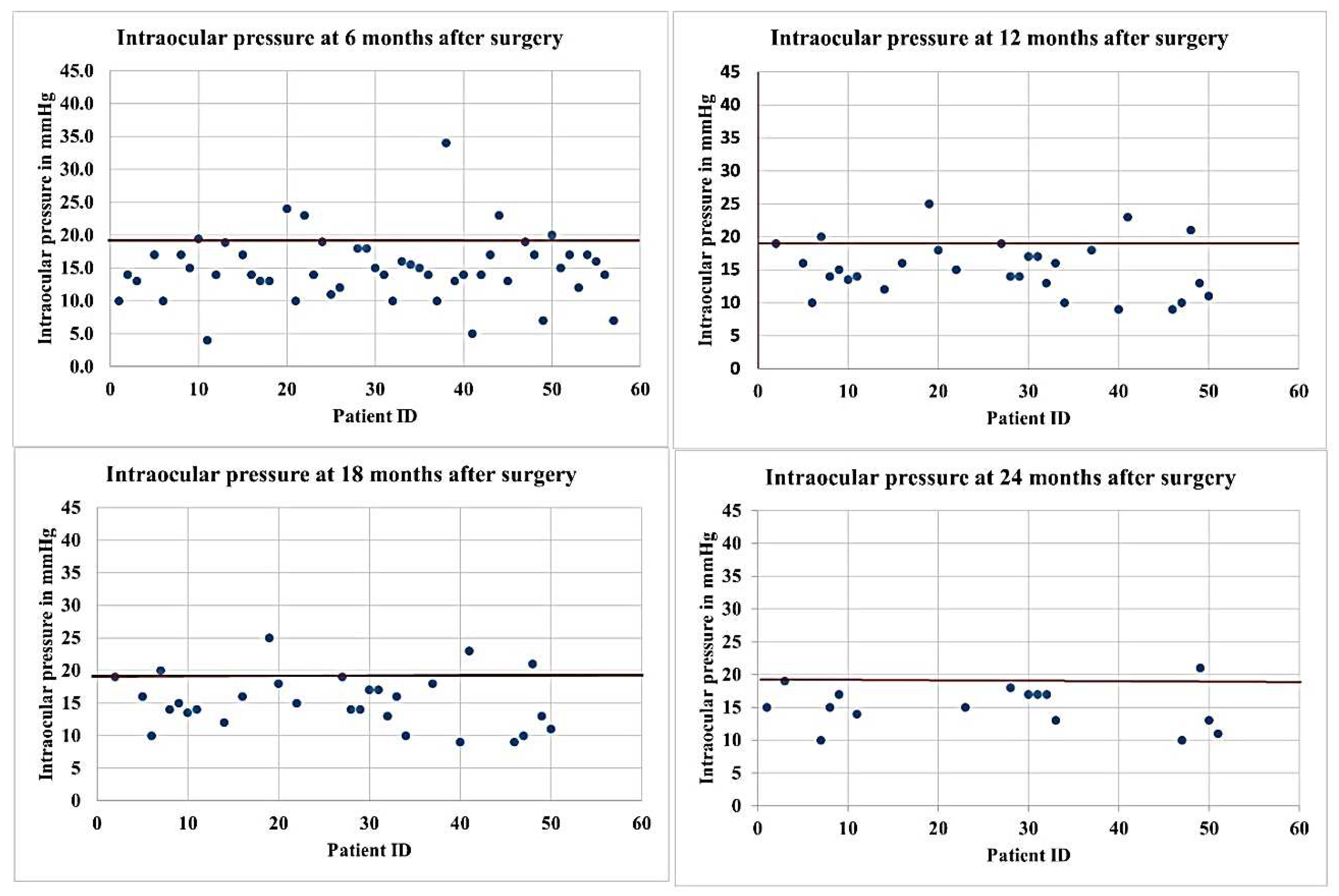
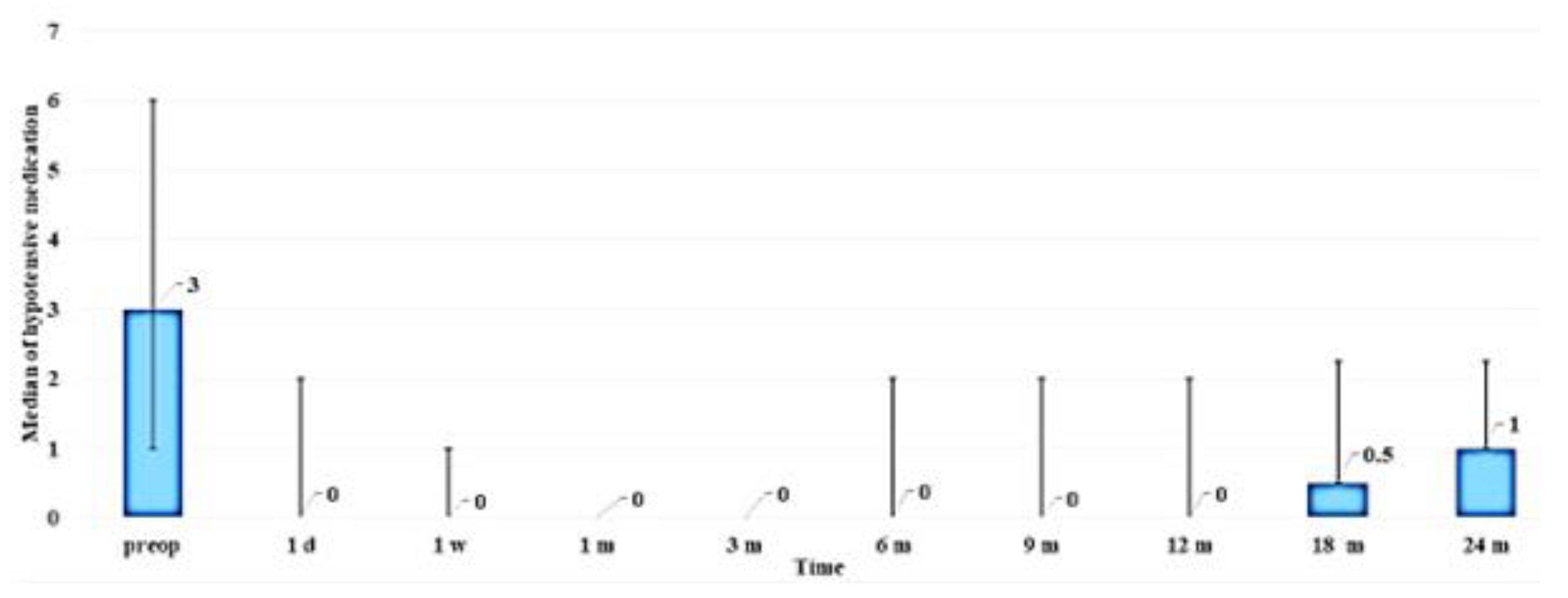
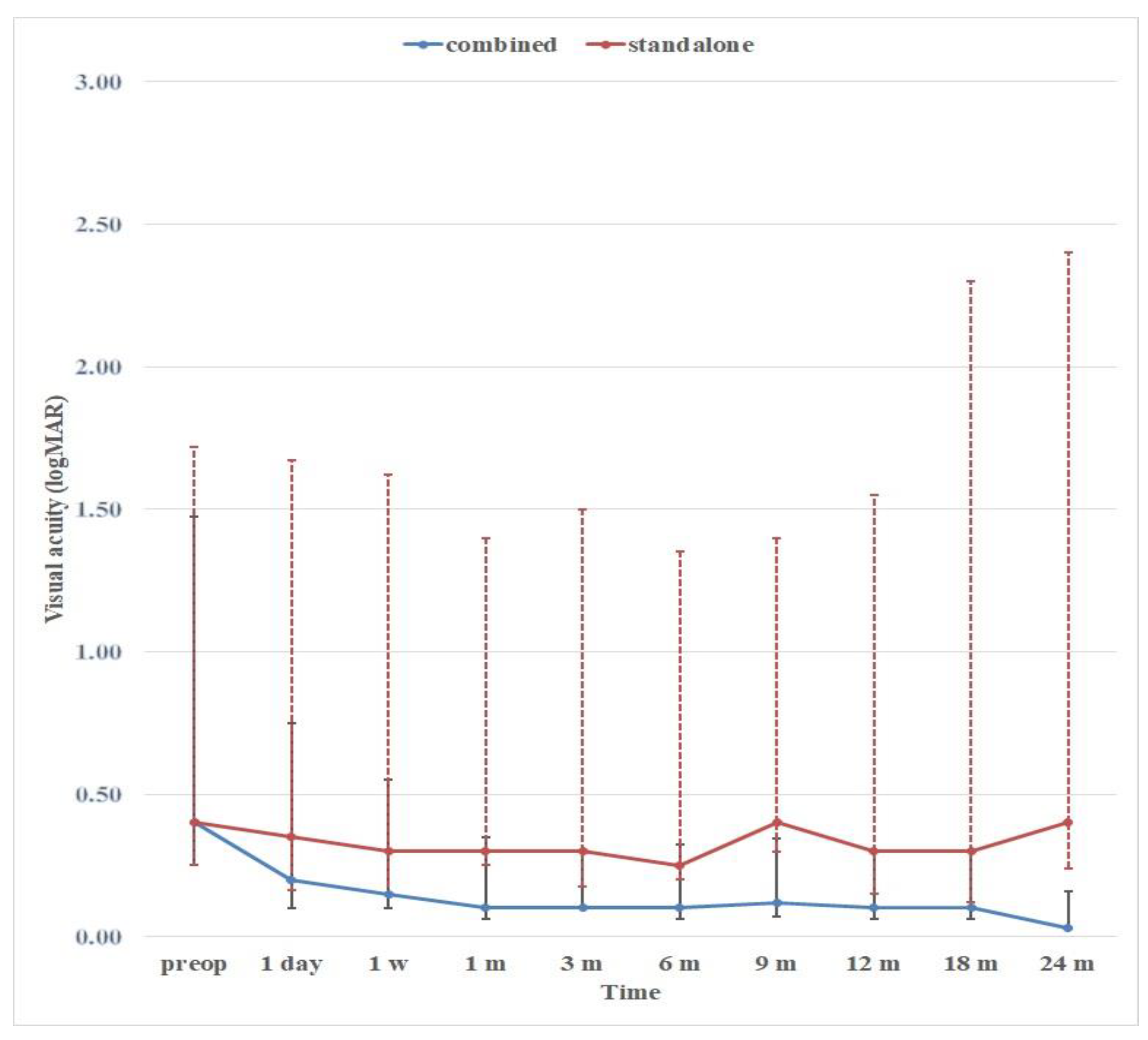
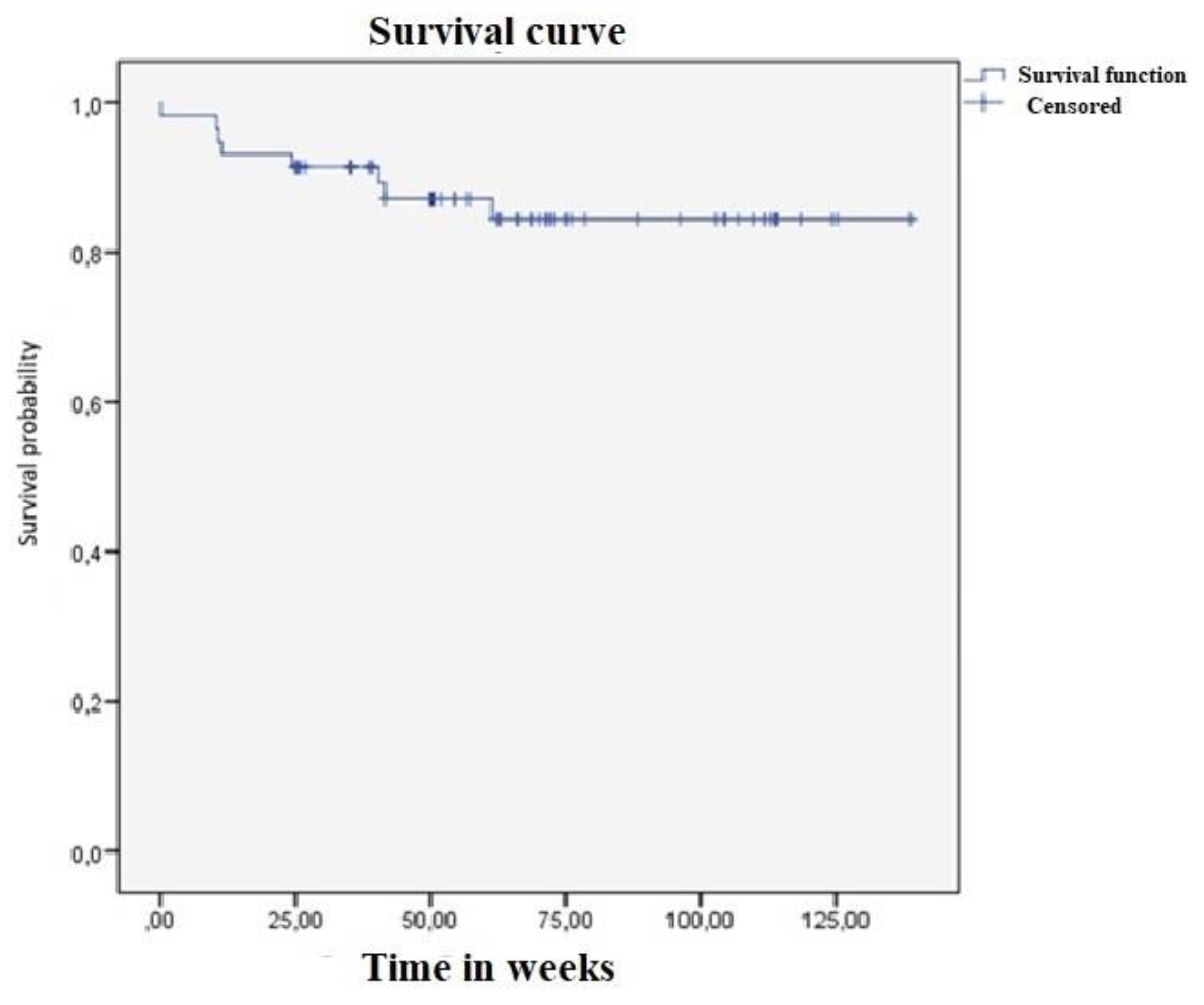
3.9. CLVs development
The postoperative development of lymphatic vessels in the conjunctiva was observed in 50% of cases (29/58). Biomicroscopically, these vessels had uneven