1. Introduction
Recombinant proteins have become increasingly popular for use in therapeutics. Recombinant protein drugs make up 23% of the total drugs approved by the FDA in 2020 (1). The majority of recombinant proteins suited for drug use are limited to soluble proteins, antibodies, insulin, and growth factors. Membrane-bound proteins, such as G-protein coupled receptors (GPCRs) are not compatible for use as injectable protein drugs due to their insoluble nature and tendency to aggregate. Isolated GPCRs also lack the necessary ability of anchoring to cell membranes coupled with signaling molecules in a live cell environment. Cellular delivery of membrane receptors could provide an alternative method to advance recombinant protein therapy in the near future.
Currently, GPCRs are not able to be engineered into an injectable protein drug. However, GPCRs are an important member of a family of membrane proteins involved in performing and maintaining normal life for almost all cells and organs (2-7). Deficiency of GPCR function is one of the major causes of human disease (8-9). Thus, many drugs were developed by targeting functional GPCRs (10).
Platelet-like particles (PLPs) prepared from immature megakaryocytes (MKs) using growth-factor induction in vitro have been reported (11). PLPs are nucleus-free cell-like particles suitable for delivery from local injection to IV infusion. Expression of the recombinant proteins anchored on PLP membranes could turn PLPs into a novel delivery vehicle of membrane-bound proteins for therapeutics. Therefore, we hypothesize that the PLPs released from the cDNA-transfected MKs, which express the GPCRs, could carry the biological function of recombinant membrane proteins. To test this hypothesis, we focused on identifying PLPs carrying recombinant GPCR functions.
Recently, we developed a “biolink” approach, which engineered several membrane-bound fusion proteins from enzymes to GPCRs (12-23). The fusion proteins, single-chain (SC) GPCR-Gα complexes: thromboxane A2 (TXA2) receptor (TP) covalently linked to G-protein αq (SC-TP-Gαq) and αs (SC-TP-Gαs) with the ability to bind TXA2 and performing signaling with a single molecule were the first to be created and characterized (23). HEK293 cells regulated cellular signing in two ways. Either Gq-mediated calcium signaling or Gs-mediated cAMP signaling upon binding to the identical TXA2 agonists (23). In this study, we used SC-TP-Gαq and SC-TP-Gαs as examples to test the hypothesis, in which the PLP system could be used as a novel nucleus-free vehicle to deliver the membrane-bound GPCR-Gα with therapeutic potentials. The study demonstrated that PLPs produced from the Meg-1 cells transfected with cDNA of SC-TP-Gαq or SC-TP-Gαs were able to carry the expressed SC-TP-Gαq or SC-TP-Gαs proteins by controlling Gq-mediated human-platelet aggregation and Gs-mediated anti-human-platelet aggregation.
2. Materials and Methods
2.1. Materials
Meg-01 cell lines were purchased from ATCC (Manassas, VA). Cell culture medium, supplements and antibiotics were purchased from Life Technologies (Grand Island, NY). TPO was purchased from R&D systems (Minneapolis, MN). Gαq and Gαs antibodies were purchased from Cayman (Ann Arbor, MI), and CD41a and CD42b antibodies were purchased from Invitrogen (Carlsbad, CA).
2.2. Cell culture
Meg-01 cells were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), and 1% Antibiotic- Antimycotic at 37 °C, in a humidified 5% CO2 incubator. Because a suspension cell line was used, the subculture was centrifuged at a speed of 200g for 5 minutes. The cell pellet was then resuspended in fresh culture medium.
2.3. Designing SC-TP-Gαq and SC-TP-Gαs
The functional SC-TP-Gαq and SC-TP-Gαs were created by linking the C-terminus of the human TP to the N-terminus of the Gαq or Gαs in a computational simulation using the crystal structures of human TP, Gαq, and Gαs (23).
2.3. cDNA synthesis and plasmid preparation
The cDNA, in which the C-terminus of TP was linked to the N-terminus of Gαq subunit (SC-TP-Gαq) or Gαs subunit (SC-TP-Gαs), was created using the PCR approach. Both full sizes of cDNAs were further sub-cloned into a pcDNA3.1 vector using Ecor1 and Xho1 restriction sites, respectively (19).
2.4. Expression of the recombinant SC-TP-Gαq and SC-TP-Gαs in MK cells
The method to establish stable cell lines expressing the SC-TP-Gαq and SC-TP-Gαs in MK cells is similar to that previously described in HEK293 cells (23). Briefly, MK cells cultured in suspension medium were transfected with the cDNA vectors by using an electroporation approach detailed by the electroporator manufacturer. After 48h of transfection, G418 (400ug/ml) was added to the medium to screen and select for MK cells expressing the recombinant proteins. The selection lasted 3-8 weeks.
2.5. Western blot
The western blot procedure followed the protocol provided by the Abcam (Cambridge, MA) website. The cells were washed with cold PBS, scratched, and harvested into a 1.5mL Eppendorf tube. They were collected by centrifugation and a 200µL lysis buffer (1 x RIPA buffer, 1mM PMSF, 1 x Protease inhibitor) was added and mixed with the cell pellet. The cell lysates were incubated on ice for 30 min while vortexing on and off. After vortexing, they were centrifuged at 16,000 rpm for 20 min at 4°C. The supernatant was decanted into a new tube. 10µl of lysate was used to perform a protein assay (Pierce 660nm Protein Assay, Thermo Scientific). 30µg of protein was subjected to electrophoresis with lab-made gradient SDS-PAGE gel (4% stacking gel and 10% separation gel). The samples on the gel were transferred onto a PVDF membrane (Bio Rad). COX-1, Gαs and Gαq antibody was diluted at 1:300 in 1% bio-grade milk. β-actin and secondary antibodies from ThermoFisher Scientific were diluted at 1:2500 in 1% bio-grade milk.
2.6. Flow cytometry analysis
A sensitive and reliable flow cytometry-based platelet aggregation assay was further modified and used to determine the PLP activity (20). Either the platelet-rich plasma (PRP) or PLP was incubated with the FITC-conjugated anti-CD41a antibody or APC-conjugated anti-CD42b antibody for 10 min at a dilution ratio of 1:10. The stained samples were mixed gently and arachidonic acid (AA) was added to a final concentration of 3µM to produce TXA2. After 10 minutes, the sample was injected into the flow cytometry machine for analysis of the TXA2-induced platelet aggregation through SC-TP-Gαq signaling, and anti-platelet aggregation through SC-TP-Gαs signaling.
3. Results
3.1. Bio-engineering of SC-TP-Gαq and SC-TP-Gαs for Controlling of Platelet Functions
TXA2, produced by activated platelets is a potent endogenous factor involved in platelet aggregation and vasoconstriction. TXA2 binds to the extracellular domain of its GPCR, TP, which is noncovalently coupled with intracellular Gαq. This signaling pathway is directly involved in blood clotting, thrombosis, and stroke. In physiological conditions, the effect of TXA2 is countered by PGI2, which binds to another GPCR, IP, and couples to intracellular Gαs to trigger cAMP signaling. This results in anti-platelet aggregation and vasodilation. The two ligands (TXA2 and PGI2) and two receptors (TP and IP) are directly involved in regulating platelet and vascular functions. Recently, we developed a method using a single ligand, TXA2, to control both Ca++ and cAMP signaling through newly engineered SC-TP-Gαq and SC-TP-Gαs, expressed in HEK293 cells (23). However, the previous study using cancer cell lines expressing SC-TP-Gαq and SC-TP-Gαs don’t have any therapeutic potential. In this study, we further describe the details of the SC-TP-Gα design, and use MK-PLP cells with therapeutic potential to carry either the overexpressed SC-TP-Gαq or SC-TP-Gαs to regulate platelet functions (
Figure 1).
