Submitted:
05 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
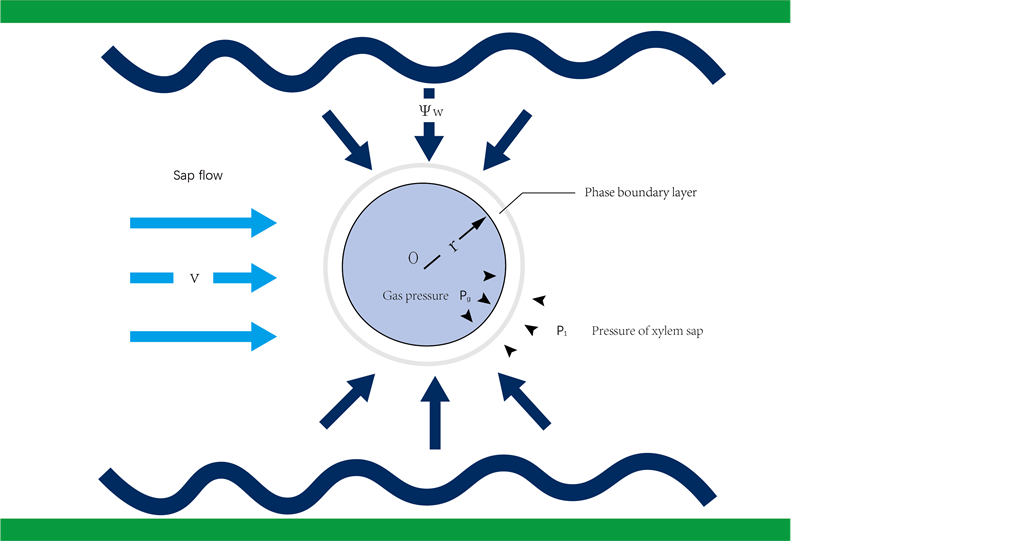
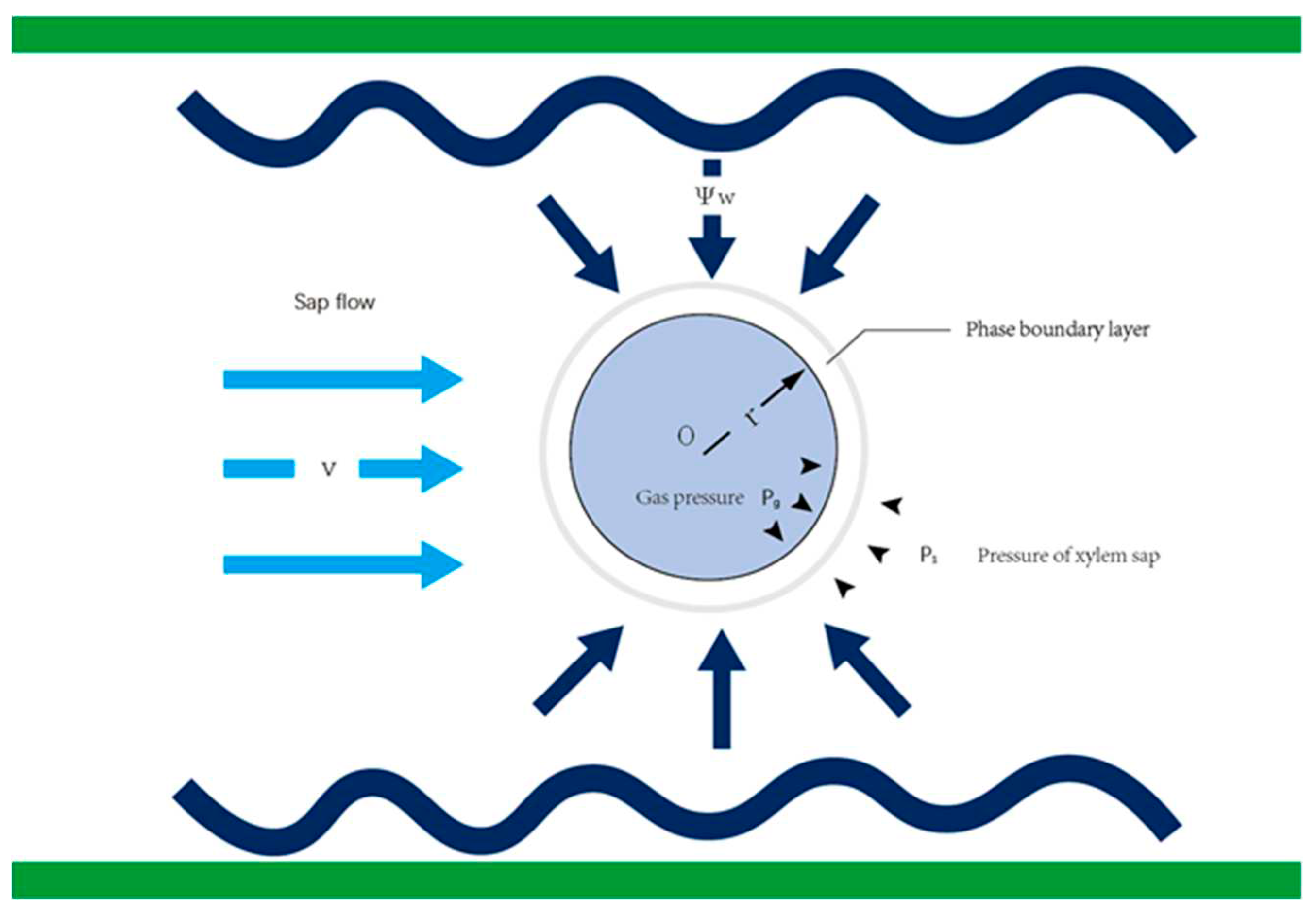
2.2. Theory
2.3. Sample Processing
2.3.1. Physiological indicators
2.3.2. Quantifying Hydraulic Conductivity Loss for Xylem Vulnerability Curve Generation
2.3.3. Small Flow Method
3. Results
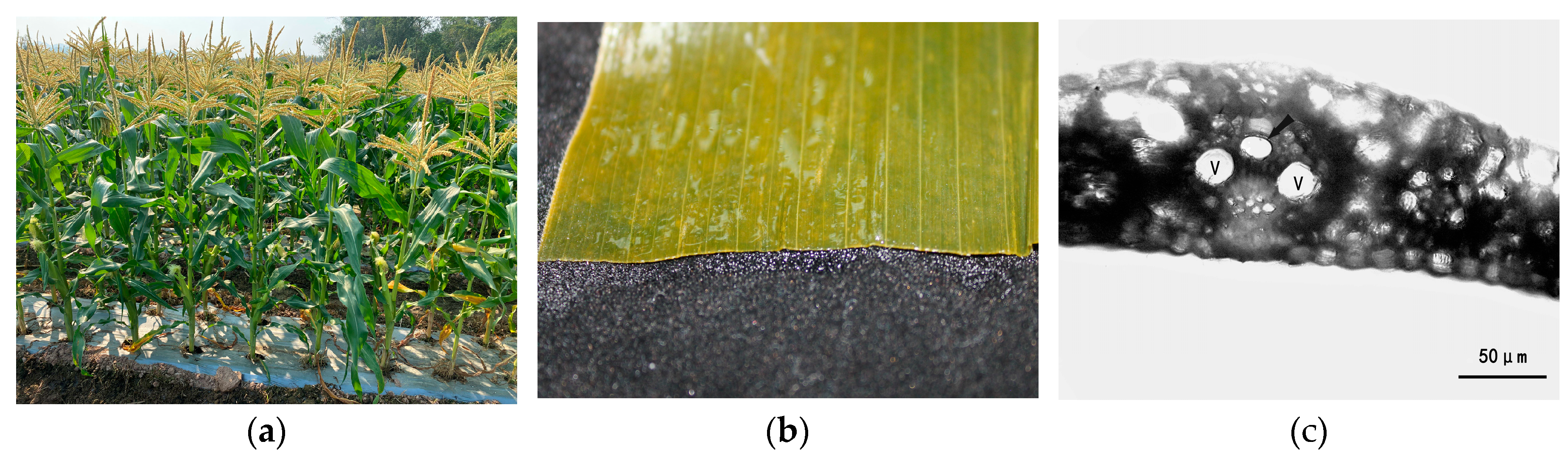
3.1. Physiological Parameters of Maize
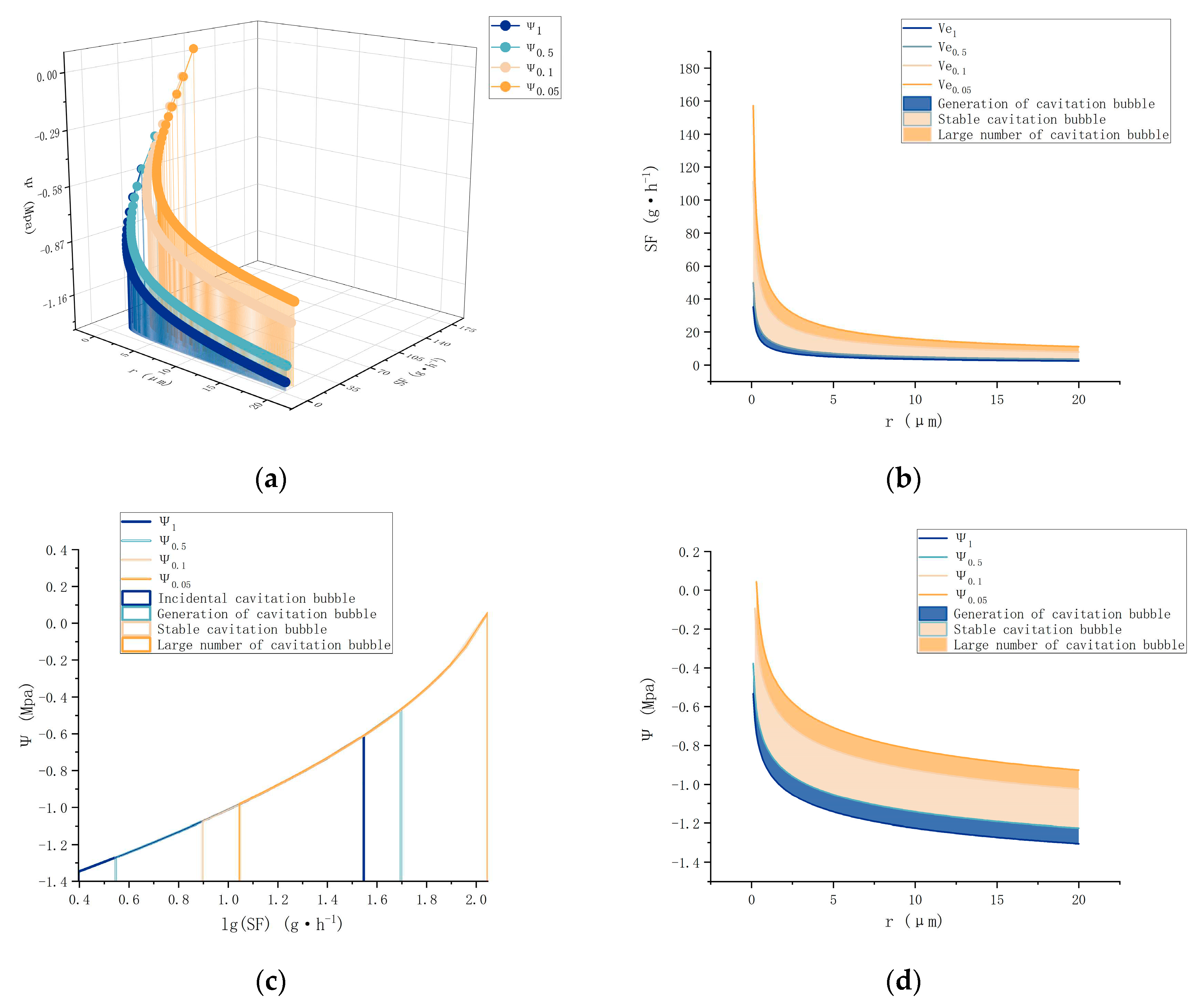
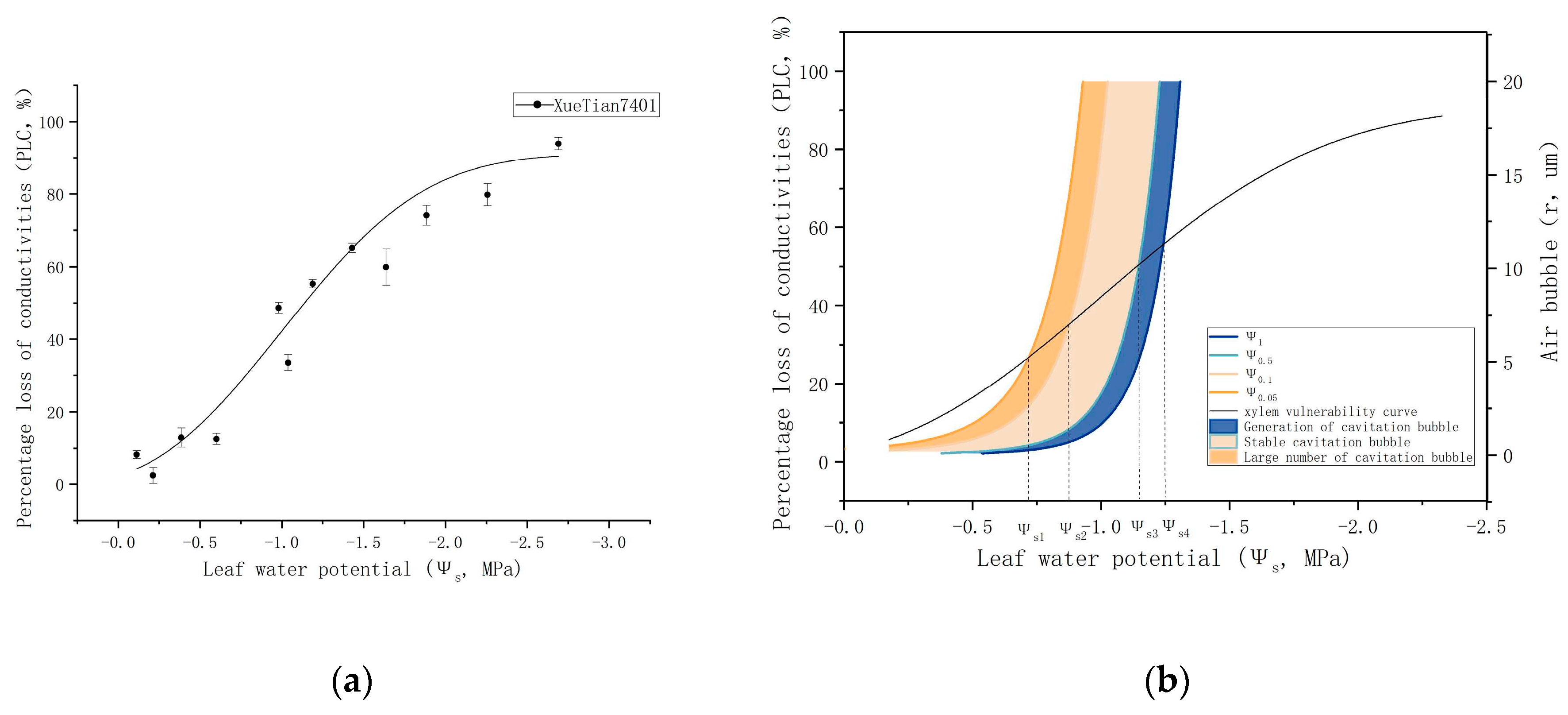
3.2. Establishing the Bubble Radius-Sap Flow Rate-Water Potential Model
3.3. Comparison of Cavitation Emergence in Leaf PLC and Model Predictions
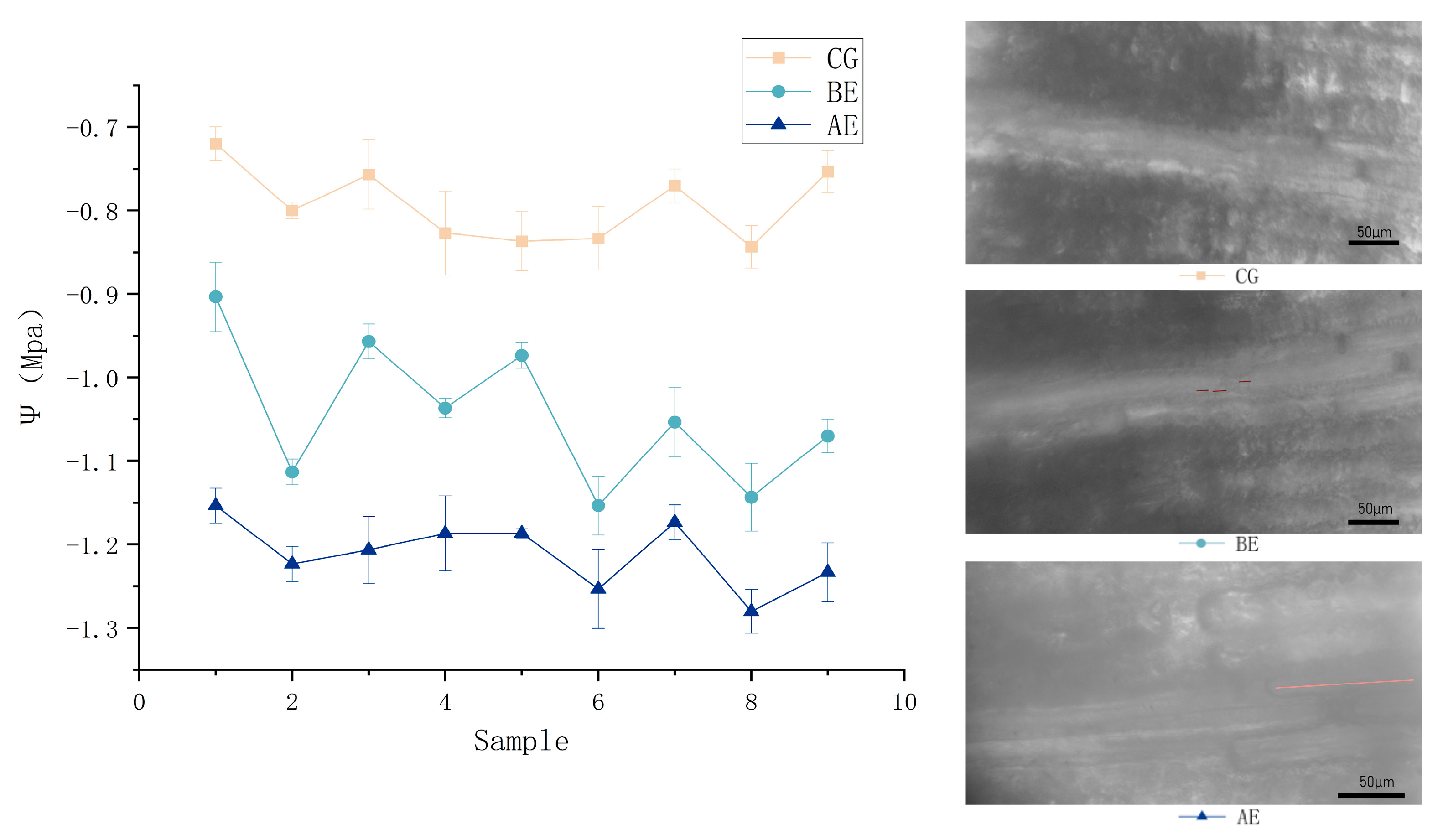
3.4. Observations of Leaf Conditions at Different Water Potentials during Cavitation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops That Feed the World 6. Past Successes and Future Challenges to the Role Played by Maize in Global Food Security. Food Sec. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Jones-Garcia, E.; Krishna, V.V. Farmer Adoption of Sustainable Intensification Technologies in the Maize Systems of the Global South. A Review. Agron. Sustain. Dev. 2021, 41, 8. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N.Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Prediction of Moisture Content in Corn Leaves Based on Hyperspectral Imaging and Chemometric Analysis. Trans. ASABE 2015, 531–537. [CrossRef]
- Katerji, N. Comparison of Corn Yield Response to Plant Water Stress Caused by Salinity and by Drought. Agricultural Water Management 2003. [Google Scholar] [CrossRef]
