1. Introduction
Reductive amination is an important and versatile organic reaction that finds extensive applications in sustainable synthetic processes and has been intensively investigated both in both academia and industry [
1,
2]. Mechanistically, the reaction begins with a condensation step in which the carbonyl compound reacts with ammonia or an amine to form the corresponding imine, followed by reduction of the imine to the alkylamine product [
3]. Many of these reduction steps require the presence of a catalyst to activate the reducing agent [
4] and transition-metals (such as Ru, Rh, Pd, Ag, Ir, Pt, and Au) have been used extensively [
5] also in the preparation of active pharmaceutical ingredients (APIs) [
6,
7]. Despite its cost, Rh also shows remarkable activity for such reaction as shown in recent literature [
8,
9,
10,
11] and has the potential to be employed in robust hydrogenation catalysts with low metal loading. A Rh/Al
2O
3-catalyzed reductive amination of furfural and other aldehydes, especially those associated with biomass, was recently reported by Chatterjee, Kawanami, and coworkers using an aqueous solution of NH
3 [
12]
. A very high selectivity to furfurylamine (∼92%) was documented by the authors in only 120 min at 80 °C with the chance to reuse the catalyst several times.
Moreover, reductive amination has attracted considerable attention in recent years in sustainable organic synthesis due to its environmental advantages and broad synthetic utility [
13,
14]. Apart from its versatility, as primary, secondary, and tertiary alkylamines are readily available, reductive amination usually exhibits good atom economy, which means that most of the atoms present in the starting materials end up in the desired product. This minimizes waste generation and maximizes resource utilization. In addition, reductive amination can often be carried out under mild conditions with remarkable energy saving [
15]. In this framework, microwave heating (MW) is a mature technology that could be exploited to boost the sustainability of reductive amination protocols. Indeed, MW promotes the remarkable reduction of reaction time, yields enhancement, and cleaner reactions compared to conventional thermal heating. MW irradiation is, therefore, a suitable tool to overcome the limitations encountered in challenging organic reactions, such as direct reductive amination in the presence of heterogeneous catalysts and gaseous reagents. In particular, the use of metallic nanoparticles as catalysts also allows a positive synergy with microwaves (MW) heating [
16,
17], an alternative technology that allows faster heating rate and less side-reaction on the surface of the reactor, [
18] which with convective heating are usually hotter than the bulk, at least in the laboratory scale. In this work we present a Rh catalysed optimized protocol for the fast and sustainable synthesis of ractopamine (a β-adrenergic compound) under MW-assisted protocol.
Ractopamine hydrochloride is the common name for the 4-[3-[[2-hydroxy-2-(4-hydroxyphenyl) ethyl] amino] butyl] phenol hydrochloride. It occurs in four stereoisomers (RR, RS, RS, SS) and it is a bioactive molecule defined as a phenyl ethanolamine β-adrenoceptor agonist (β-agonists) [
19]. The β-agonists redirects nutrients away from fat deposition to muscle deposition involving the modulation of metabolic pathways and signals in muscle and lipid cells to enhance protein accretion. Other mechanisms also include regulation of hormone release and modification of blood flow [
20]. Ractopamine :HCl is currently used as a feed additive to improve the feed efficiency and the carcass leanness in swine and cattle, as allowed in the USA, Canada, Japan and Mexico [
21].
The synthetic method of relevant ractopamine hydrochloride has been introduced following several route in existing literature. In particular, two methods are introduced in Lilly Co., Eli.’s patent [
22]. The first method involves the synthesis of ractopamine:HCl using raspberry ketone and right-hydroxymandelic acid as raw materials. The latter involve the use of
p-hydroxyphenylethanol amine instead of hydroxymandelic acid. These synthetic pathways do not lead to complete conversions and high selectivity, leading to the formation of numerous by-products. Additionally, the reaction conditions, separation, and purification processes are all quite challenging. Another synthetic approach was proposed by the Chengdu Organic Chemistry Institute, Chinese Academy of Sciences, in their patent [
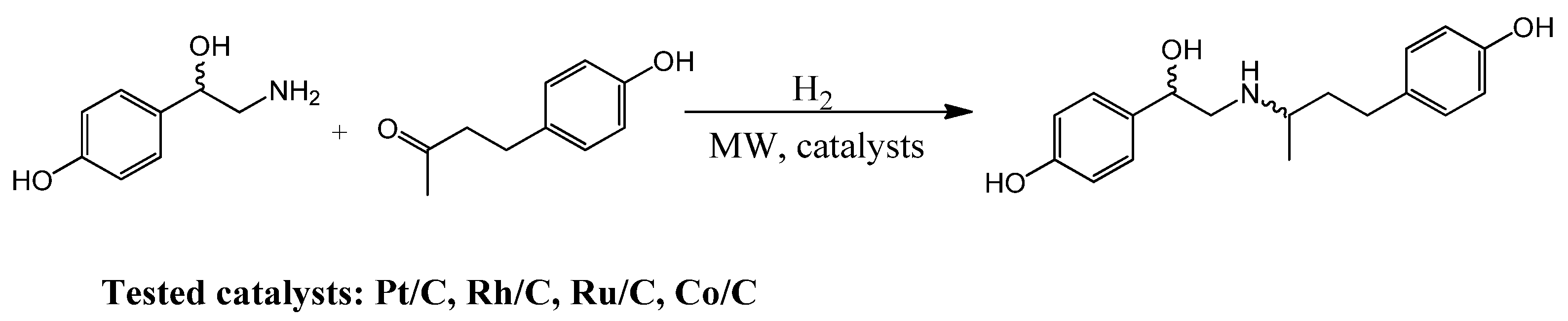
23] and developed by Jie Shaoing. Starting from raspberry ketone, this method included a ketoxime reaction followed by a condensation reaction with ω-bromo-parahydroxyacetophenone and triethylamine under low-temperature conditions. Subsequently, a catalytic hydrogenation step was required to yield the ractopamine:HCl. However, ractopamine is currently synthetized via reductive amination between raspberry ketone and octopamine (
Scheme 1) [
24] in which the hydrogenation step can be easily catalysed by heterogeneous metal-based catalyst [
25,
26] such as Pt [
27,
28,
29] or Pd [
30,
31], which can be easily recovered and reused
In this piece of work, we present a study on the MW-assisted synthesis of ractopamine, in which Pt/C, Ru/C and Rh/C (all 5 wt.%) were tested as heterogenous catalysts. Their activity was studied at different temperatures and hydrogen pressure. Moreover, the reaction was carried out in different solvents and different ketone substrates were considered for the reaction with octopamine under optimized conditions to highlight the positive influence of dielectric heating and heterogeneous catalysis.
