1. Introduction
The COVID-19 pandemic has brought to light how crucial it is to comprehend the molecular and cellular processes that underlie viral pathogenesis [
1]. SARS-CoV-2, the virus that causes COVID-19, has spread quickly over the world, killing millions of people while seriously disrupting economies, cultures, and healthcare systems [
1,
2]. The spike (S) protein, which facilitates viral entrance into host cells and interacts with the host immune system, is one of the main components of SARS-CoV-2 pathogenicity [
3]. The discovery and dissemination of SARS-CoV-2 variants with S protein mutations, however, have raised questions regarding the effectiveness of available treatments and vaccines as well as the possibility that these variants could avoid immune detection and result in more severe disease [
4,
5].
During viral entrance and fusion with host cell membranes, the SARS-CoV-2 S protein, a highly dynamic and complex molecule, undergoes conformational changes [
6]. The S protein is a crucial factor in inducing a protective immune response and is a main target for antibodies that work to neutralize pathogens [
6,
7]. Therefore, in order to create effective vaccines and treatments against SARS-CoV-2, it is crucial to comprehend the structural and functional effects of S protein mutations [
7].
There are a number of SARS-CoV-2 variants with S protein mutations that have been linked to enhanced transmissibility, higher viral loads, and less neutralization by antibodies induced by existing vaccinations, according to recent investigations [
8,
9]. These results have underlined the necessity of tracking SARS-CoV-2 development and modifying current therapies and vaccines to remain effective against novel variations. Furthermore, knowing the molecular processes underlying S protein mutations and how they affect viral pathogenesis can help in the development of novel antiviral treatments as well as pandemic preparedness techniques in the future [
10].
The development of viable vaccines and therapies as well as the ongoing COVID-19 pandemic are significantly impacted by the appearance of SARS-CoV-2 variants with S protein mutations [
11]. In order to create measures to reduce the severity and spread of COVID-19 and get ready for upcoming pandemics, it is imperative to comprehend the structural and functional effects of these mutations [
12,
13].
By summarizing the current understanding of the SARS-CoV-2 spike protein, providing an overview of the evolution and classification of spike protein mutations, evaluating their impact on viral infectivity and pathogenesis, analysing their structural and functional consequences, exploring immune evasion mechanisms associated with these mutations, and discussing their implications for COVID-19 vaccines and therapeutics, the review seeks to provide a thorough analysis of the topic. By offering a useful resource for academics and clinicians engaged in COVID-19 research, the review aims to contribute to the creation of efficient strategies for managing the current COVID-19 pandemic.
2. Structural and Functional Features Of SARS-CoV-2 Spike Protein
The development of vaccines and treatments against COVID-19 must focus on the S protein of SARS-CoV-2 because it is a highly dynamic and complex molecule that is essential for viral entrance into host cells and interacts with the host immune system [
14,
15].
2.1. Spike Protein Structure
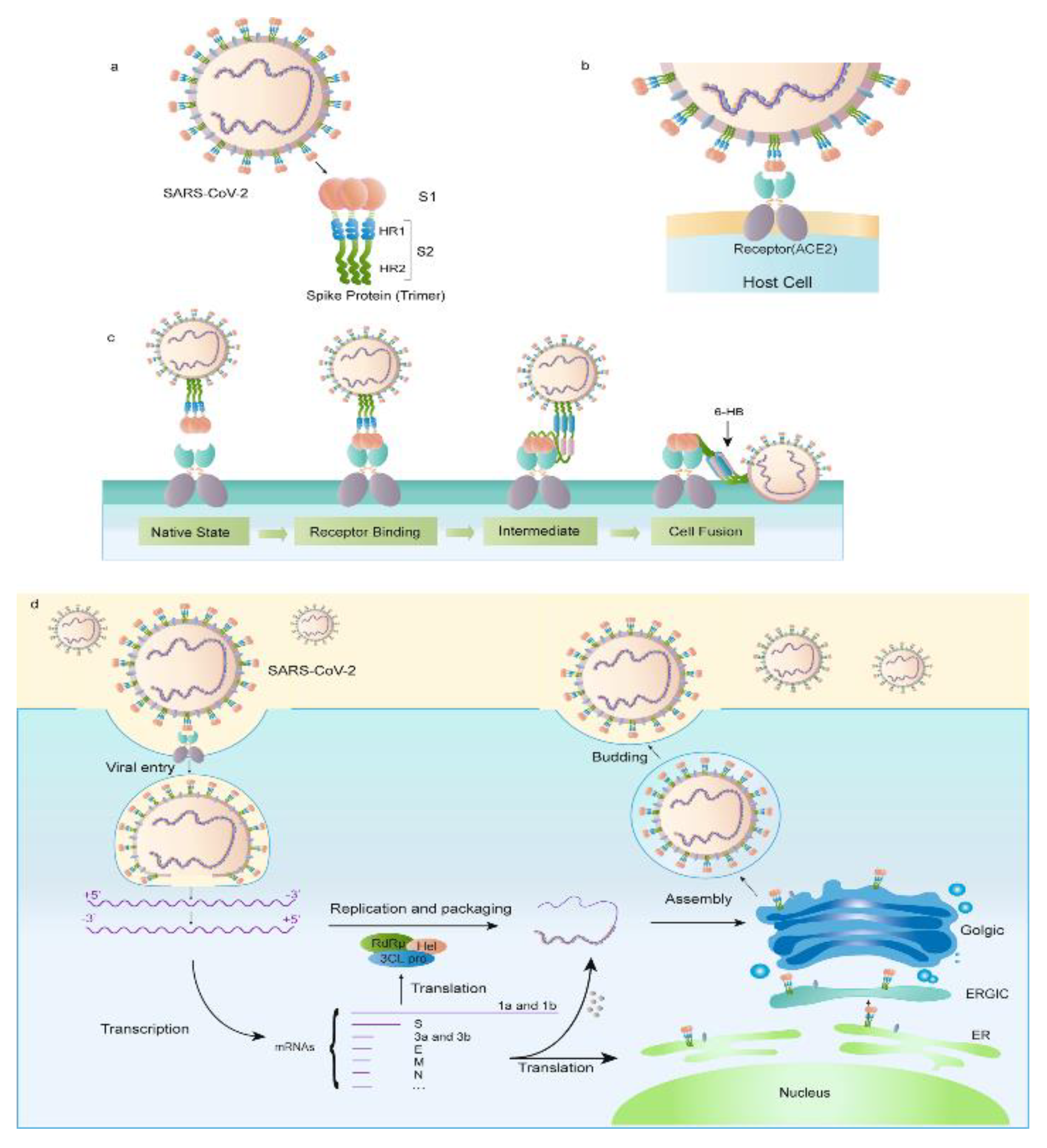
The SARS-CoV-2 virus's S protein, which is its most thoroughly studied component, is essential for the virus's entry and multiplication inside the host [
16]. S1, S2, and a transmembrane domain make up the three components of the S protein, a complex glycosylated protein. The angiotensin-converting enzyme 2 (ACE2) receptor on host cells is bound by the receptor-binding domain (RBD) of the S1 subunit, which facilitates viral entrance (See
Figure 1) [
16,
17]. The transmembrane domain secures the protein to the viral membrane, and the S2 subunit includes the fusion peptide that helps the viral and host cell membranes fuse [
18]. During viral entry, the highly dynamic S protein changes shape from a closed conformation to an open conformation, exposing the RBD for ACE2 binding [
19].
Neutralizing antibodies' main target is the S protein, which also stimulates a strong immunological response. Therefore, for the development of efficient vaccines and treatments against SARS-CoV-2, understanding the structure and function of the S protein is crucial [
20]. Recent research has revealed the emergence of numerous SARS-CoV-2 variants with S protein mutations, which are linked to both greater transmissibility and decreased neutralization by antibodies induced by existing vaccines [
21,
22]. In order to create novel vaccines and treatments against SARS-CoV-2 and to ensure that existing vaccines are still effective against new variants, more research into the structure and function of the S protein and its variants is required [
23].
2.2. Spike Protein Function
SARS-CoV-2's S protein is essential for the virus to enter host cells. The ACE2 receptor on the surface of the host cell is bound by the S protein, causing conformational changes that enable the fusion of the viral and host cell membranes and result in viral entrance [
24]. By preventing neutralizing antibodies from recognizing the viral surface, the S protein helps the virus evade the host immune system. Strong immunological responses are triggered by the S protein, which is also the main target of neutralizing antibodies [
25]. The S protein is a crucial target for the creation of potent vaccines and treatments against SARS-CoV-2 because antibodies against it can thwart viral entrance and decrease viral proliferation [
26].
There are a number of SARS-CoV-2 variants with S protein mutations, and these alterations have been linked to both greater transmissibility and decreased neutralization by antibodies induced by existing vaccinations. Investigations are still ongoing into how these alterations affect viral pathogenesis and infectivity functionally [
21,
22]. For the creation of efficient SARS-CoV-2 vaccines and therapies as well as for the management of the continuing COVID-19 pandemic, it is essential to comprehend the functional characteristics of the S protein and its variations [
5]. More investigation is required to determine how S protein mutations affect viral entrance, replication, and immune evasion as well as to create new methods to combat these alterations.
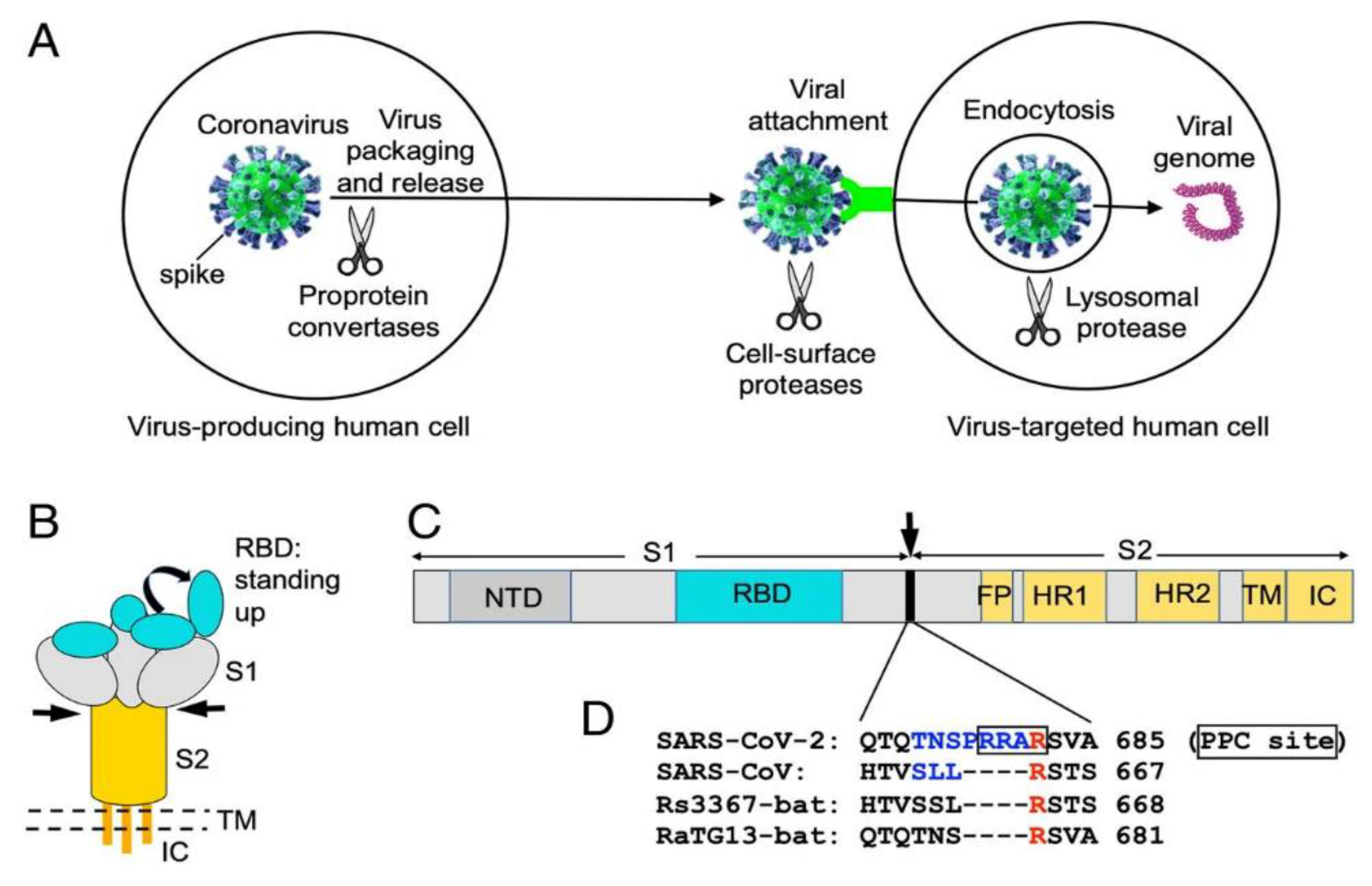
2.3. Receptor Recognition and Host Cell Entry Mechanisms
Angiotensin-converting enzyme 2 (ACE2) receptor identification and binding by the SARS-CoV-2 spike (S) protein facilitates viral entry into host cells [
27]. The S1 subunit of the S protein's receptor-binding domain (RBD) preferentially binds to the ACE2 receptor on the surface of the host cell, causing conformational changes that enable the fusion of the viral and host cell membranes and result in viral entrance (See
Figure 2) [
28]. The affinity of the RBD for ACE2 has been demonstrated to be a crucial factor of viral infectivity and pathogenicity. The S protein is highly selective for the ACE2 receptor [
29].
The S protein of the SARS-CoV-2 virus has a stronger affinity for the ACE2 receptor than the S protein of the original SARS-CoV virus, according to structural analyses [
30]. This stronger affinity could be a factor in SARS-CoV-2's higher transmissibility when compared to SARS-CoV [
31,
32]. Additionally, the S protein has a furin cleavage site that enables host proteases to cleave the S protein, facilitating viral entrance into host cells. During viral entry, the S protein goes through conformational changes [
33,
34]. It switches from a closed shape to an open conformation, exposing the RBD for ACE2 binding [
34,
35].
