1. Introduction
Protection and preservation of the natural environment have become one of the major scientific challenges that humanity is facing today. The occurrence and accumulation of carbon-based nanomaterials in the aquatic environment is undeniable, however, most researchers and innovation advisors do not perceive graphene as posing a risk to the environment based on the “just carbon” approach [
1]. Due to industrial and domestic applications of synthetic nanoparticles they are released into the aquatic and terrestrial ecosystems, which release is expected to constantly increase as their production and application rate is growing steadily [
2]
In the 21th century, graphene-family materials (GFMs) have been hailed as ‘miracle’ materials and a group of revolutionary two-dimensional carbon-based nanomaterials of exciting and unique characteristics [
3]. Graphene-family materials are a group of two-dimensional materials that are composed of sp
2-bonded carbon atoms arranged in a hexagonal lattice structure. The most well-known material in this family is graphene, which is a single layer of graphite [
4,
5]. Graphene has remarkable properties, such as high mechanical strength, excellent thermal and electrical conductivity, and unique optical properties, making it a promising material for various applications, ranging from electronics to energy storage and beyond [
6]. Graphene can be produced using several methods, including mechanical exfoliation, chemical vapour deposition, epitaxial growth, and reduction of graphene oxide [
6,
7]. Apart from graphene, other members of the graphene-family materials include few-layer graphene (FLG), graphene oxide (GO), reduced graphene oxide (rGO), and graphene derivatives with various functional groups. Few-layer graphene refers to a stack of a few graphene layers, typically less than 10 layers [
8]. Graphene oxide is obtained by oxidising graphene, resulting in the introduction of oxygen-containing functional groups, which makes it hydrophilic and easier to process. Reduced graphene oxide is obtained by reducing graphene oxide, which restores some of the graphene-like properties [
8]. Graphene derivatives with functional groups, such as graphene with nitrogen, sulfur, or fluorine atoms attached to the carbon lattice, are also part of the graphene-family materials [
6].
According to the recently updated European Commission (EC) definition [
9], graphene is considered a nanomaterial. Graphene and its specific nanoforms are subjected to the Commission Regulation 2020/878 - Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) [
10], which obliges chemical manufacturers or importers to provide specific information and chemical safety assessments for the nanoforms of chemical substances. GFMs are registered under the CAS number 1034343-98-0 and EC number 801-282-5, and they are classified based on the EC Regulation 1272/2008 on the classification, labelling and packaging of substances and mixtures (CLP) as “harmful to aquatic life with long-lasting effects following chronic exposure (H412) [
11,
12].
The graphene industry is growing due to the rise in demand for various applications of graphene globally. The global graphene market size was valued at USD 620 million in 2020 and is projected to reach USD 1,479 by 2025 [
13]. In order to reach the goals of the 2030 Agenda for Sustainable Development of the United Nations [
14] on sustainable development and environmental safety, current knowledge gaps have to be identified and need to be further investigated to better understand the environmental impacts of engineered nanomaterials (ENMs), in particular, GFMs [
15].
Graphene-based materials (GBMs) and their fragments can be released into the environment through various pathways during different stages, including manufacturing, use, and end-of-life. Graphene-based materials can enter the environment through intentional applications, such as in environmental remediation, agricultural practices, and consumer products, as well as unintentional releases during production, use, and disposal [
16]. Although GFM-based products are mostly used in the form of polymer composites, they are likely to degrade in the aquatic environment due to abiotic and biotic degradation processes, resulting in the release of GFMs [
17]. Based on the probabilistic material flow analysis of GFMs in Europe from 2004 to 2030, both in the consumption and end-of-life phases, it is estimated that over 50% will be incinerated and oxidised in waste plants, while 16% will be sent to landfills, and 12% will be exported from Europe. Additionally, approximately 1.4% of annual GBM production is predicted to end up in the environment. Projections also indicate that the expected release concentrations in 2030 will be 1.4 ng/L in surface water and 20 μg/kg in soil treated with sludge [
18].
So far, reviews on GFMs in the environment have focused on their source, fate and occurrence, environmental concentrations [
16], properties [
8] and characterisation by different analytical methods [
19], their effects on organisms [
2,
4,
20] and the applicability of existing standard ecotoxicity methods for their environmental risk assessment [
3,
21,
22] as well as on their environmental applications and potentials in wastewater treatment [
23,
24].
The available reviews reported on environmental concentrations and ecotoxicological impacts on aquatic organisms, however critical evaluation of current research trends related to the test systems applied in terms of ecological complexity, hence environmental relevance was not carried out. Numerous studies have demonstrated that the transformation of GFMs can increase their toxicity through various mechanisms [
25,
26].
Thus, the aim of this paper was (i) to overview and understand current research trends in GFMs’ ecotoxicity assessment connected to their environmental risk characterisation and (ii) to further discuss the limitations and knowledge gaps about their environmental effects and the role of aquatic micro- and mesocosms in the life-cycle oriented environmental risk assessment of GFMs, (iii) as well as to identify promising areas for future research.
2. Bibliometric analysis
As a first step, a bibliometric analysis was carried out on February 14, 2023, using the Thompson Reuters database ISI (The Institute for Scientific Information) Web of Science in order to overview the temporal distribution of scientific research in connection with GFMs and related toxicity from 1990 to 2023. To this purpose, the combination of the keywords ‘graphene’ and ‘toxic’ was used in any title, abstract or text words and a total number of 5221 candidate publications were identified.
As the prime objective of this review was to investigate current research trends in connection with the environmental and ecotoxic effects of GFMs, separate searches were conducted using the following keywords in combination with ‘graphene’ - along with the ‘AND’ operation – ‘communities’, ‘soil’, ‘water OR aquatic’, ‘wastewater’, ‘microcosm’, ‘mesocosm’.
As a second step, a deeper bibliometric analysis and a systematic and meticulous search of online databases including Web of Science (
https://apps.webofknowledge.com/), Science Direct (
https://www.sciencedirect.com/), PubMed (
https://pubmed.ncbi.nlm.nih.gov/), Scopus (
https://www.scopus.com/), complemented with Google Schoolar (https://
https://scholar.google.com/) were conducted to retrieve relevant papers on the ecotoxicity of any forms of GFMs as recommended by Qualhato
et al. [
27]. The abstracts of all candidate articles were read until February of 2023, in addition, to have an extensive overview of the appropriate publications for the analyses, we also reviewed the reference section of each paper. Reviews were excluded after preliminary analysis. The abstracts were screened with the following exclusion criteria: articles that were not written in English, protocols, technical reports and papers that did not fit the study aim. From all candidate papers, finally 185 papers were retained and subjected to further analysis.
The applied test organisms in all relevant ecotoxicity studies were categorised as protozoa, algae, cnidaria, rotifera, planaria, nematoda, annelida, mollusca, crustacea, amphibia, fish and plants, while bacterial species with the exception of Aliivibrio fischeri were not included in the results reported here. Considering that an article could report effects of GFMs on more than one test organism, the term ‘study’ was defined as series of observations on a particular test organism, e.g. if one article reported only on a particular algal species it was considered to be one study, but if it reported an algal test organism, a crustacean and a fish, it was considered to be three. This way, the number of studies presented in the results represents the number of interactions of the particular tested GFMs with all the applied test organisms in the particular paper, not the total number of publications.
