Submitted:
03 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
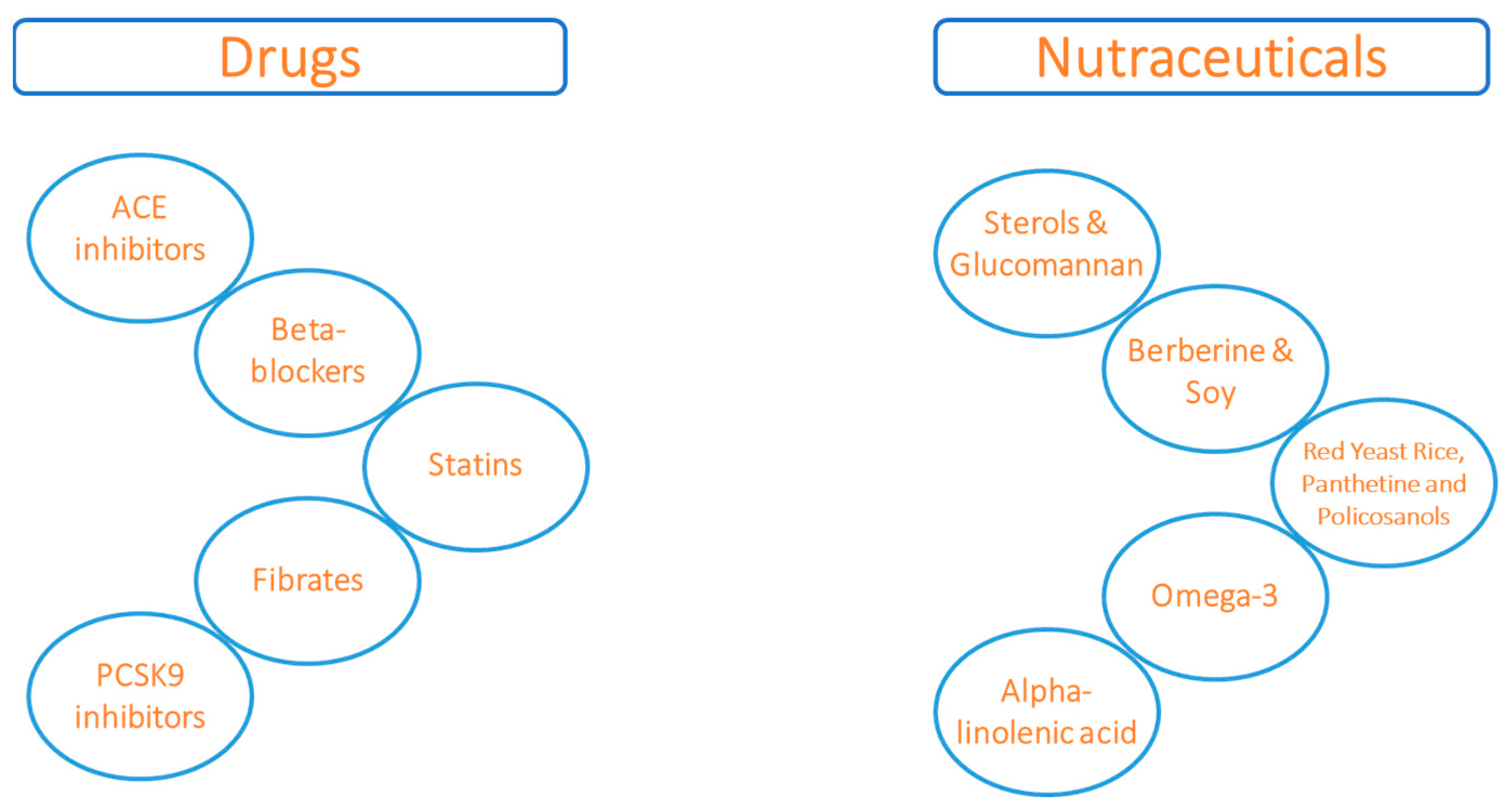
1. Introduction
Functional Effects of ALA
Risk Factors for Cardiovascular Disease
Cardioprotective Effect
2. Discussion
3. Conclusions
4. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; et al. Heart disease and stroke statistics – 2023 update: a report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Shi, Y.; Grimsgaard, S.; Alraek, T.; Fønnebø, V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin. Med. 2006, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: an overview. Br. J. Pharmacol. 2016, 174, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Hiemstra, H.; Lin, Y.; Vermeer, M.A.; Duchateau, G.S.; Trautwein, E.A. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations – A meta-analysis of randomized controlled studies. Atherosclerosis 2013, 230, 336–346. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Fat type in phytosterol products influence their cholesterol-lowering potential: A systematic review and meta-analysis of RCTs. Prog. Lipid Res. 2016, 64, 16–29. [Google Scholar] [CrossRef]
- Gordon, R.Y.; Cooperman, T.; Obermeyer, W.; Becker, D.J. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med 2010, 170, 1722–1727. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zhao, Z.-X.; Huang, M.; Feng, R.; He, C.-Y.; Ma, C.; Luo, S.-H.; Fu, J.; Wen, B.-Y.; Ren, L.; et al. Effect of Berberine on promoting the excretion of cholesterol in high-fat diet-induced hyperlipidemic hamsters. J. Transl. Med. 2015, 13, 278. [Google Scholar] [CrossRef]
- Cho, S.-J.; Juillerat, M.A.; Lee, C.-H. Cholesterol Lowering Mechanism of Soybean Protein Hydrolysate. J. Agric. Food Chem. 2007, 55, 10599–10604. [Google Scholar] [CrossRef]
- Potter, S.M. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J. Nutr. 1995, 125, 606S–611S. [Google Scholar] [PubMed]
- Cambiagi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The role of Alpha-linolenic acid and its oxylipins in human cardiovascular diseases. Int J Mol Sci 2023, 24, 6110. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef] [PubMed]
- Greupner, T.; Kutzner, L.; Pagenkopf, S.; Kohrs, H.; Hanh, A.; Schebb, N.H.; Schuchardt, J.P. Effects of a low and a high dietary LA/ALA ration on long-chain PUFA concentrations in red blood cells. Food Funct 2018, 19, 4742–4754. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ration in cardiovascular disease and other chronic diseases. Exp Biol Med 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010, 8, 1461. [Google Scholar]
- Hamilton, J.S.; Klett, E.L. Linoleic acid and the regulation of glucose homeostasis: A review of the evidence. Prostaglandins, Leukot. Essent. Fat. Acids 2021, 175, 102366. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Dong, X.; Tao, Z.; Lu, L.; Zou, X. Effects of parental dietary linoleic acid on growth performance, antioxidant capacity, and lipid metabolism in domestic pigeons (Columba livia). Poult. Sci. 2020, 99, 1471–1482. [Google Scholar] [CrossRef]