There have been a number of SARS-CoV-2 variations with changes to the S protein, and some of these changes have been linked to heightened transmissibility and diminished neutralization by antibodies induced by existing vaccinations [
21]. For the creation of efficient vaccines and treatments against SARS-CoV-2, it is crucial to comprehend the effects of these mutations on receptor recognition and host cell entry mechanisms [
36]. More investigation is required to determine the functional effects of these mutations on viral pathogenesis and infectivity as well as to find new ways to target the S protein and stop viral entry into host cells.
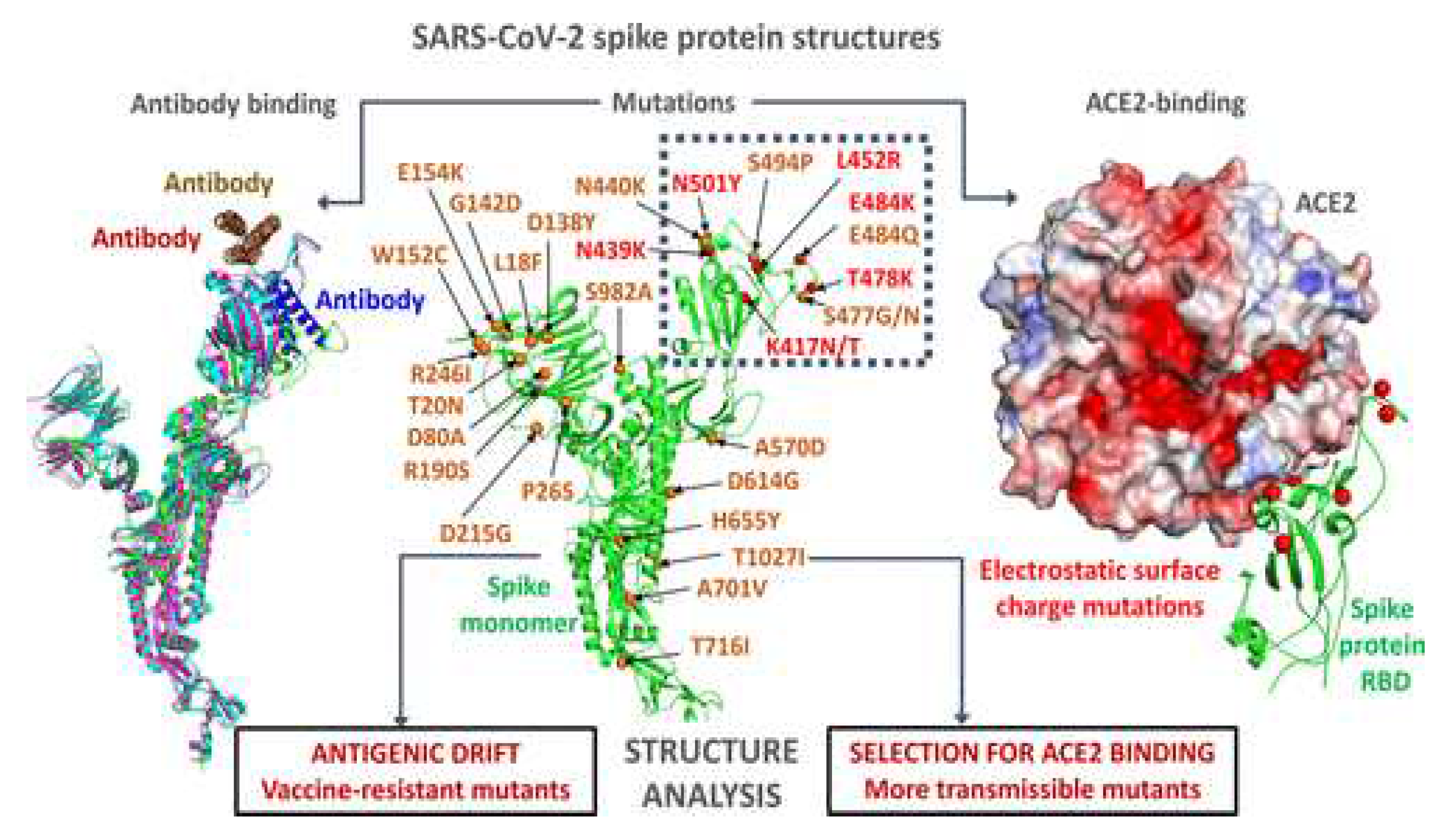
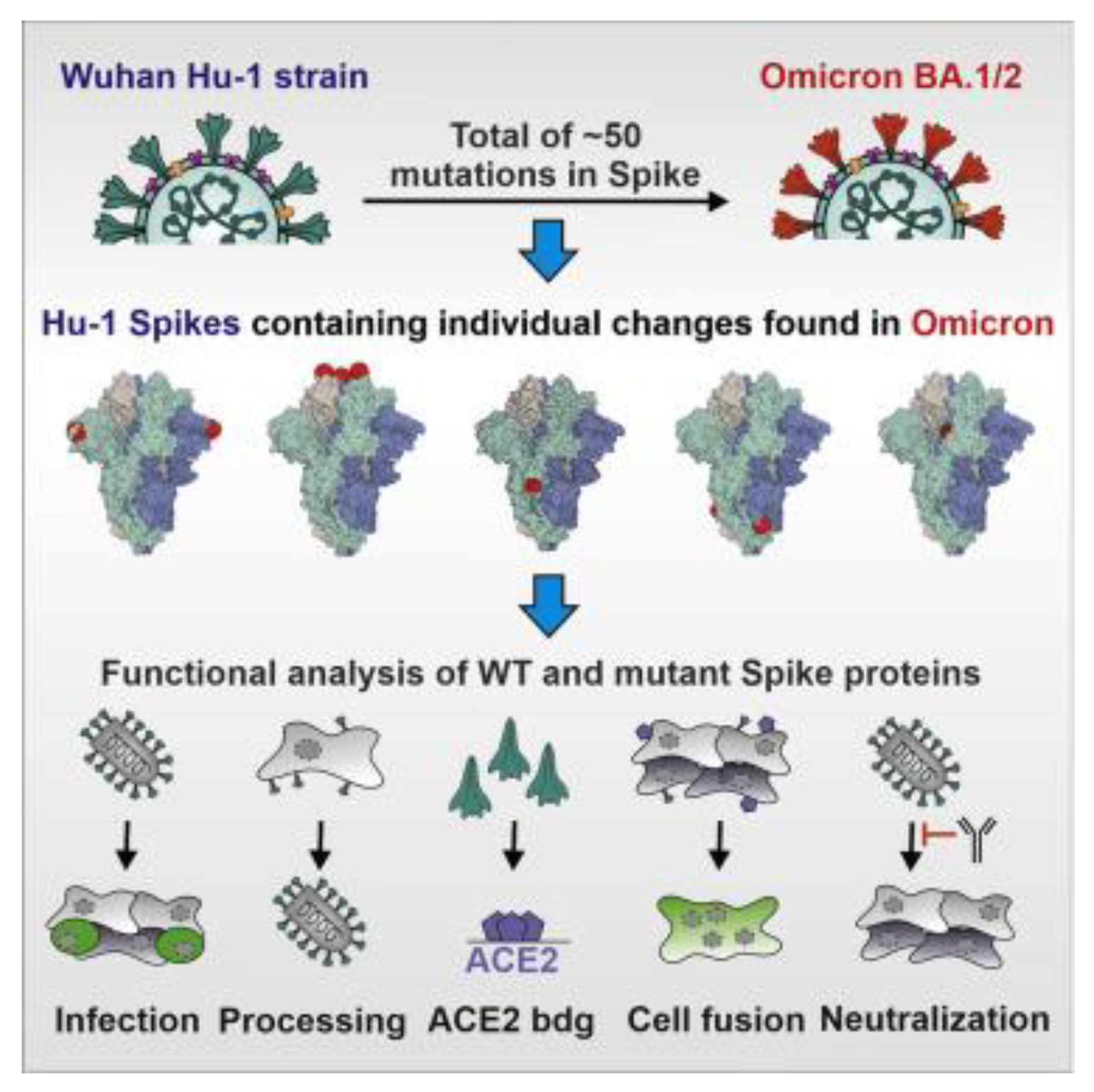
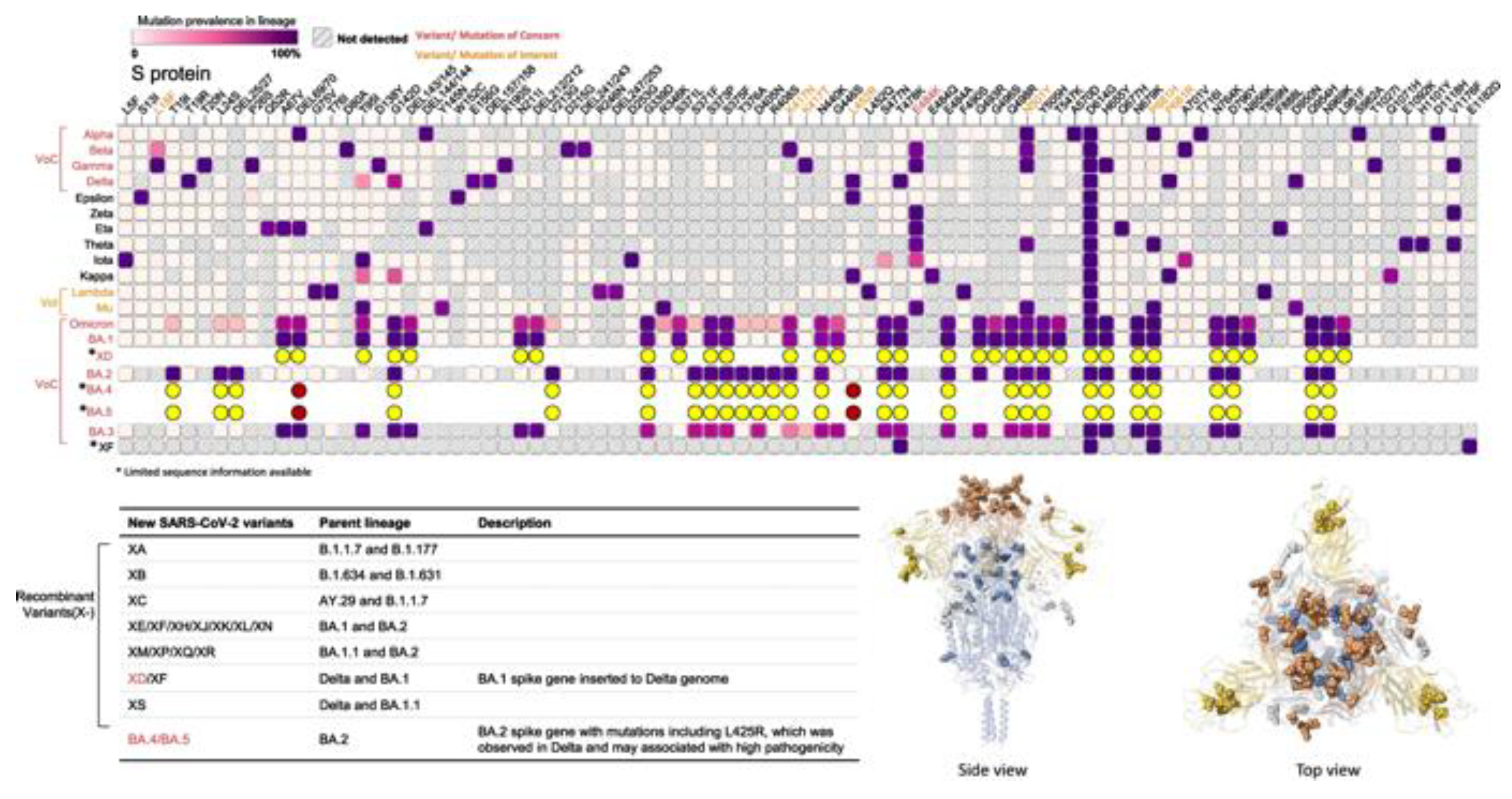
3. Evolutionary Dynamics of SARS-CoV-2 Spike Protein Mutations
As a result of the ongoing COVID-19 pandemic brought on by the SARS-CoV-2 virus, new variations with mutations in the spike [S] protein (See
Figure 3), the main target for present vaccines and therapies, have emerged and spread quickly [
5].
3.1. Emergence and Spread of Spike Protein Mutations
The appearance and propagation of SARS-CoV-2 variants with S protein mutations is extremely concerning since these changes may have an impact on the pathophysiology, immune evasion, and infectiousness of the virus [
37]. Replication errors, recombination events, and selection pressure from the host immune system are only a few of the factors that can lead to mutations in the S protein [
38]. It is expected that more S protein changes will occur when the virus spreads and multiplies in human populations, which could result in the creation of novel variations that are more transmissible, virulent, or resistant to current therapies and vaccines [
39,
40].
There have already been several SARS-CoV-2 variations with S protein mutations that have appeared. These variants have been linked to both greater transmissibility and decreased neutralization by antibodies produced by current vaccinations [
21,
22]. Among the most researched variants with S protein mutations are the B.1.1.7 variant, which was discovered in the UK, the B.1.351 variant, which was discovered in South Africa, and the P.1 variant, which was discovered in Brazil [
41,
42]. These variants have mutations in the RBD of the S protein, which have been found to decrease the S protein's affinity for the ACE2 receptor and to lessen the potency of some antibodies' neutralizing effects [
43].
For an understanding of the evolutionary processes of the virus and the creation of efficient pandemic control measures, continuing surveillance and monitoring of SARS-CoV-2 variants with S protein mutations is essential.
3.2. Classification and Nomenclature of Spike Protein Mutations
For monitoring and comprehending the appearance and transmission of variants with possible implications for viral infectivity, pathogenesis, and immune evasion, the classification and nomenclature of SARS-CoV-2 spike protein mutations are crucial [
44,
45]. The geographical origin of the variant and the mutations found in the S protein have been used by the WHO to build a methodology for the classification and designation of SARS-CoV-2 variants [
46,
47]. The Greek alphabet is used to name variations, beginning with Alpha for the B.1.1.7 variety that was initially discovered in the UK [
48].