3. Results and discussion
3.1. Temporal distribution of studies
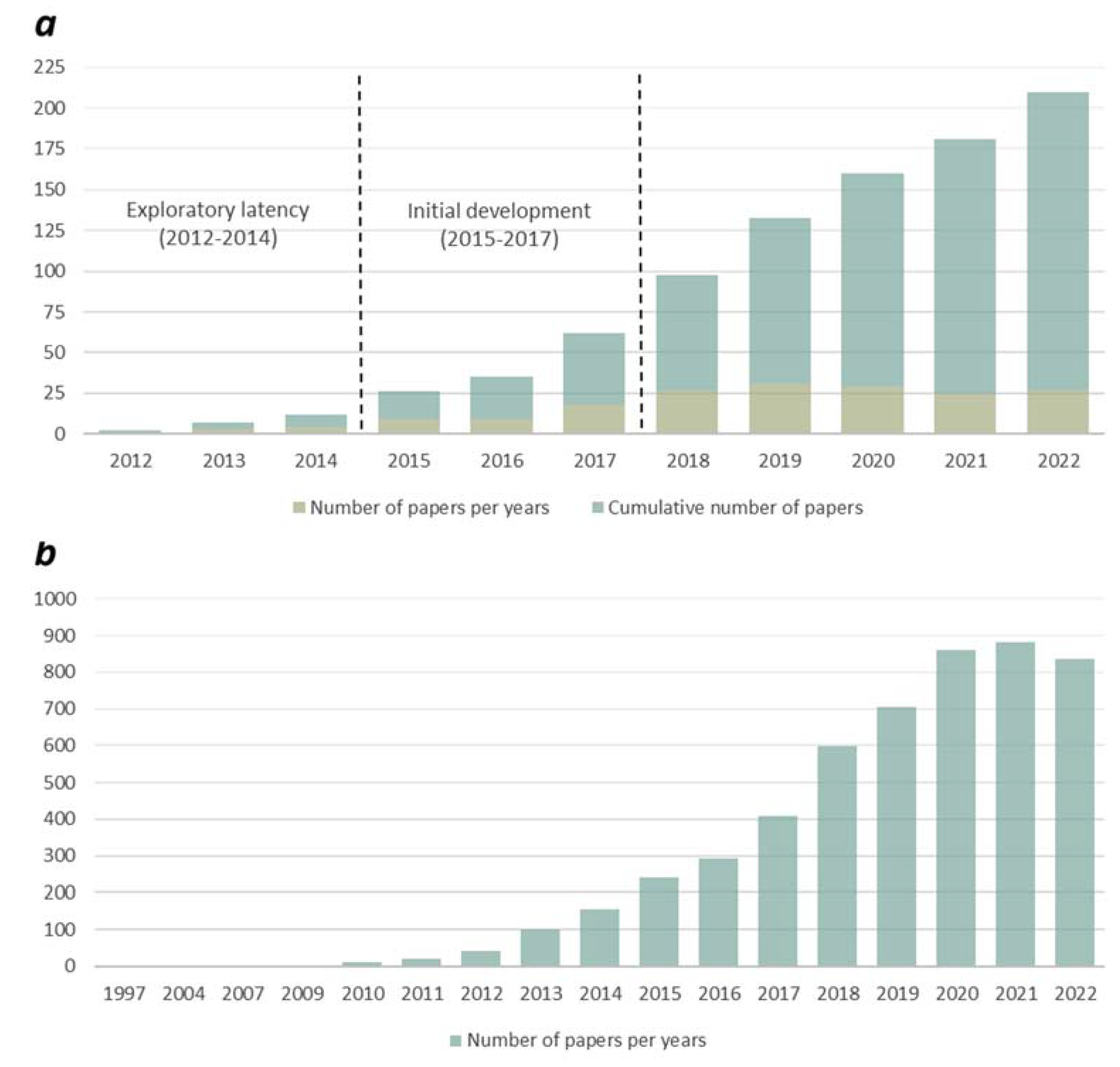
Results from our initial search showed that the number of scientific publications mentioning ‘graphene’ and ‘toxic’ has grown steadily from 2010 (
Fig. 1b). In 2022, there were 835 publications, roughly 2 times the amount from 5 years ago and roughly 20 times the amount from 10 years ago (
Fig. 1b). In the case of the strictly ecotoxicological effect-related papers a phase of exploratory latency can be found between 2012 and 2014, followed by a phase of initial development of exploratory interest between 2015 and 2017 (
Fig. 1a). The trend of ecotoxicity-themed papers on GFMs effects is also worth to be highlighted, as their number seems to be stagnant from 2018 to date (typically 25–31 papers/year) (Fig.1a).
From a biological or environmental impact assessment point of view, researches are mainly focused on the investigation of GFMs on different microbial communities in the aquatic and soil ecosystems. Taking into consideration that the use of tests with increased environmental relevance in nano-ecotoxicology has been recommended to overcome the limitations of standardised protocols [
21,
28], terrestrial or aquatic microcosm and mesocosm studies are still under-represented in risk analysis of engineered nanomaterials, specifically in the case of carbon-based nanomaterials, such as the representatives of the graphene-family. The number of results for the search terms ‘graphene’ and ‘microcosm’ was found to be 8 and even less (only 2) in the case of ‘graphene’ and ‘mesocosm’ (
Table 1).
3.2. Target organism groups reported in ecotoxicity studies with GFMs
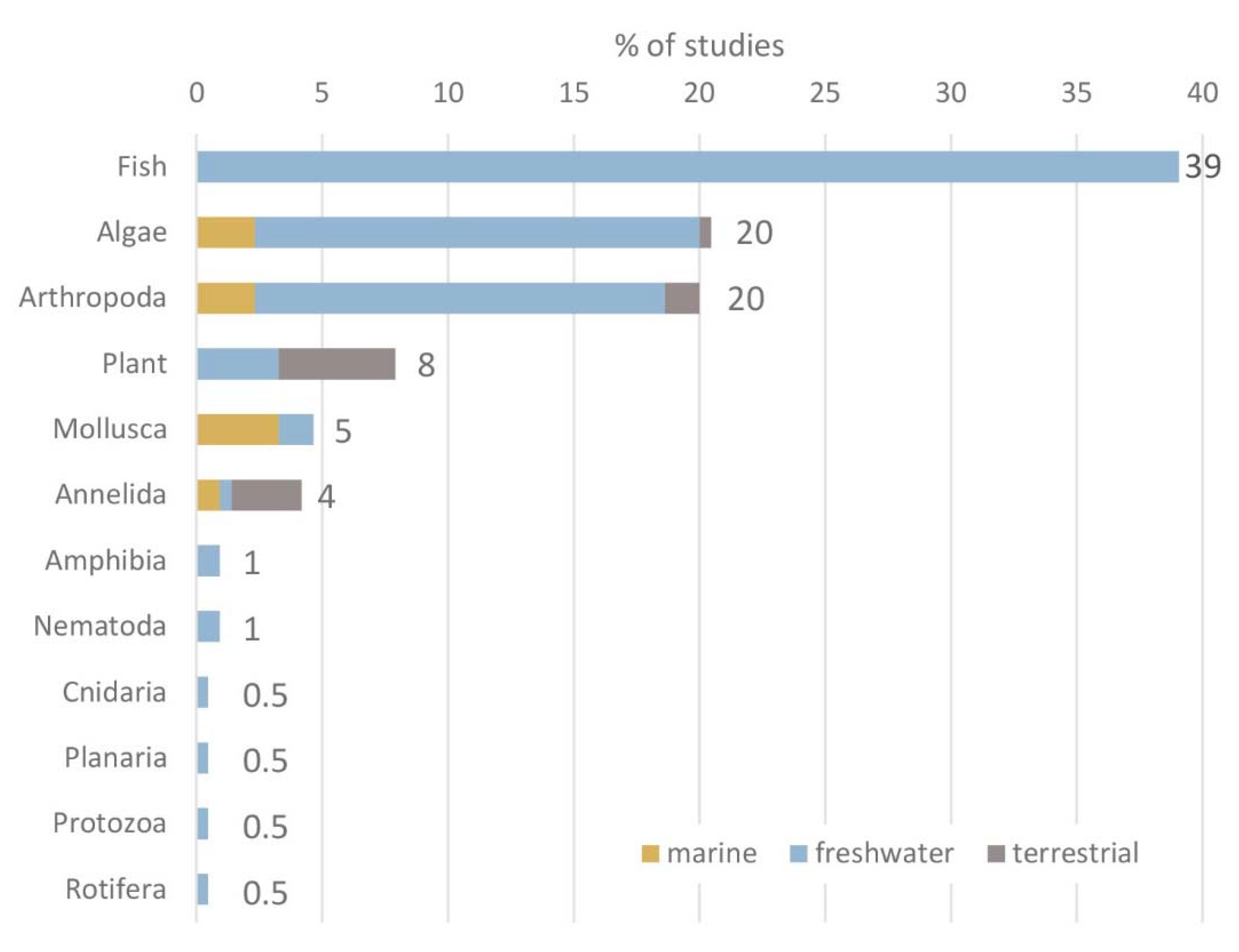
Test organisms applied in 185 papers on the ecotoxicological effects of GFMs were categorised into 12 distinctive groups of organisms. Literature analysis revealed that the three groups of organisms that are dominating ecotoxicological research on GFMs are fish (39%), algae (20%) and arthropoda (20%) predominantly applying small, planktonic crustaceans. Aquatic and terrestrial plant species represent 8% of all applied test organisms, while the remaining 8 groups of organisms account for the remaining 13% (
Fig. 2).
