Submitted:
02 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
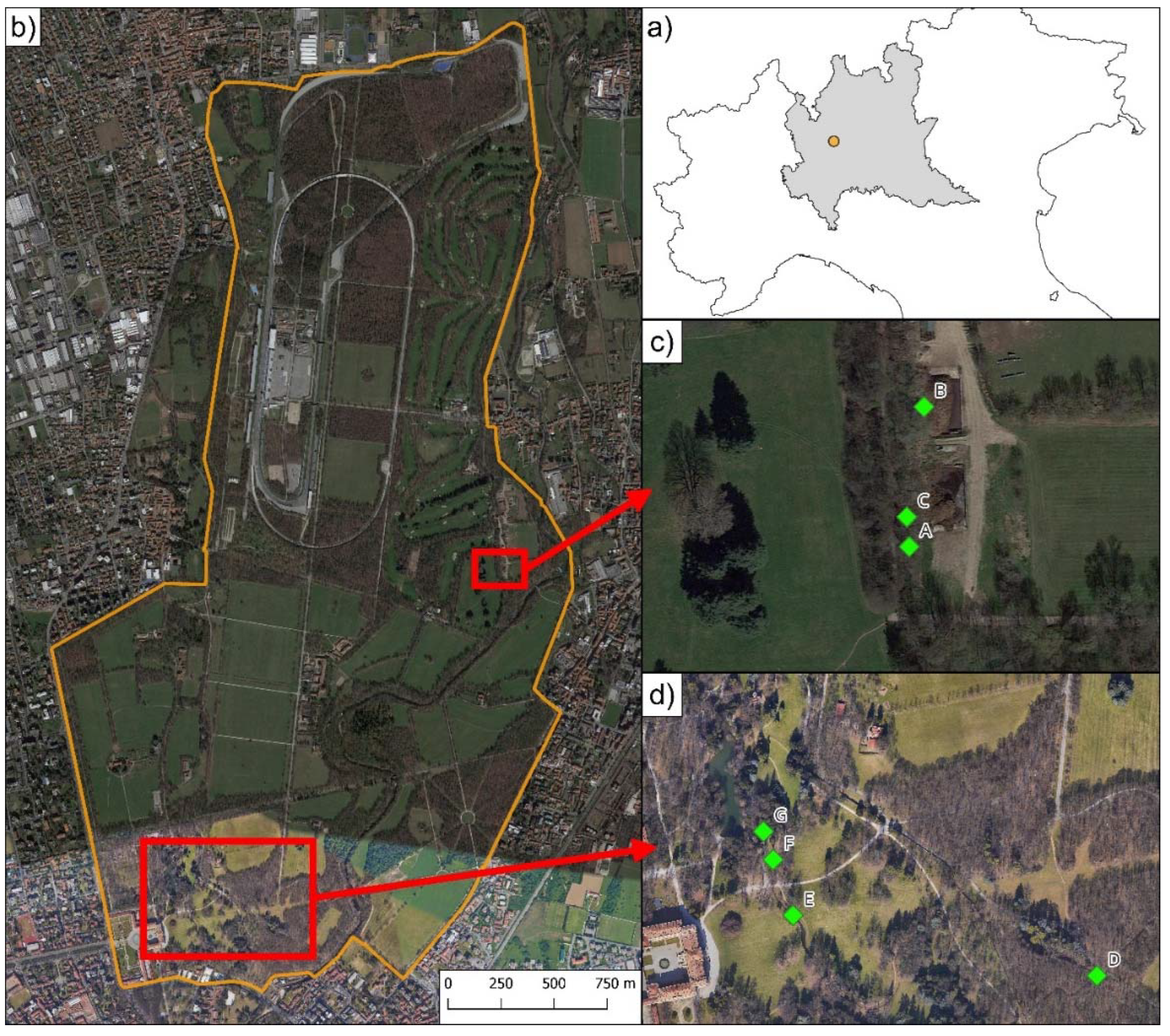
