Submitted:
04 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
- Importance of plant diseases
- Bio-Pesticides
- a)
- Microbial biopesticides
- b) Macrobial biopesticides
- c) Semiochemicals
- d) Plant Incorporated Protectants (PIP)
- e) Botanical biopesticides (Plant Extracts)
- Mechanisms of pest control of biopesticides
- Global Research Progress on the Use of Plant Biopesticides to Control Crop Diseases
- Regulation and Policy Framework of Biopesticides
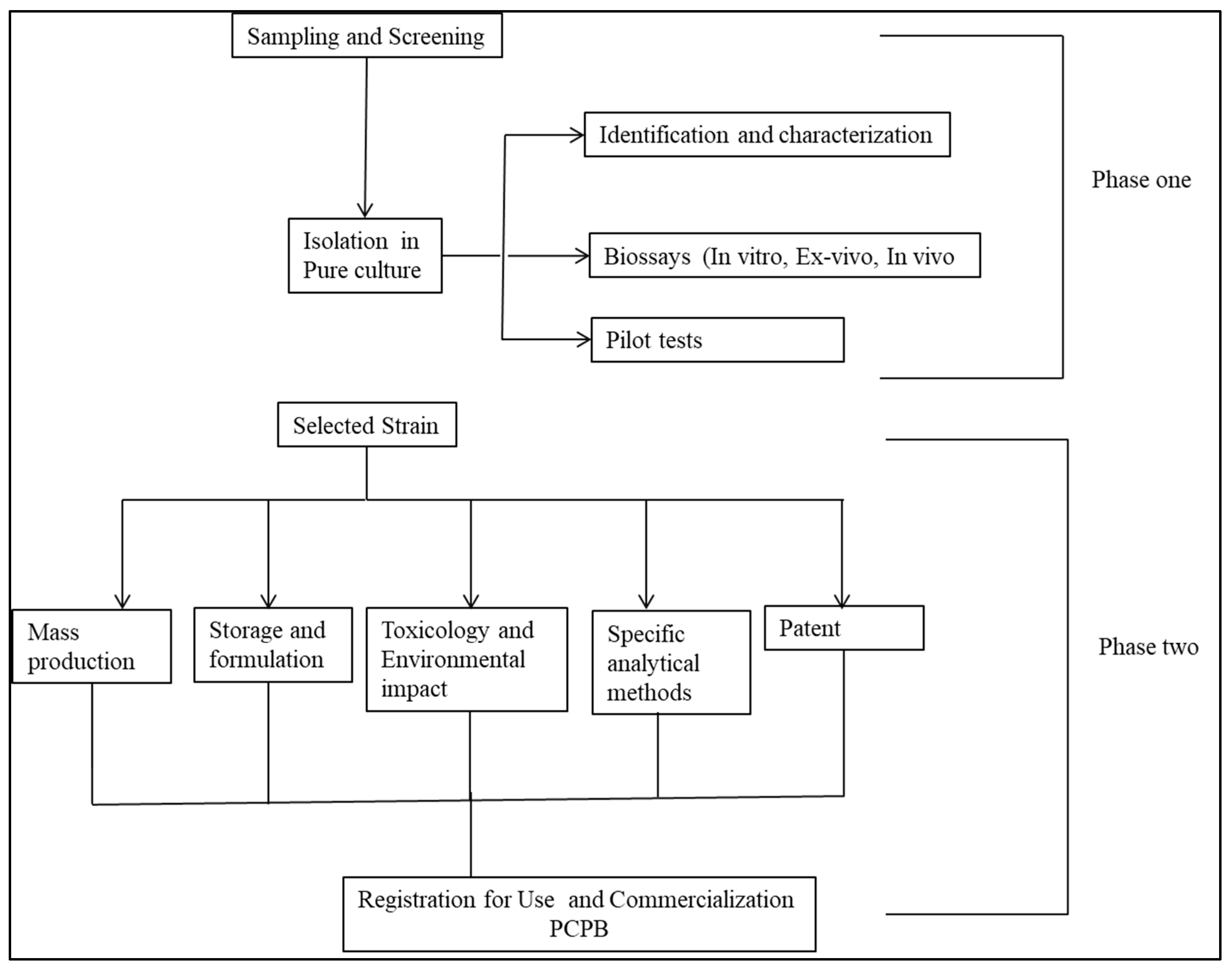
- Biopesticides development process
- Analysis of the Kenyan Legal Framework on Biocontrol Agents
- a)
- Registration process
- b) Current status of registration of biopesticides
- Formulation of Botanical biopesticides
- Safety, toxicity, and application of Botanical biopesticides
- Challenges and Drawbacks to Uptake of Botanical Biopesticides
- Opportunities for the Growth of the Botanical Biopesticides Industry
- Policy Framework and business environment
- Research and development
Conclusions
Acknowledgements
References
- AATF [African Agricultural Technology Foundation].2013. A Guide to the Development of Regulatory Frameworks for Microbial Biopesticides in Sub-Saharan Africa. Nairobi: African Agricultural Technology Foundation.