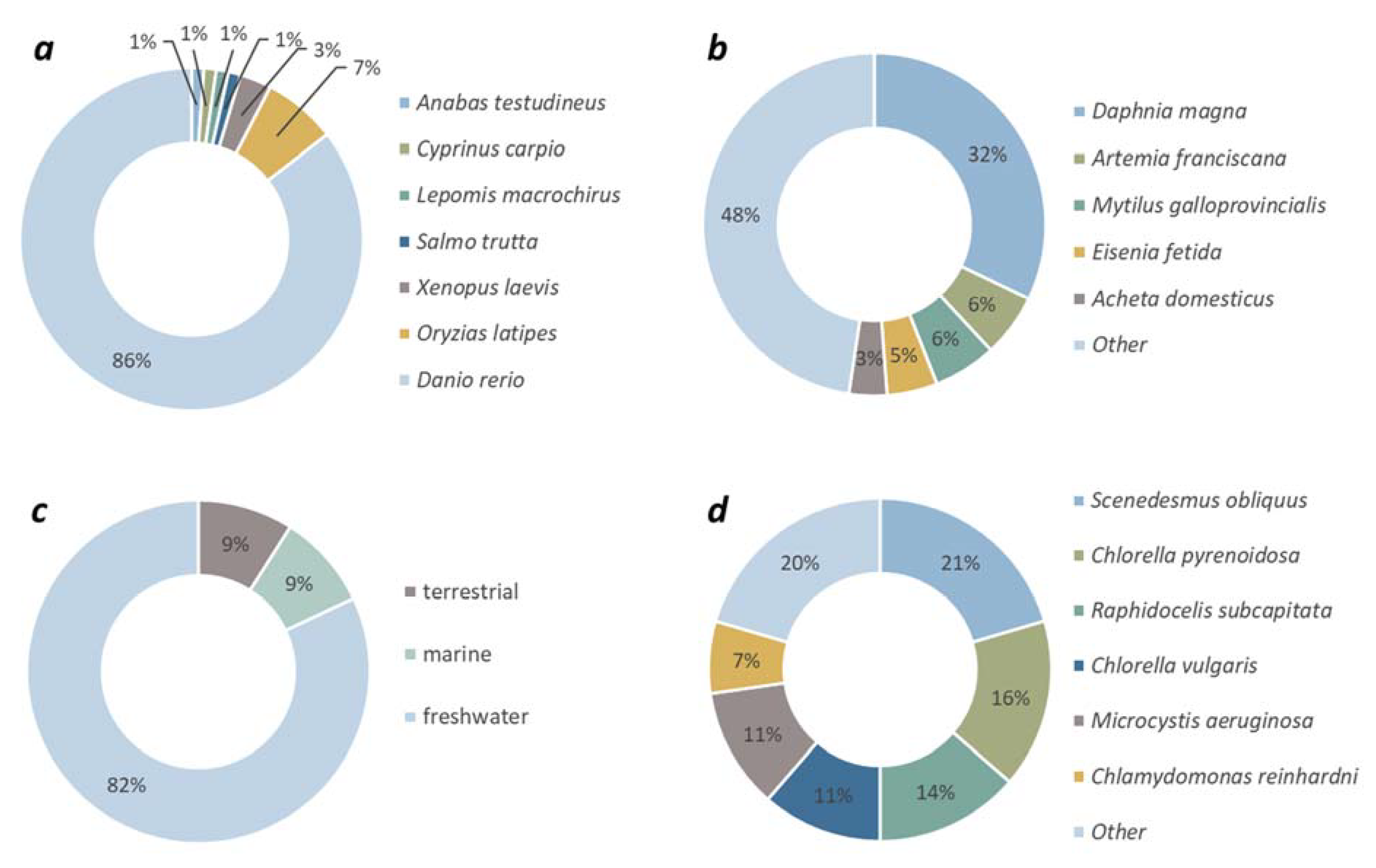
To date, ecotoxicity studies with GFMs have been carried out predominantly using freshwater species (82%), while marine or terrestrial organisms have been used in 8% of all studies individually (
Fig. 3c). The majority (86%) of studies using vertebrate species as target organism applied fish and within the group of fish test organisms,
Danio rerio was the most investigated species as 86% of all studies with aquatic vertebrates was carried out with zebrafish (
Fig. 3a). Amongst invertebrate species, the toxic effects of GFMs was investigated with
Daphnia magna in 32% of cases. Besides the planktonic crustacean,
D. magna, the brine shrimp,
Artemia salina, the mussel,
Mytilus galloprovincialis, the earthworm,
Eisenia fetida and the house cricket,
Acheta domesticus were applied in several cases (
Fig. 3b). Species used in only one or two cases accounted for 48% of all invertebrate studies. Focusing on the group of algae, five species were applied predominantly:
Scenedesmus obliquus, Chlorella pyrenoidosa, Raphidocelis subcapitata, Chlorella vulgaris, Microcystis aeruginosa and
Chlamydomonas reinhardtii. 80% of all algal species belonged to green algae, 13% belonged to cyanobacteria and 7% belonged to diatoms.
3.3. Ecotoxicological effects of GFMs in multispecies test systems
Scientific literature on the ecotoxicological effects of GFMs in multispecies test systems can be divided into two main domains: (i) studies conducted with bacterial communities and (ii) studies modelling complex ecosystems of test organisms with the hierarchical trophic organisation. These complex test systems may include bacterial species but principally do not rely on them exclusively.
This review was written with the ultimate aim to overview the state-of-the-art knowledge and latest findings on GFMs’ toxicity in complex ecosystem studies, namely micro- and mesocosm studies or any ecotoxicity studies modelling trophic transfer of GFMs. Although, authors did not intend to give a detailed overview of the current scientific literature on the effects of GFMs on microbial communities, a brief summary was provided in order to give a more contrasting picture of the underrepresentation of complex ecosystem studies within the literature of multispecies test systems. As the current knowledge on the interactions of graphene-based materials with microbial communities has been summarised in the latest review of Braylé
et al. [
29] a brief summary of their findings and bibliometric analysis is given in
Section 3.3.1.
3.3.1. Ecotoxicological effects of GFMs on bacterial communities
The growing number of studies about the effects of GFMs on microbial communities in different environmental compartments indicates the emergence of this topic. Braylé
et al. [
29] also highlighted, that scientific literature on the interactions between GFMs and microbial communities is restricted to laboratory experiments mimicking environmental bioprocesses under controlled conditions. The absence of studies carried out in full-scale bioreactors or
in natura systems was attributed to the lack of reliable GFMs quantification methods. It was also revealed, that approximately half of the studies reported on GFMs effects in bioprocess test systems. The efficiency of wastewater treatment relies primarily on the functions of a diverse microbial community, however WWTPs are ultimate repositories for GFMs [
30]. Therefore, any potential toxic effects of GFMs needs to be investigated essentially on wastewater microbial communities. Results confirmed the potential toxicity of high concentrations of GO and it was also revealed that the composition and dynamics of the microbial communities in the activated sludge changed under GFMs exposure [
31]. Furthermore, the impairment in microbial metabolic activity or shift in microbial diversity in WWTPs was reported by several articles [
30,
31,
32,
33].
Considering the different environmental compartments, studies on soil microbial communities were highly represented (27%). The effects of GFMs have been studied on bacterial communities from different aspects in recent years. Soil is a critical sink to GFMs after entering the environment as GFMs are able to migrate along the soil profile under the action of surface runoff and precipitation, which highly increase the possibility of their transformation in the terrestrial environment [
34]. The physiology and structure of the microbial community are used as important indexes to characterise the adverse effects of toxic substances, as well as the influence of GFMs on soil properties [
35]. Several authors investigated the effect of graphene on the bacterial community of soil ecosystems [
36], however there is still an ongoing debate over whether and how graphene affects soil microbial community and enzyme activity [
37]. It was reported that graphene may change the metabolism of soil microorganisms to a certain extent by adversely affecting the cell membrane integrity and by inhibiting crucial enzyme activites such as dehydrogenase, phosphatase, urease and hydrogen peroxidase in a concentration-dependent manner [
38]. However, other studies reported that the soil bacterial community was unaffected by graphene [
39]. Positive effects of graphene on bacterial diversity indexes in a Cd-contaminated Haplic Cambisols in Northeast China was reported as the species and abundance of bacteria varied with GO concentration, and GO significantly increased bacterial growth at 25 and 250 mg/L [
40].
Microbial communities from surface water bodies (river, lake, estuary, aquarium) were used for investigating the potential adverse effects of GFMs in small-scale batch incubation systems, however the effects of GFMs on water bodies were slightly understudied [
29].
In summary, most studies indicated the negative impacts of GFMs on the growth of bacterial communities, generally due to oxidative stress. In the presence of GFMs, several activities were found to be influenced, such as biochemical cycles and bioprocess yields due to the disruption of community structure. Results are heterogenous, as environmental factors have a great influence on the fate and toxicity of GFMs. This way, studies carried out with different community compositions, in different environmental compartments and also under different conditions are likely to produce contradictory results [
29].