2. Methods
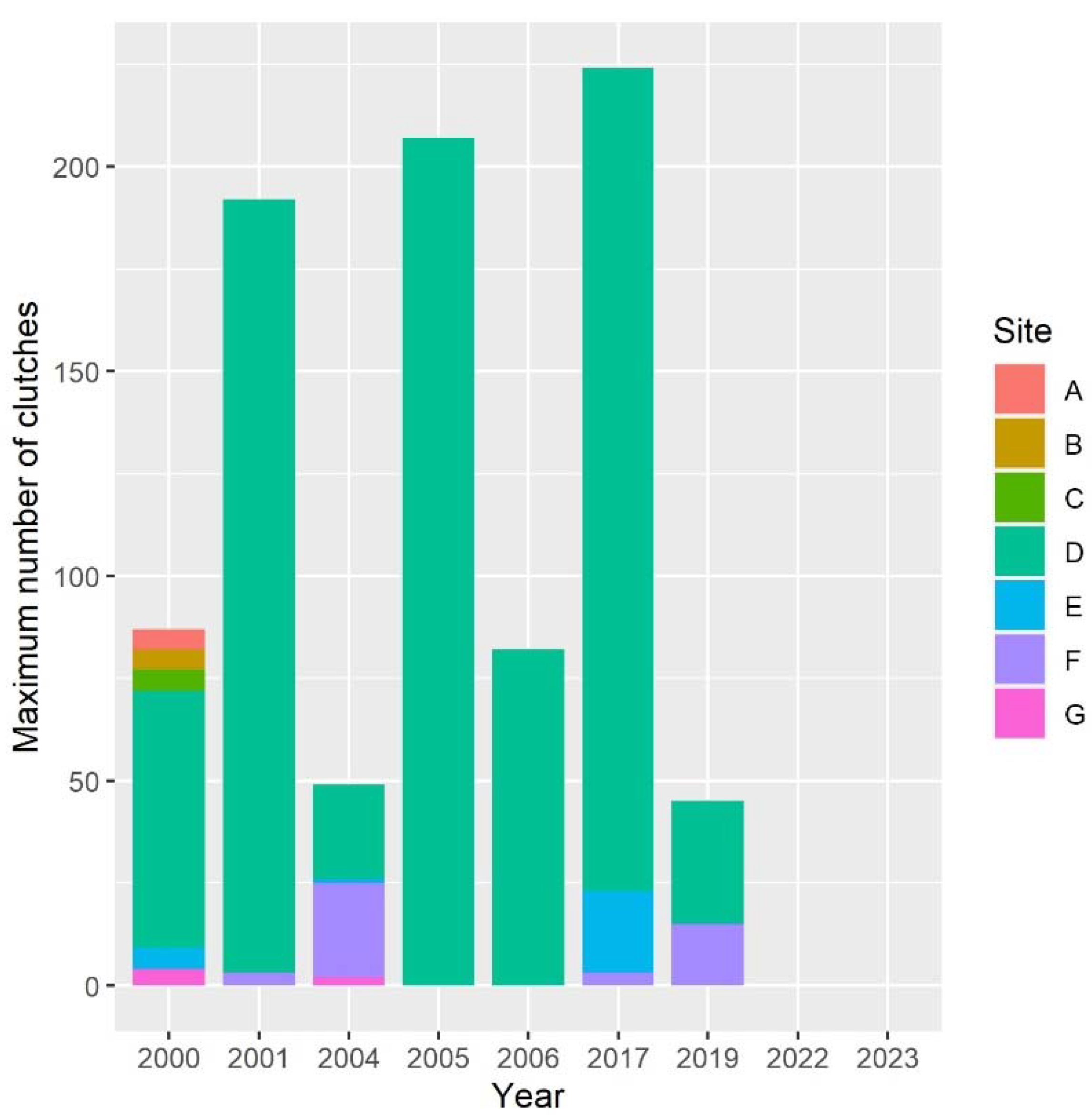
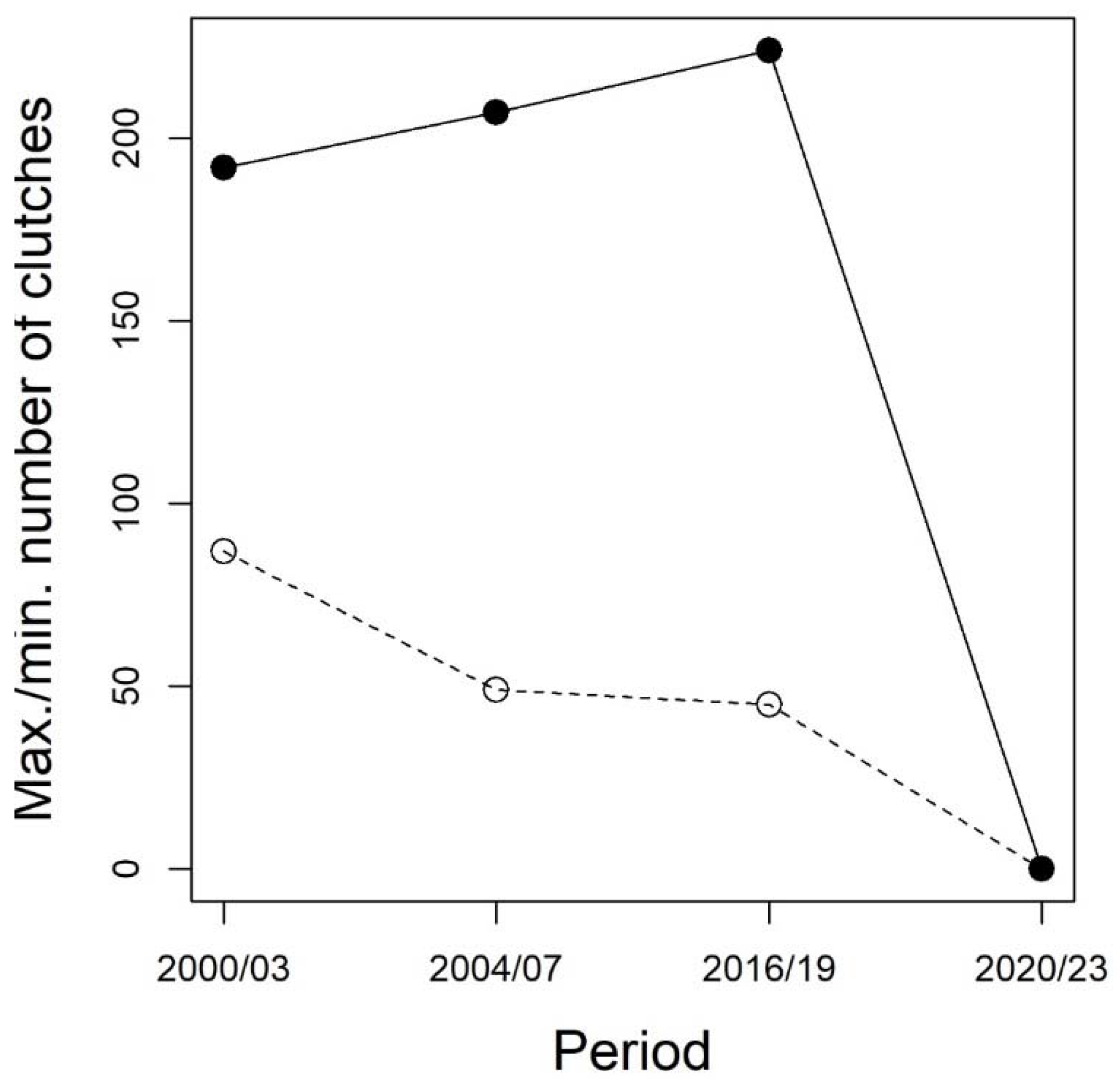
