1. Introduction
Articular cartilage is a highly specialized tissue that is essential for smooth joint movement and load transmission [
1]. Healthy articular cartilage is an avascular and aneural tissue that is mainly composed of a proteoglycan rich extracellular matrix (ECM), collagen type II and chondrocytes [
2]. Cartilage has limited ability to self-heal when damaged and the current clinical cell-based therapies available include Articular Chondrocytes Implantation (ACI) and Matrix-Associated Autologous Chondrocyte Implantation (MACI) [
3]. ACI is a two-step surgical procedure, in which a piece of cartilage is taken from a non-load bearing area of the damaged knee joint, from either the intercondylar notch or the superior ridge of the medial or lateral femoral condyle of the patient [
4]. Next chondrocytes are enzymatically isolated from the cartilage and expanded in vitro in monolayer for 4 to 6 weeks until a sufficient number of cells is available to be implanted into the defect site. Given that chondrocytes represent only 2% of articular cartilage volume, the expansion procedure is essential in order to augment the limited number of isolated chondrocytes [
5,
6]. However, the main disadvantage of this procedure is that chondrocytes when expanded in vitro in 2D tend to lose their original phenotype and acquire a more fibroblastic appearance [
7]. The dedifferentiation process is characterised by morphological changes as well as shifts in protein synthesis and gene expression, decreased cell proliferation, increased apoptosis, and cell senescence [
8]. De-differentiated chondrocytes are characterized by reduced production of cartilage-specific constituents such as collagen type II, aggrecan, and proteoglycan; whilst the production of non-specific cartilage constituents such as collagen type I are increased and this leads to biomechanically inferior articular cartilage [
9,
10]. De-differentiated chondrocytes showed reduced ability to form cartilage tissue
in vivo and de-differentiation was found to elevate the postoperative failure rate in patients after ACI [
11]. MACI is a tissue engineering strategy to repair articular cartilage that involves the use of a biopolymer membrane as a temporary scaffold to support chondrocytes adhesion, proliferation and to improve matrix deposition [
12,
13]. However, MACI still fails to prevent the formation of fibrocartilage and the integration of the cells into surrounding healthy hyaline cartilage is unsatisfactory [
14].
Current strategies to prevent de-differentiation involve culturing chondrocytes in high cell density with the addition of growth factors (e.g. TGF-β) [
8,
15,
16,
17]. Bianchi et al., (2017) showed that when passaged chondrocytes were cultured in vitro in high density in the presence of TGF-β3 they formed a cartilage-like tissue without acquiring a hypertrophic phenotype. However, a later in vivo study showed that using TGF-β3 treated chondrocytes did not enhance cartilage repair instead this led to the formation of granulation tissue. Interestingly, the authors showed that when using dedifferentiated chondrocytes, not cultured with TGF-β3, the fibrocartilaginous tissue that was produced contained more collagen type II and aggrecan compared to the tissue formed by TGF-β3 treated chondrocytes [
19]. A study by Chen et al. (2017) investigated the effect of TGF-β1 on cultured human chondrocytes and revealed that genes involved in chondrocytes hypertrophy (COL10A1), blood vessel formation (Endothelial cell-specific molecule 1 (ESM1), vascular endothelial growth factor receptor 2 (KDR/VEGFR2) and vascular growth factor (VEGF)) were significantly upregulated. It is also important to consider that cells in healthy joint tissues are not subject to high levels of active TGF-β [
20,
21]. On the other hand, permanent and high levels of active TGF-β were detected in OA joints [
22]. Exposing chondrocytes to high and sustained levels of TGF-β has been shown to preferentially activate the SMAD 1/5/8 pathway which drives chondrocytes in the direction of hypertrophy. As a consequence the effect of TGF-β on articular cartilage will lead to the expression of hypertrophic markers and the production of collagen type I [
23,
24,
25,
26,
27] resulting in the development of fibrocartilage rather than hyaline cartilage. As chondrocytes are the only cell type approved for cell-based therapies for articular cartilage repair, strategies to prevent or limit their de-differentiation are required. Previous studies have provided evidence that the three-dimensional (3D) environment has a positive impact on various chondrogenic markers [
28,
29,
30,
31,
32,
33]. Consequently, utilizing scaffolds and hydrogels to promote the re-differentiation of de-differentiated chondrocytes appears to be a promising approach for preserving the chondrocyte phenotype [
34,
35,
36,
37,
38,
39,
40,
41].
Hydrogels are hydrophilic, crosslinked polymeric networks that exhibit a unique combination of properties similar to the natural ECM, such as high water content, biodegradability and biocompatibility. Furthermore, hydrogels offer a suitable environment for cell migration, proliferation, and adhesion. Our previous study showed the potential of collagen and alginate hydrogels with a stiffness of 5.75 kPa in promoting chondrogenesis in ovine mesenchymal stem cells. Remarkably, this chondrogenic differentiation was achieved without the addition of growth factors, leading to minimal collagen type I deposition.[
42] These findings strongly suggest that collagen and alginate hydrogels with a stiffness of 5.75 kPa possess the capability to serve as effective scaffolds for facilitating chondrocyte re-differentiation, eliminating the reliance on exogenous growth factors. In this study we investigated the effect of hydrogels on the re-differentiation of chondrocytes which is important as chondrocytes are the only cell type approved for cell-based therapies for articular cartilage repair.
2. Materials and Methods
2.1. Materials
Collagen from calf skin, glacial acetic acid (≥ 99%), sodium hydroxide ≥ 97.0% pellets, bovine serum albumin, low viscosity alginic acid sodium salt from brown algae, hematoxylin Solution Mayer′s, eosin Y solution (alcoholic), collagenase from Clostridium histolyticum, DPX mountant for histology, calcium chloride anhydrous BioReagent, ≥ 96.0% were purchased from Merck Life Science UK Limited, Gillingham, UK. Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) GlutaMAX™ Supplement, Foetal bovine serum (FBS), Hank's Balanced Salt Solution (HBSS), Gibco™ phosphate buffered saline (PBS, pH 7.4), Gibco™ Trypsin-EDTA (0.25%), phenol red, Gibco™ Penicillin-Streptomycin (10,000 U/mL), Invitrogen™ Ethidium Homodimer-1 (EthD-1), Invitrogen™ Calcein, AM, cell-permeant dye, phalloidin Dylight 550, Invitrogen™ CellTrace™ CFSE Cell Proliferation Kit, for flow cytometry, Molecular Probes™ PrestoBlue™ cell viability reagent, Epredia 125 ML OCT embedding cryoembedding matrix, Anti-Mouse IgG (whole molecule) F(ab′)2 fragment–FITC, Invitrogen™ DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride), 2-Methylbutane (isopentane), Epredia™ SuperFrostTM Microscope Slides, Ground 90°, 99+%, Extra Dry, AcroSeal™, Fisherbrand™ RNase-free disposable pellet pestles were purchased from Fisher, Loughborough, UK. Mouse anti sheep CD44 antibody, clone 25.32, Mouse anti sheep CD45 antibody, clone 1.11.32, SsoAdvanced Universal SYBR Green Supermix were purchased from Bio-Rad, Watford, UK. Anti-aggrecan antibody [6-B-4], anti-collagen II antibody, rabbit monoclonal [EPR7785] to Collagen I, goat anti-rabbit IgG H&L (Alexa Fluor® 594), recombinant anti-collagen VI antibody [EPR17072] were purchased from Abcam, Cambridge, UK. RNeasy Micro Kit, QIAzol Lysis Reagent, QIAshredder were purchased from Qiagen, Manchester UK. RTqPCR specific primers were purchased from Eurofins Genomics, Ebersberg, Germany.
2.2. Collagen alginate (col/alg) hydrogel preparation
Col/alg hydrogels were prepared as previously described [
42]. Briefly, a 1% w/v collagen type I collagen solution in acetic acid (2% v/v) was prepared and after adjusting the pH to 7.4 it was diluted with DMEM to a final concentration of 0.5% w/v. A 5% w/v alginate solution was prepared in calcium-free phosphate buffer solution (PBS 1X, pH = 7.4). The two solutions were then mixed in a 1:1 ratio and crosslinked by adding 50 µl of solution to 250 µl of 60 mM CaCl
2. Further incubation for 3 hours at 37 °C allowed complete collagen gelation.
2.3. Ovine chondrocytes isolation
Primary chondrocytes were isolated from sheep articular cartilage fragments (project licence number P16F4AA0A). Briefly, cartilage fragments were washed in PBS and digested overnight at 37 °C in collagenase from Clostridium histolyticum solution (1.5 mg/ml in DMEM/F12, supplemented with penicillin/streptomycin (1%)) under continuous agitation. The next day, collagenase solution was rinsed with PBS over a 70 μm cell strainer and the filtrate containing the cells was collected. Next, cells were harvested by centrifugation and plated (passage 0 – P0) at a density of 20,000 cells/cm2 in tissue culture flasks (25 cm2). Cells were cultured in a humidified atmosphere at 37 °C, 21% O2, and 5% CO2. After reaching confluency the cells were passaged 1:2 until P4. Chondrocytes at P4 were used for molecular analysis and for encapsulation procedures.
2.4. Hydrogel cytocompatibility
2.4.1. Cell encapsulation
Chondrocytes at passage 4 were used for all experiments. All experiments were performed in triplicate and chondrocytes isolated from articular cartilage from three different sheep were used for each experiment. Cells were incubated with trypsin-EDTA for 5 minutes, centrifuged, and resuspended in 1 mL of DMEM supplemented with 10% (v/v) FBS and counted using a haemocytometer. One million cells were pelleted by centrifugation (Eppendorf® Centrifuge 5702, Eppendorf UK LTD, Stevenage, UK). The supernatant was removed and the col/alg mixture was added to cell pellet and mixed gently to avoid bubble formation. Hydrogel formation was then achieved as described above.