- Adongo, J.O.; Omolo, J.O.; Cheplogoi, P.K.; Otaye, D.O. In vitro inhibition of the grey mould fungus - Botrytis cinerea by Phaseolinone and Phomenone compounds isolated from Xylaria species. The First International Conference on Pesticidal Plants 2013, 48. [Google Scholar]
- Ahmad, I., Owais, M., Shahid, M., & Aqil, F. (2010). Combating fungal infections: Problems and remedy. In Combating Fungal Infections: Problems and Remedy (Issue June). [CrossRef]
- Alburo, R.; Olofson, H. Agricultural history and the use of botanical insecticides in Argao, Cebu. Philippine Quarterly of Culture & Society 1987, 15, 151–172. [Google Scholar]
- Al-Huqail, A.A.; Behiry, S.I.; Salem MZ, M.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z.M. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) H. L. Wendl. Flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Ambikapathy, V. , Gomathi, S., & Panneerselvam. Effect of antifungal activity of some medicinal plants against Pythium debaryanum (Hesse). Pelagia Research Library Asian Journal of Plant Science and Research 2011, 1, 131–134. [Google Scholar]
- Arjjumend, H.; Koutouki, K. Science of biopesticides and critical analysis of Indian legal frameworks regulating biocontrol agents. International Journal of Agriculture, Environment and Biotechnology 2018, 11, 563–571. [Google Scholar] [CrossRef]
- Arora, N. K., Verma, M., Prakash, J., & Mishra, J. (2016). Regulation of biopesticides: global concerns and policies. In Bioformulations: for sustainable agriculture (pp. 283-299). Springer, New Delhi.
- Baysal, Ö.; Zeller, W. Extract of Hedera helix induces resistance on apple rootstock M26 similar to Acibenzolar-S-methyl against Fire Blight (Erwinia amylovora). Physiological and Molecular Plant Pathology 2004, 65, 305–315. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sharma, D.; Jadon, N.; Agrawal, P.K. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii. Archives of Clinical Microbiology 2015, 6, 1–9. [Google Scholar]
- Branco, S. Fungal Diversity – An Overview. The Dynamical Processes of Biodiversity - Case Studies of Evolution and Spatial Distribution, December 2011, 1–17. 20 December 2011; 1–17. [CrossRef]
- Chandler, D.; Bailey, A.S.; Mark Tatchell, G.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philosophical Transactions of the Royal Society B: Biological Sciences 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Chaudhary, R.; Prashant, S.; Chaudhary, F.; Chudhari, M.; Prajapati, V.P. Antifungal activity of plant extracts on uredospores germination of leaf rust of wheat antifungal activity of plant extracts on uredospores germination of leaf rust of wheat. Trends in Biosciences 2015, 8, 2739–2742. [Google Scholar]
- Chiriac, I.P.; Ulea, E. Antibacterial activity of some plant extracts and different pesticides against an Erwinia amylovora (Burrill.) Winslow et al. strain isolated from a nursery stock. Research Journal of Agricultural Science 2012, 44, 19–23. [Google Scholar]
- Choudhury, D.; Dobhal, P.; Srivastava, S.; Saha, S.; Kundu, S. Role of botanical plant extracts to control plant pathogens. Indian Journal of Agricultural Research 2018, 52, 341–346. [Google Scholar] [CrossRef]
- DAFF (2010) Department of Agriculture, Forestry and Fisheries. Act No. 36 of 1947, Guidelines on the data required for registration of biological/biopesticides remedies in South Africa, Republic of South Africa.
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture (Switzerland) 2018, 8. [Google Scholar] [CrossRef]
- Draz, I.S.; Elkhwaga, A.A.; Elzaawely, A.A.; El-Zahaby, H.M.; Ismail, A.-W.A. Application of plant extracts as inducers to challenge leaf rust of wheat. Egyptian Journal of Biological Pest Control 2019, 29, 4–11. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chemistry Central Journal 2017, 11, 1–10. [Google Scholar] [CrossRef]
- FAO (2012) Food and Agriculture Organization. Guidance for harmonizing pesticide regulatory management in Southeast Asia RAP publication 2012/13.Food and Agriculture Organization of the United Nations, Regional Office for Asia and the Pacific, Bangkok, p 480.