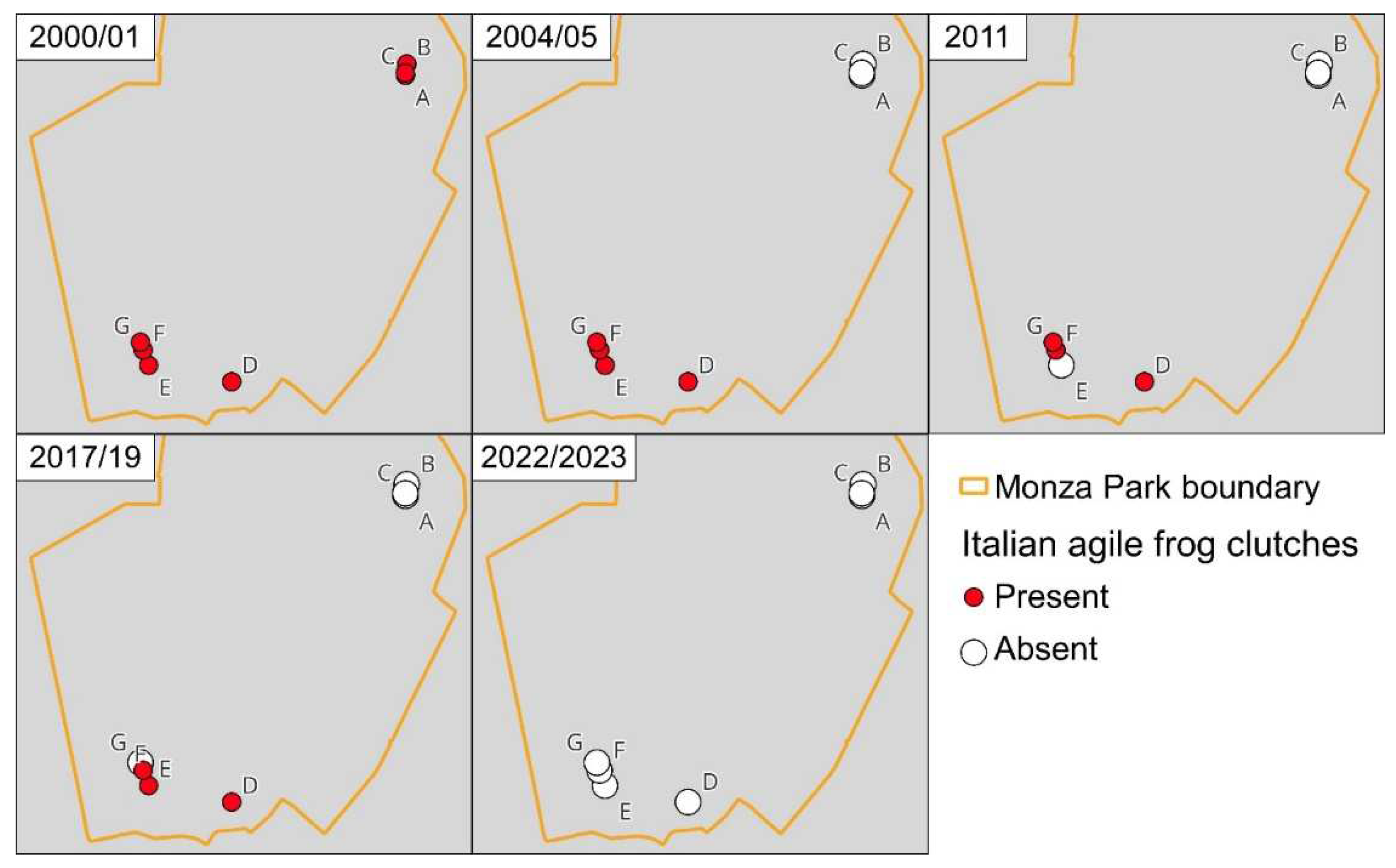
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Barinaga, M. Where have all the froggies gone? Science 1990, 247, 1033-1034.
