Submitted:
07 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Methods
Statistical Analysis
Results
Sensitization to Allergens
Serum-specific IgE Analysis
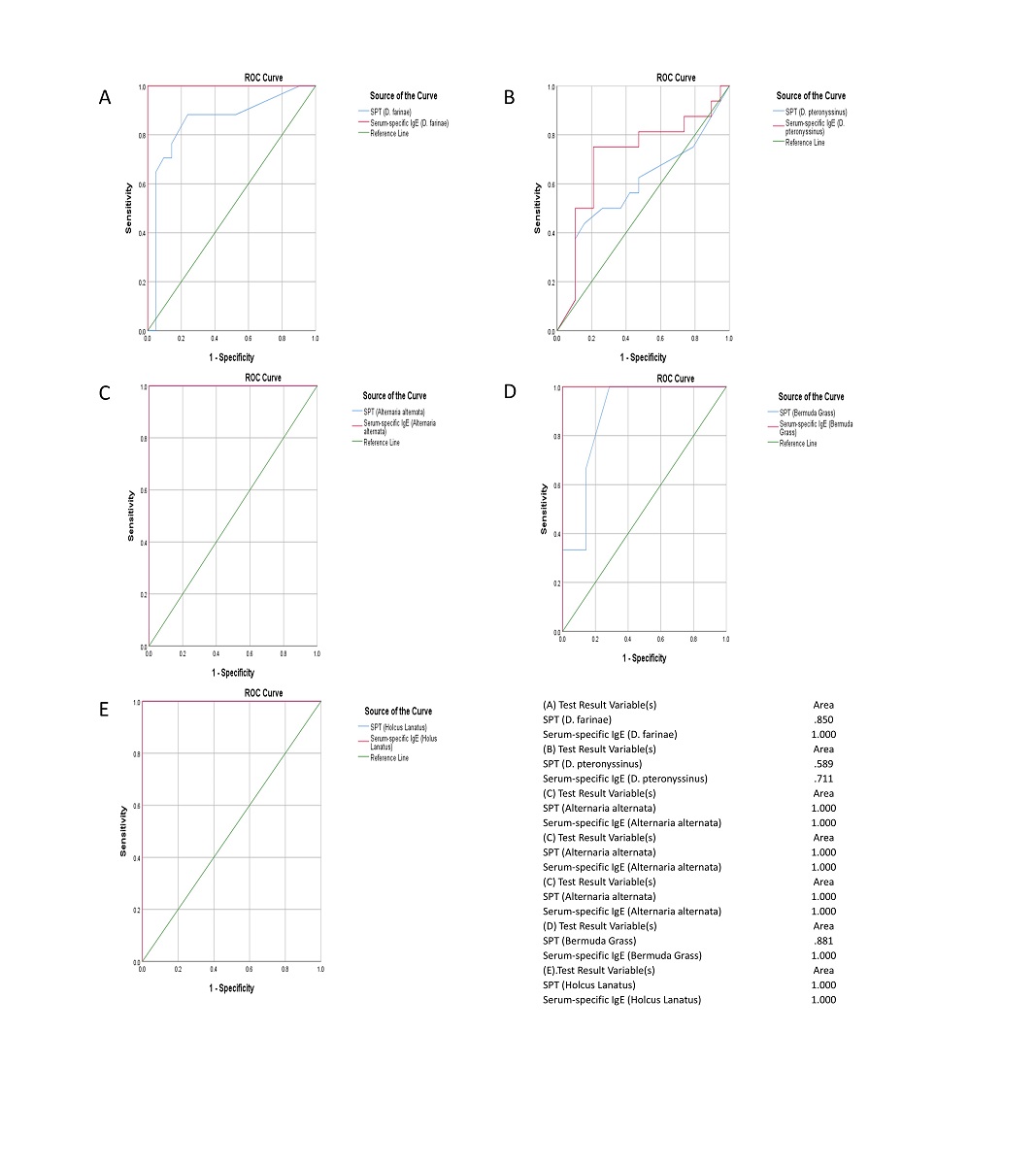
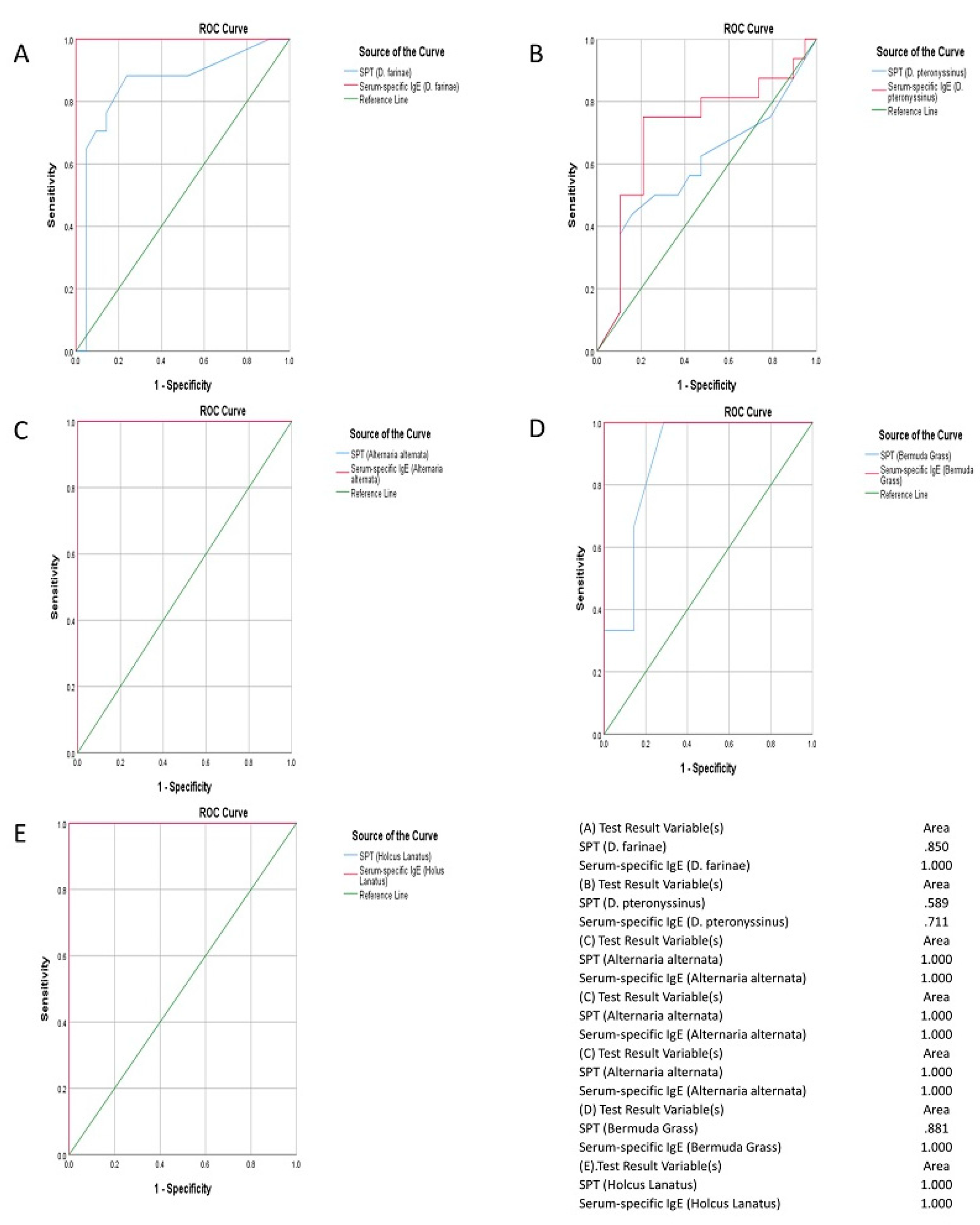
Comparison of Serum-specific IgE Test with Skin Prick Test in the Diagnosis of Allergy
Discussion
Conflict of Interest
References
- Mallol, J.; Crane, J.; von Mutius, E.; et al. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesis. Allergologia et immunopathologia. 2013, 41, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, H.C. Fifty years later: emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. Journal of Allergy and Clinical Immunology. 2016, 137, 1631–1645. [Google Scholar] [CrossRef]
- Ring, J. What is Allergy. Global Atlas of Allergy 2014, 1, 2–3. [Google Scholar]
- Joseph, N.; Palagani, R.; Shradha, N.H.; et al. Prevalence, severity and risk factors of allergic disorders among people in south India. African health sciences 2016, 16, 201–209. [Google Scholar] [CrossRef]
- Kang, S.Y.; Song, W.J.; Cho, S.H.; Chang, Y.S. Time trends of the prevalence of allergic diseases in Korea: a systematic literature review. Asia Pacific Allergy. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Dara, P.K.; Kumari, P.; Meena, H.; Sharma, B.S. Prevalence of various oculo-respiratory allergic conditions and their comorbid association: A cross-sectional observational study in children (6–18 years) from Jaipur. Indian Journal of Allergy, Asthma and Immunology. 2018, 32, 15. [Google Scholar] [CrossRef]
- Jaggi, V.; Dalal, A.; Ramesh, B.R.; et al. Coexistence of allergic rhinitis and asthma in Indian patients: The CARAS survey. Lung India: Official Organ of Indian Chest Society. 2019, 36, 411. [Google Scholar] [CrossRef]
- Kumar, R. Allergy Testing. Manual of Workshop on Respiratory Allergy: Diagnosis and Management 2016, 4, 59–108. [Google Scholar]