2.4.2. Live/dead staining
Ethidium homodimer-1 and calcein-AM were used to evaluate the viability of chondrocytes encapsulated into the hydrogels at day 1, 7 and 14 following manufacturer's instructions. Briefly, cells washed with HBBS were treated with 2 μM calcein-AM and 4 μM ethidium homodimer-1 for 1 hour at 37 °C. Samples were rinsed two times with HBSS and imaged with a confocal microscope (LSM 880, Zeiss, Oberkochen, Germany) at 488 and 543 nm.
2.4.3. Cell proliferation
Chondrocytes were labelled with CellTrace carboxyfluorescein succinimidyl ester (CFSE). Cells treated with trypsin-EDTA (5 min), centrifuged, and resuspended in 1 mL of PBS 1X (without Ca2+ and Mg2+) were incubated with 1mL CellTrace CFSE (5 and 10 μM) in PBS 1X (20 min at 37 °C). Further incubation with DMEM/F12 supplemented with 5% (v/v) FBS (5 min at 37 °C) was used to remove unconjugated CellTrace. Cells were finally centrifuged, resuspended in DMEM/F12 supplemented with 10% (v/v) FBS and analysed as described below.
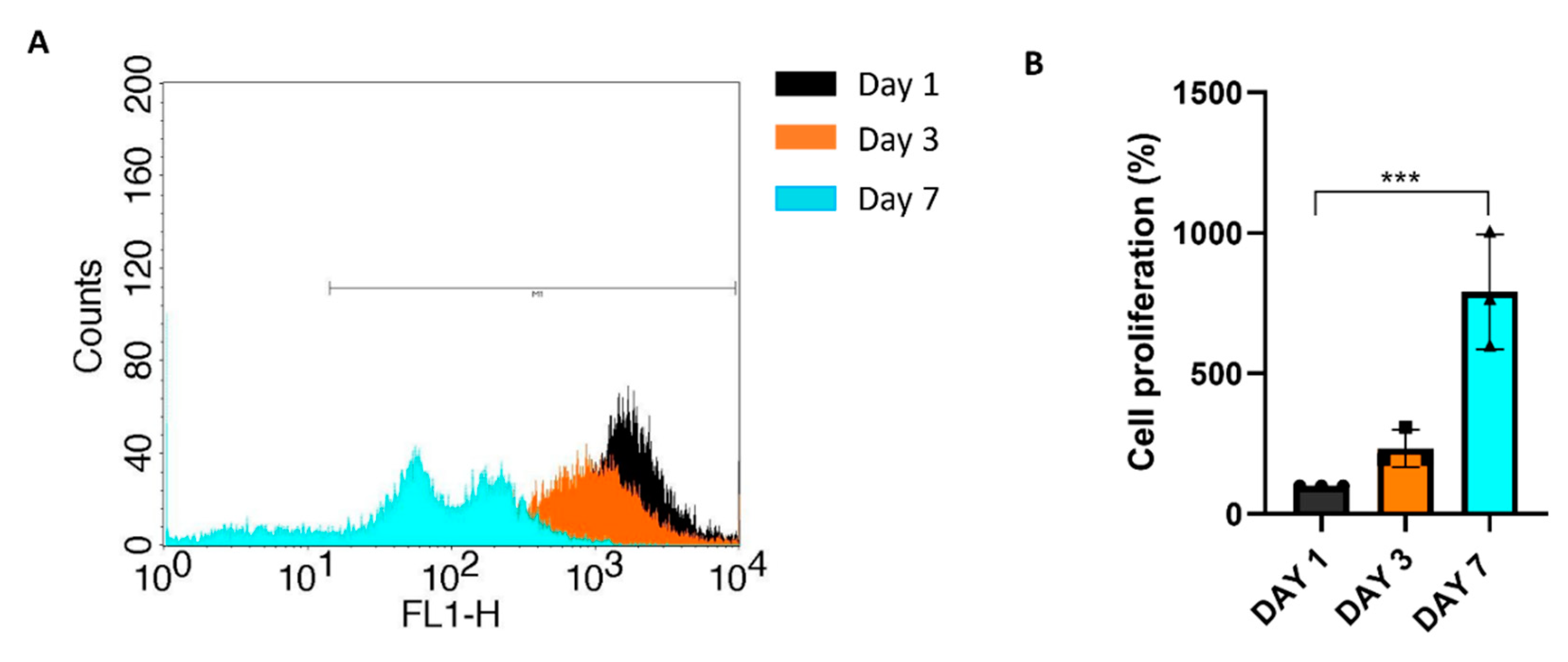
The evaluation of the efficacy of cell labelling was carried out by seeding CFSE+ chondrocytes (20,000 cells/cm2) in 24-well plates in DMEM supplemented with 10% (v/v) FBS and allowing them to grow for 7 days. The remaining percentage of labelled cells was quantified on days 1, 3, and 7 by flow cytometry. CFSE+ chondrocytes were detected using the 488 nm laser channel (FL1). Non-stained cells were included as a control. Cells were analysed in FACS Calibur (BD Biosciences, Wokingham, UK) and analysed using CellQuest software (BD Biosciences, Wokingham, UK).
A PrestoBlue assay was employed to assess the effect of CFSE on the metabolic activity of chondrocytes. Cells were seeded (20,000 cells/cm2) in a 24-well plate in DMEM supplemented with 10% (v/v) FBS and cultivated for 7 days. On day 1, 3 and 7 cells were analysed following the manufacturer's instruction. Non-stained cells were used as control in each experiment.
CFSE+ chondrocytes embedded into the col/alg hydrogels were cultivated for 1, 3 and 7 days then suspended in a 0.1 M EDTA and 0.5 M sodium citrate solution at 37 °C for 10 min. Samples were then centrifuged at 300 g for 5 min and resuspended in 500 µL of PBS. Cells were analysed in FACSCalibur flow cytometer collecting at least 10,000 events. CFSE+ chondrocytes were detected using the blue 488 nm laser channel (FL1) and non-stained cells were included as a control. CellQuest software (San Jose, California USA) was used for data analysis.
2.5. Cell morphology analysis
Cell morphology was assessed at P1, P4 and after embedding into col/alg hydrogels, using Phalloidin Dylight 550 (Sigma-Aldrich Company, Gillingham, UK), which selectively labels F-actin, was used. For 2D studies chondrocytes at P1 and P4 were seeded at a density of 30,000 cells/cm2 on a 12 mm coverslip. After 24 h cells were washed with PBS three times and fixed with 500 μL of 4% paraformaldehyde for 10 minutes. On day 7 hydrogels were washed with PBS three times and fixed with 500 μL of 4% paraformaldehyde for 1 h. Next chondrocytes were permeabilized with 0.2% Triton X-100 in PBS for 10 minutes and non-specific biding sites were blocked by incubating the cells or hydrogels in 2% BSA in PBS for 30 minutes. Chondrocytes, both in 2D and in the hydrogels were stained with Phalloidin Dylight 550 (2 units/ml, stock solution 300 units/ml in methanol) for 1 hour. Nuclei counterstaining was performed by incubating cells with DAPI (1:2000 dilution) for 15 min. Subsequently, chondrocytes were washed twice with PBS and imaged using confocal laser scanning microscope LSM880 at 405 and 543 nm. The aspect ratio of chondrocytes was measured using Image J software (National Instruments, Austin, US), for each sample three different images in three different areas were taken and the length and width of each cell in the area was measured. The aspect ratio was measured as the ratio of the length of a cell to its width.
2.6. RNA extraction and RTq-PCR
Hydrogels were homogenized with a pellet pestle (Thomas Fisher Scientific, USA) in 0.7 ml qiazol and centrifuged at 18,000 g for 2 min at room temperature. The supernatant was transferred into a fresh Qiashredder column (Qiagen, Switzerland) and centrifuged for 2 min at 18,000 g at room temperature. Total RNA was extracted using the RNeasy Micro Kit (Qiagen). RNA concentration and quality were measured using a NanoDrop (ND-1000; NanoDrop Technologies, Wilmington, DE, USA). Total RNA (250 ng from each sample) was reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™) on a thermal cycler (Biorad). qRT-PCR was performed in a QuantStudio™ 5 Real-Time PCR System (Applied Biosystems™) using SsoAdvanced Universal SYBR Green Supermix and ovine specific primers reported in
Table 1.
2.7. Immunostaining and histology
Hydrogels were fixed (day 21, 500 μL of 4% paraformaldehyde, 1 h) and rinsed three times with PBS. Fixed gels were incubated overnight in 30% w/v sucrose solution at 4°C to prevent freeze damage and then placed in optimal cutting temperature (OCT) compound (Tissue Tek, Torrance, CA) and then were frozen in an isopentane bath previously chilled in liquid nitrogen and stored at −80°C for at least one day before sectioning. Sections (20 μm) were cut with a cryostat (Leica CM3050 S Cryostat, Leica Biosystems, Milton Keynes, UK) and mounted on SuperFrost
TM Microscope Slides (Fisher Scientific, Loughborough, UK) and left to dry for 30 min. Slides were fixed in ice cold acetone (10 min at -20°C) and left to dry (30 min) before staining. Antigen retrieval was performed using TRIS/EDTA buffer (10 mM Tris base, 1 mM EDTA solution, 0.05% Tween 20, pH 9.0) at 95°C for 10 min. Samples were rinsed in PBS and incubated in a blocking buffer containing 2.5% w/v of BSA (30 min) before incubation with primary antibodies overnight at 4°C. Incubation with secondary antibodies for 1h at room temperature was performed after rinsing 3 times with PBS. Nuclei counterstaining was performed with DAPI (1:2000 dilution, 15 min). A full list of antibodies and their dilutions is reported in
Table 2. Samples were then washed three times with PBS and mounted with aqueous fluorescent mounting media and imaged using a confocal laser scanning microscope LSM880 at 405,488, 594 nm channels. For histology, slides were fixed with 70% ethanol and rinsed with deionized water. Slides were then stained for 90 seconds with filtered 0.1% Mayers Haematoxylin (Fisher Scientific, Loughborough, UK) followed by washing in tap water, 1% acetic alcohol and 70% ethanol. Slides were then stained with eosin (3 min) followed by washes in 70% and 100% ethanol and xylene. Samples were mounted with DPX and imaged using EVOS FL Auto 2 Cell Imaging System (Fisher Scientific, Loughborough, UK).