- Vitt, L.J.; Caldwell, J.P.; Wilbur, H.M.; Smith, D.C. Amphibians as harbingers of decay. Bioscience 1990, 40, 418. [CrossRef]
- Wake, D. Declining amphibian populations. Science 1991, 253, 860. [CrossRef]
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Bohm, M.; Brooks, T.M.; Butchart, S.H.M.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world's vertebrates. Science 2010, 330, 1503-1509. [CrossRef]
- Salvidio, S. Detecting amphibian population cycles: The importance of appropriate statistical analyses. Biol. Conserv. 2009, 142, 455–461. [CrossRef]
- Pechmann, J.H.K.; Scott, D.E.; Semlitsch, R.D.; Caldwell, J.P.; Vitt, L.J.; Gibbons, J.W. Declining amphibian populations: the problem of separating human impacts from natural fluctuations. Science 1991, 253, 892-895. [CrossRef]
- Pounds, J.A.; Crump, M.I. Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv. Biol. 1994, 8, 72-85. [CrossRef]
- Pechmann, J.H.K.; Wilbur, H.M. Putting declining amphibian populations in perspective - Natural fluctuations and human impact. Herpetologica 1994, 50, 65-84.
- Pounds, J.A.; Fogden, M.P.L.; Campbell, J.A. Biological response to climate change on a tropical mountain. Nature 1999, 398, 611-615. [CrossRef]
- Ochoa-Ochoa, L.M.; Whittaker, R.J.; Ladle, R.J. The Demise of the Golden Toad and the Creation of a Climate Change Icon Species. Conserv. Soc. 2013, 11, 291-319. [CrossRef]
- IUCN SSC Amphibian Specialist Group. Incilius periglenes. In The IUCN red list of threatened species 2020; 2020; p. e.T3172A54357699.
- Andreone, F.; Garner, T.W.J.; Schmidt, B.R.; Corti, C.; Vogrin, M. Rana latastei. In Threatened amphibians of the world, Stuart, S.N., Hoffman, M., Chanson, J.S., Cox, N.A., Berridge, R.J., Ramani, P., Young, B.E., Eds.; Lynx Editions, IUCN and Conservation International: Barcelona, 2008; p. 506.
- IUCN SSC Amphibian Specialist Group. Rana latastei. In The IUCN red list of threatened species 2022; 2022; p. e.T19156A215527729.
- Boulenger, G.A. Etude sur les grenouilles rousses Ranae temporariae et description d'espèces nouvelles ou méconues. Bulletin de la Société Zoologique de France Paris 1879, 4, 158-193.
- Scali, S. Amphibian and reptiles of Groane Regional Park (Lombardy, NW Italy). First census and ecological notes. Scientia herpetologica 1995, 307-311.
- Ficetola, G.F.; De Bernardi, F. Amphibians in an human-dominated landscape: the community structure is related to habitat features and isolation. Biol. Conserv. 2004, 119, 219-230. [CrossRef]
- Ficetola, G.F.; De Bernardi, F. Supplementation or in situ conservation? Evidence of local adaptation in the Italian agile frog Rana latastei and consequences for the management of populations. Anim. Conserv. 2005, 8, 33-40.
- Bernini, F.; Bonini, L.; Ferri, V.; Gentilli, A.; Razzetti, E.; Scali, S., (Eds.) Atlante degli Anfibi e dei Rettili della Lombardia. Provincia di Cremona: Cremona, 2004.
