1. Introduction
In recent years, there has been a lot of research on the molecular and proteomic mechanisms by which plants interact with their surroundings. Plants are severely harmed by toxic compounds like arsenic (As), which alters their physiology and metabolic activities (Wiszniewska, 2021). A longer length of submersion in water increases the likelihood that rice plants may accumulate arsenic (Zakaria et al., 2021). The toxicity of arsenic prevents seed germination, reduces photosynthesis, inhibits the growth of the root shoot, and has a negative impact on the protein composition of plants (Akhtar et al., 2021). By increasing osmolality, lowering water potential, decreasing leaf area and transpiration, and creating reactive oxygen species (ROS) through oxidative bursts of the lipid membrane, protein, and DNA, these dangerous metalloid impair osmoregulation in plants (Kaur et al., 2021).
High temperatures, as well as biotic and abiotic pressures, among other harsh climatic variables, have all been shown to negatively affect plants. Arsenic buildup has a negative impact on rice plants' protein levels. Arsenic stress results in protein aggregation and/or disintegration in the endoplasmic reticulum, which affects plant viability (Angulo-Bejarano et al., 2021). Rice is a model plant for molecular study due to its small genome and well-organized database. SDS-PAGE was used to examine the protein expression pattern in rice after exposure to arsenic. The early finding of Altaf et al., (2021) demonstrated that arsenic stress modifies gene expression levels, and altered metabolite levels result in an imbalance in protein levels, which has a deleterious influence on metabolism. It was also investigated the protein expression and banding pattern change under cadmium stress (Priyanka et al., 2021). The protein content of plants was dramatically boosted by iron oxide nanoparticles, which have a direct role in protein synthesis (Lee et al., 2021). When treated to silver nanoparticles, Pisum sativum L. dramatically increased its protein and carbohydrate content (Awan et al., 2020). Different proteins may build up or be generated in plants under stressful circumstances (Tariq et al., 2021).
Arsenic complexes, storage in a different plant compartment, and interaction with metal-binding proteins like metallothioneine (MTs) and phytochelatein are only a few of the various arsenic stress adaption mechanisms employed by plants (Li et al., 2020). Consequently, metallothioneins (MTs), which are produced by stressed plants, are a great biomarker. The stress protein metallothioneins (MTs), which was found, belongs to a class of proteins with a cysteine concentration of 20–30% (Maluin et al., 2021). According to (Ahmad et al., 2020) consequently, stressed plants develop metallothioneins (MTs), which are excellent biomarkers. The stress protein metallothioneins (MTs), which was found, belongs to a class of proteins with a cysteine concentration of 20–30% (Delrue et al., 2021). Studies show that arsenic stress causes DNA strand breakage, changes in gene expression, and chromosome aberration in rice plants. Stress-related genes and transcription factors (TFs) expressed more highly in plants under stressful conditions. Transcription factors (TFs) have DNA-binding domains, oligomerization sites, and signal localization (Tariq et al., 2021).
Arsenate responsive transcriptional factor (WRKY6) is reported to control the expression of arsenate and phosphate in Arabidopsis (Kerchev et al., 2020). R2R3 MYB transcriptional factor expression is increased in response to arsenic III treatment, and this transcriptional regulation of arsenic responses is important. In plants, the MYB protein, which has a conserved DNA binding domain, is made up of a variety of transcriptional factors. Stress dramatically increases the expression of the OsARMI gene in arsenic (III). Under various biotic or abiotic stress situations, these transcriptional factors are controlled (Muzamil et al., 2020). Arsenic-responsive genes' expression was decreased as a result of iron-oxide nanoparticles' restriction of arsenic in rice roots and reduction of arsenic flow in plants (Zhou et al., 2021). The largest superfamily of transcriptional regulators is called WRKY. WRKY proteins differ from other proteins due to the WRKY domain, which has 60 amino acids and a conserved amino acid sequence (WRKY GQK) that adapts to the zinc-binding motif. WRKY proteins have been divided into three groups (I.II.III) and subgroups (IIa.IIb). Transcriptional factors (WRKY) are involved in the formation and regulation of plant growth, participation in the defence system against pathogens, and response to biotic and abiotic stress conditions. These transcriptional factors can be positively or negatively regulated in plants. These rice IIa subfamily members are designated as WRKY62, WRKY28, WRKY71, and WRKY76. OsWRKY28, a transcriptional factor, was up-regulated in rice under arsenate (V) stress (Jaskulak et al., 2019). It was reported that 14-3-3 proteins bind to a variety of signalling molecules and transcriptional regulators. These proteins play a part in regulating the growth and response of plants to diverse biotic and abiotic stressors. According to Khan et al., (2022), OsGF14b, OsGF14C, OsGF14e, and OsGF14f, together known as the "14-3-3" genes, react to stimuli in distinct ways. The treatment with iron oxide nanoparticles changed the expression of the OsGF14 gene in rice plants (Kerchev et al., 2020). The goal of this study was to pinpoint the molecular changes brought on by arsenic exposure in rice plants. Therefore, we investigated the effect of iron oxide NPs on protein pattern variation using SDS PAGE and variations in gene expression for MYB, WRKY, and OsGF14 in rice under arsenic stress.
2. Materials and Methods
2.1. Seeds Collection
'Super Basmati' (SB) rice (Oryza sativa L.) cultivar seeds were received from the NARC (National Agricultural Research Centre) in Islamabad, Pakistan, and iron-oxide nanoparticles were biologically produced from Bacillus subtilis. The seeds were sterilised for three minutes with 1 percent sodium hypochlorite before being gently washed in distilled water to remove any sodium hypochlorite residue (Khan et al., 2021).
2.2. Experimental Procedure
After germination, some seeds were cultivated on petri plates before being transferred to a plastic hydroponic tray with Hoagland solution. In a greenhouse, plants were allowed to grow for 21 days using the previously described method (Akhtar et al., 2021). Following that, seedlings were treated for one week with As2O3 (0, 5, 10, and 15 mg/L), iron oxide nanoparticles (5, 10, and 15 mg/L), or a combined treatmenys (As2O3 + iron oxide NPs). The protein content and gene expression of harvested leaves, including those for MYB, WRKY, and OsGF14, were examined.
2.3. Low Molecular Weight Polypeptide (Metallothioneines)
The cysteine-rich polypeptide metallothioneins was quantified using a spectrophotometric assay in accordance with the method described in a previously published procedure (Khan et al., 2021). Plant samples were homogenized in a solution containing 20 mM Tris HCL (pH 8.6), 0.1% beta-mercaptoethanol, and 0.5 M sucrose. After completely crushing the combination, the MT-containing supernatant was recovered by centrifuging it for 30 minutes at 10,000 rpm. Cold ethanol and chloroform were combined with one millilitre of supernatant, and the mixture was centrifuged for ten minutes at 6,000 rpm. The supernatant was mixed with three millilitres of cooled ethanol, and the mixture was then kept at a low temperature for 60 minutes. 10 minutes of a second centrifugation at 6000 rpm. After rinsing the pellet with a buffer solution, centrifuge at 6000 rpm for 10 minutes (ethanol: chloroform at a ratio of 87:12). A pH 7 solution of 5 mM Tris HCL and 1 mM EDTA was used to suspend dry pellets. The fraction was re-suspended and kept at 25°C for 30 minutes while mixed with 420ml of 0.43 mM NBT and 0.2 M phosphate buffer. At 412 nm, the absorbance was measured using a typical GSH curve.