3. Results
3.1. Chondrocytes characterization
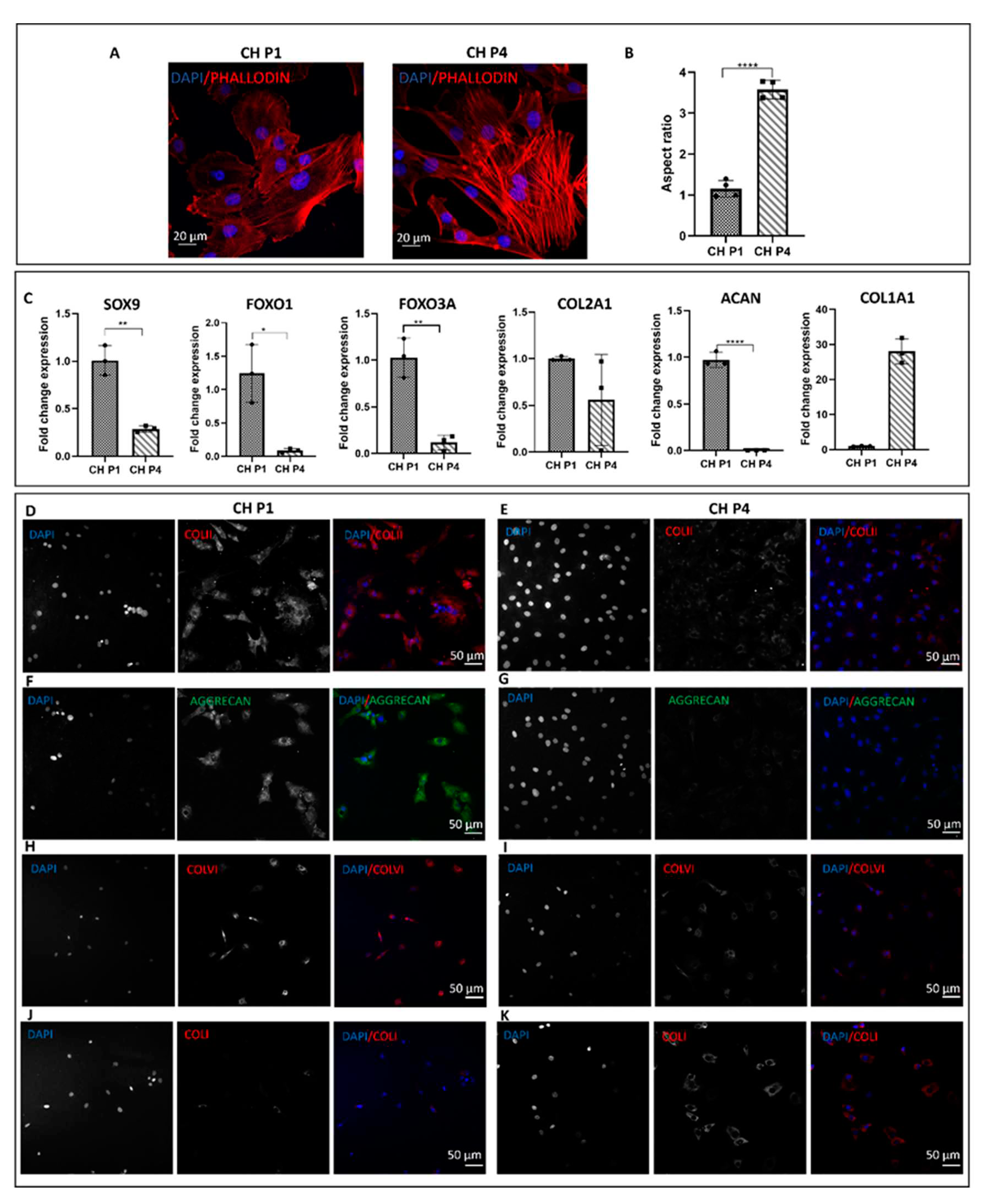
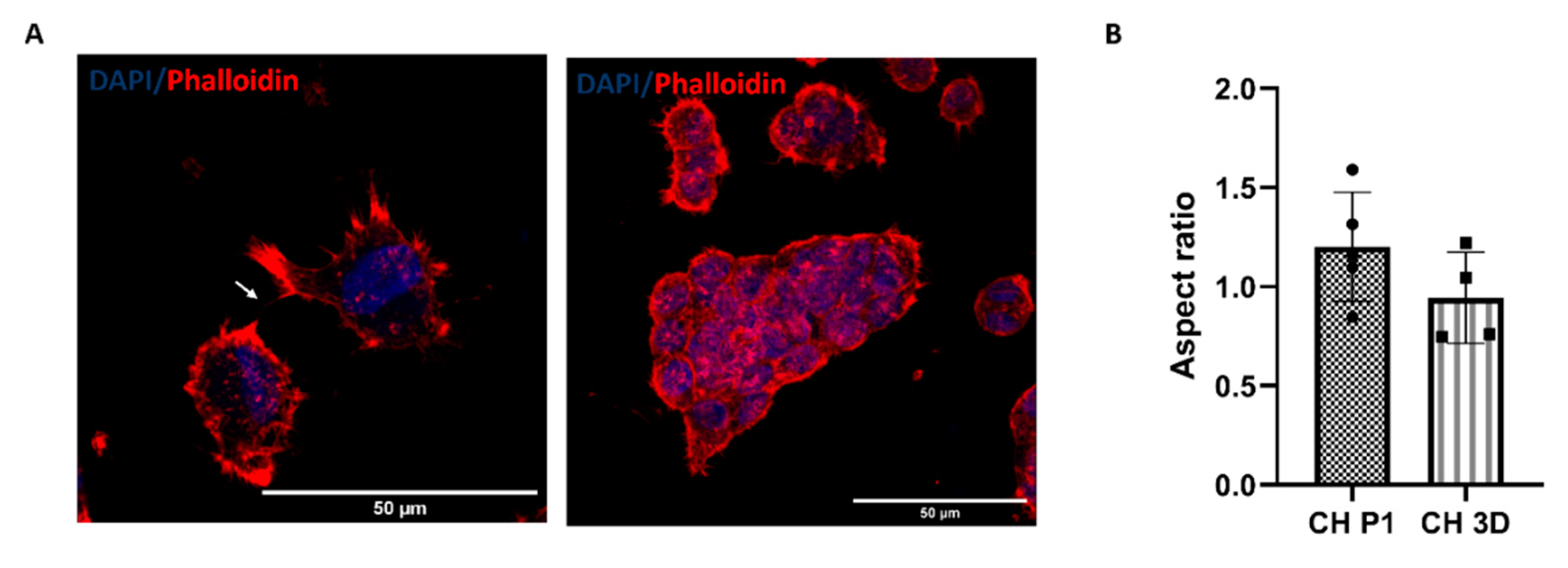
Chondrocytes were characterized at passages P1 and P4. The morphology of chondrocytes was assessed using phalloidin and DAPI staining. At P1, the chondrocytes exhibited the characteristic rounded morphology (
Figure 1A) and had an aspect ratio of 1.15 (
Figure 1B). However, when chondrocytes were grown in a 2D environment, they lost their original morphology and at P4 acquired a spindle-shaped appearance with abundant stress fibers and an aspect ratio of 3.57 (
Figure 1A,B). Molecular analysis was conducted to examine the expression of typical chondrogenic markers at different passages. At P1, the chondrocytes expressed markers such as SOX9, FOXO1, FOXO3A, ACAN, and COL2A1, indicative of their chondrogenic phenotype. However, in 2D, as passages increased, there was a significant reduction in the expression of chondrogenic markers and a notable increase in the expression of COL1A1, a marker associated with chondrocyte hypertrophy (
Figure 1C). Furthermore, passaging had an impact on the deposition of the cartilage extracellular matrix with a decrease in the deposition of components such as collagen type II and aggrecan, which are essential for maintaining cartilage structure and function. Immunofluorescence staining confirmed that at P1, the chondrocytes exhibited positive staining for collagen type II, aggrecan (p < 0.0001), and collagen type VI (
Figure 1D–K). However, at P4, a reduction in the deposition of ECM proteins was observed, accompanied by an increased deposition of collagen type I (
Figure 1D–K). This shift in matrix composition suggests a phenotypic change towards a more fibroblastic cell type.