- Li, X.; Yamada, H.; Morita, S.; Yamashita, Y.; Kim, Y.; Kometani, T.; Narang, N.; Furuta, T.; Kim, M. Effects of Free Linoleic Acid and Oleic Acid in Sesame Meal Extract as Pancreatic Lipase Inhibitors on Postprandial Triglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Volunteers. Nutrients 2023, 15, 1748. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; El-Sayed, E.M. Dietary conjugated linoleic acid and medium-chain triglycerides for obesity management. J. Biosci. 2021, 46, 12. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2019, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.M.; Kroon, P.A.; et al. Systematic review on polyphenol intake and health outcomes is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [PubMed]
- Ros, E.; Hu, F.B. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef] [PubMed]
- United States Department od Agriculture. USDA National Nutrient Database for Standard Reference; USDA: Washington, DC, USA, 2015. [Google Scholar]
- Institute of Medicine FaNB. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients); National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Alvares, A.Q.; Da Silva, V.A.; Góes, A.J.S.; Silva, M.S.; De Oliveira, G.G.; Bastos, I.V.G.A.; Neto, AG.D.C.; Alves, A.J. The fatty acid composition of vegetable oils and their potential use in wound care Adv Ski. Wound Care 2019, 32, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar] [CrossRef]
- Chan, R.L.; Olshan, A.F.; Savitz, D.A.; Herring, A.H.; Daniels, J.L.; Peterson, H.B.; Martin, S.L. Maternal influences on nausea and vomiting in early pregnancy. Matern. Child Heal. J. 2009, 15, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Lutomski, J.; McCarthy, F.P.; A Greene, R. Hyperemesis gravidarum: current perspectives. Int. J. Women's Heal. 2014, 6, 719–725. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan diets: practical advice for athletes and exercisers. J Int Soc Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; KhazáAi, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation: The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers—An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Syren, M.-L.; Turolo, S.; Marangoni, F.; Milani, G.P.; Edefonti, A.; Montini, G.; Agostoni, C. The polyunsaturated fatty acid balance in kidney health and disease: A review. Clin. Nutr. 2018, 37, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Lammardo, A.; Bonvissuto, M.; Giovannini, M.; Galli, C.; Riva, E. Polyunsaturated fatty acids in maternal plasma and in breast milk. Prostaglandins, Leukot. Essent. Fat. Acids 2002, 66, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Riva, E.; Agostoni, C. Fatty acids in pediatric nutrition. Pediatr Clin North Am 1995, 42, 861–877. [Google Scholar] [CrossRef]
- Available online: https://www.efsa.europa.eu/it/efsajournal/pub/1252.