Quantification of Low Molecular Weight Polypeptide
Plant material was boiled in protein extraction buffer 0.5 M, which contains a small amount of bromophenol blue, 1 M Tris HCL pH 6.8, 100 percent beta-mercaptoethanol, 80 percent glycerol, and 10 percent SDS. For 5 to 10 minutes, the pH of the solution was held at 7.4. following the previously published protocol (Akhtar et al., 2022). A process previously outlined by Laemmli (1970) was followed to estimate protein using Bradford's method. Low molecular weight proteins (MTs) were separated in a 17 percent SDS twin micro gel electrophoresis apparatus (USA) for 2.5 hours at 80 V. The gel was stained for 20 minutes with 20% methanol and Coomassie blue dye (R-250, Sigma). After adding 5% acetic acid to the sample, the bands were compared on the electro photogram to a common protein marker using the previously reported methodology (Akhtar et al., 2020).
2.4. Protein Extraction
In order to determine protein extraction, determined by following method of Akhtar et al., 2022). A 15 mg leaf sample was first homogenised in 400 l of protein-extraction buffer, which contained 2.5% SDS, 5% 2-Beta mercaptoethanol, 0.5 M of Tris HCl, 10% Glycerol, and a trace amount of bromophenol blue. The extract was then incubated at 40°C for 24 hours. The mixture was centrifuged for 10 minutes at 13000 rpm with the supernatant retained at 4 °C. Using the procedure of Bradford, (1976), the protein was validated by contrasting the protein sample with the reference protein BSA (Bovine serum albumin).
SDS PAGE
Proteins were analysed using SDS PAGE in accordance with the methodology established by the method of Akhtar
et al., 2022). Proteins were analysed using SDS PAGE in line with the methodology for (
Table 1). After the comb had set, protein samples and 5 l of protein leader were added to each well. Both gel plates were placed in the gel tank and run at 120 volts for 120 minutes. Staining (40 min) and destination (24 hrs) were carried out for simple band visualisation.
2.5. Gene Expression Analysis
2.5.1. Extraction of RNA and cDNA Synthesis
According to the method by the following method of Akhtar et al., 2022, the triazole technique was used to extract the RNA. One millilitre of trizol was added to fresh plant leaves, which were then vortexed for 15 minutes and heated for 10 minutes at room temperature. The sample was centrifuged at 13000 rpm for 10 minutes. 200 l of chloroform received 2 ml of supernatant before being vortexed for 15 minutes and then incubated for 3 minutes at room temperature. The sample was centrifuged once again for 15 minutes at 4 degrees Celsius and 13000 rpm. A new tube was used to collect the supernatant, and 5 ml of isopropanol was added before the mixture was incubated at 37°C for 10 min. Following a ten-minute, 13000 rpm centrifugation of the mixture, the pellet was washed with ethanol before being dried.
2.5.2. Polymerase Chain Reaction (PCR) for Gene-Specific Families
Using primers for gene-specific families including MYB, WRKY, and OsGF14, the cDNA was amplified. The PCR condition was initially denatured for 5 min at 95°C during the heat cycle, then extended for 12 min at 72°C after 30 cycles of 95°C for 20 sec, 60°C for 30 sec, and 72°C for 40 sec. Using the gel documentation system, bands were observed on a 1.5% agarose gel after the PCR product was generated.
2.6. Statistical Analysis
There were two runs of the experiment. Analysis of variance was performed to compare the "means" to see if there were any significant differences. The "Duncan" multiple range test was performed in conjunction with statistics 9 software to determine the significance of the difference between treatments (version 10).
3. Results
3.1. Low Molecular Weight Polypeptide (Metallothioneines)
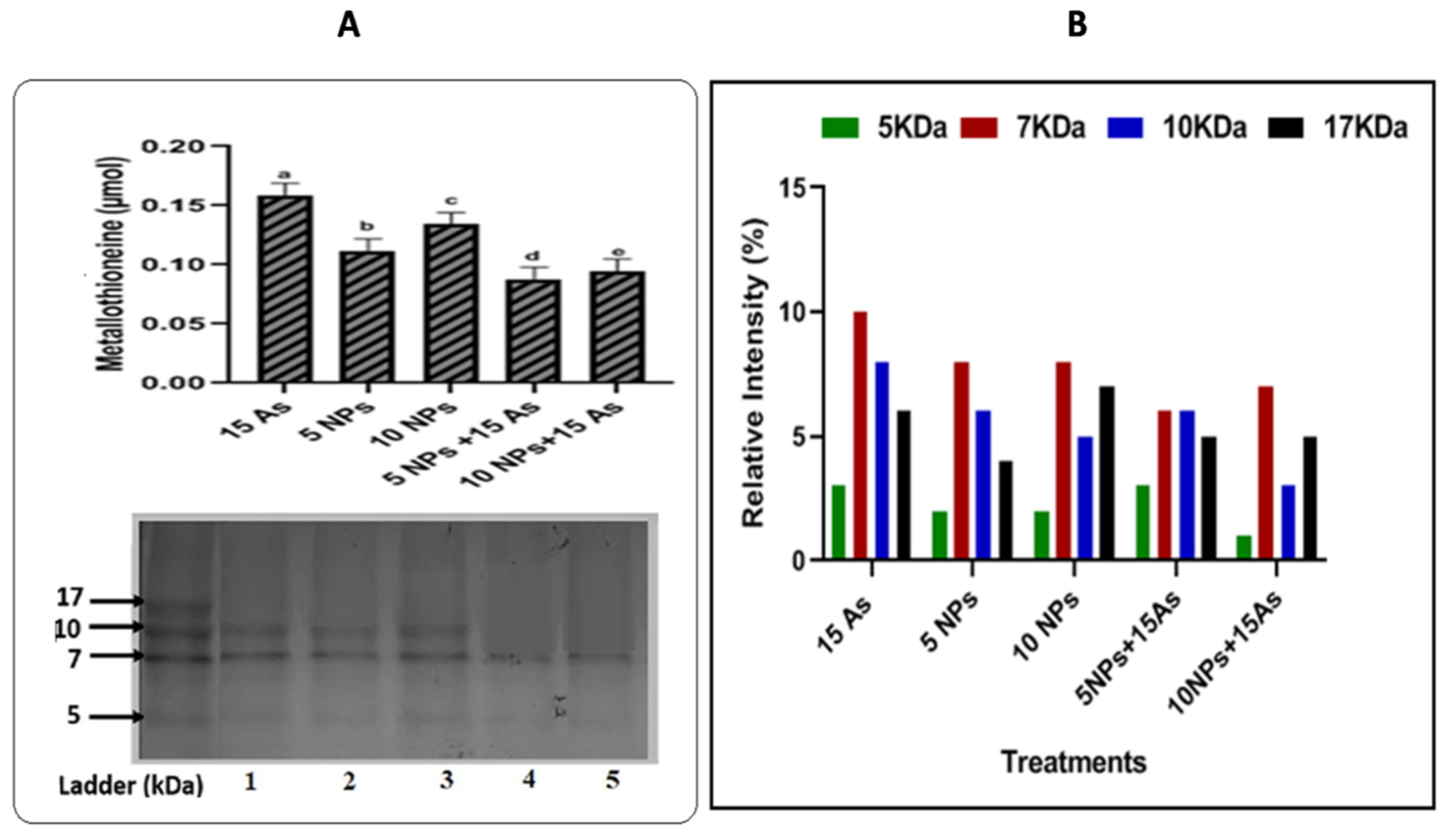
The concentration of MTs in the control and arsenic (As) treated samples was calculated using the standard glutathione curve as a guide. As initially reported in the literature by Palmiter (2004) and Tsugama et al. (2011), 1 Mol of MTs equals 20 Mol of GSH. Plants cultivated with arsenic, iron oxide NPs, and As + iron oxide NPs all exhibited MTs concentrations that could be seen (
Figure 1). Iron oxide NPs decreased the content of MTs in plants grown in arsenic-contaminated water, which was observed. MT levels in arsenic-treated plants were as high as 15 ppm (0.159 mol). Iron oxide NPs considerably decreased the MTs (0.112 mol) and (0.134 mol) with relation to 15 ppm arsenic in the treated plant at 5 and 10 ppm (MTs: 0.11 mol and 0.134 mol). However, a combined strategy (5 As + 5 iron oxide NPs) reduced the. For the investigation of MT expression, SDS PAGE profile was used. There was an observed maximum optical density for each band. Different gel concentrations were used to view the polypeptides. On a 17% SDS PAGE, electrophoresis revealed numerous polypeptide bands. The combined treatment of NPs and arsenic (5 iron oxide NPs + 15As) demonstrated low bands intensity (6.36%) at 7kDa in contrast to 15ppm of As treated plants (10.2%). The polypeptide density raised in iron oxide NPs-treated plants whereas it was lacking in polypeptide bands in arsenic-treated plants, demonstrating the protective role iron nanoparticles play in arsenic-contaminated water (
Figure 1A,B).