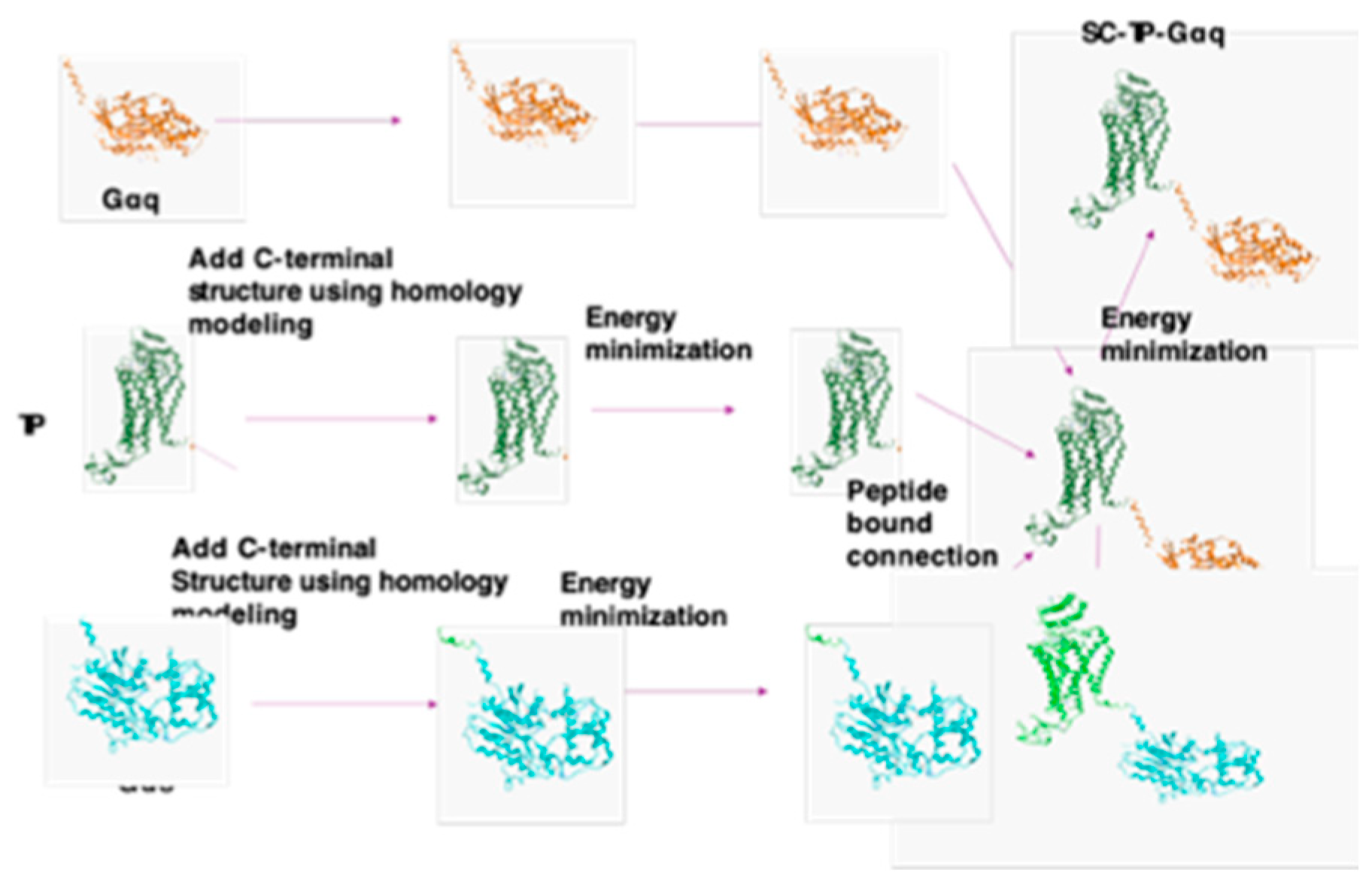
3.2. Structural designs and modeling of the bio-engineered SC-TP-Gαq and SC-TP-Gαs
3D structures of human TP, Gαq and Gαs are available, however, both lack complete terminal structures (24-26). In order to understand how the expressed SC-TP-Gαq and SC-TP-Gαs perform dual functions (binding to a ligand and G-protein mediating signaling) on a single polypeptide chain, complete 3D structural models are needed. Thus, we started from construction of the terminal structure of TP and Gαq/s first. The C-terminal, 10-residues of TP, and N-terminal, 12-residues of Gαq or Gαs which lack 3D-structural coordinates, were created by homology modeling (
Figure 1). After energy minimization, the C-terminus of TP was linked to the N-terminus of Gαq or Gαs. Energy minimization for the entire linked single-chain structure, and the final structures of SC-TP-Gαq and SC-TP-Gαs were obtained. The detailed steps are summarized in
Figure 1.
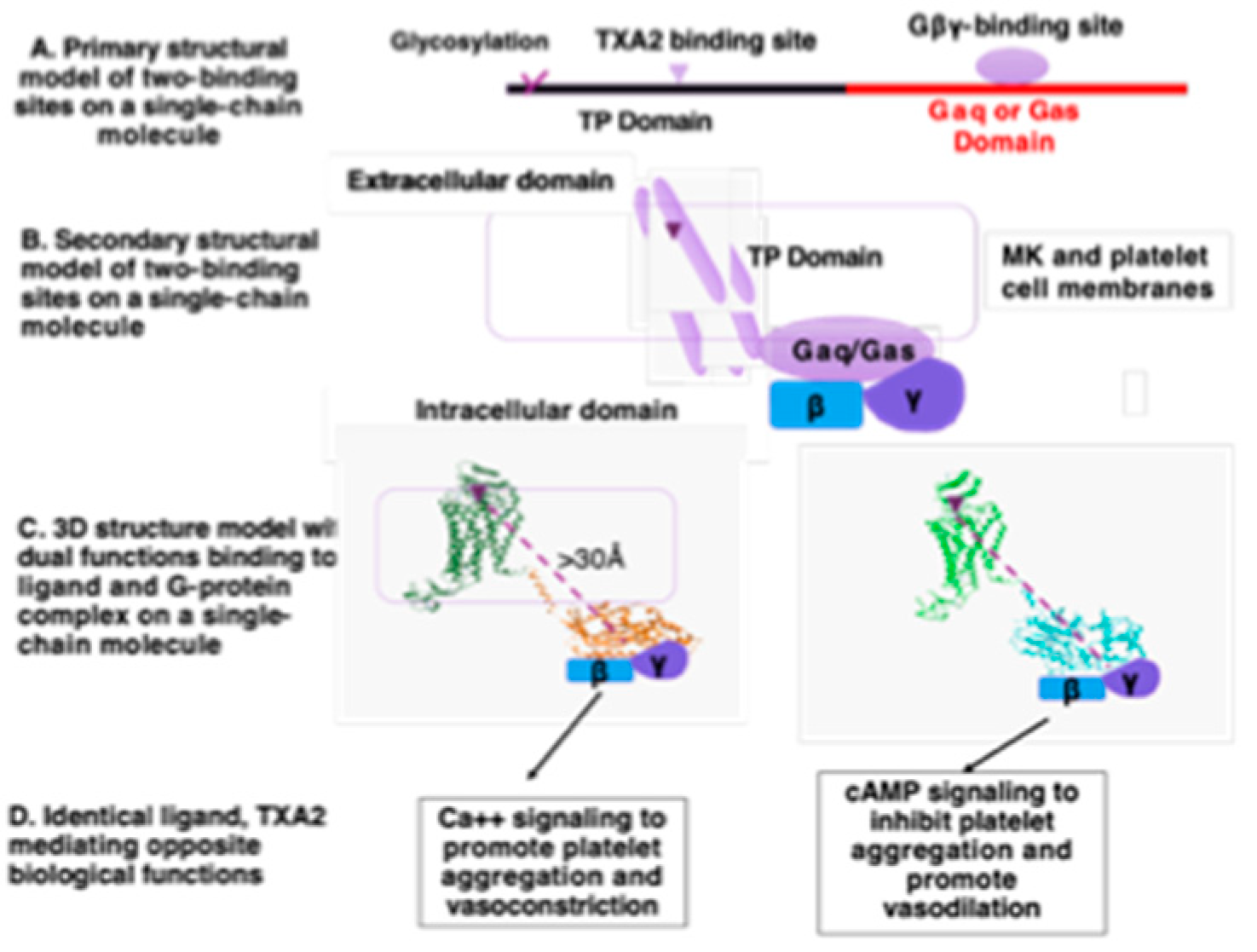
3.3. Structural and functional relationship of the two domains of TP and Gα within a single polypeptide-chain of SC-TP-Gαq and SC-TP-Gαs in respect to the cell membrane
The primary structures of the designed single-chain SC-TP-Gαq and SC-TP-Gαs contain two-functional sites: ligand and G-protein binding within a single polypeptide chain (
Figure 2A). MK cells must be correctly formed to have dual function, including proper mRNA translation, single-chain protein expression, and post-translational modification. After membrane incorporation, SC-TP-Gα binds to TXA2 on extracellular/membrane domains and Gby on intracellular domains (
Figure 2B). The membrane separating the two binding sites to avoid steric hindrance between the ligand and Gby binding sites on MK and platelet cell membranes is illustrated in
Figure 2B. The distance between the ligand and Gby binding sites is greater than 30Å, which avoids steric hindrance, assuming proper SC-TP-Gα protein folding and membrane incorporation (
Figure 2C). In this setting, TXA2 mediating Ca++ signaling (via SC-TP-Gαq) and cAMP signaling (via TP-GSC-TP-Gαs) become possible.