- Falaschi, M.; Giachello, S.; Lo Parrino, E.; Muraro, M.; Manenti, R.; Ficetola, G.F. Long-term drivers of survival and colonization dynamics in spatially-structured amphibian populations. Conserv. Biol. 2021, 35, 1530–1539. [CrossRef]
- Falaschi, M.; Muraro, M.; Gibertini, C.; Delle Monache, D.; Lo Parrino, E.; Faraci, F.; Belluardo, F.; Di Nicola, M.R.; Manenti, R.; Ficetola, G.F. Explaining declines of newt abundance in northern Italy. Freshw. Biol. 2022. [CrossRef]
- Ficetola, G.F.; Siesa, M.E.; Manenti, R.; Bottoni, L.; De Bernardi, F.; Padoa-Schioppa, E. Early assessment of the impact of alien species: differential consequences of an invasive crayfish on adult and larval amphibians. Divers. Distrib. 2011, 17, 1141-1151. [CrossRef]
- Manenti, R.; Falaschi, M.; Delle Monache, D.; Marta, S.; Ficetola, G.F. Network-scale effects of invasive species on spatially-structured amphibian populations. Ecography 2020, 43, 119-127. [CrossRef]
- Dalpasso, A.; Ficetola, G.F.; Giachello, S.; Lo Parrino, E.; Manenti, R.; Muraro, M.; Falaschi, M. Similar species, different fates: abundance dynamics in spatially structured populations of common and threatened frogs. Divers. Distrib. 2022, 28, 770-781.
- Engelhardt, E.K.; Bowler, D.E.; Hof, C. European Habitats Directive has fostered monitoring but not prevented species declines. Conservation Letters 2023, 16, e12948.
- Barbieri, F.; Mazzotti, S. Rana latastei Boulenger, 1879. In Fauna d'Italia, Vol. XLII: Amphibia, Lanza, B., Andreone, F., Bologna, M.A., Corti, C., Razzetti, E., Eds.; Calderini: Bologna, 2007; pp. 412-416.
- Muraro, M.; Romagnoli, S.; Barzaghi, B.; Falaschi, M.; Manenti, R.; Ficetola, G.F. Invasive predators induce plastic and adaptive responses during embryo development in a threatened frog. NeoBiota 2021, 70, 69-86. [CrossRef]
- Seglie, D.; Marzona, E.; Ficetola, G.F.; Giacoma, C. Comportamento riproduttivo e vocale della rana di Lataste, Rana latastei (Amphibia: Anura). In Herpetologia Sardiniae, Corti, C., Ed.; Belvedere: Latina, 2008; pp. 439-443.
- Falaschi, M.; Gibertini, C.; Lo Parrino, E.; Muraro, M.; Barzaghi, B.; Manenti, R.; Ficetola, G.F. Assessing population trends of species with imperfect detection: Double count analyses and simulations confirm reliable estimates in brown frogs. Animals 2022, 12, 2085. [CrossRef]
- Melotto, A.; Manenti, R.; Ficetola, G.F. Rapid adaptation to invasive predators overwhelms natural gradients of intraspecific variation. Nat. Commun. 2020, 11, 3608.
- Ficetola, G.F.; Padoa-Schioppa, E.; Wang, J.; Garner, T.W.J. Polygyny, census and effective population size in the threatened frog, Rana latastei. Anim. Conserv. 2010, 13 (suppl. 1), 82-89. [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1.; IUCN: Gland, Switzerland and Cambridge, UK, 2001; p. ii+30.
- Lodé, T.; Holveck, M.-J.; Lesbarrères, D. Asynchronous arrival pattern, operational sex ratio and occurrence of multiple paternities in a territorial breeding anuran, Rana dalmatina Biol. J. Linn. Soc. 2005, 86, 191–200.
- Cayuela, H.; Griffiths, R.A.; Zakaria, N.; Arntzen, J.W.; Priol, P.; Léna, J.-P.; Besnard, A.; Joly, P. Drivers of amphibian population dynamics and asynchrony at local and regional scales. J. Anim. Ecol. 2020, 89, 1350-1364. [CrossRef]
- Guarino, F.M.; Lunardi, S.; Carlomagno, M.; Mazzotti, S. A skeletochronological study of growth, longevity, and age at sexual maturity in a population of Rana latastei (Amphibia, Anura). J. Biosciences 2003, 28, 101-108.