3.2. Denatured Sodium Dodecyl Sulfate SDS-PAGE
A variety of arsenic treatments were applied to the total extracted protein, either alone or in combination with iron oxide nanoparticles (NPs). To gauge the expression of these proteins, SDS PAGE was employed. Protein banding intensity in D.W. 5, 10, and 15ppm NPs was higher than it was in samples that had been exposed to arsenic (
Figure 2A,B). Several protein bands with various intensities at molecular weights (26, 34, 43, 55, 72, 95, 103, 180, and 190KDa) were seen in plants cultivated in distilled water. The protein bands were shown to be dense after being exposed to 5 ppm of iron oxide NPs, with banding intensity peaks at 55, 72, and 180 kDa markers (17.2, 4.3, and 15.3%), respectively. Plants possess RNA polymerases II, nucleases, peroxidases, and lipoxygenases.
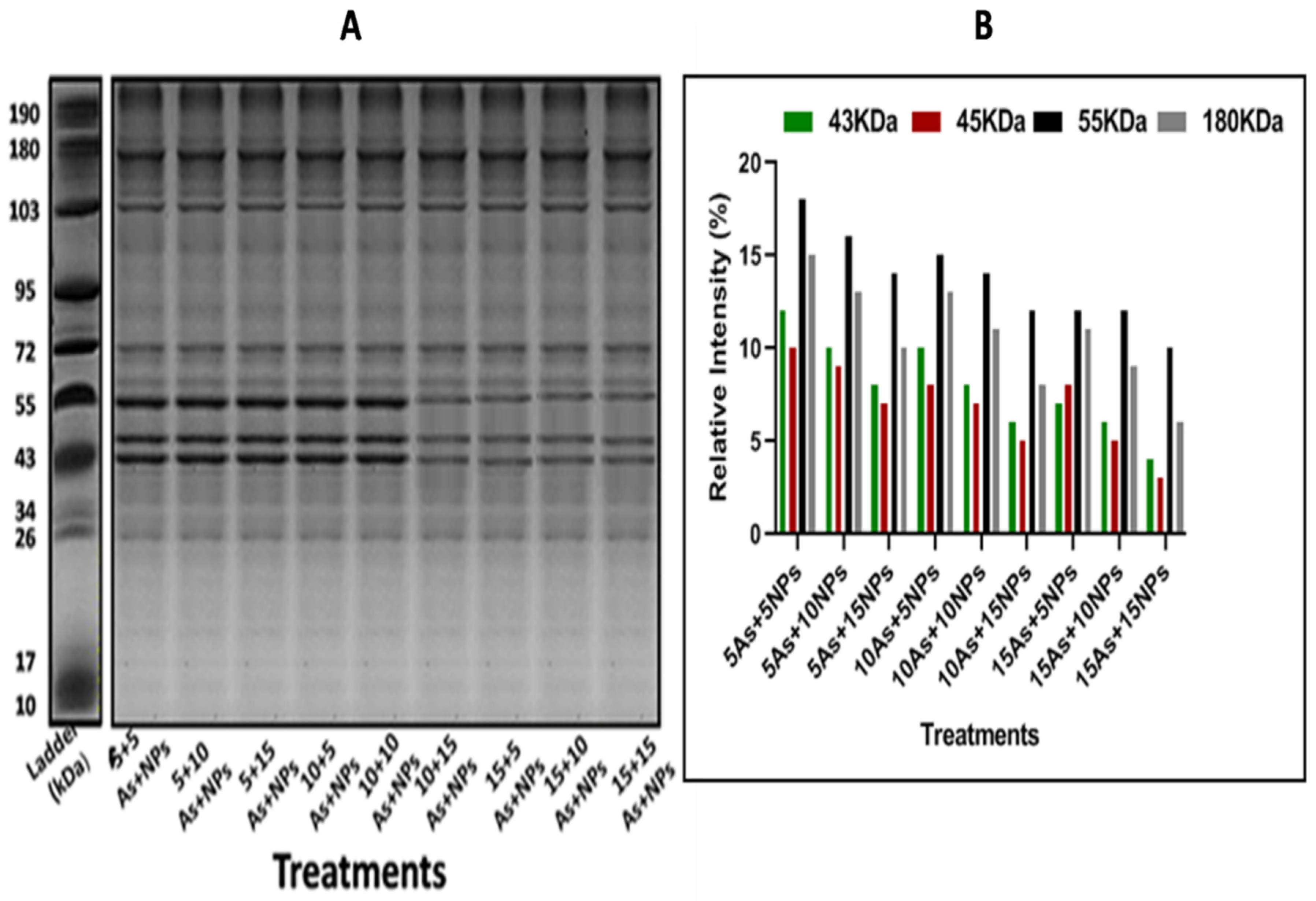
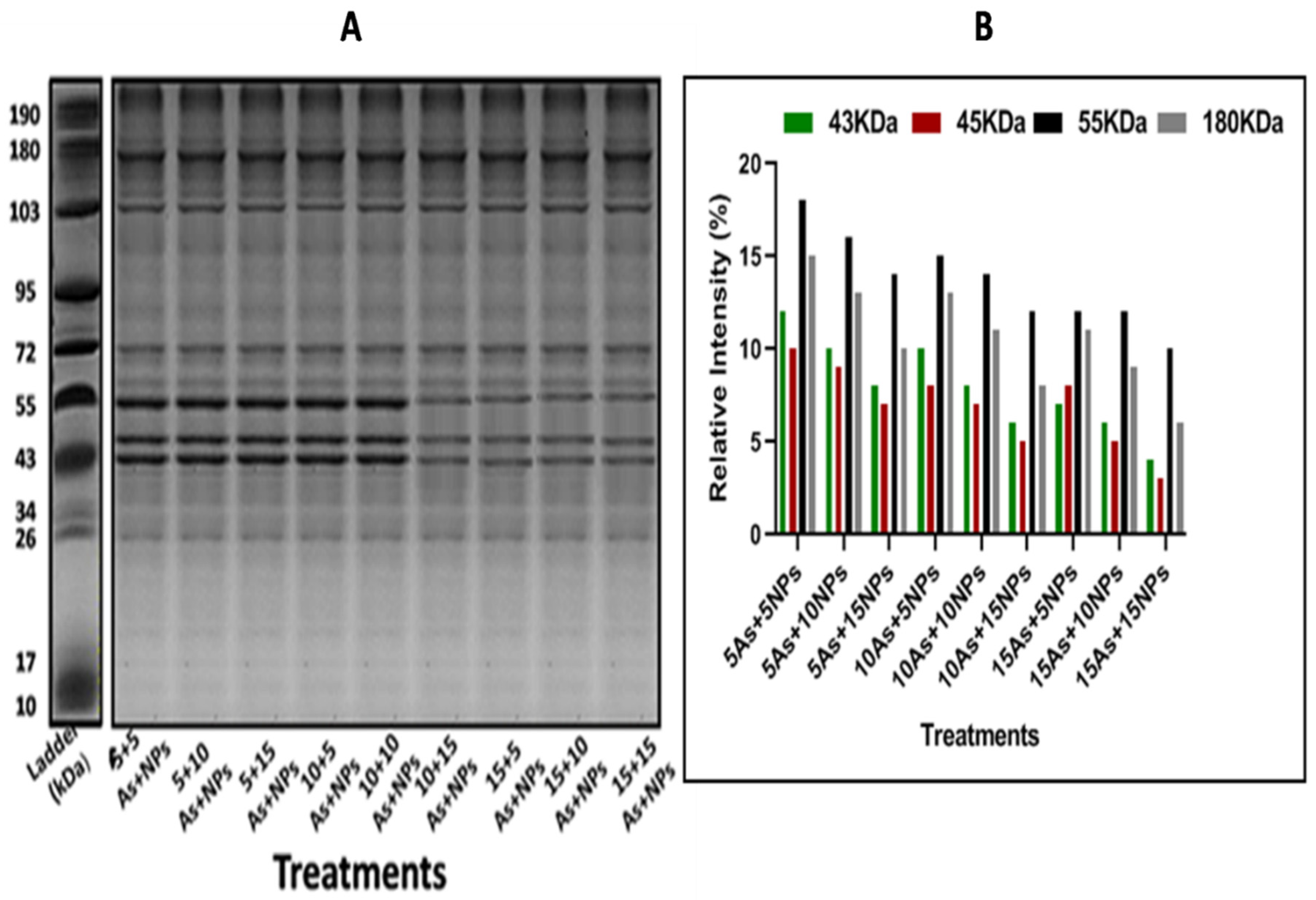
In plants raised in arsenic-contaminated water, protein banding expression was altered. At 15ppm of arsenic treatment, it was found that protein banding expression and intensity were low (8.3, 2.2, and 7.5KDa) at various marker sizes (55, 72, and 180KDa) (