3.3.2. Ecotoxicological effects of GFMs in test systems with hierarchical trophic organisation
As standardized single-species-based assays fail to represent toxicological pathways implying interactions between organisms, the use of micro- and mesocosm test systems is essential in the investigation of environmentally realistic effects of GFMs. In the current literature, the effects of GFMs in complex test systems of higher ecological relevance based on the approach of hierarchical trophic organisation of the applied organisms are understudied. After a thorough bibliometric survey, only nine articles were found that reported on the effects of GFMs in micro- or mesocosm test systems, or at least applied a trophic-transfer mimicking exposure pathway. Four articles were considered to apply real microcosm approach [
41,
42,
43,
44]. However, in one instance, authors indicated that a mesocosm experiment was carried out, the test could be considered rather a microcosm experiment as the assembled test systems contained 1500 g of soil per pot and were incubated under laboratory conditions [
44] and e.g. were not assembled as an isolated part of the natural ecosystem or placed out to the natural environment. Four additional articles were discussed that applied any form of trophic chain exposure approach [
22,
45,
46,
47]. Freshwater ecosystems were mostly represented, while only one marine and one soil microcosm study was carried out. The presence of natural or artificial sediment phase was also scarce in these studies.
Table 2 summarises the most important methodological parameters of scientific papers that reported on the effects of GFMs in trophic transfer studies or in micro- or mesocosm experiments.
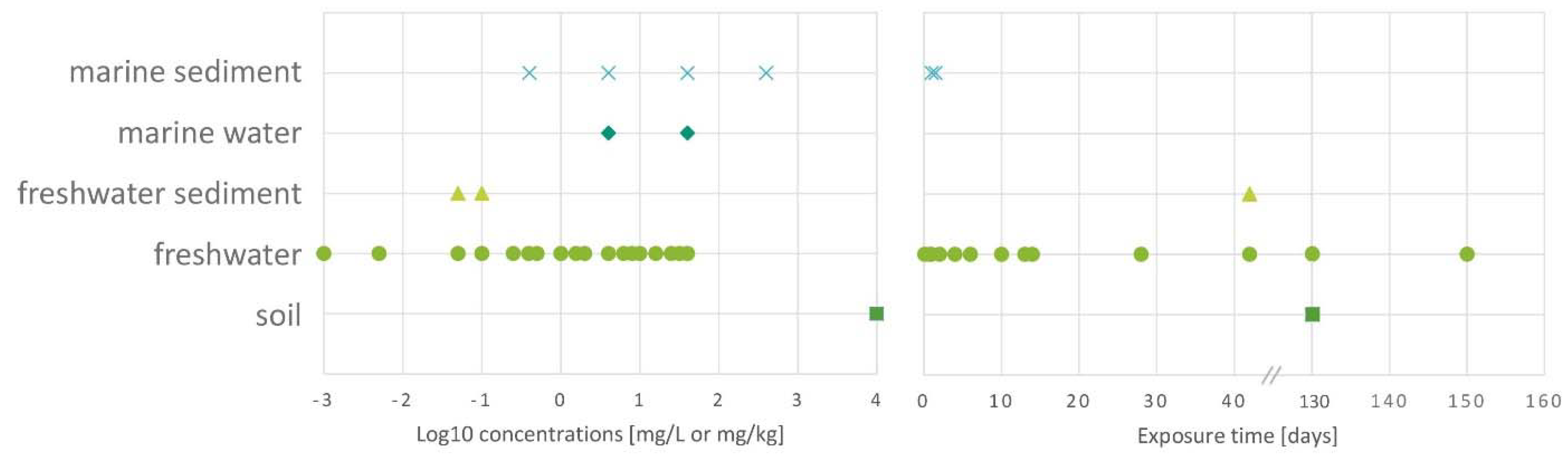
The number of studies investigating a particular type of GFM in test systems with the hierarchical trophic organisation was quite imbalanced toward GO in the current literature, as graphene multilayer nanoflakes, graphene and rGO were investigated in one article, FLG was applied in 2 papers, while GO was tested in 5 scientific articles. The tested concentrations of GFMs are heterogenous in the different environmental compartments (
Fig. 4.): the lowest concentration tested in freshwater or marine water phase was 1 ng/L, which is comparable to the predicted environmental concentration reported by Hong
et al. [
18] in surface waters. The lowest tested concentration in freshwater or marine sediment was 50 ng/L, while the lowest concentration tested in sediments was 5 orders of magnitude lower than the lowest concentration tested in soil. The concentration of GFMs in complex soil systems (10,000 mg/kg) was 5 orders of magnitude higher than the predicted environmental concentration reported in soil [
18].
3.3.3. Micro- and mesocosm approaches for GFMs ecotoxicity characterisation
Evariste
et al. [
41] was the first, who investigated the effect of GO in recirculated, large-volume microcosm test systems in environmentally relevant concentrations (0.05 and 0.1 mg/L) combining a water and a sediment compartment. As uncontaminated test medium, commercial natural spring water was used. A reconstituted trophic chain was applied in order to model the interactions between pelagic and benthic microbial communities with aquatic organisms from higher trophic levels (decomposers, primary and secondary consumers). Redox potential, pH, dissolved oxygen, dissolved organic carbon and nitrogen products (NO
3+, NO
2-, NH
4+) were monitored in the test system. Test species were gradually introduced to the test system: 3 weeks after the development of the primary producers (bacterial community and the freshwater diatom,
Nitzschia palea), primary and secondary consumers (
Chironomus riparius midge,
Pleurodeles waltii amphibian) were introduced to the test systems. Later,
Xenopus laevis tadpoles were also added to the assembled microcosms. At the end of the experiment, the bacterial community was characterised by DNA isolation. In the case of
C. riparius, mortality, growth and teratogenicity were assessed, while for
Pleurodeles larvae, mortality, growth and genotoxic potential were determined. It was found that bacterial communities were affected by GO exposure; in addition, bacterial communities from the sediment were shown to be more impacted compared to those from the water compartment. Evidence was found for the genotoxic effect of GO in the
Pleurodeles individuals. However, no toxicity was observed for chironomids, indirect effects were highlighted, resulting in changes in the decomposition of organic matter in the system.
In another study by Evariste
et al. [
42] the effect of GO and rGO was assessed toward a biofilm composed of the diatom
N. palea associated to a bacterial consortium. GO and rGO was applied at 0.1, 1, and 10 mg/L concentrations in the short- (48 h) and in the long-term (144 h). Significant inhibition of bacterial growth by GO was reported from 1 mg/L concentration level, as well as influence on the taxonomic composition of diatom-associated bacterial consortium. Different effects of rGO were observed, as rGO exerted a weaker toxicity toward the bacterial consortium, whereas it influenced more strongly the diatom physiology. Studying the interactions between the bacterial consortium and the diatom allowed authors to conclude, that diatoms benefited from diatom-bacteria interactions resulting in potential to maintain or recover its carbon-related metabolic activities when exposed to GBMs.
The effect of multilayer graphene-nanosheets (8–12 nm) was investigated in a microcosm experiment using the marine benthic worm,
Hediste diversicolor [
43]. The experiment was categorised as microcosm experiment, since natural sea sediment and sea water was applied to the experiments containing a natural phytoplankton community, however the effect of graphene-nanosheets on the phytoplankton community was not investigated or discussed. Limited toxic effect of graphene was found, however significant elevation of catalase activity indicated the activation of defence mechanisms at the early stage of exposure, while cellular damage biomarkers (SOD, GST, GSH, MDA, CBO) remained unaffected. Based on the acetylcholinesterase (AChE) activity neurotoxic effect was not expressed. Behavioural changes were reported as in the graphene-containing test systems, individulas were buried deeper in the sediment indicating an escape reaction and avoidance behaviour [
43].
In one instance, the impact of graphene and graphene oxide was investigated in soil on the abundance and diversity of soil nematodes (29 genera) after growing tall fescue plant (
Festuca arundinacea) for 130 d using a laboratory pot experiment, however data were not available on the effects concerning the soil microbial community [
44]. Authors considered the test systems as mesocosms containing 1500 g top soil and 50 g compost with 1 m/m% GFM contamination. Findings highlighted that the addition of GFMs had a negative influence on the composition and diversity of the nematode community, simplifying the community structure. Graphene or graphene oxide had no significant effects on the plant shoot biomass, however both of them promoted the root growth of tall fescue.
