Submitted:
04 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
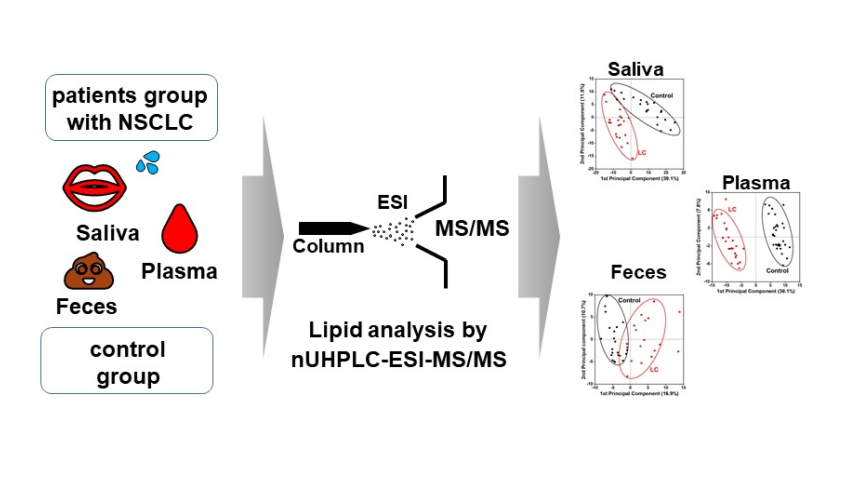
2. Materials and Methods
2.1. Materials and reagents
2.2. Human samples
2.3. Lipid extraction
2.4. Lipid analysis with nUHPLC-ESI-MS/MS
2.5. Method validation
3. Results
3.1. Identification and target-based lipid quantification
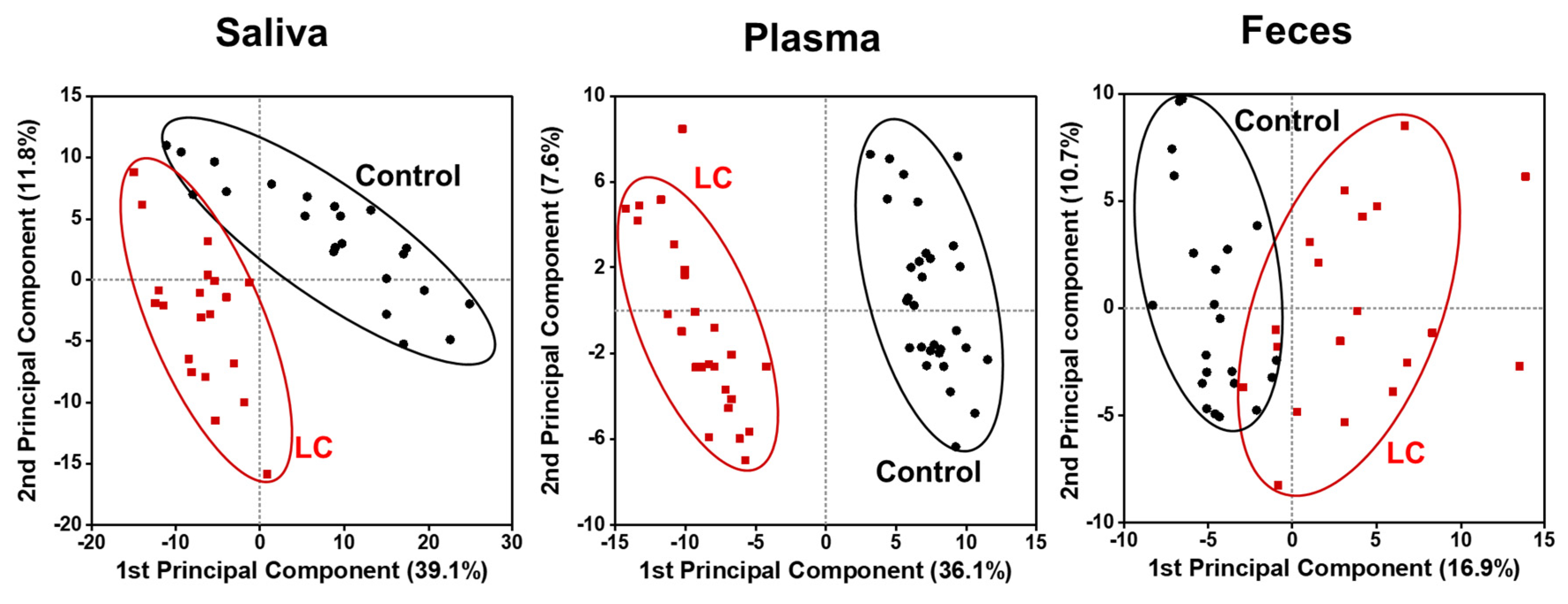
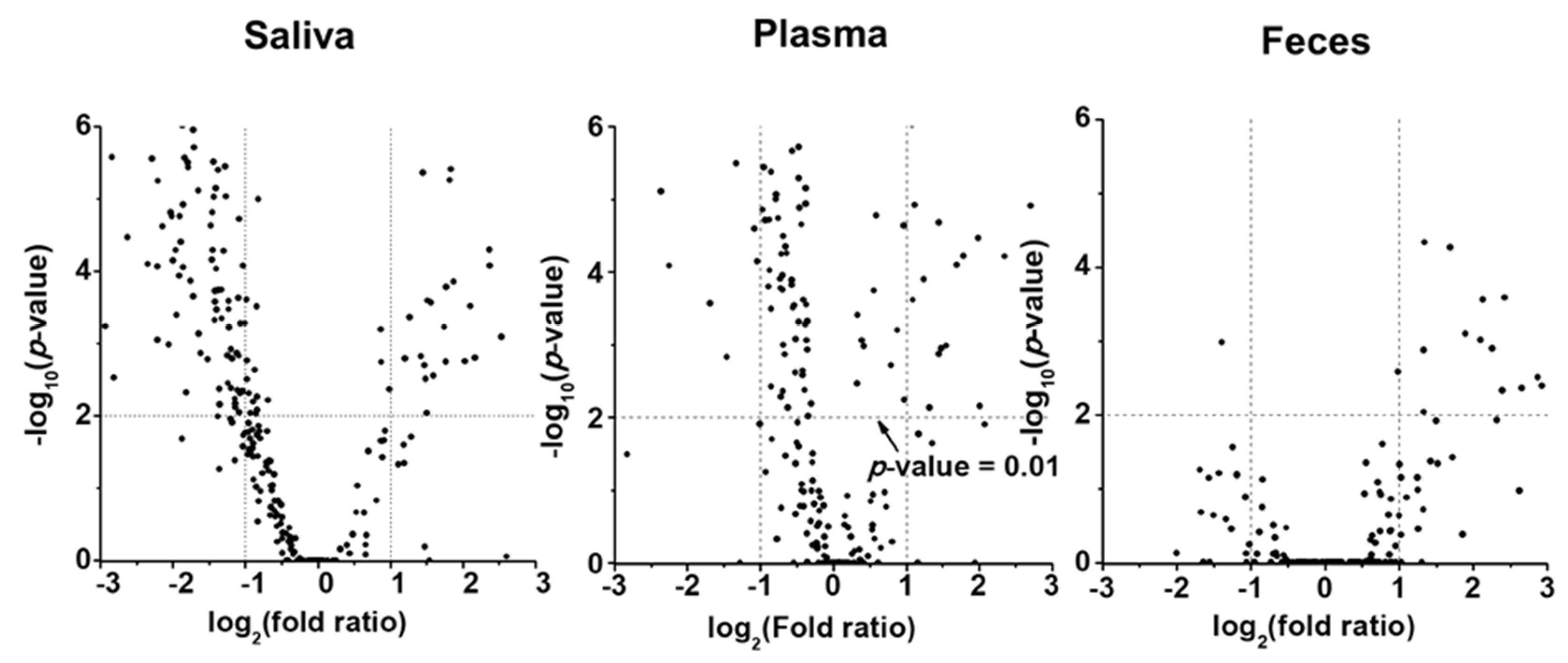
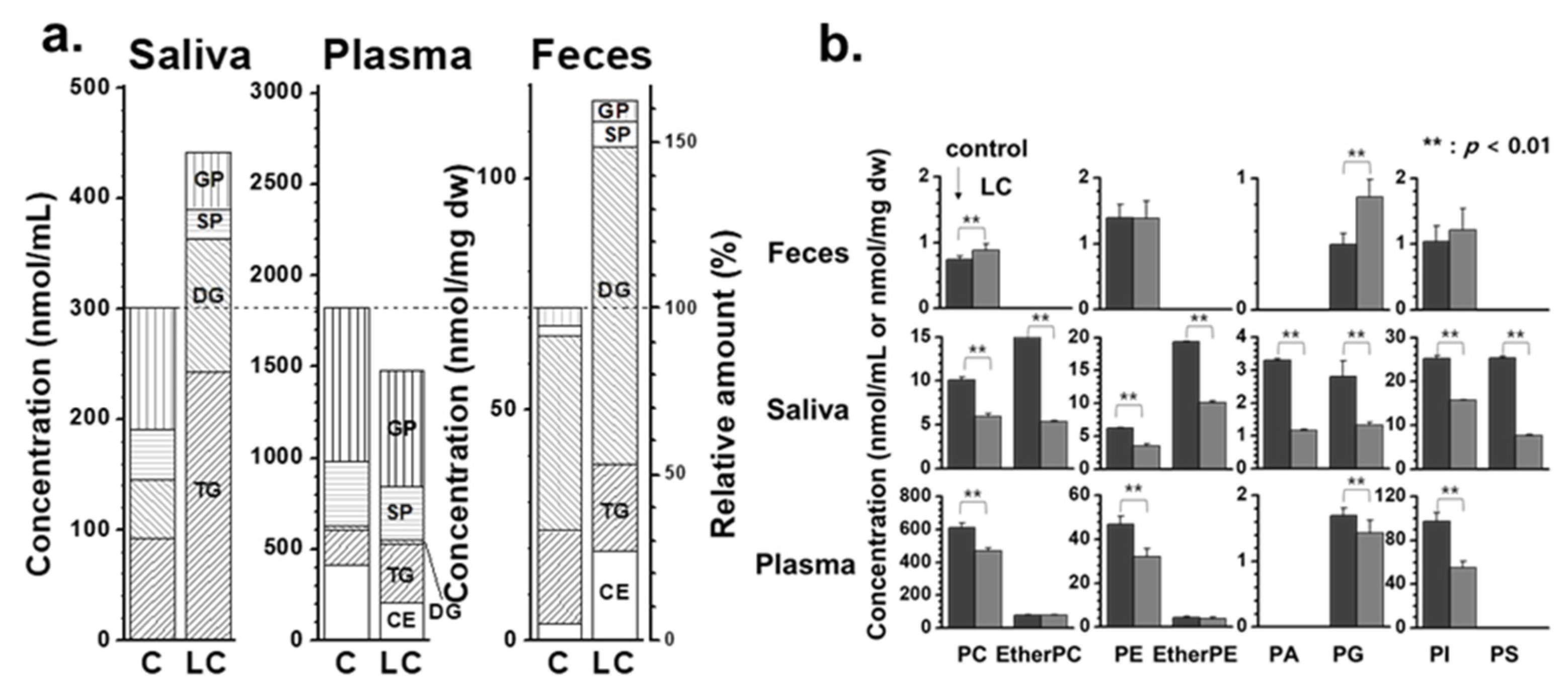
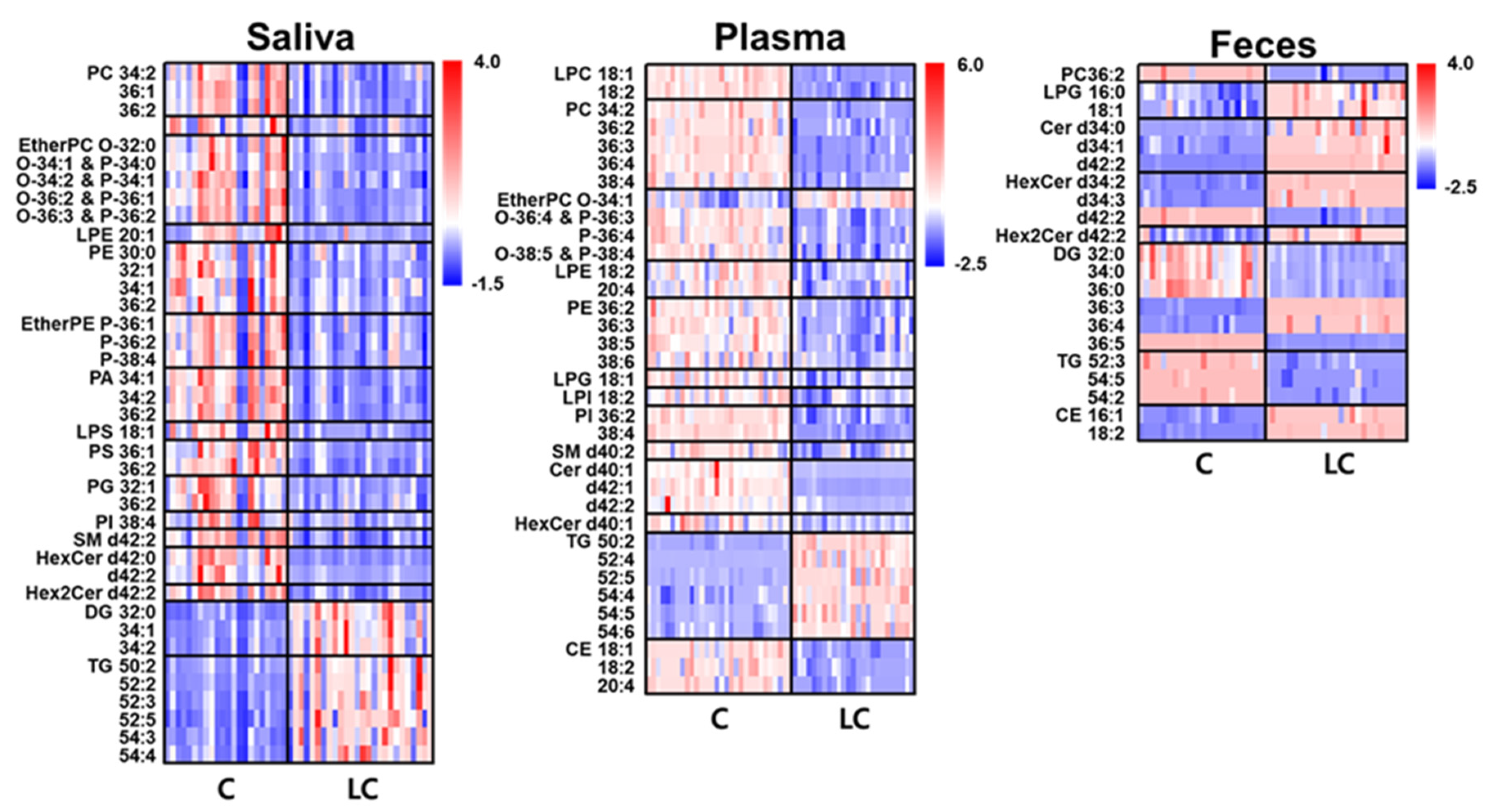
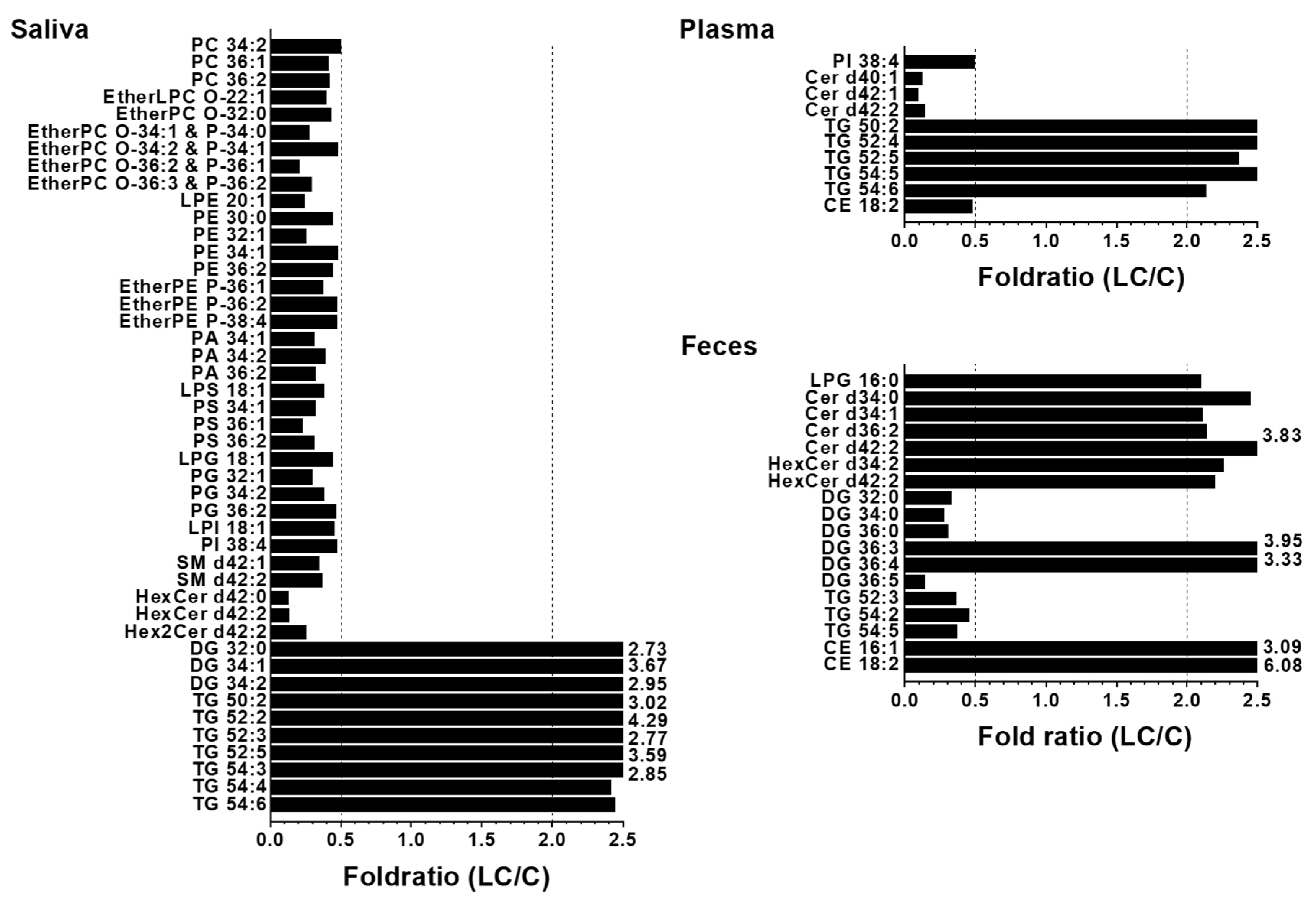
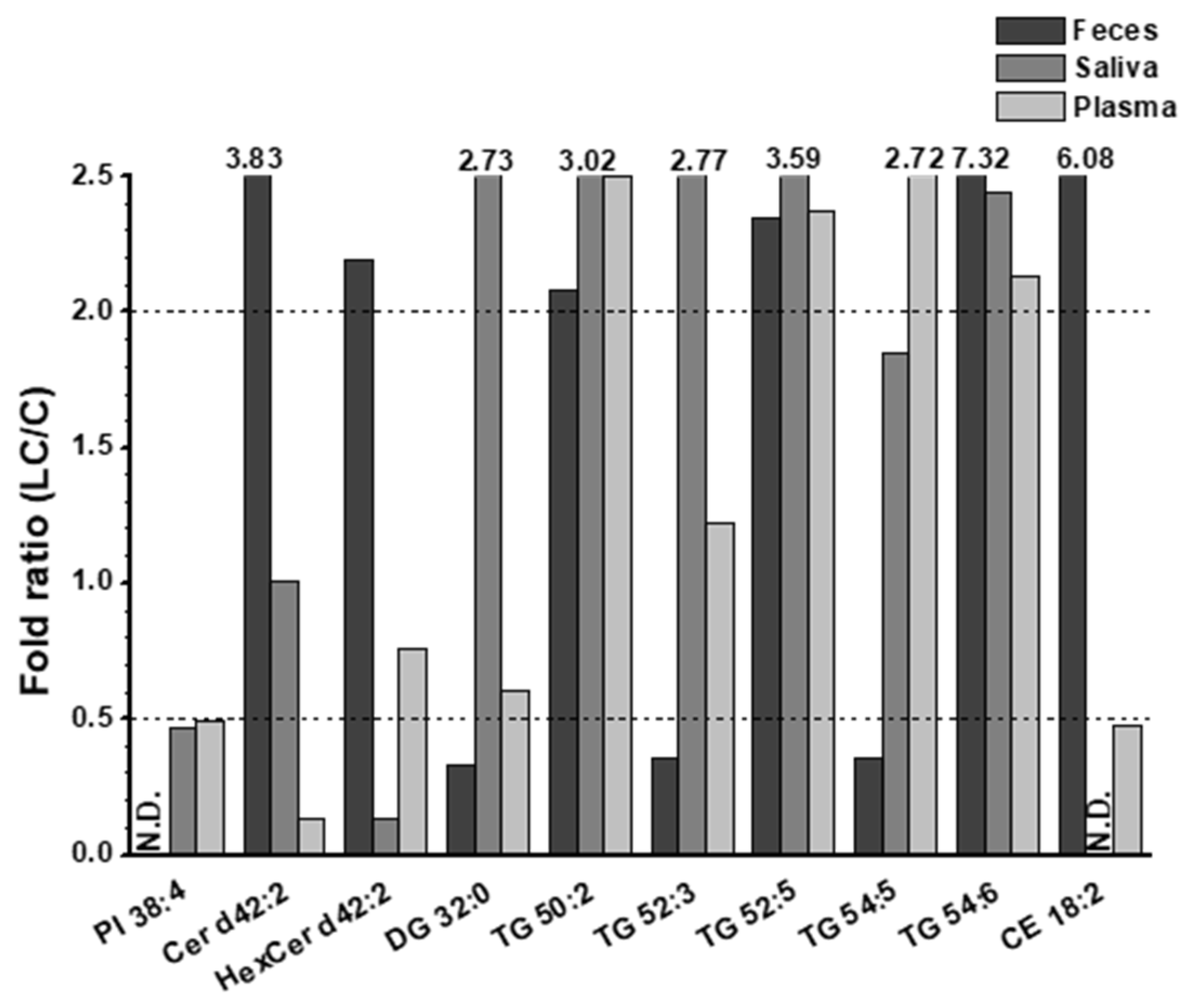
3.2. Alterations in lipid profiles of Saliva, plasma, and fecal samples in NSCLC patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018, 68, 394-424. [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. The Lancet 2021, 398, 535-554. [CrossRef]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol 2017, 7. [CrossRef]
- Holčapek, M.; Liebisch, G.; Ekroos, K. Lipidomic Analysis. Analytical Chemistry 2018, 90, 4249-4257. [CrossRef]
- Knittelfelder, O.L.; Weberhofer, B.P.; Eichmann, T.O.; Kohlwein, S.D.; Rechberger, G.N. A versatile ultra-high performance LC-MS method for lipid profiling. Journal of Chromatography B 2014, 951-952, 119-128. [CrossRef]
- García-Cañaveras, J.C.; Donato, M.T.; Castell, J.V.; Lahoz, A. A Comprehensive Untargeted Metabonomic Analysis of Human Steatotic Liver Tissue by RP and HILIC Chromatography Coupled to Mass Spectrometry Reveals Important Metabolic Alterations. Journal of Proteome Research 2011, 10, 4825-4834. [CrossRef]