- Yang, L.; Yuan, J.; Liu, L.; Shi, C.; Wang, L.; Tian, F.; Liu, F.; Wang, H.; Shao, C.; Zhang, Q.; et al. Alpha-linolenic acid inhibits human renal cell carcinoma cell proliferation through PPAR- γ activation and COX-2 inhibition. Oncol Lett 2016, 6, 197–202. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Derosa, G.; Di Gregori, V.; Bove, M.; Gaddi, A.V.; Borghi, C. Omega 3 Polyunsaturated Fatty Acids Supplementation and Blood Pressure Levels in Hypertriglyceridemic Patients with Untreated Normal-High Blood Pressure and With or Without Metabolic Syndrome: A Retrospective Study. Clin. Exp. Hypertens. 2010, 32, 137–144. [Google Scholar] [CrossRef]
- Skilton, M.R.; Pahkala, K.; Viikari, J.S.; Rönnemaa, T.; Simell, O.; Jula, A.; Niinikoski, H.; Celermajer, D.S.; Raitakari, O.T. The Association of Dietary Alpha-Linolenic Acid with Blood Pressure and Subclinical Atherosclerosis in People Born Small for Gestational Age: The Special Turku Coronary Risk Factor Intervention Project Study. J. Pediatr. 2015, 166, 1252–1257. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Weighell, W.; Edel, A.L.; LaVallee, R.; Dibrov, E.; Pinneker, R.; Maddaford, T.G.; Ramjiawan, B.; Aliani, M.; Guzman, R.; et al. Potent Antihypertensive Action of Dietary Flaxseed in Hypertensive Patients. Hypertension 2013, 62, 1081–1089. [Google Scholar] [CrossRef]
- Pan, A.; Chen, M.; Chowdhury, R.; Wu, J.H.Y.; Sun, Q.; Campos, H.; Mozaffarian, D.; Hu, F.B. Alpha-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 2012, 1262–1273. [Google Scholar] [CrossRef]
- Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; Sitlani, C.; Psaty, B.M.; Rimm, E.B.; Song, X.; McKnight, B.; Spiegelman, D.; King, I.B.; et al. Plasma phospholipid and dietary a-linolenic acid, mortality, CHD and stroke: the Cardiovascular Health Study. Br J Nutr 2014, 112, 1206–1213. [Google Scholar] [CrossRef]
- Saito, S.; Fukuhara, I.; Osaki, N.; Nakamura, H.; Katsuragi, Y. Consumption of alpha-linolenic acid-enriched diacylglycerol reduces visceral fat area in overweight and obese subjects: A randomized, double-blind controlled, parallel-group designed trial. J Oleo Sci 2016, 65, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Vedtofte, M.S.; Jakobsen, M.U.; Lauritzen, L.; Heitmann, B.L. The role of essential fatty acids in the control of coronary heart disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. Nutr. 2014, 100, 443S–448S. [Google Scholar] [CrossRef]
- Del Bo', C.; Deon, V.; Abello, F.; Massini, G.; Porrini, M.; Riso, P.; Guardamagna, O. Eight-week hempseed oil intervention improves the fatty acid composition of erythrocyte phospholipids and the omega-3 index, but does not affect the lipid profile in children and adolescents with primary hyperlipidemia. Food Res. Int. 2018, 119, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Qiu, B.; Jia, M.; Liu, W.; Guo, X.-F.; Li, N.; Xu, Z.-X.; Du, F.-L.; Xu, T.; Li, D. Effects of α-linolenic acid intake on blood lipid profiles:a systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 61, 2894–2910. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Paukste, E.; Nikbakht, E.; Khosravi-Boroujeni, H. Sesame fractions and lipid profiles: a systematic review and meta-analysis of controlled trials. Br. J. Nutr. 2016, 115, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Lowry, J.R.; Marshall, N.; Wenzel, T.J.; Murray, T.E.; Klegeris, A. The dietary fatty acids α-linolenic acid (ALA) and linoleic acid (LA) selectively inhibit microglial nitric oxide production. Mol. Cell. Neurosci. 2020, 109, 103569. [Google Scholar] [CrossRef]
- Erdinest, N.; Shohat, N.; Moallem, E.; Yahalom, C.; Mechoulam, H.; Anteby, I.; Ovadia, H.; Solomon, A. Nitric oxide secretion in human conjunctival fibroblasts is inhibited by alpha linolenic acid. J. Inflamm. 2015, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Cavina, M.; Battino, M.; Gaddi, A.V.; Savo, M.T.; Visioli, F. Supplementation with alpha-linolenic acid and inflammation: a feasibility trial. Int. J. Food Sci. Nutr. 2020, 72, 386–390. [Google Scholar] [CrossRef]
- Bemelmans, W.J.; Lenfrandt, J.D.; Feskens, E.J.; Vam Healst, P.L.; Broer, J.; Meyboom-de Jong, B.; May, J.F.; Tervaert, J.W.; Smith, A.J. Increased alpha-linolenic acid intake lowers C-reactive protein, but has no effects on markers of atherosclerosis. Eur J Clin Nutr 2004, 58, 1083–1089. [Google Scholar] [CrossRef]
- USDA and US Department of Health and Human Services (DHHS). Dietary Guidelines for Americans, 2020-2025. 9th ed. Washington (CD):USDA and US DHHS; 2020.
