Submitted:
07 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
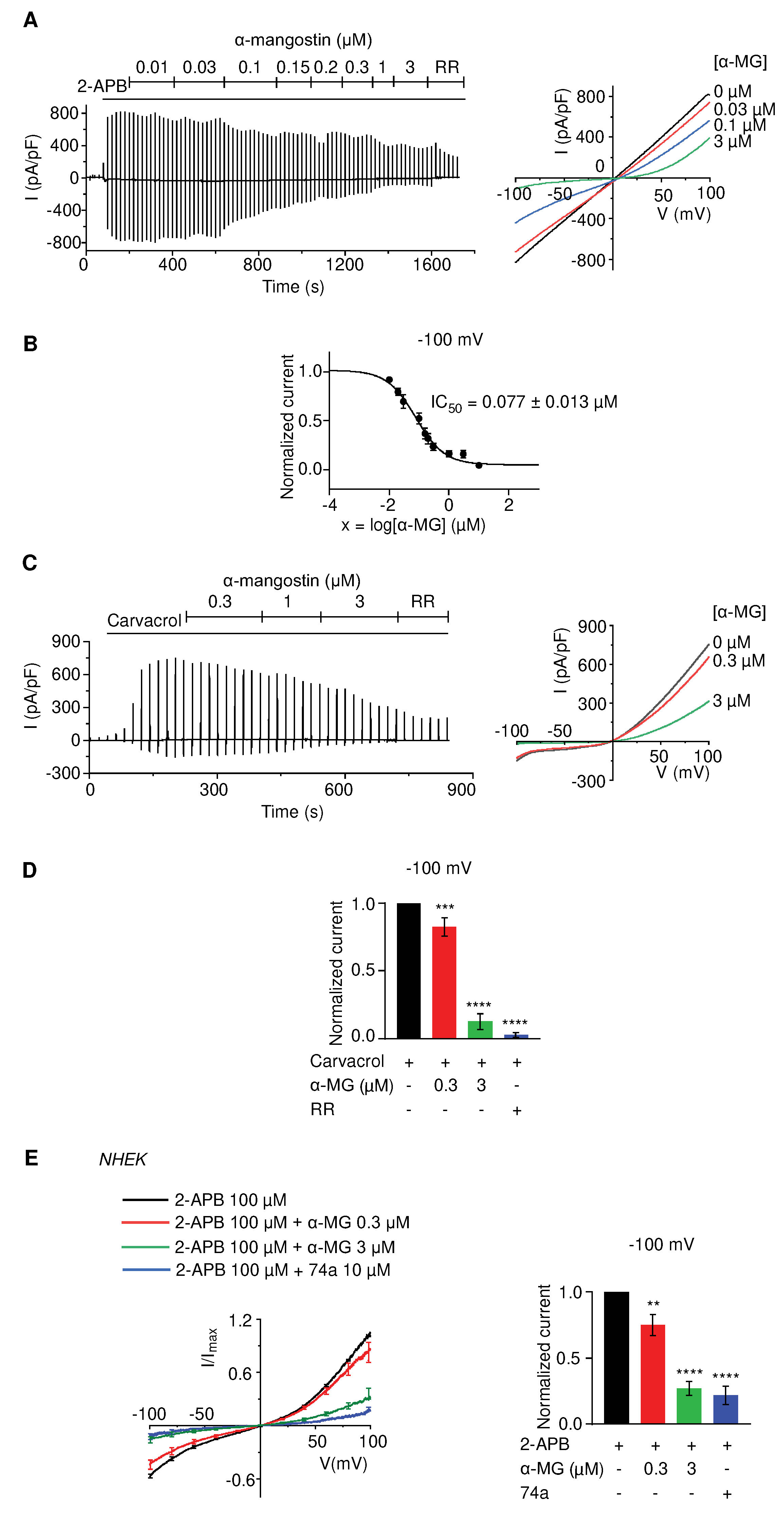
2.1. Alpha-mangostin significantly inhibits TRPV3 current
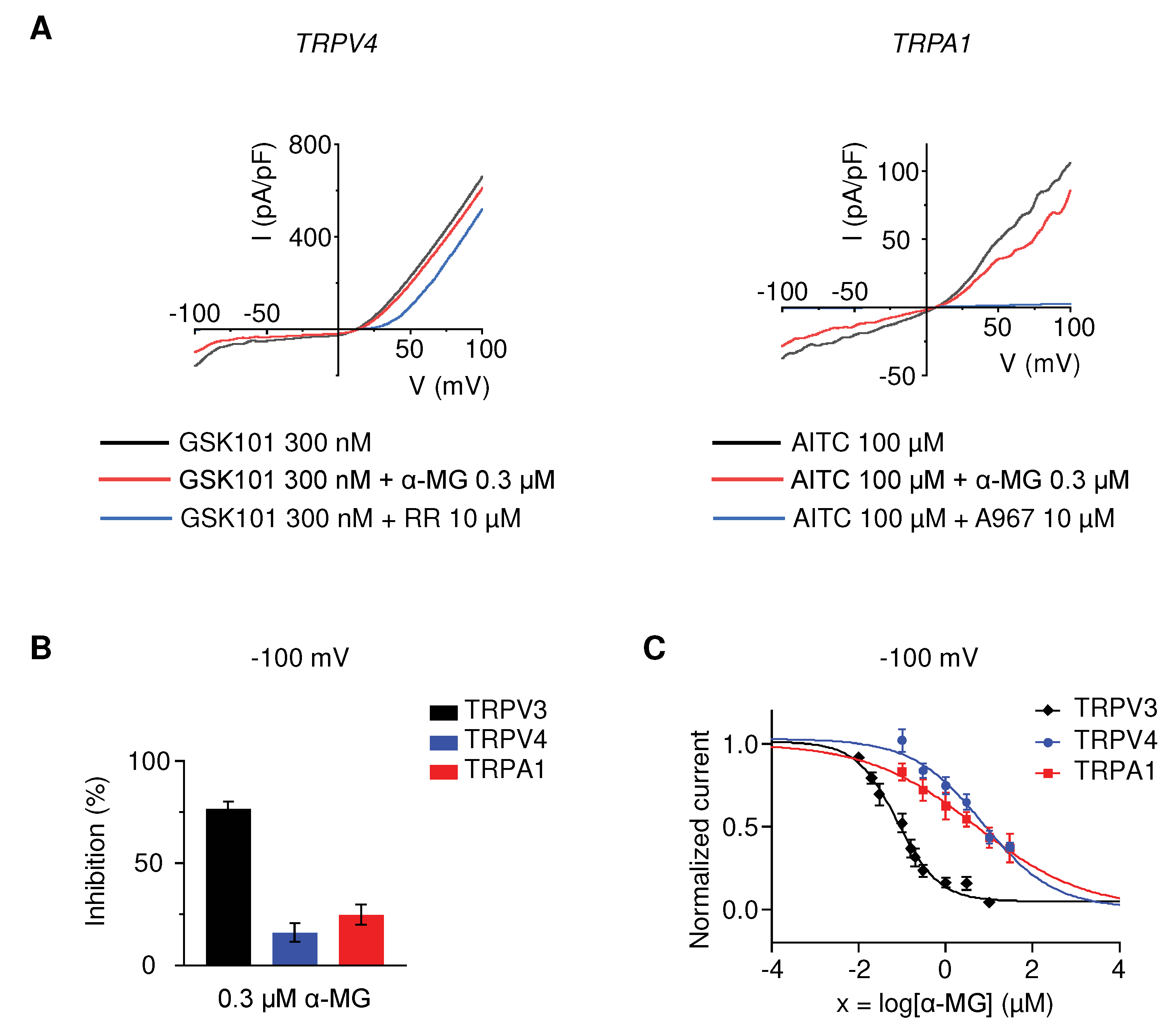
2.2. Alpha-mangostin is a potent TRPV3 inhibitor
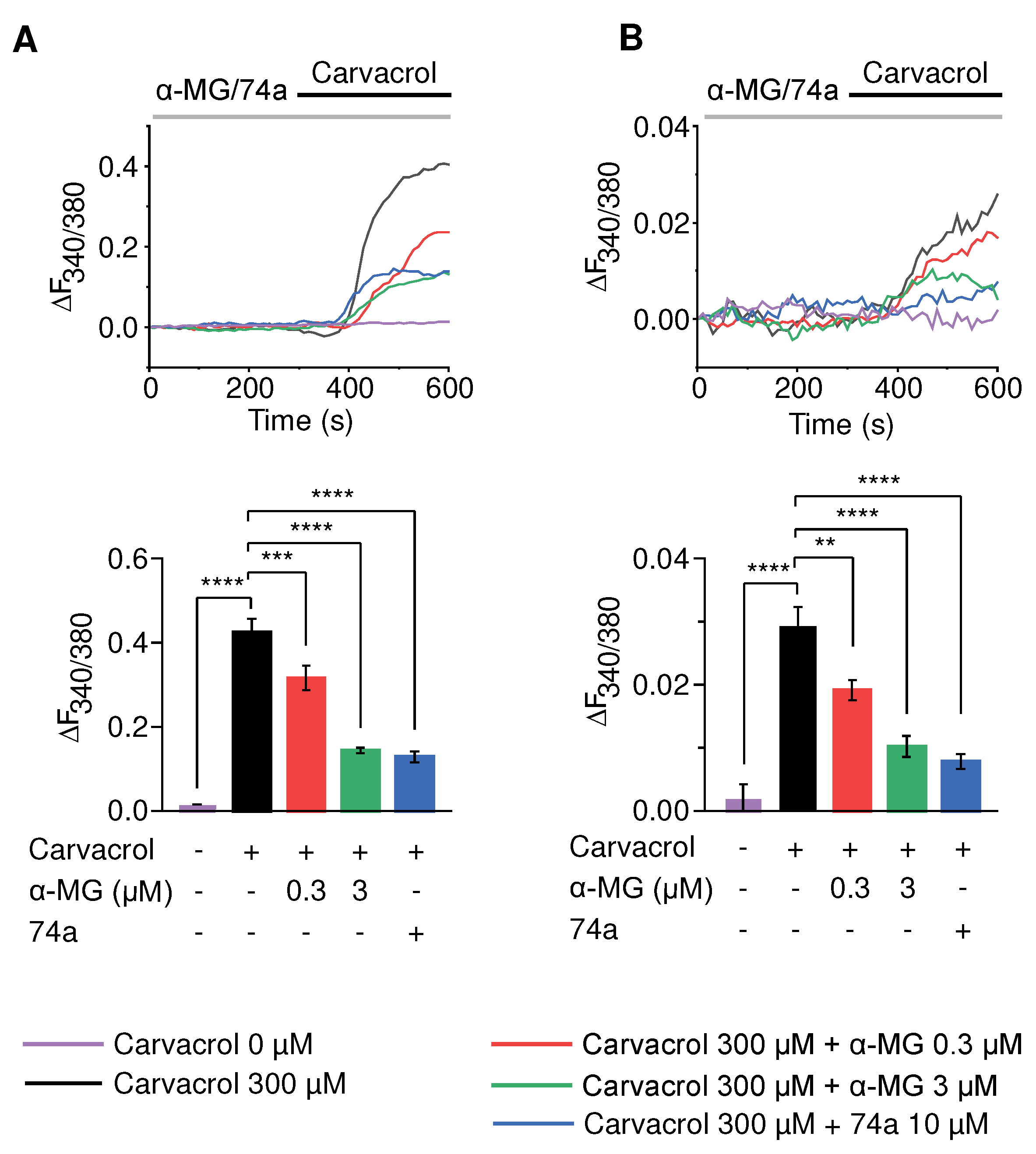
2.3. Alpha-mangostin noticeably inhibits Ca2+ influx mediated through TRPV3 channels
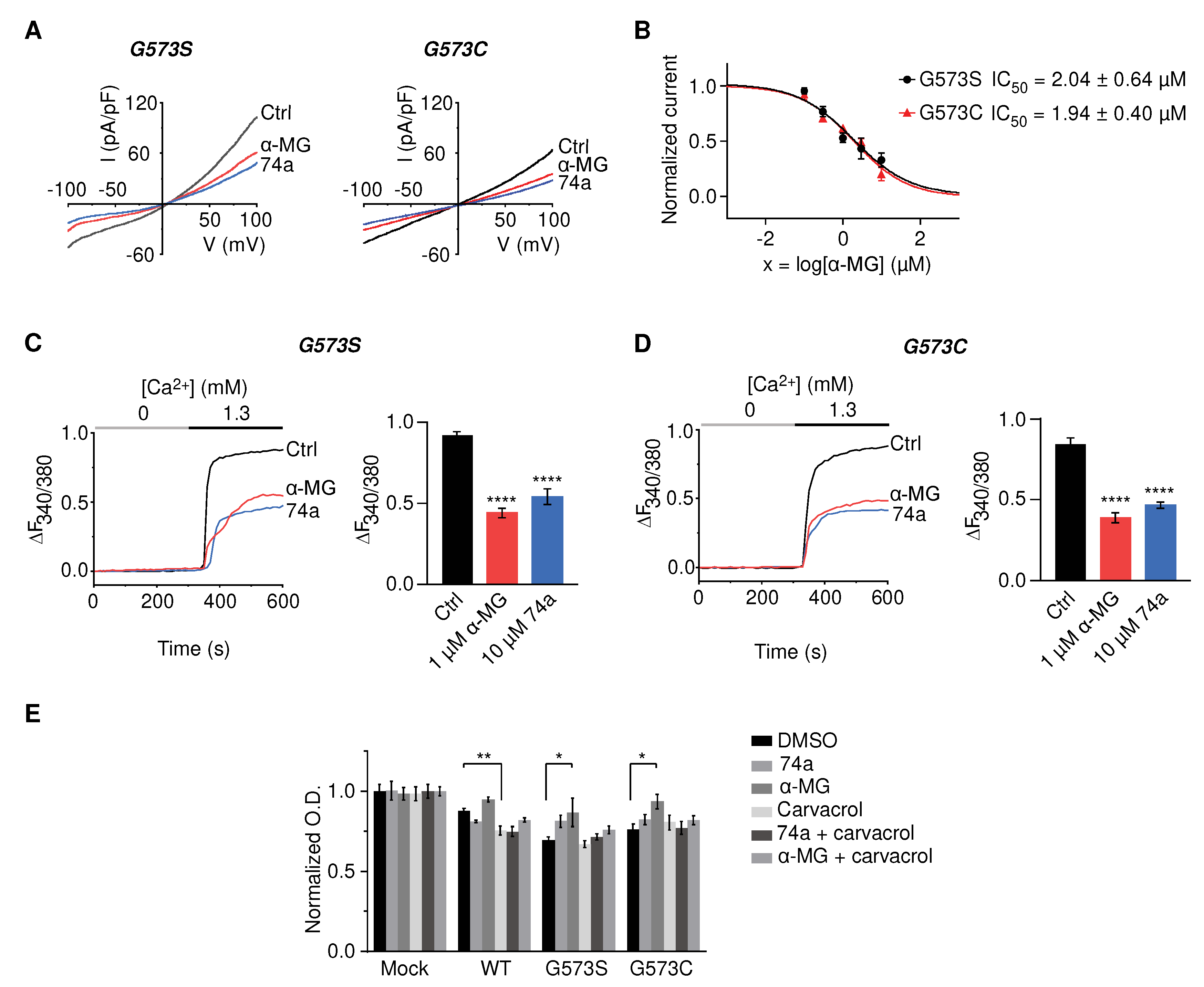
2.4. Alpha-mangostin inhibits channel activity and rescues HEK 293T cells transfected with TRPV3 mutations (G573S, G573C) from cell death
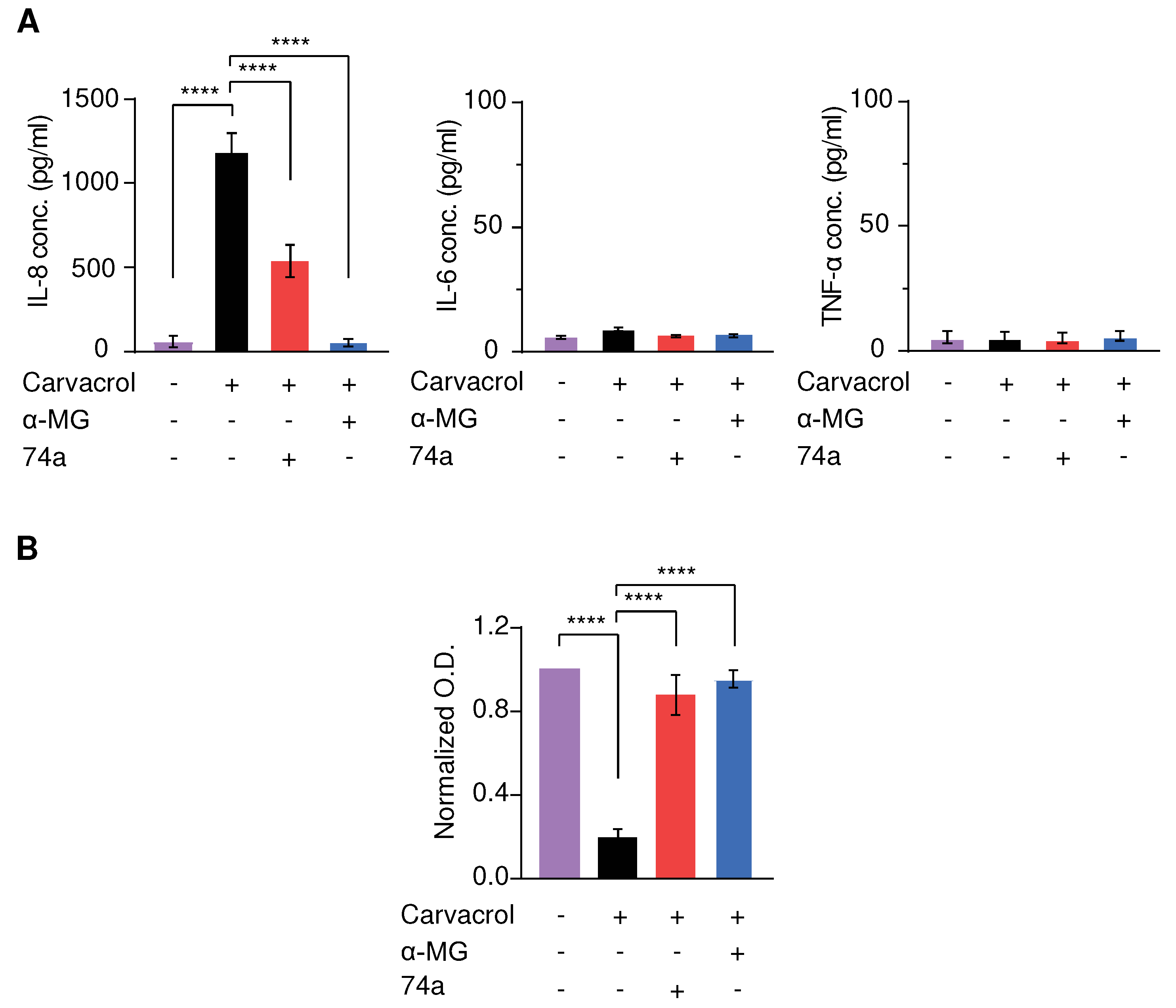
2.5. Alpha-mangostin inhibits cytokine release and rescues keratinocytes from cell death caused by TRPV3 agonists
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell culture
4.3. Construct of cDNA and transfection
4.4. Electrophysiological recording
4.5. Calcium imaging
4.6. Viability assay
4.7. Cytokine assay
4.8. Statistical analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lee, S.E.; Lee, S.H. Skin barrier and calcium. Ann Dermatol 2018, 30, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, H. Thermally activated TRPV3 channels. Curr Top Membr 2014, 74, 325–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Xue, C.; Chen, H.; Xue, Y.; Zhao, F.; Zhu, M.X.; Cao, Z. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol Toxicol 2021, 37, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.L.; Huang, S.M.; et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.Q.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J 2015, 29, 182–192. [Google Scholar] [CrossRef]