The reference amino acid and the variant amino acid are listed after the amino acid position in the S protein in the nomenclature of S protein mutations [
49]. with instance, the S protein's D614G mutation designates a replacement of aspartic acid (D) with glycine (G) at position 614 [
49,
50]. This mutation, which affects the S1 subunit of the S protein, has been linked to an increase in the transmission and infectivity of viruses [49-51]. In order to track and address the current pandemic, communication and cooperation among researchers, public health professionals, and politicians must be made easier thanks to the classification and nomenclature of SARS-CoV-2 spike protein mutations.
3.3. Genomic and Phylogenetic Analyses of Spike Protein Mutations
To comprehend the evolution and transmission of the virus and to create efficient pandemic control measures, genomic and phylogenetic investigations of SARS-CoV-2 spike protein mutations are crucial [
52]. The sequencing of the viral genome and identification of the S protein mutations are steps in the genomic study [
52,
53]. Based on the mutations found in the genomes of the various SARS-CoV-2 strains, a tree representing the evolutionary relationships between them is built during the phylogenetic analysis [
54]. These studies can shed light on the development, dispersal, and diversity of SARS-CoV-2 variants, particularly those with S protein mutations.
Some mutations in the S protein have independently evolved in several lineages, according to genomic and phylogenetic analysis, pointing to convergent evolution or positive selection pressure [
55]. For instance, the D614G mutation in the S protein, which independently appeared in several lineages and quickly rose to become the global dominant variety, may have an advantage in terms of fitness [
55,
56]. Other S protein mutations, however, have only been detected in certain lineages, raising the possibility of a founder effect or genetic drift [55-57]. Public health officials can implement targeted actions to stop future spread by identifying probable transmission clusters and sources of introduction for novel variants with the aid of genomic and phylogenetic investigations.
4. Impact of Spike Protein Mutations on Viral Infectivity and Pathogenesis
SARS-CoV-2 spike protein mutations can significantly affect the pathogenesis and infectivity of the virus, potentially increasing the disease's severity, transmissibility, and immune evasion (See
Figure 4) [
58,
59].
4.1. Effects on Viral Replication and Transmission
SARS-CoV-2 spike protein mutations can change how the virus interacts with host cells, which can impact viral reproduction and transmission [
60,
61]. For instance, changes to the S protein's receptor-binding domain (RBD) may alter the virus' affinity and specificity for the host cell receptor ACE2, resulting in a change in the amount of viral entry and reproduction [61-63]. In addition to affecting viral assembly and release, mutations in other parts of the S protein, such as the S2 subunit or the furin cleavage site, can also impact viral fusion and entry into host cells [
18]. The S protein's stability, immunogenicity, and antigenicity can all be affected by these changes, which may have an impact on the effectiveness of treatments and vaccinations.
The B.1.1.7 variation, which was initially discovered in the UK, and the B.1.617.2 variant, which was initially discovered in India, are two SARS-CoV-2 variants having mutations in the S protein that have been linked to increased transmissibility and/or viral loads [
42,
64]. These variants have changes to the S protein's RBD and/or other areas, which could boost viral entrance and reproduction in host cells [
14]. For the development of efficient preventive strategies, such as vaccinations, antivirals, and non-pharmaceutical treatments, it is essential to comprehend the consequences of spike protein mutations on viral reproduction and transmission.
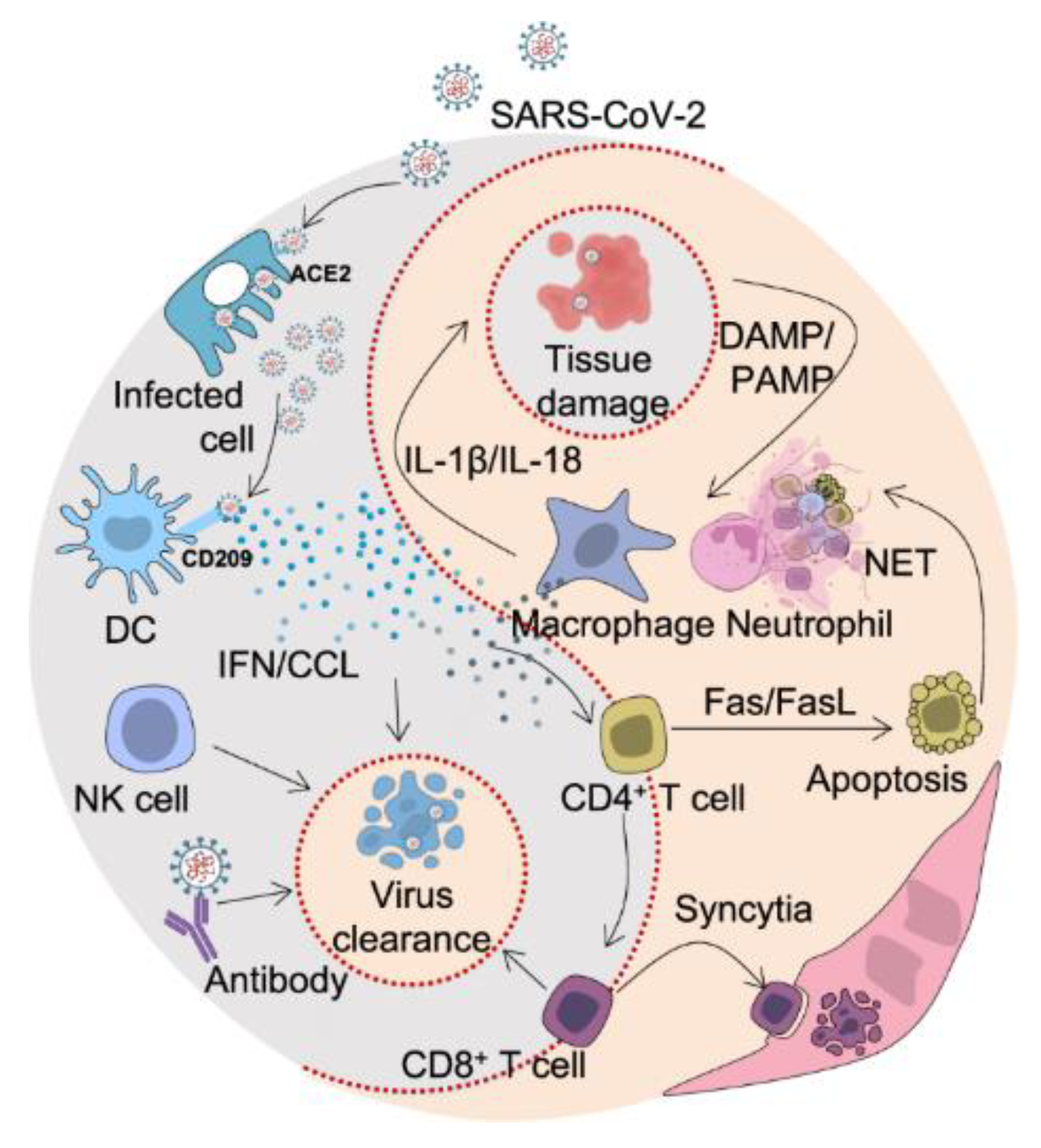
4.2. Modulation of Host Immune Responses
SARS-CoV-2 spike protein mutations can alter host immune responses, possibly resulting in immune evasion or hyperinflammatory reactions [
65]. Antibodies against the S protein are essential for neutralizing the virus and avoiding infection because the S protein is a significant target of the host immune response [
65,
66]. However, changes to the S protein's antigenic structure may lessen the protein's ability to elicit immunological recognition and neutralizing antibodies [
67]. For instance, changes to the RBD of the S protein may impact how neutralizing antibodies bind to the protein and diminish their effectiveness, increasing immune evasion [
67,
68]. The identification and binding of antibodies and T cells can be affected by mutations in other parts of the S protein, thereby decreasing the effectiveness of cellular immunity [
69].
By causing hyperinflammatory reactions or immune system dysregulation, spike protein mutations can also have an impact on the host immunological response [
65,
68]. For instance, mutations in the S protein's furin cleavage site can increase the S protein's cleavage and activation, increasing viral entrance and replication and perhaps triggering hyperinflammatory reactions [
70]. A dysregulation of the immune response and the potential to exacerbate inflammation and tissue damage can result from mutations in other parts of the S protein that impact the binding and activation of innate immune receptors [
71].
For effective vaccines and treatments that can induce protective immunity against SARS-CoV-2 variations, it is essential to comprehend how host immune responses are modulated by spike protein mutations. It is crucial for developing targeted therapeutics to lessen inflammation and tissue damage in severe COVID-19 instances as well as for discovering potential biomarkers of disease severity.
4.3. Association with Disease Severity and Clinical Outcomes
The severity of the disease and clinical outcomes are also related to SARS-CoV-2 spike protein mutations, which may have an impact on COVID-19 disease progression [
62]. The B.1.1.7 variant, originally discovered in the UK, and the B.1.351 variant, first discovered in South Africa, are two SARS-CoV-2 variants having mutations in the S protein that have been linked to increased disease severity and death [
72]. Additionally, several S protein variations have been linked to an increased risk of reinfection, possibly as a result of worse immune recognition [
62,
72].
By changing the virus's pathogenicity and tropism in various tissues and organs, spike protein mutations can also have an impact on clinical outcomes [
73]. For instance, changes in the S protein may alter the virus's affinity and selectivity for various cell types and tissues, which may influence the disease's severity and clinical presentation [
14]. The interaction between the virus and host immune cells can also be impacted by mutations in the S protein, which could result in dysregulated immunological responses, hyperinflammation, and tissue damage [
74].
Effective COVID-19 management and treatment methods require an understanding of the relationship between spike protein mutations and disease severity and clinical outcomes [
75]. On the basis of the genetic makeup of the virus and the host, it can also help with the development of targeted therapies and individualized treatment plans.
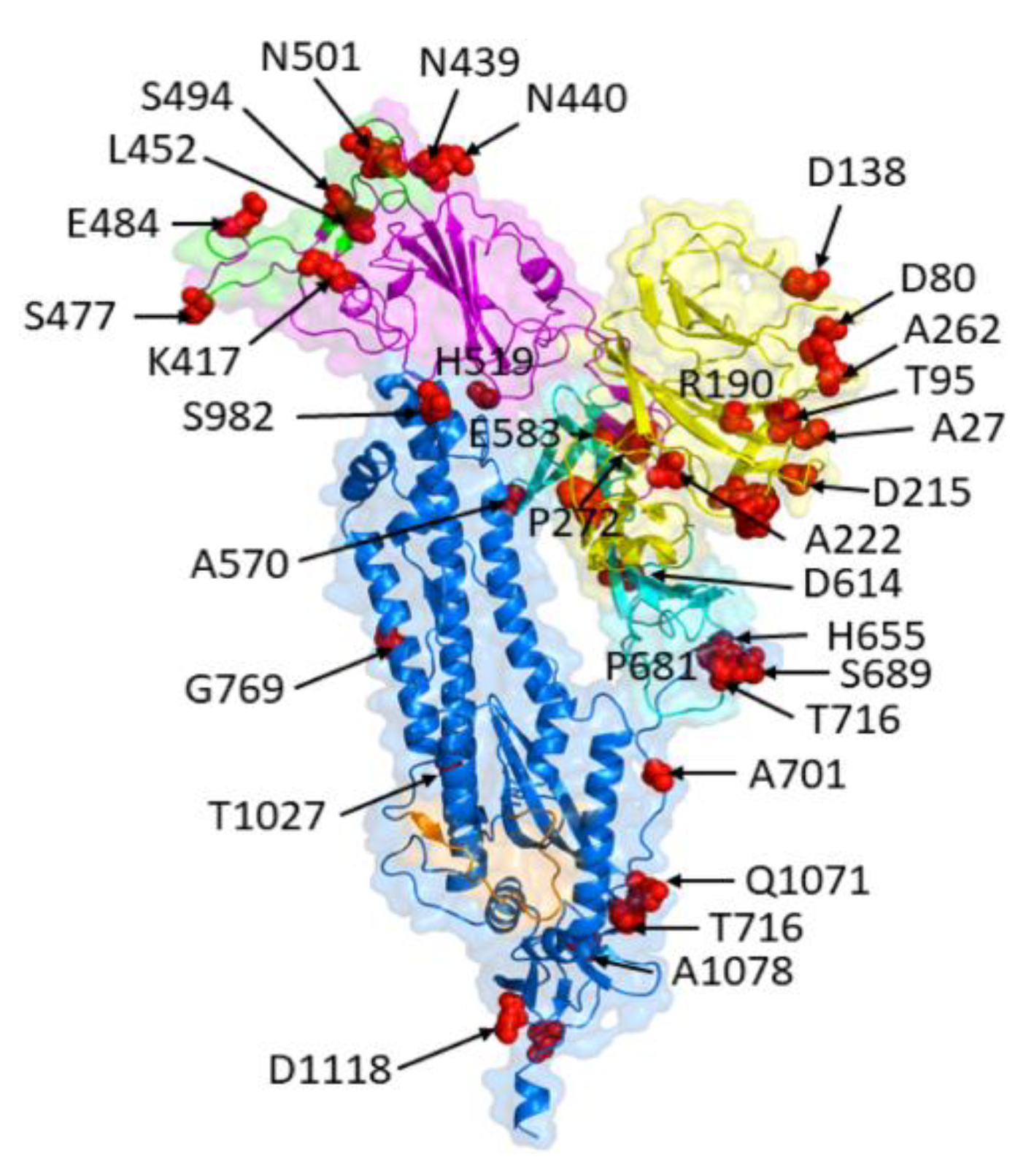
5. Structural Consequences of Spike Protein Mutations
Since SARS-CoV-2's spike protein is a primary target for vaccine development and treatment interventions, changes to this protein's structure may have an impact on the effectiveness of these measures (See
Figure 5).
5.1. Alterations in Spike Protein Conformation and Stability
The shape and stability of the SARS-CoV-2 spike protein can change due to mutations, which may have an impact on how the virus interacts with host cells and is recognized by the immune system [
76,
77]. The receptor-binding domain (RBD) of the host receptor ACE2 is found in the S1 subunit of the spike protein, which is made up of two subunits, S1 and S2 [
78,
79]. Changes in the RBD's conformation and its binding affinity for ACE2 may result from mutations, which may have an impact on viral entry and transmission [
80]. For instance, the RBD mutation (N501Y) in the B.1.1.7 variety, which was initially discovered in the UK, has been linked to higher binding affinity for ACE2 and greater transmissibility [
81,
82].
