1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic started in late 2019 when the severe acute respiratory syndrome virus (SARS-CoV-2) quickly spread from China, posing an unprecedented health crisis globally [
1,
2,
3]. The virus mainly affects the respiratory system, resulting in a broad range of symptoms from mild ones such as flu-like sickness, to severe ones, such as breathing difficulties, and even death due to sepsis, acute cardiac damage, heart failure, and multi-organ dysfunction [
4,
5]. Thus, developing immunity against the virus, including herd immunity, is crucial.
There are four structural proteins encoded by the genome of the virus; the spike (S), nucleocapsid (N), membrane (M), and envelope (E) proteins, similar to SARS-CoV-1 [
1,
4,
6,
7]. The S-protein, particularly its receptor-binding domain (RBD), is essential for infecting host cells by starting cell penetration when the domain attaches to the angiotensin-converting enzyme 2 (ACE2) receptor. In contrast, other structural proteins form the ribonucleoprotein core responsible for driving viral assembly [
1,
8,
9,
10]. Natural infection indeed elicited adaptive immune responses to the virus’ structural proteins, with T cells and antibodies being the key components [
11]. Many studies have reported SARS-CoV-2 patients exhibited T cell immunity and neutralising antibodies [
12,
13,
14,
15,
16]. The antibody frequently targets the receptor binding domain (RBD) of the spike protein to prevent the virus from interacting with the ACE2 receptor, and starting a productive infection [
11,
15,
17]. Neutralising antibodies are probably an essential correlate of COVID-19 protection [
17,
18,
19,
20] and are associated with protective immunity against second infection [
21].
In a large population, herd immunity can be achieved by vaccination besides the natural infection [
11]. Several types of vaccines have been produced, including the inactivated SARS-CoV-2 vaccine, which was used in 40 countries [
22]. The levels of neutralizing antibodies produced by the SARS-CoV-2 vaccines have been compared to those in naturally infected people [
17,
23]. This raises the question of whether or not vaccines is needed to boost immunity in people with past infections. It was suggested that hybrid immunity will be developed by vaccination in people with pre-existing immunity [
24]. It was reported that neutralization titers in vaccinated individuals were markedly higher than those in unvaccinated individuals with pre-infection acrosss several variants of SARS-CoV-2 [
25]. Several studies also revealed that neutralizing antibody titer induced by vaccination was higher among people with past infection than naive individuals[
26,
27,
28]. However, it was suggested that one vaccine dose of inactivated vaccines or mRNA vaccine is enough for subjects with pre-existing immunity to boost the antibody titer [
26,
27,
29,
30]. On the contrary, a study reported that infection-acquired immunity was higher in unvaccinated individuals than subsequently vaccinated[
31].
Therefore, it is essential to clarify whether the vaccination is needed for people with previous infection, to save the cost and the burden of vaccine production, especially in Indonesia, where no domestic vaccine produced has yet been distributed. The aim of this study was to compare the antibody titer between vaccinated individuals with and without pre-exisiting immunity and unvaccinated convalescent individuals. In addition, it evaluated the kinetics of virus-neutralizing antibodies in a prospective cohort. The result showed that vaccination does not increase the antibody titer against RBD in individuals with pre-exisiting immunity. Moreover, the capacity of viral neutralization of vaccine serum is not as good as that of the convalescent serum.
2. Materials and Methods
A prospective cohort involving COVID-19-recovered individuals and vaccine recipients visiting Tadjuddin Chalid Hospital and Wahidin Soedirohusodo Hospital in Makassar, the capital city of South Sulawesi province in Indonesia, from April 2021 to December 2021 was conducted. The inclusion criteria for COVID-19 survivors were age above 17 years old, and being confirmed recovered from COVID-19 by having a converted swab PCR test result from positive to negative. We recruited survivors without a vaccination history and persons with breakthrough infection. Blood was withdrawn at days 0, 30, and 90 after the negative PCR result, except for the breakthrough infections which was withdrawn at day 0 only.