2. Materials and Methods
The heterogeneous Pt/C, Ru/C and Rh/C 5 wt.% catalysts were all purchased from Merk. Octopamine HCl, raspberry ketone and ractopamine HCl were supplied from Huvepharma Italia. The other ketone substrates were purchased from Merck KGaA (Darmstadt, Germany). The batch reaction with conventional heating was performed in a 300 mL PolyBlock reactor equipped with a PTFE impeller for stirring. The reactor has a minimum working volume of 41 mL and operates up to 100 bar and 250 °C. A sampling valve on the bottom allows the removal of the crude reaction mixture for analysis. The reactor was flushed three times with 2 bar of N2 before the appropriate pressure of H2 was introduced. The MW-assisted reductive amination reactions were performed in a MW autoclave (SynthWAVE, Milestone srl - Bergamo) in 15 ml glass vials provided with magnetic stirring (500 rpm) all inside a 1 L PTFE reaction chamber filled with 200 mL of brine solution used to guarantee a regular MW absorption. The reaction chamber was flushed three times with 2 bar of N2 before the appropriate H2 pressure was introduced. A heating ramp of 2 min was used, with a maximum MW power of 800 W.
After the reaction the crude was recovered and filtered over paper to recover the catalyst. The filtrate was dried under vacuum, redissolved in pyridine and derivatised with N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) prior to GC-MS analysis.
The GC-MS equipment was an Agilent Technologies 6850 Network GC System fitted with a 5973 Network Mass Selective Detector, a 7683B Automatic Sampler and a capillary column Mega 5MS (length 30 m; i.d. 0.25 mm; film thickness 0.25 μm, Mega Srl, Legnano, Italy). The HPLC-DAD equipment used for the diastereomeric ratio determination was an Agilent 1100 using an Hypersil ODC C18 column (5 μm particles diameter and 4.6 mm x 250mm). The mobile phases were an ammonium hydroxide orthophosphate buffer (A) and acetonitrile (B). The peaks were recorded at 226 nm.
3. Results
Optimization of ractopamine synthesis was primarily investigated using a commercial Pt/C (5 wt.%) catalyst as a reference material for the hydrogenation step. The reductive amination between octopamine and raspberry ketone was first carried out in batch at lab scale under conventional heating (
Scheme 1). A KOH solution in MeOH was prepared and reagents were slowly added. The pH was adjusted to 6.5 with glacial acetic acid. The hydrogenation step was carried out at 50 °C with 5 bar H
2. Under these conditions, 13 hours were required to achieve complete coupling and hydrogenation of the reagents with a final yield of 98.9% in ractopamine:HCl. Using these batch conditions as benchmark, we moved on to the MW experiments (
Table 1). First, the effect of temperature and H
2 pressure on ractopamine yield was investigated. It was found that increasing the temperature from 50 to 80 °C increased the yield, but at the expense of selectivity. In contrast, increasing the H
2 pressure from 5 to 10 bar resulted in an increase in both conversion and selectivity at 50 °C.
Table 1.
Influence of temperature and hydrogen pressure.
Table 1.
Influence of temperature and hydrogen pressure.
| Entry |
T
(°C) |
H2
(bar) |
Conversion
(%) |
Selectivity
(%) |
Yield
(%) |
| 1 |
50 |
5 |
10.0 |
63.2 |
6.3 |
| 2 |
80 |
5 |
87.2 |
47.0 |
41.0 |
| 3 |
50 |
10 |
91.6 |
94.5 |
86.6 |
The main by-product observed is imine, which is formed from the coupling between raspberry ketone and octopamine but is not hydrogenated. HPLC analysis (
Appendix A,
Figure 1A,
Table 1A) to determine the diastereomeric ratio (
RS-SR /SS-RR) gave a value of 47/53, which falls within the reference ratio of the commercial product (RS-SR 45-49%). After setting 50 °C and 10 bar H
2 as the optimal reaction conditions, we compared the activity of Pt/C 5 wt% with other heterogeneous catalyst based on Rh and Ru with the same support and metal loading (
Table 2). Results show that Pt and Rh have similar activities, while Ru, despite comparable conversions, does not promote the hydrogenation step and thus leads only to an imine intermediate. Rh/C was also tested with 5 bar H
2. In this case, the activity was higher than Pt/C, but both the conversion and selectivity decreased compared to the reaction carried out at 10 bar. The crude reaction product using Rh/C was also within the acceptable diastereomeric ratio and with the same value of 47/53 as the Pt/C test (
Appendix A,
Figure 2A,
Table 2A).