Mutations in the spike protein can alter its stability and antigenicity in addition to impacting receptor binding. Approximately two-thirds of the spike protein's surface is covered with glycans, indicating that it is extensively glycosylated [
83,
84]. The spike protein's structural flexibility and stability may be impacted by mutations in the glycosylation pattern. This may lessen the effectiveness of vaccinations and therapeutic antibodies by interfering with the recognition and binding of neutralizing antibodies [
85]. For instance, the RBD and N-terminal domain (NTD) alterations in the original South African B.1.351 variation have been linked to decreased neutralization by certain monoclonal antibodies and convalescent plasma [
86,
87].
Creating efficient COVID-19 preventive and treatment plans requires an understanding of the structural effects of spike protein mutations. This entails creating treatments and vaccines that can target the spike protein in its many conformations and patterns of glycosylation, as well as keeping an eye on the emergence and dissemination of novel variants with mutations that can alter the effectiveness of these interventions.
5.2. Effects on Spike Protein Interactions with Host Factors
The interactions of the spike protein with host components like antibodies, immune cells, and other host proteins may impact the pathogenicity and infectiousness of viruses [
88,
89]. Mutations may result in the loss of the spike protein's epitopes, or regions of the protein that neutralizing antibodies may identify [
90,
91]. By reducing the potency of therapeutic antibodies and vaccinations that target specific epitopes, this may lead to breakthrough infections and decreased protection against reinfection [
59]. For instance, the E484K mutation seen in the B.1.351 and P.1 variants has been connected to decreased neutralization by certain monoclonal antibodies and convalescent plasma [
21,
92].
Spike protein mutations can alter not only antibody recognition but also how the protein interacts with immune cells like T cells and natural killer cells [
93]. Spike protein mutations may impact how antigen-presenting cells process and display viral antigens, thereby lowering T cell activation and compromising the antiviral immune response [
94,
95]. The spike protein's interactions with host proteins important in viral entry and replication, such as the ACE2 receptor and host proteases, can also be impacted by mutations [
18,
96]. This may have an impact on the effectiveness of viral entrance and replication, thereby influencing viral pathogenesis and the severity of the disease.
For the purpose of creating efficient COVID-19 prevention and treatment techniques, it is essential to comprehend the consequences of spike protein mutations on how it interacts with host factors. In addition to creating treatments that can specifically target the spike protein and host components involved in viral entry and replication, this involves keeping an eye on the development and dissemination of novel variations with mutations that may influence viral infectivity and pathogenicity.
5.3. Influence on Spike Protein Cleavage and Processing
The SARS-CoV-2 spike protein is a glycoprotein that goes through a lot of cleavage and processing when it enters host cells [
97]. To allow for viral entrance, the spike protein has a polybasic cleavage site (RRAR) that is digested by host proteases such furin and TMPRSS2 [
98]. The effectiveness of cleavage and processing can be changed by mutations in the spike protein, which may have an impact on the pathogenicity and infectiousness of viruses [
99,
100].
The D614G mutation in the spike protein, which has been found in several SARS-CoV-2 variations and is linked to increased infectivity and transmissibility, is one of the most prominent changes in the protein [
32]. It has been demonstrated that the D614G mutation, which affects the receptor-binding domain (RBD) of the spike protein, makes the spike trimer more stable and makes it easier for host proteases to digest the spike protein. These modifications could be a factor in the D614G variant's heightened contagiousness and transmissibility [
101].
It has also been demonstrated that other mutations in the spike protein alter cleavage and processing. For instance, the B.1.1.7 variant's P681H mutation has been linked to improved furin cleavage efficiency, which may help explain why the variety is more contagious [
102,
103]. On the other hand, it has been demonstrated that alterations in the polybasic cleavage site, such as the E484K mutation present in most variants of concern, impair cleavage efficiency and impact viral entrance [
104].
For the purpose of creating efficient COVID-19 therapies, it is essential to comprehend the effects of spike protein mutations on cleavage and processing. Protease inhibitors are one example of a therapeutic strategy that targets these processing steps and may be useful in inhibiting viral entrance and replication. Additionally, keeping an eye on the development and dissemination of novel variants with mutations that impact cleavage and processing can reveal crucial details about the pathogenesis and evolution of SARS-CoV-2.
6. Functional Consequences of Spike Protein Mutations
A SARS-CoV-2 mutation in the spike protein can have substantial functional ramifications because it is essential for viral entrance and pathogenesis [
28].
6.1. Changes in Spike Protein Receptor Binding Affinity and Specificity
To enable viral entrance, the SARS-CoV-2 spike protein interacts to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells [
24]. Alterations to the spike protein's binding affinity and specificity for ACE2 can have an impact on the pathogenicity and transmission of viruses [
82]. For instance, the RBD of the spike protein has several changes in the B.1.1.7 variation, including N501Y, which has been demonstrated to increase the binding affinity for ACE2 and improve viral infectivity [
105]. Similar to the B.1.351 variant, the B.1.351 variant features many mutations in the RBD, including E484K, which decreases the binding affinity for some monoclonal antibodies and might have an impact on how well the host immune system neutralizes the virus [
21,
64].
Mutations in the spike protein can alter ACE2 binding as well as result in the development of novel receptor binding capacities [
106]. According to research, the P681R mutation in the B.1.617 variation confers improved binding to the host protease furin, which may make it easier for the virus to enter host cells that don't express a lot of ACE2 [
107]. This mutation has been found in a number of variants that are cause for concern, underscoring the possibility that the development of new receptor-binding capacities may be what propels the evolution and appearance of SARS-CoV-2 variants [
108].
It is essential to comprehend how receptor binding affinity and selectivity are affected by spike protein mutations in order to create potent COVID-19 therapies. Neutralizing antibodies and ACE2 decoys are examples of therapeutic approaches that target the interaction between the spike protein and ACE2 and may be useful in limiting viral entrance and replication [
109]. Additionally, keeping an eye on the appearance and spread of novel variations with mutations that impair receptor binding will shed light on the pathogenesis and evolution of SARS-CoV-2 [
11].
6.2. Effects on Spike Protein Fusion and Membrane Fusion
SARS-CoV-2 enters host cells by the spike protein-mediated fusion of the viral and host cell membranes, which is an important stage in the process [
18,
97]. This procedure can be impacted by mutations in the spike protein, changing the pathogenicity and infectivity of the virus [
110]. For instance, as shown by the enhanced cleavage efficiency seen with the D614G mutation, alterations in the S1/S2 furin cleavage region of the spike protein can impact the proteolytic processing necessary for membrane fusion [
111]. This mutation has been linked to increased viral transmissibility and infectivity [
112].
Mutations in the spike protein can directly alter the conformational changes necessary for membrane fusion in addition to having an impact on proteolytic processing. Multiple SARS-CoV-2 variants have the E484K mutation, which has been found to stabilize the spike protein's prefusion conformation and improve the effectiveness of membrane fusion [
18,
113]. Additionally, this mutation has been linked to a decreased susceptibility to neutralizing antibodies and perhaps a decreased effectiveness of vaccination [
114].
For the purpose of creating efficient countermeasures against COVID-19, it is essential to comprehend how spike protein mutations affect membrane fusion. Fusion inhibitors and other therapeutic approaches that focus on the fusion process may be useful in limiting viral entry and reproduction [
115]. Additionally, keeping an eye on the development and dissemination of novel variations with mutations that interfere with membrane fusion can reveal vital details about the pathogenesis and evolution of SARS-CoV-2.
6.3. Modulation of Spike Protein Proteolytic Activation and Inactivation
The spike protein's proteolytic activation and inactivation are crucial processes in SARS-CoV-2's entry and egress from host cells [
116]. Numerous protease cleavage sites found in the spike protein are crucial for viral pathogenicity and infectivity [
117]. The effectiveness of proteolytic processing and the activation and inactivation of the spike protein can both be altered by mutations in these cleavage sites [
118].
The D614G mutation, which has been demonstrated to improve the efficacy of furin cleavage at the S1/S2 region of the spike protein, is one illustration of such a mutation. This results in increased viral transmissibility and infectivity [
119]. The S686G mutation, among others, can disrupt cleavage at the S2' site and may have an impact on viral entrance and pathogenesis [
120].
On the other hand, mutations that hinder the spike protein's activation and proteolytic processing can affect viral pathogenicity [
52]. For example, changes near the furin cleavage site, like the P681H mutation, have been linked to a reduction in furin-mediated cleavage and, possibly, a reduction in viral entrance and pathogenicity [
121].
For the development of efficient therapeutic approaches against COVID-19, it is essential to comprehend the effects of spike protein mutations on proteolytic processing. It may be possible to thwart viral entrance and reproduction by using protease inhibitors that target the proteases responsible for cleaving spike proteins. Furthermore, keeping an eye on the appearance and dissemination of novel variants with mutations that alter proteolytic processing can reveal crucial details about the pathogenesis and evolution of SARS-CoV-2.
7. Immune Evasion Mechanisms Associated with Spike Protein Mutations
Understanding the immune evasion mechanisms linked to these changes is crucial because SARS-CoV-2 spike protein alterations can affect the virus' capacity to avoid host immunological responses and establish infection (See
Figure 6) [
122].
7.1. Impact on Antibody Binding and Neutralization
It is crucial to take SARS-CoV-2 spike protein mutations into account when creating efficient vaccines and treatments since they can drastically affect how well antibodies can attach to and kill the virus [
123]. The portion of the spike protein's receptor-binding domain (RBD) that directly engages the host cell receptor ACE2 is the principal target of antibodies [
124]. Reduced binding affinity and neutralizing potency can result from mutations in this area that change how the RBD interacts with antibodies and its structure [
125]. For instance, research has revealed that the B.1.351 [South African] variety possesses RBD mutations that have been demonstrated to up to 10-fold reduce antibody binding and neutralization compared to the original strain [
126].
Additionally, it has been determined that antibodies can specifically target the N-terminal domain (NTD) of the spike protein [
127]. A reduction in the binding of several monoclonal antibodies has been linked to mutations in the NTD, raising worries that these mutations may aid immune evasion [
128]. For instance, it has been demonstrated that a mutation in the NTD seen in the P.1 (Brazilian) variation reduces antibody binding and neutralization [
129]. Antibodies can also target the S2 subunit of the spike protein in addition to the RBD and NTD [
128,
129]. The stability of the protein and the capacity of antibodies to recognize it can both be impacted by mutations in this area [
92].
These results emphasize the significance of tracking spike protein mutations for their effect on antibody binding and neutralization, particularly in the context of developing vaccines and antibody-based treatments. To find mutations that may be able to avoid immune recognition and impair the effectiveness of vaccinations and therapeutic antibodies, it is crucial to continuously monitor developing variants. Additionally, the creation of vaccines and treatments that focus on many spike protein areas may assist to lessen the effects of mutations in a single region and provide all-around protection from SARS-CoV-2 and its variants.
7.2. Influence on T-cell Responses and Immune Memory
Mutations in the SARS-CoV-2 spike protein can also impact immunological memory and T-cell responses [
130]. T-cells are essential for the adaptive immune response to viral infections because they can identify and destroy infected cells [
131]. SARS-CoV-2 has the ability to subvert the host immune system, and mutations in the spike protein have been demonstrated to impact T-cell identification of infected cells [
132]. According to Mengist at al., 2021, some mutations in the spike protein can impair the ability of T cells to recognize infected cells, which could result in a weakened immune response and increased viral reproduction.
The long-term immunity to SARS-CoV-2 may also be affected by spike protein mutations. By producing memory T-cells that can quickly react to re-infection with the same virus, T-cells can produce long-lasting immunity against viral infections [
130]. However, memory T-cells may be less able to identify and react to the virus upon re-infection if spike protein mutations lead to modifications to the viral antigens identified by T-cells [
72]. Because of this, research into the effects of spike protein mutations on T-cell responses and immunological memory is crucial, especially in light of the development of long-lasting immunity and the potential need for booster shots.
Spike protein mutations may affect immunological memory and T-cell responses, according to a number of studies. The T-cell response to various SARS-CoV-2 variants, including those with spike protein mutations, was recently examined, and it was discovered that some variants were less successfully detected by T-cells than the original Wuhan strain [
133]. According to another study, people infected with variations bearing the E484K mutation in the spike protein exhibited weaker T-cell responses than people infected with versions lacking the mutation [
134]. These results imply that mutations in the spike protein can influence T-cell identification of infected cells and may have consequences for the long-term immune system.
All things considered, the effect of spike protein mutations on T-cell responses and immunological memory is a significant area of research that calls for additional study. The creation of efficient vaccines and therapies for SARS-CoV-2 can be guided by an understanding of the potential effects of these mutations on the adaptive immune response.
7.3. Implications for Vaccine Efficacy and Escape
The discovery of novel variations with spike protein mutations has sparked worries about the possible effects on vaccine effectiveness [
4]. Vaccines that target the spike protein produce neutralizing antibodies that stop the contact between the virus and host cells, so preventing viral infection [
135]. However, changes to the spike protein may lessen how well vaccine-induced antibodies work. According to numerous investigations, some variations with particular spike protein mutations, like the E484K mutation, can evade neutralization by convalescent plasma or monoclonal antibodies [
136]. Additionally, in vitro research has shown that vaccine recipients' sera have less neutralizing activity against some of the variations, such as the Beta and Gamma versions [
137].
The possibility of immunity conferred by vaccines escaping is a serious worry for vaccine developers. Some vaccine producers have already started making changes to their products to better fit the new versions. Moderna and Pfizer-BioNTech, for instance, have stated that their mRNA vaccines are successful against the variants tested, but they are also creating booster doses that focus on the spike protein mutations seen in the Beta and Gamma variants [
138]. Additionally, Novavax has stated that it is creating a variation-specific booster because its protein-based vaccination is less effective against the Beta version [
139].
It is crucial to remember that vaccines trigger a wide range of immunological responses, including T cell-mediated immunity, which may offer some amount of defense against new variations [
140,
141]. T cell responses have the ability to identify and eradicate virus-infected cells, which may help to reduce the severity of sickness and regulate viral reproduction [
142]. According to several research, spike protein mutations have less of an impact on T cell responses brought on by vaccination or naturally occurring infection than they do on antibody responses [
143]. As a result, T cell responses brought on by vaccination may offer some degree of cross-protection against variations that include spike protein mutations.
