1. Introduction
Global emission and production of plastic materials are significantly growing, causes the accumulation into the natural environment. Consequently, plastic particles are present in food [
1], drinking water [
2,
3] or human lungs [
4]. The knowledge about the side effects caused by the plastics present in the human is still very limited. In this paper we aim to investigate the effect of the presence of microplastics on the rheological properties of artificial saliva and mucus.
Micro and nanoplastics can be divided into primary and secondary ones. Microplastics are considered to be primary when they are directly emitted as such into the environment, and secondary when they are degradation products from larger plastic objects (macroplastics) already in the environment. For example, macroplastics can break down due to UV-light, thermal changes and oxidative weathering, resulting in secondary microplastics [
5]. Every plastic product can end up in the environment and is therefore potentially a source of microplastics.
There are several ways micro and nanoparticles can entry organisms. In therapeutic and diagnostic applications nanoparticles are injected directly into the blood stream. They may also enter the organism with breathing air. In general, the size of the inhaled particle determines the location of its deposition, i.e., how deep a particle can enter into the airways. Particles with an aerodynamic diameter above 100 µm will deposit in the upper airways, whereas particles in the range of 10 to 100 µm will deposit in the oropharynx [
6]. Particles with a diameter between 5 and 10 µm will deposit in the central airways mucus layer, upon which ciliary movement of the endothelium will remove the particles from the airways [
6]. Only particles below approximately 5 µm will be able to reach the alveoli, upon which clearance from the lung by macrophage phagocytosis usually takes place [
6,
7]. Particles with a diameter between approximately 0.2 and 0.5 µm are not deposited to a great degree, while particles in the 5 to 100 nm range mainly deposit in the alveoli and even smaller nanoparticles deposit in the trachea-bronchial region and the upper airways [
7]. Inhaled nanoparticles can even cross the alveolar-capillary barrier and directly affect the cardiovascular system [
8].
Exposure via ingestion to microplastics present in the various environmental compartments may occur either directly or indirectly via the food chain or drinking water. Direct oral exposure to nanoplastics suspended in the air may occur when deposited inhaled nanoplastics are removed from the lungs by the mucociliary escalator, end up in the oropharynx and are eventually ingested.
The ways the micro and nanoparticles enter the human body described above show that they have contact with various body fluids (saliva, nasal and bronchial mucus, lymph, blood). The main component of all mentioned above fluids is water. They also contain various amount of proteins (enzymes, antibodies, mucins, etc.), lipids and minerals (sodium, potassium, chloride, bicarbonate), what is related to their functions and leads to the differences in their rheological properties.
The most important functions of body fluids are well known, but the effect of their rheological properties on functions is not always sufficiently recognized. Appropriate "viscosity" of body fluids is necessary to create a protecting thin layer on the surface of the mouth, eyes, nose, and respiratory tract against ingress of pathogens. The effects of microplastics on the rheological properties of saliva, and mucus have not been sufficiently recognized so far, however several papers investigating the effect of nanoparticles on the rheological properties of body fluids were recently published.
In this study, both saliva and mucus models were used to investigate the effect of two microplastics, namely PE (polyethylene) and PS (polystyrene), presence on their rheology. On this basis, the potential impact of microplastics on the functions of saliva and mucus was estimated. According to the authors’ knowledge, no study of this kind has taken place beforehand.
2. Materials and Methods
2.1. Saliva
The properties of saliva (composition, rheology, and flow) are strongly affected by factors like conditions of their collection, handling and preservation, age, gender, health status, and even emotional stress of saliva donor [
9] therefore, we decided to use artificial saliva in our study.
Artificial saliva was obtained based on the model described by Christersson et al. [
10]. The following components were dissolved in deionized water - benzalkonium chlorides ((Sigma Aldrich, Poznań, Poland) [0.02 g/l], EDTA (Sigma Aldrich, Poznań, Poland) [0.5 g/l], NaF (Chempur, Piekary Śląskie, Poland) [0.0042 g/l], xsylitol (Sigma Aldrich, Poznań, Poland) [20 g/l], methylparaben (Sigma Aldrich, Poznań, Poland) [1 g/l], mucins (type II) (Sigma-Aldrich, Poznań, Poland) [35 g/l] and then placed on a magnetic stirrer (500 rpm) for 2 h. The pH of the solution (7.00) was determined using NaOH or HCl. The sample was stored and sealed at 4 °C.
2.2. Mucus
Similarly, we have decided to use artificial mucus in our research, so to obtain reproducible results. From the number of mucus models tested in our previous studies [
11,
12,
13] we have decided to use the simplest model [
13] for further research. The mucins type II (Sigma Aldrich, Poznań, Poland) (200 g/l) and NaN
3 (POCH, Gliwice, Poland) (0.01 g/l) were dissolved in deionized water and then placed on a magnetic stirrer (500 rpm) for 2 h. After this time, the pH of the solution (7.4) was adjusted with NaOH or HCl. The mucus was stored in a closed chamber at 4 °C.
2.3. Microplastics
The microplastic particles used in experiments were manufactured by Cosphereic LLC (Goleta, USA). Three types of PE microspheres with diameters from the range 0.74 – 4.99 mm, 38 – 45 mm and 96 – 106 mm were used. The particles have the form of dry powder and are hydrophobic. The density of the particles was 0.96 g/cm3.
In our studies we have also used two types of PS particles with diameters from the range 9.5 – 11.5 mm and 38 – 48 mm. The density of the particles was 1.07 g/cm3.
2.4. Methodology
We investigated the rheological properties of artificial saliva and mucus with microplastics. The PE and PS particles were added to artificial saliva or mucus (prepared the day before measurement) in the concentrations 6 particles/ml, what corresponds to the concentration of microplastics observed in bottled water [
14]. The appropriate concentration of PE and PS particles in the model of saliva and mucus was obtained assuming (based on the datasheet of PE and PS particles) number of particles in 1 g of the product (
Table 1). After that, the plastic microparticles suspension was stirred for 15 min. The artificial saliva and mucus were warmed in a water bath to room temperature before adding the particles. The rheological properties (flow curve and the dependence of viscosity as a function of shear stress) were examined with oscillation rheometer (MCR102, Anton Paar, Graz, Austria) equipped with a Peltier system in a plate-plate system for a 1-mm-wide gap. The tests were carried out at the temperatures of 36.6 °C and 40 °C, what corresponds to the case of healthy and ill human.