- Nilahyane, A.; Islam, M.A.; Mesbah, A.O.; Herbert, S.K.; Garcia y Garcia, A. Growth, Water Productivity, Nutritive Value, and Physiology Responses of Silage Corn to Water Stress. Agron. J. 2020, 112, 1625–1635. [Google Scholar] [CrossRef]
- Mahmoud, M.A.B.; Sharp, R.E.; Oliver, M.J.; Finke, D.L.; Ellersieck, M.R.; Hibbard, B.E. The Effect of Western Corn Rootworm (Coleoptera: Chrysomelidae) and Water Deficit on Maize Performance under Controlled Conditions. Journal of Economic Entomology 2016, 109, 684–698. [Google Scholar]
- Jansen, S.; Schenk, H.J. On the Ascent of Sap in the Presence of Bubbles. American Journal of Botany 2015, 102, 1561–1563. [Google Scholar] [CrossRef]
- Ponomarenko, A.; Vincent, O.; Pietriga, A.; Cochard, H.; Badel, E.; Marmottant, P. Ultrasonic Emissions Reveal Individual Cavitation Bubbles in Water-Stressed Wood. J. R. Soc. Interface 2014, 11, 20140480. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Morales-Sillero, A.; Martin-Palomo, M.J.; Mayr, S.; Beikircher, B.; Fernandez, J.E. Shoot Hydraulic Characteristics, Plant Water Status and Stomatal Response in Olive Trees under Different Soil Water Conditions. Plant Soil 2013, 373, 77–87. [Google Scholar] [CrossRef]
- Miyashita, K.; Tanakamaru, S.; Maitani, T.; Kimura, K. Recovery Responses of Photosynthesis, Transpiration, and Stomatal Conductance in Kidney Bean Following Drought Stress. Environ. Exp. Bot. 2005, 53, 205–214. [Google Scholar] [CrossRef]
- Schenk, H.J.; Steppe, K.; Jansen, S. Nanobubbles: A New Paradigm for Air-Seeding in Xylem. Trends Plant Sci. 2015, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- TOGNETTIt, R.; Borghett, M. Formation and Seasonaloccurrence of Xylem Embolism in Alms Cordata. [CrossRef]