- FRAC. (2018). List of plant pathogenic organisms resistant to disease control agents (Issue May).
- Freire, E.S.; Campos, V.P.; Pinho RS, C.; Oliveira, D.F.; Faria, M.R.; Pohlit, A.M.; Noberto, N.P.; Rezende, E.L.; Pfenning, L.H.; Silva, J.R.C. Volatile substances produced by Fusarium oxysporum from coffee rhizosphere and other microbes affect Meloidogyne incognita and Arthrobotrys conoides. Journal of Nematology 2012, 44, 321–328. [Google Scholar]
- Fyhrquist, P. (2007). Traditional medicinal uses and biological activities of some plant extracts of African Combretum Loefl ., Terminalia L . and Pteleopsis Engl . species ( Combretaceae ). In Africa. University of Helsinki.
- Gan-Mor, S.; Matthews, G.A. Recent developments in sprayers for application of biopesticides—An overview. Biosystems Engineering 2003, 84, 119–125. [Google Scholar] [CrossRef]
- Gašić; S; Tanović, B. Biopesticide formulations, possibility of application and future trends. Pesticidi i fitomedicina 2013, 28, 97–102.
- Glare, T.R.; Caradus, J.; Gelernter, W.D.; Jackson, T.A.; Keyhani, N.O.; Köhl, J.; Marrone, P.G.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.R.; Gwynn, R.L.; Moran-Diez, M.E. Development of biopesticides and future opportunities. Microbial-Based Biopesticides 2016, 211–221. [Google Scholar]
- Glare, T.R.; Gwynn, R.L.; Moran-Diez, M.E. Development of biopesticides and future opportunities. Microbial-based biopesticides: methods and protocols 2016, 211–221. [Google Scholar]
- Goss, E.M.; Tabima, J.F.; Cooke, D.E.L. The Irish potato famine pathogen Phytophthora infestans originated in central Mexico rather than the Andes. Proceedings of the National Academy of Sciences of the United States of America. 2014, 111, 8791–8796. [Google Scholar] [CrossRef]
- Goyal, A.; Manoharachary, C. (2014). Future challenges in crop protection against fungal pathogens. In Fungal biology, (pp. xiii, 364 pages).
- Guest PJ (2015) Global biopesticide regulations – challenges, opportunities and future prospects. Available at https://www.agra-net.net/agra/agra-europe/policy-and-legislation/environment/analysis-global-biopesticide-regulations-challenges-opportunities-andfuture-prospects-477586.htm.
- Gupta, S., & Dikshit, A. K. (2010). Biopesticides: An ecofriendly approach for pest control. Journal of Biopesticides, 3(1 SPEC.ISSUE), 186–188.
- Gurjar, M.S.; Ali, S.; Akhtar, M.; Singh, K.S. Efficacy of plant extracts in plant disease management. Agricultural Sciences 2012, 03, 425–433. [Google Scholar] [CrossRef]
- Gwa, V. I., A. O., N., & Ekefan, E. J. Antifungal Effect of Five Aqueous Plant Extracts on Mycelial Growth of Penicillium Expansum Isolated from Rotted Yam Tubers in Storage. Acta Scientific Agriculture 2018, 2, 65–70. [Google Scholar]
- Handley, J. (2019). Pesticides - A brief history and analysis. Pitchcare Articles.
- Hindorf, H.; Omondi, C.O. A review of three major fungal diseases of Coffea arabica L. in the rainforests of Ethiopia and progress in breeding for resistance in Kenya. Journal of Advanced Research 2011, 2, 109–120. [Google Scholar] [CrossRef]
- Holmes, K., Chaudhary, M., Babendreier, D., Bateman, M., Grunder, J., Mulaa, M., Durocher-Granger, L., & Faheem, M. (2019). Biopesticides manual: guidelines for selecting, sourcing and using biocontrol agents for key pests of tobacco. In CAB International (Issue 5). [CrossRef]
- Hynes, R. K., Bailey, K., Boyetchko, S. M., Erlandson, M., & Peng, G. (2011). Formulation development and delivery of biopesticides. In Soils and Crops Workshop. https://core.ac.uk/display/226159631?utm_source=pdf&utm_medium=banner&utm_campaign=pdf-decoration-v1.