The appropriate volume of model saliva and mucus, pure and with microplastics, was applied with an automatic pipette to the bottom plate of the rheometer. Before the rheological measurement, all analyzed samples were subjected to the same preparation procedure, considering the mixing time, stirrer speed, and temperature. In addition, to ensure the exact ordering of mucin chains in all samples, the rheometer operation included two intervals following one another at the time of rheological measurement. In the first interval, the sample was exposed to a constant shear rate for 2 minutes (to organize the mucin chains), while in the second, the actual measurement was carried out. The shear rate range during the second interval was 1 – 200 s
-1, what corresponds to typical activities involving saliva (
4 s−1 corresponds to movement of particles across the tongue, 60 s−1 corresponds to swallowing and 160 s−1 to speech, whilst shear rates of 10–500 s−1 have been proposed to reflect the shear during eating. [
15]
. This rang is also typical for the processes occurring in human respiratory mucus except sneezing and coughing [
16]
.
Like natural saliva and mucus, the saliva and mucus model contains mucins. Mucin chains have a length of 10 - 40 MDa and a diameter of 3 - 10 nm. The degree of mixing is essential in rheological measurements for compounds with a chain structure. Depending on the degree of mixing, long chains can be more or less tangled and more or less arranged, directly translating into apparent viscosity. Less entanglement and better alignment of the chains lower the apparent viscosity. The sample preparation procedure used by us and the two-interval measurement procedure eliminated the potential impact of unequal mixing of the analyzed samples on the rheological measurement result. Each measurement was repeated at least three times, and the presented results are the arithmetic mean of the obtained results.
The least squares method was used to obtain constants in Ostwald – de Waele Power law rheological model:
where
t is shear stress,
m - apparent viscosity,
k - flow consistency index and