calibers and were visible in the form of a plexus or solitary vessels running parallel to the limbus or perpendicular to it. The lymphatic nature was confirmed by OCT [
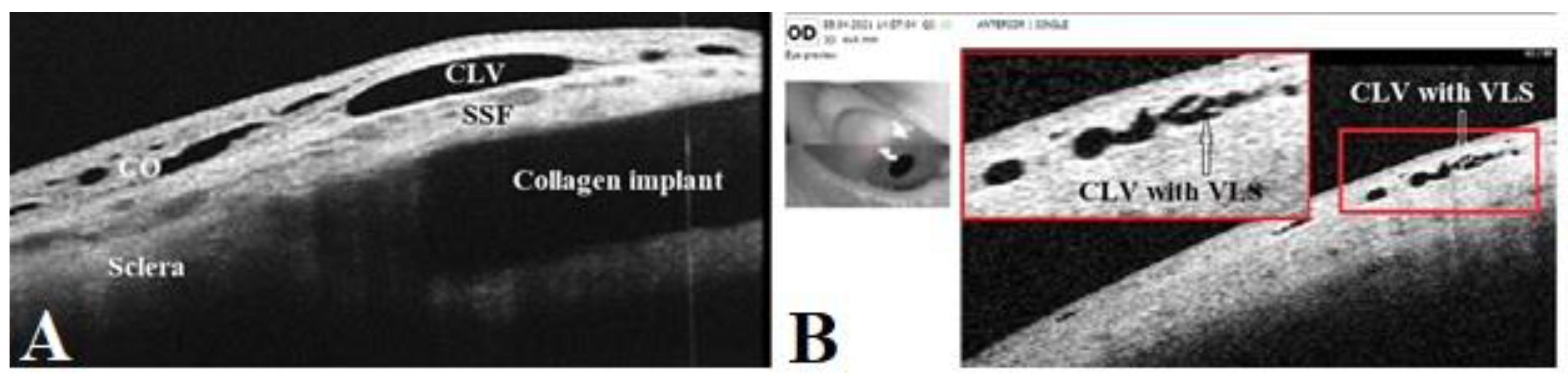
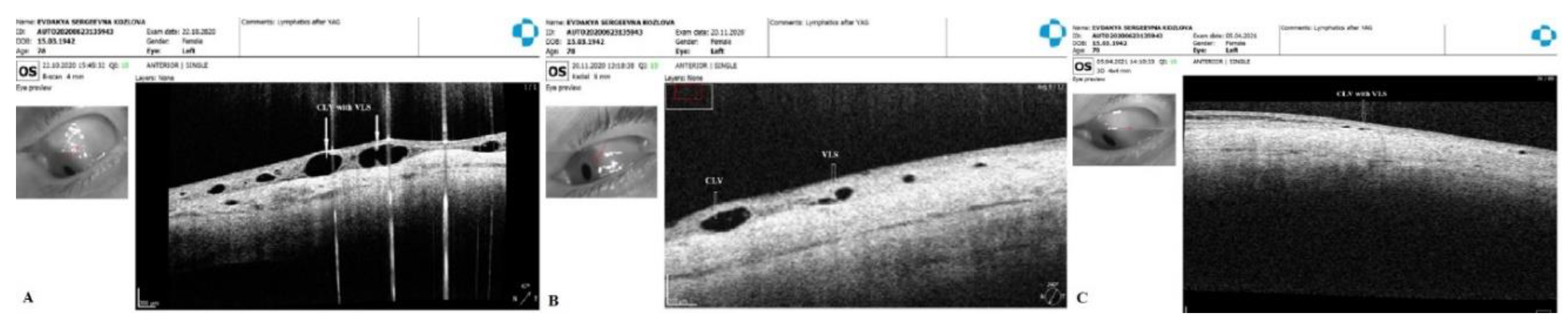
21]. Sausage-type vessels with valve like structures (VLS) in their lumens were evident.