An
in situ macrocosm experiment was carried out in the eutrophic Lake Xingyun, southwestern China, evaluating the impacts of graphene on the photocatalysis of phytoplankton under environmental conditions. Significant changes in the community structure was found as
Microcystis were significantly reduced, while the abundances of
Anabaena and
Aphanizomenon species greatly increased after graphene photocatalysis treatment. The abundances of
Chlorophyta,
Euglenophyta,
Pyrrophyta and
Cryptophyta species significantly increased, whereas in the case of eutrophic diatom species decreased abundances were observed in the treated area [
48].
3.3.4. Trophic transfer studies for GFMs ecotoxicity characterisation
The effect of
14C-labeled few layer graphene (FLG) was determined modelling trophic transfer in the aquatic food chain by Dong
et al. [
45]. Direct uptake from FLG suspension was determined in the case of
Eserichia coli, Tetrahymena termophila, Daphnia magna and
Danio rerio. In trophic transfer experiments the transfer of FLG from bacteria to protozoa, from protozoa to Daphnia and from Daphnia to zebrafish was quantified along with the determination of biomagnification factors. Different concentration of FLG in the range of 1–1000 µg/L was tested depending on the experimental procedure of the different organisms. It was found that the test organisms had high potential of accumulating graphene via direct uptake from culture medium. It was also revealed that in the case of the food chain from
E. coli to
T. thermophila, there is a high potential of trophic transfer of FLG, while for the food chain from
T. thermophila to
D. magna and from
D. magna to
D. rerio the likeliness is much lower. The main finding of the study was that in the case of
T. thermophila, D. magna, and
D. rerio the burden measured for dietary uptake was higher than that via waterborne exposure in a similar nominal concentration, indicating that trophic transfer is a nonnegligible route for the bioaccumulation of graphene in organisms.
Su
et al. [
46] investigated the effect of algal food on the uptake and distribution of
14C-labeled few-layer graphene (∼158 μg/L) in the freshwater snail,
Cipangopaludina cathayensis. It was found, that in the presence of algae cells the accumulation of the few-layer graphene was significantly enhanced with a bioaccumulation factor of 2.7 (48 h exposure). The snail retained more than 90% of the accumulated few-layer graphene in the intestine; in addition, the accumulated graphene was able to pass through the intestinal wall and enter the intestinal epithelial cells. However, without the presence of algae cells, 1.3% of the few-layer graphene was transferred to hepatocytes, this phenomenon was not observed in the absence of the algae cells. The main finding of their studies is that algae cells may act as carriers enhancing the bioavailability of the few-layer graphene to the snails.
Malina
et al. [
47] studied the interaction of three differently oxidised GO with planktonic and benthic crustaceans. As the importance of the applied feeding strategy was observed, a pre-treatment with algae was introduced prior to the ecotoxicity tests with the aim to mimick environmentally more realistic conditions. As a result of the algal pretreatment of GO, a complete mitigation of acute toxicity of GOs to all organisms was observed. The eradication of oxidative stress caused by GOs was also discovered in the algae-pretreatment test systems indicating that the pre-exposition of algal food is a crucial factor in GO's overall environmental fate, hence toxicity.
The effect of GO was tested on the green algae,
Raphidocelis subcapitata at 1, 2, 4, 8, 16 and 32 mg/L exposure concentration for 96 h, applying two different algae media, the modified Keating algae culture medium (MA-MS) and the algae medium recommended by the OECD 201 test guideline [
22]. Differences in the aggregation of GO were experienced in the different algae growth media. Hetero-aggregates were more prominent in the OECD medium than in the MA-MS medium. After 96 h, the growth rate of
R. subcapitata was determined. In the OECD medium IC
50 (Inhibition Concentration) was determined to be 4.96 mg/L GO while in the MA-MS medium it was 7.1 mg/L GO. In the second phase of experiments,
Paratya australiensis shrimps were exposed to 2 or 8 mg/L GO for 14 days and no sign of stress, food avoidance or accumulation of GO in the gut due to the consumption of heteroaggregates were observed [
22].
Although Lourerio
et al. [
49] investigated the effect of pegylated graphene oxide on the representatives of a trophic chain,
Raphidocelis subcapitata,
D. magna and
D. rerio, real trophic transfer interactions were not assessed by this study. Considering the bioaccumulation of different representatives of the graphene family in a broader context, we found that the extent of bioaccumulation by different organisms and the effect of GFMs on one of the primary food sources of planktonic animals in the aquatic ecosystems, e.g. the entrapment of algal cells by GFMs [
50].was reported by several authors [
51,
52,
53,
54,
55,
56,
57].
All these results of ecotoxicity studies in test systems with hierarchical trophic organisation provided heterogeneous results on GFMs ecotoxicity, depending on the test species, environmental compartments and exposure conditions. The effects varied within a wide range e.g. from complete mitigation of GO toxicity by algal pre-treatment [
47] to enhanced bioaccumulation by algae acting as a carrier [
46]. The authors addressed the challenges of characterising the tested GFM in complex environmental compartments which might become impossible under particular circumstances [
29,
43].
4. Current status, knowledge gaps and future needs
The environmental risk assessment (ERA) of engineered nanomaterials has been mainly focused on the investigation of pristine forms [
58]. Environmental research on aged nanomaterials is still very limited, especially in the case of ERA studies carried out in test systems of higher environmental relevance, such as microcosms and mesocosms [
28]. Although, the use of micro- and mesocosms for studying the environmental fate and effects of engineered nanomaterials (ENMs), particularly in aquatic ecosystems is increasing [
59], evaluating current scientific literature of GFMs, the lack of ecotoxicological studies of higher environmental relevance is striking even at first glance, however these complex test systems would allow for long-term characterisation of ecosystem responses to GFMs contamination and also could offer advantages over standard operating procedures as they allow for realistic contamination scenarios that incorporate natural complexity and synergies between contaminants and natural components.
Freixa
et al. [
60] published a comprehensive review article about the ecotoxicological effects of carbon-based nanomaterials in aquatic organisms based on approximately 100 articles from the past decade highlighting the following research needs: (1) using sublethal endpoints instead of the conventionally applied lethality, (2) investigation of the long term chronic effects instead of acute toxicity tests, (3) testing of environmentally relevant concentrations, (4) conduction of multispecies experiments assessing the exposure via the trophic chain. Although, there was a significant improvement in the first three research needs, the assessment of GFMs toxicity in the trophic chain is still in its infancy.
Various mesocosm designs consistently showed that ENMs entering the aquatic environment tend to be predominantly removed from the water column and accumulate in sediments in general [
61]. Statistical analysis indicates that this accumulation is likely to occur on the long term, regardless of ENM physicochemical properties [
28]. Consequently, this general scenario of potential risk is also relevant in the case of GFMs, especially for benthic species exposed to less reactive GFM nanoparticle species such as homo/hetero-aggregated and sulfidised forms but also with higher accumulation potential. On the other hand, the risk of planktonic species is associated with lower concentrations of potentially more reactive GFMs [
62].
Future studies of hierarchical trophic organisation in complex test systems could address current knowledge gaps, by identifying the most environmentally relevant GFMs and determining realistic dosing strategies, however there are some issues that may discourage the scientific community to carry out micro- and mesocosm studies with GFMs. Micro- and mesocosm studies could generate large and heterogeneous datasets that are difficult to reproduce.
The physicochemical characterization of GFMs in complex environmental compartments presents several challenges, including aggregation and sedimentation, surface modifications, matrix interferences, sample preparation, and the lack of standardised methods [
63]. This issue has been raised related to microcosm studies, as no relevant quantification analysis could be performed [
43]. Improving the understanding of GFMs transformation during exposure is crucial, as during different experimental conditions (e.g. the media used for ecotoxicity studies) [
64], the physical exclusion of algae from the water column by GO have proved to have implications on the food chain [
22].
These difficulties of GFMs characterisation may discourage authors from using complex test systems. Researchers are hesitant to conduct tests on GFMs in complex systems due to challenges in accurately characterising its physicochemical properties. This is particularly true in systems involving soil phases or sediment phases in water [
43]. This issue also has been previously observed in studies involving microbial communities [
29].
Trophic transfer studies can be considered a promising approach in between single-species standard ecotoxicity test methods and complex microcosm test systems. Applying the trophic transfer approach, it has been proven, that GO is not a hazardous material in complex aquatic environments because its acute toxicity can be successfully mitigated through the interaction with algae even at very high concentrations (25 mg/L) [
47]. Differences in the accumulation potential of GFMs were found in the case of crustacea and fish:
D. magna had a much greater capability of accumulating graphene at similar concentration and exposure duration than
D. rerio, which might be attributed to the difference in body size, organs complexity and feeding behaviours [
45]. Despite the fact, that bioaccumulation or biomagnification studies seem to offer a better understanding of GFMs’ effects, they are typically performed with algae, small planktonic crustaceans or fish, therefore bioaccumulation studies on other groups of organisms (e.g. echinodermata, cnidaria and porifera) would be essential to better determine the relevance of studies on the frequently applied test organisms.