- Pratt, R.B.; Ewers, F.W.; Lawson, M.C.; Jacobsen, A.L.; Brediger, M.M.; Davis, S.D. Mechanisms for Tolerating Freeze–Thaw Stress of Two Evergreen Chaparral Species: Rhus Ovata and Malosma Laurina (Anacardiaceae). Am. J. Bot. 2005, 92, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, T.; Fukuda, K. Seasonal Changes in the Occurrence of Embolisms among Broad-Leaved Trees in a Temperate Region. Botany 2018, 96, 873–881. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Slot, J.C.; Visser, E.; Naidoo, S.; Sovic, M.; Conrad, A.O.; Bonello, P. How Abiotic Stress Plays Ally to Pathogenic Attack in Trees. In Proceedings of the PHYTOPATHOLOGY; AMER PHYTOPATHOLOGICAL SOC 3340 PILOT KNOB ROAD, ST PAUL, MN 55121 USA, 2021; Vol. 111, pp. 63–63. [Google Scholar]
- Simova-Stoilova, L.; Vassileva, V.; Feller, U. Selection and Breeding of Suitable Crop Genotypes for Drought and Heat Periods in a Changing Climate: Which Morphological and Physiological Properties Should Be Considered? Agriculture 2016, 6, 26. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Cochard, H.; Mencuccini, M.; Delzon, S.; Badel, E. Direct Observation and Modelling of Embolism Spread between Xylem Conduits: A Case Study in Scots Pine. Plant Cell Environ. 2016, 39, 2774–2785. [Google Scholar] [CrossRef]
- Holtta, T.; Juurola, E.; Lindfors, L.; Porcar-Castell, A. Cavitation Induced by a Surfactant Leads to a Transient Release of Water Stress and Subsequent “run Away” Embolism in Scots Pine (Pinus Sylvestris) Seedlings. J. Exp. Bot. 2012, 63, 1057–1067. [Google Scholar] [CrossRef]