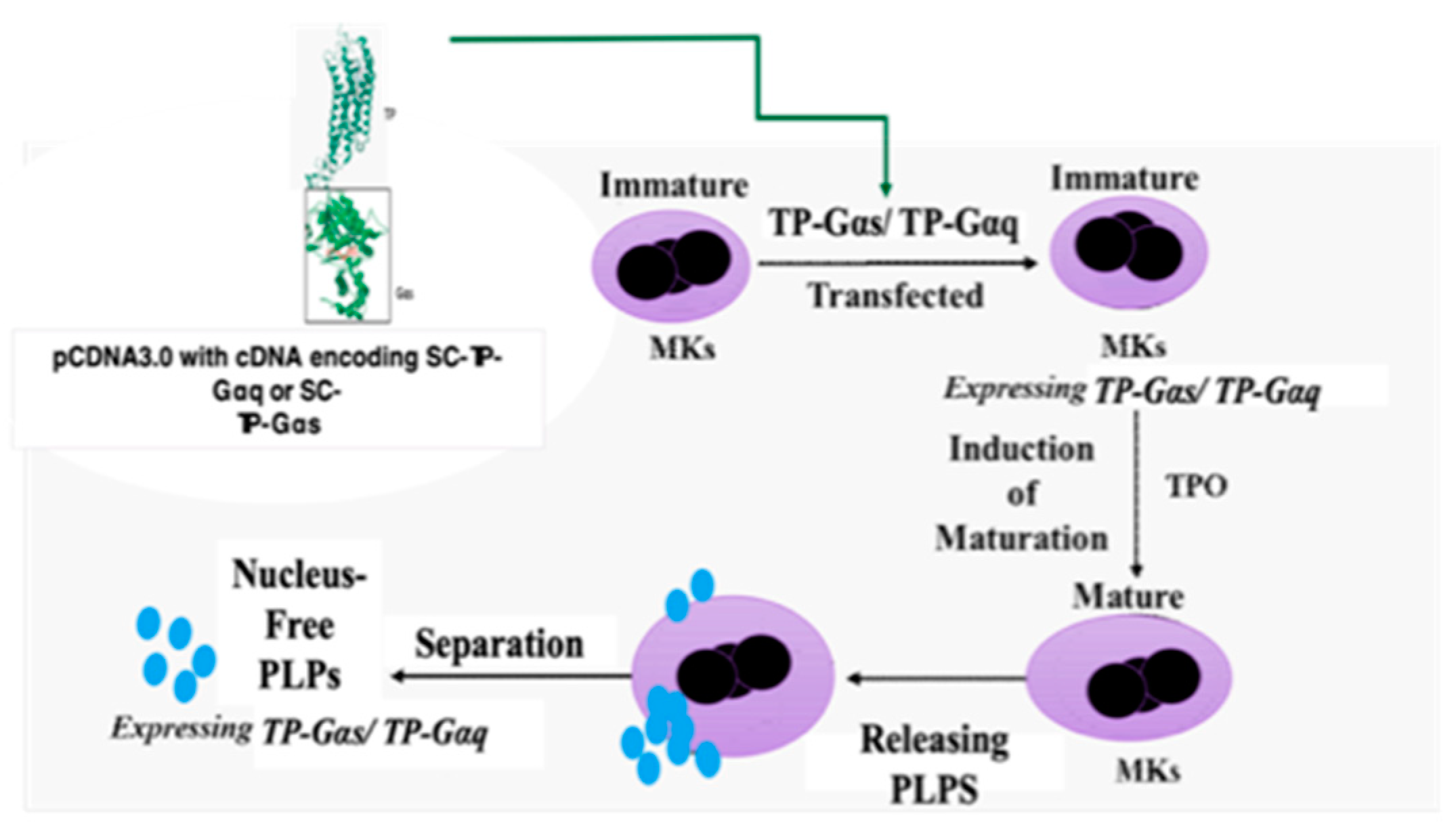
3.4. Design of nucleus-free PLPs to deliver recombinant SC-TP-Gαq or SC-TP-Gαs to regulate platelet functions
For non-self-derived cell based recombinant protein and gene therapy, one of the major issues is contamination of the donor cell-nucleus’ genetic materials. To solve this, we used the nucleus-free cellular delivery system of PLPs. The procedures to produce the clinically applicable PLPs from human pluripotent cells and MK cells have been previously reported (24). However, the use of PLPs as a carrier for recombinant GPCR and G-protein complex has not been developed. The procedures involving transfection of cDNA plasmids of SC-TP-Gαq and SC-TP-Gαs to the immature MKs and production of PLPs expressing the recombinant SC-TP-Gαq and SC-TP-Gαs is described in the
Figure 3.
We expect that bio-engineered SC-TP-Gαq and SC-TP-Gαs with dual functions could be expressed on MK cells first, and then passed to PLPs. In this case, PLPs with membrane bound SC-TP-Gαq should bind to the endogenous TXA2 and Gby within the same molecule and further promote Gαq-Ca++ signaling as an anti-bleeding agent (
Figure 2, left). In contrast, PLPs with SC-TP-Gαs on the surface should bind to the same TXA2 and Gαs, but redirect the PLPs to Gαs-cAMP signaling as an anti-thrombotic agent (
Figure 2D, right). This design is the first to explore the method using identical endogenous ligands to regulate two opposite signaling mechanisms on platelets.
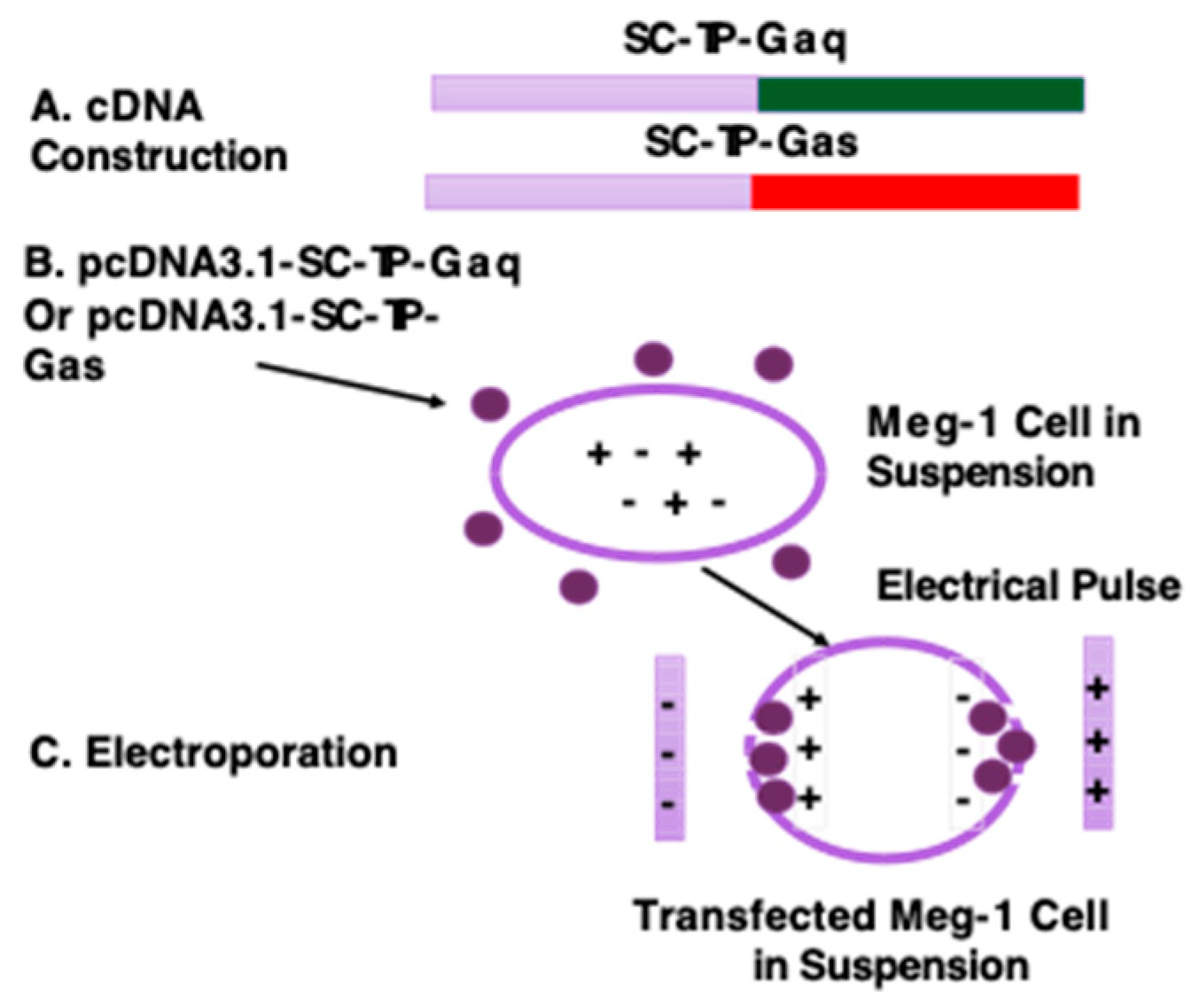
3.5. Construction of cDNA vectors and transfection of MK cells using electroporation
cDNA encoding the human SC-TP-Gαq or SC-TP-Gαs (
Figure 4A) was created and further sub-cloned into human cell expression-vector, pcDNA3.1 using the Ecor1 and Xho1 sites built in the vector. The constructed vector is suitable for transfection of human MKs in suspension. Over-expression of SC-TP-Gα on MK cells is the initial step to generate platelets carrying the expressed SC-TP-Gαq and SC-TP-Gαs to control platelet function. Here, we chose a MK cell line, Meg-01, as a model to generate PLPs (11) (
Figure 4B). The Meg-01 cell line was derived from a patient with chronic myelogenous leukemia, which has similar properties to human MKs. The well-characterized Meg-01 cell line obtained from ACCT was cultured in a suspension medium with the pcDNA3.1 vector (
Figure 4B). Transfection of Meg-1 cells using each cDNA vector were performed by a sandwich electroporation system with modification suitable for Meg-1 cells (27) (
Figure 4C). The best ratios of cDNA concentrations and electric pulse doses were established by a titration study (data not shown).