- Zhao, Y.Y.; Miao, H.; Cheng, X.L.; Wei, F. Lipidomics: Novel insight into the biochemical mechanism of lipid metabolism and dysregulation-associated disease. Chem Biol Interact 2015, 240, 220-238. [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J 2012, 279, 2610-2623. [CrossRef]
- Lee, G.B.; Lee, J.C.; Moon, M.H. Plasma lipid profile comparison of five different cancers by nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. Analytica Chimica Acta 2019, 1063, 117-126. [CrossRef]
- Marien, E.; Meister, M.; Muley, T.; Fieuws, S.; Bordel, S.; Derua, R.; Spraggins, J.; Van de Plas, R.; Dehairs, J.; Wouters, J.; et al. Non-small cell lung cancer is characterized by dramatic changes in phospholipid profiles. Int J Cancer 2015, 137, 1539-1548. [CrossRef]
- Noreldeen, H.A.A.; Du, L.; Li, W.; Liu, X.; Wang, Y.; Xu, G. Serum lipidomic biomarkers for non-small cell lung cancer in nonsmoking female patients. J Pharm Biomed Anal 2020, 185, 113220. [CrossRef]
- Siqueira, W.L.; Zhang, W.; Helmerhorst, E.J.; Gygi, S.P.; Oppenheim, F.G. Identification of Protein Components in in vivo Human Acquired Enamel Pellicle Using LC−ESI−MS/MS. Journal of Proteome Research 2007, 6, 2152-2160. [CrossRef]
- Freitas, D.; Le Feunteun, S. Oro-gastro-intestinal digestion of starch in white bread, wheat-based and gluten-free pasta: Unveiling the contribution of human salivary α-amylase. Food Chemistry 2019, 274, 566-573. [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W.; Smirnov, V.; Petukhov, A.; Gegechkori, V.; Kuzina, V.; Gorpinchenko, N.; Ramenskaya, G. Analytical Strategies in Lipidomics for Discovery of Functional Biomarkers from Human Saliva. Dis Markers 2019, 2019, 6741518. [CrossRef]