- Vedtofte, M.S.; Jakobsen, M.U.; Lauritzen, L.; O'Reilly, E.J.; Virtamo, J.; Knekt, P.; Colditz, G.; Hallmans, G.; Buring, J.; Steffen, L.M.; et al. Association between the intake of α-linolenic acid and the risk of CHD. Br. J. Nutr. 2014, 112, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hou, R.; Xi, Y.; Kowalski, A.; Wang, T.; Yu, Z.; Hu, Y.; Chandrasekar, E.K.; Sun, H.; Ali, M.K. The association and dose–response relationship between dietary intake of α-linolenic acid and risk of CHD: a systematic review and meta-analysis of cohort studies. Br. J. Nutr. 2018, 119, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Morrow, D.A.; Scirica, B.M.; Furtado, J.D.; Guo, J.; Mozaffarian, D.; Sabatine, M.S.; O’donoghue, M.L. Plasma Omega-3 Fatty Acids and the Risk of Cardiovascular Events in Patients After an Acute Coronary Syndrome in MERLIN-TIMI 36. J. Am. Hear. Assoc. 2021, 10, e017401. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Mediterranean Diet and Survival Among Patients With Coronary Heart Disease in Greece. Arch. Intern. Med. 2005, 165, 929–935. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Renaud, S.; Salen, P.; Monjaud, I.; Mamelle, N.; Martin, J.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Guasch-Ferrè, M.; Hu, F.B.; Sanchez-Tainta, A.; Bullò, M.; Serra-Mir, M.; Lopez-Sabater, C.; Sorlì, J.V.; Aròs, F.; Fiol, M.; et al. Dietary alpha-linolenic acid, marine omega-3 fatty acids, and mortality in a population with high fish consumption: findings from the PREvenciòn con DIeta MEDiterranea (PREDIMED) study. J Am Heart Assoc 2016, 5, e002543. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Wei, J.; Hou, R.; Xi, Y.; Kowalski, A.; Wang, T.; Yu, Z.; Hu, Y.; Chandrasekar, E.K.; Sun, H.; Ali, M.K. The association and dose–response relationship between dietary intake of α-linolenic acid and risk of CHD: a systematic review and meta-analysis of cohort studies. Br. J. Nutr. 2018, 119, 83–89. [Google Scholar] [CrossRef]
- Djoussè, L.; Rautaharju, P.M.; Hopkins, P.N.; Whitsel, E.A.; Arnett, D.K.; Eckfeldt, J.H.; Province, M.A.; Ellison, R.C. ; and Investigators of the NHLBI Family Heart Study. Dietary linolenic acid and adjusted QT and JT intervals in the National Heart, Lung, and Blood Institute Family Heart Study. J Am Coll Cardiol 2005, 45, 1716–1722. [Google Scholar] [PubMed]
- Geleijnse, J.M.; Giltay, E.J.; Schouten, E.G.; de Goede, J.; Griep, L.M.O.; Teitsma-Jansen, A.M.; Katan, M.B.; Kromhout, D. Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: Design and baseline characteristics of the Alpha Omega Trial. Am. Hear. J. 2010, 159, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Geleijnse, J.M.; de Goede, J.; Griep, L.M.O.; Mulder, B.J.; de Boer, M.-J.; Deckers, J.W.; Boersma, E.; Zock, P.L.; Giltay, E.J. n-3 Fatty Acids, Ventricular Arrhythmia–Related Events, and Fatal Myocardial Infarction in Postmyocardial Infarction Patients With Diabetes. Diabetes Care 2011, 34, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Daien, C.; Czernichow, S.; Letarouilly, J.-G.; Nguyen, Y.; Sanchez, P.; Sigaux, J.; Beauvais, C.; Desouches, S.; Le Puillandre, R.; Rigalleau, V.; et al. Dietary recommendations of the French Society for Rheumatology for patients with chronic inflammatory rheumatic diseases. Jt. Bone Spine 2021, 89, 105319. [Google Scholar] [CrossRef]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; et al. Effect of omega-3 dosage on cardiovascular outcomes: An updated meta-analysis and meta-regression of interventional trials. Mayo Clin Proc 2021, 96, 304–313. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do Omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol 2006, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.-M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on alpha-linolenic acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. Omega-3 and Omega-3 polyunsaturated fatty acids, obesity and cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