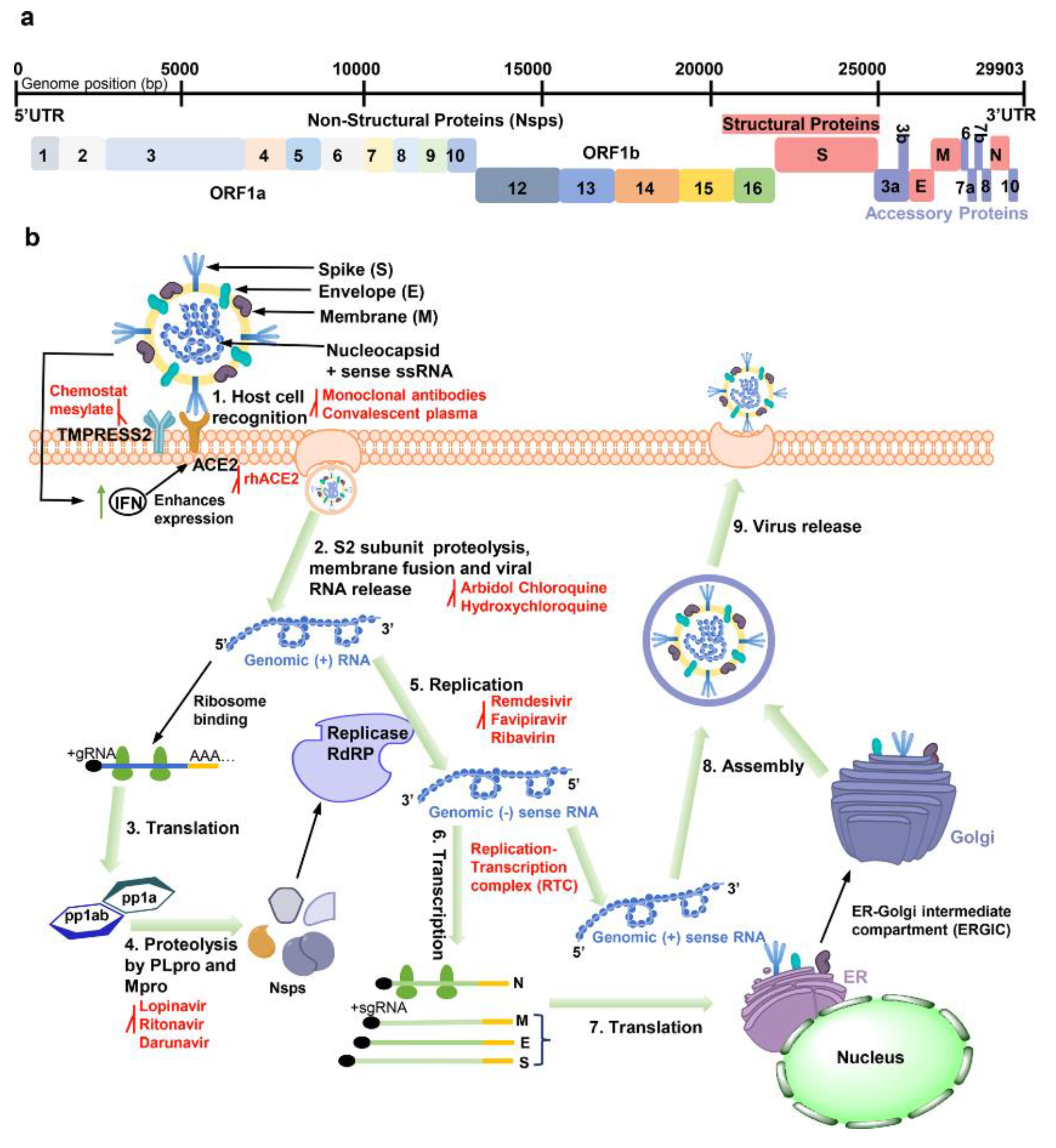
8. Implications for Covid-19 Vaccines and Therapeutics
The outbreak of SARS-CoV-2 and the subsequent COVID-19 pandemic have had a major effect on economy and health around the world [
144]. As a result, an enormous effort has been made to create efficient medicines and vaccines to combat the virus. The spike protein has been the main target for the development of vaccines and treatments because it facilitates viral entrance into host cells [
145,
146]. However, there are worries about the possible effects on vaccination and therapy efficacy due to the virus's continuing evolution, especially the appearance of novel spike protein mutations. Therefore, it is essential to assess how these alterations may affect the creation and application of COVID-19 vaccines and treatments (See
Figure 7).
8.1. Design and Development of Spike Protein-Based Vaccines
Due to its crucial function in viral entrance into host cells, the spike protein of SARS-CoV-2 has been a primary target for the development of COVID-19 vaccines. Typically, entire inactivated virus, live attenuated virus, viral vectors, or mRNA technology are used in the design and development of spike protein-based vaccines [
147]. The selection of a platform is influenced by a variety of elements, including cost-effectiveness, scalability, efficacy, and safety.
Utilizing virus particles that have been inactivated or have undergone attenuation and are expressing the spike protein can result in a broad immune response against several viral antigens [
148]. Another strategy is to introduce the spike protein gene into host cells using viral vectors like adenovirus or vesicular stomatitis virus to start an immunological reaction against the virus [
148,
149]. A more recent strategy makes use of mRNA technology to transport the spike protein's genetic code to host cells, enabling those cells to manufacture the protein and trigger an immune response [
150,
151].
Regardless of the platform chosen, the design of a vaccine based on a spike protein must consider the possible effects of mutations on the vaccination's efficacy [
152]. In order to maintain effectiveness, the vaccine's ability to trigger a protective immune response against the virus may be affected by the introduction of new spike protein mutations, necessitating design changes or booster shots [
85]. For the successful development and application of spike protein-based vaccines against COVID-19, constant viral surveillance and vaccine efficacy monitoring are therefore essential.
8.2. Evaluation of Spike Protein Mutations on Vaccine Efficacy
Concerns have been raised about the effect of SARS-CoV-2 spike protein mutations on the effectiveness of vaccines [
153]. The impact of spike protein mutations on the capacity of vaccine-induced antibodies to neutralize the virus has been examined in a number of studies [
154]. These investigations have demonstrated that specific spike protein mutations can lessen the binding of vaccine-induced neutralizing antibodies [
155]. It has been discovered that changes to the spike protein's receptor-binding domain (RBD) have an impact on how well antibodies attach to and kill viruses [
155,
156].
Researchers have carried out tests using serum samples from vaccine recipients to evaluate the effect of spike protein mutations on vaccine effectiveness [
157]. These investigations have demonstrated that vaccines in use now offer some defence against problematic variations, including those with spike protein mutations [
158]. For some variations, the level of protection could be lessened. For instance, it has been demonstrated that the B.1.351 variation, which carries a number of mutations in the spike protein, decreases the effectiveness of several vaccines [
21].
Updated versions of various vaccinations have been created by vaccine producers to address the potential impact of spike protein mutations on vaccine efficacy [
159]. These modified vaccinations aim to boost protection against these variants by incorporating the spike protein changes discovered in variants of concern. Clinical trials are being conducted now to judge the security and effectiveness of these upgraded vaccines [
160].
In addition to vaccinations, COVID-19 treatment options may also include monoclonal antibodies that target the spike protein [
23]. The effectiveness of these treatments has, however, been questioned in light of the appearance of spike protein mutations [
23,
161]. Monoclonal antibodies' capacity to attach to the spike protein and so neutralize the virus has been demonstrated in studies to be affected by various mutations [
162]. Therefore, ongoing surveillance of spike protein mutations is crucial for the development of effective vaccines and therapies to combat COVID-19.
8.3. Therapeutic Targeting of Spike Protein Mutations
Research into the therapeutic targeting of SARS-CoV-2 spike protein mutations is crucial since it may lessen the severity of the disease and enhance patient outcomes [
28,
18]. The primary target of several monoclonal antibody treatments and currently available COVID-19 vaccines is the spike protein [
158]. The usefulness of these vaccines and treatments has, however, come under scrutiny due to the appearance of novel spike protein mutations [
23]. In order to create novel treatments and enhance the effectiveness of current ones, it is crucial to comprehend how these mutations affect the spike protein's structure and function [
142].
The creation of broad-spectrum antivirals that target conserved areas of the spike protein is one method to address the problems brought on by spike protein mutations [
154]. A different tactic is to combine monoclonal antibodies that are directed against various spike protein epitopes [
160]. Researchers are also investigating the use of mRNA-based vaccinations, which can be quickly updated to target new viral strains [
82].
It's also crucial to remember that there are other factors to consider in addition to how well vaccinations and treatments work against spike protein mutations [
10]. In the struggle against COVID-19, other elements including vaccination hesitancy, vaccine distribution and access, and global equality in vaccine distribution also play a crucial role [
161]. Therefore, combatting the pandemic requires a comprehensive strategy that incorporates vaccine efforts, public health initiatives, and research on the creation of novel therapies and preventative measures.
9. Future Directions and Concluding Remarks
The current COVID-19 pandemic has brought attention to the importance of continuing research on SARS-CoV-2 and the mutations in its spike protein. It is crucial to look into the potential effects of these changes on viral infectivity, pathogenicity, and immune evasion as new virus varieties continue to appear and the world's immunization efforts change [
162].
9.1. Unresolved Questions and Future Research Directions
There is still a lot to learn about the SARS-CoV-2 virus and its spike protein alterations as the COVID-19 pandemic develops. Many concerns are still unsolved despite tremendous advances in understanding the structural and functional effects of these alterations. The long-term effects of spike protein mutations on viral infectivity, transmissibility, and pathogenesis are a crucial field for further study. Determining if and how new mutations will continue to appear and spread, as well as how they may affect the creation and effectiveness of vaccines and medicines, will be crucial. Further study is required to understand the mechanisms causing immune evasion and to create fresh methods of thwarting this phenomenon. There is a pressing need for continued collaboration and communication among scientists, public health officials, and policymakers to ensure that the most up-to-date information is available to guide ongoing efforts to control the spread of COVID-19.
9.2. Significance and Implications of Spike Protein Mutations
A global health emergency has been caused by the development and spread of SARS-CoV-2. Understanding the effects of spike protein mutations is essential for the creation of potent vaccines and treatments as the virus continues to evolve. Spike protein mutations have been linked to alterations in immune evasion, pathogenesis, and receptor binding, all of which can affect the severity of a disease and the effectiveness of a vaccination. The structural and functional effects of spike protein mutations and their implications for viral pathogenesis and infectiousness have been covered in this review. We have also discussed the difficulties in developing vaccines and the possibility for therapeutic targeting of mutations in spike proteins (See
Figure 8).
The rapid development of the virus, which might result in the introduction of new versions with distinct spike protein mutations, is one of the major obstacles in the fight against COVID-19. For efficient communication between scientists and medical experts, the classification and nomenclature of these mutations must be standardized. The development of tailored therapies can be aided by understanding the emergence and dissemination of these mutations using genomic and phylogenetic analysis.
Additionally, the intensity of the illness and the clinical results are significantly impacted by the effect of spike protein mutations on viral proliferation, transmission, and host immune responses. Identifying high-risk patients and creating focused therapies can be made easier by understanding the relationship between spike protein mutations and disease severity. Additionally, the effectiveness of vaccines and immunological memory may be affected by the manipulation of host immune responses by spike protein mutations.
The advent of vaccination-resistant variations emphasizes how crucial it is to assess how spike protein mutations affect vaccine effectiveness. The possibility for mutations to affect vaccination efficacy must be taken into consideration throughout the design and development of spike protein-based vaccines. A further option for the prevention and treatment of COVID-19 is the therapeutic targeting of spike protein mutations.
In conclusion, the study of spike protein mutations is crucial for understanding the evolution, pathogenesis, and control of SARS-CoV-2. Further research is needed to unravel the unresolved questions surrounding the impact of spike protein mutations on viral infectivity, immune evasion, and vaccine efficacy.
9.3. Concluding Remarks and Recommendations.
The structural and functional characteristics of the SARS-CoV-2 spike protein are examined in this review paper, along with the evolutionary dynamics of spike protein mutations and their effects on viral pathogenesis, vaccination effectiveness, and viral infectivity. The article describes how changes to the spike protein's shape, receptor binding affinity and specificity, and interactions with host components can all have an impact on viral reproduction and transmission, control of host immunological responses, and immune evasion strategies. The effectiveness of vaccines and the creation and development of new treatments may both be impacted by these changes, according to the article.
The review concludes that the spike protein is a significant factor in the pathogenesis of SARS-CoV-2 and the creation of efficient vaccines and treatments. Continuous monitoring of spike protein mutations is required to spot new variants that might bypass vaccine-induced immunity and to direct the creation of fresh vaccinations and medications. The authors advise combining vaccines that target several viral components as well as continuing to create broadly protective vaccines that specifically target conserved spike protein domains. The significance and ramifications of spike protein mutations in SARS-CoV-2 pathogenesis and vaccine development are explained in detail by this review.