- Jalander, V.; Gachande, B.D. Effect of aqueous leaf extracts of Datura Sp. against two plant pathogenic fungi. International Journal of Food, Agriculture and Veterinary 2012, 2, 131–134. [Google Scholar]
- Jangam, S.S.; Chaudhari, P.S.; Chaudhari, S.V.; Baheti, K.G. Herbal Plants for Insect Pest Management. International Journal of Scientific & Engineering Research 2014, 5, 882–884. [Google Scholar]
- Kabaluk JT, Antonet MS, Mark SG, Stephanie GW (2010) The use and regulation of microbial pesticides in representative jurisdictions worldwide. IOBC Global. www.IOBC-Global.org.
- Kachhawa, D. Microorganisms as a biopesticides. Journal of Entomology and Zoology Studies 2017, 5, 468–473. [Google Scholar]
- Kansiime, M., Mulema, J., Karanja, D., Romney, D., & Day, R. (2017). Crop pests and disease management in Uganda: status and investment needs (Issue March).
- Kimani V (2014) Bio-pesticides development, use and regulation in Kenya regional experts, workshop on development, regulation and use of bio-pesticides in East Africa, Nairobi. Presented at the Regional Experts Workshop on Development, Regulation and Use of Bio-pesticides in East Africa, Nairobi, Kenya, 22–23 May 2014. 22–23 May.
- Koche, D.; Shirsat, R.; Kawale, M. An overview of major classes of phytochemicals: Their type and role in disease prevention. Hislopia Journal 2016, 9, 2016. [Google Scholar]
- Kokoskova, B., Pavela, R., & Pouvova, D. Effectiveness of plant essential oils against Erwinia amylovora, Pseudomonas syringae pv. syringae and associated saprophytic bacteria on/in host plants. Effectiveness of Plant Essential Oils Against Erwinia Amylovora, Pseudomonas syringae pv. syringae and Associated Saprophytic Bacteria on/in Host Plants 2011, 133-139.
- Koul O (2011) Microbial biopesticides: opportunities and challenges. CAB Rev: Perspect Agric Vet Sci Nutr Nat Resour 6, No. 056.
- Koul, O. Microbial biopesticides: Opportunities and challenges. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2011, 6. [CrossRef]
- Kurmukov, A.G. Phytochemistry of medicinal plants. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan 2013, 1, 13–14. [Google Scholar] [CrossRef]
- Kutawa, A.B.; Muhammad, A.; Abdullahi, G.; State, A.; Musa, H. Biopesticides for Pests Control : a Review Biopesticides for Pests Control : a Review. Journal of Biopesticides and Agriculture 2016, 3. [Google Scholar]
- Mandakini, H.T.; Manamgoda, D.S. (2021). Microbial Biopesticides: Development and Application. In Microbial Technology for Sustainable Environment (pp. 167-189). Springer, Singapore.
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M. Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Pathology 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Marrone, P.G. Barriers to adoption of biological control agents and biological pesticides. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2007, 2(January 2010). 20 January. [CrossRef]
- Mburu, H. , Cortada, L., Haukeland, S., Ronno, W., Nyongesa, M., Kinyua, Z.,... & Coyne, D. Potato cyst nematodes: a new threat to potato production in East Africa. Frontiers in Plant Science 2020, 11, 670. [Google Scholar] [PubMed]
- Mikiciński, A.; Sobiczewski, P.; Berczyński, S. Efficacy of fungicides and essential oils against bacterial diseases of fruit trees. Journal of Plant Protection Research 2012. [CrossRef]
- Mokrini, F.; Viaene, N.; Waeyenberge, L.; Dababat, A.A.; Moens, M. Root-lesion nematodes in cereal fields: importance, distribution, identification, and management strategies. Journal of Plant Diseases and Protection 2019, 126, 1–11. [Google Scholar] [CrossRef]
- Monteiro AC, A.; de Resende ML, V.; Valente TC, T.; Ribeiro Junior, P.M.; Pereira, V.F.; da Costa, J.R.; da Silva JA, G. Manganese phosphite in coffee defence against Hemileia vastatrix, the coffee rust fungus: Biochemical and Molecular Analyses. Journal of Phytopathology 2016, 164, 1043–1053. [Google Scholar] [CrossRef]
- Muleta, D. (2007). Microbial Inputs in Coffee ( Coffea arabica L .) Production Systems , Southwestern Ethiopia. Swedish University of Agricultural Sciences/Uppsala 2007. 2007.
- Murtaza, G.; Mukhtar, M.; Sarfraz, A. A Review: Antifungal Potentials of Medicinal Plants. Journal of Bioresource Management 2015, 2. [Google Scholar] [CrossRef]
- Ngadze, E. In vitro and greenhouse evaluation of fungicidal properties of botanical extracts against Rhizoctonia solani and Phythopthora infestans. Proceedings of the First International Conference on Pesticidal Plants 2013, 1, 204–208. [Google Scholar]
- Ngaruiya, P. N. 2004. Overview of registration of pesticides in Kenya. In registration for biocontol agents in Kenya. Workshop Proceedings of the PCPB/KARI/DFID CPP Workshop held in Nakuru, Kenya 14th-16th 2003.