- Borbíró, I.; Lisztes, E.; Tóth, B.I.; Czifra, G.; Oláh, A.; Szöllosi, A.G.; Szentandrássy, N.; Nánási, P.P.; Péter, Z.; Paus, R.; et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J Invest Dermatol 2011, 131, 1605–1614. [Google Scholar] [CrossRef]
- Singh, A.K.; McGoldrick, L.L.; Demirkhanyan, L.; Leslie, M.; Zakharian, E.; Sobolevsky, A.I. Structural basis of temperature sensation by the TRP channel TRPV3. Nat Struct Mol Biol 2019, 26, 994–998. [Google Scholar] [CrossRef]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of pain and itch by TRP channels. Neurosci Bull 2018, 34, 120–142. [Google Scholar] [CrossRef]
- Asakawa, M.; Yoshioka, T.; Matsutani, T.; Hikita, I.; Suzuki, M.; Oshima, I.; Tsukahara, K.; Arimura, A.; Horikawa, T.; Hirasawa, T.; Sakata, T. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol 2006, 126, 2664–2672. [Google Scholar] [CrossRef]
- Yoshioka, T.; Imura, K.; Asakawa, M.; Suzuki, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Horikawa, T.; Arimura, A. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol 2009, 129, 714–722. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, Q.; Lee, M.; Cao, X.; Zhang, J.; Ma, D.; Chen, L.; Hu, X.; Wang, H.; Wang, X.; et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet 2012, 90, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, G.; Sun, X.; Wang, K. Inhibition of the warm temperature-activated Ca(2+)-permeable transient receptor potential vanilloid TRPV3 channel attenuates atopic dermatitis. Mol Pharmacol 