]
- Oehmen, A.; Keller-Lehmann, B.; Zeng, R.J.; Yuan, Z.; Keller, J. Optimisation of poly-β-hydroxyalkanoate analysis using gas chromatography for enhanced biological phosphorus removal systems. Journal of Chromatography A 2005, 1070. [Google Scholar] [CrossRef]
- Dai, H.-H.; Gao, J.-F.; Wang, Z.-Q.; Zhao, Y.-F.; Zhang, D. Behavior of nitrogen, phosphorus and antibiotic resistance genes under polyvinyl chloride microplastics pressures in an aerobic granular sludge system. Journal of Cleaner Production 2020, 256. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polymer Engineering & Science 2020, 60. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Ding, W.; Zhang, Z.; Nghiem, L.D.; Sun, J.; Wang, Q. Do Microplastics Affect Biological Wastewater Treatment Performance? Implications from Bacterial Activity Experiments. ACS Sustainable Chemistry & Engineering 2019, 7. [Google Scholar] [CrossRef]
- Li, L.; Song, K.; Yeerken, S.; Geng, S.; Liu, D.; Dai, Z.; Xie, F.; Zhou, X.; Wang, Q. Effect evaluation of microplastics on activated sludge nitrification and denitrification. Science of the Total Environment 2020, 707. [Google Scholar] [CrossRef]
- Gabriella, C.; Cristina, P.; Simone, C.; Marcella, L.; Rosabruna, L.F.; Angelina, L.G.; Giulia, M.; Carmen, R.; Giovanna, M.; Ciro, R.A.; et al. Effects of microplastics on trophic parameters, abundance and metabolic activities of seawater and fish gut bacteria in mesocosm conditions. Environmental science and pollution research international 2018, 25. [Google Scholar] [CrossRef]
- J, S.A.; David, J. Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, Part III: Anaerobic sources of reducing equivalents. Water environment research : a research publication of the Water Environment Federation 2003, 75. [Google Scholar] [CrossRef]
- Francesca, D.P.; Simona, C.; Caterina, L.; Luca, V.; Maria, S.; Loris, P.; Stefania, D.V.; Stefano, A.; Simona, R. Plastisphere in lake waters: Microbial diversity, biofilm structure, and potential implications for freshwater ecosystems. Environmental pollution (Barking, Essex : 1987) 2022, 310. [Google Scholar] [CrossRef]
- Zhou, Y.; Pijuan, M.; Zeng, R.J.; Lu, H.; Yuan, Z. Could polyphosphate-accumulating organisms (PAOs) be glycogen-accumulating organisms (GAOs)? Water Research 2008, 42. [Google Scholar] [CrossRef] [PubMed]
- Rikke, K.; Thu, N.H.T.; Marc, S.A.; Lund, N.J.; Reinhard, W.; Quy, L.V.; Jon, M.S.; Steve, P.; J, S.R.; Alexandra, C.; et al. A metabolic model for members of the genus Tetrasphaera involved in enhanced biological phosphorus removal. The ISME journal 2013, 7. [Google Scholar] [CrossRef]
- Adrian, O.; J, Z.R.; Zhiguo, Y.; Jürg, K. Anaerobic metabolism of propionate by polyphosphate-accumulating organisms in enhanced biological phosphorus removal systems. Biotechnology and bioengineering 2005, 91. [Google Scholar] [CrossRef]
- Lanham, A.B.; Oehmen, A.; Saunders, A.M.; Carvalho, G.; Nielsen, P.H.; Reis, M.A.M. Metabolic versatility in full-scale wastewater treatment plants performing enhanced biological phosphorus removal. Water Research 2013, 47. [Google Scholar] [CrossRef]
- Z, G.A.; A, S.; B, N.J.; D, S.H.; L, B.L. Functionally relevant microorganisms to enhanced biological phosphorus removal performance at full-scale wastewater treatment plants in the United States. Water environment research : a research publication of the Water Environment Federation 2008, 80. [Google Scholar] [CrossRef]
- Qiu, G.; Zuniga-Montanez, R.; Law, Y.; Thi, S.S.; Nguyen, T.Q.N.; Eganathan, K.; Liu, X.; Nielsen, P.H.; Williams, R.B.H.; Wuertz, S. Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources. Water Research 2019, 149. [Google Scholar] [CrossRef]
- Annalisa, O.-H.; Varun, S.; B, T.N.; Guangyu, L.; Dongqi, W.; L, B.J.; Charles, B.; Paul, D.; Peter, S.; Adrienne, M.; et al. Survey of full-scale sidestream enhanced biological phosphorus removal (S2EBPR) systems and comparison with conventional EBPRs in North America: Process stability, kinetics, and microbial populations. Water environment research : a research publication of the Water Environment Federation 2020, 92. [Google Scholar] [CrossRef]