- Turunen, S.; Puurunen, J.; Auriola, S.; Kullaa, A.M.; Kärkkäinen, O.; Lohi, H.; Hanhineva, K. Metabolome of canine and human saliva: A non-targeted metabolomics study. Metabolomics 2020, 16, 90. [CrossRef]
- de Oliveira, L.R.P.; Martins, C.; Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; de Oliveira Torres, R.; Soares, A.L.; Almeida, F.C.L.; Valente, A.P.; de Souza, I.P.R. Salivary Metabolite Fingerprint of Type 1 Diabetes in Young Children. Journal of Proteome Research 2016, 15, 2491-2499. [CrossRef]
- Sun, Y.; Liu, S.; Qiao, Z.; Shang, Z.; Xia, Z.; Niu, X.; Qian, L.; Zhang, Y.; Fan, L.; Cao, C.-X.; et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Analytica Chimica Acta 2017, 982, 84-95. [CrossRef]
- Lee, G.B.; Caner, A.; Moon, M.H. Optimisation of saliva volumes for lipidomic analysis by nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. Analytica Chimica Acta 2022, 1193, 339318. [CrossRef]
- Karu, N.; Deng, L.; Slae, M.; Guo, A.C.; Sajed, T.; Huynh, H.; Wine, E.; Wishart, D.S. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database. Analytica Chimica Acta 2018, 1030, 1-24. [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans With Nonalcoholic Fatty Liver Disease. Clinical Gastroenterology and Hepatology 2013, 11, 868-875.e863. [CrossRef]
- Jia, P.; Wang, S.; Xiao, C.; Yang, L.; Chen, Y.; Jiang, W.; Zheng, X.; Zhao, G.; Zang, W.; Zheng, X. The anti-atherosclerotic effect of tanshinol borneol ester using fecal metabolomics based on liquid chromatography-mass spectrometry. Analyst 2016, 141, 1112-1120. [CrossRef]