- Ngouegni, Y.Y.; Tsopmbeng Noumbo, G.R.; Keuete Kamdoum, E.; Nchongboh, C.G. Antifungal activities of plant extracts against coffee berry disease caused by Colletotrichum kahawae L. International Journal of Current Research in Biosciences and Plant Biology 2017, 4, 60–66. [Google Scholar] [CrossRef]
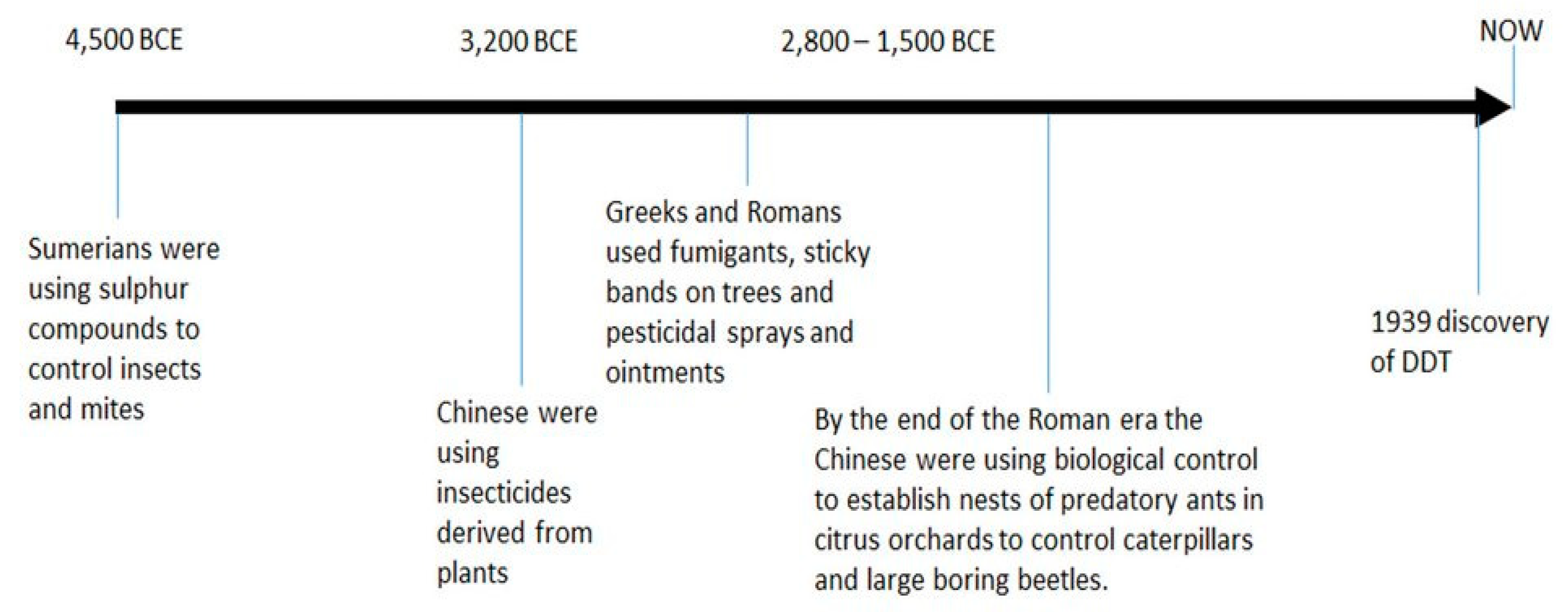
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A short history of insecticides. Journal of Plant Protection Research 2015, 55, 221–226. [Google Scholar] [CrossRef]
- Paradza, V.; Icisishahayo, D.; Ngadze, E. Assessing effectiveness of botanical extracts from garlic and neem on controlling potato soft rot pathogens. Proceedings of the First International Conference on Pesticidal Plants 2013, 1, 73–77. [Google Scholar]
- Parveen, S.; Wani, A.H.; Ganie, A.A.; Pala, S.A.; Mir, R.A. Antifungal activity of some plant extracts on some pathogenic fungi. Archives of Phytopathology and Plant Protection 2014, 47, 279–284. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects - A review. Plant Protection Science 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Pharmacopeia, B. British pharmacopeia. British Pharmacopeia. 1998, 137–138. [Google Scholar]
- Pulawska, J. Crown gall of stone fruits and nuts—Economic significance and diversity of its causal agent tumorigenic Agrobacterium spp. Journal of Plant Pathology 2010, 92, 87–98. [Google Scholar]
- Raghavendra, K.V.; Gowthami, R.; Lepakshi, N.M.; Dhananivetha, M.; Shashank, R. Use of botanicals by farmers for integrated pest management of crops in karnataka. Asian Agri-History 2016, 20, 173–180. [Google Scholar]
- Rahman, M.; Hasan, M.F.; Das, R.; Khan, A. The determination of antibacterial and antifungal activities of polygonum hydropiper (l.) root extract. Advances in Biological Research 2009, 3, 53–56. [Google Scholar]
- Rahman, S.; Biswas, S.K.; Barman, N.C.; Ferdous, T. Plant extract as selective pesticide for integrated pest management. Biotechnological Research 2016, 2, 6–10. [Google Scholar]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: a primer for the natural products research community. Journal of Natural Products 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial biopesticides in agroecosystems. Agronomy 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Salhi, N.; Mohammed Saghir, S.A.; Terzi, V.; Brahmi, I.; Ghedairi, N.; Bissati, S. Antifungal activity of aqueous extracts of some dominant algerian medicinal plants. BioMed Research International 2017, 2017. [Google Scholar] [CrossRef]
- Sen, A.; Batra, A. Determination of antimicrobial potentialities of different solvent extracts of the medicinal plant: Phyllanthus amarus Schum. and Thonn. International Journal of Green Pharmacy 2012, 6, 50–56. [Google Scholar] [CrossRef]