- Omelyanyuk, M.; Ukolov, A.; Pakhlyan, I.; Bukharin, N.; El Hassan, M. Experimental and Numerical Study of Cavitation Number Limitations for Hydrodynamic Cavitation Inception Prediction. Fluids 2022, 7, 198. [Google Scholar] [CrossRef]
- Zhao, L.; He, Z.; Zhao, W.; Yang, Q. Extensive Investigation of the Sap Flow of Maize Plants in an Oasis Farmland in the Middle Reach of the Heihe River, Northwest China. J Plant Res 2016, 129, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Bibi, F.; Rahman, A. An Overview of Climate Change Impacts on Agriculture and Their Mitigation Strategies. Agriculture 2023, 13, 1508. [Google Scholar] [CrossRef]
- Lens, F.; Gleason, S.M.; Bortolami, G.; Brodersen, C.; Delzon, S.; Jansen, S. Functional Xylem Characteristics Associated with Drought-Induced Embolism in Angiosperms. New Phytol. 2022, 236, 2019–2036. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Dominguez, C.M.; Carins Murphy, M.R.; Lucani, C.; Brodribb, T.J. Mapping Xylem Failure in Disparate Organs of Whole Plants Reveals Extreme Resistance in Olive Roots. New Phytol 2018, 218, 1025–1035. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Parent, B. Modelling the Coordination of the Controls of Stomatal Aperture, Transpiration, Leaf Growth, and Abscisic Acid: Update and Extension of the Tardieu–Davies Model. Journal of Experimental Botany 2015, 66, 2227–2237. [Google Scholar] [CrossRef]
- Venturas, M.D.; Sperry, J.S.; Love, D.M.; Frehner, E.H.; Allred, M.G.; Wang, Y.; Anderegg, W.R.L. A Stomatal Control Model Based on Optimization of Carbon Gain versus Hydraulic Risk Predicts Aspen Sapling Responses to Drought. New Phytol 2018, 220, 836–850. [Google Scholar] [CrossRef]