The time of CLVs development differed from case to case. In six eyes, CLVs developed the following day after Nd:YAG laser trabeculotomy was performed, in seven eyes after one week, and in 14 eyes after one month. In summary, in 93.1% eyes (27 out of 29 eyes), CLVs developed within one month. In two eyes, CLVs developed late, at six months in one eye and at nine months in another eye.
The CLVs lasted for different periods of time. The time of CLV development and the duration of CLV visibility via biomicroscopy are presented in
Table 6. On OCT, CLVs with VLS in their lumens were diagnosed in most cases—in 82.8% eyes (48/58).
No relationship was established between Nd:YAG laser trabeculotomy and the development of CLVs. CLVs were observed to develop irrespective of Nd:YAG laser trabeculotomy (Figures 9,10). Of thirty-nine eyes that underwent Nd:YAG laser trabeculotomy, CLVs developed in 46.1% eyes (18/39 eyes), whereas in eyes without laser trabeculotomy, they developed in 52.6% eyes (10/19 eyes), suggesting that the role of laser trabeculotomy was limited to provide AH resistance-free flow from the AC to the SCS.
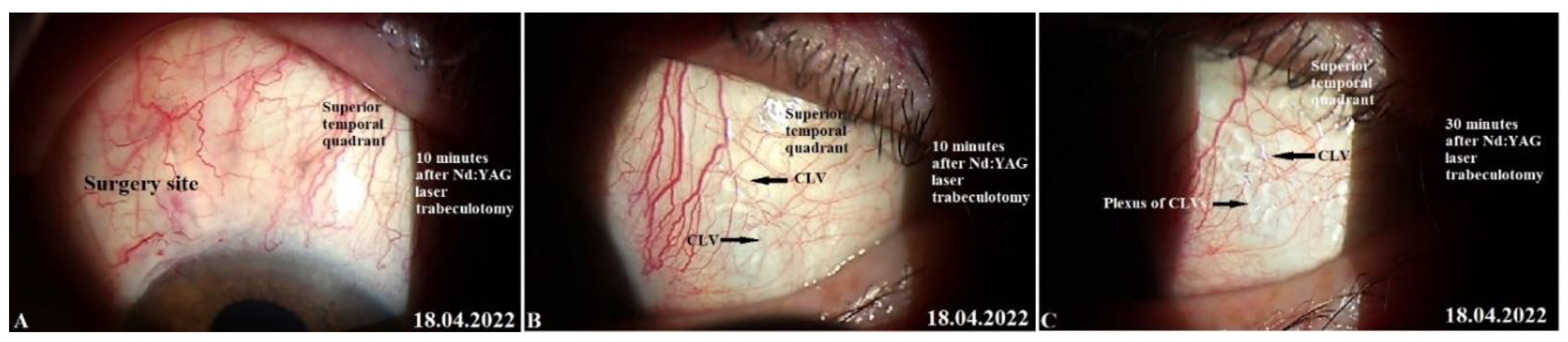
Figure 9.
Slit lamp view of the surgery site and superior quadrants in a patient’s left eye in whom CLVs developed immediately after Nd:YAG laser trabeculotomy. (A) 10 minutes after Nd:YAG laser trabeculotomy, surgery site and nearby areas free from CLVs; some swelling at the surgery site persists. (B) 10 minutes after Nd:YAG laser trabeculotomy, slit lamp view of the superior temporal quadrant showing a CLVs plexus (black arrows). (C) 30 minutes after the procedure, slit lamp view of the superior temporal quadrant after massaging the area with the lid margin; a well-developed CLVs plexus is observed far from the surgery site. CLV=conjunctival lymphatic vessel; Nd:YAG laser = neodymium yttrium aluminum garnet laser.
Figure 9.
Slit lamp view of the surgery site and superior quadrants in a patient’s left eye in whom CLVs developed immediately after Nd:YAG laser trabeculotomy. (A) 10 minutes after Nd:YAG laser trabeculotomy, surgery site and nearby areas free from CLVs; some swelling at the surgery site persists. (B) 10 minutes after Nd:YAG laser trabeculotomy, slit lamp view of the superior temporal quadrant showing a CLVs plexus (black arrows). (C) 30 minutes after the procedure, slit lamp view of the superior temporal quadrant after massaging the area with the lid margin; a well-developed CLVs plexus is observed far from the surgery site. CLV=conjunctival lymphatic vessel; Nd:YAG laser = neodymium yttrium aluminum garnet laser.
Figure 10.
Slit lamp view of the right eye of a patient in whom CLVs developed without Nd:YAG laser trabeculotomy. (A) Slit lamp view of the superior temporal quadrant of the eyeball on day 21 after surgery showing a plexus of CLVs located away from the surgery site (arrow with black borders). (B) The same view with a red-free filter. (C) Same site as in A and B, an Enface image (OCT angio) demonstrating sausage-shaped lymphatic vessels with uneven calibers (arrows with black borders). CLV = conjunctival lymphatic vessel.
Figure 10.
Slit lamp view of the right eye of a patient in whom CLVs developed without Nd:YAG laser trabeculotomy. (A) Slit lamp view of the superior temporal quadrant of the eyeball on day 21 after surgery showing a plexus of CLVs located away from the surgery site (arrow with black borders). (B) The same view with a red-free filter. (C) Same site as in A and B, an Enface image (OCT angio) demonstrating sausage-shaped lymphatic vessels with uneven calibers (arrows with black borders). CLV = conjunctival lymphatic vessel.
Interesting facts were uncovered when the site of CLV development was analyzed as per the operated eye. The results are presented in
Table 7. In cases where surgery was performed on the right eye, CLVs developed more commonly in the nasal quadrant, and in cases where surgery was performed on the left eye, CLVs developed more commonly in the temporal quadrant. Out of 17 cases where glaucoma surgery was performed in the right eyes of patients, in 16 cases (94.1%), CLVs developed in the nasal quadrant. Of these, in five eyes, CLVs developed in both quadrants. The quantitative analysis of CLVs identified on OCT presented the same pattern—in the superior nasal quadrant, they were identified in 20 out of 24 eyes (83.3%). Nearly the same pattern of CLV development was noticed in the left eyes of patients. In cases where surgery was performed on the left eyes (12 eyes), CLVs were predominant in the temporal area in nine eyes (75.0%). The same was true for OCT—CLVs were identified in the temporal area (60.7%) in 17 out of 28 eyes.
In all except one case, CLVs developed in an area located away from the surgery site. CLVs developed from the sclera, indicating the existence of intrascleral microchannels located across the sclera connecting the SCS to the CLVs (
Figure 9C). Clinically, we observed a few patients in whom CLVs first appeared in one quadrant; with time, they disappeared, but new CLVs developed in another quadrant (pl. refer to case 2).
To define the relationship between CLV development and the decrease in IOP, all cases were divided into two subgroups: subgroup I included cases with CLVs identified via a slit lamp and subgroup II included cases without CLV identification. In each subgroup, the success rate was analyzed. The results are presented in
Table 8. The number of cases in both subgroups was equal. The analysis showed that the rate of complete success was higher in subgroup II, achieving 75.9% versus 37.9% in subgroup I (p=0.000368). The qualified success rate was equal in both subgroups. The instillation of hypotensive medications to achieve target IOP values was required by more patients in subgroup I—by 44.8% patients versus 13.8% patients in subgroup II (p=5.13X10
-5). There was no difference in failure rates in both subgroups. These results suggest that the development of CLVs after surgery has a poor prognostic value for IOP control. If the fluid flow from the SCS to CLVs through intrascleral microchannels is smooth and resistance-free, no CLV development is evident. However, if any resistance exists in the flow, the fluid accumulates in lymphatics, resulting in their engorgement and longer visibility period.
Below, we present a few clinical cases to demonstrate the effectiveness and safety of the proposed technique, the role of Nd:YAG laser trabeculotomy, the impact of CLVs development on IOP decrease, and the possible mechanism of the hypotensive effect produced by the technique without forming a filtration bleb.
3.10. Case reports
Case 1. Patient K, a 78-year-old female patient with advanced stage glaucoma in her right eye and moderate-stage glaucoma in her left eye underwent combined surgery in both eyes. Glaucoma was diagnosed five years ago and, since then, the patient has been on four classes of hypotensive medications in both eyes (analog of prostaglandins F2α, beta blocker, carbonic anhydrase inhibitor, and selective α 2-adrenomimetic). Her IOP prior to surgery was 27.0 mmHg in both eyes. The patient had a history of intravitreal injections of antiVEGF (sol. Lucentis—three injections) for wet macular degeneration in her right eye. Her preoperative best-corrected visual acuity (logMAR) was 0.1 in both eyes.