- Sharma, K.R.; Raju SV, S.; Deepak, K.J.; Sudeshna, T. Biopesticides: An effective tool for insect pest mangement and current scenario in India. Indian Journal of Agriculture and Allied Sciences 2018, 4, 59–62. [Google Scholar]
- Sharma, S.; Malik, P. Biopestcides : Types and Applications. International Journal of Advances in Pharmacy 2012, 1, 508–515. [Google Scholar]
- Sherkhane, A.S.; Suryawanshi, H.H.; Mundada, P.S.; Shinde, B.P. Control of bacterial blight disease of pomegranate using silver nanoparticles. Journal of Nanomedical and. Nanotechnology 2018, 9, 500. [Google Scholar]
- Shomari, S.H.; Menge, D.S.N. Investigations on the performance of potential botanicals against cashew powdery mildew disease in Tanzania. Proceedings of the First International Conference on Pesticidal Plants 2013, 1, 11–15. [Google Scholar]
- Signh, S.; Singh, B.; Singh, A.P. Nematodes: A threat to sustainability of agriculture. Procedia Environmetal Sciences 2015, 29, 215–216. [Google Scholar]
- Singh, D. (2014). Advances in plant biopesticides. In D. Singh (Ed.), Advances in Plant Biopesticides (pp. 1–401). Springer.
- Smiley, R. Root-lesion nematodes: Biology and management in Pacific Northwest wheat cropping systems.Bharti, V., & Ibrahim, S. Biopesticides: Production, formulation and application systems. International Journal of Current Microbiology and Applied Science 2020, 9, 3931–3946. [Google Scholar]
- Speckbacher, V., & Zeilinger, S. (2018). Secondary Metabolites of Mycoparasitic Fungi. Secondary Metabolites - Sources and Applications. [CrossRef]
- Stadlinger N, Mmochi AJ, Kumblad L (2013) Weak governmental institutions impair the management of pesticide import and sales in Zanzibar. Ambio.
- Stefani, E. Economic significance and control of bacterial spot/canker of stone fruits caused by Xanthomonas arboricola pv. pruni. Journal of Plant Pathology 2010, 92, 99–103. [Google Scholar]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Bio Rev 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Suurbaar, J.; Mosobil, R.; Donkor, A.M. Antibacterial and antifungal activities and phytochemical profile of leaf extract from different extractants of Ricinus communis against selected pathogens. BMC Research Notes 2017, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P. , Batista, D., Diniz, I., Vieira, A., Silva, D. N., Loureiro, A., Tavares, S., Pereira, A. P., Azinheira, H. G., Guerra-Guimarães, L., Várzea, V., & Silva, M. do C. The coffee leaf rust pathogen Hemileia vastatrix: one and a half centuries around the tropics. Molecular Plant Pathology 2017, 18, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Teicher, H. (Undated). Formulating biopesticides: the labcoat guide to pesticides & biopesticides. https://biocomm.eu/2017/12/11/formulating-biopesticides-labcoat-guide-pesticides-biopesticides/.