3.2. Cytocompatibility of col/alg hydrogels
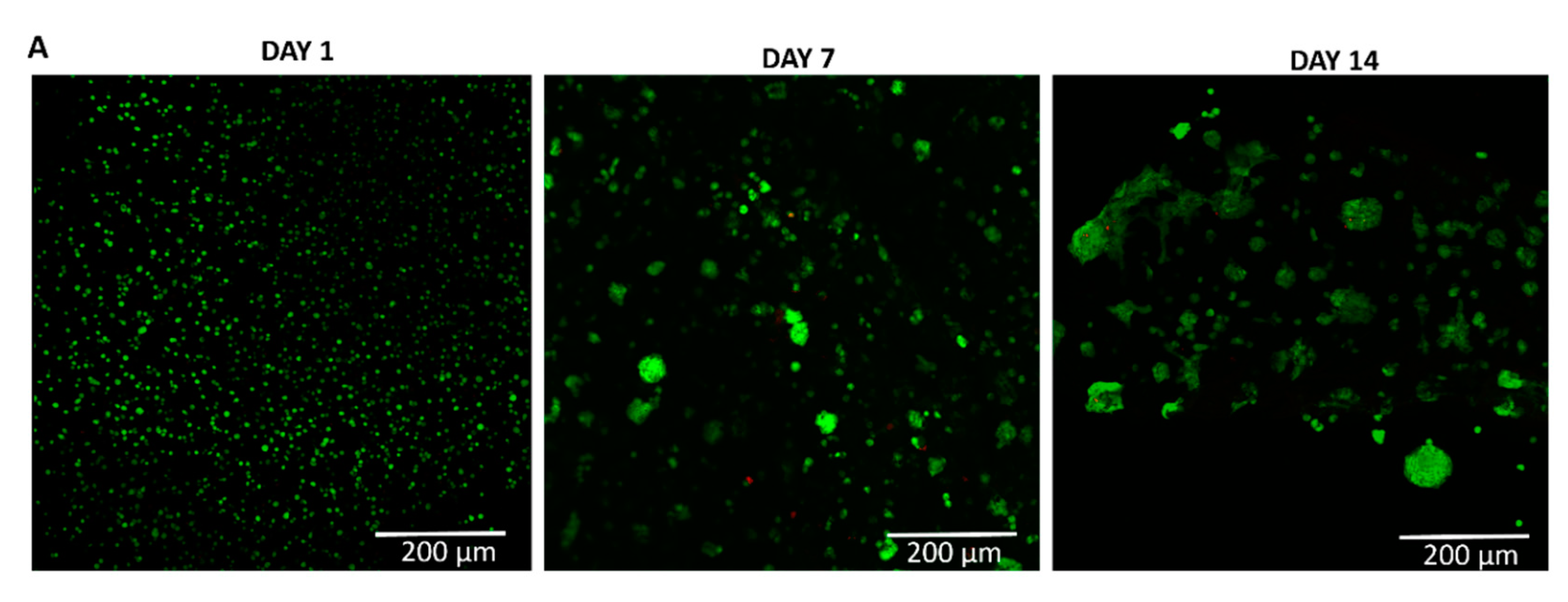
To evaluate the viability of chondrocytes at P4 within the collagen/alginate (col/alg) hydrogels, a live/dead assay was conducted at different time points (days 1, 7, and 14). The results, shown in
Figure 2, demonstrate that embedding P4 chondrocytes into the col/alg hydrogels did not have a detrimental effect on cell viability, as the cells remained viable within the hydrogels for up to 14 days. The distribution of chondrocytes within the col/alg hydrogels was evaluated over time. On day 1, the chondrocytes appeared to be uniformly dispersed throughout the hydrogels. However, as the culture period progressed, distinct changes became apparent. From day 7 onwards, chondrocytes started to rearrange themselves and form spherical colonies within the hydrogels. This was associated with cells migration which overall affected cell density in some areas of the hydrogels.
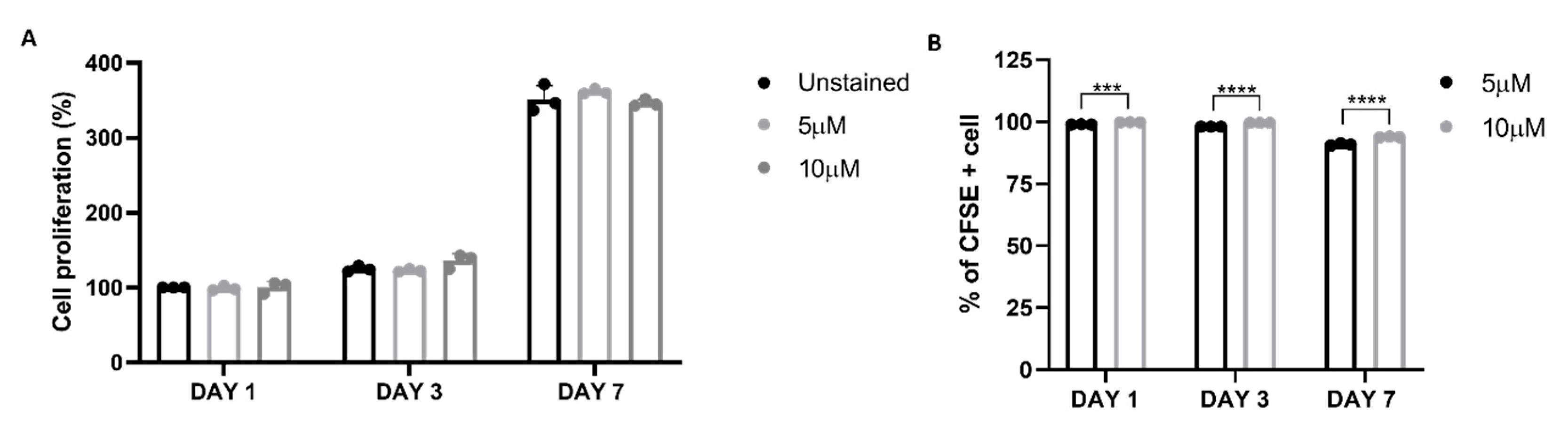
Cell proliferation was assessed using CFSE Celltrace staining, where a reduction of fluorescence overtime indicates the proliferation of chondrocytes. Initially, cell labelling optimization was conducted in 2D monolayer culture using two concentrations of CellTrace dye (5 and 10 µM). The viability and proliferation of cells labelled with CellTrace were compared to the unstained control, and it was determined that the use of CellTrace did not have an adverse effect on cell viability and proliferation, even at the highest concentration tested (
Figure 3A). To evaluate labelling efficacy the percentage of CFSE-positive cells was monitored for seven days using flow cytometry. After 7 days over 90% of cells were positive for CFSE staining, however the difference between the two staining concentrations was significant and the 10 µM was used to assess the proliferation of dedifferentiated chondrocytes into the col/alg hydrogels.
P4 chondrocytes were labelled with CFSE and then embedded into the col/alg hydrogel. The proliferation of these cells within the hydrogel was monitored over a 7-day period. Flow cytometry analysis revealed a decrease in fluorescence intensity from day 1 to day 7, indicating that the cells embedded in the hydrogel were undergoing proliferation (
Figure 4 A,B)
3.3. Morphology of P4 chondrocytes within col/alg hydrogels
Phalloidin and DAPI staining were used to evaluate the cellular morphology of P4 chondrocytes within the col/alg hydrogels after 7 days of culture.
Figure 5 shows that the chondrocytes regained their characteristic rounded morphology and did not present abundant stress fibers when cultured within the col/alg hydrogels. Furthermore, chondrocytes started to form interconnection and self-assembled into clusters. This observation highlights the ability of the col/alg hydrogels to support cellular interactions. The formation of interconnections and clusters suggests the initiation of cell-cell communication and the establishment of tissue-like structures, which are important aspects of chondrocyte re-differentiation and the development of functional cartilage tissue.
Quantitative analysis revealed that after 7 days of culture, the chondrocytes in the col/alg hydrogels exhibited an aspect ratio of 1.08, which was not significantly different from their original aspect ratio at P1. This indicates that the chondrocytes within the hydrogels maintained their characteristic rounded shape, resembling their native morphology.