- Nagaraju, K. Approach to an Allergic Child. Manual of Pediatric Allergy (second edition) 2021, 1, 1–7. [Google Scholar]
- Simpson, C.R.; Newton, J.; Hippisley-Cox, J.; Sheikh, A. Incidence and prevalence of multiple allergic disorders recorded in a national primary care database. J R Soc Med. 2008, 101, 558–563. [Google Scholar] [CrossRef]
- Ghaffari, J.; Khademloo, M.; Saffar, M.; Raflei, A.; Masiha, F. Hypersensitivity to house dust mite and cockroach is the most common allergy in north of Iran. Iran J Immunol 2010, 7, 234–239. [Google Scholar]
- Almogren, A. Airway allergy and skin reactivity to aeroallergens in Riyadh. Saudi Med J 2009, 3, 392–396. [Google Scholar]
- Madden, K.J.; Forrester, T.E.; Hambleton, I.R.; et al. Skin test reactivity to aeroallergens in Jamaicans: Relationship to Asthma. West Indian Med J 2006, 55, 142–147. [Google Scholar] [CrossRef]
- Kumar, R.; Sharan, N.; Kumar, M.; Bisht, I.; Gaur, S.N. Pattern of skin sensitivity to various aeroallergens in patients of bronchial asthma and/or allergic rhinitis in India. Ind J Allergy Asthma Immunol 2012, 26, 66–72. [Google Scholar]
- Wagner, N.; Rudert, M. Sensitivity and specificity of standardised allergen extracts in skin prick test for diagnoses of IgE-mediated respiratory allergies. Clin Transl Allergy 2019, 9, 8. [Google Scholar] [CrossRef]
- Boechat, J.L.; Moore, D.; Cortes, V.; et al. Prevalence, clinical features and severity of allergic rhinitis in elderly: preliminary results. World Allergy Organization Journal. BioMed Central. 2015, 8, A276. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organization Journal. 2014, 7, 12. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.B.; Salvi, S.; et al. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. The Clinical Respiratoy Journal. 2018, 12, 547–556. [Google Scholar] [CrossRef]