- Sperry, J.S.; Venturas, M.D.; Anderegg, W.R.L.; Mencuccini, M.; Mackay, D.S.; Wang, Y.; Love, D.M. Predicting Stomatal Responses to the Environment from the Optimization of Photosynthetic Gain and Hydraulic Cost: A Stomatal Optimization Model. Plant, Cell & Environment 2017, 40, 816–830. [Google Scholar] [CrossRef]
- Mencuccini, M. Modelling Water Fluxes in Plants: From Tissues to Biosphere. New Phytologist 2019. [Google Scholar] [CrossRef]
- Fan, D.-Y.; Dang, Q.-L.; Xu, C.-Y.; Jiang, C.-D.; Zhang, W.-F.; Xu, X.-W.; Yang, X.-F.; Zhang, S.-R. Stomatal Sensitivity to Vapor Pressure Deficit and the Loss of Hydraulic Conductivity Are Coordinated in Populus Euphratica, a Desert Phreatophyte Species. Front. Plant Sci. 2020, 11, 1248. [Google Scholar] [CrossRef]
- Phillips, N.G.; Oren, R.; Licata, J.; Linder, S. Time Series Diagnosis of Tree Hydraulic Characteristics. Tree Physiology 2004, 24, 879–890. [Google Scholar] [CrossRef]
- 36 10.1093@treephys@tpz070.Pdf.
- Giles, A.L.; Rowland, L.; Bittencourt, P.R.L.; Bartholomew, D.C.; Coughlin, I.; Costa, P.B.; Domingues, T.; Miatto, R.C.; Barros, F.V.; Ferreira, L.V.; et al. Small Understorey Trees Have Greater Capacity than Canopy Trees to Adjust Hydraulic Traits Following Prolonged Experimental Drought in a Tropical Forest. Tree Physiology 2022, 42, 537–556. [Google Scholar] [CrossRef]
- Wason, J.; Bouda, M.; Lee, E.F.; McElrone, A.J.; Phillips, R.J.; Shackel, K.A.; Matthews, M.A.; Brodersen, C. Xylem Network Connectivity and Embolism Spread in Grapevine( Vitis Vinifera L.). Plant Physiology 2021, 186, 373–387. [Google Scholar] [CrossRef]