- Bjerrum, J.T.; Wang, Y.; Hao, F.; Coskun, M.; Ludwig, C.; Günther, U.; Nielsen, O.H. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics 2015, 11, 122-133. [CrossRef]
- Gregory, K.E.; Bird, S.S.; Gross, V.S.; Marur, V.R.; Lazarev, A.V.; Walker, W.A.; Kristal, B.S. Method Development for Fecal Lipidomics Profiling. Analytical Chemistry 2013, 85, 1114-1123. [CrossRef]
- Van Meulebroek, L.; De Paepe, E.; Vercruysse, V.; Pomian, B.; Bos, S.; Lapauw, B.; Vanhaecke, L. Holistic Lipidomics of the Human Gut Phenotype Using Validated Ultra-High-Performance Liquid Chromatography Coupled to Hybrid Orbitrap Mass Spectrometry. Anal Chem 2017, 89, 12502-12510. [CrossRef]
- Ertl, V.M.; Horing, M.; Schott, H.F.; Blucher, C.; Kjolbaek, L.; Astrup, A.; Burkhardt, R.; Liebisch, G. Quantification of diacylglycerol and triacylglycerol species in human fecal samples by flow injection Fourier transform mass spectrometry. Anal Bioanal Chem 2020, 412, 2315-2326. [CrossRef]
- Coleman, M.J.; Espino, L.M.; Lebensohn, H.; Zimkute, M.V.; Yaghooti, N.; Ling, C.L.; Gross, J.M.; Listwan, N.; Cano, S.; Garcia, V.; et al. Individuals with Metabolic Syndrome Show Altered Fecal Lipidomic Profiles with No Signs of Intestinal Inflammation or Increased Intestinal Permeability. Metabolites 2022, 12. [CrossRef]
- Gao, B.; Zeng, S.; Maccioni, L.; Shi, X.; Armando, A.; Quehenberger, O.; Zhang, X.; Starkel, P.; Schnabl, B. Lipidomics for the Prediction of Progressive Liver Disease in Patients with Alcohol Use Disorder. Metabolites 2022, 12. [CrossRef]