2019, 96, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qi, H.; Wu, H.; Qu, Y.; Wang, K. Antipruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca(2+)-permeable TRPV3 channel. J Dermatol Sci 2020, 97, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Szöllősi, A.G.; Vasas, N.; Angyal, Á.; Kistamás, K.; Nánási, P.P.; Mihály, J.; Béke, G.; Herczeg-Lisztes, E.; Szegedi, A.; Kawada, N.; et al. Activation of TRPV3 regulates inflammatory actions of human epidermal keratinocytes. J Invest Dermatol 2018, 138, 365–374. [Google Scholar] [CrossRef]
- Zhao, J.; Munanairi, A.; Liu, X.Y.; Zhang, J.; Hu, L.; Hu, M.; Bu, D.; Liu, L.; Xie, Z.; Kim, B.S.; et al. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J Invest Dermatol 2020, 140, 1524–1532. [Google Scholar] [CrossRef]
- Yamamoto-Kasai, E.; Imura, K.; Yasui, K.; Shichijou, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Yoshioka, T. TRPV3 as a therapeutic target for itch. J Invest Dermatol 2012, 132, 2109–2112. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab J Chem 2016, 9, 317–329. [Google Scholar] [CrossRef]
- Mohan, S.; Syam, S.; Abdelwahab, S.I.; Thangavel, N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: An in silico, in vitro and in vivo approach. Food Funct 2018, 9, 3860–3871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yotsumoto, H.; Tian, Y.; Sakamoto, K. α-mangostin suppressed melanogenesis in B16F10 murine melanoma cells through GSK3β and ERK signaling pathway. Biochem Biophys Rep 2021, 26, 100949. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Tanaka, A.; Nishikawa, S.; Oida, K.; Matsuda, A.; Jung, K.; Amagai, Y.; Matsuda, H. Suppressive effect of mangosteen rind extract on the spontaneous development of atopic dermatitis in NC/Tnd mice. J Dermatol 2013, 40, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Kono, T.; Sauder, D.N.; McKenzie, R.C. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol 1993, 101, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Deng, W.; He, G.; Gan, X.; Gao, S.; Chen, Y.; Gao, Y.; Xu, K.; Qi, J.; Lin, H.; et al. Alpha- and gamma-mangostins exhibit anti-acne activities via multiple mechanisms. Immunopharmacol Immunotoxicol 2018, 40, 415–422. [Google Scholar] [CrossRef]
- Nilius, B.; Bíró, T. TRPV3: A ‘more than skinny’ channel. Exp Dermatol 2013, 22, 447–452. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Humeau, M.; Boniface, K.; Bodet, C. Cytokine-mediated crosstalk between keratinocytes and T cells in atopic dermatitis. Front Immunol 2022, 13, 801579. [Google Scholar] [CrossRef]
- Su, W.; Qiao, X.; Wang, W.; He, S.; Liang, K.; Hong, X. TRPV3: Structure, diseases and modulators. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol 2012, 132, 1253–1262. [Google Scholar] [CrossRef]
- Scott, V.E.; Patel, H.; Wetter, J.; Edlmayer, R.; Neelands, T.; Miller, L.; Huang, S.; Gauld, S.; Todorovic, V.; Gomtsian, A.; et al. 534 Defining a mechanistic link between TRPV3 activity and psoriasis through IL-1α and EGFR signaling pathways. J Invest Dermatol 2016, 136, S 94, 534. [Google Scholar] [CrossRef]
