Submitted:
04 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Structure and Role of the Blood-Brain Barrier
3. MSCs as a Therapeutic Option in CNS Diseases
- Neuroprotective effect: MSCs have demonstrated to have an important neuroprotective effect, as they secrete neurotrophic growth factors such as glial cell-derived neurotrophic factor, VEGF, brain-derived neurotrophic factor and nerve growth factor (NGF) [79], as well as anti-apoptotic factors like Bcl-2 [80]. These factors enable MSCs to promote nervous regeneration, inhibit neuronal apoptosis, and induce endogenous neurogenesis. For example, Oh et al. [81] demonstrated that intravenous injection of MSCs increased hippocampal neurogenesis and differentiation of neural progenitor cells into mature neurons in Aβ-treated mice (AD model) by augmenting the Wnt signaling pathway. Additionally, MSCs may inhibit stroke-associated apoptosis through the Bcl-2 pathway in neurons and astrocytes from rats [82]. Besides, MSCs can transfer healthy mitochondria to damaged cells, protecting neural stem cells from neurotoxic agents. MSCs may transfer this organelle in various ways, including gap junctions, cell fusion, microvesicles, and through tunnelling nanotube formation [83]. Mitochondria play a crucial role in maintaining metabolic homeostasis, and defects such as membrane leakage, electrolyte imbalances, activation of pro-apoptotic pathways, and mitophagy have been implicated in the pathogenesis of various CNS disorders [84]. It has been demonstrated that the ability of MSCs to transfer healthy mitochondria to damaged cells protects neural stem cells from neurotoxic agents [85], and has garnered significant attention in the field of cellular therapy for CNS disorders.
- Immunomodulatory role: MSCs can interact with the immune system and participating in both innate and adaptive immunity due to their significant immunoregulatory functions. This indicates that, depending on the environment in which MSCs are introduced, they can modulate the response. Thus, in an inflammatory