- Cífková, E.; Brumarová, R.; Ovčačíková, M.; Dobešová, D.; Mičová, K.; Kvasnička, A.; Vaňková, Z.; Šiller, J.; Sákra, L.; Friedecký, D.; et al. Lipidomic and metabolomic analysis reveals changes in biochemical pathways for non-small cell lung cancer tissues. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2022, 1867, 159082. [CrossRef]
- Eggers, L.F.; Müller, J.; Marella, C.; Scholz, V.; Watz, H.; Kugler, C.; Rabe, K.F.; Goldmann, T.; Schwudke, D. Lipidomes of lung cancer and tumour-free lung tissues reveal distinct molecular signatures for cancer differentiation, age, inflammation, and pulmonary emphysema. Scientific Reports 2017, 7, 11087. [CrossRef]
- Fan, Y.; Noreldeen, H.A.A.; You, L.; Liu, X.; Pan, X.; Hou, Z.; Li, Q.; Li, X.; Xu, G. Lipid alterations and subtyping maker discovery of lung cancer based on nontargeted tissue lipidomics using liquid chromatography-mass spectrometry. J Pharm Biomed Anal 2020, 190, 113520. [CrossRef]
- Cesbron, N.; Royer, A.L.; Guitton, Y.; Sydor, A.; Le Bizec, B.; Dervilly-Pinel, G. Optimization of fecal sample preparation for untargeted LC-HRMS based metabolomics. Metabolomics 2017, 13, 99. [CrossRef]
- Koelmel, J.P.; Kroeger, N.M.; Ulmer, C.Z.; Bowden, J.A.; Patterson, R.E.; Cochran, J.A.; Beecher, C.W.W.; Garrett, T.J.; Yost, R.A. LipidMatch: An automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinformatics 2017, 18, 331. [CrossRef]
- Ros-Mazurczyk, M.; Jelonek, K.; Marczyk, M.; Binczyk, F.; Pietrowska, M.; Polanska, J.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Widlak, P. Serum lipid profile discriminates patients with early lung cancer from healthy controls. Lung Cancer 2017, 112, 69-74. [CrossRef]