Table 2.
heterogeneous catalyst screening.
Table 2.
heterogeneous catalyst screening.
| Entry |
Catalyst |
H2
(bar) |
Conversion
(%) |
Selectivity
(%) |
Yield
(%) |
| 1 |
Pt/C |
10 |
91.6 |
94.5 |
86.6 |
| 2 |
Rh/C |
10 |
81.0 |
91.9 |
74.4 |
| 3 |
Ru/C |
10 |
73.0 |
n.d. |
- |
| 4 |
Rh/C |
5 |
47.0 |
80.4 |
37.8 |
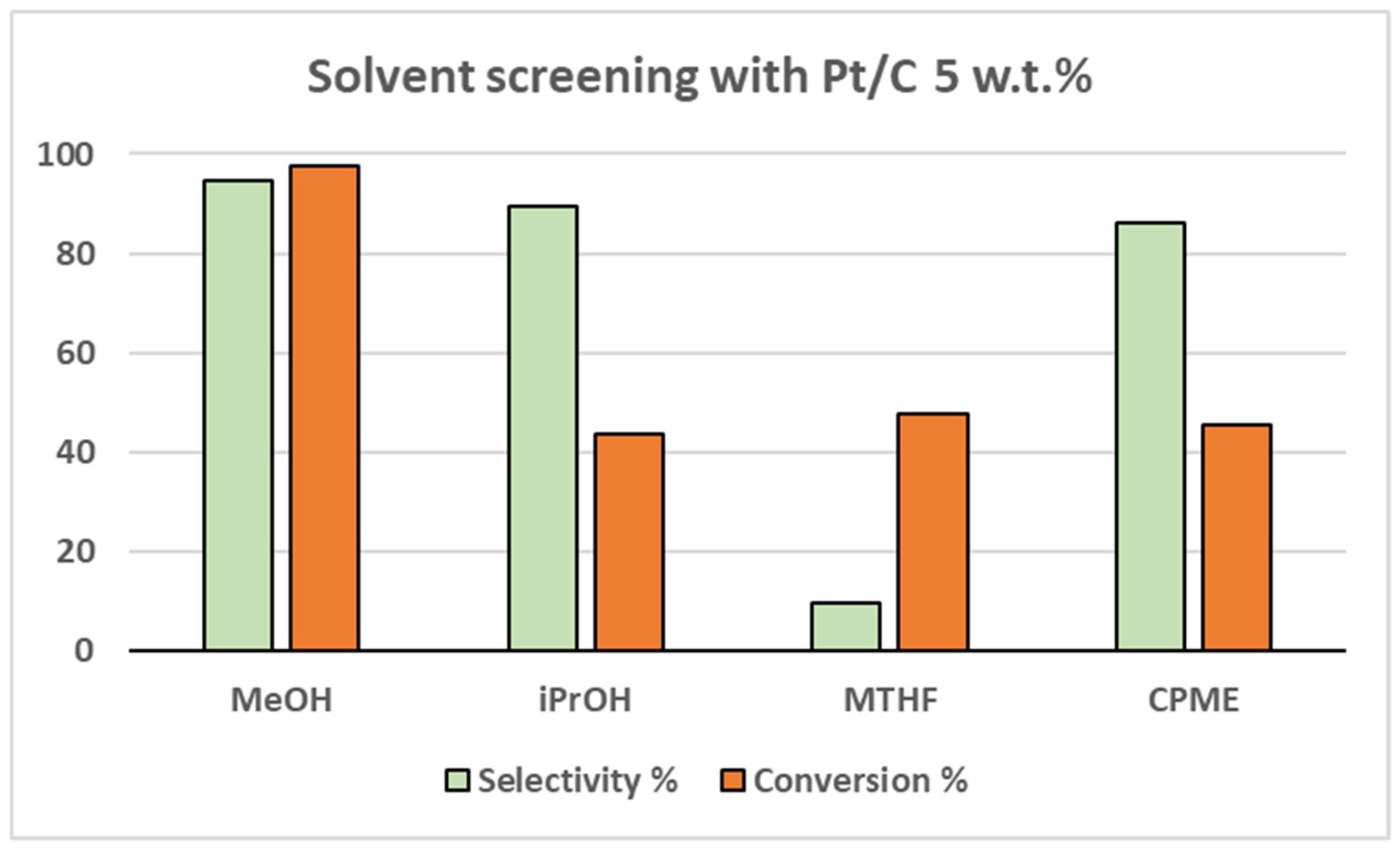
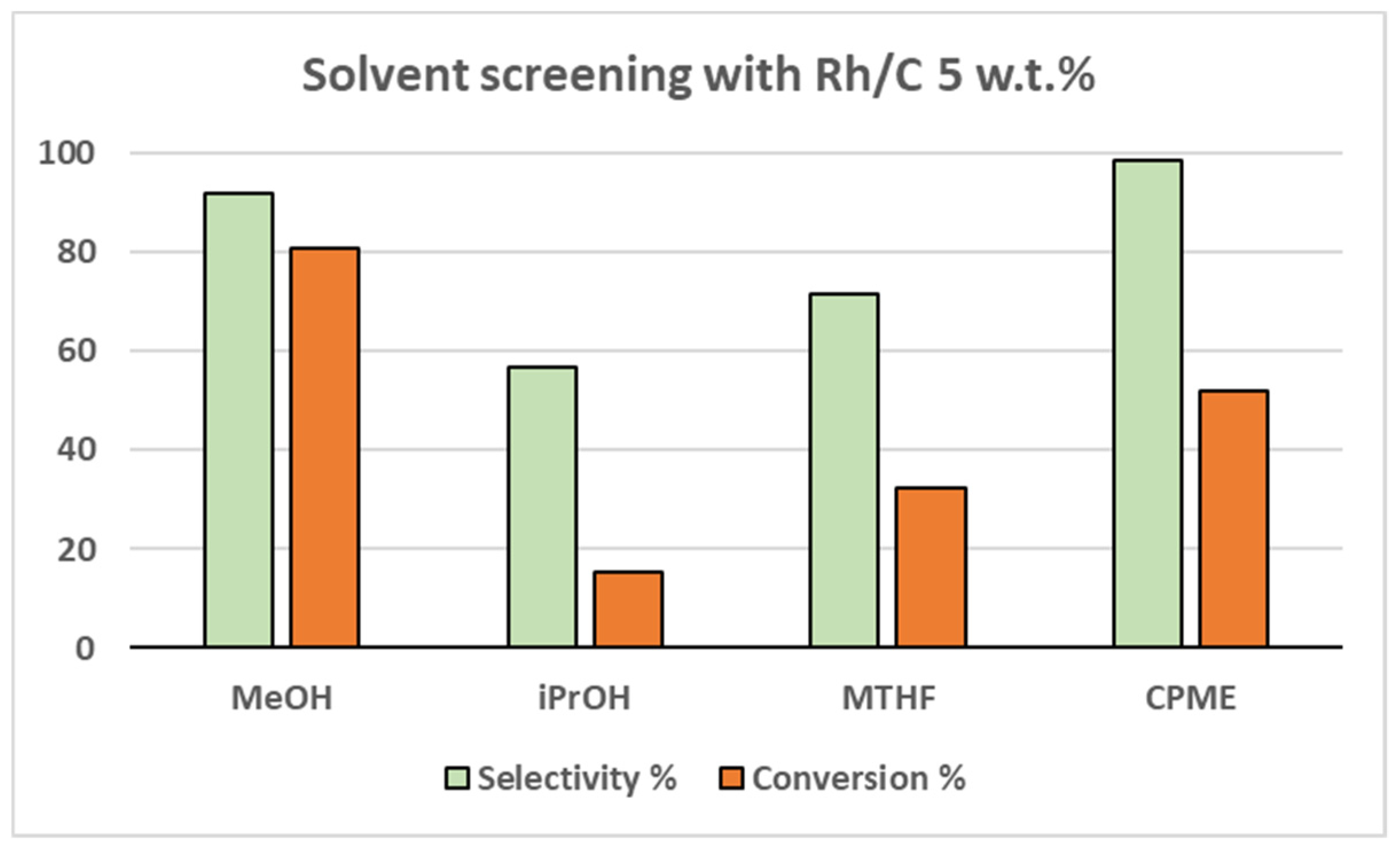
Different solvents were also tested for the reaction in order to scout for greener and safer alternatives to methanol (
Figure 1 and
Figure 2). Isopropanol (
iPrOH), 2-methyltetrahydrofuran (MTHF) and cyclopenthylmethylether (CPME) were thus selected. However, the methanolic solution proved to be the optimal solvent for both catalysts. The second-best result was achieved with CPME both over Pt/C (39.1% yield) and Rh/C (51.3% yield).
Figure 1.
Solvent screening over Pt/C 5 wt.%.
Figure 1.
Solvent screening over Pt/C 5 wt.%.
Figure 2.
solvent screening over Rh/C 5 wt.%.
Figure 2.
solvent screening over Rh/C 5 wt.%.
Finally, to broaden the scope of our study, other ketones were used as substrates for the reaction (
Table 3). Acetophenone, cyclohexanone and 2-butanone were selected to provide an array of cyclic, linear, aromatic and aliphatic substrates. While acetophenone gave low selectivity in the tested conditions, both cyclohexanone and 2-butanone gave excellent results over both Pt/C and Rh/C. Also, using 10 bar of H
2 was a disadvantage since selectivity was lower than with 5 bar only.
Table 3.
conversion of ketone substrates over Pd/C and Rh/C 5 wt.%.