environment, MSCs exhibit anti-inflammatory behavior. By expressing different molecules such as transforming growth factor β, indoleamine 2,3-dioxygenase, prostaglandin E2, nitric oxide, and interleukin-10 (IL-10), they can interact with immune cells either through direct cell-to-cell contact or via paracrine activity [86,87,88,89,90]. MSCs can also modulate the macrophage/microglia polarization upregulating the ratio of anti- versus pro-inflammatory responses [91], suppress Th1 and Th17 responses, enhance the maturation of DCs from monocytes, and enhance the Th2 response and the generation of Forkhead Box P3 positive Treg population. Moreover, some studies reported that the secretion of IL-6 by MSCs can inhibit astrocyte apoptosis, increase the neuroprotective population of astrocytes, and reduce neuron damage post-injury [92].
- Regulation of protein clearance: treatment with MSCs has been shown to induce the secretion of neprilysin in vitro and in vivo, improving the endogenous machinery for the degradation of Aβ-plaques and enhancing the clearance of these aggregates [93]. This is particularly relevant as abnormal protein aggregation is one of the major hallmarks of neurodegenerative diseases like PD and AD [94].
4. MSCs as Promising Modulators of the BBB in Neurodegenerative Diseases
4.1. Alzheimer´s disease
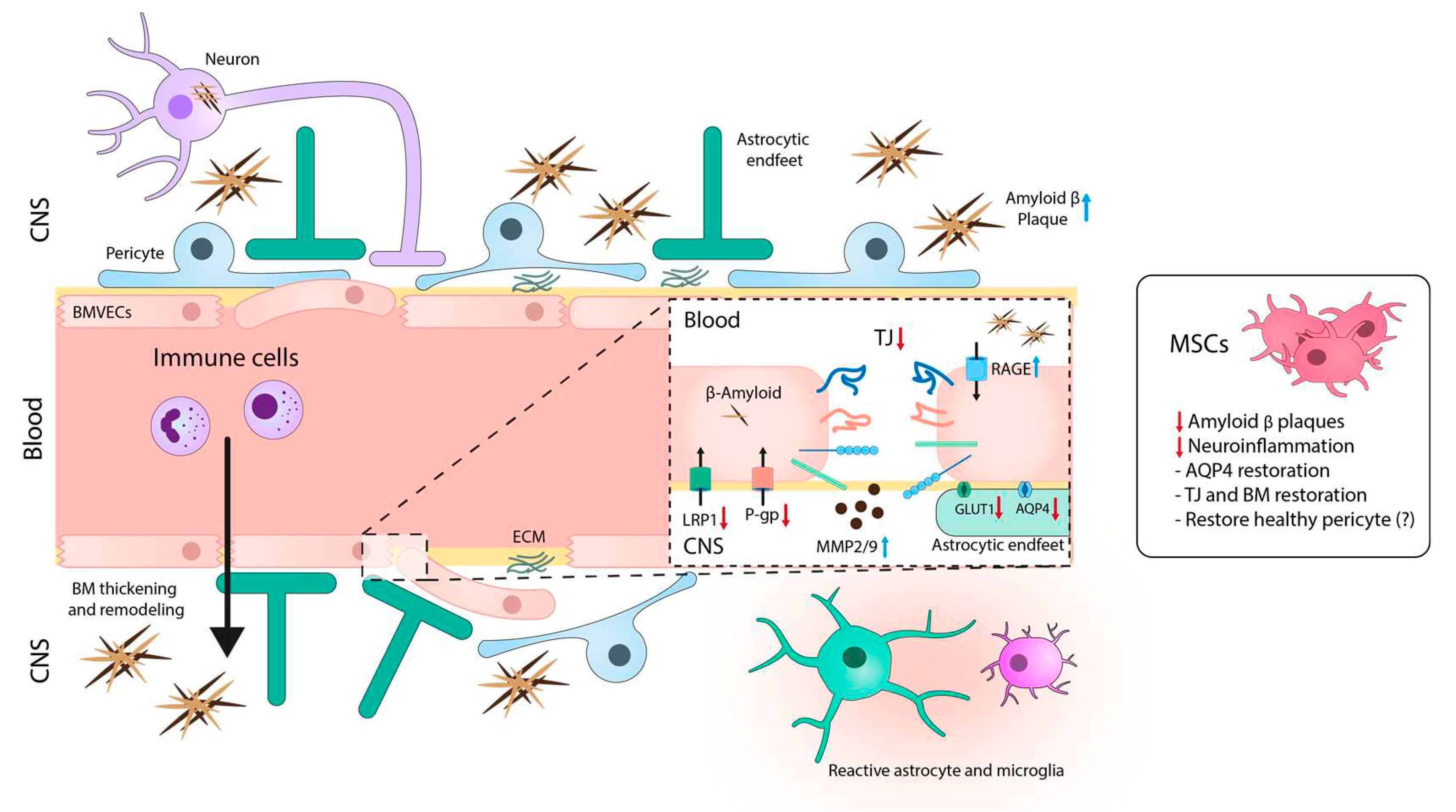
4.1.1. Dysfunctional BBB in AD
4.1.2. Therapeutic Opportunities for MSCs Targeting BBB in AD
4.2. Parkinson´s disease
4.2.1. Dysfunctional BBB in PD
4.2.2. Therapeutic Opportunities for MSCs Targeting BBB in PD
4.3. Multiple sclerosis
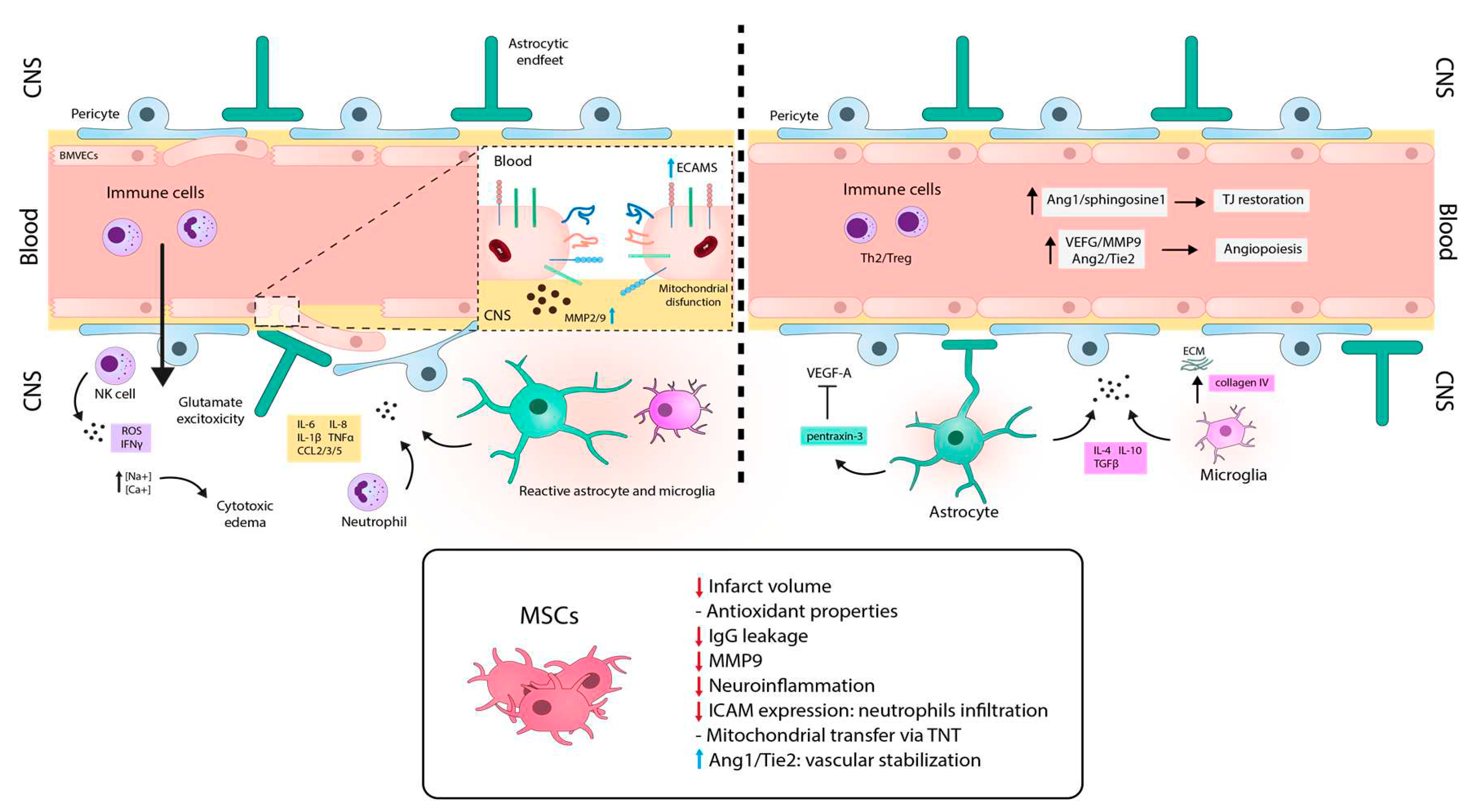
4.3.1. Dysfunctional BBB in MS
4.3.2. Therapeutic Opportunities for MSCs Targeting BBB in MS
4.4. Stroke
4.4.1. Dysfunctional BBB in Brain Ischemia.
4.4.2. Therapeutic Opportunities for MSCs Targeting BBB in Brain Ischemia.
5. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- WHO reveals reading cause of death and disability worldwide: 2000-2019. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 6 May 2023).