We recruited vaccine recipients without a history of COVID-19 (naïve vaccine) and those with a COVID-19 pre-infection. The inclusion criteria for vaccine recipients were age over 17 years old, and completed two doses of the inactivated whole SARS-CoV-2 virus vaccine CoronaVac® from Sinovac at a 4-week interval. Blood was taken at 0, 30, and 90 days after the second immunization dose.
Collected blood was centrifuged at the Hasanuddin University Medical Research Centre (HUMRC) of Hasanuddin University Hospital for serum separation. All sera were kept at -80°C before being subjected to any experiments.
Indirect ELISAs were done using the commercial human embryonic kidney (HEK293) HPLC-verified (Sino Biological, #40591-V08H) RBD protein as the antigens. The 96-well microplate (Corning, #3590) was coated with 0,2μg/mL of the antigen dissolved in phosphate-buffered saline (PBS), pH 7.4 per well, incubated overnight at 4°C. The plates were blocked with 1% bovine serum albumin (BSA) in PBS (pH 7.4), washed with PBS-T, and incubated with serum samples diluted 1:100 in PBS containing 1% BSA. After washing, the sera samples were incubated with horseradish peroxidase (HRP)-conjugated monoclonal antibodies recognizing an Fc domain of human IgG. After incubation, the plates were washed, then 100 μL/well of the substrate was added to each well, and the plates were incubated for 30 min at room temperature for color development. The absorbance was measured at 414 nm on a microplate reader. For this experiment, we also used 30 samples from pre-pandemic era as negative controls.
Neutralizing activity of the serum was examined using a VSV-based pseudovirus, as previously described [
32]. Briefly, the pseudovirus was engineered to express the Wuhan-Hu-1 SARS-CoV-2 Spike protein on the viral surface, in which the luciferase gene was incorporated in the viral genome. The serum was diluted with the medium, and the virus was added in triplicate. The final dilution rate of the serum was 1:100. The mixture of the virus and the serum was incubated with human embryonic kidney (HEK) 293T cells that expressed human ACE2 and human TMPRSS2. The cells were examined by Luciferase Assay System (Promega, Madison, WI, USA) after 24 hours incubation.
Statistical analysis was performed using GraphPad Prism version 9.0 for Mac OS. Kolmogorov-Smirnov test was used to analyze the normality of the distribution of the antibody titers. One-way ANOVA followed by Tukey’s multiple comparison test was used for normally distributed data, whereas Kruskal-Wallis test with a post hoc Dunn’s multiple comparison test was used to compare differences in antibody titers between groups. A p-value of < 0.05 was considered statistically significant. Spearman’s test was used to analyze the correlations between optical densities (ODs) and the percentage of internalization.