- Um, J.Y.; Kim, H.B.; Kim, J.C.; Park, J.S.; Lee, S.Y.; Chung, B.Y.; Park, C.W.; Kim, H.O. TRPV3 and Itch: The Role of TRPV3 in Chronic pruritus according to Clinical and Experimental Evidence. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yin, M.Z.; Roh, J.W.; Kim, H.J.; Choi, S.W.; Wainger, B.J.; Kim, W.K.; Kim, S.J.; Nam, J.H. Multi-target modulation of ion channels underlying the analgesic effects of α-mangostin in dorsal root ganglion neurons. Phytomedicine 2023, 115, 154791. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luo, A.; Kamau, P.M.; Takomthong, P.; Hu, J.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br J Pharmacol 2021, 178, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm Sin B 2022, 12, 723–734. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Qi, H.; Ma, Q.; Zhou, Q.; Wang, W.; Wang, K. Pharmacological inhibition of the temperature-sensitive and Ca2+-permeable transient receptor potential vanilloid TRPV3 channel by natural forsythoside B attenuates pruritus and cytotoxicity of keratinocytes. J Pharmacol Exp Ther 2019, 368, 21–31. [Google Scholar] [CrossRef]
- Sun, X.Y.; Sun, L.L.; Qi, H.; Gao, Q.; Wang, G.X.; Wei, N.N.; Wang, K. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol Pharmacol 2018, 94, 1164–1173. [Google Scholar] [CrossRef]
- Yang, N.N.; Shi, H.; Yu, G.; Wang, C.M.; Zhu, C.; Yang, Y.; Yuan, X.L.; Tang, M.; Wang, Z.L.; Gegen, T.; et al. Osthole inhibits histamine-dependent itch via modulating TRPV1 activity. Sci Rep 2016, 6, 25657. [Google Scholar] [CrossRef]