- Banks, W.A.; Reed, M.J.; Logsdon, A.F.; Rhea, E.M.; Erickson, M.A. Healthy aging and the blood-brain barrier. Nat Aging 2021, 1, 243–254. [Google Scholar] [CrossRef]
- Farrall, A.J.; Wardlaw, J.M. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Elahy, M.; Jackaman, C.; Mamo, J.C.; Lam, V.; Dhaliwal, S.S.; Giles, C.; Nelson, D.; Takechi, R. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing 2015, 12, 2. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Martinez, G.R.; Hu, A.; Choi, J.; Keep, R.F.; Andjelkovic, A.V. Decline in sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol Dis 2019, 126, 105–116. [Google Scholar] [CrossRef]
- Verheggen, I.C.M.; de Jong, J.J.A.; van Boxtel, M.P.J.; Postma, A.A.; Jansen, J.F.A.; Verhey, F.R.J.; Backes, W.H. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience 2020, 42, 1751–1764. [Google Scholar] [CrossRef]
- Verheggen, I.C.M.; de Jong, J.J.A.; van Boxtel, M.P.J.; et al. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience 2020, 42, 1183–1193. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Morin, A.M.; Cipp, L.J.; Haspel, H.C. Glucose transport is reduced in the blood-brain barrier of aged rats. Brain Res 1991, 551, 145–149. [Google Scholar] [CrossRef]
- Banks, W.A.; Moinuddin, A.; Morley, J.E. Regional transport of TNF-alpha across the blood-brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol Aging 2001, 22, 671–676. [Google Scholar] [CrossRef]
- Staff, N.P.; Jones, D.T.; Singer, W. Mesenchymal stromal cell therapies for neurodegenerative diseases. Mayo Clin. Proc. 2019, 94, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal stem cells for neurological disorders. Adv Sci (Weinh) 2021, 8, 2002944. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015, 7, a020412. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 2006, 1, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Uwamori, H.; Ono, Y.; Yamashita, T.; Arai, K.; Sudo, R. Comparison of organ-specific endothelial cells in terms of microvascular formation and endothelial barrier functions. Microvasc Res 2019, 122, 60–70. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Greene, C.; Munnich, A.; et al. The CLDN5 gene at the blood-brain barrier in health and disease. Fluids Barriers CNS 2023, 20. [Google Scholar] [CrossRef]
- Wong, A. D; Ye, M.; Levy, A. F.; Rothstein, J. D.; Bergles, D. E.; Searson, P. C. The blood-brain barrier: an engineering perspective. Front Neuroeng 2013, 6, 7. [Google Scholar] [CrossRef]
- Oldendorf, W.H.; Cornford, M.E.; Brown, W.J. The large apparent work capability of the blood-brain barrier: A study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1977, 1, 409–417. [Google Scholar] [CrossRef]
- Paolinelli, R.; Corada, M.; Orsenigo, F.; Dejana, E. The Molecular Basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol Res 2011, 63, 165–171. [Google Scholar] [CrossRef]
- Artus, C.; Glacial, F.; Ganeshamoorthy, K.; et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab 2014, 34, 433–440. [Google Scholar] [CrossRef]
- Ghandour, M.S.; Langley, O.K.; Varga, V. Immunohistological localization of γ glutamyltranspeptidase in cerebellum at light and electron microscope levels. Neurosci Lett 1980, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Vorbrodt, A.W.; Lossinsky, A.S.; Wisniewski, H.M. Localization of alkaline phosphatase activity in endothelia of developing and mature mouse blood-brain barrier. Dev Neurosci 1986, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.L. Sodium transport in capillaries isolated from rat brain. J Neurochem 1983, 41, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, E.; van Kammen, C.M.; de Theije, C.G.M.; et al. A quantitative method for microstructural analysis of myelinated axons in the injured rodent brain. Sci Rep 2017, 7, 16492. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol Rev 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Badaut, J.; Bix, G.J. Vascular neural network phenotypic transformation after traumatic injury: potential role in long-term sequelae. Transl Stroke Res 2014, 5, 394–406. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 2018, 21, 1318–31. [Google Scholar] [CrossRef]
- Parker, K.R.; Migliorini, D.; Perkey, E.; Yost, K.E.; Bhaduri, A.; Bagga, P.; Haris, M.; Wilson, N.E.; Liu, F.; Gabunia, K.; et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 2020, 183, 126–142.e17. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.M.; Tome, M.E.; Davis, T.P. Primer on cerebrovascular diseases (Second Edition). In Primer on Cerebrovascular Diseases (Second Edition); Academic Press 2017, 2017; p. Chapter 9-Development and Maintenance of the Blood-Brain Barrier, pag. 53 ISBN 978-0-12-803059-2.
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Fallier-Becker, P.; Sperveslage, J.; Wolburg, H.; Noell, S. The Impact of agrin on the formation of orthogonal arrays of particles in cultured astrocytes from wild-type and agrin-null mice. Brain Res 2011, 1367, 2–12. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A 2009, 106, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guillamon, M.; Delgado, P.; Ortega, L.; et al. Neuronal TIMP-1 release accompanies astrocytic MMP-9 secretion and enhances astrocyte proliferation induced by beta-amyloid 25-35 fragment. J Neurosci Res 2009, 7, 2115–2125. [Google Scholar] [CrossRef]
- Owens, T.; Bechmann, I.; Engelhardt, B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 2008, 67, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ivars, F.; Anderson, P.; Hallmann, R.; Vestweber, D.; Nilsson, P.; Robenek, H.; Tryggvason, K.; Song, J.; Korpos, E.; et al. Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med 2009, 15, 519–527. [Google Scholar] [CrossRef]
- Korpos, E.; Wu, C.; Song, J.; Hallmann, R.; Sorokin, L. Role of the extracellular matrix in lymphocyte migration. Cell Tissue Res 2010, 339, 47–57. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement Membrane and blood–brain barrier. Stroke Vasc Neurol 2018, 4, 78–82. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Transport across the blood-brain barrier. J Cereb Blood Flow Metab 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. Bioimpacts 2016, 6, 225–248. [Google Scholar] [CrossRef]