3.6. Transient Gene Expression of SC-TP-Gαq and SC-TP-Gαs on MK Cell Lines
We used three steps to generate PLPs carrying SC-TP-Gαq and SC-TP-Gαs from immature MKs. A). Transient expression; B) generation of a stable expression cell-line; and C) induction of immature MKs to release PLPs. The transient gene expression, which is the first step toward successful production of the engineered PLPs, was characterized by microscopy and Western blot analysis. The effects of the heterogeneity of MK cells were not significantly changed by comparison before and after the cDNA transfection (
Figure 5A). When comparing Meg-01 over- expression of SC-TP-Gαq or SC-TP-Gαs in suspension, both the immature and unaggregated properties were similar including the different signaling molecules introduced into the cells (
Figure 5B). Transient expression of SC-TP-Gαq and SC-TP-Gαs on immature Meg-01 cells was confirmed by Western blot analysis (
Figure 5C). These results indicated that expression of SC-TP-Gαq and SC-TP-Gαs in Meg-1 cells has been successfully obtained. The transfected immature Meg-1 cells are suitable to generate cell lines with stable expression of SC-TP- Gαq or SC-TP-Gαs.
3.7. Screening of the immature Meg-01 cells stably expressing SC-TP-Gαq to generate PLPs carrying SC-TP-Gαq
The immature Meg-1 cells transfected with the cDNA of SC-TP-Gαq shown in
Figure 5 were used for screening of the cell line stably expressing SC-TP-Gαq. First, the transfected immatureeg-01 cells with transient expression of SC-TP-Gαq (
Figure 5) were treated with the culture medium containing G-418 as a selecting reagent (
Figure 6A). After three weeks, the untransfected Meg-01 cells were killed by the G-418, and the cell line stably expressing SC-Gαq (MK-SC-TP-Gαq cell line) remained in the suspension. A typical stable cell is shown in figure 6A. Next, the immature MK-SC-TP-Gαq cell line was induced to maturation by addition of megakaryocyte growth and development factors (MGDFs) and TPO (11) (
Figure 6B). The images collected from the steps to generate PLPs carrying SC-TP-Gαq are summarized in
Figure 6A,B. The typical mature-Meg-1 cells, which releases the PLPs carrying the expressed SC-TP-Gαq (PLP-SC-TP-Gαq) are shown in
Figure 6B. The immature stable Meg-1 line and mature PLPs expressing the recombinant SC-TP-Gαq were confirmed by Western blot analysis (
Figure 6C).
3.8. Screening of Meg-01 cells stably expressing SC-TP-Gαs to generate PLPs carrying SC-TP-Gαs
The immature Meg-1 cells transfected with the cDNA of SC-TP-Gαs shown in
Figure 5 were used for screening of the cell line stably expressing SC-TP-Gαs. First, the transfected Meg-01 cells expressing SC-TP-Gαs (
Figure 5) were treated with the culture medium containing G-418 for three weeks. The untransfected Meg-01 cells were killed by the G-418, and the cell line stably expressing the SC-TP-Gαs (MK-SC-TP-Gαs) was obtained. The immature MK-SC-TP-Gαs cell line was further induced to maturation with the release of PLPs by the addition of MGDFs and TPO. The typical mature-Meg-1, which releases PLPs carrying the expressed SC-TP-Gαs (PLP-SC-TP-Gαq), is shown in
Figure 7a. PLP-SC-TP-Gαs expressing the recombinant SC-TP-Gαs was confirmed by Western analysis (
Figure 7b).
3.9. Establishing highly sensitive flow cytometry using double stained human platelets for identification of the biological activities of PLP-SC-TP-Gαq and PLP-SC-TP-Gαs
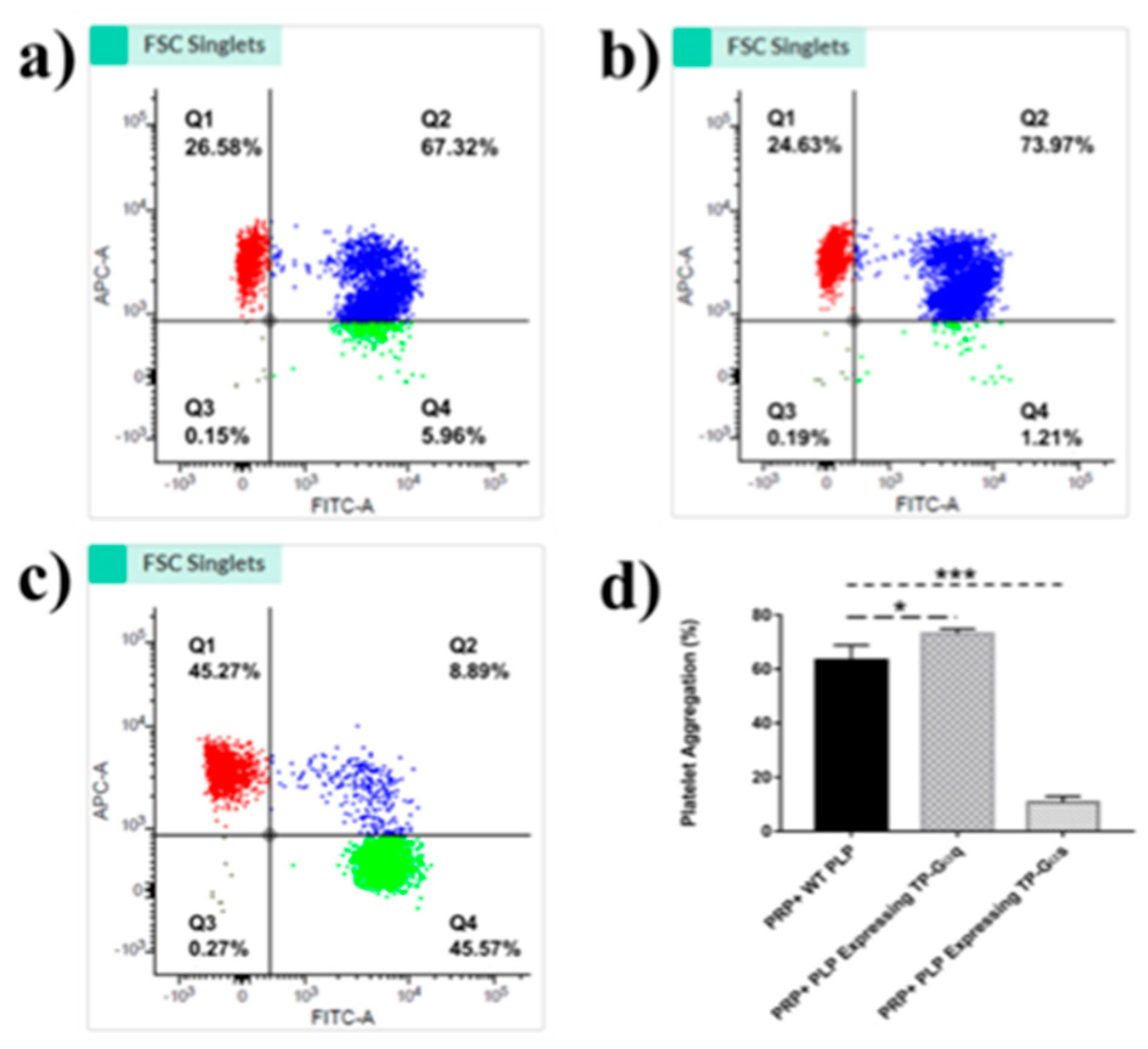
Flow cytometry was used to determine the biological activities of PLP-SC-TP-Gαq and PLP-SC-TP-Gαs by monitoring their effects on human platelet aggregation. In this assay, two antibodies, anti-platelets markers CD41a and CD42b were labeled with FITC and APC to generate FITC-CD41a antibody and APC-CD 42b antibody, respectively. Human platelet rich plasma (PRP) was prepared from human platelets obtained from a local blood bank. Half of the PRP sample was stained with the FITC-CD41a antibody, and the other half with the APC-CD42b antibody. The samples were mixed, and 3µM of arachidonic acid was added to induce aggregation. The sample was analyzed by flow cytometry. The signals double-stained by both CD41a and CD 42b were used to determine platelet aggregation (
Figure 8). The positive and negative results for each staining were analyzed using three groups of samples. In the first group, platelets without any staining treatments (FITC-, APC-), were used as the negative control. The area with cells present was defined as the Q3 Quadrant (
Figure 8a). The second group was comprised of a mixture of FITC-CD41a stained and negative platelets. After analysis by flow cytometry, two populations were present (
Figure 6b. Left: FITC-; Right: FITC+). The area containing FITC positive cells was defined as the Q4 Quadrant (
Figure 8b). The third group was comprised of APC-conjugated CD 42b stained platelets mixed with negative platelets. Following flow cytometry analysis, two populations were present: the upper population of APC-positive platelets in the area defined as Q1 Quadrant (
Figure 8c) and the Q2 Quadrant which described the double-staining population, indicating the aggregation of platelets.