3. Results
3.1. Antibody titer elicited by vaccination and natural infection
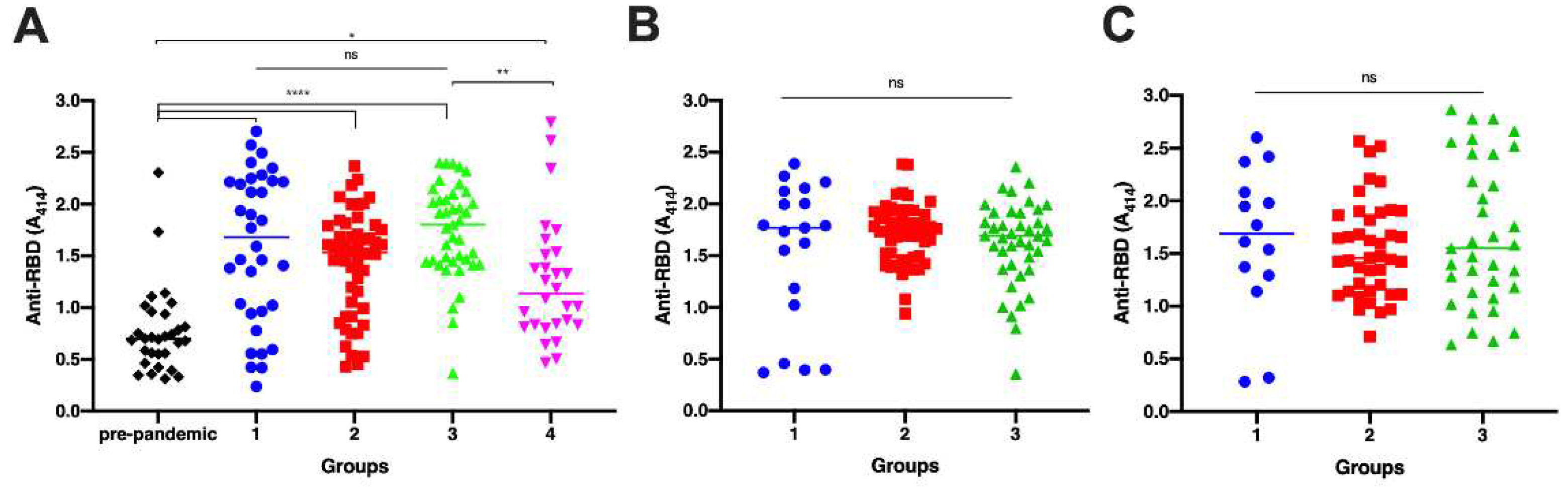
We collected 386 samples from 136 subjects at baseline (
Table 1), consisting of 32 unvaccinated survivors (Group 1), 52 vaccinated persons with a pre-infection (Group 2), 41 naïve vaccine recipients (Group 3), and 28 persons with breakthrough infections (Group 4). The study was completed on day 90 by 90 subjects consisting of 14 covid-19 unvaccinated survivors, 41 vaccine recipients with pre-infection, and 35 naïve vaccine recipients. The characteristics of the study subjects were shown in
Table 2.
Analysis of anti-RBD antibody titers between groups showed a significant difference on day 0 between all groups to pre-pandemic sera (
Figure 1A;
p<0.0001). In addition, the antibody titer of persons with breakthrough infections was significantly lower than that of vaccinated naïve individuals, whereas no significant differences were observed among other seropositive groups (
p = 0.0065). The antibody titers on day 30 did not show a significant difference between groups statistically (
Figure 1B;
p = 0.2535), similar to those on day 90 (
Figure 1C;
p = 0.6249).
These data inferred the antibody titers of survivors were not boosted by vaccination on day 28 after first dose-injection. On the other hand, breakthrough infection did not increase the antibody titer elicited by vaccines. Since no baseline data for group 4, we assumed the breakthrouh infection occured among vaccinated people with low antibody titer (non-responders).