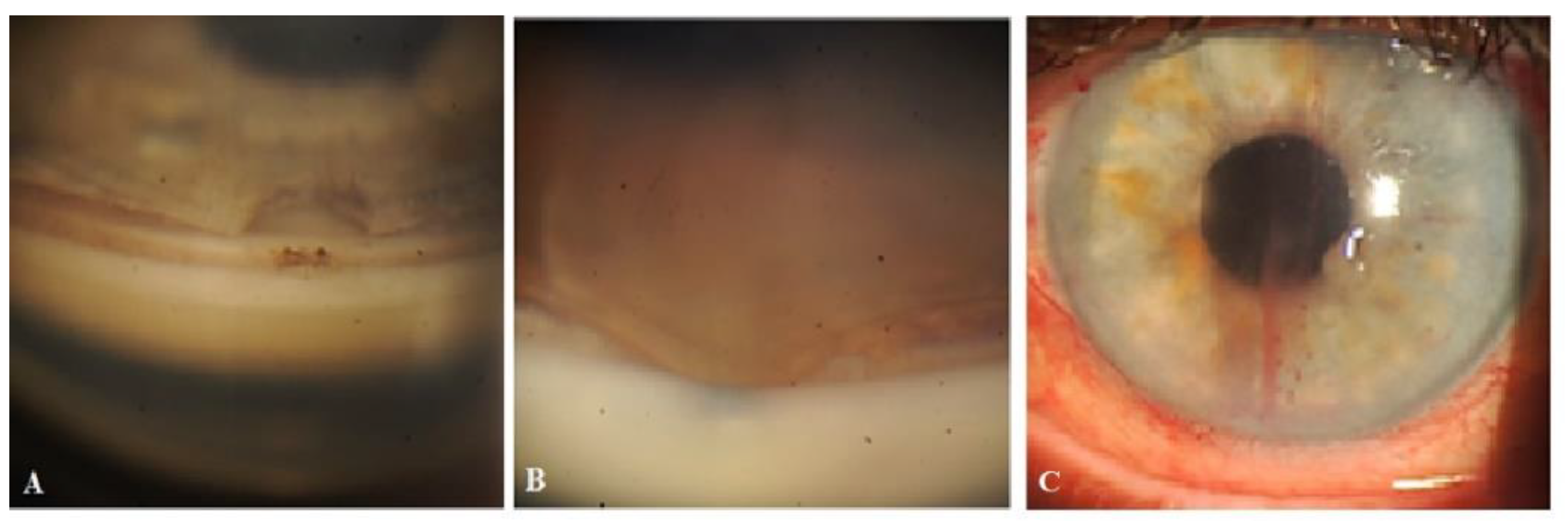
Right-eye surgery. On 14 April 2020, under retrobulbar anesthesia (2% solution of lidocaine hydrochloride), the patient underwent a combined procedure. Prior to surgery, no washout of hypotensive medications was performed. First, phacoemulsification with an implantation of a foldable acrylic IOL in the capsular bag was performed, followed by glaucoma surgery, as previously described. Intraoperatively, no complications were encountered. Postoperatively, the patient followed a standard protocol. On day 16, conjunctival sutures were removed. On day 21, the patient’s IOP on hypotensive medications was 23 mmHg and she underwent Nd:YAG laser trabeculotomy in the operated area.
IOP change. Immediately following the laser trabeculotomy procedure, some swelling was observed at the surgery site (not a bleb according to OCT), and to avoid the blockage of the trabeculotomy opening by iris tissue, the instillation of 1% pilocarpine eye drops twice daily was advised for three days, and the patient was advised to cease the instillation of other hypotensive medications. On the following follow-up visit on day 10, after the trabeculotomy, no filtration bleb was observed at the surgery site, and the patient’s IOP was 13 mmHg off medications. The patient was further followed up at predetermined intervals up to 2.5 years. At 3, 6, 12, 15, and 30 months, the patient’s IOP was 9, 13, 11, 16, and 9 mmHg, respectively, off medication. The absence of bleb was confirmed by OCT at all follow-up visits.
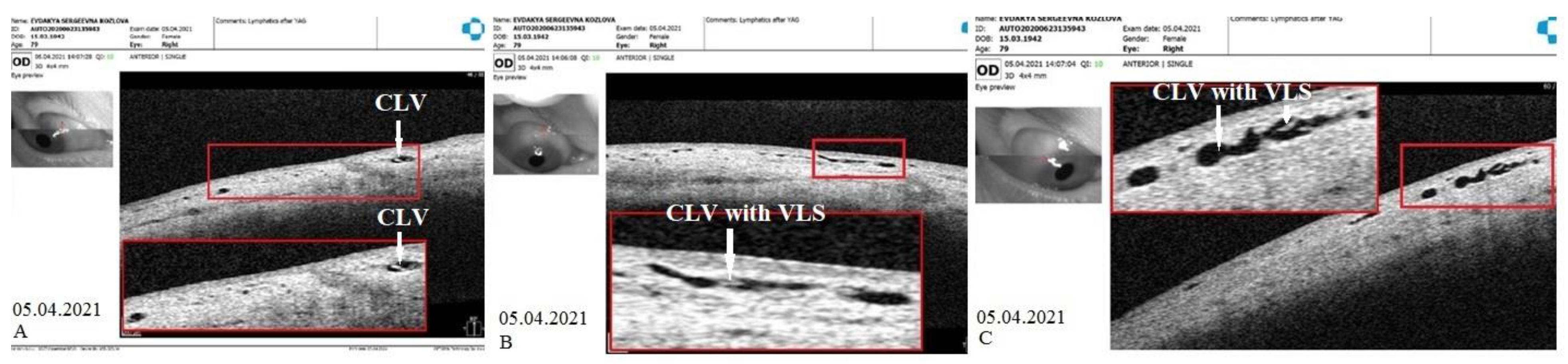
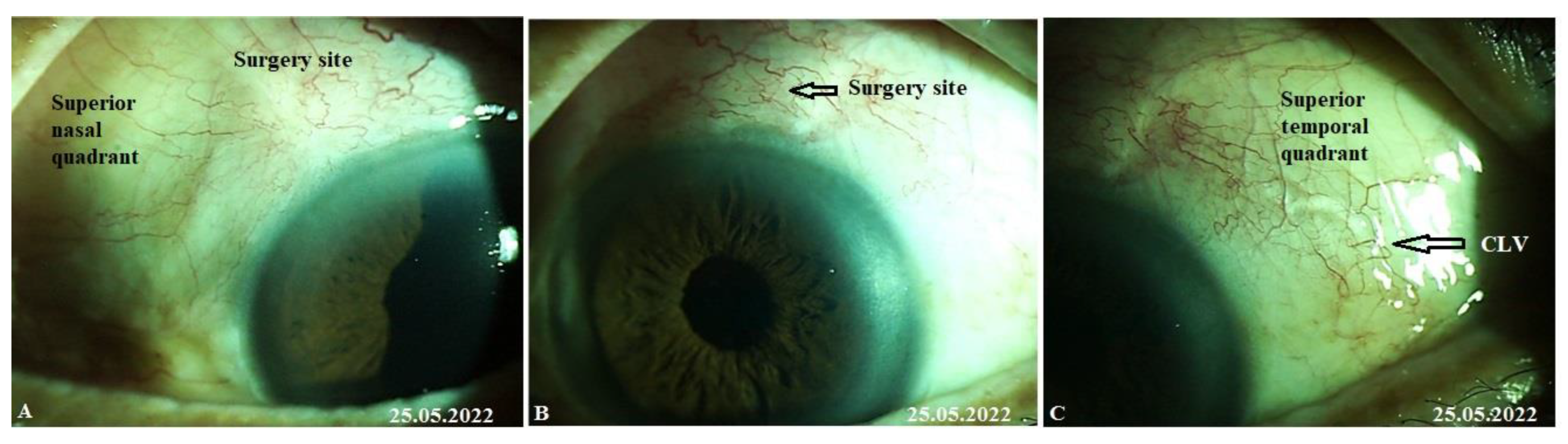
CLVs development. The following day after the trabeculotomy, a well-developed plexus of CLVs was identified biomicroscopically in the superior nasal quadrant. With the passage of time, these vessels reduced in number and size (
Figure 11,
Figure 12 and
Figure 13). At the last follow-up visit, at 2.5 years, no CLVs were identified biomicroscopically. However, on OCT, CLVs were identifiable in the nasal and temporal quadrants until the last follow-up visit.
Left-eye surgery. On 23 September 2020, the patient underwent an identical-to-right-eye combined procedure on her left eye. No preoperative hypotensive medication washout was performed. First, phacoemulsification with the implantation of a foldable acrylic IOL in the capsular bag was performed, followed by glaucoma surgery. Surgery was uneventful. Postoperatively, on day 7, conjunctival sutures were removed. On days 1, 6, and 13, the patient’s IOP values on two classes of medications were 18, 13, and 25 mmHg, respectively. On day 13, the patient underwent Nd:YAG laser trabeculotomy at the surgery site.
IOP change. Immediately after the trabeculotomy, some swelling appeared at the surgery site (absence of bleb confirmed by OCT), and the IOP decreased to 11 mmHg. The patient was advised to discontinue the installation of hypotensive medication and was followed up for a period of 24 months with an interval of 3 months between visits. At 6, 12, 18, and 24 months, the patient’s IOP values were 15, 14, 10, and 10 mmHg, respectively, off medication.
