1. Introduction
The idiopathic scoliosis (IS) is a developmental deformity of the spine and trunk in three planes, the most expressed is the lateral spine curvature in thoracolumbar vertebrae and the rotation along the axis. The results of epidemiological studies show significant incident rate discrepancy from 0.93% to 12% in the world population [
1,
2]. Untreated scoliosis can lead to significant trunk deformities, changes in the biomechanics of the chest, and the development of internal organ pathologies. Abnormal curvature of the spine, most often and fast developing from the age of four years, affects the anatomical relationships of the spinal cord in the spinal central canal, leads to changes in the activity of the grey matter nerve centers, conduction of nerve impulses in the axons of the lateral funiculi in the white matter, changes in the conduction of spinal roots, development of neuropathy in the peripheral nervous system and neurogenic changes in the muscular system [
3]. In addition to the pathologies mentioned above, the aesthetic factor of a deformed body figure is one of the main reasons for the patient and his family to seek the most effective ways of treating IS, which they expect from a spine surgeons [
4].
The conservative treatment by means of the physiotherapy [
5] and Cheneau-brace [
6,
7,
8] application can be useful for the prevention of scoliotic curve progression and sometimes it’s slowing down or limitation in patients with IS. However, many factors influence the effect of such therapy and the surgical implantation of the deformity corrective instrumentation is necessary in majority of progressive IS cases [
9], especially when its lateral main angle exceeds 40 degrees [
10].
Spinal surgery involves a wide spectrum of procedures during which the spinal cord, nerve roots, and key blood vessels are frequently at risk for injury. Neurologic complications may occur in 6.3% patients through various mechanisms, including direct trauma to the spinal cord, ischemia, and stretch during IS deformity correction [
11]. Intraoperative neuromonitoring provides a safe and useful warning mechanism to minimize spinal cord injury that may arise during scoliosis correction surgery in pediatric patients [
12]. This procedure utilizes methods of clinical neurophysiology to assess the afferent and efferent neural impulse transmission in the spinal cord tracts based on the electrical or magnetic stimulation of the sensory and motor pathways [
13]. Combined somatosensory-evoked (SEP) and neurogenic motor-evoked (MEP) potentials monitoring during IS surgery represents a contemporary standard of care [
14] that enables abandonment of the need for an intraoperative Vauzelle and Stagnar "wake-up" test popular until the end of the 1980s [
15]. During critical intraoperative procedures which may be iatrogenic for the spinal cord structures or its vascularity, the reliable data obtained from neurophysiological recordings are immediately reported to the surgeon, who changes, repeats or abandons the last performed procedure [
16,
17].
The value of motor evoked potentials (MEPs) recordings in evaluating efferent trans-mission within spinal cord tracts during neuromonitoring associated with spine surgeries is undeniable. However, the vast majority of studies devoted to the surgical correction of idiopathic scoliosis when neuromonitoring procedures have been used describe ambiguously or with little detail MEPs parameters that generally should prove the absence of side effects caused by either implant positioning or corrective maneuvers (distraction, derotation). Usually, the researchers provide data on the percentages of changes which should be considered critical in intraoperative MEPs recordings [
18], or they focus on the selection of the most dangerous elements of the surgical procedure that may affect the occurrence of iatrogenic side effects [
19,
20]. The morphology and parameters of MEPs recorded intraoperatively have not been presented, compared or discussed in detail in the literature or the relevant studies were performed on the small population of IS patients [
21]. In an extensive review of this issue, Chang et al. [
22] did not show details of parameter variability, but found that during spinal deformity surgery, combined MEP and SEP monitoring shows high sensitivity and specificity for detection of the neural transmission deficits. Most papers are concentrated on the variability of MEPs depending on the number of applied pedicle screws for mounting the corrective spinal instrumentation, maneuvers and the type of instrumentation used in IS surgery [
20]. Waveform MEPs deterioration has been shown to commonly occur during rotation maneuvers and more frequently in patients with a larger preoperative lateral spinal curvature. Significant relationship between the number of spinal levels fused and the MEPs waveform deterioration was presented [
23].
Another problem constitutes the evaluation of asymmetry in the spinal transmission of impulses in patients with IS, which seems to be an important neurological indicator for the surgeon undertaking the decision to introduce the treatment at the theatre. Clinical studies usually do not present clearly such a symptom, while functional evaluation with neurophysiological methods provides revealing subtle but sometimes controversial results. A trend towards increased asymmetries in side-to-side differences in the spinal efferent transmission and cortical latencies was detected, probably representing subclinical involvement of the corticospinal tracts secondary to mechanical compression, according to the conclusion of Kimiskidis et al. [
24]. Luc et al. [
25] claim that there is no difference in latencies in MEPs examinations of patients with scoliosis on the right and left side when recorded from the tibialis anterior muscle, which is most often considered the key muscle for neuromonitoring, also in the undertaken work. It seems that the answer to this question may be provided by a comparison of results of clinical neurophysiology studies in patients with IS verifying the bilateral efferent transmission of the neural impulses from the upper motor neuron level to the effector (MEP), the conduction of motor impulses in the peripheral nervous system (electroneurography, ENG) and assessing the contractile properties of the muscles themselves (electromyography, sEMG).
The paravertebral muscles in patients with scoliosis have been the subject of most electromyographical studies in IS patients [
26,
27], while the effects of disease progression and its surgical and conservative treatment are described in preliminary clinical neurophysiology observations following the examination of the proximal and distal muscles in lower extremities [
28,
29]. In this paper we describe the results of the studies with the methodology of the MEPs recordings with the surface electrodes from the tibialis anterior muscle bilaterally, which is more and more widely used not only in pre- and post-operative diagnostic purposes but also has been proven to be precise enough for intraoperative monitoring in comparison to the standard needle electrodes [
30,
31]. Our previous pilot results on improving the neuromonitoring methodology are fully compatible with their observations [
32].
It has not yet been documented whether the results of MEPs recordings induced by the transcranial single magnetic stimulus can be compared with MEPs induced with the trains of electrical stimuli which are applied intraoperatively for diagnostic evaluation of the spinal neural transmission. The results provided by Glasby et al. [
33] suggest that these measurements may be used comparatively and semi-quantitatively to compare pre-, intra-, and post-operative spinal cord function in spinal deformity surgery. It should be, however, remembered that trains of stimuli applied transcranially during neuromonitoring may cause the temporal and perhaps the spatial summation of the efferent impulses to the spinal motoneurones, which are mediated polisynaptically, and therefore the latencies and amplitudes parameters of MEPs may show the variability [
34]. This study also attempts to determine whether the constant level of optimal anesthesia during surgical treatment of scoliosis affects the parameters of MEPs recorded during neuromonitoring procedures. The results of the study by Lo et al. [
35] confirm that in susceptible individuals, MEPs may rarely occur unpredictably, independent of surgical or anesthetic intervention. However they did not provide specific results for the recorded parameters of MEPs.
Is there any relationship between pre- and intraoperative motor evoked potentials recordings and does IS surgical correction improve directly the spinal efferent transmission? This study aims to compare the results of surface recorded electromyography (EMG), electroneurography (ENG), and especially motor evoked potentials, not only before and after scoliosis correction but also at three stages of the intraoperative treatment. The review of the literature does not indicate studies on the simultaneous comparison of the MEP results in the same patients treated surgically for idiopathic scoliosis that was recorded pre-, intra-, and postoperatively.
2. Materials and Methods
2.1. Participants and Study Design
The total number of 353 girls with the idiopathic scoliosis were included in this retrospective study (
Table 1). They were treated surgically at Wiktor Dega Orthopedic and Rehabilitation Hospital in Poznań, Poland.
All the clinical studies before and after treatment (including analysis of anterior-posterior and lateral X-rays) as well as the surgeries were performed by the same team of four experienced spine surgeons; neurological status and anesthesia were evaluated and proceeded by the same neurologist and anesthesiologist, respectively. Two clinical neurophysiologists have performed pre- and postoperatively the same set of three diagnostic tests. They comprised (1) bilateral tibialis anterior (TA) muscle electromyography during maximal contraction with the surface electrodes (sEMG,) (2) peroneal nerve electroneurography (ENG) recorded from extensor digitorum brevis (EXT) muscle after electrical stimulation at ankle, and (3) motor evoked potential (MEP) recordings from tibialis anterior muscles following transcranial magnetic stimulation (TMS). The same neurophysiological examinations have been performed on the group of eighty healthy girls (
Table 1) to obtain the reference values for comparison.