- Shen, F. Analysis of Cavitation Processes in Xylem. JAMP 2020, 08, 1767–1778. [Google Scholar] [CrossRef]
- Shen, F.; Gao, R.; Liu, W.; Zhang, W. Physical Analysis of the Process of Cavitation in Xylem Sap. Tree Physiology 2002, 22, 655–659. [Google Scholar] [CrossRef]
- Shen, F.; Cheng, Y.; Zhang, L.; Gao, R.; Shao, X. Experimental Study of the Types of Cavitation by Air Seeding Using Light Microscopy. Tree Physiol 2015, 35, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Huang, Jitang. Principles and Applications of Cavitation and Cavitation; Tsinghua University Press, 1991; ISBN 7-302-00670-9. [Google Scholar]
- Sperry, J.S.; Wang, Y.; Wolfe, B.T.; Mackay, D.S.; Anderegg, W.R.L.; McDowell, N.G.; Pockman, W.T. Pragmatic Hydraulic Theory Predicts Stomatal Responses to Climatic Water Deficits. New Phytol 2016, 212, 577–589. [Google Scholar] [CrossRef]
- Borghetti, M.; Grace, J.; Raschi, A. Water Transport in Plants under Climatic Stress; Cambridge University Press, 1993; ISBN 0-521-44219-2. [Google Scholar]
- Tyree, M.T.; Salleo, S.; Nardini, A.; Assunta Lo Gullo, M.; Mosca, R. Refilling of Embolized Vessels in Young Stems of Laurel. Do We Need a New Paradigm? Plant physiology 1999, 120, 11–22. [Google Scholar] [CrossRef]
- Yang, S.; Tyree, M.T. A Theoretical Model of Hydraulic Conductivity Recovery from Embolism with Comparison to Experimental Data on Acer Saccharum. Plant, Cell & Environment 1992, 15, 633–643. [Google Scholar] [CrossRef]