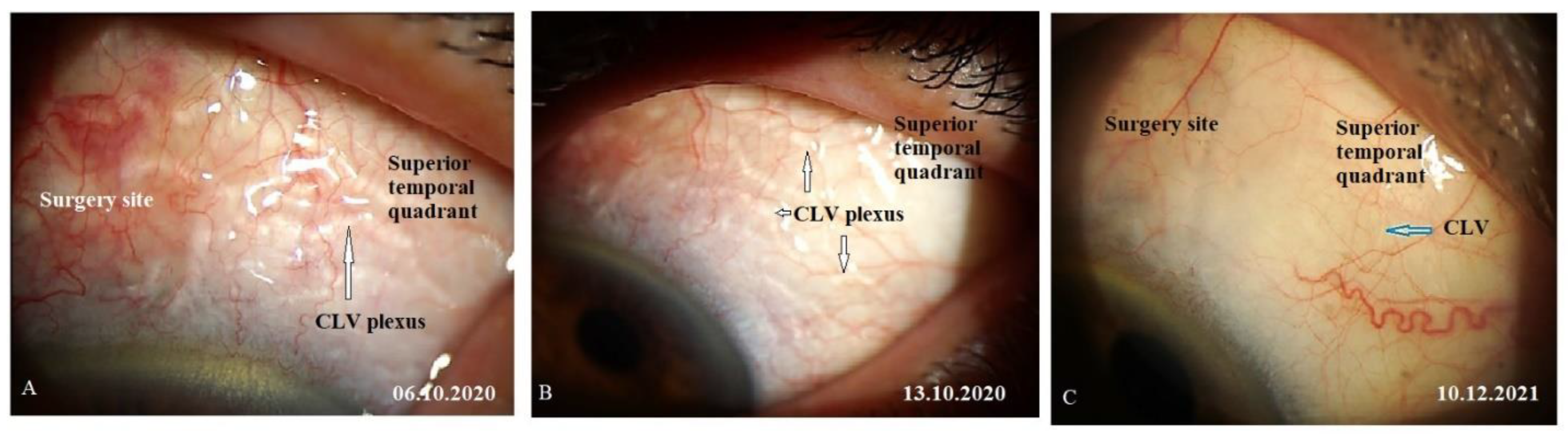
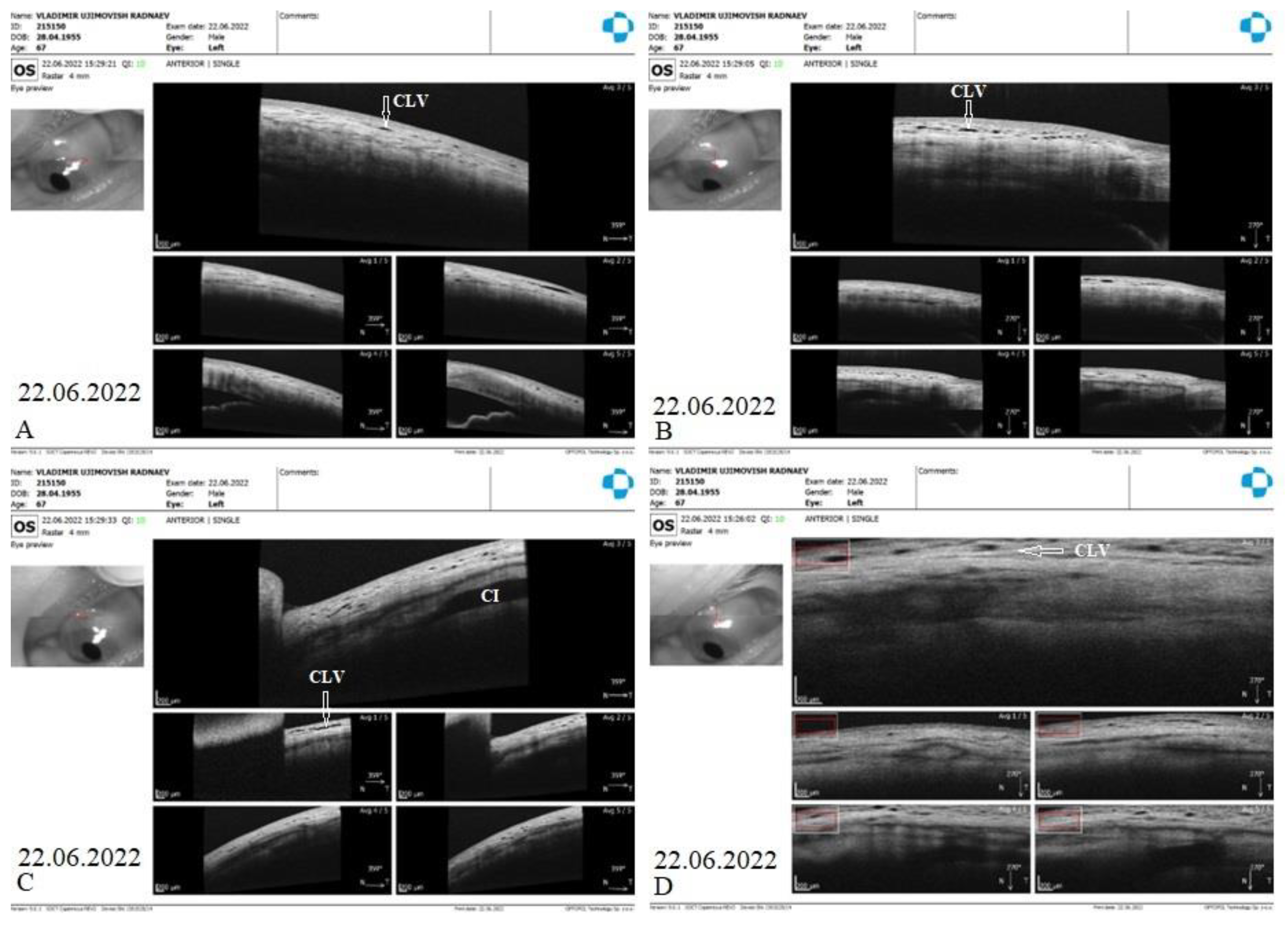
CLVs development. The day after the laser trabeculotomy, a plexus of CLVs with uneven calibers and filled with transparent fluid was observed in the superior temporal quadrant (
Figure 14A). During the subsequent follow-up visit, one week after the trabeculotomy (on 13 October 2020), CLVs were reduced in size and number (
Figure 14B). By month 14, only an isolated CLV could be observed in the temporal quadrant (Fig.14C). The surgery site and superior nasal and temporal quadrants, which were investigated by OCT, showed CLVs with VLSs in their lumens (
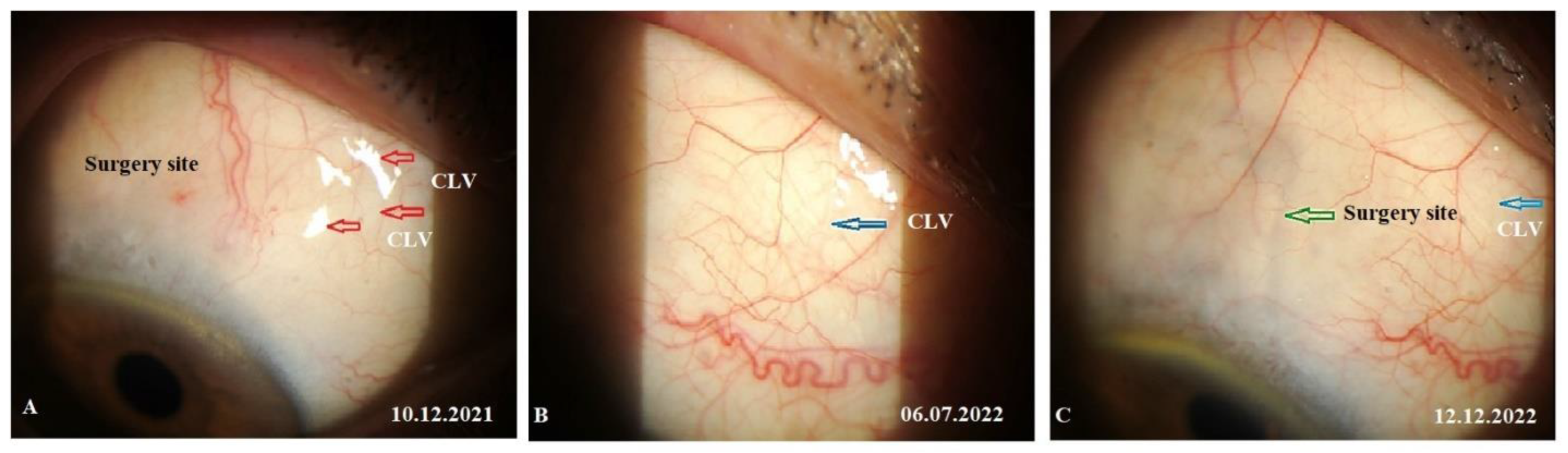
Figure 15A-C). At the final follow-up visit, CLVs were not visible biomicroscopically (
Figure 16A-C).
By reporting this case, the role of Nd:YAG laser trabeculotomy in providing the resistance-free flow of AH from the AC to the SCS was emphasized in our study. The patient underwent combined surgery in both eyes, and laser trabeculotomies were performed after surgery on both eyes. Prior to the trabeculotomy, sufficient time was allowed for the conjunctiva to heal, thus preventing AH flow to the subconjunctival space, which was shown by OCT. Until the last follow-up visit, the patient presented a significant decrease in IOP without a filtering bleb. Postoperatively, CLVs, which were identified biomicroscopically, developed in both eyes. Notably, once the proper AH flow from the SCSs to CLVs was established, these vessels reduced in size and number, leaving a few (identified on OCT) to maintain the flow.