Intraoperative neurophysiological monitoring was performed by the same neurophysiologists, and included recordings of MEPs from muscles of upper and lower extremities bilaterally. MEPs were induced following the transcranial electrical stimulation (TES). For the purposes of this paper, the results of MEPs from the tibialis anterior muscle are presented, as the key-muscle most often described in scientific reports for comparison of the parameters on the results of neuromonitoring during scoliosis surgery. Results from the neuromonitoring recordings have been chosen for analysis in T0 – intraoperative observation period before surgery onset, T1 - intraoperative observation period after pedicle screws’ implantation, T2 - intraoperative observation period after corrective rods’ implantation, derotation with the convex rod, apical translation, segmental derotation, distraction on the concave side, and compression on the convex side.
Exclusion criteria for TES applied during the neuromonitoring included epilepsy, cortical lesions, convexity skull vault defects, raised intracranial pressure, cardiac disease, proconvulsant medications or anesthetics, intracranial electrodes, vascular clips or shunts, and cardiac pacemakers or other implanted biomedical devices [
16].
Ethical considerations were in agreement with the Helsinki Declaration. Approval was received from the Bioethical Committee of University of Medical Sciences in Poznań, Poland (including studies on healthy people), decisions No 942/21. Each subject (and her parent/legal guardian) was informed about the aim of the study and gave written consent for examinations and data publication.
2.2. Anaesthesia and Spine Surgery
The spine surgeries and recordings of MEPs following trains of the applied transcranial electrical stimulation (TES) were performed under Propofol/Remifentanil anaesthesia (induction dose of Remifentanil 0.5 µg/kg and Propofol 2 mg/kg, and later Remifentanil 0.5-2.0 µg/kg/h and Propofol 2-4 mg/kg/h in continuous infusion) with one-time dose neuromuscular blockade (0.5 mg/kg of rocuronium bromide) at the beginning of the procedure. The level of anaesthesia was continuously monitored and ascertained in Bispectral Index Monitor (BIS, GE, Heathcare, Helsinki, Finland); it was kept constant from 40 to 65 during all applied surgery procedures and neuromonitoring MEPs recordings [
38]. The arterial blood pressure 80 to 100, the temperature and %SpO
2, CO
2 partial pressures were continuously monitored and kept within the physiological limits during surgery. Inhalational anaesthetics were not routinely applied [
39].
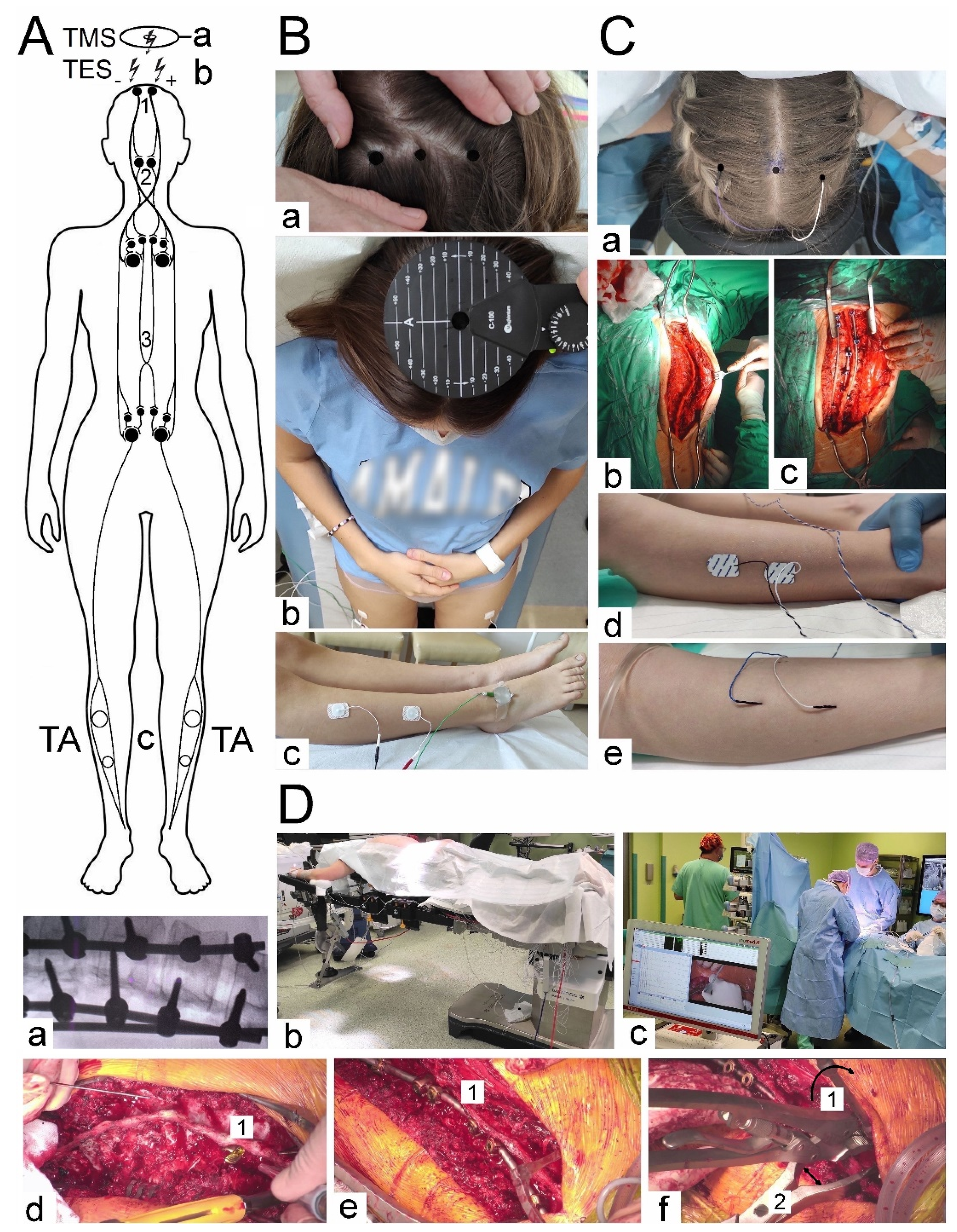
At the beginning of the scoliotic spine surgery, the patient was positioned prone on the operation table (
Figure 1 Db). The whole spine was prepped and draped. A posterior midline skin incision was performed. The paraspinal muscles were dissected subperiosteally. The spine was exposed bilaterally from the midline along the spinous processes, laminas to the tip of transverse processes (
Figure 1 Cb). The surgeon cauterized the paravertebral muscles as the spine was exposed to control the bleeding. The spinous processes with supraspinous ligament were preserved for further anatomical wound closure. Removed pieces of the bones from processes and released spine joints were collected and then used as autografts for the fine fusion. Pedicle screws were placed bilaterally with free-hand technique (from 8 to 16, 12 on average) (
Figure 1 Dd). All patients had implanted corrective instrumentation system (Nova Spine, Amiens, France). Polyaxial and monoaxial screws with 5.5 mm rods made of titanium alloy were used (
Figure 1 Cc and De). The deformity was corrected by combining the following manoeuvers: convex rod rotation, apical translation, segmental derotation, distraction on the concave side, and compression on the convex side (
Figure 1 Df). To obtain spine fusion, decortication was performed, and locally harvested bone grafts were used. The wound was closed in layers over a subfascial drain. The location, angle and depth of the pedicle screw implantation were controlled with the X-ray C-arm for intraoperative imaging (
Figure 1 Da).
(B,C) Photographs illustrating methodology of the pre- and postoperative MEPs (Bb) and sEMG (Bc) recordings with pairs of electrodes placed bilaterally over the surface of TA in healthy volunteers and in patients with scoliosis. “Hot spot” stimulating points were detected and marked preoperatively (Ba) following TMS (Bb) for TES (Ca) purposes performed intraoperatively with needle electrodes and recorded from TA with surface electrodes (Cd) or occasionally with needle electrodes (Cd). Cb - a view of the thoracolumbar spine prepared before the scoliosis correction. Cd – two implanted rods for distraction and derotation procedures of the scoliosis correction
(D) (a) – Intraoperative X-ray coronal image of the thoracic spine with the implanted screws to the vertebrae and two rods. (b) - a view of the patient in the theatre in the prone position with the prepared back area for the surgical approach. (c) - a view of the neuromonitoring device in the theatre with the distance from the surgery table. Certain steps of the scoliosis surgery: Dd – pedicle screw (1) implantation, De - corrective rod (1) implantation, Dc – correction manoeuvres, derotation (1) and distraction (2) of the spine curvature.