3.4. Gene expression of P4 chondrocytes embedded into col/alg hydrogels
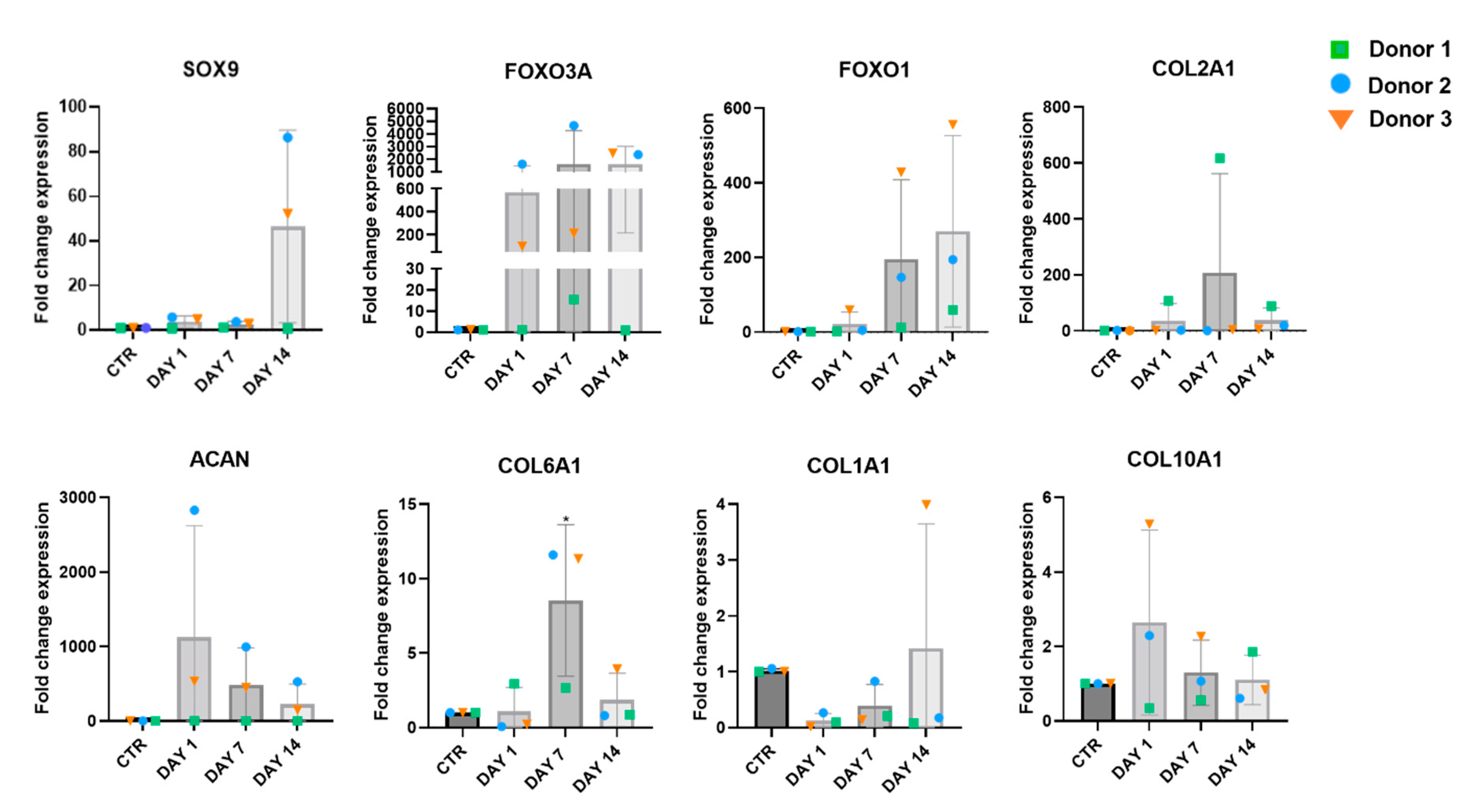
The gene expression analysis was performed on chondrocytes embedded into col/alg hydrogels on day 1, 7 and 14. P4 chondrocytes grown in 2D were used as control. Although the changes in the expression of the chondrogenic markers SOX9, FOXO1 and FOXO3A was not significant, a trend could be observed on day 14 (
Figure 6). In addition, the 3D culture affected the expression of ECM proteins, where at day 7 a significant increase in the expression COL6A1 was measured. Compared to the 2D controls cells, an increase of COL2A1 expression was measured in chondrocytes in the hydrogel at day 7 and 14, indicating the potential of the 3D culture to promote the production of cartilage-specific ECM protein. The 3D culture influenced the expression of hypertrophic markers, on day 1 and at day 7 a reduction in the expression of COL1A1 was observed. On day 1 an increase in gene expression of COL10A1 was observed, however on day 7 and 14 the expression of COL10A1 remained comparable to the 2D control. This may suggest that the 3D culture system stabilizes the expression of COL10A1 over time, potentially preventing further progression of chondrocyte hypertrophy.
3.5. Histology and immunostaining of P4 chondrocytes into col/alg hydrogels
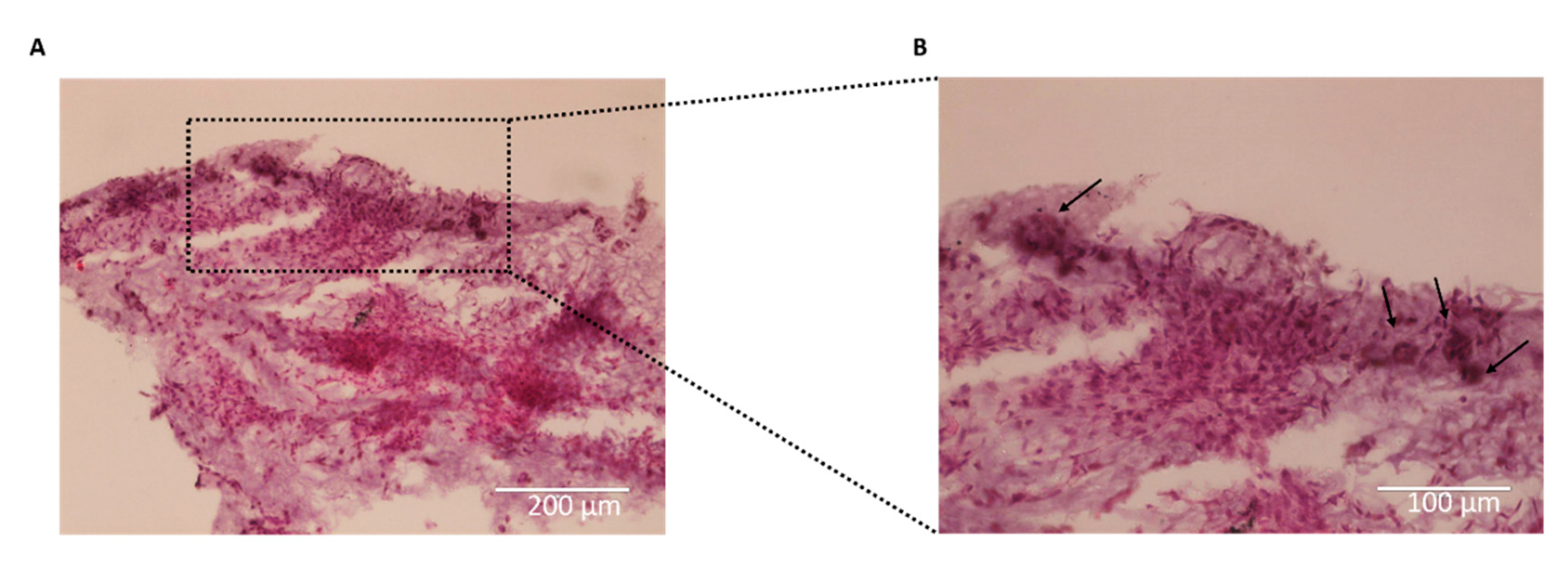
On day 21, H&E staining of the hydrogels containing chondrocytes revealed extensive colonization of the entire hydrogel, with the formation of large spherical cell clusters (
Figure 3.7). The presence of chondrocyte aggregates was still evident within the hydrogels indicating their sustained growth and maturation (
Figure 3.7B). However, it was observed that the aggregates were undergoing fusion, gradually merging together to form a more tissue-like structure. This observation suggests the development of a more organized and integrated chondrocyte network within the hydrogel, indicative of tissue formation and maturation.
Figure 7.
Haematoxylin and eosin (H&E) staining of P4 chondrocytes embedded into hydrogels on day 21. (A) Chondrocytes embedded into col/alg hydrogels at day 21 stained with H&E scale bar 200 µm; (B) magnification of images A, arrow indicates the presence of chondrocytes aggregates within the tissue, scale bar 100 µm.
Figure 7.
Haematoxylin and eosin (H&E) staining of P4 chondrocytes embedded into hydrogels on day 21. (A) Chondrocytes embedded into col/alg hydrogels at day 21 stained with H&E scale bar 200 µm; (B) magnification of images A, arrow indicates the presence of chondrocytes aggregates within the tissue, scale bar 100 µm.
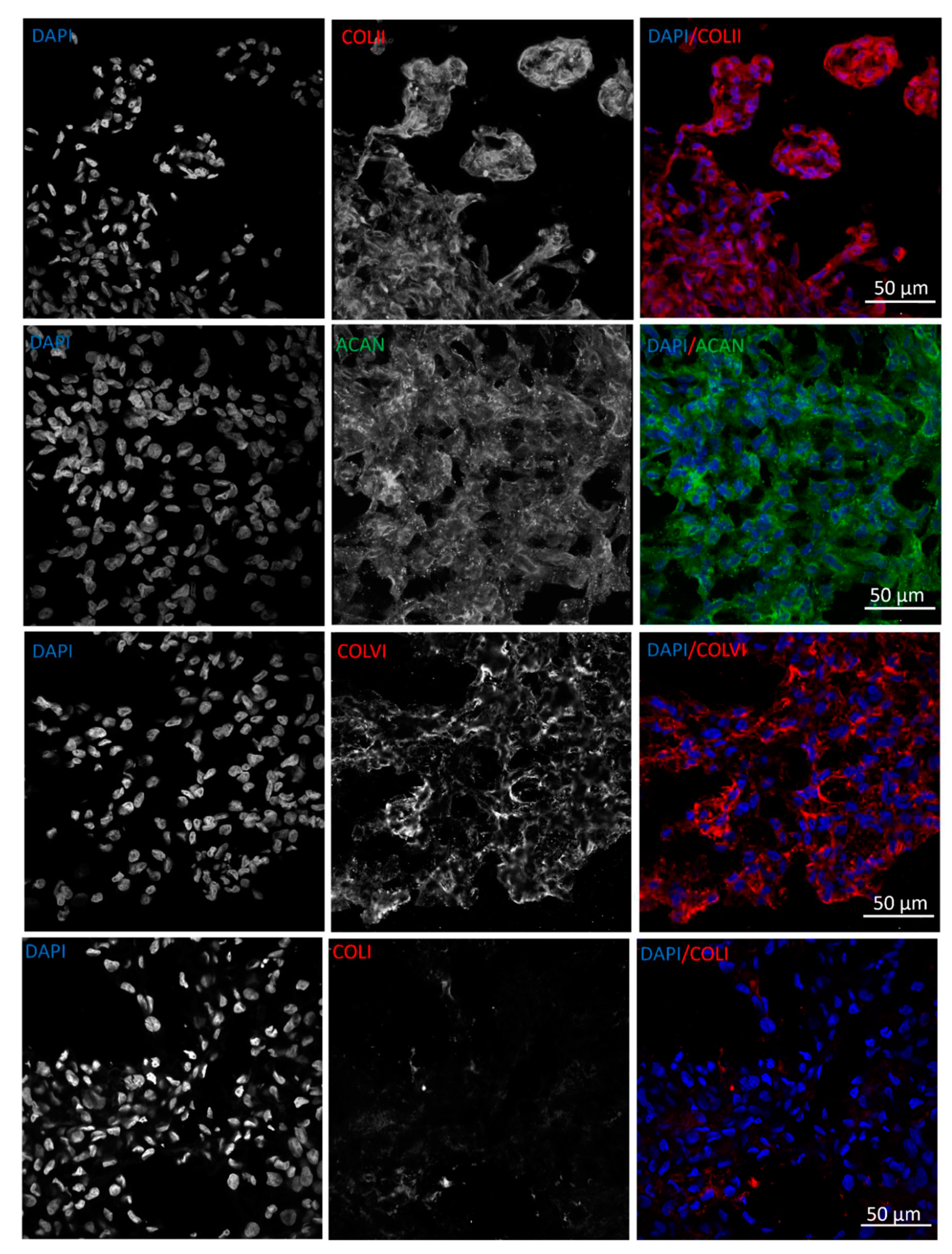
The ability of chondrocytes to deposit cartilaginous ECM when embedded into col/alg hydrogels without any growth factor was evaluated at day 21 by immunofluorescence staining of collagen type II, aggrecan, collagen type VI as cartilaginous markers, and collagen type I as a hypertrophic marker. The results demonstrated that when embedded in col/alg hydrogels, P4 chondrocytes regained their capacity to deposit matrix components, as indicated by positive immunofluorescence staining for aggrecan, collagen type II, and collagen type VI (
Figure 8). This suggests that the chondrocytes were able to produce and secrete cartilaginous ECM proteins within the hydrogel environment. Furthermore, on day 21, chondrocytes aggregated within the hydrogels began to exhibit tissue-like characteristics, indicating their progressive maturation and organization. Importantly, immunostaining analysis revealed no evidence of collagen type I deposition after 21 days, suggesting that the chondrocytes maintained a non-hypertrophic phenotype.