n - flow behavior index. Equation 1 describes the flow curve, while Equation 2 is derived from equation 1 by comparison with relation defining apparent viscosity:
Analysis were carried out using Matlab Curve Fitting Toolbox. Nonlinear least-squares was the most appropriate for estimating model coefficients in our case.
A nonlinear model has the matrix form
where
y is an
n-by-1 vector of response data,
β -
m-by-1 vector of coefficients,
X -
n-by-
m design matrix,
f is a nonlinear function of
β and
X,
ε is an
n-by-1 vector of unknown errors.
Curve Fitting Toolbox uses the following iterative approach to calculate the coefficients:
Initialize the coefficient values.
Calculate the fitted curve for the current set of coefficients. The fitted response value ŷ is given by ŷ =f(X,b) and is calculated using the Jacobian of f(X,β). The Jacobian of f(X,β) is defined as a matrix of partial derivatives taken with respect to the coefficients in β.
Adjust the coefficients using a Trust-region algorithm [
17].
The regression was performed for the Equation 1, however the regression for Equation 2 give exactly the same values of flow behaviour and flow consistency indexes, however the calculated values of correlation coefficient were obviously different.
We have performed regression for several more advanced rheological model of pseudoplastic fluids, but have not found better agreement with experimental data. The tested models are summarized in
Table 1.
Table 2.
Rheological models.
Table 2.
Rheological models.
| Model |
Formula |
Parameters |
| Prandtl |
|
A [Pa], C [s-1] |
| Powell - Eyring |
|
A [s-1], B [Pa], C [Pas] |
| Williamson |
|
A [Pa], B [s-1], m∞ [Pas] |
| Sisko |
|
A [Pas], B [Pasn], n [-] |
3. Results and discussion
3.1. Rheological properties of saliva
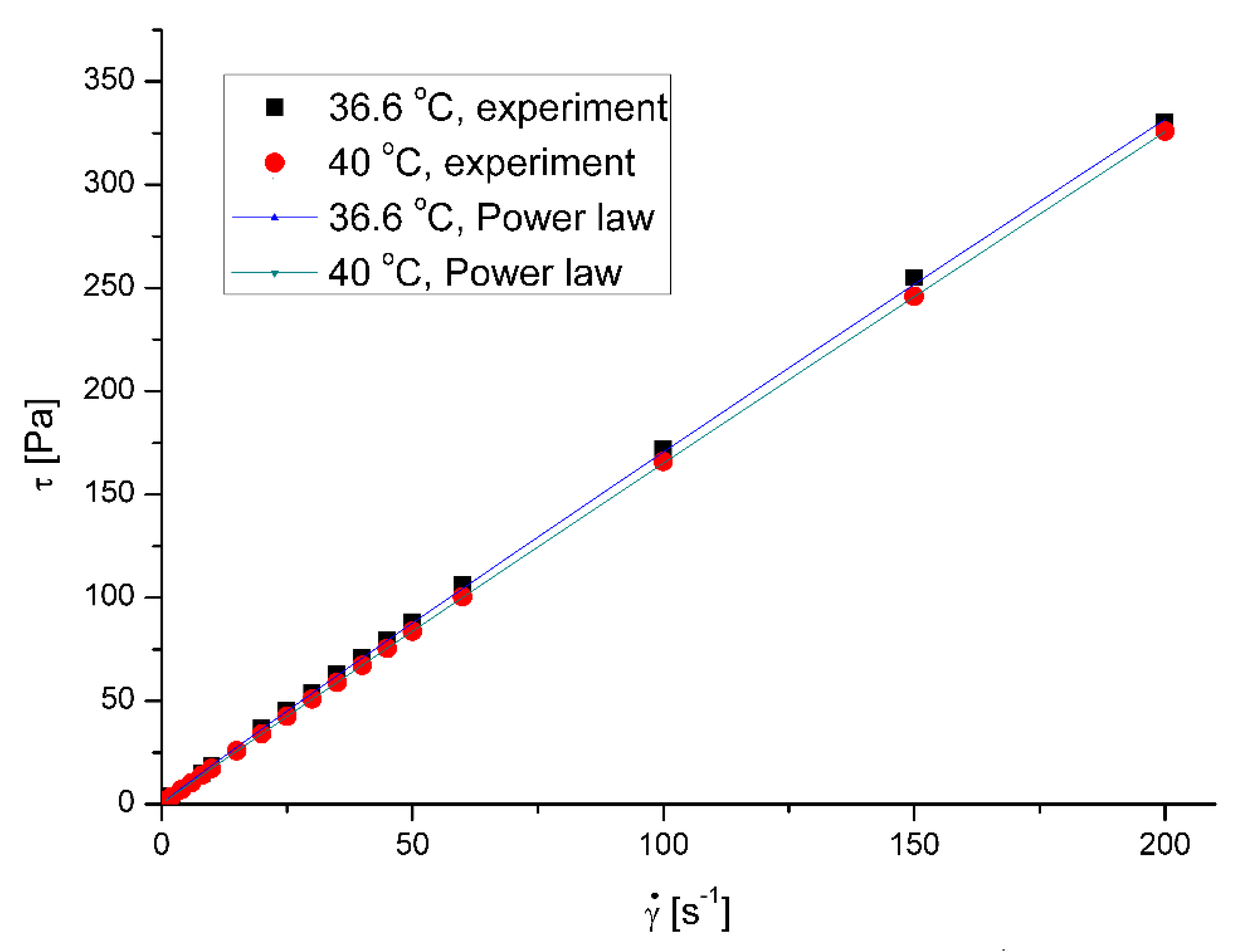
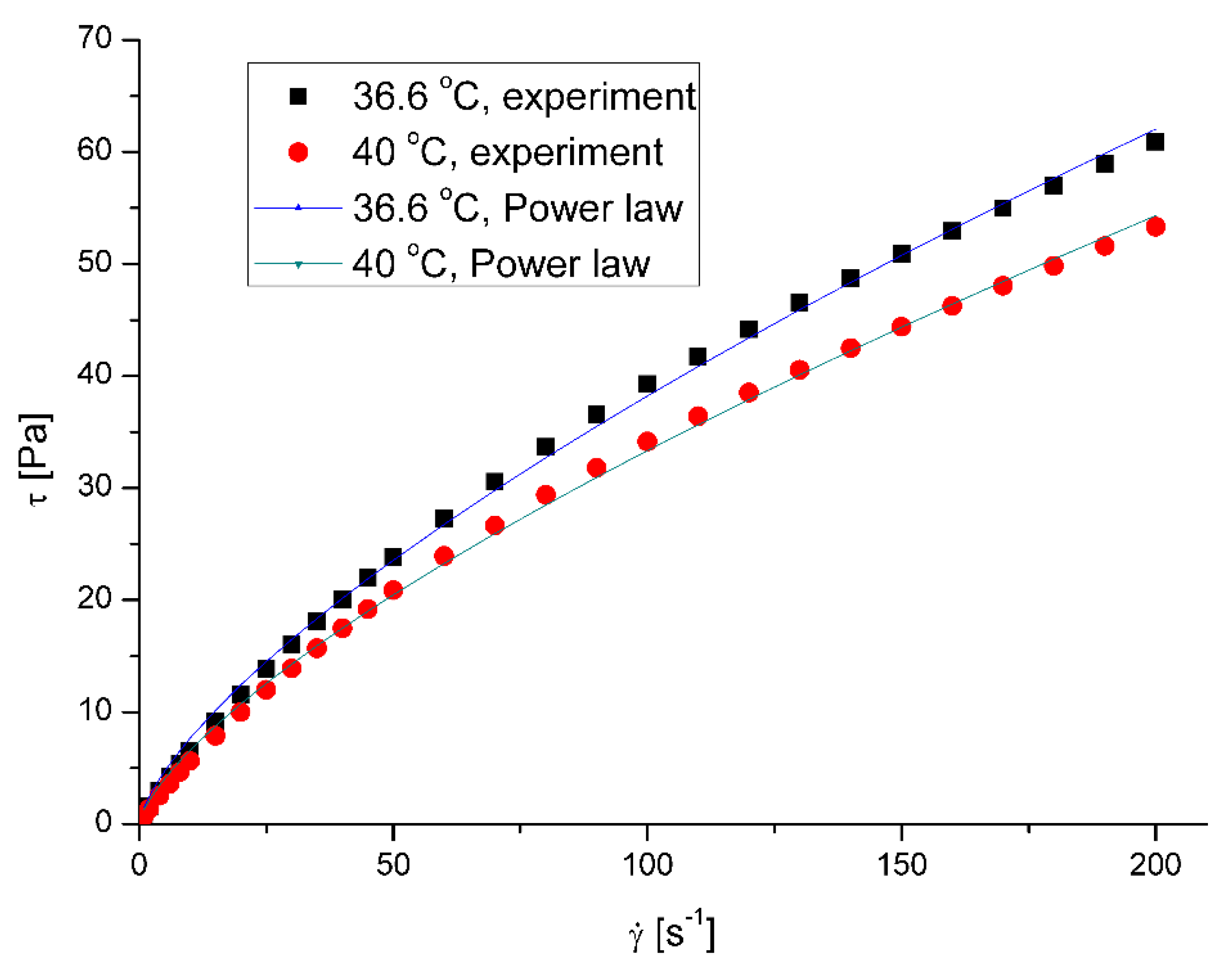
The saliva model behaves slightly like a pseudoplastic fluid. The viscosity decreases with the increase of the shear rate at all examined temperatures. However, the flow curves – shear stress,
τ, vs shear rate,
, (
Figure 1) are almost straight lines, which is a characteristic of Newtonian fluids.