Abbreviations: TMS – transcranial magnetic stimulation, TES – transcranial electrical stimulation, TA – tibialis anterior muscle, MEP- motor evoked potential, sEMG – surface electromyography; A-P – anterior-posterior
2.3. Neurophysiological Recordings
Figure 1 presents the methodological principles of the neurophysiological studies. The examinations were performed in an air-conditioned room with a controlled temperature 22°C. Surface electromyography (sEMG) recordings were performed bilaterally from the tibialis anterior muscle before and after the surgery to evaluate the motor unit recruitment during the attempt of a 5-second maximal contraction (
Figure 1 Bc). The sEMG recordings were performed using the KeyPoint Diagnostic System (Medtronic A/S, Skøvlunde, Den-mark) with patients in a supine position during the test. For measurements, we applied standard, disposable Ag/AgCl surface recording electrodes (5 mm
2 of an active surface) with an active electrode placed on the muscle belly, a reference electrode placed on the distal tendon of the same muscle, and a ground electrode placed on the distal part of the examined muscle—according to the Guidelines of the International Federation of Clinical Neurophysiology—European Chapter [
40,
42,
43,
44]. Patients were instructed to contract the muscles under examination and make the strongest possible contraction of the muscles for 5 seconds. Three attempts were performed each time, separated by a 1-minute rest period. The neurophysiologists selected independently the best attempt for analysis—the one with the highest mean amplitude measured peak-to-peak with reference to the isoelectric line. The output measures were the amplitude measured in μV and the frequency of muscle motor unit action potential recruitment measured in Hz. A frequency index (FI, 3–0) was scored based on the calculations of motor unit action potential recruitment during maximal contraction in sEMG recording: 3 = 95–70 Hz—normal; 2 = 65–40 Hz—moderate abnormality; 1 = 35–10 Hz—severe abnormality; 0 = no contraction. sEMG recordings in both controls and patients were performed at a base time of 80 ms/D and an amplification of 20–1000 μV/D. We set the upper 10 kHz and the lower 20 Hz filters in the recorder.
Bilateral electroneurography (ENG) was performed to assess the transmission of neural impulses in the motor peripheral fibers of the peroneal nerves. The aim was assessing whether there are significant differences in nerve conduction that can negatively affect the evaluation of muscle function or the efferent transmission measurements. The procedure involved delivering rectangular pulses of 0.2 ms duration at a frequency of 1 Hz and an intensity ranging from 0 to 80 mA using bipolar stimulating electrodes placed over the skin along the anatomical passages of the nerves at the ankle. The compound muscle action potentials M-waves (CMAP) and F-waves were recorded from the extensor digitorum brevis muscles (EXT). Recordings of these potentials verified transmission of neuronal impulses in the motor fibers peripherally and within L5 ventral spinal roots, respectively. The recordings were performed at the amplification of 500–5000 µV/D and a time base of 5-10 ms/D, and compared to normative values recorded in the healthy volunteers with the patients. The outcome measures were the parameters of amplitudes (in µV) and latencies (in ms) for M–waves, interlatencies of recorded M-F waves (in ms), and frequencies for F-waves (normally not less than 14 during evoking 20 positive, successive recordings of M - waves). The measurements were performed at an amplification of 5–5000 µV and a time base of 2–10 ms. The normative values recorded in healthy volunteer subjects were then compared with the test results of the patients. More details on the methodology of acquisition and interpretation of ENG studies are described in other papers published by members of our team [
41,
42].
Motor evoked potentials (MEPs) were elicited by transcranial magnetic single stimulus (TMS, biphasic, 5 ms lasting) using the magnetic circular coil (C-100, 12 cm in diameter) placed over the scalp in the area of M1 motor cortex targeted with an angle for the corona radiate excitation, where the fibres of the corticospinal tract for upper and lower extremities origin (
Figure 1Bb), and recorded with surface electrodes from TA muscles bilaterally (
Figure 1 Bc). The MagPro X100 magnetic stimulator (Medtronic A/S, Skovlunde, Denmark) was used for the MEPs testing. The magnetic field stream delivered from the coil at the strength 70-80% of resting motor threshold (RMT; 0,84-0,96 T) excited all neural structures up to 3-5 cm deep. The latencies and amplitudes parameters were analyzed as the primary outcome measure to assess the primary motor cortex’s output and evaluate the efferent transmission of neural impulses to effectors via spinal cord descending tracts (
Figure 1 A). Attempts of consecutive trackings searched the optimal stimulation location (a hot spot in the area where TMS elicited the largest MEP amplitude,
Figure 1 Ba) distanced 5mm each other. The amplitude was measured from peak to peak of the signal, the latency from the stimulus application marked by the artefact in the recording to the onset of the positive inflection of potential. The patients and healthy volunteers did not report the stimulation as painful, but they felt the little spread of current to the lower extremities, they were always awake and cooperating. MEPs were recorded using the 8-channel KeyPoint Diagnostic System (Medtronic A/S, Skovlunde, Denmark). Standard disposable Ag/AgCl surface electrodes with an active surface of 5 mm
2 were used. The ground electrode was located on the leg, near knee. The recorder’s low-pass filter was set to 20Hz, high-pass filter to 10kHz and the time base at 10ms/D, the amplification of signals was set between 200-5000µV. A bandwidth of 10Hz to 1000Hz and digitalization at 2000 samples per second and channel were used during recordings. The resistance between the surface of electrode and the skin was decreased with electroconductive gel. The methodology of MEPs recordings has been described in details elsewhere [
40,
42,
43,
44].
Neuromonitoring sessions were performed in the theatre at the same temperature of 22° C using the ISIS system (Inomed Medizintechnik, Emmendinger, Germany) (
Figure 1 Dc). Motor evoked potentials were induced as a result of transcranial electrical stimulation (
Figure 1 A; TES, b) in areas of the cortical motor fields for innervation of the thumb and selected muscles of the lower extremity (
Figure 1 Ca) by a sequence of four stimuli (duration of a single pulse 500 µs) with an intensity of 40-170 mA via bipolar subcutaneous electrodes. Stimulating electrodes were positioned over the skull according to the 10-system: Cz-C3 3-6 cm to the left and Cz to C4 – distance 3-6 cm to the right [Deletis 2007, Legatt et al]. The impendence of scalp electrodes was about 0.8 kΩ. Particular attention was paid to ensure that the level of anesthesia (indications of BIS) and the strength of electrical stimuli (in mA) adjusted at the beginning of the surgery did not change and were maintained at the appropriate level throughout the applied corrective procedures. The needle ground electrode was applied in the area of the iliac crest. We have used our experience in the utilization of the surface electrodes for MEPs recording from TA muscles according to the previous descriptions [
32]. Their impedance measured at the beginning of the neuromonitoring sessions was 10-20 kΩ. The recorded potentials were characterized by a variable amplitude from 100 to 2000 µV and latencies in the range from 27 to 40 ms depending on the conduction distance. Potentials did not require averaging. The following standard settings of measurements were applied: filters hardware high-pass [Hz] 30; software high-pass [Hz] 0.5; software low-pass [Hz] 2000; stimulation frequency [Hz] at 0.5-2.4 ms intervals. Before starting the surgery, after implanting the stimulating (
Figure 1 Ca) and recording (
Figure 1 Cd) electrodes in the supine position of the patient (
Figure 1Db), the electrodes impedances were checked, the correct values for needle electrodes (
Figure 1 Ce) were in the range from 0.1 to 5.0 kΩ, indicating proper connections with the recorder amplifier.
After the patient was transferred to the operating table in the prone position, the MEPs with reference amplitude and latency values were recorded (reference values, T0) for comparison with these which were recorded in the subsequent stages of the surgery (T1 and T2). Amplitudes (in µV) and latencies (in ms) of MEPs were the outcomes measurements. All results of MEPs obtained in patients intraoperatively were also compared to the preoperatively recorded following the magnetic stimulation, aiming to verify the compatibility of the patient’s neurophysiological status regarding the neural efferent impulses transmission. Neuromonitoring was carried out at every stage of surgical correction of scoliosis, and each change in the amplitude or latency parameter of the recorded MEPs induced by TES and recorded bilaterally from the muscles of the upper (abductor pollicis brevis) and lower (rectus femoris, tibialis anterior, and abductor halluccis) extremities was reported to the surgical team. For the purposes of this paper, the results of MEPs recorded from the tibialis anterior muscle are presented. A list of the most common reasons for such fluctuations was selected, and their frequencies calculated. For example, pilot observations indicated that overheating of the tissues accompanying the cauterization before T1 could affect the conduction of nerve impulses in the spinal cord pathways within the white matter funiculi. The surgeon was warned in these cases, and the surgical area was rinsed with the 0.9% NaCl solution at 36.6° C. Calculations were made on how often such activity caused the latency parameter fluctuation in MEPs recorded from the anterior tibial muscles.