- Veerapaneni, V.; Jayaraj, B.S.; Lokesh, K.S.; et al. Prevalence of allergic rhinitis, atopic dermatitis and asthma among school children in Hyderabad, India. Journal of Allergy and Clinical Immunology. 2017, 139, AB205. [Google Scholar] [CrossRef]
- Yoo, B.; Park, Y.; Park, K.; Kim, H. A 9-year trend in the prevalence of allergic disease based on national health insurance data. Journal of Preventive Medicine and Public Health. 2015, 48, 301. [Google Scholar] [CrossRef]
- Bousquet, J.; Heinzerling, L.; Bachert, C.; et al. Global Allergy and Asthma European Network; Allergic Rhinitis and its impact on Asthma. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012, 67, 18–24. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients, n (%) | |
|---|---|
| Patients enrolled in the study | 267 |
| Year 2017 | 80 (29.9) |
| Year 2018 | 106 (39.7) |
| Year 2019 | 66 (24.7) |
| Year 2020 | 15 (5.6) |
| Patients completed the study | 256 (95.9) |
| Patients analysed in the study | 256 (95.9) |
| Patients withdrawn from the study | 11 (4.1) |
| Reason for withdrawal | - |
| Lost to follow-up | 11 (4.1) |
| Investigator-executed withdrawal | 0 |
| SPT positive allergens | Number of Patients, n (%) (N=256) |
|---|---|
| Mites | - |
| D. farinae | 173 (67.6) |
| D. pteronyssinus | 174 (68.0) |
| Acarus siro | 93 (36.3) |
| Lepidoglyphus destructor | 96 (37.5) |
| Mould | - |
| Aspergillus fumigatus | 66 (25.8) |
| Alternaria alternata (Alternaria tenuis) | 70 (27.3) |
| Helminthosporium halodes | 46 (18.0) |
| Penicillum notatum | 29 (11.3) |
| Cladosporium herbarum | 54 (21.1) |
| Rhizopus nigricans | 37 (14.5) |
| Fusarium moniliforme | 31 (12.1) |
| Botrytis cinerea | 46 (18.0) |
| Pollen (Grasses) | - |
| Bermuda Grass (Cynodon dactylon) | 74 (28.9) |
| Barley (Hordeum vulgare) | 8 (3.1) |
| Orchard Grass (Dactylis glomerata) | 51 (19.9) |
| Timothy Grass (Phleum pratense) | 65 (25.4) |
| Rye grass (Lolium perenne) | 57 (22.3) |
| Kentucky Blue Grass (Poa pratensis) | 52 (20.3) |