Author Contributions
Conceptualization, A.G.A.M. and H.K.; writing—original draft preparation, A.G.A.M.; writing—review and editing, S.C.U. and N.A.M; supervision, RK and H.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the University of Kwazulu-Natal.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the College of Health Sciences of the University of Kwazulu-Natal for the support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef]
- Funk, C.D. , Laferrière, C. and Ardakani, A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Frontiers in pharmacology 2020, 11, p937. [Google Scholar] [CrossRef]
- Wang, K. , Chen, W., Zhou, Y.S., Lian, J.Q., Zhang, Z., Du, P., Gong, L., Zhang, Y., Cui, H.Y., Geng, J.J. and Wang, B. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. biorxiv, 2020. [Google Scholar]
- Islam, F. , Dhawan, M., Nafady, M.H., Emran, T.B., Mitra, S., Choudhary, O.P. and Akter, A. Understanding the omicron variant [B. 1.1. 529] of SARS-CoV-2: Mutational impacts, concerns, and the possible solutions. Annals of medicine and surgery, 1037. [Google Scholar]
- Araf, Y. , Akter, F., Tang, Y.D., Fatemi, R., Parvez, M.S.A., Zheng, C. and Hossain, M.G. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. Journal of medical virology 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- Singh, R. , Bhardwaj, V.K., Sharma, J., Kumar, D. and Purohit, R. Identification of potential plant bioactive as SARS-CoV-2 Spike protein and human ACE2 fusion inhibitors. Computers in Biology and Medicine 2021, 136, 104631. [Google Scholar] [CrossRef]
- Kyriakidis, N.C. , López-Cortés, A., González, E.V., Grimaldos, A.B. and Prado, E.O. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Shrestha, L.B. , Foster, C., Rawlinson, W., Tedla, N. and Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA. 1 to BA. 5: Implications for immune escape and transmission. Reviews in Medical Virology 2022, 32, pe2381. [Google Scholar] [CrossRef]
- Khan, N.A. , Al-Thani, H. and El-Menyar, A. The emergence of new SARS-CoV-2 variant [Omicron] and increasing calls for COVID-19 vaccine boosters-The debate continues. Travel medicine and infectious disease 2022, 45, 102246. [Google Scholar] [CrossRef]
- Dhawan, M. , Saied, A.A., Mitra, S., Alhumaydhi, F.A., Emran, T.B. and Wilairatana, P. Omicron variant [B. 1.1. 529] and its sublineages: What do we know so far amid the emergence of recombinant variants of SARS-CoV-2? Biomedicine & Pharmacotherapy, 2022; 113522. [Google Scholar]
- Khandia, R. , Singhal, S., Alqahtani, T., Kamal, M.A., Nahed, A., Nainu, F., Desingu, P.A. and Dhama, K. Emergence of SARS-CoV-2 Omicron [B. 1.1. 529] variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environmental research 2022, 209, 112816. [Google Scholar] [CrossRef]
- Telenti, A. , Arvin, A., Corey, L., Corti, D., Diamond, M.S., García-Sastre, A., Garry, R.F., Holmes, E.C., Pang, P.S. and Virgin, H.W. After the pandemic: perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef]
- Ghaebi, M. , Osali, A., Valizadeh, H., Roshangar, L. and Ahmadi, M.. Vaccine development and therapeutic design for 2019-nCoV/SARS-CoV-2: Challenges and chances. Journal of cellular physiology 2020, 235, 9098–9109. [Google Scholar] [CrossRef]
- Ovsyannikova, I.G. , Haralambieva, I.H., Crooke, S.N., Poland, G.A. and Kennedy, R.B. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunological reviews 2020, 296, 205–219. [Google Scholar] [CrossRef]
- Reis, C.A. , Tauber, R. and Blanchard, V. Glycosylation is a key in SARS-CoV-2 infection. Journal of Molecular Medicine 2021, 99, 1023–1031. [Google Scholar] [CrossRef]
- Kadam, S.B. , Sukhramani, G.S., Bishnoi, P., Pable, A.A. and Barvkar, V.T. SARS-CoV-2, the pandemic coronavirus: Molecular and structural insights. Journal of basic microbiology 2021, 61, 180–202. [Google Scholar] [CrossRef]
- Zhong, P. , Xu, J., Yang, D., Shen, Y., Wang, L., Feng, Y., Du, C., Song, Y., Wu, C., Hu, X. and Sun, Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal transduction and targeted therapy 2020, 5, 256. [Google Scholar] [CrossRef]
- Jackson, C.B. , Farzan, M., Chen, B. and Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nature reviews Molecular cell biology 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Choi, Y.K. , Cao, Y., Frank, M., Woo, H., Park, S.J., Yeom, M.S., Croll, T.I., Seok, C. and Im, W. Structure, dynamics, receptor binding, and antibody binding of the fully glycosylated full-length SARS-CoV-2 spike protein in a viral membrane. Journal of Chemical Theory and Computation 2021, 17, 2479–2487. [Google Scholar] [CrossRef]
- Pang, N.Y.L. , Pang, A.S.R., Chow, V.T. and Wang, D.Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Military Medical Research 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Boehm, E. , Kronig, I., Neher, R.A., Eckerle, I., Vetter, P. and Kaiser, L. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clinical Microbiology and Infection 2021, 27, 1109–1117. [Google Scholar] [CrossRef]
- Gómez, C.E. , Perdiguero, B. and Esteban, M. Emerging SARS-CoV-2 variants and impact in global vaccination programs against SARS-CoV-2/COVID-19. Vaccines 2021, 9, 243. [Google Scholar] [CrossRef]
- Seyran, M. , Takayama, K., Uversky, V.N., Lundstrom, K., Palù, G., Sherchan, S.P., Attrish, D., Rezaei, N., Aljabali, A.A., Ghosh, S. and Pizzol, D. The structural basis of accelerated host cell entry by SARS-CoV-2. The FEBS journal 2021, 288, 5010–5020. [Google Scholar] [CrossRef]
- Bachmann, M.F. , Mohsen, M.O., Zha, L., Vogel, M. and Speiser, D.E. SARS-CoV-2 structural features may explain limited neutralizing-antibody responses. npj Vaccines 2021, 6, 2. [Google Scholar] [CrossRef]
- Chen, T.H. , Tsai, M.J., Chang, C.S., Xu, L., Fu, Y.S. and Weng, C.F. The exploration of phytocompounds theoretically combats SARS-CoV-2 pandemic against virus entry, viral replication and immune evasion. Journal of Infection and Public Health 2023, 16, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Almehdi, A.M. , Khoder, G., Alchakee, A.S., Alsayyid, A.T., Sarg, N.H. and Soliman, S.S. SARS-CoV-2 spike protein: pathogenesis, vaccines, and potential therapies. Infection 2021, 49, 855–876. [Google Scholar] [CrossRef] [PubMed]
- Bian, J. and Li, Z. Angiotensin-converting enzyme 2 [ACE2]: SARS-CoV-2 receptor and RAS modulator. Acta Pharmaceutica Sinica B 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. and Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nature Reviews Microbiology 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.T. , Serrano, M.L., Pujol, F.H. and Rangel, H.R.. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. EXCLI journal 2020, 19, 410. [Google Scholar] [PubMed]
- Ozono, S. , Zhang, Y., Ode, H., Sano, K., Tan, T.S., Imai, K., Miyoshi, K., Kishigami, S., Ueno, T., Iwatani, Y. and Suzuki, T. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nature communications 2021, 12, 848. [Google Scholar] [CrossRef]
- Wicht, O. , Li, W., Willems, L., Meuleman, T.J., Wubbolts, R.W., van Kuppeveld, F.J., Rottier, P.J. and Bosch, B.J.. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. Journal of virology 2014, 88, 7952–7961. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, A. , Pepi, L.E., Rouhani, D.S., Heiss, C. and Azadi, P. Glycosylation of SARS-CoV-2: structural and functional insights. Analytical and bioanalytical chemistry, 2021; 1–15. [Google Scholar]
- Casalino, L. , Gaieb, Z., Goldsmith, J.A., Hjorth, C.K., Dommer, A.C., Harbison, A.M., Fogarty, C.A., Barros, E.P., Taylor, B.C., McLellan, J.S. and Fadda, E. Beyond shielding: the roles of glycans in the SARS-CoV-2 spike protein. ACS central science 2020, 6, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.I. , Porter, J.R., Ward, M.D., Singh, S., Vithani, N., Meller, A., Mallimadugula, U.L., Kuhn, C.E., Borowsky, J.H., Wiewiora, R.P. and Hurley, M.F. SARS-CoV-2 simulations go exascale to predict dramatic spike opening and cryptic pockets across the proteome. Nature chemistry 2021, 13, 651–659. [Google Scholar] [CrossRef]
- Shang, J. , Wan, Y., Luo, C., Ye, G., Geng, Q., Auerbach, A. and Li, F. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B. , Jahanafrooz, Z., Abbasi, A., Goli, M.B., Sadeghi, M., Mottaqi, M.S. and Zamani, M. Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microbial Pathogenesis 2021, 154, 104831. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L. , Charitos, I.A., Carretta, D.M., De Nitto, E. and Lovero, R. The human coronaviruses [HCoVs] and the molecular mechanisms of SARS-CoV-2 infection. Journal of Molecular Medicine 2021, 99, 93–106. [Google Scholar] [CrossRef]
- Xu, W. , Wang, M., Yu, D. and Zhang, X.. Variations in SARS-CoV-2 spike protein cell epitopes and glycosylation profiles during global transmission course of COVID-19. Frontiers in Immunology 2020, 11, p565278. [Google Scholar] [CrossRef]
- Sanches, P.R. , Charlie-Silva, I., Braz, H.L., Bittar, C., Calmon, M.F., Rahal, P. and Cilli, E.M. Recent advances in SARS-CoV-2 Spike protein and RBD mutations comparison between new variants Alpha [B. 1.1. 7, United Kingdom], Beta [B. 1.351, South Africa], Gamma [P. 1, Brazil] and Delta [B. 1.617. 2, India]. Journal of virus eradication 2021, 7, 100054. [Google Scholar]
- Singh, J. , Samal, J., Kumar, V., Sharma, J., Agrawal, U., Ehtesham, N.Z., Sundar, D., Rahman, S.A., Hira, S. and Hasnain, S.E. Structure-function analyses of new SARS-CoV-2 variants B. 1.1. 7, B. 1.351 and B. 1.1. 28.1: clinical, diagnostic, therapeutic and public health implications. Viruses 2021, 13, 439. [Google Scholar]
- Khan, A. , Gui, J., Ahmad, W., Haq, I., Shahid, M., Khan, A.A., Shah, A., Khan, A., Ali, L., Anwar, Z. and Safdar, M. The SARS-CoV-2 B. 1.618 variant slightly alters the spike RBD–ACE2 binding affinity and is an antibody escaping variant: a computational structural perspective. RSC advances 2021, 11, 30132–30147. [Google Scholar]
- Thakur, S. , Sasi, S., Pillai, S.G., Nag, A., Shukla, D., Singhal, R., Phalke, S. and Velu, G.S.K. SARS-CoV-2 mutations and their impact on diagnostics, therapeutics and vaccines. Frontiers in medicine, 2022; 9. [Google Scholar]
- Souza, P.F. , Mesquita, F.P., Amaral, J.L., Landim, P.G., Lima, K.R., Costa, M.B., Farias, I.R., Belém, M.O., Pinto, Y.O., Moreira, H.H. and Magalhaes, I.C. The spike glycoproteins of SARS-CoV-2: A review of how mutations of spike glycoproteins have driven the emergence of variants with high transmissibility and immune escape. International Journal of Biological Macromolecules, 2022. [Google Scholar]
- Tegally, H. , Moir, M., Everatt, J., Giovanetti, M., Scheepers, C., Wilkinson, E., Subramoney, K., Makatini, Z., Moyo, S., Amoako, D.G. and Baxter, C. Emergence of SARS-CoV-2 omicron lineages BA. 4 and BA. 5 in South Africa. Nature medicine 2022, 28, 1785–1790. [Google Scholar] [CrossRef]
- Tegally, H. , Wilkinson, E., Lessells, R.J., Giandhari, J., Pillay, S., Msomi, N., Mlisana, K., Bhiman, J.N., von Gottberg, A., Walaza, S. and Fonseca, V. Sixteen novel lineages of SARS-CoV-2 in South Africa. Nature medicine 2021, 27, 440–446. [Google Scholar] [CrossRef]
- Das, S. Samanta, S., Banerjee, J., Pal, A., Giri, B., Kar, S.S. and Dash, S.K. Is Omicron the end of pandemic or start of a new innings? Travel Medicine and Infectious Disease, 2022; 102332. [Google Scholar]
- Sanyaolu, A. , Okorie, C., Marinkovic, A., Haider, N., Abbasi, A.F., Jaferi, U., Prakash, S. and Balendra, V. The emerging SARS-CoV-2 variants of concern. Therapeutic advances in infectious disease 2021, 8, 20499361211024372. [Google Scholar]
- Laha, S. , Chakraborty, J., Das, S., Manna, S.K., Biswas, S. and Chatterjee, R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infection, genetics and evolution 2020, 85, 104445. [Google Scholar] [CrossRef]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiology and immunology 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Yin, C. Genotyping coronavirus SARS-CoV-2: methods and implic ations. Genomics 2020, 112, 3588–3596. [Google Scholar] [CrossRef]
- Sahin, E. , Bozdayi, G., Yigit, S., Muftah, H., Dizbay, M., Tunccan, O.G., Fidan, I. and Caglar, K. Genomic characterization of SARS-CoV-2 isolates from patients in Turkey reveals the presence of novel mutations in spike and nsp12 proteins. Journal of Medical Virology 2021, 93, 6016–6026. [Google Scholar] [CrossRef]
- Zhang, L. , Wang, S., Ren, Q., Yang, J., Lu, Y., Zhang, L. and Gai, Z. Genome-wide variations of SARS-CoV-2 infer evolution relationship and transmission route. medRxiv, 2020; 2020-04. [Google Scholar]
- Martin, D.P. , Weaver, S., Tegally, H., San, J.E., Shank, S.D., Wilkinson, E., Lucaci, A.G., Giandhari, J., Naidoo, S., Pillay, Y. and Singh, L. The emergence and ongoing convergent evolution of the SARS-CoV-2 N501Y lineages. Cell 2021, 184, 5189–5200. [Google Scholar] [CrossRef]
- Rochman, N.D. , Wolf, Y.I., Faure, G., Mutz, P., Zhang, F. and Koonin, E.V. Ongoing global and regional adaptive evolution of SARS-CoV-2. Proceedings of the National Academy of Sciences 2021, 118, e2104241118. [Google Scholar] [CrossRef]
- Korber, B. , Fischer, W.M., Gnanakaran, S., Yoon, H., Theiler, J., Abfalterer, W., Foley, B., Giorgi, E.E., Bhattacharya, T., Parker, M.D. and Partridge, D.G. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. BioRxiv.