- Schulze, C.; Firth, J.A. Immunohistochemical localization of adherens junction components in blood-brain barrier microvessels of the rat. J Cell Sci 1993, 104 Pt 3, 773–782. [Google Scholar] [CrossRef]
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The Control of Vascular Integrity by Endothelial Cell Junctions: Molecular Basis and Pathological Implications. Dev Cell 2009, 16, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Tietz, S.; Engelhardt, B. Brain Barriers: Crosstalk between complex tight junctions and adherens junctions. J Cell Biol 2015, 209, 493–506. [Google Scholar] [CrossRef]
- Ueda, H.; Baba, T.; Terada, N.; Kato, Y.; Fujii, Y.; Takayama, I.; Mei, X.; Ohno, S. Immunolocalization of dystrobrevin in the astrocytic endfeet and endothelial cells in the rat cerebellum. Neurosci Lett 2000, 283, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Vestweber, D. The Role of VE-Cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci 2013, 116, 119–144. [Google Scholar] [CrossRef]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.; et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef]
- Turowski, P.; Martinelli, R.; Crawford, R.; Wateridge, D.; Papageorgiou, A.-P.; Lampugnani, M.G.; Gamp, A.C.; Vestweber, D.; Adamson, P.; Dejana, E.; et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 2008, 121, 10–1242. [Google Scholar] [CrossRef]
- Dickstein, D.L.; Biron, K.E.; Ujiie, M.; Pfeifer, C.G.; Jeffries, A.R.; Jefferies, W.A. Aβ peptide immunization restores blood-brain barrier integrity in Alzheimer disease. FASEB J 2006, 20, 426–433. [Google Scholar] [CrossRef]
- Wessel, F.; Winderlich, M.; Holm, M.; Frye, M.; Rivera-Galdos, R.; Vockel, M.; Linnepe, R.; Ipe, U.; Stadtmann, A.; Zarbock, A.; et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-Cadherin. Nat Immunol 2014, 15, 223–230. [Google Scholar] [CrossRef]
- Takeichi, M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 2014, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.J.; Weber, P.A.; Cao, F.; Chang, H.C.; Lampe, P.; Goldberg, G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res 2000, 33, 369–398. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insight into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed]
- Ezan, P.; André, P.; Cisternino, S.; Saubaméa, B.; Boulay, A.-C.; Doutremer, S.; Thomas, M.-A.; Quenech’du, N.; Giaume, C.; Cohen-Salmon, M. Deletion of astroglial connexins weakens the blood–brain barrier. J Cereb Blood Flow Metab 2012, 32, 1457. [Google Scholar] [CrossRef] [PubMed]
- Kotini, M.; Barriga, E.H.; Leslie, J.; et al. Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat Commun 2018, 9, 3846. [Google Scholar] [CrossRef]
- Devraj, K.; Klinger, M.E.; Myers, R.L.; Mokashi, A.; Hawkins, R.A.; Simpson, I.A. GLUT-1 Glucose transporters in the blood-brain barrier: Differential phosphorylation. J Neurosci Res 2011, 89, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Pines, G.; Danbolt, N.C.; Bjørås, M.; Zhang, Y.; Bendahan, A.; Eide, L.; Koepsell, H.; Storm-Mathisen, J.; Seeberg, E.; Kanner, B.I. Cloning and expression of a rat brain L-Glutamate transporter. Nature 1992, 360, 464–467. [Google Scholar] [CrossRef]
- Huttunen, J.; Peltokangas, S.; Gynther, M.; Natunen, T.; Hiltunen, M.; Auriola, S.; Ruponen, M.; Vellonen, K. S.; Huttunen, K. M. L-Type Amino Acid Transporter 1 (LAT1/Lat1)-Utilizing prodrugs can improve the delivery of drugs into neurons, astrocytes and microglia. Sci Rep 2019, 9, 12860. [Google Scholar] [CrossRef]
- Mann, G.E.; Yudilevich, D.L.; Sobrevia, L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 2003, 83, 183–252. [Google Scholar] [CrossRef]
- O’Kane, R.L.; Martı́nez-López, I.; DeJoseph, M.R.; Viña, J.R.; Hawkins, R.A. Na+-Dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier: A mechanism for glutamate removal. J Biol Chem 1999, 274, 31891–31895. [Google Scholar] [CrossRef]
- Simpson, I.A.; Carruthers, A.; Vannucci, S.J. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J Cereb Blood Flow Metab 2007, 27, 1766–1791. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des 2014, 20, 1487–1498. [Google Scholar] [CrossRef]
- Leclerc, M.; Bourassa, P.; Tremblay, C.; Caron, V.; Sugère, C.; Emond, V.; Bennett, D.A.; Calon, F. Cerebrovascular insulin receptors are defective in Alzheimer’s Disease. Brain 2023, 146, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL Receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 2008, 88, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Du Yan, S.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates Amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 2003, 9, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Generoso, J.S.; Collodel, A.; Dominguini, D.; Faller, C.J.; Tardin, F.; Bhatti, G.S.; Petronilho, F.; Dal-Pizzol, F.; Barichello, T. Receptor for advanced glycation end products (RAGE) mediates cognitive impairment triggered by Pneumococcal Meningitis. Neurother 2021, 18, 640–653. [Google Scholar] [CrossRef]
- Ahmed Juvale, I.I.; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-Glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef]
- Wei, W.; Bodles-Brakhop, A.M.; Barger, S.W. A Role for P-Glycoprotein in clearance of Alzheimer Amyloid β-Peptide from the brain. Curr Alzheimer Res 2016, 13, 615–620. [Google Scholar] [CrossRef]
- Bourassa, P.; Alata, W.; Tremblay, C.; Paris-Robidas, S.; Calon, F. Transferrin receptor-mediated uptake at the blood-brain barrier is not impaired by Alzheimer’s Disease neuropathology. Mol Pharmaceutics 2019, 16. [Google Scholar] [CrossRef]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In vitro differentiation potential of mesenchymal stem cells. Transfus Med Hemotherapy 2008, 35, 228–238. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm 2005, 2, 8. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011, 9, 12. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat Tissue: An underappreciated source of stem cells for biotechnology. Trends in Biotechnol 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and clinical applications of Wharton’s Jelly-derived mesenchymal stromal cells. Curr Res. Transl Med 2020, 68, 5–16. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov (accessed on 26 July 2023).