- Yu, N.; Zhao, C.; Ma, B.; Li, S.; She, Z.; Guo, L.; Zhang, Q.; Zhao, Y.; Jin, C.; Gao, M. Impact of ampicillin on the nitrogen removal, microbial community and enzymatic activity of activated sludge. Bioresource Technology 2019, 272, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Yang, L.; Changwei, N.; Yueyue, W.; Bing, W.; Yaohui, L.; Liming, G.; Zichao, W. Long-term exposure of polytetrafluoroethylene-nanoplastics on the nitrogen removal and extracellular polymeric substances in sequencing batch reactor. Enzyme and Microbial Technology 2023, 166. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiology and Biochemistry 2017, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fei, X.; Chi, Y.; Jiao, X.; Wang, L. Integrated temperature and DO effect on the lab scale A2O process: Performance, kinetics and microbial community. International Biodeterioration & Biodegradation 2018, 133. [Google Scholar] [CrossRef]
- Qian, Y.; Dang-Cong, P. Nitrite oxidizing bacteria (NOB) dominating in nitrifying community in full-scale biological nutrient removal wastewater treatment plants. AMB Express 2017, 7. [Google Scholar] [CrossRef]
- Agrawal, S.; Seuntjens, D.; Cocker, P.D.; Lackner, S.; Vlaeminck, S.E. Success of mainstream partial nitritation/anammox demands integration of engineering, microbiome and modeling insights. Current Opinion in Biotechnology 2018, 50, 214–221. [Google Scholar] [CrossRef]
- Wei, W.; Hao, Q.; Chen, Z.; Bao, T.; Ni, B.-J. Polystyrene nanoplastics reshape the anaerobic granular sludge for recovering methane from wastewater. Water Research 2020, 182. [Google Scholar] [CrossRef]
- Qidong, T.; Minghuo, W.; Yuelin, Z.; Jingzhe, L.; Jinxuan, L.; Hao, Z.; Yuanyuan, Q.; Xuwang, Z. Performance and bacterial community profiles of sequencing batch reactors during long-term exposure to polyethylene terephthalate and polyethylene microplastics. Bioresource technology 2021, 347. [Google Scholar] [CrossRef]
- Hans-Curt, F.; Jost, W. The biofilm matrix. Nature reviews. Microbiology 2010, 8. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Y.; Esquivel-Elizondo, S.; Sørensen, S.J.; Rittmann, B.E. How Microbial Aggregates Protect against Nanoparticle Toxicity. Trends in Biotechnology 2018, 36. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Cheng, Y.-F.; Liu, Y.-Y.; Zhang, Q.; Zhu, B.-Q.; Jin, R.-C. Deciphering the evolution characteristics of extracellular microbial products from autotrophic and mixotrophic anammox consortia in response to nitrogen loading variations. Environment International 2019, 124. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Minggang, Z.; Aifeng, L.; Ling, W.; Xin, P.; Jun, L.; Xiangbin, R. Effects of emerging contaminants and heavy metals on variation in bacterial communities in estuarine sediments. The Science of the total environment 2022, 832. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.-T.; Huang, Q.-S.; Ni, B.-J. Polyethylene terephthalate microplastics affect hydrogen production from alkaline anaerobic fermentation of waste activated sludge through altering viability and activity of anaerobic microorganisms. Water Research 2019, 163. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Yupeng, H.; Dehui, K.; Wei, Y.; Wei, T.; Qingkong, C.; Sisi, Q.; Xiaofei, Z.; Deqiang, Z. Factors Impacting Microplastic Biofilm Community and Biological Risks Posed by Microplastics in Drinking Water Sources. Water, Air, & Soil Pollution 2022, 233. [Google Scholar] [CrossRef]
- Hui, D.; Qianqian, F.; Dazhen, L.; Yuqing, Z.; Jianxiong, H.; Dan, F.; Yuanyuan, Z.; Gan, D.; Huamei, Y.; Chengjun, G. Microplastic-associated biofilm in an intensive mariculture pond: Temporal dynamics of microbial communities, extracellular polymeric substances and impacts on microplastics properties. Journal of Cleaner Production 2021, 319. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Mielczarek, A.T.; Kragelund, C.; Nielsen, J.L.; Saunders, A.M.; Kong, Y.; Hansen, A.A.; Vollertsen, J. A conceptual ecosystem model of microbial communities in enhanced biological phosphorus removal plants. Water Research 2010, 44. [Google Scholar] [CrossRef] [PubMed]