3.10. Determination of the effects of the nucleus-free PLP-SC-TP-Gαq and PLP-SC-TP-Gαs on regulating platelet aggregation using the double-stained flow cytometry
Based on the signaling cascades mediated by SC-TP-Gαq and SC-TP-Gαs, we hypothesized that the PLPs expressing SC-TP-Gαq could further promote platelet aggregation, while the PLPs expressing SC-TP-Gαs could inhibit platelet aggregation. To test our hypothesis, three groups of platelet mixtures were analyzed by flow cytometry to determine the effects of SC-TP-Gαq and SC-TP-Gαs on platelet aggregation activities. The first group contained a mixture of 200µL human PRP and 200µL nucleus-free PLPs released by WT Meg-01 cells. After inducing aggregation by AA, roughly 67% percent aggregation was observed in the Q2 double-staining Quadrant (
Figure 9a). The second group was comprised of 200µL PRP mixed with 200µL nucleus-free PLP-SC-TP-Gαq. The aggregative activity was induced by the addition of AA, and an increased aggregation percentage (~74%) was identified (
Figure 9b). This is explained by the ability of PLP SC-TP-Gαq to mediate calcium signaling, which could further increase the percentage of platelet aggregation. The last group was comprised of a mixture of 200µL PRP with 200µL nucleus-free PLP-SC-TP-Gαs. The aggregative reaction was also triggered by the addition of AA, which dramatically decreased the aggregation percentage to 9% (
Figure 9c). This resulted from cAMP signaling mediated by SC-TP-Gαs which inhibited platelet aggregation. Three trials were conducted in each group. Statistical analysis of the results led to the conclusion that the PLP-SC-TP-Gαq protein complex has biological activity to promote platelet aggregation, which might be beneficial in anti-bleeding. In contrast, PLP-SC-TP-Gαs has activity to dramatically prevent platelet aggregation, which may be beneficial in treating thrombotic diseases (
Figure 9d).
3.11. Working model of PLP-SC-TP-Gαq and PLP-SC-TP-Gαs regulation of human platelet aggregation
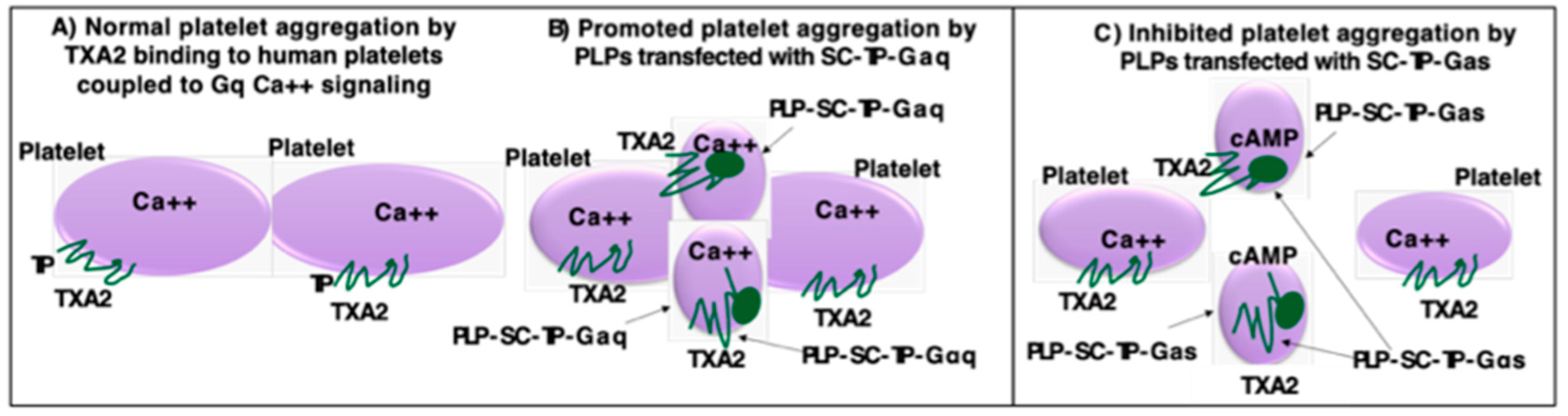
A working model is described in
Figure 10. In the presence of PLP-SC-TP-Gαq, TXA2 produced from AA as a substrate catalyzed by COX-1 coupled to TXAS in human platelets binds to TP (
Figure 10A) and SC-TP-Gαq on PLPs (
Figure 10B). This triggers Ca++ signaling on both cells leading to overlapping platelet aggregation (
Figure 10B). In contrast, only part of TXA2 binds to TP on human platelets, triggering platelet aggregation. Another part of TXA2 binds to SC-TP-Gαs on PLPs triggering cAMP mediated anti-platelet aggregation, effectively inhibiting human platelet aggregation (
Figure 10C). In other words, SC-TP-Gαs expressed on PLPs inhibited platelet aggregation through dual effects: one is to decrease the amount of TXA2 binding to human platelets, and the other is to trigger cAMP signaling to prevent human platelet aggregation.
4. Discussion
While the therapeutic potentials of nucleus-free PLPs have been previously reported, it is limited to the applications of mimicking platelet functions. Here, we broaden the application by testing PLPs as a recombinant protein-drug delivery system. Specifically, we focused on establishing the unique therapeutic potentials as a nucleus-free cell-recombinant membrane protein therapeutic agent, and a novel cell-based GPCR-G-protein signaling therapy. In this study, we discovered advantages to the PLP-based recombinant membrane-protein delivery system. First, PLPs do not have nuclei. This prevents potential contamination of nucleic acid and genetic DNAs. PLPs contain less proteins and enzymes compared to other therapeutic cells, which limits unwanted side effects. Because of the small size of PLPs, they are suitable for many applications, including IM, IP and IV infusion. In contrast to other cell-based delivery systems, such as intramuscular injection of stem cells, IV infusion of PLPs do not cause possible acute-inflammatory reactions. In other cell-based delivery systems, the elimination of aggregative cells creates an issue for IV infusion. However, PLPs (suspension cells) are fully suitable for IV infusion. Sources for these clinical PLPs are no longer limited. Recently, a research group established a megakaryocyte cell line which can produce clinically-applicable PLPs (11,12).
As a class of recombinant protein-drug delivery carriers, PLPs are necessary to express the desired recombinant proteins within the cells. However, PLPs themselves do not have an existing system to make recombinant proteins. In this study, transfection of PLP precursor cells, MKs were designed to solve this issue. Immature MKs were transfected using cDNA plasmids to express the desired recombinant proteins. After maturation of MKs, the expressed recombinant proteins within the cells were passed to the released PLPs. We successfully utilized the Meg-01-PLP system as a model in the study. After the establishment of stable immature Meg-01 cell lines expressing the desired recombinant proteins, MKs were induced to maturation to release PLPs with the expressed recombinant protein. Therefore, we demonstrated that the nucleus-free PLPs carrying the recombinant protein have potential to become a novel tool to deliver recombinant proteins in therapeutics.
Currently, membrane proteins have limited potential in therapeutics due to their insolubility and aggregation potential. However, membrane proteins, such as GPCRs are directly involved in conducting cellular activities. We hypothesized that the PLP-recombinant protein approach is suitable to carry many recombinant proteins, including membrane-bound proteins. Here, we used SC-TP-Gαq and SC-TP-Gαs with seven transmembrane domain proteins as a model to test the PLP system’s ability to transport membrane proteins. The study confirmed that the PLP-recombinant membrane protein system has potential to regulate cellular functions. For example, PLPs expressing SC-TP-Gαs could serve as a “cleaner” to continually absorb the excessive TXA2 in the circulation with anti-thrombotic effects. PLPs carrying SC-TP-Gαq could be used to promote platelet aggregation as an anti-bleeding agent in diverse bleeding situations. By applying PLP- SC-TP-Gαq to the bleeding site, TXA2, released by the bleeding tissue, could trigger downstream Gαq-calcium signaling. This mimics the natural signaling cascades mediated by TP promoting platelet aggregation and vasoconstriction. Conversely, under the same TXA2 stimulation, PLP-SC-TP-Gαs is able to specifically convert downstream signaling into Gαs-cAMP signaling, which opposes natural signaling activities mediated by the TP receptor. This inhibits platelet aggregation, mediates vasodilation, promotes anti-thrombotic effects, and reduces the risk of strokes and various thrombotic diseases.
In conclusion, these findings show that nucleus-free PLPs derived from the transfected MKs can be used as a delivery vehicle for recombinant membrane proteins by controlling cell signaling. Thus, the PLP-recombinant protein system has potential for development into a novel and advanced therapeutic tool to carry a variety of insoluble membrane proteins. For example, it could be used as a therapeutic agent to counter vascular thrombosis by introducing PLP-SC-TP-Gαs into the vascular system. Furthermore, this research suggests that the PLP system can be manipulated as a regulatory and therapeutic tool to control and even reverse the existing functions of GPCRs, which may become a future method to regulate functions of cellular GPCRs, not including traditional drugs as agonist and antagonist of the GPCRs.
Author Contributions
Renzhong Lu and Yan Li, Anna Xu, Bridgette King performed the experiments, analyzed the data, and prepared the figures. Ke-He Ruan designed the experiments. Renzhong Lu and K.-H.R. wrote the manuscript. Anna Xu, Bridgette King edited the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by NIH Grants (RO1 HL56712 and HL79389 for KHR) and American Heart Association grants (10GRNT4470042 and 14GRNT20380687 for KHR).
Informed Consent Statement
Kehe Ruan: give my consent for the publication of identifiable details, which can include photograph(s) and/or case history and/or details within the text to be published in the above Journal and Article.
Data Availability Statement
All of data published are available for public.
Acknowledgments
This work was supported by NIH Grants (RO1 HL56712 and HL79389 for KHR) and American Heart Association grants (10GRNT4470042 and 14GRNT20380687 for KHR).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Nature Review Drug Discovery. NEWS 2021, 20, 5.