- Cova, L.; Armentero, M.-T.; Zennaro, E.; Calzarossa, C.; Bossolasco, P.; Busca, G.; Lambertenghi Deliliers, G.; Polli, E.; Nappi, G.; Silani, V.; et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s Disease. Brain Res 2010, 1311, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, N.; Ong, L.-L.; Nesselmann, C.; Klopsch, C.; Ladilov, Y.; Furlani, D.; Piechaczek, C.; Moebius, J.M.; Lützow, K.; et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 2007, 25, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, H.N.; Park, H.-J.; Shin, J.Y.; Lee, P.H. Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in an Alzheimer’s Disease model. Cell Transplant 2015, 24, 1097–1109. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Tuazon, J.P.; Lee, J.-Y.; Corey, S.; Kvederis, L.; Kingsbury, C.; Kaneko, Y.; Borlongan, C.V. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen Res 2019, 14, 597. [Google Scholar] [CrossRef]
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 2018, 25, 31. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J. D.; Gorick, C. M.; Kumar, J. S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M. R.; Price, R. J.; Tvrdik, P.; Kalani, M. Y. S. Mitochondrial dysfunction in neurological disorders: Exploring mitochondrial transplantation. NPJ Regen 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Boukelmoune, N.; Chiu, G.S.; Kavelaars, A.; Heijnen, C.J. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Q.; Wang, Z.; Tong, H.; Ma, L.; Zhang, Y.; Shan, F.; Meng, Y.; Yuan, Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s Jelly as sources of cell immunomodulatory therapy. Hum Vaccines Immunother 2016, 12, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC Suppression correlates with cytokine induction of indoleamine 2,3-Dioxygenase and bystander M2 macrophage differentiation. Mol Ther 2012, 20, 187–195. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, R.; Wang, Y.; Huang, W.; Hu, B.; Zhu, G.; Zhang, R.; Li, F.; Han, J.; Li, Y. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res 2019, 1724, 146422. [Google Scholar] [CrossRef]
- Gu, Y.; He, M.; Zhou, X.; Liu, J.; Hou, N.; Bin, T.; Zhang, Y.; Li, T.; Chen, J. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by supressing apoptosis in astrocyte. Sci Rep 2016, 6, 18587. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, D.H.; Kim, J.H.; Lee, D.; Jeon, H.B.; Kwon, S.-J.; Kim, S.M.; Yoo, Y.J.; Lee, E.H.; Choi, S.J.; et al. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces Amyloid-β plaques. Cell Death Differ. 2012, 19, 680–691. [Google Scholar] [CrossRef]
- Sarkar, S. Protein aggregation in neurodegenerative disorders: A cause or consequence? Adv. Tech. Biol. Med. 2015, 02. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Rüster, B.; Göttig, S.; Ludwig, R.J.; Bistrian, R.; Müller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef]
- Yilmaz, G.; Vital, S.; Yilmaz, C.E.; Stokes, K.Y.; Alexander, J.S.; Granger, D.N. Selectin-mediated recruitment of bone marrow stromal cells in the postischemic cerebral microvasculature. Stroke 2011, 42, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Mitkari, B.; Kerkelä, E.; Nystedt, J.; Korhonen, M.; Mikkonen, V.; Huhtala, T.; Jolkkonen, J. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol 2013, 239, 158–162. [Google Scholar] [CrossRef]
- Lee, N.K.; Yang, J.; Chang, E.H.; Park, S.E.; Lee, J.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L. Intra-arterially delivered mesenchymal stem cells are not detected in the brain parenchyma in an Alzheimer’s Disease mouse model. PLOS ONE 2016, 11, e0155912. [Google Scholar] [CrossRef]
- Albert, M.S. Changes in cognition. Neurobiol Aging 2011, 32 Suppl 1(0 1), S58–S63. [Google Scholar] [CrossRef]
- Whitaker, K.W.; LaFerla, F.M.; Steinbusch, H.W.M.; Lemere, C.A.; Bovenkamp, D.E. BrightFocus Alzheimer’s fast track 2019. Mol Neurodegener 2019, 14, 48. [Google Scholar] [CrossRef]
- Terry, R. D. Neuropathological changes in Alzheimer disease. Prog Brain Res 1994, 101, 383–390. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 2008, 9, 768–778. [Google Scholar] [CrossRef]
- Carrano, A.; Hoozemans, J.J.M.; van der Vies, S.M.; van Horssen, J.; de Vries, H.E.; Rozemuller, A.J.M. Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neurodegener Dis 2012, 10, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Marco, S.; Skaper, S.D. Amyloid Beta-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci Lett 2006, 401, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Chung, H.C.; Shah, G.N. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging 1997, 18, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Graven, C.; Dederen, P.J.; Tanila, H.; van Groen, T.; Kiliaan, A.J. Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res 2007, 1181, 93–103. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Vitek, M.P.; Colton, C.A. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience 2009, 159, 1055–1069. [Google Scholar] [CrossRef]
- de Jong, G.I.; Jansen, A.S.; Horvath, E.; Gispen, W.H.; Luiten, P.G. Nimodipine effects on cerebral microvessels and sciatic nerve in aging rats. Neurobiol Aging 1992, 13, 73–81. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Pax, A.B. Increased collagen content of cerebral microvessels in Alzheimer’s disease. Brain Res 1995, 705, 349–352. [Google Scholar] [CrossRef]
- Christov, A.; Ottman, J.; Hamdheydari, L.; Grammas, P. Structural changes in Alzheimer’s disease brain microvessels. Curr Alzheimer Res 2008, 5, 392–395. [Google Scholar] [CrossRef]
- Jung, S.S.; Zhang, W.; Van Nostrand, W.E. Pathogenic a beta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem 2003, 85, 1208–1215. [Google Scholar] [CrossRef]
- Asahina, M.; Yoshiyama, Y.; Hattori, T. Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer’s disease brain. Clin Neuropathol 2001, 20, 60–63. [Google Scholar] [PubMed]
- Miller, M.C.; Tavares, R.; Johanson, C.E.; Hovanesian, V.; Donahue, J.E.; Gonzalez, L.; Silverberg, G.D.; Stopa, E.G. Hippocampal RAGE immunoreactivity in early and advanced alzheimer’s disease. Brain Res 2008, 1230, 273–280. [Google Scholar] [CrossRef] [PubMed]