2.4. Statistical Analysis
Data were analyzed with Statistica, version 13.1 (StatSoft, Kraków, Poland). Descriptive statistics were reported as minimal and maximal values (range), with mean and standard deviation (SD). The normality distribution and homogeneity of variances was studied with Shapiro–Wilk tests and the homogeneity of variances were studied with Levene’s tests. Frequency sEMG index, recorded F-wave frequencies and BIS data were of the ordinal scale type, while amplitudes and latencies were of the interval scale type. However, they did not represent a normal distribution; therefore, the non-parametric tests had to be used. None of the collected data represented a normal distribution or were of the ordinal scale type; therefore, the Wilcoxon’s signed-rank test was used to compare the differences between results obtained before and after surgeries, as well as to compare results at the beginning (T0), during (T1) and in the end (T2) of the surgical procedures. In the cases of independent variables the non-parametric Mann–Whitney test was used. Any p-values of ≤ 0.05 were considered statistically significant. The cumulative data from parameters of MEPs recordings performed on both sides were used for comparison of the relationships between BIS read-outs in T0, T1 and T2. The results from all neurophysiological tests performed on patients were also calculated from the group of healthy subjects (control group) to achieve the normative parameters used to compare the health status between the patients and the controls. Results did not reveal any significant difference in values of parameters recorded in neurophysiological tests on the left and right sides in controls. Attention was paid to matching patients and healthy controls’ demographic and anthropometric properties, including gender, age, height, weight and BMI. The statistical software was used to determine the required sample size using the primary outcome variable of sEMG and MEPs amplitudes recorded from TA muscles before and after treatment with a power of 80% and a significance level of 0.05 (two-tailed). The mean and standard deviation (SD) were calculated using the data from the first hundred patients, and the sample size software estimated that more than two hundred patients were needed for the purposes of this study.
3. Results
During neuromonitoring in T0, the impedance of the stimulating electrodes distributed with the 10-20 systems inserted under the skin over the skull was 0.8 ± 0.2 kΩ. The impedance of the surface disposable bipolar recording electrodes from muscle groups was in a range from 10 to 20 kΩ (mean of 13.2 ± 1.3 kΩ).
The coincidence of the positioning of the electrodes stimulating the transcranial motor centers for the innervation of more lower than the upper muscles using measurements of the 10-20 system with the method of determining the "hot spots" during the recording of the largest amplitude preoperative MEPs was calculated at 86%.
Data in
Table 2 indicate that during surgeries the events evoking the fluctuation of intraoperatively recorded MEPs parameters (more amplitudes than latencies) and reported to surgeons were associated the most frequently with pedicle screw implantation, corrective rods implantation, derotation with convex rod and distraction on concave side. Heating of the spine associated with cauterization was the most frequent reason for latency fluctuation in the MEPs evoked TES recordings. Among 353 neuromonitoring cases described in this paper, none of the listed incidents reported to surgeons with their immediate reaction resulted in a significant postoperative neurological deficit or motor function.
Data on parameters of sEMG and ENG recordings indicate (
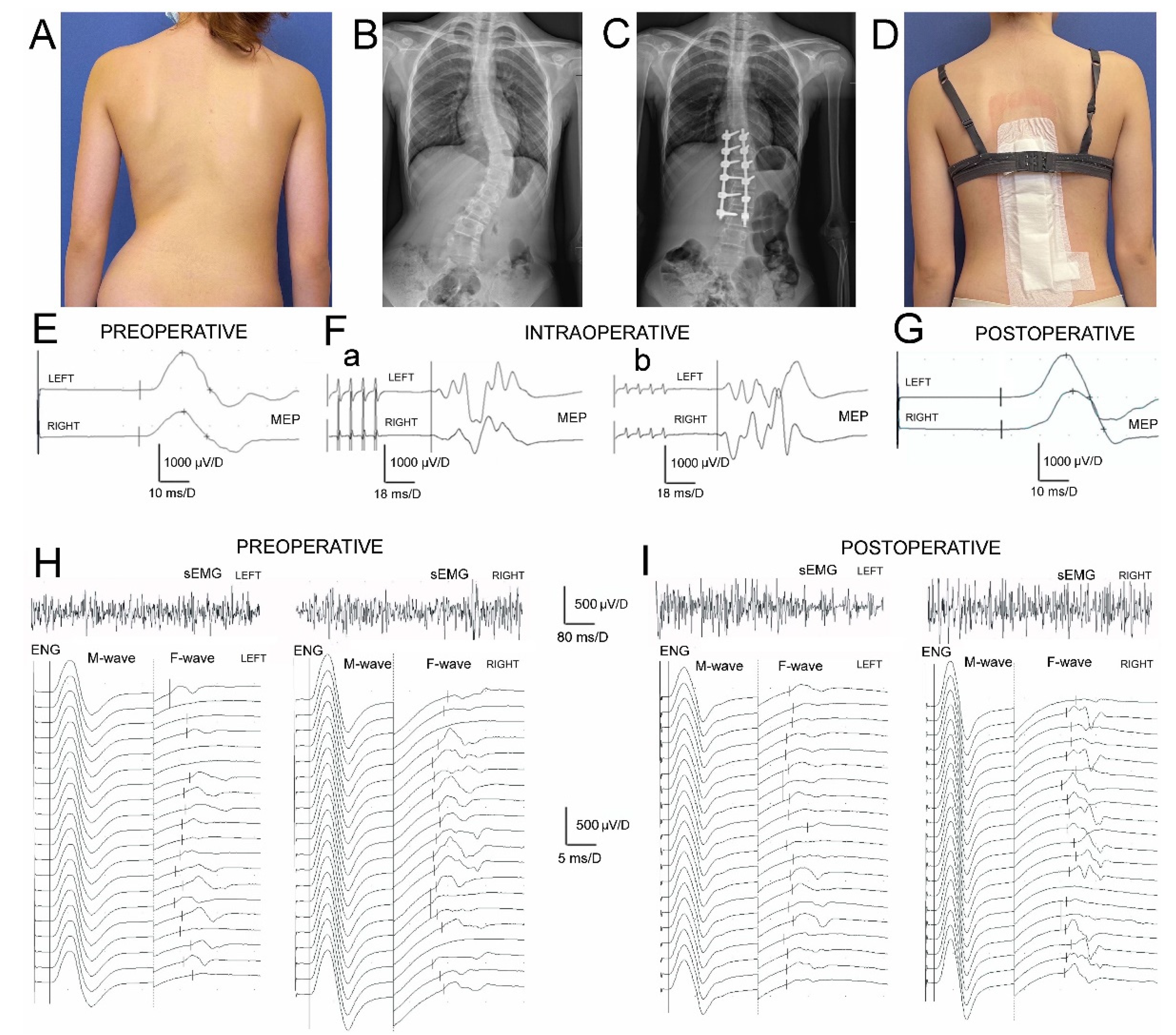
Table 3), that muscle motor units activity and conduction of the motor impulses in peroneal nerve fibers peripherally in IS patients were significantly different from the healthy controls similarly before and after the surgery. The difference in MEPs amplitudes before surgery (
Figure 2 E) was at p=0.009 bilaterally and after the treatment (
Figure 2 G) it was at p=0.02-0.01, indicating a slight improvement in the efferent transmission of neural impulses with the fibers of the spinal tracts postoperatively.
Preoperatively, results of all neurophysiological studies in IS patients (
Figure 2 E-Fa, H) were significantly asymmetrical and recorded worse on the concave side suggesting greater neuro-logical motor deficits at p=0.04. One week postoperatively this asymmetry has been recorded as significantly reduced (
Figure 2 Fb, I).
The surgeries in IS patients brought a significant increase of amplitudes at p=0.04 but not FI in sEMG recordings bilaterally (
Figure 2 H and I; upper traces), what point at improvement in activity of muscle motor units still with the signs of the neurogenic type of injury. Decreased values of M-waves amplitudes and latencies recorded in ENG examinations (
Figure 2 H and I; bottom traces) indicated the symptoms of peroneal motor fibers injury of the axonal type and improved only on the concave side at about p=0.04. They were in parallel with the significant increase in the values of F-waves parameters (p=0.04) which suggests that surgeries might result in the lumbar ventral roots decompression. During ENG stimulation studies, the strength of the current to evoke the maximal M-wave in healthy volunteers ranged from 18 to 40 mA with a mean of 27.7 ± 2.4 mA, while in patients at 38-65 mA (mean of 43.7 ± 2.6 mA) preoperatively and at 32-63 mA (mean 42.9 ± 2.2 mA) postoperatively.
There were no detected significant differences in amplitudes or latencies of MEPs induced with TMS or TES comparing the parameters recorded preoperatively (one day before surgery) and intraoperatively in T0. The amplitudes of TES evoked MEPs increased gradually at p=0.04 in the subsequent periods (T1 and T2) of observation. The significant reduction of MEPs latency at p=0.05 was observed only at the end of the IS surgery.
The total time of the surgical procedures from transferring the patient to the operation table in a prone position to the final suturing of the wound over the surgical field ranged from 4,5 to 5,5 hours (5 hours on average). The additional half an hour should be added to consider the total time of the patient’s anesthesia.