| Rye (Secale cereale) | 51 (19.9) |
| Wheat (Triticum sativum) | 34 (13.3) |
| Zea mays (Corn) | 32 (12.5) |
| Holcus lanatus (Velvet grass) | 14 (5.5) |
| Pollen (Weeds) | - |
| Lambs Quarter (Chenopodium album) | 65 (25.4) |
| Ragweed (Ambrosia artemisiifolia) | 58 (22.7) |
| Engl. Plantain (Plantago lanceolata) | 81 (31.6) |
| Nettle (Urtica dioica) | 44 (17.2) |
| Mugwort (Artemisia vulgaris) | 81 (31.6) |
| Taraxacum vulgare (Dandelion) | 12 (4.7) |
| Pollen (Trees) | - |
| Locust Black (Robinia pseudoacacia) | 50 (19.5) |
| Mountaineous Pollen (Trees) | - |
| Alder (Alnus glutinosa) | 35 (13.7) |
| Birch (Betula alba) | 46 (18.0) |
| Poplar (Populus alba) | 50 (19.5) |
| Salix capera (Willow) | 14 (5.5) |
| Quercus robur (Oak) | 12 (4.7) |
| Cottonwood (Populas deltoids) | 1 (0.4) |
| Animal Epithelia | - |
| Cow Epithhelia | 29 (11.3) |
| Cat Epithelia | 21 (8.2) |
| Fruits | - |
| Banana | 39 (15.2) |
| Orange | 45 (17.6) |
| Food - Flours & Seeds | - |
| Corn | 68 (26.6) |
| Wheat | 51 (19.9) |
| Gluten | 27 (10.5) |
| Food - Nuts | - |
| Ground nut | 37 (14.5) |
| Walnut | 41 (16.0) |
| Hazelnut | 1 (0.4) |
| Vegetables | - |
| Spinach | 9 (3.5) |
| Asparagus | 13 (5.1) |
| Spices and Pulses | - |
| Aniseed | 7 (2.7) |
| Milk/Egg | - |
| Milk | 34 (13.3) |
| Egg | 31 (12.1) |
| SIgE values (IU/mL) | Number of Patients, n (%) (N = 256) |
| 0.1-2 | 22 (8.6) |
| 2-20 | 23 (9.0) |
| >20 | 32 (12.5) |
| Allergens | Number of Patients, n (%) (N=256) |
|
|---|---|---|
| SPT | Serum-specific IgE | |
| Mites | - | - |
| D. farinae | 173 (67.6) | 36 (14.1) |
| D. pteronyssinus | 174 (68.0) | 32 (12.5) |
| Mould | - | - |
| Aspergillus fumigatus | 66 (25.8) | 12 (4.7) |
| Alternaria alternata (Alternaria tenuis) | 70 (27.3) | 11 (4.3) |
| Cladosporium herbarum | 54 (21.1) | 6 (2.3) |
| Pollen (Grasses) | - | - |
| Bermuda Grass (Cynodon dactylon) | 74 (28.9) | 8 (3.1) |