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Chenoweth, A.; Crescioli, S.; Reichert, J.M. Antibodies to Watch in 2022. mAbs 2022, 14, 2014296. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Biopharmaceutical Benchmarks. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J. Refreshing the Biologic Pipeline. Nat. Biotechnol. 2021, 39, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Ruan, C.H.; King, B.; Ruan, K.H. Recent Advances and Near Future of Insulin Production and Therapy. Future Med. Chem. 2019, 11, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.; Kibble, E.; El-Jawhari, J.J. Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration. Bioengineering 2022, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Guan, Q.; Cheng, H.; Zhu, Z.; Jiang, C.; Guo, P.; Tai, Y.; Sun, H.; Wang, M.; Wei, W.; et al. Functions of G Protein-Coupled Receptor 56 in Health and Disease. Acta Physiol. 2022, e13866. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, C.Y. G Protein-Coupled Receptors as Potential Targets for Nonalcoholic Fatty Liver Disease Treatment. World J. Gastroenterol. 2021, 27, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Scully CC, G.; de Graaf, C.; Brown AJ, H.; Maguire, J.J. Advances in Therapeutic Peptides Targeting G Protein-Coupled Receptors. Nat. Rev. Drug Discovery 2020, 19, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Sim, X.; Poncz, M.; Gadue, P.; French, D.L. Understanding Platelet Generation from Megakaryocytes: Implications for In Vitro–Derived Platelets. Blood 2016, 127, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takayama, N.; Hirata, S.; Seo, H.; Endo, H.; Ochi, K.; Fujita, K.I.; Koike, T.; Harimoto, K.I.; Dohda, T.; et al. Expandable Megakaryocyte Cell Lines Enable Clinically Applicable Generation of Platelets from Human Induced Pluripotent Stem Cells. Cell Stem Cell 2014, 14, 535–548. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.Y.; Ling, Q.L.; So, S.P.; Ruan, K.H. A Novel Single-Chain Enzyme Complex with Chain Reaction Properties Rapidly Producing Thromboxane A2 and Exhibiting Powerful Anti-Bleeding Functions. J. Cell. Mol. Med. 2019, 23, 8343–8354. [Google Scholar] [CrossRef]
- Ruan, D.T.; Tang, N.; Akasaka, H.; Lu, R.; Ruan, K.H. Engineering ‘Enzymelink’ for Screening Lead Compounds to Inhibit mPGES-1 While Maintaining Prostacyclin Synthase Activity. Future Med. Chem. 2021, 13, 1091–1103. [Google Scholar] [CrossRef]
- Ling, Q.L.; Mohite, A.J.; Murdoch, E.; Akasaka, H.; Li, Q.Y.; So, S.P.; Ruan, K.H. Creating a Mouse Model Resistant to Induced Ischemic Stroke and Cardiovascular Damage. Sci. Rep. 2018, 8, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.H.; Mohite, A.J.; So, S.P. Resistant to Thrombosis, Induced Stroke and Heart Arrest by Incorporation of a Single Gene of PGI2-Synthesizing COX-1-PGIS In Vivo: Implication against Human Heart Disease. Int. J. Cardiol. 2013, 168, 2960–2961. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xi, Y.; Terry, T.; So, S.P.; Mohite, A.; Zhang, J.; Wu, G.; Liu, X.; Cheng, J.; Ruan, K.H.; et al. Engineered Endothelial Progenitor Cells That Overexpress Prostacyclin Protect Vascular Cells. J. Cell. Physiol. 2012, 227, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.H.; Cervantes, V.; So, S.P. Engineering of a Novel Hybrid Enzyme: An Anti-Inflammatory Drug Target with Triple Catalytic Activities Directly Converting Arachidonic Acid into the Inflammatory Prostaglandin E2. Protein Eng. Des. Sel. 2009, 22, 733–740. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Procedure for the structural design of SC-TP-Gα expression in MK-PLPs. A). Modeling of unavailable 3D-structures of the C-terminal region of TP (PDB ID: 6IIU) and N-terminal region of Gαq (PDB ID: 4GNK) and Gαs (PDB ID:) by adding the terminal structures to the TP and Gαq/s; B) Covalently linked TP C-terminus to Gαq or s N-terminus to form SC-TP-Gαq and SC-TP-Gαs. Energy minimization was applied to each step to obtain final structures. The 3D structures used for the models are crystal structures of human TP (PDB ID: 6IIU (21), Gαq (PDB ID: 4GNK (22) and Gαs (GDP-bound Gs heterotrimer (23). The PLPs carrying SC-TP-Gαq (left) and SC-TP-Gαs (right) were schematically presented.
Figure 1.
Procedure for the structural design of SC-TP-Gα expression in MK-PLPs. A). Modeling of unavailable 3D-structures of the C-terminal region of TP (PDB ID: 6IIU) and N-terminal region of Gαq (PDB ID: 4GNK) and Gαs (PDB ID:) by adding the terminal structures to the TP and Gαq/s; B) Covalently linked TP C-terminus to Gαq or s N-terminus to form SC-TP-Gαq and SC-TP-Gαs. Energy minimization was applied to each step to obtain final structures. The 3D structures used for the models are crystal structures of human TP (PDB ID: 6IIU (21), Gαq (PDB ID: 4GNK (22) and Gαs (GDP-bound Gs heterotrimer (23). The PLPs carrying SC-TP-Gαq (left) and SC-TP-Gαs (right) were schematically presented.
Figure 2.
A Illustration of the designs and models of SC-TP-Ga with dual function within a single peptide-chain. A). Model illustrating the primary structure of SC-TP-Gα; B). Model of the secondary structure of SC-TP-Gα; C). Distance of the two binding sites on the SC-TP-Gα; D). Schematic presentation of the production of nucleus-free PLPs expressing ST-TP-Gαq or SC-TP-Gαs with opposite biological function to regulate platelet functions.
Figure 2.
A Illustration of the designs and models of SC-TP-Ga with dual function within a single peptide-chain. A). Model illustrating the primary structure of SC-TP-Gα; B). Model of the secondary structure of SC-TP-Gα; C). Distance of the two binding sites on the SC-TP-Gα; D). Schematic presentation of the production of nucleus-free PLPs expressing ST-TP-Gαq or SC-TP-Gαs with opposite biological function to regulate platelet functions.
Figure 3.
Design of nucleus-free PLPs generated from MK cells to deliver recombinant SC-TP-Gα proteins with therapeutic potentials.
Figure 3.
Design of nucleus-free PLPs generated from MK cells to deliver recombinant SC-TP-Gα proteins with therapeutic potentials.
Figure 4.
Procedure for transfection of the Meg-1 cell in suspension using electroporation. (A). cDNAs of SC-TP-Gαq or SC-TP-Gαs were constructed using a PCR approach. (B). pcDNA3.1 vector with cDNA of SC-TP-Gαq or SC-TP-Gαs was prepared. (C). pcDNA3.1 vector was transfected into the Meg-1 cell in suspension by electric pulse.
Figure 4.
Procedure for transfection of the Meg-1 cell in suspension using electroporation. (A). cDNAs of SC-TP-Gαq or SC-TP-Gαs were constructed using a PCR approach. (B). pcDNA3.1 vector with cDNA of SC-TP-Gαq or SC-TP-Gαs was prepared. (C). pcDNA3.1 vector was transfected into the Meg-1 cell in suspension by electric pulse.
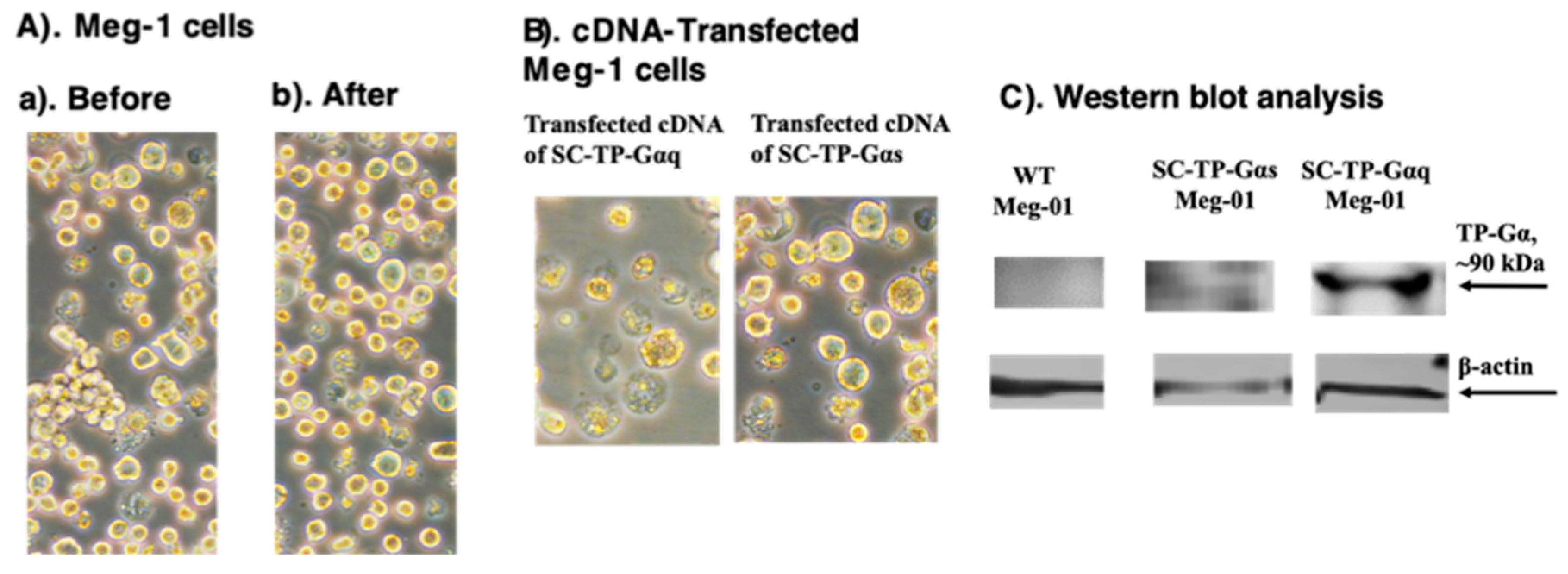
Figure 5.