- Fan, Y. , Li, X., Zhang, L., Wan, S., Zhang, L. and Zhou, F. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal transduction and targeted therapy 2022, 7, 141. [Google Scholar] [CrossRef]
- Hossain, A. , Trishna, S.A., Rashid, A.A., Khair, S. and Alam, A.R.U. Unique mutations in SARS-CoV-2 omicron subvariants' non-spike proteins: Potential impact on viral pathogenesis and host immune evasion. Microbial pathogenesis, 1056. [Google Scholar]
- Wu, S. , Tian, C., Liu, P., Guo, D., Zheng, W., Huang, X., Zhang, Y. and Liu, L. Effects of SARS-CoV-2 mutations on protein structures and intraviral protein–protein interactions. Journal of medical virology 2021, 93, 2132–2140. [Google Scholar] [CrossRef]
- Akkiz, H. Implications of the novel mutations in the SARS-CoV-2 genome for transmission, disease severity, and the vaccine development. Frontiers in medicine 2021, 8, 636532. [Google Scholar] [CrossRef]
- Jackson, C.B. , Zhang, L., Farzan, M. and Choe, H. Functional importance of the D614G mutation in the SARS-CoV-2 spike protein. Biochemical and Biophysical Research Communications 2021, 538, 108–115. [Google Scholar] [CrossRef]
- Lazarevic, I. , Pravica, V., Miljanovic, D. and Cupic, M. Immune evasion of SARS-CoV-2 emerging variants: what have we learnt so far? Viruses 2021, 13, 1192. [Google Scholar] [CrossRef]
- Liu, J. , Liu, Y., Xia, H., Zou, J., Weaver, S.C., Swanson, K.A., Cai, H., Cutler, M., Cooper, D., Muik, A. and Jansen, K.U. BNT162b2-elicited neutralization of B. 1.617 and other SARS-CoV-2 variants. Nature 2021, 596, 273–275. [Google Scholar] [CrossRef]
- Prompetchara, E. , Ketloy, C. and Palaga, T., 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pacific journal of allergy and immunology.
- Catanzaro, M. , Fagiani, F., Racchi, M., Corsini, E., Govoni, S. and Lanni, C., 2020. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal transduction and targeted therapy.
- Grant, O.C. , Montgomery, D., Ito, K. and Woods, R.J., 2020. Analysis of the SARS-CoV-2 spike protein glycan shield reveals implications for immune recognition. Scientific reports.
- Shah, V.K. , Firmal, P., Alam, A., Ganguly, D. and Chattopadhyay, S., 2020. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Frontiers in immunology, 1949. [Google Scholar]
- Amor, S. , Fernández Blanco, L. and Baker, D., 2020. Innate immunity during SARS-CoV-2: evasion strategies and activation trigger hypoxia and vascular damage. Clinical & Experimental Immunology.
- Abraham, C. and Medzhitov, R., 2011. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology, 1729. [Google Scholar]
- Groves, D.C. , Rowland-Jones, S.L. and Angyal, A., 2021. The D614G mutations in the SARS-CoV-2 spike protein: Implications for viral infectivity, disease severity and vaccine design. Biochemical and biophysical research communications, 104–107.
- Harrison, A.G. , Lin, T. and Wang, P., 2020. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends in immunology, 1115. [Google Scholar]
- Mangalmurti, N. and Hunter, C.A., 2020. Cytokine storms: understanding COVID-19. Immunity.
- Maneikis, K. , Šablauskas, K., Ringelevičiūtė, U., Vaitekėnaitė, V., Čekauskienė, R., Kryžauskaitė, L., Naumovas, D., Banys, V., Pečeliūnas, V., Beinortas, T. and Griškevičius, L., 2021. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. The Lancet Haematology.
- Khan, S. , Liu, J. and Xue, M., 2020. Transmission of SARS-CoV-2, required developments in research and associated public health concerns. Frontiers in medicine.
- Kumar, S. , Thambiraja, T.S., Karuppanan, K. and Subramaniam, G., 2022. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. Journal of medical virology, 1649. [Google Scholar]
- Ghimire, D. , Han, Y. and Lu, M., 2022. Structural Plasticity and Immune Evasion of SARS-CoV-2 Spike Variants. Viruses.
- Kadam, S.B. , Sukhramani, G.S., Bishnoi, P., Pable, A.A. and Barvkar, V.T., 2021. SARS-CoV-2, the pandemic coronavirus: Molecular and structural insights. Journal of basic microbiology.
- Ou, J. , Zhou, Z., Dai, R., Zhang, J., Zhao, S., Wu, X., Lan, W., Ren, Y., Cui, L., Lan, Q. and Lu, L., 2021. V367F mutation in SARS-CoV-2 spike RBD emerging during the early transmission phase enhances viral infectivity through increased human ACE2 receptor binding affinity. Journal of Virology, 2021. [Google Scholar]
- Villoutreix, B.O. , Calvez, V., Marcelin, A.G. and Khatib, A.M., 2021. In silico investigation of the new UK [B. 1.1. 7] and South African [501y. v2] SARS-CoV-2 variants with a focus at the ace2–spike rbd interface. International journal of molecular sciences.
- Meng, B. , Kemp, S.A., Papa, G., Datir, R., Ferreira, I.A., Marelli, S., Harvey, W.T., Lytras, S., Mohamed, A., Gallo, G. and Thakur, N., 2021. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B. 1.1. 7. Cell reports, 2021. [Google Scholar]
- Kathiravan, M.K. , Radhakrishnan, S., Namasivayam, V. and Palaniappan, S., 2021. An overview of spike surface glycoprotein in severe acute respiratory syndrome–coronavirus. Frontiers in Molecular Biosciences, 2021. [Google Scholar]
- Isobe, A. , Arai, Y., Kuroda, D., Okumura, N., Ono, T., Ushiba, S., Nakakita, S.I., Daidoji, T., Suzuki, Y., Nakaya, T. and Matsumoto, K., 2022. ACE2 N-glycosylation modulates interactions with SARS-CoV-2 spike protein in a site-specific manner. Communications Biology.
- Mengist, H.M. , Kombe, A.J.K., Mekonnen, D., Abebaw, A., Getachew, M. and Jin, T., 2021, June. Mutations of SARS-CoV-2 spike protein: Implications on immune evasion and vaccine-induced immunity. In Seminars in immunology [Vol. 55, p. 101533]. Academic Press.
- Wang, P. Nair, M.S., Liu, L., Iketani, S., Luo, Y., Guo, Y., Wang, M., Yu, J., Zhang, B., Kwong, P.D. and Graham, B.S., 2021. Antibody resistance of SARS-CoV-2 variants B. 1.351 and B. 1.1. 7. Nature, 593[7857], pp.130-135.
- Wang, R. Zhang, Q., Ge, J., Ren, W., Zhang, R., Lan, J., Ju, B., Su, B., Yu, F., Chen, P. and Liao, H., 2021. Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity, 54[7], pp.1611-1621.
- Vigerust, D.J. and Shepherd, V.L., 2007. Virus glycosylation: role in virulence and immune interactions. Trends in microbiology, 15[5], pp.211-218.
- Hossain, A. Trishna, S.A., Rashid, A.A., Khair, S. and Alam, A.R.U., 2022. Unique mutations in SARS-CoV-2 omicron subvariants' non-spike proteins: Potential impact on viral pathogenesis and host immune evasion. Microbial pathogenesis, p.105699.
- Sun, C. , Chen, L., Yang, J., Luo, C., Zhang, Y., Li, J., Yang, J., Zhang, J. and Xie, L., 2020. SARS-CoV-2 and SARS-CoV spike-RBD structure and receptor binding comparison and potential implications on neutralizing antibody and vaccine development. Biorxiv, pp.2020-02.
- Baum, A. , Fulton, B.O., Wloga, E., Copin, R., Pascal, K.E., Russo, V., Giordano, S., Lanza, K., Negron, N., Ni, M. and Wei, Y., 2020. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science, 369[6506], pp.1014-1018.
- Zhou, D. , Dejnirattisai, W., Supasa, P., Liu, C., Mentzer, A.J., Ginn, H.M., Zhao, Y., Duyvesteyn, H.M., Tuekprakhon, A., Nutalai, R. and Wang, B., 2021. Evidence of escape of SARS-CoV-2 variant B. 1.351 from natural and vaccine-induced sera. Cell, 184[9], pp.2348-2361.
- Yewdell, J.W. , 2021. Antigenic drift: understanding COVID-19. Immunity, 54[12], pp.2681-2687.
- Chung, J.Y. , Thone, M.N. and Kwon, Y.J., 2021. COVID-19 vaccines: The status and perspectives in delivery points of view. Advanced drug delivery reviews, 1–25.
- Farzi, R. , Aghbash, P.S., Eslami, N., Azadi, A., Shamekh, A., Hemmat, N., Entezari-Maleki, T. and Baghi, H.B., 2022. The role of antigen-presenting cells in the pathogenesis of COVID-19. Pathology-Research and Practice, 233, p.153848.
- Senapati, S. , Banerjee, P., Bhagavatula, S., Kushwaha, P.P. and Kumar, S., 2021. Contributions of human ACE2 and TMPRSS2 in determining host–pathogen interaction of COVID-19. Journal of Genetics, 1–16.
- Papa, G. , Mallery, D.L., Albecka, A., Welch, L.G., Cattin-Ortolá, J., Luptak, J., Paul, D., McMahon, H.T., Goodfellow, I.G., Carter, A. and Munro, S., 2021. Furin cleavage of SARS-CoV-2 Spike promotes but is not essential for infection and cell-cell fusion. PLoS pathogens, 17[1], p.e1009246.
- Essalmani, R. , Jain, J., Susan-Resiga, D., Andréo, U., Evagelidis, A., Derbali, R.M., Huynh, D.N., Dallaire, F., Laporte, M., Delpal, A. and Sutto-Ortiz, P., 2022. Distinctive roles of furin and TMPRSS2 in SARS-CoV-2 infectivity. Journal of Virology, 96[8], pp.e00128-22.
- Licitra, B.N. , Millet, J.K., Regan, A.D., Hamilton, B.S., Rinaldi, V.D., Duhamel, G.E. and Whittaker, G.R., 2013. Mutation in spike protein cleavage site and pathogenesis of feline coronavirus. Emerging infectious diseases, 19[7], p.1066.
- Meng, B. , Abdullahi, A., Ferreira, I.A., Goonawardane, N., Saito, A., Kimura, I., Yamasoba, D., Gerber, P.P., Fatihi, S., Rathore, S. and Zepeda, S.K., 2022. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature, 603[7902], pp.706-714.
- Verkhivker, G.M. , Agajanian, S., Oztas, D.Y. and Gupta, G., 2021. Comparative perturbation-based modeling of the SARS-CoV-2 spike protein binding with host receptor and neutralizing antibodies: Structurally adaptable allosteric communication hotspots define spike sites targeted by global circulating mutations. Biochemistry, 60[19], pp.1459-1484.
- Meng, B. , Kemp, S.A., Papa, G., Datir, R., Ferreira, I.A., Marelli, S., Harvey, W.T., Lytras, S., Mohamed, A., Gallo, G. and Thakur, N., 2021. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B. 1.1. 7. Cell reports, 35[13], p.109292.
- Lubinski, B. , Jaimes, J.A. and Whittaker, G.R., 2022. Intrinsic furin-mediated cleavability of the spike S1/S2 site from SARS-CoV-2 variant B. 1.1. 529 [Omicron]. BioRxiv, pp.2022-04.
- Thakur, V. , Bhola, S., Thakur, P., Patel, S.K.S., Kulshrestha, S., Ratho, R.K. and Kumar, P., 2021. Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe. Infection, pp.1-16.
- Barton, M.I. , MacGowan, S.A., Kutuzov, M.A., Dushek, O., Barton, G.J. and Van Der Merwe, P.A., 2021. Effects of common mutations in the SARS-CoV-2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. Elife, 10, p.e70658.
- Ren, W. , Ju, X., Gong, M., Lan, J., Yu, Y., Long, Q., Kenney, D.J., O’Connell, A.K., Zhang, Y., Zhong, J. and Zhong, G., 2022. Characterization of SARS-CoV-2 variants B. 1.617. 1 [Kappa], B. 1.617. 2 [Delta], and B. 1.618 by cell entry and immune evasion. MBio, 13[2], pp.e00099-22.
- Nelson, G. , Buzko, O., Spilman, P., Niazi, K., Rabizadeh, S. and Soon-Shiong, P., 2021. Molecular dynamic simulation reveals E484K mutation enhances spike RBD-ACE2 affinity and the combination of E484K, K417N and N501Y mutations [501Y. V2 variant] induces conformational change greater than N501Y mutant alone, potentially resulting in an escape mutant. BioRxiv, pp.2021-01.
- Kalita, P. , Tripathi, T. and Padhi, A.K., 2023. Computational Protein Design for COVID-19 Research and Emerging Therapeutics. ACS Central Science.
- Asghar, A. , Imran, H.M., Bano, N., Maalik, S., Mushtaq, S., Hussain, A., Varjani, S., Aleya, L., Iqbal, H.M. and Bilal, M., 2022. SARS-COV-2/COVID-19: scenario, epidemiology, adaptive mutations, and environmental factors. Environmental Science and Pollution Research, 29[46], pp.69117-69136.
- Yao, H. , Lu, X., Chen, Q., Xu, K., Chen, Y., Cheng, L., Liu, F., Wu, Z., Wu, H., Jin, C. and Zheng, M., 2020. Patient-derived mutations impact pathogenicity of SARS-CoV-2. MedRxiv, pp.2020-04.
- Nguyen, H.T. , Zhang, S., Wang, Q., Anang, S., Wang, J., Ding, H., Kappes, J.C. and Sodroski, J., 2021. Spike glycoprotein and host cell determinants of SARS-CoV-2 entry and cytopathic effects. Journal of virology, 95[5], pp.e02304-20.
- Lubinski, B. , Frazier, L.E., Phan, M.V., Bugembe, D.L., Tang, T., Daniel, S., Cotten, M., Jaimes, J.A. and Whittaker, G.R., 2021. Spike protein cleavage-activation mediated by the SARS-CoV-2 P681R mutation: a case-study from its first appearance in variant of interest [VOI] A. 23.1 identified in Uganda. bioRxiv pp.2021-06.
- Gobeil, S.M.C. , Janowska, K., McDowell, S., Mansouri, K., Parks, R., Stalls, V., Kopp, M.F., Manne, K., Li, D., Wiehe, K. and Saunders, K.O., 2021. Effect of natural mutations of SARS-CoV-2 on spike structure, conformation, and antigenicity. Science, 373[6555], p.eabi6226.