Figure 2A,B). According to the literature, the plant lacked lipoxygenases and RNA polymerase II, although it did have low levels of nucleases and peroxidases. It was discovered that treatments with smaller concentrations of iron nanoparticles produced the strongest banding. At the combined treatment 5NPs+15As, protein banding expression and intensity were low (7.6, 8.1, 12.3, and 11.3) at marker sizes 43, 45, 55, and 180KDa (
Figure 3A,B).
3.3. Gene Expression Analysis
The expression profiles of the arsenic sensitive genes MYB, WRKY, and OsGF14 transcriptional factor were determined in rice plants grown in iron oxide NPs and arsenic alone or in combination treatments (As+ iron oxide NPs).
3.3.1. Expression Profile of Arsenic Responsive MYB Transcriptional Factor
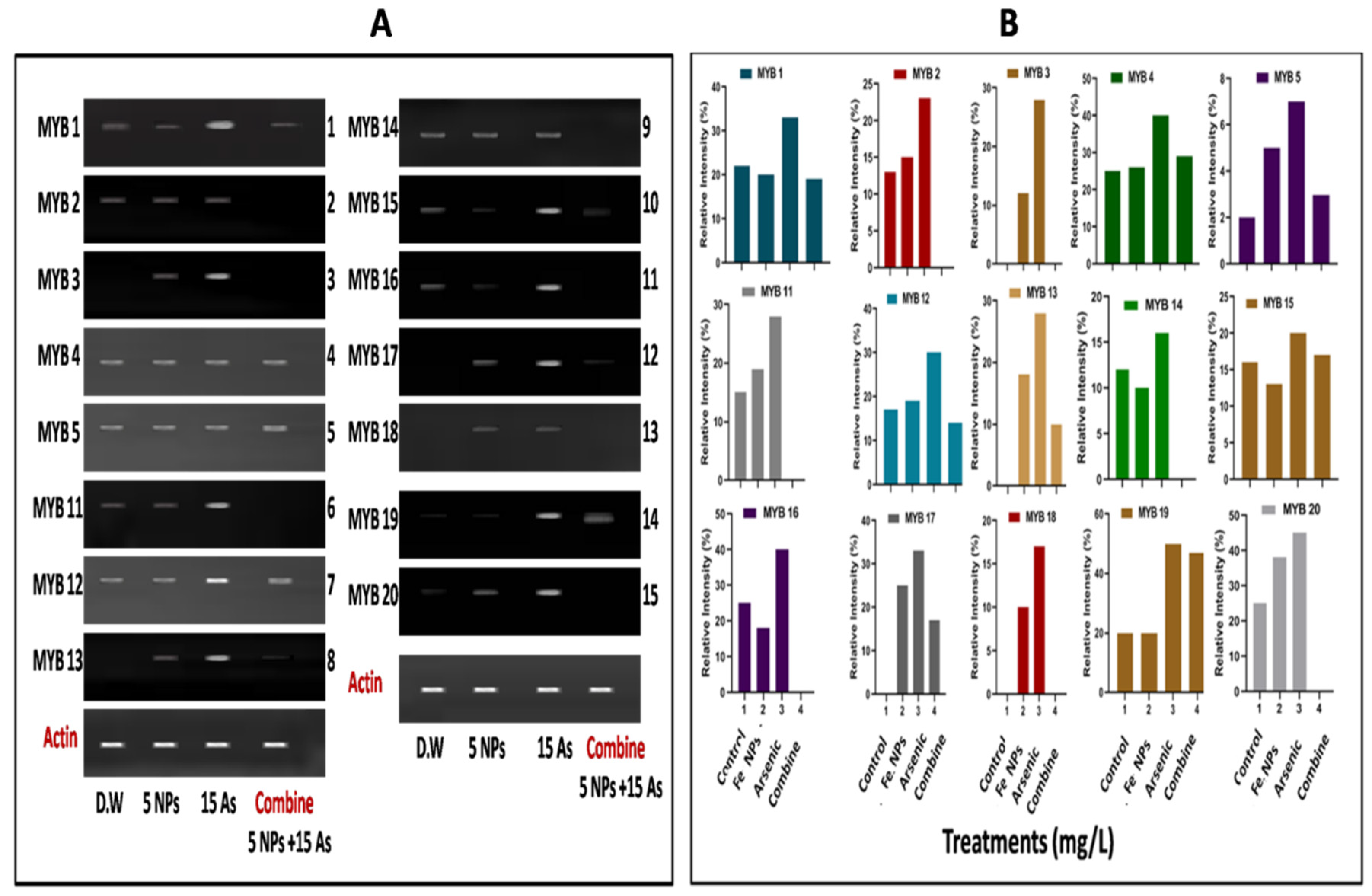
The 20 MYB gene families have a big impact on the differentiation and plant growth processes in the rice plant. In the current study, we looked at the expression of 15 MYB genes in plants growing in distilled water, water that had been contaminated with arsenic, water that had iron oxide nanoparticles, and a combined treatment (As+ iron oxide nanoparticles). RT-PCR was utilised to analyse the transcriptional profile of arsenic responsive transcriptional factors including MYB in leaves using control and treated plants. The "MYB genes" (MYB 1, MYB 2, MYB 3, MYB 4, MYB 5, MYB 11, MYB 12, MYB 13, MYB 14, MYB 15, MYB 16, MYB 17, MYB 18, MYB 19 and MYB 20) were shown to be up-regulated in arsenic-treated plants in (32.2, 22, 29, 40, 7, 29, 30 (
Figure 4A,B).