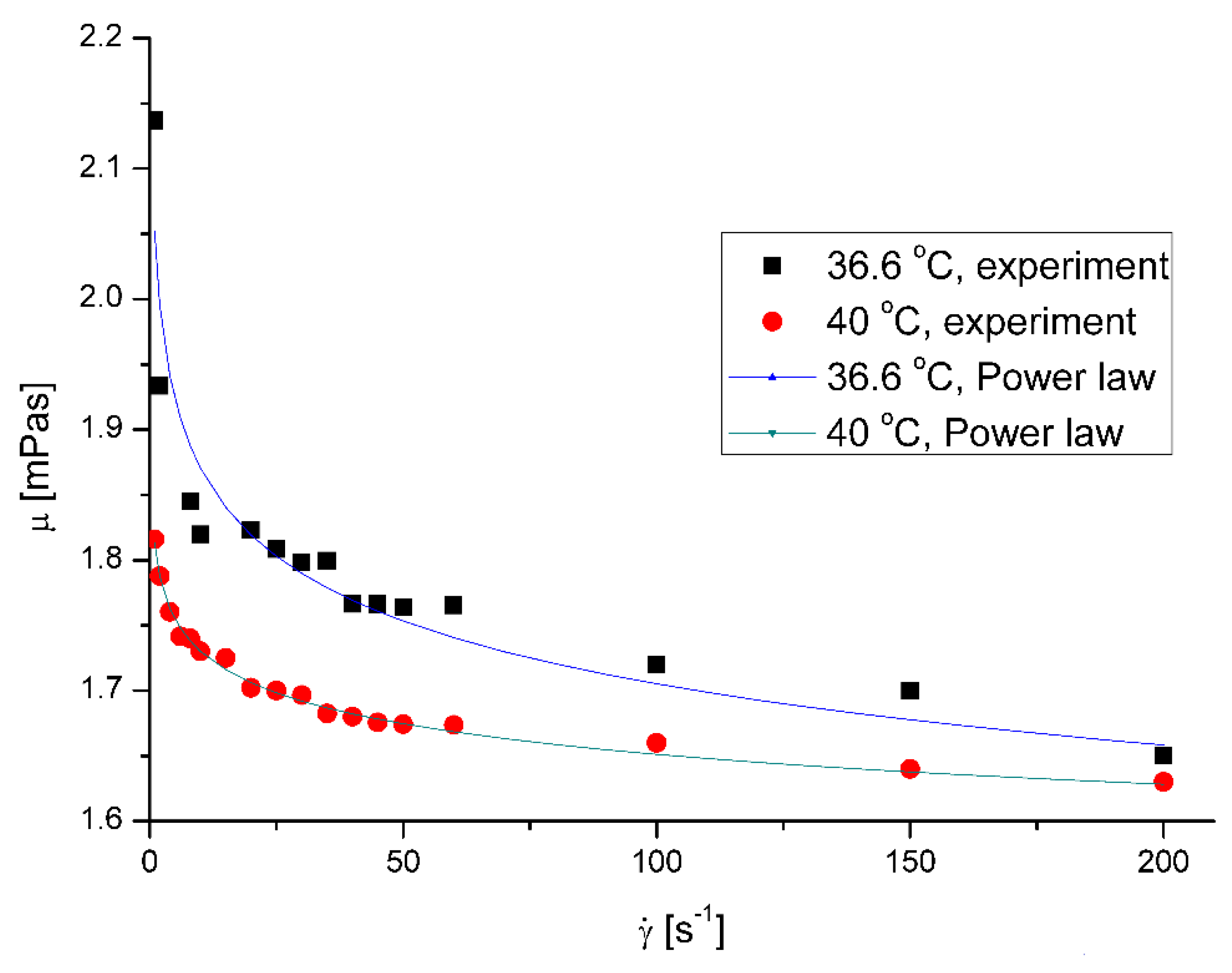
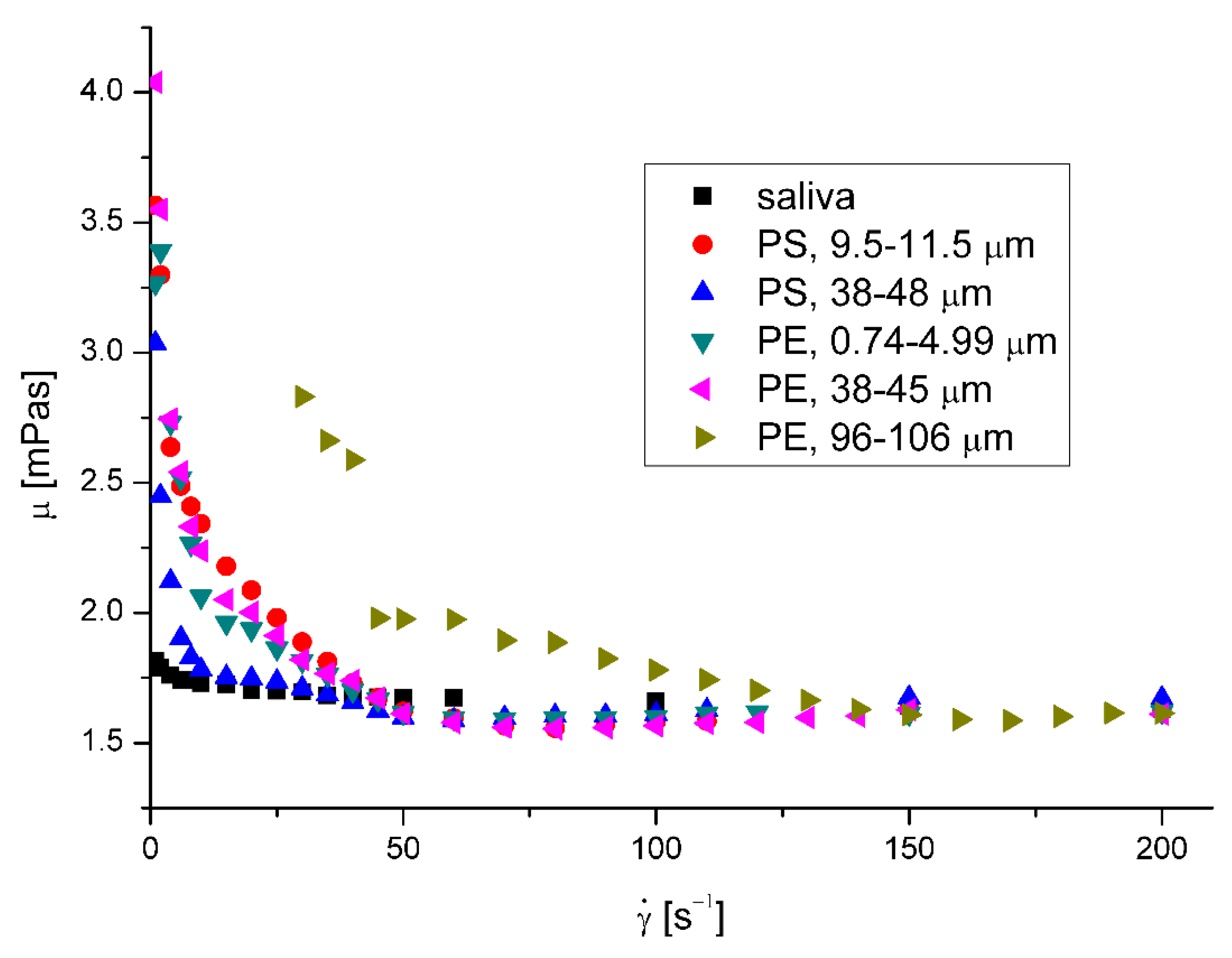
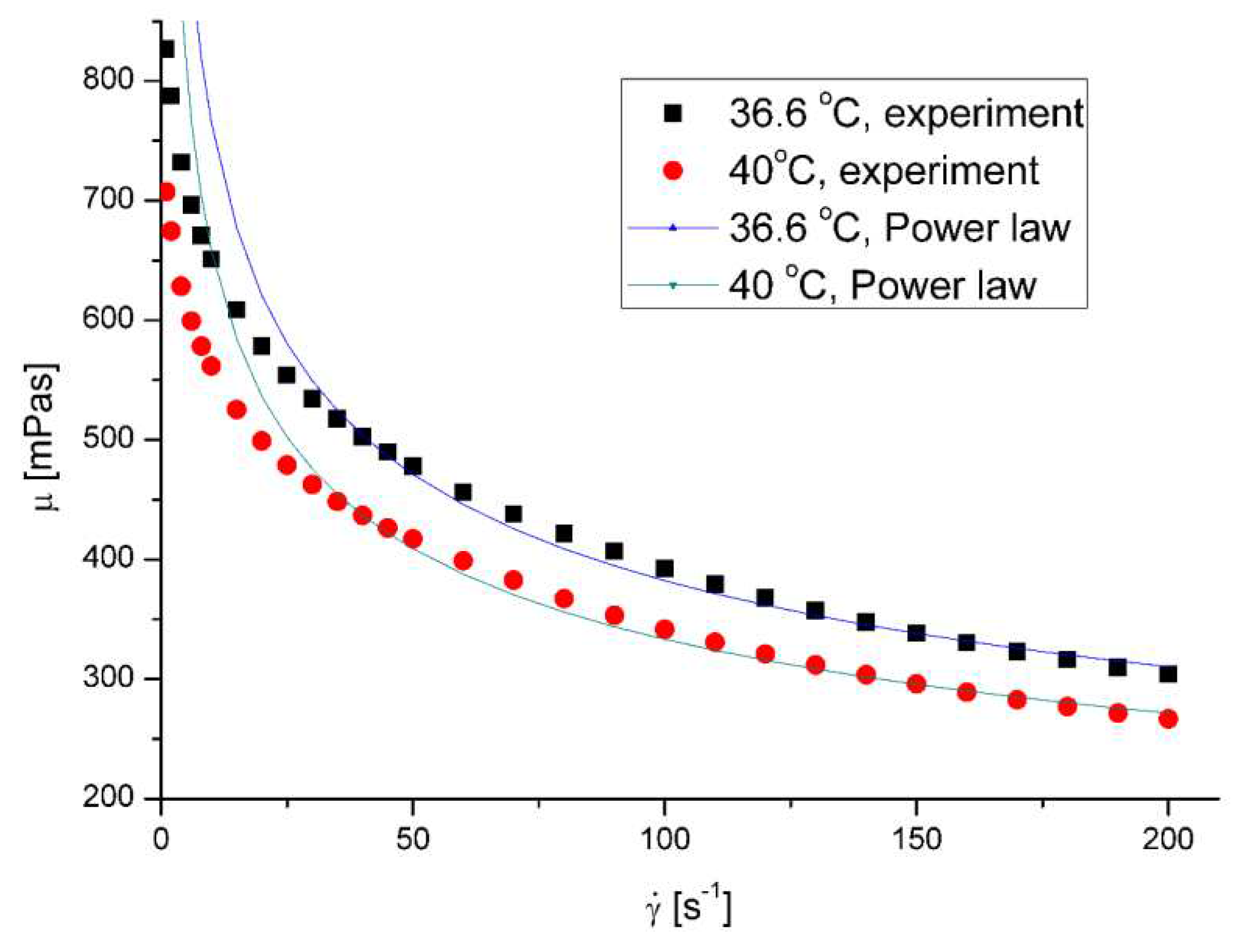
Figure 2 presents the effect of shear rate on the saliva apparent viscosity,
μ. Figure 3 Figure 4 present the effect of microplastics on the apparent viscosity of saliva at 36.6 °C and 40 °C, respectively. The presence of miocroplastics in artificial saliva increased the viscosity for low shear rates. The effect was visibly weaker with increasing shear rate, what is typical behavior for solid particles suspensions in fluids [
18]. The less squares method regression to Ostwald – de Waele Power law mode shows that estimated flow behavior index of pure saliva,
n, is equal to 0.9598 and 0.9797 for the temperatures 36.6 °C and 40 °C, respectively.
The increase of fluid velocity and the presence of microparticles also caused non-Newtonian saliva behavior, dropping noticeably the value of the flow index. (
Table 3)
Biesbrock et al. [
19] and Yas and Radhi [
20] found that there are relationships between the viscosity of saliva and the extent and incidence of dental caries and periodontal disease. While the salivary viscosity increases, dental caries also increases. They did not define if dental caries increases because the viscosity of saliva increases or the viscosity increases because the dental caries increases. However, the relationship between salivary viscosity and dental caries is sufficient to assume that an increase in the viscosity of saliva due to the presence of microplastics may have adverse health consequences.
Moreover, while the saliva viscosity increases, the bacteria co-aggregation decreases that leads to disruption in oral clearance and, consequently, may even cause the increases in the likelihood of aspiration pneumonia and cardiovascular diseases especially in the elderly [
21].