- Stohr, A.; Losch, R. Xylem Sap Flow and Drought Stress of Fraxinus Excelsior Saplings. Tree Physiology 2004, 24, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Fiscus, E.L.; Wullschleger, S.; Dixon, M. Detection of Xylem Cavitation in Corn under Field Conditions. Plant Physiology 1986, 82, 597–599. [Google Scholar] [CrossRef] [PubMed]
- McCully, M.; Huang, C.; Ling, L. Daily Embolism and Refilling of Xylem Vessels in the Roots of Field-Grown Maize. The New Phytologist 1998, 138, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Sperry, J.S. Vulnerability of Xylem to Cavitation and Embolism. Annual review of plant biology 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Xiong, D.; Nadal, M. Linking Water Relations and Hydraulics with Photosynthesis. Plant J 2020, 101, 800–815. [Google Scholar] [CrossRef]
- McCully, M.E. Root Xylem Embolisms and Refilling. Relation to Water Potentials of Soil, Roots, and Leaves, and Osmotic Potentials of Root Xylem Sap. Plant physiology 1999, 119, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Sutherland, R.A. Stomatal Control of Xylem Embolism. Plant Cell Environ 1991, 14, 607–612. [Google Scholar] [CrossRef]
- Cochard, H.; Delzon, S.; Badel, E. X-Ray Microtomography (Micro-CT): A Reference Technology for High-Resolution Quantification of Xylem Embolism in Trees: A Reference Method for Xylem Embolism. Plant Cell Environ 2015, 38, 201–206. [Google Scholar] [CrossRef]
- Jia, Y.; Xiao, W.; Ye, Y.; Wang, X.; Liu, X.; Wang, G.; Li, G.; Wang, Y. Response of Photosynthetic Performance to Drought Duration and Re-Watering in Maize. Agronomy-Basel 2020, 10, 533. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, S.; Ma, J.; Huo, Z.; Zhang, C. Irrigation Management for Spring Maize Grown on Saline Soil Based on SWAP Model. Field Crop. Res. 2016, 196, 85–97. [Google Scholar] [CrossRef]