3.3.2. Expression Profile of WRKY Transcriptional Factor
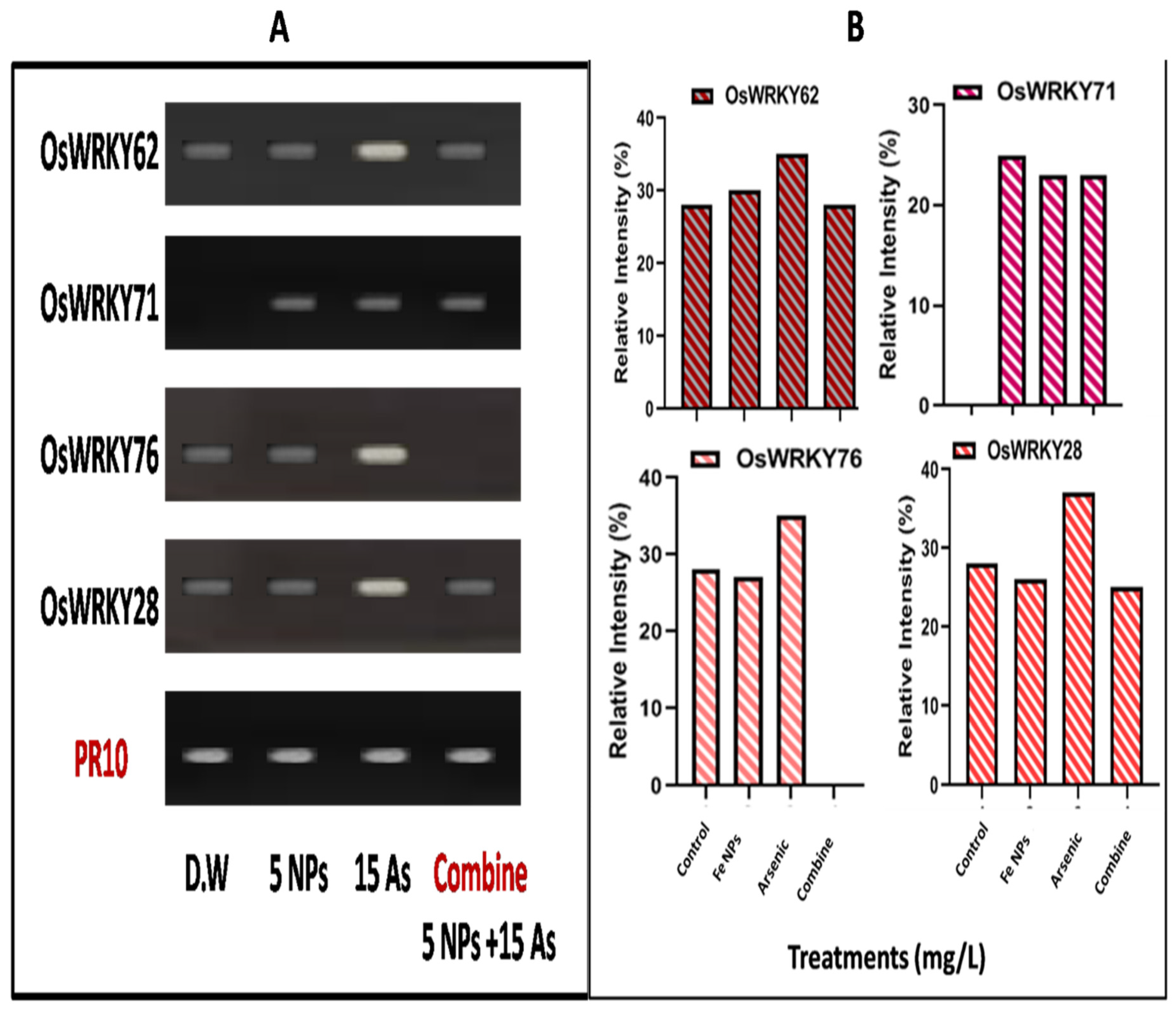
Many biological processes in plants depend on the WRKY transcriptional factors, but their reaction to biotic and abiotic stresses is particularly important. In the current study, we investigated the expression of four WRKY genes in plants growing in distilled water, water contaminated with arsenic, iron oxide nanoparticles, and a combined treatment (As+ iron oxide nanoparticles). Using RT-PCR, the WRKY transcriptional profile in untreated and treated plant leaves was compared. As compared to plants grown in distilled water, where banding intensities were, respectively, 28.1, 27.2, and 27.3%, arsenic-treated plants had an up-regulation of "WRKY genes" together with greater banding intensities (WRKY 62, 71, 76, and 28) (
Figure 5A,B).