- Korolkova, Y.; Makarieva, T.; Tabakmakher, K.; Shubina, L.; Kudryashova, E.; Andreev, Y.; Mosharova, I.; Lee, H.S.; Lee, Y.J.; Kozlov, S. Marine cyclic guanidine alkaloids Monanchomycalin B and Urupocidin A act as inhibitors of TRPV1, TRPV2 and TRPV3, but not TRPA1 receptors. Mar Drugs 2017, 15. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Ogurtsova, E.K.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Tabakmakher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Lee, H.S.; et al. Pulchranins B and C, new acyclic guanidine alkaloids from the Far-Eastern marine sponge Monanchora pulchra. Nat Prod Commun 2013, 8, 1229–1232. [Google Scholar] [CrossRef]
- Larkin, C.; Chen, W.; Szabó, I.L.; Shan, C.; Dajnoki, Z.; Szegedi, A.; Buhl, T.; Fan, Y.; O’Neill, S.; Walls, D.; et al. Novel insights into the TRPV3-mediated itch in atopic dermatitis. J Allergy Clin Immunol 2021, 147, 1110–1114e5. [Google Scholar] [CrossRef]
- Pan-In, P.; Wongsomboon, A.; Kokpol, C.; Chaichanawongsaroj, N.; Wanichwecharungruang, S. Depositing α-mangostin nanoparticles to sebaceous gland area for acne treatment. J Pharmacol Sci 2015, 129, 226–232. [Google Scholar] [CrossRef]
- Yamanoi, Y.; Lei, J.; Takayama, Y.; Hosogi, S.; Marunaka, Y.; Tominaga, M. TRPV3-ANO1 interaction positively regulates wound healing in keratinocytes. Commun Biol 2023, 6, 88. [Google Scholar] [CrossRef]
- Tan, Y.F.; Koay, Y.S.; Zulkifli, R.M.; Abdul Hamid, M. In vitro hair growth and hair tanning activities of mangosteen pericarp extract on hair dermal papilla cells. J Herb Med 2022, 36. [Google Scholar] [CrossRef]