| Year | Month | Mean month air temperature (°C) | Humidity range (%) | Precipitation (mm) | Mean wind speed (m s−1) | Mean daily net radiation (W m−2) |
|---|---|---|---|---|---|---|
| 2021 | March | 21.91 | 18.1-99.9 | 1.39 | 1.6 | 259.3 |
| 2022 | March | 23.12 | 25.4-99.9 | 79.33 | 1.6 | 206.1 |
| 2023 | March | 20.69 | 23.1-99.9 | 0.13 | 1.5 | 197.4 |
| Year | Number of leaves | Number of veins | Average leaf radius (μm) | Maize leaf length(cm) |
|---|---|---|---|---|
| 2021 | 12 | 45 | 66.02 ±2.10 | 74.95 ±1.21 |
| 9 | 38 | 42.01 ±3.33 | 56.50 ± 0.53 | |
| 11 | 43 | 54.19 ±0.35 | 64.40 ±3.18 | |
| 6 | 35 | 32.79 ±1.3 | 47.90 ±2.26 | |
| 9 | 39 | 51.43 ±3.77 | 67.40 ±1.30 | |
| 15 | 53 | 97.20 ±2.43 | 93.70 ± 2.11 | |
| 12 | 48 | 62.36 ±1.27 | 66.40 ± 1.57 | |
| Average value | 10.57 | 43 | 58.00 | 67.30 |
| 2022 | 11 | 45 | 69.03 ±0.98 | 78.40 ±0.43 |
| 7 | 34 | 27.64 ±1.85 | 41.55 ±3.53 | |
| 8 | 35 | 33.98 ±1.24 | 49.60 ±1.73 | |
| 10 | 38 | 41.72 ±3.79 | 56.10 ±3.12 | |
| 12 | 47 | 69.29 ±1.43 | 75.35 ±1.83 | |
| 7 | 29 | 23.12 ±1.26 | 40.75 ±0.86 | |
| 10 | 40 | 49.11 ±2.36 | 62.75 ±1.48 | |
| Average value | 9.29 | 38.29 | 44.84 | 57.80 |
| 2022 | 12 | 49 | 73.5 ±1.24 | 72.5 ±1.32 |
| 11 | 46 | 53.15 ±1.57 | 59.05 ±1.2 | |
| 7 | 36 | 34.80 ±0.74 | 49.40 ±2.10 | |
| 12 | 41 | 53.50 ±0.69 | 66.70 ±1.63 | |
| 8 | 37 | 35.69 ±3.1 | 49.30 ±3.41 | |
| 10 | 27 | 41.30 ±1.45 | 78.15 ±0.76 | |
| 9 | 35 | 38.50 ±1.15 | 56.20 ±0.84 | |
| Average value | 9.86 | 38.71 | 47.21 | 62.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