4. Discussion
Data presented in this work demonstrated that chondrocytes grown on flat tissue culture plastic develop a hypertrophic phenotype but when grown in 3D in soft col/alg hydrogel there is a change in gene expression with spherical cells that aggregate into clusters and produce extracellular matrix. These characteristics indicate that when chondrocytes are grown in soft col/alg hydrogel they re-differentiate into chondrocytes and regain the ability to deposit ECM composed of collagen II and aggrecan. Importantly the change in the phenotype of these cells is accomplished without using growth factors. The main drawback in current cell based therapies that is the de-differentiation of chondrocytes [
8,
43]. The loss of chondrocytes’ original phenotype leads to the production of fibrocartilage which has inferior mechanical properties compared to hyaline cartilage [
34]. One of the main strategies to re-establish chondrocytes original phenotype is the use of growth factors, such as TGF-β, furthermore growth factors could promote the expansion of chondrocytes obtained from elderly patients [
44,
45,
46,
47]. However, the use of growth factors raises the issue of their clinical biosafety. As they induce rapid proliferation of cells that may favour damage accumulation in DNA, uncontrolled proliferation, and genomic instability; thus increasing the risk of tumorigenic transformation [
4,
48,
49]. Furthermore, the use of TGF-β for re-differentiating chondrocytes has shown to promote the expression of hypertrophic markers, which will eventually lead to hypertrophy and cartilage mineralization [
17,
19,
50].
Finding strategies to reprogram chondrocytes without the use of growth factors, preventing hypertrophy is vital in order to repair and regenerate cartilage tissue. We previously demonstrated that soft col/alg hydrogels supported the differentiation of oMSCs and limited the deposition of collagen type I [
42].The effect of soft col/alg hydrogels on P4 chondrocytes was investigated in order to evaluate if they can support the reprogramming of chondrocytes to their original phenotype without the use of growth factors. Chondrocytes were characterised at P1 and P4 and as expected, when expanded in 2D, they lost their original phenotype, acquiring a spindle shape morphology and lost the ability to deposit ECM at P4. At P4 chondrocytes were embedded into col/alg hydrogels and their response in terms of viability, proliferation, gene expression and ECM deposition were evaluated. The results reported in this work showed that chondrocytes were viable and proliferated within the hydrogel and chondrocytes regained their original morphology and formed aggregates after only 7 days of culture. In accordance with other studies, de-differentiated chondrocytes started re-expressing chondrogenic markers upon the restoration of a spheroidal shape [
51,
52,
53]. In this study, after 7 days of culture it was possible to observe an increase in the expression of FOXO3A, COL6A1 and ACAN, whilst the expression of COL1A1 and COL10A1 decreased. On day 7 chondrocytes started to self-assemble suggesting that they were able to migrate within the hydrogel. It is well known that chondrocyte aggregates promote cell differentiation and ECM synthesis by mimicking the
in vivo environment which allows cell-to-cell contact and cell-to-ECM contact [
43]. The use of spheroids to repair cartilage defects in the knee is in clinical use where spheroids measuring 500–800 μm in diameter are generated from autologous chondrocytes and injected
in vivo [
54]. While spheroids have been used in clinical settings for repairing cartilage defects, they can present challenges such as the development of a necrotic core due to limited nutrient diffusion and difficulties in handling during surgery [
55]. Additionally, the production of spheroids and their subsequent encapsulation into hydrogels can be time-consuming and costly. Conversely, in our study P4 chondrocytes in col/alg hydrogels were able to migrate, self-assemble and form aggregates in just 7 days of culture. Furthermore, an important observation was that the chondrocyte aggregates formed within the col/alg hydrogels did not exhibit any signs of a necrotic core, as confirmed by the live/dead staining. This is a significant finding, as it indicates that the aggregates remained viable and metabolically active throughout the culture period.
The chondrocytes aggregates were able to support ECM synthesis by day 21. At day 21, unless colonized by chondrocytes, the col/alg hydrogel are usually resorbed however when cultured with chondrocytes the gel is replaced by ECM creating a coherent structure [
42]. This demonstrates the potential of col/alg hydrogels to create a microenvironment that promotes cell-cell interactions and ECM deposition, leading to the
in vivo development of functional cartilaginous tissue. One of the most remarkable findings in this study was the notable absence of collagen type I expression on day 21, which strongly indicates that the utilization of col/alg hydrogels facilitated the re-differentiation of chondrocytes and effectively preventing chondrocyte hypertrophy and the formation of fibrocartilage. This result underscores the potential of col/alg hydrogels as a promising approach to promote chondrocyte reprogramming and facilitate the regeneration of cartilaginous tissue. It is worth noting that the de-differentiation process of chondrocytes from P1 to P4 in this study occurred over a period of 4 to 6 weeks, which aligns with the current estimated time required for the growth of articular chondrocytes in autologous chondrocyte implantation (ACI) procedures. To further explore the potential of soft col/alg hydrogels, additional ex vivo and in vivo studies should be conducted. These studies would allow for the evaluation of the long-term deposition of extracellular matrix (ECM) and the integration of the generated tissue with the native cartilage. Furthermore, investigating the effect of col/alg hydrogels on maintaining chondrocyte phenotype could be considered as an alternative approach to traditional 2D culture methods for in vitro expansion of chondrocytes. The main limitation in the use of soft col/alg hydrogels in the repair of articular cartilage is that this type of hydrogels will not be able to withstand the loads that cartilage is subject to. A pre-culture of chondrocytes will be required before implantation in order to allow the formation of ECM, which would be expected to increase the Young’s modulus of the hydrogel-chondrocyte combination. A possible solution would be to increase the mechanical properties of the col/alg hydrogels, however this may affect the stiffness of the gel and alter the phenotypic behavior of the cells.
5. Conclusions
In this study, the effect of col/alg hydrogels on the reprogramming of P4 chondrocytes was investigated. Overall, the results indicate that the 3D culture in the soft col/alg hydrogel provides a more favourable microenvironment for chondrocytes, leading to a shift in gene expression towards a chondrogenic phenotype and the formation of cell aggregates capable of producing cartilaginous extracellular matrix. These findings highlight the potential of col/alg hydrogels to support chondrocyte re-differentiation and the deposition of cartilaginous matrix in the absence of exogenous growth factors, further emphasizing their suitability as a scaffold for cartilage tissue engineering applications.