A). Suspension culture of Meg-1 cells before and after cDNA-transfection. B). Suspension culture of Meg-01 Cells transfected with SC-TP-Gαq (left) and SC-TP-Gαs (right). C). Western blot analysis. The expressed SC-TP-Gαs (lane 2), SC-TP-Gαq (lane 3) on Meg-01 cells are shown.
Figure 5.
A). Suspension culture of Meg-1 cells before and after cDNA-transfection. B). Suspension culture of Meg-01 Cells transfected with SC-TP-Gαq (left) and SC-TP-Gαs (right). C). Western blot analysis. The expressed SC-TP-Gαs (lane 2), SC-TP-Gαq (lane 3) on Meg-01 cells are shown.
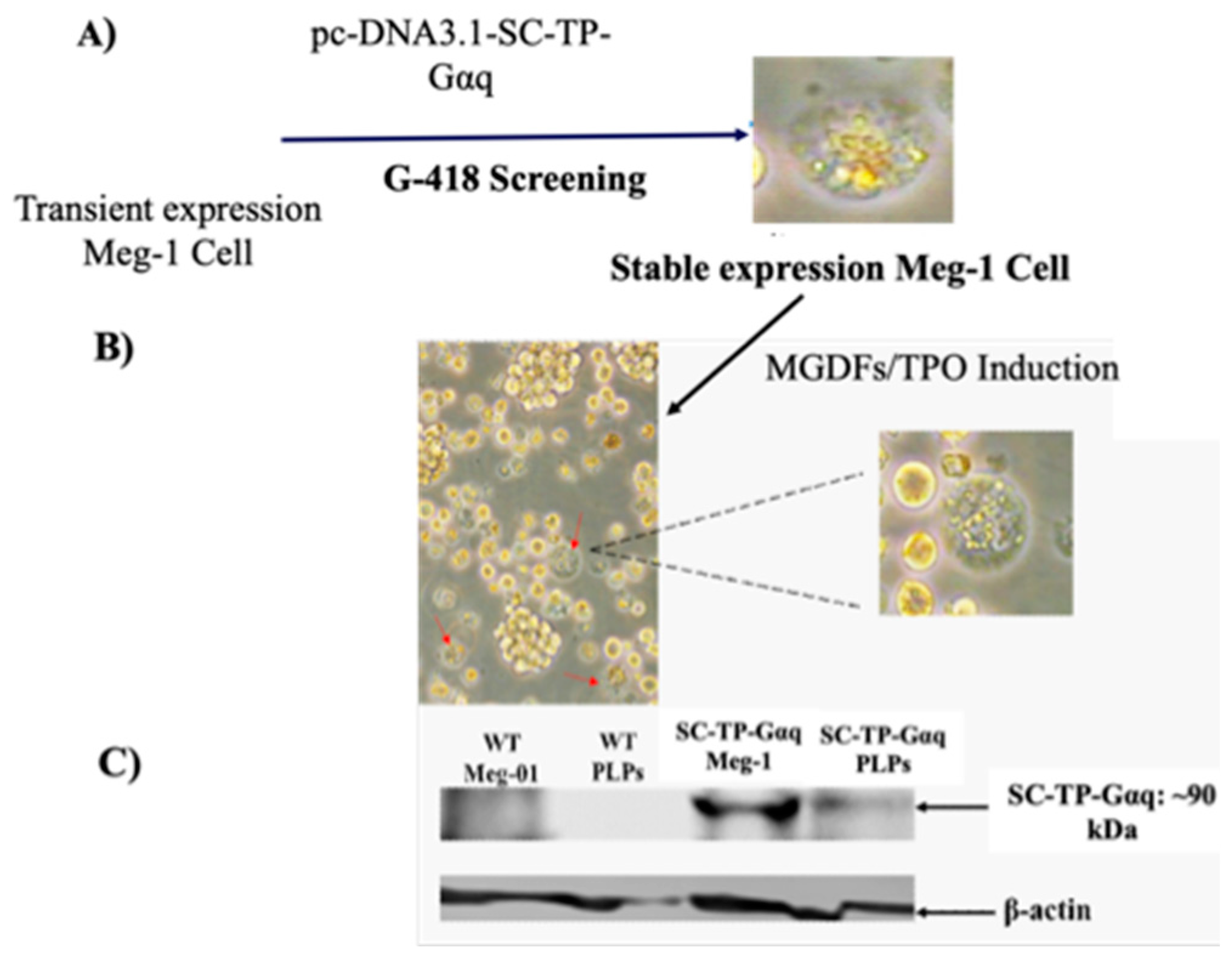
Figure 6.
Production of PLPs expressing SC-TP-Gαq from immature Meg-1 cells. (A). Stable cell line screening; (B). PLP production; and (C) Western blot analysis using 10% SDS-PAGE and anti-human TP antibody.
Figure 6.
Production of PLPs expressing SC-TP-Gαq from immature Meg-1 cells. (A). Stable cell line screening; (B). PLP production; and (C) Western blot analysis using 10% SDS-PAGE and anti-human TP antibody.
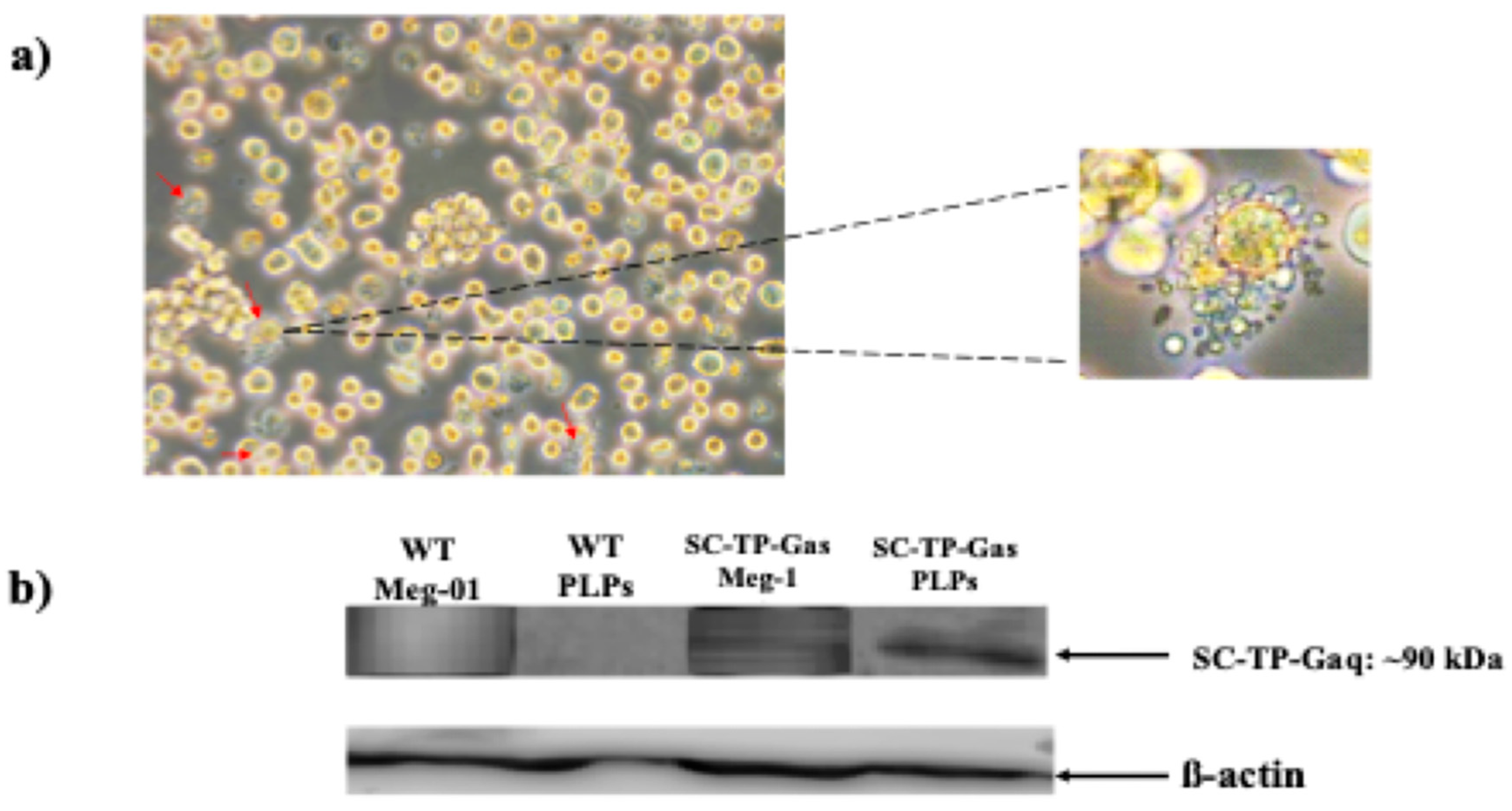
Figure 7.