3.3.3. Expression Profile of OsGF14 Gene Family
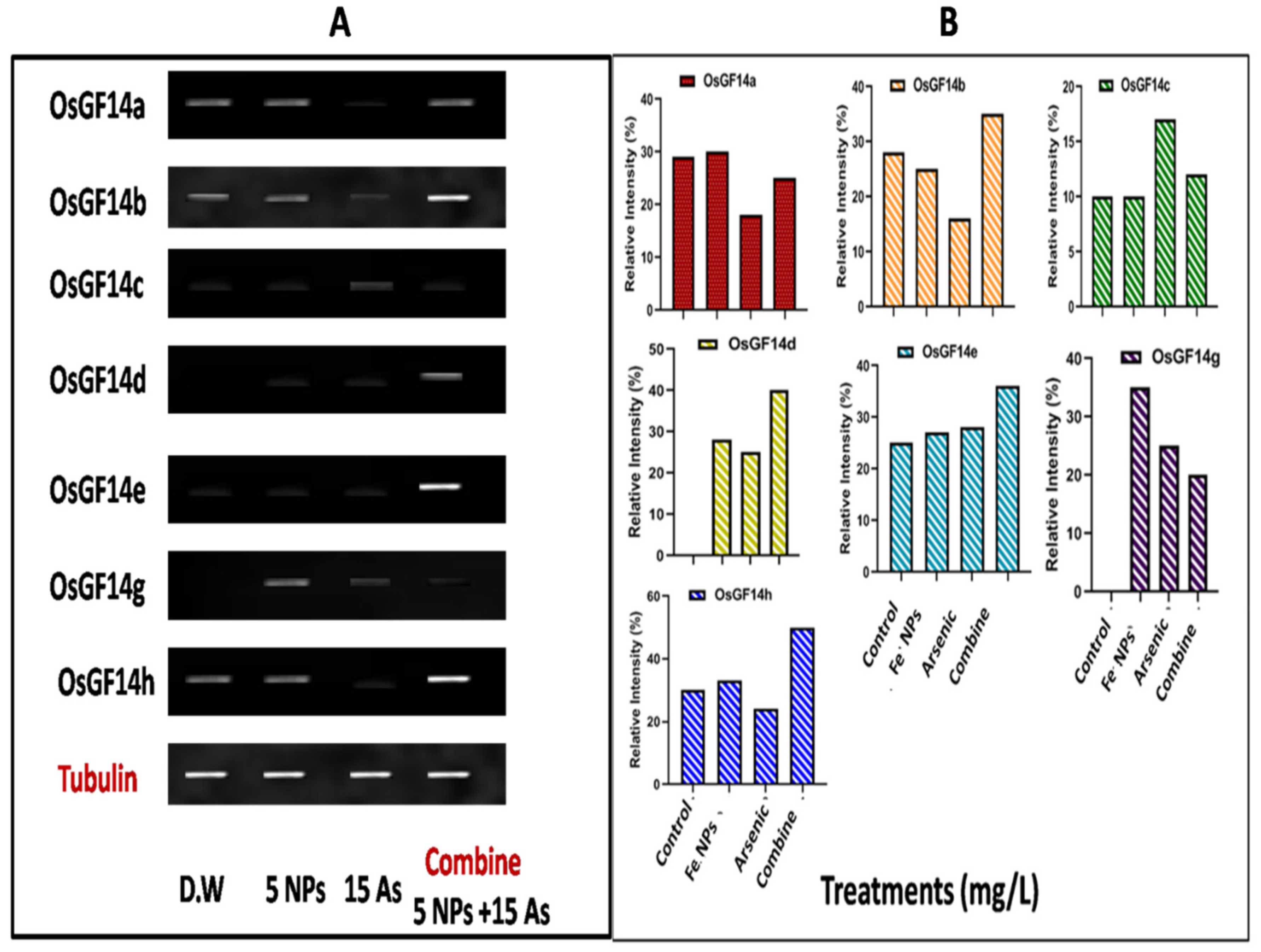
The widely present protein OsGF14 was essential for signalling, regulating plant growth, metabolism, and adaptation to numerous biotic and abiotic stresses. We investigated the effects of iron oxide nanoparticles generated by
Bacillus subtilis on the OsGF14 expression profile in rice plants (
Oryza sativa L.) growing in iron oxide NPs and arsenic (As) polluted water in the current study. OsGF14 a, b, c, d, e, g, and h, as well as banding intensity (15.2, 13.1, 17.1, 22, 25.1, 26.2 and 20.3%), in the arsenic-treated plants were up-regulated. Expression was less than when arsenic alone was treated when iron oxide nanoparticles were also added. Additionally, administration of iron oxide NPs resulted in down-regulation and a (
Figure 6A,B).
4. Discussions
Due to rapid industrialisation, human activity, and agricultural practises, arsenic levels in drinking and irrigation water have considerably grown. Arsenic stress causes major proteome and molecular changes that have an impact on plant growth. As a result, the demand for more efficient arsenic adsorbents is growing. Particularly nanoparticles have drawn a lot of interest because of their distinctive morphological, textural, and structural properties (Javaid et al. 2020). It has been demonstrated that iron oxide NPs promote nutritional reserves and increase molecular activity to sustain growth in arsenic stress. The direct enhancement of nutrient intake and water molecule transport from the plant's roots to its higher sections by these iron ions also improved cell integrity (Mitra et al., 2021)
The formation of metallothioneins (MTs) by plants under arsenic stress is a well-known phenomenon (Gu et al., 2020). Recent research revealed that a plant grown in arsenic-tainted water contained a sizable amount of MTs. Our findings support (Ashraf et al., 2021) According to early research; plants under arsenic stress have higher MTs levels. High-intensity polypeptide in the 5-18KDa range was verified by SDS-PAGE profiling. Even while a higher MTs content in Brassica plants under Cd stress was confirmed, according to a previous study (Hossain et al., 2021). Iron oxide NPs have shown a significant tolerance to arsenic in contaminated water by lowering the MTs level.
Stress caused by arsenic in rice plants affects a variety of physiological systems. Proteomic analysis is a useful technique for monitoring changes in protein levels. In this work, we subjected arsenic-stressed rice leaves to an SDS PAGE. We found that proteins are down-regulated in arsenic stress (
Figure 1,
Figure 2 and
Figure 3). A significant change in the banding pattern was seen under arsenic stress, which restricts protein synthesis and inhibits plant growth due to their phytotoxicity (Wang
et al., 2021). Arsenic may disconnect the P flow and interfere with ATP-dependent functions as well as energy flow. This decrease in protein synthesis, inhibition of enzyme activity, drop in phosphorus content, arsenic accumulation, changed protein folding, and damaged protein structure all contribute to a decrease in the rate of protein synthesis in plants (Maluin
et al. 2021). However, because Rubisco subunits are destroyed in plants under arsenic stress, the severe down-regulation was noticed (Ashraf
et al., 2021). RT-PCR was utilised to evaluate the rice arsenic sensitive transcriptional factor (MYB) under various treatments. However, it has been shown that under arsenic stress, other expression patterns exist. Results showed that iron oxide NPs downregulated the expression of the gene whereas arsenic treatment upregulated it. The current results support earlier suggestions that rice damage from arsenic could be mitigated (Bidi
et al., 2021).