Table 3.
conversion of ketone substrates over Pd/C and Rh/C 5 wt.%.
| Entry |
Catalyst |
Substrate |
H2
(bar) |
Conversion
(%) |
Selectivity
(%) |
Yield
(%) |
| 1 |
Pt/C |
Acetophenone |
5 |
n.d. |
n.d. |
- |
|
| 2 |
10 |
89.6 |
29.2 |
26.2 |
|
| 3 |
Cyclohexanone |
5 |
>99 |
>99 |
>99 |
|
| 4 |
10 |
>99 |
>99 |
>99 |
|
| 5 |
2-Butanone |
5 |
>99 |
80.2 |
80.2 |
|
| 6 |
10 |
>99 |
96.3 |
96.3 |
|
| 7 |
Rh/C |
Acetophenone |
5 |
>99 |
8.2 |
8.2 |
|
| 8 |
10 |
>99 |
8.0 |
8.0 |
|
| 9 |
Cyclohexanone |
5 |
>99 |
82.7 |
82.7 |
|
| 10 |
10 |
>99 |
22.1 |
22.1 |
|
| 11 |
2-Butanone |
5 |
>99 |
92.4 |
92.4 |
|
| 12 |
10 |
>99 |
50.2 |
50.6 |
|
5. Conclusions
The effect of temperature and hydrogen pressure was studied for the synthesis of ractopamine by reductive amination. Increasing the temperature from 50 to 80 °C had a positive effect on the conversion, but at the expense of selectivity, while increasing the H2 pressure from 5 to 10 bar improved the yield from 6.3 to 86.6%. Different heterogeneous catalysts were tested and both Pt/C and Rh/C (5 wt.%) were found to be suitable for the reaction, which was completed in 3 hours instead of 13 hours as in the batch tests using conventional heating. Finally, various ketone substrates were coupled with octopamine, demonstrating the flexibility of the setup that allows yields up to 99% even at 5 bar of H2.
Author Contributions
“Conceptualization, G.C. and E.C.G..; formal analysis, F.B. and E.C.; investigation, F.B., M.C. and G.G.; data curation, G.G. and E.C.; writing—original draft preparation, F.B. and M.C.; writing—review and editing, E.C.G. and G.C.; supervision, E.C.G. and G.C. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The R&D department of Huvepharma Italy is warmly acknowledged for its technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
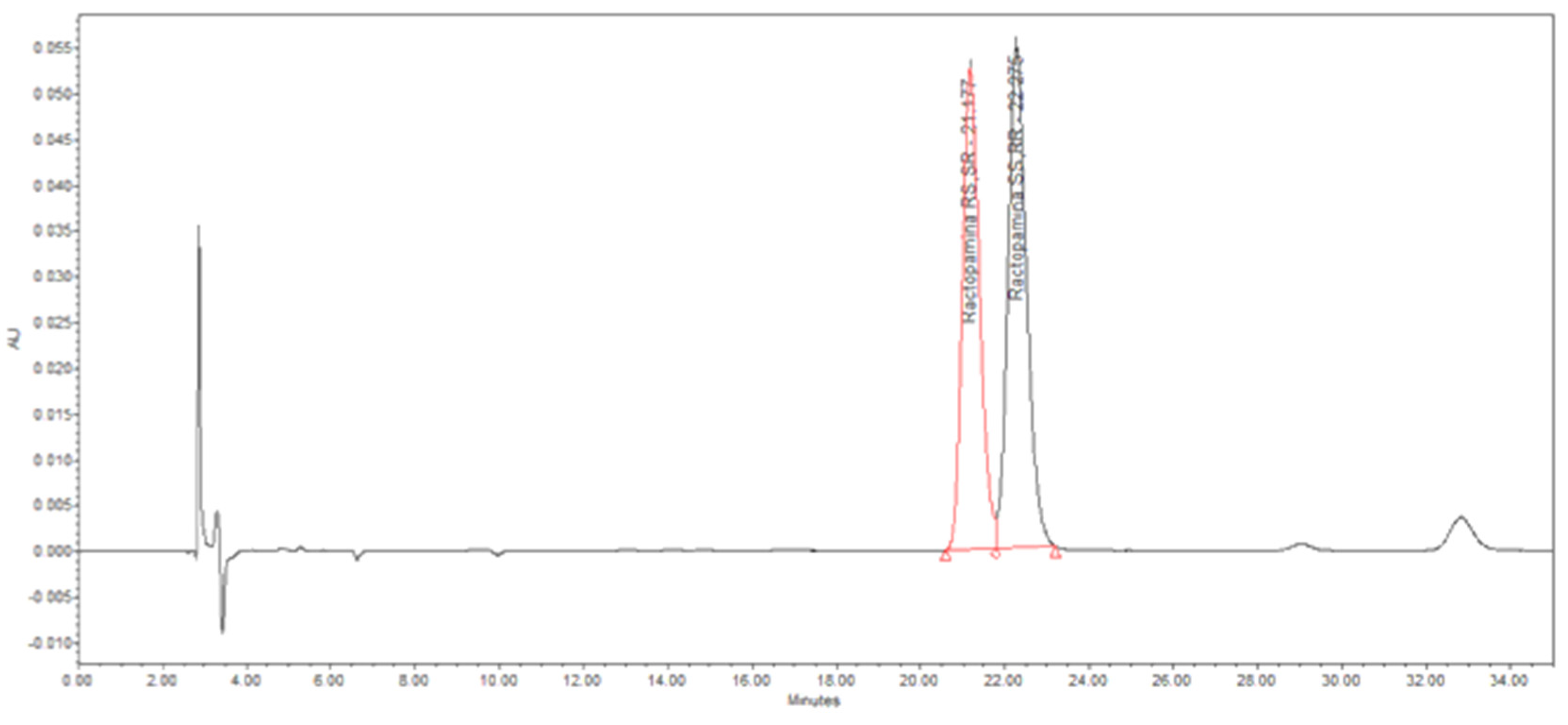
The best reaction conditions for the MW-assisted synthesis of ractopamine (50 °C under MW 180 min and H
2 (10 bar), using Pt/C (5% w/w) (
Table 2, entry 1) were studied in detail and products characterized by HPLC-DAD. Analyses were performed with a validated HPLC method described in paragraph 2. The results (
Figure 1A and
Table 1A) indicate that the newly synthesized ractopamine:HCl is on specification [
32].