Induction of maturation of the Meg-01 cells expressing SC-TP-Gαs. (a) The pro-platelet structure was also identified under microscope. (b) The expression of SC-TP-Gαq in PLPs was also confirmed by western blot.
Figure 7.
Induction of maturation of the Meg-01 cells expressing SC-TP-Gαs. (a) The pro-platelet structure was also identified under microscope. (b) The expression of SC-TP-Gαq in PLPs was also confirmed by western blot.
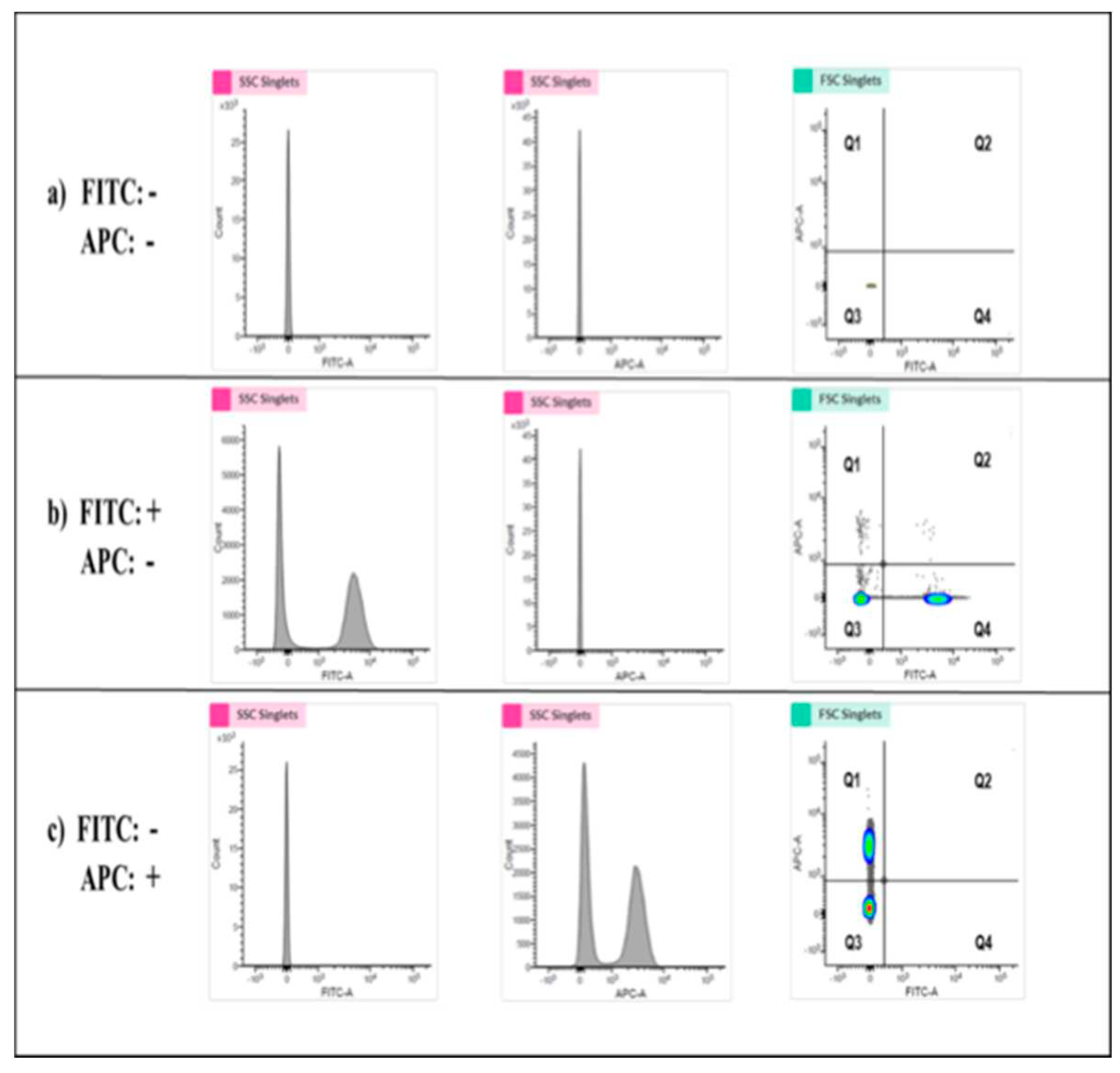
Figure 8.
Determination of the specific quadrant to indicate platelet aggregation activities. (a) The analysis of platelets without staining was shown. The Q3 Quadrant represents the negative platelets without fluorescence. (b) The platelets stained with the FITC marker was mixed with the negative platelets. The shown left population represents the FITC negative platelets, and the right population (Q4 Quadrant) represents the FITC positive but APC negative platelets. (c) The mixture of APC marker-stained platelets and negative platelets was analyzed. The bottom population represents the ACP negative platelets, and the upper one (Q3) represents the APC positive while FITC negative platelets. The Q4 Quadrant, which represents the double staining by both FITC and APC, indicates platelet aggregation activity.
Figure 8.
Determination of the specific quadrant to indicate platelet aggregation activities. (a) The analysis of platelets without staining was shown. The Q3 Quadrant represents the negative platelets without fluorescence. (b) The platelets stained with the FITC marker was mixed with the negative platelets. The shown left population represents the FITC negative platelets, and the right population (Q4 Quadrant) represents the FITC positive but APC negative platelets. (c) The mixture of APC marker-stained platelets and negative platelets was analyzed. The bottom population represents the ACP negative platelets, and the upper one (Q3) represents the APC positive while FITC negative platelets. The Q4 Quadrant, which represents the double staining by both FITC and APC, indicates platelet aggregation activity.
Figure 9.
Identification of the PLPs expressing the protein complexes in platelet aggregation assays. (a) 200µL PRP was mixed well with 200µL WT PLPs. The mixed sample was divided equally into two groups: one was stained with the FITC-conjugated CD41a antibody and the other was stained with the APC-conjugated CD 42b antibody. After staining, the two groups were mixed again thoroughly and AA was added to induce aggregation (3µM). Then the sample was analyzed by flow cytometry. About 67% of aggregation was recognized. (b) 200µL PRP was mixed completely with 200µL PLPs expressing SC-TP-Gαq. Then the above procedures were repeated. The flow cytometry analysis indicated about 74% of aggregation. (c) The mixture of 200µL PRP and 200µL PLPs expressing SC-TP-Gαs was used to repeat the above steps. Around 9% of aggregation was demonstrated in this group.
Figure 9.
Identification of the PLPs expressing the protein complexes in platelet aggregation assays. (a) 200µL PRP was mixed well with 200µL WT PLPs. The mixed sample was divided equally into two groups: one was stained with the FITC-conjugated CD41a antibody and the other was stained with the APC-conjugated CD 42b antibody. After staining, the two groups were mixed again thoroughly and AA was added to induce aggregation (3µM). Then the sample was analyzed by flow cytometry. About 67% of aggregation was recognized. (b) 200µL PRP was mixed completely with 200µL PLPs expressing SC-TP-Gαq. Then the above procedures were repeated. The flow cytometry analysis indicated about 74% of aggregation. (c) The mixture of 200µL PRP and 200µL PLPs expressing SC-TP-Gαs was used to repeat the above steps. Around 9% of aggregation was demonstrated in this group.
Figure 10.
Models of the effects of the PLP-SC-TP-Gαq and PLP-SC-TP-Gαs on platelet aggregation. (A). In the absence of PLPs: TXA2 binds to TP on the platelet surface, which couples to Gq mediating Ca++ signaling and platelet aggregation; (B). In the presence of PLPs expressing SC-TP-Gαq, TXA2 binds to TP on platelets and SC-TP-Gαq on PLPs, which enhances Ca++ signaling and overlaps platelet and PLP aggregation; and (C). In the presence of PLPs expressing SC-TP-Gαs, TXA2 binds to TP on platelets and SC-TP-Gαs on PLPs which increases cAMP signaling to inhibit platelet and PLP aggregation.
Figure 10.
Models of the effects of the PLP-SC-TP-Gαq and PLP-SC-TP-Gαs on platelet aggregation. (A). In the absence of PLPs: TXA2 binds to TP on the platelet surface, which couples to Gq mediating Ca++ signaling and platelet aggregation; (B). In the presence of PLPs expressing SC-TP-Gαq, TXA2 binds to TP on platelets and SC-TP-Gαq on PLPs, which enhances Ca++ signaling and overlaps platelet and PLP aggregation; and (C). In the presence of PLPs expressing SC-TP-Gαs, TXA2 binds to TP on platelets and SC-TP-Gαs on PLPs which increases cAMP signaling to inhibit platelet and PLP aggregation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).