The fact, that marine and terrestrial ecotoxicity studies are underrepresented in GFMs toxicity studies has been commented by several authors [
62,
65,
66], as the lack of information on the toxic effect of GFMs in the marine or terrestrial environment is considered a main knowledge gap. Besides, we know little about the transgenerational effects of GFMs [
67,
68]. Concerns have been also addressed about the uncertainty of the reliable life cycle assessment of GFMs that can be derived from current knowledge on their environmental impacts [
69].
43 studies investigating the interactive effects of GFMs and other environmental contaminants have been identified to date, Although, 5 additional papers investigated the effect of humic acid on GFMs [
70,
71,
72,
73,
74], and 1 study investigated the effect of alginate on graphene [
46], these were not considered as co-contaminant studies. However, there is a growing number of studies on the combined effects of GFMs with other environmental contaminants, further investigation is needed to be able to draw reasoned conclusions on the synergistic or mitigation effects. Gaining insights into the interactions between GFMs and environmental pollutants is of outmost importance as GFMs exhibit high absorbent capabilities for removing heavy metals and organic pollutants from water matrices depending on particular colloidal properties [
75,
76,
77,
78,
79].
5. Conclusions and future perspectives
The relevant ecotoxicity characterisation connected to the environmental risk assessment of GFMs faces several critical knowledge gaps that need to be addressed to ensure their safe and sustainable use. Standardized physicochemical methods for characterising and quantifying GFMs in complex environmental compartments and robust ecotoxicological data for a better understanding of their long-term fate and behaviour in environmentally relevant systems are urgently needed.
The identified knowledge gaps in the environmental risk assessment of GFMs have significant implications for the regulatory and scientific communities. The limited toxicological data on GFMs in complex test systems with greater environmental relevance hinder the accurate assessment of their potential ecological impacts. Additionally, the lack of understanding of the long-term environmental fate and behavior of GFMs raises concerns about their potential accumulation and persistence in environmental systems, which may have far-reaching ecological consequences.
Based on the results of this review paper, the following issues are recommended to consider:
Performing more studies on GFMs effects at environmentally relevant concentrations;
Perform more field and laboratory studies with marine and terrestrial organisms;
Assess the ecotoxicity of GFMs in more environmentally relevant conditions, such as trophic transfer studies and multispecies exposures in micro- or mesocosms;
Gaining insights into the interactive effects between GFMs and environmental pollutants;
Investigate the stability of GFMs in aquatic environments as a function of concentration. Despite the widespread use of GFMs there is limited knowledge about their actual environmental concentrations. Therefore, it is imperative to develop appropriate methods and detection techniques to accurately determine the concentrations of GFMs in the environment;
Encourage the publication of negative results.
Author Contributions
Conceptualization, I.F.K., K.L. and M.M.; methodology, I.F.K. and M.M.; formal analysis, I.F.K. and M.M; investigation, I.F.K. and M.M.; resources, I.F.K. and M.M; data curation, I.F.K. and M.M.; writing—original draft preparation, I.F.K. and M.M; writing—review and editing, I.F.K. and M.M; visualization, I.F.K.; supervision, M.M.; project administration, K.L. and M.M.; funding acquisition, K.L. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support for the research is greatly acknowledged to the Hungarian National Research, Development and Innovation Office Fund in the frame of the OTKA_K_128410, OTKA_K_143571 Research and TKP2021-EGA-02 Projects.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to Emese Vaszita for her contribution to language editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arvidsson, R.; Boholm, M.; Johansson, M.; De Montoya, M.L. “just Carbon”: Ideas About Graphene Risks by Graphene Researchers and Innovation Advisors. NanoEthics 2018, 12, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, A.M.; Olszyna, A.R. The Ecotoxicity of Graphene Family Materials: Current Status, Knowledge Gaps and Future Needs. Journal of Nanoparticle Research 2015, 17. [Google Scholar] [CrossRef]
- Malhotra, N.; Villaflores, O.B.; Audira, G.; Siregar, P.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Toxicity Studies on Graphene-based Nanomaterials in Aquatic Organisms: Current Understanding. Molecules 2020, 25, 3618. [Google Scholar] [CrossRef]
- Montagner, A.; Bosi, S.; Tenori, E.; Bidussi, M.; Alshatwi, A.A.; Tretiach, M.; Prato, M.; Syrgiannis, Z. Ecotoxicological Effects of Graphene-Based Materials. 2D Materials 2016, 4(1), 12001. [Google Scholar] [CrossRef]
- Ruíz-Santoyo, V.; Romero-Toledo, R.; Andrade-Espinoza, B.A. ; Virginia Viewpoint: How the Graphene Could Help to Decrease Sars-cov-2 Spread? Periodica Polytechnica Chemical Engineering 2021, 65, 283–291. [Google Scholar] [CrossRef]
- Nassef, B.G; Nassef, G.A.; Daha, M.A. Graphene and Its Industrial Applications – A Review. International Journal of Materials Engineering 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Commission Recommendation of 10.06.2022 on the definition of nanomaterial. Available online: https://ec.europa.eu/environment/chemicals/nanotech/pdf/C_2022_3689_1_EN_ACT_part1_v6.pdf (accessed on 04.04.2023).
- Commission Regulation (EU) 2020/878 of 18 June 2020 amending Annex II to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). 18 June.
- ECHA – European Chemicals Agency. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/24678/6/2/2 (accessed on 04.04.2023).
- ECHA – European Chemicals Agency. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.227.924 (accessed on 04.04.2023).
- Markets and Markets. Graphene market Research Report. Report code: CH 3833. Published on March, 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/graphene-market-83933068.html?gclid=Cj0KCQjwla-hBhD7ARIsAM9tQKus0cHp6Nd4POP33HdI84FZklGGRn7YTcXkSKbtMrBlg6DJPtXdZfIaAj89EALw_wcB (accessed on 04.04.2023).
- United Nations. The 2030 Agenda and the Sustainable Development Goals: An opportunity for Latin America and the Caribbean (LC/G.2681-P/Rev.3), Santiago, 2018.