Figure 1A.
HLPC Chromatogram - diastereomeric ratio determination at the best reaction conditions: 50 °C with MW for 180 min and H2 (10 bar), using Pt/C 5% as catalyst.
Figure 1A.
HLPC Chromatogram - diastereomeric ratio determination at the best reaction conditions: 50 °C with MW for 180 min and H2 (10 bar), using Pt/C 5% as catalyst.
Table 1A.
Diastereomeric ratio of the sample obtained at the best reaction conditions: 50 °C with MW for 180 min and H2 pressure at 10 bar, using Pt/C 5% w/w as catalyst.
Table 1A.
Diastereomeric ratio of the sample obtained at the best reaction conditions: 50 °C with MW for 180 min and H2 pressure at 10 bar, using Pt/C 5% w/w as catalyst.
| Name |
Retention Time |
Area |
% Area |
Specification |
| Ractopamine RS,SR |
21.177 |
1497290 |
47.00 |
RS,SR 45–49% |
| Ractopamine SS,RR |
22.275 |
1688481 |
53.00 |
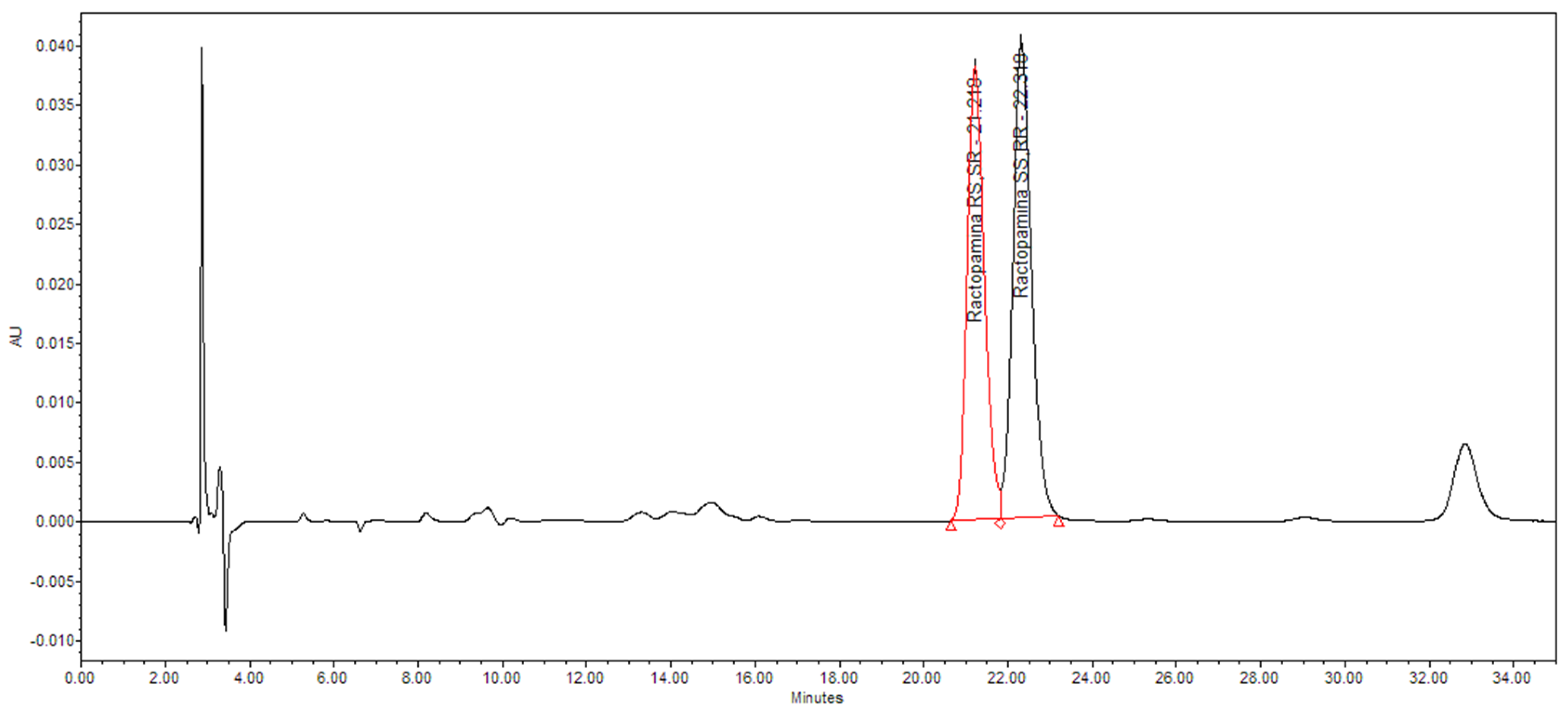
The
RS,SR diastereomeric ratio was also evaluated for the ractopamine:HCl sample synthetized under MW 180 min at 50 °C and H
2 (10 bar) with Rh/C as catalyst (
Table 2, entry 2). Analyses were performed following a validated HPLC method described in paragraph 2. and the results showed that the sample is on specification (
Figure 2A,
Table 2A). [
31]
Figure 2A.