- Barker, R.J.; Schofield, M.R.; Link, W.A.; Sauer, J.R. On the reliability of N-mixture models for count data. Biometrics 2018, 74, 369–377. [CrossRef]
- Bürkner, P.-C. Advanced Bayesian Multilevel Modeling with the R Package brms. The R Journal 2018, 10, 395-411. [CrossRef]
- IUCN Standards and Petitions Subcommittee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 15.1.; http://www.iucnredlist.org/documents/RedListGuidelines.pdf: IUCN, 2022.
- Richter, S.C.; Young, J.E.; Johnson, G.N.; Seigel, R.A. Stochastic variation in reproductive success of a rare frog, Rana sevosa: implications for conservation and for monitoring amphibian populations. Biol. Conserv. 2003, 111, 171-177.
- Marsh, D.M.; Trenham, P.C. Metapopulations dynamics and Amphibian conservation. Conserv. Biol. 2001, 15, 40-49.
- Ficetola, G.F.; Maiorano, L. Contrasting effects of temperature and precipitation change on amphibian phenology, abundance and performance. Oecologia 2016, 181, 683–693. [CrossRef]
- Curtis, R.A.; Sterling, W.B.; Ashley, D.; Andrew, B.; David, R.C. Early Spring and Early Vanishing Wetlands as Harbingers of the Future? The Climate Change Trap for Ephemeral Pond-Breeding Frogs. Northwest Science 2019, 93, 52-65. [CrossRef]
- Smith, M.A.; Green, D.M. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 2005, 28, 110-128.
- Ficetola, G.F.; Garner, T.W.J.; De Bernardi, F. Genetic diversity, but not hatching success, is jointly affected by post glacial colonization and isolation in the threatened frog, Rana latastei. Mol. Ecol. 2007, 16, 1787-1797.
- Hanski, I.; Gaggiotti, O.E., (Eds.) Ecology, genetics and evolution of metapopulations. Elsevier Academic Press: San Diego, USA, 2004; p. 696. [CrossRef]
- Lo Parrino, E.; Ficetola, G.F.; Manenti, R.; Falaschi, M. Thirty years of invasion: the distribution of the invasive crayfish Procambarus clarkii in Italy. Biogeographia 2020, 35, 43-50.
- Bonaldo, D.; Bellafiore, D.; Ferrarin, C.; Ferretti, R.; Ricchi, A.; Sangelantoni, L.; Vitelletti, M.L. The summer 2022 drought: a taste of future climate for the Po valley (Italy)? Reg. Envir. Chang. 2022, 23, 1. [CrossRef]
- Mathwin, R.; Wassens, S.; Young, J.; Ye, Q.; Bradshaw, C.J.A. Manipulating water for amphibian conservation. Conserv. Biol. 2021, 35, 24-34.
- Mathwin, R.; Wassens, S.; Gibbs, M.S.; Young, J.; Ye, Q.; Saltré, F.; Bradshaw, C.J.A. Modeling the effects of water regulation on the population viability of a threatened amphibian. Ecosphere 2023, 14, e4379.
- Henle, K.; Alard, D.; Clitherow, J.; Cobb, P.; Firbank, L.; Kull, T.; McCracken, D.; Moritz, R.F.A.; Niemelä, J.; Rebane, M.; et al. Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe–A review. Agriculture, Ecosystems & Environment 2008, 124, 60-71.
- Donati, G.F.A.; Bolliger, J.; Psomas, A.; Maurer, M.; Bach, P.M. Reconciling cities with nature: Identifying local Blue-Green Infrastructure interventions for regional biodiversity enhancement. Journal of Environmental Management 2022, 316, 115254.
- Hamer, A.J.; McDonnell, M.J. Amphibian ecology and conservation in the urbanising world: A review. Biol. Conserv. 2008, 141, 2432-2449. [CrossRef]
- European Commission. EU's biodiversity strategy for 2030; https://ec.europa.eu/environment/strategy/biodiversity-strategy-2030_en: 2021.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