| Timothy Grass (Phleum pratense) | 65 (25.4) | 5 (2.0) |
| Rye grass (Lolium perenne) | 57 (22.3) | 1 (0.4) |
| Rye (Secale cereale) | 51 (19.9) | 4 (1.6) |
| Holcus lanatus (Velvet grass) | 14 (5.5) | 6 (2.3) |
| Pollen (Weeds) | - | - |
| Lambs Quarter (Chenopodium album) | 65 (25.4) | 4 (1.6) |
| Ragweed (Ambrosia artemisiifolia) | 58 (22.7) | 2 (0.8) |
| Engl. Plantain (Plantago lanceolata) | 81 (31.6) | 7 (2.7) |
| Nettle (Urtica dioica) | 44 (17.2) | 1 (0.4) |
| Mugwort (Artemisia vulgaris) | 81 (31.6) | 10 (3.9) |
| Mountaineous Pollen (Trees) | - | - |
| Alder (Alnus glutinosa) | 35 (13.7) | 1 (0.4) |
| Birch (Betula alba) | 46 (18.0) | 1 (0.4) |
| Quercus robur (Oak) | 12 (4.7) | 2 (0.8) |
| Animal Epithelia | - | - |
| Cat Epithelia | 21 (8.2) | 1 (0.4) |
| Fruits | - | - |
| Orange | 45 (17.6) | 1 (0.4) |
| Food - Flours & Seeds | - | - |
| Corn | 68 (26.6) | 1 (0.4) |
| Wheat | 51 (19.9) | 2 (0.8) |
| Gluten | 27 (10.5) | 3 (1.2) |
| Food – Nuts | - | - |
| Hazelnut | 1 (0.4) | 1 (0.4) |
| Spices and Pulses | - | - |
| Aniseed | 7 (2.7) | 1 (0.4) |
| Milk/Egg | - | - |
| Milk | 34 (13.3) | 3 (1.2) |
| Type of allergen | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (%) |
NPV (%) |
|---|---|---|---|---|
| D. farinae (Mite) | 88.0% | 44.8% | 68.1% | 53.2% |
| D. pteronyssinus (Mite) | 87.6% | 35.7% | 73.7% | 56.9% |
| Alternaria alternate (Mould) | 55.0% | 18.4% | 64.8% | 96.4% |
| Bermuda Grass (Pollen [grasses]) | 34.7% | 74.6% | 81.3% | 56.3% |
| Holcus lanatus (Pollen [grasses]) | 13.2% | 91.8% | 66.6% | 72.9% |
| Type of allergen | p-value * | r* |
|---|---|---|
| D. farinae (Mite) | 0.001 | 0.412 |
| D. pteronyssinus (Mite) | 0.001 | 0.398 |
| Alternaria alternate (Mould) | 0.001 | 0.560 |
| Bermuda Grass (Pollen [grasses]) | 0.001 | 0.457 |
| Holcus lanatus (Pollen [grasses]) | 0.001 | 0.531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