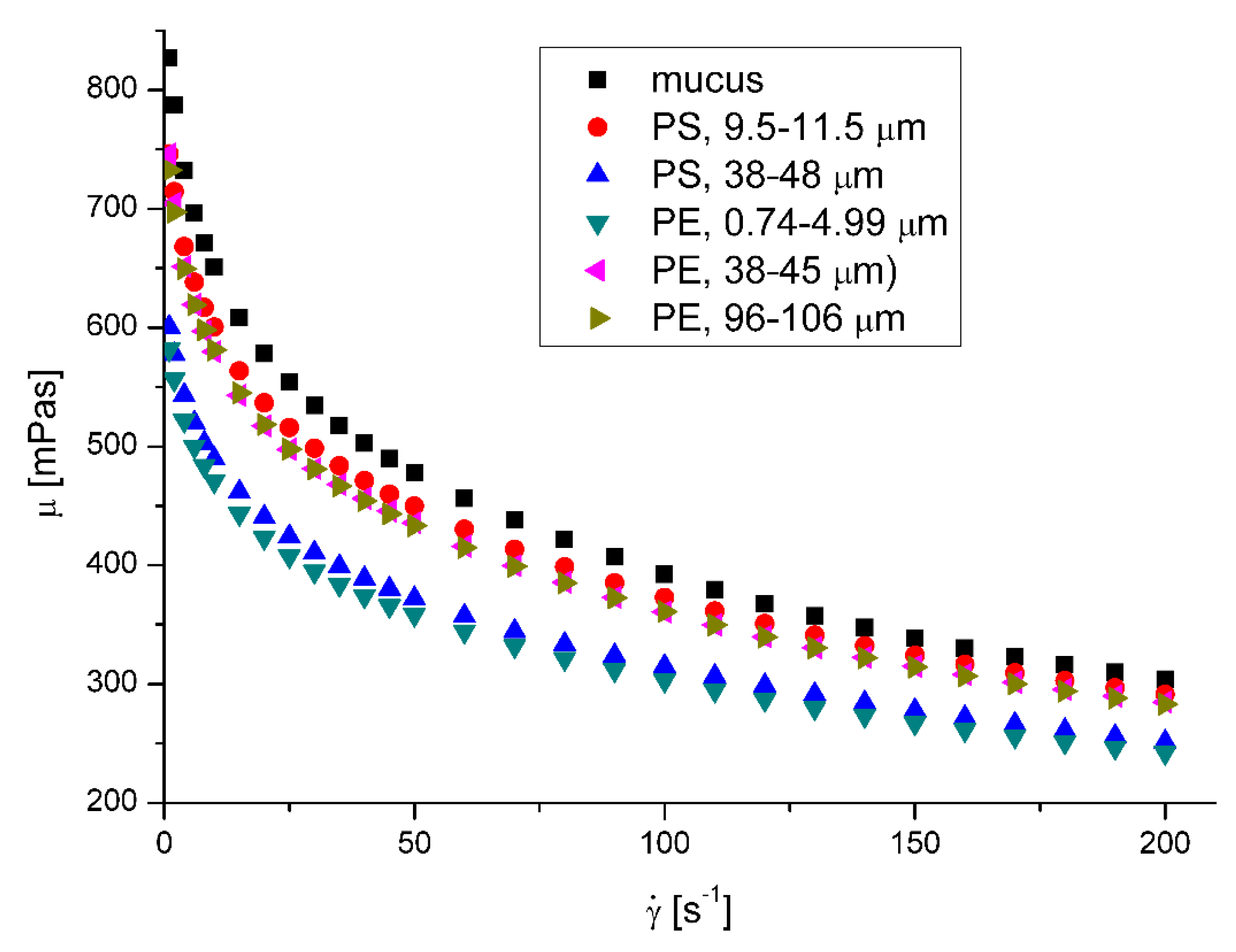
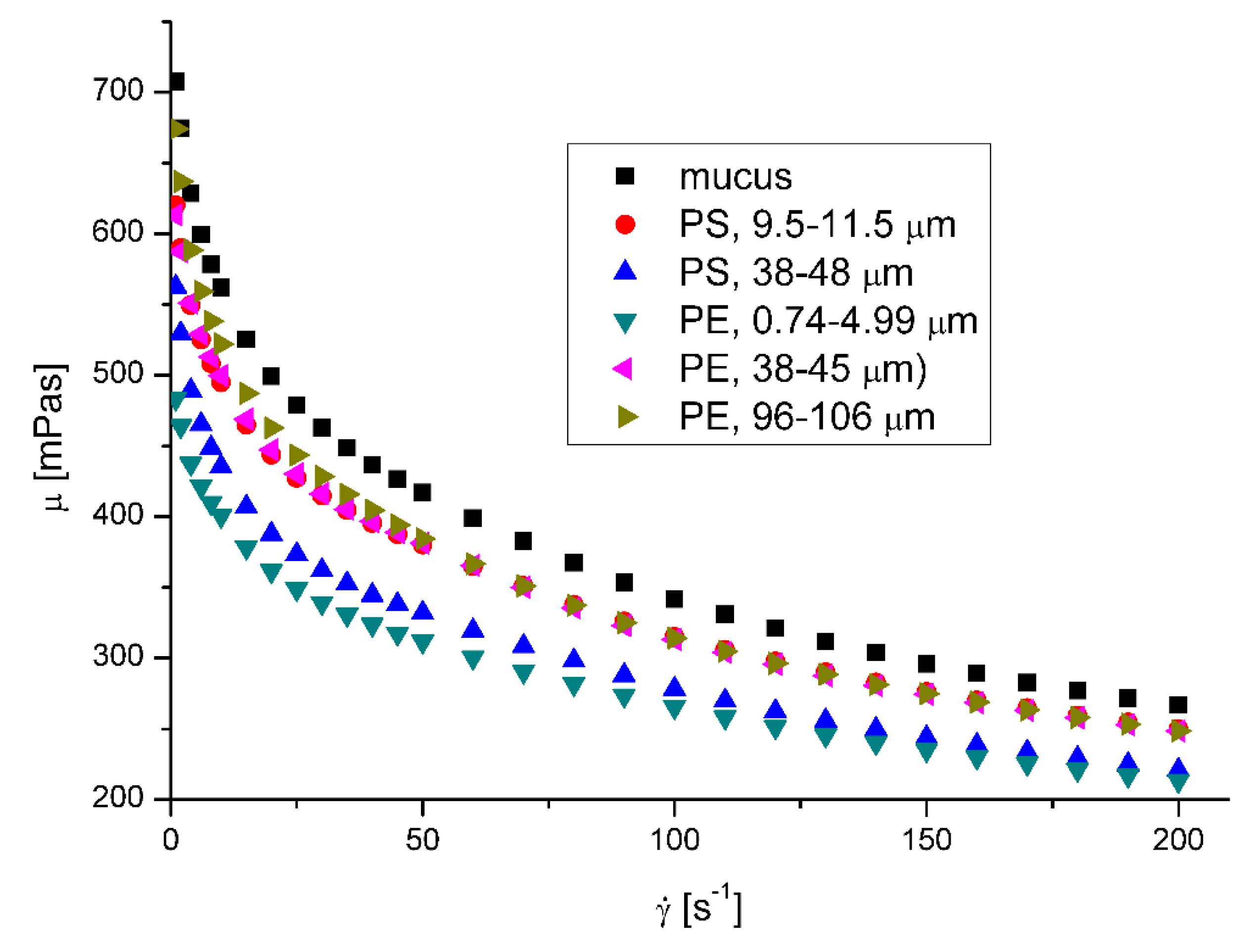
3.2. Rheological properties of mucus
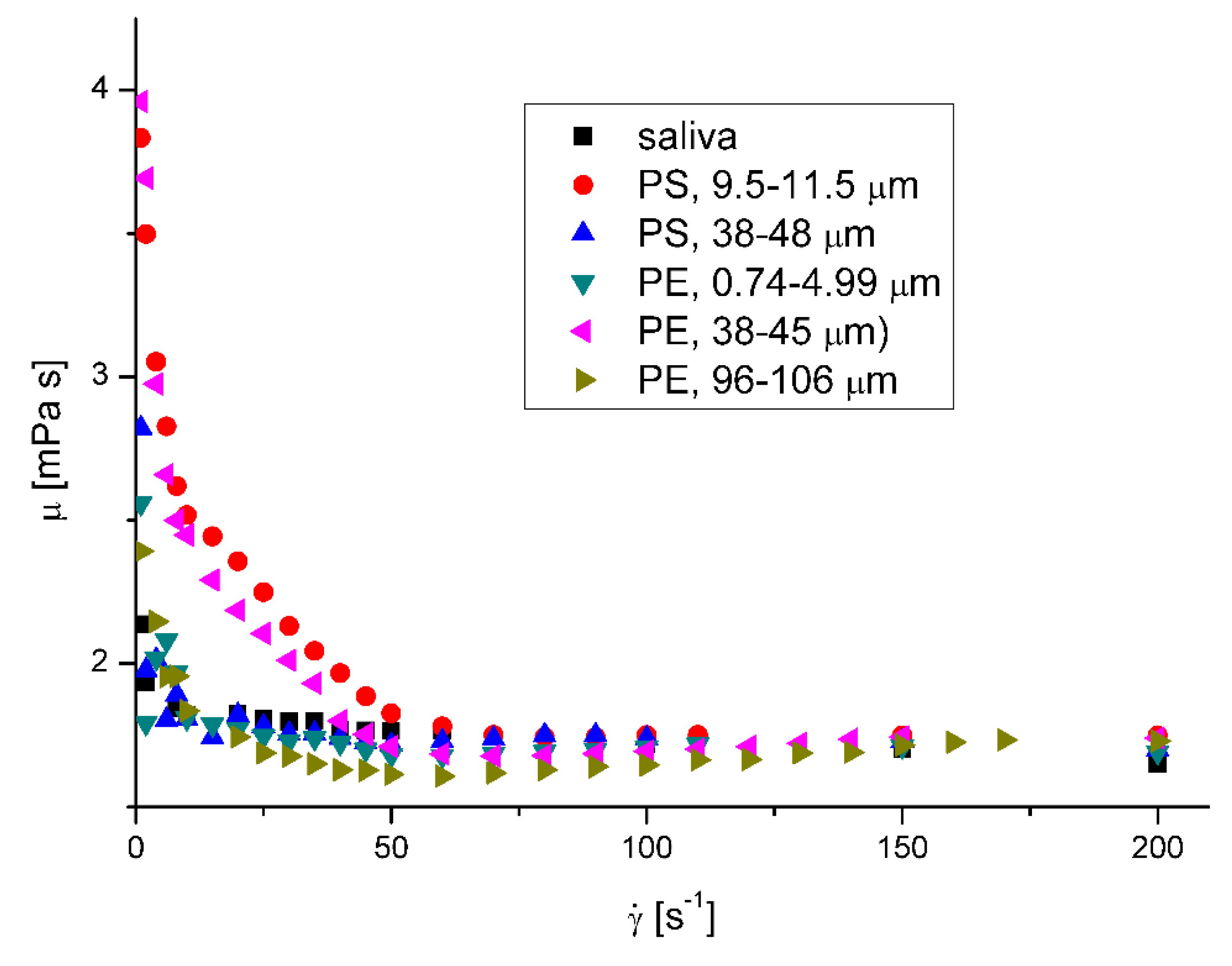
The mucus model behaves like a pseudoplastic fluid without yield stress. (
Figure 5 and
Figure 6 ) The non-Newtonian character can be observed for the clear mucus. The addition of the microplastic particles does not influence the value of the flow index (
Table 4). However, in all the cases the addition of the microplastics to mucus leads to the decrease of apparent viscosity.
The effect of nanoparticles on the apparent viscosity of artificial mucus is presented in
Figure 7 and
Figure 8 . The decrease of fluid suspension by addition of suspended particles is not typical behavior but was observed in rare cases for complex fluids [
22,
23,
24]. Ben-David et. al. [
25] have recently shown, that in jellyfish mucus the microsized plastic particles do not form suspension, but are captured by mucin network, what is potential reason of changing its mechanical properties, what may affect its mechanical properties and result in decrease of apparent viscosity of mucus. Moreover, there is no explicit relation between the decreasing effect of microplatics presence in mucus and their size, what suggests the complex mechanism of interactions. The decreasing effect of microparticles presence on the mucus viscosity was observed for the whole range of investigated shear rates. The decrease of mucus viscosity has generally negative effect on its protective function. The decrease of viscosity results in the increase of diffusion coefficient of particles and pathogens possibly present in mucus and thus increase the possibility of reaching the surface of epithelium and further transport to the body. The relation between the diffusion coefficient, D, and fluid viscosity is
D~
ma, where a usually varies from -0.5 to -1 [
26]. On the other hand lower viscosity facilitates the removal of mucus from the respiratory tracts by cough or sneezing.
4. Conclusions
The presented models of body fluids are a useful tool for estimating the threat arising from exposure to airborne particles, indicating the direction for further research. Compared to real biological fluids, they have only a few ingredients but they always have the same composition, which allows to obtain universal results. The obtained results showed that Non-Newtonian, shear thinning body fluids i.e. mucus or saliva react to the presence of plastic particles, changing their apparent viscosity. To confirm the hypothesis, that non-trivial behavior of microplastics suspension in mucus is caused by particles-mucins interactions we are going to perform Molecular Dynamics calculations. The effect caused by the presence of particles depends on the interaction of the particle-mucin, and on the concentration of mucins. We are also going to investigate the influence of micro- and nanoplastics and other airborne particles, such as Diesel Exhaust Particles (DEP) or natural dust on the rheological properties of other body fluids, particularly blood. Fluids with low mucins concentration, like saliva or tears [
27] behave like typical suspensions, while behavior of fluids with high protein concentration is much more complex.