3.2. Neutralization capacity of the antibody
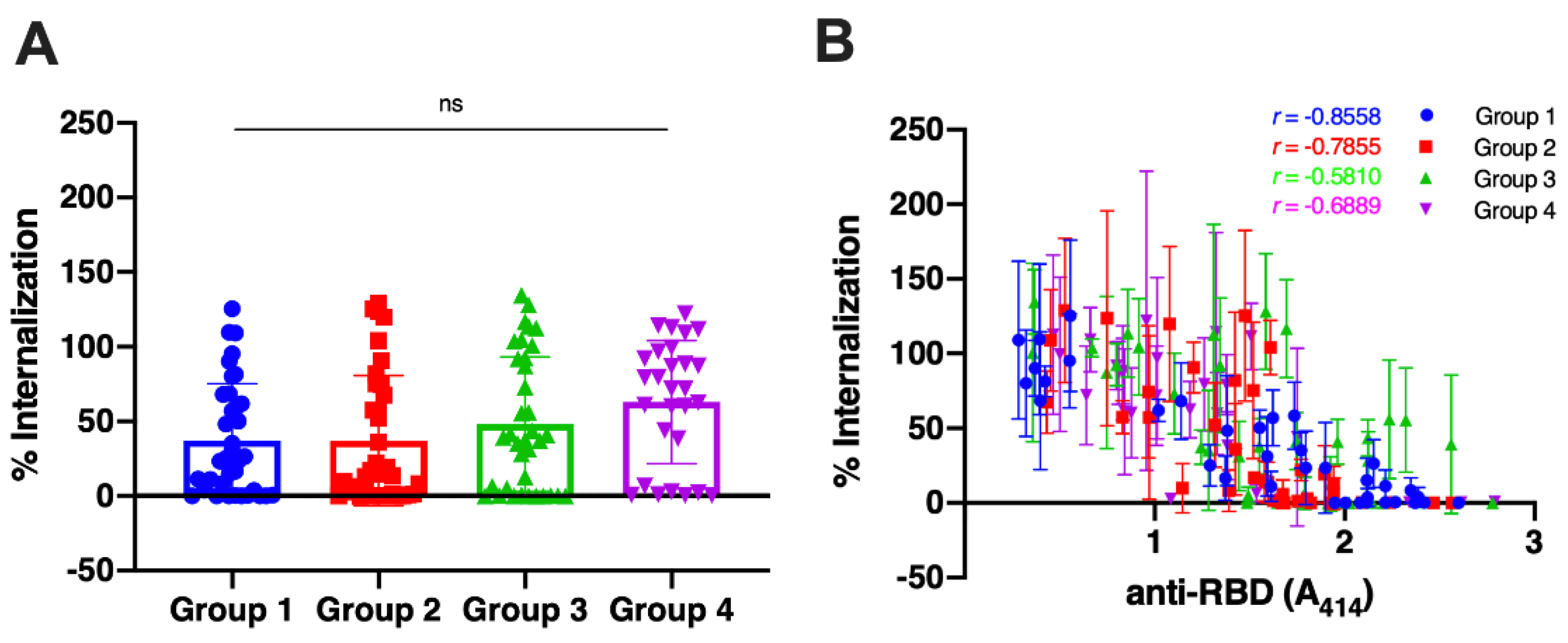
After determining the antibody titers of all samples, we randomly selected 36 samples for each group 1,2, and 3 and 28 samples for group 4 to analyze the capacity of the antibodies to neutralize the viruses. The sera were mixed with the Wuhan-Hu-1 pseudovirus, and then the percentage of virus internalization to the cells, as compared with non-serum control by 100%, was calculated by the luciferase activity. The result revealed that the antibodies of unvaccinated survivors neutralized the virus better (
Figure 2A, Group 1; percentage of internalization 37.26 ± 37.88,) than those of vaccinated individuals without pre-infection (Group 3; 48.15 ± 45.13). The mean percentage of viral internalization of the vaccinated persons with history of infection and the infected person after having two doses of vaccine were 37.02 ± 43.13 (Group 2) and 62.95 ± 41.28 (Group 4), respectively. No statistical differences among all groups.
We then analyzed the correlation of antibody titers with the virus neutralization. using Spearman’ test (
Figure 2B). The result revealed that the antibodies of unvaccinated survivors had the most robust neutralization capacity (
r = -0.8558; 95% Confidence Interval (CI), -0.9259 to -0.7288;
p < 0.0001) whereas the antibodies of vaccinated individuals without pre-infection had the lowest capacity (
r = -0.581; 95% CI, -0.7679 to -0.3028;
p = 0.0002). The correlation coefficient of the vaccinated persons with a history of infection and the infected person after having two doses of vaccine were -0.7855 (95% CI, -0.8877 to -0.6096) and -0.6889 (95% CI, -0.8481 to -0.4156). These results suggest that natural infection alone induced more effective antibodies than whole-inactivated-vaccine and no booster effect from vaccines to the existing immunity.