HPLC Chromatogram - diastereomeric ratio determination at the best reaction conditions: 50 °C with MW for 180 min and H2 (10 bar), using Rh/C 5% w/w as catalyst.
Figure 2A.
HPLC Chromatogram - diastereomeric ratio determination at the best reaction conditions: 50 °C with MW for 180 min and H2 (10 bar), using Rh/C 5% w/w as catalyst.
Table 2A.
Diastereomeric ratio of the sample obtained at the best reaction conditions: 50 °C with MW for 180 min and H2 (10 bar), using Rh/C 5% w/w as catalyst.
Table 2A.
Diastereomeric ratio of the sample obtained at the best reaction conditions: 50 °C with MW for 180 min and H2 (10 bar), using Rh/C 5% w/w as catalyst.
| Name |
Retention Time |
Area |
% Area |
Specification |
| Ractopamine RS,SR |
21.218 |
1085144 |
47.06 |
RS,SR 45–49% |
| Ractopamine SS,RR |
22.318 |
1220961 |
52.94 |
References
- Sukhorukov, A. Y. Catalytic Reductive Amination of Aldehydes and Ketones With Nitro Compounds: New Light on an Old Reaction. Front. Chem. 2020, 8, 215. [CrossRef]
- Afanasyev, O. I.; Kuchuk, E.; Usanov, D. L.; Chusov, D. Reductive Amination in the Synthesis of Pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [CrossRef]
- Li, J.; Wang, B.; Qin, Y.; Tao, Q.; Chen, L. MOF-Derived Ni@NC Catalyst: Synthesis, Characterization, and Application in One-Pot Hydrogenation and Reductive Amination. Catal. Sci. Technol. 2019, 9, 3726–3734. [CrossRef]
- Tian, Y.; Hu, L.; Wang, Y. Z.; Zhang, X.; Yin, Q. Recent Advances on Transition-Metal-Catalysed Asymmetric Reductive Amination. Org. Chem. Front. 2021, 8, 2328–2342. [CrossRef]
- Irrgang, T.; Kempe, R. Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen. Chem. Rev. 2020, 120, 9583–9674. [CrossRef]
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V. G.; Natte, K.; Kamer, P. C. J.; Beller, M.; Jagadeesh, R. V. Catalytic Reductive Aminations Using Molecular Hydrogen for Synthesis of Different Kinds of Amines. Chem. Soc. Rev. 2020, 49, 6273–6328. [CrossRef]
- Alinezhad, H.; Yavari, H.; Salehian, F. Recent Advances in Reductive Amination Catalysis and Its Applications. Curr. Org. Chem. 2015, 19, 1021–1049. [CrossRef]
- Bucciol, F.; Gaudino, E. C.; Villa, A.; Valsania, M. C.; Cravotto, G.; Manzoli, M. Microwave-Assisted Reductive Amination of Aldehydes and Ketones Over Rhodium-Based Heterogeneous Catalysts. Chempluschem 2023, 88, e202300017. [CrossRef]
- Pérez Alonso, A.; Pham Minh, D.; Pla, D.; Gómez, M. A Cooperative Rh/Co-Catalyzed Hydroaminomethylation Reaction for the Synthesis of Terpene Amines. ChemCatChem 2023, 15, e202300501. [CrossRef]
- Ortega, M.; Manrique, R.; Jiménez, R.; Parreño, M.; Domine, M. E.; Arteaga-Pérez, L. E. Secondary Amines from Catalytic Amination of Bio-Derived Phenolics over Pd/C and Rh/C: Effect of Operation Parameters. Catalysts 2023, 13, 654. [CrossRef]
- Migliorini, F.; Monciatti, E.; Romagnoli, G.; Parisi, M. L.; Taubert, J.; Vogt, M.; Langer, R.; Petricci, E. Switching Mechanistic Pathways by Micellar Catalysis: A Highly Selective Rhodium Catalyst for the Hydroaminomethylation of Olefins with Anilines in Water. ACS Catal. 2023, 13, 2702–2714. [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Reductive Amination of Furfural to Furfurylamine Using Aqueous Ammonia Solution and Molecular Hydrogen: An Environmentally Friendly Approach. Green Chem. 2016, 18, 487–496. [CrossRef]
- Fiore, A. M.; Romanazzi, G.; Dell’Anna, M. M.; Latronico, M.; Leonelli, C.; Mali, M.; Rizzuti, A.; Mastrorilli, P. Mild and Efficient Synthesis of Secondary Aromatic Amines by One-Pot Stepwise Reductive Amination of Arylaldehydes with Nitroarenes Promoted by Reusable Nickel Nanoparticles. Mol. Catal. 2019, 476, 110507. [CrossRef]
- Pedrajas, E.; Sorribes, I.; Junge, K.; Beller, M.; Llusar, R. Selective Reductive Amination of Aldehydes from Nitro Compounds Catalyzed by Molybdenum Sulfide Clusters. Green Chem. 2017, 19, 3764–3768. [CrossRef]
- Cravotto, G.; Carnaroglio, D. Microwave Chemistry, 1st ed.; De Gruyter, 2017.