- Zhang, Q.; Xu, H.; Liu, R.; Gao, P.; Yang, X.; Jin, W.; Zhang, Y.; Bi, K.; Li, Q. A Novel Strategy for Targeted Lipidomics Based on LC-Tandem-MS Parameters Prediction, Quantification, and Multiple Statistical Data Mining: Evaluation of Lysophosphatidylcholines as Potential Cancer Biomarkers. Anal Chem 2019, 91, 3389-3396. [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr 2017, 1859, 1558-1572. [CrossRef]
- Gao, L.; Wen, Z.; Wu, C.; Wen, T.; Ong, C.N. Metabolic profiling of plasma from benign and malignant pulmonary nodules patients using mass spectrometry-based metabolomics. Metabolites 2013, 3, 539-551. [CrossRef]
- Lee, G.B.; Lee, J.C.; Moon, M.H. Plasma lipid profile comparison of five different cancers by nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta 2019, 1063, 117-126. [CrossRef]
- Yang, Z.; Song, Z.; Chen, Z.; Guo, Z.; Jin, H.; Ding, C.; Hong, Y.; Cai, Z. Metabolic and lipidomic characterization of malignant pleural effusion in human lung cancer. J Pharm Biomed Anal 2020, 180, 113069. [CrossRef]
- Yang, Y.; Ma, L.; Qiao, X.; Zhang, X.; Dong, S.F.; Wu, M.T.; Zhai, K.; Shi, H.Z. Salivary microRNAs show potential as biomarkers for early diagnosis of malignant pleural effusion. Transl Lung Cancer Res 2020, 9, 1247-1257. [CrossRef]
- Zhou, X.; Tian, C.; Cao, Y.; Zhao, M.; Wang, K. The role of serine metabolism in lung cancer: From oncogenesis to tumor treatment. Front Genet 2022, 13, 1084609. [CrossRef]
- Numata, M.; Voelker, D.R. Anti-inflammatory and anti-viral actions of anionic pulmonary surfactant phospholipids. Biochim Biophys Acta Mol Cell Biol Lipids 2022, 1867, 159139. [CrossRef]