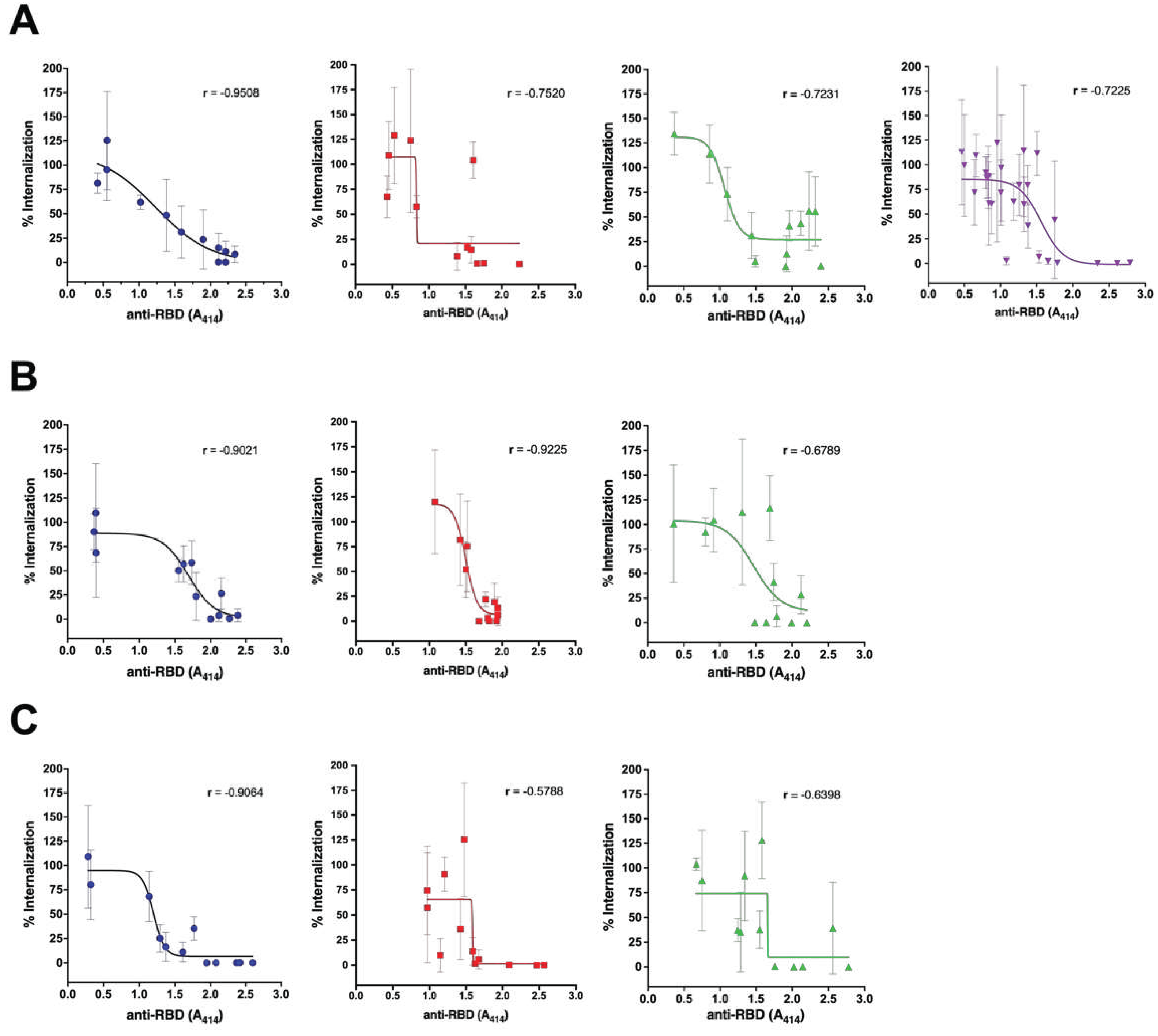
Further analysis was conducted based on the sample collection time (
Figure 3). The natural antibodies consistently had an outstanding capacity to neutralize the virus until day 90 (Group 1,
Figure 3A–C). The vaccinated naïve also shows consistent neutralization capacity until day 90, although at the lower level (Group 3,
Figure 3A–C). In the vaccinated persons with pre-infection, the highest neutralization capacity was shown on day 30 after the second dose. However, it decreased to a lower level than the vaccinated persons without pre-infection on day 90 (Group 2,
Figure 3A–C). In group 4, the proportion of the anti-RBD Ab with high level was low (
Figure 1A), and thus the neutralization activity was quite low in total (
Figure 3A).