- Nam, J.H.; Jung, H.W.; Chin, Y.W.; Yang, W.M.; Bae, H.S.; Kim, W.K. Spirodela polyrhiza extract modulates the activation of atopic dermatitis-related ion channels, Orai1 and TRPV3, and inhibits mast cell degranulation. Pharm Biol 2017, 55, 1324–1329. [Google Scholar] [CrossRef]
- Kim, H.J.; Nam, Y.R.; Nam, J.H. Flos Magnoliae inhibits chloride secretion via ANO1 inhibition in Calu-3 cells. Am J Chin Med 2018, 46, 1079–1092. [Google Scholar] [CrossRef]
- Woo, J.; Kim, H.J.; Nam, Y.R.; Kim, Y.K.; Lee, E.J.; Choi, I.; Kim, S.J.; Lee, W.; Nam, J.H. Mitochondrial dysfunction reduces the activity of KIR2.1 K(+) channel in myoblasts via impaired oxidative phosphorylation. Korean J Physiol Pharmacol 2018, 22, 697–703. [Google Scholar] [CrossRef]
- Martínez, M.; Martínez, N.A.; Silva, W.I. Measurement of the intracellular calcium concentration with Fura-2 AM using a fluorescence plate reader. Bio Protoc 2017, 7, e2411. [Google Scholar] [CrossRef]
- Tinning, P.W.; Franssen, A.J.P.M.; Hridi, S.U.; Bushell, T.J.; McConnell, G.A. 340/380 nm light-emitting diode illuminator for Fura-2 AM ratiometric Ca(2+) imaging of live cells with better than 5 nM precision. J Microsc 2018, 269, 212–220. [Google Scholar] [CrossRef]
- Woo, J.H.; Nam, D.Y.; Kim, H.J.; Hong, P.T.L.; Kim, W.K.; Nam, J.H. Nootkatol prevents ultraviolet radiation-induced photoaging via ORAI1 and TRPV1 inhibition in melanocytes and keratinocytes. Korean J Physiol Pharmacol 2021, 25, 87–94. [Google Scholar] [CrossRef]
| Natural compound | Channel inhibited | IC50 (µM) |
|---|---|---|