- Tembo, Y., Mkindi, A. G., Mkenda, P. A., Mpumi, N., Mwanauta, R., Stevenson, P. C., Ndakidemi, P. A., & Belmain, S. R. (2018). Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Frontiers in Plant Science, 9(September), 1–10. [CrossRef]
- USDA. United States Department of Agriculture. 2017a. About AMS.
- Uwague, A. Phytochemical Screening and Proximate Analysis of Sweet Orange ( Citrus Sinesis ) Fruit Wastes. IJSRST 2017, 3, 48–53. [Google Scholar]
- Yang, J., Hsiang, T., Bhadauria, V., Chen, X. L., & Li, G. (2017). Plant Fungal Pathogenesis. BioMed Research International. [CrossRef]
| Class | Sub-Class | Description | Mechanism of control | References |
| Phenolic compounds | Simple and alkylated phenols | Plant defence mechanisms against pathogens and insects. | -Membrane disruption, substrate deprivation | (Bhardwaj et al., 2015; Choudhury et al., 2018) |
| Phenolic acids | Aromatic acids that contain a phenolic ring and a carboxyl functional group | -Bind to adhesins, forms complex with the cell wall, inactivate enzymes | (Altemimi et al., 2017; Draz et al., 2019; Dulf et al., 2017; Kurmukov, 2013; Pharmacopeia, 1998) | |
| phenylpropanoids coumarins, quinines, anthraquinones, xanthones |
Aromatic plants mainly produce them and have antifungal and antibacterial properties | - Interaction with eucaryotic DNA | (Al-Huqail et al., 2019; Gurjar et al., 2012; Monteiro et al., 2016). | |
| Tannins | Astringent, polyphenolic biomolecules | Bind to and precipitate proteins, enzyme inhibition, substrate deprivation | (Gurjar et al., 2012; Koche et al., 2016; Kurmukov, 2013; Salhi et al., 2017) | |
| Terpenoids (Isoprenoids) sesquiterpenes, diterpenes, diterpenoids, triterpenoids | Essential oils prenyllipids | These are the oldest group of small molecular products synthesized by plants and the most widely spread. | Cell membrane disruption | (Bhardwaj et al., 2015; Koche et al., 2016; Kurmukov, 2013) |
| Alkaloids | Basic, naturally occurring organic compounds that contain at least one nitrogen, such as morphine or caffeine | Intercalate into cell wall | (Koche et al., 2016; Salhi et al., 2017) | |
| Flavones, flavonoids and flavonols- | Plants synthesize them in response to microbial infections. They are phenolic compounds with one carboxyl group. |
Bind to adhesins, forms complex with the cell wall, Inactivate enzymes | (Kurmukov, 2013; Salhi et al., 2017; Uwague, 2017) | |
| Lectins and Polypeptides | Carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules | Form disulfide bridges Cause agglutination |
(Freire et al., 2012) | |
| Saponins | Constitutive antifungal plant metabolites that act as natural detergents chemically related to triterpenes saponins and steroidal saponins | - antimicrobial, cholesterol-lowering, and anti-cancer. The main compound produced by cayenne pepper. |
(Kurmukov, 2013; Salhi et al., 2017) |
| Plant | Common name | Target pathogen | Reference |
|---|---|---|---|
| Datura stramonium | Datura |
Puccinia triticina Eriks Alternaria solani and Fusarium oxysporum |
(Chaudhary et al., 2015; Draz et al., 2019; M. Rahman et al., 2009), (Jalander & Gachande, 2012) |
| Acalypha wilkesiana | Acalypha | Puccinia triticina Eriks | (Draz et al., 2019) |
| Lawsonia inermis | Henna | Puccinia triticina Eriks | (Ambikapathy et al., 2011; Draz et al., 2019) |
| Melia azedarach | Chinaberry | Puccinia triticina Eriks | (Draz et al., 2019) |
| Punica granatum | Pomegranate | Puccinia triticina Eriks | (Draz et al., 2019) |
| Lantana camara | Lantana | Puccinia triticina Eriks | (Draz et al., 2019) |
| Allium cepa | Onion | Helminthosporium turcicum and Ascochyta rabiei | (Gwa et al., 2018) |
| Calotropis procera | Giant milkweed | Helminthosporium turcicum and Ascochyta rabiei | (Gwa et al., 2018) |