- Gambardella, C.; Pinsino, A. Nanomaterial Ecotoxicology in the Terrestrial and Aquatic Environment: A Systematic Review. Toxics 2022, 10, 393. [Google Scholar] [CrossRef]
- Ding, X.; Pu, Y.; Tang, M.; Zhang, T. Environmental and Health Effects of Graphene-Family Nanomaterials: Potential Release Pathways, Transformation, Environmental Fate and Health Risks. Nano Today 2022, 42, 101379. [Google Scholar] [CrossRef]
- Goodwin, D. G.; Jr, Shen, S. J.; Lyu, Y.; Lankone, R.; Barrios, A. C.; Kabir, S.; Perreault, F.; Wohlleben, W.; Nguyen, T.; Sung, L. Graphene/polymer nanocomposite degradation by ultraviolet light: The Effects of Graphene Nanofillers and Their Potential for Release. Polymer degradation and stability 2020, 182. [Google Scholar] [CrossRef]
- Hong, H.; Part, F.; Nowack, B. Prospective Dynamic and Probabilistic Material Flow Analysis of Graphene-based Materials in Europe from 2004 to 2030. Environmental Science & Technology 2022, 56, 13798–13809. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review of the Mechanical and Thermal Properties of Graphene and Its Hybrid Polymer Nanocomposites for Structural Applications. Journal of Materials Science 2019, 54, 5992–6026. [Google Scholar] [CrossRef]
- Saxena, P.; Sangela, V.; Ranjan, S.; Dutta, V.; Dasgupta, N.; Phulwaria, M.; Rathore, D.S. ; Harish Aquatic Nanotoxicology: Impact of Carbon Nanomaterials on Algal Flora. Energy, Ecology and Environment 2020, 5, 240–252. [Google Scholar] [CrossRef]
- Hjorth, R.; Holden, P.A.; Hansen, S.F.; Colman, B.P.; Grieger, K.; Hendren, C.O. The Role of Alternative Testing Strategies in Environmental Risk Assessment of Engineered Nanomaterials. Environmental Science: Nano 2017, 4, 292–301. [Google Scholar] [CrossRef]
- Markovic, M.; Andelkovic, I.; Shuster, J.; Janik, L.; Kumar, A.; Losic, D.; McLaughlin, M. J. Addressing Challenges in Providing a Reliable Ecotoxicology Data for Graphene-Oxide (GO) Using an Algae (Raphidocelis subcapitata), and the Trophic Transfer Consequence of GO-Algae Aggregates. Chemosphere 2020, 245, 125640. [Google Scholar] [CrossRef] [PubMed]
- Karthik, V.; Selvakumar, P.; Senthil Kumar, P.; Vo, D.-V.N.; Gokulakrishnan, M.; Keerthana, P.; Tamil Elakkiya, V.; Rajeswari, R. Graphene-based Materials for Environmental Applications: A Review. Environmental Chemistry Letters 2021, 19, 3631–3644. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Srinivasan, S.; Jeevanantham, S.; Vishnu, M.; Amith, K.V.; Sruthi, R.; Saravanan, R.; Vo, D.-V.N. (2022). Insights on Synthesis and Applications of Graphene-Based Materials in Wastewater Treatment: A Review. Chemosphere 2022, 298, 134284. [Google Scholar] [CrossRef]
- Hu, M.; Li, X.; Xiong, J.; Zeng, L.; Huang, Y.; Wu, Y.; Cao, G.; Li, W. Nano-Fe3C@PGC as a Novel Low-Cost Anode Electrocatalyst for Superior Performance Microbial Fuel Cells. Biosensors and Bioelectronics 2019, 142, 111594. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S.; Zhu, Z.; Wang, S.; Liu, F.; Liu, G. Effects of Oxidation Degree on Photo-Transformation and the Resulting Toxicity of Graphene Oxide in Aqueous Environment. Environmental Pollution 2019, 249, 1106–1114. [Google Scholar] [CrossRef]
- Qualhato, G.; Vieira, L. G.; Oliveira, M.; Rocha, T. L. Plastic Microfibers as a Risk Factor for the Health of Aquatic Organisms: A Bibliometric and Systematic Review of Plastic Pandemic. The Science of the total environment 2023, 870, 161949. [Google Scholar] [CrossRef] [PubMed]
- Carboni, A.; Slomberg, D.L.; Nassar, M.; Santaella, C.; Masion, A.; Rose, J.; Auffan, M. Aquatic Mesocosm Strategies for the Environmental Fate and Risk Assessment of Engineered Nanomaterials. Environmental Science & Technology 2021, 55, 16270–16282. [Google Scholar] [CrossRef]
- Braylé, P.; Pinelli, E.; Gauthier, L.; Mouchet, F.; Barret, M. Graphene-based Nanomaterials and Microbial Communities: A Review of Their Interactions, from Ecotoxicology to Bioprocess Engineering Perspectives. Environmental Science: Nano 2022, 9, 3725–3741. [Google Scholar] [CrossRef]
- Ahmed, F.; Rodrigues, D. F. Investigation of Acute Effects of Graphene Oxide on Wastewater Microbial Community: A Case Study. Journal of Hazardous Materials 2013, 256–257, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lian, S.; Qu, Y.; Li, S. , Zhang, Z.; Zhang, H.; Dai, C.; Deng, Y. Interaction of Graphene-Family Nanomaterials with Microbial Communities in Sequential Batch Reactors Revealed by High-Throughput Sequencing. Environmental Research 2020, 184, 109392. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Castro-Wallace, S.L.; Rodrigues, D.F. Acute Toxicity of Graphene Nanoplatelets on Biological Wastewater Treatment Process. Environmental Science: Nano 2017, 4, 160–169. [Google Scholar] [CrossRef]
- Sha, Y.; Liu, J.; Yu, J.; Xu, S.; Yan, W.; Li, Z.; Shahbaz, M. Effect of Graphene Oxide on the Ammonia Removal and Bacterial Community in a Simulated Wastewater Treatment Process. Journal of Environmental Engineering 2020, 146(9), 04020097. [Google Scholar] [CrossRef]
- Dong, S.; Wang, T.; Lu, K.; Zhao, J.; Tong, Y.; Mao, L. Fate of 14c-labeled Few-layer Graphene in Natural Soils: Competitive Roles of Ferric Oxides. Environmental Science: Nano 2021, 8, 1425–1436. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.-Y.; Wu, S.-H.; Wen, M.-X.; Lu, L.-M.; Ke, F.-Z.; Wu, Q.-S. Effect of Arbuscular Mycorrhizal Fungi on Rhizosphere Organic Acid Content and Microbial Activity of Trifoliate Orange Under Different Low P Conditions. Archives of Agronomy and Soil Science 2019, 65, 2029–2042. [Google Scholar] [CrossRef]
- Shi, Y.; Xia, W.; Liu, S.; Guo, J.; Qi, Z.; Zou, Y.; Wang, L.; Duan, S.-Z.; Zhou, Y.; Lin, C. Impact of Graphene Exposure on Microbial Activity and Community Ecosystem in Saliva. ACS Applied Bio Materials 2019, 2, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Duan, C.; Sang, Y.; Wu, S.; Ru, J.; Cui, X. Effects of Graphene on Bacterial Community Diversity and Soil Environments of Haplic Cambisols in Northeast China. Forests 2018, 9, 677. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Li, L.N.; Teng, Y.; Ren, W.J.; Li, Z.G.; Luo, Y.M. Effects of Graphene on Soil Enzyme Activities and Microbial Communities. Soil 2016, 48, 102–108. [Google Scholar]
- Ru, J.; Chen, G.; Liu, Y.; Sang, Y.; Song, J. Graphene Oxide Influences Bacterial Community and Soil Environments of Cd-polluted Haplic Cambisols in Northeast China. Journal of Forestry Research 2021, 32, 1699–1711. [Google Scholar] [CrossRef]
- Evariste, L.; Mottier, A.; Lagier, L.; Cadarsi, S.; Barret, M.; Sarrieu, C.; Soula, B.; Mouchet, F.; Flahaut, E.; Pinelli, E. Assessment of Graphene Oxide Ecotoxicity at Several Trophic Levels Using Aquatic Microcosms. Carbon 2020, 156, 261–271. [Google Scholar] [CrossRef]
- Evariste, L.; Braylé, P.; Mouchet, F.; Silvestre, J.; Gauthier, L.; Flahaut, E.; Pinelli, E.; Barret, M. Graphene-Based Nanomaterials Modulate Internal Biofilm Interactions and Microbial Diversity. Frontiers in Microbiology 2021, 12. [Google Scholar] [CrossRef]
- Urban-Malinga, B.; Jakubowska, M.; Hallmann, A.; Dąbrowska, A. Do the Graphene Nanoflakes Pose a Potential Threat to the Polychaete Hediste diversicolor? Chemosphere 2021, 269, 128685. [Google Scholar] [CrossRef]
- Zhao, S.; Bai, X.; Mou, M.; Duo, L. Carbon Nanomaterial Addition Changes Soil Nematode Community in a Tall Fescue Mesocosm. Pedosphere 2022, 32, 777–784. [Google Scholar] [CrossRef]
- Dong, S.; Xia, T.; Yang, Y.; Lin, S.; Mao, L. Bioaccumulation of 14c-labeled Graphene in an Aquatic Food Chain Through Direct Uptake or Trophic Transfer. Environmental Science & Technology 2018, 52, 541–549. [Google Scholar] [CrossRef]
- Su, Y.; Tong, X.; Huang, C.; Chen, J.; Liu, S.; Gao, S.; Mao, L.; Xing, B. Green Algae as Carriers Enhance the Bioavailability of 14c-labeled Few-layer Graphene to Freshwater Snails. Environmental Science & Technology 2018, 52, 1591–1601. [Google Scholar] [CrossRef]
- Malina, T.; Maršálková, E.; Holá, K.; Zbořil, R.; Maršálek, B. The Environmental Fate of Graphene Oxide in Aquatic Environment-Complete itigation of its Acute Toxicity to Planktonic and Benthic Crustaceans by Algae. Journal of hazardous materials 2020, 399, 123027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Chang, F.; Xie, P.; Liu, Q.; Duan, L.; Wu, H.; Zhang, X.; Peng, W.; Liu, F.; Xu, L. In-situ Responses of Phytoplankton to Graphene Photocatalysis in the Eutrophic Lake Xingyun, Southwestern China. Chemosphere 2021, 278, 130489. [Google Scholar] [CrossRef]