Author Contributions
Conceptualization, T.R., G.B. and M.R.; methodology, T.R., G.B. and M.R.; formal analysis, T.R. and M.R..; investigation, T.R.; resources, G.B. and M.R.; data curation, T.R.; writing—original draft preparation, T.R.; writing—review and editing, G.B. and M.R.; visualization, T.R.; supervision, G.B. and M.R.; project administration, M.R.; funding acquisition, G.B. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was supported by the award of internal University of Portsmouth TRIF funding to M.R. for the project “Development of an off-the-shelf product for cartilage regeneration” through the Health and Wellbeing theme.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lach, M.S.; Rosochowicz, M.A.; Richter, M.; Jagiełło, I.; Suchorska, W.M.; Trzeciak, T. The Induced Pluripotent Stem Cells in Articular Cartilage Regeneration and Disease Modelling: Are We Ready for Their Clinical Use? Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of Alginate Hydrogels for Next-Generation Articular Cartilage Regeneration. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Wallenborn, M.; Petters, O.; Rudolf, D.; Hantmann, H.; Richter, M.; Ahnert, P.; Rohani, L.; Smink, J.J.; Bulwin, G.C.; Krupp, W.; et al. Comprehensive high-resolution genomic profiling and cytogenetics of human chondrocyte cultures by GTG-banding, locus-specific fish, sky and SNP array. Eur. Cells Mater. 2018, 35, 225–241. [Google Scholar] [CrossRef]
- Ammendola, S.; Scotto d’Abusco, A. Oxidative stress, senescence and Mediterranean diet effects on osteoarthritis. In Aging: Oxidative Stress and Dietary Antioxidants; 2020; pp. 73–81. ISBN 9780128186985. [Google Scholar]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C. Dedifferentiation: inspiration for devising engineering strategies for regenerative medicine. npj Regen. Med. 2020, 5. [Google Scholar] [CrossRef]
- Al-Masawa, M.E.; Wan Kamarul Zaman, W.S.; Chua, K.H. Biosafety evaluation of culture-expanded human chondrocytes with growth factor cocktail: a preclinical study. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.Y.; Chae, B.C.; Jang, J.; Lee, E.A.; Son, Y. Fully Dedifferentiated Chondrocytes Expanded in Specific Mesenchymal Stem Cell Growth Medium with FGF2 Obtains Mesenchymal Stem Cell Phenotype In Vitro but Retains Chondrocyte Phenotype In Vivo. Cell Transplant. 2017, 26, 1673–1687. [Google Scholar] [CrossRef]
- Wongin, S.; Waikakul, S.; Chotiyarnwong, P.; Siriwatwechakul, W.; Kino-oka, M.; Kim, M.H.; Viravaidya-Pasuwat, K. Maintenance of human chondrogenic phenotype on a dendrimer-immobilized surface for an application of cell sheet engineering. BMC Biotechnol. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Wu, A.; Kohn, J. An Innovative Laboratory Procedure to Expand Chondrocytes with Reduced Dedifferentiation. Cartilage 2018, 9, 202–211. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Andriolo, L.; Di Matteo, B.; Balboni, F.; Marcacci, M. Clinical profiling in cartilage regeneration: Prognostic factors for midterm results of matrix-assisted autologous chondrocyte transplantation. Am. J. Sports Med. 2014, 42, 898–905. [Google Scholar] [CrossRef]
- Andriolo, L.; Reale, D.; Di Martino, A.; De Filippis, R.; Sessa, A.; Zaffagnini, S.; Filardo, G. Long-term Results of Arthroscopic Matrix-Assisted Autologous Chondrocyte Transplantation: A Prospective Follow-up at 15 Years. Am. J. Sports Med. 2020, 48, 2994–3001. [Google Scholar] [CrossRef]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Redifferentiation of articular chondrocytes by hyperacute serum and platelet rich plasma in collagen type I hydrogels. Int. J. Mol. Sci. 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-J.; Bhang, S.H.; La, W.-G.; Yang, H.S.; Seong, J.Y.; Lee, H.; Im, G.-I.; Lee, S.-H.; Kim, B.-S. Spinner-flask culture induces redifferentiation of de-differentiated chondrocytes. Biotechnol. Lett. 2011, 33, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Narcisi, R.; Quarto, R.; Ulivi, V.; Muraglia, A.; Molfetta, L.; Giannoni, P. TGF β-1 administration during Ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J. Cell. Physiol. 2012, 227, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.J.; Weber, J.F.; Waldman, S.D.; Backstein, D.; Kandel, R.A. Formation of hyaline cartilage tissue by passaged human osteoarthritic chondrocytes. Tissue Eng. - Part A 2017, 23, 156–165. [Google Scholar] [CrossRef]
- Bianchi, V.J.; Lee, A.; Anderson, J.; Parreno, J.; Theodoropoulos, J.; Backstein, D.; Kandel, R. Redifferentiated Chondrocytes in Fibrin Gel for the Repair of Articular Cartilage Lesions. Am. J. Sports Med. 2019, 47, 2348–2359. [Google Scholar] [CrossRef]
- van der Kraan, P.M. Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018, 25, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C - Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef]
- Fava, R.; Olsen, N.; Keski-Oja, J.; Moses, H.; Pincus, T. Active and latent forms of transforming growth factor β activity in synovial effusions. J. Exp. Med. 1989, 169, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Guo, Q.; Li, Y.; Wu, C.; Zhu, S.; Wang, R.; Guo, X.E.; Kim, B.C.; Huang, J.; Hu, Y.; et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat. Commun. 2021, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, S.; Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Retting, K.N.; Song, B.; Yoon, B.S.; Lyons, K.M. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 2009, 136, 1093–1104. [Google Scholar] [CrossRef]
- Finnson, K.W.; Parker, W.L.; Chi, Y.; Hoemann, C.D.; Goldring, M.B.; Antoniou, J.; Philip, A. Endoglin differentially regulates TGF-β-induced Smad2/3 and Smad1/5 signalling and its expression correlates with extracellular matrix production and cellular differentiation state in human chondrocytes. Osteoarthr. Cartil. 2010, 18, 1518–1527. [Google Scholar] [CrossRef]
- Valcourt, U.; Gouttenoire, J.; Moustakas, A.; Herbage, D.; Mallein-Gerin, F. Functions of transforming growth factor-β family type I receptors and Smad proteins in the hypertrophic maturation and osteoblastic differentiation of chondrocytes. J. Biol. Chem. 2002, 277, 33545–33558. [Google Scholar] [CrossRef]
- Kutaish, H.; Bengtsson, L.; Matthias Tscholl, P.; Marteyn, A.; Braunersreuther, V.; Guérin, A.; Béna, F.; Gimelli, S.; Longet, D.; Ilmjärv, S.; et al. Hyaline Cartilage Microtissues Engineered from Adult Dedifferentiated Chondrocytes: Safety and Role of WNT Signaling. Stem Cells Transl. Med. 2022, 11, 1219–1231. [Google Scholar] [CrossRef]
- Loverdou, N.; Cuvelier, M.; Nilsson Hall, G.; Christiaens, A.-S.; Decoene, I.; Bernaerts, K.; Smeets, B.; Ramon, H.; Luyten, F.P.; Geris, L.; et al. Stirred culture of cartilaginous microtissues promotes chondrogenic hypertrophy through exposure to intermittent shear stress. Bioeng. Transl. Med. 2023, 8, e10468. [Google Scholar] [CrossRef]
- Yue, H.; Pathak, J.L.; Zou, R.; Qin, L.; Liao, T.; Hu, Y.; Kuang, W.; Zhou, L. Fabrication of chondrocytes/chondrocyte-microtissues laden fibrin gel auricular scaffold for microtia reconstruction. J. Biomater. Appl. 2020, 35, 838–848. [Google Scholar] [CrossRef]
- Dong, L.; Liu, Q.; Gao, Y.; Jia, H.; Dai, W.; Guo, L.; Fan, H.; Fan, Y.; Zhang, X. The effect of collagen hydrogels on chondrocyte behaviors through restricting the contraction of cell/hydrogel constructs. Regen. Biomater. 2021, 8, rbab030. [Google Scholar] [CrossRef]
- S2949723X22000046.
- Cipriani, F.; Krüger, M.; de Torre, I.G.; Sierra, L.Q.; Rodrigo, M.A.; Kock, L.; Rodriguez-Cabello, J.C. Cartilage Regeneration in Preannealed Silk Elastin-Like Co-Recombinamers Injectable Hydrogel Embedded with Mature Chondrocytes in an Ex Vivo Culture Platform. Biomacromolecules 2018, 19, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, C.E.; Walimbe, T.; Panitch, A.; Liu, J.C. Incorporation of a Collagen-Binding Chondroitin Sulfate Molecule to a Collagen Type I and II Blend Hydrogel for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J. Biomed. Mater. Res. - Part A 2021, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Singh, A.; Datta, D.; Katti, D.S. Bioinspired Injectable Hydrogels Dynamically Stiffen and Contract to Promote Mechanosensing-Mediated Chondrogenic Commitment of Stem Cells. ACS Appl. Mater. Interfaces 2022. [Google Scholar] [CrossRef]
- Anand, R.; Nimi, N.; Sivadas, V.P.; Lal, L.P.M.R.; Nair, P.D. Dual crosslinked pullulan-gelatin cryogel scaffold for chondrocyte-mediated cartilage repair: Synthesis, characterization and in vitro evaluation. Biomed. Mater. 2022, 17. [Google Scholar] [CrossRef]
- Gorroñogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers (Basel). 2022, 14, 1–17. [Google Scholar] [CrossRef]
- Zhou, S.; Bei, Z.; Wei, J.; Yan, X.; Wen, H.; Cao, Y.; Li, H. Mussel-inspired injectable chitosan hydrogel modified with catechol for cell adhesion and cartilage defect repair. J. Mater. Chem. B 2022, 10. [Google Scholar] [CrossRef]
- Bachmann, B.; Spitz, S.; Schädl, B.; Teuschl, A.H.; Redl, H.; Nürnberger, S.; Ertl, P. Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation. Front. Bioeng. Biotechnol. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. Initiating human articular chondrocyte re-differentiation in a 3D system after 2D expansion. J. Mater. Sci. Mater. Med. 2017, 28. [Google Scholar] [CrossRef]
- Roncada, T.; Bonithon, R.; Blunn, G.; Roldo, M. Soft substrates direct stem cell differentiation into the chondrogenic lineage without the use of growth factors. J. Tissue Eng. 2022, 13. [Google Scholar] [CrossRef]
- De Moor, L.; Beyls, E.; Declercq, H. Scaffold Free Microtissue Formation for Enhanced Cartilage Repair. Ann. Biomed. Eng. 2020, 48, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Grogan, S.; Schäfer, D.; Heberer, M.; Mainil-Varlet, P.; Martin, I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr. Cartil. 2004, 12, 476–484. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; Blaney Davidson, E.N.; van den Berg, W.B. A role for age-related changes in TGFβ signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res. Ther. 2010, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, B.; Xu, H. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 2018, 17, 2756–2765. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, L.; Post, J.N.; Karperien, M. Co-treatment of TGF-β3 and BMP7 is superior in stimulating chondrocyte redifferentiation in both hypoxia and normoxia compared to single treatments. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Inman, G.J. Switching TGFβ from a tumor suppressor to a tumor promoter. Curr. Opin. Genet. Dev. 2011, 21, 93–99. [Google Scholar] [CrossRef]
- Grose, R.; Dickson, C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev. 2005, 16, 179–186. [Google Scholar] [CrossRef]
- Chen, J.L.; Zou, C.; Chen, Y.; Zhu, W.; Liu, W.; Huang, J.; Liu, Q.; Wang, D.; Duan, L.; Xiong, J.; et al. TGFβ1 induces hypertrophic change and expression of angiogenic factors in human chondrocytes. Oncotarget 2017, 8, 91316–91327. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Chondrocyte redifferentiation and construct mechanical property development in single-component photocrosslinkable hydrogels. J. Biomed. Mater. Res. - Part A 2014, 102, 2544–2553. [Google Scholar] [CrossRef]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular chondrocyte phenotype regulation through the cytoskeleton and the signaling processes that originate from or converge on the cytoskeleton: Towards a novel understanding of the intersection between actin dynamics and chondrogenic function. Int. J. Mol. Sci. 2021, 22, 1–60. [Google Scholar] [CrossRef]
- Tew, S.R.; Hardingham, T.E. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J. Biol. Chem. 2006, 281, 39471–39479. [Google Scholar] [CrossRef] [PubMed]
- Eschen, C.; Kaps, C.; Widuchowski, W.; Fickert, S.; Zinser, W.; Niemeyer, P.; Roël, G. Clinical outcome is significantly better with spheroid-based autologous chondrocyte implantation manufactured with more stringent cell culture criteria. Osteoarthr. Cartil. Open 2020, 2, 100033. [Google Scholar] [CrossRef] [PubMed]
- De Moor, L.; Fernandez, S.; Vercruysse, C.; Tytgat, L.; Asadian, M.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Hybrid Bioprinting of Chondrogenically Induced Human Mesenchymal Stem Cell Spheroids. Front. Bioeng. Biotechnol. 2020, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Chondrocytes (CH) characterisation at P1 and P4. (A) Phalloidin and DAPI staining of chondrocytes p1 and chondrocytes p4 (B) Aspect ratio analysis of chondrocytes at p1 and p4. Error bars denote standard deviation, n = 4. Comparison between groups was assessed by unpaired t test. P <0.0001 (C) The expression of typical chondrogenic markers evaluated at mRNA level presented as fold change using the 2−ΔΔCt method relative to chondrocytes P1. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by unpaired t test. **** = P <0.0001, ** P =0.0055; Immunofluorescence staining of (D) collagen type II, (F) aggrecan, (H) collagen type VI, (J) collagen type I of chondrocytes at passage 1 and (E, G, I, K) dedifferentiated chondrocytes.