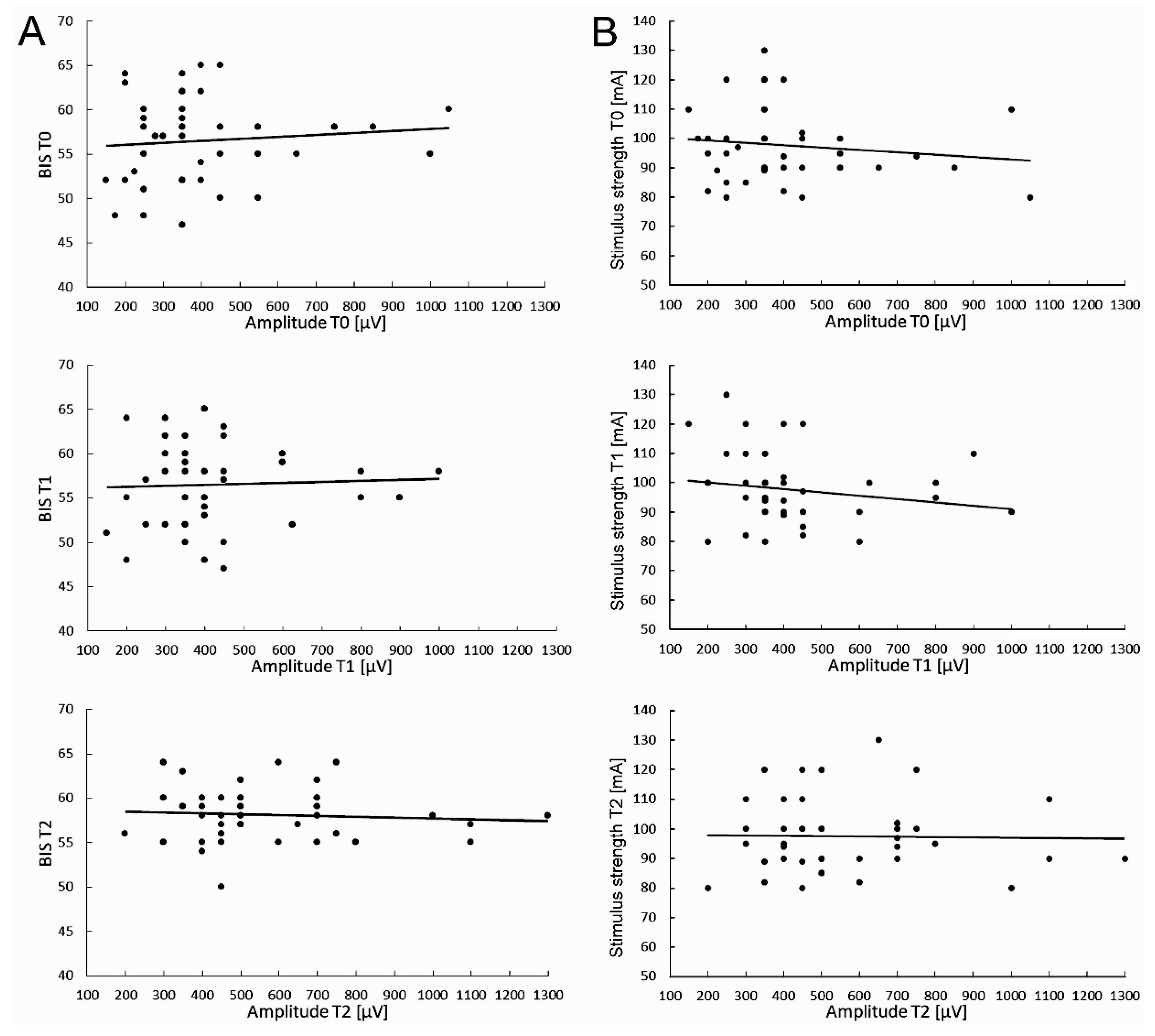
Particular attention was paid to ensuring that the level of anesthesia (BIS indications) and the strength of electrical stimuli (in mA) did not change and were maintained at the same level throughout the neuromonitoring procedure. Preliminary studies on the possible relationships between the level of anesthesia fluctuations and required TMS stimulus strength, as well the MEPs parameters changes measured in T0-T2 periods of observations were performed in 40 patients undergoing scoliosis surgeries (
Figure 3).
The value of the electrical stimulus strength for evoking the highest and stable MEP amplitude parameter was kept constant, and its value ranged from 80 to 130mA (mean of 97.6±12,4SD) (
Figure 3B).
The average value of the BIS parameter measured during about 5 hours of the surgery was 56.5±4.8 at the beginning of the scoliosis correction procedure (T0), slightly decreased to 55.3±3.7 in its middle (T1), and reached 58.1±3.0 after its completion (T2), which may suggest the only discrete changes of the anesthesia level applied to the patients (
Figure 3A). These differences were not statistically significant (at p=0.09). It should be remembered that the difference at the range of 10 in BIS measurements is clinically insignificant.
With the same periods of observation, the cumulative mean values of the MEPs amplitude parameter recorded from the anterior tibialis muscles were 409.0±58.5µV (T0), 406.6±76.5µV (T1), and 562.5±45.9µV (T2), respectively. The difference between recordings at T0 and T2 was statistically different at p=0.03.
The cumulative mean values of MEPs latencies recorded in T0 was 32.0±2.0ms, 32.9±2.2ms in T1, and 32.7±2.1 in T2, and the differences between them were statistically insignificant (at p=0.21 and p=0.35). There were not found significant relationships between BIS fluctuations (
Figure 3Aa-c) and the applied electrical stimulus strengths (
Figure 3Ba-c) for evoking the maximal MEPs amplitudes trends at three periods of observations.
The above data may suggest an improvement in the spinal conductivity of neural impulses but lack of relationship between the fluctuation of the MEP amplitude parameter and the applied level of anesthesia (
Figure 3Aa-c) or the constant electrical stimulus strength (
Figure 3 Ba-c) during surgeries of patients with IS under this study. It is not likely that they could be the factors influencing the efferent transmission in spinal pathways bilaterally recorded in MEPs tests besides the surgical procedures.
4. Discussion
The results of MEPs recordings evoked with TMS in this study indicated a slight improvement in the efferent transmission of neural impulses within the fibers of the spinal tracts in IS patients postoperatively. Results of all neurophysiological studies were significantly asymmetrical and recorded worse on the concave side; this asymmetry had been significantly reduced following IS surgery. The surgeries in IS patients brought significant improvement in the parameters of sEMG recordings, however, still reflect the consequences of the neurogenic injury of TA muscle motor units. ENG studies results proved the symptoms of the axonal type injury in peroneal motor fibers; postoperatively improved only on the concave side in parallel with the lumbar ventral roots motor conduction. MEPs parameters induced with TMS preoperatively and TES at T0 did not differ. The amplitudes of TES-evoked MEPs increased gradually in two periods of intraoperative observation (T1 and T2). Studies on the possible influence between the level of anesthesia and fluctuations of MEPs amplitudes did not reveal a direct relationship.
The compatibility between the positioning of the electrodes stimulating transcranially the motor centers for the innervation of lower rather than upper muscles using the 10-20 system measurements with the method of determining the "hot-spots" during preoperative MEP recordings was calculated at 86%. This variability is partly due to the human individual differences in the distribution of motor centers [
45], which was also reported in their pioneering works by Penfield and Jasper [
46] as "paradoxical distribution of motor centers". This suggests the 10-20 method should be routinely combined and compared preoperatively with "hot spots" induced MEPs to avoid complications during neuromonitoring in the theatre at T0, what was underlined by Garcia et al [
47]. The same applies to the general idea of pre-operative neurophysiological tests performed each time in treated patients with IS, enabling accurate recognition of changes in efferent neural transmission through MEPs recordings and the functional ability of muscle motor units to a contraction in non-invasive sEMG recordings. They also include the recognition of the degree of asymmetry of the recordings and the level of neuromere in which there are the greatest deficits in the activities of the motor centres [
48,
49].
Similarly to Gadella et al. [
30] and Duffler et al. [
31] we have observed in T0 twice as many values of impedances of surface electrodes than needle electrodes, which did not greatly influence the signal-to-noise ratio parameter, and convinced similarly to the high utility of both methods in neuromonitoring procedures. Our previous pilot results on improving the neuromonitoring methodology [
32] are fully compatible with their observations. Moreover, taking into account the fact that IS surgeries are pediatric and the consequences of neuromonitoring procedures using TES when MEPs are recorded with needle electrodes can be ecchymosis and bruises associated with the stimulation-related muscles movements, rarely the local nerve damage or infections [
50], and frequent postoperative skin reddening [
51], the recording from the muscle’s surface is more beneficial.
According to data provided by Wang et al. [
52], anesthesia can significantly affect the reliability of TES-evoked MEP monitoring. Our results of preliminary studies on the possible variability of the anesthesia level on the parameters of intraoperative recorded MEPs on 40 patients show no clear relationships. We can conclude that during our recordings, MEPs parameters changes are determined by the surgery procedures during neuromonitoring, not the anesthesia conditions if they are kept stable, which influences a decrease in the number of false-positive warnings. Our study did not confirm anesthesia-related warnings as frequent during spinal deformity surgery, contrary to the result reported by Acharya et al. [
53], when 50% of the alerts were associated with anesthetic management.