- Loureiro, S.; Gonçalves, S.F.; Gonçalves, G.; Hortiguela, M.J.; Rebelo, S.; Ferro, M.C.; Vila, M. Eco-Friendly Profile of Pegylated Nano-Graphene Oxide at Different Levels of an Aquatic Trophic Chain. Ecotoxicology and Environmental Safety 2018, 162, 192–200. [Google Scholar] [CrossRef]
- Wahid, M.H.; Eroglu, E.; Chen, X.; Smith, S.M.; Raston, C.L. Entrapment of Chlorella vulgaris Cells Within Graphene Oxide Layers. RSC Advances 2013, 3, 8180. [Google Scholar] [CrossRef]
- Guo, X.; Dong, S.; Petersen, E.J.; Gao, S.; Huang, Q.; Mao, L. Biological Uptake and Depuration of Radio-Labeled Graphene by Daphnia magna. Environmental science & technology 2013, 47, 12524–12531. [Google Scholar] [CrossRef]
- Cano, A.M. , Maul, J.D.; Saed, M.; Shah, S.A.; Green, M.J.; Cañas-Carrell, J.E. Bioaccumulation, Stress, and Swimming Impairment in Daphnia magna Exposed to Multiwalled Carbon Nanotubes, Graphene, and Graphene oxide. Environmental toxicology and chemistry 2017, 36, 2199–2204. [Google Scholar] [CrossRef]
- Mao, L.; Liu, C.; Lu, K.; Su, Y.; Gu, C.; Huang, Q.; Petersen, E.J. Exposure of Few Layer Graphene to Limnodrilus Hoffmeisteri Modifies the Graphene and Changes Its Bioaccumulation by Other Organisms. Carbon 2016, 109, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Dong, S.; Petersen, E.J.; Niu, J.; Chang, X.; Wang, P.; Lin, S.; Gao, S.; Mao, L. Biological Uptake, Distribution, and Depuration of Radio-labeled Graphene in Adult Zebrafish: Effects of Graphene Size and Natural Organic Matter. ACS Nano 2017, 11, 2872–2885. [Google Scholar] [CrossRef]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Cai, Z.; Li, B. A Mechanism Study on Toxicity of raphene Oxide to Daphnia magna: Direct Link Between Bioaccumulation and Oxidative Stress. Environmental pollution 2018, 234, 953–959. [Google Scholar] [CrossRef]
- Martínez-Álvarez, I.; Le Menach, K.; Devier, M. H.; Barbarin, I.; Tomovska, R.; Cajaraville, M. P.; Budzinski, H.; Orbea, A. Uptake and Effects of Graphene Oxide Nanomaterials Alone and in Combination with Polycyclic Aromatic Hydrocarbons in Zebrafish. The Science of the total environment 2021, 775, 145669. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Liang, D.; Liu, Y.; Zhao, Q.; Huang, P.; Li, X.; Fan, W. Accumulation, Transformation and Subcellular Distribution of Arsenite Associated with Five Carbon Nanomaterials in Freshwater Zebrafish Specific-Tissues. Journal of Hazardous Materials 2021, 415, 125579. [Google Scholar] [CrossRef] [PubMed]
- Schwirn, K.; Voelker, D.; Galert, W.; Quik, J.; Tietjen, L. Environmental Risk Assessment of Nanomaterials in the Light of New Obligations Under the REACH Regulation: Which Challenges Remain and How to Approach Them? Integrated Environmental Assessment and Management 2020, 16, 706–717. [Google Scholar] [CrossRef]
- Auffan, M.; Masion, A.; Mouneyrac, C.; de Garidel-Thoron, C.; Hendren, C. O.; Thiery, A.; Santaella, C.; Giamberini, L.; Bottero, J.-Y.; Wiesner, M. R.; Rose, J. Contribution of Mesocosm Testing to a Single-Step and Exposure-Driven Environmental Risk Assessment of Engineered Nanomaterials. NanoImpact 2019, 13, 66–69. [Google Scholar] [CrossRef]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological Effects of Carbon Based Nanomaterials in Aquatic Organisms. Science of The Total Environment 2018, 619–620, 328–337. [Google Scholar] [CrossRef]
- Espinasse, B.P.; Geitner, N.K.; Schierz, A.; Therezien, M.; Richardson, C.J.; Lowry, G.V.; Ferguson, L.; Wiesner, M.R. Comparative Persistence of Engineered Nanoparticles in a Complex Aquatic Ecosystem. Environmental Science and Technology 2018, 52, 4072–4078. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the Aquatic Environment: Adsorption, Dispersion, Toxicity and Transformation. Environmental Science & Technology 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U. Safety Assessment of Graphene-based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Avant, B.; Bouchard, D.; Chang, X.; Hsieh, H.-S.; Acrey, B.; Han, Y.; Spear, J.; Zepp, R.; Knightes, C.D. Environmental Fate of Multiwalled Carbon Nanotubes and Graphene Oxide Across Different Aquatic Ecosystems. NanoImpact 2019, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pretti, C.; Oliva, M.; Pietro, R. di, Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of Pristine Graphene to Marine Organisms. Ecotoxicology and Environmental Safety 2014, 101, 138–145. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, F.; Chen, W.; Zhu, B.; Wang, G. Toxicity Evaluation of Graphene Oxide on Cysts and Three Larval Stages of Artemia salina. Science of The Total Environment 2017, 595, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Transgenerational Effects of Reduced Graphene Oxide Modified by Au, Ag, Pd, Fe3O4, Co3O4 and SnO2 on Two Generations of Daphnia magna. Carbon 2017, 122, 669–679. [Google Scholar] [CrossRef]
- Dziewięcka, E.; Gliniak, M.; Winiarczyk, M.; Karapetyan, A.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Dziewięcki, M.; Podolec, P.; Rubiś, P. Mortality Risk in Dilated Cardiomyopathy: The Accuracy of Heart Failure Prognostic Models and Dilated Cardiomyopathy-tailored Prognostic Model. ESC Heart Failure 2020, 7, 2455–2467. [Google Scholar] [CrossRef]
- Beloin-Saint-Pierre, D.; Hischier, R. Towards a More Environmentally Sustainable Production of Graphene-based Materials. The International Journal of Life Cycle Assessment 2021, 26, 327–343. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, C.; Ouyang, S.; Hu, X.; Zhou, Q. Mitigation in Multiple Effects of Graphene Oxide Toxicity in Zebrafish Embryogenesis Driven by Humic Acid. Environmental Science and Technology 2015, 49, 10147–10154. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.L.S.S.; Franqui, L.S.; Silva, C.A.; Martinez, D.S.T. Nanotoxicity of Graphene Oxide: Assessing the Influence of Oxidation Debris in the Presence of Humic Acid. Environmental pollution 2017, 225, 118–128. [Google Scholar] [CrossRef]
- Castro, V.L.; Clemente, Z.; Jonsson, C.; Silva, M.; Vallim, J.H.; De Medeiros, A.M.Z.; Martinez, D.S.T. Nanoecotoxicity Assessment of Graphene Oxide and Its Relationship with Humic Acid. Environmental Toxicology and Chemistry 2018, 37, 1998–2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Guo, X.; Yang, R.; Si, X.; Zhou, J. Humic Acid Alleviates the Ecotoxicity of Graphene-Family Materials on the Freshwater Microalgae Scenedesmus obliquus. Chemosphere 2018, 197, 749–758. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Shi, L.; Guo, X.; Si, X.; Yang, R.; Quan, X. The Effects of Humic Acid on the Toxicity of Graphene Oxide to Scenedesmus obliquus and Daphnia magna. Science of The Total Environment 2019, 649, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Li, Y. Role of Graphene Oxide in Mitigated Toxicity of Heavy Metal Ions on Daphnia magna. RSC Advances 2018, 8, 41358–41367. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Shi, L.; Yu, J.; Yao, J.; Sun, J.; Zhao, L.; Sun, J. Enhanced Cd Accumulation by Graphene Oxide (GO) Under Cd Stress in Duckweed. Aquatic Toxicology 2020, 229, 105579. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Zhou, Q.; Liu, Z.; Li, Q. Hexavalent Chromium Amplifies the Developmental Toxicity of Graphene Oxide During Zebrafish Embryogenesis. Ecotoxicology and Environmental Safety 2021, 208, 111487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Yuan, P.; Wu, Z.; Zhaoxin, W.; Wu, W. Graphene Oxide Promoted Chromium Uptake by Zebrafish Embryos with Multiple Effects: Adsorption, Bioenergetic Flux and Metabolism. Science of the Total Environment 2022, 802, 149914. [Google Scholar] [CrossRef]
- Jurgelene, Z.; Montvydiene, D.; Semcuk, S.; Stankeviciute, M.; Sauliute, G.; Pazusiene, J.; Morkvenas, A.; Butrimiene, R.; Joksas, K.; Pakstas, V.; Kazlauskienė, N.; Karabanovas, V. The Impact of Co-Treatment with Graphene Oxide and Metal Mixture on Salmo trutta at Early Development stages: The Sorption Capacity and Potential Toxicity. Science of the Total Environment 2022, 838, 156525. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).