- Harvey, W.T. , Carabelli, A.M., Jackson, B., Gupta, R.K., Thomson, E.C., Harrison, E.M., Ludden, C., Reeve, R., Rambaut, A., COVID-19 Genomics UK [COG-UK] Consortium and Peacock, S.J., 2021. SARS-CoV-2 variants, spike mutations and immune escape. Nature Reviews Microbiology, 19[7], pp.409-424.
- Tang, T. , Bidon, M., Jaimes, J.A., Whittaker, G.R. and Daniel, S., 2020. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral research, 178, p.104792.
- Tiwari, V. , Beer, J.C., Sankaranarayanan, N.V., Swanson-Mungerson, M. and Desai, U.R., 2020. Discovering small-molecule therapeutics against SARS-CoV-2. Drug Discovery Today, 25[8], pp.1535-1544.
- Hoffmann, M. , Kleine-Weber, H. and Pöhlmann, S., 2020. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Molecular cell, 78[4], pp.779-784.
- Lubinski, B. , Fernandes, M.H., Frazier, L., Tang, T., Daniel, S., Diel, D.G., Jaimes, J.A. and Whittaker, G.R., 2022. Functional evaluation of the P681H mutation on the proteolytic activation of the SARS-CoV-2 variant B. 1.1. 7 [Alpha] spike. Iscience, 25[1], p.103589.
- Zhang, L. , Jackson, C.B., Mou, H., Ojha, A., Peng, H., Quinlan, B.D., Rangarajan, E.S., Pan, A., Vanderheiden, A., Suthar, M.S. and Li, W., 2020. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nature communications, 11[1], p.6013.
- Shiliaev, N. , Lukash, T., Palchevska, O., Crossman, D.K., Green, T.J., Crowley, M.R., Frolova, E.I. and Frolov, I., 2021. Natural and recombinant SARS-CoV-2 isolates rapidly evolve in vitro to higher infectivity through more efficient binding to heparan sulfate and reduced S1/S2 cleavage. Journal of Virology, 95[21], pp.e01357-21.
- Liu, Y. , Liu, J., Johnson, B.A., Xia, H., Ku, Z., Schindewolf, C., Widen, S.G., An, Z., Weaver, S.C., Menachery, V.D. and Xie, X., 2022. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell reports, 39[7], p.110829.
- Mistry, P. , Barmania, F., Mellet, J., Peta, K., Strydom, A., Viljoen, I.M., James, W., Gordon, S. and Pepper, M.S., 2022. SARS-CoV-2 variants, vaccines, and host immunity. Frontiers in immunology, 12, p.5400.
- Ao, D. , Lan, T., He, X., Liu, J., Chen, L., Baptista-Hon, D.T., Zhang, K. and Wei, X., 2022. SARS-CoV-2 Omicron variant: Immune escape and vaccine development. MedComm, 3[1], p.e126.
- Sternberg, A. and Naujokat, C., 2020. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life sciences, 257, p.118056.
- Wang, Y. , Liu, C., Zhang, C., Wang, Y., Hong, Q., Xu, S., Li, Z., Yang, Y., Huang, Z. and Cong, Y., 2022. Structural basis for SARS-CoV-2 Delta variant recognition of ACE2 receptor and broadly neutralizing antibodies. Nature communications, 13[1], p.871.
- Dejnirattisai, W. , Zhou, D., Supasa, P., Liu, C., Mentzer, A.J., Ginn, H.M., Zhao, Y., Duyvesteyn, H.M., Tuekprakhon, A., Nutalai, R. and Wang, B., 2021. Antibody evasion by the P. 1 strain of SARS-CoV-2. Cell, 184[11], pp.2939-2954.
- Chi, X. , Yan, R., Zhang, J., Zhang, G., Zhang, Y., Hao, M., Zhang, Z., Fan, P., Dong, Y., Yang, Y. and Chen, Z., 2020. A potent neutralizing human antibody reveals the N-terminal domain of the Spike protein of SARS-CoV-2 as a site of vulnerability. BioRxiv pp.2020-05.
- Cameroni, E. , Bowen, J.E., Rosen, L.E., Saliba, C., Zepeda, S.K., Culap, K., Pinto, D., VanBlargan, L.A., De Marco, A., di Iulio, J. and Zatta, F., 2022. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature, 602[7898], pp.664-670.
- Dan, J.M. , Mateus, J., Kato, Y., Hastie, K.M., Yu, E.D., Faliti, C.E., Grifoni, A., Ramirez, S.I., Haupt, S., Frazier, A. and Nakao, C., 2021. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science, 371[6529], p.eabf4063.
- Tay, M.Z. , Poh, C.M., Rénia, L., MacAry, P.A. and Ng, L.F., 2020. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology, 20[6], pp.363-374.
- Agerer, B. , Koblischke, M., Gudipati, V., Montaño-Gutierrez, L.F., Smyth, M., Popa, A., Genger, J.W., Endler, L., Florian, D.M., Mühlgrabner, V. and Graninger, M., 2021. SARS-CoV-2 mutations in MHC-I-restricted epitopes evade CD8+ T cell responses. Science Immunology, 6[57], p.eabg6461.
- Riou, C. , Keeton, R., Moyo-Gwete, T., Hermanus, T., Kgagudi, P., Baguma, R., Valley-Omar, Z., Smith, M., Tegally, H., Doolabh, D. and Iranzadeh, A., 2021. Escape from recognition of SARS-CoV-2 variant spike epitopes but overall preservation of T cell immunity. Science translational medicine, 14[631], p.eabj6824.
- Pretti, M.A.M. , Galvani, R.G., Scherer, N.M., Farias, A.S. and Boroni, M., 2022. In silico analysis of mutant epitopes in new SARS-CoV-2 lineages suggest global enhanced CD8+ T cell reactivity and also signs of immune response escape. Infection, Genetics and Evolution, 99, p.105236.
- Jiang, S. , Hillyer, C. and Du, L., 2020. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends in immunology, 41[5], pp.355-359.
- Yan, F. , Li, E., Wang, T., Li, Y., Liu, J., Wang, W., Qin, T., Su, R., Pei, H., Wang, S. and Feng, N., 2022. Characterization of two heterogeneous lethal mouse-adapted SARS-CoV-2 variants recapitulating representative aspects of human COVID-19. Frontiers in immunology 13.
- Noori, M. , Nejadghaderi, S.A., Arshi, S., Carson-Chahhoud, K., Ansarin, K., Kolahi, A.A. and Safiri, S., 2022. Potency of BNT162b2 and mRNA-1273 vaccine-induced neutralizing antibodies against severe acute respiratory syndrome-CoV-2 variants of concern: A systematic review of in vitro studies. Reviews in medical virology, 32[2], p.e2277.
- Hadj Hassine, I. 2022. Covid-19 vaccines and variants of concern: A review. Reviews in medical virology, 32[4], p.e2313.
- Lien, G.T.K. , Anh, N.T.B., Thuan, N.T.P. and Nguyen, H.M., 2022. Omicron: Flighty factor challenging global vaccine campaigns or the ending signal of the COVID-19 pandemic. VNUHCM Journal of Science and Technology Development, 25[2], pp.2390-2401.
- McCafferty, S. , Haque, A.A., Vandierendonck, A., Weidensee, B., Plovyt, M., Stuchlíková, M., François, N., Valembois, S., Heyndrickx, L., Michiels, J. and Ariën, K.K., 2022. A dual-antigen self-amplifying RNA SARS-CoV-2 vaccine induces potent humoral and cellular immune responses and protects against SARS-CoV-2 variants through T cell-mediated immunity. Molecular Therapy, 30[9], pp.2968-2983.
- Geers, D. Shamier, M.C., Bogers, S., den Hartog, G., Gommers, L., Nieuwkoop, N.N., Schmitz, K.S., Rijsbergen, L.C., van Osch, J.A., Dijkhuizen, E. and Smits, G., 2021. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Science immunology, 6[59], p.eabj1750.
- Moss, P. 2022. The T cell immune response against SARS-CoV-2. Nature immunology, 23[2], pp.186-193.
- Song, G. He, W.T., Callaghan, S., Anzanello, F., Huang, D., Ricketts, J., Torres, J.L., Beutler, N., Peng, L., Vargas, S. and Cassell, J., 2021. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nature communications, 12[1], p.2938.
- Pinilla, J. Barber, P., Vallejo-Torres, L., Rodríguez-Mireles, S., López-Valcárcel, B.G. and Serra-Majem, L., 2021. The economic impact of the SARS-COV-2 [COVID-19] pandemic in Spain. International journal of environmental research and public health, 18[9], p.4708.
- Suzuki, Y.J. 1and Gychka, S.G., 2021. SARS-CoV-2 spike protein elicits cell signaling in human host cells: implications for possible consequences of COVID-19 vaccines. Vaccines, 9[1], p.36.
- Zhang, Q. , Xiang, R., Huo, S., Zhou, Y., Jiang, S., Wang, Q. and Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal transduction and targeted therapy 2021, 6, 233. [Google Scholar] [CrossRef]
- Peng, X.L. , Cheng, J.S.Y., Gong, H.L., Yuan, M.D., Zhao, X.H., Li, Z. and Wei, D.X., 2021. Advances in the design and development of SARS-CoV-2 vaccines. Military Medical Research.
- Chung, J.Y. , Thone, M.N. and Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Advanced drug delivery reviews 2021, 170, 1–25. [Google Scholar] [CrossRef]
- Vanaparthy, R. , Mohan, G., Vasireddy, D. and Atluri, P. Review of COVID-19 viral vector-based vaccines and COVID-19 variants. Le infezioni in medicina 2021, 29, 328. [Google Scholar]
- Fang, E. , Liu, X., Li, M., Zhang, Z., Song, L., Zhu, B., Wu, X., Liu, J., Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduction and Targeted Therapy 2022, 7, 94. [Google Scholar] [CrossRef]
- Chavda, V.P. , Pandya, R. and Apostolopoulos, V. DNA vaccines for SARS-CoV-2: toward third-generation vaccination era. Expert review of vaccines 2021, 20, 1549–1560. [Google Scholar] [CrossRef]
- Bagherzadeh, M.A. , Izadi, M., Baesi, K., Jahromi, M.A.M. and Pirestani, M. Considering epitopes conservity in targeting SARS-CoV-2 mutations in variants: a novel immunoinformatics approach to vaccine design. Scientific Reports 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Noh, J.Y. , Jeong, H.W. and Shin, E.C. SARS-CoV-2 mutations, vaccines, and immunity: implication of variants of concern. Signal Transduction and Targeted Therapy 2021, 6, 203. [Google Scholar] [CrossRef]
- Wahid, M. , Jawed, A., Mandal, R.K., Dailah, H.G., Janahi, E.M., Dhama, K., Somvanshi, P. and Haque, S. Variants of SARS-CoV-2, their effects on infection, transmission and neutralization by vaccine-induced antibodies. Eur. Rev. Med. Pharmacol. Sci 2021, 25, 5857–5864. [Google Scholar]
- Li, Q. , Wu, J., Nie, J., Zhang, L., Hao, H., Liu, S., Zhao, C., Zhang, Q., Liu, H., Nie, L. and Qin, H. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 2020, 182, 1284–1294. [Google Scholar] [CrossRef]
- Zhu, Z. , Chakraborti, S., He, Y., Roberts, A., Sheahan, T., Xiao, X., Hensley, L.E., Prabakaran, P., Rockx, B., Sidorov, I.A. and Corti, D. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proceedings of the National Academy of Sciences 2007, 104, 12123–12128. [Google Scholar] [CrossRef]
- Thiruvengadam, R. , Awasthi, A., Medigeshi, G., Bhattacharya, S., Mani, S., Sivasubbu, S., Shrivastava, T., Samal, S., Murugesan, D.R., Desiraju, B.K. and Kshetrapal, P. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta [B. 1.617. 2] variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses. The Lancet Infectious Diseases 2022, 22, 473–482. [Google Scholar]
- Kumari, M. , Lu, R.M., Li, M.C., Huang, J.L., Hsu, F.F., Ko, S.H., Ke, F.Y., Su, S.C., Liang, K.H., Yuan, J.P.Y. and Chiang, H.L. A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. Journal of biomedical science 2022, 29, 68. [Google Scholar] [CrossRef]
- Rubin, R. COVID-19 vaccines vs variants—determining how much immunity is enough. Jama 2021, 325, 1241–1243. [Google Scholar] [CrossRef]
- Lai, C.C. , Chen, I.T., Chao, C.M., Lee, P.I., Ko, W.C. and Hsueh, P.R. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert review of vaccines 2021, 20, 1013–1025. [Google Scholar] [CrossRef]
- Cox, M. Peacock, T.P., Harvey, W.T., Hughes, J., Wright, D.W., COVID-19 Genomics UK [COG-UK] Consortium, Willett, B.J., Thomson, E., Gupta, R.K., Peacock, S.J. and Robertson, D.L., 2023. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nature Reviews Microbiology, 21[2], pp.112-124.
- Marovich, M. , Mascola, J.R. and Cohen, M.S., 2020. Monoclonal antibodies for prevention and treatment of COVID-19. Jama 2020, 1013–1025. [Google Scholar]
- Straus, M.R. , Tang, T., Lai, A.L., Flegel, A., Bidon, M., Freed, J.H., Daniel, S. and Whittaker, G.R. Ca2+ ions promote fusion of Middle East respiratory syndrome coronavirus with host cells and increase infectivity. Journal of virology 2020, 94, e00426-20. [Google Scholar] [CrossRef]
- Liu, L. , Wang, P., Nair, M.S., Yu, J., Rapp, M., Wang, Q., Luo, Y., Chan, J.F.W., Sahi, V., Figueroa, A. and Guo, X.V. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef]
- Van De Pas, R. , Widdowson, M.A., Ravinetto, R., N Srinivas, P., Ochoa, T.J., Fofana, T.O. and Van Damme, W. COVID-19 vaccine equity: a health systems and policy perspective. Expert Review of Vaccines 2022, 21, 25–36. [Google Scholar] [CrossRef]
- Moghaddar, M. , Radman, R. and Macreadie, I. Severity, pathogenicity and transmissibility of delta and lambda variants of SARS-CoV-2, toxicity of spike protein and possibilities for future prevention of COVID-19. Microorganisms 2021, 9, 2167. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).