- van Dieren, J.M.; Simons-Oosterhuis, Y.; Raatgeep, H.C.; Lindenbergh-Kortleve, D.J.; Lambers, M.E.; van der Woude, C.J.; Kuipers, E.J.; Snoek, G.T.; Potman, R.; Hammad, H.; et al. Anti-inflammatory actions of phosphatidylinositol. Eur J Immunol 2011, 41, 1047-1057. [CrossRef]
- Zhang, Y.; He, C.; Qiu, L.; Wang, Y.; Zhang, L.; Qin, X.; Liu, Y.; Zhang, D.; Li, Z. Serum unsaturated free Fatty acids: Potential biomarkers for early detection and disease progression monitoring of non-small cell lung cancer. J Cancer 2014, 5, 706-714. [CrossRef]
- Piccinini, M.; Scandroglio, F.; Prioni, S.; Buccinna, B.; Loberto, N.; Aureli, M.; Chigorno, V.; Lupino, E.; DeMarco, G.; Lomartire, A.; et al. Deregulated sphingolipid metabolism and membrane organization in neurodegenerative disorders. Mol Neurobiol 2010, 41, 314-340. [CrossRef]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 2004, 4, 604-616. [CrossRef]
- Hoppstadter, J.; Dembek, A.; Horing, M.; Schymik, H.S.; Dahlem, C.; Sultan, A.; Wirth, N.; Al-Fityan, S.; Diesel, B.; Gasparoni, G.; et al. Dysregulation of cholesterol homeostasis in human lung cancer tissue and tumour-associated macrophages. EBioMedicine 2021, 72, 103578. [CrossRef]
- Hall, Z.; Wilson, C.H.; Burkhart, D.L.; Ashmore, T.; Evan, G.I.; Griffin, J.L. Myc linked to dysregulation of cholesterol transport and storage in nonsmall cell lung cancer. J Lipid Res 2020, 61, 1390-1399. [CrossRef]
- Yu, Z.; Chen, H.; Zhu, Y.; Ai, J.; Li, Y.; Gu, W.; Borgia, J.A.; Zhang, J.; Jiang, B.; Chen, W.; et al. Global lipidomics reveals two plasma lipids as novel biomarkers for the detection of squamous cell lung cancer: A pilot study. Oncol Lett 2018, 16, 761-768. [CrossRef]