Case 2. Patient R., an 80-year-old male patient, was operated upon on his left eye for an underlying visually significant cataract and advanced-stage glaucoma. The patient had glaucoma in both his eyes, which was diagnosed seven years prior and, since then, he was on two classes of hypotensive medications (a combination of a carbonic anhydrase inhibitor and a beta blocker). Two years ago, the patient underwent cataract surgery in his right eye. Preoperatively, IOP levels on medications were 19 and 31 mmHg in his right and left eye, respectively. Best-corrected visual acuity (logMAR) values were 0.3 and 0.4 in his right and left eye, respectively.
Left-eye surgery. On 26 April 2021, the patient underwent a combined procedure under retrobulbar anesthesia (2% solution of lidocaine hydrochloride). Prior to surgery, no washout of hypotensive medications was performed. First, phacoemulsification with an implantation of a foldable acrylic IOL in the capsular bag was performed. Intraoperatively, no complications occurred. Postoperatively, the patient followed the standard protocol and continued the instillation of hypotensive medication. Conjunctival sutures were removed on day 10. His IOP was 10 mmHg on this day, and he was advised to discontinue the instillation of hypotensive medication. The patient was followed up at predetermined intervals up to 12 months. As his IOP was below the target level at all visits, no YAG laser trabeculotomy was performed.
IOP change. At 1, 3, 6, and 12 months, the patient’s IOP levels were 14, 10, 10, and 7 mmHg, respectively, off medication. Postoperative logMAR VA improved to 0 and remained at this value until the final follow-up visit.
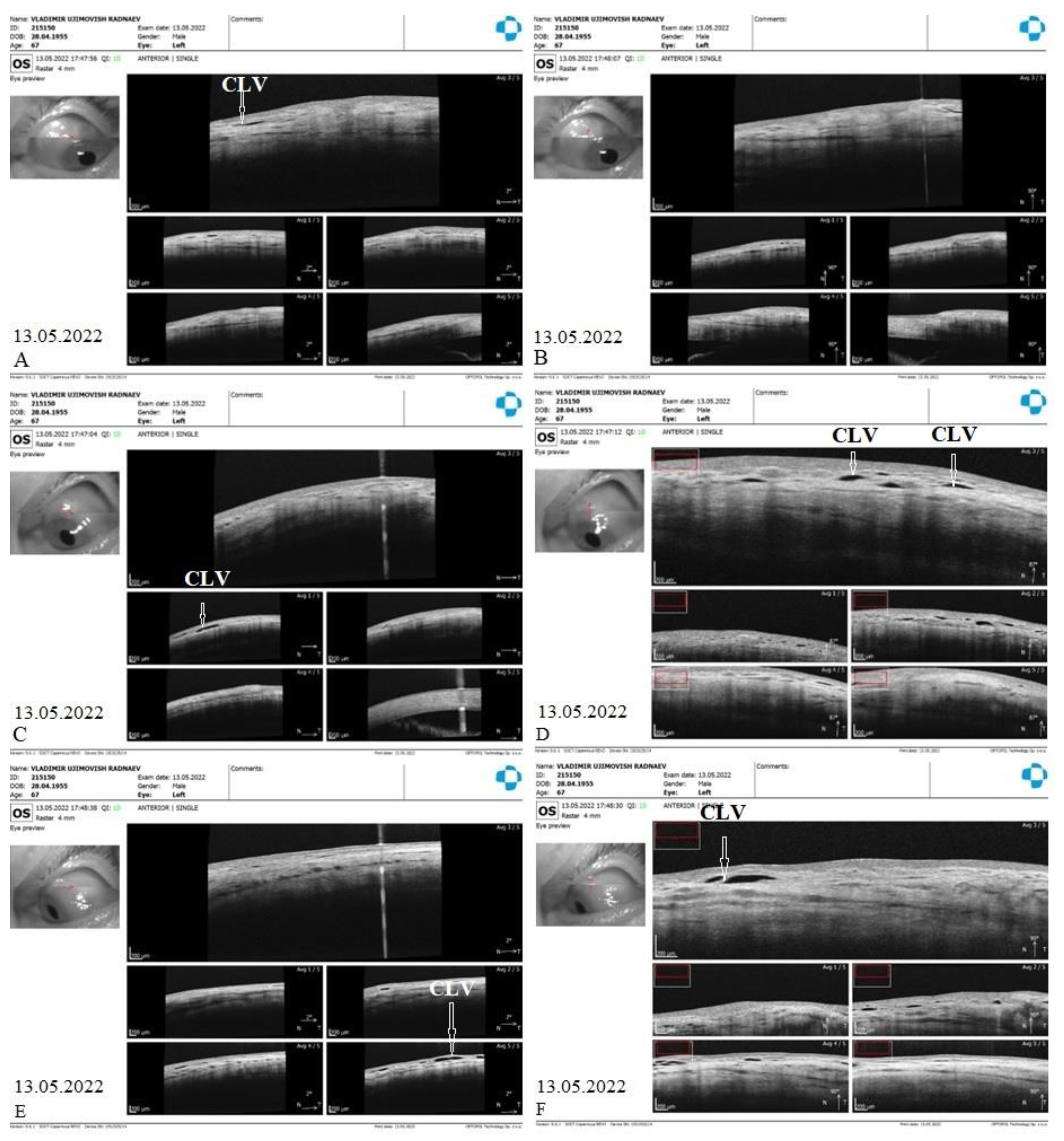
CLVs development. At one week after surgery (on 13 May 2022), slit lamp biomicroscopy revealed an absence of bleb and CLVs. On OCT, the absence of bleb at the surgery site was confirmed, and CLVs were identified in the superior nasal and temporal quadrants and at the surgery site (
Figure 17 A-F).
The patient was then consulted after another 2 weeks on 25 May 2022. On biomicroscopy, two CLVs were observed in the superior temporal quadrant (
Figure 18 A-C); the lymphatics arose directly from the sclera located at a considerable distance from the surgery site and became more prominent when the upper eye lid closed over them (see
Supplementary Materials Video S1_Case 2_month 1 after surgery: CLVs in superior temporal quadrant).
The patient’s subsequent consultation was on 22 June 2022 (1.5 months after surgery). The slit lamp showed that remnants of CLVs could be identified in the superior temporal quadrant. On OCT, CLVs were identifiable in the superior temporal quadrant and at the surgery site (
Figure 19 A-D).
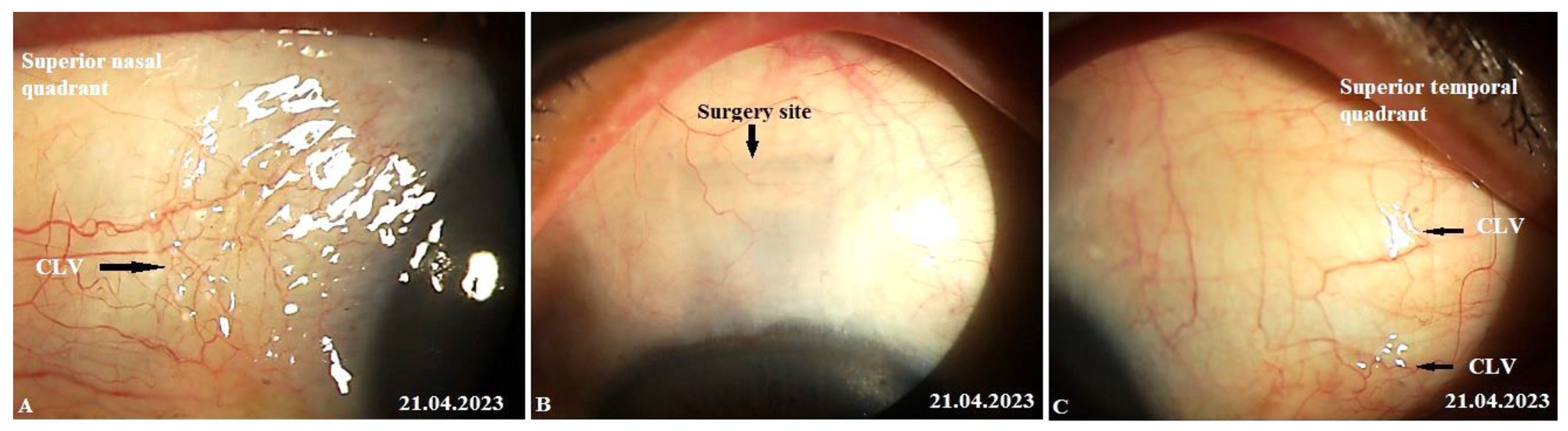
The patient’s last follow-up visit was at 12 months (on 21 April 2023). On biomicroscopy, no bleb was noticed at the surgery site and, notably, no CLVs were observed in the superior temporal quadrant; instead, close to the insertion of the internal rectus muscle and in the superior nasal quadrant, a plexus of CLVs was observed. Massaging the conjunctiva over the plexus by moving the lid margin over it caused the vessels to become engorged (
Figure 20 A-C). Pl. refer to
Supplementary Materials video S2_Case 2_month 12 after surgery: CLVs in superior nasal quadrant.