The contemporary studies of MEPs recordings in IS patients assessing the pathologies in the efferent transmission or the effectiveness of treatment provide slightly different values of latencies recorded from lower extremity muscles in comparison to our results. This may be due to the multiple routes of excitation within anatomical structures in the supraspinal and spinal systems to motoneuronal centers involving di- or trisynaptic pathways, giving the delays difference by 3-4 ms (
Figure 1 A), or the consequences of summation of the excitatory neural impulses in efferent pathways evoked by the trains of transcranial electrical stimulation. The obvious reason is the difference in the conduction distance influencing the MEPs latency parameter following TES to the recording site in lower extremities muscles, both in the population of IS patients (with different angles of primary or secondary curvatures) and healthy controls of different ages and heights ranges [
54]. However the results of MEPs parameters recorded preoperatively following TMS in this study are very similar to MEPs induced with TES, which leads to the conclusion that both methods are comparable in the sensitivity and reliability of the assessments. In the end, we believe that discrete transient changes in the latency during the whole surgery and detected especially in T1 as probably side effects and reported to the surgeon, although statistically insignificant, were more related to the heating from the cauterization. Our study, similarly to the findings of Toki et al. [
55], pointed at the lack of statistically significant difference in the MEPs latency parameters following application single versus trains of transracially applied impulses.
The comparison of the amplitude parameter of the MEPs recorded in this study from the TA muscle with the reports of other authors is different [
22,
24]. Lo et al. [
56] reported a consistent average latency parameter of about 31-32 ms, but an average amplitude parameter at of about 46.5 µV; ten times lower than that presented in the current study, assuming the same type of anesthesia used in patients with IS. On the other hand, Edmonds et al. [
21] following nitrous oxide-narcotic anesthesia application during the onset the surgery of twelve IS patients, reported similar to those presented in this study, the mean parameter of amplitude at 490 µV and the latency at 32.0 ms. Suppression of anesthesia level diminished more than half of the amplitude parameter in their study.
Analyzing the data listed in
Table 2 on sources of evoking the fluctuation of intraoperatively recorded MEPs parameters, their interpretation of the mechanism of action could be proposed. Pedicle screw implantation may cause mechanical bending of the spine along its axis, causing pressure on the paravertebral vessels or direct pressure on the structures of the lateral and ventral spinal cord funiculi. Moreover, it can be a source of stretching the bone structure of the vertebral body by the screw or occasionally direct pressing to the nerve structures. Corrective rod implantation can cause compression of a deformed spinal cord, which has been created the additional beside physiological curvatures during the pathological lateral curvature progression and rotation in the ontogenesis. Correction of spinal deformity, causing the greatest frequency of the surgeons warnings by the neurophysiologist during neuromonitoring should be, in fact, considered the most dangerous stage of scoliosis surgery, which support the similar conclusion by Morota et al. [
57] and Dormans et al. [
58].Waveform deterioration commonly occur during rotation maneuvers and more frequently in patients with a larger preoperative lateral scoliosis angle [Kobayashi].
One of the possible explanations for immediate improving the total efferent transmission recorded in sEMG, ENG and MEPs parameters following IS spine surgery may be restoring the correct anatomical and functional relationships of the nervous structures in the middle canal of the deformed spine. This applies not only to the axons in the lateral and ventral white matter funiculi, but especially to the spinal roots, which may be compressed in narrowed intervertebral foramina as a result of wedging of the adjacent vertebral bodies in main or secondary IS curvatures.
One of the study limitations can be the selection for the final analysis of only the MEPs recordings from TA muscle, although data from the other proximal and distal muscles of lower extremities bilaterally have also been collected. It is accepted that during neuromonitoring procedure, the MEPs recordings from rectus femoris, TA, calf group and abductor hallucis longus muscles provide the highest sensitivity and specificity and best predictive power for postoperative lower extremity weakness [
59]. However, the numerical data of other researchers are seldom provided for recordings from these muscles. Therefore have chosen TA because the MEPs monitoring data are the best accessible for comparison in the literature.
The results presented in this study first time provide evidence of the possibility of using pre- intra- and postoperative MEPs recordings as effective and accurate tool for detecting neurological deficits during spine surgery. Moreover, our prospective study seems to fill the gap in the validation of protocols to manage functional evaluation with neurophysiological methods on certain steps of IS patients’ treatment [
60]. In recent years, many spine surgeons now advocate MEPs monitoring for all spinal surgery since they better predict good postoperative motor outcomes than using SEPs alone. Moreover, patients with immature neural pathways or preexisting neuromuscular disease may have abnormal baseline SEP recordings what regard IS patients [
61]. Transcranial electric motor evoked potentials are exquisitely sensitive to altered spinal cord blood flow due to either hypotension or a vascular insult. Moreover, changes in transcranial electric motor evoked potentials are detected earlier than changes in somatosensory evoked potentials, facilitating more rapid identification of impending spinal cord injury [
62].
The results of our study confirm the observation of Lo et al. [
35], that MEPs abnormalities may rarely occur unpredictably, independent of surgical or anesthetic intervention. Moreover, they also support the necessity of the preoperative- MEPs recordings presented in this study because early recognition of their parameters is important to prevent false positives in the course of IS spinal surgery.
The many advances in motor system assessment achieved in the last two decades undoubtedly improve monitoring efficacy without unduly compromising safety. Further studies and experience will likely clarify existing controversies and bring new advances [
14]. Motor evoked potentials are the modality of choice for monitoring motor tract function, and negate the use of full neuromuscular blockade [
61]. The future of developing neuromonitoring methods with MEPs recordings should consider not only non-invasive methods utilizing surface electrodes but also studies exploring the approach of nerve versus muscle-recorded MEPs [
63]. These are of special importance, considering the “resistance” of nerve-recorded potentials to paralysis applied by the anesthesiologists during the intraoperative neuromonitoring of spine surgeries [
64].
In terms of basic research, especially an attempt to explain the etiopathogenesis of IS, the results presented in this study show how important may be the impact of the asymmetry and abnormalities of spinal neural transmission in the main curvature progression. It appears to be a pathology secondary to a primary cause located at the supraspinal level [
65,
66]. The clinical significance of the presented study is mainly related to the possibility of precise assessment of the surgical treatment results using functional tests of clinical neurophysiology and forecasting the need for further surgical treatment related to the natural progress of patients’ height.
5. Conclusions
Considering that MEPs amplitude parameter reflects the number of axons excited from the motor cortex and transmitting the efferent impulses via spinal descending tracts in the white matter, pre- (TMS evoked) and intraoperative (TES evoked) recordings are reliable for evaluating the patient’s neurological status before and during surgical scoliosis correction procedures. The results of this study indicate an agreement between preoperative and early-intraoperative evaluations with these both diagnostic methods. An increase of MEPs amplitude parameters recorded on both sides after scoliosis surgery proves immediate improvement of the total efferent spinal cord transmission. Considering comparative pre- and postoperative sEMG and ENG recordings it can be concluded that surgeries might directly result in the additional lumbar ventral roots decompression.
Our results of the tests on the possible variability of the anesthesia level on the parameters of intraoperative recorded MEPs show no clear relationships. We can conclude that MEPs parameters changes are determined by the surgery procedures during neuromonitoring, not the anesthesia conditions if they are kept stable, which influences a decrease in the number of false-positive neuromonitoring warnings.
Further studies on a larger population of patients with long-lasting observation postoperatively are required to confirm the presented conclusions on the direct influences of scoliosis surgery on improvement of the motor function in patients with IS.
The use of intraoperative neuromonitoring in IS surgery, very often complicated due to neurological deficits, not only offers safety for the patient but also protects the hospital from possible consequences due to the patient’s claims as a result of complications.
Author Contributions
Conceptualisation, P.D., J.H. and T.K.; methodology, P.D., J.H., K.K., M.D. and T.K.; software, J.H.; validation, P.D., J.H., K.K., M.D. and T.K.; formal analysis, P.D., J.H., K.K., M.D. and T.K.; investigation, P.D., J.H., K.K., P.J., P.G., M.T., M.D. and T.K.; resources, P.D. and J.H.; data curation, P.D., J.H., K.K. and T.K.; writing—original draft preparation, P.D. and J.H.; writing—review and editing, P.D., J.H. and T.K.; visualisation, J.H.; supervision, P.D., J.H. and T.K.; project administration, P.D. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of Poznan University of Medical Science, decision no 942/21 dated on 13 January 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the data generated or analysed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weinstein, S.L.; Dolan, L.A.; Cheng, J.C.Y.; Danielsson, A.; Morcuende, J.A. Adolescent idiopathic scoliosis. Lancet Lond Engl. 2008, 371, 1527–1537.