| Adenocallima alliaceum | garlic vine | Alternaria alternate and Fusarium oxysporum | |
| Zingiber officinale | Ginger | Penicillium expansum | (Gwa et al., 2018; Parveen et al., 2014) |
| Piper nigrum | Black pepper | Penicillium expansum | (Gwa et al., 2018) |
| Azadirachta indica | Neem |
Penicillium expansum, Pectobacterium carotovorum subspecies carotovorum, Pectobacterium atrosepticum, Dickeya dadantii, Oidium anacardia, Phytophthora infestans and Rhizoctonia infestans |
(Gwa et al., 2018; Ngadze, 2013; Paradza et al., 2013; Shomari & Menge, 2013) |
| Nicotiana tabacum | Tobacco | Penicillium expansum | (Gwa et al., 2018; Jangam et al., 2014; S. Rahman et al., 2016) |
| Acacia saligna (Labill.) H. L. Wendl. | Golden wattle | Rhizoctonia solani, Fusarium culmorum and Penicillium chrysogenum | (Al-Huqail et al., 2019) |
| Xylaria spp | Botrytis cinerea | (Adongo et al., 2013) | |
| Allium sativum | Garlic |
Pectobacterium carotovorum subspecies carotovorum, Pectobacterium atrosepticum Dickeya dadantii, Phytophthora infestans and Rhizoctonia infestans |
(Ngadze, 2013; Paradza et al., 2013) |
|
Morinda morindoides, Senna occidentalis Opuntia cactus Opuntia vulgaris |
- - - - |
Oidium anacardii | (Shomari & Menge, 2013) |
| Carica papaya | Pawpaw | Colletotrichum kahawae L., Phytophthora infestans and Rhizoctonia infestans | (Ngadze, 2013; Ngouegni et al., 2017) |
|
Tagetes minuta Vinca rosea |
Mexican marigold Periwinkle |
Phytophthora infestans and Rhizoctonia infestans | (Ngadze, 2013) |
|
Artemisia herba alba Cotula cinereal Asphodelus tenuifolius Euphorbia guyoniana |
desert or white wormwood Onion weed Euphorbia |
Fusarium graminearum and Fusarium sporotrichioides | (Salhi et al., 2017) |
|
Phyllanthus amarus Schum. and Thonn. |
- | Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, and Rhizopus stolonifera | (Sen & Batra, 2012) |
|
Lawsonia inermis L. Mimosa pudica L. Phyllanthus niruri L. Tephrosia purpurea Pens. Vinca rosea L. |
- | Pythium debaryanum | (Ambikapathy et al., 2011) |
| Cymbopogon citratus | - | Colletotrichum kahawae L., Ustilago maydis, Ustilaginoidea virens, Curvularia lunata, and Rhizopus spp | (Ngouegni et al., 2017) |
| Eucalyptus saligna | - | Colletotrichum kahawae L. | (Ngouegni et al., 2017) |
| Phenopodium ambroides | - | Rhizoctonia solani | (Singh, 2014) |
|
Chromoleana odorata Xylopia aethiopica |
- | Ustilago maydis, Ustilaginoidea virens, Curvularia lunata, and Rhizopus spp | (Singh, 2014) |
| Leonotis nepetifolia L. | - | Phoma exigua | |
| Ocimum gratissimum L. | - | Phoma exigua, Ustilago maydis, Ustilaginoidea virens, Curvularia lunata, and Rhizopus spp | (Singh, 2014) |
| Pelargonium odoratissimum | Apple geranium | Erwinia armylovora | (Chiriac and Ulea 2012) |
| Salvia officinalis | Sage | Erwinia armylovora | (Chiriac and Ulea 2012) |
| Tagetes minuta | French marigold | Erwinia armylovora | (Chiriac and Ulea 2012) |
| Hedera helix L | Ivy | Erwinia armylovora | (Baysal and Zeller, 2004) |
| Syzygium aromatcum | Clove |
Erwinia amylovora Xanthomonas arboricola pv. corylina, Xanthomonas arboricola pv. juglandis, Pseudomonas syringae pv. Syringae Agrobacterium tumefaciens |
(Mikicinski et al., 2012) |
| Origanum compactum | Oregano |
Erwinia amylovora Pseudomonas syringae pv.Syringae, Pseudomonas fluorescens, Pantoea dispersa Pantoea agglomerans |
(Kokoskova et al., 2011) |
| Thymus vulgaris | Thyme |
Erwinia amylovora Pseudomonas syringae pv.Syringae, Pseudomonas fluorescens, Pantoea dispersa, Pantoea agglomerans |
(Kokoskova et al., 2011) |
| Origanum vulgare | Wild oregano | Pseudomonas syringae pv. garcea | (Kokoskova et al., 2011) |
| Ocimum tenuiflorum | Holy basil | Xanthomonas axonopodis pv. punicae | (Sherkhane et al., 2018) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