Figure 1.
Chondrocytes (CH) characterisation at P1 and P4. (A) Phalloidin and DAPI staining of chondrocytes p1 and chondrocytes p4 (B) Aspect ratio analysis of chondrocytes at p1 and p4. Error bars denote standard deviation, n = 4. Comparison between groups was assessed by unpaired t test. P <0.0001 (C) The expression of typical chondrogenic markers evaluated at mRNA level presented as fold change using the 2−ΔΔCt method relative to chondrocytes P1. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by unpaired t test. **** = P <0.0001, ** P =0.0055; Immunofluorescence staining of (D) collagen type II, (F) aggrecan, (H) collagen type VI, (J) collagen type I of chondrocytes at passage 1 and (E, G, I, K) dedifferentiated chondrocytes.
Figure 2.
Viability of P4 chondrocytes in col/alg hydrogels. Representative images of live and dead staining using calcein-am and ethidium homodimer-1 to determine the viability of dedifferentiated chondrocytes embedded into col/alg hydrogels at day 1,7 and 14.
Figure 2.
Viability of P4 chondrocytes in col/alg hydrogels. Representative images of live and dead staining using calcein-am and ethidium homodimer-1 to determine the viability of dedifferentiated chondrocytes embedded into col/alg hydrogels at day 1,7 and 14.
Figure 3.
Validation of CFSE staining protocol on chondrocytes in 2D. (A) Viability of chondrocytes stained with CellTrace CFSE at 5 and 10 μM at different time points. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by ordinary two-way ANOVA using post hoc Tukey’s test, (B) Flow cytometry analysis of CFSE labelling efficacy of oMSC at different concentrations and time points. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by ordinary two-way ANOVA using post hoc Sidaks’s test, *** p = 0.0006, **** p < 0.0001.
Figure 3.
Validation of CFSE staining protocol on chondrocytes in 2D. (A) Viability of chondrocytes stained with CellTrace CFSE at 5 and 10 μM at different time points. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by ordinary two-way ANOVA using post hoc Tukey’s test, (B) Flow cytometry analysis of CFSE labelling efficacy of oMSC at different concentrations and time points. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by ordinary two-way ANOVA using post hoc Sidaks’s test, *** p = 0.0006, **** p < 0.0001.
Figure 4.
Chondrocytes proliferation into the col/alg hydrogels. (A) Flow cytometry histogram of the frequency distribution of CFSE stained chondrocyte at day 1,3 and 7, (B) Cell proliferation analysis of chondrocytes into col/alg hydrogels at day 1, day 3 and day 7. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by ordinary one-way ANOVA using post hoc Dunnett’s test n = 3, * p = 0.05, **** p <0.0001.
Figure 4.
Chondrocytes proliferation into the col/alg hydrogels. (A) Flow cytometry histogram of the frequency distribution of CFSE stained chondrocyte at day 1,3 and 7, (B) Cell proliferation analysis of chondrocytes into col/alg hydrogels at day 1, day 3 and day 7. Error bars denote standard deviation, n = 3. Comparison between groups was assessed by ordinary one-way ANOVA using post hoc Dunnett’s test n = 3, * p = 0.05, **** p <0.0001.
Figure 5.
P4 chondrocytes morphology into col/alg hydrogels (A) Morphological analysis of dedifferentiated chondrocytes embedded into hydrogels at day 7. (B) Aspect ratio analysis comparing chondrocytes at p1 and dedifferentiated chondrocytes embedded into hydrogels at day 7. Error bars denote standard deviation, n = 4. Comparison between groups was assessed by unpaired t test.
Figure 5.
P4 chondrocytes morphology into col/alg hydrogels (A) Morphological analysis of dedifferentiated chondrocytes embedded into hydrogels at day 7. (B) Aspect ratio analysis comparing chondrocytes at p1 and dedifferentiated chondrocytes embedded into hydrogels at day 7. Error bars denote standard deviation, n = 4. Comparison between groups was assessed by unpaired t test.
Figure 6.
Gene expression of P4 chondrocytes embedded into col/alg hydrogels at day 1, 7 and 14. The effect of the 3D culture on dedifferentiated chondrocytes compared to dedifferentiated chondrocytes in 2D, mRNA data are presented as fold change relative to 2D control. Results represent mean ± SD (n=3). Comparison between groups was assessed by ordinary one-way ANOVA using post hoc Dunnett’s test.
Figure 6.
Gene expression of P4 chondrocytes embedded into col/alg hydrogels at day 1, 7 and 14. The effect of the 3D culture on dedifferentiated chondrocytes compared to dedifferentiated chondrocytes in 2D, mRNA data are presented as fold change relative to 2D control. Results represent mean ± SD (n=3). Comparison between groups was assessed by ordinary one-way ANOVA using post hoc Dunnett’s test.
Figure 8.
Immunofluorescence images of ECM deposition at day 21. Representative confocal images of P4 chondrocytes embedded into col/alg hydrogels stained for collagen type II, aggrecan, collagen type VI and collagen type I.
Figure 8.
Immunofluorescence images of ECM deposition at day 21. Representative confocal images of P4 chondrocytes embedded into col/alg hydrogels stained for collagen type II, aggrecan, collagen type VI and collagen type I.
Table 1.
Primers employed in gene expression analysis with RTqPCR.
Table 1.
Primers employed in gene expression analysis with RTqPCR.
| Gene name |
Forward |
Reverse |
| GAPDH |
5’-AAGGCCATCACCATCTTCCA-3’ |
5’-TCACGCCCATCACAAACATG-3’ |
| SOX9 |
5’-TAAGGATGTGTGGAAGCCCG-3’ |
5’-GGGCTGAGGCAGTCTTTCAT-3’ |
| FOXO1 |
5’-GCTGCAGGACAGCAAATCG-3’ |
5’-ATGATGTCACTGTGCGGAGG-3’ |
| FOXO3A |
5’-CTGCTGACTCCATGATCCCC-3’ |
5’-CTCCAGGAGCCAAGAGCC-3’ |
| COL2A1 |
5’-TAAGGATGTGTGGAAGC-3’ |
5’-GGGCTGAGGCAGTCTTT-3’ |
| COL1A1 |
5’-GAAGACCAGGGAAGCCT-3’ |
5’-GAAGACCAGGGAAGCCT-3’ |
| ACAN |
5’-GCTGTCTCGCCAAGTGTATG-3’ |
5’-ATGGTTCAGGGATGCTGACA-3’ |
| COL10A1 |
5’-GCCACAAGGACCTACAGGAG-3’ |
5’-CAAGGAGCACAATACCCCGT-3’ |
Table 2.
List of antibodies and dilutions.
Table 2.
List of antibodies and dilutions.
| |
Antibodies |
Dilution |
| Primary antibodies |
Rabbit monoclonal [EPR7785] to Collagen I |
1:500 |
| |
Anti-Aggrecan antibody [6-B-4] |
1:300 |
| Anti-Collagen II antibody |
1:300 |
| |
Recombinant Anti-Collagen VI antibody [EPR17072] |
1:300 |
| Secondary antibodies |
Goat Anti-Rabbit IgG H&L (Alexa Fluor® 594) |
1:300 |
| |
Sheep Anti-Mouse IgG (whole molecule) F(ab′)2 fragment–FITC |
1:300 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).