- van Assema, D.M.E.; Lubberink, M.; Bauer, M.; van der Flier, W.M.; Schuit, R.C.; Windhorst, A.D.; Comans, E.F.I.; Hoetjes, N.J.; Tolboom, N.; Langer, O.; et al. Blood-brain barrier p-glycoprotein function in alzheimer’s disease. Brain 2012, 135, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Yamada, S.; Kumar, S.R.; Calero, M.; Bading, J.; Frangione, B.; Holtzman, D.M.; Miller, C.A.; Strickland, D.K.; Ghiso, J.; et al. Clearance of alzheimer’s amyloid-Ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000, 106, 1489–1499. [Google Scholar] [CrossRef]
- Deane, R.; Wu, Z.; Sagare, A.; Davis, J.; Du Yan, S.; Hamm, K.; Xu, F.; Parisi, M.; LaRue, B.; Hu, H.W.; et al. LRP/Amyloid beta-peptide interaction mediates differential brain efflux of abeta isoforms. Neuron 2004, 43, 333–344. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Hohsfield, L.A.; Humpel, C. Migration of blood cells to β-amyloid plaques in Alzheimer’s disease. Exp Gerontol 2015, 65, 8–15. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Z.; Zhang, J. Immunomodulatory role of mesenchymal stem cells in Alzheimer’s disease. Life Sci 2020, 246, 117405. [Google Scholar] [CrossRef]
- Garcia, K.; Ornellas, F.; Matsumoto, P.; Patti, C.; Mello, L.; Frussa-Filho, R.; Han, S.; Longo, B. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci 2014, 6, 30. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Park, H.; Ahn, H.; Choi, J.; Kim, H.; Lee, H.S.; Lee, S.; Park, H.-J.; Kim, S.U.; et al. Protection against RAGE-mediated neuronal cell death by SRAGE-secreting human mesenchymal stem cells in 5xFAD transgenic mouse model. Brain Behav Immun 2017, 66, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. lu; Ouyang, F. bing; Hu, L. ting; Sun, P.; Yang, J.; Sun, Y. jing; Liao, M. shi; Lan, L. fang; Pei, Z.; Fan, Y. hua Mesenchymal stem cells improve cognitive impairment and reduce Aβ deposition via promoting AQP4 polarity and relieving neuroinflammation in rats with chronic hypertension-induced cerebral small-vessel disease. Front Aging Neurosci 2022, 14, 883503. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Yamazaki, Y.; Liu, C.-C.; Bu, G.; Kanekiyo, T. Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-β pathology in amyloid model mice. Exp Neurol 2018, 300, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Lim, J.; Choi, H.; Kang, H.; Jeon, N.L.; Son, Y. Human bone marrow-derived mesenchymal stem cells play a role as a vascular pericyte in the reconstruction of human BBB on the angiogenesis microfluidic chip. Biomaterials 2021, 279, 121210. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Shinojima, N.; Hossain, A.; Gumin, J.; Yong, R.L.; Colman, H.; Marini, F.; Andreeff, M.; Lang, F.F. Isolation and perivascular localization of mesenchymal stem fells from mouse brain. Neurosurgery 2010, 67, 711. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat Rev Dis Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood–brain barrier leakage is increased in Parkinson’s disease. Front Physiol 2020, 11. [Google Scholar] [CrossRef]
- Gray, M.T.; Woulfe, J.M. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 2015, 35, 747–750. [Google Scholar] [CrossRef]
- Pienaar, I.S.; Lee, C.H.; Elson, J.L.; McGuinness, L.; Gentleman, S.M.; Kalaria, R.N.; Dexter, D.T. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis 2015, 74, 392–405. [Google Scholar] [CrossRef]
- Loeffler, D.A.; Connor, J.R.; Juneau, P.L.; Snyder, B.S.; Kanaley, L.; DeMaggio, A.J.; Nguyen, H.; Brickman, C.M.; LeWitt, P.A. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem 1995, 65, 710–724. [Google Scholar] [CrossRef]
- Li, J.Q.; Tan, L.; Yu, J.T. The role of the LRRK2 gene in parkinsonism. Mol Neurodegener 2014, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Droździk, M.; Białecka, M.; Myśliwiec, K.; Honczarenko, K.; Stankiewicz, J.; Sych, Z. Polymorphism in the p-glycoprotein drug transporter MDR1 gene: A possible link between environmental and genetic factors in parkinson’s disease. Pharmacogenetics 2003, 13, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, B.A.; Bonnet, A.M.; Agid, Y.; Hirsch, E.C. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet 1999, 353, 981–982. [Google Scholar] [CrossRef] [PubMed]
- Barcia, C.; Emborg, M.E.; Hirsch, E.C.; Herrero, M.-T. Blood vessels and parkinsonism. Front Biosci 2004, 9, 277–282. [Google Scholar] [CrossRef]
- Desai, B.S.; Monahan, A.J.; Carvey, P.M.; Hendey, B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant 2007, 16, 285–299. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S.T. Microglia and neuroinflammation: A pathological perspective. J Neuroinflammation 2004, 1, 14. [Google Scholar] [CrossRef]
- Wong, D.; Dorovini-Zis, K.; Vincent, S.R. Cytokines, nitric oxide, and CGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol 2004, 190, 446–455. [Google Scholar] [CrossRef]
- Chao, Y.X.; He, B.P.; Tay, S.S.W. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson’s disease. J Neuroimmunol 2009, 216, 39–50. [Google Scholar] [CrossRef]
- Xue, C.; Li, X.; Ba, L.; Zhang, M.; Yang, Y.; Gao, Y.; Sun, Z.; Han, Q.; Zhao, R.C. MSC-derived exosomes can enhance the angiogenesis of human brain MECs and show therapeutic potential in a mouse model of Parkinson’s disease. Aging Dis 2021, 12, 1211–1222. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, J.Y.; Kim, H.N.; Oh, S.H.; Song, S.K.; Lee, P.H. Mesenchymal stem cells stabilize the blood–brain barrier through regulation of astrocytes. Stem Cell Res Ther 2015, 6, 187. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N Engl J Med 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Pathology and disease mechanisms in different stages of multiple sclerosis. J Neurol Sci 2013, 333, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The blood–brain barrier—A key player in multiple sclerosis disease mechanisms. Biomolecules 2022, 12, 538. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, K.; Zhang, X.; Zhang, J.; Xu, B. ATP induces disruption of tight junction proteins via IL-1 beta-dependent MMP-9 activation of human blood-brain barrier in vitro. Neural Plast 2016, 2016, 8928530. [Google Scholar] [CrossRef]
- Desai, T.R.; Leeper, N.J.; Hynes, K.L.; Gewertz, B.L. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res 2002, 104, 118–123. [Google Scholar] [CrossRef]