The WRKY gene family is a plant-specific transcription factor (TF) that is important for a number of abiotic stress response pathways, including those in response to heat, salinity, alkali, and UV radiation. WRKY family members have a range of control mechanisms. In a nutshell, the WRKY protein can activate or inhibit downstream target gene transcription when combined with W-box regions (Gu
et al., 2020). The proper signalling is started and sent to the cell interior when a plant perceives stress. Reactive oxygen species (ROS) and calcium ions are exchanged during signal transduction in the majority of cells. Then, to regulate the actions of related TFs, MPKs and other protein kinases are activated. The plant reacts under stress as a result. Abiotic stresses can quickly differentially express several WRKY TFs, improving signal transmission and regulating the expression of related genes. Over time, it has been shown that WRKY TFs participate in complex regulatory networks and mechanisms connected to external abiotic stressors as well as plant growth and development (Priyanka
et al. 2021). The arsenic responsive WRKY transcriptional factor's expression profile was examined after being exposed to arsenic and iron oxide nanoparticles (
Figure 4). The genes belonging to WRKY are up-regulated in response to arsenic stress. The results showed that plants under arsenic stress upregulate the expression of the WRKY gene while plants treated with iron oxide NPs downregulate the expression of the WRKY transcriptional factor. Iron oxide NPs reduce arsenic translocation in plants and the amount of arsenic in rice roots, both of which reduce the expression of arsenic responsive genes (Delrue
et al. 2021)
Signal transmission, nutrition shortage adaptation, abiotic stress tolerance, metabolic regulation, plant-pathogen interactions, and other biological processes in plants are all known to depend on a family of ubiquitous regulators known as 14-3-3 proteins. In this study, the impact of As stress on the rice plant's (Oryza sativa) 14-3-3 gene expression profile was investigated. Multiple lines of evidence suggest that the 14-3-3 proteins of higher plants bind to various transcription factors and signalling molecules. According to past studies, 14-3-3 proteins are involved in how plants react to environmental challenges such salt, oxidative stress, pathogen attack, and food shortage. Heavy metals, in particular metalloid As, have a negative impact on plant growth because of their interaction with sulfhydryl groups, disruption of membranes, interference with ionic equilibrium, and induction of oxidative stress. Maintaining ion homeostasis depends on the regulation of plant plasma membrane H+ ATPase by 14-3-3 protein binding. The pH and voltage gradient that H+ATPases produce across the plasma membrane is what drives secondary ion and nutrient transport into and out of the cells (Ahmad et al., 2020). In rice plant cells, the OsGF14 gene's expression profile was discernible after diverse treatments. However, it was noted that different OsGF14 gene expression patterns were observed under arsenic stress conditions. It has been demonstrated that iron oxide nanoparticles and arsenic treatment have opposite effects on the OsGF14 gene's regulation. The current results showed that iron oxide NPs enhanced plant development, increased plant resistance to arsenic, and changed the expression of the OsGF14 gene (Maluin et al. 2021).
5. Conclusions
The recent study showed how iron oxide nanoparticle concentrations at lower levels regulate the remediation mechanism at the proteome and molecular levels. The application of iron oxide nanoparticles to the plant protein profile in this study's SDS PAGE analysis showed promising results. Arsenic and iron oxide nanoparticle therapy to increase the expression of plant proteins. Arsenic stress increases MYB, WRKY, and OsGF14 transcription factors, whereas iron oxide nanoparticles reduce their expression. Because arsenic only partially reached the plants, expression was decreased.
Author Contributions
S.K was the main author and wrote the manuscript, carried out the experiment and performed the numerical calculations for the suggested experiment, verified the formulation and statistical methods, contributed to sample preparation and interpretation of the results, took the lead in writing the manuscript, designed the figures, worked out almost all of the technical details, and contributed to the final version of the manuscript. N.A was involved in planning and supervised the work, the main conceptual ideas and proof outline, verified the statistical methods, encouraged to investigate, and supervised the findings of this work. E.S.R. provided critical feedback and helped to shape the research. All authors have read and agreed to the published version of the manuscript. S.U.R. provided critical feedback and helped shape the research, analysis, and manuscript. M.J. help in performed the experiments, computational framework and analyzed the data, performed the calculations, and carried out the implementation, contributed to sample preparation and interpretation of the results.
Funding
The research was partly supported by a grant No. 4372 from the Higher Education commission (HEC), Pakistan.
Acknowledgments
Quaid-i-Azam University, Islamabad and Centralized Resource Laboratory (CRL) University of Peshawar has been greatly acknowledged for characterization of Bacillus subtilis synthesized Fe3O4 NPs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Rehman, Z.U.M.; Rha, E.S.; Jamil, M. Zinc oxide nanoparticles enhance the tolerance and remediation potential of Bacillus spp. against heavy metals stress. Adsorpt. Sci. Technol. 2021, 9, 110–115. [Google Scholar] [CrossRef]
- Altaf, R.; Altaf, S.; Hussain, M.; Shah, R.U.; Ullah, R.; Ullah, M.I.; Datta, R. Heavy metal accumulation by roadside vegetation and implications for pollution control. PLoS ONE 2021, 16, e0249147. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef]
- Ashraf, I.; Ahmad, F.; Sharif, A.; Altaf, A.R.; Teng, H. Heavy metals assessment in water, soil, vegetables and their associated health risks via consumption of vegetables, District Kasur, Pakistan. SN Appl. Sci. 2021, 3, 552. [Google Scholar]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Brestic, M. Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L. ) varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Delrue, F.; Ribeiro de Jesus Cerqueira, M.; Compadre, A.; Alvarez, P.; Fleury, G.; Escoffier, C.; Sassi, J.F. Hydroponic farm wastewater treatment using an indigenous consortium. Processes 2021, 9, 519. [Google Scholar] [CrossRef]
- Gu, M.; Hao, L.; Wang, Y.; Li, X.; Chen, Y.; Li, W.; Jiang, L. The selective heavy metal ions adsorption of zinc oxide nanoparticles from dental wastewater. Chem. Phys. 2020, 534, 110750. [Google Scholar] [CrossRef]
- Hossain, S.; Hossain, S.; Islam, M.R.; Kabir, M.H.; Ali, S.; Islam, M.S.; Mahmud, Z.H. Bioremediation of hexavalent chromium by chromium resistant bacteria reduces phytotoxicity. Int. J. Environ. Res. Public Health 2020, 17, 6013. [Google Scholar] [CrossRef]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Chaabene, Z.; Kacprzak, M.; Vandenbulcke, F. Bioaccumulation, antioxidative response, and metallothionein expression in Lupinus luteus L. exposed to heavy metals and silver nanoparticles. Environ. Sci. Pollut. 2019, 26, 16040–16052. [Google Scholar] [CrossRef]
- Javaid, S.; Zaman, Q.; Sultan, K.; Riaz, U.; Aslam, A.; Saba Sharif, N.E.; Ibraheem, S. Heavy metals stress, mechanism and remediation techniques in rice (Oryza sativa L.): A review. Pure Appl. Biol. 2020, 9, 403–426. [Google Scholar] [CrossRef]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant. 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 40, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Rolly, N.K.; Al Azzawi, T.N.I.; Imran, M.; Mun, B.G.; Lee, I.J.; Yun, B.W. Lead (Pb)-induced oxidative stress alters the morphological and physio-biochemical properties of rice (Oryza sativa L. ). Agronomy 2021, 11, 409. [Google Scholar] [CrossRef]
- Lee, S. Recent Advances on nitrogen use efficiency in rice. Agronomy 2021, 11, 753. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Sun, J.; Mao, X.; Wang, J.; Liu, H.; Zou, D. Heavy metal stress-associated proteins in rice and arabidopsis: Genome-wide identification, phylogenetics, duplication, and expression profiles analysis. Front. Genet. 2020, 11, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Maluin, F.N.; Hussein, M.Z.; Nik Ibrahim, N.N.L.; Wayayok, A.; Hashim, N. Some emerging opportunities of nanotechnology development for soilless and microgreen farming. Agronomy 2021, 11, 1213. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Shadangi, S.; Mohapatra, P.K.D.; Panneerselvam, P. Rhizobacteria mediated seed bio-priming triggers the resistance and plant growth for sustainable crop production. Curr. Res. Microb. Sci. 2021, 2, 100071. [Google Scholar] [CrossRef]
- Muzammil, M.; Zahid, A.; Breuer, L. Water resources management strategies for irrigated agriculture in the Indus basin of Pakistan. Water 2020, 12, 1429. [Google Scholar] [CrossRef]
- Priyanka, N.; Geetha, N.; Manish, T.; Sahi, S.V.; Venkatachalam, P. Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol. Rep. 2021, 8, 295–302. [Google Scholar]
- Tariq, F.; Wang, X.; Saleem, M.H.; Khan, Z.I.; Ahmad, K.; Saleem Malik, I.; Ali, S. Risk assessment of heavy metals in basmati rice: Implications for public health. Sustainability 2021, 13, 8513. [Google Scholar] [CrossRef]
- Wang, S.; Liu, T.; Xiao, X.; Luo, S. Advances in microbial remediation for heavy metal treatment: A mini review. J. Leather Sci. Eng. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Wiszniewska, A. Priming strategies for benefiting plant performance under toxic trace metal exposure. Plants 2021, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Zulkafflee, N.S.; Mohd Redzuan, N.A.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Abdull Razis, A.F. Understanding potential heavy metal contamination, absorption, translocation and accumulation in rice and human health risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Rui, Y. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2021, 11, 26. [Google Scholar] [CrossRef]
Figure 1.