- Tiantian, S.; Rui, D.; Qiongpeng, D.; Ying, L.; Yongzhen, P. Rapidly achieving partial nitrification of municipal wastewater in enhanced biological phosphorus removal (EBPR) reactor: Effect of heterotrophs proliferation and microbial interactions. Bioresource Technology 2021, 340. [Google Scholar] [CrossRef]
- Bing, Y.; Song, X.; ShuangLin, G.; JiaQi, F.; JiuJiu, W.; JiHai, X.; YuanSong, W. [Microbial Community Structure and Diversity During the Enrichment of Anaerobic Ammonium Oxidation Bacteria]. Huan jing ke xue= Huanjing kexue 2020, 41. [Google Scholar] [CrossRef]
- E, L.C.; J, S.B.; W, H.N.; S, H.A.; R, H.E.; Barry, R.; S, M.D.; D, R.W.; J, H.S. Rare taxa have potential to make metabolic contributions in enhanced biological phosphorus removal ecosystems. Environmental microbiology 2015, 17. [Google Scholar] [CrossRef]
- Nierychlo, M.; Andersen, K.S.; Xu, Y.; Green, N.; Jiang, C.; Albertsen, M.; Dueholm, M.S.; Nielsen, P.H. MiDAS 3: An ecosystem-specific reference database, taxonomy and knowledge platform for activated sludge and anaerobic digesters reveals species-level microbiome composition of activated sludge. Water Research 2020, 182. [Google Scholar] [CrossRef]
- Qin, L.; Wang, D.Q.; Zhang, Z.; Li, X.X.; Chai, G.D.; Lin, Y.S.; Liu, C.; Cao, R.; Song, Y.X.; Meng, H.Y.; et al. Impact of Dissolved Oxygen on the Performance and Microbial Dynamics in Side-Stream Activated Sludge Hydrolysis Process. WATER 2023, 15. [Google Scholar] [CrossRef]
- Zafiri, C.; Kornaros, M.; Lyberatos, G. Kinetic modelling of biological phosphorus removal with a pure culture of Acinetobacter sp. under aerobic, anaerobic and transient operating conditions. Water Research 1999, 33. [Google Scholar] [CrossRef]
- Hisao, O.; Kenji, T.; Yoshiaki, T.; Kiyoshi, T. Uptake and release of phosphate by a pure culture of Acinetobacter calcoaceticus. Water Research 1985, 19. [Google Scholar] [CrossRef]
- Parnian, I.; Parin, I.; Ahmed, E. Evaluation of PAO adaptability to oxygen concentration change: Development of stable EBPR under stepwise low-aeration adaptation. Chemosphere 2022, 286. [Google Scholar] [CrossRef]
- Weon, S.-Y.; Lee, C.-W.; Lee, S.-I.; Koopman, B. Nitrite inhibition of aerobic growth of Acinetobacter sp. Water Research 2002, 36. [Google Scholar] [CrossRef]
- Chen, Y.; Su, Y.; Zheng, X.; Chen, H.; Yang, H. Alumina nanoparticles-induced effects on wastewater nitrogen and phosphorus removal after short-term and long-term exposure. Water Research 2012, 46. [Google Scholar] [CrossRef]
- Fu, S.-F.; Ding, J.-N.; Zhang, Y.; Li, Y.-F.; Zhu, R.; Yuan, X.-Z.; Zou, H. Exposure to polystyrene nanoplastic leads to inhibition of anaerobic digestion system. Science of the Total Environment 2018, 625. [Google Scholar] [CrossRef] [PubMed]
- Dan-Qi, T.; Juan, W.; Tian-Long, Z.; Jian-Guo, L.; Qun-Hui, W. [Effect of PLA/starch slow-release carbon source on biological denitrification]. Huan jing ke xue= Huanjing kexue 2014, 35. [Google Scholar]
- Chu, Z.-r.; Wang, K.; Li, X.-k.; Zhu, M.-t.; Yang, L.; Zhang, J. Microbial characterization of aggregates within a one-stage nitritation–anammox system using high-throughput amplicon sequencing. Chemical Engineering Journal 2015, 262. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, P.; Hao, B.; Yu, Z. Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Bioresource Technology 2011, 102. [Google Scholar] [CrossRef]
- Mergaert, J.; Boley, A.; Cnockaert, M.C.; Müller, W.-R.; Swings, J. Identity and Potential Functions of Heterotrophic Bacterial Isolates from a Continuous-Upflow Fixed-Bed Reactor for Denitrification of Drinking Water with Bacterial Polyester as Source of Carbon and Electron Donor. Systematic and Applied Microbiology 2001, 24. [Google Scholar] [CrossRef] [PubMed]
- Timothé, P.; Jianghao, T.; Chrystelle, B.; Cédric, C.; Cédric, M.; Julien, T.; Théodore, B.; Frédéric, B. Denitrifying bio-cathodes developed from constructed wetland sediments exhibit electroactive nitrate reducing biofilms dominated by the genera Azoarcus and Pontibacter. Bioelectrochemistry 2021, 140. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).