| Alpha-mangostin | TRPV3 | 0.077 ± 0.013 |
| TRPV1 | 0.43 ± 0.27 | |
| TRPV4 | 8.470 ± 1.725 | |
| TRPA1 | 5.092 ± 2.213 | |
| Citrusinine II [34] | TRPV3 | 12.43 ± 1.86 |
| Isochlorogenic acid A [35] | TRPV3 | 2.7 ± 1.3 |
| Isochlorogenic acid B [35] | TRPV3 | 0.9 ± 0.3 |
| Forsythoside B [36] | TRPV3 | 6.7 ± 0.7 |
| Verbascoside [14] | TRPV3 | 14.1 ± 3.3 |
| Coumarin osthole [37,38] | TRPV3 | 37.0 ± 1.9 |
| TRPV1 | - | |
| Monanchomycalin B [39] | TRPV3 | 3.25 ± 0.6 |
| TRPV1 | 6.02 ± 0.36 | |
| TRPV2 | 2.84 ± 1.01 | |
| Pulchranin A [40] | TRPV3 | 71.8 ± 9.4 |
| TRPV1 | 27.5 ± 1.4 | |
| TRPA1 | 174.2 ± 7.4 | |
| Pulchranin B [40] | TRPV3 | 117.9 ± 11.8 |
| TRPV1 | 95.4 ± 13.1 | |
| TRPA1 | > 200 | |
| Pulchranin C [40] | TRPV3 | >200 |
| TRPV1 | 182.7 ± 27.0 | |
| TRPA1 | > 200 |
| Ion channel | Bath solution (in mM) | Pipette solution (in mM) |
|---|---|---|
| TRPV3 | 139 NaCl, 5 KCl, 1.8 BaCl2, 2 MgCl2, 10 glucose, 10 sorbitol, and 10 HEPES (pH 7.4, adjusted with NaOH) | 120 CsCl, 2 MgCl2, 3 Mg-ATP, 10 EGTA, 10 HEPES, and 3.15 CaCl2 (equivalent to 150 nM free Ca2+, calculated by the free Webmax software) (pH 7.2, adjusted with CsOH) |
| TRPV3 mutants | 120 CsCl, 2 MgCl2, 3 Mg-ATP, 10 EGTA, 10 HEPES (pH 7.2, adjusted with CsOH) | |
| TRPV4 | 145 NaCl, 3.6 KCl, 10 HEPES, 5 glucose, 1 MgCl2, 1.3 CaCl2 (pH 7.4, adjusted with NaOH) | 140 CsCl, 1 MgCl2, 5 HEPES, 10 EGTA, 3 Mg-ATP (pH 7.2, titrated by CsOH) |
| TRPA1 | 150 CsCl, 10 BAPTA, 10 HEPES, 1 MgCl2 (pH 7.2, adjusted by CsOH) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