The impact of iron oxide nanoparticles produced by Bacillus subtilis on the levels of metallothioneines in rice (Oryza sativa L.) in arsenic-contaminated water, as expressed in (A) quantification and expression (B) intensity. Iron oxide nanoparticles (5, 10 ppm), 15 ppm arsenic (As), and combined treatments (15 ppm As + 5 ppm iron oxide NPs, 15 ppm As + 10 ppm iron oxide NPs). Data are shown as Mean + S.E., with different letters on each error bar denoting statistical significance at the 0.05 level.
Figure 1.
The impact of iron oxide nanoparticles produced by Bacillus subtilis on the levels of metallothioneines in rice (Oryza sativa L.) in arsenic-contaminated water, as expressed in (A) quantification and expression (B) intensity. Iron oxide nanoparticles (5, 10 ppm), 15 ppm arsenic (As), and combined treatments (15 ppm As + 5 ppm iron oxide NPs, 15 ppm As + 10 ppm iron oxide NPs). Data are shown as Mean + S.E., with different letters on each error bar denoting statistical significance at the 0.05 level.
Figure 2.
The effect of Bacillus subtilis synthesized iron oxide nanoparticles on rice (Oryza sativa L.) (A) Protein banding profile and (B) protein banding intensity in arsenic (As) contaminated water. Lane 1: (distilled water), Lane 2: (5ppm NPs), Lane 3: (10ppm NPs), Lane 4: (15ppm NPs), Lane 5: (5ppm As), Lane 6: (10 ppm As) and Lane 7: (15ppm As).
Figure 2.
The effect of Bacillus subtilis synthesized iron oxide nanoparticles on rice (Oryza sativa L.) (A) Protein banding profile and (B) protein banding intensity in arsenic (As) contaminated water. Lane 1: (distilled water), Lane 2: (5ppm NPs), Lane 3: (10ppm NPs), Lane 4: (15ppm NPs), Lane 5: (5ppm As), Lane 6: (10 ppm As) and Lane 7: (15ppm As).
Figure 3.
The Bacillus-subtilis synthesized iron oxide naoparticles effect on rice (Oryza sativa L.) (A) protein banding profile and (B) protein banding intensity in arsenic(As)-contaminated water. Lane 1: (5As+5NPs ), Lane 2: (5As+10NPs), Lane 3: (5As+15NPs), Lane 4: (10As+5NPs), Lane 5: (10As+10NPs), Lane 6: (10As+15NPs), Lane 7: (15As+5NPs). Lane 8: (15As+10NPs). Lane 9: (15As+15NPs).
Figure 3.
The Bacillus-subtilis synthesized iron oxide naoparticles effect on rice (Oryza sativa L.) (A) protein banding profile and (B) protein banding intensity in arsenic(As)-contaminated water. Lane 1: (5As+5NPs ), Lane 2: (5As+10NPs), Lane 3: (5As+15NPs), Lane 4: (10As+5NPs), Lane 5: (10As+10NPs), Lane 6: (10As+15NPs), Lane 7: (15As+5NPs). Lane 8: (15As+10NPs). Lane 9: (15As+15NPs).
Figure 4.
The (A) Myeloblastosis (MYB) gene and (B) Gene intensity in rice (Oryza sativa L.) grown in water contaminated with arsenic (As) is affected by iron oxide nanoparticles generated by Bacillus subtilis. Various methods were used, including distilled water (D.W), iron oxide nanoparticles (NPs) at 5 ppm, 15 ppm of arsenic (As), and a combination strategy.
Figure 4.
The (A) Myeloblastosis (MYB) gene and (B) Gene intensity in rice (Oryza sativa L.) grown in water contaminated with arsenic (As) is affected by iron oxide nanoparticles generated by Bacillus subtilis. Various methods were used, including distilled water (D.W), iron oxide nanoparticles (NPs) at 5 ppm, 15 ppm of arsenic (As), and a combination strategy.
Figure 5.
Rice (Oryza sativa L.) grown in water contaminated with arsenic (As) and the effects of iron oxide nanoparticles generated by Bacillus subtilis on the (A) WRKY genes and (B) relative intensity. Various methods, including combination treatment, distilled water (D.W), iron oxide nanoparticles (NPs) at 5 ppm, and 15 ppm of arsenic (As).
Figure 5.
Rice (Oryza sativa L.) grown in water contaminated with arsenic (As) and the effects of iron oxide nanoparticles generated by Bacillus subtilis on the (A) WRKY genes and (B) relative intensity. Various methods, including combination treatment, distilled water (D.W), iron oxide nanoparticles (NPs) at 5 ppm, and 15 ppm of arsenic (As).
Figure 6.
Effect of iron oxide nanoparticles produced by Bacillus subtilis on the (A) OsGF14 genes and (B) relative intensity in rice (Oryza sativa L.) grown in water contaminated with arsenic (As). Different approaches Arsenic (As) 15 ppm, iron oxide nanoparticles (5 ppm), and combined treatment (5 iron oxide NPs + 15 As) in distilled water.
Figure 6.
Effect of iron oxide nanoparticles produced by Bacillus subtilis on the (A) OsGF14 genes and (B) relative intensity in rice (Oryza sativa L.) grown in water contaminated with arsenic (As). Different approaches Arsenic (As) 15 ppm, iron oxide nanoparticles (5 ppm), and combined treatment (5 iron oxide NPs + 15 As) in distilled water.
Table 1.
Chemical composition of running and stacking gel.
Table 1.
Chemical composition of running and stacking gel.
| Running gel Composition |
Stacking gel composition |
| Component |
Quantity |
Components |
Quantity |
1.5M Tris-HCl (pH 8.8)
Distilled water (DW)
30% of Acrylamide
20% of SDS
10% of APS
TEMED |
2.6ml
3.2ml
4ml
100µl
100µl
7-10µl |
0.5M Tris-HCl (pH 6.8)
Distilled water (DW)
30% of Acrylamide
10% of APS
20% of SDS
TEMED |
2.50ml
3.975ml
600µl
50µl
100µl
5-7µl |
Table 2.
Polymerase chain reaction (PCR) cycle.
Table 2.
Polymerase chain reaction (PCR) cycle.
| |
Step |
Temperature |
Time |
Number of cycle |
| Step 1 |
Initial denaturation |
95°C |
5 min |
- |
| |
Denaturation |
95°C |
20 sec |
30 |
| Step 2 |
Annealing |
60°C |
30 sec |
- |
| |
Extension |
72°C |
40 sec |
- |
| Step 3 |
Final Extension |
72°C |
12 min. |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).