- Schanche, J. S. Microwave Synthesis Solutions from Personal Chemistry. Mol. Divers. 2003, 7, 293–300. [CrossRef]
- Bogdal, D.; Lukasiewicz, M.; Pielichowski, J.; Miciak, A.; Bednarz, S. Microwave-Assisted Oxidation of Alcohols Using MagtrieveTM. Tetrahedron 2003, 59, 649–653. [CrossRef]
- Ricciardi, L.; Verboom, W.; Lange, J. P.; Huskens, J. Reactive Extraction Enhanced by Synergic Microwave Heating: Furfural Yield Boost in Biphasic Systems. ChemSusChem 2020, 13, 3589–3593. [CrossRef]
- Bories, G.; Brantom, P.; Brufau De Barberà, J.; Chesson, A.; Cocconcelli, P. S.; Debski, B.; Dierick, N.; Gropp, J.; Halle, I.; Hogstrand, C.; De Knecht, J.; Leng, L.; Haldorsen, A.-K. L.; Lindgren, S.; Mantovani, A.; Mézes, M.; Nebbia, C.; Rambeck, W.; Rychen, G.; Von Wright, A.; Wester, P. Safety Evaluation of Ractopamine. EFSA J. 2009, 7, 1041. [CrossRef]
- Menegat, M. B.; Robert D. Goodband; Joel M. DeRouchey; Mike D. Tokach; Jason C. Woodworth; Steve S. Dritz. Feed Additives in Swine Diets. 2019, 1–14.
- Rikard-Bell, C.; Curtis, M. A.; van Barneveld, R. J.; Mullan, B. P.; Edwards, A. C.; Gannon, N. J.; Henman, D. J.; Hughes, P. E.; Dunshea, F. R. Ractopamine Hydrochloride Improves Growth Performance and Carcass Composition in Immunocastrated Boars, Intact Boars, and Gilts. J. Anim. Sci. 2009, 87, 3536–3543. [CrossRef]
- Anderson, D. B.; Schmiegel, K. K.; Veenhuizen, E. L. Novel Process for Preparing Ractopamine from P-Hydroxyacetophenone and Raspberry Ketone. GB2133986A, February 11, 2004.
- Dai Mo, C.; Aiquiao, M.; Yaozhong, J.; Yuliang, W. Animal Feed Compositions Containing Phenethanolamines. CN1116620A, January 30, 1984.
- IN2012MU02355 A "Improved Process For Manufacture Of Ractopamine Hydrochloride" Nadikuduru, S. K.; Patil B. S.; Ghan, J. B. 2012.
- Liu, J.; Song, Y.; Ma, L. Earth-Abundant Metal-Catalyzed Reductive Amination: Recent Advances and Prospect for Future Catalysis. Chemistry - An Asian Journal. John Wiley and Sons Ltd September 1, 2021, pp 2371–2391. [CrossRef]
- Irrgang, T.; Kempe, R. Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen. Chem. Rev. 2020, 120, 9583–9674. [CrossRef]
- Touchy, A. S.; Hakim Siddiki, S. M. A.; Kon, K.; Shimizu, K. I. Heterogeneous Pt Catalysts for Reductive Amination of Levulinic Acid to Pyrrolidones. ACS Catal. 2014, 4, 3045–3050. [CrossRef]
- Li, X.; Le, S. D.; Nishimura, S. Reductive Amination of 5-Hydroxymethyl-2-Furaldehyde Over Beta Zeolite-Supported Ruthenium Catalyst. Catal. Letters 2021. [CrossRef]
- Yamada, T.; Park, K.; Furugen, C.; Jiang, J.; Shimizu, E.; Ito, N.; Sajiki, H. Highly Selective Hydrogenative Conversion of Nitriles into Tertiary, Secondary, and Primary Amines under Flow Reaction Conditions. ChemSusChem 2022, 15, e202102138. [CrossRef]
- Mahato, S.; Rawal, P.; Devadkar, A. K.; Joshi, M.; Roy Choudhury, A.; Biswas, B.; Gupta, P.; Panda, T. K. Hydroboration and Reductive Amination of Ketones and Aldehydes with HBpin by a Bench Stable Pd( Ii )-Catalyst. Org. Biomol. Chem. 2022, 20, 1103–1111. [CrossRef]
- Arteaga-Pérez, L. E.; Manrique, R.; Castillo-Puchi, F.; Ortega, M.; Bertiola, C.; Pérez, A.; Jiménez, R. One-Pot Amination of Cyclohexanone-to-Secondary Amines over Carbon-Supported Pd: Unraveling the Reaction Mechanism and Kinetics. Chem. Eng. J. 2021, 417. [CrossRef]
- “Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the safety evaluation of Ractopamine.,” The EFSA Journal, vol. 1041, pp. 1–52, Apr. 2009.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).