- Eltayeb, K.; La Monica, S.; Tiseo, M.; Alfieri, R.; Fumarola, C. Reprogramming of Lipid Metabolism in Lung Cancer: An Overview with Focus on EGFR-Mutated Non-Small Cell Lung Cancer. Cells 2022, 11. [CrossRef]
| Saliva | Plasma | Feces | ||||||
|---|---|---|---|---|---|---|---|---|
| Molecular species | AUC | Molecular species | AUC | Molecular species | AUC | Molecular species | AUC | |
| a) | PC 36:1 | 0.840 | PS 36:1 | 0.902 | PI 38:4 | 1.000 | LPG 16:0 | 0.846 |
| PC 36:2 | 0.831 | PS 36:2 | 0.928 | Cer d40:1 | 1.000 | Cer d34:0 | 0.827 | |
| EtherPC O-34:1 & P-34:0 | 0.885 | PG 32:1 | 0.815 | Cer d42:1 | 1.000 | Cer d34:1 | 0.981 | |
| EtherPC O-34:2 & P-34:1 | 0.817 | SM d42:1 | 0.881 | TG 50:2 | 1.000 | Cer d34:2 | 0.875 | |
| EtherPC O-36:2 & P-36:1 | 0.913 | SM d42:2 | 0.837 | TG 52:4 | 0.969 | DG 32:0 | 0.967 | |
| EtherPC O-36:3 & P-36:2 | 0.841 | HexCer d42:0 | 0.940 | TG 54:6 | 0.864 | DG 34:0 | 0.978 | |
| PE 32:1 | 0.866 | HexCer d42:2 | 0.872 | DG 36:0 | 0.971 | |||
| EtherPE P-36:1 | 0.835 | Hex2Cer d42:2 | 0.861 | DG 36:3 | 1.000 | |||
| PA 34:1 | 0.911 | DG 32:0 | 0.928 | DG 36:4 | 0.907 | |||
| PA 34:2 | 0.872 | DG 34:1 | 0.872 | DG 36:5 | 1.000 | |||
| PA 36:2 | 0.914 | DG 34:2 | 0.810 | TG 54:2 | 1.000 | |||
| LPS 18:1 | 0.815 | TG 52:2 | 0.847 | CE 16:1 | 0.950 | |||
| PS 34:1 | 0.936 | |||||||
| b) | Cer d42:2 | 0.948 | Cer d42:2 | 1.000 | ||||
| TG 52:3 | 0.806 | TG 52:3 | 0.950 | |||||
| TG 52:5 | 0.844 | TG 52:5 | 0.927 | |||||
| TG 54:5 | 0.912 | TG 54:5 | 1.000 | |||||
| CE 18:2 | 0.930 | CE 18:2 | 1.000 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