By reporting this case, we aimed to emphasize the effectiveness of the proposed technique in decreasing the IOP level without performing Nd:YAG laser trabeculotomy. The deroofing of the SC and the thinning of its inner wall by removing a part of the JCT was sufficient for AH outflow from the AC to decrease IOP significantly. Furthermore, OCT investigations of the surgery site at different follow-up visits confirmed that the IOP decrease occurred without the formation of a filtering bleb. This indirectly indicated that the proposed technique rerouted the AH flow from the AC to the SCS without leaking into the subconjunctival space. This case also emphasized that the development of CLVs occurs irrespective of laser trabeculotomy. The deroofed SC and its thinned inner wall allowed enough AH to flow from the AC to the SCS to initiate its outflow across the sclera to CLVs. AH flowed across the sclera through some pores or microchannels. Our clinical observations in this case demonstrated that some of the AH flowed from SCSs to CLVs. Another important observation we made in this case was that CLVs first appeared in the superior temporal quadrant and lasted for a period of 12 months. Towards the end of this observation period, another CLV plexus developed in the superior nasal quadrant. This meant that as soon as the existing lymphatic pathway became unfunctional for some reason, the CLVs developed in another area where a functional natural outflow pathway from SCSs to CLVs remained. For practical purposes, this case emphasized that it is paramount to preserve the lymphatics while performing glaucoma surgery.
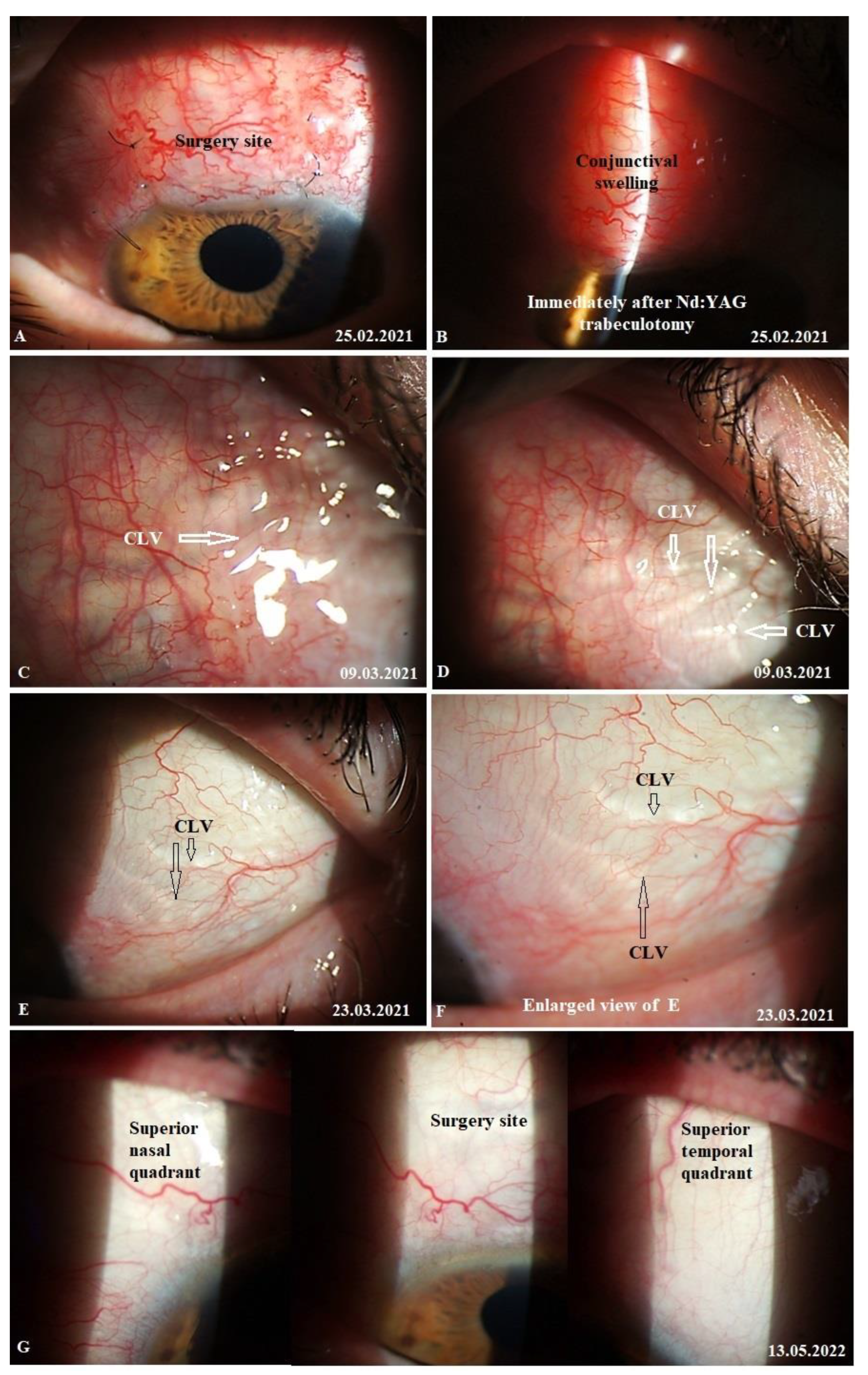
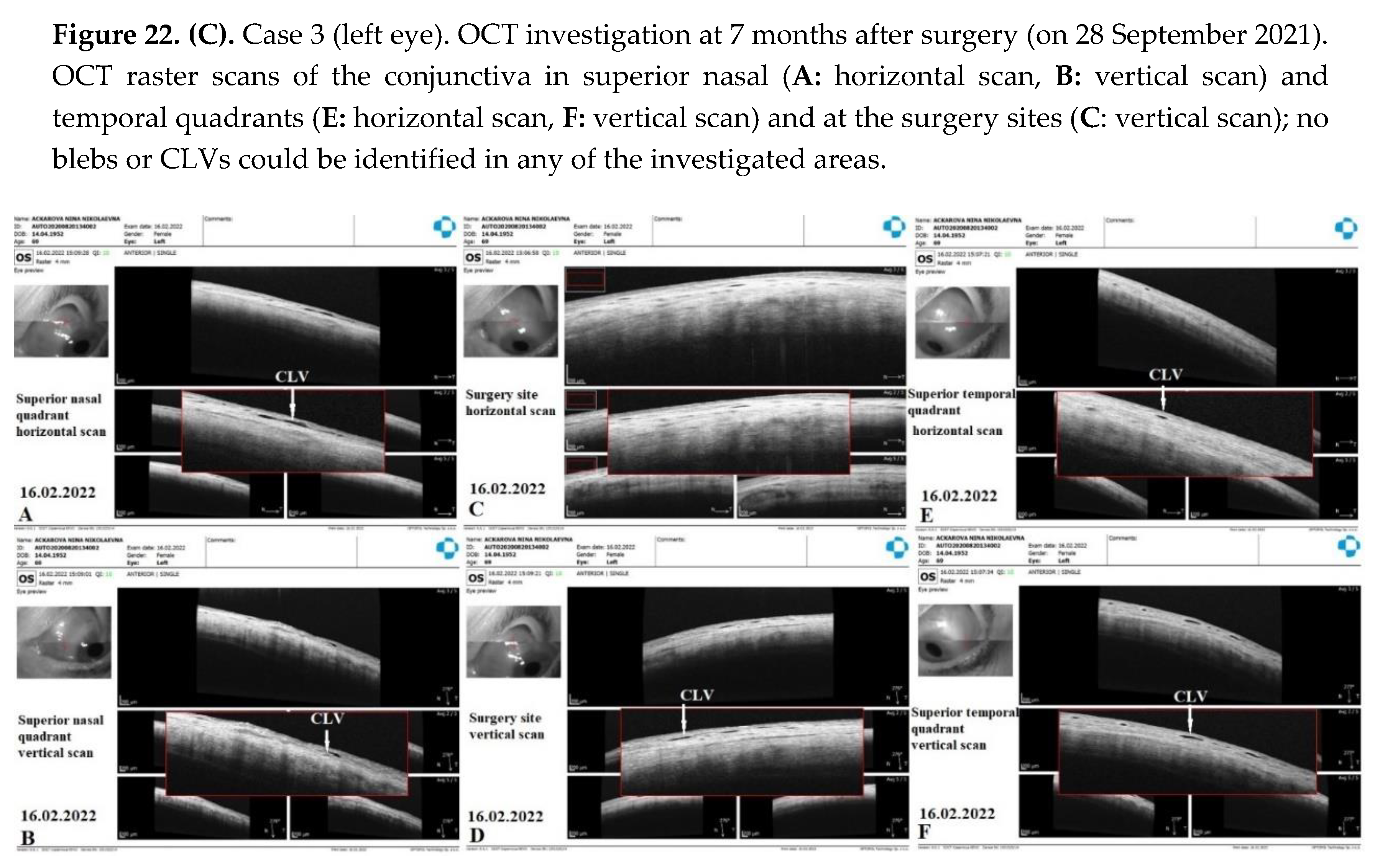
Case 3. Patient A. was a 69-year-old female patient who underwent a combined procedure for an intumescent cataract and advanced-stage OAG in her left eye, which was followed up for a period of 1.5 years. Glaucoma was diagnosed eight years prior and, since then, the patient was on hypotensive medications. Prior to surgery, the IOP in her right eye on one class of hypotensive medication (analog of prostaglandins) was 25 mmHg and on three classes of hypotensive medication (analog of prostaglandins, beta blockers, and carbonic anhydrase inhibitors) was 30 mmHg in her left eye. The preoperative best-corrected logMar VA values were 0 and 2 in her right and left eye, respectively. On 13 February 2021, the patient underwent surgery in her left eye under retrobulbar anesthesia (2% solution of lidocaine hydrochloride). Prior to surgery, no washout of hypotensive medications was performed. First, phacoemulsification with an implantation of a foldable acrylic IOL in the capsular bag was performed, followed by glaucoma surgery, as previously described. Surgery was uneventful, and no intraoperative complications were observed. Postoperatively, the patient followed a standard protocol and continued the instillation of two classes of hypotensive medications. On day 12, conjunctival sutures were removed (Fig.21A).