- Pesenti, S.; Jouve J.L.; Morin, C. et al. Evolution of adolescent idiopathic scoliosis: Results of a multicenter study at 20 years’ follow-up. Orthop Traumatol Surg Res. 2015, 101(5), 619-622. [CrossRef]
- Huber, J.; Rogala, P. Etiopathogenesis of the adolescent idiopathic scoliosis basing on neuroimaging and neurophysiological examinations with the special emphazing of motor evoked potentials (MEP). Stud Health Technol Inform. 2012, 176, 446.
- Kinel, E.; Korbel, K.; Kozinoga, M.; Czaprowski, D.; Stępniak, Ł.; Kotwicki, T. The Measurement of Health-Related Quality of Life of Girls with Mild to Moderate Idiopathic Scoliosis-Comparison of ISYQOL versus SRS-22 Questionnaire. J Clin Med. 2021, 10(21), 4806. [CrossRef]
- Negrini, S.; Antonini, G.; Carabalona, R.; Minozzi, S. Physical exercises as a treatment for adolescent idiopathic scoliosis. A systematic review. Pediatr Rehabil. 2003, 6(3-4), 227-235. [CrossRef]
- Kotwicki, T.; Cheneau, J. Biomechanical action of a corrective brace on thoracic idiopathic scoliosis: Cheneau 2000 orthosis. Disabil Rehabil Assist Technol. 2008, 3(3), 146-153. [CrossRef]
- Negrini, S.; Minozzi, S.; Bettany-Saltikov, J.; Zaina, F.; Chockalingam, N.; Grivas, T. B.; Kotwicki, T.; Maruyama, T.; Romano, M.; Vasiliadis, E. S. Braces for idiopathic scoliosis in adolescents. Spine. 2010, 35(13), 1285–1293. [CrossRef]
- Pepke, W.; Morani, W.; Schiltenwolf, M.; Bruckner, T.; Renkawitz, T.; Hemmer, S.; Akbar, M. Outcome of Conservative Therapy of Adolescent Idiopathic Scoliosis (AIS) with Chêneau-Brace. J. Clin. Med. 2023, 12, 2507. [CrossRef]
- Addai, D.; Zarkos, J.; Bowey, A.J. Current concepts in the diagnosis and management of adolescent idiopathic scoliosis. Child’s Nerv. Syst. 2020, 36, 1111–1119. [CrossRef]
- Negrini, S.; Donzelli, S.; Aulisa, A.G. et al. SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018, 13:3. [CrossRef]
- Kwan, M.K.; Loh, K.W.; Chung, W.H.; Hasan, M.S.; Chan, C.Y.W. Perioperative outcome and complications following single-staged posterior spinal fusion using pedicle screw instrumentation in adolescent idiopathic scoliosis(AIS): A review of 1057 cases from a single centre. BMC Musculoskelet. Disord. 2021, 22, 413. [CrossRef]
- Ferguson, J.; Hwang, S. W.; Tataryn, Z.; Samdani, A. F. Neuromonitoring changes in pediatric spinal deformity surgery: A single-institution experience. Journal of neurosurgery. Pediatrics, 2014, 13(3), 247–254. [CrossRef]
- Deletis, V. Basic methodological principles of multimodal intraoperative monitoring during spine surgeries. Eur Spine J. 2007, 16, 147-152. [CrossRef]
- MacDonald, D.B. Intraoperative motor evoked potential monitoring: Overview and update. J Clin Monit Comput. 2006, 20(5), 347-377. [CrossRef]
- Padberg, A. M.; Wilson-Holden, T. J.; Lenke, L. G.; Bridwell, K. H. Somatosensory- and motor-evoked potential monitoring without a wake-up test during idiopathic scoliosis surgery. An accepted standard of care. Spine. 1998, 23(12), 1392–1400. [CrossRef]
- MacDonald, D.B. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002, 19(5), 416-429. [CrossRef]
- Pastorelli, F.; Di Silvestre, M.; Plasmati, R.; Michelucci,R.; Greggi, T.; Morigi, A.; Bacchin, M,R.; Bonarelli, S.; Cioni, A.; Vommaro, F.; Fini, N.; Lolli, F.; Parisini, P. The prevention of neural complications in the surgical treatment of scoliosis: The role of the neurophysiological intraoperative monitoring. Eur Spine J. 2011, 20, 105-14. [CrossRef]
- Hausmann, O.; N, Böni.; T., Pfirrmann, C.W.; Curt, A.; Min, K. Preoperative radiological and electrophysiological evaluation in 100 adolescent idiopathic scoliosis patients. European spine journal : Official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2003, 12(5), 501–506. [CrossRef]
- Lyon, R.; Lieberman, J.A.; Grabovac, M.T.; Hu, S. Strategies for managing decreased motor evoked potential signals while distracting the spine during correction of scoliosis. Journal of Neurosurgical Anesthesiology. 2004, 16(2), 167-170. [CrossRef]
- Yoshida, G.; Imagama, S.; Kawabata, S. et al. Adverse Events Related to Transcranial Electric Stimulation for Motor-evoked Potential Monitoring in High-risk Spinal Surgery. Spine 2019, 44(20), 1435-1440. [CrossRef]
- Edmonds, H.L.; Jr. Paloheimo, M.P.; Backman, M.H.; Johnson, J.R.; Holt, R.T.; Shields, C.B. Transcranial magnetic motor evoked potentials (tcMMEP) for functional monitoring of motor pathways during scoliosis surgery. Spine 1989, 14(7), 683-686. [CrossRef]
- Chang, S.H.; Park, Y.G.; Kim, D.H.; Yoon, S.Y. Monitoring of Motor and Somatosensory Evoked Potentials During Spine Surgery: Intraoperative Changes and Postoperative Outcomes. Ann Rehabil Med. 2016, 40(3), 470-80. [CrossRef]
- Kobayashi, K.; Imagama, S.; Ito, Z. et al. Transcranial motor evoked potential waveform changes in corrective fusion for adolescent idiopathic scoliosis. J Neurosurg Pediatr. 2017, 19(1), 108-115. [CrossRef]
- Kimiskidis, V. K.; Potoupnis, M.; Papagiannopoulos, S. K.; Dimopoulos, G.; Kazis; D. A.; Markou; K.; Zara, F.; Kapetanos, G.; Kazis, A. D. Idiopathic scoliosis: A transcranial magnetic stimulation study. Journal of Musculoskeletal & Neuronal Interactions 2007, 7(2), 155–160.
- Luc, F.; Mainard, N.; Payen, M. et al. Study of the latency of transcranial motor evoked potentials in spinal cord monitoring during surgery for adolescent idiopathic scoliosis. Neurophysiol Clin. 2022, 52(4), 299-311. [CrossRef]
- Zetterberg, C.; Björk, R.; Ortengren, R.; Andersson, G.B. Electromyography of the paravertebral muscles in idiopathic scoliosis. Measurements of amplitude and spectral changes under load. Acta Orthop Scand. 1984, 55(3), 304-309. [CrossRef]
- Cheung, J.; Halbertsma, J.P.; Veldhuizen, A.G.; Sluiter, W.J.; Maurits, N.M.; Cool, J.C.; van Horn, J.R. A preliminary study on electromyographic analysis of the paraspinal musculature in idiopathic scoliosis. Eur. Spine J. 2005, 14(2), 130-7. [CrossRef]
- Huber, J.; Głowacki, M.; Rogala, P.; Wilusz, A.; Szulc, A. The juvenile idiopathic scoliosis in clinical electromyographical and electroneurographical examinations, Electroencephal Clin Neurophysiol. 1997, 103(1), 101.
- Rogala, P.; Huber, J.; Kubaszewski, Ł. Possible origin of adolescent idiopathic scoliosis (ais) basing on results of clinical neurophysiological investigations. Orthop Procs. 2008 90-b, 443-444.