All data suggest that vaccination with whole-inactivated vaccine does not effectively induce antibody titer with a strong neutralization capacity for persons with history of COVID-19 natural infection.
4. Discussion
In this study, we compare the capacity of the RBD antibodies elicited by COVID-19 natural infection to those elicited by inactivated vaccines. We also investigated whether vaccination boosted the antibody titer in people with pre-existing immunity. We found that there is no difference between the antibody titer of unvaccinated survivors compared with vaccinated individuals, either with or without pre-infection. A similar result was reported by a study on 35,768 healthcare workers in the UK, which found that the humoral responses of unvaccinated survivors remained consistently higher than the survivors who received two doses of BNT162b2[
31]. However, another mRNA-based vaccine, the mRNA-1273, induced a higher titer of neutralizing antibodies than natural infection[
33]. Therefore, the induction of antibody titer among individuals with pre-existing immunity might depends on the vaccine type.
In the current study we analysed the correlation of the neutralization capacity of sera with the anti-RBD titers since the neutralizing epitopes on the RBD of the spike protein are highly immunogenic, primarily the domain that bind with ACE2 receptor. Thus it was suggested that a single mutation could not avoid human polyclonal antibody neutralization[
21]. However, several mutations have changed the RBD conformation that may disturb the antigen recognition[
34]. Since its emergence, SARS-CoV-2 has undergone mutations causing variants of concern and variants of interest, some highly transmissible and are capable of escaping the antibody neutralization either from natural infection or vaccines[
21,
24,
34,
35,
36,
37]. Therefore, the limitation of this study is that it merely investigated the neutralization activity to the original Wuhan-Hu strain. Nonetheless, since the whole-inactivated vaccines generated from the original strain as well, we still can infer that this vaccine type may not be as powerful as natural infection in inducing antibodies. We predict that even the inactivated vaccines is generated using the later strains of SARS-CoV-2, people with pre-existing immunity to these strains may not need a vaccine shot. Indeed, future studies are needed to address this concern.
Our study also revealed that the expected hybrid immunity was not elicited by the breakthrough infection or whole inactivated vaccination to pre-infected persons, contrary to a study reporting that vaccination with mRNA-1273 increased the neutralizing antibodies 25 times higher in the pre-infected persons compared with vaccinated persons without pre-infection, and even 100 times higher than the natural infection alone [
24]. This contradictive result suggests that inactivated vaccines might not be not as potent as mRNA-based vaccines in inducing neutralizing antibodies.
Another limitation of this study was no baseline data for both group, infection and vaccine. However, a study has shown that the neutralizing antibodies peaked at 120 days after onset and are still detectable for over one year[
22].
Since the global campaign of COVID-19 vaccination, Indonesia has achieved excellent coverage but the interest in taking booster doses has waned due to the decrease in cases recently; hence the WHO recommended that the Indonesian government incorporate the COVID-19 vaccinations into routine services[
38]. If so, to decrease the demand of vaccines and to save resources, priotizing persons without history of natural infection should be considered as our data suggest that vaccination did not produce a better neutralizing antibodies in persons with a history of infection.