IOP changes. On day 12, the patient’s IOP on medication was 18 mmHg. On the same day, sutures were removed and Nd:YAG laser trabeculotomy was performed. Immediately after the trabeculotomy, the patient’s IOP decreased to 16 mmHg and the patient was advised to stop the instillation of hypotensive medications. On the following two follow-up visits, the patient had elevated IOP levels up to 26 mmHg. Repeat-laser trabeculotomy was attempted two more times with an interval of 7 days. After the third trabeculotomy attempt, the patient’s IOP decreased to 7 mmHg off medication. The IOP remained below the target level until 3 months, when an increase in the IOP to 31 mmHg was noticed. A 4th YAG trabeculotomy was attempted to decrease the IOP; however, this failed. The patient was advised to restart the instillation of hypotensive medications. At 6, 9, 12, and 15 months, the patient’s IOP levels were 34, 24, 24, and 27 mmHg, respectively, on three classes of hypotensive medications. A repeat glaucoma surgery was advised. On 29 May 2022, a diode laser cyclophotodestruction procedure was performed.
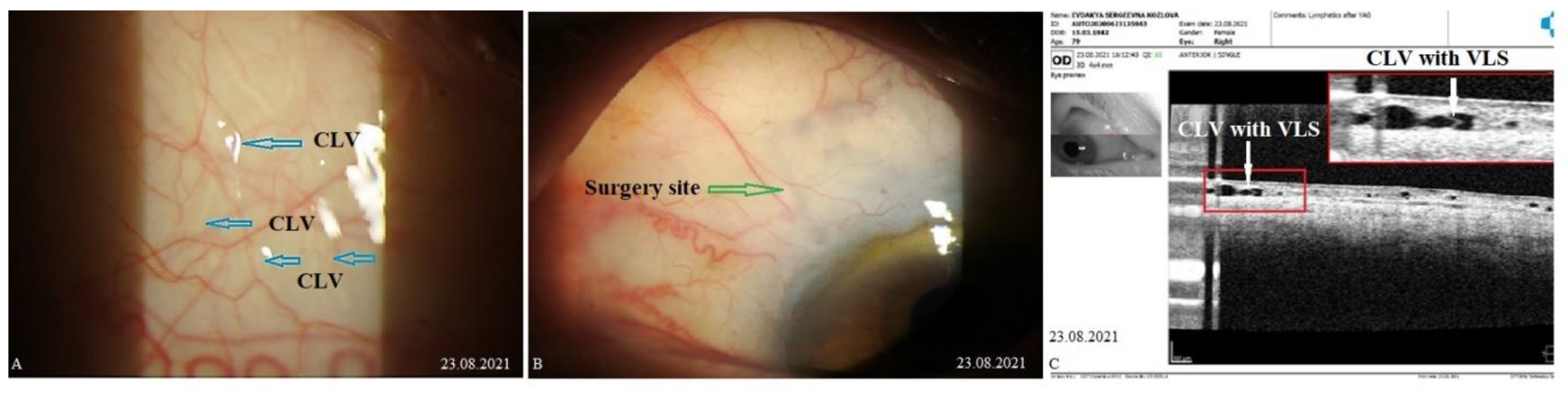
CLVs development. Immediately after the first Nd:YAG laser trabeculotomy, some conjunctival swelling appeared at the surgery site (
Figure 21B). After 1 week, the slit lamp images showed overfilled CLVs with uneven calibers in the form of a plexus, which became visible at the surgery site (
Figure 21C). Their course from the surgery site could be detected towards the superior temporal quadrant (
Figure 21D). CLVs remained visible biomicroscopically up to months 2 after the trabeculotomy. With time, their number and size decreased considerably (
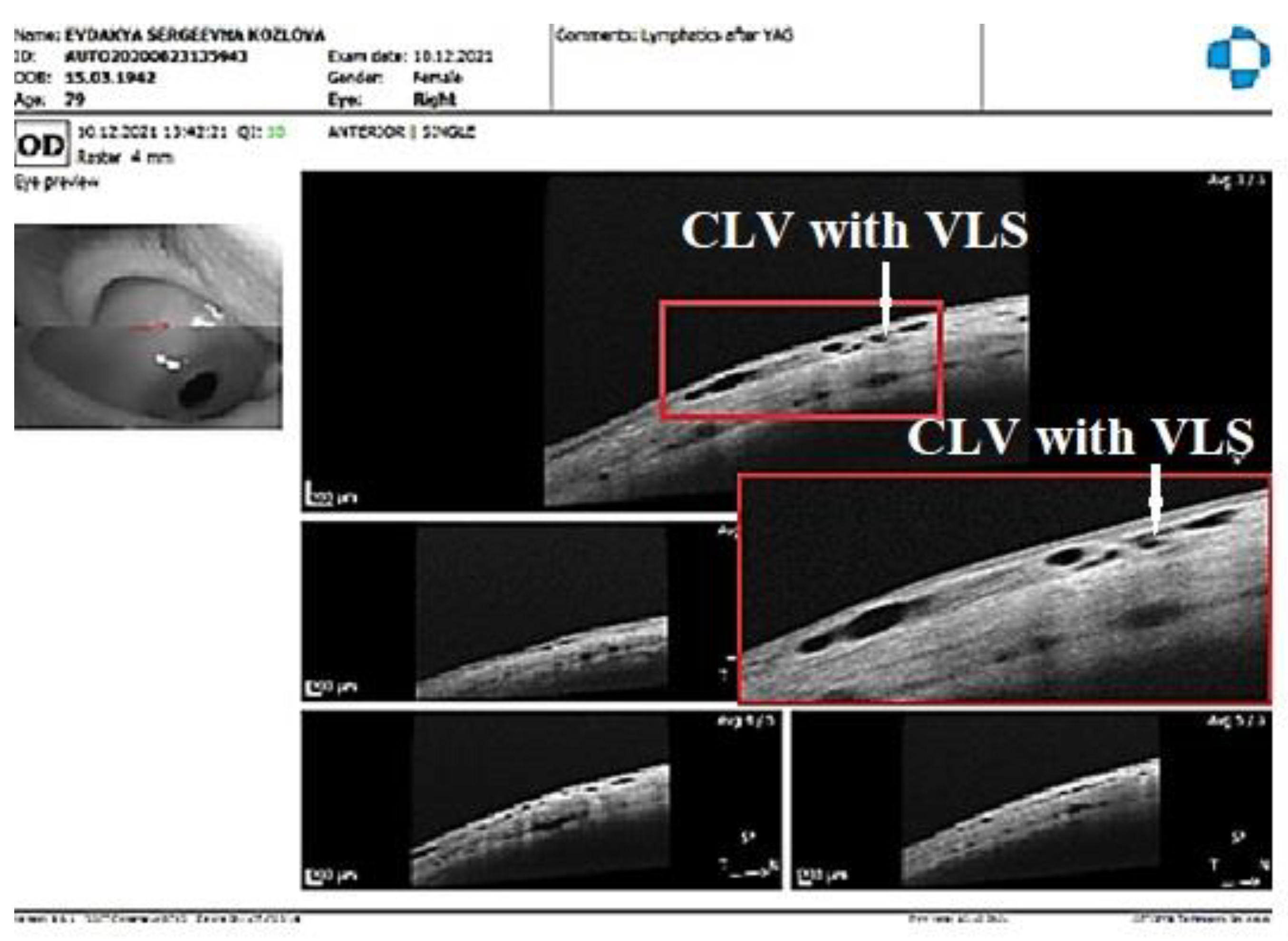
Figure 21E, F). At 7 months, the CLVs completely disappeared (Fig.21G). At each follow-up visit, the patient’s surgery site and nearby quadrants were evaluated by OCT (
Figure 22A-D). CLVs with VLSs were present in the conjunctiva over the surgery site and in both superior quadrants at all follow-up visits.
This study demonstrated the activation of AH flow from SCSs to CLVs after Nd:YAG laser trabeculotomy was performed. Similar to the previous cases, outflow activation occurred without the formation of a filtering bleb (the absence of bleb was confirmed by OCT). The appearance of engorged CLVs with uneven calibers over the surgery site and in the superior temporal quadrant within a period of 7 days after laser trabeculotomy indicated that, with the proposed technique, the functional status of conjunctival lymphatics played a considerable role in IOP regulation. Overfilled engorged CLVs pointed out the possible resistance in the fluid pathway or the non-satisfactory functional status of the conjunctival lymphatic system. This case emphasizes the necessity for early surgical intervention in glaucoma patients when conjunctival lymphatics are still active and functional.