- Gadella, M.C.; Dulfer, S.E.; Absalom, A.R. et al. Comparing Motor-Evoked Potential Characteristics of Needle versus Surface Recording Electrodes during Spinal Cord Monitoring-The NERFACE Study Part I. J Clin Med. 2023, 12(4), 1404. [CrossRef]
- Dulfer, S.E.; Gadella, M.C.; Tamási, K. et al. Use of Needle Versus Surface Recording Electrodes for Detection of Intraoperative Motor Warnings: A Non-Inferiority Trial. The NERFACE Study Part II. J Clin Med. 2023, 12(5), 1753. [CrossRef]
- Daroszewski, P.; Garasz, A.; Huber, J. et al. Update on neuromonitoring procedures applied during surgery of the spine - observational study. Reumatologia 2023, 61(1), 21-29. [CrossRef]
- Glasby, M.A.; Tsirikos, A.I.; Henderson, L. et al. Transcranial magnetic stimulation in the semi-quantitative, pre-operative assessment of patients undergoing spinal deformity surgery. Eur Spine J. 2017, 26(8), 2103-2111. [CrossRef]
- Weber, M.; Eisen, A.A. Magnetic stimulation of the central and peripheral nervous systems. Muscle and Nerve 2002, 25(2), 160-175. [CrossRef]
- Lo, Y.L.; Tan, Y.E.; Raman, S.; Teo, A.; Dan, Y.F.; Guo, C.M. Systematic re-evaluation of intraoperative motor-evoked potential suppression in scoliosis surgery. Scoliosis Spinal Disord. 2018, 13, 12. [CrossRef]
- Lenke, L.G.; Betz, R.R.; Haher, T.R. et al. Multisurgeon assessment of surgical decision-making in adolescent idiopathic scoliosis: Curve classification, operative approach, and fusion levels. Spine 2001, 26(21), 2347-2353. [CrossRef]
- Ovadia, D. Classification of adolescent idiopathic scoliosis (AIS). J Child Orthop. 2013, 7(1), 25-28. [CrossRef]
- Medical Advisory Secretariat. Bispectral index monitor: An evidence-based analysis. Ont Health Technol Assess Ser. 2004, 4(9), 1-70.
- Soghomonyan, S.; Moran, K.R.; Sandhu, G.S.; Bergese, S.D. Anesthesia and evoked responses in neurosurgery. Front Pharmacol. 2014, 14, 5- 74. [CrossRef]
- Wincek, A.; Huber, J.; Leszczyńska, K.; Fortuna, W.; Okurowski, S.; Chmielak, K.; Tabakow, P. The Long-Term Effect of Treatment Using the Transcranial Magnetic Stimulation rTMS in Patients after Incomplete Cervical or Thoracic Spinal Cord Injury. J. Clin. Med. 2021, 10, 2975. [CrossRef]
- Huber, J.; Leszczyńska, K.; Wincek, A.; Szymankiewicz-Szukała, A.; Fortuna, W.; Okurowski, S. Tabakow, P. The Role of Peripheral Nerve Electrotherapy in Functional Recovery of Muscle Motor Units in Patients after Incomplete Spinal Cord Injury. Appl. Sci. 2021, 11, 9764. [CrossRef]
- Leszczyńska, K.; Huber, J. The Role of Transcranial Magnetic Stimulation, Peripheral Electrotherapy, and Neurophysiology Tests for Managing Incomplete Spinal Cord Injury. Biomedicines 2023, 11, 1035. [CrossRef]
- Leszczyńska, K.; Huber, J. Unveiling the Correlations between Clinical Assessment of Spasticity and Muscle Strength and Neurophysiological Testing of Muscle Activity in Incomplete Spinal Cord Injury Patients: The Importance of a Comprehensive Evaluation. Appl Sci. 2023, 13, 7609. [CrossRef]
- Wesołek, A.; Daroszewski, P.; Huber, J. Neurophysiological Evaluation of the Functional State of Muscular and Nervous Systems in High-Maneuvering Jet Fighters. Appl Sci. 2023, 13, 1120. [CrossRef]
- Legatt, A.D.; Emerson, R.G.; Epstein, C.M. et al. ACNS Guideline: Transcranial Electrical Stimulation Motor Evoked Potential Monitoring. J Clin Neurophysiol. 2016 33(1), 42-50. [CrossRef]
- Penfield, W.; Jasper, H. (Eds.) Epilepsy and the Functional Anatomy of the Human Brain. Little, Brown, Boston. 1954. [CrossRef]
- Garcia, M.A.C.; Souza, V.H.; Lindolfo-Almas, J.; Matsuda, R.H.; Nogueira-Campos, A.A. Motor potential evoked by transcranial magnetic stimulation depends on the placement protocol of recording electrodes: A pilot study. Biomed Phys Eng Express. 2020, 6(4). [CrossRef]
- Virk, S.; Klamar, J.; Beebe, A.; Ghosh, D.; Samora, W. The Utility of Preoperative Neuromonitoring for Adolescent Idiopathic Scoliosis. International Journal of Spine Surgery. 2019, 13(4), 317–320. [CrossRef]
- Hudec, J.; Prokopová, T.; Kosinová, M.; Gál, R. Anesthesia and Perioperative Management for Surgical Correction of Neuromuscular Scoliosis in Children: A Narrative Review. J. Clin. Med. 2023, 12, 3651. [CrossRef]
- Darcey, T.M.; Kobylarz, E.J.; Pearl, M.A. et al. Safe use of subdermal needles for intraoperative monitoring with MRI. Neurosurg Focus 2016, 40(3), E19. [CrossRef]
- Sanders, A.; Andras, L.; Lehman, A.; Bridges, N.; Skaggs, D.L. Dermal Discolorations and Burns at Neuromonitoring Electrodes in Pediatric Spine Surgery. Spine 2017, 42(1), 20-24. [CrossRef]
- Wang, A.C.; Than, K.D.; Etame, A.B.; La Marca, F.; Park, P. Impact of anesthesia on transcranial electric motor evoked potential monitoring during spine surgery: A review of the literature. Neurosurg Focus. 2009, 27(4), E7. [CrossRef]
- Acharya, S.; Palukuri, N.; Gupta, P.; Kohli, M. Transcranial Motor Evoked Potentials during Spinal Deformity Corrections-Safety, Efficacy, Limitations, and the Role of a Checklist. Front Surg. 2017, 4, 8. [CrossRef]
- Jones, S.J.; Harrison, R.; Koh, K.F.; Mendoza, N.; Crockard, H.A. Motor evoked potential monitoring during spinal surgery: Responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996, 100(5), 375-383. [CrossRef]
- Toki, T.; Fujita, N.; Ichikawa, T.; Ochi, N.; Yokota, I.; Sudo, H.; Morimoto, Y. Factors Affecting Transcranial Motor-Evoked Potential Measurements Using Single-Train Stimulation with an Increased Number of Pulses during Adolescent Scoliosis Surgery: A Prospective Observational Study. J. Clin. Med. 2023, 12, 4433. [CrossRef]
- Lo, Y.L.; Dan, Y.F.; Tan, Y.E. et al. Intra-operative monitoring in scoliosis surgery with multi-pulse cortical stimuli and desflurane anesthesia. Spinal Cord 2004, 42(6), 342-345. [CrossRef]
- Morota, N.; Deletis, V.; Constantini, S.; Kofler, M.; Cohen, H.; Epstein, F.J. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 1997, 41, 1327–1336. [CrossRef]
- Dormans, J. P. MD, FACS. Establishing a Standard of Care for Neuromonitoring During Spinal Deformity Surgery. Spine 2010, 35(25), 2180-2185. [CrossRef]
- Miller, S.M.; Donegan, S.W.; Voigt, N.; Eltorai, A.E.M.; Nguyen, J.; Machan, J.T.; Daniels, A.H.; Shetty, T. Transcranial motor-evoked potentials for prediction of postoperative neurologic and motor deficit following surgery for thoracolumbar scoliosis. Orthop Rev. 2019, 12, 11(1), 7757. [CrossRef]
- Charalampidis, A.; Jiang, F.; Wilson, J. R. F.; Badhiwala, J. H., Brodke; D. S.; Fehlings, M. G. Use of Intraoperative Neurophysiological Monitoring in Spine Surgery. Global Spine Journal 2020, 10, 104- 114. [CrossRef]
- Glover, C.D.; Carling, N.P. Neuromonitoring for scoliosis surgery. Anesthesiol Clin. 2014, 32(1), 101-114. [CrossRef]
- Schwartz, D.M.; Auerbach, J.D.; Dormans, J.P. et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007, 89(11), 2440-2449. [CrossRef]
- Gonzalez, A. A.; Jeyanandarajan, D.; Hansen, C.; Zada, G.; Hsieh, P. C. Intraoperative neurophysiological monitoring during spine surgery: A review. Neurosurgical Focus 2009, 27(4). [CrossRef]
- Garasz, A.; Huber, J.; Grajek, M.; Daroszewski, P. Motor evoked potentials recorded from muscles versus nerves after lumbar stimulation in healthy subjects and patients with disc-root conflicts. Int J Artif Organs. 2023, 46(5), 303-313. [CrossRef]
- Geissele, A.E.; Kransdorf, M.J.; Geyer, C.A.; Jelinek, J.S.; Van Dam, B.E. Magnetic resonance imaging of the brain stem in adolescent idiopathic scoliosis. Spine 1991, 16(7), 761-763. [CrossRef]
- Goldberg, C.J.; Dowling, F.E.; Fogarthy, E.E. Adolescent idiopathic scoliosis and cerebral asymmetry: An examination of a non-spinal perceptual system. Spine 1995, 20, 1695–1697. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).