Author Contributions
Conceptualization, R.P. and A.M.; methodology, A.P., U.M. and R.P.; validation, R.P.; data curation, U.M and R.P.; writing—original draft preparation, R.P. and A.P.; writing—review and editing, A.P. and A.M; visualization, R.P.; supervision, A.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naseer, B.; Srivastava, G.; Qadri, O.; Faridi, S.; Ul Islam, R.; Younis, K. Importance and health hazards of nanoparticles used in the food industry. Nanotechnol. Rev. 2018, 7, 623–641. [Google Scholar] [CrossRef]
- Tiede, K.; Foss Hansen, S.; Westerhoff, P.; Fren, G.; Hankin, S.; Aitken, R.; Chaudhry, Q.; Boxall, A. How important is drinking water exposure for the risks of engineered nanoparticles to consumers? Nanotoxicol. 2016, 10, 102–110. [Google Scholar] [CrossRef]
- Almaiman, L.; Aljomah, A.; Bineid, M.; Aljeldah, F.M.; Aldawsari, F.; Liebmann, B.; Lomako, I.; Sexlinger, K.; Alarfaj, R. The occurance nd dietary intake related to the presence of microplastics in drinking water in Saudi Arabia. Environ. Monit. Assess. 2021, 193, 390. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Enviorn. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Salvador Cesa, F.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Sci. Total Environ., 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Tena, A.F.; Clarà, P.C. Deposition of inhaled particles in the lungs. Archivos de Bronconeumología. 2012, 48, 240–246. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I.; Williams III, R.O. Influence of particle size on regional lung deposition–what evidence is there? Int.l J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Dick Vethaak, A.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Eniorn. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Techn. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Christersson, C.E.; Lindh, L.; Arnebrant, T. Film-forming properties and viscosities of saliva substitutes and human whole saliva. Eur. J. Oral. Sci., 2000, 108, 418–425. [Google Scholar] [CrossRef]
- D'Angelo, I.; Casciaro, B.; Miro, A.; Quaglia, F.; Mangoni, M.L.; Ungaro, F. Overcoming barriers in Pseudomonas aeruginosa lung infections: engineered nanoparticles for local delivery of a cationic antimicrobial peptide. 2015, Colloids Surf. B: Biointerfaces. 2015, 135, 717–725. [Google Scholar] [CrossRef]
- Dawson, M.; Krauland, E.; Wirtz, D.; Henes, J. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnol. Prog. 2004, 20, 851–857. [Google Scholar] [CrossRef]
- McGill, S.L.; Smith, H.D.C. Disruption of the mucus barrier by topically applied exogenous particles. Mol. Pharm. 2010, 7, 2280–2288. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water, Water Res. 141.
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.J.; Gershkovich, P. Characterisation of human saliva as a platform for oral dissolution medium development. Euro. J. Pharm. and Biopharm. 2015, 91, 16–24. [Google Scholar] [CrossRef]
- Ren, S.; Cai, M.; Shi, Y.; Luo, Z.; Wang, T. Influence of cough airflow characteristics on respiratory mucus clearance. Phys. Fluids. 2022, 34, 041911. [Google Scholar] [CrossRef]
- Xu, C. Nonlinear Least Squares: Trust Region Methods. In Encyclopedia of Optimization, 2nd ed.; Floudas, C.A., Pardalos, P.M., Eds.; Springer: New York, USA, 2008; pp. 2630–2637. [Google Scholar]
- Liu, Y.; Zhang, Q.; Liu, R. Effect of particle size distribution and shear rate on relative viscosity of concentrated suspensions. Rheologica Acta. 2021, 60, 763–774. [Google Scholar] [CrossRef]
- Biesbrock, A.R.; Dirksen, T.; Schuster, G. Effects of tung oil on salivary viscosity and extent and incidence of dental caries in rats. Caries. Res. 1992, 26, 117–123. [Google Scholar] [CrossRef]
- Yas, B.A.; Radhi, N.J. Salivary viscosity in relation to oral health status among a group of 20-22 years old dental students. Iraqi J. Comm. Med. 2013, 3, 219–224. [Google Scholar]
- Kitada, K.; Oho, T. Effect of saliva viscosity on the co-aggregation between oral streptococci and Actinomyces naeslundii. Gerodontology. 2011, 29, e981–e987. [Google Scholar] [CrossRef]
- Michalczuk, U.; Przekop, R. , Moskal, A. The effect of selected nanoparticles on rheological properties of human blood. Bull. Polish Acad. Sci. Techn. Sci. 2022, 70, e140437. [Google Scholar]
- Xu, M.; Liu, H.; Zhao, H.; Li, W. How to decrease the viscosity of suspension with the second fluid and nanoparticles? Sci. Rep. 2013, 3, 3137. [Google Scholar] [CrossRef] [PubMed]
- Gvaramia, M.; Mangiapia, G.; Pipich, V.; Appavou, M.-S.; Jaksch, S.; Holderer, O.; Rukhadze, M.D.; Frielinghaus, H. Tunable viscosity modification with diluted particles: when particles decrease the viscosity of complex fluids. Colloid Polymer Sci. 2019, 297, 1507–1517. [Google Scholar] [CrossRef]
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Sammar, M.; Angel, D.L.; Dror, H.; Lahovitski, H.; Booth, A.M.; Sabbah, I. Mechanism of nanoplastics capture by jellyfish mucin and its potential as a sustainable water treatment technology. Sci. Total Enviorn. 2023, 869, 161824. [Google Scholar] [CrossRef] [PubMed]
- Poling, B.E.; Prausnitz, J.M.; O’Connel, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, USA, 2001; p. 11. [Google Scholar]
- Penconek, A.; Michalczuk, U.; Moskal, A. The Effect of Airborne Particles on Human Body Fluids. In Practical Aspects of Chemical Engineering, 1st ed.; Ochowiak, M., Woziwodzki, S., Mitkowski, P.T., Doligalski, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 305–313. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).