- Förster, C.; Burek, M.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Drenckhahn, D. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol 2008, 586, 1937–49. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Wang, M.M.; Jankovic, I.; Andjelkovic, A.V. ; Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem 2009, 284, 19053–19066. [Google Scholar] [CrossRef]
- Nagyoszi, P.; Wilhelm, I.; Farkas, A.E.; Fazakas, C.; Dung, N.T.K.; Haskó, J.; Krizbai, I.A. Expression and regulation of Toll-like receptors in cerebral endothelial cells. Neurochem Int 2010, 57, 556–564. [Google Scholar] [CrossRef]
- Racke, M.K.; Drew, P.D. Toll-like receptors in multiple sclerosis. Curr Top Microbiol Immunol 2009, 336, 155–168. [Google Scholar] [CrossRef]
- Sheikh, M. H.; Henson, S. M.; Loiola, R. A.; Mercurio, S.; Colamatteo, A.; Maniscalco, G. T.; De Rosa, V.; McArthur, S.; Solito, E. Immuno-metabolic impact of the multiple sclerosis patients' sera on endothelial cells of the blood-brain barrier. J Neuroinflammation 2020, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, G.; Mohácsik, P.; Balkhi, M.Y.; Gereben, B.; Lechan, R.M. Endotoxin-induced inflammation down-regulates l-type amino acid transporter 1 (LAT1) expression at the blood–brain barrier of male rats and mice. Fluids Barriers CNS 2015, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Goralski, K.B. A Critical Overview of the Influence of inflammation and infection on p-glycoprotein expression and activity in the brain. Expert Opin Drug Metab Toxicol 2008, 4, 1245–1264. [Google Scholar] [CrossRef]
- Gelati, M.; Corsini, E.; Dufour, A.; Massa, G.; Giombini, S.; Solero, C.L.; Salmaggi, A. High-dose methylprednisolone reduces cytokine-induced adhesion molecules on human brain endothelium. Can. J Neurol. Sci. 2000, 27, 241–244. [Google Scholar] [CrossRef]
- Ge, S.; Jiang, X.; Paul, D.; Song, L.; Wang, X.; Pachter, J.S. Human ES-derived MSCs correct TNF-α-mediated alterations in a blood–brain barrier model. Fluids Barriers CNS 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Heon Ryu, C.; Jun, J.A.; Kim, S.M.; Jeong, C.H.; Jeun, S.-S. Interferon β-secreting mesenchymal stem cells combined with minocycline attenuate experimental autoimmune encephalomyelitis. J Neuroimmunol 2014, 274, 20–27. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Du, B.; Wang, Y.; Yang, G.-Y.; Bi, X. Mesenchymal stem cells attenuated blood-brain barrier disruption via downregulation of aquaporin-4 expression in EAE mice. Mol Neurobiol 2020, 57, 3891–3901. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Peng, L.; Hu, G.; Yao, Q.; et al. Microglia autophagy in ischemic stroke: A double-edged sword. Front Immunol 2022, 13, 1013311. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of blood-brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front Neurol 2020, 11, 594672. [Google Scholar] [CrossRef]
- Magee, P.N.; Stoner, H.B.; Barnes, J.M. The experimental production in oedema in the central nervous system of the rat by triethyltin compounds. J Pathol 1957, 73, 107–124. [Google Scholar] [CrossRef]
- Liebeskind, D.S.; Jüttler, E.; Shapovalov, Y.; Yegin, A.; Landen, J.; Jauch, E.C. Cerebral edema associated with large hemispheric infarction. Stroke 2019, 50, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rosenberg, G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Menon, D.K.; Peters, A.M.; Ballinger, J.R.; Barber, R.W.; Balan, K.K.; Lynch, A.; Xuereb, J.H.; Fryer, T.; Guadagno, J.V.; Warburton, E.A. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke 2004, 35, 1659–1664. [Google Scholar] [CrossRef]
- Krizbai, I.A.; Bauer, H.; Bresgen, N.; Eckl, P.M.; Farkas, A.; Szatmári, E.; Traweger, A.; Wejksza, K.; Bauer, H.-C. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol 2005, 25, 129–139. [Google Scholar] [CrossRef]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci 2016, 10, 56. [Google Scholar] [CrossRef]
- Qiu, Y.M.; Zhang, C.L.; Chen, A.Q.; Wang, H.L.; Zhou, Y.F.; Li, Y.N.; Hu, B. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy? Front Immunol 2021, 12, 678744. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; Shi, M.J.; Tian, Y.; Fan, W.; Zhao, B.Q. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun 2020, 11, 2488. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.; Ander, B.P.; Stamova, B.; Zhan, X.; Sharp, F.R. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015, 35, 888–901. [Google Scholar] [CrossRef]
- Kataoka, H.; Kim, S.W.; Plesnila, N. Leukocyte-endothelium interactions during permanent focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2004, 24, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.W.; Chang, W.N.; Shaw, C.F.; Jan, C.R.; Huang, C.R.; Chen, S.D.; Chuang, Y.C.; Lee, L.H.; Lu, C.H. The value of leukocyte adhesion molecules in patients after ischemic stroke. J Neurol 2009, 256, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Liu, Q.; Wu, W.; Yin, J.-X.; Bai, X.-F.; Shen, R.; Wang, Y.; Chen, J.; La Cava, A.; Poursine-Laurent, J.; et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. PNAS USA 2014, 111, 2704–2709. [Google Scholar] [CrossRef]
- Kurzepa, J.; Kurzepa, J.; Golab, P.; Czerska, S.; Bielewicz, J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci 2014, 124, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Stabile, E.; Kinnaird, T.; Shou, M.; Devaney, J.M.; Epstein, S.E.; Burnett, M.S. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol 2004, 43, 474–82. [Google Scholar] [CrossRef]
- Liu, R.; Pan, M.-X.; Tang, J.-C.; Zhang, Y.; Liao, H.-B.; Zhuang, Y.; Zhao, D.; Wan, Q. Role of neuroinflammation in ischemic stroke. Neurol Neuroimmunol Neuroinflamm 2017, 4, 158–166. [Google Scholar] [CrossRef]
- Krupinski, J.; Kaluza, J.; Kumar, P.; Kumar, S.; Wang, J.M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994, 25, 1794–1798. [Google Scholar] [CrossRef]
- Navarro-Sobrino, M.; Rosell, A.; Hernández-Guillamon, M.; Penalba, A.; Boada, C.; Domingues-Montanari, S.; Ribó, M.; Alvarez-Sabín, J.; Montaner, J. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis 2011, 216, 205–211. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 2018, 163–164, 144–171. [Google Scholar] [CrossRef]
- Shindo, A.; Maki, T.; Mandeville, E.T.; Liang, A.C.; Egawa, N.; Itoh, K.; Itoh, N.; Borlongan, M.; Holder, J.C.; Chuang, T.T.; et al. Astrocyte-derived pentraxin 3 supports blood-brain barrier integrity under acute phase of stroke. Stroke 2016, 47, 1094–1100. [Google Scholar] [CrossRef]