Author Contributions
Conceptualization, M.I, I.I, S.Y., and Y.Y; methodology, M.I, A.A.H, A.S, H.H, K.Y, S.Y, S.Y; software, A.A.H, Y.Y.; validation, A.B., Y.Y.; formal analysis, S.N, M.I, A.A.H, A.B., Y.Y.; investigation, S.N, M.I, A.A.H, K.A., K.H.Z; resources, M.I., S.Y., D.S, P.B.S.A, Y.Y.; data curation, M.I., A.A.H, K.A.; writing—original draft preparation, SN., M.I., A.A.H, Y.Y; writing— review and editing, M.I., K.H.Z, I.I., I.D, Y.Y.; visualization, A.A.H. Y.Y; supervision, M.I, I.I., Y.Y.; project administration, K.A.; funding acquisition, M.I., Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly funded by Indonesian Directorate General of Higher Education (DGHE)-Japan Society for the Promotion of Science (JSPS) joint research projects to Y.Y (No.105/E4.4/KU/2021) and M.I (JPJSBP-120218101) and Internal Research Funding to Y.Y (No.915/UN4.22/PT.01.03/2021) from the Institute for Research and Community Services (LPPM) of Hasanuddin University. The APC was funded by Hasanuddin University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Hasanuddin University (Approval Number 753/UN4.6.4.5.31/PP36/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data presented in this study will be made available to any researchers on request to the corresponding author.
Acknowledgments
We would like to express our sincere gratitude to all volunteers involved in this study. We are also grateful for the laboratory assistance of Handayani Halik at Hasanuddin University Medical Research Centre in sera sample processing. The authors also thank all staff who supported the sample collection in Wahidin Soedirohusodo Hospital. We thank Syahruddin at Tadjuddin Chalid Hospital for the support during the sample collection and lab work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Luo, J.; Brakel, A.; Krizsan, A.; Ludwig, T.; Mötzing, M.; Volke, D.; Lakowa, N.; Grünewald, T.; Lehmann, C.; Wolf, J.; et al. Sensitive and specific serological ELISA for the detection of SARS-CoV-2 infections. Virology Journal 2022, 19, 50. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, L.; Wang, X.; Luo, N.; Li, L. Clinical Outcomes in 55 Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Who Were Asymptomatic at Hospital Admission in Shenzhen, China. The Journal of Infectious Diseases 2020, 221, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Raj, R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochemistry and Biophysics Reports 2021, 25, 100847. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, G.; Ma, H.; Zhao, D.; Yang, Y.; Liu, M.; Mohammed, A.; Zhao, C.; Yang, Y.; Xie, J.; et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochemical and Biophysical Research Communications 2020, 527, 618–623. [Google Scholar] [CrossRef]
- Chang, C.-k.; Sue, S.-C.; Yu, T.-h.; Hsieh, C.-M.; Tsai, C.-K.; Chiang, Y.-C.; Lee, S.-j.; Hsiao, H.-h.; Wu, W.-J.; Chang, W.-L.; et al. Modular organization of SARS coronavirus nucleocapsid protein. Journal of Biomedical Science 2006, 13, 59–72. [Google Scholar] [CrossRef]
- McBride, R.; Van Zyl, M.; Fielding, B.C. The Coronavirus Nucleocapsid Is a Multifunctional Protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.-h.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.A.P.M.; et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nature Communications 2021, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. The Lancet Infectious Diseases 2020, 20, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Fafi-Kremer, S.; Bruel, T.; Madec, Y.; Grant, R.; Tondeur, L.; Grzelak, L.; Staropoli, I.; Anna, F.; Souque, P.; Fernandes-Pellerin, S.; et al. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. eBioMedicine 2020, 59. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Science Immunology 2020, 5, eabe0367. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- L'Huillier, A.G.; Meyer, B.; Andrey, D.O.; Arm-Vernez, I.; Baggio, S.; Didierlaurent, A.; Eberhardt, C.S.; Eckerle, I.; Grasset-Salomon, C.; Huttner, A.; et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clinical Microbiology and Infection 2021, 27, e781–e784. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford Katharine, H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.-L.; Jerome Keith, R.; Bloom Jesse, D.; Greninger Alexander, L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. Journal of Clinical Microbiology 2020, 58, 10–1128. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nature Communications 2020, 11, 4704. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. New England Journal of Medicine 2020, 384, 533–540. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nature Microbiology 2022, 7, 423–433. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. New England Journal of Medicine 2020, 383, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Hybrid immunity. Science 2021, 372, 1392–1393. [Google Scholar] [CrossRef]
- Seaman, M.S.; Siedner, M.J.; Boucau, J.; Lavine, C.L.; Ghantous, F.; Liew, M.Y.; Mathews, J.I.; Singh, A.; Marino, C.; Regan, J.; et al. Vaccine breakthrough infection leads to distinct profiles of neutralizing antibody responses by SARS-CoV-2 variant. JCI Insight 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. New England Journal of Medicine 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Jia M; Wang X; Gong W; Zhong J; Leng Z; Ren L; Feng L; Guo L; Gao L; Liang X; et al. Humoral responses after inactivated COVID-19 vaccination in individuals with and without prior SARS-CoV-2 infection: A prospective cohort study. J Med Virol. 2022, 94, 5746–5757. [Google Scholar] [CrossRef]
- Ma, M.-L.; Shi, D.-W.; Li, Y.; Hong, W.; Lai, D.-Y.; Xue, J.-B.; Jiang, H.-W.; Zhang, H.-N.; Qi, H.; Meng, Q.-F.; et al. Systematic profiling of SARS-CoV-2-specific IgG responses elicited by an inactivated virus vaccine identifies peptides and proteins for predicting vaccination efficacy. Cell Discovery 2021, 7, 67. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nature Medicine 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Favresse, J.; Gillot, C.; Di Chiaro, L.; Eucher, C.; Elsen, M.; Van Eeckhoudt, S.; David, C.; Morimont, L.; Dogné, J.-M.; Douxfils, J. Neutralizing Antibodies in COVID-19 Patients and Vaccine Recipients after Two Doses of BNT162b2. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. New England Journal of Medicine 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Osawa, H.; Hashimoto, H.; Mizuno, T.; Hasyim, A.A.; Abe, Y.-i.; Okahashi, Y.; Ogawa, R.; Iyori, M.; Shida, H.; et al. A replication-competent smallpox vaccine LC16m8Δ-based COVID-19 vaccine. Emerging Microbes & Infections 2022, 11, 2359–2370. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. New England Journal of Medicine 2020, 384, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dai, T.; Wang, B.; Zhang, L.; Zeng, L.-h.; Huang, J.; Yan, H.; Zhang, L.; Zhou, F. The way of SARS-CoV-2 vaccine development: success and challenges. Signal Transduction and Targeted Therapy 2021, 6, 387. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Kimura, I.; Shirakawa, K.; Takaori-Kondo, A.; Nakada, T.-a.; Kaneda, A.; Nakagawa, S.; Sato, K. Neutralization of the SARS-CoV-2 Mu Variant by Convalescent and Vaccine Serum. New England Journal of Medicine 2021, 385, 2397–2399. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nature Reviews Microbiology 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Chen, L.-L.; Chua, G.T.; Lu, L.; Chan, B.P.-C.; Wong, J.S.-C.; Chow, C.C.-K.; Yu, T.-C.; Leung, A.S.-Y.; Lam, S.-Y.; Wong, T.-W.; et al. Omicron variant susceptibility to neutralizing antibodies induced in children by natural SARS-CoV-2 infection or COVID-19 vaccine. Emerging Microbes & Infections 2022, 11, 543–547. [Google Scholar] [CrossRef]
- Tanoto, R. COVID-19 vaccination post introduction evaluation (cPIE) in Indonesia. Available online: https://www.who.int/indonesia/news/detail/05-07-2023-covid-19-vaccination-post-introductionevaluation-(
cpie)-in-indonesia (accessed on 25 July 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).