- Lin, R.; Cai, J.; Nathan, C.; Wei, X.; Schleidt, S.; Rosenwasser, R.; Iacovitti, L. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol Dis 2015, 74, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Chopp, M.; Roberts, C.; Liu, X.; Wei, M.; Nejad-Davarani, SP.; Wang, X.; Zhang, Z.G. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS One 2014, 9, 12e113972. [Google Scholar] [CrossRef]
- Do, P.T.; Wu, C.-C.; Chiang, Y.-H.; Hu, C.-J.; Chen, K.-Y. Mesenchymal stem/stromal cell therapy in blood–brain barrier preservation following ischemia: molecular mechanisms and prospects. Int J Mol Sci 2021, 22, 10045. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Cai, J.; Qiu, Y.; Zheng, H.; Lai, X.; Sui, X.; Wang, Y.; Lu, Q.; Zhang, Y.; et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics 2018, 8, 5929–5944. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.-Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J Neuroinflammation 2018, 15, 135. [Google Scholar] [CrossRef]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Liu, K.; Guo, L.; Zhou, Z.; Pan, M.; Yan, C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res 2019, 123, 74–80. [Google Scholar] [CrossRef]
- Zacharek, A.; Chen, J.; Li, A.; Cui, X.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/TIE2 and VEGF/FLK1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab 2007, 27, 1684–1691. [Google Scholar] [CrossRef]
- Gregorius, J.; Wang, C.; Stambouli, O.; Hussner, T.; Qi, Y.; Tertel, T.; Börger, V.; Mohamud Yusuf, A.; Hagemann, N.; Yin, D.; et al. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res Cardiol 2021, 116, 40. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, X.; Li, L.; Fang, Y.; Yang, Y.; Gu, J.; Xu, J.; Chu, L. Exosomes derived from bone marrow mesenchymal stem cells promote angiogenesis in ischemic stroke mice via upregulation of miR-21-5p. Biomolecules 2022, 12, 883. [Google Scholar] [CrossRef]
- Xia, Y.; Ling, X.; Hu, G.; Zhu, Q.; Zhang, J.; Li, Q.; Zhao, B.; Wang, Y.; Deng, Z. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res Ther 2020, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Kvistad, C.E.; Kråkenes, T.; Gjerde, C.; Mustafa, K.; Rekand, T.; Bø, L. Safety and clinical efficacy of mesenchymal stem cell treatment in traumatic spinal cord injury, multiple sclerosis and ischemic stroke – A systematic review and meta-analysis. Front Neurol 2022, 13, 891514. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Liu, D.D.; Shen, M.; Kevadiya, B.D.; Ganguly, A.; Primavera, R.; Chetty, S.; Yarani, R.; Thakor, A.S. Mesenchymal stromal cells for the treatment of Alzheimer’s disease: Strategies and Limitations. Front Mol Neurosci 2022, 15, 1011225. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]



| Transporter | Cargo | Location | Description | Source |
|---|---|---|---|---|
| Glucose Transporter 1(GLUT-1) | Glucose | Abluminal and luminal side | Main glucose transporter of BMVECs. Also expressed in astrocytes but not in neurons. Na+ dependent transporters | [40] [56] |
| Large neutral amino acid transporter 1(LAT1) | Large neutral amino acids | Abluminal and luminal side | Abluminal side LAT1 transport is dependent of Na+ concentration. Bidirectional transport | [40] [57] |
| Cationic amino acid transporter 1 and 3 (CAT1/3) | Cationic amino acids | Abluminal and luminal side | CAT-1 is pH and Na+ independent but sensitive to changes in membrane potential | [58] |
| Na+-dependent transporters for glutamate exist on astrocytes 1 and 2 (EAAT1/2) | Glutamate | Abluminal side | Expressed in astrocytes. Possible protective mechanism against glutamate neurotoxicity | [59] |
| Monocarboxylate transporters (MCT1) | Monocarboxylic acids (lactate, pyruvate and acetoacetate and β-hydroxybutyrate) | Abluminal and luminal side | Intracerebral transport. Located in BMVECs and astrocytes. The transport mechanism is a H+ cotransporter or a monocarboxylate exchanger | [60] [61] |
| Insulin receptor (IR) | Insulin | Abluminal and luminal | Located in BMVECs. Insulin binding activates IR by phosphorylation of beta-chain region. Impaired phosphorylation response in AD | [62] |
| Low-density lipoprotein receptor–related protein 1 (LRP1) | APO2 and APO3 | Mainly in the abluminal side | Located in BMVECs. LRP1 binds to Aβ aggregates and mediates their clearance from brain to blood. LRP1 level diminished in AD patients leads to aggregates accumulation. | [26] [63] |
| Receptors for advanced glycation end-products (RAGE) | Advanced glycation end products (AGE), high mobility group box-1 (HMGB-1) protein | Mainly at the luminal side | Located in BMVECs, microglia and astrocytes. Upregulated in AD. It mediates the influx of Aβ into the brain | [64] [65] |
| P-glycoprotein, ATP-binding cassette 1(P-gp, ABCB1) | Xenobiotics and drugs | Expressed in the luminal side | P-gp is a unilateral efflux pump from blood to brain. It uses ATP in the active transport of substances. It is crucial in the ADMET properties of pharmaceutical drugs. In AD, P-gp is involved in accumulation of Aβ peptides in the CNS. | [66] [67] |
| Transferrin receptor protein (Tfr) | Transferrin (apo- and holo-transferrin) | Abluminal and luminal side | Primary iron transporting system. Highly enriched in BMVECs. Studied as a targeted transporter of therapeutics to the brain. | [68] |
| Components in the clinical trials | Categories | Studies (%) |
|---|---|---|
| MSC type | Bone Marrow | 27 (30) |
| Umbilical Cord | 24 (26.67) | |
| Adipose | 14 (15.56) | |
| Neural Progenitor derived | 4 (4.44) | |
| Embryonic | 1 (1.11) | |
| Exosomes | 1 (1.11) | |
| Not indicated | 20 (20.22) | |
| Disease | Multiple Sclerosis | 35 (38.89) |
| Ischemic Stroke | 25 (27.78) | |
| Alzheimer | 17 (18.89) | |
| Parkinson | 13 (14.44) | |
| Modality | Autologous | 41 (45.56) |
| Allogenic | 21 (23.33) | |
| Not indicated | 28 (31.11) | |
| Route | Intravenous | 48 (53.33) |
| Intrathecal | 8 (8.89) | |
| Intravenous/Intrathecal | 3 (3.33) | |
| Intraventricular | 1 (1.11) | |
| Intra-striatal | 1 (1.11) | |
| Intracerebral | 1 (1.11) | |
| Nasal | 1 (1.11) | |
| Not indicated | 27 (30) | |
| Target | Score | 76 (76.77) |
| Immune | 13 (13.13) | |
| Neurological | 10 (10.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





